Introduction
Endometriosis (Ems) is a common gynecological
disease that manifests in ~5-20% of women experiencing pelvic pain,
20-50% of infertile women and 6-10% of women of reproductive age
(1). Despite major developments
in the treatment of Ems over the past decades (2), such as laparoscopic surgery, the
recurrence rate remains high. The presence of functional
endometrial-like tissues outside the uterine cavity is the main
pathological characteristic of this disease, and the major reason
for its formation is the excessive invasiveness of endometrial
stromal cells (ESCs) (3).
Therefore, therapeutic strategies that inhibit the invasion of ESCs
may be beneficial in Ems.
MicroRNAs (miRNAs) are single-stranded non-coding
RNAs that bind to the target mRNAs and interfere with the
translation process (4).
Aberrantly expressed miRNAs have been found in endometriotic
tissues from patients with Ems, suggesting that miRNAs may play
important roles in the development of Ems (5,6).
For example, Liang et al demonstrated that miR-200c was
downregulated in ectopic endometrial tissues, and overexpression of
miR-200c suppressed the growth of endometriotic lesions in a rat
model of Ems (7). Notably,
several studies have demonstrated that the proliferative and
invasive abilities of ESCs are regulated by miRNAs. Meng et
al observed that inhibition of miR-126-5p enhanced the
migration and invasion of ESCs by promoting the expression of
breast cancer anti-estrogen resistance protein 3 (8). Zhang et al reported that
miR-141-3p overexpression suppressed the proliferation and
migration, and promoted the apoptosis of ESCs via targeting
Krüppel-like factor 12 (9);
however, the effects of miRNAs on the invasiveness of ESCs remain
elusive.
A large number of studies have demonstrated the
involvement of the nuclear factor (NF)-κB signaling pathway in the
modulation of the invasion of ESCs in Ems (10,11). Inhibitor of NF-κB kinase subunit β
(IKKβ) is an important regulator of the NF-κB pathway, in which
IKK-mediated phosphorylation of IκB may facilitate NF-κB entering
the nucleus and activating the expression of specific genes
(12). It has been previously
reported that miRNAs are key regulators of ESC function through the
NF-κB signaling pathway. For example, Zhang et al
demonstrated that miR-138 expression affected the growth of ESCs
through the NF-κB signaling pathway (13). However, whether miRNAs affect the
invasive ability of ESCs through the IKKβ/NF-κB pathway remains
largely unknown.
In the present study, the miRNA expression profile
was examined in endometriotic tissues from patients with Ems. The
functions of miR-16 in suppressing the invasiveness of ESCs were
determined, and the potential underlying mechanism was
investigated. Taken together, these findings may provide
theoretical evidence for the development of new treatment methods
targeting miRNAs for Ems treatment.
Materials and methods
Patients and samples
A total of 20 eutopic and ectopic endometrial
tissues were collected from patients who underwent laparoscopic
surgery or hysterectomy at the Department of Gynecology of the
Maternal and Child Care Service Center of Dongguan City Guangdong
Province between October 2016 and October 2017. Control endometrium
was obtained from 20 patients without Ems. All participants were
legally considered adults, and no minors were included in the
present study. Written informed consent was obtained from each
patient and the protocol of the present study was approved by the
Ethics Committee of the Maternal and Child Care Service Center of
Dongguan City Guangdong Province.
Cell culture and transfection
Primary endometrial stromal cells were isolated
using three eutopic endometrial tissues, as previously described
(14). The cells were maintained
in DMEM/F12 supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) in a 5% CO2 incubator at 37°C.
When cells in 6-well plates had grown to ~80%
confluence, miR-16 mimics (20 nmol/l), miR-16 inhibitor (20
nmol/l), si-IKKβ (30 nM) or 2 µg pcDNA-IKKβ were transfected
into cells at 37°C for 48 h, using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). The miR-16 mimics,
mimics negative control (NC), miR-16 inhibitor and inhibitor NC
were obtained from RiBoBio Co., Ltd. IKKβ overexpressing vector
pcDNA-IKKβ and pcDNA vector were constructed by Qiagen, Inc. In
addition, IKKβ siRNA-1, -2, -3 and corresponding negative control
siRNA (si-Scramble) were purchased from RiboBio Co., Ltd.
miRNA microarray
Total RNA was extracted from three eutopic and three
ectopic endometrium tissues by miRNeasy isolation kit (Qiagen,
Inc.) according to the manufacturer's protocol. Total RNA (200 ng)
was labeled with fluorescence dye Hy3 or Hy5 using the miRCURY
Hy3/Hy5 Power Labeling kit (Exiqon). After hybridization on the
miRCURY™ LNA Array (v.18.0, Exiqon), data obtained from Axon
GenePix 4000B microarray scanner (Axon Instruments; Molecular
Devices, LLC) were imported into the GenePix Pro 6.0 program (Axon
Instruments; Molecular Devices, LLC) for analysis (15). The bioinformatics analysis was
performed by RiboBio Co., Ltd. Finally, the heatmap of miRNAs with
the most marked differences was created using hierarchical
clustering in GeneSpring GX software, version 7.3 (Agilent
Technologies, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from endometrial tissues and
cells using the TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Reverse transcription of miR-16 and IKKβ was
performed using the PrimeScript RT reagent kit (Takara Bio, Inc.)
and a reverse transcription kit (Invitrogen; Thermo Fisher
Scientific, Inc.), respectively, at 42°C for 1 h. miR-16 and IKKβ
expressions were measured using the Exiqon SYBR Green Master Mix
(Exiqon) on a LightCycler 480 instrument (Roche Diagnostics). The
primers used were as follows: miR-16 F:
5′-TAGCAGCACGTAAATATTGGCG-3′ and R: 5′-TGCGTGTCGTGGAGTC-3′; U6 F:
5′-TGCGGGTGCTCGCTTCGCAGC-3′ and R: 5′-CCAGTGCAGGGTCCGAGGT-3′; IKKβ
F: 5′-TGTCAGTGGAAGCCCGGATAG-3′ and R:
5′-AGGTTATGTGCTTCAGCCACCAG-3′; and GAPDH F:
5′-AGGTCGGTGTGAACGGATTTG-3′ and R: 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
The reaction mixtures were denatured at 95°C for 3 min, followed by
40 two-step cycles of 95°C for 10 sec and 60°C for 30 sec. The
expression of miR-16 and IKKβ in the tissues was normalized to the
expression of U6 and GAPDH, respectively. The RT-qPCR assays were
performed in triplicate and the relative expression levels were
calculated based on the 2−ΔΔCq method (16).
Cell invasion
Transwell chambers (8-µm pore; BD
Biosciences) coated with Matrigel (BD Biosciences) were used for
the invasion assay. Briefly, primary ESC suspension containing
8×104 cells was added in the top chamber with DMEM/F12,
while DMEM/F12 containing 20% FBS was added to the lower chamber.
After 24 h of incubation, the cells were stained with 0.1% crystal
violet solution for 10 min at room temperature and photographed
with an inverted micro-scope (IX71, Olympus Corporation) at a
magnification of ×200.
Wound healing assay
After achieving 90% confluence, the transfected
primary ESC layer was scratched using a 10-µl pipette tip
and the cells were cultured for another 24 h in DMEM/F12.
Subsequently, the wound area was captured at 0 and 24 h, and the
distance between the edges of the scratch was calculated using
ImageJ software, version 1.46 (Rawak Software, Inc.).
Bioinformatics analysis and dual
luciferase reporter assay
miRNA target prediction tools, including TargetScan
7.0 (http://targetscan.org/) and miRanda
(http://miranda.org) were used to search for the
putative targets of miR-16. A sequence containing miR-16 predicted
target within the IKKβ 3′-untranslated region (UTR)
CUCUUUUUAUUUCACUGCUGCUA or a mutant sequence
lacking any complementarity with the miR-16 seed sequence
CUCUUUUUAUUUCACACAGCAGA were inserted into pGL3
control vector (Promega Corporation) to generate the reporter
vector pGL3-IKKβ 3′-UTR wild-type (wt) and pGL3-IKKβ 3′-UTR mutant
(mut). When ESCs reached 60-70% conf1uence in a 24-well plate, 100
ng of Luciferase plasmid was co-transfected with 50 ng of
Renilla plasmid (Ambion) and 650 ng of miR-16 mimics or
mimics NC using Lipofectamine 2000. At 48 h post-transfection, the
luciferase activities were analyzed using the Dual Luciferase
Reporter Assay system (Promega Corporation).
Western blot analysis
Western blotting was performed as previously
described (17). Briefly, 40
µg extracted protein samples were separated by 12% SDS-PAGE
(w/v) and transferred onto a PVDF membrane (EMD Millipore).
Subsequently, the membranes were blocked with 5% skimmed milk for 2
h at room temperature, followed by incubation with primary
anti-bodies against IKKβ (cat. no. ab178870; 1:500, Abcam), IκB-α
(cat. no. sc-373893; 1:1,000, Santa Cruz Biotechnology, Inc.),
p-IκB-α (cat. no. 2859; 1:1,000, Cell Signaling Technologies,
Inc.), anti-nuclear p-p65 (cat. no. 3033; 1:1,000, Cell Signaling
Technologies, Inc.), anti-histone H3 (cat. no. sc-8654; 1:1,000,
Santa Cruz Biotechnology, Inc.) and β-actin antibody (cat. no.
4970; 1:1,000, Cell Signaling Technologies, Inc.) at 4°C overnight,
followed by HRP-conjugated goat anti-rabbit IgG (cat. no. 205718;
1:10,000, Abcam). The protein bands were developed using an ECL kit
(Cytiva) and blot bands were quantified with ImageJ software,
version 1.46 (Rawak Software, Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism, version 5.0 (GraphPad Software, Inc.). Data are presented as
means ± standard deviation. When only two groups were compared,
Wilcoxon test was conducted. Differences between multiple groups
were analyzed using one-way ANOVA followed by Tukey's post hoc
test. To determine the correlation between the expression of miR-16
and IKKβ, the data were analyzed using Spearman's analysis.
P<0.05 was considered to indicate statistically significant
differences.
Results
miR-16 is downregulated in ectopic
endometrium
A number of studies have confirmed the aberrant
expression patterns of miRNAs in ectopic as well as eutopic
endometrial tissues from patients with Ems, which are different
compared with those of healthy individuals (18,19). Therefore, using a micro-array
assay, we analyzed the differentially expressed miRNAs between
eutopic and ectopic endometrial tissues from patients with Ems. The
results demonstrated that 24 miRNAs were downregulated and 35
miRNAs were upregulated in ectopic endometrial tissues compared
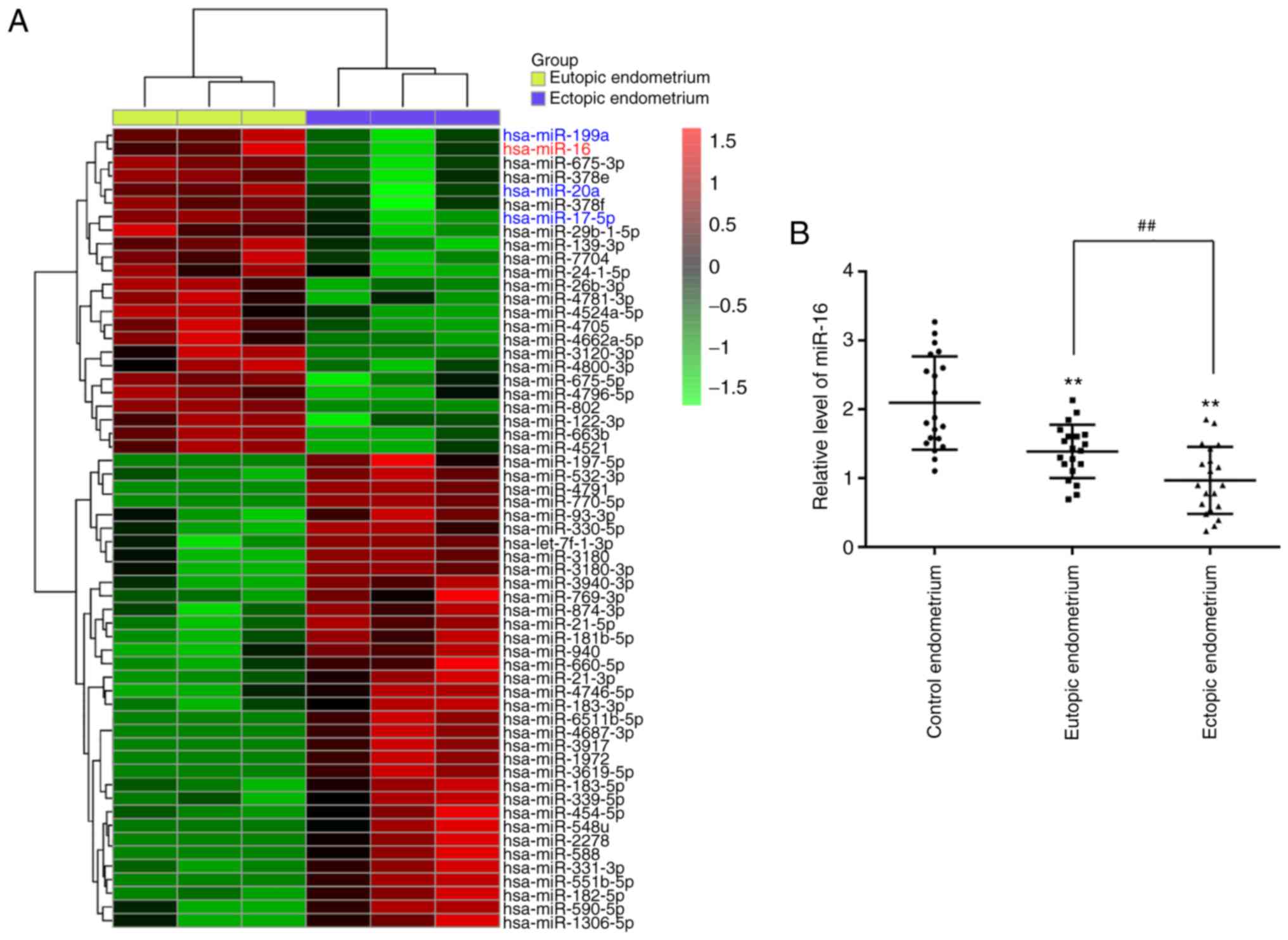
with eutopic endometrial tissues (Fig. 1A). Interestingly, some miRNAs,
including miR-16, miR-199a, miR-20a and miR-17-5p, have also been
found to be downregulated in previous studies, consistently with
our results (20-22). In the present study, miR-16 was
selected for further investigation as its expression level was the
lowest in the ectopic endometrium. Moreover, previous studies
indicated that miR-16 participates in the regulation of cancer cell
invasiveness (23-25); however, the effect of miR-16 on
the invasiveness of ESCs remains unknown.
Next, RT-qPCR was performed to verify the results of
the miRNA microarray assay in 20 paired eutopic and ectopic
endometrial tissues from patients with Ems. As shown in Fig. 1B, miR-16 was also found to be
downregulated in eutopic and ectopic endometrial tissues from
patients with Ems compared with the control endometrium group. In
addition, miR-16 was found to be significantly lower in ectopic
endometrial tissues compared with eutopic endometrial tissues. All
data suggest that miR-16 may play a key role in the pathogenesis of
Ems.
miR-16 inhibits the invasion and
migration of ESCs
Previous studies have reported that the invasion of
ESCs is a major factor leading to the formation of endometriotic
lesions (26,27). To investigate the role of miR-16
in the invasiveness of ESCs, miR-16 mimics and miR-16 inhibitor
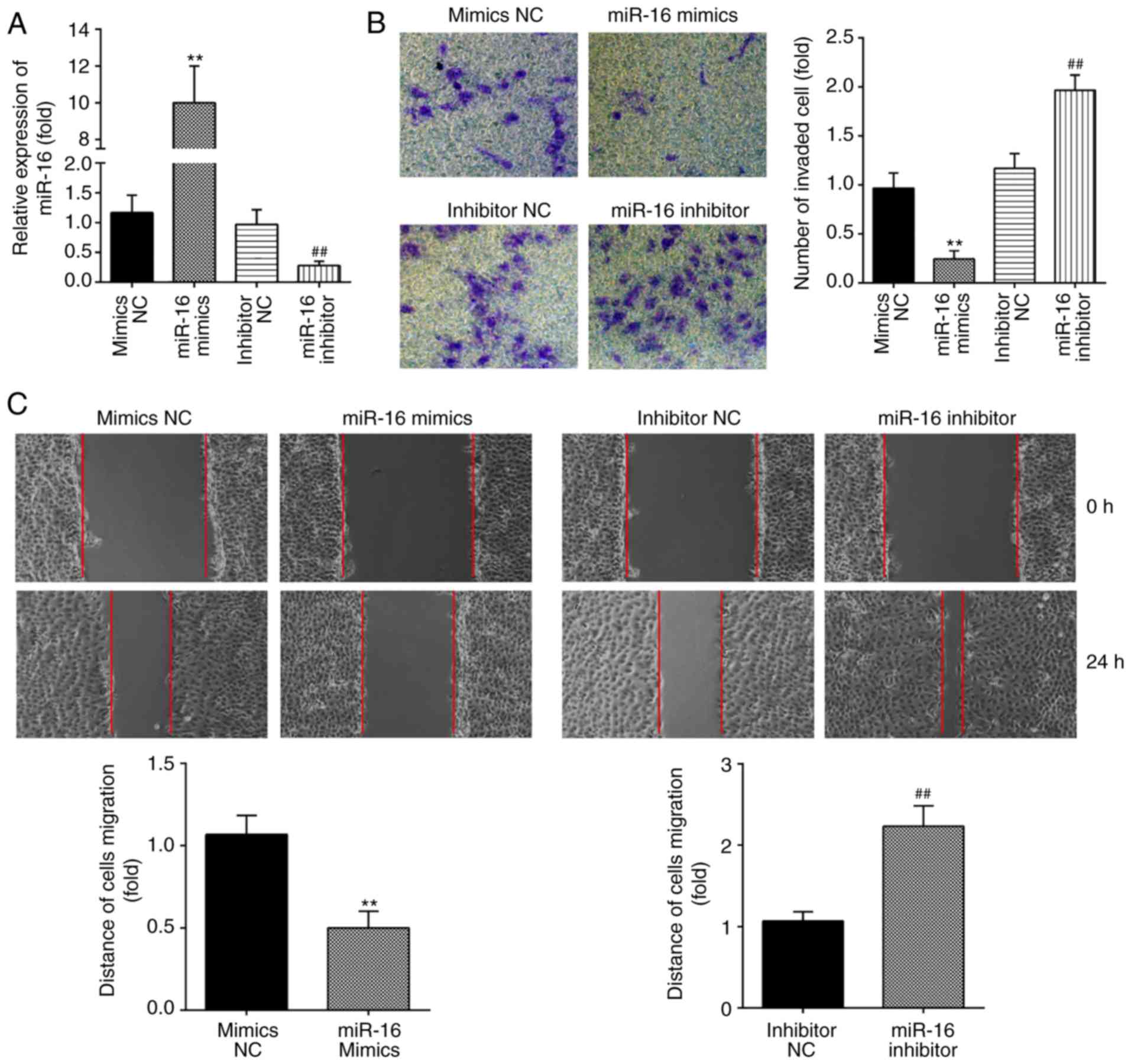
were added to primary ESCs, and RT-qPCR was performed to evaluate
the transfection efficiency. As shown in Fig. 2A, the levels of miR-16 were
markedly increased following miR-16 mimics transfection, and
decreased following miR-16 inhibitor transfection. Subsequently,
Transwell and wound healing assays were performed to assess the
changes in the invasive and migratory abilities of ESCs. It was
observed that miR-16 overexpression markedly suppressed the
invasive and migratory abilities of ESCs. By contrast, inhibition
of miR-16 enhanced the migration and invasion of ESCs (Fig. 2B and C). Collectively, these
findings indicate that miR-16 upregulation suppresses the
invasiveness of ESCs.
IKKβ is a direct target of miR-16
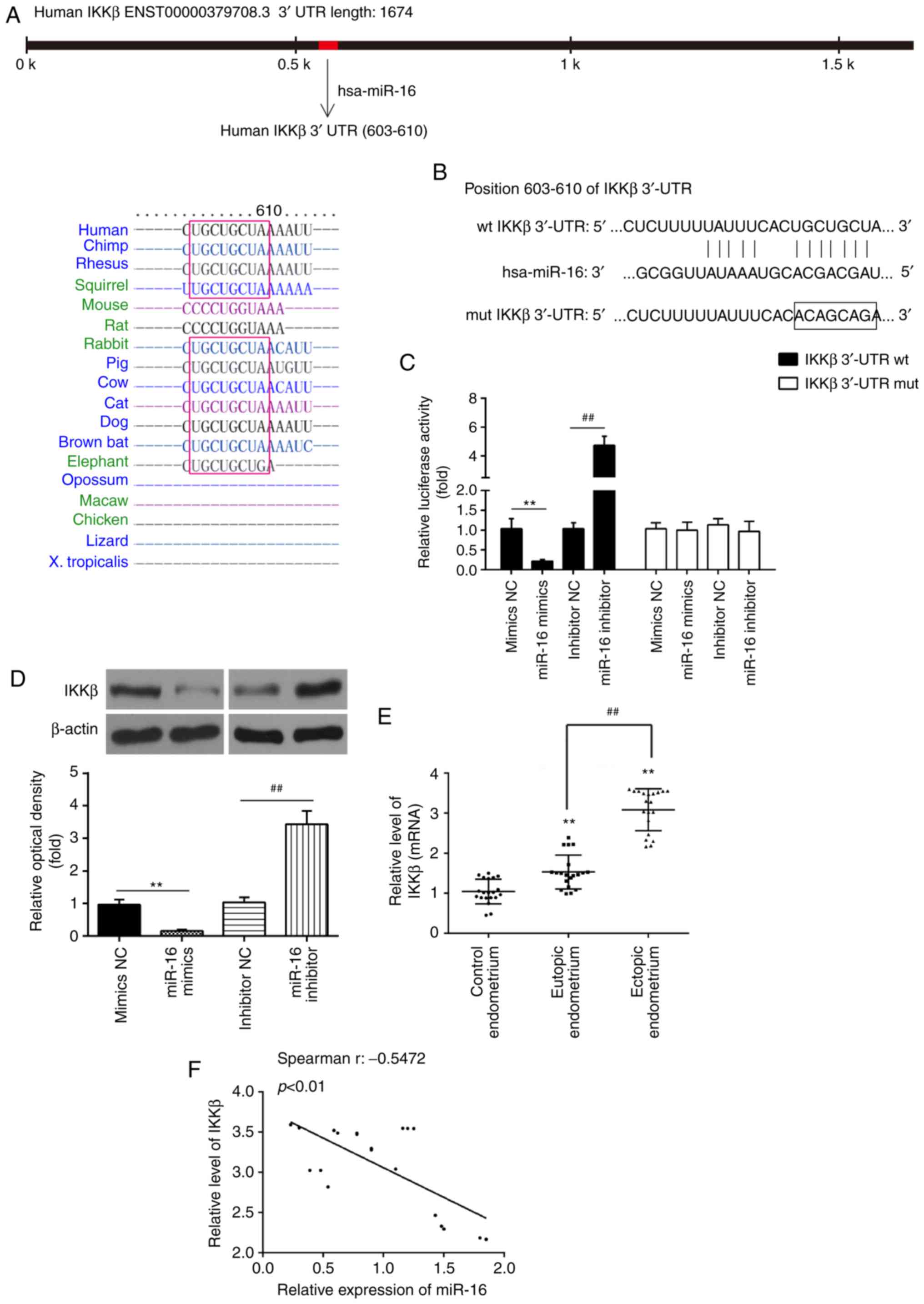
Through bioinformatics prediction using TargetScan
7.0 (http://targetscan.org/) and miRanda
(http://miranda.org), a putative target site of miR-16
was found in the 3′-UTR of IKKβ mRNA (Fig. 3A and B). To determine whether
miR-16 targets IKKβ, a luciferase reporter assay was performed. The
results revealed that miR-16 mimics markedly inhibited the
luciferase activity in the IKKβ-3′UTR-wt reporter, and miR-16
inhibitor caused increased luciferase activity; however, no change
was observed in cells with co-transfection of IKKβ 3′-UTR-mut with
miR-16 (Fig. 3C). To determine
whether IKKβ is regulated by miR-16, the protein level of IKKβ was
measured by western blotting. As shown in Fig. 3D, IKKβ was markedly decreased
following miR-16 mimics transfection, while its expression was
increased by miR-16 inhibitor in primary ESCs. In addition,
compared with control endometrial tissues, the mRNA level of IKKβ
was significantly increased in eutopic and ectopic endometrial
tissues (Fig. 3E). In addition,
IKKβ was found to be significantly higher in ectopic endometrial
tissues compared with its expression in eutopic endometrial
tissues. Spearman's analyses were used for correlation analysis and
the results indicated that miR-16 expression was inversely
correlated with IKKβ expression in ectopic endometrial tissues
(r=-0.5472, P<0.01; Fig. 3F).
These data indicate that miR-16 directly targets IKKβ and
suppresses its translation in primary ESCs.
Knockdown of IKKβ suppresses ESC
migration and invasion
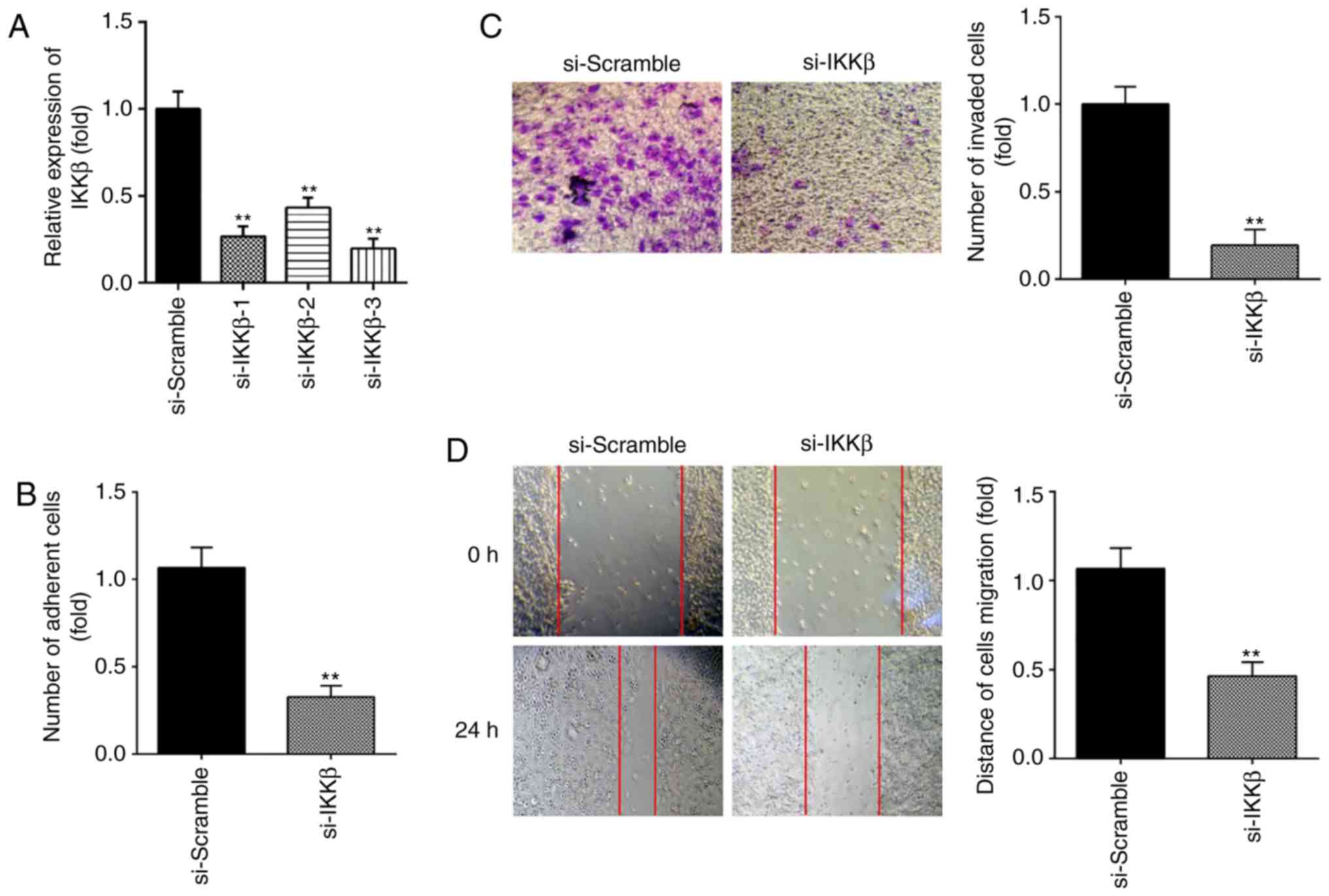
Next, to determine the effect of IKKβ on ESC
migration and invasion, si-IKKβ was transfected into primary ESCs
to downregulate the expression of IKKβ. As shown in Fig. 4A, IKKβ protein expression was
notably decreased following si-IKKβ transfection. si-IKKβ-3 was
selected in subsequent assays for its more effective inhibition. It
was observed that inhibition of IKKβ markedly suppressed the
invasive and migratory abilities of ESCs, similar to the effects of
miR-16 mimics (Fig. 4B-D).
Collectively, these findings indicate that knockdown of IKKβ
inhibits ESC migration and invasion.
miR-16 inhibits the invasion and
migration of ESCs by targeting IKKβ
As mentioned above, IKKβ knockdown inhibited ESC
migration and invasion; however, whether miR-16 inhibited ESC
invasion and migration via inhibiting IKKβ remained unclear. To
this end, the IKKβ overexpression vector pcDNA-IKKβ and miR-16
mimics were co-transfected into primary ESCs and incubated for 48
h. The results of western blotting demonstrated that IKKβ was
significantly upregulated in the miR-16 mimics + pcDNA-IKKβ group
compared with the miR-16 mimics group, whereas the expression level
of IKKβ in the pcDNA-IKKβ group was markedly higher compared with
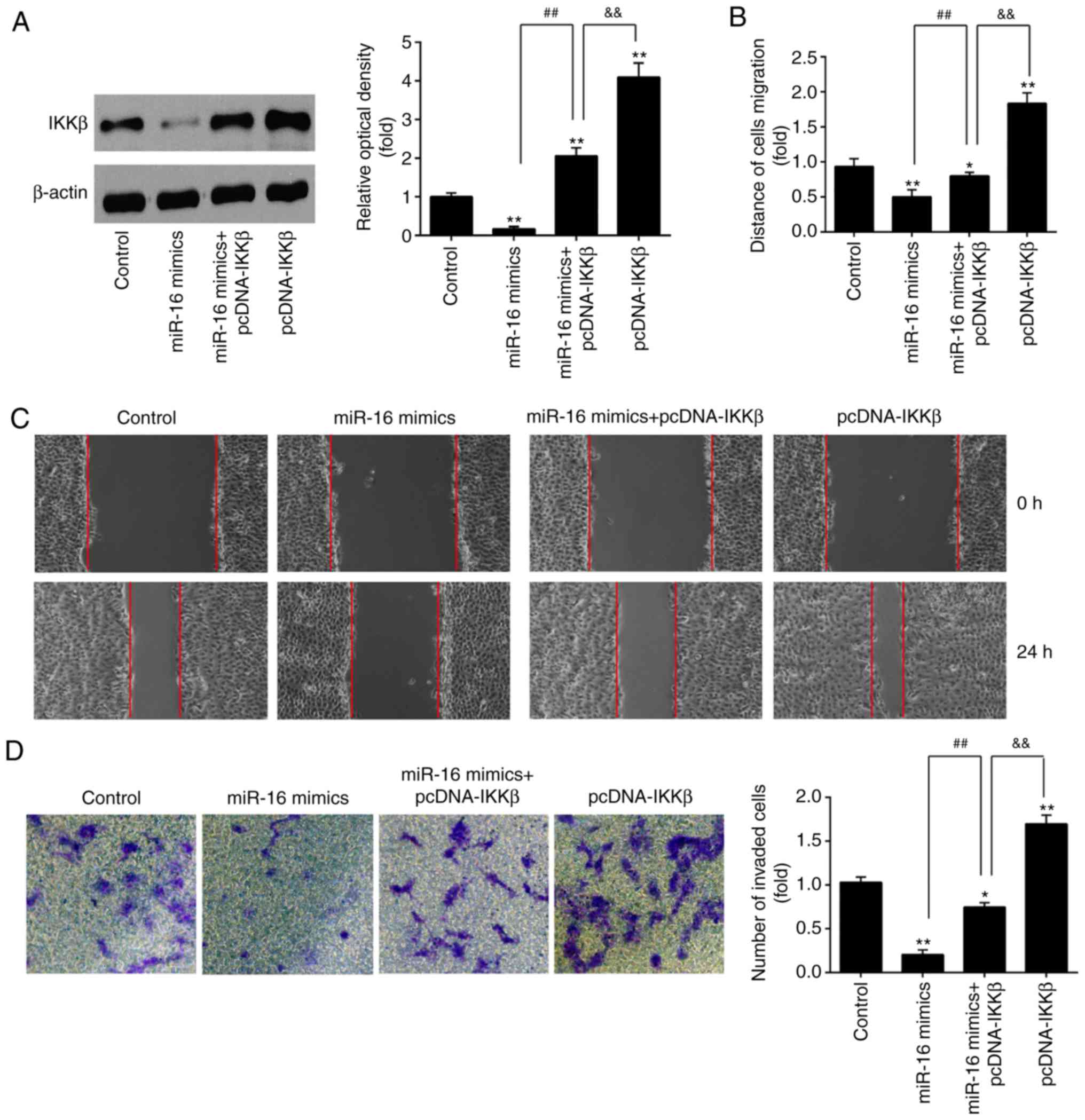
that in the miR-16 mimics + pcDNA-IKKβ group (Fig. 5A). These results demonstrated that
IKKβ over-expression reversed the inhibitory effects of miR-16
mimics on cell migration and invasion (Fig. 5B-D).
Furthermore, the effect of IKKβ overexpression on
the migration and invasion of ESCs was evaluated. It was observed
that the invasion and migration of ESCs was markedly enhanced in
the pcDNA-IKKβ alone group (Fig.
5B-D). However, following miR-16 mimics co-transfection, the
promoting effects of pcDNA-IKKβ on cell migration and invasion were
notably reversed. These data suggest that miR-16 may inhibit ESC
invasion and migration by suppressing IKKβ expression.
miR-16 blocks the activation of the NF-κB
pathway via suppression of IKKβ
It is well known that IKKβ is an important regulator
of the NF-κB pathway, and activation of NF-κB signaling is
implicated in ESC function, including cell invasion and
proliferation (28-30). To determine whether miR-16 affects
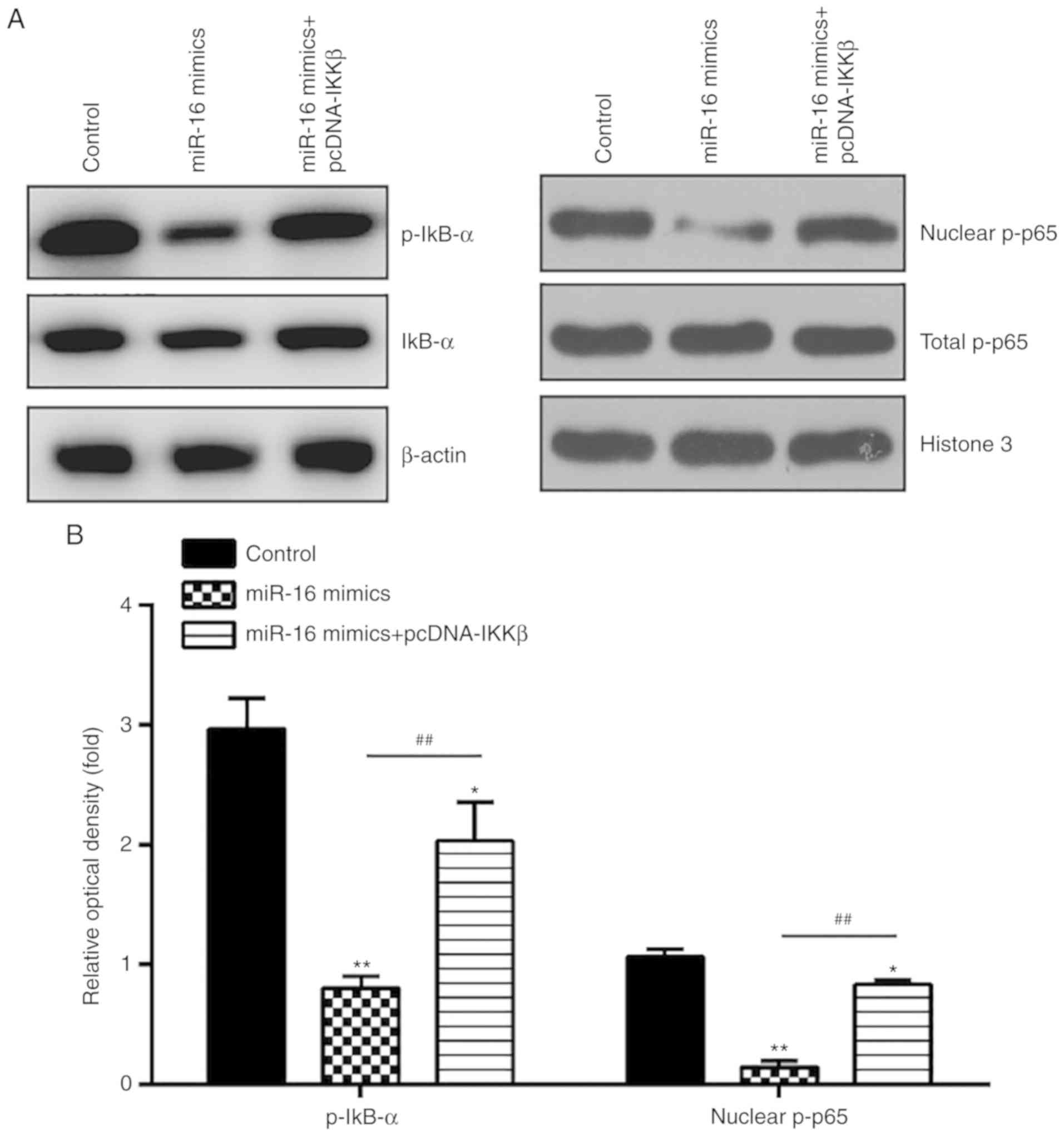
the activation of the NF-κB pathway in primary ESCs, the
expressions of key proteins in this pathway, namely nuclear p-p65,
p-IκB-α and IκB-α, were detected by western blotting. The results
demonstrated that miR-16 mimics significantly reduced the
expression levels of p-IκB-α and nuclear p-p65, whereas IKKβ
overexpression reversed the suppressive effects of miR-16 on this
pathway (Fig. 6A and B). These
data indicate that miR-16 inhibits NF-κB pathway activation by
suppressing IKKβ.
Discussion
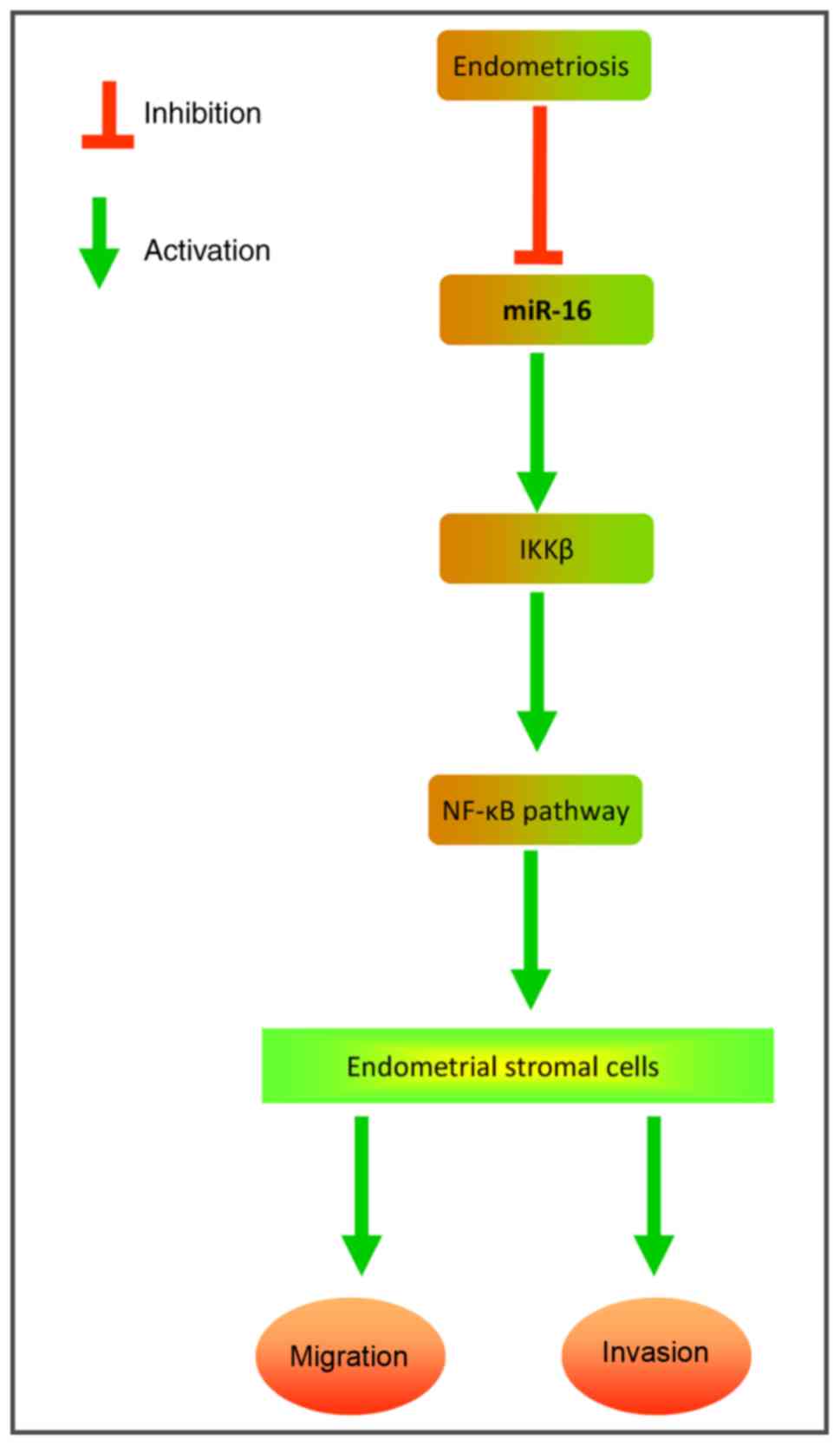
The present study demonstrated that miR-16 is
downregulated in eutopic and ectopic endometrial tissues from
patients with Ems compared with the endometrium of healthy
individuals. Moreover, miR-16 overexpression attenuated ESC
adhesion, migration and invasion in vitro. Notably, our data
demon-strated that the upregulation of miR-16 exerted anti-invasive
effects by suppressing the IKKβ/NF-κB pathway in primary ESCs
(Fig. 7). These findings suggest
that miR-16 may serve as a potential therapeutic target for the
management of Ems.
Several miRNAs have been previously identified to
contribute to the development of Ems (31-33). For example, Jia et al
reported that miR-17-5p, miR-20a and miR-22 are downregulated in
plasma samples from patients with Ems, and investigated their
prognostic value (20). Ma et
al observed that injection of miR-142-3p significantly
attenuated ectopic endometriotic lesions in vivo (34). Li et al also found that
miR-451a inhibition reduced established Ems lesions in a murine
model (35). Zhu et al
demonstrated that miR-488 plays a protective role in mice with
artificial Ems by targeting Frizzled-7 (36). All these findings outline a
potential molecular target for Ems treatment. In the present study,
using a miRNA microarray, a number of miRNAs were found to be
markedly deregulated. In particular, we focused on miR-16, the
expression of which was the most notably decreased in endometriotic
tissues, which was in agreement with a previous study (22), indicating that miR-16 may be
involved in the initiation and progression of Ems.
It has been demonstrated that increased adhesion and
invasion of ESCs are the major causes of the formation of
endometriotic lesions (37,38). Accumulating evidence has
demonstrated that miR-16 expression is downregulated in various
types of human cancer, and the invasion and migration of cancer
cells were suppressed by the upregulation of miR-16. For example,
Li et al observed that miR-16 overexpression exerts an
anti-invasive effect on ovarian cancer via the regulation of the
Wnt/β-catenin pathway (23). Jiao
et al demonstrated that miR-16 overexpression in
osteosarcoma cells suppressed their migration and invasion by
targeting RAB23 (25). However,
the effects of miR-16 on the migration and invasion of ESCs were
not extensively investigated. The present study demonstrated that
miR-16 upregulation markedly suppressed the migration and invasion
of primary ESCs. However, the precise mechanism remains
unclear.
IKKβ, one of the catalytic subunits of the IKK
complex, has been reported to be a critical regulator of the
activation of NF-κB signaling pathway (39). It has previously been demonstrated
that downregulating IKKβ activity may inhibit the activation of
NF-κB signaling and attenuate ESC migration and invasion (21). Although IKKβ was shown to be a
target of miR-16 in nasal epithelial cells (40), it has not been determined whether
IKKβ is also a direct target of miR-16 in ESCs. In the present
study, IKKβ was proven to be a target of miR-16, and its
translation was suppressed by miR-16 in ESCs. Additionally, it was
observed that the levels of IKKβ were upregulated and inversely
correlated with miR-16 levels in ectopic endometrial tissues.
Furthermore, the knockdown of IKKβ mimicked the effects of miR-16
overexpression on the migration and invasion of ESCs, whereas
overexpression of IKKβ markedly reversed these effects, indicating
that miR-16 may exert its anti-invasive effects by suppressing IKKβ
expression in ESCs. Numerous in vitro and in vivo
studies have suggested that NF-κB plays an important role in
regulating key cell processes in Ems, such as cell migration,
invasion and angiogenesis (41,42). Constitutive activation of the
NF-κB pathway has been found in peritoneal endometriotic lesions
and inhibition of NF-κB may reduce Ems development and persistence
(11,43). Given the association between IKKβ
and the NF-κB pathway, it is possible that miR-16 attenuates ESC
invasiveness partly through IKKβ/NF-κB pathway inhibition. Indeed,
it was observed that miR-16 overexpression decreased IκB-α
phosphorylation and lowered NF-κB nuclear translocation, which
suggests that miR-16 may block NF-κB pathway activation through
downregulating IKKβ protein expression; this may be the underlying
basis of miR-16-mediated suppression of ESC invasiveness.
It is generally accepted that the establishment and
development of Ems require retrograde menstruation or endometrial
fragments, which contain aberrantly activated of eutopic ESCs. This
is followed by the migration, invasion, implantation and
proliferation of these ESCs on the surface or inner part of pelvic
tissues or organs, which occurs simultaneously with a controlled
local inflammatory response and angiogenesis (44). Multiple studies have suggested
that ESCs in patients with Ems possess different properties
compared with the ESCs of healthy controls (45,46). Ectopic ESCs exhibit increased
invasive capacity compared with that of their eutopic counterparts
from patients with Ems and those from non-endometriotic controls
(47,48). Therefore, identification of the
underlying mechanisms that alter the invasiveness of ESCs may be a
major step toward better understanding the pathogenesis of Ems. In
the present study, it should be noted that miR-16 overexpression
inhibited the migration and invasion of ESCs, which are dependent
on the downregulation of the downstream molecule IKKβ. The adhesion
ability of ESCs in the pathological process of Ems does not appear
to be as important as their invasive capacity (8). Therefore, the ESC adhesion ability
was not evaluated in the present study.
There were certain limitations to the present study:
i) The sample size was relatively small; ii) whether other
signaling pathways are also regulated by miR-16 remains to be
determined in future studies; and iii) the exact mechanism
underlying the effect of miR-16 in ESC invasion remains to be fully
elucidated. Thus, further research is required to address these
issues.
In conclusion, we herein demonstrated that miR-16 is
down-regulated in endometriotic tissues, and miR-16 overexpression
inhibited primary ESC migration and invasion through regulation of
the IKKβ/NF-κB pathway. These findings may help with the
development of novel strategies targeting the miR-16/IKKβ/NF-κB
signaling pathway in the prophylaxis of Ems. In the future, we aim
to investigate the role of miR-16 in the prevention and treatment
of Ems in vivo.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors' contributions
XW, RR and MS performed the experiments, contributed
to data analysis and wrote the manuscript. XW, RR and MS analysed
the data. JL conceptualized the study design, contributed to data
analysis and experimental materials. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All individuals provided informed consent for the
use of their tissue specimens for clinical research. The present
study was approved by the Maternal and Child Care Service Center of
Dongguan city Guangdong Province Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ponandai-Srinivasan S, Andersson KL,
Nister M, Saare M, Hassan HA, Varghese SJ, Peters M, Salumets A,
Gemzell-Danielsson K and Lalitkumar PGL: Aberrant expression of
genes associated with stemness and cancer in endometria and
endometrioma in a subset of women with endometriosis. Hum Reprod.
33:1924–1938. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duffy JM, Arambage K, Correa FJ, Olive D,
Farquhar C, Garry R, Barlow DH and Jacobson TZ: Laparoscopic
surgery for endometriosis. Cochrane Database Syst Rev.
3:CD0110312014.
|
|
3
|
Vercellini P, Vigano P, Somigliana E and
Fedele L: Endometriosis: Pathogenesis and treatment. Nat Rev
Endocrinol. 10:261–275. 2014. View Article : Google Scholar
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toloubeydokhti T, Pan Q, Luo X, Bukulmez O
and Chegini N: The expression and ovarian steroid regulation of
endometrial micro-RNAs. Reprod Sci. 15:993–1001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burney RO, Hamilton AE, Aghajanova L, Vo
KC, Nezhat CN, Lessey BA and Giudice LC: MicroRNA expression
profiling of eutopic secretory endometrium in women with versus
without endometriosis. Mol Hum Reprod. 15:625–631. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang Z, Chen Y, Zhao Y, Xu C, Zhang A,
Zhang Q, Wang D, He J, Hua W and Duan P: miR-200c suppresses
endometriosis by targeting MALAT1 in vitro and in vivo. Stem Cell
Res Ther. 8:2512017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng X, Liu J, Wang H, Chen P and Wang D:
MicroRNA-126-5p downregulates BCAR3 expression to promote cell
migration and invasion in endometriosis. Mol Cell Endocrinol.
494:1104862019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Yan J and Pan X: miR-141-3p
affects apoptosis and migration of endometrial stromal cells by
targeting KLF-12. Pflugers Arch. 471:1055–1063. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takenaka Y, Taniguchi F, Miyakoda H, Takai
E, Terakawa N and Harada T: Lipopolysaccharide promoted
proliferation and invasion of endometriotic stromal cells via
induction of cyclo-oxygenase-2 expression. Fertil Steril.
93:325–327. 2010. View Article : Google Scholar
|
|
11
|
Gonzalez-Ramos R, Donnez J, Defrere S,
Leclercq I, Squifflet J, Lousse JC and Van Langendonckt A: Nuclear
factor-kappa B is constitutively activated in peritoneal
endometriosis. Mol Hum Reprod. 13:503–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan MH, Lin-Shiau SY and Lin JK:
Comparative studies on the suppression of nitric oxide synthase by
curcumin and its hydrogenated metabolites through down-regulation
of IkappaB kinase and NFkappaB activation in macrophages. Biochem
Pharmacol. 60:1665–1676. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang A, Wang G, Jia L, Su T and Zhang L:
Exosome-mediated microRNA-138 and vascular endothelial growth
factor in endo-metriosis through inflammation and apoptosis via the
nuclear factor-kB signaling pathway. Int J Mol Med. 43:358–370.
2019.
|
|
14
|
Brosens JJ, Hayashi N and White JO:
Progesterone receptor regulates decidual prolactin expression in
differentiating human endometrial stromal cells. Endocrinology.
140:4809–4820. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei D, Shen B, Wang W, Zhou Y, Yang X, Lu
G, Yang J and Shao Y: MicroRNA199a-5p functions as a tumor
suppressor in oral squamous cell carcinoma via targeting the
IKKβ/NFkB signaling pathway. Int J Mol Med. 43:1585–1596.
2019.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Dai L, Gu L, Ding C, Qiu L and Di W: TWEAK
promotes ovarian cancer cell metastasis via NF-kappaB pathway
activation and VEGF expression. Cancer Lett. 283:159–167. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Gu L, Ni J, Hu P, Hu K and Shi YL:
MiR-183 regulates ITGB1P expression and promotes invasion of
endometrial stromal cells. Biomed Res Int.
2015:3402182015.PubMed/NCBI
|
|
19
|
Pan ML, Chen LR, Tsao HM and Chen KH: Risk
of gestational hypertension-preeclampsia in women with preceding
endometriosis: A nationwide population-based study. Plos One.
12:e01812612017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia SZ, Yang Y, Lang J, Sun P and Leng J:
Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women
with endometriosis. Hum Reprod. 28:322–330. 2013. View Article : Google Scholar :
|
|
21
|
Dai L, Gu L and Di W: MiR-199a attenuates
endometrial stromal cell invasiveness through suppression of the
IKKβ/NF-kB pathway and reduced interleukin-8 expression. Mol Hum
Reprod. 18:136–145. 2012. View Article : Google Scholar
|
|
22
|
Braza-Boils A, Salloum-Asfar S,
Mari-Alexandre J, Arroyo AB, González-Conejero R, Barceló-Molina M,
García-Oms J, Vicente V, Estellés A, Gilabert-Estellés J and
Martínez C: Peritoneal fluid modifies the microRNA expression
profile in endometrial and endometriotic cells from women with
endometriosis. Hum Reprod. 30:2292–2302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li N, Yang L, Sun Y and Wu X: MicroRNA-16
inhibits migration and invasion via regulation of the Wnt/β-catenin
signaling pathway in ovarian cancer. Oncol Lett. 17:2631–2638.
2019.PubMed/NCBI
|
|
24
|
Jin W, Chen F, Wang K, Song Y, Fei X and
Wu B: miR-15a/miR-16 cluster inhibits invasion of prostate cancer
cells by suppressing TGF-β signaling pathway. Biomed Pharmacother.
104:637–644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiao ZH, Wang JD and Wang XJ: MicroRNA-16
suppressed the invasion and migration of osteosarcoma by directly
inhibiting RAB23. Eur Rev Med Pharmacol Sci. 22:2598–2605.
2018.PubMed/NCBI
|
|
26
|
Lee MY, Kim SH, Oh YS, Heo SH, Kim KH,
Chae HD, Kim CH and Kang BM: Role of interleukin-32 in the
pathogenesis of endometriosis: In vitro, human and transgenic mouse
data. Hum Reprod. 33:807–816. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chatterjee K, Jana S, DasMahapatra P and
Swarnakar S: EGFR-mediated matrix metalloproteinase-7 up-regulation
promotes epithelial-mesenchymal transition via ERK1-AP1 axis during
ovarian endometriosis progression. FASEB J. 32:4560–4572. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aggarwal BB and Sung B: NF-kB in cancer: A
matter of life and death. Cancer Discov. 1:469–471. 2011.
View Article : Google Scholar
|
|
29
|
Julien S, Puig I, Caretti E, Bonaventure
J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A
and Larue L: Activation of NF-kappaB by Akt upregulates Snail
expression and induces epithelium mesenchyme transition. Oncogene.
26:7445–7456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsuchiya Y, Osaki K, Kanamoto M, Nakao Y,
Takahashi E, Higuchi T and Kamata H: Distinct B subunits of PP2A
regulate the NF-kB signalling pathway through dephosphorylation of
IKKβ, IkBα and RelA. FEBS Lett. 591:4083–4094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu H, Zhong Q, Xia Y, Li E, Wang S and Ren
R: MicroRNA-2861 targets STAT3 and MMP2 to regulate the
proliferation and apoptosis of ectopic endometrial cells in
endometriosis. Pharmazie. 74:243–249. 2019.PubMed/NCBI
|
|
32
|
Zhang H, Li G, Sheng X and Zhang S:
Upregulation of miR33b promotes endometriosis via inhibition of
Wnt/β-catenin signaling and ZEB1 expression. Mol Med Rep.
19:2144–2152. 2019.PubMed/NCBI
|
|
33
|
Teague EM, Print CG and Hull ML: The role
of microRNAs in endometriosis and associated reproductive
conditions. Hum Reprod Update. 16:142–165. 2010. View Article : Google Scholar
|
|
34
|
Ma L, Li Z, Li W, Ai J and Chen X:
MicroRNA-142-3p suppresses endometriosis by regulating
KLF9-mediated autophagy in vitro and in vivo. RNA Biol.
16:1733–1748. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li M, Zhou Y and Taylor HS: miR-451a
inhibition reduces established endometriosis lesions in mice.
Reprod Sci. 26:1506–1511. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu H, Cao XX, Liu J and Hua H:
MicroRNA-488 inhibits endometrial glandular epithelial cell
proliferation, migration, and invasion in endometriosis mice via
Wnt by inhibiting FZD7. J Cell Mol Med. 23:2419–2430. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bulun SE: Endometriosis. N Engl J Med.
360:268–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mei J, Jin LP, Ding D, Li MQ, Li DJ and
Zhu XY: Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix
metallopro-teinase-9 expression and decreases proliferation,
adhesion and invasion of endometrial stromal cells. Mol Hum Reprod.
18:467–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karin M: The beginning of the end: IkappaB
kinase (IKK) and NF-kappaB activation. J Biol Chem.
274:27339–27342. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao Y and Yu Z: MicroRNA16 inhibits
interleukin13induced inflammatory cytokine secretion and mucus
production in nasal epithelial cells by suppressing the IkB kinase
β/nuclear factor-kB pathway. Mol Med Rep. 18:4042–4050.
2018.PubMed/NCBI
|
|
41
|
Zhang JJ, Xu ZM, Zhang CM, Dai HY, Ji XQ,
Wang XF and Li C: Pyrrolidine dithiocarbamate inhibits nuclear
factor-kB pathway activation, and regulates adhesion, migration,
invasion and apoptosis of endometriotic stromal cells. Mol Hum
Reprod. 17:175–181. 2011. View Article : Google Scholar
|
|
42
|
Zhang JJ, Xu ZM, Dai HY, Ji XQ, Duan YY,
Zhang CM and Qin DY: Application of the nuclear factor-kB inhibitor
pyrrolidine dithiocarbamate for the treatment of endometriosis: An
in vitro study. Fertil Steril. 94:2942–2944. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huber AV, Huber JC, Kolbus A, Imhof M,
Nagele F, Loizou D, Kaufmann U and Singer CF: Systemic HCG
treatment in patients with endometriosis: A new perspective for a
painful disease. Wien Klin Wochenschr. 116:839–843. 2004.
View Article : Google Scholar
|
|
44
|
Iwase A, Kotani T, Goto M, Kobayashi H,
Takikawa S, Nakahara T, Nakamura T, Kondo M, Bayasula, Nagatomo Y
and Kikkawa F: Possible involvement of CD10 in the development of
endometriosis due to its inhibitory effects on CD44-dependent cell
adhesion. Reprod Sci. 21:82–88. 2014. View Article : Google Scholar :
|
|
45
|
Lessey BA and Young SL: Integrins and
other cell adhesion molecules in endometrium and endometriosis.
Semin Reprod Endocrinol. 15:291–299. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vercellini P, Aimi G, Panazza S, Vicentini
S, Pisacreta A and Crosignani PG: Deep endometriosis conundrum:
Evidence in favor of a peritoneal origin. Fertil Steril.
73:1043–1046. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Agic A, von Wussow U, Starzinski-Powitz A,
Diedrich K, Altevogt P and Hornung D: Inhibition of cell
proliferation, adhesion, and invasion with an anti-L1-cell adhesion
molecule monoclonal antibody in an in vitro endometriosis model.
Fertil Steril. 94:1102–1104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu H, Zhang Z, Xiong W, Zhang L, Xiong Y,
Li N, He H, Du Y and Liu Y: Hypoxia-inducible factor-1α promotes
endometrial stromal cells migration and invasion by upregulating
autophagy in endometriosis. Reproduction. 153:809–820. 2017.
View Article : Google Scholar : PubMed/NCBI
|