Introduction
Asthma is one of the most common chronic diseases
and more than 300 million individuals worldwide currently suffer
from asthma (1). It was estimated
that the number will reach 400 million by 2025 (2). Asthma has been considered a
heterogeneous disease that comprises different phenotypes and
shares similar clinical manifestations and typical features of
airway inflammation, airway hyperresponsiveness (AHR) and airway
remodeling (3,4). Although corticosteroids and
bronchodilators can control disease symptoms (5,6),
asthma is not readily preventable or curable (7,8).
Therefore, it is necessary to identify early effective primary
prevention and intervention strategies.
Interleukin 27 (IL-27) is a novel cytokine composed
of the subunit p28 (IL-27p28) and Epstein Barr virus-induced gene 3
(9). This molecule is primarily
produced by activated antigen-presenting cells and exhibits
pleiotropic effects on T-helper cell responses (9,10).
For example, it induces T-helper 1 (Th1) cell differentiation
(11,12) and directly suppresses T-helper 2
(Th2) production and T-helper 17 (Th17) differentiation (13,14). Th2-type cytokines play a pivotal
role in airway inflammation and AHR, which are the hallmarks of
asthma (15,16). Therefore, IL-27 may play an
important role in the pathogenesis of asthma.
Several studies have found that high levels of IL-27
combined with activation of the type-2 signature are associated
with increasing severity of asthma (17-19). IL-27 may be stimulated as a
counter-regulatory cytokine to suppress Th2 inflammation (17-19). In addition, Miyazaki et al
(20) reported that IL-27R (-/-)
mice challenged with ovalbumin (OVA) exhibited increased asthmatic
phenotypes, suggesting that IL-27 plays a pivotal role in the
inhibition of lung inflammation and AHR. Jirmo et al
(21) demonstrated that IL-27 is
critical for the control of allergic asthma. Therefore, IL-27 is a
novel, promising preventative agent for alleviating asthma
development and exacerbation. A series of studies based on the
OVA-induced mouse model have shown that preventative intranasal
administration of IL-27 reduced airway inflammation and improved
pathological changes, whereas IL-27 does not reduce airway
inflammation and improve pathological changes when administered in
a therapeutic mode following OVA challenge (22,23). However, Yoshimoto et al
(17) reported that intranasal
administration of IL-27 could inhibit OVA-induced airway
hyperresponsiveness and inflammation in OVA-immunized mice. The
biological impact of IL-27 on asthma remains elusive and previous
studies have shown contradictory pro-inflammatory and
anti-inflammatory effects (24,25). Furthermore, it has not been
examined whether IL-27 plays a role in airway remodeling of chronic
asthma.
In the present study, the effects of intranasal
administration of IL-27 on airway inflammation and AHR were
investigated in OVA-immunized mouse models of acute asthma. In
addition, the effects of IL-27 on airway remodeling in mouse models
of chronic asthma were explored. Finally, the effects of IL-27
stimulation on the expression levels of signal transducer and
activator of transcription (STAT)1, STAT3, T-box transcription
factor (T-bet), and GATA binding protein-3 GATA3) were studied. The
data revealed that the effect of IL-27 was dependent on STAT1 and
STAT3 pathways
Materials and methods
Mice
A total of 24 healthy female BALB/c mice were
purchased from the Experimental Animal Center of Shandong
University and used between 6-8 weeks of age. The animals were kept
under specific pathogen-free and standard conditions including 12 h
light/dark cycle, room temperature of 22°C, relative humidity of
60% and free access to food and water. The health and wellbeing of
animals were monitored via daily observations of behaviour and
condition. The mice were randomly assigned to three study groups:
PBS group (PBS, n=8), OVA group (OVA, n=8) and IL-27 group (OVA +
IL-27, n=8). All procedures on mice were performed in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (26). In
addition, all protocols were approved by the Ethics Committee for
Laboratory Animals Care and Use in Shandong Qianfoshan Hospital,
Shandong University (Shandong, China).
Experimental model for OVA-induced acute
asthma
OVA sensitization and challenge were accomplished
using a modified protocol as previously described by Reddy et
al (27) and Venkayya et
al (16). Briefly, in the OVA
and OVA + IL-27 groups, the mice received intraperitoneal (i.p.)
injections containing 100 µg OVA (Sigma-Aldrich; Merck
KGaA), 2 mg Alum (Thermo Fisher Scientific, Inc.) and 100 µl
PBS (Invitrogen; Thermo Fisher Scientific, Inc.) on days 0 and 7.
The mice received aerosol challenge with 50 µl PBS and 100
µg OVA intranasally (i.n.) on days 14-18 under light
isoflurane anesthesia, which was performed for 1 min with a
concentration of 3% isoflurane (28). The mice were sensitized with
sterile endotoxin-free PBS/Alum and challenged with PBS i.n. on the
same days in the PBS group. In the OVA + IL-27 group, the mice were
treated i.n. with 50 µl PBS and 50 ng IL-27 twice a day on
days 6 and 7. On days 0, 7 and 14-18, the mice received i.n. 50
µl PBS and 1 µg IL-27 1 h prior to OVA sensitization
and the subsequent challenge. The mice were treated with 50
µl PBS alone in the OVA and PBS groups (see the protocol
scheme in Fig. 1A). Each group
included eight mice.
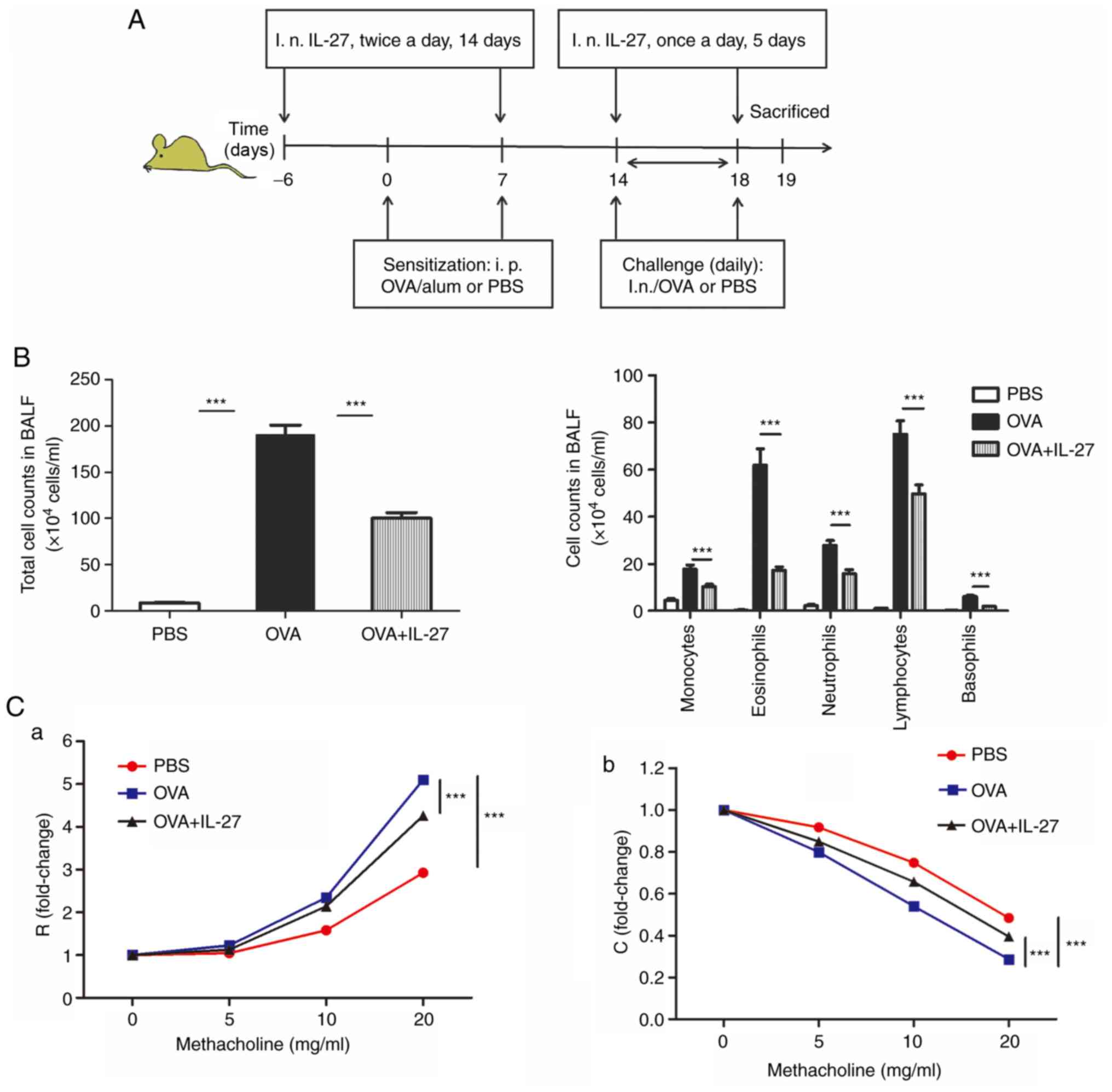 | Figure 1Intranasal administration of IL-27
alleviates airway inflammation and AHR in an acute model of
experimental allergic asthma. (A) Protocol of OVA-induced allergic
asthma and administration of IL-27. (B) The total cell number and
the differential cell counts in the BALF samples. The total cell
number and the numbers of macrophagocytes, neutrophil, basophils,
eosinophils and lymphocytes were decreased in the OVA+IL-27 group.
(C) AHR following methacholine challenge was measured in an
invasive lung function assay. Treatment with IL-27 exhibited
significant improvement in (a) lung resistance and (b) lung
compliance. The data are expressed as the mean ± standard error of
the mean of three independent experiments, with eight animals per
group. ***P<0.001. IL-27, interleukin 27; OVA,
ovalbumin; AHR, airway hyperresponsiveness; BALF, bronchoalveolar
lavage fluid; i.n, intranasal; R, lung resistance; C, lung
compliance. |
Experimental model of OVA-induced chronic
asthma
The mice were sensitized and challenged with OVA
using a modified protocol described by Kirstein et al
(29). In brief, a mixture
containing 20 µg OVA/1 mg Alum/200 µl PBS was
delivered subcutaneously to mice on days 0, 7, 14 and 21 to the OVA
and OVA + IL-27 groups, while 1 mg Alum/200 µl PBS was
administered to the PBS group. On days 26 and 28 and on the
following 4 weeks, the mice received i.n challenge of 20 mg either
OVA in PBS (50 µl; OVA and OVA + IL-27 groups) or 50
µl PBS alone (PBS group) following induction of anesthesia.
The administration of OVA and IL-27 from day 28 was performed twice
every week. In the OVA + IL-27 group, the mice were treated i.n.
with 50 µl PBS and 50 ng IL-27 twice a day on days 6 and 7.
On days 0, 7, 26-28, 35, 38, 42, 49, 52, 56 and 59 the mice
received i.n. 50 µl PBS and 20 ng IL-27 1 h prior to OVA
sensitization and prior to the subsequent challenge. The mice were
treated with 50 µl PBS alone in the OVA and PBS groups
(Fig. 2A). Each group included
eight mice.
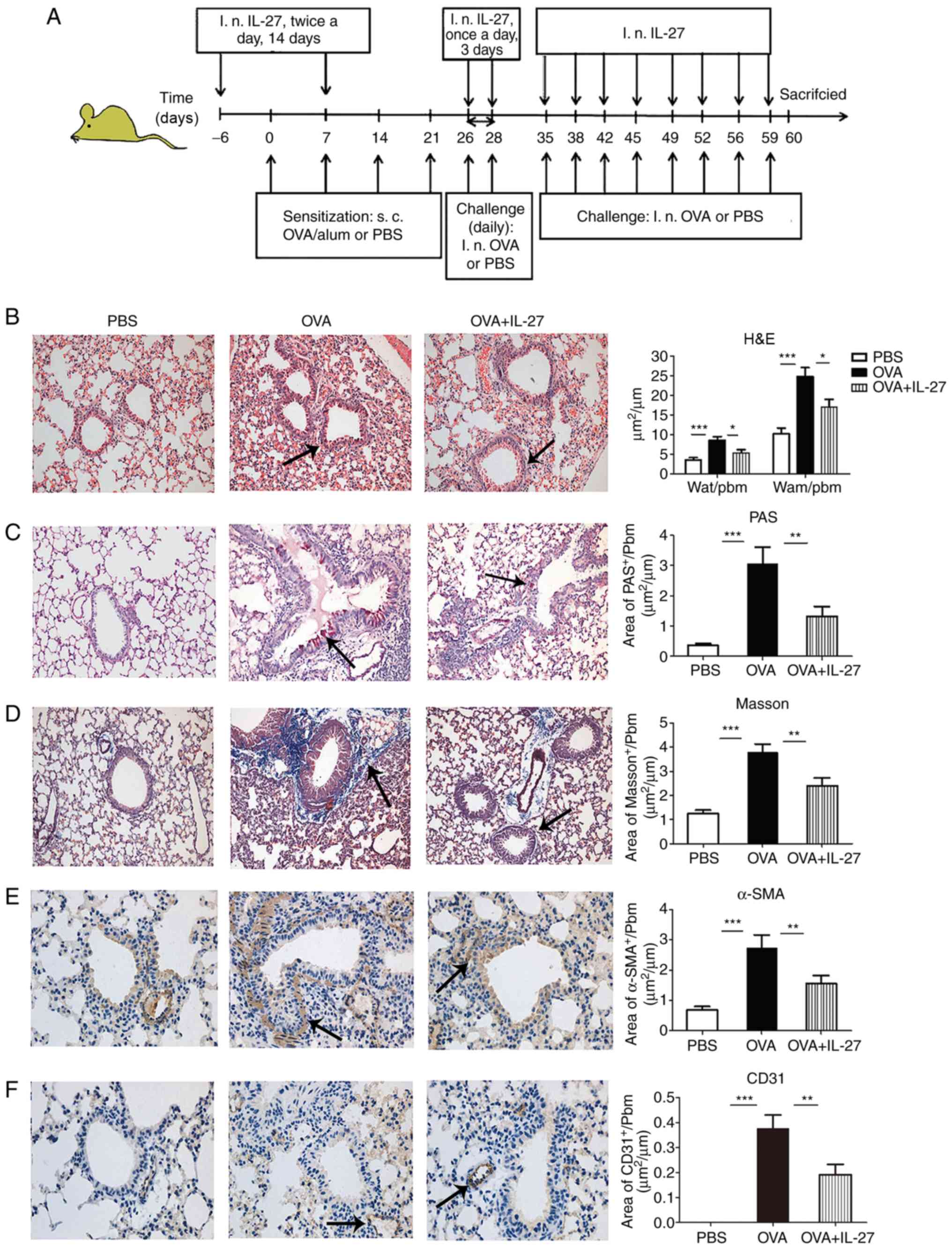 | Figure 2Intranasal administration of IL-27
prevents OVA-induced airway remodeling in a chronic experimental
model of asthma. (A) Protocol of OVA-induced allergic asthma and
administration of IL-27. (B) Representative photomicrographs of
H&E-stained lung sections from each group (×200 magnification).
Significantly reduced basal membrane thickening and hyperplasia of
airway smooth muscle cells (indicated by the arrow) were observed
in IL-27-treated mice compared with OVA-exposed mice. (C)
Representative photomicrographs of PAS-stained (indicated by the
arrow) lung sections from each group (×200 magnification).
Significantly reduced airway mucus production was observed in
IL-27-treated mice compared with OVA-exposed mice. (D)
Representative photomicrographs of Masson's trichrome-stained
sections from each group (×200 magnification). Significantly
reduced subepithelial fibrosis (indicated by the arrow) was
observed in IL-27-treated mice compared with OVA-exposed mice. (E)
Representative photomicrographs of α-SMA-immunostained sections
from each group (×400 magnification). Significantly reduced
peribronchial α-SMA-immunostained area (indicated by the arrow) was
observed in IL-27-treated mice compared with the corresponding area
from OVA-exposed mice. (F) Representative photomicrographs of
CD31-immunostained sections from each group (×400 magnification).
Significantly reduced peribronchial CD31-immunostained area
(indicated by the arrow) was observed in IL-27-treated mice
compared with the area of OVA-exposed mice. The data are expressed
as the mean ± standard error of the mean of three independent
experiments with eight animals per group. *P<0.05,
**P<0.01 and ***P<0.001. IL-27,
interleukin 27; OVA, ovalbumin; H&E, hematoxylin and eosin;
PAS, periodic-acid Schiff; α-SMA, α-smooth muscle actin; Pbm,
perimeter of basement membrane; Wat, total area of airway wall;
Wam, area of smooth muscle; d, day; i.n, intranasal. |
Measurement of airway responsiveness
Airway responses and dynamic lung compliance to
methacholine challenge were assessed 24 h following the last OVA
challenge, as previously reported (30). Briefly, the mice were anesthetized
with pentobarbital sodium (50 mg/kg, i.p. injection) and the depth
of anesthesia was determined by mice's responses to nociceptive
stimulation and movement (31).
No side effects of anesthesia were observed in 3 mice groups. After
the mice were fully anaesthetized (immobility and absence of the
withdrawal reflex of the right paw), a cannula was subsequently
inserted into the trachea, and the mice were connected to the
flexiVent system (Scireq). The mice were mechanically ventilated
with a tidal volume of 5 ml/kg at a rate of 150 breaths/min with a
positive end-expiratory pressure of 3 cm H2O (32). In the plethysmograph chamber, a
thermostat-controlled warming pad was built to retain the
temperature at 37°C. The mice were initially challenged with
aerosol saline followed by challenge with increasing concentrations
of acetyl-β-methylcholine chloride (methacholine; 0, 5, 10, and 20
mg/ml; Sigma-Aldrich; Merck KGaA) for 10 sec at each dose. Airway
resistance and lung compliance were calculated as percentage
increase over baseline (saline challenge).
Bronchoalveolar lavage (BAL)
At 24 h following the final OVA challenge and
immediately after the measurement of airway responsiveness, mice
were euthanized by cervical dislocation and death was confirmed
according to lack of breathing, pulse, corneal reflex and response
to firm toe pinch (33). BAL
fluid (BALF) was collected and processed as previously described
(34). In brief, the trachea was
cannulated and 4×1 ml PBS-EDTA (0.05 M; Merck KGaA) was instilled
in the right lung and recovered by gentle manual aspiration.
Subsequently, the BALF was centrifuged (80 × g for 10 min at 4°C)
and the cell pellets were resuspended in 1 ml PBS-EDTA. Total and
differential cell counts were determined on cytospin slide
preparations and stained with Wright-Giemsa at room temperature for
8 min. A total of 200 cells per slide were counted per sample using
a light microscope at ×400 magnification (DP73; Olympus
Corporation) (35).
Enzyme-linked immunosorbent assay
(ELISA)
The expression levels of IL-4 (cat. no. M4000B),
IL-5 (cat. no. M5000), IL-13 (cat. no. DY413), IL-17 (cat. no.
M1700) and interferon (IFN)-γ (cat. no. DY485) in the serum and
supernatant of BALF were determined using ELISA kits (R&D
Systems, Inc.) as previously described, according to the
manufacturer's protocol (36).
Reverse transcription-quantitative PCR
(RT-qPCR)
The left lungs of mice were removed immediately
after collection of BALF and total RNA was extracted from the lung
tissues using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
concentration of RNA was assessed by a Nanodrop™ ND-1000
spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA synthesis
was performed using a Superscript III First-Strand Synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols at 50°C for 50 min. qPCR for mRNA
detection was conducted using an ABI 7000 PCR instrument (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The PCR reaction system consisted of 1 µl
cDNA, 0.5 µl of each forward and reverse primer, 10
µl SYBR Green Mix (Beijing Solarbio Science & Technology
Co., Ltd.) and 8 µl ddH2O. The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 94°C for 5 min, followed by 40 cycles of 10 sec at
94°C and 20 sec at 60°C and a final extension of 30 sec at 72°C.
β-actin was used as the reference control gene. The primer
sequences used for this analysis and the expected size of the PCR
products are listed in Table I.
The relative abundance of the mRNA transcripts was calculated using
the 2−∆∆Cq method (37). Each sample was tested in
triplicate and at least three wells were used for each group.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′–3′) | Product size
(bp) |
|---|
| STAT1 | F:
CACCCTTGCTTACTCTACTGC | 122 |
| R:
TTGAATGACTAAACGCCTGA |
| STAT3 | F:
ACCTCCAGGACGACTTTG | 130 |
| R:
AGGGCTGTGAGCATCTGT |
| GATA3 | F:
CCTTTATTCCTCCGTGTCTGC | 106 |
| R:
ATCTTTGCGGGATAGTTTAGC |
| T-bet | F:
TCCCATTCCTGTCCTTCACCG | 142 |
| R:
ATGCTGCCTTCTGCCTTTCCA |
| β-actin | F:
CTGTGCCCATCTACGAGGGCTAT | 155 |
| R:
TTTGATGTCACGCACGATTTCC |
Western blot analysis
Western blot analysis was performed as described
previously (38). Briefly, the
proteins were extracted from mouse lung tissues and loaded on 5%
SDS polyacrylamide gels. A bicinchoninic acid protein assay kit
(cat. no. 23225; Pierce; Thermo Fisher Scientific, Inc.) was used
to determine protein concentration. Following electrophoresis, the
proteins were transferred to PVDF membranes (EMD Millipore). The
membranes were blocked in 5% (w/v) nonfat dry milk in TBS-Tween-20
(0.15%) at 37°C for 1 h. Subsequently, membranes were incubated
with primary antibodies against STAT1 (cat. no. sc-464),
phosphorylated (p)-STAT1 (cat. no. sc-8394), STAT3 (cat. no.
sc-482), p-STAT3 (cat. no. sc-8059), GATA3 (cat. no. sc-9009),
T-bet (cat. no. sc-21003) and β-actin (cat. no. sc-47778; all from
Santa Cruz Biotechnology, Inc.) separately. The dilutions of the
primary antibodies were 1:500 and the incubations were performed
overnight at 4°C. Subsequently, the membranes were incubated with
goat anti-rabbit immunoglobulin G-horseradish peroxidase secondary
antibody (cat. no. sc-2004; 1:5,000; Santa Cruz Biotechnology,
Inc.) at 37°C for 45 min. β-actin was used as the internal control.
The protein bands were visualized using an ECL detection reagent
kit (GE Healthcare) according to the manufacturer's instructions.
Densitometry was performed using Gel-Pro analyzer software (version
4.0; Media Cybernetics, Inc.).
Lung histology
The lung tissues (5-µm thick) were
subsequently formalin-fixed and paraffin-embedded, as described
previously (39). The lung
sections were prepared and stained with hematoxylin and eosin
(H&E), Masson's trichrome and periodic acid-Schiff (PAS) stain
according to a standard protocol (39,40). Two pathologists who were blinded
to the group assignment of mice independently evaluated and scored
three differently stained tissue sections and the average scores
were calculated. H&E-stained lung sections were used mainly for
the assessment of basal membrane thickening and hyperplasia of
airway smooth muscle cells. Three bronchioles with 150–200
µm inner diameter were selected in each slide. The perimeter
of basement membrane (Pbm), total area of airway wall (Wat) and
area of smooth muscle (Wam) were measured by morphometric analysis
(Image-Pro Plus 6.0; Media Cybernetics, Inc.) and the ratios of Wat
to Pbm (Wat/Pbm) and Wam to Pbm (Wam/Pbm) were calculated (40). Masson's trichrome-stained sections
were used to identify subepithelial fibrosis. The epithelial
basement membranes with diameters of >250 µm were
selected and both the collagen fiber area (stained in blue) beneath
the basement membrane and Pbm were measured using Image-Pro Plus
6.0. The mean score of the fibrotic area divided by Pbm was
calculated (41). PAS-stained
sections were used for evaluating goblet cell hyperplasia. The
bronchioles with 1.0–2.5 mm-long epithelial basement membranes were
selected, and the area of goblet cells was measured as the area of
PAS-positive staining using Image-Pro Plus 6.0. Subsequently, the
ratio of the area of PAS-positive staining to Pbm was calculated
(42). In addition, myofibroblast
activation and angiogenesis were assessed by immunohistochemical
analysis for α-smooth muscle actin (α-SMA) (1:200; cat. no.
sc-15320; Santa Cruz Biotechnology, Inc.) and CD31(1:100; cat. no.
sc-28188; Santa Cruz Biotechnology, Inc.). The areas of
peribronchial a-SMA-positive immunostaining and CD31-positive
immunostaining in the submucosa were outlined and determined by
Image-Pro Plus 6.0 and the expression levels of these markers were
quantified by the area of α-SMA and CD31-positive staining relative
to Pbm (43).
Statistical analysis
The data are expressed as the mean ± SEM.
Statistical analysis was performed using GraphPad Prism 5 software
(GraphPad Software, Inc.). One-way ANOVA with Bonferroni's multiple
comparisons test was used to determine statistical significance
between experimental groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Intranasal administration of IL-27
alleviates allergic airway inflammation in an acute experimental
model of asthma
The total cell number from BALF samples in the PBS
group was significantly lower compared with the OVA group.
Moreover, the total cell number in OVA + IL-27 group significantly
decreased compared with the OVA group (Fig. 1B). Furthermore, the numbers of
other inflammatory cells from BALF in the OVA+IL-27 group, such as
macrophagocytes, lymphocytes, basophils and neutrophils, were also
significantly reduced compared with the OVA group (Fig. 1B).
Intranasal administration of IL-27
ameliorates AHR in an acute experimental model of asthma
Baseline airway responsiveness (at 0 mg/ml
methacholine) did not reveal significant differences among the
three groups (Fig. 1C-a and C-b).
In OVA-challenged mice, airway responsiveness (R) to acetylcholine
was significantly enhanced compared with that PBS-challenged mice
(Fig. 1C-a). In the OVA + IL-27
group, IL-27 reversed methacholine-induced AHR as reflected by a
significant reduction in airway resistance and a significant
increase in lung compliance (C) compared with the OVA group
(Fig. 1C-b).
Intranasal administration of IL-27
suppresses production of IL-4, IL-5, IL-13 and IL-17, whereas it
induces IFN-γ production in an acute experimental model of
asthma
To determine whether IL-27 ameliorates inflammation
and whether AHR can reflect altered cytokine profiles, the
concentration levels of the latter markers were examined in both
BALF and serum samples derived from mice. The OVA group exhibited
significantly higher levels of Th2 cytokines (IL-4, IL-5 and IL-13)
and the Th17 cytokine (IL-17), while significantly lower levels of
the Th1 cytokine (IFN-γ) were observed compared with in the PBS
group (Fig. 3). Compared with the
OVA group, intranasal administration of IL-27 led to a significant
reduction of IL-4, IL-5, IL-13 and IL-17, and a significant
induction of IFN-γ (Fig. 3).
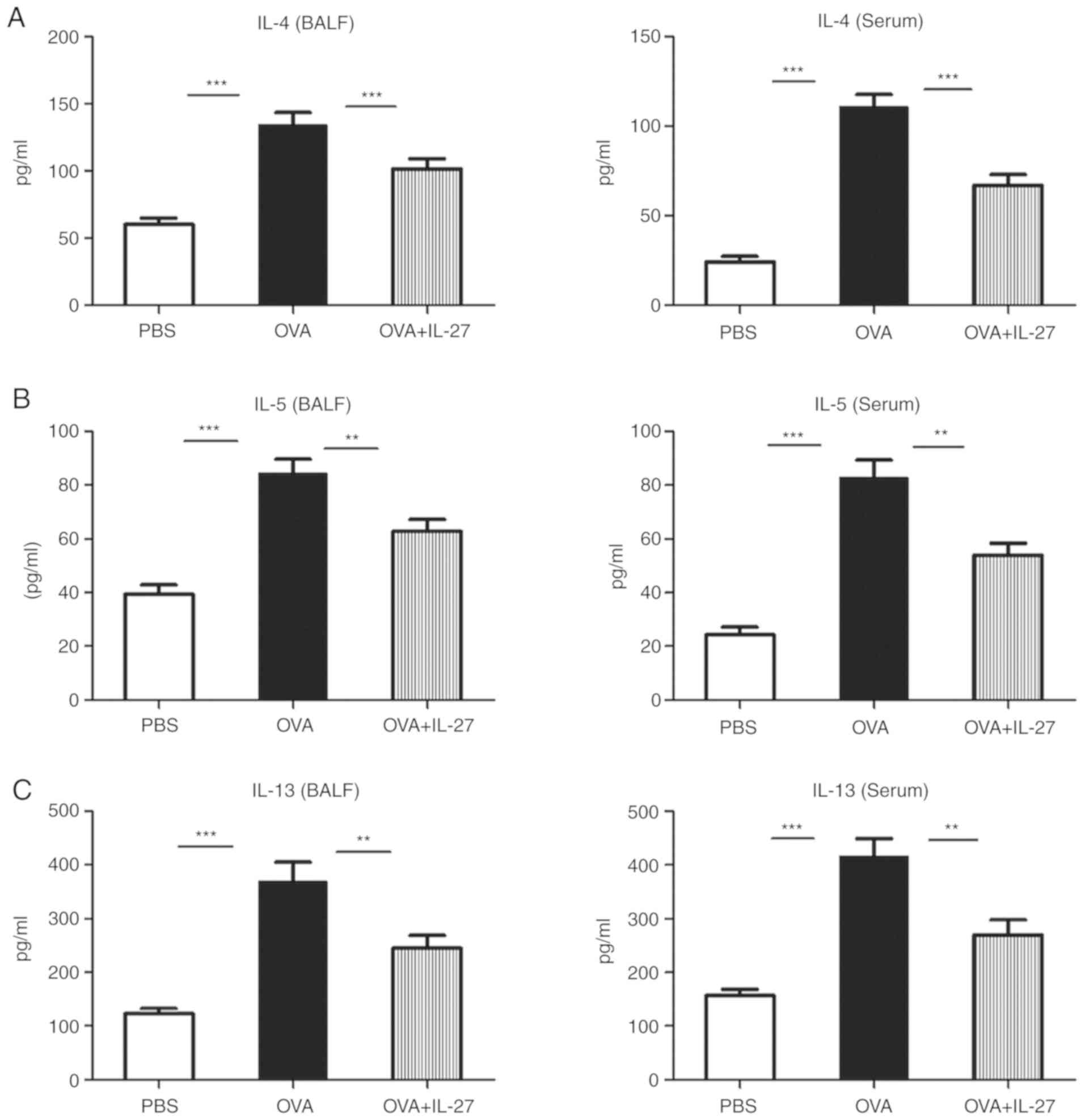 | Figure 3Intranasal administration of IL-27
reduces Th2 and Th17 cytokine levels and enhances the production of
IFN-γ. The concentration levels of Th1, Th2 and Th17 cytokines in
serum and BALF samples were measured by ELISA. The levels of (A)
IL-4, (B) IL-5, (C) IL-13 and (D) IL-17 were lower, whereas those
of (E) IFN-γ were higher in the OVA+IL-27 group compared with the
OVA group. The data are expressed as the mean ± standard error of
the mean of three independent experiments, with eight animals per
group. *P<0.05, **P<0.01 and
***P<0.001. IL, interleukin; Th, T-helper; BALF,
bronchoalveolar lavage fluid; OVA, ovalbumin; IFN, interferon. |
Intranasal administration of IL-27
prevents OVA-induced airway remodeling in a chronic experimental
model of asthma
To investigate whether IL-27 plays a role in the
development of airway remodeling, airway epithelium, peribronchial
interstitial tissue, airway smooth muscle cells and bronchial
vasculature were used to evaluate IL-27 levels in a chronic
experimental model of asthma. The representative sections of each
group were stained with H&E, Masson's trichrome and PAS, as
well as with antibodies specific for α-SMA and CD31 detection
(Fig. 2). The OVA + IL-27 group
exhibited significantly reduced peribronchial and perivascular
inflammatory infiltrates, basal membrane thickening (Fig. 2B), mucus secretion (Fig. 2C), collagen deposition (Fig. 2D), number of airway smooth muscle
cells (Fig. 2E) and vascular size
(Fig. 2F) compared with the
corresponding indices noted in the OVA group. Significant
differences were noted for Wat/Pbm and Wam/Pbm (Fig. 2B), PAS area (Fig. 2C), Masson's trichrome (Fig. 2D), α-SMA (Fig. 2E) and CD31-positive stained/Pbm
(Fig. 2F) between PBS and OVA
groups and OVA and OVA + IL-27 groups.
Intranasal administration of IL-27 in
mice activates the STAT1 and STAT3 pathways
The involvement of the possible signaling pathways
that include IL-27 was assessed. Both mRNA and protein levels of
STAT1, STAT3, GATA3 and T-bet were detected in lung tissues using
RT-qPCR and western blotting analyses. Compared with the OVA group,
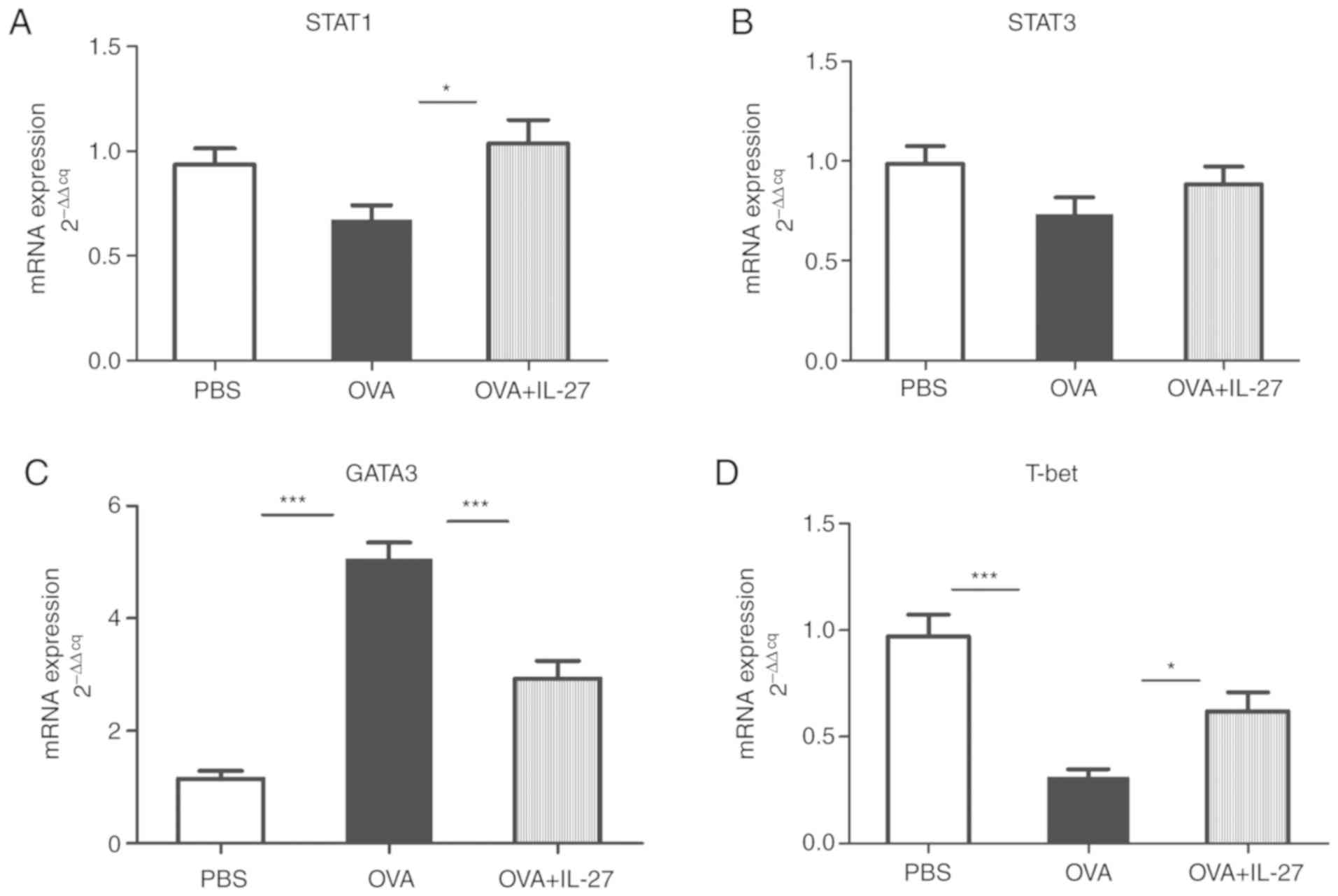
the mRNA expression levels of STAT1 were significantly upregulated
in the OVA + IL-27 group while STAT3 levels exhibited no
significant changes (Fig. 4A and
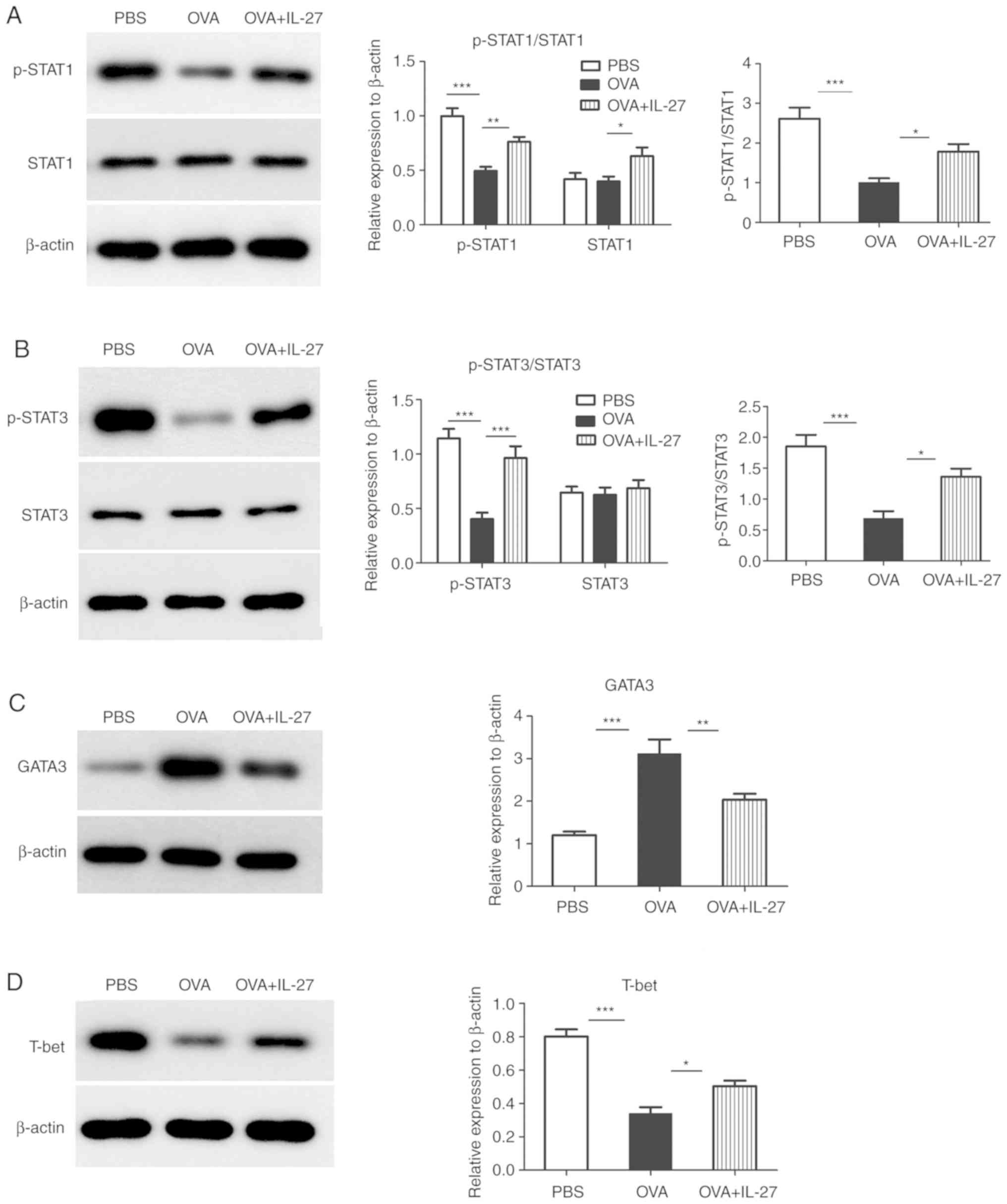
B). Accordingly, the total and phosphorylated levels of STAT1
and STAT3 proteins and their ratios demonstrated similar changes
(Fig. 5A and B). In addition, the
mRNA and protein expression levels of GATA3 were increased in the
OVA group compared with the PBS group, whereas these were reduced
in the OVA+IL-27 group compared with the OVA group (Figs. 4C and 5C). In contrast to these findings,
compared with the PBS group, the expression levels of T-bet mRNA
and protein were lower in the OVA group and higher in the OVA+IL-27
group compared with the OVA group (Figs. 4D and 5D).
Discussion
In the current study, the data indicated that
intranasal administration of IL-27 significantly inhibited airway
inflammation and AHR in the acute mouse models of experimental
asthma. This finding is consistent with a study reported by Su
et al (22) highlighting
that preventative administration of IL-27 could reduce airway
inflammation and improve AHR in asthmatic mice. In addition, the
present study demonstrated that intranasal administration of IL-27
attenuated significant airway remodeling in chronic mouse models of
experimental asthma. To the best of our knowledge, this is the only
study which examined the effects of IL-27 on airway remodeling
using a chronic asthma model. The data indicated that
phosphorylation of STAT1 and STAT3 was impaired in the lung tissues
of asthmatic mice and could be reversed by IL-27
administration.
As a pleiotropic cytokine, IL-27 is involved in the
regulation of immune responses of certain inflammatory cells, such
as Th cells, dendritic cells and macrophages (44). Therefore, IL-27 may be implicated
in the pathogenesis of autoimmune and inflammatory diseases
including asthma (45). Chronic
airway inflammation is one of the main characteristics of asthma
and plays a critical role in the pathogenesis of this disorder
(46). Various types of cells,
such as eosinophils, T cells, mast cells and neutrophils, are
involved in the inflammatory response to allergens during asthma
progression (20). AHR is one of
the key features characteristic of asthma and one of the main
factors responsible for the pathogenesis of this disease (47). AHR is characterized by increased
airway resistance and decreased airway compliance (48). The results of the present study
highlighted that intranasal administration of IL-27 could reduce
inflammatory cell numbers in BALF samples and reverse
methacholine-induced AHR. This indicated that IL-27 could
effectively attenuate airway inflammation and AHR in experimental
asthma. Furthermore, the serum and BALF concentration levels of the
Th1 cytokines (IFN-γ) increased, whereas levels of Th2 cytokines
(IL-4, IL-5, and IL-13) decreased in the OVA+IL-27 group compared
with the OVA group, which suggested that enhanced production of Th2
cytokines and/or decreased production of Th1 cytokines may
contribute to the effects of IL-27 on allergic asthma in this
experimental model. These findings concur with a previous study,
which demonstrated that preventative administration of IL-27
diminished BALF concentration levels of Th2 cytokines, such as IL-5
and IL-13 (22). IL-27 activates
naive CD4+ T cells and natural killer (NK) cells to
stimulate production of IFN-γ (49). Moreover, it can upregulate the
expression levels of IL-12Rb2 and T-bet to promote Th1-effector
function (49). Both IFN-γ and
T-bet play a role in the suppression of AHR and eosinophil lung
infiltration, indicating that IL-27 may have regulatory effects on
asthmatic immune responses and promote the development of Th1
(50). In addition, IL-27 can
directly suppress Th2 cell differentiation and conversely induce
these cells to develop into Th1 cells that produce IFN-γ (17). Under Th2 polarization conditions,
naive IL-27 receptor WSX-1-/- CD4 T cells increased
production of Th2 cytokines (14). Previous studies showed that
certain cytokines, such as IL-4, IL-5 and IL-13, play important
roles in the initiation of airway inflammation, AHR and in variable
airflow obstruction (15,51). Administration of IL-27 attenuated
allergen-induced AHR and airway inflammation, which are hallmarks
of allergic asthma (17,22,23). These studies are in concordance
with the present work and demonstrated that IL-27 significantly
suppresses Th2 cytokine production.
IL-17 is secreted by multiple cell types, such as
Th17, NK cells, mast cells, neutrophils and γδ T cells (52). IL-17 cytokines are also key
players in several immune responses (52). Serum and BALF IL-17 levels were
significantly downregulated in the OVA+IL-27 group compared with
those noted in the OVA group. These findings suggested that IL-17
plays a role in allergic asthma and that IL-27 acts in a manner
antagonistic to IL-17, which is in accordance with previously
reported data (21,53). Moreover, a previous study revealed
that high levels of IL-17 were found in induced sputum and
bronchial biopsies obtained from severe asthmatics and were
associated with poor patient response to steroids (54). IL-27 inhibited the differentiation
of Th17 cells by suppressing the expression of retinoic
acid-related orphan receptor (ROR)γ and RORα (55). In addition, IL-27 has a direct
effect on effector T cells and inhibited the development of
IL-17-producing Th cells (56).
Furthermore, IL-27 inhibited the expression of
granulocyte-macrophage colony-stimulating factor, which is a
regulator of Th17 cells (57).
Airway remodeling is a central feature of asthma
(42). This condition includes
epithelial changes, thickening of basement membrane, hypertrophy
and hyperplasia of airway smooth muscle, angiogenesis, increased
deposition of collagen proteins and subepithelial fibrosis
(58). Airway remodeling is the
main etiological factor of airflow limitation, bronchial
hyperreactivity and mucus production (51). In the present study, it was
initially shown that IL-27 could reduce the number of
mucus-containing airway goblet cells (PAS-positive), the thickening
of the basal membrane and the hyperplasia of airway smooth muscle
cells. Previous studies have shown that in airway epithelial cells,
both goblet cell hyperplasia and mucus overproduction can be
induced by IL-13 (59,60). In addition, certain Th2 cytokines
exhibit a negative effect on epithelial hypertrophy in a murine
model of asthma (41,61). Therefore, IL-27 may modulate
goblet cell proliferation, mucus production, and thickening of
airway wall via a pathway including IL-13 and other cytokines.
Subsequently, subepithelial fibrosis was examined,
which is an important feature of airway remodeling in asthma
(62). The data indicated that
OVA induced the activation of myofibroblasts (those expressing
α-SMA) in the experimental model of chronic asthma, whereas IL-27
inhibited this process. Furthermore, IL-27 decreased the area of
Masson's-stained sections, which reflected reduced deposition of
collagen, which is an ingredient of the extracellular matrix (ECM)
(62). It was reported that
higher numbers of fibroblasts are associated with severe asthma.
The accumulation of peribronchial myofibroblasts was detected in a
mouse model of chronic asthma (63,64). Myofibroblasts and fibroblasts are
the major sources of ECM proteins and deposition of the ECM, along
with other components, such as fibronectin and glycoproteins,
contributed to subepithelial fibrosis (62). IL-27 was found to suppress
differentiation of myofibroblasts and fibroblasts and thereby
alleviated the process of pulmonary fibrosis (65). Therefore, IL-27 may play a pivotal
role in subepithelial fibrosis in the experimental model of chronic
asthma.
Finally, the level of angiogenesis was assessed, and
the vascular surface area was quantified by immunohistochemical
staining of CD31. Therefore, the extent of airway vascularity was
increased in OVA-induced asthmatic mice compared with PBS-induced
subjects. These data are consistent with previous reports (66,67). Notably, the degree of angiogenesis
was reduced in the OVA+IL-27 group. Lung inflammation may
contribute to neovascularization and inflammation stimulated by
effector cells, such as eosinophils, mast cells and macrophages,
which are the major sources of various angiogenic and
lymphangiogenic factors (66).
Vascular endothelial growth factor (VEGF) may be an important
player in the development of angiogenesis in asthma, whereas IL-27
can suppress both VEGFA mRNA expression and protein secretion
(68). This suggested that IL-27
may control vascular remodeling. It was shown that IL-27 can
inhibit angiogenesis in the tumor microenvironment in various
malignancies such as prostate and lung cancer, which is a known
anti-tumor mechanism (69). Taken
together, the findings suggested that IL-27 can attenuate airway
remodeling in a chronic mouse model of asthma.
It was that IL-27 can activate multiple signaling
cascades, including Janus kinase (JAK) 1, JAK2 and tyrosine
kinase-2 (70). STAT enzymes are
the downstream proteins of JAKs (70). A previous in vitro study
revealed that the effects of IL-27 on epithelial cells are
dependent on STAT1 and STAT3 signaling pathways (71). IL-27 can exert its effects on T
cells by inducing the phosphorylation of STAT1/STAT3/STAT5. In the
current study, it was shown that both the STAT1 and STAT3 pathways
were suppressed in the OVA group, whereas the opposite effects were
noted in the OVA+IL-27 group, which indicated that the STAT1 and
STAT3 pathways were impaired in the OVA-induced mice model of
asthma. This impairment was reversed following IL-27 treatment. The
STAT1 pathway is critically important for IL-27 signaling, which
results in the induction of Th1 differentiation and the inhibition
of Th2 differentiation (44).
Following IL-27-stimulated activation of STAT1 signaling, the
expression of T-bet was induced. T-bet serves as the main regulator
of Th1 differentiation and of the expression of intercellular
adhesion molecule 1 (ICAM-1)/lymphocyte function-associated antigen
1 (LFA-1) (12). The latter
molecules interact and induce extracellular signal-regulated kinase
activation (ERK) (67). ERK is
located downstream of ICAM-1/LFA-1 and plays an important role in
modulating the polarizing of naive T-helper cells toward Th1
subsets (12). Accordingly, IL-27
promotes early Th1 commitment by enhanced expression of IL-12Rβ2
and production of IFN-γ (12,72). Formation of the IL-12 receptor
complex with IL-12 Rβ1 and IL-12 Rβ2 leads to the cells response to
IL-12, which is indispensable for Th1 initiation (72). In addition, IL-27 was reported to
activate T-bet and lead to IFN-γ secretion via the
p38/mitogen-activated protein kinase signaling pathway, which is
independent of JAK/STAT (11).
Moreover, STAT1 is required for the suppression of the Th2-specific
transcription factor, GATA-3 (73). In the presence of IL-2 and IL-4,
naïve Th cells express GATA-3 at a high level and develop into Th2
cells (16). By suppressing
GATA-3, IL-27 represses Th2 lineage commitment, which is dependent
on STAT1 (73). Moreover, T-bet
can directly interact with GATA-3, which interferes with the
binding of GATA-3 to its target DNA, and causes inhibition of Th2
differentiation (74). In the
present study, IL-27 upregulated T-bet expression and downregulated
GATA-3 expression. These findings are in concordance with previous
reports (12,23).
In addition to STAT1 activation, IL-27 can increase
STAT3 activation in naive CD4+T cells. Augmented activation of
STAT3 is found to serve as a counterbalance to activation of STAT1
(18). The activation of STAT3
and STAT1 are reciprocally regulated and their balance in
phosphorylation levels is key to maintaining appropriate immune
responses (75). IL-27-induced
activation of the STAT3 signaling pathway is important for
proliferation and differentiation of T-cells (45,70). The expression levels of T-bet and
GATA-3 can be modulated by STAT3 along with STAT1, leading to the
positive or negative regulation of Th1 and Th2 differentiation
(73). By activation of the STAT1
and STAT3 pathways, IL-27 also inhibited γδ T cells from producing
Th2-related cytokines, such as IL-5 and IL-13 (76) in the immune response against
tumors. STAT3 activation further plays an important role in Th17
cell differentiation (77).
Activated STAT3 directly binds to IL-17A and IL-17F promoters and
increased IL-17 gene transcription (77). In addition to the activation of
STAT3, the induction of RORγt, a master regulator of Th17 cell
differentiation, is required for the expression of genes encoding
IL-17 in naïve CD4+ Th cells (78). IL-27 can modulate the activity of
RORγt and plays an important role in the development of Th17 cell
differentiation (78). These
findings are in alignment with the evidence of the present study,
which indicated that the STAT1 and STAT3 pathways were suppressed
in the OVA group and activated in the OVA+IL-27 group. This
evidence demonstrated that IL-27 upregulated the phosphorylation of
STAT1 and STAT3 proteins and restored the STAT1 and STAT3 signaling
pathways in the asthma mouse model used.
In summary, the present study demonstrated that
intranasal administration of IL-27 could alleviate airway
inflammation and AHR by restored both the STAT1 and STAT3 pathways
in an experimental mouse model. The present study provided
additional evidence that IL-27 could ameliorate airway remodeling
in mice with chronic allergen exposure in vivo. These
findings will add insight into the multifaceted role of IL-27 in
asthma. Additional studies are required to ensure the potential
therapeutic applications of IL-27 in asthma. The exact mechanism of
IL-27 in therapeutic action requires further investigation.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL, JL and CZ contributed to the conception and
design of the present research. ZZ, JL, XJ, HP and FS performed the
experiments, collected and analyzed the data. JL, XJ and YJ
supervised the methods of all the experiments. JL, ZZ, HP, XJ and
FS wrote the initial draft of the manuscript. DL, JL, XJ and YJ
revised the manuscript. CZ critically reviewed the article. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All protocols were approved by the Ethics Committee
for Laboratory Animal Care and Use in Shandong Qianfoshan Hospital,
Shandong University (approval no. 2019-S-306). All procedures on
mice were performed in accordance with the National Institutes of
Health Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors thank Dr Xianchen Liu for critical
appraisal of the manuscript.
References
|
1
|
Godar M, Blanchetot C, de Haard H,
Lambrecht BN and Brusselle G: Personalized medicine with biologics
for severe type 2 asthma: Current status and future prospects.
MAbs. 10:34–45. 2018. View Article : Google Scholar :
|
|
2
|
Dominguez-Ortega J, Phillips-Anglés E,
Barranco P and Quirce S: Cost-effectiveness of asthma therapy: A
comprehensive review. J Asthma. 52:529–537. 2015. View Article : Google Scholar
|
|
3
|
Eder W, Ege MJ and von Mutius E: The
asthma epidemic. N Engl J Med. 355:2226–2235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wenzel SE: Asthma phenotypes: The
evolution from clinical to molecular approaches. Nat Med.
18:716–725. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnes CB and Ulrik CS: Asthma and
adherence to inhaled corticosteroids: Current status and future
perspectives. Respir Car. 60:455–468. 2015. View Article : Google Scholar
|
|
6
|
Conner JB and Buck PO: Improving asthma
management: The case for mandatory inclusion of dose counters on
all rescue bronchodilators. J Asthma. 50:658–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szefler SJ: Advances in pediatric asthma
in 2012: Moving toward asthma prevention. J Allergy Clin Immunol.
131:36–46. 2013. View Article : Google Scholar
|
|
8
|
Petronella SA and Conboy-Ellis K: Asthma
epidemiology: Risk factors, case finding, and the role of asthma
coalitions. Nurs Clin North Am. 38:725–735. 2003. View Article : Google Scholar
|
|
9
|
Pflanz S, Timans JC, Cheung J, Rosales R,
Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E,
et al: IL-27, a heterodimeric cytokine composed of EBI3 and p28
protein, induces proliferation of naive CD4+ T cells.
Immunity. 16:779–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hunter CA: New IL-12-family members: IL-23
and IL-27, cytokines with divergent functions. Nat Rev Immunol.
5:521–531. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meka RR, Venkatesha SH, Dudics S, Acharya
B and Moudgil KD: IL-27-induced modulation of autoimmunity and its
therapeutic potential. Autoimmun Rev. 14:1131–1141. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Owaki T, Asakawa M, Fukai F, Mizuguchi J
and Yoshimoto T: IL-27 induces Th1 differentiation via p38
MAPK/T-bet- and intercellular adhesion
Molecule-1/LFA-1/ERK1/2-dependent pathways. J Immunol.
177:7579–7587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang RX, Yu CR, Mahdi RM and Egwuagu CE:
Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune
uveitis by inhibiting autoreactive Th1/Th17 cells and promoting
expansion of regulatory. T cells J Biol Chem. 287:36012–36021.
2012. View Article : Google Scholar
|
|
14
|
Artis D, Villarino A, Silverman M, He W,
Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, et al:
The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive
elements of type 2 immunity. J Immunol. 173:5626–5634. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wills-Karp M: Immunologic basis of
antigen-induced airway hyperresponsiveness. Annu Rev Immunol.
17:255–281. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Venkayya R, Lam M, Willkom M, Grünig G,
Corry DB and Erle DJ: The Th2 lymphocyte products IL-4 and IL-13
rapidly induce airway hyperresponsiveness through direct effects on
resident airway cells. Am J Respir Cell Mol Biol. 26:202–208. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshimoto T, Yoshimoto T, Yasuda K,
Mizuguchi J and Nakanishi K: IL-27 suppresses Th2 cell development
and Th2 cytokines production from polarized Th2 cells: A novel
therapeutic way for Th2-mediated allergic inflammation. J Immunol.
179:4415–4423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie M, Mustovich AT, Jiang Y, Trudeau JB,
Ray A, Ray P, Hu H, Holguin F, Freeman B and Wenzel SE: IL-27 and
type 2 immunity in asthmatic patients: Association with severity,
CXCL9, and signal transducer and activator of transcription
signaling. J Allergy Clin Immunol. 135:386–394. 2015. View Article : Google Scholar
|
|
19
|
Fujita H, Teng A, Nozawa R,
Takamoto-Matsui Y, Katagiri-Matsumura H, Ikezawa Z and Ishii Y:
Production of both IL-27 and IFN-gamma after the treatment with a
ligand for invariant NK T cells is responsible for the suppression
of Th2 response and allergic inflammation in a mouse experimental
asthma model. J Immunol. 183:254–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyazaki Y, Inoue H, Matsumura M,
Matsumoto K, Nakano T, Tsuda M, Hamano S, Yoshimura A and Yoshida
H: Exacerbation of experimental allergic asthma by augmented Th2
responses in WSX-1-deficient mice. J Immunol. 175:2401–2407. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jirmo AC, Daluege K, Happle C, Albrecht M,
Dittrich AM, Busse M, Habener A, Skuljec J and Hansen G: IL-27 is
essential for suppression of experimental allergic asthma by the
TLR7/8 agonist R848 (Resiquimod). J Immunol. 197:4219–4227. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su X, Pan J, Bai F, Yuan H, Dong N, Li D,
Wang X and Chen Z: IL-27 attenuates airway inflammation in a mouse
asthma model via the STAT1 and GADD45γ/p38 MAPK pathways. J Transl
Med. 14:2832016. View Article : Google Scholar
|
|
23
|
Liu X, Li S, Jin J, Zhu T, Xu K, Liu C,
Zeng Y, Mao R, Wang X and Chen Z: Preventative tracheal
administration of interleukin-27 attenuates allergic asthma by
improving the lung Th1 microenvironment. J Cell Physiol.
234:6642–6653. 2019. View Article : Google Scholar
|
|
24
|
Villarino AV, Huang E and Hunter CA:
Understanding the pro- and anti-inflammatory properties of IL-27. J
Immunol. 173:715–720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirase T, Hara H, Miyazaki Y, Ide N,
Nishimoto-Hazuku A, Fujimoto H, Saris CJM, Yoshida H and Node K:
Interleukin 27 inhibits atherosclerosis via immunoregulation of
macrophages in mice. Am J Physiol Heart Circ Physiol.
305:H420–H429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Department of Health and Human Services:
Guide for the care and use of laboratory animals. (NIH Pub no
85-23). Washington, DC: 1985
|
|
27
|
Reddy AT, Lakshmi SP and Reddy RC: Murine
model of allergen induced asthma. J Vis Exp. e37712012.PubMed/NCBI
|
|
28
|
Harikrishnan VS, Hansen AK, Abelson KS and
Sørensen DB: A comparison of various methods of blood sampling in
mice and rats: Effects on animal welfare. Lab Anim. 52:253–264.
2018. View Article : Google Scholar
|
|
29
|
Kirstein F, Nieuwenhuizen NE, Jayakumar J,
Horsnell WGC and Brombacher F: Role of IL-4 receptor alpha-positive
CD4(+) T cells in chronic airway hyperresponsiveness. J Allergy
Clin Immunol. 137:1852–1862e9. 2016. View Article : Google Scholar
|
|
30
|
Hoymann HG: Lung function measurements in
rodents in safety pharmacology studies. Front Pharmacol. 3:1562012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Overmyer KA, Thonusin C, Qi NR, Burant CF
and Evans CR: Impact of anesthesia and euthanasia on metabolomics
of mammalian tissues: Studies in a C57BL/6J mouse model. PLoS One.
10:e01172322015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin TR, Gerard NP, Galli SJ and Drazen
JM: Pulmonary responses to bronchoconstrictor agonists in the
mouse. J Appl Physiol. 64:2318–2323. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leary S, Underwood W, Anthony R, Cartner
S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA,
Meyer R, et al: AVMA guidelines for the euthanasia of animals: 2013
edition. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf.
2013
|
|
34
|
Cataldo DD, Tournoy KG, Vermaelen K,
Munaut C, Foidart JM, Louis R, Noël A and Pauwels RA: Matrix
metalloproteinase-9 deficiency impairs cellular infiltration and
bronchial hyperresponsiveness during allergen-induced airway
inflammation. Am J Pathol. 161:491–498. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Polte T, Behrendt AK and Hansen G: Direct
evidence for a critical role of CD30 in the development of allergic
asthma. J Allergy Clin Immunol. 118:942–948. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Albrecht M, Chen HC, Preston-Hurlburt P,
Ranney P, Hoymann HG, Maxeiner J, Staudt V, Taube C, Bottomly HK
and Dittrich AM: T(H)17 cells mediate pulmonary collateral priming.
J Allergy Clin Immunol. 128:168–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
38
|
Loke WS, Freeman A, Garthwaite L,
Prazakova S, Park M, Hsu K, Thomas PS and Herbert C: T-bet and
interleukin-27: Possible TH 1 immunomodulators of sarcoidosis.
Inflammopharmacology. 23:283–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kelly-Welch AE, Melo ME, Smith E, Ford AQ,
Haudenschild C, Noben-Trauth N and Keegan AD: Complex role of the
IL-4 receptor alpha in a murine model of airway inflammation:
Expression of the IL-4 receptor alpha on nonlymphoid cells of bone
marrow origin contributes to severity of inflammation. J Immunol.
172:4545–4555. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tang X, Nian H, Li X, Yang Y, Wang X, Xu
L, Shi H, Yang X and Liu R: Effects of the combined extracts of
Herba Epimedii and Fructus Ligustrilucidi on airway remodeling in
the asthmatic rats with the treatment of budesonide. BMC Complement
Altern Med. 17:3802017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Komai M, Tanaka H, Masuda T, Nagao K,
Ishizaki M, Sawada M and Nagai H: Role of Th2 responses in the
development of allergen-induced airway remodelling in a murine
model of allergic asthma. Br J Pharmacol. 138:912–920. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kohan M, Breuer R and Berkman N:
Osteopontin induces airway remodeling and lung fibroblast
activation in a murine model of asthma. Am J Respir Cell Mol Biol.
41:290–296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eifan AO, Orban NT, Jacobson MR and Durham
SR: Severe persistent allergic rhinitis. Inflammation but no
histologic features of structural upper airway remodeling. Am J
Respir Crit Care Med. 192:1431–1439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Iwasaki Y, Fujio K, Okamura T and Yamamoto
K: Interleukin-27 in T cell immunity. Int J Mol Sci. 16:2851–2863.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma N, Fang Y, Xu R, Zhai B, Hou C, Wang X,
Jiang Z, Wang L, Liu Q, Han G and Wang R: Ebi3 promotes T- and
B-cell division and differentiation via STAT3. Mol Immunol.
107:61–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hamid Q and Tulic M: Immunobiology of
asthma. Annu Rev Physiol. 71:489–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
O'Byrne PM and Inman MD: Airway
hyperresponsiveness. Chest. 123(3 Suppl): S411–S416. 2003.
View Article : Google Scholar
|
|
48
|
Kaczka DW, Ingenito EP, Israel E and
Lutchen KR: Airway and lung tissue mechanics in asthma. Effects of
albuterol. Am J Respir Crit Care Med. 159:169–178. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tait Wojno ED, Hunter CA and Stumhofer JS:
The immunobiology of the interleukin-12 family: Room for discovery.
Immunity. 50:851–870. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tumes DJ, Papadopoulos M, Endo Y, Onodera
A, Hirahara K and Nakayama T: Epigenetic regulation of T-helper
cell differentiation, memory, and plasticity in allergic asthma.
Immunol Rev. 278:8–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jacobsen EA, Doyle AD, Colbert DC, Zellner
KR, Protheroe CA, LeSuer WE, Lee NA and Lee JJ: Differential
activation of airway eosinophils induces IL-13-mediated allergic
Th2 pulmonary responses in mice. Allergy. 70:1148–1159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pappu R, Ramirez-Carrozzi V and Sambandam
A: The interleukin-17 cytokine family: Critical players in host
defence and inflammatory diseases. Immunology. 134:8–16. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vultaggio A, Nencini F, Pratesi S, Maggi
L, Guarna A, Annunziato F, Romagnani S, Parronchi P and Maggi E:
The TLR7 ligand 9-benzyl-2-butoxy-8-hydroxy adenine inhibits IL-17
response by eliciting IL-10 and IL-10-inducing cytokines. J
Immunol. 186:4707–4715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chesné J, Braza F, Mahay G, Brouard S,
Aronica M and Magnan A: IL-17 in severe asthma. Where do we stand?
Am J Respir Crit Care Med. 190:1094–1101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Diveu C, McGeachy MJ, Boniface K,
Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan
TK, de Waal Malefyt R, et al: IL-27 blocks RORc expression to
inhibit lineage commitment of Th17 cells. J Immunol. 182:5748–5756.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Muallem G, Wagage S, Sun Y, DeLong JH,
Valenzuela A, Christian DA, Harms Pritchard G, Fang Q, Buza EL,
Jain D, et al: IL-27 limits type 2 immunopathology following
Parainfluenza Virus infection. PLoS Pathog. 13:e10061732017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Young A, Linehan E, Hams E, O'Hara Hall
AC, McClurg A, Johnston JA, Hunter CA, Fallon PG and Fitzgerald DC:
Cutting edge: Suppression of GM-CSF expression in murine and human
T cells by IL-27. J Immunol. 189:2079–2083. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Russell RJ and Brightling C: Pathogenesis
of asthma: Implications for precision medicine. Clin Sci (Lond).
131:1723–1735. 2017. View Article : Google Scholar
|
|
59
|
Tanabe T, Fujimoto K, Yasuo M, Tsushima K,
Yoshida K, Ise H and Yamaya M: Modulation of mucus production by
interleukin-13 receptor alpha2 in the human airway epithelium. Clin
Exp Allergy. 38:122–134. 2008. View Article : Google Scholar
|
|
60
|
Tanabe T and Rubin BK: Airway goblet cells
secrete Pro-inflammatory cytokines, chemokines, and growth factors.
Chest. 149:714–720. 2016. View Article : Google Scholar
|
|
61
|
Foster PS, Ming Y, Matthei KI, Young IG,
Temelkovski J and Kumar RK: Dissociation of inflammatory and
epithelial responses in a murine model of chronic asthma. Lab
Invest. 80:655–662. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fernandes DJ, Bonacci JV and Stewart AG:
Extracellular matrix, integrins, and mesenchymal cell function in
the airways. Curr Drug Targets. 7:567–577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Benayoun L, Druilhe A, Dombret MC, Aubier
M and Pretolani M: Airway structural alterations selectively
associated with severe asthma. Am J Respir Crit Care Med.
167:1360–1368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Miller M, Cho JY, McElwain K, McElwain S,
Shim JY, Manni M, Baek JS and Broide DH: Corticosteroids prevent
myofibroblast accumulation and airway remodeling in mice. Am J
Physiol Lung Cell Mol Physiol. 290:L162–169. 2006. View Article : Google Scholar
|
|
65
|
Dong Z, Zhao X, Tai W, Lei W, Wang Y, Li Z
and Zhang T: IL-27 attenuates the TGF-β1-induced proliferation,
differentiation and collagen synthesis in lung fibroblasts. Life
Sci. 146:24–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Detoraki A, Granata F, Staibano S, Rossi
FW, Marone G and Genovese A: Angiogenesis and lymphangiogenesis in
bronchial asthma. Allergy. 65:946–958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hoshino M, Nakamura Y and Hamid QA: Gene
expression of vascular endothelial growth factor and its receptors
and angiogenesis in bronchial asthma. J Allergy Clin Immunol.
107:1034–1038. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang Q, da Cunha AP, Li S, Hao Q, Kainz
V, Huang Q and Wu HY: IL-27 regulates HIF-1α-mediated VEGFA
response in macrophages of diabetic retinopathy patients and
healthy individuals. Cytokine. 113:238–247. 2019. View Article : Google Scholar
|
|
69
|
Di Carlo E, Sorrentino C, Zorzoli A, Di
Meo S, Tupone MG, Ognio E, Mincione G and Airoldi I: The antitumor
potential of Interleukin-27 in prostate cancer. Oncotarget.
5:10332–10341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kamiya S, Owaki T, Morishima N, Fukai F,
Mizuguchi J and Yoshimoto T: An indispensable role for STAT1 in
IL-27-induced T-bet expression but not proliferation of naive CD4+
T cells. J Immunol. 173:3871–3877. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Diegelmann J, Olszak T, Goke B, Blumberg
RS and Brand S: A novel role for interleukin-27 (IL-27) as mediator
of intestinal epithelial barrier protection mediated via
differential signal transducer and activator of transcription
(STAT) protein signaling and induction of antibacterial and
anti-inflammatory proteins. J Biol Chem. 287:286–298. 2012.
View Article : Google Scholar
|
|
72
|
Takeda A, Hamano S, Yamanaka A, Hanada T,
Ishibashi T, Mak TW, Yoshimura A and Yoshida H: Cutting edge: Role
of IL-27/WSX-1 signaling for induction of T-bet through activation
of STAT1 during initial Th1 commitment. J Immunol. 170:4886–4890.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lucas S, Ghilardi N, Li J and de Sauvage
FJ: IL-27 regulates IL-12 responsiveness of naive CD4+ T cells
through Stat1-dependent and -independent mechanisms. Proc Natl Acad
Sci USA. 100:15047–15052. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hwang ES, Szabo SJ, Schwartzberg PL and
Glimcher LH: T helper cell fate specified by kinase-mediated
interaction of T-bet with GATA-3. Science. 307:430–433. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Regis G, Pensa S, Boselli D, Novelli F and
Poli V: Ups and downs: The STAT1:STAT3 seesaw of Interferon and
gp130 receptor signalling. Semin Cell Dev Biol. 19:351–359. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Morandi F, Prigione I and Airoldi I: Human
TCRγδ+ T cells represent a novel target for IL-27 activity. Eur J
Immunol. 42:1547–1552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hwang ES: Transcriptional regulation of T
helper 17 cell differentiation. Yonsei Med J. 51:484–491. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ivanov II, McKenzie BS, Zhou L, Tadokoro
CE, Lepelley A, Lafaille JJ, Cua DJ and Littman DR: The orphan
nuclear receptor RORgammat directs the differentiation program of
proinflammatory IL-17+ T helper cells. Cell.
126:1121–1133. 2006. View Article : Google Scholar : PubMed/NCBI
|