Introduction
Cardiovascular disease (CVD) is one of the leading
causes of mortality, with an annual mortality rate of >17.3
million worldwide (1).
Atherosclerosis (AS) constitutes a large subgroup of CVD and is the
biggest cause of heart attack and stroke, accompanied by
inflammation, and characterized by lipid accretion and fibrous cap
and necrotic core formation (2).
The formation of AS lesions involves a diverse range of cells,
including endothelial cells (ECs), smooth muscle cells (SMCs), and
macrophages (3). It is well-known
that vascular SMCs (VSMCs) are the major components of the arterial
wall. Previous studies have also demonstrated that the abnormal
proliferation and migration of VSMCs are important
patho-physiological processes in the development of atherosclerosis
and that adequate VSMC function is crucial in protecting the vessel
wall against atherosclerosis (4,5).
The abnormal proliferation, apoptosis, autophagy, and migration of
VSMCs are closely associated to AS advancement (6,7).
Depending on this, VSMCs were selected in the present study to
explore the role of CTBP1-AS2 on cell function. Autophagy also
serves an important role in AS (8). Oxidized low-density lipoprotein
(ox-LDL), a primary factor in the promotion of AS progression,
causes VSMC proliferation, invasion, and apoptosiss (9). Therefore, the regulatory mechanisms
involved in AS were explored using ox-LDL-stimulated VSMCs as an AS
cell model.
Long non-coding RNAs (lncRNAs) measure 200
nucleotides (nt) long and are a category of non-coding (nc)RNAs
that serve as core modulators in manifold complex diseases,
including AS (10,11). lncRNAs associated with AS have
received extensive attention due to their involvement in the
inflammatory response, lipid metabolism, cell proliferation,
adhesion, apoptosis and migration (12). For example, lncRNA p21 exhibits an
overtly decreased expression in AS plaques of Apolipoprotein
E-/- mice and strengthens p53 activity to curb VSMCs and
mononuclear macrophage cells of mice to proliferate and boost their
apoptosis (13). The
downregulation of lncRNA RNCR3 accelerates AS progression by
inhibiting the migration and proliferation of ECs and VSMCs,
thereby resulting in their apoptosis in vitro (14). lncRNA CTPB1-AS2 is an
lncRNA that acts a novel modulator of cardiomyocyte hypertrophy by
regulating TLR4 and an oncogene in papillary thyroid cancer
(15). However, its role in AS
has not been explored.
MicroRNAs (miRNAs) are small ncRNAs measuring ~22 nt
that post-transcriptionally modulate genes (16). Numerous miRNAs are pivotal
modulators in AS pathological processes, including immune
responses, lipid and cholesterol biosynthesis, endotheliocyte
vascular and biological functions, cholesterol effluence, and
lipoprotein metabolism (17).
miR-195-5p is located on chromosome 17p13.1 and belongs to the
miR-15a/b/16/195/497 family (18). miR-195-5p is aberrantly expressed
and serve a role as tumor suppressor in a number of types of cancer
in humans, including prostate (19) and breast cancer (20), and hepatocellular carcinoma
(21); however, its functions in
AS is rarely known.
In the present study, lncRNA CTPB1-AS2 was
downregulated among patients with AS, and miR-195-5p was identified
as a potential target gene. The study aimed to investigate the
possible effects and molecular characteristics of CTBP1-AS2
in VSMC progression, to determine the potential targets of AS
treatment.
Materials and methods
Clinical samples
The present study was approved by The First
Affiliated Hospital of Anhui Medical University. All samples were
collected between February 2015 and October 2019. The subjects,
aged between 50-70 years (mean ± standard deviation, 61.21±1.302
years), including 21 males and 3 females, provided written informed
consent. Then, 10 ml blood samples were collected from 24 patients
with untreated AS and 24 healthy volunteers (age range, 50-70
years; mean ± standard deviation, 58.88±1.081 years; 20 males and 4
females) into anticoagulant-free centrifuge tubes in the hospital.
The healthy volunteers also provided written informed consent. The
enrollment criteria for healthy volunteers were as follows:
Individuals without AS, inflammatory diseases, malignant tumors,
autoimmune diseases, or recent infection (< 1 month). The
collected blood samples were preserved at room temperature for 1 h
and centrifuged to extract serum at 1,006 x g, 4°C for 5 min.
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was
used for RNA isolation from the serum.
Cell culture
Human aorta VSMCs (HA-VSMCs) were preserved in F-12
K medium acquired from American Type Culture Collection, and the
medium contained 10% fetal bovine saline (Invitrogen; Thermo Fisher
Scientific, Inc.), 0.05 mg/ml ascorbic acid, 0.01 mg/ml
transferrin, 0.01 mg/ml insulin, 10 ng/ml sodium selenite, 10 mM
TES and 10 mM HEPES (Sigma-Aldrich; Merck KGaA), and 0.03 mg/ml
endothelial cell growth supplement purchased from Cell Applications
Inc., in a humid incubator under 5% CO2 and 37°C.
Cell transfection and treatment
Full length CTBP1-AS2 sequences were
amplified via PCR and subcloned into pcDNA3.1 vectors (Invitrogen;
Thermo Fisher Scientific, Inc.) to generate pcDNA-CTBP1-AS2
overexpression (OE) plasmids. The small interfering RNAs (siRNAs)
specific to CTBP1-AS2, miR-195-5p mimic, and miR-195-5p
inhibitor and their corresponding negative controls si negative
control (NC), mimic NC, and inhibitor NC were designed and
synthesized by Shanghai GenePharma Co. Ltd.
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was utilized for cell transfection following the
manufacturer's protocol. HA-VSMCs were treated with ox-LDL (0, 25,
50 and 100 μg/ml; Beijing Biosynthesis Biotechnology Co.,
Ltd.) for 1 day to determine its effects on CTBP1-AS2 and
miR-195-5p expression levels. ROC-325 (MedChemExpress), a novel
inhibitor of autophagy, was dissolved in double distilled water
(ddH2O) and applied to inhibit cell autophagy. Following
treatment with 5 μM ROC-325 for 24 h, the levels of
autophagy proteins LC3I/II and Beclin1 were examined.
Reverse transcription-quantitative
(RT-q)PCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for total RNA isolation following
the manufacturer's guidelines. Then, 1 μg RNA from each
serum or cell sample was reverse transcribed into first-strand cDNA
using RT primers (miR-195-5p and U6 small nucleolar RNA) or random
primers (CTBP1-AS2 and β-actin) and M-MLV reverse
transcriptase from Invitrogen; Thermo Fisher Scientific, Inc.
CTBP1-AS2, autophagy related 14 (ATG14), GAPDH, miR-195-5p
and U6 expression levels were examined using qPCR primers and SYBR
Green Real-Time PCR Master Mix provided by Toyobo Life Sciences.
The 2−ΔΔCq method (22) was used to calculate the relative
gene expression normalized by GAPDH and U6. The sequences of the
primers were listed below: CTBP1-AS2 forward, 5′-GAG CCC TGA
TTT ACC GTC CG-3′ and reverse, 5′-AAGGGGAGACGTAGGCTTCT-3′; ATG14
forward, 5′-CATAACAACCCCGCCTACAC-3′ and reverse,
5′-TGCGTTCAGTTTCCTCACTG-3′; miR-195-5p forward,
5′-GGGGTAGCAGCACAGAAAT-3′ and reverse, 5′-TCCAGTGCGTGTCGTGGA-3′; U6
forward, 5′-CTCGCTTCGGCAGCAGCACATATA-3′ and reverse,
5′-AAATATGGAACGCTTCACGA-3′; GAPDH forward,
5′-GAAGAGAGAGACCCTCACGCTG-3′ and reverse,
5′-ACTGTGAGGAGGGGAGATTCAGT-3′.
Western blot analysis
RIPA lysis buffer (Beyotime Institute of
Biotechnology) and a Pierce bicinchoninic acid Protein Assay kit
(Thermo Fisher Scientific, Inc.) were used to isolate and quantify
whole proteins, respectively. Then, proteins (50 μg) were
separated using 10% SDS-PAGE and transferred onto PVDF membranes
(EMD Millipore). The membranes were blocked with fat-free milk (5%)
for 1 h at room temperature and incubated at 4°C overnight with
primary antibodies against the following: Proliferating cell
nuclear antigen (PCNA; cat. no. ab92552; 1:1,000), GAPDH (cat. no.
ab181602; 1:2,500), proliferation marker protein Ki-67 (cat. no.
ab92742; 1:2,000), Beclin-1 (cat. no. ab62557; 1:1,000) and
microtubule-associated proteins 1A/1B light chain 3B (LC3; cat. no.
ab51520; 1:1,000). All primary antibodies were from Abcam. The
membranes were then incubated at room temperature for 1 h with
horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit
secondary antibody (cat. nos. ab97040 and ab97080, respectively;
1:3,000) from Abcam. Clarity Max™ Western ECL Substrate
provided by Bio-Rad Laboratories, Inc., was used to amplify certain
protein signals. Image J (V1.8.0.112; National Institutes of
Health) was used to perform the densitometric analysis.
CCK-8 experiment
Subsequent to seeding onto 96-well plates at a
density of ~1x104/well overnight, HA-VSMCs were
transfected for 0, 24, 48 or 72 h, and then 10 μl Cell
Counting Kit-8 solution (MedChemExpress) was added to each well and
incubated at 37°C for 3 h. Cell proliferation was evaluated by
measuring the absorbance (A450).
Colony formation experiment
Cells in the logarithmic growth phase were digested
into a single cell layer using 0.25% trypsin and then suspended in
F-12 K medium. Each group was inoculated with 200 cells per dish.
Following cultivation for 15 days in complete medium, the
transfected HA-VSMCs were fixed using 75% methanol for 15 min at
room temperature and stained with 0.1% crystal violet solution
(Sigma-Aldrich; Merck KGaA) at room temperature for 20 min.
Finally, after washing, images of the cells were captured and cells
were counted using light microscopy (magnification, x10), and the
colony-forming efficiency was calculated using the following
formula: Colony forming efficiency=clone number/inoculated cell
number x100%.
Intracellular protein segregation
A Cytoplasmic and Nuclear RNA Purification kit
(Norgen Biotek Corp.) was used for separation and purification of
RNAs in the nucleus and cytoplasm of HA-VSMCs according to the
manufacturer's guidelines. Next, CTBP1-AS2, GAPDH and U6
expression levels in nuclear and cytoplasmatic segments were
independently examined via RT-qPCR.
Bioinformatics analysis
DIANA tools-miRGenv3 (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=mirgenv3%2Findex)
was applied to predict the target gene of CTBP1-AS2.
Luciferase reporter assay
PCR amplification was implemented for specific
segments of CTBP1-AS2 and ATG14 3′ untranslated regions
(UTRs) with miR-195-5p binding sites, and these segments were
created into psiCHECK-2 vector (Promega Corporation). Therefore,
wild-type lncRNA CTBP1-AS2 and ATG14 reporter plasmids were
generated. A QuikChange Multi Site-Directed Mutagenesis kit
(Stratagene; Agilent Technologies, Inc.) was used to generate
mutated lncRNA CTBP1-AS2 and ATG14 reporters with mutational
miR-195-5p binding sites. HA-VSMCs were then transfected with
miRNAs or plasmids and the created luciferase reporters,
independently. After 48 h, a dual-luciferase reporter assay kit
(Promega Corporation) was utilized to detect cell luciferase
activity according to the protocols of the manufacturer.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used for statistical
analysis of all data. Each experiment was repeated a minimum of
three times, and data are presented as mean ± standard error of the
mean. Pearson's analysis was performed to analyze the correlation
between miR-195-5p and lncRNA CTBP1-AS2. Comparisons between
groups were performed using Student's t-test or one-way ANOVA
followed by Bonferroni's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
CTBP1-AS2 level is decreased and
miR-195-5p is increased in AS serum and HA-VSMCs treated with
ox-LDL
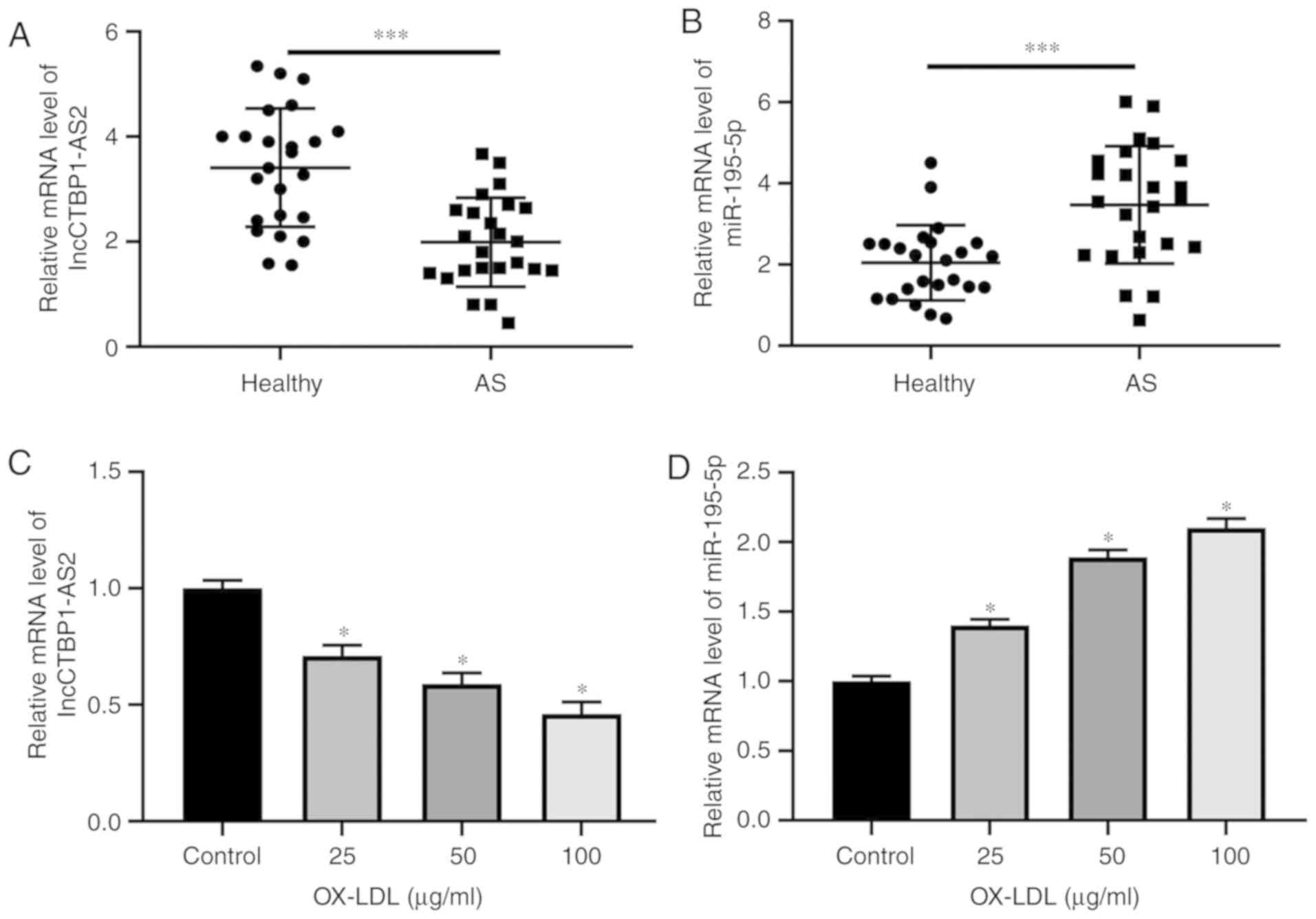
CTBP1-AS2 and miR-195-5p expression levels in the
serum of patients with AS and HA-VSMCs treated with ox-LDL were
examined. RT-qPCR experiments revealed that CTBP1-AS2 level
decreased significantly (Fig. 1A)
and miR-195-5p level increased significantly (Fig. 1B) in the serum of patients with AS
compared with that of healthy volunteers. Treatment with ox-LDL
dose-dependently decreased CTBP1-AS2 expression levels
(Fig. 1C) and increased
miR-195-5p expression levels (Fig.
1D) in HA-VSMCs. CTBP1-AS2 and miR-195-5p may be pivotal
factors in AS advancement.
OE of CTBP1-AS2 inhibits proliferation in
HA-VSMCs treated with ox-LDL and triggers their autophagy
The effects of CTBP1-AS2 on the proliferation
and autophagy of HA-VSMCs treated with ox-LDL was detected by the
use of OE-CTBP1-AS2. The RT-qPCR results indicated that
OE-CTBP1-AS2 markedly increased CTBP1-AS2 expression
compared with the negative control (NC) in HA-VSMCs treated with
ox-LDL (Fig. 2A). The CCK-8 assay
results suggested that CTBP1-AS2 markedly decreased the
level of proliferation in HA-VSMCs treated with ox-LDL compared
with the NC (Fig. 2B). This
result was corroborated by the results from the colony formation
experiment (Fig. 2C). PCNA and
Ki-67 expression levels in HA-VSMCs treated with ox-LDL were
examined, and their expression levels exhibited a marked decrease
subsequent to CTBP1-AS2 overexpression (Fig. 2D and E). The overexpression of
CTBP1-AS2 markedly promoted autophagy, and resulted in
significant increases in the lipid-modified LC3/GAPDH ratio and in
the Beclin-1 protein expression level in HA-VSMCs compared with the
NC (Fig. 2F). Rescue experiments
were conducted to determine the association between VSMC
proliferation and autophagy. As demonstrated in Fig. 2G, treating cells with 5 μM
ROC-325, an inhibitor of autophagy, in combination with
CTBP1-AS2 OE plasmids partly reversed the ox-LDL-mediated
increase in cell proliferation. CTBP1-AS2 inhibited the
proliferation of HA-VSMCs treated with ox-LDL by increasing the
levels of autophagy.
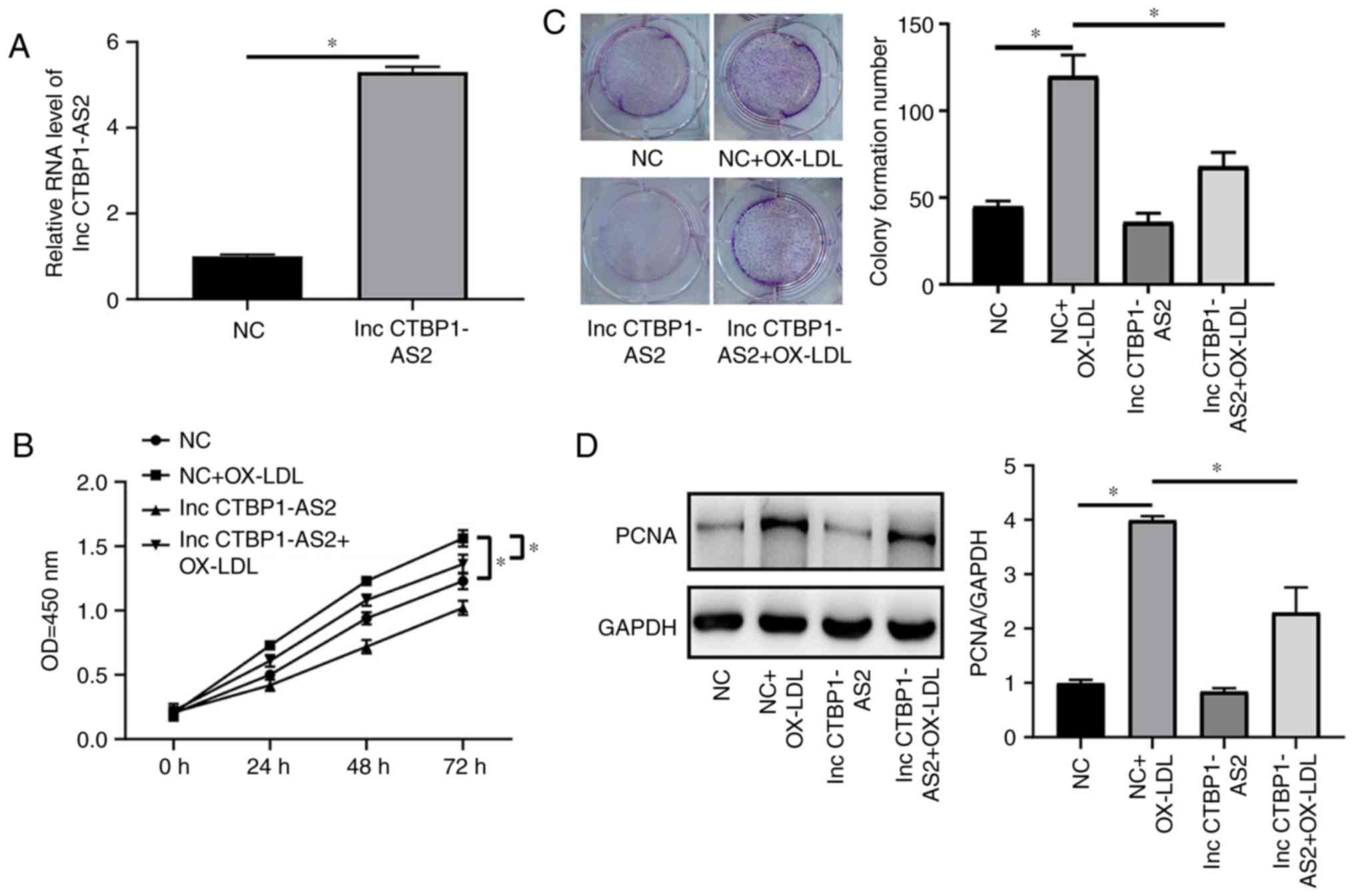 | Figure 2Overexpression of CTBP1-AS2 inhibits
the level of proliferation in HA-VSMCs treated with ox-LDL and
triggers their autophagy. CTBP1-AS2-treated HA-VSMCs were
treated with 50 μg/ml ox-LDL, and (A) CTBP1-AS2
expression, (B) cell formation, (C) colony formation, (D) PCNA and
(E) Ki-67 expression, and (F) autophagy-associated proteins LC3 and
Beclin-1 expression were measured. (G) The proliferation of cells
co-transfected with CTBP1-AS2 and ROC-325 was detected. Data
are presented as mean ± standard error of the mean of at least 3
independent experiments. *P<0.05. HA-VSMCs, human
aorta vascular smooth muscle cells; ox-LDL, oxidized low-density
lipoprotein; PCNA, proliferating cell nuclear antigen; Ki-67,
proliferation marker protein Ki-67; LC3, microtubule-associated
proteins 1A/1B light chain 3B; LC3I, cytoplasmic LC3; LC3II,
lipid-modified LC3; NC, negative control; OD, optical density; lnc,
long non-coding RNA. |
CTBP1-AS2 prevents miR-195-5p expression
by direct interaction
Bioinformatics analysis was used to ascertain the
potential targets of CTBP1-AS2. Complementary sites between
CTBP1-AS2 and miR-195-5p were identified (Fig. 3A). As demonstrated in Fig. S1, other potential target miRNAs
were also detected; however, no significant difference between the
patients with AS and the controls was identified (Fig. S1A-F). CTBP1-AS2 was
primarily located in the cytoplasm of HA-VSMCs, as verified by the
intracellular segregation experiment, indicating the potential
interaction between CTBP1-AS2 and miR-195-5p due to their
shared location (Fig. 3B). The
luciferase reporter assay verified that treatment with the
miR-195-5p mimic decreased the luciferase activity in wild-type
CTBP1-AS2 reporter plasmid by ~48% compared with that in the
scramble control (Fig. 3C).
Nevertheless, miR-195-5p did not affect the luciferase activity of
the mutated CTBP1-AS2 reporter plasmid, which had mutated
assumed binding sites between CTBP1-AS2 and miR-195-5p
(Fig. 3C). An RT-qPCR assay was
implemented to determine the effects of CTBP1-AS2 on
miR-195-5p expression. CTBP1-AS2 upregulation decreased
miR-195-5p expression, whereas si-CTBP1-AS2 transfection
significantly increased miR-195-5p expression (Fig. 3D and E). miR-195-5p expression
exhibited an inverse correlation with CTBP1-AS2 levels in
the serum of 24 patients with AS (Fig. 3F). These data confirmed that
CTBP1-AS2 inhibited miR-195-5p expression by direct
reciprocal action.
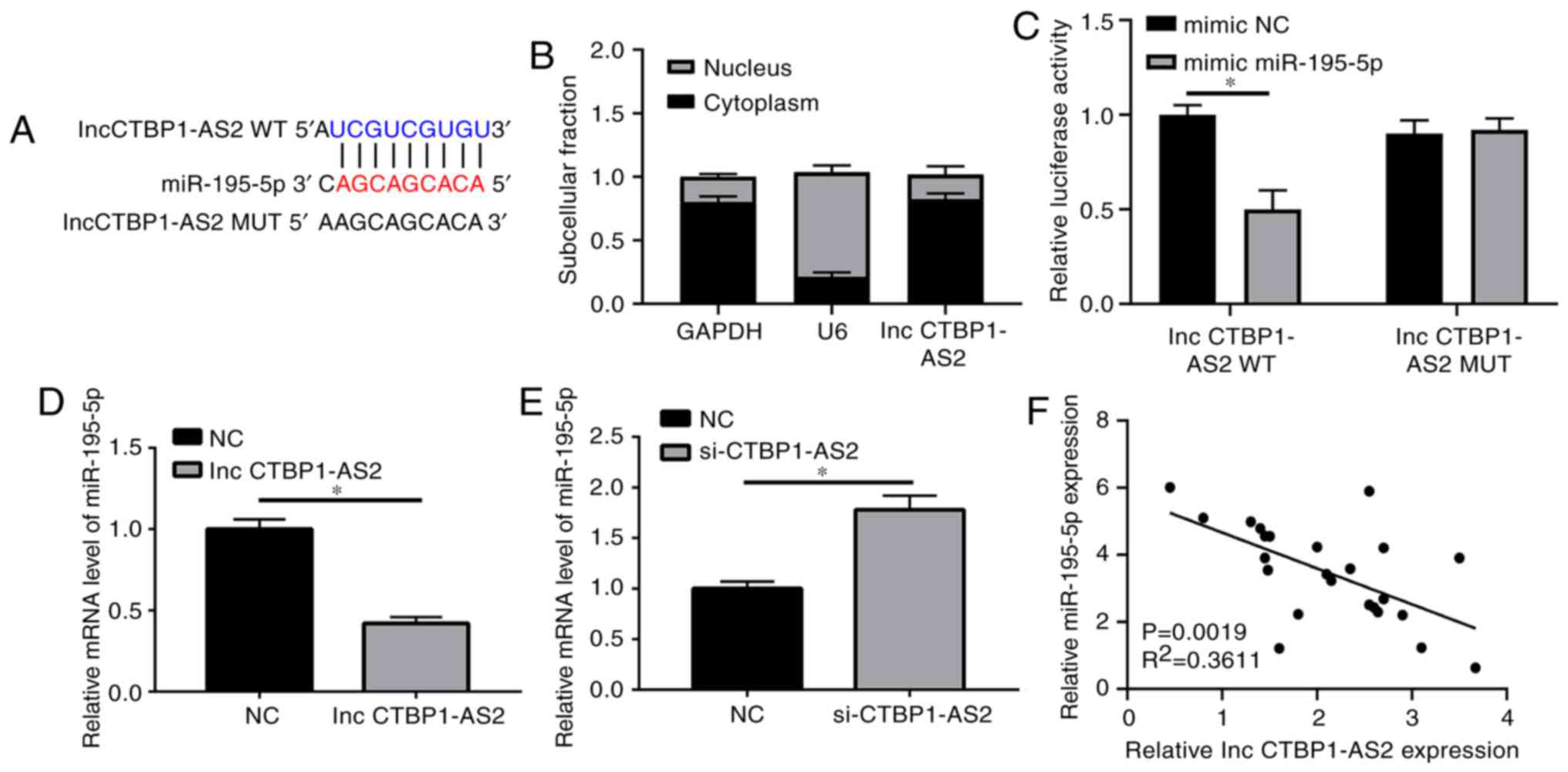 | Figure 3CTBP1-AS2 prevents miR-195-5p
expression by direct interaction. (A) Predicted binding sites
between CTBP1-AS2 and miR-195-5p, and the mutated sites in
the MUT CTBP1-AS2 reporter plasmid. GAPDH, U6 and
CTBP1-AS2 expression levels in the cytoplasm and nucleus of
HA-VSMCs. GAPDH was used as a transcript control in the cytoplasm
and U6 as a transcript control in the nucleus. (B) The percentage
fractions of GAPDH, U6 and CTBP1-AS2 proteins in the
cytoplasm and nucleus. (C) Luciferase activity of HA-VSMCs treated
with WT or MUT CTBP1-AS2 reporter plasmids and miR-195-5p or
mimic NC. (D) Decreased miR-195-5p expression following
upregulation of CTBP1-AS2 expression, and (E) increased
miR-195-5p expression following downregulation of CTBP1-AS2
expression. (F) CTBP1-AS2 was inversely correlated with
miR-195-5p expression in patients with atherosclerosis. Data are
presented as mean ± standard error of the mean of at least 3
independent experiments. *P<0.05. miR, microRNA; WT,
wild-type; MUT, mutant; HA-VSMCs, human aorta vascular smooth
muscle cells; NC, negative control; si, small interfering RNA; lnc,
long non-coding RNA. |
miR-195-5p mimic counteracts the effects
of CTBP1-AS2 on the levels of proliferation and autophagy in
HA-VSMCs treated with ox-LDL
The RT-qPCR assay results demonstrated that the
miR-195-5p mimic induced miR-195-5p expression and promoted
miR-195-5p expression via CTBP1-AS2 in HA-VSMCs treated with
ox-LDL (Fig. 4A). Co-treatment
with miR-195-5p and ox-LDL promoted HA-VSMCs to proliferate and
form colonies (Fig. 4B and C).
The CCK-8, colony formation and western blot assays results showed
that the effect of CTBP1-AS2 on cell growth was remarkably
abrogated by the miR-195-5p mimic, as demonstrated by increased
cell proliferation (Fig. 4B),
colony formation (Fig. 4C), and
PCNA and Ki-67 expression levels (Fig. 4D). miR-195-5p significantly
counteracted CTBP1-AS2-mediated autophagy, as shown by the
increased level of the autophagy-associated proteins (Fig. 4E). miR-195-5p can modulate the
CTBP1-AS2-mediated effects by inhibiting proliferation and
promoting autophagy in HA-VSMCs treated with ox-LDL.
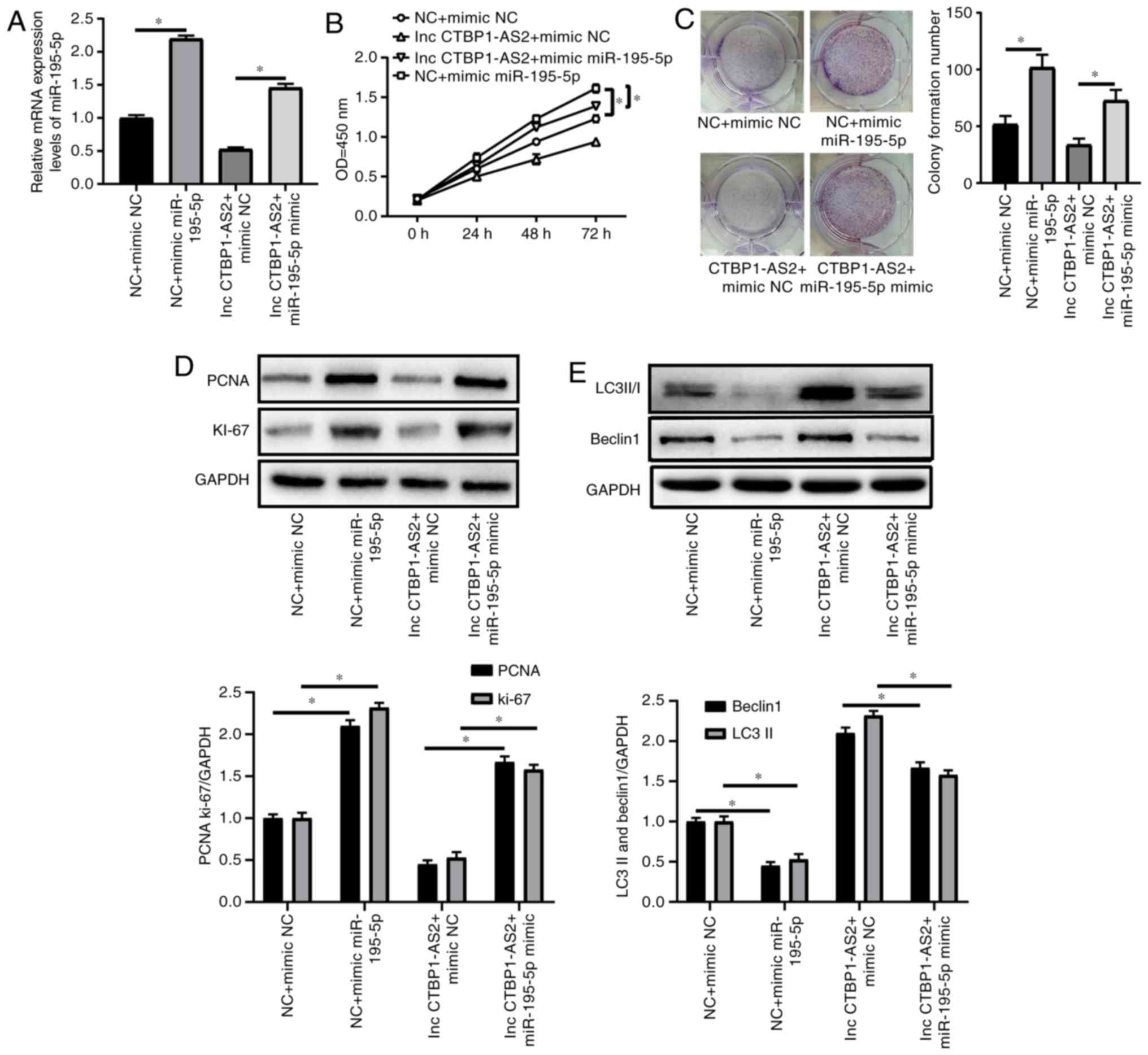 | Figure 4miR-195-5p mimic counteracts the
effects of CTBP1-AS2 on the levels of proliferation and autophagy
in HA-VSMCs treated with ox-LDL. (A-C) HA-VSMCs were transfected
with si NC + mimic NC, si NC + mimic miR-195-5p, CTBP1-AS2 +
mimic NC, or CTBP1-AS2 and mimic miR-195-5p and then treated
with ox-LDL (50 μg/ml) for 1 day, and (A) miR-195-5p, (B)
cell proliferation (C) and colony formation were assessed. (D and
E) HA-VSMCs were transfected with si NC + mimic NC, si NC + mimic
miR-195-5p, CTBP1-AS2 + mimic NC, or CTBP1-AS2 and
mimic miR-195-5p and then treated with ox-LDL (50 μg/ml) for
1 day, and the levels of (D) PCNA and Ki-67, and (E) LC3 and
Beclin-1 expression were detected. Data are presented as mean ±
standard error of the mean of at least 3 independent experiments.
*P<0.05. miR, microRNA; HA-VSMCs, human aorta
vascular smooth muscle cells; NC, negative control; si, small
interfering RNA; PCNA, proliferating cell nuclear antigen; Ki-67,
proliferating marker protein Ki-67; LC3, microtubule-associated
proteins 1A/1B light chain 3B; LC3I, cytoplasmic LC3; LC3II,
lipid-modified LC3; OD, optical density; lnc, long non-coding
RNA. |
ATG14 is a target of miR-195-5p
Multiple studies have revealed that lncRNAs modulate
target mRNA expressions via their function as competing endogenous
RNAs (ceRNAs) of miRNAs (23-25). Bioinformatics methods were used to
find the potential targets of miR-195-5p. The results revealed that
potential binding sequences of miR-195-5p were present in the ATG14
3′UTR (Fig. 5A). Concomitantly,
the relative expression levels of other potential target genes in
AS were also detected, but no significant difference was observed
(Fig. S1G-L). Subsequently, the
dual luciferase reporter assay showed that miR-195-5p evidently
inhibited the luciferase activity of the wild-type ATG14 reporter
plasmid, but did not change that of the mutated ATG14 reporter
(Fig. 5B). The data from the
clinical samples indicated that ATG14 exhibited a high expression
level in the serum of healthy volunteers compared with those of
patients with AS (Fig. 5C). The
RT-qPCR assay data demonstrated that the ox-LDL-mediated decrease
in ATG14 was dose-dependent (Fig.
5D). The RT-qPCR results also showed that ATG14 expression was
positively correlated with CTBP1-AS2 expression (Fig. 5E) but inversely correlated with
miR-195-5p expression (Fig. 5F)
in the serum of 24 patients with AS. In addition, CTBP1-AS2
induced ATG14 expression, and si-CTBP1-AS2 decreased ATG14
expression in HA-VSMCs treated with ox-LDL (Fig. 5G and H). miR-195-5p upregulation
decreased ATG14, but its inhibitor increased ATG14 (Fig. 5I and J). Western blot analysis
demonstrated that decreased CTBP1-AS2 or miR-195-5p markedly
inhibited ATG14 expression compared with their respective control
(si-NC or mimic-NC). Ectopically expressed CTBP1-AS2
abrogated the miR-195-5p-mediated inhibition of ATG14 expression in
HA-VSMCs when the cells were treated with ox-LDL (Fig. 5K). Collectively, these results
suggested that CTBP1-AS2 promoted ATG14 expression by
serving as a ceRNA for miR-195-5p in HA-VSMCs.
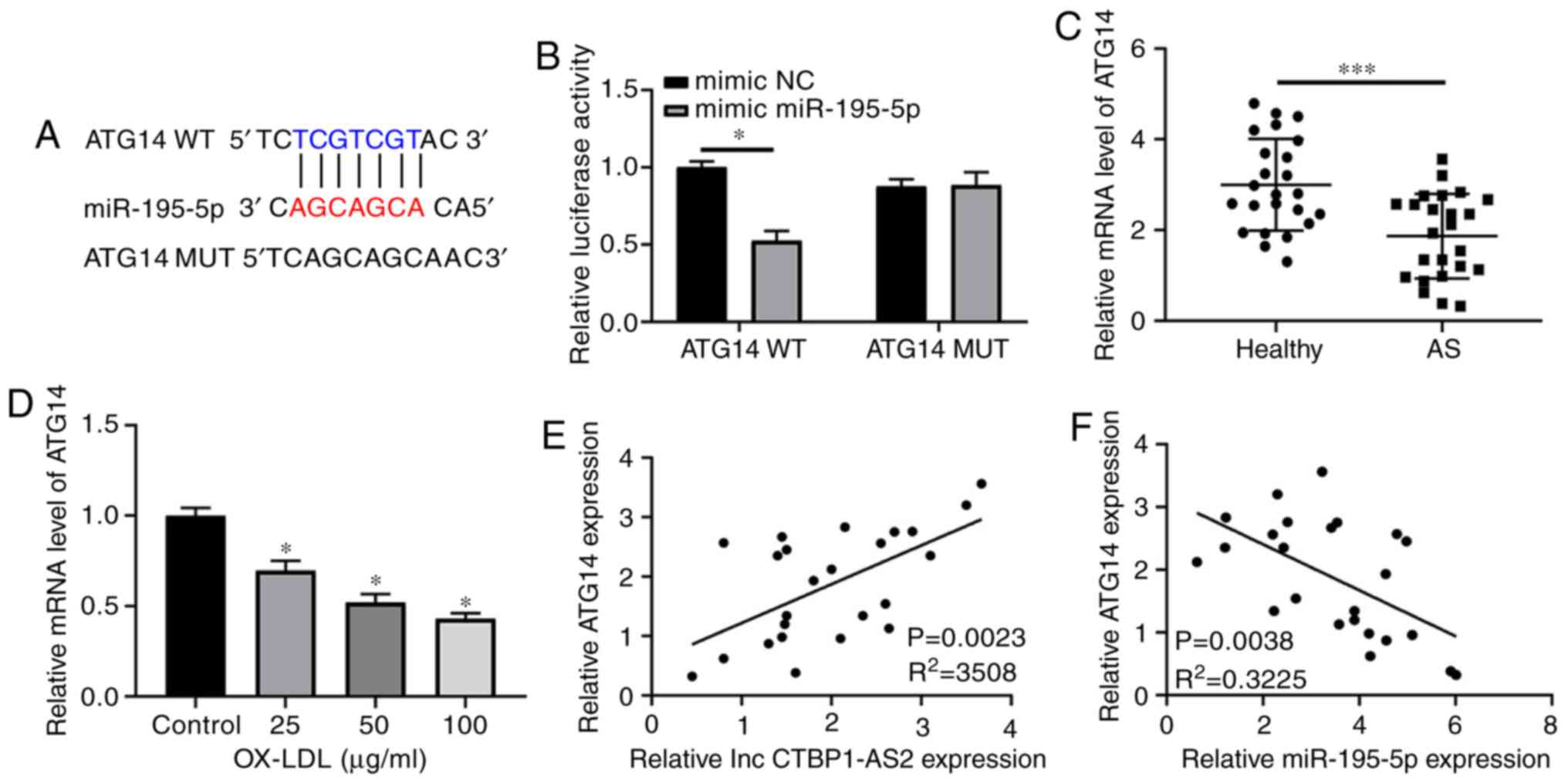 | Figure 5ATG14 is a target of miR-195-5p. (A)
Predicted binding sequences between miR-195-5p and ATG14 3′
untranslated region and the mutated sites in mutated ATG14 reporter
plasmid. (B) Luciferase activity for 48 h after co-transfection
with WT or MUT ATG14 reporter and mimic miR-195-5p/NC into
HA-VSMCs. (C) ATG14 expression in the serum of 24 healthy
volunteers and 24 patients with AS. (D) HA-VSMCs were treated with
ox-LDL (0, 25, 50 or 100 μg/ml) for 1 day, and ATG14 levels
were measured. (E and F) Correlation analysis between ATG14 and (E)
CTBP1-AS2 or (F) miR-195-5p in the serum of 24 patients with AS.
(G) Increased ATG14 expression following the increase in CTBP1-AS2
expression, and (H) decreased ATG14 expression following the
downregulation of CTBP1-AS2 expression. (I) Downregulation of
miR-195-5p increased ATG14 expression levels. (J) Upregulation of
miR-195-5p decreased ATG14 levels. (K) Effects of CTBP1-AS2
knockdown (si CTBP1-AS2), miR-195-5p overexpression
(miR-195-5p), and CTBP1-AS2 overexpression on ATG14 protein
expression assessed using western blot analysis at 48 h after
transfection in HA-VSMCs treated with ox-LDL. Data are presented as
mean ± standard error of the mean of at least 3 independent
experiments. *P<0.05 and ***P<0.001.
ATG14, autophagy related 14; miR, microRNA; WT, wild-type; MUT,
mutant; NC, negative control; HA-VSMCs, human aorta vascular smooth
muscle cells; ox-LDL, oxidized low-density lipoprotein; si, small
interfering RNA; lnc, long non-coding RNA. |
Discussion
Despite substantial advances in research
investigating its diagnosis and treatment methods, AS remains a
leading threat to human health with high incidence and mortality
rates worldwide (26). ncRNAs,
including miRNAs and lncRNAs, exert pivotal functions in AS
development (27,28). In the present study,
bioinformatics analysis was used to identify the possible targets
of CTBP1-AS2 and further investigate its molecular
mechanism. The results suggested that miR-195-5p may act as a
downstream gene of CTBP1-AS2. miR-195-5p, belonging to the
miR-195 family, modulates core processes in cells, including cell
cycle progression, proliferation, invasion, migration and
differentiation (29,30). miR-195 regulates the proliferation
and migration of VSMCs, induces cytokine secretion mode, and
prevents neointimal generation in the cardiovascular system
(31).
Ox-LDL can trigger AS by promoting EC activation and
dysfunction, VSMC behavior, foam cell generation and other
mechanisms (32). As a core
vascular cell type, VSMCs are implicated in arterial wall
reconstruction, which are important for maintaining blood flow in
vessels (33,34). As such, the present study was
designed to determine the role of CTBP1-AS2 in AS regulation
by controlling miR-195-5p in HA-VSMCs treated with ox-LDL. A
decreased level of CTBP1-AS2 and increased level miR-195-5p
in the serum of patients with AS were detected. Furthermore, when
VSMCs were treated with ox-LDL, the decrease of CTBP1-AS2
and increase of miR-195-5p were ox-LDL dose-dependent.
Autophagy is a self-degradative process mediated by
lysosomes and has a critical effect on maintaining cell homeostasis
(35); it is implicated in
multiple states of vascular diseases, such as hypertension,
atherosclerosis and restenosis (36). Autophagy restrained the formation
of ox-LDL-provoked VSMCs (37).
In the present study, it was identified that CTBP1-AS2 was
downregulated in AS and over-expressed CTBP1-AS2 could promote
autophagy in VSMCs. According to a previous study (38), enhanced autophagy can inhibit AS,
and inhibition of autophagy may aggravate the occurrence of AS
(35). Therefore, the low
expression levels of CTBP1-AS2 may promote the development of AS by
inhibiting autophagy. In addition, the abnormal proliferation of
VSMCs is an important pathophysiological process in the development
of atherosclerosis, and the inhibition of VSMCs proliferation may
delay disease progression (39).
In the present study, it was also identified that the upregulated
CTBP1-AS2 impaired the proliferation of VSMCs; therefore, the
downregulation of CTBP1-AS2 may accelerate the progression of AS by
promoting the proliferation of VSMCs. The results of the present
study also demonstrated that excessive autophagy may trigger
autophagy-mediated cell death. However, a systematic review
reported that active autophagy is able to induce autophagic death
of VSMCs, resulting in decreased synthesis of collagen leading to
the destabilization of plaque (8). These contradicting results may be
due to the fact that the present study explored the progress of AS
from a different perspective. The present study focused on the
association between proliferation and autophagy of VSMCs, however
the aforementioned systematic review may focus on the relationship
between autophagy and the synthesis of collagen, which is related
to the stability of plaque. However, the role of autophagy in AS is
not clear at present (8,40). In addition, it may exert its roles
through different ways. The results of the present study provide
new insights concerning the association between autophagy and AS.
This topic should also be investigated in more detail to improve
understanding on the mechanism of autophagy during AS progression.
This may help find a balance point where autophagy inhibits the
proliferation of VSMCs but does not cause the destabilization of
plaques in the development of AS.
The mechanism of CTBP1-AS2 in AS was verified
by determining whether its effects of the proliferation and
autophagy of HA-VSMCs was mediated through miR-195-5p. According to
the results of the present study, the anti-proliferation and
pro-autophagy effects modulated by CTBP1-AS2 were markedly
inhibited by the decrease in miR-195-5p expression in HA-VSMCs
treated with ox-LDL.
miRNAs modulate the translation or stability of
target mRNAs. Therefore, the possible target mRNAs of miR-195-5p
were predicted, and the role of ATG14 as the potential target of
miR-195-5p was verified by the luciferase reporter assay. ATG14 is
an essential autophagy-specific regulator of the class III
phosphatidylinositol 3-kinase complex (41). Autophagy occurs in developing
atherosclerotic plaques (42).
ATG14 mRNA expression was demonstrated to exhibit a positive
correlation with that of CTBP1-AS2 but a negative
correlation with that of miR-195-5p in the serum of patients with
AS. miR-195-5p upregulation decreased the ATG14 protein expression
level, and CTBP1-AS2 increased the level slightly.
Therefore, CTBP1-AS2 may increase ATG14 expression via its
role as a ceRNA for miR-195-5p in HA-VSMCs.
However, there were certain limitations in the
present study; for example, only the proliferation and autophagy of
VSMCs were detected in the cell function experiments without cell
cycle and apoptosis. However, it is important to note that, to the
best of our knowledge, there have only been few studies
investigating the association between proliferation and autophagy
of VSMCs; therefore, the present study aimed to reveal the
association between proliferation and autophagy of VSMCs. Finally,
as there was no access to a working flow cytometer, the levels of
apoptosis could not be examined. When this has been resolved, it
will be included in our future studies.
The results of the present study demonstrated the
involvement of CTBP1-AS2/miR-195-5p/ATG14 regulatory axis in
the proliferation and autophagy of HA-VSMCs and suggest the
potential use of CTBP1-AS2 in AS prevention. However, the
use as a drug target and mechanisms of CTBP1-AS2 in VSMCs
and AS should be studied further in in vivo animal
models.
Supplementary Data
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and WHG performed the experiments and generated
data. CXZ analyzed the data. SLG designed the experiments. YW and
WHG wrote the manuscript. SLG and CXZ revised the manuscript. All
authors gave final approval of the version to be published and
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The First
Affiliated Hospital of Anhui Medical University (Hefei, China). All
patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Laslett LJ, Alagona P Jr, Clark BA III,
Drozda JP Jr, Saldivar F, Wilson SR, Poe C and Hart M: The
worldwide environment of cardiovascular disease: Prevalence,
diagnosis, therapy, and policy issues: A report from the American
college of cardiology. J Am Coll Cardiol. 60(25 Suppl): S1–S49.
2012. View Article : Google Scholar
|
|
2
|
Homburg PJ, Rozie S, van Gils MJ, Jansen
T, de Weert TT, Dippel DW and van der Lugt A: Atherosclerotic
plaque ulceration in the symptomatic internal carotid artery is
associated with nonlacunar ischemic stroke. Stroke. 41:1151–1156.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chong H, Wei Z, Namuhan, Sun G, Zheng S,
Zhu X, Xue Y, Zhou Q, Guo S, Xu J, et al: The PGC-1α/NRF1/miR-378a
axis protects vascular smooth muscle cells from FFA-induced
proliferation, migration and inflammation in atherosclerosis.
Atherosclerosis. 297:136–145. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Li F, Wang X, Gong J, Xian Y,
Wang G, Zheng Z, Shang C, Wang B, He Y, et al: MiR-145 alleviates
Hcy-induced VSMC proliferation, migration, and phenotypic switch
through repression of the PI3K/Akt/mTOR pathway. Histochem Cell
Biol. March 2–2020.Epub ahead of print.
|
|
6
|
Wang Q, Li DC, Li ZF, Liu CX, Xiao YM,
Zhang B, Li XD, Zhao J, Chen LP, Xing XM, et al: Upregulation of
miR-27a contributes to the malignant transformation of human
bronchial epithelial cells induced by SV40 small T antigen.
Oncogene. 30:3875–3886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang P, Xu TY, Guan YF, Zhao Y, Li ZY, Lan
XH, Wang X, Yang PY, Kang ZM, Vanhoutte PM and Miao CY: Vascular
smooth muscle cell apoptosis is an early trigger for hypothyroid
atherosclerosis. Cardiovasc Res. 102:448–459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grootaert MOJ, Moulis M, Roth L, Martinet
W, Vindis C, Bennett MR and De Meyer GRY: Vascular smooth muscle
cell death, autophagy and senescence in atherosclerosis. Cardiovasc
Res. 114:622–634. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pirillo A, Norata GD and Catapano AL:
LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm.
2013:1527862013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Yan CC, Zhang X and You ZH: Long
non-coding RNAs and complex diseases: From experimental results to
computational models. Brief Bioinform. 18:558–576. 2017.
|
|
11
|
Li H, Zhu H and Ge J: Long noncoding RNA:
Recent updates in atherosclerosis. Int J Biol Sci. 12:898–910.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Zheng L, Wang Q and Hu YW: Emerging
roles and mechanisms of long noncoding RNAs in atherosclerosis. Int
J Cardiol. 228:570–582. 2017. View Article : Google Scholar
|
|
13
|
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen
C, Cai Y, Huang H, Yang Y, Liu Y, et al: LincRNA-p21 regulates
neointima formation, vascular smooth muscle cell proliferation,
apoptosis, and atherosclerosis by enhancing p53 activity.
Circulation. 130:1452–1465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shan K, Jiang Q, Wang XQ, Wang YN, Yang H,
Yao MD, Liu C, Li XM, Yao J, Liu B, et al: Role of long non-coding
RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell
Death Dis. 7:e22482016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo X, He S, Hu Y, Liu J and Chen X:
Sp1-induced LncRNA CTBP1-AS2 is a novel regulator in cardiomyocyte
hypertrophy by interacting with FUS to stabilize TLR4. Cardiovasc
Pathol. 42:21–29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Georges M, Coppieters W and Charlier C:
Polymorphic miRNA-mediated gene regulation: Contribution to
phenotypic variation and disease. Curr Opin Genet Dev. 17:166–176.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giral H, Kratzer A and Landmesser U:
MicroRNAs in lipid metabolism and atherosclerosis. Best Pract Res
Clin Endocrinol Metab. 30:665–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He JF, Luo YM, Wan XH and Jiang D:
Biogenesis of MiRNA-195 and its role in biogenesis, the cell cycle,
and apoptosis. J Biochem Mol Toxicol. 25:404–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo J, Wang M and Liu X: MicroRNA-195
suppresses tumor cell proliferation and metastasis by directly
targeting BCOX1 in prostate carcinoma. J Exp Clin Cancer Res.
34:912015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Zhang X, Zou C, Kung HF, Lin MC,
Dress A, Wardle F, Jiang BH and Lai L: MiR-195 inhibits tumor
growth and angiogenesis through modulating IRS1 in breast cancer.
Biomed Pharmacother. 80:95–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang M, Zhang J, Tong L, Ma X and Qiu X:
MiR-195 is a key negative regulator of hepatocellular carcinoma
metastasis by targeting FGF2 and VEGFA. Int J Clin Exp Pathol.
8:14110–14120. 2015.
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Ding W, Xu Y, Tao E, Mo M, Xu W,
Cai X, Chen X, Yuan J and Wu X: Long non-coding RNA RHPN1-AS1
promotes tumorigenesis and metastasis of ovarian cancer by acting
as a ceRNA against miR-596 and upregulating LETM1. Aging (Albany
NY). 12:4558–4572. 2020. View Article : Google Scholar
|
|
25
|
Zhao D, Zhang H, Long J and Li M: LncRNA
SNHG7 functions as an oncogene in cervical cancer by sponging
miR-485-5p to modulate JUND expression. Onco Targets Ther.
13:1677–1689. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weber C and Noels H: Atherosclerosis:
Current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Salisbury D and Sallam T: Long
noncoding RNAs in atherosclerosis: JACC review topic of the week. J
Am Coll Cardiol. 72:2380–2390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou T, Ding JW, Wang XA and Zheng XX:
Long noncoding RNAs and atherosclerosis. Atherosclerosis.
248:51–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang K, Sun Y, Tao W, Fei X and Chang C:
Androgen receptor (AR) promotes clear cell renal cell carcinoma
(ccRCC) migration and invasion via altering the
circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett.
394:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qu Q, Chu X and Wang P: MicroRNA-195-5p
suppresses osteosarcoma cell proliferation and invasion by
suppressing naked cuticle homolog 1. Cell Biol Int. 41:287–295.
2017. View Article : Google Scholar
|
|
31
|
Wang YS, Wang HY, Liao YC, Tsai PC, Chen
KC, Cheng HY, Lin RT and Juo SH: MicroRNA-195 regulates vascular
smooth muscle cell phenotype and prevents neointimal formation.
Cardiovasc Res. 95:517–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakajima K, Nakano T and Tanaka A: The
oxidative modification hypothesis of atherosclerosis: The
comparison of atherogenic effects on oxidized LDL and remnant
lipoproteins in plasma. Clin Chim Acta. 367:36–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Vascular smooth muscle cell in atherosclerosis. Acta Physiol
(Oxf). 214:33–50. 2015. View Article : Google Scholar
|
|
34
|
Johnson JL: Emerging regulators of
vascular smooth muscle cell function in the development and
progression of atherosclerosis. Cardiovasc Res. 103:452–460. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng Y, Wang B, Zhou H, Dang S, Jin M,
Shi Y, Hao L, Yang Z and Zhang Y: Autophagy is required for the
maintenance of liver progenitor cell functionality. Cell Physiol
Biochem. 36:1163–1174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Meyer GR, Grootaert MO, Michiels CF,
Kurdi A, Schrijvers DM and Martinet W: Autophagy in vascular
disease. Circ Res. 116:468–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li BH, Yin YW, Liu Y, Pi Y, Guo L, Cao XJ,
Gao CY, Zhang LL and Li JC: TRPV1 activation impedes foam cell
formation by inducing autophagy in oxLDL-treated vascular smooth
muscle cells. Cell Death Dis. 5:e11822014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He K, Sun H, Zhang J, Zheng R, Gu J, Luo M
and Shao Y: Rab7mediated autophagy regulates phenotypic
transformation and behavior of smooth muscle cells via the
Ras/Raf/MEK/ERK signaling pathway in human aortic dissection. Mol
Med Rep. 19:3105–3113. 2019.PubMed/NCBI
|
|
39
|
Bennett MR, Sinha S and Owens GK: Vascular
smooth muscle cells in atherosclerosis. Circ Res. 118:692–702.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hassanpour M, Rahbarghazi R, Nouri M,
Aghamohammadzadeh N, Safaei N and Ahmadi M: Role of autophagy in
atherosclerosis: Foe or friend? J Inflamm (Lond). 16:82019.
View Article : Google Scholar
|
|
41
|
Sun Q, Fan W, Chen K, Ding X, Chen S and
Zhong Q: Identification of barkor as a mammalian autophagy-specific
factor for Beclin 1 and class III phosphatidylinositol 3-kinase.
Proc Natl Acad Sci USA. 105:19211–19216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martinet W and De Meyer GR: Autophagy in
atherosclerosis: A cell survival and death phenomenon with
therapeutic potential. Circ Res. 104:304–317. 2009. View Article : Google Scholar : PubMed/NCBI
|