Introduction
Multiple myelomas (MM) is a type of plasma cell
tumour and the second most common haematological malignancy. To
date, no curative treatments for MM have been reported (1). MM is characterised by the
uncontrolled growth of monoclonal plasma cells in the bone marrow,
leading to anaemia, bone lesions, hypercalcaemia, renal failure,
and other complications (2). MM
cells are located at multiple sites in the bone, and the mechanisms
through which MM cells migrate from the primary lesion to distant
sites have been studied in detail (3). Angiogenesis is involved in MM
progression and is promoted via the production of angiogenic
cytokines by plasma cells within the bone marrow microenvironment.
Several anti-angiogenic therapeutic strategies, including
thalidomide treatment, have been evaluated in patients with myeloma
(4). However, the mechanisms of
angiogenesis in the pathogenesis of myeloma remain unclear, and
effective biomarkers for anti-angiogenic therapy have not been
identified.
MicroRNAs (miRNAs or miRs) are a large group of
non-coding RNAs. A single miRNA can control multiple genes and
molecular pathways. Recently, miRNAs have emerged as instrumental
regulators of cellular processes that enable the development and
dissemination of MM (5-7). The functions of miRNAs in MM vary.
Several miRNAs, including miR-15a and miR-16, are
markedly downregulated in MM, suggesting tumour-suppressive roles.
By contrast, miR-21 and miR-221 are highly expressed
and function as oncogenes (oncomiRs) in MM. In addition, several
miRNAs, such as those belonging to the miR-34 family, are
transcriptional targets of p53 and mediate its tumour-suppressive
functions. miR-34a/b/c, miR-124-1, miR-194-2,
miR-192, miR-203, miR-152 and
miR-10b-5p are frequently methylated in MM (8).
Recently, the discovery of exosome-mediated transfer
of tumour-suppressive miRNAs has demonstrated the need for the
deeper understanding of the mechanisms through which tumour cells
and the microenvironment communicate. Exosome-associated
miR-9 is involved in nasopharyngeal carcinoma tumorigenesis,
suggesting potential roles for exosome-based therapies in cancer
treatment (9). However, the
mechanisms through which miRNAs regulate MM, particularly migration
and angiogenesis, remain unclear.
Accordingly, in the present study, the expression of
miRNAs was evaluated in patients with MM and the effects of
miR-144-3p on the proliferation, migration and angiogenesis
of MM cells were examined.
Materials and methods
Patient sample collection
Bone marrow samples were collected from 15 patients
with MM and 10 patients with non-haematological diseases at
Shengjing Hospital of China Medical University from February, 2015
to November, 2017. The basis clinical information of the study
subjects is presented in Table SI. Samples were extracted using
CD138 magnetic beads (Miltenyi Biotec GmbH). The purity of the
CD138+ plasma cells was at least 90% (data not shown).
Mononuclear cells were extracted from bone marrow using
Ficoll-Hypaque (lymphocyte separation fluid; Beijing Solarbio
Science & Technology Co., Ltd.) density gradient
centrifugation. The present study was approved by the Research
Ethics Committee of 0Shengjing Hospital of China Medical University
(approval no. 2019PS270K) and all patients provided informed
consent.
Cell lines and cell culture
The human MM cell lines U266 and RPMI-8226 were
purchased from the Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences. The cells were stored in RPMI-1640
medium (Cellgro, Mediatech; Corning, Inc.) containing 10% foetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and cultured
at 37°C in a conventional cell culture incubator with a 5%
CO2 atmosphere.
Cell transfection
A miR-144-3p mimic, miR negative control
(miR-NC), siRNA against myocyte enhancer factor 2A (si-MEF2A) and
siRNA negative control (si-NC) were purchased from Hanbio
Technology, Ltd. The miR-144-3p mimic and its control
sequence were as follows: miR-144-3p mimic sense, 5′-UAC AGU
AUA GAU GAU GUA CU-3′ and antisense, 5′-AGU ACA UCA UCU AUA CUG
UA-3′; and negative control sense, 5′-UCA CAA CCU CCU AGA AAG AGU
AGA -3′ and antisense, 5′-UCU ACU CUU UCU AGG AGG UUG UGA -3′. The
siRNA sequences were as follows: si-MEF2A sense, 5′-CCA GAC CCU GAU
ACU UCA UdT dT-3′ and antisense, 5′-AUG AAG UAU CAT GGG GCU -3′;
and si-NC sense, 5′-UUC UCC GAA CGU GUC ACG UdT d-3′ and antisense,
5′-ACG UGA CAC GUU CGG AGA AdT d-3′. The MEF2A coding sequence was
inserted into the pcDNA3.1 vector (Kingsray Biotechnology Co.,
Ltd.), and an MEF2A overexpression plasmid (pcDNA-MEF2A) was
constructed in the cells. Briefly, the transfection mass and
concentration of plasmid (Genescript Biotech Corporation) and small
molecule RNA (Hanbio Technology, Ltd.) in the 24-well plate/6-well
plate were 0.5 µg/5 µg and 100 nM, respectively and
were transfected into the cells using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol, with a final transfection concentration of
100 nM. The transfection efficiency was monitored by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) at
48 h following transfection.
Total RNA extraction RT-qPCR
Total RNA was extracted from each sample using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The quantity and
quality of the extracted RNA were measured with a NanoDrop
spectrophotometer (NanoVne, GE Healthcare). The reverse
transcription of miRNA was performed using a tailing reverse kit
(Sangon Biotech), and mRNA was reversed transcribed into
first-strand cDNA using a PrimeScriptTM RT kit (Takara Bio, Inc.).
The conditions of reverse transcription were as follows: 42°C for 2
min, 37°C for 15 min and 85°C for 5 sec. The expression of MEF2A
and miR-144-3p was detected with SYBR Premix Ex Taq™ (Takara
Bio, Inc.) using the Bio-Rad CFX96 Real-Time PCR System (Bio-Rad
Laboratories, Inc.). The denaturing, annealing and extension
conditions of each PCR cycle were 40 cycles of 95°C for 5 sec and
60°C for 34 sec, respectively. All primers were designed and
synthesised by Sangon Biotech. The primer sequences were as
follows: miR-144-3p forward, 5′-GCG CGC GTA CAG TAT AGA
TGA-3′ and reverse, 5′-AGT GCA GGG TCC GAG GTA TT-3′; U6 forward,
5′-GCT TCG GCA GCA CAT ATA CTA AAA T-3′ and reverse, 5′-CGC TTC ACG
AAT TTG CGT GTC AT-3′; MEF2A forward, 5′-ACG TCC AGT GTG GCA TGG
AG-3′ and reverse, 5′-AGG CTG GTT TCC ACC CAG AG-3′; and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward, 5′-CCA
CCC ATG GCA AAT TCC ATG GCA -3′ and reverse, 5′-TCT AGA CGG CAG GTC
AGG TCC ACC -3′. The expression of the target genes was calculated
using the 2−ΔΔcq method (10).
Cell proliferation assay
A Cell Titer-Glo chemiluminescence cell viability
assay (Promega Corp.) was used to detect cell proliferation
(11). The transfected MM cells
were seeded into 96-well plates (10,000 cells/well). Following
culture at 37°C in a 5% CO2 incubator for various
periods of time (12, 24, 36, 48 and 60 h), luminescence was added
to each well and the substrate was detected at 25 µl and
incubated at 37°C for 1 h. The absorbance (OD) value was measured
with a Multifunctional enzyme labelling instrument (Synergy2,
BioTek Instruments, Inc.) at a wavelength of 560 nm.
Apoptosis and cell cycle analyses
At 48 h following transfection, at least
105 cells were collected from each group and washed
twice with PBS. According to the instructions provided with the
apoptotic kit (BD Biosciences), dyes were added to the cells and
protected from light for 10 min, after which flow cytometry (ACEA
Biosciences) was performed to analyse the cell cycle distribution
with nova express software (ACEA Biosciences). Cells from the
different groups were collected, with at least 105 cells
for each group. The cells were then washed twice with PBS and fixed
with 75% ethanol overnight, according to the instructions provided
with the cell cycle kit (KeyGEN Biotech). Dyes were added
sequentially, the samples were protected from light for 30 min, and
flow cytometric analysis was then performed.
Transwell assay
Cell suspensions were prepared using serum-free
medium and diluted to 105 cells/ml. Subsequently, 200
µl of the cell suspension was added to the supporting
chambers of 24-well plates (Corning, Inc.). The medium in the lower
well was Dulbecco's modified Eagle's medium/F12 (HyClone; GE
Healthcare Life Sciences) containing 10% foetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.). Following incubation for
24 h, the cells were fixed with paraformaldehyde (Bioshap),
subjected to crystal violet staining (Beyotime Institute of
Biotechnology) at room temperature for 30 min and imaged using an
inverted microscope (Eclipse Ci; Nikon Corp.). ImageJ software
(version 1.6; National Institutes of Health) was used to analyse
the number of cells in each image and perform statistical
analysis.
Dual-luciferase reporter assay
miR-144-3p target gene prediction was
performed using the online bioinformatics databases, databases,
StarBase (http://starbase.sysu.edu.cn/), miRDB (http://mirdb.org/miRDB/), TargetScan (http://www.targetscan.org/), PicTar (https://pictar.mdc-berlin.de/) and miRanda (http://www.microrna.org/microrna/home.do) (12-14). The results of the above 5
databases were integrated, the potential mRNA targets of
differentially expressed miR-144-3p were identified, the
possible binding sites of MEF2A and miR-144-3p were
predicted, and the double luciferase reporter gene was analysed.
The 3′ untranslated region (UTR) of MEF2A containing the
miR-144-3p target sequence was inserted into the pmirGLO
vector (Promega Corp.) to obtain the luciferase reporter plasmid
wild-type MEF2A (MEF2A-WT) and antisense mutation of
the predicted binding site to obtain the mutant MEF2A-MUT
vector. 293T cells (FH0244; Shanghai Fuheng Biotechnology Co.,
Ltd.) were co-transfected with wild-type or mutant 3′UTR containing
and miR-144-3p mimics or NC control. At 36 h following
transfection, the luciferase activity was detected, and the results
were analysed according to the manufacturer's protocol using the
Dual-Luciferase ®Reporter Assay system (E1901; Promega
Corp.) experimental procedures.
Proliferation of and tube formation in
human umbilical vein endothelial cells (HUVECs)
At 48 h following transfection, the cell
supernatants were collected and centrifuged at 500 × g at room
temperature for 10 min. The pellets were removed, and supernatants
were obtained. HUVECs (obtained from the Cell Resource Center,
China; 3142C0001000000138; 2×105 cells/ml) were added to
96-well plates containing mixed medium [tumour cell supernatant (45
µl) + fresh ECM medium (45 µl)]. The cells were
cultured in a 5% CO2 incubator at 37°C for 24 h. A Cell
Counting kit-8 (CCK-8; Beyotime Institute of Biotechnology) was
used to detect HUVEC proliferation. To determine tube formation
rates, 96-well plates were precooled at-20°C for 20 min, and the
wells were inoculated with 50-70 µl Matrigel [BD Matrigel
(Growth factor reduced, cat. no. 356231); BD Biosciences]. The
plates were then incubated in a cell incubator for 30 min. HUVECs
(2×105 cells/ml; 10 µl/well) were seeded into the
wells of the Matrigel-coated plate with 90 µl mixed medium
[tumour cell culture supernatant (45 µl) + fresh ECM medium
(45 µl); ScienCell]. Tube formation was observed at 1, 4 and
7 h using an inverted microscope (IX71, Nikon Corp.), and the
number of tubes was counted in each group.
Western blot analysis
Total protein was extracted from the MM cells using
radio-immunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) with phenylmethyl-sulphonyl fluoride (Beyotime
Institute of Biotechnology). The proteins were isolated by
centrifugation at 12,000 × g for 15 min at 4°C. Protein
concentrations were quantified using a bicinchoninic acid assay
(Beyotime Institute of Biotechnology). All protein samples were
boiled with Loading Buffer (Beyotime Institute of Biotechnology) at
100°C for 10 min. Equal amounts of protein (30 µg) in each
sample were separated by 10% sodium dodecyl sulphate polyacrylamide
gel electrophoresis for 2 h at a constant voltage (110 V) and then
transferred onto polyvinylidene fluoride membranes (EMD Millipore).
The membranes were blocked for 1 h at room temperature in
Tris-buffered saline containing 10% non-fat dried milk and
incubated overnight at 4°C with the following primary antibodies:
Rabbit polyclonal anti-MEF2A (1:500; cat. no. 9736; Cell Signaling
Technology, Inc.), rabbit polyclonal anti-vascular endothelial
growth factor (VEGF; 1:500; cat. no. 2463; Cell Signaling
Technology, Inc.), and rabbit monoclonal antibody anti-GAPDH
(1:1,000; cat. no. ab181602; Abcam). The membranes were washed and
then incubated at room temperature for 1 h with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG,
1:2,000; cat. no. ab205718; Abcam). Chemiluminescent detection was
performed using an ECL kit (32132×3; Thermo Fisher Scientific,
Waltham, Inc.). Bands were analysed using ImageJ software (version
1.6) to verify the relative expression levels of the target
proteins.
Statistical analysis
Data analysis was performed using GraphPad Prism 6.0
software (GraphPad, Inc.). Data are presented as the means ±
standard deviation (SD). A Student's t-test was used for
comparisons between 2 groups, and a one-way analysis of variance
was used for comparisons between multiple groups with Tukey's post
hoc test. All experiments were repeated independently at least 3
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-144-3p expression is lower in MM than
in normal cells
To explore the mechanisms through which miRNAs
regulate MM, small RNA expression profiling was first performed in
exosomes from bone marrow supernatants from patients with MM and
healthy donors. miR-144-3p expression varied between groups,
suggesting that miR-144-3p was involved in the pathogenesis
of MM (Fig. S1; GEP data were deposited in NCBI SRA; accession ID
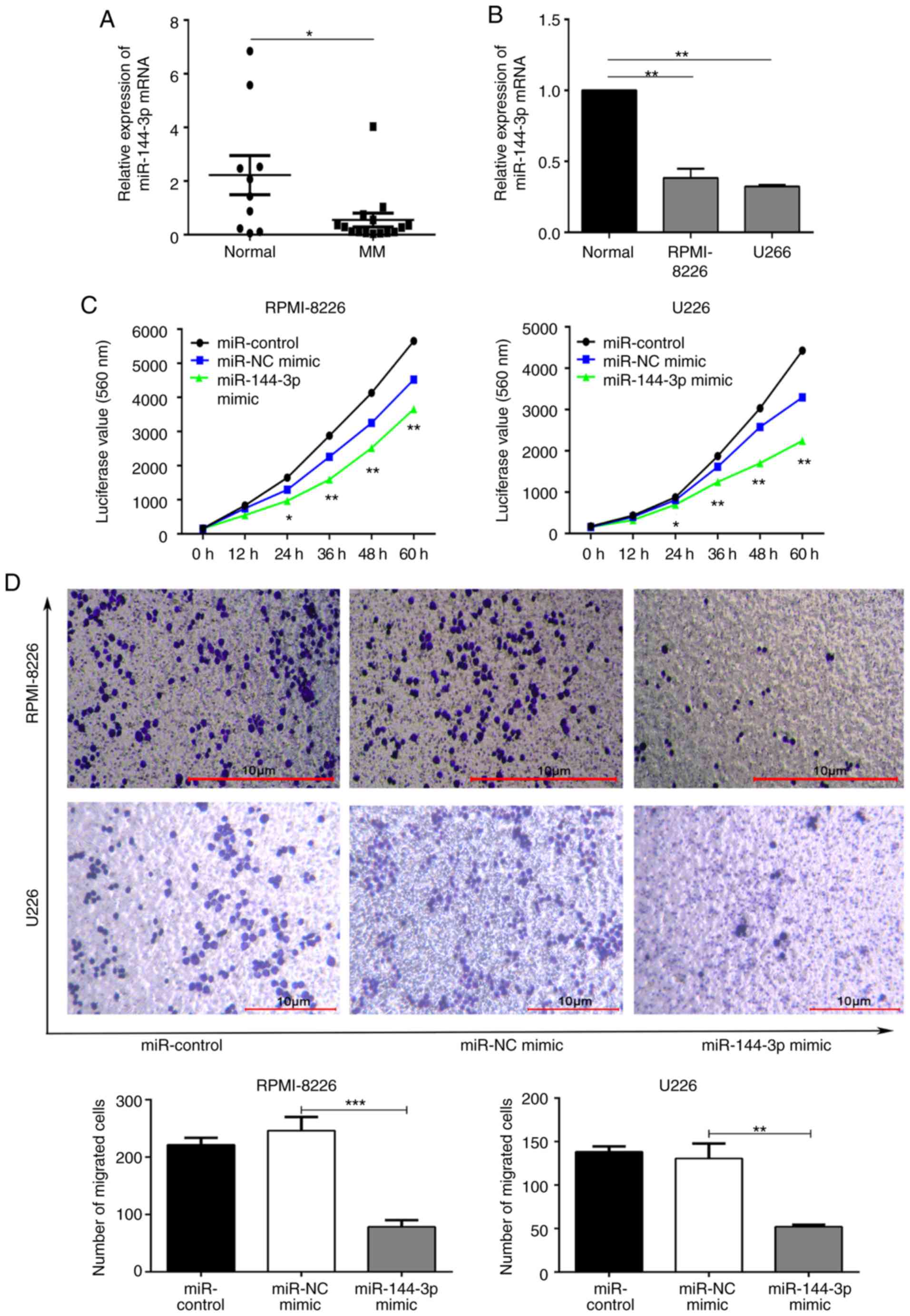
no. SRP239379). The expression of miR-144-3p in 15 patients
with primary MM and 10 normal donors was detected by RT-qPCR. The
results revealed that miR-144-3p expression in patients with
primary MM was significantly lower than that in normal bone marrow
mono-nuclear cells (P<0.05; Fig.
1A). A similar pattern was observed (P<0.01; Fig. 1B) in the MM cell lines, RPMI8226
and U266. These data suggest that miR-144-3p expression is
decreased in MM. This finding was consistent with the results of
the small RNA sequencing of bone marrow exosomes from patients with
MM.
Recovery of miR-144-3p inhibits the
proliferation and migration, and induces cell cycle arrest and
apoptosis of MM cells
Subsequently, the role of miR-144-3p in MM
was examined. miR-144-3p expression in RPMI8226 and U266
cells was induced by transfection with miR-144-3p mimic or
miR-NC as a negative control. RT-qPCR was used to detect the
expression of miR-144-3p (Fig. S2). Compared with the
negative control, the proliferation rate was significantly
inhibited by the expression of miR-144-3p in the RPMI-8226
and U266 cells (P<0.01; Fig.
1C). The proportion of cells in the G0/G1
phase was increased, whereas that in the S phase was decreased in
the miR-144-3p mimic group. These data demonstrate that this
miRNA plays a role in the cell cycle arrest of MM cells (P<0.05;
Fig. S3A). Moreover, the results revealed that the recovery of
miR-144-3p expression induced the apoptosis (P<0.05; Fig.
S3B) and inhibited the migration of MM cells (P<0.001; Fig. 1D). Overall, these data indicate
that miR-144-3p affects MM cell progression.
MEF2A is a target ofmiR-144-3p in MM
cells
miRNAs specifically bind to the 3′UTRs of mRNAs to
promote their degradation or inhibit translation. Bioinformatics
databases (StarBase, miRDB, TargetScan, PicTar and miRanda) were
used to identify the potential binding sites of miR-144-3p,
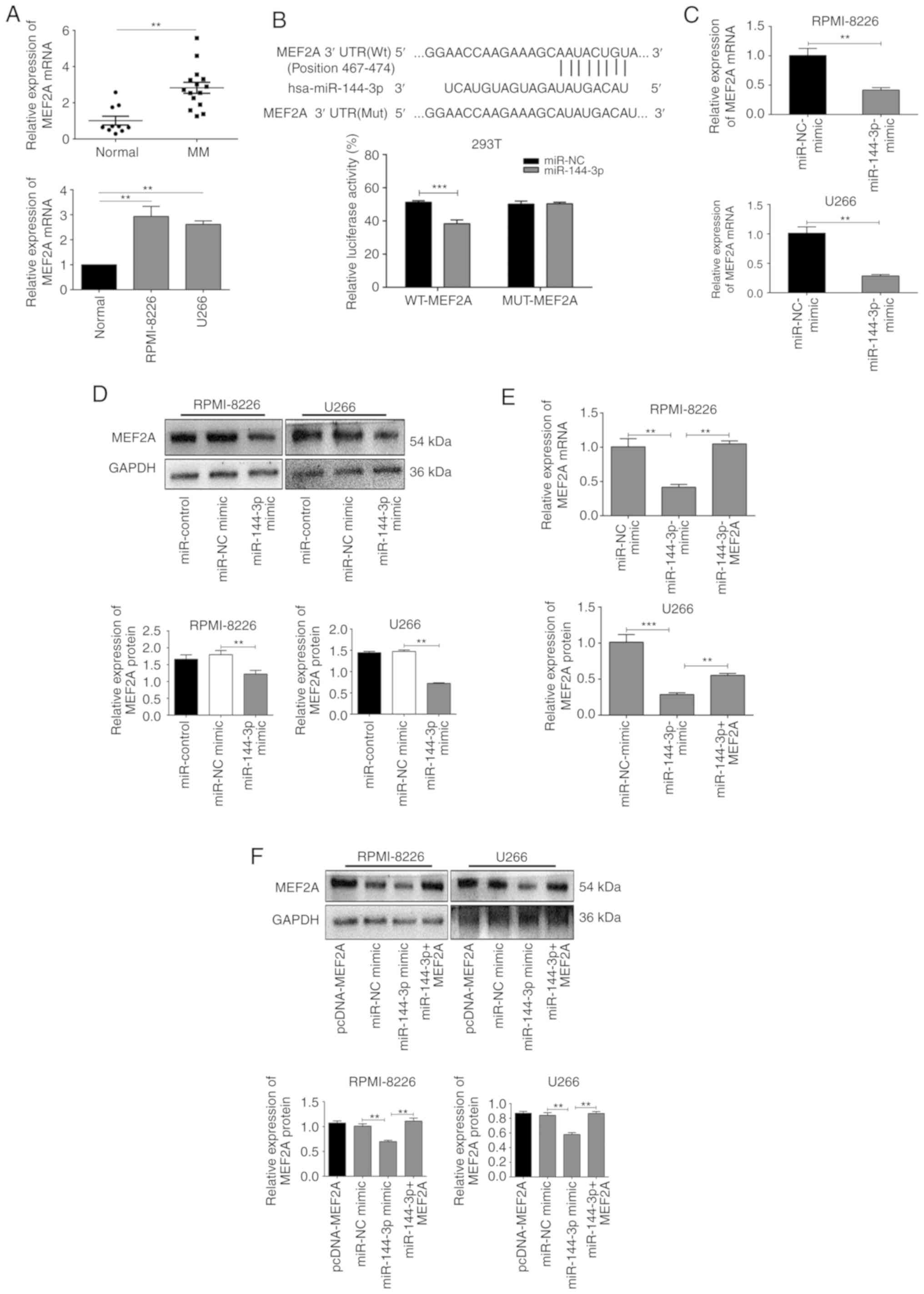
and MEF2A was found to be a possible target (Fig. 2B). To confirm these findings, the
expression of MEF2A was evaluated in patients with primary MM, in
MM cell lines, and in healthy donor cells. The results revealed
that MEF2A expression was significantly upregulated in MM
cells compared to normal cells (P<0.05; Fig. 2A). Moreover, luciferase reporter
assays revealed that the luciferase activity of wild-type
MEF2A-3′UTR in 293T cells transfected with miR-144-3p was
significantly lower than that in cells transfected with miR-NC
(P<0.001); importantly, miR-144-3p expression did not
affect mutant MEF2A (P>0.05; Fig.
2B). It was also found that the transfection of cells with
miR-144-3p mimic decreased the mRNA (P<0.01; Fig. 2C) and protein expression of MEF2A
in the RPMI-8226 and U266 cells (P<0.01; Fig. 2D). Finally, MEF2A rescue
experiments revealed that the inhibition of MEF2A expression by the
miR-144-3p mimic was reversed by transfection with a MEF2A
overexpression plasmid (P<0.01; Fig. 2E and F). Collectively, these
results indicated that miR-144-3p directly targeted the
3′UTR of MEF2A, resulting in MEF2A inhibition in MM
cells.
MEF2A regulates proliferation and
migration in MM cells
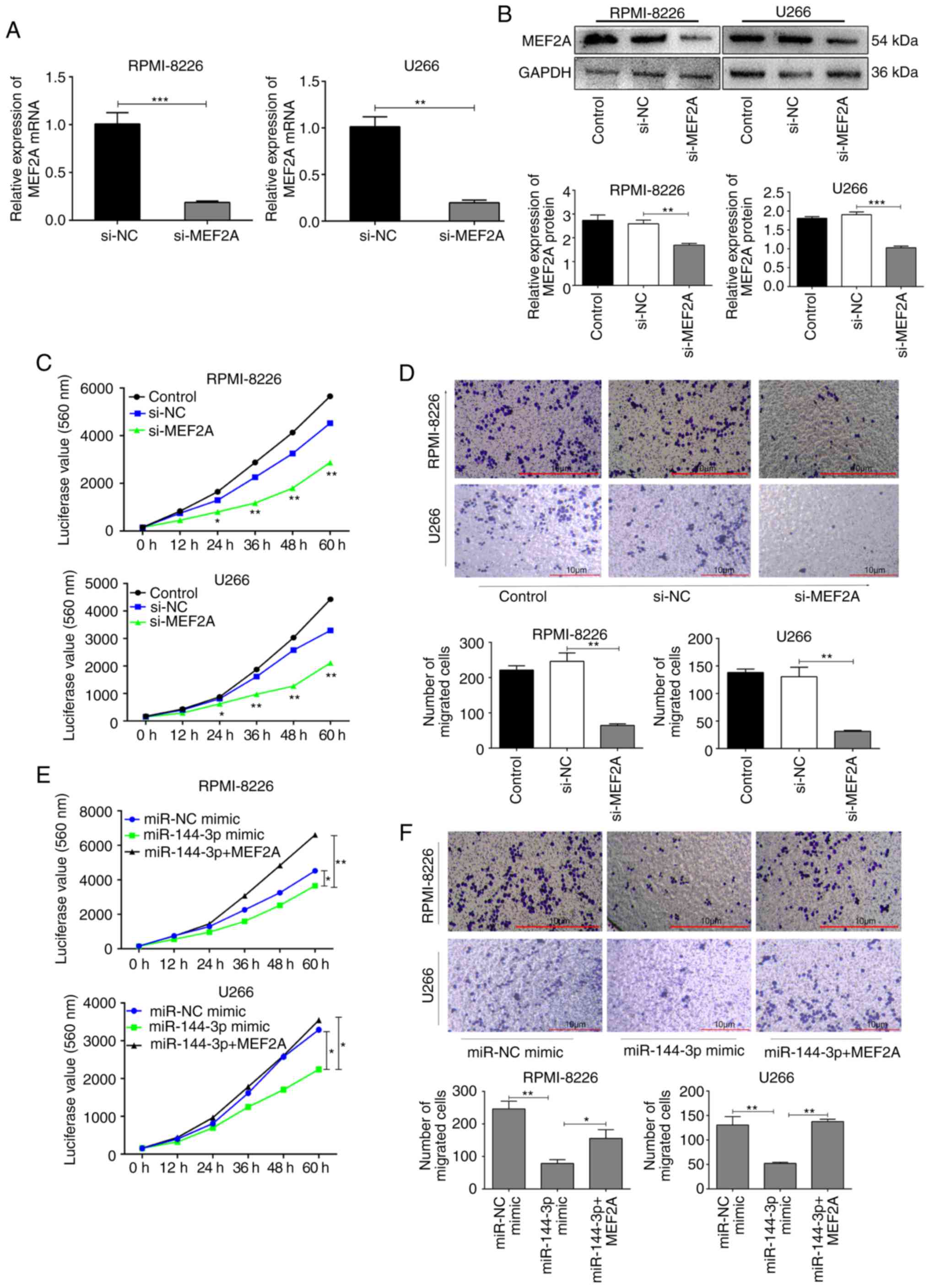
To further explore the biological function of MEF2A,
MEF2A was knocked down using short hairpin RNA in RPMI-8226 and
U266 cells (Fig. S4). The knockdown efficiency was verified by
RT-qPCR (P<0.01; Fig. 3A) and
western blot analysis (P<0.001; Fig. 3B). The proliferation and migration
of the cells in which MEF2A was knocked down were inhibited
compared to the control cells (P<0.01; Fig. 3C and D). By contrast, the
overexpression of MEF2A partly reversed the inhibitory effects of
miR-144-3p on the proliferation (P<0.01; Fig. 3E) and migration (P<0.05;
Fig. 3F) of RPMI-8226 and U266
cells. Moreover, the inhibition of MEF2A also partly induced the
cell cycle arrest and apoptosis of MM cells (Fig. S5), whereas the
overexpression of MEF2A reversed these effects (Fig. S6).
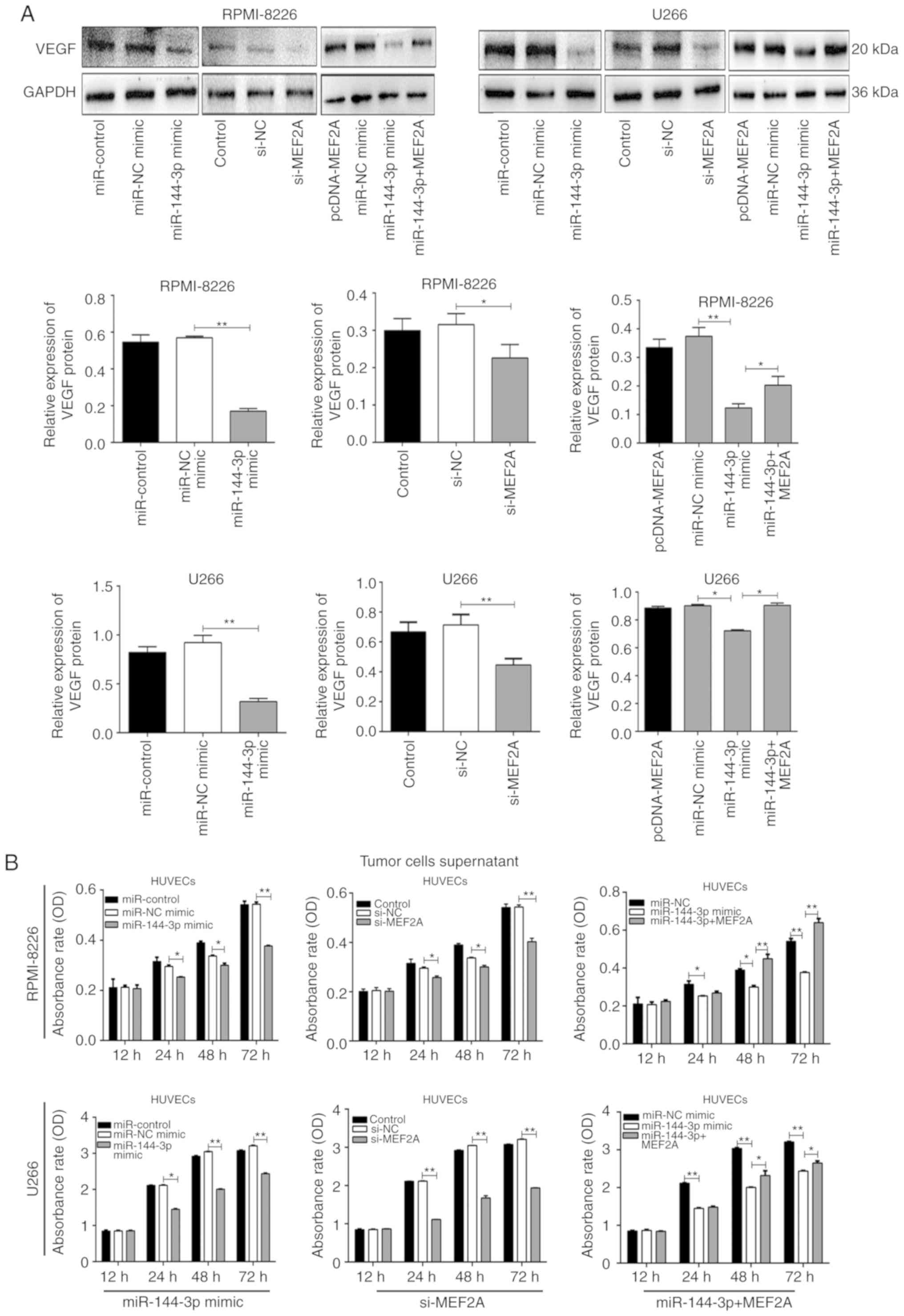
miR-144-3p regulates VEGF expression by
targeting MEF2A in MM cells
MEF2A is a DNA-binding protein that regulates
transcription. In endothelial cells, MEF2A is involved in sprouting
angiogenesis (15). However, the
association between MEF2A and VEGF, which regulates angiogenesis,
remains unclear. In the present study, it was found that both
miR-144-3p overexpression and MEF2A knockdown reduced VEGF
expression in MM cells (P<0.01; Fig. 4A). Moreover, co-transfection with
pcDNA-MEF2A and miR-144-3p partially restored the expression
of VEGF. These data suggest that the reduced expression of
miR-144-3p in MM increases VEGF expression by targeting
MEF2A.
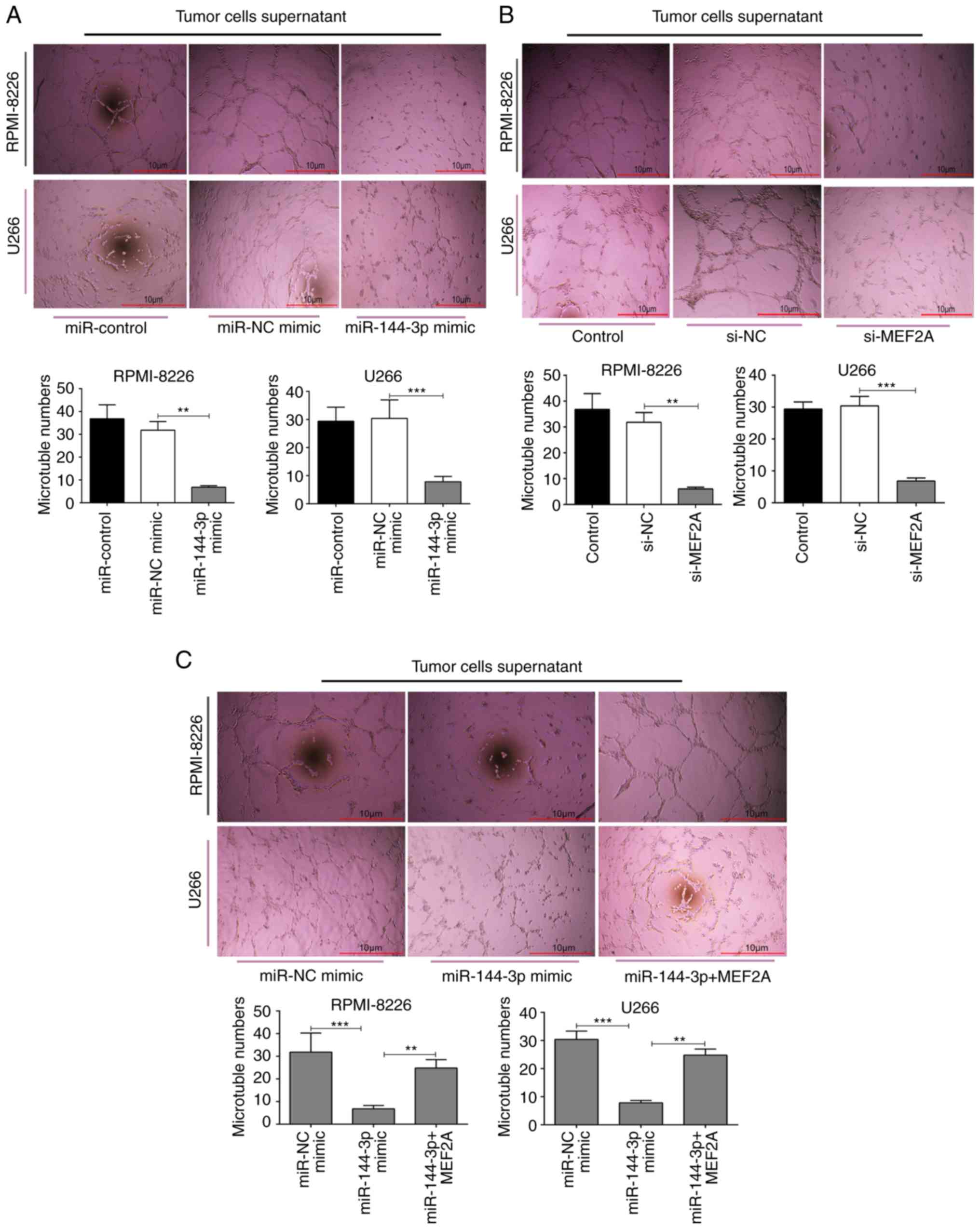
miR-144-3p inhibits angiogenesis by
targeting MEF2A in MM
Angiogenesis is a feature of MM and is induced by
plasma cells via angiogenic factors released by cells within the
tumour microenvironment (4). In
the present study, to explore the function of miR-144-3p in
angiogenesis, MM cell culture supernatants were prepared and HUVECs
were cultured with the different supernatants. The results revealed
that the overexpression of miR-144-3p and the knockdown of
MEF2A inhibited the proliferation (P<0.01; Fig. 4B) and disrupted the tubular
structure of HUVECs (P<0.01; Fig.
5A and B). pcDNA-MEF2A partly restored the inhibitory effects
of miR-144-3p on tube formation in HUVECs (P<0.01;
Fig. 5C).
Discussion
MM is a biologically heterogeneous disease of plasma
cells. In recent years, researchers have focused on the roles of
non-coding RNAs, such as miRNAs, long non-coding RNAs, siRNAs and
piwi-interacting RNAs, in the pathology of MM. Al Masri et
al demonstrated that miR-125b, miR-133a,
miR-1, miR-124a, miR-15 and miR-16 were
downregulated in MM cell lines and samples from patients with MM
compared to their normal counterparts (16). In the present study, a lower
expression of miR-144-3p was observed in MM compared with normal
cells, and the recovery of miR-144-3p expression inhibited the
proliferation, and induced the cell cycle arrest and apoptosis of
MM cells. Further analysis revealed that miR-144-3p exerted these
effects by downregulating MEF2A, suggesting that this
miR-144-3p/MEF2A interaction is involved in the mechanism of MM
cell proliferation. Zhao et al demonstrated similar results
and that miR-144-3p inhibits cell proliferation and induces
apoptosis in MM by targeting c-Met (17). Tianhua et al found that the
long non-coding RNA Sox2 overlapping transcript (SOX2OT) promoted
MM progression via the microRNA-144-3p/c-MET axis (18). However, whether MEF2A interacts
with the c-MET pathway to regulate MM proliferation and migration
warrants further investigation.
However, the regulation of migration and
angiogenesis by miR-144-3p in MM has not yet been investigated to
date, at least to the best of our knowledge. Raimondi et al
found that miR-199a-5p expression increased the adhesion of
MM cells to bone marrow stromal cells under hypoxic conditions
(19). These results indicate
that miRNA affects the migration of MM. In the present study, it
was found that miR-144-3p may directly target the 3′UTR of
MEF2A in MM cells to inhibit the metastasis of MM cells. The
MEF2 family of transcription factors includes 4 members, i.e.,
MEF2A, MEF2B, MEF2C and MEF2D, which play key roles in regulating
differentiation responses. The roles of MEF2A in promoting muscle
differentiation (20) and heart
development (21) have been
well-established. However, the involvement of MEF2A in cancer has
not yet been investigated in detail. In the present study, MEF2A
was identified as a target gene regulated by miR-144-3p and
it was found that MEF2A expression may promote HUVEC
proliferationand induce angiogenesis in MM.
Angiogenesis depends on the balance of positive and
negative angiogenic modulators within the vascular
microenvironment. MM angiogenesis mainly depends on the release of
growth factors, such as VEGF, by neoplastic cells. VEGF is specific
for endothelial cells and stimulates the growth of blood vessels
(22). Additionally, Roccaro
et al (23) demonstrated
that miR-15a/-16, which was downregulated in MM plasma
cells, exerted anti-angiogenic effects by reducing VEGF secretion
from MM cells, thereby suppressing the pro-angiogenic effects of MM
plasma cells on endothelial cells by targeting the stromal
cell-derived factor 1-α/C-X-C chemokine motif receptor 4
pathway (24). The present study
demonstrated a novel possible mechanism through which
miR-144-3p targets MEF2A to decrease VEGF expression,
thereby blocking angiogenesis in MM.
The BM microenvironment exerts marked effects on MM
progression. Gupta et al found that the targeting of stromal
versican, which plays a key role in matrix remodelling, malignant
transformation and tumour progression, by miR-144/199 may inhibited
MM via the downregulation of the FAK/STAT3 signalling pathway
(25). This indicates that
miR-144 can be used to regulate the BM microenvironment. In the
present study, it was found that miR-144-3p expression was
lower in exosomes from bone marrow supernatants in patients with MM
than in healthy donors. The effects of miR-144-3p on MM
cells were then evaluated. However, as miR-144-3p can be
transferred through exosomes from MM cells to other cells in the
micro-environment, the regulatory effects of miR-144-3p on
the BM microenvironment remain unclear. Thus, additional studies
and in vivo experiments are warranted to evaluate this
mechanism.
In conclusion, the findings of the present study
demonstrated that miR-144-3p inhibited the proliferation,
migration and angiogenesis of MM cells, and induced cell cycle
arrest and apoptosis by targeting MEF2A in MM. Considering the
molecular and biological complexity of angiogenesis, these results
provide useful insight into the development of novel and effective
anti-MM drugs.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272629), the
Natural Science Foundation of Liaoning Province (2019-ZD-0792), the
Special Fund for Clinical Medical Research of the Chinese Medical
Doctor Association (grant no. 20111210) and the Natural Science
Foundation of Liaoning Province (grant no. 20180551257).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AL, FT, HW, HM and YZ conceived and designed the
experiments. FT performed the experiments and wrote the manuscript.
FT and AL analysed the data and critically revised the manuscript.
FT, HM and YZ completed the post-production image processing. AL
and HW supervised all research and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Shengjing Hospital of China Medical University
(approval no. 2019PS270K) and all patients provided informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liang B, Yin JJ and Zhan XR: MiR-301a
promotes cell proliferation by directly targeting TIMP2 in multiple
myeloma. Int J Clin Exp Pathol. 8:9168–9174. 2015.PubMed/NCBI
|
|
2
|
Brigle K and Rogers B: Pathobiology and
diagnosis of multiple myeloma. Semin Oncol Nurs. 33:225–236. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao Q, Luo F, Ma J and Yu X: Bone
metastasis-related Micrornas: New targets for treatment? Curr
Cancer Drug Targets. 15:716–725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ribatti D and Vacca A: New insights in
anti-angiogenesis in multiple myeloma. Int J Mol Sci. 19:20312018.
View Article : Google Scholar :
|
|
5
|
D'Ario M, Griffiths-Jones S and Kim M:
Small RNAs: Big impact on plant development. Trends Plant Sci.
22:1056–1068. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baek D, Villén J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biswas S: MicroRNAs as therapeutic agents:
The future of the battle against cancer. Curr Top Med Chem.
18:2544–2554. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Misiewicz-Krzeminska I, Krzeminski P,
Corchete LA, Quwaider D, Rojas EA, Herrero AB and Gutiérrez NC:
Factors regulating microRNA expression and function in multiple
myeloma. Noncoding RNA. 5:92019.
|
|
9
|
Lu J, Liu QH, Wang F, Tan JJ, Deng YQ,
Peng XH, Liu X, Zhang B, Xu X and Li XP: Exosomal miR-9 inhibits
angiogenesis by targeting MDK and regulating PDK/AKT pathway in
nasopharyngeal carcinoma. J Exp Clin Cancer Res. 37:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
He Y, Li J, Ding N, Wang X, Deng L, Xie Y,
Ying Z, Liu W, Ping L, Zhang C, et al: Combination of enzastaurin
and ibrutinib synergistically induces anti-tumor effects in diffuse
large B cell lymphoma. J Exp Clin Cancer Res. 38:862019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krek A, Grun D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, Piedade ID, Gunsalus KC, Stoffel M and
Rajewsky N: Combinatorial microRNA target predictions. Nat Genet.
37:495–500. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ritchie W: MicroRNA target prediction.
Methods Mol Biol. 1513:193–200. 2017. View Article : Google Scholar
|
|
14
|
van Iterson M, Bervoets S, de Meijer EJ,
Buermans HP, Hoen PA, Menezes RX and Boer JM: Integrated analysis
of microRNA and mRNA expression: Adding biological significance to
microRNA target predictions. Nucleic Acids Res. 41:e1462013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li M, Linseman DA, Allen MP, Meintzer MK,
Wang X, Laessig T, Wierman ME and Heidenreich KA: Myocyte enhancer
factor 2A and 2D undergo phosphorylation and caspase-mediated
degradation during apoptosis of rat cerebellar granule neurons. J
Neurosci. 21:6544–6552. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al Masri A, Price-Troska T, Chesi M, Chung
TH, Kim S, Carpten J, Bergsagel PL and Fonseca R: MicroRNA
expression analysis in multiple myeloma. Blood. 106:15542005.
View Article : Google Scholar
|
|
17
|
Zhao Y, Xie Z, Lin J and Liu P: MiR-144-3p
inhibits cell proliferation and induces apoptosis in multiple
myeloma by targeting c-Met. Am J Transl Res. 9:2437–2446.
2017.PubMed/NCBI
|
|
18
|
Tianhua Y, Dianqiu L, Xuanhe Z, Zhe Z and
Dongmei G: Long non-coding RNA Sox2 overlapping transcript (SOX2OT)
promotes multiple myeloma progression via microRNA-143-3p/c-MET
axis. J Cell Mol Med. 24:5185–5194. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raimondi L, Amodio N, Di Martino MT,
Altomare E, Leotta M, Caracciolo D, Gullà A, Neri A, Taverna S,
D'Aquila P, et al: Targeting of multiple myeloma-related
angiogenesis by miR-199a-5p mimics: In vitro and in vivo anti-tumor
activity. Oncotarget. 5:3039–3054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Estrella NL, Desjardins CA, Nocco SE,
Clark AL, Maksimenko Y and Naya FJ: MEF2 transcription factors
regulate distinct gene programs in mammalian skeletal muscle
differentiation. J Biol Chem. 290:1256–1268. 2015. View Article : Google Scholar :
|
|
21
|
Ewen EP, Snyder CM, Wilson M, Desjardins D
and Naya FJ: The Mef2A transcription factor coordinately regulates
a costamere gene program in cardiac muscle. J Biol Chem.
286:29644–29653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ribatti D, Nico B, Crivellato E, Roccaro
AM and Vacca A: The history of the angiogenic switch concept.
Leukemia. 21:44–52. 2007. View Article : Google Scholar
|
|
23
|
Roccaro AM, Sacco A, Thompson B, Leleu X,
Azab AK, Azab F, Runnels J, Jia X, Ngo HT, Melhem MR, et al:
MicroRNAs 15a and 16 regulate tumor proliferation in multiple
myeloma. Blood. 113:6669–6680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van de Donk NW, Lokhorst HM, Nijhuis EH,
Kamphuis MM and Bloem AC: Geranylgeranylated proteins are involved
in the regulation of myeloma cell growth. Clin Cancer Res.
11:429–439. 2005.PubMed/NCBI
|
|
25
|
Gupta N, Kumar R, Seth T, Garg B and
Sharma A: Targeting of stromal versican by miR-144/199 inhibits
multiple myeloma by downregulating FAK/STAT3 signalling. RNA Biol.
17:98–111. 2020. View Article : Google Scholar
|