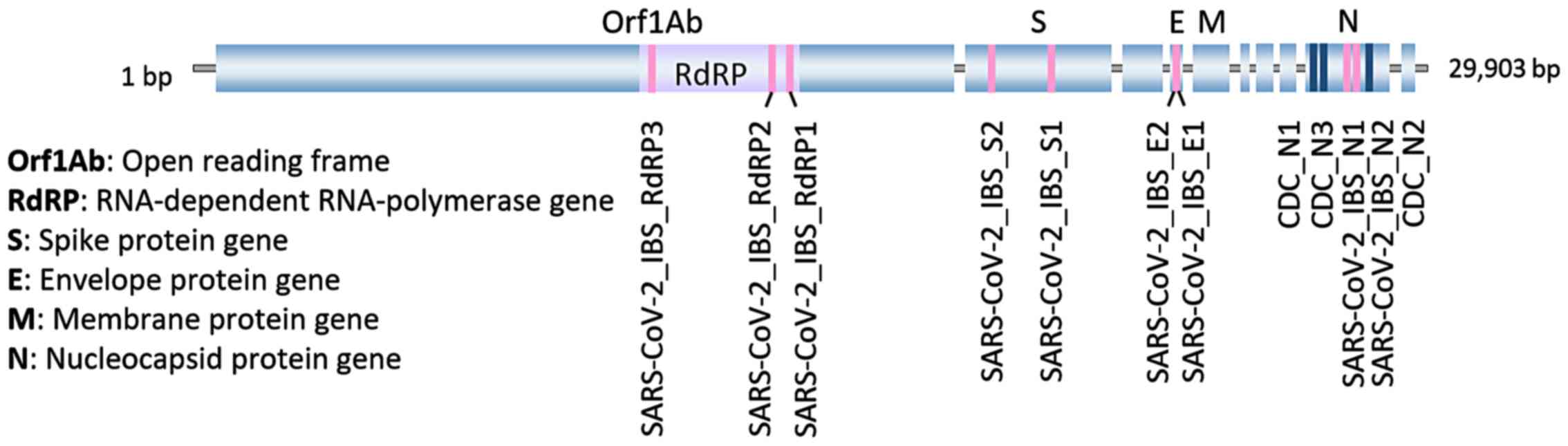
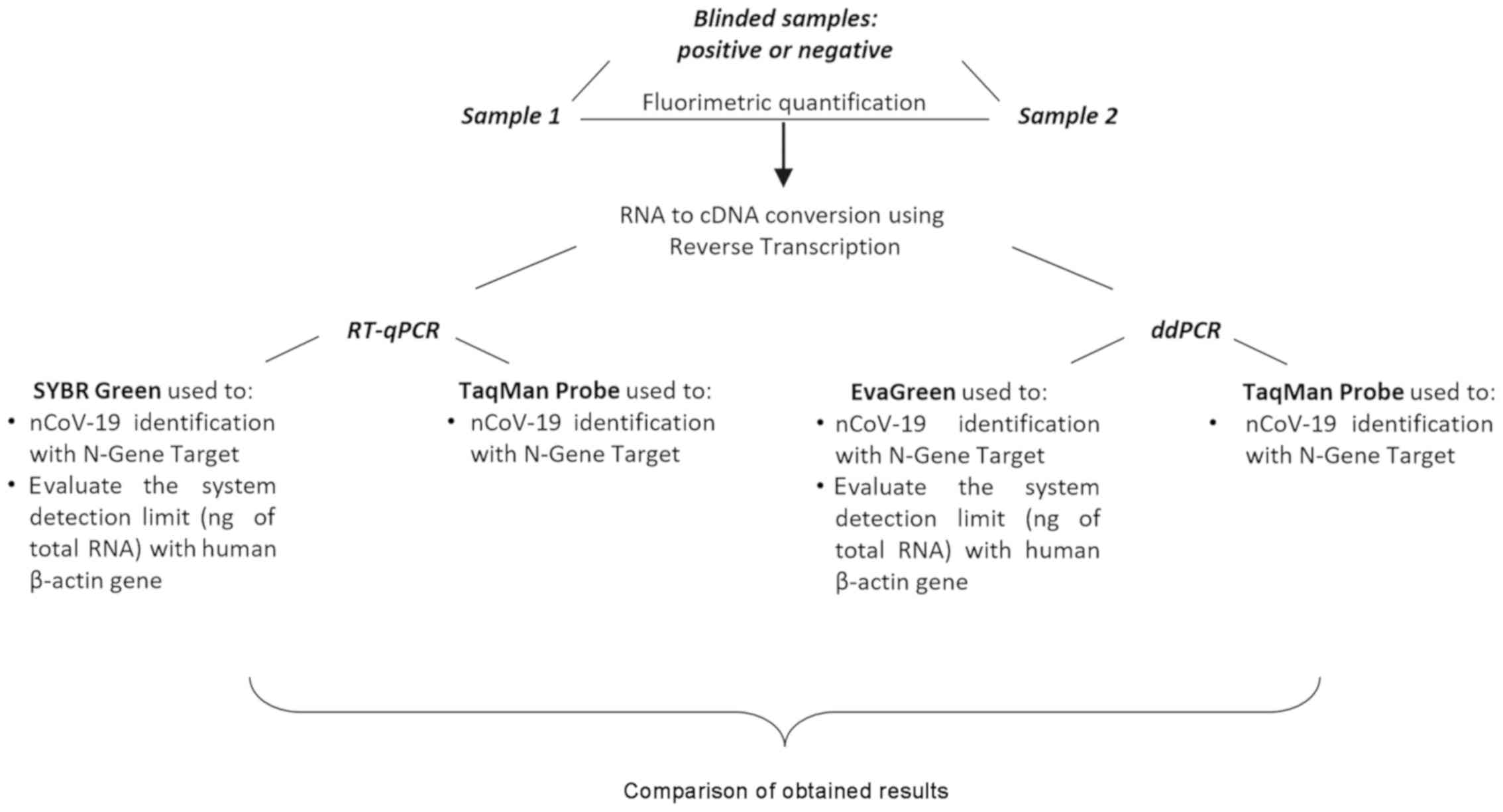
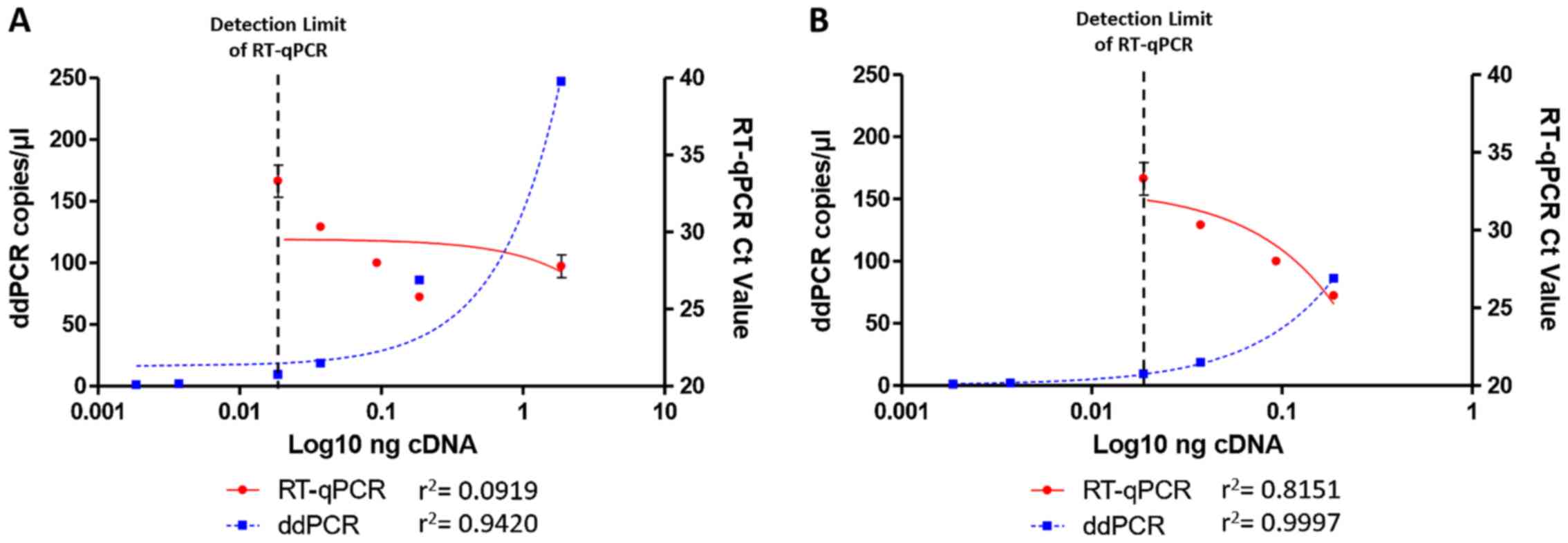
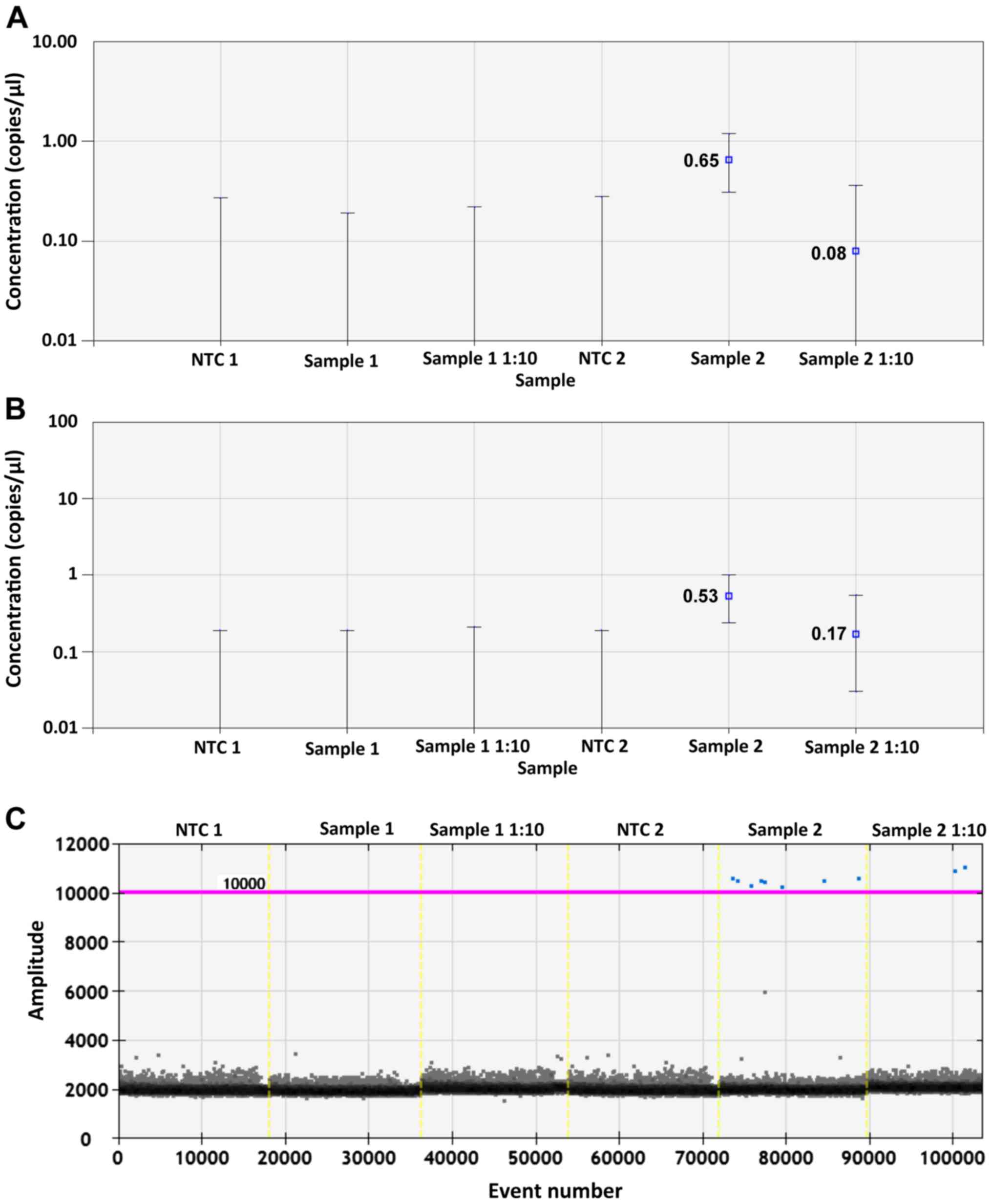
|
1
|
Chakraborty I and Maity P: COVID-19
outbreak: Migration, effects on society, global environment and
prevention. Sci Total Environ. 728:1388822020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re-emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI
|
|
3
|
Mackenzie JS and Smith DW: COVID-19-A
novel zoonotic disease: A review of the disease, the virus, and
public health measures. Asia Pac J Public Health. May 30–2020.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goumenou M, Sarigiannis D, Tsatsakis A,
Anesti O, Docea AO, Petrakis D, Tsoukalas D, Kostoff R, Rakitskii
V, Spandidos DA, et al: COVID-19 in Northern Italy: An integrative
overview of factors possibly influencing the sharp increase of the
outbreak (Review). Mol Med Rep. 22:20–32. 2020.PubMed/NCBI
|
|
5
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F,
Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16.
2020.PubMed/NCBI
|
|
6
|
Corman VM, Landt O, Kaiser M, Molenkamp R,
Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML,
et al: Detection of 2019 novel coronavirus (2019-nCoV) by real-time
RT-PCR. Euro Surveill. 25:20000452020. View Article : Google Scholar :
|
|
7
|
World Health Organization (WHO): All
technical guidance on COVID-19 - select topic from drop down menu.
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance.
|
|
8
|
Won J, Lee S, Park M, Kim TY, Park MG,
Choi BY, Kim D, Chang H, Kim VN and Lee CJ: Development of a
Laboratory-safe and low-cost detection protocol for SARS-CoV-2 of
the Coronavirus Disease 2019 (COVID-19). Exp Neurobiol. 29:107–119.
2020. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Yao H, Xu X, Zhang P, Zhang M,
Shao J, Xiao Y and Wang H: Limits of detection of six approved
RT-PCR kits for the novel SARS-coronavirus-2 (SARS-CoV-2). Clin
Chem. 66:977–979. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tahamtan A and Ardebili A: Real-time
RT-PCR in COVID-19 detection: Issues affecting the results. Expert
Rev Mol Diagn. 20:453–454. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lippi G, Simundic AM and Plebani M:
Potential preanalytical and analytical vulnerabilities in the
laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin
Chem Lab Med. 58:1070–1076. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang YW, Schmitz JE, Persing DH and
Stratton CW: Laboratory diagnosis of COVID-19: Current issues and
challenges. J Clin Microbiol. 58:e00512–e00520. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taylor SC, Carbonneau J, Shelton DN and
Boivin G: Optimization of Droplet Digital PCR from RNA and DNA
extracts with direct comparison to RT-qPCR: Clinical implications
for quantification of Oseltamivir-resistant subpopulations. J Virol
Methods. 224:58–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hindson CM, Chevillet JR, Briggs HA,
Gallichotte EN, Ruf IK, Hindson BJ, Vessella RL and Tewari M:
Absolute quantification by droplet digital PCR versus analog
real-time PCR. Nat Methods. 10:1003–1005. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hindson BJ, Ness KD, Masquelier DA,
Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY,
Hiddessen AL, Legler TC, et al: High-throughput droplet digital PCR
system for absolute quantitation of DNA copy number. Anal Chem.
83:8604–8610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Filetti V, Falzone L, Rapisarda V,
Caltabiano R, Eleonora Graziano AC, Ledda C and Loreto C:
Modulation of microRNA expression levels after naturally occurring
asbestiform fibers exposure as a diagnostic biomarker of
mesothelial neoplastic transformation. Ecotoxicol Environ Saf.
198:1106402020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salemi R, Falzone L, Madonna G, Polesel J,
Cinà D, Mallardo D, Ascierto PA, Libra M and Candido S: MMP-9 as a
candidate marker of response to BRAF inhibitors in melanoma
patients with BRAFV600E mutation detected in circulating-free DNA.
Front Pharmacol. 9:8562018. View Article : Google Scholar :
|
|
18
|
Battaglia R, Palini S, Vento ME, La
Ferlita A, Lo Faro MJ, Caroppo E, Borzì P, Falzone L, Barbagallo D,
Ragusa M, et al: Identification of extracellular vesicles and
characterization of miRNA expression profiles in human blastocoel
fluid. Sci Rep. 9:842019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Whale AS, Huggett JF, Cowen S, Speirs V,
Shaw J, Ellison S, Foy CA and Scott DJ: Comparison of microfluidic
digital PCR and conventional quantitative PCR for measuring copy
number variation. Nucleic Acids Res. 40:e822012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Department of Health and Human Services,
Public Health Service, Centres for Disease Control and Prevention
(CDC): 2019-Novel Coronavirus (2019-nCoV) Real-time rRT-PCR Panel.
Primers and Probes. Division of Viral Diseases. CDC; Atlanta, GA:
2020, https://www.who.int/docs/default-source/coronaviruse/uscdcrt-pcr-panel-primer-probes.pdf?sfvrsn=fa29cb4b_2.
Accessed March 15, 2020.
|
|
21
|
Fresta CG, Fidilio A, Lazzarino G, Musso
N, Grasso M, Merlo S, Amorini AM, Bucolo C, Tavazzi B, Lazzarino G,
et al: Modulation of pro-oxidant and pro-inflammatory activities of
M1 macrophages by the natural dipeptide carnosine. Int J Mol Sci.
21:E7762020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Musso N, Caronia FP, Castorina S, Lo Monte
AI, Barresi V and Condorelli DF: Somatic loss of an EXT2 gene
mutation during malignant progression in a patient with hereditary
multiple osteochondromas. Cancer Genet. 208:62–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
World Health Organization (WHO):
Emergencies diseases novel coronavirus 2019. https://www.who.int/.
|
|
24
|
Carter LJ, Garner LV, Smoot JW, Li Y, Zhou
Q, Saveson CJ, Sasso JM, Gregg AC, Soares DJ, Beskid TR, et al:
Assay techniques and test development for COVID-19 diagnosis. ACS
Cent Sci. 6:591–605. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao AT, Tong YX and Zhang S:
False-negative of RT-PCR and prolonged nucleic acid conversion in
COVID-19 Rather than recurrence. J Med Virol. https://doi.org/10.1002/jmv.25855.
|
|
26
|
Li Y, Yao L, Li J, Chen L, Song Y, Cai Z
and Yang C: Stability issues of RT-PCR testing of SARS-CoV-2 for
hospitalized patients clinically diagnosed with COVID-19. J Med
Virol. 92:903–908. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dingle TC, Sedlak RH, Cook L and Jerome
KR: Tolerance of droplet-digital PCR vs real-time quantitative PCR
to inhibitory substances. Clin Chem. 59:1670–1672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong L, Wang X, Wang S, Du M, Niu C, Yang
J, Li L, Zhang G, Fu B, Gao Y, et al: Interlaboratory assessment of
droplet digital PCR for quantification of BRAF V600E mutation using
a novel DNA reference material. Talanta. 207:1202932020. View Article : Google Scholar
|
|
29
|
Pinheiro LB, O'Brien H, Druce J, Do H, Kay
P, Daniels M, You J, Burke D, Griffiths K and Emslie KR:
Interlaboratory reproducibility of Droplet Digital Polymerase Chain
Reaction using a new DNA reference material format. Anal Chem.
89:11243–11251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rutsaert S, Bosman K, Trypsteen W, Nijhuis
M and Vandekerckhove L: Digital PCR as a tool to measure HIV
persistence. Retrovirology. 15:162018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yucha RW, Hobbs KS, Hanhauser E, Hogan LE,
Nieves W, Ozen MO, Inci F, York V, Gibson EA, Thanh C, et al:
High-throughput characterization of HIV-1 reservoir reactivation
using a single-cell-in-Droplet PCR assay. EBioMedicine. 20:217–229.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trypsteen W, Kiselinova M, Vandekerckhove
L and De Spiegelaere W: Diagnostic utility of droplet digital PCR
for HIV reservoir quantification. J Virus Erad. 2:162–169.
2016.PubMed/NCBI
|