Introduction
Quinazoline alkaloid tryptanthrin (TR) is one of the
most widespread and well known cytotoxic and antimicrobial agents
(1), obtained for the first time
more than 140 years ago by sublimation of natural indigo under
reduced pressure. Although TR was obtained by this method and by
oxidation of indigo long before its finding in different biological
sources, it was later isolated, using a chromatographic separation
technique, from extracts of numerous indigoid higher plants (genera
Couroupita, Isatis, Polygonum,
Strobilanthes and others), fungi (Candida lipolitica,
Schizophyllum commune, Leucopaxillus cerealis)
(1), marine bacteria (2) as well as yeasts of the genus
Malassezia, known as a part of the normal skin flora
(3).
Therapeutic application of tryptanthrin-containing
topical preparations in Chinese folk medicine (4) confirmed its potent anti-inflammatory
action as well as anti-tumour and antimicrobial activities,
particularly against mycobacteria and pathogenic protozoa,
including malaria plasmodia (5-8). A
tryptanthrin-containing oral and topical preparation has been
developed using chitosan as carrier of TR, known as 'Kourochitin'.
It has been shown that this preparation has anti-inflammatory,
antimicrobial, wound healing, anti-tumour, anti-allergic and other
biological activities (9-12).
The peculiarities of TR biological action include an
absence of membrane activity, ability to exhibit an
immunosuppressive effect and an increase in intracellular ROS level
(13). Oxidative stress induced
by TR in various tumour cell lines is one of the assumed reasons
for its cytotoxic activity and the ability to cause cell death via
apoptosis mechanisms (1,14). Interest in TR and its natural and
synthetic derivatives was stimulated, not only by its promising
biomedical properties, but also by its possibility of obtaining
good yields using different synthetic methods; the coupling of
isatin or its sodium salt with isatoic anhydride (1), oxidation of isatin (15), its treatment with PCl5
or POCl3 (16), and
condensation of anthranilic acid with different aromatic
derivatives (1) are examples of
this.
At the same time, the main imperfections of TR and
of the majority of its analogues and derivatives include very poor
solubility in biological liquids, relatively high toxicity and an
immunosuppressive effect when administered systemically. Based on
this, we designed synthesis of new therapeutically promising,
water-soluble and less toxic derivatives. The strategy of the study
was to obtain a water-soluble analog of TR, known as mostotrin
(MT), closely related to its mother compound by its flat structure,
in which the CO group has been replaced by an isosteric C=N group.
This modification led to significant changes of biological
activities MT in comparison with TR, particularly in enhancing
antitumor properties. Herein, we report the synthesis and
biological properties MT, namely the antibacterial, cytotoxic and
antitumour activity.
Materials and methods
Obtaining mostotrin from
tryptanthrin
TR (0.508 g, 2 mmol), produced by experimental plant
of PIBOC (Russia) by oxidation of isatin, and 20 ml of ice acetic
acid, was placed in a two-neck flask equipped with a dropping
funnel and reflux condenser and heated at 90°C over 1 h.
Subsequently, Girard reagent T (0.458 g, 2.7 mmol), in 4 ml of ice
acetic acid, was added dropwise for 10 min to the formed
suspension. The reaction mixture was heated and stirred at the same
temperature for 4 h. The solvent was removed under reduced pressure
from the obtained red-brown solution at 60°C. The residue was
treated with water (15 ml), stirred and filtered. The resulting
solution was concentrated on a rotary evaporator in a vacuum and,
consequently, butanol was added in small portions as a defoamer.
Ethanol (20 ml) was added to the concentrate. After cooling at 5°C
for 12 h, the precipitate of the product MT was separated by
filtration (yield 67%), m.p. >248°C (decomp.) and twice
crystallised from ethanol. Its solubility was determined in
experiments to obtain an aqueous solution of 200 mg/ml
concentration using warm water (approximately 40°C); the obtained
solution was stable for at least 1 month without turbidity or
sedimentation. For comparison, the mother compound (TR) was
sparingly soluble. Approximately 39 mg was separated by filtration
and drying to a constant weight (3 replicates) after boiling 40 mg
of TR in 400 ml of water, corresponding to a solubility of
0.0025±0.0005 mg/ml. This corresponds with information about its
solubility, of 2.5 µg/ml, from the company producing this
preparation (Enzo-Life Science; tryptanthrin, inhibitor of
prostaglandin and leukotriene synthesis; CAS 13220-57-0). Thus, MT
was at least 5 orders of magnitude more soluble in water than its
mother compound (TR).
1H and 13C NMR spectra were
measured on Bruker Avance 700 and 400 spectrometers (Bruker
Corporation) at 700 and 100 MHz, respectively, using D2O
or DMSO-d6 as the solvent. Signals were assigned using a DEPT-135
pulse sequence. The 1H NMR spectrum was [700 MHz,
(D2O, d6-acetone as exterior standard, 2.219
ppm)]: 7.83 d (1H), 7.76 d (1H), 7.66 t (1H), 7.748 m (NH), 7.45 t
(1H), 7.395 d (1H), 7.36 t (1H), 7.32 d (1H), 7.18 t (1H), 4.645 s
(2H), 3.495 s (9H). The 13C NMR spectrum was (DEPT,
(CD3)2SO, 39.5 ppm): 168.4, 166.5, 157.7,
145.7, 145.5, 140.1, 135.3, 132.3, 129.3, 128.15, 126.9, 126.85,
122.2, 121.95, 121.3, 117.6, 116.4, 62.0 (CH2), 53.6
(CH3)3.
The IR spectra were recorded in KBr on a Spectrum
BX-II spectrometer (Perkin Elmer). IR spectrum (KBr): 3430 (NH),
2925 (CH2), 1691 (C=O), 1632 cM−1 (C=N)
(SI).
Mass spectra were recorded using an Agilent 6510
Q-TOF apparatus (Agilent Technologies), with a sample concentration
of 0.01 mg/ml in methanol. ESI HRMS, positive mode, m/z: 362.1610,
calculated for cation
C20H20N5O2 362.1612;
ESI HRMS/MS of 362.1611, positive mode, m/z: 260.0822
(M+-HCl-COCH2-N(CH3)3)
(SI).
The samples were dissolved in MeOH (c 0.001 mg/ml)
and Sorbfil Si gel plates 4.5×6.0 cm, 5-17 µm (Sorbpolimer)
were used for thin-layer chromatography. Melting points were
determined on melting point apparatus Leuca VM HB.
X-ray analysis of mostotrin
Analysis was carried out using a diffractometer,
Bruker Kappa APEX2 (Bruker Corporation) (MoKa-radiation, graphite
monochromator) at 173°K. Data collection, editing and refinement of
the unit cell parameters were carried out using APEX2 programs
(Bruker Corporation) (17).
Calculations on the definition and refinement of the structure were
made using the SHELXTL/PC programmes, Georg-August Universitat
Gottingen, Department of Structural Chemistry, Gottingen, Germany
(18). Absorption of X-rays in
the sample was taken into account by equivalent reflections. The
structure was determined by the direct method and refined by the
least square method in the anisotropic approximation of
non-hydrogen atoms. Hydrogen atoms were placed in geometrically
calculated positions and refined in the rider model to study their
participation in the formation of intramolecular bonds. The CIF
file, containing information concerning the structure of MT, was
deposited in the CCDC, no. 1964205.
Studies on binding MT and TR to DNA
Spectra for the studies concerned with binding to
DNA were carried out in 10 µM phosphate buffer (pH 8.0), 100
mM KCl solution at 22°C. Calf thymus DNA (Sigma) (ctDNA) was used
in experiments. Fluorescence spectra were recorded with Cary
Eclipse Fluorescence Spectrophotometer (Agilent Technologies) for
10 µM of compounds with increased DNA concentration. The
excitation wavelength was 380 nm, and emission was recorded in the
range of 400-650 nm. Circular dichroism spectra were recorded with
a Jasco J715 spectropolarimeter (JASCO Corporation). DNA
concentration was 100 µM (base pairs), and concentrations of
the studied compound were 10 and 50 µM (19). Binding parameters were calculated
from the dependence of fluorescence intensity on DNA concentration
using the McGhee-von Hippel equation (20). The binding isotherm reflecting an
anti-cooperative binding of MT to DNA was plotted in the Scatchard
coordinates.
Antimicrobial action
Strains of microorganisms, including Escherichia
coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853,
Bacillus cereus ATCC 10702, Candida parapsilosis ATCC
22019, Mycobacterium spp. R (fast growing nog9), were
obtained from the museum of the Gause Institute of New Antibiotics,
Moscow, Russia. Aqueous solutions of MT and DOX, or their solutions
in PBS, as well as solutions of TR in DMSO (Sigma-Aldrich) were
used in experiments. To reactivate after cryoconservation, test
strains of micro-organisms were seeded on agar nutrient medium as
follows: Bacterial strains on CASO-Agar (Sifin), and Candida
parapsilosis with a titre of 2.5×103 CFU/ml on
RPMI-1640 medium (PanEco) with the addition of 0.2% glucose or on
Saburo (Sigma-Aldrich). The bacterial strains were cultivated for
18-20 h, with the exception of Mycobacterium spp., which was
cultivated for 3-5 days, and Candida parapsilosis which was
cultivated for 48 h at 35±2°C. After cultivation, the biomasses of
microorganisms were diluted in a physiological solution
(Sigma-Aldrich) to a turbidity of 0.5 units, according to the
McFarland turbidity standard, which was measured on a McFarland
Densitometer (Biosan). Subsequently, 10 µl portions of the
studied compounds were added to the wells with microorganisms at
the concentration range of 0.06-128 µg/ml. The plates with
the tested strains were incubated in a normal atmosphere at a
temperature of 35±2°C for 16-24 h for bacterial cultures (in the
case of Mycobacterium spp. R=96 h), and 24-48 h for
Candida spp. The growth rate of microorganisms in each well
was measured by the turbidity/absorption of the cell inoculum using
a microplate reader at a wavelength between 405 and 530 nm.
Antimicrobial activities of the tested compounds were determined as
the minimum inhibitory concentration (MIC). The analysis was
carried out in accordance with the recommendations of Russian GOST
R ISO 20776-1-2010 and GOST R ISO 16256-2015 rules and the standard
protocol described within.
Cytotoxic activity against tumour
cells
The K562 human leukaemia, HCT116 colon carcinoma,
B16-F0 murine melanoma, MCF-7 human breast adenocarcinoma and
MDA-MB-231 human breast cancer cell lines were purchased from the
American Type Culture Collection (ATCC). The reagents were
purchased from Sigma-Aldrich, unless specified otherwise.
An aqueous solution of MT or its solution in PBS, as
well as solutions of TR and doxorubicin (DOX) in DMSO, were used in
the experiments. Cultures of the corresponding cells in the
logarithmic growth phase were placed in a 96-well plate (Nalge NUNC
International) at 5×103 cells per well in 190 µl
of Eagle's culture medium (PanEco) and RPMI-1640 medium (PanEco),
with the addition of 5-10% fetal bovine serum (HyClone), 2 mM
L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin
and incubated for 24 h at 37°C, 5% CO2. The cells were
processed with 10 µl of each solution of the studied
compounds, prepared by successive double dilutions, added in a
range of final concentrations from 0.1 to 50 µM (total 10
concentrations: 0.1; 0.2; 0.4; 0.8; 1.6; 3.2; 6; 12; 25 and 50
µM) and with 0.1% DMSO (vehicle control). Then, the treated
cells were incubated for 72 h (each concentration was studied with
three replications). At the end of the incubation, 10 µl of
an aqueous solution of 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT, PanEco; 5 mg/ml) was introduced into the
wells. After staining, the culture medium was removed, the cells
were suspended in 100 µl of DMSO, and the optical density of
the solutions was measured on a microplate ELx800 photometer
(BioTek) at a wavelength of 570 nm. The number of living cells in
each sample (N, %) was calculated as a percentage of the control
using the formula N=(IE/IK) ×100%, where IE is the optical density
in each experimental well and IK is the optical density in the
control wells (samples not treated with compound 1 or 2, the values
N of which are taken as 100%). The cytotoxic effect of the
compounds was characterised by the concentration of half-maximal
inhibition (IC50), which was calculated based on the
obtained values of N.
Animals
Animal procedures were performed using female CD-1
(total 145 animals) or BALB/C (total 28 animals) albino mice,
weighing 25±2 g, 8-10 weeks of age, originally obtained from the
vivarium of the Institute of Bioorganic Chemistry, Pushchino,
Russia. The mice were housed in standard animal facility in cages
with an ambient temperature of 22±2°C, a controlled humidity of 50%
and a 12-h light/dark cycle and were provided with free access to
standard food and water.
Toxicity
Toxicity assessment was performed using CD-1 albino
mice, weighing 25±2 g. MT was administered once intraperitoneally
in doses of 500, 250, 125 and 50 mg/kg/mice (5 mice in each group).
Within 24 h after injection, mortality and changes in basic
physiological parameters, such as body temperature, breathing
frequency, wool characteristics, physical activity (motility,
coordination and speed of movement), behavioural responses
(excitation or inhibition, eating and drinking behaviours,
grooming), were visually registered in each group of animals.
LD50 values were calculated 24 h after administration
using the formula:
LD50=LD100−∑Zd/n
where, LD100 is the maximum dose that causes the death
of all animals in the group, Z is the arithmetic average of the
number of animals in which a mortality is noted under the influence
of two adjacent doses, d is the interval between two adjacent
doses, and n is the number of animals in each group (
21).
Ethics approval
The animal study was performed in accordance with
the European Convention for the Protection of Vertebrate Animals,
Directives 86/609/EEC (Council of Europe European Convention for
the Protection of Vertebrate Animals used for Experimental and
Other Scientific Purposes. Strasbourg: 1986, Accessed August 28,
2018) 18.III.1986. Council of Europe, ETS No. 123, European
Convention for the humane methods for the animal welfare and
maintenance [Directive 2010/63/EU on the protection of animals used
for scientific purposes EN. Official Journal of the European Union,
L 276/33-276/79 (20.10.2010)], the National Standard of the Russian
Federation R 53434-2009 'Good Laboratory Practice' (National state
standard GOST P 53434-2009 the Russian Federation standard 'The
principles of Good Laboratory Practice' approved and put into
effect by the Order of the Federal Agency for Technical Regulation
and Metrology of December 2, 2009, No 544) and approved by Ethic
Committee of Animal Experimentation of G.B Elyakov Pacific
Institute of Bioorganic Chemistry of the Russian Academy of
Science.
Murine model of ascite Ehrlich's
adenocarcinoma
Ascitic Ehrlich carcinoma cells (EACCs) were
maintained in the vivarium of PIBOC (Pacific Institute of
Bioorganic Chemistry) using CD-1 albino mice by serial
intraperitoneal (i.p.) passages with 7-10 day intervals. The mice
were euthanised 7 days after inoculation, and the ascitic fluid was
collected under aseptic conditions from tumour-bearing mice by
needle aspiration from the peritoneal cavity. EACCs were obtained
after washing three times with normal saline, followed by
centrifugation (Eppendorf Centrifuge 5804) at 1,500 × g for 10 min
at 24°C. Tumour cell counts were carried out using the trypan blue
dye exclusion method. Cell viability was examined microscopically.
For the study on anti-tumour activity, a suspension of these tumour
cells (3×106 cells/mouse) in 0.5 ml of 1%
phosphate-buffered saline (PBS) (Sigma-Aldrich) was injected i.p.
into CD-1 mice. One day after tumour inoculation, treatment was
started with the studied compounds. The compounds and saline (for
negative control) were injected i.p. as 0.5 ml aqueous solutions
over 5 days (one injection per day). MT was used both in
monotherapy at doses of 2.5, 5, 10, 25 and 50 mg/kg and in
combination (with 5 or 10 mg/kg of MT +0.25 mg/kg antitumour drug,
Doxorubicin-Teva, (DOX) (Pharmachemie). The animals were divided
into the following groups, n=9 per gropu: i) Contr(-), negative
control; ii) DOX, 0.25 mg/kg; iii) MT, 2.5 mg/kg; iv) MT, 5 mg/kg;
v) MT, 10 mg/kg; vi) MT, 25 mg/kg; vii) MT, 50 mg/kg; viii) Dox
0.25 mg/kg + MT, 5 mg/kg; ix) DOX 0.25 mg/kg + MT 10 mg/kg.
Mean survival time (MST) and the percentage of
increase in life span (% ILS) were calculated using the formulae:
MST=Survival time (days) of each mouse in a group/Total number of
mice and % ILS=MST of treated mice/MST of control group ×100%.
Murine model of solid Ehrlich's
adenocarcinoma
EACCs were obtained as described in the previous
section. Experiments were performed using BALB/C albino mice. Mice
were inoculated with 0.2 ml containing 1.5×106 viable
EACCs in the right hind limb (thigh) subcutaneously. The animals
were randomised and divided into 4 groups (n=7): i) Cont(-),
negative control; ii) DOX, 0.25 mg/kg; iii) MT, 10 mg/kg; iv) DOX,
0.25 mg/kg+MT, 10 mg/kg. The treatment started when the primary
tumour reached a size of 57-60 mm3. All tested remedies
and saline (for negative control) were injected i.p. as 0.5 ml
portions of aqueous solutions for 5 days (one injection a day).
Tumour volume was measured from the 10th day of tumour induction,
and then every 4 days for a period of 17 days. Tumour growth was
assessed by measuring the volume of the solid tumour using a
digital calliper, and was calculated using the formula:
V=π/6xLxWxH,
where V, tumour volume; L, tumour length; W, tumour width; H,
tumour height.
Evaluation of the chemotherapeutic efficacy of the
tested remedies was carried out by inhibition tumour growth (TGI,
%) in the experimental groups. TGI was calculated as described in
(22) using the formula:
TGI=(1−Ve/Vc)×100.
where, Ve and Vc represent the median tumour volume in experimental
and control groups, respectively. On the termination day (22nd day
of tumour induction) experimental animals were euthanised by
cervical dislocation under ether anaesthesia, and the tumour mass
was removed for visual assessment of the tumour.
Anti-inflammatory action
Lipopolysaccharide (LPS) from the bacterium E.
coli (Sigma-Aldrich), at the dose of 0.1 mg/kg, was used for
simulation of early stages of system inflammation in female CD-1
mice. A matching volume of i.p.-injected PBS was used as a vehicle
control. The positive control was provided by application of
Dexamethasone, a corticosteroid anti-inflammatory drug, injected
i.p. at a dose of 10 mg/kg 1.5 h after LPS application. All the
mice were divided into 5 groups (n=7): i) Cont(in), intact animals;
ii) Cont(-), animals treated only with LPS (negative control); iii)
Cont(+), animals treated with LPS and Dexamethasone 1.5 h later,
injected i.p. at a dose of 10 mg/kg (positive control); iv) MT,
animals treated with LPS and 1.5 h later with MT injected i.p. at a
dose of 10 mg/kg; v) TR, treated with LPS and 1.5 h later with TR
injected i.p. at a dose of 10 mg/kg. Levels of cytokines (INF-γ,
IL-1 and IL-2) as indicators of system inflammation, were
determined in the plasma of mice by immunoassay (BD Bioscience
OptEIA). Animals were terminally anaesthetised with sodium
pentobarbital (40 mg per mouse i.p., Euthatal, Merial Animal
Health). The thoracic cavity was opened, and blood collected in
heparinised tubes directly from the right atrium of the heart. This
whole blood was centrifuged at 1.5 × g for 15 min at 24°C to remove
cells; the plasma was then aliquoted and stored at -20°C. These
samples were diluted appropriately and then analysed for INF-γ,
IL-1 and IL-2.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software v.5.01 (GraphPad Prism Software Inc.) and MS Excel
2013 (Microsoft Corporation). The data for all in vitro and
in vivo assays are presented as mean ± SEM (standard error
of the mean). Statistical analysis among various groups was
conducted by a one-way analysis of variance with Tukey's post hoc
test. Statistical analysis for the survival curves in the animal
treatment study was carried out using the log-rank test (Mantel-Cox
test). Statistically significant differences were considered if
P<0.05. In the antimicrobial action test, the MICs were
determined from independent triplicate assays and were based on a
serial 2-fold plus or minus system; to be considered valid, MIC
determinations for each of the 3 replicates had to be within plus
or minus 1 dilution of each other. If necessary, additional
replicates were run until 3 replicates were obtained within these
limits.
Results
Chemical synthesis and X-ray
analysis
In order to improve the physicochemical properties
and biological activity of TR, we performed chemical modifications
of its structure. In particular, the reaction of TR and/or its
derivatives was used with hydrazides of acids, which bear a
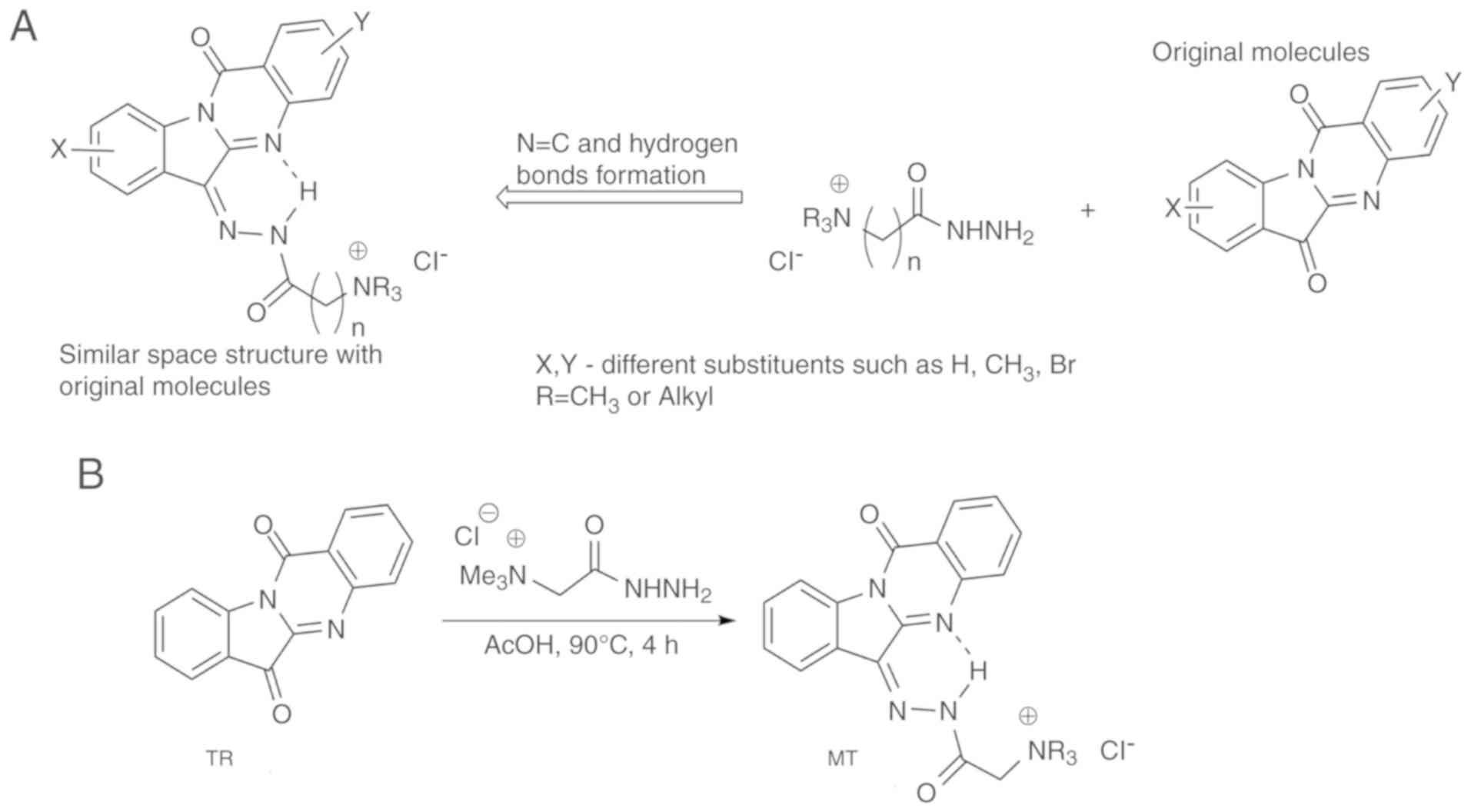
positive charged fragment remote from the hydrazide group. Fig. 1A and B describe the strategy of
syntheses of water-soluble derivatives of TR and the synthetic path
to the compound MT, respectively. It was expected that the product
would contain a nearly planar arrangement, similar to TR itself
(23) due to an intramolecular
hydrogen bond between the NH group in the side chain and the
nitrogen atom in the core moiety (Fig. 1). Commercially available Girard
reagent T, 1-trimethylammonium-3-hydrazin-ylpropan-2-one chloride,
was determined to be suitable for this synthesis. Compound MT was
synthesised by heating TR and Girard reagent in ice acetic acid
(Fig. 1B).
The conditions of synthesis, as well as the NMR, MS
and IR spectroscopic data of MT used to establish its structure,
are described in the experimental section. These spectra are given
in Figs. S1-4. The molecular
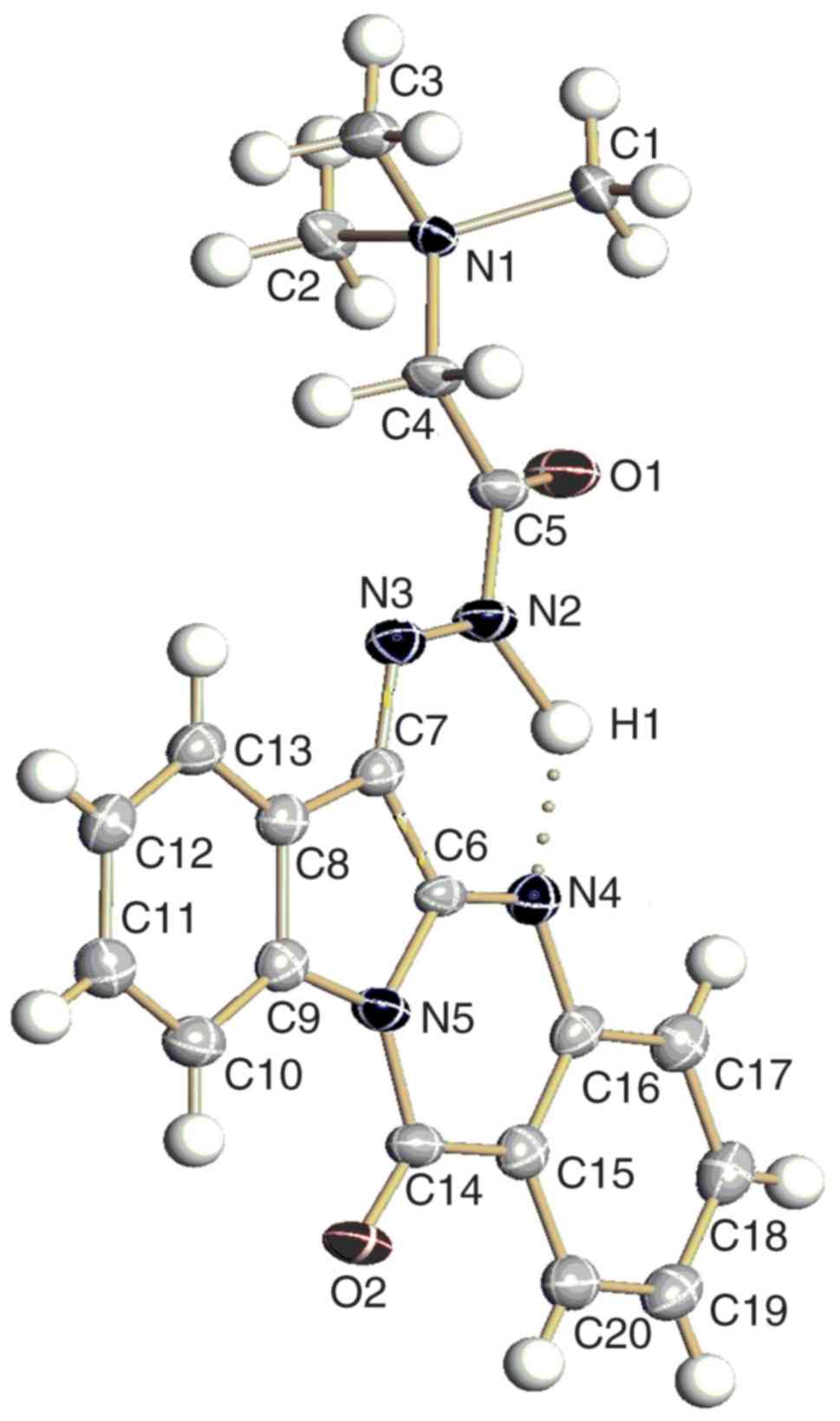
structure of MT was also confirmed by single crystal X-ray analysis
(Table SI) and the results are
shown in Fig. 2. The crystalline
molecular structure revealed the elemental unit to consist of twice
repeated couples of oppositely oriented molecules of MT, four
chloride ions and four water molecules (Fig. S5). The positions of hydrogen
atoms were determined from the difference in electron density and
were not specified. The atom, designated as H1 in Fig. 2, participates in the formation of
intramolecular hydrogen bonds in 1.
X-ray analysis showed that compound MT, when
compared with that of the initial compound TR, has an additional
six-membered pseudo-cycle, with five covalent and one hydrogen
bonds (Fig. 2, Table SII).
The interatomic distance of the abovementioned
intramolecular bond (Table SII)
emphasised this hydrogen bond to be strong because its length
(1.75Å) was insignificantly more than the sum of Van-der-Waals
radiuses of nitrogen and hydrogen atoms (1.55+1.09 Å). The plane
obtained by our compound is as flat as TR (23) and has a similar electron density
distribution, caused by the replacement of one carbonyl group for
an isosteric C=N group.
Antimicrobial action
Parent TR and its derivatives showed a
broad-spectrum of antimicrobial and antifungal activities (1). To evaluate the antimicrobial
potential of the newly synthesised MT, we compared the
antimicrobial activities of the compounds MT and TR against
gram-negative and gram-positive bacteria, including
Mycobacterium spp., and the fungus Candida
parapsilosis (Table I).
 | Table IExperimental values of the minimum
inhibitory concentration of MT and RT in relation to the pathogenic
strains of microorganisms. |
Table I
Experimental values of the minimum
inhibitory concentration of MT and RT in relation to the pathogenic
strains of microorganisms.
| Test
microorganisms | MIC (µg/ml)
|
|---|
| MT | TR |
|---|
| Staphylococcus.
aureus ATCC 29213 | 64 | 32 |
| Staphylococcus.
aureus (MRSA) 88 | 128 | 32 |
| Enterococcus
faecalis (VRE) 583 | 128 | 64 |
| Bacillus
cereus ATCC 10702 | 8 | 8 |
| Escherichia
coli ATCC 25922 | >128 | 8 |
| Pseudomonas
aeruginosa ATCC 27853 | >128 | >128 |
| Mycobacterium
spp. | 2 | 2 |
| Candida
parapsilosis ATCC 22019 | 128 | 128 |
The obtained MIC values against Bacillus
cereus ATCC 10702 and Mycobacterium spp. showed a potent
antimycobacterial action of MT and TR, but no significant
differences were revealed between the compounds. The antifungal
activities of both MT and TR against C. parapsilosis were
rather moderate. However, MT, in contrast with TR, is less active
in relation to the majority of other bacteria and the fungus.
Therefore, it should be noted that both MT and TR had high
antibacterial activity against the drug-resistant tuberculosis
bacteria Mycobacterium spp.
Cytotoxic activities against tumour
cells
In numerous earlier studies it was shown that TR
itself, as well as some of its derivatives and/or analogues,
demonstrates cytotoxic action against various lines of human and
animal tumour cells in vitro (1,24-26). In continuation of our search for
more active and available cytotoxic compounds of this structural
family (9), we evaluated the
cytotoxic effect of the synthesised compound MT against the cell
lines given in Table II. A wide
range of tumour cell lines of various tissue origins was chosen for
the MTT test. This allowed the rating of the cytotoxic activity of
the new derivative MT in comparison with its paternal alkaloid TR
but also the selection of cell lines sensitive to the new cytotoxic
agent.
 | Table IIAntiproliferative activities of MT
and TR in comparison with the antitumor drug doxorubicin. |
Table II
Antiproliferative activities of MT
and TR in comparison with the antitumor drug doxorubicin.
| Test compounds | IC50,
µM
|
|---|
| HCT-116 | MCF-7 | B16-F0 | K-562 | MDA-MB-231 |
|---|
| MT | 5.0±0.4b | 11.0±0.9a | >50 | 1.0±0.1b | 46.0±2.7 |
| TR | >50 | >50 | 48.3±3.9 | 42.4±3.2 | 21.2±1.7 |
| DOX | 0.11±0.02 | 0.61±0.05 | 0.55±0.05 | 0.10±0.01 | 0.52±0.05 |
DOX, well-established against various types of
cancer, was used as a positive control. It was demonstrated that MT
showed higher activity, when compared with TR, against HCT-116,
K-562 and MCF-7 tumour cell lines. By contrast, TR did not show
activity against these cell lines at the investigated range of
concentrations (50 µM and below) but was more active against
the MDA-MB-231 cell line. MT and TR did not show activity in
relation to B16-F0.
In this way, compound MT was 5- to 40-fold more
active against human tumour cells in comparison with TR, with the
exception of MDA-MB-231 cells. Interestingly, compound MT exhibited
the highest cytotoxic activity to the chronic myelogenous leukaemia
cell line (IC50, µM=1.0±0.1) (Table II). The high sensitivity of K-562
cells to the cytotoxic effect of MT may be associated with the
biochemical proliferation features of these undifferentiated
progenitor cells (27) and the
presence of selective molecular targets for MT. Thus, the higher
antiproliferative activity of MT against the majority of tested
tumour cell lines and the much lower toxicity, in comparison with
TR, suggested a probable augmentation of MT antitumour potential
in vivo.
Studies on binding of MT and TR to
DNA
To connect the shown cytotoxic activity with the
physicochemical properties of MT, its chemical structure was taken
into consideration. The planar structure of MT suggests this
compound was able to interact with DNA and/or intercalate between
DNA nucleotides in double-stranded DNA, and this may be connected
with the mechanism of its biological action as has been shown for
some other clinically established drugs (28). In order to determine whether the
compounds MT and/or TR interact with ctDNA, a titration of the
studied compounds with different concentrations of DNA was
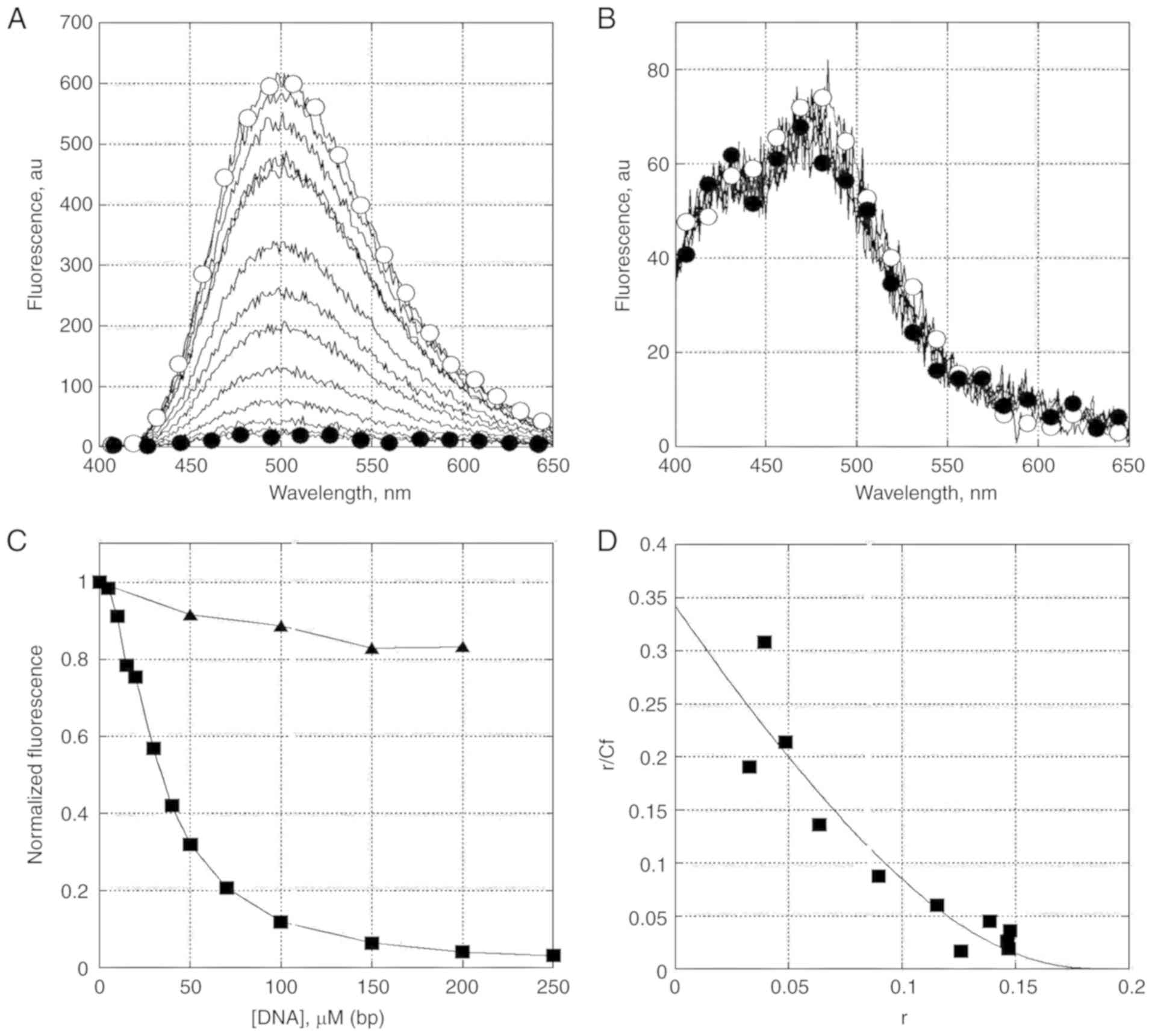
performed. In this experiment, it was shown that both MT and TR
have intrinsic fluorescence, with a maximum near 500 nm. Using
changes in fluorescence as a convenient method to indicate the
formation of DNA complexes with the studied compounds, we initially
established that the fluorescence of MT was markedly stronger when
compared with TR. Apparently, the increase in fluorescence of MT is
due to the replacement of a ketone oxygen in the precursor TR by
nitrogen, linked to the side chain of MT. Notably, TR in contrast
to MT, did not change in fluorescence in our experiments when DNA
was added to its solution. Fluorescence quenching of the studied
compound MT in this experiment showed its capability to be bound
with DNA (Fig. 3A-C).
Binding parameters can be calculated from the
dependence of fluorescence intensity on DNA concentration. The
binding isotherm was plotted in the Scatchard coordinates (Fig. 3D). The isotherm reflects an
anti-cooperative binding of MT to DNA. The approximation of the
McGhee-von Hippel equation allowed us to determine the binding
parameters. Approximately 5 base pairs on DNA were occupied by MT.
The binding constant for MT was approximately 3×105
M−1. At the same time, it should be noted that changes
in the fluorescence intensities and spectra were not observed in
experiments with TR. Therefore, the latter seems to have a much
weaker interaction with DNA under the given conditions.
Circular dichroism spectra changed upon
binding of MT
In order to clarify the conformation of the complex
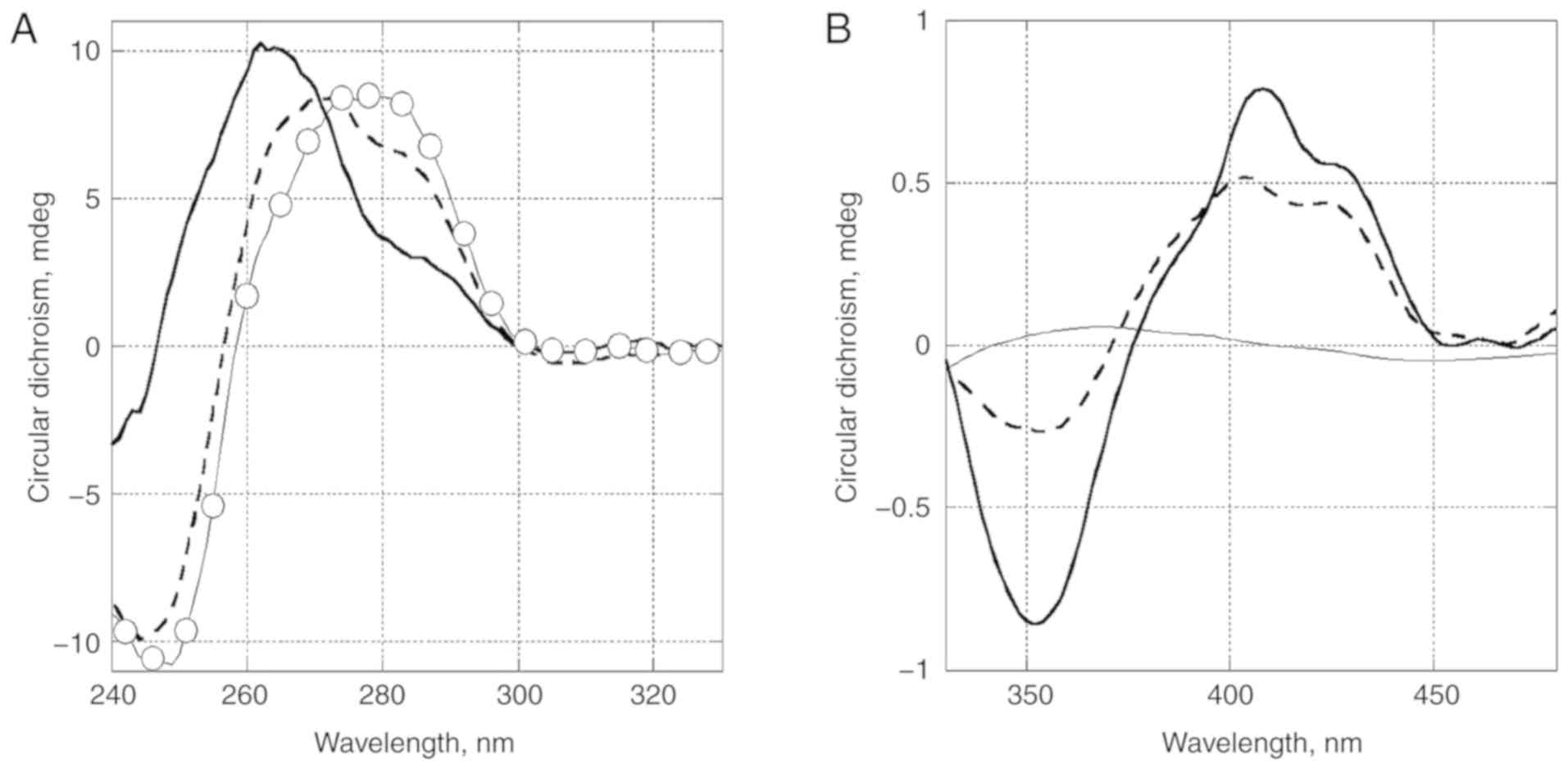
formed by MT and DNA, circular dichroism (CD) spectra of DNA
(Fig. 4A) and the studied
compound in complexes with DNA (Fig.
4B) were obtained. A change of CD spectrum in the region of DNA
absorbance (Fig. 4A) was
associated with conformational changes in DNA. It is of interest
that the spectrum became similar to that of DNA A-form (positive
band of DNA at 280 nm shifted to 260 nm).
These changes could also be caused by an induced CD
spectrum of the ligand. The long wavelength absorbance in the CD
spectrum reflected the ligand binding mode (Fig. 4B). The positive band at low DNA
occupancy (ratio of concentrations of DNA to MT is 10:1)
demonstrated a groove binding was more likely than intercalation. A
higher DNA occupancy led to the increased amplitude of a negative
band near 350 nm, which apparently came from a close interaction of
bonded nearby molecules. CD spectrum changes by TR at the same
conditions were not detected.
Thus, the binding of MT with DNA, in contrast with
the initial compound TR used for its synthesis, was confirmed in
our experiments. Further attention should be paid to this specific
binding in future studies on the molecular mechanisms of action of
MT.
Toxicity
Following these in vitro results, the
examination of general toxicity in vivo, of the novel
compound MT in comparison with TR, was carried out. The acute
toxicity of MT was evaluated at doses from 50 to 500 mg/kg/mice (a
single injection) via i.p. administration. A slight toxic effect of
MT at doses from 125 to 250 mg/kg was observed during the first
15-60 min after administration, with the following external signs
of intoxication: Decreased body temperature, shortness of breath,
decreased mobility and general physical activity. These occurred
without subsequent mortality. In these experimental groups,
mortality was not detected and after 24 h, the animals showed a
full recovery. However, at the dose of 500 mg/kg, all the animals
in the experimental group died within 30-60 min, which is the
reason for not using higher doses. The calculation established the
LD50 of MT to be 375 mg/kg, making it possible to
classify this compound as moderately toxic, according to the Hodge
and Sterner toxicity scale. The LD50 of TR, as
previously determined by us in the same animals, was determined to
be 75 mg/kg. Therefore, we concluded that MT is approximately
5-fold less toxic than TR (unpublished data).
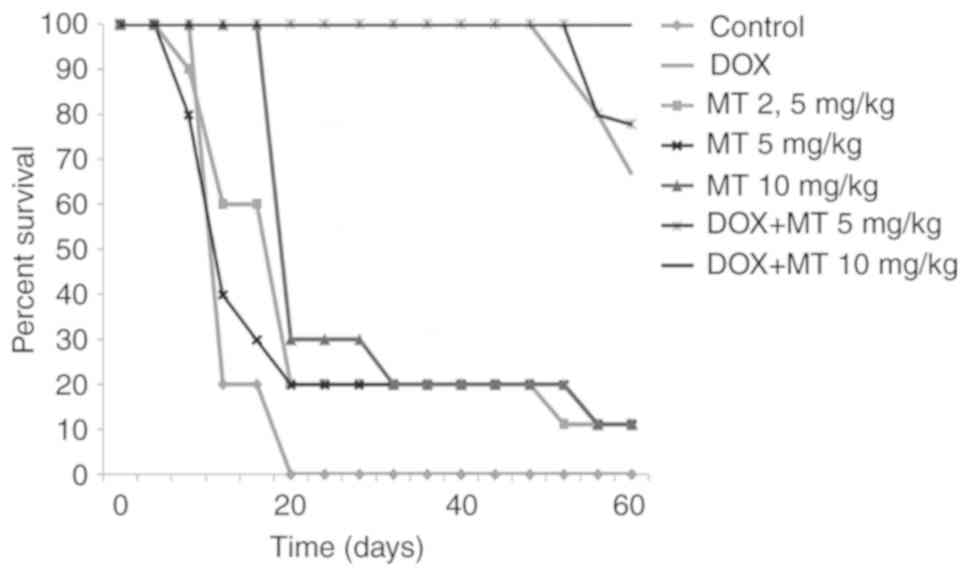
Antitumour activity in vivo using a
murine model of the ascitic form of Ehrlich adenocarcinoma
In our experiments, DOX was used as a positive
control and along with compound MT for combinational treatment. The
anti-tumour activity of the studied compound MT was dose-dependent
and its overall activity was less than the activity of DOX. At
doses of 50 and 25 mg/kg, this compound provided survival rates of
37.5 and 25%, respectively (data not shown). Active doses, which
provided both survival and life expectancy, ranged from 2.5 to 10
mg/kg, with maximum effects at 10 mg/kg. The obtained results are
given in the Table III and
Fig. 5. It should be noted that
joint application of MT+DOX, at doses of 0.25+5 mg/kg, resulted in
78% of survival on the 60th day, while increasing the dose of MT in
this combination to 10 mg/kg provided 100% survival of mice, in
contrast with the use of DOX alone. Thus, an enhancement of the
anti-tumour action of DOX by MT was clearly established (Table III).
 | Table IIIEvaluation of antitumor activity at
treatment with DOX, MT and combinations of DOX and MT. |
Table III
Evaluation of antitumor activity at
treatment with DOX, MT and combinations of DOX and MT.
| Group | Average life
expectancy, days | Longer life
expectancy, % | Survival, % |
|---|
| Control(-) | 17±0.17 | - | 0 |
| DOX (0.25
mg/kg) | 57.55±0.2a | 338±0.5 | 67 |
| MT (2.5 mg/kg) | 26±0.45 | 152.9±1.07 | 10 |
| MT (5 mg/kg) | 25.7±0.47 | 151.2±1.11 | 10 |
| MT (10 mg/kg) | 29.1±0.45 | 171.2±1.06 | 10 |
| DOX+MT (0.25 + 5
mg/kg) | 58.9±0.16a | 346.5±0.4 | 78 |
| DOX+MT (0.25 + 10
mg/kg) | 60±0a | 352.9±0b | 100 |
Moreover, the examination of surviving animals
treated with DOX revealed more significant signs of secondary
cancer development in comparison with animals treated with a
combination of MT and DOX. In fact, tumours were observed in 70% of
the surviving animals treated by DOX, but only in 10% of those
treated by the combination DOX+MT (Table SIII).
Anti-tumour activity in vivo using the
murine model of solid Ehrlich's adenocarcinoma
In this experiment, MT treatment hardly changed the
tumour size, whereas treatment with DOX alone led to some
inhibition of the tumour growth. In the DOX and MT co-treatment
group, chemotherapeutic efficacy was higher. On the 22nd day of the
experiment, tumour size was markedly decreased and tumour growth
inhibition (TGI) was slightly >20% (Table SIV, Fig. S5). Consequently, solid Ehrlich's
adenocarcinoma is less sensitive to the action of MT and DOX in
comparison with the ascitic form.
Anti-inflammatory action
It is well known that TR and its derivatives are
potent anti-inflammatory and immunosuppressive agents (1,2).
Thus, we investigated the anti-inflammatory action of the compound
MT in comparison with that of TR, using the murine model of
systemic inflammation induced by the lipopolysaccharide (LPS) from
E. coli. LPS is the exogenous ligand of Toll-like receptors
and is responsible for the activation of key inflammatory pathways.
It increases the expression of the genes responsible for the
synthesis of, mainly, cytokines, which are involved in the
development of the inflammatory and autoimmune processes (29). The results of the analysis of
cytokine content in treated and untreated mice are given in
Fig. 6.
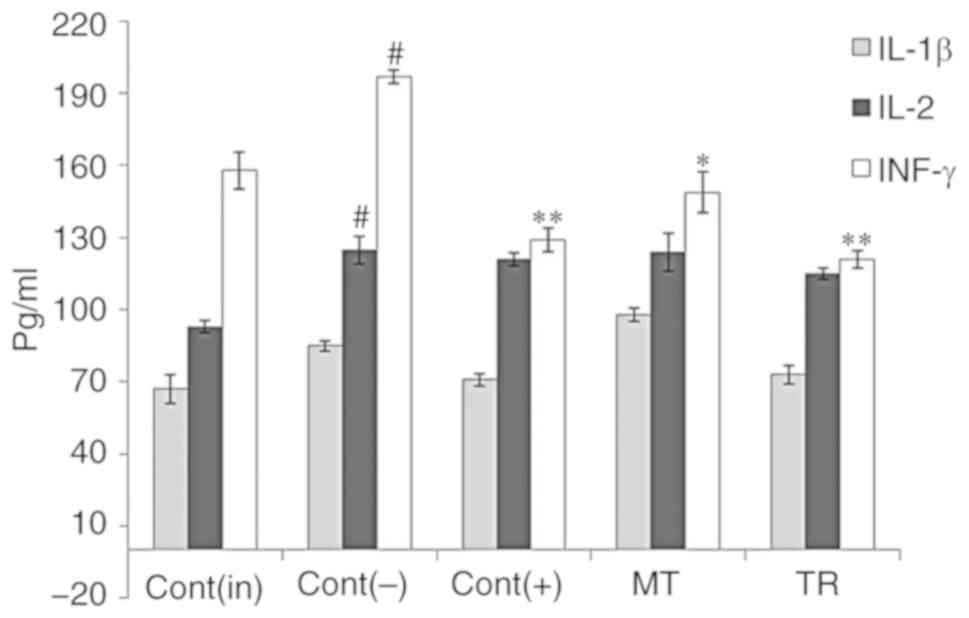 | Figure 6Effect of TR and MT on the levels of
the cytokines IL-1β, 2 and IFN-γ in blood serum. Cont(in), intact
control; Cont(-), negative control; Cont(+), positive control
(treatment with anti-inflammatory drug dexamethasone); MT, group
treated with mostotrin; TR, group of animals treated with
tryptanthrin. Columns show the concentrations of pro-inflammatory
cytokines, in pg/ml. Results are presented as mean ± SEM, n=7 for
each group. P-values are labelled as *P<0.05,
**P<0.01 vs. negative control group, and #P<0.05
vs. intact group (Tukey's test). |
It was found that TR, unlike MT, decreased the
levels of cytokines in the serum of experimental animals measured
1.5 h after their administration, as for treatment with
Dexamethasone (Fig. 6). Under the
same conditions, MT hardly influenced the levels of IL-1β and 2,
and showed a lower inhibition of IFN-γ levels than TR. Thus, MT is
a weaker immunosuppressive agent in comparison with TR.
Discussion
In the present study, we designed and performed the
synthesis of so-called MT, a new water-soluble TR derivative. To
the best our knowledge, MT is the first compound of the TR series
with such properties. Its X-ray analysis has shown that the
obtained compound MT contains an additional pseudo-cycle formed by
an intramolecular hydrogen bond, in contrast with TR. The
evaluation of its physiological action demonstrates that it has
5-fold lower toxicity than TR. Moreover, this modification led to
significant changes of antimicrobial, cytotoxic, antitumor and
immunosuppressive activities of MT in comparison with TR.
Parent TR showed a broad spectrum of antimicrobial
and antifungal activities (1).
The efficiency of TR against the pathogenic fungus Trichophyton
mentagrophytes was comparable to that of the antibiotic
griseofulvin (30). An oxime of
TR was reported to be highly active against the bacterium E.
coli (31). Compound TR also
inhibits the growth of Helicobacter pylori, the main
microbial factor responsible for the development of gastric ulcers
(32). Some derivatives of TR are
much more active than TR against pathogenic bacteria and fungi
(33). The greatest attention to
compounds belonging to this class has been caused by their
anti-tuberculosis properties (34), as well as their efficacy against
the flagellated protozoa Trypanosoma brucei, which is
transmitted by tsetse flies and cause a sleeping sickness in humans
and a related disease in cattle (35). In contrast to TR, antibacterial
activity of MT in relation to the majority of chosen bacteria and
the fungus was lower, but MT had high antibacterial activity
against Mycobacterium spp. According to Kamal et al
(36), an in silico
molecular docking study demonstrated that TR and its derivatives
can inhibit the activity of the enoyl-ACP reductase (InhA), an
enzyme essential for the survival of Mycobacterium spp. InhA
is one of the key enzymes involved in the synthesis of mycolic
acid, which is an important component of Mycobacterium spp.
cell walls (37). Thus, InhA may
be suggested as a potential antimicrobial target of compound
MT.
Previous findings have shown that TR itself, as
well as some of its derivatives and/or analogues, demonstrate
cyto-toxic action against various lines of human and animal tumour
cells in vitro (1,24-26). In the evaluation of cytotoxic
activity of MT and TR in the present study it was demonstrated that
MT showed higher anti-proliferative activity, when compared with
TR, against HCT-116, K-562 and MCF-7 tumour cell lines. Thus, MT
exhibits antimicrobial activity and shows cytotoxic effects against
different human tumour cell lines in vitro. Of those strains
of microorganisms and tumour cell cultures tested, mycobacteria and
leukaemia cells are most sensitive to the action of MT. The DNA
binding capability of MT (unlike TR) may be connected with a
possible mechanism of its cytotoxic action.
MT possesses in vivo anti-tumour action on
Ehrlich's carcinoma models either alone or, notably, in combination
with DOX. In the ascitic form of Ehrlich adenocarcinoma it was
shown that treatment with a combination of MT and DOX was more
effective in comparison with monotherapy of MT and DOX. Thus, solid
Ehrlich's adenocarcinoma is less sensitive to the action of MT and
DOX in comparison with the ascitic form. It is well known that the
ascitic form of Ehrlich's adenocarcinoma responds significantly
better to i.p. treatment with anti-tumour drugs than the solid form
(38). To understand the
mechanism of action of MT against various tumour types in future
studies the collection of histopathological samples would be
valuable. We consider that the results concerning joint application
of MT with DOX (in vivo experiments) are of biomedical
significance. The obtained data were also compared with previous
results on the examination of anti-tumour activity of the parent
compound TR and remedies based on it, such as the so-called
'Courochitin' (11,26). Of note, the latter also inhibited
tumour growth in mice with ascitic Ehrlich carcinoma at oral
administration, particularly in combination with the antitumour
drug 'Cyclophosphan' (11), but
its effect was less significant. It also was noted that
modification of the structure of TR plays an important role in the
development of new anti-tumour agents, with high efficacy against
DOX- and etoposide-resistant tumour cells (26).
It should be noted that MT is a weaker
immunosup-pressive agent in comparison with TR. It is well known
that anti-inflammatory and immunosuppressive activities of TR and
its derivatives are believed to be the result of their multiple
action on Toll-like receptors and signalling pathways involved in
the development of inflammatory reactions, for example, nuclear
factor-κB (NF-κB) and signal transducer and activator of
transcription (STAT), as well as its activity on the expression
level of enzymes such as cyclooxygenase-2 (COX-2) and
5-lipooxygenase (5-LOX) (39-41). Consequently, in the murine model
of systemic inflammation induced by LPS TR, TR inhibited the
release of IL-1β, IL-2 and INF-γ. In particular, a pronounced
decrease in the level of INF-γ was detected. INF-γ is known as an
important pro-inflammatory cytokine, which activates the NF-κB and
JAK-STAT path-ways, and also COX-2 and 5-LOX activity (42,43). By contrast, MT exerts a lower
immunosuppressive effect via inhibition of proinflammatory cytokine
levels in plasma compared to TR. This may be an important positive
factor for the treatment of cancer and/or tuberculosis with MT, but
these findings require further confirmation. Thus, MT may be
considered a promising anti-tumour and antimicrobial hit
compound.
Supplementary Data
Funding
G.B. Elyakov Pacific Institute of Bioorganic
Chemistry funding from the Russian Ministry of Science and Higher
Education.
Availability of data and materials
The analysed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AP, AK, AS and VS conceived and designed the
experiments and wrote the manuscript; AK, OS, TM, NG, LD, DK, PD,
AG and AU performed the experiments and analysed the data. All the
authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
The animal study was performed in accordance with
the European Convention for the Protection of Vertebrate Animals,
Directives 86/609/EEC [Council of Europe European Convention for
the Protection of Vertebrate Animals used for Experimental and
Other Scientific Purposes. Strasbourg: 1986, Accessed August 28,
2018] 18.III.1986. Council of Europe, ETS No. 123, European
Convention for the humane methods for the animal welfare and
maintenance [Directive 2010/63/EU on the protection of animals used
for scientific purposes EN. Official Journal of the European Union,
L 276/33-276/79 (20.10.2010)], the National Standard of the Russian
Federation R 53434-2009 'Good Laboratory Practice' (National state
standard GOST P 53434-2009 the Russian Federation standard 'The
principles of Good Laboratory Practice' approved and put into
effect by the Order of the Federal Agency for Technical Regulation
and Metrology of December 2, 2009, No. 544) and approved by Ethics
Committee of Animal Experimentation of G.B Elyakov Pacific
Institute of Bioorganic Chemistry of the Russian Academy of
Science.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Jahng Y: Progress in the studies on
tryptanthrin, an alkaloid of history. Arch Pharm Res. 36:517–535.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagner-Döbler I, Rheims H, Felske A,
El-Ghezal A, Flade-Schröder D, Laatsch H, Lang S, Pukall R and
Tindall BJ: Oceanibulbus indolifex gen. nov., sp. nov., a North Sea
alphap-roteobacterium that produces bioactive metabolites. Int J
System Evol Microbial. 54:1177–1184. 2004. View Article : Google Scholar
|
|
3
|
Vlachos C, Schulte BM, Magiatis P, Adema
GJ and Gaitanis G: Malassezia-derived indoles activate the aryl
hydrocarbon receptor and inhibit Toll-like receptor-induced
maturation in mono-cyte-derived dendritic cells. Br J Dermatol.
167:496–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng HM, Wu YC, Wang Q, Song M, Wu J,
Chen D, Li K, Wadman E, Kao ST, Li TC, et al: Clinical efficacy and
IL-17 targeting mechanism of Indigo naturalis as a topical agent in
moderate psoriasis. BMC Complement Altern Med. 17:4392017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaur R, Manjal SK, Rawal RK and Kumar K:
Recent synthetic and medicinal perspectives of tryptanthrin. Bioorg
Med Chem. 25:4533–4552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitcher LA and Baker W: Tuberculosis: A
search for novel therapy starting with natural products. Med Res
Revs. 18:363–374. 1998. View Article : Google Scholar
|
|
7
|
Krivogorsky B, Grundt P, Yolken R and
Jones-Brando L: Inhibition of Toxoplasma gondii by indirubin and
tryptanthrin analogs. Antimicrob Agents Chemother. 52:4466–4469.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitscher LA, Wong WC, De Meulenaere T,
Sulko J and Drake S: Antimicrobial agents from plants. New
synthesis and bioactivity of tryptanthrin
(indolo[2,1-b]quinazoline-6,12-dione) and its derivatives.
Heterocycles. 15:1017–1021. 1981. View Article : Google Scholar
|
|
9
|
Popov AM, Gafurov YM, Moskovkina TV,
Kachanov AV, Krivoshapko ON, Petrovicheva SE and Stonik VA:
Kourokhitin, a potential drug containing two active substances.
Dokl Biochem Biophys. 426:131–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Popov AM, Nedashkovskaya OI, Gafurov YM
and Moskovkina TV: Antimicrobial activity of «Kourochitin»
preparation. Russ J Biopharm. 3:19–22. 2011.
|
|
11
|
Popov AM, Shtoda YP, Krivoshapko ON,
Gafurov YM and Moskovkina TV: Wound healing activity of different
ointment forms of quinazoline alkaloid tryptanthrin. Russ J
Biopharm. 4:21–24. 2012.
|
|
12
|
Popov AM, Krivoshapko ON, Tsybulsky AV,
Shtoda YP, Klimovich AA, Gafurov YM and Artyukov AA: Therapeutic
activity of preparation «Kourochitin» at modeling allergic
dermatitis. Russ J Biopharm. 7:24–30. 2015.
|
|
13
|
Popov AM, Osipov AN, Korepanova EA,
Krivoshapko ON, Shtoda YP and Klimovich AA: Study of antioxidant
and membranotropic activities of quinazoline alkaloid tryptanthrin
using different model systems. Biofizika. 60:700–707. 2015.In
Russian. PubMed/NCBI
|
|
14
|
Klimovich AA, Popov AM, Krivoshapko ON,
Shtoda YP and Tsybulsky AV: A comparative assessment of the effects
of alkaloid tryptanthrin, rosmarinic acid, and doxorubicin on the
redox status of tumour and immune cells. Biophysics. 62:588–594.
2017. View Article : Google Scholar
|
|
15
|
Moskovkina TV, Denisenko VA, Kalinovskii
AI and Stonik VA: Synthesis of substituted tryptanthrins via
oxidation of isatin and its derivatives. Russ J Org Chem.
49:1740–1743. 2013. View Article : Google Scholar
|
|
16
|
Moskovkina TV, Kalinovskii AI and
Makhan'kov VV: Synthesis of tryptanthrin (couroupitine) derivatives
by reaction of substituted isatins with phosphoryl chloride. Rus J
Org Chem. 48:123–126. 2012. View Article : Google Scholar
|
|
17
|
Bruker: APEX2. Bruker AXS Inc; Madison,
WI: 2012
|
|
18
|
Sheldrick GM: SHELXT-integrated
space-group and crystal-structure determination. Acta Crystallogr A
Found Adv. 71:3–8. 2015. View Article : Google Scholar :
|
|
19
|
Tikhomirov AS, Tsvetkov VB, Kaluzhny DN,
Volodina YL, Zatonsky GV, Schols D and Shchekotikhin AE: Tri-armed
ligands of G-quadruplex on heteroarenefused anthraquinone
scaffolds: Design, synthesis and pre-screening of biological
properties. Eur J Med Chem. 159:59–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGhee JD and von Hippel PH: Theoretical
aspects of DNA-protein interactions: Co-operative and
non-co-operative binding of large ligands to a one-dimensional
homogeneous lattice. J Mol Biol. 86:469–489. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akhila JS, Deepa S and Alwar MC: Acute
toxicity studies and median lethal dose. Curr Sci. 93:917–920.
2007.
|
|
22
|
Elsherbinya NM, Younisb NN, Shaheenc MA
and Elseweidy MM: The synergistic effect between vanillin and
doxorubicin in Ehrlich ascites carcinoma solid tumour and MCF-7
human breast cancer cell line. Pathol Res Pract. 212:767–777. 2016.
View Article : Google Scholar
|
|
23
|
Fedeli W and Mazza F: Crystal structure of
tryptanthrin (indolo[2,1-b]quinazoline-6,12-dione. J Chem Soc
Perkin Trans. 2:1621–1623. 1974. View Article : Google Scholar
|
|
24
|
Kimoto T, Hino K, Koya-Miyata S, Yamamoto
Y, Takeuchi M, Nishizaki Y, Micallef MJ, Ushio S, Iwaki K, Ikeda M
and Kurimoto M: Cell differentiation and apoptosis of monocytic and
promyelocytic leukemia cells (U-937 and HL-60) by tryptanthrin, an
active ingredient of Polygonum tinctorium Lour. Pathol Int.
51:315–325. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu ST, Chern JW, Chen TM, Chiu YF, Chen HT
and Chen YH: Cytotoxicity and reversal of multidrug resistance by
trypt-anthrin-derived indoloquinazolines. Acta Pharmacol Sin.
31:259–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jao CW, Lin WC, Wu YT and Wu PL:
Isolation, structure eluci-dation, and synthesis of cytotoxic
tryptanthrin analogues from Phaius mishmensis. J Nat Prod.
71:1275–1279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duncan M, DeLuca T, Kuo HY, Yi M, Mrksich
M and Miller WM: SIRT1 is a critical regulator of K562 cell growth,
survival, and differentiation. Exp Cell Res. 344:40–52. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deo KM, Pages BJ, Ang DL, Gordon CP and
Aldrich-Wright JR: Transition metal intercalators as anticancer
agents-recent advances. Int J Mol Sci. 17:18182016. View Article : Google Scholar
|
|
29
|
Ito W, Takeda M, Ueki S, Tanigai T, Kayaba
H and Chihara J: Effect of the hepatocyte growth factor on allergic
inflammatory cells. Int Arch Allergy Immunol. 152(Suppl 1):
S96–S100. 2010. View Article : Google Scholar
|
|
30
|
Honda G, Tabata M and Tsuda M: The
antimicrobial specificity of tryptanthrin. Planta Med. 37:172–174.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bandekar PP, Roopnarine KA, Parekh VJ,
Mitchell TR, Novak MJ and Sinden RR: Antimicrobial activity of
tryptanthrins in Escherichia coli. J Med Chem. 53:3558–3565. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kataoka M, Hirata K, Kunikata T, Ushio S,
Iwaki K, Ohashi K, Ikeda M and Kurimoto M: Antibacterial action of
tryptanthrin and kaempferol, isolated from the indigo plant
(Polygonum tinctorium Lour.), against Helicobacter pylori-infected
Mongolian gerbils. J Gastroenterol. 36:5–9. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawakami J, Matsushima N, Ogawa Y,
Kakinami H, Nakane A, Kitahara H, Nagaki M and Ito S: Antibacterial
and antifungal activities of tryptanthrin derivatives. Trans Mat
Res Soc Japan. 36:603–606. 2011. View Article : Google Scholar
|
|
34
|
Hwang JM, Oh T, Kaneko T, Upton AM,
Franzblau SG, Ma Z, Cho SN and Kim P: Design, synthesis, and
structure-activity relationship studies of tryptanthrins as
antitubercular agents. J Nat Prod. 76:354–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scovill J, Blank E, Konnick M, Nenortas E
and Shapiro T: Antitrypanosomal activities of Tryptanthrins.
Antimicrob Agents Chemother. 46:882–883. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kamal A, Reddy BV, Sridevi B, Ravikumar A,
Venkateswarlu A, Sravanthi G, Sridevi JP, Yogeeswari P and Sriram
D: Synthesis and biological evaluation of phaitanthrin congeners as
anti-myco-bacterial agents. Bioorg Med Chem Lett. 25:3867–3872.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chollet A, Maveyraud L, Lherbet C and
Bernardes-Génisson V: An overview on crystal structures of InhA
protein: Apo-form, in complex with its natural ligands and
inhibitors. Eur J Med Chem. 146:318–343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sugiura K: Relative sensitivity of the
solid and ascites forms of sarcoma 180 and Ehrlich carcinoma to
inhibitory compounds. Ann N Y Acad Sci. 76:575–585. 1958.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishihara T, Kohno K, Ushio S, Iwaki K,
Ikeda M and Kurimoto M: Tryptanthrin inhibits nitric oxide and
prostaglandin E(2) synthesis by murine macrophages. EurJ Pharmacol.
407:197–204. 2000. View Article : Google Scholar
|
|
40
|
Pergola C, Jazzar B, Rossi A, Northoff H,
Hamburger M, Sautebin L and Werz O: On the inhibition of
5-lipoxygenase product formation by tryptanthrin: Mechanistic
studies and efficacy in vivo. Br J Pharmacol. 165:765–776. 2012.
View Article : Google Scholar :
|
|
41
|
Han NR, Kim HM and Jeong HJ: Thymic
stromal lymphopoietin is regulated by the intracellular calcium.
Cytokine. 59:215–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hernandez-Santana YE, Giannoudaki E, Leon
G, Lucitt MB and Walsh PT: Current perspectives on the
interleukin-1 family as targets for inflammatory disease. Eur J
Immunol. 49:1306–1320. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pathania AS, Kumar S, Guru SK, Bhushan S,
Sharma PR, Aithagani SK, Singh PP, Vishwakarma RA, Kumar A and
Malik F: The synthetic tryptanthrin analogue suppresses STAT3
signaling and induces caspase dependent apoptosis via ERK Up
regulation in human leukemia HL-60 cells. PLoS One. 9:e1104112014.
View Article : Google Scholar : PubMed/NCBI
|