Introduction
Oxidative stress refers to the increase in free
radicals or the weakening of the body's antioxidant protective
ability following stimulation with harmful factors, leading to an
imbalance in the oxidation and antioxidant systems (1). The excessive accumulation of
reactive oxygen species (ROS) damages biological molecules, such as
nucleic acids, proteins and lipids, resulting in the occurrence and
development of cardiovascular diseases, Alzheimer's disease and
other chronic degenerative diseases. Antioxidants can alleviate or
inhibit cellular damage by neutralizing free radicals (2,3).
Autophagy is a process through which the body
removes aged, damaged or defective proteins and organelles. During
autophagy, the degradable contents of the cytoplasm are
encapsulated in subcellular bilayer vesicles and then transported
to lysosomes for degradation (4).
Recent studies have suggested that autophagy exerts protective
effects against neurodegeneration, in which autophagic deficiency
was associated with a decline in learning and memory (5). The importance of autophagy in the
reduction of oxidative stress has also been recognized, and several
autophagy inducers have been tested for their therapeutic potential
(6). Previous studies have
indicated that the autophagic flux is inhibited under conditions of
oxidative stress in degenerative diseases (6,7);
thus, it was hypothesized that the induction of autophagy may be a
promising antioxidant approach.
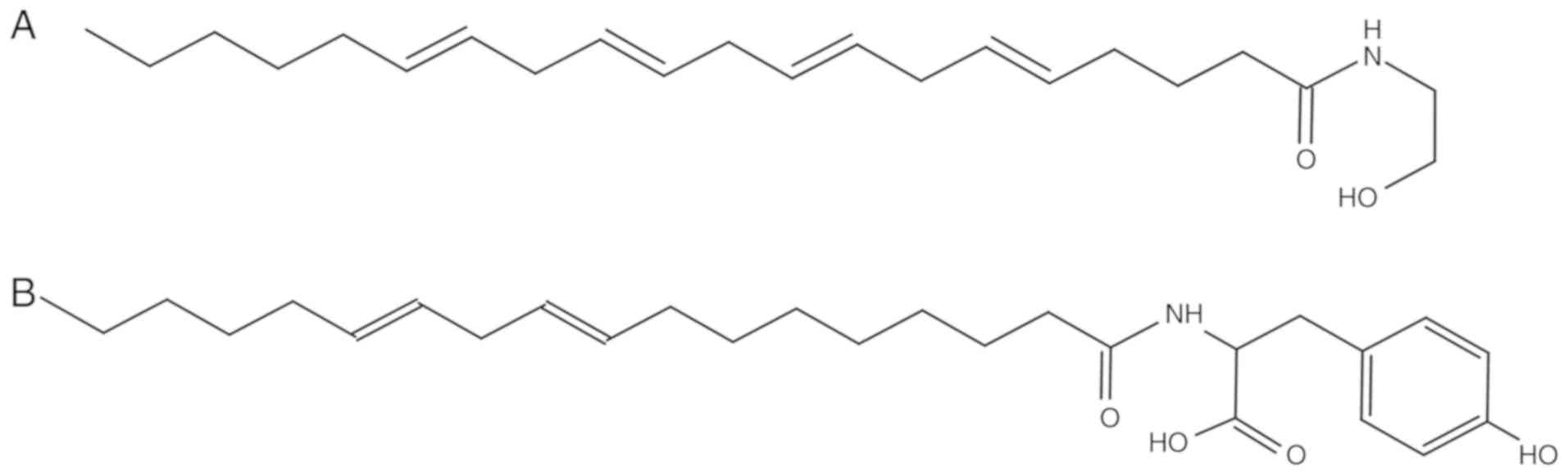
It has been demonstrated that as the ligand for the
cannabinoid type 1 (CB1) and 2 (CB2) receptors, anandamide (AEA)
exerts neuroprotective effects by inhibiting oxidative stress and
free radical formation (8).
Additionally, cannabinoids and activating cannabinoid receptors
induce autophagy in cardiovascular disease (9-11).
However, the rapid metabolic inactivation of AEA in vivo has
limited its further application (12); thus, the development of novel AEA
analogs is of considerable importance. Based on the chemical
structure of AEA (Fig. 1), its
analogue, N-linoleyl tyrosine (NITyr), was previously synthesized
in our laboratory (13). To the
best of our knowledge, the present study aimed to demonstrate for
the first time, the effects and potential mechanisms of NITyr on
autophagy in rat pheochromocytoma (PC12) cells under conditions of
oxidative stress.
Materials and methods
Materials
NITyr was previously independently synthesized
(13). The following additional
reagents were used in the present study: Polyclonal antibodies
against LC3, beclin-1, autophagy-related protein (ATG) 5 and the
CB1 receptor (1:1,000, cat. nos. 14600-1-AP, 11306-1-AP, 10181-2-AP
and 17978-1-AP, respectively; ProteinTech Group, Inc.); CB2
receptor rabbit polyclonal antibody (1:500; cat. no. 101550, Cayman
Chemical Company); ATG13 rabbit polyclonal antibody (1:1,000; cat.
no. 500690, Chengdu Zen Bioscience Co., Ltd.); GAPDH rabbit
polyclonal antibody (1:2,000; cat. no. 60004-1-AP, ProteinTech
Group, Inc.); horseradish peroxidase (HRP)-conjugated goat
anti-mouse/anti-rabbit IgG(H+L) (1:10,000; cat. no. AB0102, cat.
no. AB0101, Abways Technology, Inc.); Alexa Fluor 594-conjugated
goat anti-rabbit IgG (H+L) (1:500; cat. no. AB0141, Abways
Technology, Inc.); CB1 receptor antagonist AM251 (cat. no. S2819,
Selleck Chemicals); CB2 receptor antagonist AM630 (cat. no.
SML0327, Sigma-Aldrich, Merck KGaA); 3-meth-yladenine (3MA; cat.
no. 19389, CSNpharm, Inc.); hydrogen peroxide
(H2O2; cat. no. 20180610, Chengdu Jinshan
Chemical Reagent Co., Ltd.); and Dulbecco's modified Eagle's medium
(DMEM; cat. no. SH30809, HyClone; Cytiva).
Cell culture and experimental
groupings
PC12 cells were cultured in DMEM supplemented with
10% fetal calf serum, 5,000 U/ml penicillin and 5 mg/ml
streptomycin. The cells were cultured at 37°C in an incubator
supplemented with 5% CO2, and sub-cultured every 2 days.
Subsequently, the cells were seeded and pre-incubated with
combinations of NITyr (0.5, 1 or 5 µmol/l), 3MA (3 mmol/l), AM251
(3 µmol/l) and AM630 (3 µmol/l) for 24 h. This was followed by the
addition of 250 µmol/l H2O2 and the cells
were incubated for a further 24 h. Following the drug treatments,
the cells were stained with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazodium bromide (MTT),
4′,6-diamidino-2-phenylindole (DAPI) or 2′,7-dichlorofluorescin
diacetate (DCFH-DA), and western blot analysis and
immunofluorescence evaluation were performed. The experimental
groups were as follows: i) Control; ii) 50, 100, 250 or 500 µmol/l
H2O2; iii) H2O2+ 0.5, 1
or 5 µmol/l NITyr, respectively; iv) H2O2 + 1
µmol/l NITyr and 3 µmol/l AM251; v)
H2O2 + 1 µmol/l NITyr and 3
µmol/l AM630; vi) H2O2 + 3
µmol/l AM251; and vii) H2O2 + 3
µmol/l AM630; viii) control + 5 µmol/L NITyr. The
concentrations of AM251 and AM630 were determined according to the
published literature and previous experimental findings (14,15).
Cell viability assay
PC12 cells were seeded into 96-well plates at a
density of 5×104 cells/100 µl, and cultured in an
incubator at 37°C (5% CO2) for 24 h. The cells were then
treated with various concentrations of H2O2
(50, 100, 250 and 500 µmol/l) and NITyr (0.5, 1 and 5
µmol/l) for 12, 24 and 48 h, respectively. Subsequently, 10
µl MTT solution (5 mg/ml) were added to each well and the
plates were incubated for a further 4 h at 37°C. The formamide was
dissolved using dimethyl sulfoxide (100 µl/well), and the
absorbance of each sample was detected at 560 nm using a VICTOR
Nivo™ multimode plate reader (PerkinElmer, Inc.).
DAPI and DCFH-DA staining
Intracellular DNA damage and ROS generation were
assessed using DAPI (1:1,000; Beyotime Institute of Biotechnology)
and DCFH-DA probes (1:1,000; Dalian Meilun Biology Technology Co.,
Ltd.), respectively. Following drug treatment, PC12 cells (at a
density of 2.5×105cells/well) were incubated in
serum-free DMEM containing DAPI at room temperature for 10 min, or
DCFH-DA at 37°C for 20 min. The 6-well plate was then rinsed 3
times in PBS (5 min each) and immediately analyzed using a
fluorescence microscope (magnification ×20 and ×40; Olympus
Corporation). The relative fluorescence intensity of DCFH-DA was
analyzed using a fluorometer (Thermo Fisher Scientific, Inc.).
Immunofluorescence assay
PC12 cells were seeded onto coverslips at a density
of 25×104 cells/well and placed into 6-well plates.
Following drug treatment, the cells were treated according to a
previously described immunofluorescence protocol (16). The coverslips were rinsed 3 times
with PBS for 5 min each time, immobilized with 4% paraformaldehyde
for 15 min and permeabilized with 0.5% Triton X-100 for 20 min in
room temperature. The cells were rinsed again and blocked with 5%
bovine serum albumin (BSA) for 1 h at room temperature. Each
coverslip was then incubated with the corresponding primary
antibodies (LC3, beclin-1, ATG5, ATG13, CB1 and CB2) diluted in
Tris-buffered saline with Tween-20 (TBST) (1% BSA) overnight at
4°C, and subsequently washed with 1X TBST (3 times for 3 min each)
prior to incubation with an Alexa Fluor 594-conjugated secondary
antibody for 1 h at room temperature in the dark. Subsequently, 1
µg/ml DAPI was added to detect the cell nuclei, and the
cells were finally washed 5 times with 1X TBST for 8 min each time
and observed using a BX63 fluorescence microscope (magnification
×20 and ×40; Olympus Corporation).
Western blot analysis
PC12 cells were seeded at a density of
1×106cells/well and cultured in 6-well plates for 24 h.
Following drug treatment, the cells were treated according to the
following western blotting protocol: The cells were washed 3 times
with PBS for 5 min each time, and then incubated on ice for 30 min
in prepared lysis buffer (RIPA lysis buffer, protease inhibitor and
EDTA at a 100:1:1 ratio; all Beyotime Institute of Biotechnology,
Inc.). Following lysis, the cells were centrifuged at 12,000 × g
for 20 min at 4°C, and the supernatants were collected. The protein
concentration was then detected and adjusted according to the
instructions of the Easy II Protein Quantitative Kit (BCA, TransGen
Biotech Co., Ltd.), and the samples were then denatured at 100°C
for 6 min. To detect proteins with different molecular weights, 50
µg protein per lane were separated using 10% polyacrylamide
gels (Tris-HCl system), and then transferred onto polyvi-nylidene
difluoride membranes (Merck Millipore Ltd.). The membranes were
blocked with 5% BSA on a shaking platform at 37°C for 1 h, and then
incubated with the corresponding primary antibodies (LC3, beclin-1,
ATG5, ATG13, CB1 and CB2) overnight at 4°C; a GAPDH rabbit
polyclonal antibody was used to detect the internal control.
Subsequently, the membranes were washed with TBST 3 times for 5 min
each, and then incubated with secondary HRP-conjugated antibodies
at room temperature for 1 h. Finally, the membranes were washed
with TBST as aforementioned, and treated with chemiluminescent HRP
substrate (EMD Millipore) for protein band detection. Images were
captured using a ChemiDoc system (Bio-Rad Laboratories, Inc.), and
the gray values of the proteins were quantified using Image J
software 1.8.0 (NIH) with GAPDH as the comparative internal
control.
Statistical analyses
SPSS statistical software 17.0 (SPSS, Inc.) was used
for statistical experimental analysis and all data are expressed as
the means ± standard deviation. For comparisons between groups, the
data were evaluated by one-way ANOVA followed by Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
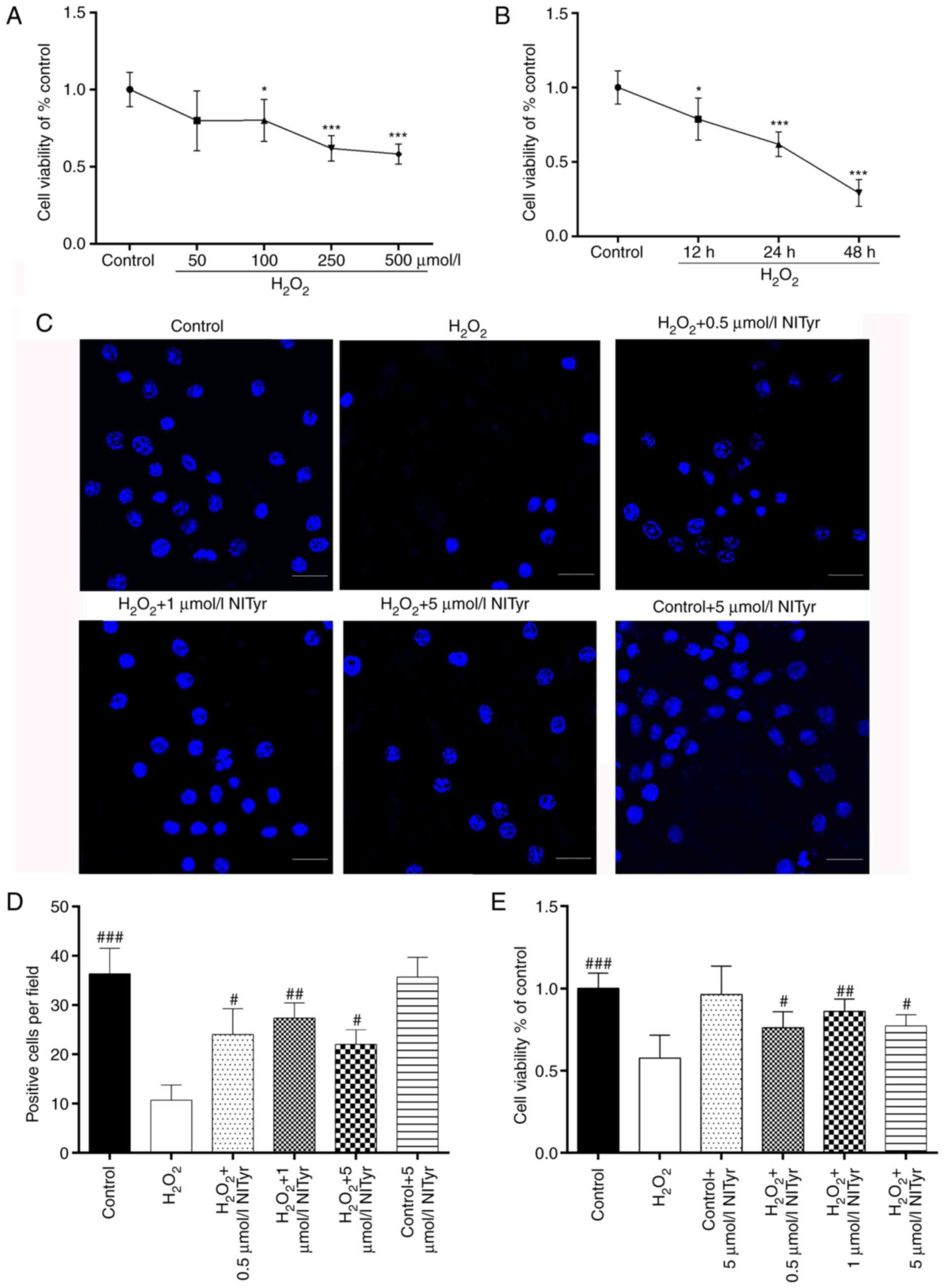
NITyr protects PC12 cells against
H2O2 insults
Following exposure to H2O2,
cell viability was assessed by MTT assay and was shown to be
significantly decreased in a time- and concentration-dependent
manner. The cell survival rates decreased gradually as a result of
exposure to 100, 250 and 500 µmol/l
H2O2, respectively (P<0.05 and P<0.001,
respectively; Fig. 2A).
Ultimately, the concentration of 250 µmol/l
H2O2 was selected to induce cellular damage,
as this induced a plateau in the cell viability results, and 500
µmol/l induced further cellular damage without an obvious
difference in viability. Following exposure to 250 µmol/l
H2O2, the cell survival rates decreased
gradually at the 12, 24 and 48 h time points, respectively
(Fig. 2B). As exposure to
H2O2 for 48 h resulted in considerable
cellular injury, the following experiments were conducted with 250
µmol/l H2O2 for 24 h. Additionally,
the numbers of the nuclei in the H2O2-treated
group were decreased compared with those in the control group
(Fig. 2C and D). However,
treatment with NITyr (0.5, 1 and 5 µmol/l) conferred a
benign effect against H2O2-induced injury,
with optimal recovery occurring at the concentration of 1
µmol/l NITyr (F=14.841, P<0.05 and P<0.01; Fig. 2E), and NITyr alone did not affect
cellular viability compared with the control group. In addition,
the results of DAPI staining indicated that detrimental changes in
cellular morphology were prevented by various concentrations of
NITyr (Fig. 2D), which was
consistent with the results of MTT.
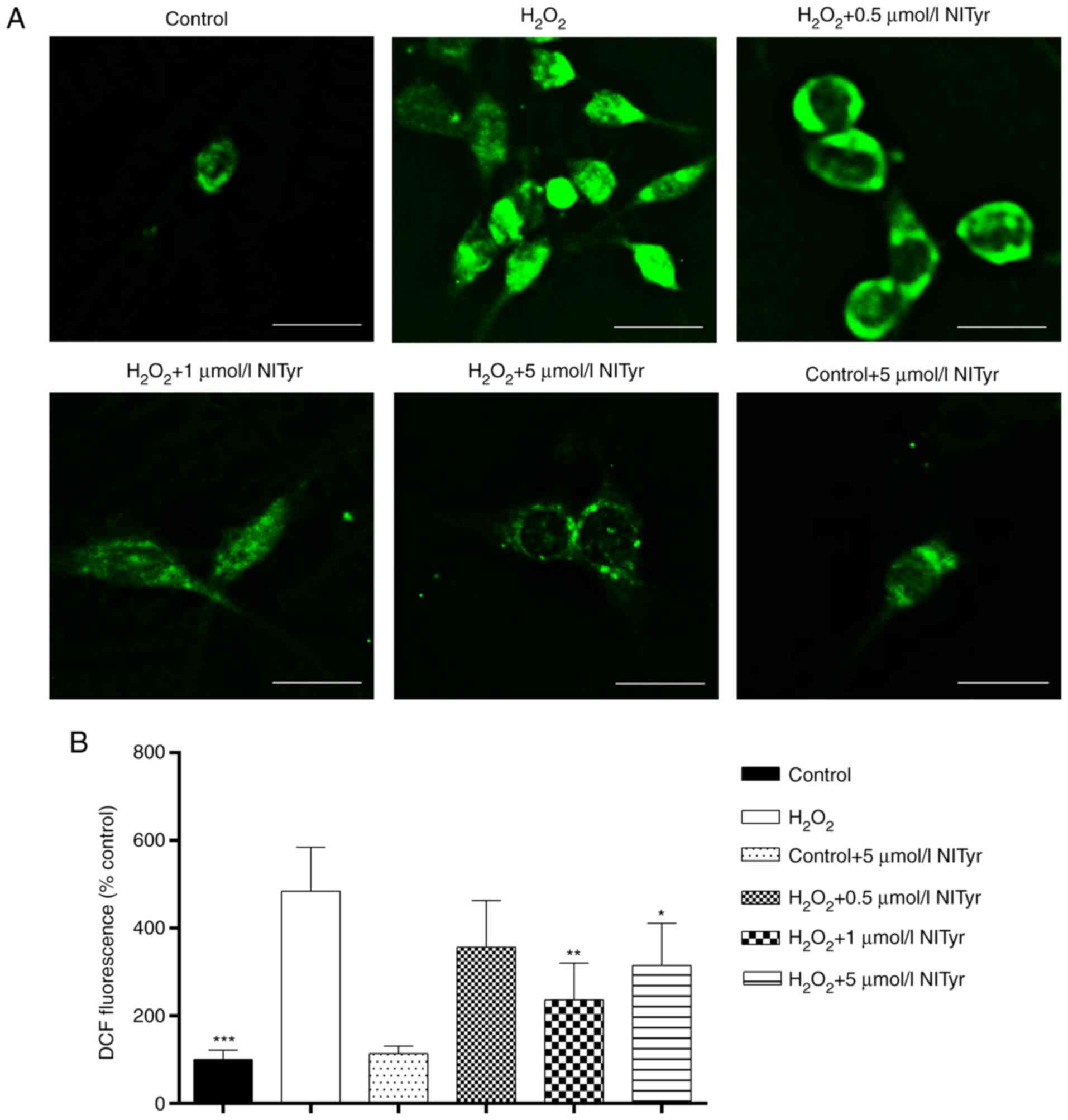
NITyr inhibits ROS-mediated
H2O2-induced cellular injury
Considering the importance of ROS in
H2O2-induced cytotoxicity, the levels of ROS
in PC12 cells were evaluated following pre-treatment with 0.5, 1 or
5 µmol/l NITyr. As shown in Fig. 3A, an increased number of green
dots (representing DCFH-DA fluorescence) was observed in the PC12
cells exposed to H2O2 compared with the
control cells. NITyr effectively decreased the number of green dots
induced by H2O2, indicating a reduction in
ROS production. Furthermore, the relative fluorescence intensity of
DCFH-DA was detected by fluorometry, and the elevation in
H2O2-induced fluorescence was inhibited in
the NITyr group (0.5, 1 and 5 µmol/l) with optimal rescue
occurring at 1 µmol/l (F=16.052, P<0.01 and P<0.05;
Fig. 3B); thus, the concentration
of 1 µmol/l NITyr was used in the following experiments to
investigate the underlying mechanisms of NITyr.
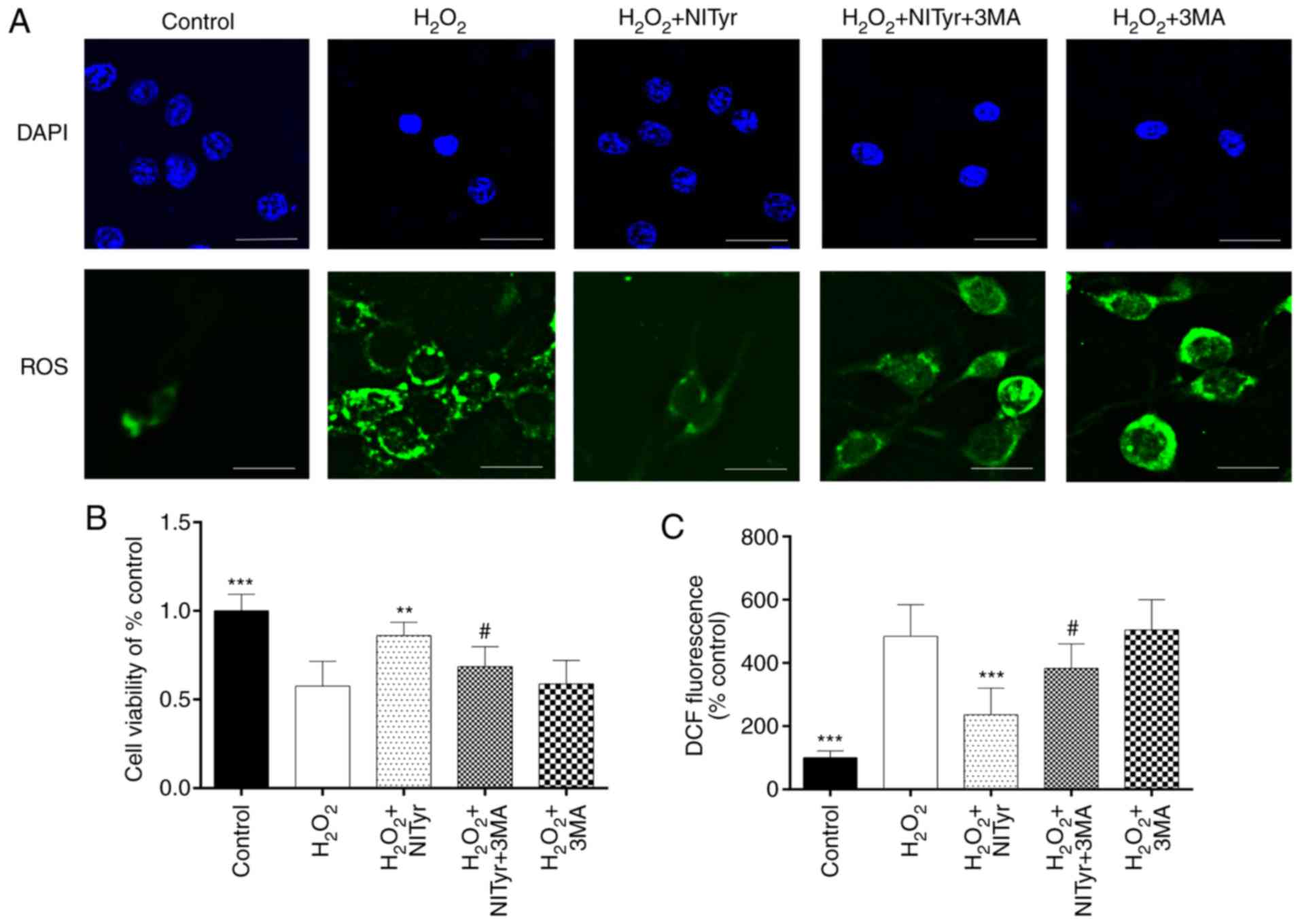
3MA attenuates the effects of NITyr on
cell viability and ROS levels
As shown in Fig.
4A, pre-treatment with 3MA following H2O2
exposure had no significant effect on the number of cell nuclei or
ROS levels compared with H2O2 exposure, and
the DCFH-DA fluorescence intensity of the 3MA group approached that
of the H2O2 group. 3MA combined with NITyr
exerted a weaker effect on cell viability and ROS levels than
treatment with 1 µmol/l NITyr following
H2O2 exposure. Moreover, the relative DCFH-DA
fluorescence intensity and cell viability were detected by
fluorometry and MTT assay, respectively. The results were
consistent with those of DCFH-DA and DAPI staining (F=15.704,
F=27.591, P<0.05; Fig. 4B and
C).
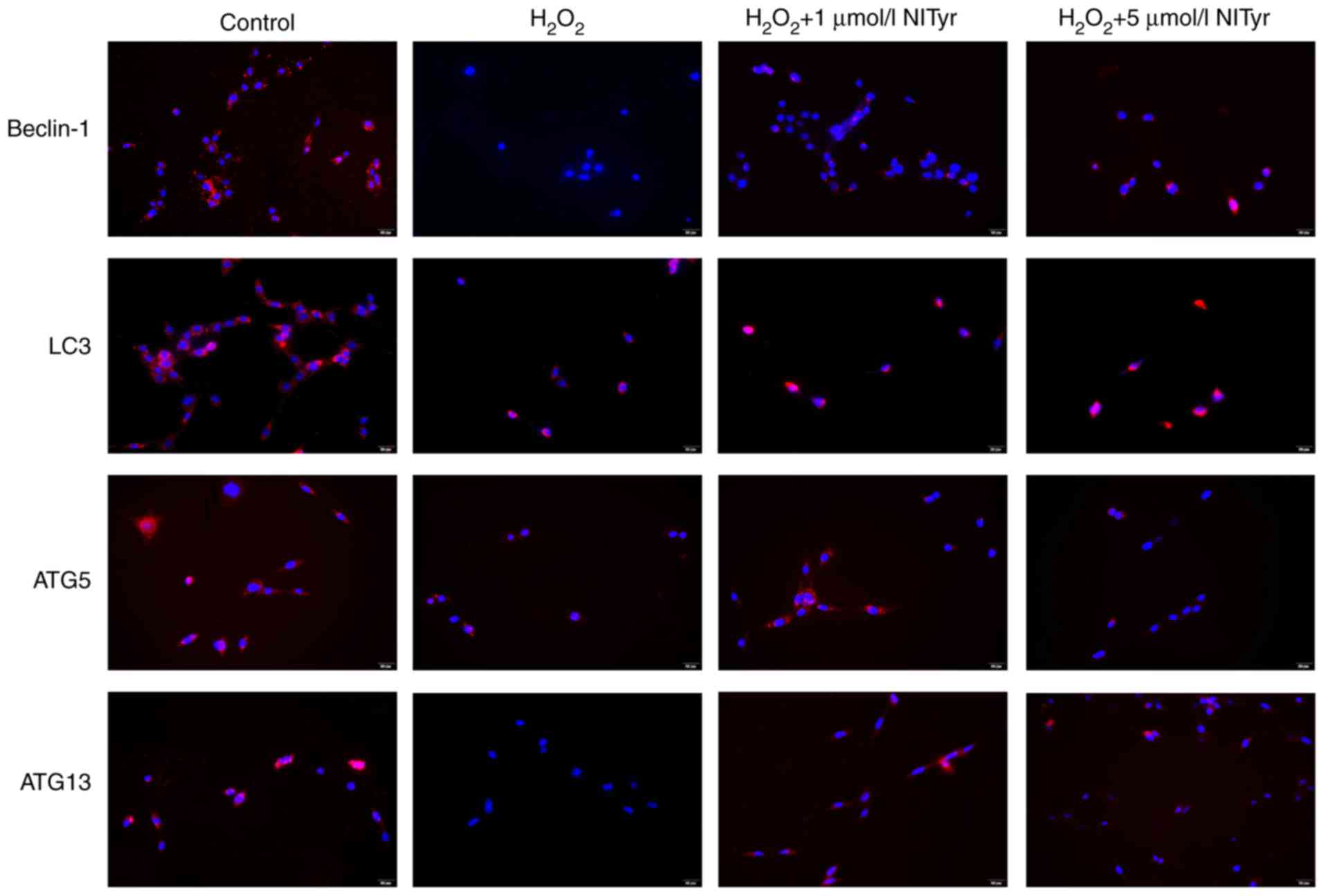
Effects of NITyr on the expression of
autophagy-related proteins
As shown in Fig.
5, compared with the control group, cell numbers in the
H2O2 group were notably decreased (as
indicated by the decreased level of blue fluorescence) and
shriveling of the nuclei was apparent. Reduced levels of red
fluorescence indicate decreased protein expression of LC3,
beclin-1, ATG5 and ATG13, which was reversed by treatment with
NITyr.
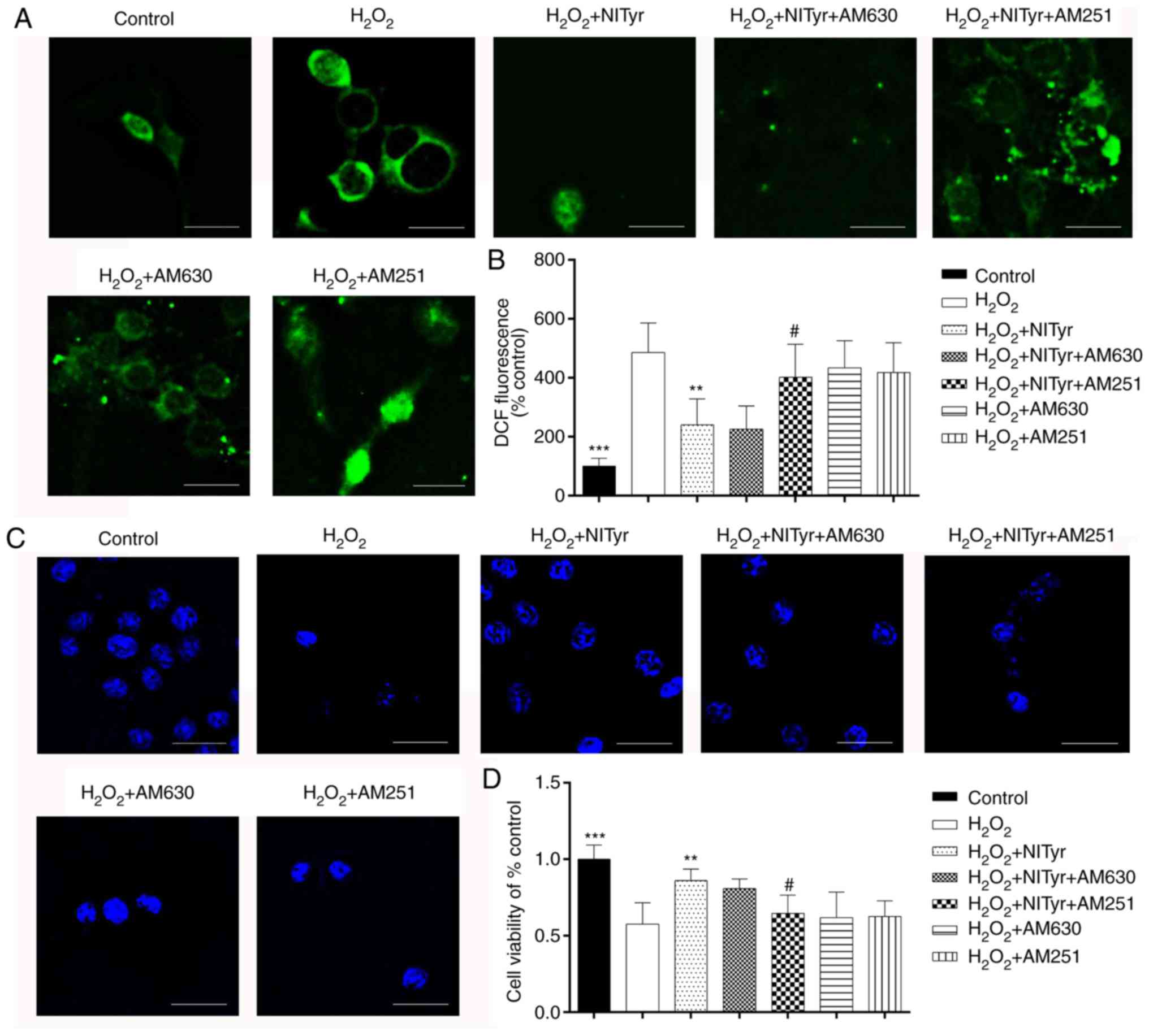
Effects of CB receptor antagonists on
cell viability and ROS production
Compared with H2O2 exposure,
pre-treatment with the CB1 receptor antagonist, AM251, or the CB2
antagonist, AM630, following H2O2 exposure
had negligible effects on cell viability and ROS levels (Fig. 6A and C). However, AM251, but not
AM630, diminished the effects of NITyr (1 µmol/l) on cell
viability and ROS generation. Moreover, the relative DCFH-DA
fluorescence intensity and cell viability were detected by
fluorometry and MTT assay, respectively, and the results were
consistent with those of DCFH-DA and DAPI staining
(F(DCFH-DA)=11.502, F(DAPI)=14.973,
P<0.05; Fig. 6B and D).
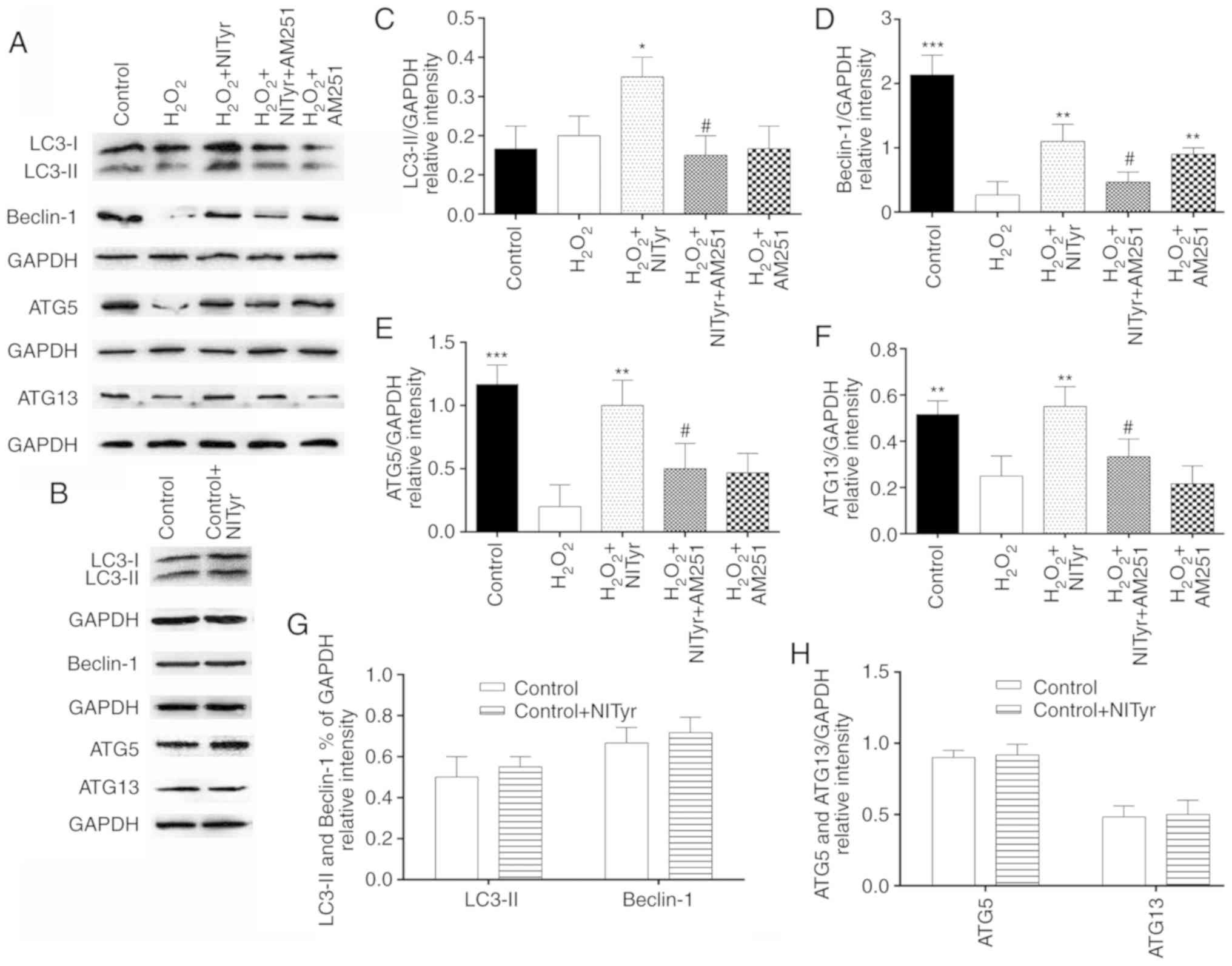
Effects of the CB1 receptor antagonist,
AM251, on autophagy- related proteins
According to the aforementioned experimental
results, the PC12 cells were treated with a combination of 1
µmol/l NITyr and 3 µmol/l AM251. Compared with the
H2O2 group, negligible effects on the protein
expression of LC3-II, ATG5 and ATG13 (P>0.05), but significant
effects on beclin-1 protein expression (P<0.05) were observed in
the AM251 group following H2O2 exposure
(Fig. 7A and C-F). When used in
combination with AM251, 1 µmol/l NITyr exerted diminished
effects on autophagy-related protein expression than when used
alone (F(LC3-II)=5.786, F(Beclin-1)=533.174,
F(ATG-5)=15.479, F(ATG-13)=11.639, P<0.05;
Fig. 7A and C-F). In addition,
compared with the control group, negligible effects of NITyr were
observed on the protein expression of LC3-II, Beclin-1, ATG5 and
ATG13 (P>0.05; Fig. 7B, G and
H).
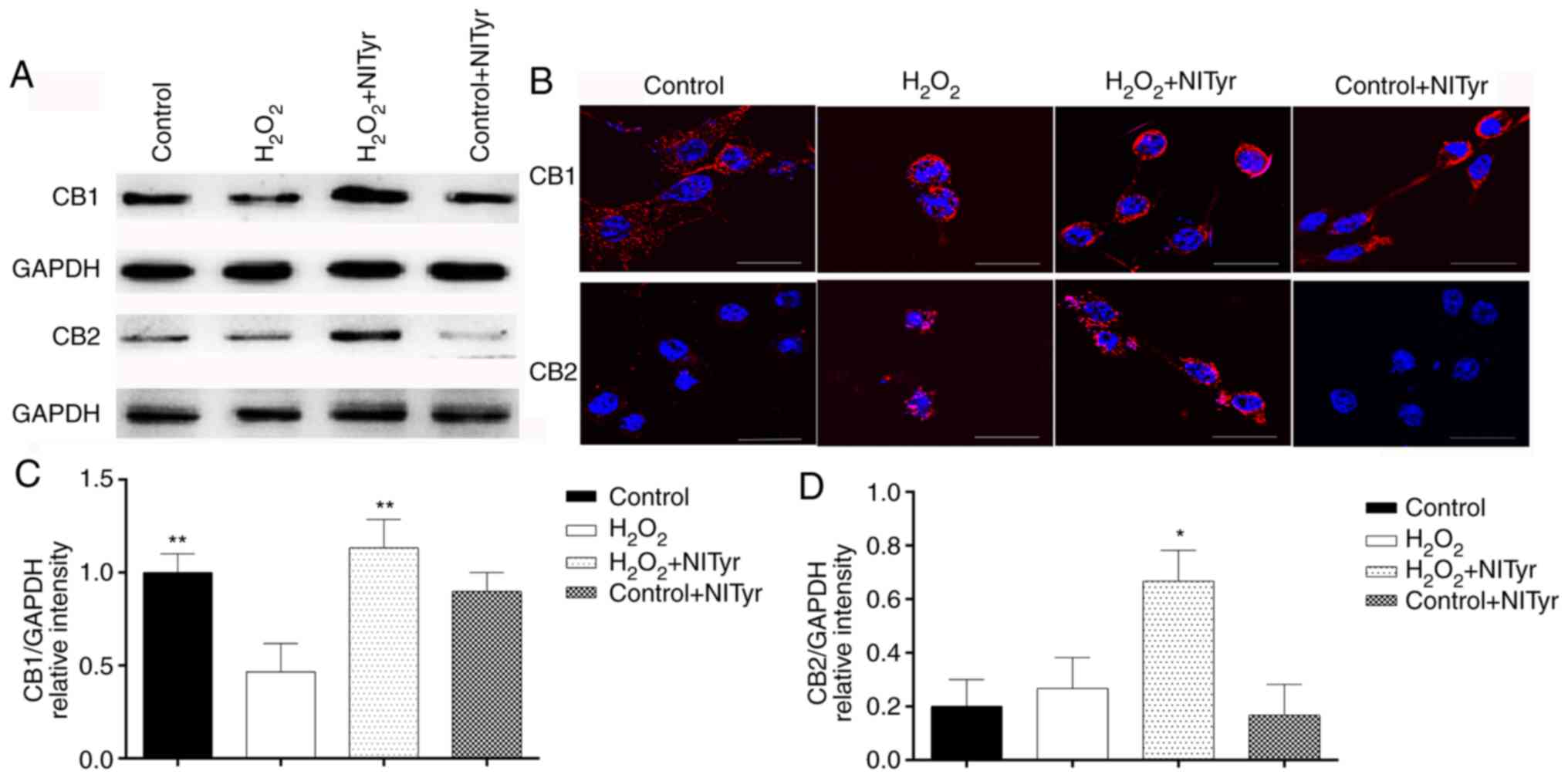
Effects of NITyr on CB1 and CB2 receptor
expression
Significant effects on CB1 and CB2 protein
expression were observed in the H2O2 + NITyr
group compared with the H2O2 group
(P<0.05; Fig. 8A, C and D). In
addition, negligible effects were observed on the protein
expression of CB1 and CB2 in the control + NITyr group compared
with the control group (P>0.05). As shown in Fig. 8B, red fluorescence represents the
protein expression of the CB1 and CB2 receptors. Compared with the
control group, CB1, but not CB2 receptor expression levels were
downregulated in the H2O2 group. Furthermore,
pre-treatment with 1 µmol/l NITyr increased the intensity of
red fluorescence compared with H2O2 exposure
alone.
Discussion
AEA has been shown to exert benign antioxidant
activity, but its short half-life is a limitation to its clinical
application (8,17). In previous studies, the saturated
fatty acyl amino acid, NSTyr, was synthesized based on the
structure of AEA, and this compound was found to possess strong
antioxidant properties (18-20). However, NSTyr must be used at a
considerably higher concentration than AEA to elicit a moderate
antioxidant effect (21). Based
on the level of activity, the structure of AEA was compared with
that of NSTyr, and AEA was found to possess unsaturated bonds,
while NSTyr did not. The method for NSTyr synthesis was
subsequently improved to include the incorporation of unsaturated
bonds. Due to the strong antioxidant activities of AEA and NSTyr,
it was speculated that NITyr also possessed antioxidant properties.
Therefore, the aim of the present study was to determine the
potential antioxidant effects of NITyr, and the underlying
mechanisms through which these effects are elicited.
Due to a high demand for oxygen in brain tissue, the
central nervous system is particularly vulnerable to hypoxia, and
cell membranes are prone to attack by oxygen free radicals. In
general, oxidative stress has been confirmed to be elevated in
neurodegenerative diseases (22,23). There is also increasing evidence
that excessive intracellular H2O2 is toxic to
the cell membrane and contributes to oxidative stress through
virous ways, such as formation of reactive oxygen species, etc.
Therefore, in the present study, PC12 cells were stimulated with
H2O2 to establish an oxidative damage model
(23). The fact that oxidative
stress activates contradictory signaling pathways of survival and
death implies that there must be sophisticated crosstalk between
these opposite signals that dictate cells fate. A number of Akt
substrates have been identified as elements of the initiation and
execution phases of apoptosis. Akt appears to be a substrate of
caspase-3 in vitro.So Akt signaling pathway is not only
related to cell survival, but also to cell apoptosis (24,25). Combined with the literature, it is
hypothesized that under the early stimulation of low concentration
of H2O2, cells will activate their own
defense mechanism to resist damage via AKT activation, resulting in
cells proliferation. However, under the long stimulation of high
concentration of H2O2, the defense mechanism
initiated by cells is not sufficient to resist the injury; thus,
cell viability is reduced. Therefore, the activation of the Akt
pathway induced by H2O2 leads to cell
proliferation or cell death, which may be related the concentration
and time of H2O2 stimulation (26). In a previous study, NITyr
activated the Akt signaling pathway to delay cell injury in the
ischemia-reperfusion model (13).
NITyr was demonstrated to markedly suppress
H2O2-induced cellular damage and ROS
generation, and this protective effect was amplified with NITyr
treatement. The optimum concentration of NITyr was 1 µmol/l,
and a plateau effect was reached at 5 µmol/l. Collectively,
the results of the present study indicate that
H2O2 promotes cellular injury, and that NITyr
inhibits ROS-induced damage resulting from
H2O2 exposure.
Autophagy is a highly conserved catabolic process
for the removal of damaged organelles that can result in the
production of intracellular ROS (27). The presence of excess ROS
stimulates an autophagic response, which in turn restores
intracellular ROS levels. Decreased autophagy increases the
accumulation of damaged organelles and ROS, which are involved in
the pathogenesis of various diseases, including neurodegenerative
conditions (28-30). According to the initial results of
the present study, NITyr plays a considerable role in preventing
H2O2-mediated cellular injury and ROS
elevation, and therefore, the role of autophagy in these processes
was investigated. When autophagy occurs, LC3 facilitates the
formation of the autophagic membrane. A small segment of the
cytoplasmic form of LC3 (LC3-I) is enzymatically degraded, and as
such, LC3-I is transformed into membranous LC3 (LC3-II). The level
of LC3-II, which is widely used to indicate overall autophagic
degradation, has been significantly associated with the number of
autophagosomes (31), and
beclin-1 promotes the localization of autophagic proteins to
autophagic vesicles (32). ATG13
is essential for autophagic vesicle formation (33), and ATG5 is a key regulator
involved in the membrane extension of phagocytes into these
vesicles (34). Thus, LC3,
beclin-1, ATG5 and ATG13 play important roles in the formation and
extension of autophagic vesicles. In the present study, NITyr was
found to upregulate the protein expression levels of LC3-II,
beclin-1, ATG5 and ATG13, indicating that it induces autophagy. To
further elucidate the association between autophagy and oxidative
stress, PC12 cells were treated with the autophagy inhibitor, 3MA,
which was found to diminish the effects of NITyr on ROS generation
and cellular viability. However, 3MA alone with
H2O2 exposure did not affect cellular
viability and the ROS levels. These results indicate that NITyr
protects against cytotoxicity and excessive ROS production by
activating autophagy.
AEA is an endogenous ligand of the cannabinoid
receptor which has been reported to play an attenuative role in the
pathogenesis and progression of neurodegenerative diseases
(35). The CB1 receptor may be
indirectly involved in oxidative stress (36). Conversely, the CB2 receptor is
directly involved in counteracting oxidative stress (37). Additionally, AEA analogs have been
associated with autophagy (9-11),
and the protective functions of CB1 have also been linked to
autophagy in various diseases (38). As an AEA analogue, NITyr may play
a similar role to AEA. In the present study, PC12 cells were
pre-treated with selective antagonists of the CB1 and CB2 receptors
(AM251 and AM630, respectively), and AM251 pre-treatment blocked
the protective effects of NITyr on
H2O2-stimulated PC cells, while AM630 had no
such effect. Moreover, AM251 and AM630 alone following
H2O2 exposure did not affect cellular
viability and the ROS levels. Therefore, the role of the CB1
receptor in NITyr-associated protection was the primary focus of
the following experiments. Further experiments revealed that AM251
diminished the effects of NITyr on autophagy-related proteins, and
confirmed that NITyr induced autophagy via the CB1 receptor,
resulting in an antioxidant effect. Moreover, CB2 receptor
expression in the presence of H2O2 remained
the same as that under the control conditions, while the expression
of the CB1 receptor was downregulated; these findings suggest that
the CB2 receptor is highly stable, and that the CB1 receptor is
more sensitive to oxidative stimuli. NITyr increased the expression
of both the CB1 and CB2 receptor, whereas the CB2 receptor
antagonist, AM630, was unable to inhibit the effects of NITyr. This
may be due to the fact that the CB2 receptor is not highly
expressed in PC12 cells, and that it is more prone to inflammatory
stimulation, but not oxidative stress. It was further confirmed
that the CB1 receptor may be the antioxidant target of NITyr.
To the best of our knowledge, the present study
demonstrates for the first time that NITyr alleviates
H2O2-induced injury and oxidative stress in
PC12 cells by promoting autophagy. Furthermore, NITyr significantly
maintained intracellular ROS homeostasis, reduced cellular injury
and enhanced autophagy via the CB1 receptor. These findings suggest
that NITyr inhibits oxidative stress through the CB1/ROS pathway
with the involvement of autophagy. NITyr may therefore serve as a
potential antioxidant by regulating the CB1 receptor.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81803514), the National
Student Innovation Training Program (grant no. 202013705073), the
Fund Project of Sichuan Provincial Department of Education (grant
no. 18ZB0165), and the Fund Project of Development and Regeneration
Key Laboratory of Sichuan Province (grant no. SYS15-007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RY and SL designed and supervised the experiments.
XL, YW and DZ performed the experiments, and YX and YL analyzed the
data. YZ guided the immunofluorescence experiment, the data
analysis and wrote the manuscript. SL provided approval of the
final published version. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
References
|
1
|
Sies H: Oxidative stress: A concept in
redox biology and medicine. Redox Biol. 4:180–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tönnies E and Trushina E: Oxidative
stress, synaptic dysfunction, and Alzheimer's disease. J Alzheimers
Dis. 57:1105–1121. 2017. View Article : Google Scholar
|
|
3
|
Salim S: Oxidative stress and the central
nervous system. J Pharmacol Exp Ther. 360:201–205. 2017. View Article : Google Scholar :
|
|
4
|
Ravanan P, Srikumar IF and Talwar P:
Autophagy: The spotlight for cellular stress responses. Life Sci.
188:53–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Munck D, De Meyer DR and Martinet W:
Autophagy as an emerging therapeutic target for age-related
vascular pathologies. Expert Opin Ther Targets. 24:131–145. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Filomeni G, De Zio D and Cecconi F:
Oxidative stress and autophagy: The clash between damage and
metabolic needs. Cell Death Differ. 22:377–388. 2015. View Article : Google Scholar :
|
|
7
|
Fang C, Gu L, Smerin D, Mao S and Xiong X:
Interrelation between reactive oxygen species and autophagy in
neurological disorders. Oxi Med Cell Longev. 2017:84951602017.
|
|
8
|
Martín Giménez VM, Noriega SE, Kassuha DE,
Fuentes LB and Manucha W: Anandamide and endocannabinoid system: An
attractive therapeutic approach for cardiovascular disease. Ther
Adv Cardiovasc Dis. 12:177–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Y, Tao Y and Hu J: Cannabinoid receptor
2 deletion deteriorates myocardial infarction through the
down-regulation of AMPK-Mtor-p70S6K signaling-mediated autophagy.
Biosci Rep. 39:BSR201806502019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu A, Hu P, Lin J, Xia W and Zhang R:
Activating cannabinoid receptor 2 protects against diabetic
cardiomyopathy through autophagy induction. Front Pharmacol.
9:12922018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Q, Zhang M, Liu X, Zhang J and Wang H:
CB2R orchestrates neuronal autophagy through regulation of the mtor
signaling pathway in the hippocampus of developing rats with status
epilepticus. Int J Mol Med. 45:475–484. 2020.PubMed/NCBI
|
|
12
|
Willoughby KA, Moore SF, Martin BR and
Ellis EF: The biodis-position and metabolism of anandamide in mice.
J Pharmacol Exp Ther. 282:243–247. 1997.PubMed/NCBI
|
|
13
|
Cheng L, Li J, Zhou Y, Zheng Q, Ming X and
Liu S: N-linoleyltyrosine protects against transient cerebral
ischemia in gerbil via CB2 receptor involvement in PI3K/Akt
signaling pathway. Biol Pharm Bull. 42:1867–1876. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu Y, Zhou KY, Wang ZJ, Lu Y and Yin M:
N-stearoyl-l-tyrosine inhibits the cell senescence and apoptosis
induced by H2O2 in HEK293/Tau cells via the CB2 receptor. Chem Biol
Interact. 272:135–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dyall SC, Mandhair HK, Fincham RE, Kerr
DM, Roche M and Molina-Holgado F: Distinctive effects of
eicosapentaenoic and docosahexaenoic acids in regulating neural
stem cell fate are mediated via endocannabinoid signaling pathways.
Neuropharmacology. 107:387–395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo Z, Yuan Y, Guo Y, Wang H, Song C and
Huang M: Nischarin attenuates apoptosis induced by oxidative stress
in PC12 cells. Exp Ther Med. 17:663–670. 2019.PubMed/NCBI
|
|
17
|
Maccarrone M: Metabolism of the
endocannabinoid anan-damide: Open questions after 25 years. Front
Mol Neurosci. 29:1662017. View Article : Google Scholar
|
|
18
|
Zhang YB, Kan MY, Yang ZH, Ding WL, Yi J,
Chen HZ and Lu Y: Neuroprotective effects of N-steroyltyrosine on
transient global cerebral ischemia in gerbils. Brain Res.
1287:146–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang SQ, Yin S, Liu S, Le KJ, Yang RL, Liu
JH, Wang XL, Zheng ZX, Zheng L, Lin Q and Lu Y: N-stearoyltyrosine
dipotassium ameliorates high-fat diet-induced obesity in C57BL/6
mice. Eur J Pharm Sci. 74:18–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu S, Tang SQ, Cui HJ, Yin S, Yin M, Zhao
H, Meng LH, Wang ZJ and Lu Y: Dipotassium N-stearoyltyrosinate
ameliorated pathological injuries in triple-transgenic mouse model
of Alzheimer's disease. J Pharmacol Sci. 132:92–99. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao LY, Lin Q, Niu YY, Deng KM, Zhang JH
and Lu Y: Synthesis of Lipoamino acids and their activity against
cerebral ischemia injury. Molecules. 14:4051–4064. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh A, Kukreti R, Saso L and Kukreti S:
Oxidative stress: A key modulator in neurodegenerative diseases.
Molecules. 22:15832019. View Article : Google Scholar
|
|
23
|
Chen L, Wu X, Shen T, Wang X, Wang S, Wang
J and Ren D: Protective effects of ethyl gallate on H2O2-induced
mitochondrial dysfunction in PC12 cells. Metab Brain Dis.
34:545–555. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jia J, Ma L, Wu MC, Zhang L, Zhang X, Zhai
Q, Jiang Q, Wang Q and Xiong L: Anandamide protects HT22 cells
exposed to hydrogen peroxide by inhibiting cb1 receptor-mediated
type 2 NADPH oxidase. Oxid Med Cell Longev. 2014:8935162014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang ZH, Liu JL, Wu L, Yu Z and Yang HT:
Concentration-dependent wrestling between detrimental and
protective effects of H2O2 during myocardial ischemia/reperfusion.
Cell Death Dis. 5:e12972014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martin D, Salinas M, Fujita N, Tsuruo T
and Cuadrado A: Ceramide and reactive oxygen species generated by
H2O2 induce caspase-3-independent degradation of Akt/protein kinase
B. J Biol Chem. 277:42943–42952. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Denton D and Kumar S: Autophagy-dependent
cell death. Cell Death Differ. 26:605–616. 2019. View Article : Google Scholar :
|
|
28
|
Kaushal GP, Chandrashekar K and Juncos LA:
Molecular interactions between reactive oxygen species and
autophagy in kidney disease. Int J Mol Sci. 20:37912019. View Article : Google Scholar :
|
|
29
|
Wang H, Gao N, Li Z, Yang Z and Zhang T:
Autophagy alleviates melamine-induced cell death in PC12 cells via
decreasing ROS levels. Mol Neurobiol. 53:1718–1729. 2016.
View Article : Google Scholar
|
|
30
|
Cao S, Li S, Li Q, Zhang F, Sun M, Wan Z
and Wang S: Protective effects of salvianolic acid B against
hydrogen peroxide-induced apoptosis of human umbilical vein
endothelial cells and underlying mechanisms. Int J Mol Med.
44:457–468. 2020.
|
|
31
|
Cao Y, Chen J, Ren G, Zhang Y, Tan X and
Yang L: Punicalagin prevents inflammation in LPS-induced RAW264.7
macrophages by inhibiting Fox3a/Autophagy signaling pathway.
Nutrients. 11:27942019. View Article : Google Scholar
|
|
32
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alers S, Wesselborg S and Stork B: ATG13:
Just a companion, or an executor of the autophagic program?
Autophagy. 10:944–956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arakawa S, Honda S, Yamaguchi H and
Shimizu S: Molecular mechanisms and physiological roles of
Atg5/Atg7-independent alternative autophagy. Proc Jpn Acad Ser B
Phys Biol Sci. 93:378–385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moreira-Silva D, Carrettiero DC, Oliveira
AS, Rodrigues S, Dos Santos-Lopes J, Canas PM, Cunha PA, Almeida MC
and Ferreira TL: Anandamide effects in a streptozotocin-induced
Alzheimer's disease-like sporadic dementia in rats. Front Neurosci.
12:6532018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marsicano G, Moosmann B, Hermann H, Lutz B
and Behl C: Neuroprotective properties of cannabinoids against
oxidative stress: Role of the cannabinoid receptor CB1. J
Neurochem. 80:448–456. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parlar A, Arslan SQ, Doğan MF, Çam SA,
Yalçin A, Elibol E, Özer MK, Üçkardeş F and Kara H: The exogenous
administration of CB2 specific agonist, GW405833, inhibits
inflammation by reducing cytokine production and oxidative stress.
Exp Ther Med. 16:4900–4908. 2018.PubMed/NCBI
|
|
38
|
Gugliandolo A, Pollastro F, Bramanti P and
Mazzon E: Cannabidiol exerts protective effects in an in vitro
model of Parkinson's disease activating AKT/Mtor pathway.
Fitoterapia. 143:1045532020. View Article : Google Scholar : PubMed/NCBI
|