Introduction
Glaucoma describes a group of eye diseases that are
characterized by idiopathic optic nerve injury and is regarded as
the primary cause of irreversible visual impairment (1). It is generally accepted that the
abnormal elevation of intraocular pressure (IOP), which is one of
the critical risk factors associated with retinal ganglion cell
(RGC) damage, is primarily responsible for the occurrence and
development of glaucoma (2).
However, since RGC failure is also observed under normal IOP, and
appropriate IOP control can only inhibit RGC degeneration induced
by ocular hypertension (3), novel
neuro-protective approaches for RGCs are required as an additional
strategy in managing glaucoma.
There are several hypotheses into the possible
mechanisms underlying RGC cell death in glaucoma, including
oxidative stress, glutamate excitotoxicity and nitric oxide-induced
injury (4). In addition,
accumulating evidence is suggesting that mitochondrial dysfunction
can also result in the death of RGCs (5-7).
Mitochondria are subcellular organelles that are enclosed in two
layers of separated membranes and are considered a cornerstone of
metabolism in the cell by generating energy (8). Therefore, mitochondrial dysfunction
induces detrimental effects on cells, particularly those with
higher energy demands, including RGCs, cardiomyocytes and skeletal
muscle cells (9-12). Mitochondria continuously undergo
morphological changes. This dynamic process, which is caused by the
constant cycle of fusion and fission, is under strict sophisticated
regulation by a group of dynamin-related GTPases (13,14). Among these mitochondrial dynamins,
optical atrophy 1 (Opa1) is of particular interest. Opa1 is a
mitochondrial protein that is encoded in the nucleus, which can not
only regulate mitochondrial fusion but can also inhibit programmed
cell death by regulating cytochrome c release (15).
Trans 3,5,4'-trihydroxystilbene, commonly referred
to as resveratrol, is an important activator of sirtuin1 (Sirt1)
that is abundantly present in giant knotweed, wine and grapes
(16,17). Over recent decades, resveratrol
has been garnering significant research attention due to its
numerous potentially beneficial properties being reported,
including anti-apoptotic, anti-inflammatory and antioxidant
(18,19). Additionally, no apparent adverse
effects of resveratrol treatment have been observed in previous
animal models and clinical human studies, suggesting that
resveratrol has promising prospects for future therapeutic use
(20). Previous studies in rat
models of glaucoma have also shown that resveratrol can exert
protective effects on RGCs (21).
However, the precise mechanisms of the effects of resveratrol on
RGCs have not been fully characterized (22).
The present study investigated the potential effects
of resveratrol on ischemia/reperfusion (I/R)-induced retina injury
and serum deprivation-induced R28 cell apoptosis. The results
showed that resveratrol protected RGCs against apoptosis by
regulating the expression of Opa1 and the activity of superoxide
dismutase (SOD). Additionally, it was found that resveratrol
influenced the long Opa1 isoform to short Opa1 isoform
(L-Opa1/S-Opa1) ratio by regulating the expression of proteins that
post-translationally process Opa1 in the retina, including
overlapping activity with m-AAA protease (Oma1) and yeast
mitochondrial DNA escape 1 (Yme1). Although further study may be
warranted to confirm the relationship between resveratrol and
post-transcriptional proteases of Opa1, the present study
contributes evidence that resveratrol can exert protective effects
on RGCs.
Materials and methods
Animals
All experiments concerning animal studies were
performed in accordance with the Association for Research in Vision
and Ophthalmology Statement for the Use of Animals in Ophthalmic
and Vision Research (arvo.
org/About/policies/statement-for-the-use-of-animals-in-ophtha
lmic-and-vision-research/). All procedures concerning animal use
were approved and monitored by the Institutional Animal Care and
Use Committee of Nanchang University (approval no. 2016NC-020-02).
Male Sprague-Dawley (SD) rats, 2-3 months of age were provided by
the Center of Laboratory Animal Science of Nanchang University and
were used for transient retinal I/R studies. The rats were provided
free access to sterile water and food and housed in a controlled
environment with a 12-h light/dark cycle at 23°C and 60%
humidity.
R28 cell culture
All reagents required for cell culture were provided
by Biological Industries. R28 retinal neuronal-like cells were
kindly provided by Dr Guotong Xu (Tongji University School of
Medicine). R28 cells were maintained in low-glucose DMEM containing
100 mg/ml streptomycin, 100 U/ml penicillin and 10% FBS, in a
humidified incubator with 5% CO2 at 37°C as described
previously (23). Cells were
sub-cultured every 3-4 days, and they had a doubling time of 18-20
h.
Transient retinal I/R model
A total of 66 rats were randomly separated into four
groups: i) Sham group (n=18, eyes/group); ii) I/R group (n=30,
eyes/group); iii) I/R + vehicle group (n=18, eyes/group); iv) and
I/R + resveratrol group (n=18 eyes/group). The right eye was used
for the I/R procedure in the I/R, I/R + vehicle and I/R +
resveratrol groups. The sham procedure which cannulated and
maintained the eye at normal IOP was performed on the left eye of
the rats in the I/R group and was used as the control (24). To minimize the suffering of rats
during I/R injury, rats were anesthetized by an intraperitoneal
injection of 10% chloral hydrate (400 mg/kg) prior to I/R injury
and did not exhibit symptoms of discomfort, including pain or
peritonitis following chloral hydrate administration. To induce
ocular ischemia, the IOP was elevated (~110 mm Hg) in one eye of
the rats by cannulating the anterior chambers through a canula
equipped with a raised reservoir for 60 min. Completion of retinal
ischemia was judged by observing the blanching of the iris and
retina. Resveratrol (Sigma-Aldrich; Merck KGaA) was dissolved and
diluted in 25% ethanol to a concentration of 10 mg/ml. The I/R +
resveratrol group were injected intraperitoneally with 25 mg/kg
resveratrol 1 day before, at the time of and 1 day after I/R retina
injury, whilst the I/R + vehicle group were treated with 25%
ethanol. After 1, 3 and 7 days of retinal ischemia, the rats were
euthanized by an intraperitoneal injection of 2% sodium
pentobarbital (100 mg/kg), and the heartbeat, breathing and corneal
reflex were observed. After verification of animal death through a
lack of heartbeat, breathing or respiration, the eyeballs were then
harvested from each rat.
Serum deprivation and drug treatment
Retinal neuronal R28 cells were seeded at the
desired density into tissue culture dishes. A total of 8 groups of
cells, which were seeded into 6-well plates at a density of
1.5x106 cells/well, were washed three times with PBS.
For serum deprivation treatment, three groups of cells were
incubated in serum-free DMEM for 12, 24 or 48 h respectively. Cells
in the control group were incubated with supplemented DMEM. For
resveratrol treatment studies, resveratrol was dissolved in DMSO to
a stock concentration of 10 mM, and three groups of cells were then
treated with 4 µM resveratrol dissolved in serum-free DMEM for 12,
24 or 48 h (25). The cells were
incubated with supplemented DMEM containing 4 µM DMSO, and was
considered the vehicle group. Untreated cells and treated cells
were incubated in serum-free DMEM in the same manner as the normal
controls and serum deprivation controls, respectively.
Protein preparation
Retinal tissue was separated from the retinal
pigment epithelium and choroid before lysing using RIPA buffer
containing PMSF (Beijing Solarbio Science & Technology Co.,
Ltd.). The lysates were then centrifuged at 15,000 x g at 4°C for
15 min, following which, the supernatant was collected to obtain
tissue proteins.
For R28 cells, whole cell lysates were obtained by
adding PMSF-containing RIPA buffer, followed by centrifugation at
10,000 x g for 20 min at 4°C. The supernatant, which contained the
cellular proteins, was collected. The concentration of proteins
were determined using a Bradford assay (Beyotime Institute of
Biotechnology) according to the manufacturer's protocols.
Western blot analysis
The sample protein (30 µg) was separated using
7.5-15% SDS-PAGE and transferred onto a nitrocellulose membrane.
Blots were blocked in 5% non-fat dry milk in TBS/Tween-20 for 1 h
at room temperature before incubation with the appropriate primary
antibodies against Sirt1 (1:1,000; cat. no. 9475; Cell Signaling
Technology, Inc.), Opa1 (1:1,000; cat. no. 612606; BD Biosciences)
or β-tubulin (1:1,000; cat. no. HC101-01; TransGen Biotech)
overnight at 4°C. The blots were then incubated with a horseradish
peroxidase-conjugated anti-rabbit IgG secondary antibody (1:2,500;
cat. no. 7074; Cell Signaling Technology, Inc.) or horseradish
peroxidase-conjugated anti-mouse IgG secondary antibody (1:2,500;
cat. no. 7076; Cell Signaling Technology, Inc.) for 1 h at room
temperature. Finally, protein bands on the membrane were visualized
using enhanced chemiluminescent western blot detection reagent (EMD
Millipore). Densitometry analysis was performed using ImageJ
version 1.46 (National Institutes of Health).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
According to the manufacturer's protocols, RNA was
extracted from the rat retinas using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). RNA concentration and
purity were determined using spectrophotometry (Tiangen Biotech
Co., Ltd.; cat. no. OSE-260). The extracted RNA (200 ng/sample) was
then reverse-transcribed into cDNA using the Quantscript RT kit
(Tiangen Biotech Co., Ltd.). Specific oligonucleotide primers for
each gene, including Sirt1, Opa1, Oma1, Yme1 and β-actin (Table I), were designed and synthesized
by Tiangen Biotech Co., Ltd.. Subsequently, qPCR was performed
using RealMasterMix (SYBR Green) kit (Tiangen Biotech Co., Ltd.)
with the following thermocycling conditions: 95°C for 20 sec;
followed by 39 cycles of 58°C for 20 sec and 68°C for 30 sec. All
samples were tested in triplicate, and the mean of the reactions
was used for calculating the expression levels. All the data were
collected from the linear range of each amplification. The results
obtained were analyzed using the 2-ΔΔCq method (26) and expression levels were
normalized to the average of the β-tubulin mRNA levels from the
same samples.
 | Table ISequences of the primers used in the
present study. |
Table I
Sequences of the primers used in the
present study.
| Gene | Primer sequence,
5'-3' |
|---|
| Opa1 | |
| Forward |
CAGCTGGCAGAAGATCTCAAG |
| Reverse |
CATGAGCAGGATTTTGACACC |
| Yme1 | |
| Forward |
AGGATGCAATGCCAATCAAT |
| Reverse |
TGCCACTCTTCCTCCCATAC |
| Oma1 | |
| Forward |
ACCAGTGCAAAAGCTCCTTG |
| Reverse |
TGTTCCTCTTCACGCTGTCT |
| Sirt1 | |
| Forward |
GGCACCGATCCTCGAACAAT |
| Reverse |
CGCTTTGGTGGTTCTGAAAGG |
| β-actin | |
| Forward |
CCCGCGAGTACAACCTTCTT |
| Reverse |
CGACGAGCGCAGCGATA |
| β-actin
nuclear | |
| encoded
variant | |
| Forward |
CTGCTCTTTCCCAGATGAGG |
| Reverse |
CCACAGCACTGTAGGGGTTT |
| COII | |
| Forward |
TGAGCCATCCCTTCACTAGG |
| Reverse |
TGAGCCGCAAATTTCAGAG |
Immunocytochemistry
R28 cells cultured in dishes were fixed with PBS
containing 4% sucrose and 4% paraformaldehyde (PFA) for 20 min at
room temperature. Cells were then blocked with PBS containing 5%
BSA and 0.2% Triton X-100 (PBSTX) for 30 min at room temperature.
Cells were incubated at room temperature with primary antibodies
against Opa1 (1:50) diluted in PBSTX with 1% BSA for 4-6 h. The
fixed cells were then washed and incubated with the appropriate
Alexa Fluor® 488-conjugated donkey anti-Mouse IgG (H+L)
secondary antibody (1:200; cat. no. R37144; Thermo Fisher
Scientific, Inc.) dissolved in PBSTX containing 1% BSA for 45 min
at room temperature. Subsequently, the cells were incubated with
DAPI (Vector Laboratories, Inc.) and sealed using anti-fading
buffer (Bioworld Technology, Inc.).
Immunohistochemistry, and hematoxylin and
eosin (H&E) staining
Under anesthesia, 0.1 M PBS (pH 7.4) containing
ice-cold 4% PFA was injected into the left cardiac ventricle of the
rats for fixation before the eyeballs of the rats were removed and
embedded in 4% PFA at 4°C for 1 day. After fixation, the eyeballs
were dehydrated, embedded in paraffin and cut into 5-µm sections.
The central part of the eyeballs, through the optic nerve, were
selected for immunohistochemical analysis. Each section was heated
with 10 mM sodium citrate buffer (pH 6.0) at the secondary boiling
point (100°C) for 10 min and then cooled for 30 min for antigen
retrieval. Blocking, primary and secondary antibody labelling, and
all washing steps were performed as mentioned above. Staining was
observed using a confocal microscopy at a magnification of x20
(Axio-Imager_LSM-800, ZEISS, Germany). H&E staining was
observed using an inverted fluorescence microscope at a
magnification of x20 (Nikon Corporation). The thicknesses of the
inner retina [RGC + inner plexiform layer (IPL) + inner nuclear
layer (INL)] were measured in the H&E sections using the
Image-pro Plus version 6.0 (Media Cybernetics) at every quarter
point of each retinal cross-section, which were then averaged. Each
group contained four eyeballs to be measured. A total of 10 images
were obtained from each retinal section of every eyeball for
measuring the average.
RGC labeling and quantification
Fluoro-Gold (Fluorochrome, LLC) was injected into
the superior colliculi of the rats to retro-gradely label the RGCs.
The rats were anaesthetized with an intraperitoneal injection of
10% chloral hydrate and placed in a small stereotaxic instrument
(RWD Life Science Co., Ltd.). The skull was then opened to identify
the bregma spot and mark the injection site of the superior
colliculus. A hole was then drilled at the designated point using a
25-gauge needle. In total, 1.5 µl 5% Fluoro-Gold solution dissolved
in 0.9% sodium chloride were injected into the superior colliculus
of both hemispheres using a Hamilton syringe before the skin
incision was sutured. The rats started to receive resveratrol
treatment 7 days after Fluoro-Gold injection, specifically at 1 day
before, at the time of and 1 day after I/R retina injury before
being sacrificed 7 days after retinal I/R injury. The eyes were
then enucleated and dissected at the corneal limbus, following
which, the lenses were removed and the posterior segments were
fixed in 4% PFA at 4°C for 1 h. The retina, with a distinctive
'four-leafed' shape, was then tiled onto glass slides. RGCs labeled
with Fluoro-Gold in these four microscopic fields of the retina
were viewed under a fluorescence microscope at x20 magnification
(IX71, Olympus Corporation). The selected fields were located at ~2
mm radially from the optic disc to account for the variations in
retinal ganglion cell density. The number of RGCs were counted by
two individuals in a double-blinded manner, where the averages of
the two were taken as the final count.
Apoptosis assay
Apoptosis analysis in retinal cells was performed as
described previously (25,27).
The preparation of sections (5 µm) and the treatment of R28 cells
were described above, following which a TUNEL assay was performed
using In Situ Cell Death Detection kit, Fluorescein (Roche
Applied Science) to detect apoptotic cells in accordance with
manufacturer's protocol. Tissue sections were assessed using a
confocal microscope at a magnification of x20 (Axio-Imager_
LSM-800, ZEISS, Germany). Digital images were obtained (SPOT;
Diagnostic Instruments, Inc.) and Photoshop versions 5.5 and 7.0
(Adobe Systems, Inc.) was used to compile images.
Cell counting Kit-8 (CCK-8) assay
The viability of the cells was assessed using a
CCK-8 assay (BestBio Science). Cells in the logarithmic growth
phase were seeded into 96-well culture plates at a density of
~2.5-3x104 cells in 100 µl culture medium. After the
cells had adhered, they were serum starved or treated with
resveratrol before being treated with 10 µl CCK-8 Solution. An
automatic microplate reader (Multiskan MK3; Thermo Fisher
Scientific, Inc.) was used to measure the optical density value
(OD) in each well at a wavelength of 450 nm. Cell viability was
calculated from the OD values in each well using the following
formula: [(Treated cell OD-blank OD)/(control cell OD-blank
OD)]x100%.
SOD activity analysis
R28 cells were seeded into 60-mm tissue culture
dishes, serum starved and treated with or without resveratrol.
Whole cell lysates were obtained by sonication at 20% amplitude, 20
kHz, 130 W for 1 min, five times each on ice, with saline and then
centrifuged at 10,000 x g for 15 min at 4°C. The supernatant was
then assayed using an SOD kit (Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's protocol. The OD value
of each sample was measured using a Multiskan MK3 reader at a
wavelength of 450 nm. The SOD value was calculated according to the
following formulas: SOD inhibition rate (%)=[(AControl
group-AControl blank group)-(AMeasurement
group-AMeasurement blank group)]/(AControl
group-AControl blank group)x100%; and, SOD
activity (U/mg)=SOD inhibition rate (%)/50%x[reaction system (0.24
ml)/dilution factor (0.02 ml)]/sample protein concentration
(mgprot/ml).
Mitochondrial (mt)DNA copy number
(mitCN)
Ezup column animal genomic DNA extraction kit
(Sangon Biotech Co., Ltd.) was used to collect retinal DNA.
Relative mitCNs in the retinal samples were then measured by
RT-qPCR and corrected using nuclear DNA. The amplification of
mitochondrial cytochrome c oxidase subunit II (COII) and β-actin
(nuclear-encoded gene) were examined. The specific sequences of the
primers used are shown in Table
I.
Statistical analysis
Statistical analysis was performed using Microsoft
Excel (Office 365; Microsoft Corporation) and statistical analysis
was performed using GraphPad Prism version 6 (GraphPad Software,
Inc.). Results are presented as the mean ± standard deviation.
Comparisons were performed using an ANOVA followed by a post-hoc
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Resveratrol protects the retina and RGCs
from I/R injury in rats
In the present study, general histopathological
changes in the retina 7 days after injury induction were first
investigated using H&E staining to evaluate the effects of
resveratrol on the retina following retinal I/R injury. Significant
thinning of the retina was observed after I/R injury, particularly
in the GCL, IPL and INL. Administration of resveratrol prevented
this reduction in retinal thickness (Fig. 1A). The quantity of RGCs was
assessed using retrograde Fluoro-Gold labeling. RGCs were labeled
with Fluoro-Gold 1 week before I/R, and the number of RGCs were
counted 7 days after I/R or I/R + resveratrol. A significant
reduction in the number of RGCs was observed 7 days after I/R,
which was significantly reversed by the administration of
resveratrol (Fig. 1B).
Statistical analysis of inner retinal thickness (Fig. 1C) and RGC counts (Fig. 1D) were performed.
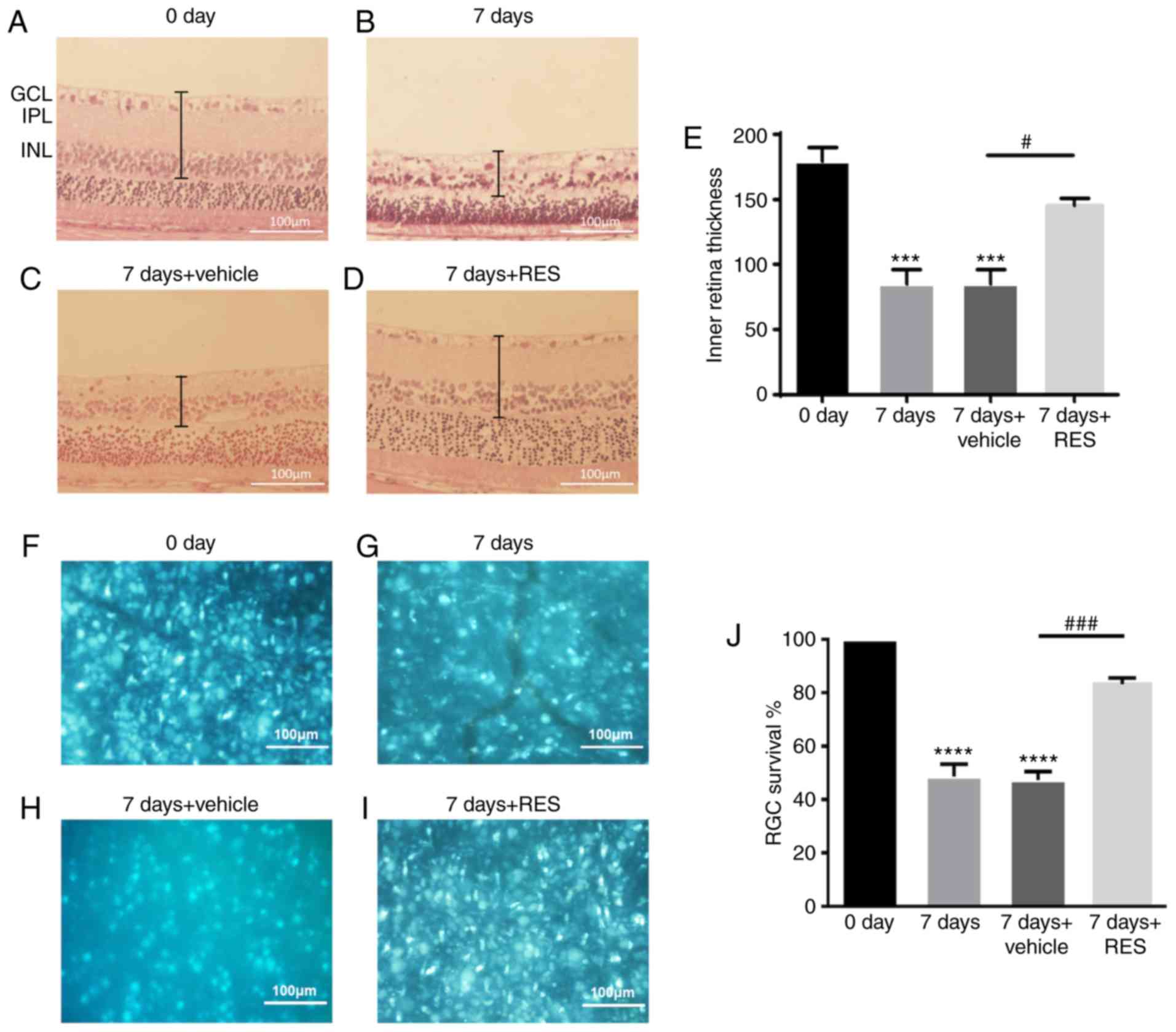 | Figure 1H&E staining and Fluoro-Gold
retrograde marker. (A-D) H&E staining was observed using an
inverted fluorescence microscope. Magnification x20. (E)
Quantitative analysis of inner retina (GCL+IPL+INL) thickness
variation. (F-I) Tiled retinas were examined 7 days after I/R and
I/R + RES and were observed using an inverted fluorescence
microscope. Magnification x40. (J) Quantitative analysis of
Fluoro-Gold staining of RGCs. n=3 per group. Scale bar, 100 µm.
***P<0.001, ****P<0.0001 vs. 0 day;
#P<0.05, ###P<0.001 vs. 7 days +
vehicle group. RES, resveratrol; GCL, ganglion cell layer; IPL,
inner plexiform layer; INL, inner nuclear layer; H&E,
hematoxylin and eosin; I/R, ischemia/reperfusion; RGC, retinal
ganglion cell. |
To investigate the effects of resveratrol on the
retina further, TUNEL staining was performed 1, 3 and 7 days after
I/R injury to explore the effects of resveratrol on apoptosis.
Compared with the control group (day 0), there was an increase in
the number of TUNEL-positive cells observed in the GCL and INL of
the I/R group on day 1 (Fig. 2).
A strong TUNEL-positive fluorescent signal was observed in the ONL
of the I/R group on day 3, whilst almost no fluorescence was
observed in the retina 7 days after I/R. Resveratrol treatment
reduced the levels of cell apoptosis on days 1, 3 and 7 after
I/R.
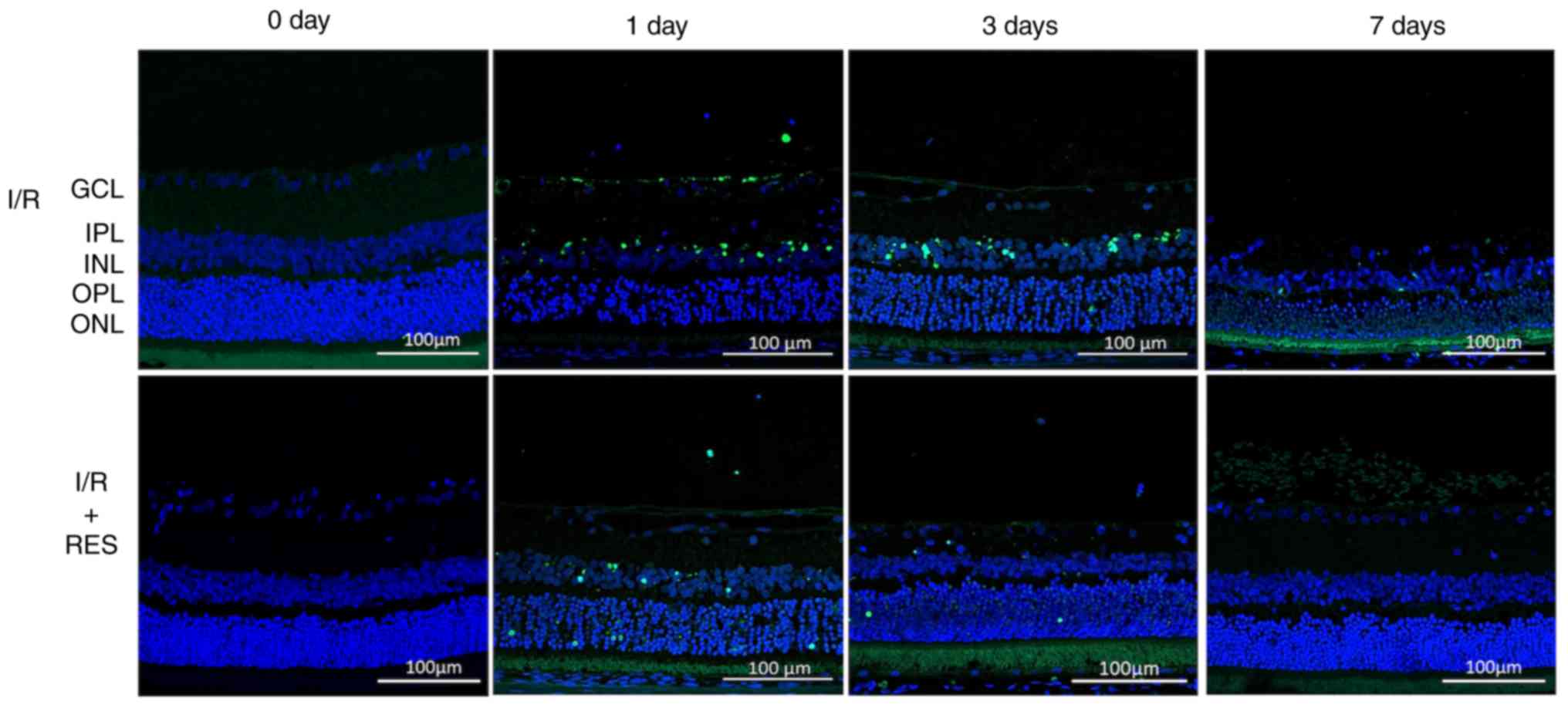 | Figure 2TUNEL staining to detect apoptosis.
Retinal paraffin section TUNEL staining observed using a confocal
microscope. In the normal retinal paraffin section, no fluorescence
was observed following TUNEL staining. TUNEL staining of I/R 1 d
retinal paraffin sections exhibited strong fluorescence in GCL and
INL. In the TUNEL staining of I/R 3 d retinal paraffin sections,
ONL exhibited a stronger fluorescence signal. TUNEL staining of I/R
7 days retinal paraffin sections showed no obvious fluorescence
signal. I/R 0 and 7 days retinal paraffin sections showed no
significant fluorescence changes following RES treatment. RES
significantly attenuated the fluorescence signal induced by
apoptosis in GCL at I/R 1 and 3 days. Scale bar, 100 µm. Blue,
DAPI; green, TUNEL-positive cells. RES, resveratrol; INL, inner
nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell
layer; I/R, ischemia/reperfusion. |
Resveratrol regulates Opa1 protein and
mRNA expression following retinal I/R injury
Resveratrol has been previously demonstrated to be a
critical activator of Sirt1 (28). To verify the effect of resveratrol
on Sirt1 regulation, the expression profile of Sirt1 was examined.
Four time points following I/R (0, 1, 3 and 7 days) assessed, and
the expression of the Sirt1 protein was found to be significantly
lower only 7 days after I/R (Fig.
3A). Subsequently, to assess the effect of resveratrol on Sirt1
expression in the I/R model, rats were sacrificed 7 days after I/R
injury with or without resveratrol treatment. Intraperitoneal
injections of resveratrol partially restored the expression of the
Sirt1 protein and mRNA expression (Fig. 3B and C). Immunofluorescence
staining showed that Sirt1 was primarily expressed in GCL, INL and
ONL of the normal retina, and was found to be reduced 7 days after
I/R injury. Resveratrol treatment partially restored Sirt1
expression following I/R (Fig.
3D).
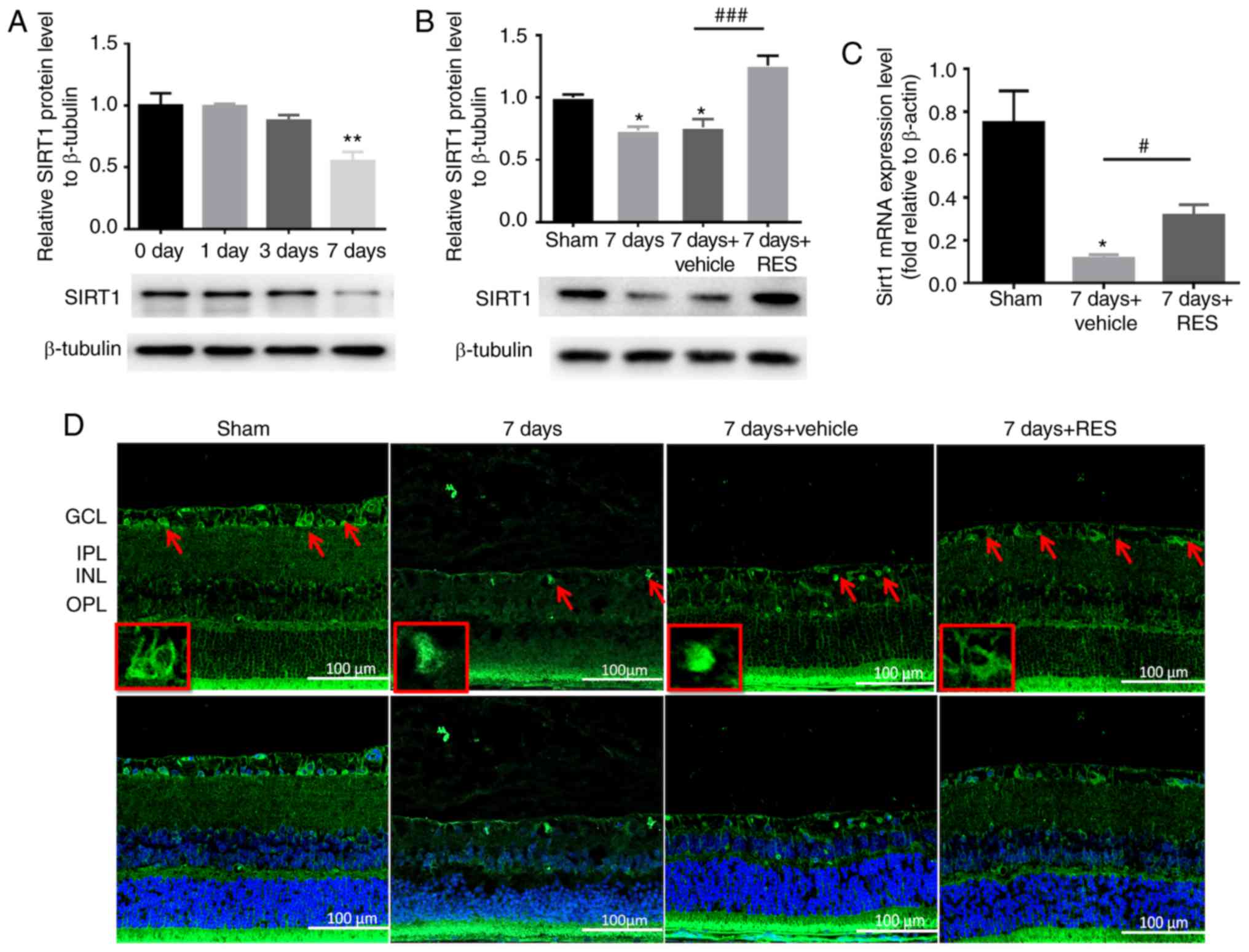 | Figure 3RES affects Sirt1 protein and gene
expression following I/R injury. (A) Sirt1 protein expression
gradually decreased over time, and this decrease was statistically
significant at I/R 7 days. n=3 rats per group.
**P<0.01 vs. 0 day group. (B and C) Intraperitoneal
injection of RES restored Sirt1 protein and gene expression to some
extent. n=3 rats per group. *P<0.05 vs. sham group;
#P<0.05, ###P<0.001 vs. 7 days +
vehicle group. (D) Confocal microscopy was used to observe the
localization of Sirt1 in the retina. Magnification, x20. Sirt1 is
primarily expressed in GCL, INL and ONL of normal retina. The
expression of Sirt1 in the retina was significantly reduced 7 days
after I/R injury. Resveratrol partly restores Sirt1 expression. Red
arrow indicates the expression of Sirt1 in GCL. Scale bar, 100 µm.
RES, resveratrol; GCL, ganglion cell layer; IPL, inner plexiform
layer; INL, inner nuclear layer; I/R, ischemia/reperfusion; Sirt1,
sirtuin1. |
The effect of resveratrol on Opa1 expression was a
primary focus of the present study. It was found that at the
selected time points after I/R injury, Opa1 protein expression also
exhibited a decreasing trend, which was statistically significant
only 7 days after I/R (Fig. 4A).
Therefore, to investigate the effect of resveratrol on Opa1
expression in the I/R model, the rats were sacrificed 7 days after
I/R injury with or without resveratrol treatment. Intraperitoneal
injection of resveratrol was shown to partially restore Opa1
protein and gene expression (Fig. 4B
and C). The localization of Opa1 expression were then
elucidated by immunofluorescence staining in the adult rat retinal
tissues. It was observed that positive Opa1 expression (green
fluorescence) was primarily present in the GCL, IPL, INL and outer
plexiform layer. On day 7 after I/R injury, the expression of Opa1
was found to be reduced in all layers of the retina, which was
significantly reversed by resveratrol treatment in various layers
of the retina (Fig. 4D).
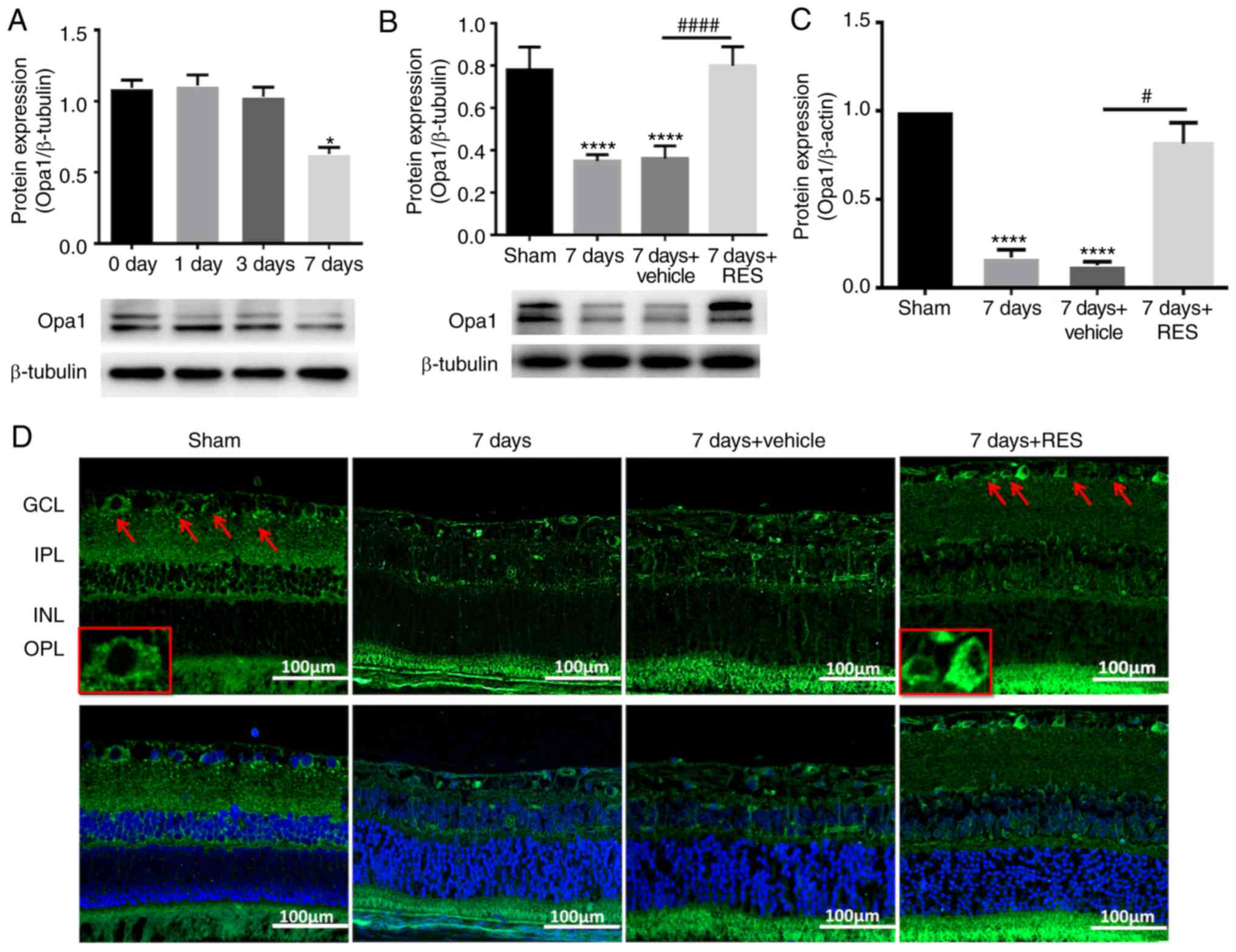 | Figure 4RES influences the expression and
localization of OPA1 following I/R injury. (A) Protein expression
levels of OPA1 gradually decreased, and this decrease was
statistically significant at I/R 7 days. n=3 rats per group.
*P<0.05 vs. 0 day group. (B and C) Intraperitoneal
injection of RES restored OPA1 protein and gene expression to some
extent. n=3 rats per group. ****P<0.0001 vs. sham
group; #P<0.05, ####P<0.0001 vs. 7 days
+ vehicle group. Confocal microscopy was used to observe the
localization of OPA1 in the retina. Magnification, x20. (D) OPA1 is
primarily expressed in GCL, IPL, INL and OPL of the normal retina.
Expression of OPA1 was not observed in any layer of the retina 7
days after I/R injury. Resveratrol restored OPA1 expression. Red
arrow indicates the expression of OPA1 in GCL. Scale bar, 100 µm.
RES, resveratrol. GCL, ganglion cell layer; IPL, inner plexiform
layer; INL, inner nuclear layer. I/R, ischemia/reperfusion; OPA1,
optic atrophy 1. |
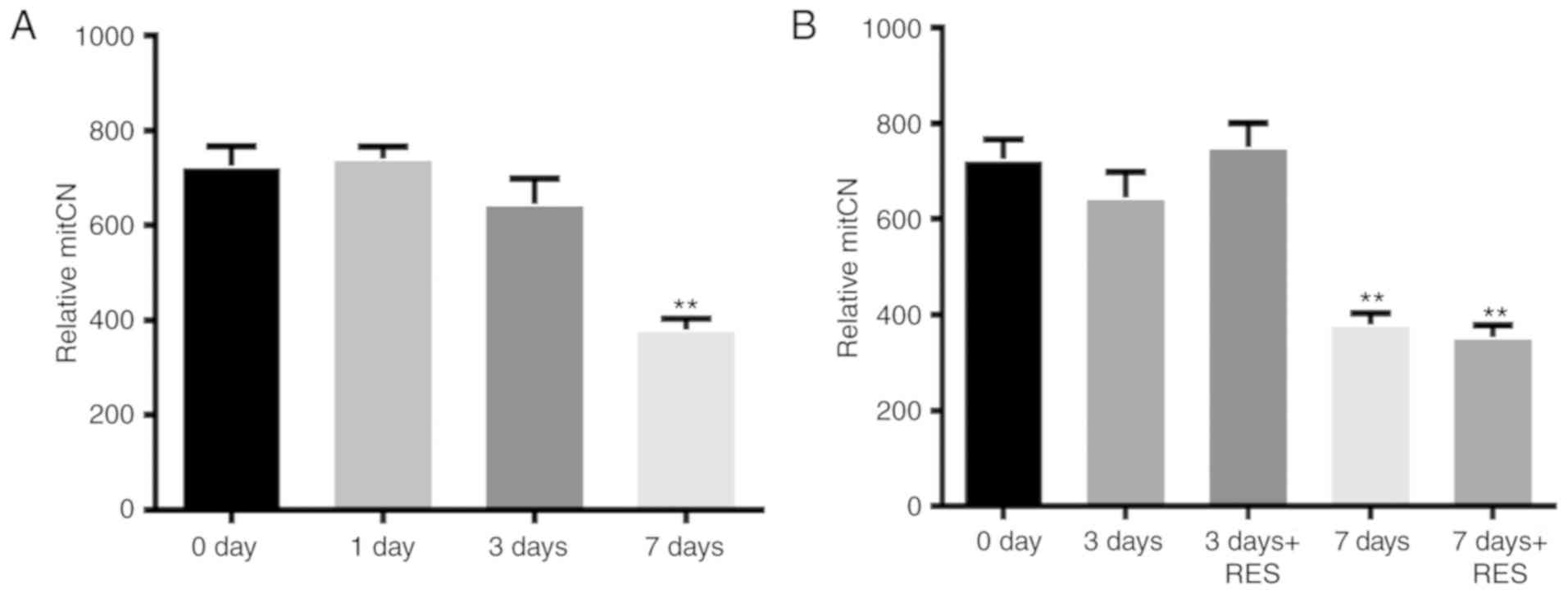
In addition, to investigate the effects of
resveratrol on mtDNA in the I/R model, mitCN was measured at the
selected time points (0, 1, 3 and 7 days). A significant reduction
(~50%) was observed in the mitCN in the retina 7 days after I/R
compared with that in the sham group (Fig. 5A). However, resveratrol treatment
did not reverse this change (Fig.
5B).
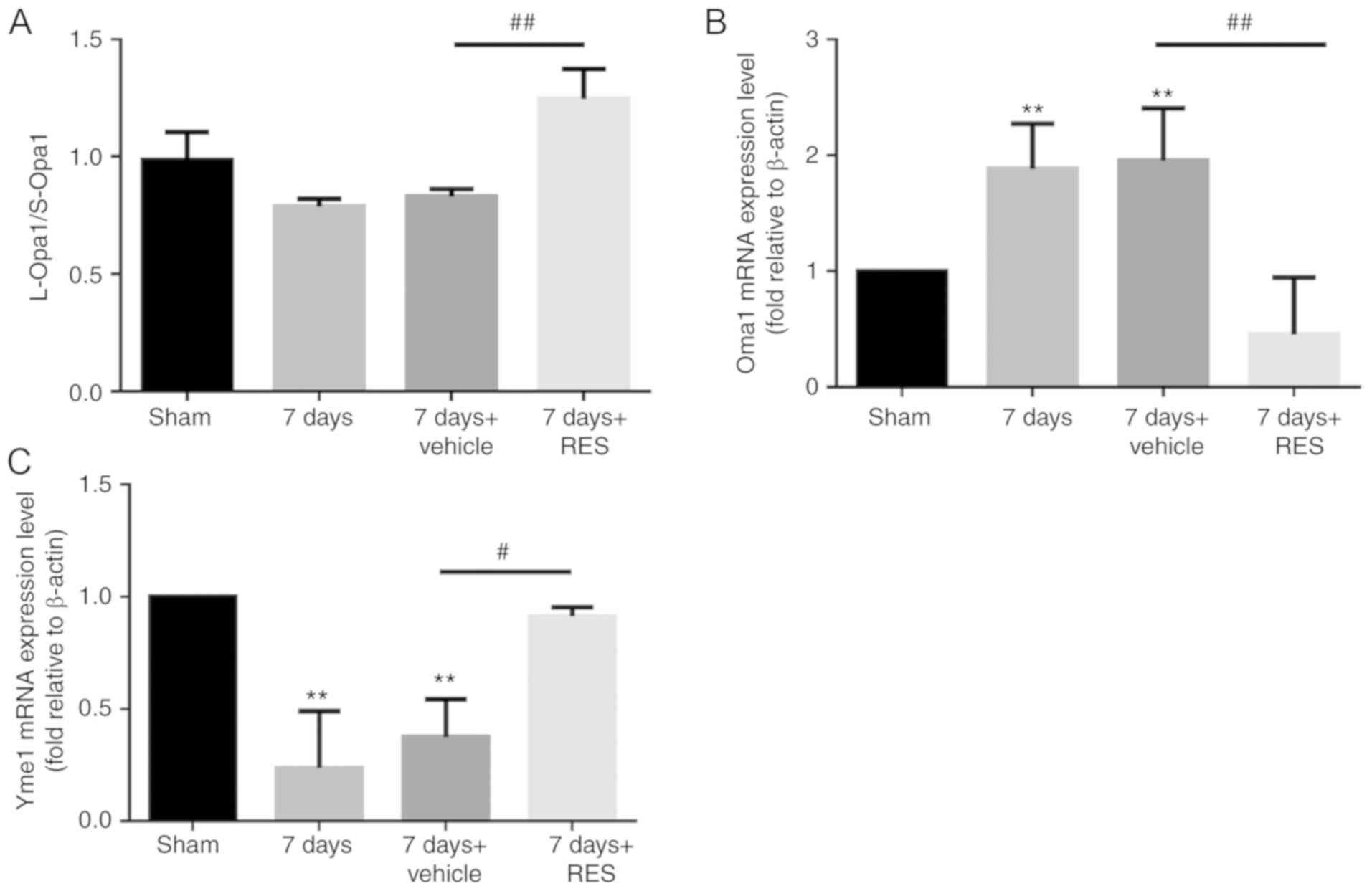
Resveratrol regulates the ratio of
L-Opa1/S-Opa1
In mammalian cells, strict regulation of the
L-Opa1/S-Opa1 ratio is required for a physiologically functioning
mitochondrial network. In the present study, it was found that
resveratrol increased the L-Opa1/S-Opa1 ratio (Fig. 6A). To investigate the detailed
underlying mechanism, RT-qPCR was performed to study the influence
of resveratrol on Oma1 and Yme1, two post-transcriptases that have
previously been reported to regulate Opa1 (29,30). The expression of Oma1 was found to
be increased 7 days after I/R, which was reversed by resveratrol
(Fig. 6B). The expression profile
of Yme1 expression was opposite to that of Oma1, with significant
reductions 7 days after I/R, and upregulation after resveratrol
treatment (Fig. 6C).
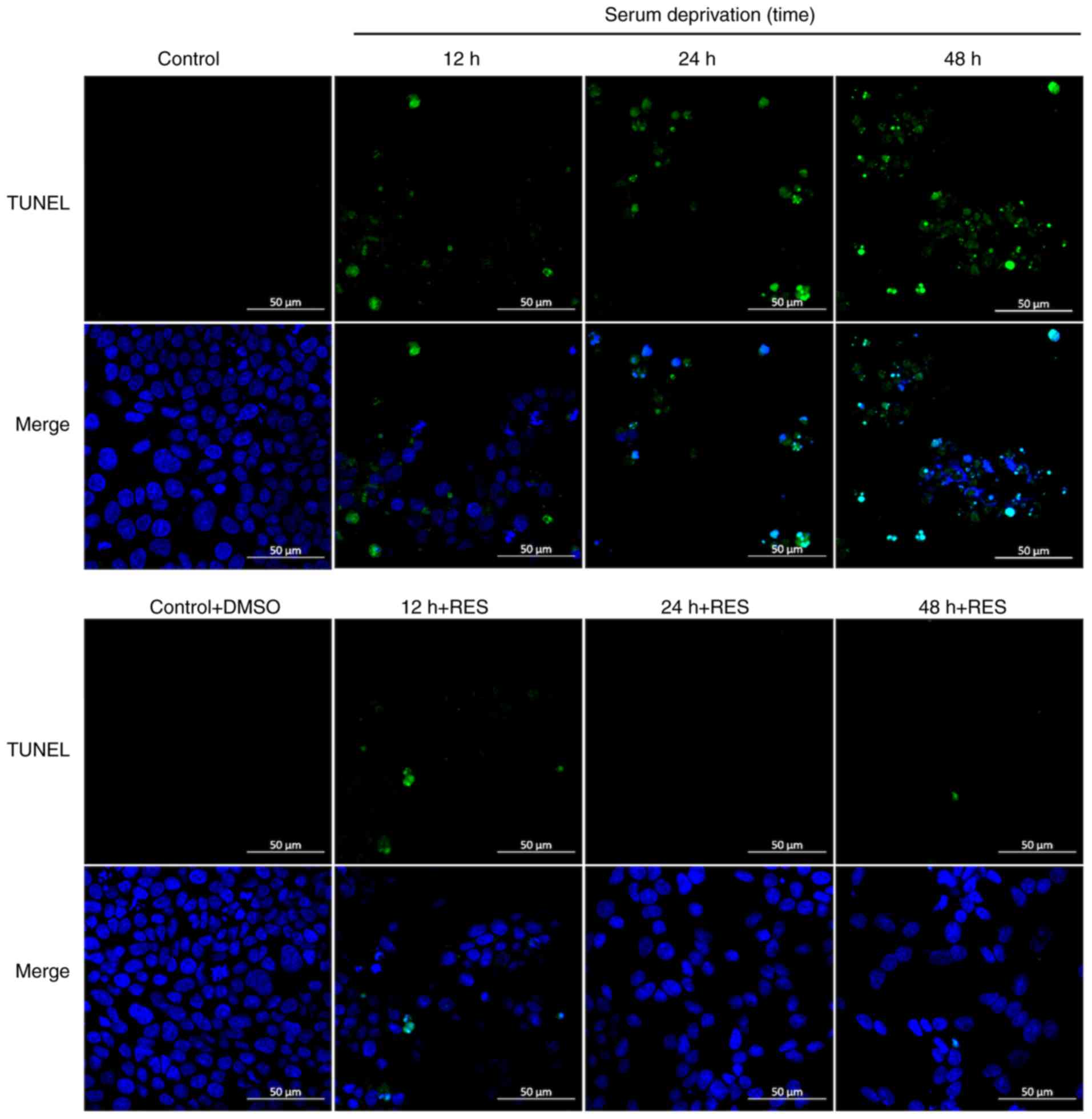
Effects of resveratrol on apoptosis,
activity and the vitality of SOD in R28 cells
The effects of resveratrol on apoptosis was next
explored using TUNEL staining 12, 24 and 48 h after serum
deprivation. Compared with those in the control and solvent control
group, a higher number of TUNEL-positive cells were observed in the
serum deprivation group (12, 24 and 48 h), suggesting that serum
deprivation can cause R28 cell apoptosis. Consistent with
observations in vivo, resveratrol treatment also reversed
this change (Fig. 7). The
viability of R28 cells was then determined using a CCK-8 assay
(Fig. 8A-C). Compared with that
in the control group, the viability of R28 cells was found to be
significantly reduced in the solvent control group (DMSO for 12, 24
and 48 h). Cell viability in cells 12 h after serum deprivation was
similar to that in the solvent control group. However, cells
exhibited lower cell viability compared with that in the solvent
control group 24 and 48 h after serum deprivation, suggesting that
the effect of serum deprivation on cell viability is
time-dependent. Resveratrol treatment was demonstrated to partially
reverse this reduction in cell viability. To investigate the
relationship between SOD activity and apoptosis, the activity of
SOD in R28 cells was also examined. After 24 h of serum
deprivation, the activity of SOD was found to be significantly
reduced, and this was attenuated by resveratrol treatment (Fig. 8D).
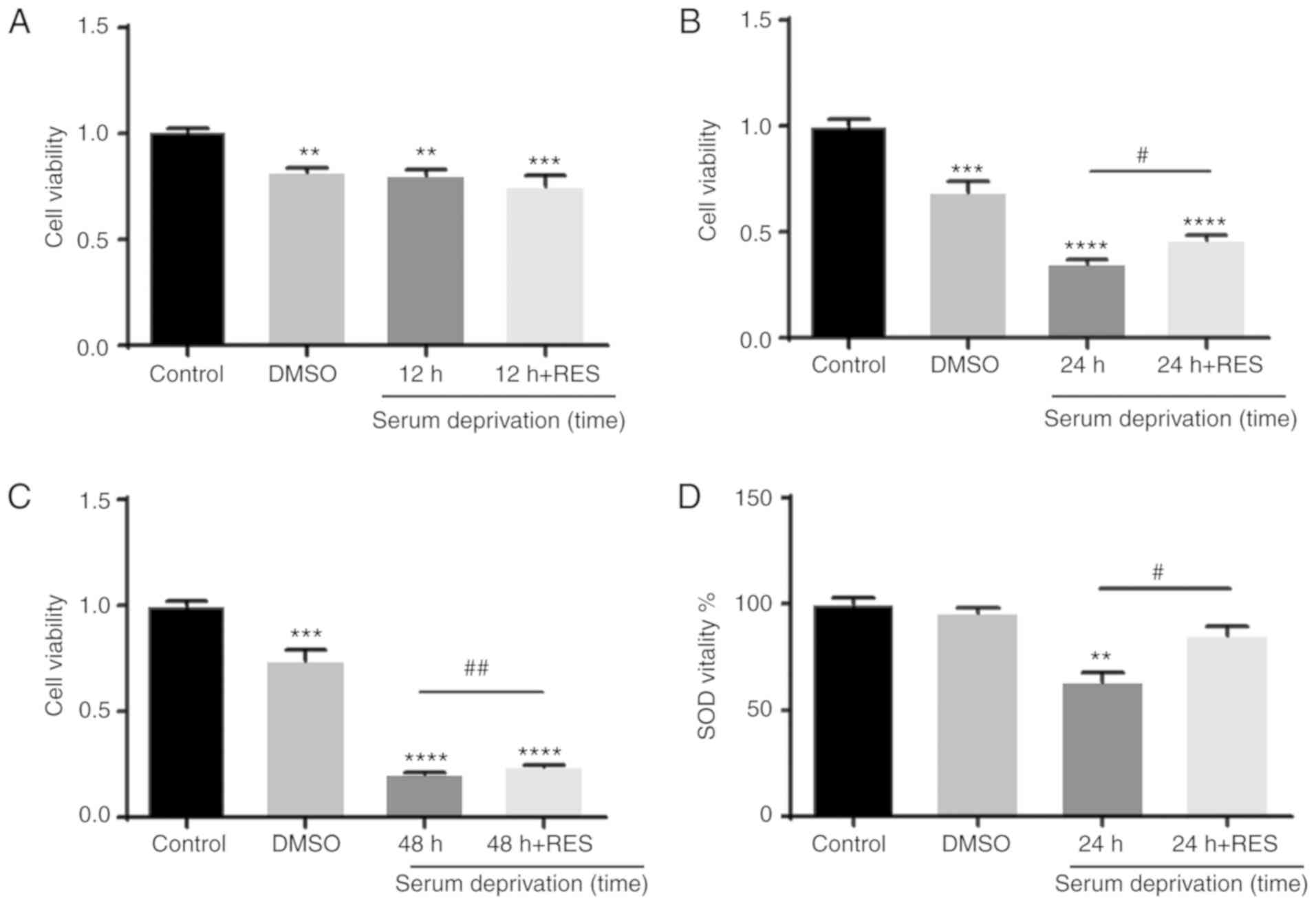 | Figure 8Resveratrol affects the activity of
SOD in R28 cells. The viability of R28 cells was detected using a
Cell Counting Kit-8 assay. (A) Compared with the control group, the
viability of R28 cells was significantly reduced in the solvent
control group (treated with DMSO for 12 h), a similar reduction was
also observed in the cells that had been serum deprived cells for
12 h. Compared with the cells that had been serum deprived cells
for 12 h, there was no significant increase in R28 cell viability
when treated with RES and serum deprived for 12 h.
**P<0.01, ***P<0.001 vs. control group.
(B) Cell viability of R28 cells was reduced in the solvent control
group (treated with DMSO for 24 h), a more significant reduction
was observed in the cells that had been serum deprived cells for 24
h. Compared to the cells that had been serum deprived for 24 h, RES
treatment partially restored the viability of R28 cells.
***P<0.001, ****P<0.0001 vs. control
group; #P<0.05 vs. 24 h of serum deprivation. (C)
Serum deprivation for 48 h resulted in similar results to that of
24 h serum deprivation. ***P<0.001,
****P<0.0001 vs. control group.
##P<0.01 vs. 48 h. (D) SOD activity in R28 cells was
detected using a SOD kit. SOD activity was significantly reduced
after 24 h of serum deprivation, and the decrease in activity was
attenuated by RES treatment. **P<0.01, vs. control
group; #P<0.05 vs. 24 h of serum deprivation. RES,
resveratrol; SOD, superoxide dismutase. |
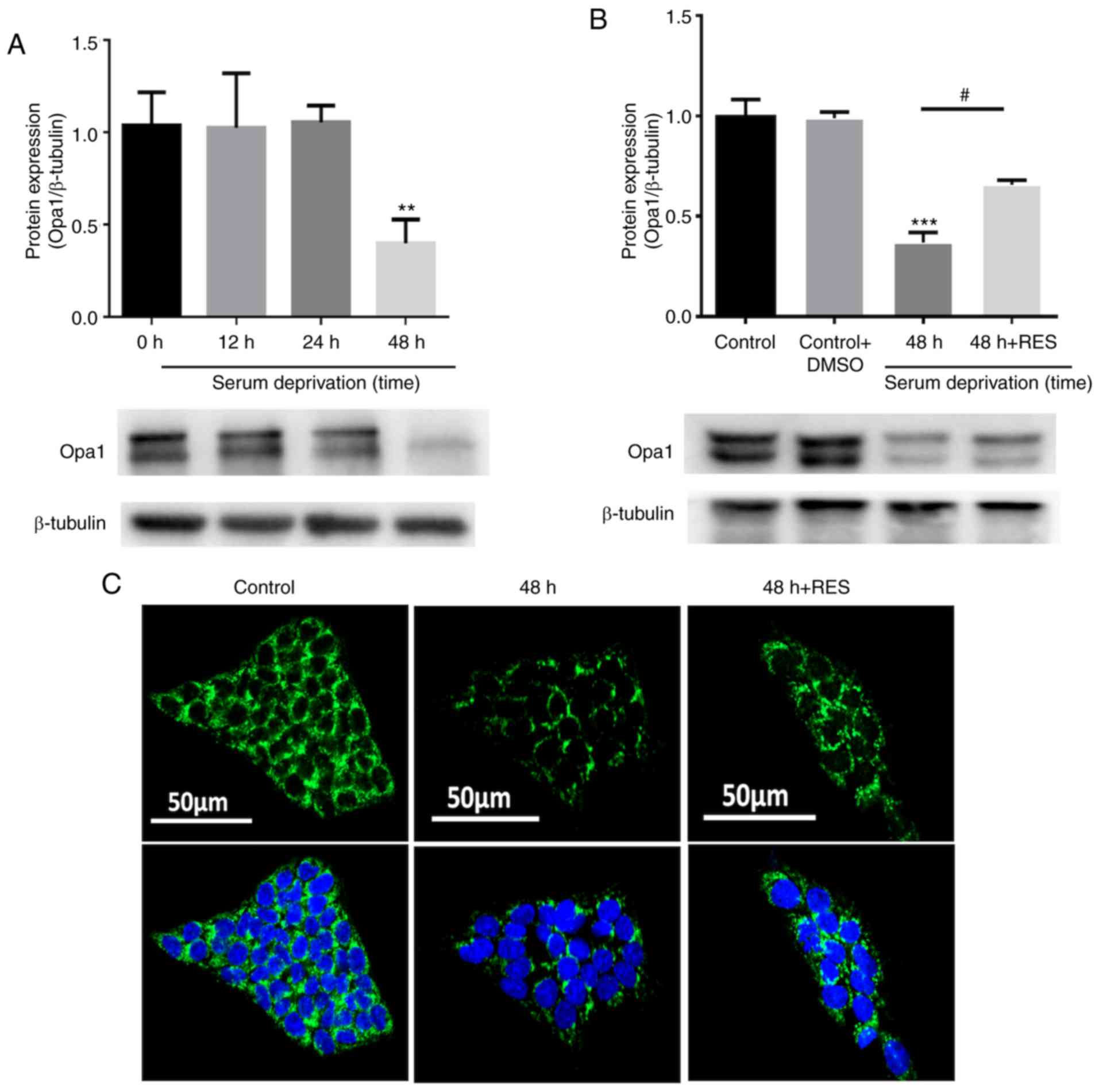
Effects of resveratrol on Opa1 expression
and localization in R28 cells
Compared with the control cells, Opa1 expression was
shown to be significantly reduced 24 and 48 h after serum
deprivation (Fig. 9A), which was
partially attenuated by resveratrol treatment (Fig. 9B). Immunostaining of R28 cells was
subsequently performed to determine the localization of Opa1. Opa1
was found to be expressed in the cytoplasm, but neither serum
deprivation nor resveratrol treatment resulted in a significant
alteration in Opa1 localization (Fig.
9C).
Discussion
Irreversible damage to neurons in the retina results
in deterioration of vision and serves a central role in a number of
optical diseases, including glaucoma, age-associated macular
degeneration and diabetic retinopathy (31). However, minimal success has been
achieved to halt or reverse the onset and development of these
types of vision loss by clinical interventions (32). Therefore, to explore potential
therapeutic targets for such diseases in the eye, particular
research attention should be paid to the underlying molecular
mechanism of retinal neuronal injury induction. In the present
study, the potential effects of resveratrol on RGC following I/R
injury and in vitro serum deprivation models were
investigated, with a particular focus on the effects of resveratrol
on the expression of Opa1 in vitro and in vivo.
Resveratrol treatment was found to inhibit retinal damage, RGC loss
and apoptosis following I/R injury and in in vitroserum
deprivation models. In addition, resveratrol also altered the
expression profiles of Oma1 and Yme1 at the mRNA level, two primary
proteins responsible for the post-transcriptional cleavage of Opa1,
thereby increasing the L-Opa1/S-Opa1 ratio. The present study also
showed that resveratrol partially reversed the reduction in Opa1
expression and the SOD activity caused by I/R. Thus, the results
suggest that resveratrol treatment can protect RGCs by increasing
Opa1 expression and regulating the expression of the
post-transcriptional proteases of Opa1 in the ischemic retina.
The present study contributes information on the
influence of resveratrol treatment on the ischemic retina.
Resveratrol has attracted significant attention due to its
previously reported neuroprotective, antitumorigenic and
antioxidant properties (33).
Zhang et al (34)
previously reported that resveratrol can alleviate retinal damage
and RGC-5 cell apoptosis by activating the 5'-AMP-activated protein
kinase/peroxisome proliferator-activated receptor γ-coactivator 1
signaling pathway in addition to restoring RGC malfunction. Lindsey
et al (35) also suggested
that long term dietary resveratrol supplements can alter the
expressional patterns of endoplasmic reticulum chaperones to
protect against RGC dendrite loss. In the present study, a retinal
I/R animal model was used, where increased IOP resulted in retinal
circulatory disturbances and increased levels of reactive oxygen
species and free radicals that attack cell structures and proteins
during reperfusion, resulting in significant tissue damage
(36,37). Results from the present study
suggested that resveratrol treatment significantly reduced retinal
damage, RGC loss and apoptosis in I/R injury models, consistent
with previous studies. Additionally, the same conclusions were
drawn from the in vivo experiments. R28 cells have been
previously used for in vitro and in vivo retinal cell
behavior experiments (38). Serum
deprivation was induced to measure the degree of apoptosis and
explore potential protective interventions (39). Serum deprivation-induced damage
has been reported to result from the lack of nutrients in the cell
culture (39-42). In the present study, it was found
that serum deprivation resulted in reduced R28 cell viability along
with reduced apoptosis. Resveratrol treatment significantly
improved the viability of R28 cells and partially prevented
apoptosis. In particular, two notable observations were made based
on the TUNEL staining in the retina. Although the levels of cell
apoptosis in the GCL and INL layers were reduced, apoptotic cells
could be observed in the ONL in I/R+RES group. Such multifaceted
effects of resveratrol in the different retinal layers may be
associated with the cellular distribution of the adenosine receptor
subtypes. Adenosine A2 receptors are expressed predominantly in the
GCL and INL (43), whilst
adenosine A3 receptors are primarily found in the ONL (44). Activated A2 receptors can mediate
the protective effects on ischemic neurons, whereas A3 receptors
have been previously documented to serve a noxious role in
triggering neuron apoptosis. Therefore, it is hypothesized that
resveratrol is a non-selective adenosine receptor agonist that can
induce apoptosis in the ONL whilst inhibiting this process in the
INL and GCL, although the detailed mechanism requires further study
(45-47). In addition, it is worth noting
that TUNEL staining in the retina was negative 7 days after I/R.
The possible cause of this may be that TUNEL stained the 3' end of
the cleaved DNA during apoptosis (48). Apoptosis is a gradual and phased
process, such that following progression to a certain stage,
apoptotic bodies are gradually engulfed by neighboring cells or
phagocytic cells in vivo(49).
Mitochondrial dysfunction is considered to be the
pivotal pathological change in the process of RGC loss during
glaucoma development, which has consistently been the primary focus
of research on its etiology (50). A number of proteins have been
reported to participate in maintaining mitochondrial function. Opa1
is well known for its role in the regulation of mitochondrial
dynamics, where it inhibits mitochondrial cristae remodeling and
cytochrome c release (13,51).
Cytochrome c released into the cytosol can recruit pro-apoptotic
signals and trigger downstream apoptotic events by binding between
the two tryptophan (W) and aspartate (D)-rich WD domains of the
apoptotic protease activating factor (Apaf-1), thereby irreversibly
activating the apoptotic cascade (52). Cristae morphogenesis, which is
induced by the reduction of Opa1 expression, may increase the
probability of cytochrome c release (53,54). In previous studies, Opa1 has been
reported to serve a neuroprotective role in the retina by
modulating mitochondrial function (55). Additionally, Opa1 has also been
found to be significantly downregulated in the retina of Wistar
rats following I/R injury (13).
Opa1 mutations have been proposed to result in RGC dendropathy,
increasing the susceptibility of RGCs to apoptosis and
vulnerability to oxidative stress (56). A substantial number of studies
focusing on the molecular mechanism by which Opa1 protects against
RGC loss have been performed. Hu et al (57) hypothesized that during
experimental glaucoma, Opa1 exerted a protective effect on RGCs by
promoting mitochondrial fusion and enhancing Parkin-mediated
mitophagy (57). This previous
study also suggested that the Opa-1-mediated downregulation of Bax,
a pro-apoptotic member of the Bcl-2 family, coupled with the
activation of Bcl-2, can alleviate ischemia-induced RGC loss
(57). In the present study, at
the selected I/R time points, the expression of the Opa1 protein
also exhibited a decreasing trend in the retina of SD rats.
Consistent with these findings, serum deprivation also resulted in
the reduction of Opa1 expression in R28 cells. These observations
support the notion that Opa1 may protect against RGC apoptosis.
Resveratrol has been previously reported to induce mitochondrial
biogenesis and improve mitochondrial function (58,59). In addition, it has also been shown
to significantly reverse rotenone-induced reductions in Opa1
protein and mRNA expression in PC12 cells (60). Similar results were obtained
following resveratrol treatment in the I/R retinal injury and serum
deprivation models used in the present study. These observations
suggest that resveratrol may confer therapeutic potential for the
treatment of RGC apoptosis by increasing Opa1 expression.
Results from the present study also provided
evidence that resveratrol can upregulate the L-Opa1/S-Opa1 ratio by
modulating the proteolytic processing of Opa1. In mammals, L-Opa1
is anchored onto the inner membrane, whereas S-Opa1 is soluble
(61). S-Opa1 is generated by the
cleavage of L-Opa1 in the transmembrane domain (61). Mitochondrial fusion is determined
by the tightly regulated L-Opa1/S-Opa1 ratio, where L-Opa1
facilitated mitochondrial inner membrane fusion and S-Opa1 promoted
mitochondrial fission. Excessive mitochondrial fission can induce
mitochondrion fragmentation, resulting in mitochondrial dysfunction
and cell death (62-66). Under physiological conditions,
Oma1 is degraded by sufficient ATP and Yme-1 serves a pivotal role
in promoting mitochondrial fusion whilst inhibiting apoptosis by
generating L-Opa1. By contrast, during conditions of high oxidative
stress, Oma1 can promote mitochondrial fragmentation by rapidly
converting L-Opa1 into S-Opa1, which triggers the ubiquitination of
proteins in the outer membrane to be removed by mitophagy (67-69). In the retina, 7 days after I/R
injury, the mRNA expression levels of Yme1 were found to be
significantly reduced, whilst that of Oma1 was found to be
increased, and this was reversed by resveratrol treatment. This
observation suggested that resveratrol administration may increase
the L-Opa1/S-Opa1 ratio by regulating the post-transcriptional
cleavage of Opa1 in the ischemic retina. To the best of our
knowledge, this was the first time that resveratrol was
demonstrated to influence the L-Opa1/S-Opa1 ratio in ischemic
retina. However, whether these changes in Opa1 post-transcriptional
cleavage are direct effects of resveratrol administration warrant
further investigation.
To assess whether resveratrol can effectively
improve mitochondrial quality, the mitCN in the retina was measured
using RT-qPCR. Accumulating evidence provided by previous
genotyping studies demonstrated that depletion of mtDNA, which is
one of the biomarkers of mitochondrial quality, can be detected in
patients with glaucoma (70,71). In addition, it has been previously
reported that Opa1 is pivotal in maintaining mitCNs and
mitochondrial morphology (72).
Supporting this, an increased number of mtDNA-deficient cells with
mitochondrial fragmentation is observed in organisms encoding the
mutated version of the Opa1 gene (73). The present study reported a ~50%
decrease in the mitCN within the retina 7 days after I/R,
consistent with the results from previous studies. However, no
notable changes were detected after resveratrol treatment, contrary
to that of Chen et al(74), who demonstrated that resveratrol
could maintain mitCNs in a serum deprivation model of RGCs. A
possible explanation for this striking difference in mitCNs
following resveratrol treatment in vivo and in vitro
may be that, compared with the cell culture model, the animal model
is considerably more complex as a number of uncontrollable factors
which can influence cell behavior and gene expression, such as
endogenous growth factors, should be taken into consideration. When
investigating the protective mechanisms of resveratrol on the
ischemic retina, monolayer culture models alone cannot be
considered as a reliable tool compared with animal studies
(75-77). Therefore, it is crucial that
further research into this specific mechanism is performed in
vivo.
In vitro data in the present study
demonstrated that resve-ratrol supplementation can effectively
reduce ROS production. Excessive production of reactive oxygen
species can also be regarded as the main cause of turbulence in
bioenergetic homeostasis and mitochondrial dysfunction. Oxidative
stress can lead to the suppression of pro-survival signaling and
pro-apoptotic signaling activation, in turn activating apoptosis
downstream of caspase activation (78). SOD is one of the ROS scavengers
that rapidly converts superoxide radicals into
H2O2 to efficiently prevent deteriorations in
mitochondrial function and apoptosis (79). It has been previously reported
that resveratrol can induce superoxide dismutase in the liver to
inhibit ethanol-induced oxidative stress (80). To initially investigate the
relationship among SOD activity, apoptosis and the regulatory role
of resveratrol in RGCs, SOD activity of R28 cells was first
assessed. After 24 h of serum deprivation, the activity of SOD was
found to be reduced, and this was attenuated by resveratrol
treatment.
These present findings may confer important
implications for the development of potential therapeutic
strategies against glaucomatous RGC loss. However, the present
study has several limitations. The underlying molecular pathway
between resveratrol treatment and Opa1 post-transcriptional
cleavage requires further elucidation. Additionally, in the present
study, resveratrol was injected intraperitoneally into the rats.
Since intraperitoneal injection of resveratrol is not a suitable
method for delivery into humans, an optimized method of resveratrol
administration should also be developed for its prospective
clinical application (81).
In summary, the present study found that treatment
with resveratrol exhibited protective effects on I/R-induced
retinal injury and serum deprivation-induced R28 cell apoptosis. In
addition, resveratrol has also been found to exert protective
effects on RGCs. Mechanistically, resvera-trol was shown to
regulate the expression of Opa1 and the activity of SOD. However,
it remains necessary to further investigate the relationship
between apoptosis, Opa1 and SOD activity to provide clearer
evidence of the protective effects of resveratrol on RGCs, in
particular in association with glaucoma.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81271425, 81860170 and
81260148) and the Natural Science Foundation of Jiangxi (grant no.
20181ACG70010).
Availability of data and materials
The data sets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YP, MQ, PH and XZ conceived the study. YP, MQ, PH,
KJ, RX, NS and XP performed the experiments. YP, MQ, PH and XZ
analyzed the data. YP, MQ and XZ drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed in accordance with
the ARVO Statement for the Use of Animals in Ophthalmic and Vision
Research and were approved and monitored by the Institutional
Animal Care and Use Committee of Nanchang University (approval no.
2016NC-020-02).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
We are grateful to Dr Lingfeng Li, Dr Hongdou Luo
and Dr Weiwei Sheng (Affiliated Eye Hospital of Nanchang
University, Jiangxi Research Institute of Ophthalmology and Visual
Science) for their guidance.
References
|
1
|
Bonomi L: Epidemiology of angle-closure
glaucoma. Acta Ophthalmol Scand Suppl. 236:11–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia-Valenzuela E, Shareef S, Walsh J
and Sharma SC: Programmed cell death of retinal ganglion cells
during experimental glaucoma. Exp Eye Res. 61:33–44. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quigley HA, Nickells RW, Kerrigan LA,
Pease ME, Thibault DJ and Zack DJ: Retinal ganglion cell death in
experimental glaucoma and after axotomy occurs by apoptosis. Invest
Ophthalmol Vis Sci. 36:774–786. 1995.PubMed/NCBI
|
|
4
|
Almasieh M, Wilson AM, Morquette B, Cueva
Vargas JL and Di Polo A: The molecular basis of retinal ganglion
cell death in glaucoma. Prog Retin Eye Res. 31:152–181. 2012.
View Article : Google Scholar
|
|
5
|
Williams PA, Harder JM, Foxworth NE,
Cochran KE, Philip VM, Porciatti V, Smithies O and John SW: Vitamin
B3 modulates mitochondrial vulnerability and prevents
glaucoma in aged mice. Science. 355:756–760. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang C, Zhang P, Wang W, Xu Y, Wang M,
Chen X and Dong X: Long-term blue light exposure induces RGC-5 cell
death in vitro: Involvement of mitochondria-dependent apoptosis,
oxidative stress, and MAPK signaling pathways. Apoptosis.
19:922–932. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Das A, Bell CM, Berlinicke CA,
Marsh-Armstrong N and Zack DJ: Programmed switch in the
mitochondrial degradation pathways during human retinal ganglion
cell differentiation from stem cells is critical for RGC survival.
Redox Biol. 34:1014652020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giacomello M, Pyakurel A, Glytsou C and
Scorrano L: The cell biology of mitochondrial membrane dynamics.
Nat Rev Mol Cell Biol. 21:204–224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee S, Van Bergen NJ, Kong GY,
Chrysostomou V, Waugh HS, O'Neill EC, Crowston JG and Trounce IA:
Mitochondrial dysfunction in glaucoma and emerging bioenergetic
therapies. Exp Eye Res. 93:204–212. 2011. View Article : Google Scholar
|
|
10
|
Schober MS, Chidlow G, Wood JP and Casson
RJ: Bioenergetic-based neuroprotection and glaucoma. Clin Exp
Ophthalmol. 36:377–385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dhingra A, Jayas R, Afshar P, Guberman M,
Maddaford G, Gerstein J, Lieberman B, Nepon H, Margulets V, Dhingra
R and Kirshenbaum LA: Ellagic acid antagonizes Bnip3-mediated
mitochondrial injury and necrotic cell death of cardiac myocytes.
Free Radic Biol Med. 112:411–422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geng J, Wei M, Yuan X, Liu Z, Wang X,
Zhang D, Luo L, Wu J, Guo W and Qin ZH: TIGAR regulates
mitochondrial functions through SIRT1-PGC1 α pathway and
translocation of TIGAR into mitochondria in skeletal muscle. FASEB
J. 33:6082–6098. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Y, Xue W, Song Z, Huang K and Zheng L:
Restoration of Opa1-long isoform inhibits retinal injury-induced
neurodegeneration. J Mol Med (Berl). 94:335–346. 2016. View Article : Google Scholar
|
|
14
|
Youle RJ and van der Bliek AM:
Mitochondrial fission, fusion, and stress. Science. 337:1062–1065.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Frezza C, Cipolat S, Martins de Brito O,
Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS,
Danial NN, De Strooper B and Scorrano L: OPA1 controls apoptotic
cristae remodeling independently from mitochondrial fusion. Cell.
126:177–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chong ZZ, Shang YC, Wang S and Maiese K:
SIRT1: new avenues of discovery for disorders of oxidative stress.
Expert Opin Ther Targets. 16:167–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmed T, Javed S, Javed S, Tariq A, Šamec
D, Tejada S, Nabavi SF, Braidy N and Nabavi SM: Resveratrol and
Alzheimer's disease: Mechanistic insights. Mol Neurobiol.
54:2622–2635. 2017. View Article : Google Scholar
|
|
19
|
Pandey AK, Bhattacharya P, Shukla SC, Paul
S and Patnaik R: Resveratrol inhibits matrix metalloproteinases to
attenuate neuronal damage in cerebral ischemia: A molecular docking
study exploring possible neuroprotection. Neural Regen Res.
10:568–575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Richard T, Pawlus AD, Iglésias ML, Pedrot
E, Waffo-Teguo P, Mérillon JM and Monti JP: Neuroprotective
properties of resveratrol and derivatives. Ann N Y Acad Sci.
1215:103–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abu-Amero KK, Kondkar AA and Chalam KV:
Resveratrol and ophthalmic diseases. Nutrients. 8:2002016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zuo L, Khan RS, Lee V, Dine K, Wu W and
Shindler KS: SIRT1 promotes RGC survival and delays loss of
function following optic nerve crush. Invest Ophthalmol Vis Sci.
54:5097–5102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krishnamoorthy RR, Agarwal P, Prasanna G,
Vopat K, Lambert W, Sheedlo HJ, Pang IH, Shade D, Wordinger RJ,
Yorio T, et al: Characterization of a transformed rat retinal
ganglion cell line. Brain Res Mol Brain Res. 86:1–12. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu B, Chen H, Johns TG and Neufeld AH:
Epidermal growth factor receptor activation: An upstream signal for
transition of quiescent astrocytes into reactive astrocytes after
neural injury. J Neurosci. 26:7532–7540. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bennet D and Kim S: Effects of agmatine
and resveratrol on RGC-5 cell behavior under light stimulation.
Environ Toxicol Pharmacol. 38:84–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Chintala SK, Zhang X, Austin JS and Fini
ME: Deficiency in matrix metalloproteinase gelatinase B (MMP-9)
protects against retinal ganglion cell death after optic nerve
ligation. J Biol Chem. 277:47461–47468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lagouge M, Argmann C, Gerhart-Hines Z,
Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P,
Elliott P, et al: Resveratrol improves mitochondrial function and
protects against metabolic disease by activating SIRT1 and
PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anderson CJ, Kahl A, Fruitman H, Qian L,
Zhou P, Manfredi G and Iadecola C: Prohibitin levels regulate OMA1
activity and turnover in neurons. Cell Death Differ. 27:1896–1906.
2020. View Article : Google Scholar
|
|
30
|
Griparic L, Kanazawa T and van der Bliek
AM: Regulation of the mitochondrial dynamin-like protein Opa1 by
proteolytic cleavage. J Cell Biol. 178:757–764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Osborne NN, Casson RJ, Wood JP, Chidlow G,
Graham M and Melena J: Retinal ischemia: Mechanisms of damage and
potential therapeutic strategies. Prog Retin Eye Res. 23:91–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao H, Zhang HL, Shou J, Chen L, Shen Y,
Tang Q, Huang J and Zhu J: Towards retinal ganglion cell
regeneration. Regen Med. 7:865–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Semba RD, Ferrucci L, Bartali B,
Urpí-Sarda M, Zamora-Ros R, Sun K, Cherubini A, Bandinelli S and
Andres-Lacueva C: Resveratrol levels and all-cause mortality in
older community-dwelling adults. JAMA Intern Med. 174:1077–1084.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Feng Y, Wang Y, Wang J, Xiang D,
Niu W and Yuan F: Resveratrol ameliorates disorders of
mitochondrial biogenesis and dynamics in a rat chronic ocular
hypertension model. Life Sci. 207:234–245. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lindsey JD, Duong-Polk KX, Hammond D,
Leung CK and Weinreb RN: Protection of injured retinal ganglion
cell dendrites and unfolded protein response resolution after
long-term dietary resveratrol. Neurobiol Aging. 36:1969–1981. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Szabo ME, Droy-Lefaix MT, Doly M and
Braquet P: Free radical-mediated effects in reperfusion injury: A
histologic study with superoxide dismutase and EGB 761 in rat
retina. Ophthalmic Res. 23:225–234. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Belforte N, Sande PH, de Zavalia N,
Fernandez DC, Silberman DM, Chianelli MS and Rosenstein RE:
Ischemic tolerance protects the rat retina from glaucomatous
damage. PLoS One. 6:e237632011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seigel GM: Review: R28 retinal precursor
cells: The first 20 years. Mol Vis. 20:301–306. 2014.PubMed/NCBI
|
|
39
|
Charles I, Khalyfa A, Kumar DM,
Krishnamoorthy RR, Roque RS, Cooper N and Agarwal N: Serum
deprivation induces apoptotic cell death of transformed rat retinal
ganglion cells via mitochondrial signaling pathways. Invest
Ophthalmol Vis Sci. 46:1330–1338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee SB, Kim JJ, Kim TW, Kim BS, Lee MS and
Yoo YD: Serum deprivation-induced reactive oxygen species
production is mediated by Romo1. Apoptosis. 15:204–218. 2010.
View Article : Google Scholar
|
|
41
|
Liu Q, Ju WK, Crowston JG, Xie F, Perry G,
Smith MA, Lindsey JD and Weinreb RN: Oxidative stress is an early
event in hydrostatic pressure induced retinal ganglion cell damage.
Invest Ophthalmol Vis Sci. 48:4580–4589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kang KD, Andrade da Costa BL and Osborne
NN: Stimulation of prostaglandin EP2 receptors on RGC-5 cells in
culture blunts the negative effect of serum withdrawal. Neurochem
Res. 35:820–829. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kvanta A, Seregard S, Sejersen S, Kull B
and Fredholm BB: Localization of adenosine receptor messenger. RNAs
in the rat eye Exp Eye Res. 65:595–602. 1997. View Article : Google Scholar
|
|
44
|
Grillo SL, McDevitt DS, Voas MG, Khan AS,
Grillo MA and Stella SJ Jr: Adenosine receptor expression in the
adult zebrafish retina. Purinergic Signal. 15:327–342. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Castillo A, Tolón MR, Fernández-Ruiz J,
Romero J and Martinez-Orgado J: The neuroprotective effect of
cannabidiol in an in vitro model of newborn hypoxic-ischemic brain
damage in mice is mediated by CB(2) and adenosine receptors.
Neurobiol Dis. 37:434–440. 2010. View Article : Google Scholar
|
|
46
|
Dai SS, Zhou YG, Li W, An JH, Li P, Yang
N, Chen XY, Xiong RP, Liu P, Zhao Y, et al: Local glutamate level
dictates adenosine A2A receptor regulation of neuroinflammation and
traumatic brain injury. J Neurosci. 30:5802–5810. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Von Lubitz DK, Lin RC, Popik P, Carter MF
and Jacobson KA: Adenosine A3 receptor stimulation and cerebral
ischemia. Eur J Pharmacol. 263:59–67. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kressel M and Groscurth P: Distinction of
apoptotic and necrotic cell death by in situ labelling of
fragmented DNA. Cell Tissue Res. 278:549–556. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hass DT and Barnstable CJ: Mitochondrial
uncoupling protein 2 knock-out promotes mitophagy to decrease
retinal ganglion cell death in a mouse model of glaucoma. J
Neurosci. 39:3582–3596. 2019.PubMed/NCBI
|
|
51
|
Olichon A, Guillou E, Delettre C, Landes
T, Arnauné-Pelloquin L, Emorine LJ, Mils V, Daloyau M, Hamel C,
Amati-Bonneau P, et al: Mitochondrial dynamics and disease OPA1.
Biochim Biophys Acta. 1763:500–509. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shalaeva DN, Dibrova DV, Galperin MY and
Mulkidjanian AY: Modeling of interaction between cytochrome c and
the WD domains of Apaf-1: Bifurcated salt bridges underlying
apopto-some assembly. Biol Direct. 10:292015. View Article : Google Scholar
|
|
53
|
Olichon A, Baricault L, Gas N, Guillou E,
Valette A, Belenguer P and Lenaers G: Loss of OPA1 perturbates the
mitochondrial inner membrane structure and integrity, leading to
cytochrome c release and apoptosis. J Biol Chem. 278:7743–7746.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Varanita T, Soriano ME, Romanello V,
Zaglia T, Quintana-Cabrera R, Semenzato M, Menabò R, Costa V,
Civiletto G, Pesce P, et al: The OPA1-dependent mitochondrial
cristae remodeling pathway controls atrophic, apoptotic, and
ischemic tissue damage. Cell Metab. 21:834–844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang L, He Z, Zhang Q, Wu Y, Yang X, Niu
W, Hu Y and Jia J: Exercise pretreatment promotes mitochondrial
dynamic protein OPA1 expression after cerebral ischemia in rats.
Int J Mol Sci. 15:4453–4463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Williams PA, Morgan JE and Votruba M: Opa1
deficiency in a mouse model of dominant optic atrophy leads to
retinal ganglion cell. dendropathy Brain. 133:2942–2951. 2010.
View Article : Google Scholar
|
|
57
|
Hu X, Dai Y, Zhang R, Shang K and Sun X:
Overexpression of optic atrophy type 1 protects retinal ganglion
cells and upregu-lates Parkin expression in experimental glaucoma.
Front Mol Neurosci. 11:3502018. View Article : Google Scholar
|
|
58
|
Jardim FR, de Rossi FT, Nascimento MX, da
Silva Barros RG, Borges PA, Prescilio IC and de Oliveira MR:
Resveratrol and brain mitochondria:. A review Mol Neurobiol.
55:2085–2101. 2018. View Article : Google Scholar
|
|
59
|
de Oliveira MR, Nabavi SF, Manayi A,
Daglia M, Hajheydari Z and Nabavi SM: Resveratrol and the
mitochondria: From triggering the intrinsic apoptotic pathway to
inducing mitochondrial biogenesis, a mechanistic view. Biochim
Biophys Acta. 1860:727–745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Peng K, Tao Y, Zhang J, Wang J, Ye F, Dan
G, Zhao Y, Cai Y, Zhao J, Wu Q, et al: Resveratrol regulates
mitochondrial biogenesis and fission/fusion to attenuate
rotenone-induced neurotoxicity. Oxid Med Cell Longev.
2016:67056212016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee H, Smith SB, Sheu SS and Yoon Y: The
short variant of optic atrophy 1 (OPA1) improves cell survival
under oxidative stress. J Biol Chem. 295:6543–6560. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
MacVicar T and Langer T: OPA1 processing
in cell death and disease-the long and short of it. J Cell Sci.
129:2297–2306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lang A, Anand R, Altinoluk-Hambüchen S,
Ezzahoini H, Stefanski A, Iram A, Bergmann L, Urbach J, Böhler P,
Hänsel J, et al: SIRT4 interacts with OPA1 and regulates
mitochondrial quality control and mitophagy. Aging (Albany NY).
9:2163–2189. 2017. View Article : Google Scholar
|
|
64
|
Alavi MV: Targeted OMA1 therapies for
cancer. Int J Cancer. 145:2330–2341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ishihara N, Fujita Y, Oka T and Mihara K:
Regulation of mitochondrial morphology through proteolytic cleavage
of OPA1. EMBO J. 25:2966–2977. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Song Z, Chen H, Fiket M, Alexander C and
Chan DC: OPA1 processing controls mitochondrial fusion and is
regulated by mRNA splicing, membrane potential, and Yme1L. J Cell
Biol. 178:749–755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Rainbolt TK, Lebeau J, Puchades C and
Wiseman RL: Reciprocal degradation of YME1L and OMA1 adapts
mitochondrial proteo-lytic activity during stress. Cell Rep.
14:2041–2049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Stiburek L, Cesnekova J, Kostkova O,
Fornuskova D, Vinsova K, Wenchich L, Houstek J and Zeman J: YME1L
controls the accumulation of respiratory chain subunits and is
required for apoptotic resistance, cristae morphogenesis, and cell
proliferation. Mol Biol Cell. 23:1010–1023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rainbolt TK, Saunders JM and Wiseman RL:
YME1L degradation reduces mitochondrial proteolytic capacity during
oxidative stress. EMBO Rep. 16:97–106. 2015. View Article : Google Scholar :
|
|
70
|
Singh LN, Crowston JG, Lopez Sanchez MIG,
Van Bergen NJ, Kearns LS, Hewitt AW, Yazar S, Mackey DA, Wallace DC
and Trounce IA: Mitochondrial DNA variation and disease
susceptibility in primary open-angle glaucoma. Invest Ophthalmol
Vis Sci. 59:4598–4602. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kumar M, Tanwar M, Faiq MA, Pani J, Shamsi
MB, Dada T and Dada R: Mitochondrial DNA nucleotide changes in
primary congenital glaucoma patients. Mol Vis. 19:220–230.
2013.PubMed/NCBI
|
|
72
|
Garcia I, Innis-Whitehouse W, Lopez A,
Keniry M and Gilkerson R: Oxidative insults disrupt OPA1-mediated
mitochondrial dynamics in cultured mammalian cells. Redox Rep.
23:160–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kondadi AK, Anand R and Reichert AS:
Functional interplay between cristae biogenesis, mitochondrial
dynamics and mitochondrial DNA integrity. Int J Mol Sci.
20:43112019. View Article : Google Scholar :
|
|
74
|
Chen S, Fan Q, Li A, Liao D, Ge J, Laties
AM and Zhang X: Dynamic mobilization of PGC-1α mediates
mitochondrial biogenesis for the protection of RGC-5 cells by
resveratrol during serum deprivation. Apoptosis. 18:786–799. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim MJ, Chi BH, Yoo JJ, Ju YM, Whang YM
and Chang IH: Structure establishment of three-dimensional (3D)
cell culture printing model for bladder cancer. PLoS One.
14:e2236892019.
|
|
76
|
Liang T, Tao Q, Guan R, Cao G, Shen H, Liu
Z and Xia Q: Antioxidant and antiproliferative activities of
cyanidin-3-O-glu-coside (C3G) liposome in Caco-2 cells cultivated
in 2D and 3D cell culture models. J Food Sci. 84:1638–1645. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Burd A, Kwok CH, Hung SC, Chan HS, Gu H,
Lam WK and Huang L: A comparative study of the cytotoxicity of
silver-based dressings in monolayer cell, tissue explant, and
animal models. Wound Repair Regen. 15:94–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li D, Ni S, Miao KS and Zhuang C: PI3K/Akt
and caspase pathways mediate oxidative stress-induced chondrocyte
apoptosis. Cell Stress Chaperones. 24:195–202. 2019. View Article : Google Scholar :
|
|
79
|
Fukai T and Ushio-Fukai M: Superoxide
dismutases: Role in redox signaling, vascular function, and
diseases. Antioxid Redox Signal. 15:1583–1606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen WM, Shaw LH, Chang PJ, Tung SY, Chang
TS, Shen CH, Hsieh YY and Wei KL: Hepatoprotective effect of
resveratrol against ethanol-induced oxidative stress through
induction of superoxide dismutase in vivo and in vitro. Exp Ther
Med. 11:1231–1238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tomé-Carneiro J, Larrosa M,
González-Sarrías A, Tomás-Barberán FA, García-Conesa MT and Espín
JC: Resveratrol and clinical trials: The crossroad from in vitro
studies to human evidence. Curr Pharm Des. 19:6064–6093. 2013.
View Article : Google Scholar : PubMed/NCBI
|