Introduction
The ovaries are known to be unique and crucial
female organs which reproduce generations of eggs and secrete sex
hormones for the female secondary sexual characteristics. However,
the number of ovarian follicles secreted during a lifetime is
limited. Follicles consist of several cells, including oocytes,
granulosa cells and theca cells. The oocyte is surrounded by layers
of granulosa cells and forms a direct connection with granulosa
cells. It has been suggested that granulosa cells not only provide
nutrient to oocytes, but also protect oocytes from oxidative stress
during follicle development (1,2),
and that reactive oxygen species (ROS)-mediated oxidative stress
results in granulosa cell apoptosis to promote abnormal follicular
development (3).
It has been reported that sirtuin 1 (SIRT1), a
well-known NAD+ dependent deacetylase (class III histone
deacetylase), influences not only histone proteins, but also
non-histone proteins, and regulates various biological processes
involving aging, apoptosis or the stress response (4,5).
In addition, SIRT1 is known to induce the phosphorylation of
extracellular signal-regulated kinase (ERK) and inhibit the
translocation of nuclear factor (NF)-κB into the nucleus, leading
to suppression of granulosa cell apoptosis (6). It has been shown that various
microRNAs (miRNAs or miRs) such as miRNA-590-3P or miRNA-494
suppress SIRT1 activity to promote apoptosis (7,8).
It has been suggested that SIRT1 deacetylates lysine 382 of p53,
thereby reducing p53 transcriptional activity and p53-mediated
apoptosis, whereas p53 expression suppresses the transcription of
SIRT1 gene induced by c-Myc (9-11).
T-LAK cell originated protein kinase (TOPK), a type
of serine/threonine kinase, has been shown to be strongly expressed
in various solid cancers, including colorectal cancer (12-14), lung cancer (15,16), gastric cancer (17), prostate cancer (18,19), ovarian carcinoma (20), nasopharyngeal carcinoma (21) and esophageal squamous cell
carcinoma (22). TOPK consists of
322 amino acids and has a functional similarity with MKK3/6, which
activates p38 (23,24). However, it has been reported that
TOPK phosphorylates ERK in EGF-stimulated HCT116 colorectal cancer
cells or T47D cells (25,26) and phosphorylates JNK1 in
ras-induced cell transformation or UVB-mediated signaling (27). In addition, TOPK has been shown to
contribute to an increased metastasis of breast cancer cells by
modulating the expression of MMP9 through the regulation of NF-κB
activity in LPS signaling (28).
On the other hand, it has been reported that TOPK directly binds to
the DNA-binding domain of p53 and suppresses p53 transcriptional
activity (29,30). However, whether TOPK affects
oxidative stress-induced granulosa cell apoptosis remains to be
determined.
In the present study, it was first revealed that
TOPK inhibition increased p53-mediated granulosa cell apoptosis
through SIRT1 downregulation upon H2O2
treatment. It was demonstrated that p53 negatively regulated SIRT1
transcriptional activity in response to H2O2,
which was aggravated by TOPK inhibition. It was also found that
TOPK inhibition promoted p53 stability, leading to an increase in
H2O2-induced granulosa cell apoptosis. These
findings provide evidence that TOPK suppresses
H2O2-induced granulosa cell apoptosis through
the regulation of the p53/SIRT1 axis.
Materials and methods
Cell culture and reagents
Human granulosa COV434 cells were purchased from
Sigma-Aldrich; Merck KGaA. HeLa cells were purchased from the
American Type Culture Collection (ATCC) and maintained in DMEM
supplemented with 10% FBS, 2 mM L-glutamine, and 1% penicillin and
streptomycin (Thermo Fisher Scientific, Inc.). COV434 cells were
maintained in high glucose Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine,
1% penicillin and streptomycin at 37°C in a CO2
incubator with 5% CO2. Rabbit anti-TOPK (ab75987),
rabbit anti-SIRT1 (ab32441), mouse anti-p53 (ab26), rabbit
anti-phospho-p53 (Ser-15) (ab1431) and rabbit anti-acetyl p53
(K382) (ab75754) antibodies were purchased from Abcam. Rabbit
anti-cleaved poly(ADP-ribose) polymerase (PARP) antibody (#9541)
was purchased from Cell Signaling Technology, Inc. Mouse
anti-β-actin antibody (A1975) was purchased from Sigma-Aldrich.
OTS514, Z-VAD-FMK were purchased from Selleckchem;
2′,7′-dichloroflurescin diacetate, Nutlin-3, Pifithrin-μ,
resveratrol and Ex527 were purchased Sigma-Aldrich; Merck KGaA.
Annexin V-FITC antibody and propidium iodide were purchased from
Thermo Fisher Scientific, Inc. The luciferase assay kit was
purchased from Promega Corporation.
Plasmids and transfection
The SIRT1 promoter linked luciferase reporter gene,
SIRT1-Luc construct was generated. Briefly, an approximately 1 kb
promoter region containing nucleotides -1 to -1,026 of the human
SIRT1 gene was amplified using the Takara Ex Taq polymerase (Takara
Bio, Inc.) and human genomic DNA (Promega Corporation) as a
template. The amplified product was cloned into the NheI and
XhoI sites of the pGL3-basic vector (cat. no. E1751, Promega
Corporation). The specific primer sequences were as follows:
Forward, 5′-CCGGCTAACCCATACTAGGCTTAAGG-3′ and reverse,
5′-CCGCTCGAGCTTCCAACTGCCTCTCTGGC-3′. p53-GFP was a gift from Geoff
Wahl (Addgene plasmid #11770). The pGL2-p53 promoter-Luc construct
was a gift from Wafik El-Deiry (Addgene plasmid #16292) and the
pGL2-p21 promoter-Luc plasmid was a gift from Martin Walsh (addgene
plasmid #33021). The pcDNA3.1 or pEGFP-N1 vector was purchased from
Invitrogen; Thermo Fisher Scientific, Inc. (cat. no. V79020) or
Clontech (cat. no. 6085-1), respectively, and the V5-TOPK construct
was previously described (31).
Briefly, total RNA from HeLa cells was prepared, and cDNA synthesis
and PCR were performed using Superscript III reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) and primers,
5′-CGCGGATCCCGATGGAAGGGATCAGTAATTT-3′ harboring the BamHI
site (forward) and 5′-CGCTCGAGCGGGACATCTGTTTCCAGAGCTT-3′ harboring
the XhoI site (reverse). The PCR product digested with
BamHI/XhoI was inserted into the
BamHI/XhoI sites of pcDNA6/V5-His ABC (cat. no.
V22020, Invitrogen; Thermo Fisher Scientific, Inc.), generating the
V5-TOPK construct. Non-target siRNA and p53 siRNA were purchased
from Dharmacon. COV434 cells growing in 12-well plates were
transfected with 1 µg of pcDNA3.1 or V5-TOPK, 2 µg of
pGL3-SIRT1 promoter-Luc, 1 µg of pEGFP-N1, pGL2-p53
promoter-Luc or pGL2-p21 promoter-Luc plasmids or 30 nM of
non-target or p53 siRNA using Lipofectamine 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to manufacturer's
instructions. At 24 h following transfection, cells were lysed, and
luciferase assay was performed using cell lysate.
Flow cytometric analysis
A total of 1×106 of COV434 cells growing
on 60 mm dishes were pre-treated with inhibitors or activators,
OTS514 (20 nM), Z-VAD-FMK (50 µM), resveratrol (20
µM), Ex527 (30 nM), Pifithrin-μ (1 µM), or Nutlin-3
(10 µM) for 2 h and H2O2 (0.1 mM) was
then added for 24 h. Apoptosis analysis was performed using Annexin
V-FITC and propidium iodide according to manufacturer's
instructions (Thermo Fisher Scientific, Inc.), and data were
acquired using a FACScalibur (BD Biosciences).
2′,7′-Dichloroflurescin diacetate
assay
COV434 cells growing on 60 mm dishes at 80%
confluency were pre-treated with OTS514 (20 nM) for 2 h, and
incubated at 37°C with H2O2 (0.1 mM) for 24
h. The conditioned medium was removed, and fresh medium containing
10 µM H2DCF-DA was added. The cells were then incubated at
37°C for 30 min, and then washed and trypsinized. Cells were
resuspended using conditioned medium. DCF fluorescence was measured
using a FACScalibur (BD Biosciences).
TUNEL assay
A total of 2×106 of COV434 cells were
seeded in 6-well plates and pre-treated with inhibitor or
activator, OTS514 (20 nM), Z-VAD-FMK (50 µM), resveratrol
(20 µM), Ex527 (30 nM), Pifithrin-µ (1 µM), or
Nutlin-3 (10 µM), for 2 h, and then
H2O2 (0.1 mM) was added for 24 h. Apoptosis
was determined using the DeadEnd™ Fluorometric TUNEL System (G3250,
Promega Corporation). The cells were fixed using 4% formaldehyde
for 25 min at 4°C, and then permeabi-lized by 0.2% Triton X-100 for
5 min at room temperature. The cells were labeled using 50
µl of TdT reaction mix and incubated for 60 min at 37°C and
then data analysis was performed using a laser-scanning confocal
microscope Zeiss LSM 710 (Zeiss AG).
Western blot analysis
Briefly, cells were harvested and lysed using lysis
buffer containing 0.5% Triton X-100, 1 mM EDTA, 50 mM Tris-HCl, pH
7.4 and 40 mM NaCl. Briefly, 30 µg of cell lysate was
separated on 8 or 10% SDS-PAGE and transferred to nitrocellulose
membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked
with 5% skim milk in Tris-buffered saline and Tween-20 (TBST) for 1
h and then incubated with anti-TOPK (1:5×106 in 5% Skim
milk), p53 (1:1,000 in TBST), phospho-p53 (1:1,000 in TBST),
acetyl-p53 (1:1,000 in 5% Skim milk), cleaved PARP (1:1,000 in
TBST), SIRT1 (1:1,000 in TBST) and β-actin (1: 20,000 in 5% Skim
milk) antibodies at 4°C for overnight. The membranes were washed 3
times for 5 min using TBST, and then incubated at room temperature
with goat anti-rabbi IgG, polyclonal (1:4,000 in 5% Skim milk,
ADI-SAB-300-J, Enzo Life Sciences) or goat anti-Mouse polyclonal
(1:4,000 in 5% Skim milk, ADI-SAB-100-J, Enzo Life Sciences)
antibodies for 2 h. After washing, the blots were analyzed with
SuperSignal West Pico chemiluminescent substrate (Pierce; Thermo
Fisher Scientific, Inc.) and X-ray film. Each protein level was
determined using Image J software (ver.1.52a; National Institute of
Health).
Luciferase assay
A total of 1×105 of COV434 cells were
seeded in 12-well plates. After 24 h, 1 µg of SIRT1, p53 or
p21 promoter-luciferase reporter constructs plus 0.5 µg of
the pRL-SV40 gene were transfected into the COV434 cells
using Lipofectamine 3000 reagent. At 24 h following transfection,
the cells were pre-treated with OTS514 (20 nM), resveratrol (20
µM), Ex527 (30 nM), Pifithrin-µ (1 µM) or Nutlin-3
(10 µM) for 2 h, and then incubated at 37°C with 0.1 mM
H2O2 for 24 h. Cells were harvested and
lysed. Firefly and Renilla luciferase activities were
measured using Dual-Luciferase® Reporter assay system
(E1910, Promega Corporation).
Protein stability assay
COV434 cells were seeded in 6-well plates. After 24
h, the cells were pre-treated with OTS514 (20 nM) for 2 h, and then
treated with H2O2 (0.1 mM) for 24 h at 37°C.
24 h after H2O2 treatment, cells were
incubated with cycloheximide (10 µg/ml) (Sigma-Aldrich) for
0, 2, or 8 h, and then were harvested, lysed and subjected to
western blot analysis.
Statistical analysis
Results are presented as the means ± standard
deviation (SD) for at least 3 independent experiments in duplicate.
Statistical analysis was carried out by one-way ANOVA with Tukey's
test or two-way ANOVA followed by Bonferroni's correction. P-values
<0.05 were considered to indicate statistically significant
differences.
Results
TOPK inhibition increases
H2O2-induced human COV434 granulosa cell
apoptosis
Previous studies have demonstrated that oxidative
stress induces granulosa cell apoptosis and SIRT1 downregulation
mediated by oxidative stress contributes to this apoptosis
(32-34). In addition, it has been suggested
that TOPK functions as a survival effector in several cancer cells
(35-37). Based on these findings, the
present study wished to determine whether TOPK inhibition affects
H2O2-induced granulosa cell apoptosis or
SIRT1 expression. COV434 cells were treated with
H2O2, OTS514 or H2O2
plus OTS514 for 24 h. Treatment of human COV434 granulosa cells
with the TOPK inhibitor, OTS514, increased the number of
TUNEL-positive cells induced by H2O2
stimulation (Fig. 1A), and
significantly elevated the H2O2-induced
cleavage of PARP (Fig. 1B). Of
note, OTS514 treatment dose-dependently decreased TOPK expression
upregulated by H2O2 and abolished the
expression of SIRT1 downregulated by H2O2.
Furthermore, the total p53 level, as well as its phosphorylation
and acetylation were markedly increased upon
H2O2 stimulation, and these effects were
enhanced by OTS514 co-treatment. These data suggest that TOPK plays
an important role in the regulation of p53 or SIRT1 expression in
response to H2O2.
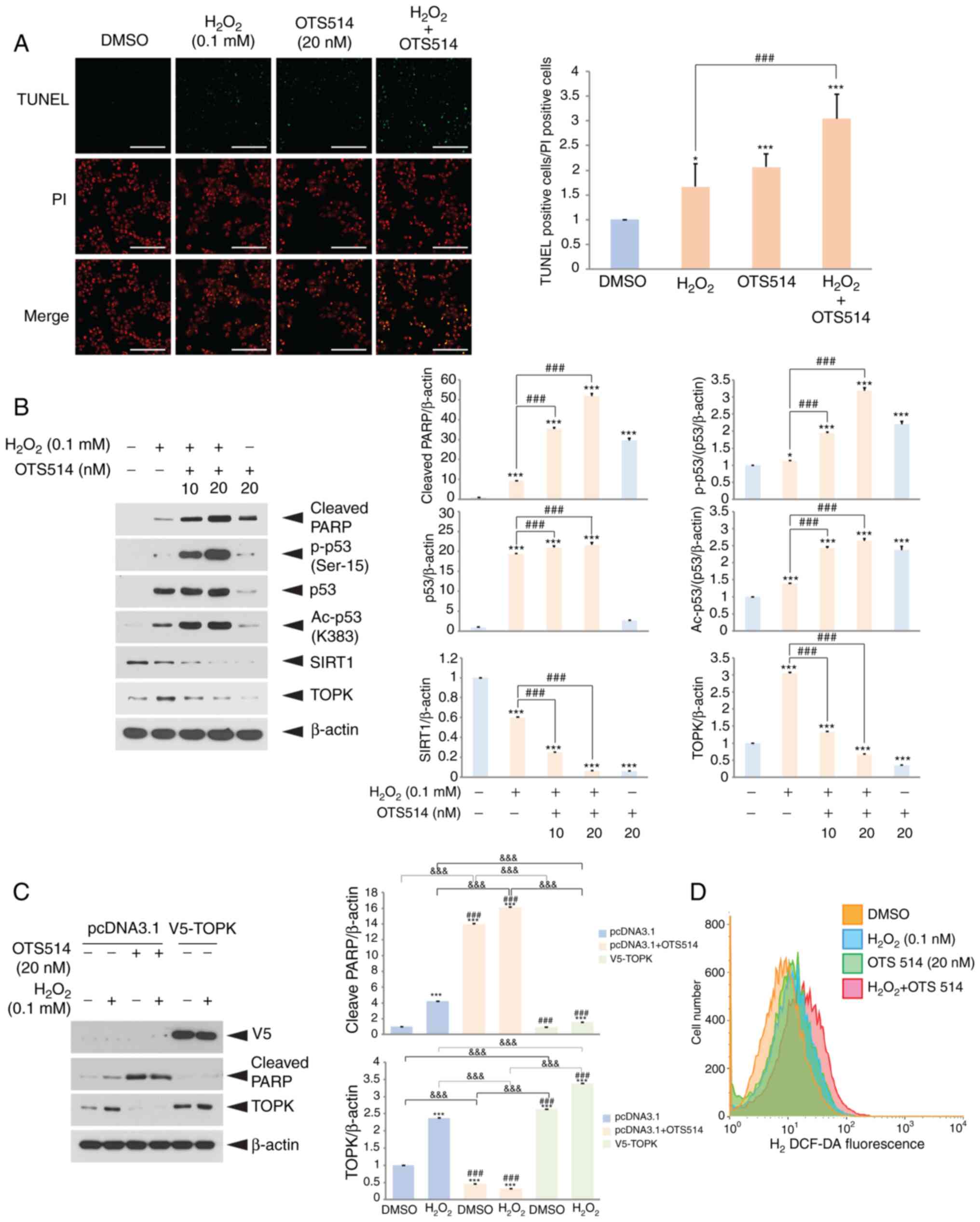 | Figure 1TOPK inhibition aggravates
H2O2-induced human COV434 granuolsa cell
apoptosis and decreases the endogenous SIRT1 level. (A) COV434
cells were treated with DMSO, H2O2, OTS514 or
H2O2 plus OTS514 for 24 h, fixed and then
examined for apoptosis by TUNEL assay. Cells were treated with
OTS514 for 2 h prior to H2O2 exposure. Nuclei
were stained with propidium iodide (PI). The ratio of
TUNEL-positive cells to PI-positive cells is shown. Scale bar, 100
µm. (B) COV434 cells were treated with DMSO (−),
H2O2 or H2O2 plus
OTS514 for 24 h. Cell lysates were separated on SDS-PAGE, and then
subjected to western blot analysis using indicated antibodies. The
β-actin level was used as a loading control. (C) COV434 cells were
transfected with empty vector, pcDNA3.1 or TOPK overexpression
vector, V5-TOPK for 24 h, and then treated with a combination of
H2O2 or OTS514 for 24 h. Western blot
analysis was performed using indicated antibodies. (D) COV434 cells
were incubated with DMSO, H2O2 or
H2O2 in combination with OTS514 for 24 h.
H2DCF-DA (10 µM) was added and incubated for 30 min. DCF
fluorescence was measured by flow cytometry. Representative images
of 3 independent experiments and graphs for quantitation are shown.
*P<0.05, ***P<0.001 vs. DMSO.
###P<0.001 vs. H2O2 or
H2O2 control.
&&&P<0.001 (two-way ANOVA with
Bonferroni's correction). |
Subsequently, the effects of TOPK overexpression on
the cleavage of PARP induced by H2O2 were
examined. For this purpose, the COV434 cells were transfected with
empty vector or TOPK overexpression vector for 24 h, and then
incubated with a combination of H2O2 or
OTS514 for 24 h. TOPK overexpression blocked the
H2O2-induced cleavage of PARP (Fig. 1C). Furthermore, the effects of
TOPK inhibition on endogenous ROS generation were investigated by
2′,7′-dichlo-roflurescin diacetate assay. The results revealed that
targeting TOPK by OTS514 treatment enhanced the
H2O2-induced ROS generation in granulosa
cells, accelerating H2O2-induced the
apoptosis (Fig. 1D).
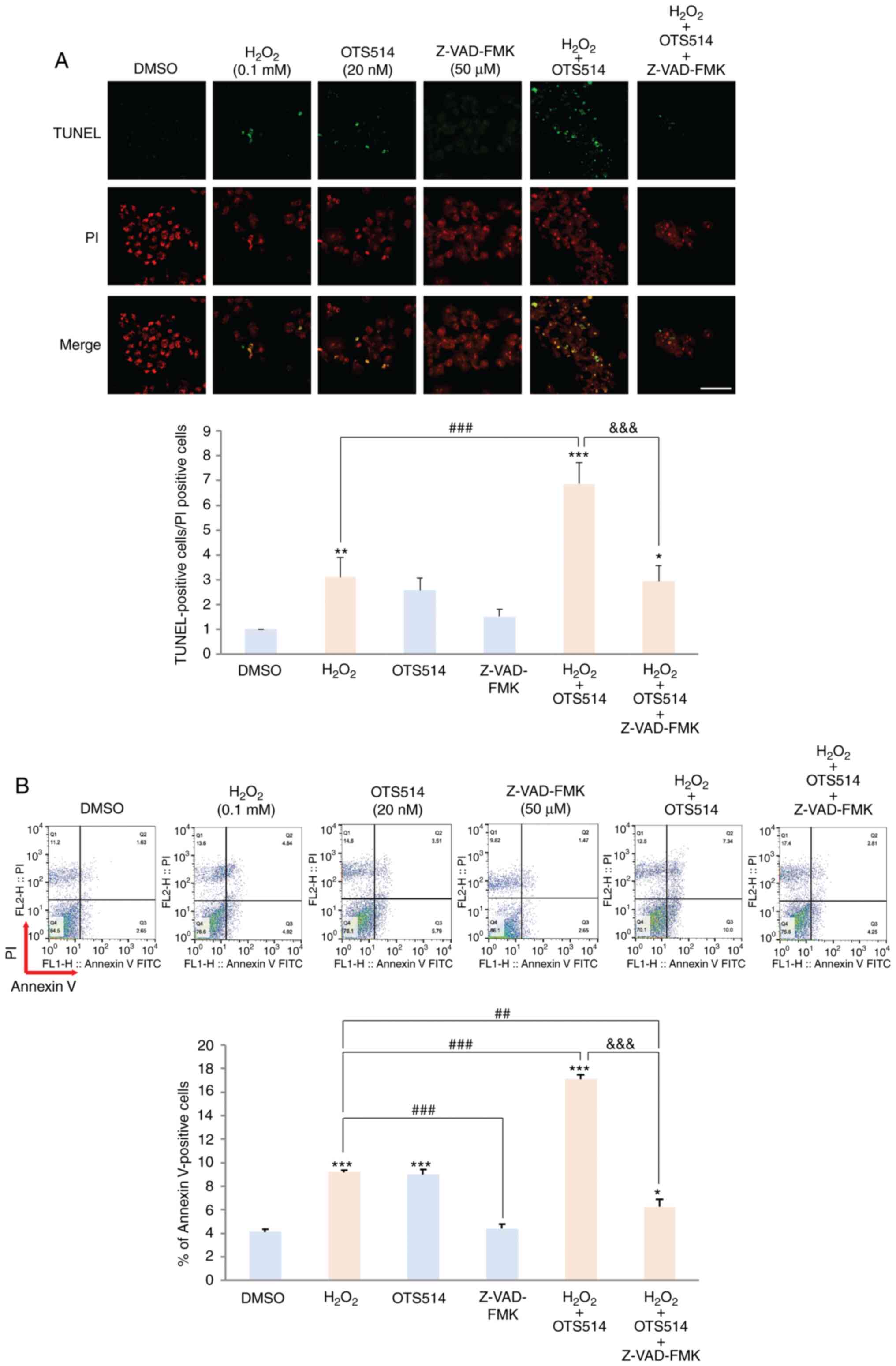
Caspase inhibitor blocks granulosa cell
apoptosis induced by co-treatment with H2O2
and OTS514
The present study then examined whether granulosa
cell apoptosis induced by H2O2 and OTS514 is
dependent on caspase-dependent apoptotic signaling pathways. COV434
cells were exposed to H2O2 only,
H2O2 plus OTS514 or
H2O2 plus OTS514 in combination with
Z-VAD-FMK for 24 h. Co-treatment of the COV434 cells with OTS514
and H2O2 markedly increased the number of
TUNEL-positive cells, compared to the cells stimulated with
H2O2 only. However, co-treatment with the
cell-permeable, irreversible pan-caspase inhibitor, Z-VAD-FMK,
significantly decreased the number of TUNEL-positive cells
(Fig. 2A). Consistent with this
finding, flow cytometric analysis indicated that Z-VAD-FMK
effectively diminished the number of Annexin V-positive cells
induced by treatment with H2O2 and OTS514
(Fig. 2B). These findings suggest
that caspase-dependent apoptosis pathways play a pivotal role in
granulosa cell apoptosis induced by H2O2 and
OTS514.
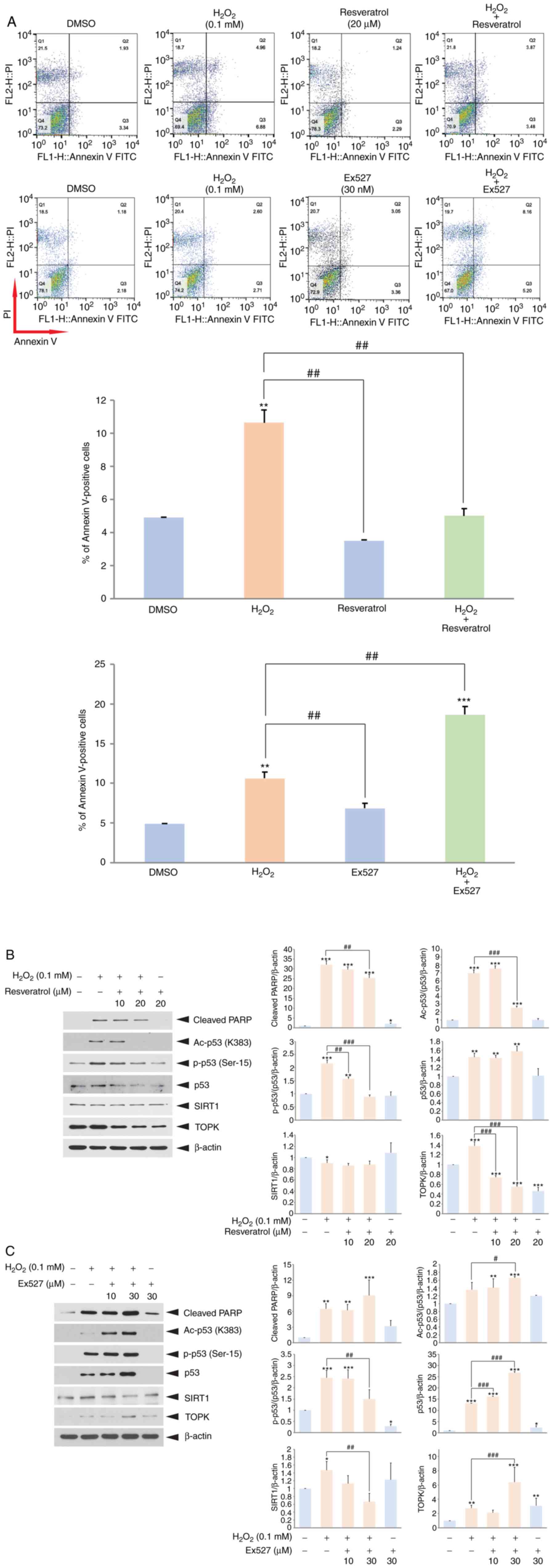
SIRT1 plays an important role in the
regulation of H2O2-induced granulosa cell
apoptosis
Subsequently, whether SIRT1 regulates
H2O2-induced granulosa cell apoptosis was
investigated. For this purpose, the SIRT1 activator, resveratrol,
or the SIRT1 inhibitor, Ex527, were employed. The COV434 cells were
incubated with H2O2 only, or
H2O2 plus resveratrol or Ex527. As was
expected, co-treatment with resveratrol decreased
H2O2-induced apoptosis (Fig. 3A), whereas co-treatment with Ex527
increased apoptosis. In addition, western blot analysis revealed
that co-treatment with resveratrol decreased the level of cleaved
PARP level, as well as the acetylated p53 level induced by
H2O2 (Fig.
3B); however, co-treatment with Ex527 increased the level of
cleaved PARP and acetylated p53 level (Fig. 3C). These findings demonstrate that
SIRT1 activity affects H2O2-induced granulosa
cell apoptosis and they p53 level or activity is a key factor for
the suppression of apoptosis.
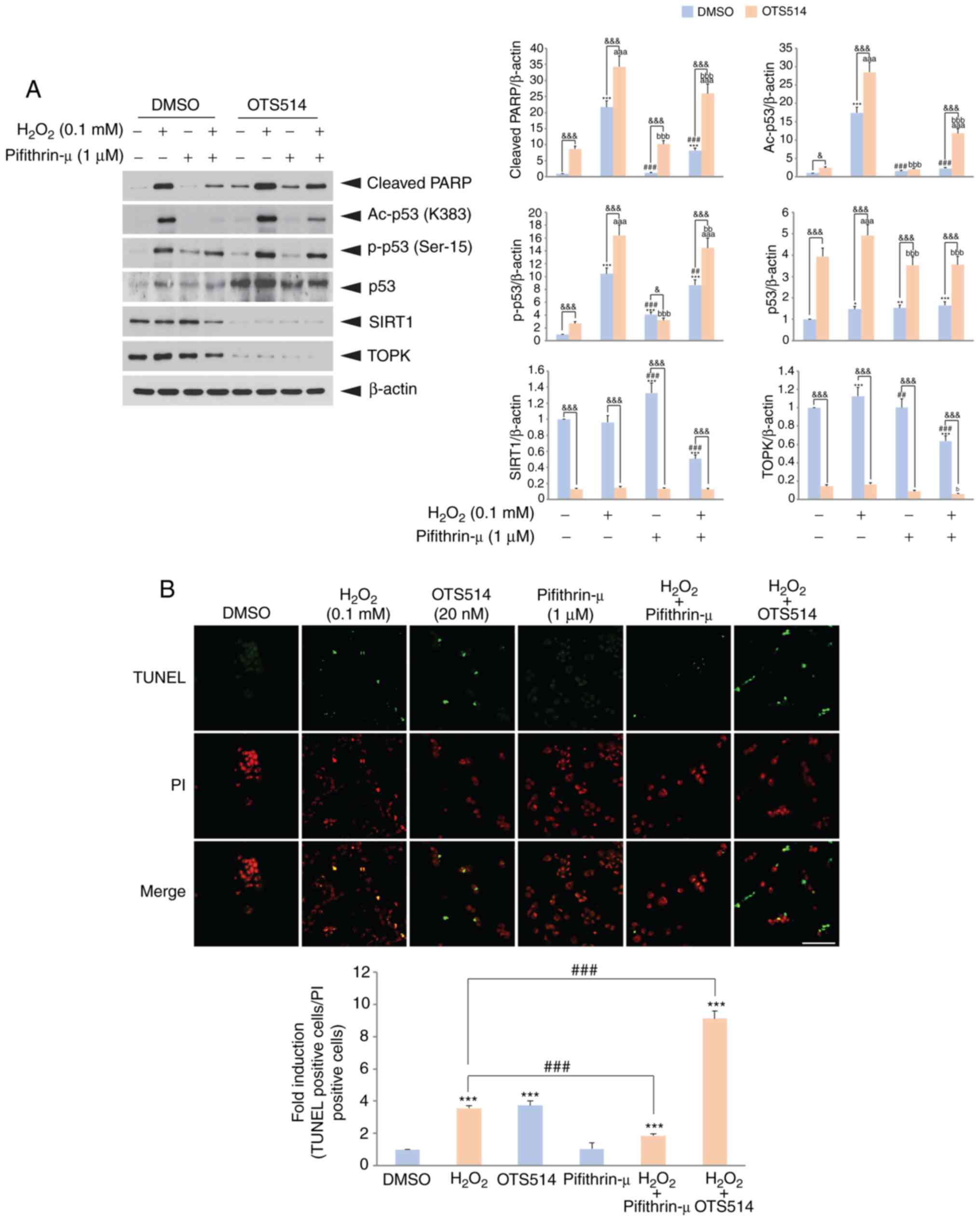
p53 inhibition suppresses
H2O2 or OTS514-induced granulosa cell
apoptosis
It has been suggested that tumor suppressor p53 is
involved in granulosa cell apoptosis in response to oxidative
stress or cAMP-mediated signaling (33,38). The present study then wished to
determine whether p53 affects COV434 cell apoptosis upon
H2O2 and OTS514 treatment. For this purpose,
COV434 cells were treated with a combination of
H2O2, OTS514 or the p53 inhibitor,
Pifithrin-µ. The inhibition of p53 by Pifithrin-µ treatment
decreased the H2O2-induced increase in the
level of cleaved PARP (Fig. 4A).
Co-treatment with OTS514 decreased TOPK expression, elevated the
p53 level and decreased SIRT1 expression. In addition, TUNEL assay
revealed that the inhibition of p53 decreased the
H2O2-induced COV434 cell apoptosis by
approximately 2-fold. However, co-treatment with OTS514 increased
cell apoptosis (Fig. 4B). Taken
together, these results demonstrate that p53 plays a key role in
H2O2 or OTS514-induced granulosa cell
apoptosis.
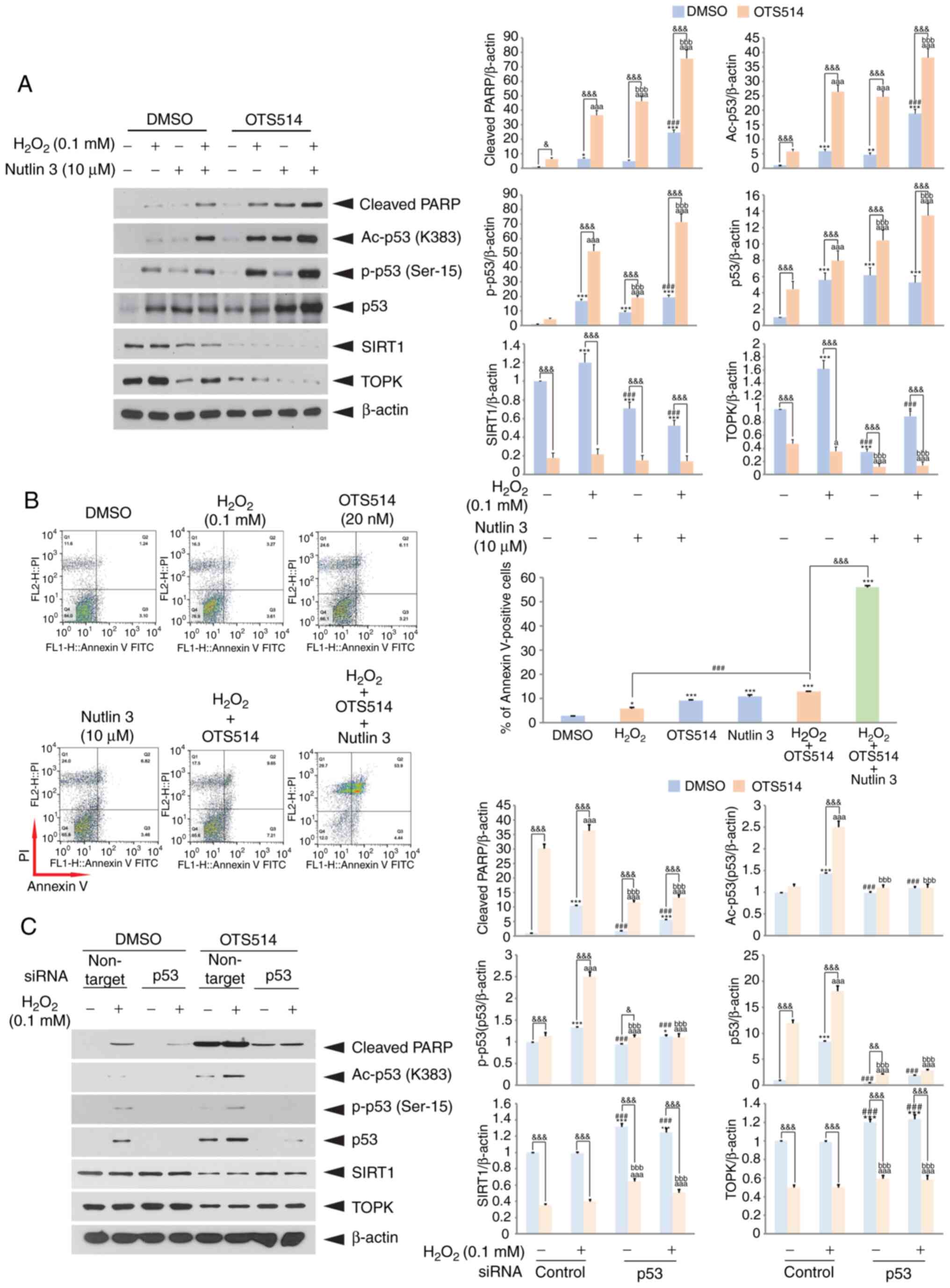
Inhibition of Mdm2 accelerates
H2O2- and OTS514-induced granulosa cell
apoptosis
To examine the effects of Mdm2 inhibition on
H2O2 and OTS514-induced granulosa cell
apoptosis, the Mdm2 antagonist, Nutlin 3, that inhibits the
interaction of Mdm2 and p53 was employed. Treatment of the COV434
cells with Nutlin 3 elevated the levels of cleaved PARP, total p53,
acetylated p53 or the phosphorylated p53 level, whereas it
decreased the SIRT1 level upon H2O2 and
OTS514 treatment (Fig. 5A).
Moreover, flow cytometric analysis indicated that the inhibition of
the interaction of Mdm2 and p53 markedly increased the number of
Annexin V-positive cells (Fig.
5B). Subsequently, whether p53 knockdown affects the
H2O2-induced decrease in SIRT1 expression was
examined. As was expected, the results revealed that p53 knockdown
markedly restored SIRT1 expression to decrease the
H2O2-induced cleavage of PARP (Fig. 5C). These findings suggest that
both the level and activity of p53 may function as important
factors in the regulation of granulosa cell apoptosis.
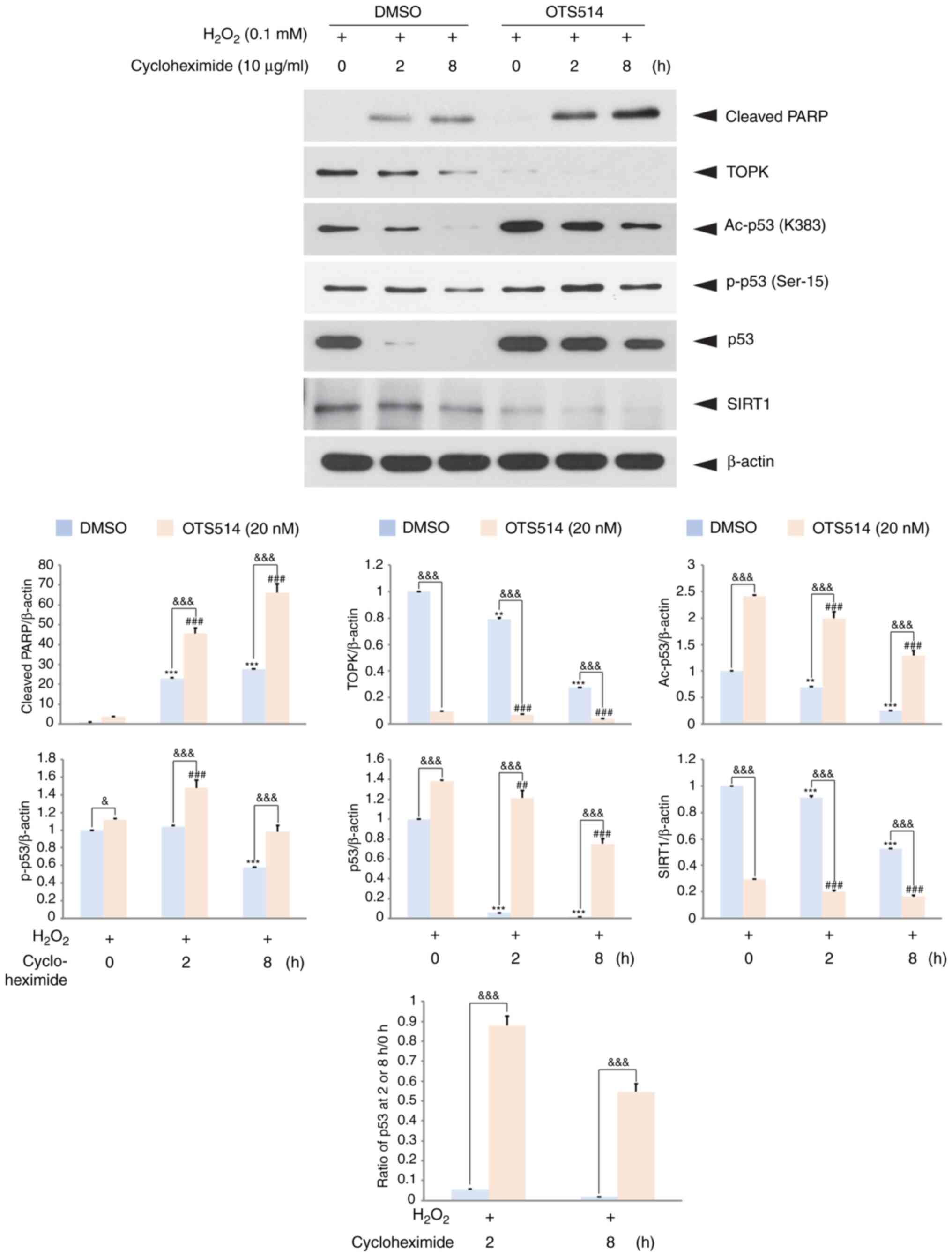
To examine whether p53 stability is affected by TOPK
inhibition in H2O2-exposed granulosa cells, a
cycloheximide chase experiment was then performed. The COV434 cells
were treated with H2O2, OTS514 and
cycloheximide, an inhibitor of protein biosynthesis. The level of
acetylated p53, as well as the total p53 or cleaved PARP level in
the H2O2- and OTS514-treated cells was
elevated, while the TOPK or SIRT1 level was almost abolished 8 h
following cycloheximide treatment compared with the cells
stimulated with H2O2 only (Fig. 6). These findings suggest that TOPK
inhibitor positively regulates p53 stability in
H2O2-stimulated granulosa cells.
TOPK inhibition decreases the SIRT1
transcriptional level downregulated by H2O2,
but increases the H2O2-induced p53
transcriptional level
It has been suggested that p53 negatively regulates
SIRT1 expression. It has also been suggested that p53 negatively
regulates SIRT1 expression (11).
The present study thus examined the effect of TOPK inhibition on
the SIRT1 or p53 transcriptional level, and the effect of p53
expression on the SIRT1 transcriptional level upon
H2O2 stimulation. COV434 cells were
transfected with SIRT1 or a p53 promoter-driven luciferase reporter
construct. At 24 h following transfection, the cells were incubated
with a combination of H2O2 or OTS514. The
results indicated that stimulation of the COV434 cells with
H2O2 resulted in a decrease in the SIRT1
transcriptional level and co-treatment with OTS514 promoted this
decrease (Fig. 7A). On the other
hand, co-treatment with OTS514 promoted the level of p53 or the p53
target, p21 transcriptional level induced by
H2O2 (Fig.
7B). Notably, the expression of GFP-p53 diminished the SIRT1
transcriptional level downregulated by H2O2
(Fig. 7A). These findings
demonstrate that upon H2O2 exposure, TOPK
inhibition suppresses the SIRT1 transcriptional level and enhances
the p53 transcriptional level, and that p53 negatively regulates
SIRT1 expression. In addition, schematic model indicated that TOPK
inhibition enhanced p53-mediated granulosa cell apoptosis through
SIRT1 downregulation in response to H2O2
(Fig. 7C), suggesting the role of
TOPK in the SIRT1/p53 regulatory axis.
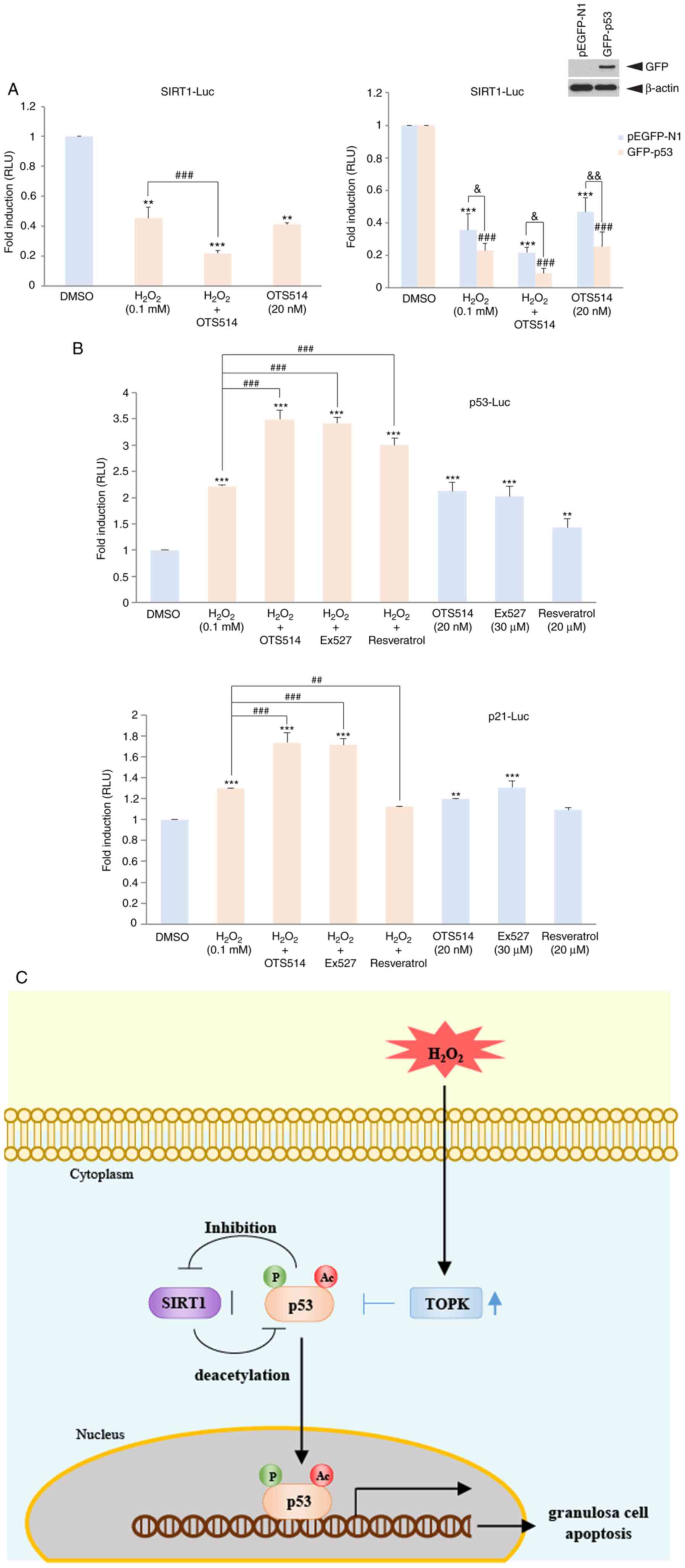 | Figure 7TOPK inhibition mitigates
H2O2-downregulated SIRT1 transcriptional
activity, but promotes p53 or p21 transcriptional activity
upregulated by H2O2. (A) COV434 cells were
transfected with SIRT1 promoter driven luciferase reporter
construct (SIRT1-LUC) alone or SIRT1-LUC together with
GFP-p53 or pEGFP-N1 plus pRL-SV40 gene. At 24 h following
transfection, cells were treated with DMSO,
H2O2, OTS514 or H2O2
plus OTS514 for 24 h. (B) COV434 cells were transfected with
p53-luciferase (p53-LUC) or p21-luciferase (p21-LUC)
plus pRL-SV40 gene. At 24 h post-transfection, cells were
incubated in combination of H2O2 with OTS514,
EX527 or resveratrol for 24 h. Firefly luciferase activity was
estimated by normalization against Renilla luciferase
activity. Relative luciferase unit (RLU) is indicated.
**P<0.01, ***P<0.001 vs. DMSO.
##P<0.01, ###P<0.001 vs. H2O2.
&P<0.05, &&P<0.01 (two-way
ANOVA with Bonferroni's correction). TOPK inhibition mitigates
H2O2-downregulated SIRT1 transcriptional
activity, but promotes p53 or p21 transcriptional activity
upregu-lated by H2O2. (C) Schematic model for
role of TOPK-p53-SIRT axis in regulation of granulosa cell
apoptosis. TOPK is increased in response to
H2O2, but TOPK downregulated by TOPK
inhibitor OTS514 results in elevation of activity and expression of
p53, which leads to the suppression of SIRT1 expression, thereby
promoting p53-mediated granulosa cell apoptosis. |
Discussion
Granulosa cells are well known to be a class of
cells that constitute a follicle for oocyte maturation. It has been
proposed that granulosa cells and oocytes form gap junctions
through connexins, which transfer low molecular weight molecules,
such as cGMP, cAMP and calcium, and that granulosa cells transfer
nutrients and regulatory factors for the growth and differentiation
of oocytes (39-43). In addition, it seems that
granulosa cells protect oocytes from oxidative stress involving ROS
during follicle development. ROS have been reported to carry out
functions that induce follicle rupture, and to regulate the
expression of genes which induce oocyte maturation; however,
accumulated ROS levels have been shown to cause oxidative stress
that damages oocytes and granulosa cells (44-47). ROS mainly produced from the
mitochondria cause mitochondrial damage to increase Bax expression,
decrease Bcl-2 expression, and to release cytochrome c that
forms the apoptosome, leading to the activation of the caspase
cascade (48,49).
In this study, it was determined whether TOPK can
affect oxidative stress-induced human granulosa cell apoptosis. It
has been suggested that OTS514, a thieno[2,3-c]quinolone compound,
strongly suppresses the growth of TOPK-positive cancer cells,
including ovarian cancer, lung cancer or leukemia with low IC50
values ranging from 1.5 to 14 nM (50-52). OTS514 was shown to inhibit TOPK
expression as well as TOPK kinase activity. It has been suggested
that OTS514 suppresses Forkhead box protein M1 (FOXM1) and FOXM1
transactivates the c-Myc promoter (52,53). Furthermore, c-Myc has been shown
to positively regulate TOPK expression (54). In addition, FOXM1 enhances cyclin
B1 that phosphorylates TOPK on the Thr-9 residue (55). Collectively, it seems that OTS514
inhibits TOPK activity and expression by suppressing FOXM1 or
c-Myc. Based on these reports, an inhibitor was employed in the
presents study to suppress TOPK expression or activity. The
inhibition of TOPK activity or expression by the TOPK inhibitor,
OTS514, exacerbated H2O2-induced COV434
granulosa cell apoptosis, which is dependent on caspase signaling
pathways. It was also found that ROS production was markedly
increased by co-treatment with H2O2 and TOPK
inhibitor than with H2O2 only in COV434
granulosa cells. consistent with these findings, it has been
previously demonstrated that TOPK inhibits the accumulation of
H2O2 in RPMI7951 melanoma cells (56), induces the expression of manganese
superoxide dismutase (MnSOD), an essential antioxidant enzyme
localized in the mitochondria, in PC12 cells (57), and induces the expression of Nrf2,
an antioxidant molecule, to reduce ROS (58). Collectively, the present study
provides evidence that TOPK negatively regulates oxida-tive
stress-induced granulosa cell apoptosis, thereby positively
contributing to follicular development.
It has been suggested that oxidative stress induces
tumor suppressor p53 to increase the expression of p53 target
proteins, including p21, Bax and PUMA (9,59,60). In granulosa cells, oxidative
stress has also been shown to result in PUMA expression, leading to
the promotion of apoptosis through p53 induction (33). The findings of the presents study
demonstrated that TOPK inhibition by OTS514 elevated the expression
of total p53, acetylated p53 or the phosphorylated p53 level in
granulosa cells. In addition, it was found that the inhibition of
p53 degradation by Nutlin 3 accelerated
H2O2-mediated granulosa cell apoptosis. It
appears that the increase in the phosphorylated or acetylated p53
level, as well as the total p53 level contributed to the apoptosis.
In agreement with these findings, it has been reported that TOPK
binds to the p53 DNA binding domain, leading to the inhibition of
expression of p53 and p53 target protein, p21, and thereby the
induction of cell cycle arrest (29,30). Moreover, it has been proposed that
the phosphorylation of p53 at the Ser-15 residue decreases its
interaction with Mdm2, but increases its interaction with CBP/p300,
thereby promoting p53 acetylation and activity (61,62). It is well known that p53-mediated
apoptosis is due to the p53 translocation into the mitochondria.
The present study confirmed that p53 inhibition by Pifithrin-µ, an
inhibitor of p53 binding to the mitochondria alleviates granulosa
cell apoptosis induced by H2O2 and OTS514.
Previous studies have demonstrated that mitochondrial p53
accumulation promotes the opening of the mitochondrial permeability
transition pore (MPTP), which is a protein channel for the
regulation of mitochondrial membrane permeability (63-65). Mitochondrial p53 is known to
induce Bak and Bax oligomerization and to suppress the
anti-apoptotic activity of Bcl-xL and Bcl-2 (63,66,67).
On the other hand, it has been suggested that p53
deacetylation is mostly caused by HDACs, but SIRT1 specifically
deacetylates p53 at the K382 residue (60,68,69). The present study found that the
regulation of p53 acetylation by SIRT1 activator or inhibitor
affected H2O2-induced granulosa cell
apoptosis, and that H2O2-induced apoptosis
was further decreased by resveratrol and Pifithrin-µ, and increased
by Ex527 and Nutlin 3. Furthermore, TOPK inhibition diminished
SIRT1 transcriptional activity downregulated by
H2O2 treatment and p53 expression promoted
the inhibitory effect of TOPK, which may be due to an increase in
p53 expression induced by TOPK inhibition. In agreement with these
findings, previous studies have suggested that p53 inhibits SIRT1
transcription by directly binding to the SIRT1 promoter, and that
TOPK functions as a suppressor for p53 (11,30).
It has been suggested that SIRT1 binds to p53 and
deacetylates the C-terminal p53 residue, Lys382, to negatively
regulate the transcriptional activity of p53 (70,71). In the present study, the SIRT1
activator, resveratrol, or the SIRT1 inhibitor, Ex527, decreased or
increased H2O2-induced TOPK expression,
respectively. Although research on the regulation of the TOPK
promoter remains elusive, p53 may transcriptionally modulate TOPK
expression (16). Therefore,
these findings may be due to the change in the
H2O2 induction of SIRT1-mediated p53
transactivation, activity regulating TOPK expression. Taken
together, these findings provide evidence for a novel role of TOPK
in the p53/SIRT1 regulatory axis that mediates granu-losa cell
apoptosis. Therefore, TOPK may be a novel target molecule for the
improvement of follicular development or oocyte maturation.
Acknowledgements
The authors would like to thank the laboratory
(Protein Expression Laboratory, Department of Biochemstry, Konyang
University) members for their helpful discussion about this
work.
Abbreviations:
|
TOPK
|
T-lymphokine-activated killer
cell-originated protein kinase
|
|
SIRT1
|
sirtuin1
|
|
ROS
|
reactive oxygen species
|
|
H2O2
|
hydrogen peroxide
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
HDAC
|
histone deacetylase
|
|
MnSOD
|
manganese superoxide dismutase
|
|
Bax
|
Bcl-2-associated X protein
|
Funding
The present study was supported by the National
Research Foundation of Korea (NRF) grants funded by the Korea
government (MSIP) (NRF-2019R1I1A3A01063191). The present study was
also supported by the Priority Research Centers Program through the
NRF funded by the MEST (NRF-2017R1A6A1A03015713).
Availability of materials and data
The data that support the findings of the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JHP and SAP conducted experiments, collected data,
analyzed data and wrote the manuscript. YJL and NRJ performed
experiments. JS provided some reagents and made suggestions during
the performing of the experiments and data analysis. SMO designed
and supervised the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Adeldust H, Zeinoaldini S, Kohram H, Amiri
Roudbar M and Daliri Joupari M: In vitro maturation of ovine oocyte
in a modified granulosa cells co-culture system and
alpha-tocopherol supplementation: Effects on nuclear maturation and
cleavage. J Anim Sci Technol. 57:272015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tripathi A, Shrivastav TG and Chaube SK:
An increase of granu-losa cell apoptosis mediates aqueous neem
(Azadirachta indica) leaf extract-induced oocyte apoptosis in rat.
Int J Appl Basic Med Res. 3:27–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Devine PJ, Perreault SD and Luderer U:
Roles of reactive oxygen species and antioxidants in ovarian
toxicity. Biol Reprod. 86:272012.
|
|
4
|
Tatone C, Di Emidio G, Vitti M, Di Carlo
M, Santini S, D'Alessandro AM, Falone S and Amicarelli F: Sirtuin
functions in female fertility: Possible role in oxidative stress
and aging. Oxid Med Cell Longev. 2015:6596872015.PubMed/NCBI
|
|
5
|
Yang H, Yan B, Liao D, Huang S and Qiu Y:
Acetylation of HDAC1 and degradation of SIRT1 form a positive
feedback loop to regulate p53 acetylation during heat-shock stress.
Cell Death Dis. 6:e17472015.PubMed/NCBI
|
|
6
|
Han Y, Luo H, Wang H, Cai J and Zhang Y:
SIRT1 induces resistance to apoptosis in human granulosa cells by
activating the ERK pathway and inhibiting NF-κB signaling with
anti-inflammatory functions. Apoptosis. 22:1260–1272.
2017.PubMed/NCBI
|
|
7
|
Abdolvahabi Z, Nourbakhsh M, Hosseinkhani
S, Hesari Z, Alipour M, Jafarzadeh M, Ghorbanhosseini SS, Seiri P,
Yousefi Z, Yarahmadi S and Golpou P: MicroRNA-590-3P suppresses
cell survival and triggers breast cancer cell apoptosis via
targeting sirtuin-1 and deacetylation of p53. J Cell Biochem.
120:9356–9368. 2019.
|
|
8
|
Yu X, Zhang S, Zhao D, Zhang X, Xia C,
Wang T, Zhang M, Liu T, Huang W and Wu B: SIRT1 inhibits apoptosis
in in vivo and in vitro models of spinal cord injury via
microRNA-494. Int J Mol Med. 43:1758–1768. 2019.PubMed/NCBI
|
|
9
|
Han MK, Song EK, Guo Y, Ou X, Mantel C and
Broxmeyer HE: SIRT1 regulates apoptosis and Nanog expression in
mouse embryonic stem cells by controlling p53 subcellular
localization. Cell Stem Cell. 2:241–251. 2008.PubMed/NCBI
|
|
10
|
Kume S, Haneda M, Kanasaki K, Sugimoto T,
Araki SI, Isono M, Isshiki K, Uzu T, Kashiwagi A and Koya D: Silent
information regulator 2 (SIRT1) attenuates oxidative stress-induced
mesangial cell apoptosis via p53 deacetylation. Free Radic Biol
Med. 40:2175–2182. 2006.PubMed/NCBI
|
|
11
|
Yuan F, Liu L, Lei Y and Tang P: P53
inhibits the upregulation of sirtuin 1 expression induced by c-Myc.
Oncol Lett. 14:4396–4402. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zlobec I, Molinari F, Kovac M, Bihl MP,
Altermatt HJ, Diebold J, Frick H, Germer M, Horcic M, Montani M, et
al: Prognostic and predictive value of TOPK stratified by KRAS and
BRAF gene alterations in sporadic, hereditary and metastatic
colorectal cancer patients. Br J Cancer. 102:151–161. 2010.
View Article : Google Scholar :
|
|
13
|
Zykova TA, Zhu F, Wang L, Li H, Bai R, Lim
DY, Yao K, Bode AM and Dong Z: The T-LAK cell-originated protein
kinase signal pathway promotes colorectal cancer metastasis.
EBioMedicine. 18:73–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su TC, Chen CY, Tsai WC, Hsu HT, Yen HH,
Sung WW and Chen CJ: Cytoplasmic, nuclear, and total PBK/TOPK
expression is associated with prognosis in colorectal cancer
patients: A retrospective analysis based on immunohistochemistry
stain of tissue microarrays. PLoS One. 13:e02048662018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shih MC, Chen JY, Wu YC, Jan YH, Yang BM,
Lu PJ, Cheng HC, Huang MS, Yang CJ, Hsiao M and Lai JM: TOPK/PBK
promotes cell migration via modulation of the PI3K/PTEN/AKT pathway
and is associated with poor prognosis in lung cancer. Oncogene.
31:2389–2400. 2012. View Article : Google Scholar
|
|
16
|
Lei B, Qi W, Zhao Y, Li Y, Liu S, Xu X,
Zhi C, Wan L and Shen H: PBK/TOPK expression correlates with mutant
p53 and affects patients' prognosis and cell proliferation and
viability in lung adenocarcinoma. Hum Pathol. 46:217–224. 2015.
View Article : Google Scholar
|
|
17
|
Ohashi T, Komatsu S, Ichikawa D, Miyamae
M, Okajima W, Imamura T, Kiuchi J, Kosuga T, Konishi H, Shiozaki A,
et al: Overexpression of PBK/TOPK relates to tumour malignant
potential and poor outcome of gastric carcinoma. Br J Cancer.
116:218–226. 2017. View Article : Google Scholar :
|
|
18
|
Sun H, Zhang L, Shi C, Hu P, Yan W, Wang
Z, Duan Q, Lu F, Qin L, Lu T, et al: TOPK is highly expressed in
circulating tumor cells, enabling metastasis of prostate cancer.
Oncotarget. 6:12392–12404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pirovano G, Ashton TM, Herbert KJ, Bryant
RJ, Verrill CL, Cerundolo L, Buffa FM, Prevo R, Harrap I, Ryan AJ,
et al: TOPK modulates tumour-specific radiosensitivity and
correlates with recurrence after prostate radiotherapy. Br J
Cancer. 117:503–512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikeda Y, Park JH, Miyamoto T, Takamatsu N,
Kato T, Iwasa A, Okabe S, Imai Y, Fujiwara K, Nakamura Y, et al:
T-LAK cell-originated protein kinase (TOPK) as a prognostic factor
and a potential therapeutic target in ovarian cancer. Clin Cancer
Res. 22:6110–6117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang MY, Lin ZR, Cao Y, Zheng LS, Peng LX,
Sun R, Meng DF, Xie P, Yang JP, Cao L, et al: PDZ binding kinase
(PBK) is a theranostic target for nasopharyngeal carcinoma: Driving
tumor growth via ROS signaling and correlating with patient
survival. Oncotarget. 7:26604–26616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohashi T, Komatsu S, Ichikawa D, Miyamae
M, Okajima W, Imamura T, Kiuchi J, Nishibeppu K, Kosuga T, Konishi
H, et al: Overexpression of PBK/TOPK contributes to tumor
development and poor outcome of esophageal squamous cell carcinoma.
Anticancer Res. 36:6457–6466. 2017. View Article : Google Scholar
|
|
23
|
Abe Y, Matsumoto S, Kito K and Ueda N:
Cloning and expression of a novel MAPKK-like protein kinase,
lymphokine- activated killer T-cell-originated protein kinase,
specifically expressed in the testis and activated lymphoid cells.
J Biol Chem. 275:21525–21531. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsumoto S, Abe Y, Fujibuchi T, Takeuchi
T, Kito K, Ueda N, Shigemoto K and Gyo K: Characterization of a
MAPKK-like protein kinase TOPK. Biochem Biophys Res Commun.
325:997–1004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu F, Zykova TA, Kang BS, Wang Z, Ebeling
MC, Abe Y, Ma WY, Bode AM and Dong Z: Bidirectional signals
transduced by TOPK-ERK interaction increase tumorigenesis of HCT116
colorectal cancer cells. Gastroenterology. 133:219–231. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aksamitiene E, Kholodenko BN, Kolch W,
Hoek JB and Kiyatkin A: PI3K/Akt-Sensitive MEK-independent
compensatory circuit of ERK activation in ER-positive PI3K-mutant
T47D breast cancer cells. Cell Signal. 22:1369–1378. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oh SM, Zhu F, Cho YY, Ki WL, Bong SK, Kim
HG, Zykova T, Bode AM and Dong Z: T-Lymphokine-activated killer
cell-originated protein kinase functions as a positive regulator of
c-Jun-NH2-kinase 1 signaling and H-ras-induced cell transformation.
Cancer Res. 67:5186–5194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seol MA, Park JH, Jeong JH, Lyu J, Han SY
and Oh SM: Role of TOPK in lipopolysaccharide-induced breast cancer
cell migration and invasion. Oncotarget. 8:40190–40203. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nandi AK, Ford T, Fleksher D, Neuman B and
Rapoport AP: Attenuation of DNA damage checkpoint by PBK, a novel
mitotic kinase, involves protein-protein interaction with tumor
suppressor p53. Biochem Biophy Res Commun. 358:181–188. 2007.
View Article : Google Scholar
|
|
30
|
Hu F, Gartenhaus RB, Eichberg D, Liu Z,
Fang HB and Rapoport AP: PBK/TOPK interacts with the DBD domain of
tumor suppressor p53 and modulates expression of transcriptional
targets including p21. Oncogene. 29:5464–5474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park JH, Yoon DS, Choi HJ, Hahm DH and Oh
SM: Phosphorylation of IκBα at serine 32 by T-lymphokine-activated
killer cell-originated protein kinase is essential for
chemoresistance against doxorubicin in cervical cancer cells. J
Biol Chem. 288:3585–3593. 2013. View Article : Google Scholar
|
|
32
|
Weng Q, Liu Z, Li B, Liu K, Wu W and Liu
H: Oxidative stress induces mouse follicular granulosa cells
apoptosis via JNK/FoxO1pathway. PLoS One. 11:e01678692016.
View Article : Google Scholar
|
|
33
|
Yang H, Xie Y, Yang D and Ren D: Oxidative
stress-induced apoptosis in granulosa cells involves JNK, p53 and
puma. Oncotarget. 8:25310–25322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang M, Zhang Q, Hu Y, Xu L, Jiang Y,
Zhang C, Ding L, Jiang R, Sun J, Sun H and Yan G: MiR-181a
increases FoxO1 acetylation and promotes granulosa cell apoptosis
via SIRT1 downregulation. Cell Death Dis. 8:e30882017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Joel M, Mughal AA, Grieg Z, Murrell W,
Palmero S, Mikkelsen B, Fjerdingstad HB, Sandberg CJ, Behnan J,
Glover JC, et al: Targeting PBK/TOPK decreases growth and survival
of glioma initiating cells in vitro and attenuates tumor growth in
vivo. Mol Cancer. 14:1212015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim DJ, Li Y, Reddy K, Lee MH, Kim MO, Cho
YY, Lee SY, Kim JE, Bode AM and Dong Z: Novel TOPK inhibitor
HI-TOPK-032 effectively suppresses colon cancer growth. Cancer Res.
72:3060–3068. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu M and Xu S: PBK/TOPK overexpression and
survival in solid tumors: A PRISMA-compliant meta-analysis.
Medicine (Baltimore). 98:e147662019. View Article : Google Scholar
|
|
38
|
Keren-Tal I, Suh BS, Dantes A, Lindner S,
Oren M and Amsterdam A: Involvement of p53 expression in
cAMP-mediated apoptosis in immortalized granulosa cells. Exp Cell
Res. 218:283–295. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Heller DT, Cahill DM and Schultz RM:
Biochemical studies of mammalian oogenesis: Metabolic cooperativity
between granu-losa cells and growing mouse oocytes. Dev Biol.
84:455–464. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brower PT and Schultz RM: Intercellular
communication between granulosa cells and mouse oocytes: Existence
and possible nutritional role during oocyte growth. Dev Biol.
90:144–153. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bruzzone R, White TW and Paul DL:
Connections with connexins: The molecular basis of direct
intercellular signaling. Eur J Biochem. 238:1–27. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kumar NM and Gilula NB: The gap junction
communication channel. Cell. 84:381–388. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grazul-Bilska AT, Reynolds LP and Redmer
DA: Gap junctions in the ovaries. Biol Reprod. 57:947–957. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi
KW and Oh KS: Detection of reactive oxygen species (ROS) and
apoptosis in human fragmented embryos. Hum Reprod. 13:998–1002.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hensley K, Robinson KA, Gabbita SP,
Salsman S and Floyd RA: Reactive oxygen species, cell signaling,
and cell injury. Free Radic Biol Med. 28:1456–1462. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Goud AP, Goud PT, Diamond MP, Gonik B and
Abu-Soud HM: Reactive oxygen species and oocyte aging: Role of
superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic
Biol Med. 44:1295–1304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li D, Ueta E, Kimura T, Yamamoto T and
Osaki T: Reactive oxygen species (ROS) control the expression of
Bcl-2 family proteins by regulating their phosphorylation and
ubiquitination. Cancer Sci. 95:644–650. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alarifi S, Ali H, Alkahtani S and Alessia
MS: Regulation of apoptosis through bcl-2/bax proteins expression
and DNA damage by nano-sized gadolinium oxide. Int J Nanomedicine.
12:4541–4551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alachkar H, Mutonga M, Malnassy G, Park
JH, Fulton N, Woods A, Meng L, Kline J, Raca G, Odenike O, et al:
T-LAK cell-originated protein kinase presents a novel therapeutic
target in FLT3-ITD mutated acute myeloid leukemia. Oncotarget.
6:33410–33425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Matsuo Y, Park JH, Miyamoto T, Yamamoto S,
Hisada S, Alachkar H and Nakamura Y: TOPK inhibitor induces
complete tumor regression in xenograft models of human cancer
through inhibition of cytokinesis. Sci Transl Med. 6:259ra1452014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Park JH, Inoue H, Kato T, Zewde M,
Miyamoto T, Matsuo Y, Salgia R and Nakamura Y: TOPK (T-LAK
cell-originated protein kinase) inhibitor exhibits growth
suppressive effect on small cell lung cancer. Cancer Sci.
108:488–496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wierstra I and Alves J: FOXM1c
transactivates the human c-myc promoter directly via the two TATA
boxes P1 and P2. FEBS J. 273:4645–4667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu F, Gartenhaus RB, Zhao XF, Fang HB,
Minkove S, Poss DE and Rapoport AP: C-Myc and E2F1 drive PBK/TOPK
expression in high-grade malignant lymphomas. Leukemia Res.
37:447–454. 2013. View Article : Google Scholar
|
|
55
|
Leung TW, Lin SS, Tsang AC, Tong CS, Ching
JC, Leung WY, Gimlich R, Wong GG and Yao KM: Over-expression of
foxM1 stimulates cyclin B1 expression. FEBS Lett. 507:59–66. 2001.
View Article : Google Scholar
|
|
56
|
Zykova TA, Zhu F, Vakorina TI, Zhang J,
Higgins LA, Urusova DV, Bode AM and Dong Z: T-LAK cell-originated
protein kinase (TOPK) phosphorylation of prx1 at ser-32 prevents
UVB-induced apoptosis in RPMI7951 melanoma cells through the
regulation of Prx1 peroxidase activity. J Biol Chem.
285:29138–29146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhao H, Wang R, Tao Z, Gao L, Yan F, Gao
Z, Liu X, Ji X and Luo Y: Ischemic postconditioning relieves
cerebral ischemia and reperfusion injury through activating T-LAK
cell-originated protein kinase/protein kinase b pathway in rats.
Stroke. 45:2417–2424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu Y, Liu H, Cao H, Song B and Zhang W
and Zhang W: PBK/TOPK mediates promyelocyte proliferation via
Nrf2-regulated cell cycle progression and apoptosis. Oncol Rep.
34:3288–3296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hsieh TC, Juan G, Darzynkiewicz Z and Wu
JM: Resveratrol increases nitric oxide synthase, induces
accumulation of p53 and p21(WAF1/CIP1), and suppresses cultured
bovine pulmonary artery endothelial cell proliferation by
perturbing progression through S and G2. Cancer Res. 59:2596–2601.
1999.
|
|
60
|
Luo J, Nikolaev AY, Imai S, Chen D, Su F,
Shiloh A, Guarente L and Gu W: Negative control of p53 by Sir2alpha
promotes cell survival under stress. Cell. 107:137–148. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shieh SY, Ikeda M, Taya Y and Prives C:
DNA damage-induced phosphorylation of p53 alleviates inhibition by
MDM2. Cell. 91:325–334. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lambert PF, Kashanchi F, Radonovich MF,
Shiekhattar R and Brady JN: Phosphorylation of p53 serine 15
increases interaction with CBP. J Biol Chem. 273:33048–33053. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Marchenko ND and Moll UM: Mitochondrial
death functions of p53. Mol Cell Oncol. 1:e9559952014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen LJ, Gao YQ, Li XJ, Shen DH and Sun
FY: Melatonin protects against MPTP/MPP+ -induced mitochondrial DNA
oxidative damage in vivo and in vitro. J Pineal Res. 39:34–42.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yu W, Zhang X, Liu J, Wang X, Li S, Liu R,
Liao N, Zhang T and Hai C: Cyclosporine A suppressed glucose
oxidase induced P53 mitochondrial translocation and hepatic cell
apoptosis through blocking mitochondrial permeability transition.
Int J Biol Sci. 12:198–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wolff S, Erster S, Palacios G and Moll UM:
P53's mitochondrial translocation and MOMP action is independent of
puma and bax and severely disrupts mitochondrial membrane
integrity. Cell Res. 18:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sykes SM, Mellert HS, Holbert MA, Li K,
Marmorstein R, Lane WS and McMahon SB: Acetylation of the p53
DNA-binding domain regulates apoptosis induction. Mol Cell.
24:841–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Brooks CL and Gu W: The impact of
acetylation and deacetylation on the p53 pathway. Protein Cell.
2:456–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Islam S, Abiko Y, Uehara O and Chiba I:
Sirtuin 1 and oral cancer. Oncol Lett. 17:729–738. 2019.PubMed/NCBI
|
|
70
|
Ong AL and Ramasamy TS: Role of
sirtuin1-p53 regulatory axis in aging, cancer and cellular
reprogramming. Ageing Res Rev. 43:64–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
van Leeuwen I and Lain S: Sirtuins and
p53. Adv Cancer Res. 102:171–195. 2009. View Article : Google Scholar : PubMed/NCBI
|