Introduction
Cervical cancer is the fourth most common
gynecological malignant tumor that affects females, and results in
~57,000 new cases annually with 311,000 deaths worldwide (1,2).
In China, the incidence of cervical cancer has increased rapidly
over the past 20 years (3).
Surgery, radiotherapy and chemotherapy are currently the main
treatment methods for cervical cancer (4). Although advances in these therapies
have prolonged patient survival, the limitations of effective
regimens are the resistance of tumor cells and their severe side
effects (5). Thus, there is an
urgent need for new anticancer drugs with fewer side effects that
may improve survival rates.
Voltage-dependent anion channel (VDAC) family
proteins in the mitochondrial outer membrane play an important role
in cell survival and death (6).
VDACs interact with members of the Bcl-2 family to participate in
the release of cytochrome c and the subsequent activation of
caspase-9 (7,8). Furthermore, VDACs are involved in
caspase-independent apoptosis (9). Among the three isomers (VDAC1, VDAC2
and VDAC3), VDAC1 shows the highest expression in mammalian
mitochondria and plays an important role in apoptosis (10,11). VDAC1 upregulation promotes
apoptosis in multiple cell lines (12,13). VDAC1 is also a target of various
pro-apoptotic compounds such as curcumin, arsenic trioxide and
cannabinoids (14-16).
A previous study showed that a VDAC1-based peptide
induces apoptosis by releasing bound hexokinase 2 (HK2) from the
mitochondria (17). HK2 is a
crucial enzyme in the glycolytic pathway and antagonizes apoptosis
through its direct interaction with VDAC1 (18). HK2 is overexpressed in a variety
of tumor cell types, and HK2 is transported to the mitochondria and
interacts with VDAC1, which promotes the development of anaerobic
metabolism to compensate for the higher energy requirements in
tumor cells (19). Most tumor
cells still show active glucose uptake and glycolysis under aerobic
conditions, a phenomenon known as the Warburg effect, and metabolic
dysregulation affects tumor growth and cell death (20,21). Several compounds, such as
3-bromopyru-vate and methyl jasmonates, interact with HK2, causing
HK2 to separate from mitochondria and trigger apoptosis (22,23).
20(S)-Ginsenoside Rh2 [20(S)-GRh2], a
protopanaxadiol-type ginsenoside extracted from
Panaxginseng, is an active component with an anti-tumor
effect that inhibits tumor cell growth and induces tumor cell
apoptosis (24,25). Previous studies showed that
20(S)-GRh2 has safety advantages (such as reverse multidrug
resistance, balanced immunity and enhanced chemotherapy) (24) and shows no side effects,
indicating 20(S)-GRh2 may be a promising new antitumor drug
(26). Several reports
demonstrated that 20(S)-GRh2 stimulates apoptosis and induces
mitochondrial dysfunction by activating caspases, upregulating
various pro-apoptotic proteins such as Bax and downregulating
Bcl-XL (27-29). Therefore, 20(S)-GRh2 may stimulate
HeLa cell apoptosis by affecting the mitochondria and regulating
energy metabolism.
The present study tested this hypothesis and the
results showed that 20(S)-GRh2 exposure activated
mitochondrion-dependent apoptosis and suppressed mitochondrial
oxidative phosphorylation and glycolysis. To understand its
underlying mechanism, it was found that VDAC1 was significantly
upregulated in response to 20(S)-GRh2 treatment, while HK2 was
downregulated and separated from mitochondria. Bax transport from
the cytosol to the mitochondria was promoted by 20(S)-GRh2 and
knockdown of VDAC1 reduced 20(S)-GRh2-induced Bax translocation and
apoptosis. The results provided a more comprehensive understanding
of the mechanistic association between cancer mitochondrial
metabolism and tumor growth control by 20(S)-GRh2 and may
facilitate the development of new therapeutic methods for cervical
cancer.
Materials and methods
Materials and antibodies
20(S)-GRh2 (98% pure) was purchased from Shanghai
Yanye Biotechnology Co., Ltd. The following primary antibodies were
used in this study: Anti-HK2 rabbit polyclonal antibody (1:1,000;
cat. no. 22029-1-AP; ProteinTech Group, Inc.), anti-VDAC1 rabbit
monoclonal antibody (1:1,000; cat. no. AF1027; Beyotime Institute
of Biotechnology), anti-prohibitin rabbit monoclonal antibody
(1:1,000; cat. no. AF1126; Beyotime Institute of Biotechnology),
anti-cytochrome c rabbit monoclonal antibody (1:1,000; cat.
no. AF2047; Beyotime Institute of Biotechnology), anti-Bax rabbit
monoclonal antibody (1:1,000; cat. no. D2E11; Cell Signaling
Technology, Inc.), anti-Bcl-2 rabbit polyclonal antibody (1:1,000;
cat. no. abs131701, Absin) and anti-β-actin mouse monoclonal
antibody (1:1,000; cat. no. BS6007M; Bioworld Technology,
Inc.).
Cell culture and transfection
HeLa cells were obtained from the Cell Bank of the
Chinese Academy of Sciences and cultured in DMEM (HyClone; Cytiva)
with 10% FBS (HyClone; Cytiva), 100 µg/ml penicillin and 100
µg/ml streptomycin (HyClone; Cytiva) at 37°C in a humidified
atmosphere with 5% CO2. Cells were transfected with a
final concentration of 100 nM small interfering RNA (siRNA)
targeting VDAC1 using ribo-FECT CP reagent (Guangzhou Ribobio Co.,
Ltd.) according to the manufacturer's instructions. Following
incubation for 24 h, HeLa cells were treated with 20(S)-GRh2 (35
and 45 µM) for 24 h at 37° before subsequent
experimentation. The target sequence in negative controls for RNA
interference is 5′-AAT TCT CCG AAC GTG TCA CGT-3′, and the target
sequences in VDAC1 for RNA interference were 5′-GGA GAC CGC TGT CAA
TCT T-3′ (siVC1-1) and 5′-GCT GCG ACA TGG ATT TCG A-3′
(siVC1-2).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from HeLa cells treated with
20(S)-GRh2 using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). RNA purity was measured using a
BioSpec-nano spectrophotometer (Shimadzu Corporation), and total
RNA was reverse transcribed using a PrimeScript RT Reagent Kit with
gDNA Eraser (Takara Bio, Inc.) according to the manufacturer's
instructions. RT-qPCR was performed using a SYBR-Green assay
(Takara Bio, Inc.) on a CFX Connect Real-Time system (Bio-Rad
Laboratories, Inc.). Fold-changes in the expression of each gene
were calculated using the 2-ΔΔCq method (30). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30
sec. GAPDH mRNA was used as an internal reference. The following
primer pairs were used for the qPCR: GAPDH forward, 5′-CTG GGC TAC
ACT GAG CAC C-3′ and reverse, 5′-AAG TGG TCG TTG AGG GCA ATG-3′
(Harbin Xinhai Genetic Testing Co., Ltd.); VDAC1 forward, 5′-CTG
ACC TTC GAT TCA TCC TTC TC-3′ and reverse, 5′-CTC CCG CTT GTA CCC
TGT C-3′ (Harbin Xinhai Genetic Testing Co., Ltd.) and HK2 forward,
5′-AGC CAC CAC TCA CCC TAC-3′ and reverse, 5′-CCA TTG TCC GTT ACT
TTC AC-3′ as well as forward, 5′-AGC CAC CAC TCA CCC TAC-3′ and
reverse, 5′-CCC ATT GTC CGT TAC TTT C-3′ (Comate Bioscience Co.,
Ltd.).
Cell viability assessment
The Cell Counting Kit-8 (CCK-8) assay (Wuhan Boster
Biological Technology Co., Ltd.) was used to assess cell viability
according to the manufacturer's instructions. HeLa cells were
treated with 20(S)-GRh2 at 37°C for 24 h at various concentrations
(35, 45, 55 and 65 µM) to determine the IC50
values. In addition, cells were treated with IC50
concentrations at 37°C at various timepoints (6, 12, 24, 36 and 48
h). Following treatment, 20 µl CCK-8 solution was added to
each well, and the plate was incubated at 37°C for 1 h. Absorption
at a wavelength of 450 nm was recorded using an Infinite M200 pro
reader (Tecan Group, Ltd.) and the cell viability was
calculated.
Cell apoptosis analysis
Cells were treated with various concentrations (35
and 45 µM) of 20(S)-GRh2 at 37°C for 24 h. Cells were then
suspended in 1X Binding Buffer and incubated with 5 µl
Annexin V-FITC and 5 µl PI (BD Biosciences) at room
temperature in the dark for 15 min. The cells were analyzed with a
Flow Sight flow cytometer (Amnis Corporation) and data were
analyzed using IDEAS software v6.1 (Amnis Corporation). Apoptotic
cells were also analyzed by Hoechst 33342 staining (Beyotime).
Briefly, following 20(S)-GRh2 (35 and 45 µM) treatment,
cells were incubated with Hoechst 33342 at 37°C in the dark for 30
min. Cells treated with 45 µM DMSO waere used as the control
group. Images were captured under an EVOS FL Auto fluorescence
microscope (Thermo Fisher Scientific, Inc.) at ×295
magnification.
Determination of the mitochondrial
membrane potential (MMP)
MMP alterations were measured using a JC-1 MMP Assay
kit (Beijing Solarbio Science & Technology Co., Ltd.). HeLa
cells were treated with 20(S)-GRh2 (35 and 45 µM) at 37°C
for 24 h and then washed with cold PBS. A total of 10 µM
carbonyl cyanide 3-chlorophenylhydrazone (CCCP) was used to induce
a decrease in mitochondrial membrane potential for 20 min as a
positive control. JC-1 working solution was added to the cell
culture medium, and cells were incubated at 37°C in the dark for 20
min. Cells were imaged using an EVOS FL Auto fluorescence
microscope (Thermo Fisher Scientific, Inc.) at ×295
magnification.
MMP was also measured using the cationic dye
rhodamine 123 (Beyotime Institute of Biotechnology). Briefly, HeLa
cells were pre-treated with 20(S)-GRh2 (35 and 45 µM) at
37°C for 24 h and then incubated with 2 µM rhodamine 123 at
37°C in the dark for 30 min. Fluorescence intensity was detected
using the Flow Sight flow cytometer (Amnis Corporation) and data
were analyzed using IDEAS software v6.1 (Amnis Corporation).
Western blotting
Cells treated with 20(S)-GRh2 (35 and 45 µM)
at 37°C for 24 h were lysed in RIPA buffer (Beyotime Institute of
Biotechnology) pre-cooled at 4°C. Mitochondrial and cytoplasmic
proteins were extracted using a cell mitochondrial isolation kit
(Beyotime Institute of Biotechnology). Protein quantification was
performed using the BCA method (Beyotime Institute of
Biotechnology). Equal amounts of protein (30 µg) were
subjected to 12% SDS-PAGE and then transferred onto a
nitrocellulose membrane (Pall Corporation). The membranes were
blocked with 5% skim milk powder and PBS for 1 h at room
temperature, then incubated with primary antibodies overnight at
4°C, followed by incubation with goat polyclonal secondary antibody
to mouse IgG-H&L (HRP) (1:1,000; cat. no. 115-005-00; Jackson
ImmunoResearch Laboratories, Inc.) and goat anti-rabbit IgG H&L
(HRP) (1:1,000; cat. no. 111-005-003; Jackson ImmunoResearch
Laboratories, Inc.) for 1 h at 37°C. Development was performed with
an ECL kit (Beyotime Institute of Biotechnology). Imaging and
densitometric analysis were performed using an iBright FL1000
imaging system (Invitrogen; Thermo Fisher Scientific, Inc.).
β-actin and prohibitin were used as loading controls.
Measurement of cellular ATP levels
Total ATP levels were determined using a
Chemiluminescence ATP assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Briefly, the culture solution was removed, followed by addition of
200 µl lysate to each well of a six-well plate and
centrifuged at 12,000 × g to collect the cell supernatant. In
addition, 100 µl ATP detection working solution was added to
the 96-well assay plates and incubated at room temperature for 3
min. Subsequently, 20 µl sample was added to the wells and
analyzed on the Infinite M200 pro reader (Tecan Group, Ltd.).
Meanwhile, added gradient diluions of ATP standard solution were
added to 96-well plates to generate a standard curve, and ATP
concentration was calculated according to the standard curve.
Determination of reactive oxygen species
(ROS) levels
To detect intracellular ROS levels, the
ROS-sensitive probe 2′,7′-dichlorodihydrofluorescein diacetate
(H2DCFDA; Beyotime Institute of Biotechnology) was used.
Cells were treated with the indicated concentrations of 20(S)-GRh2
(35 and 45 µM) for 24 h and then incubated with 10 µM
H2DCFDA in the dark for 30 min at 37°C. The fluorescence
intensity of the cells was measured with a Flow Sight flow
cytometer (Amnis Corporation).
Mitochondrial oxidative phosphorylation
(OXPHOS) and glycolysis assays
A total of 5×103 HeLa cells were seeded
into the B-G pores of an XF 8 Cell Culture Microplate (Agilent
Technologies, Inc.) in 80 µl medium. Equal volumes of media
were then added into the A and H pores, followed by overnight
incubation at 37°C with 5% CO2. Only the calibration
solution was left on both sides of the culture chamber. HeLa cells
were treated with 20(S)-GRh2 (35 and 45 µM) for 24 h. The
probe plate of the Seahorse XFp sensor was incubated overnight in
the calibration solution the day before the experiment. Cells were
washed with 180 µl DMEM without bicarbonate and then
incubated at 37°C for 1 h before measurement.
Oxygen consumption rates (OCR) and extracellular
acidification rates (ECAR) were measured using an XFp Extracellular
Flux Analyzer (Agilent Technologies, Inc.) according to
manufacturer′s instructions. The OCR was continuously recorded for
12 cycles, with each cycle consisting of 3 min of mixing and 3 min
of measurement. Basal respiration was measured during cycles 1-3.
Further measurement of OCR was run as follows: As 4-6 cycles of
oligomycin injection (oligo, ATP synthase inhibitor, 5 µM),
7-9 cycles of carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
(FCCP, proton, 5 µM) injection and 10-12 cycles of
rotenone/antimycin A (AA/Rot, complex I and III inhibitor, 2.5
µM) injection [all, XFp Cell Mito Stress Test kit (Agilent
Technologies, Inc.)]. Further measurement of ECAR was run as
follows: 4-6 cycles of glucose (substrate for hexokinase, 10 mM)
injection, 7-9 cycles of oligomycin (oligo, ATP synthase inhibitor,
5 µM) injection and 10-12 cycles of 2-Deoxy-D-glucose (2-DG,
competitive inhibitor of hexokinase, 50 mM) injection. The use of
these inhibitors permits the determination of key aspects of
mitochondrial function (31).
Caspase activity assays
Assays for caspase-3 (cat. no. C1115), -8 (cat. no.
C1151) and -9 (cat. no. C1157) activities were performed using kits
according to the manufacturer′s instructions (Beyotime Institute of
Biotechnology). Briefly, cells were washed with PBS, collected by
centrifugation (600 × g, 5 min) at 4°C and resuspended in ice cold
lysis buffer. A total of 100 µl lysate was used per
2×106 cells and incubated on ice for 20 min. The protein
concentration in the supernatant was measured with a Bradford assay
kit (Tiangen Biotech Co., Ltd.). Subsequently, 50 µl cell
lysate supernatant was mixed with 10 µl Ac-DEVD-pNA (2 mM)
for caspase-3, Ac-IETD-pNA (2 mM) for caspase-8 and Ac-LEHD-pNA (2
mM) for caspase-9 in 40 µl assay buffer for 4 h at 37°C, and
then analyzed with an Infinite M200 pro reader (Tecan Group,
Ltd.).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software 6 (GraphPad Software, Inc.). In quantitative
analyses shown in histograms, values were obtained from three
independent experiments and expressed as the mean ± SD. One-way
ANOVA followed by Dunnett′s post-hoc test was used to compare
between two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
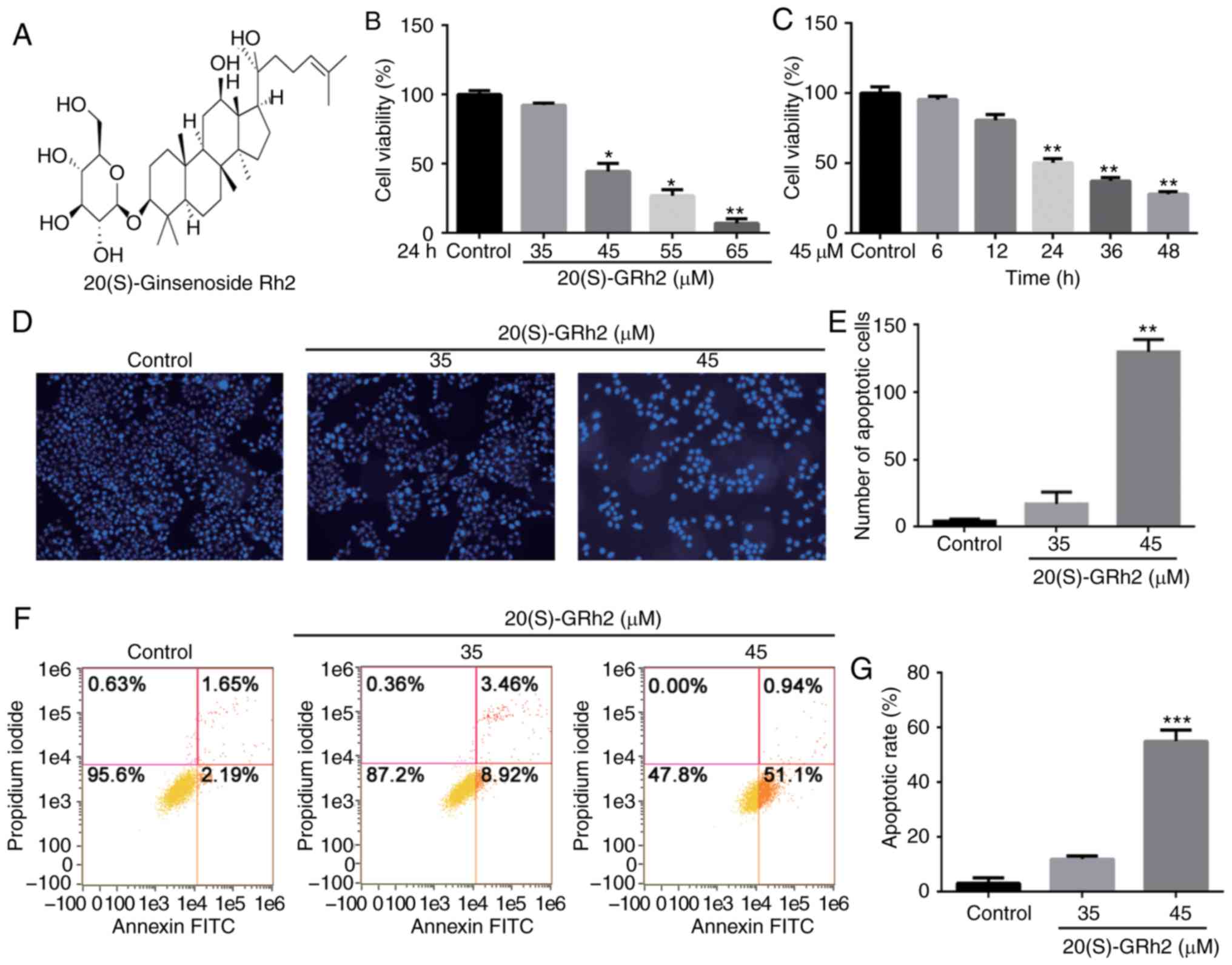
20(S)-GRh2 inhibits cell viability and
induces apoptosis in HeLa cells
The CCK-8 method was used to examine the effect of
20(S)-GRh2 (Fig. 1A) on HeLa cell
viability. Cells were treated with various doses of 20(S)-GRh2 for
different timepoints. The results showed that 20(S)-GRh2 reduced
the viability of HeLa cells in dose- and time-dependent manners
(Fig. 1B and C). The
IC50 of 20(S)-GRh2 was calculated to be ~45 µM,
and 35 and 45 µM for further experiments.
It was next examined whether 20(S)-GRh2 inhibited
cell viability via inducing apoptosis. Hoechst staining showed an
increase in the amount of condensed and fragmented nuclei in cells
treated with 45 µM 20(S)-GRh2 compared with the control
group (Fig. 1D and E), indicating
that 20(S)-GRh2 induced apoptosis in HeLa cells. Annexin V and PI
double staining was used to detect the apoptotic rate of HeLa cells
treated with 20(S)-GRh2. The percentages of apoptotic cells upon
treatment with 0, 35 and 45 µM 20(S)-GRh2 were 3.84, 12.38
and 52.04%, respectively (Fig. 1F and
G). Taken together, these findings suggested that 20(S)-GRh2
reduces cell viability by inducing apoptosis in HeLa cells.
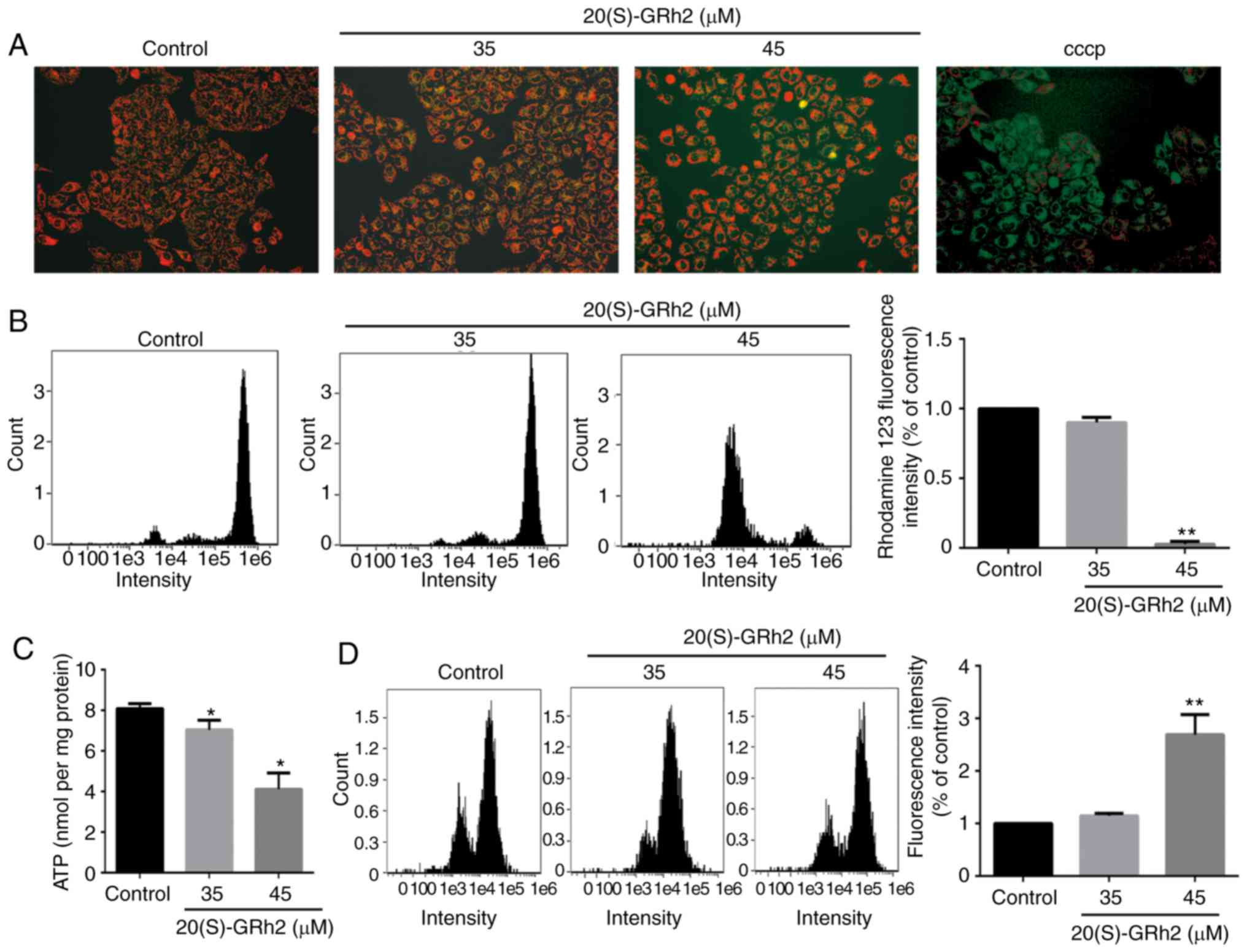
20(S)-GRh2 reduces MMP, decreases ATP
generation and induces ROS in HeLa cells
Mitochondria play a central role in the growth and
apoptosis of cells (32).
Therefore, it was next assessed whether 20(S)-GRh2-induced
apoptosis was associated with mitochondrial dysfunction. The
effects of 20(S)-GRh2 on the MMP, an indicator of mitochondrial
functions, were assessed using JC-1 and rhodamine-123 staining.
Fluorescence microscopy showed that cells exposed to 20(S)-GRh2
showed increased conversion of JC-1 aggregates (red) to JC-1
monomers (green). Following treatment with 10 µM CCCP as the
positive control, the cells showed obvious green fluorescence,
indicating that 10 µM CCCP almost completely depleted the
MMP of HeLa cells (Fig. 2A). Flow
cytometry showed that 45 µM 20(S)-GRh2 treatment
significantly reduced the fluorescence intensity of rhodamine-123
(Fig. 2B). MMP and ATP generation
are indicators of mitochondrial function (33), and thus the effects of 20(S)-GRh2
on the cellular ATP levels were assessed. Compared with the control
group, 20(S)-GRh2 exposure reduced cellular ATP levels in HeLa
cells by 14% (35 µM) and 49% (45 µM) (Fig. 2C). The decrease in MMP and ATP
levels showed that 20(S)-GRh2 induced mitochondrial dysfunction in
HeLa cells.
It was hypothesized that 20(S)-GRh2-induced
mitochondrial dysfunction may cause increased levels of ROS. To
test this hypothesis, ROS levels in 20(S)-GRh2-treated HeLa cells
were measured. Low concentrations of 20(S)-GRh2 (35 µM) had
no significant effect on cellular ROS, whereas a high concentration
of 20(S)-GRh2 (45 µM) significantly increased cellular ROS
by 2.69-fold compared with control cells (Fig. 2D).
20(S)-GRh2 inhibits mitochondrial OXPHOS
and glycolysis
Since the mitochondrial electron transfer chain is
the main target site of ROS (34), the present study hypothesized that
20(S)-GRh2 might affect mitochondrial ATP generation, which is
involved in 20(S)-GRh2-induced cell death. The XFp extracellular
flux analyzer was then used to assess the role of 20(S)-GRh2 on
mitochondrial OXPHOS and glycolysis, the two major energy
metabolism pathways, in real-time. OXPHOS was measured by
quantifying the OCR of the medium and glycolysis was assessed by
quantifying the ECAR of the medium. Treatment with 20(S)-GRh2 (35
and 45 µM) caused a decrease in the OCR and altered cellular
responses to typical mitochondrial complexes inhibitors (including
oligomycin, an ATP synthase inhibitor; FCCP, a mitochondrial
uncoupler and AA/Rot, inhibitors of complex I and III), suggesting
that the mitochondrial OXPHOS capacity was inhibited (Fig. 3A). HeLa cells had a significantly
reduced OCR linked to ATP production and maximum respiratory
capacity following 20(S)-GRh2 (35 and 45 µM) treatment
(Fig. 3B). However, in HeLa cells
treated with 20(S)-GRh2, the OCR associated with non-mitochondrial
respiration was also reduced (Fig.
3A). This result indicated that 20(S)-GRh2 may also inhibit the
oxygen consumption of the non-mitochondrial respiratory pathway,
but the exact molecular mechanism was unclear.
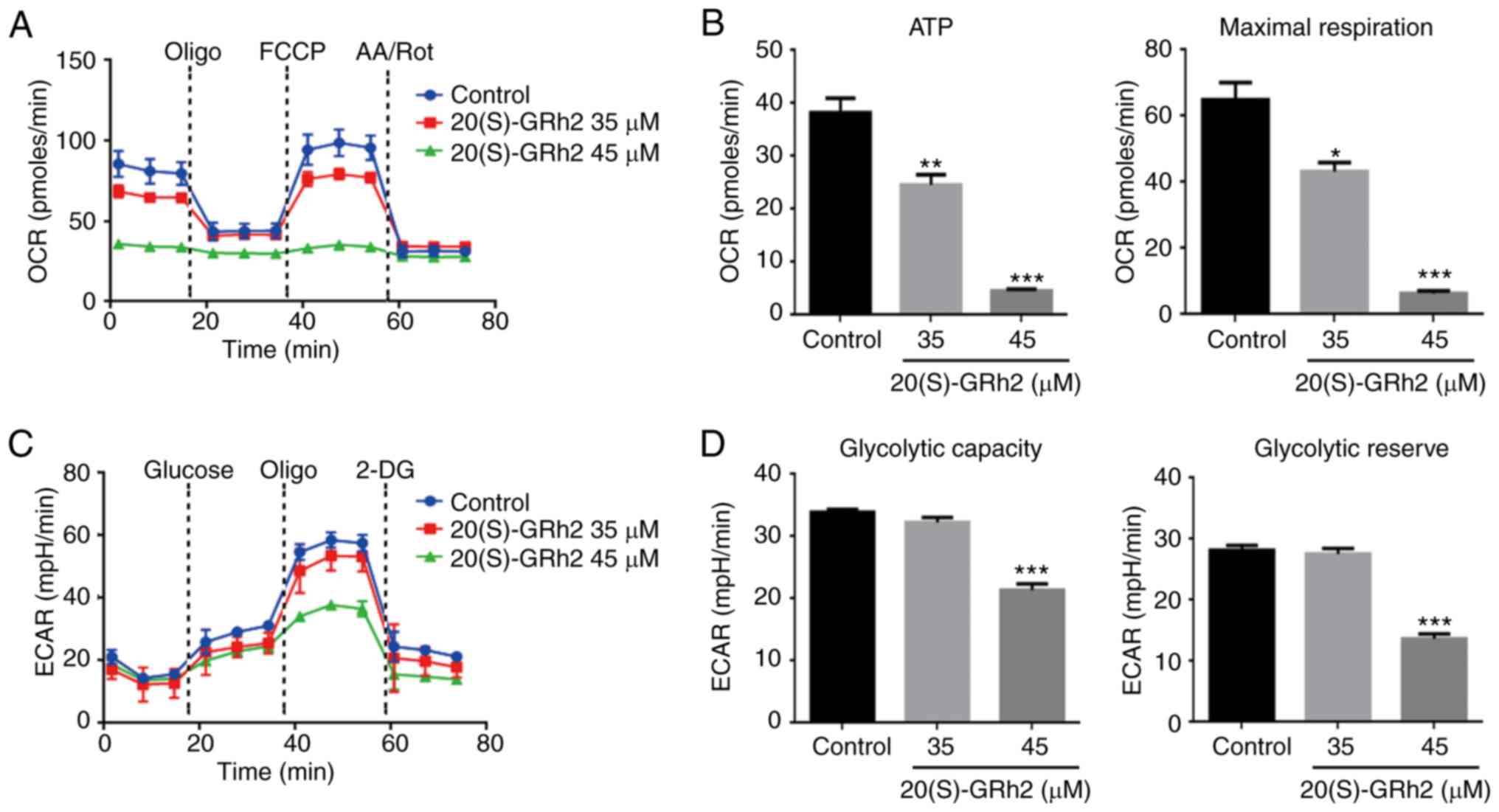 | Figure 3Effects of 20(S)-GRh2 on
mitochondrial respiration and glycolysis. HeLa cells were treated
with 20(S)-GRh2 at indicated concentrations for 24 h and the
dynamics of OCR and ECAR were measured. (A) Graphical
representation of the OCR measurement over time; sequential
additions are indicated as: Oligo, FCCP and AA/Rot. (B) The effects
of 20(S)-GRh2 on the ATP-linked OCR and maximal respiration
capacity-linked OCR calculated from the OCR curves. Data analyzed
using one-way ANOVA and presented as the mean ± standard deviation.
n=3. *P<0.05; **P<0.01 and
***P<0.001 vs. control. (C) Graphical representation
of the ECAR measurement over time; sequential additions are
indicated as: Glucose, Oligo and 2-DG. (D) The effects of
20(S)-GRh2 on the glycolytic capacity-linked ECAR and glycolytic
reserve-linked ECAR calculated from the ECAR curves. Data were
analyzed using one-way ANOVA and presented as the mean ± standard
deviation. n=3. ***P<0.001 vs. control. 20(S)-GRh2,
20(S)-ginsenoside Rh2; OCR, oxygen consumption rate; ECAR,
extracellular acidification rate; Oligo, oligomycin; FCCP, carbonyl
cyanide 4-(trifluoromethoxy) phenylhydrazone; AA/Rot, antimycin
A/rote-none; 2-DG, 2-Deoxy-D-glucose; mpH/min, the rate of mpH
change per minute. |
Numerous cancer cells break down glucose by aerobic
glycolysis to obtain the energy needed for growth in a process
known as the Warburg effect (35). It was next examined whether
20(S)-GRh2 also induced this effect on glycolysis in HeLa cells.
The results showed that both basal and stimulated ECAR reduced
after 45 µM 20(S)-GRh2 treatment (Fig. 3C). 20(S)-GRh2 (45 µM)
significantly reduced the ECAR linked to glycolytic capacity and
the glycolytic reserve in HeLa cells (Fig. 3D). At 35 µM, 20(S)-GRh2 had
no effect on ECAR (Fig. 3C) but
inhibited OCR (Fig. 3A) and
decreased cellular ATP levels (Figs.
2C and 3B), suggesting that
20(S)-GRh2 decreased ATP production primarily by inhibition of
OXPHOS.
Mitochondrial pathway is involved in
20(S)-GRh2-induced HeLa cell apoptosis
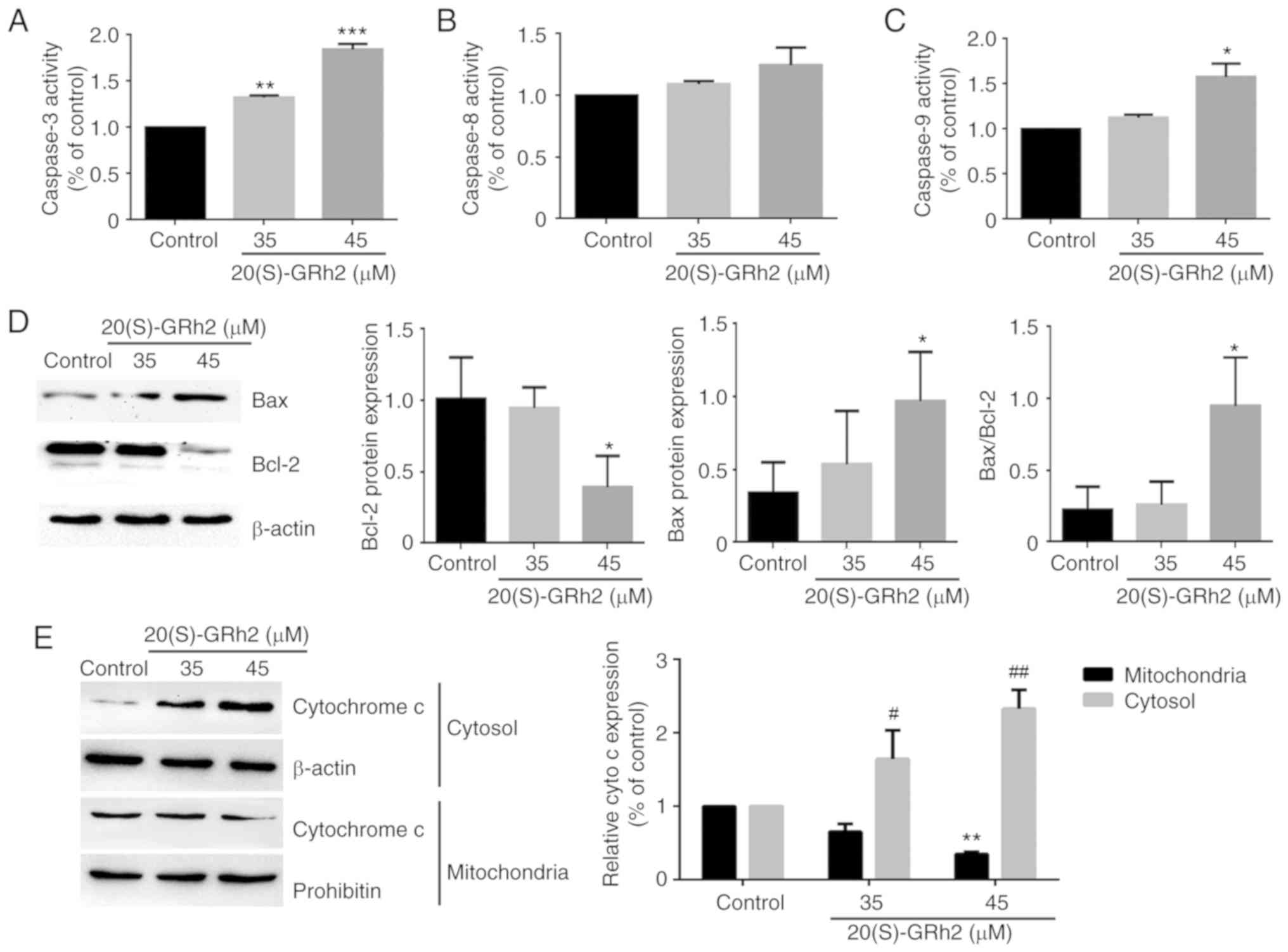
To further assess the apoptotic process induced by
20(S)-GRh2, the activation of caspase-3, a critical marker of
apoptosis, was investigated. As shown in Fig. 4A, 20(S)-GRh2 treatment
significantly increased caspase-3 activity in a dose-dependent
manner. Next, the upstream regulators of caspase-3 involved in
20(S)-GRh2-induced apoptosis were determined.
Mitochondrion-associated caspase-9 activity was significantly
increased by 45 µM 20(S)-GRh2 but did not affect death
receptor-associated caspase-8 activity in HeLa cells (Fig. 4B and C). Since caspase-9 is
activated by Bcl-2 family proteins (36), it was investigated whether
20(S)-GRh2 affected the expression of Bcl-2 and Bax proteins. 45
µM 20(S)-GRh2 induced Bax upregulation and Bcl-2
downregulation (Fig. 4D). Bcl-2
and Bax induces the release of cytochrome c from the
mitochondria into the cytoplasm (37). Western blot analysis showed that
20(S)-GRh2 significantly promoted the release of cytochrome
c into the cytoplasm, which coincided with caspase-9
activation (Fig. 4E). Once
cytochrome c is released into the cytoplasm, it activates
caspase-9, triggering a cascade causing mitochondrion-mediated
apoptosis (38); hence, the
results indicated that 20(S)-GRh2 induces apoptosis via the
mitochondrial pathway (Fig.
4E).
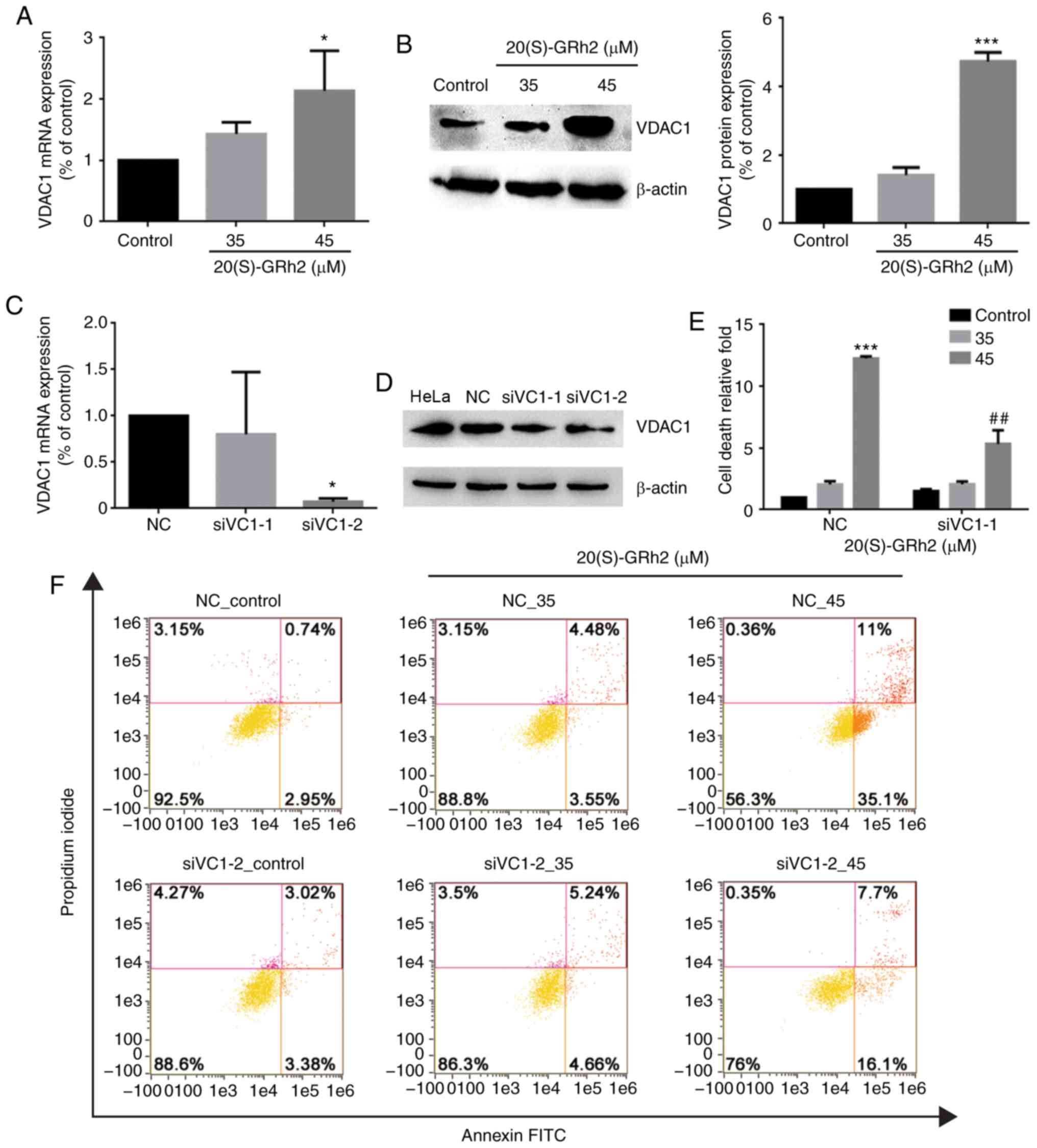
Upregulation of VDAC1 plays a role in
20(S)-GRh2-induced apoptosis
VDAC1 plays an important role in regulating
mitochondrial energy metabolism and mitochondrion-mediated
apoptosis (39). Therefore, the
role of VDAC1 in 20(S)-GRh2-induced apoptosis was assessed. VDAC1
expression in HeLa cells was significantly increased by 45
µM 20(S)-GRh2 treatment (Fig.
5A and B), indicating that induction of VDAC1 may be involved
in 20(S)-GRh2-mediated induction of apoptosis in HeLa cells. To
determine the role of VDAC1 in HeLa cell apoptosis induced by
20(S)-GRh2, a synthetic siRNA targeting VDAC1 to knockdown VDAC1 in
HeLa cells was used (Fig. 5C and
D). Annexin V staining showed that 20(S)-GRh2-induced apoptosis
was suppressed in VDAC1-silenced cells (Fig. 5E and F). These results showed that
20(S)-GRh2 induced apoptosis in HeLa cells by regulating VDAC1.
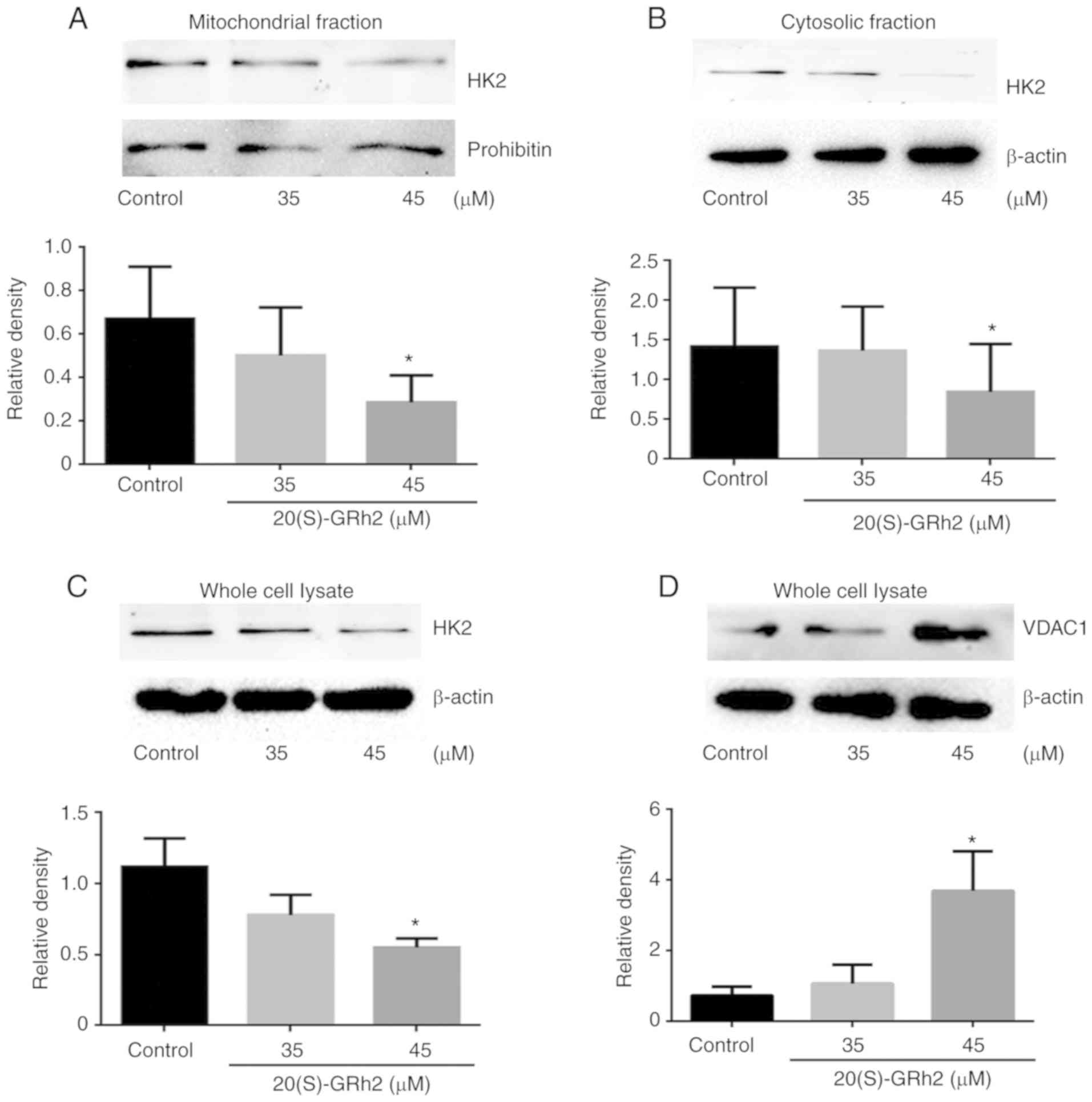
20(S)-GRh2-induced reduction of HK2
results in VDAC1 upregulation
To further elucidate the molecular mechanism
underlying 20(S)-GRh2-induced VDAC1 upregulation on HeLa cell
apoptosis, known VDAC1-binding proteins were investigated. HK2
interacts with VDAC1 and plays major roles in tumor cell
metabolism, including mitochondrial ATP synthesis and glucose
metabolism (40). HK2 also
inhibits tumor cell apoptosis by inhibiting changes in
mitochondrial membrane permeability (41). Therefore, HK2 expression was
measured. It was found that the expression of mitochondrial HK2 and
cytosolic HK2 in HeLa cells treated with 45 µM 20(S)-GRh2
was decreased which was consistent with the pattern of total HK2
(Fig. 6A-C). By contrast, the
expression levels of VDAC1 protein was increased in response to 45
µM 20(S)-GRh2 (Fig. 6D),
indicating that 20(S)-GRh2 induces changes in the expression levels
of HK2 and VDAC1 that are closely related to its induction of
apoptosis.
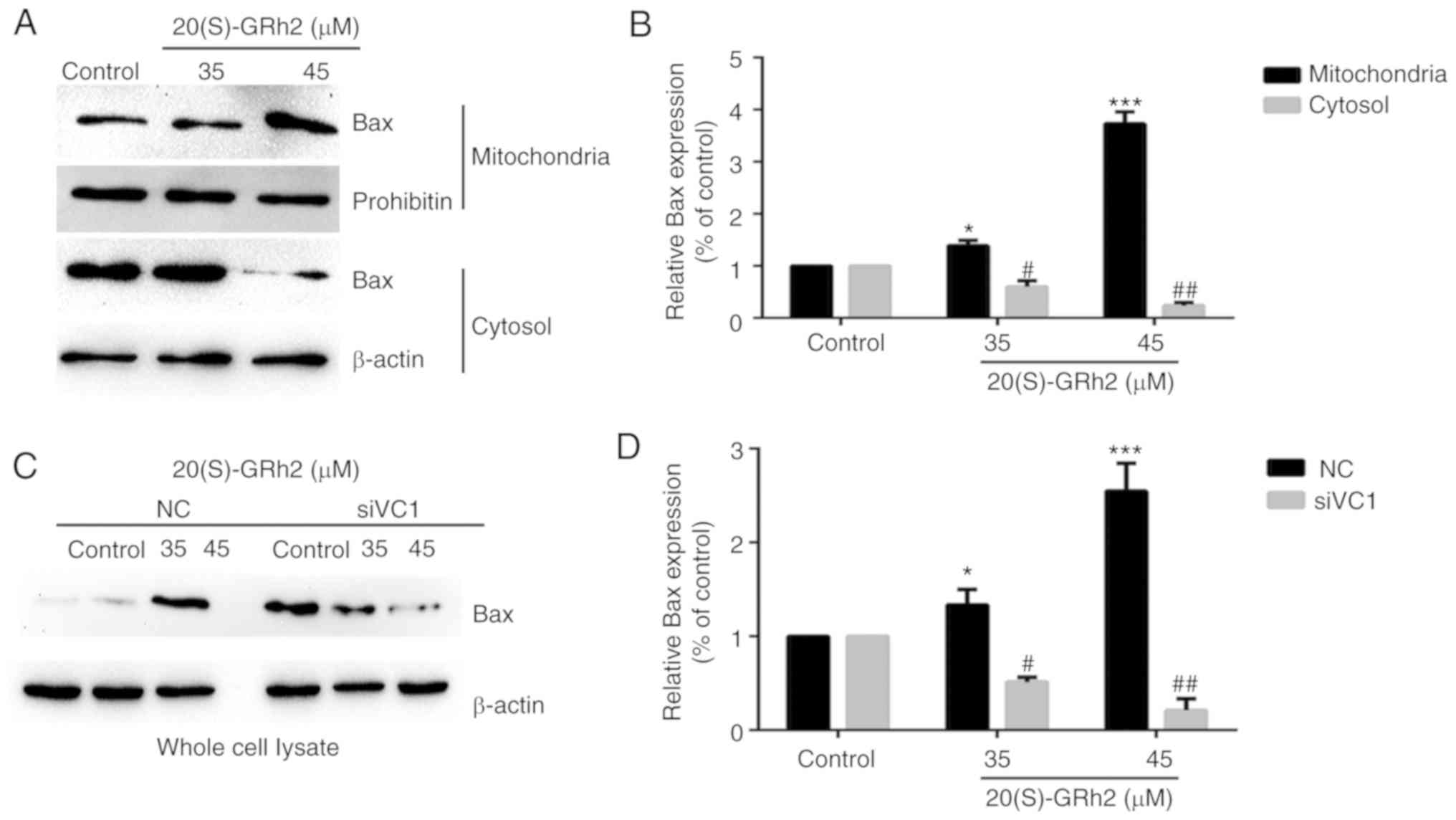
Bax expression is involved in
20(S)-GRh2-induced apoptosis
It was found that 20(S)-GRh2 treatment induced Bax
upregulation and promoted Bax translocation to the mitochondria
(Fig. 7A and B). Bax expression
was significantly suppressed when VDAC1 was silenced by siRNA
(Fig. 7C and D). The
aforementioned results showed that the separation of HK2 from the
mitochondria promoted the translocation of Bax to mitochondria and
the release of cytochrome cinto the cytoplasm.
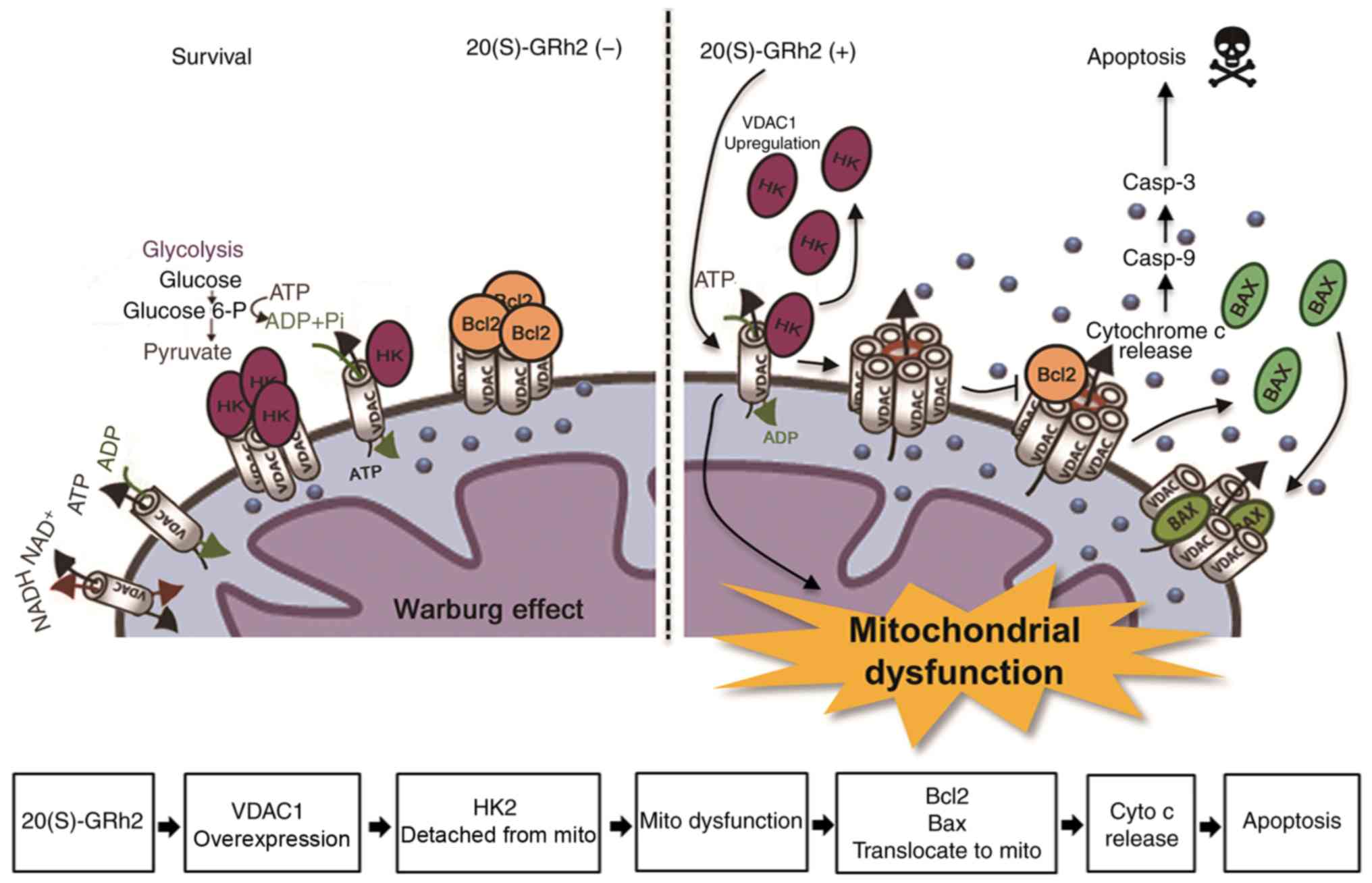
We hypothesize that treatment of HeLa cells with
20(S)-GRh2 causes metabolic stress leading to upregulation of
VDAC1, the translocation of Bax to the mitochondria and subsequent
apoptosis of cancer cells (Fig.
8).
Discussion
The present study found that 20(S)-GRh2, a
pharmacologically active component of Panax ginseng, exerted
significant cytotoxicity in HeLa cells. Exposure to 20(S)-GRh2
altered mitochondrial function in HeLa cells, leading to
mitochondrion-related apoptosis, confirming that 20(S)-GRh2 induces
apoptosis of HeLa cells, as reported previously (25,42). In addition, cell death induced by
20(S)-GRh2 was associated with the regulation of energy metabolism.
Treatment with 20(S)-GRh2 significantly inhibited OXPHOS and
glycolysis. The dual targeting of mitochondrial and glycolysis
pathways by 20(S)-GRh2 may explain why ROS generation, MMP collapse
and ATP reduction were observed in HeLa cells treated with
20(S)-GRh2.
In addition, the OCR that corresponds to
non-mitochondrial O2 consumption was also slightly
affected by 20(S)-GRh2. Mitochondria consume >95% of the
O2 of aerobic higher organisms; however, 10% of cellular
oxygen uptake in mammal cells is due to non-mitochondrial
respiration (43). It has been
proposed that an increase in non-mitochondrial oxygen consumption
might serve as a protective mechanism to remove ROS when it is
present at potentially harmful concentrations (44). This suggestion provides a
plausible explanation for the decrease in non-mitochondrial oxygen
consumption observed in the present study, as it would help
increase in ROS generation and oxidative stress to further induce
apoptosis.
The mechanism by which 20(S)-GRh2 regulates cell
death and energy metabolism was next elucidated. 20(S)-GRh2
influenced the protein levels of VDAC1 and HK2, which are important
regulators of glucose metabolism (45,46), and the changes in VDAC1 and HK2
protein levels were inversely associated. Treatment with 20(S)-GRh2
inhibited mitochondrial metabolism, leading to dissociation of HK2
from mitochondrial VDAC1, which regulated the transfer of Bax to
the mitochondria and allowed cytochrome c to enter the
cytosol via the outer mitochondrial membrane. These events led to
apoptosis induced by metabolic stress. A loss of the MMP during
20(S)-GRh2-induced apoptosis and the opening of mitochondrial
permeability transition pores were observed. The present findings
are the first to show that this mechanism is associated with
20(S)-GRh2-induced apoptosis. These results also showed that VDAC1
may be a target in the process of HeLa cell apoptosis induced by
20(S)-GRh2.
By transferring HK2 to the mitochondria, an acidic
anti-hypoxic microenvironment suitable for tumor cell survival can
be formed and maintained (47).
The interaction between HK2 and VDAC1 provides metabolic
preponderance for cancer cells by enhancing anaerobic glycolysis,
which is called the Warburg effect (48). When HK2 separates from the
mitochondria, cells become sensitive to many apoptotic factors
(49). Many anticancer compounds
that target the mitochondria have been shown to release HK2 from
the mitochondria (50-52). The present study found that
20(S)-GRh2 disaggregated HK2 from the mitochondria in HeLa cells.
However, whether desorption of HK2 was due solely to the direct
interaction of 20(S)-GRh2 with VDAC1 was unclear, and an
interaction between 20(S)-GRh2 and HK2 cannot be ruled out. Thus,
20(S)-GRh2-induced cell death is a cumulative effect of VDAC1
blockade and desorption of HK2 from mitochondria. ROS production,
MMP collapse and ATP decrease may be the result of VDAC shutdown.
The wide range of antitumor activities of 20(S)-GRh2 includes
inhibiting tumor growth, restraining tumor progression and
strengthening chemotherapeutic responses (24). Previous studies have shown that
20(S)-GRh2 inhibits the growth of many tumor cell types and its
potential mechanism has been confirmed. Ginsenoside Rh2 also
inhibited proliferation and promotes apoptosis of H1299 cells by
inducing endoplasmic reticulum stress mediated by ROS (53). The synergistic effect of autophagy
and β-catenin signal transduction inhibited the proliferation and
migration of human liver cancer HepG2, Hep3B and Huh7 cells, and
restrained tumor growth in HepG2-xenografted mice (54,55). Ginsenoside Rh2 inhibited cell
proliferation, promoted apoptosis and reversed the resistance of
colon cancer to the chemotherapeutic drug oxaliplatin (56). The p53 tumor suppressor is also
activated in response to ginsenosides (25,57). Ginsenoside Rh2 also induced
apoptosis of HCT-116 and SW-480 cells via p53 activation (58).
In conclusion, the present findings have revealed
VDAC1 as a new target of 20(S)-GRh2. These results have provided
further understanding of the metabolic mechanism of ginsenoside Rh2
against cancer and have validated the potential clinical value for
20(S)-GRh2 or anticancer drugs based on 20(S)-GRh2 in cervical
cancer in vitro. However, the present study only studied a
human papillomavirus (HPV)-positive cervical cancer cell. Based on
evidence suggesting the heterogeneity in cervical cancer-related
pathways in terms of HPV status, we are currently examining the
effects of 20(S)-GRh2 in a comprehensive panel of human cervical
cancer-derived cell lines, including HPV-positive and HPV-negative
cell lines. Future research will examine the efficacy of 20(S)-GRh2
in an in vivo system.
Funding
This work was supported by the National Key Research
and Development program of China (grant no. 2017YFC1702104) and
grants from the National Natural Foundation of China (grant no.
81703663); and Key Project At Central Government Level: The Ability
Establishment of Sustainable Use For Valuable Chinese Medicine
Resources (grant no. 2060302).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors′ contributions
ML and JW managed data collection and designed the
experiments. YL, JQ, SL and SW performed the experiments. YL and ML
wrote the manuscript. DZ and XB contributed to data analysis,
supervised the experiments and helped draft the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Chen S and Wang J: HAND2-AS1 inhibits
invasion and metastasis of cervical cancer cells via
microRNA-330-5p-mediated LDOC1. Cancer Cell Int. 19:3532019.
View Article : Google Scholar :
|
|
2
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar :
|
|
3
|
Di J, Rutherford S and Chu C: Review of
the cervical cancer burden and population-based cervical cancer
screening in China. Asian Pac J Cancer Prev. 16:7401–7407. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwata T, Miyauchi A, Suga Y, Nishio H,
Nakamura M, Ohno A, Hirao N, Morisada T, Tanaka K, Ueyama H, et al:
Neoadjuvant chemotherapy for locally advanced cervical cancer. Chin
J Cancer Res. 28:235–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marchetti C, Fagotti A, Tombolini V,
Scambia G and De Felice F: Survival and toxicity in neoadjuvant
chemotherapy plus surgery versus definitive chemoradiotherapy for
cervical cancer: A systematic review and meta-analysis. Cancer
Treat Rev. 83:1019452020. View Article : Google Scholar
|
|
6
|
Shoshan-Barmatz V, Ben-Hail D, Admoni L,
Krelin Y and Tripathi SS: The mitochondrial voltage-dependent anion
channel 1 in tumor cells. Biochim Biophys Acta. 1848:2547–2575.
2015. View Article : Google Scholar
|
|
7
|
Abu-Hamad S, Zaid H, Israelson A, Nahon E
and Shoshan-Barmatz V: Hexokinase-I protection against apoptotic
cell death is mediated via interaction with the voltage-dependent
anion channel-1: Mapping the site of binding. J Biol Chem.
283:13482–13490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shoshan-Barmatz V, Israelson A, Brdiczka D
and Sheu SS: The voltage-dependent anion channel (VDAC): Function
in intracellular signalling, cell life and cell death. Curr Pharm
Des. 12:2249–2270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adachi M, Higuchi H, Miura S, Azuma T,
Inokuchi S, Saito H, Kato S and Ishii H: Bax interacts with the
voltage-dependent anion channel and mediates ethanol-induced
apoptosis in rat hepatocytes. Am J Physiol Gastrointest Liver
Physiol. 287:G695–G705. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zaid H, Abu-Hamad S, Israelson A, Nathan I
and Shoshan-Barmatz V: The voltage-dependent anion channel-1
modulates apoptotic cell death. Cell Death Differ. 12:751–760.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto T, Yamada A, Watanabe M,
Yoshimura Y, Yamazaki N, Yoshimura Y, Yamauchi T, Kataoka M, Nagata
T, Terada H and Shinohara Y: VDAC1, having a shorter N-terminus
than VDAC2 but showing the same migration in an SDS-polyacrylamide
gel, is the predominant form expressed in mitochondria of various
tissues. J Proteome Res. 5:3336–3344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li B, Chen X, Yang W, He J, He K, Xia Z,
Zhang J and Xiang G: Single-walled carbon nanohorn aggregates
promotes mitochondrial dysfunction-induced apoptosis in
hepatoblastoma cells by targeting SIRT3. Int J Oncol. 53:1129–1137.
2018.PubMed/NCBI
|
|
13
|
Nagakannan P, Islam MI, Karimi-Abdolrezaee
S and Eftekharpour E: Inhibition of VDAC1 protects against
glutamate-induced oxytosis and mitochondrial fragmentation in
hippocampal HT22 cells. Cell Mol Neurobiol. 39:73–85. 2019.
View Article : Google Scholar
|
|
14
|
Tewari D, Ahmed T, Chirasani VR, Singh PK,
Maji SK, Senapati S and Bera AK: Modulation of the mitochondrial
voltage dependent anion channel (VDAC) by curcumin. Biochim Biophys
Acta. 1848:151–158. 2015. View Article : Google Scholar
|
|
15
|
Watanabe M, Funakoshi T, Unuma K, Aki T
and Uemura K: Activation of the ubiquitin-proteasome system against
arsenic trioxide cardiotoxicity involves ubiquitin ligase Parkin
for mitochondrial homeostasis. Toxicology. 322:43–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rimmerman N, Ben-Hail D, Porat Z, Juknat
A, Kozela E, Daniels MP, Connelly PS, Leishman E, Bradshaw HB,
Shoshan-Barmatz V and Vogel Z: Direct modulation of the outer
mitochondrial membrane channel, voltage-dependent anion channel 1
(VDAC1) by cannabidiol: A novel mechanism for cannabinoid-induced
cell death. Cell Death Dis. 4. pp. e9492013, View Article : Google Scholar
|
|
17
|
Liu Y, Cheng H, Zhou Y, Zhu Y, Bian R,
Chen Y, Li C, Ma Q, Zheng Q, Zhang Y, et al: Myostatin induces
mitochondrial metabolic alteration and typical apoptosis in cancer
cells. Cell Death Dis. 4:e4942013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pastorino JG and Hoek JB: Regulation of
hexokinase binding to VDAC. J Bioenerg Biomembr. 40:171–182. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dolder M, Wendt S and Wallimann T:
Mitochondrial creatine kinase in contact sites: Interaction with
porin and adenine nucleotide translocase, role in permeability
transition and sensitivity to oxidative damage. Biol Signals
Recept. 10:93–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rai Y, Yadav P, Kumari N, Kalra N and
Bhatt AN: Hexokinase II inhibition by 3-bromopyruvate sensitizes
myeloid leukemic cells K-562 to anti-leukemic drug, daunorubicin.
Biosci Rep. 39:BSR201908802019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo X, Zhang X, Wang T, Xian S and Lu Y:
3-Bromopyruvate and sodium citrate induce apoptosis in human
gastric cancer cell line MGC-803 by inhibiting glycolysis and
promoting mitochondria-regulated apoptosis pathway. Biochem Biophys
Res Commun. 475:37–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu
L, Zhao M, Liu Q, Cheng Z, Zou J, et al: Naturally occurring
anti-cancer compounds: Shining from Chinese herbal medicine. Chin
Med. 14:482019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo XX, Li Y, Sun C, Jiang D, Lin YJ, Jin
FX, Lee SK and Jin YH: p53-dependent Fas expression is critical for
ginsenoside Rh2 triggered caspase-8 activation in HeLa cells.
Protein Cell. 5:224–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S, Guo W, Gao Y and Liu Y: Ginsenoside
Rh2 inhibits growth of glioblastoma multiforme through mTor. Tumour
Biol. 36:2607–2612. 2015. View Article : Google Scholar
|
|
27
|
Popovich DG and Kitts DD:
Structure-function relationship exists for ginsenosides in reducing
cell proliferation and inducing apoptosis in the human leukemia
(THP-1) cell line. Arch Biochem Biophys. 406:1–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo XX, Guo Q, Li Y, Lee SK, Wei XN and
Jin YH: Ginsenoside Rh2 induces human hepatoma cell apoptosisvia
bax/bak triggered cytochrome C release and caspase-9/caspase-8
activation. Int J Mol Sci. 13:15523–15535. 2012. View Article : Google Scholar
|
|
29
|
Choi S, Oh JY and Kim SJ: Ginsenoside Rh2
induces Bcl-2 family proteins-mediated apoptosis in vitro and in
xenografts in vivo models. J Cell Biochem. 112:330–340. 2011.
View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Hu M, Crawford SA, Henstridge DC, Ng IH,
Boey EJ, Xu Y, Febbraio MA, Jans DA and Bogoyevitch MA: p32 protein
levels are integral to mitochondrial and endoplasmic reticulum
morphology, cell metabolism and survival. Biochem J. 453:381–391.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao L, Zhu L and Guo X: Valproic acid
attenuates Aβ 25-35-induced neurotoxicity in PC12 cells through
suppression of mitochondria-mediated apoptotic pathway. Biomed
Pharmacother. 106:77–82. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liberti MV and Locasale JW: The Warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qu C, Zhang S, Wang W, Li M, Wang Y, van
der Heijde-Mulder M, Shokrollahi E, Hakim MS, Raat NJH,
Peppelenbosch MP and Pan Q: Mitochondrial electron transport chain
complex III sustains hepatitis E virus replication and represents
an antiviral target. FASEB J. 33:1008–1019. 2019. View Article : Google Scholar :
|
|
35
|
Pavithra PS, Mehta A and Verma RS:
Induction of apoptosis by essential oil from P. Missionis in skin
epidermoid cancer cells. Phytomedicine. 50:184–195. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abbaszadeh H, Valizadeh A, Mahdavinia M,
Teimoori A, Pipelzadeh MH, Zeidooni L and Alboghobeish S:
3-Bromopyruvate potentiates TRAIL-induced apoptosis in human colon
cancer cells through a reactive oxygen species- and
caspase-dependent mitochondrial pathway. Can J Physiol Pharmacol.
97:1176–1184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan X, Yan D, Wang D, Wu X, Zhao W, Lu Q
and Yan H: Mitochondrion-mediated apoptosis induced by acrylamide
is regulated by a balance between Nrf2 antioxidant and MAPK
signaling pathways in PC12 cells. Mol Neurobiol. 54:4781–4794.
2017. View Article : Google Scholar
|
|
38
|
Camara AKS, Zhou Y, Wen PC, Tajkhorshid E
and Kwok WM: Mitochondrial VDAC1: A key gatekeeper as potential
therapeutic target. Front Physiol. 8:4602017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xue YN, Yu BB, Li JL, Guo R, Zhang LC, Sun
LK, Liu YN and Li Y: Zinc and p53 disrupt mitochondrial binding of
HK2 by phosphorylating VDAC1. Exp Cell Res. 374:249–258. 2019.
View Article : Google Scholar
|
|
40
|
Li M, Shao J, Guo Z, Jin C, Wang L, Wang
F, Jia Y, Zhu Z, Zhang Z, Zhang F, et al: Novel
mitochondrion-targeting copper(II) complex induces HK2 malfunction
and inhibits glycolysis via Drp1-mediating mitophagy in HCC. J Cell
Mol Med. 24:3091–3107. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shoshan-Barmatz V, Maldonado EN and Krelin
Y: VDAC1 at the crossroads of cell metabolism, apoptosis and cell
stress. Cell Stress. 1:11–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ham YM, Lim JH, Na HK, Choi JS, Park BD,
Yim H and Lee SK: Ginsenoside-Rh2-induced mitochondrial
depolarization and apoptosis are associated with reactive oxygen
species- and Ca2+-mediated c-Jun NH2-terminal kinase 1 activation
in HeLa cells. J Pharmacol Exp Ther. 319:1276–1285. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brand MD and Nicholls DG: Assessing
mitochondrial dysfunction in cells. Biochem J. 435:297–312. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bishop T and Brand MD: Processes
contributing to metabolic depression in hepatopancreas cells from
the snail Helix aspersa. J Exp Biol. 203:3603–3612. 2000.PubMed/NCBI
|
|
45
|
Bustamante MF, Oliveira PG,
Garcia-Carbonell R, Croft AP, Smith JM, Serrano RL, Sanchez-Lopez
E, Liu X, Kisseleva T, Hay N, et al: Hexokinase 2 as a novel
selective metabolic target for rheumatoid arthritis. Ann Rheum Dis.
77:1636–1643. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park SH, Lee AR, Choi K, Joung S, Yoon JB
and Kim S: TOMM20 as a potential therapeutic target of colorectal
cancer. BMB Rep. 52:712–717. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pastorino JG and Hoek JB: Hexokinase II:
The integration of energy metabolism and control of apoptosis. Curr
Med Chem. 10:1535–1551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yuan S, Fu Y, Wang X, Shi H, Huang Y, Song
X, Li L, Song N and Luo Y: Voltage-dependent anion channel 1 is
involved in endostatin-induced endothelial cell apoptosis. FASEB J.
22:2809–2820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shoshan-Barmatz V and Ben-Hail D: VDAC, a
multi-functional mitochondrial protein as a pharmacological target.
Mitochondrion. 12:24–34. 2012. View Article : Google Scholar
|
|
50
|
Arbel N, Ben-Hail D and Shoshan-Barmatz V:
Mediation of the antiapoptotic activity of Bcl-xL protein upon
interaction with VDAC1 protein. J Biol Chem. 287:23152–23161. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zheng Y, Shi Y, Tian C, Jiang C, Jin H,
Chen J, Almasan A, Tang H and Chen Q: Essential role of the
voltage-dependent anion channel (VDAC) in mitochondrial
permeability transition pore opening and cytochrome c release
induced by arsenic trioxide. Oncogene. 23:1239–1247. 2004.
View Article : Google Scholar
|
|
52
|
Tajeddine N, Galluzzi L, Kepp O, Hangen E,
Morselli E, Senovilla L, Araujo N, Pinna G, Larochette N, Zamzami
N, et al: Hierarchical involvement of Bak, VDAC1 and Bax in
cispl-atin-induced cell death. Oncogene. 27:4221–4232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li H, Huang N, Zhu W, Wu J, Yang X, Teng
W, Tian J, Fang Z, Luo Y, Chen M and Li Y: Modulation the crosstalk
between tumor-associated macrophages and non-small cell lung cancer
to inhibit tumor migration and invasion by ginsenoside Rh2. BMC
Cancer. 18:5792018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li Q, Li B, Dong C, Wang Y and Li Q:
20(S)-Ginsenoside Rh2 suppresses proliferation and migration of
hepatocellular carcinoma cells by targeting EZH2 to regulate
CDKN2A-2B gene cluster transcription. Eur J Pharmacol. 815:173–180.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang J, Li W, Yuan Q, Zhou J, Zhang J,
Cao Y, Fu G and Hu W: Transcriptome analyses of the
anti-proliferative effects of 20(S)-ginsenoside Rh2 on HepG2 cells.
Front Pharmacol. 10:13312019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ma J, Gao G, Lu H, Fang D, Li L, Wei G,
Chen A, Yang Y, Zhang H and Huo J: Reversal effect of ginsenoside
Rh2 on oxaliplatin-resistant colon cancer cells and its mechanism.
Exp Ther Med. 18:630–636. 2019.PubMed/NCBI
|
|
57
|
Li B, Zhao J, Wang CZ, Searle J, He TC,
Yuan CS and Du W: Ginsenoside Rh2 induces apoptosis and
paraptosis-like cell death in colorectal cancer cells through
activation of p53. Cancer Lett. 301:185–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang CZ, Zhang B, Song WX, Wang A, Ni M,
Luo X, Aung HH, Xie JT, Tong R, He TC and Yuan CS: Steamed American
ginseng berry: Ginsenoside analyses and anticancer activities. J
Agric Food Chem. 54:9936–9942. 2006. View Article : Google Scholar : PubMed/NCBI
|