Introduction
Idiopathic short stature (ISS) is characterized by a
standing height of <2 standard deviation scores (SDSs;
equivalent to the 2.3rd percentile or commonly regarded as the
<3rd percentile) for a given age, sex and population, in the
absence of systemic disease, a psychological disorder, nutritional
imbalance, overt hormone deficiency or chromosomal abnormality
(1). The pathogenesis of ISS has
been investigated for >10 years; however, its exact etiology
remains unclear (2-5). Currently, the primary treatment for
ISS is recombinant human growth hormones; however, this treatment
is not effective in all children with ISS (6) and treatment responses vary
considerably between studies (7-10).
Therefore, the pathogenesis of ISS requires further elucidation to
improve methods for treating the condition.
Longitudinal bone growth is driven by growth plates
(11), that consist of three
distinct zones: Resting, proliferative and hypertrophic. Cells
within the resting zone are known as chondrocyte-progenitor cells,
which subsequently divide into chondrocytes to form the
proliferative zone. Chondrocytes then proliferate and differentiate
into the hypertrophic zone, where blood vessels, osteoblasts and
osteoclasts convert cartilage into bone, resulting in longitudinal
bone growth (11-13). Numerous studies have shown that
non-coding (nc)RNAs regulate the growth plate (14-16) and thus, may affect longitudinal
bone growth. For example, it has been reported that microRNAs
(miRNA/miR) could regulate the endochondral ossification of the
growth plate and longitudinal bone growth (17,18).
Circular (circ)RNAs are an identified group of
ncRNAs, that are widely expressed and highly conserved in mammalian
cells (19-21). circRNAs are highly stable compared
with that in other non-coding miRNAs and long ncRNAs (lncRNAs), due
to their closed circular structure (19-21). Several studies have shown that
circRNAs could regulate cell proliferation, differentiation and
apoptosis in different types of cells, such as chondrocytes, tumor
cells, cardiomyocytes and endothelial cells (22-26), whilst their increased or decreased
expression levels has been associated with a number of diseases,
including cancer, diabetes, preeclampsia, osteoarthritis, and
cardiovascular and neurological diseases (23-26). However, to the best of our
knowledge, the role of circRNAs in ISS pathogenesis has not yet
been addressed.
The present study compared the circRNA expression
patterns of patients with ISS and healthy individuals to identify
differentially expressed circRNAs that regulate ISS pathogenesis
and their target miRNAs.
Materials and methods
Study subject characteristics
A total of 152 patients with ISS and
age-/sex-matched control individuals were recruited from The Second
Affiliated Hospital of Nanchang University, China. Patients
exhibiting the following were included in the study: i) A
small-for-gestational age (birth weight or length <10th
percentile) during their neonatal period; ii) hormonal
abnormalities [e.g. growth hormone (GH) deficiency, with a peak
level of ≤6 ng/ml] or pubertal or thyroid disorders; iii) exposure
to chronic conditions and environmental factors that influence
human growth, such as nephrotic syndrome and inflammatory bowel
disease; iv) skeletal anomalies or dysmorphic features; and/or v)
cytogenetically detected chromosomal aberrations, such as
chondrodysplasia and Down's syndrome; and vi) whole exon
sequencing. A total of 152 blood samples were obtained from the
study participants between October 2016 and March 2018, and were
immediately frozen in liquid nitrogen, before storage at -80°C
until further analysis. For circRNA microarray analysis, 4 specimen
pairs were used, whilst all 76 pairs were used to validate circRNA
expression using reverse transcription-quantitative PCR (RT-qPCR).
Human specimen collection was approved by the Human Research Ethics
Committee of the Second Affiliated Hospital of Nanchang University.
As all the participants were under the age of 12 years, written
informed consent was provided by their parents or legal guardians
before their enrollment.
Sample labeling and hybridization for
microarray
The 4 blood sample pairs were used for microarray
analysis, with the Arraystar Human circRNA microarray v2
(Arraystar, Inc.). The RNA was extracted from the samples using
TRIzol® (Thermo Fisher Scientific, Inc.) and were
prepared for microarray hybridization using an Eastep®
Super RNA simple total RNA kit (Promega Corporation) according to
the manufacturer's protocol. Total RNA was digested using RNase R
(Epicentre; Illumina, Inc.) to enrich the sample for circRNAs and
eliminate linear RNAs. The circRNA samples were amplified by PCR
and transcribed into fluorescent cy3-UTP-labeled cRNA using a
random priming approach with the Arraystar super RNA labeling kit
(Arraystar, Inc.). The total RNA concentration of each sample was
measured using a NanoDrop ND-1000 Spectrophotometer (Nanodrop
Technologies; Thermo Fisher Scientific, Inc.). Labeled cRNA samples
were hybridized onto the Arraystar human circRNA array v2 (8×15 K;
Arraystar, Inc.). After washing the slides using gene expression
wash buffer 1 (cat. no. 5188-5325; Agilent Technologies, Inc.),
reactions were performed using an Agilent G2505C microarray scanner
(Agilent Technologies, Inc.).
Microarray analysis of differentially
expressed circRNAs
To obtain raw expression data, the array images were
processed using Agilent feature extraction software (v11.0.1.1;
Agilent Technologies, Inc.). circRNA expression values were
quantile normalized and analyzed using the Quantile algorithm and
3.11 version of limma packages (https://www.bioconductor.org/packages/release/bioc/html/limma.html)
in R 3.6.0 software (https://www.R-project.org/). The obtained circRNA
expression profiles were divided into ISS and control groups.
Volcano plot filtering was used to identify circRNAs, that were
significantly differently expressed between the ISS and control
groups, and the Benjamini-Hochberg method was used to determine the
false discovery rate (FDR)-corrected P-value. Statistical
significance was set at a fold-change (FC) value of >2.0 and
FDR-corrected P<0.05. circRNA expression patterns between the
two sample groups were distinguished using hierarchical clustering,
while scatter plots were used to assess significantly
differentially expressed circRNAs between the two groups, both
using the R program.
Bioinformatics and in silico
analyses
As miRNAs play significant roles in regulating
longitudinal bone growth and circRNAs have been found to act as
miRNA sponges by altering gene expression levels (19-21,27), the CircInteractome database
(http://circinteractome.nia.nih.gov/)
was used to predict the target genes of circRNAs. In addition, the
miR-140-3p target genes were predicted using Starbase database
(http://starbase.sysu.edu.cn/) and KEGG
pathway analyses was performed using the Database for Annotation,
Visualization and Integrated Discovery (DAVID; v6.8; https://david.ncifcrf.gov/). The signaling pathways
were automatically identified and ranked in the webpage of DAVID
according to P<0.05 and enrichment factor.
RT-qPCR validation of key circRNAs
Total RNA was extracted from the frozen specimens
using an Eastep® Super RNA simple total RNA kit (Promega
Corporation). After cDNA synthesis (PrimeScript™ RT reagent kit and
PrimeScript™ RT reagent kit with gDNA Eraser; Takara Bio, Inc.),
circRNA expression levels were quantified using RT-qPCR with the
primer sequences in Table I, and
an ABI Q6 PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The RT-qPCR protocol used for circRNA-0079201 (TB
Green® Premix Ex Taq™ II; Takara Bio, Inc.) was as
follows: Initial denaturation at 95°C for 10 min, followed by 40
cycles of 95°C for 10 sec, and 60°C for 34 sec. GAPDH was used as
the internal control. Relative mRNA expression levels of the target
genes were calculated using the 2−ΔΔCq method (28) and normalized against GAPDH, an
internal control used to reduce errors, due to differences in RNA
concentration and transcription efficiency. IQCE is the host gene
of hsa_circRNA_0079201. For verifying the upregulation of
hsa_circRNA_0079201, but not linear IQCE, RNase R (Epicentre;
Illumina, Inc.) was used to degrade linear RNA.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR of circRNA, miRNA and mRNA
expression levels. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR of circRNA, miRNA and mRNA
expression levels.
| Primer name | Sequence
(5′-3′) |
|---|
|
circRNA_0079201F |
TGTGGTGCTTCAGGCAGCTT |
|
circRNA_0079201R |
GCTTGTACACCTTCCACTGGG |
|
circRNA_0000423F |
TTGAATTGAAATGCAGAGCCAATG |
|
circRNA_0000423R |
GGGTGGCCTTTTCCAACTGT |
|
circRNA_0051239F |
GGGTGGGGCCTGTAGGATA |
|
circRNA_0051239R |
AGGACAGCATGCCCCTGATA |
|
circRNA_0087631F |
CACAGCTGAGAAGTAGTCAGAACT |
|
circRNA_0087631R |
AGTTGGCTCCACATCACTGG |
|
circRNA_0064644F |
AGGTACTCAGAGGCAGGACT |
|
circRNA_0064644R |
TAACGTATGGCGGATGAGGC |
| ActinF |
AGCACAGAGCCTCGCCTTTG |
| ActinR |
CTTCTGACCCATGCCCACCA |
| miR-140-3pF |
GGCGTACCACAGGGTAGAA |
| miR-140-3pR |
GTCGTATCCAGTGCAGGGTCCGAGGTATTGCACTGGATACGACCCGTG |
| miR-564F |
GGCATAGGCACGGTGUC |
| miR-564R |
TGGTGTCGTGGAGTCG |
| miR-635F | TATAGCA
TATGCAGGGTG |
| miR-635R | CGCATTCGGAGTGC
GAGTT |
| U6F |
CTCGCTTCGGCAGCACA |
| U6R |
AACGCTTCACGAATTTGCGT |
| SMAD2F |
ATGTCGTCCATCTTGCCATT |
| SMAD2R |
TTTTCTTCCTGCCCATTCTG |
| IHHF |
GACTCATTGCCTCCCAGAACTG |
| IHHR |
CCAGGTAGTAGGGTCACATTGC |
| RUNX2F |
ACTTCCTGTGCTCCGTGCTG |
| RUNX2R |
TCGTTGAACCTGGCTACTTGG |
| COL10F |
GCAGCATTACGACCCAAGAT |
| COL10R |
CATGATTGAACTCCCTGAAG |
| GAPDHF |
GGAGCGAGATCCCTCCAAAAT |
| GAPDHR |
GGCTGTTGTCATACTTCTCATGG |
Cell culture
Human chondrocytes were purchased from the Procell
Cell Resource Center and cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified incubator with 5% CO2.
Hsa_circRNA_0079201 overexpression vector
and miR-140-3p mimics
The hsa_circRNA_0079201 plasmid
(pHBLV-CMV-Cicr-MCS-EF1-zsgreen-T2A-puro) was successfully
constructed by Hanbio Biotechnology Co., Ltd. The
hsa_circRNA_0079201 overexpression vector was named as
overcircRNA_0079201. Chondrocytes were seeded (5×105) in
a 6-well plate and cultured until 80% confluence. The
overcircRNA_0079201 (5 µl) was then added to the 6-well
plate using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.). An empty vector was used as the control
group. The transfection efficiency of the hsa_circRNA_0079201
vector was evaluated using a fluorescence microscope and RT-qPCR.
The final concentration of hsa_circRNA_0079201 plasmid was 50 nM.
The miR-140-3p mimics were obtained from Hanbio Biotechnology Co.,
Ltd. The 5 µl miR-140-3p mimics (sense 5′-UAC CAC AGG GUA
GAA CCA CGG-3′ and antisense 5′-CCG UGG UUC UAC CCU GUG GUA-3′) or
negative control (NC) (sense 5′-UUU GUA CUA CAC AAA AGU ACU G-3′,
and antisense 5′-CAG UAC UUU UGU GUA GUA CAA A-3′) were transfected
into the cells in a 6-well plate using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) basing on the
manufacturer's instructions. The transfection efficiency of the
miR-140-3p mimics was evaluated using RT-qPCR. The final
concentration of miR-140-3p mimics/NC was 50 nM. The cells were
harvested for further experimentation 72 h following transfection.
All the experiments were conducted independently three times.
Cell proliferation and
5′ethynyl-2′-deoxyuridine (EdU) assays
Chondrocytes transfected with overcircRNA_0079201
were seeded in a 6-well plate (5×105) and cultured until
70-80% confluent. The proliferation rate was assessed using a Cell
Counting Kit-8 (CCK-8; TransGen Biotech Co., Ltd.) kit in
accordance with the manufacturer's protocol. Briefly,
2×103 chondrocytes were seeded in 96-well plates and
incubated at 37°C in a humidified incubator with 5% CO2,
and their proliferation was examined once every 24 h, for 96 h, 4
times in total. After 24 h, 10 µl CCK-8 was added to each
well, the cells were cultured for 2 h at 37°C in a humidified with
incubator 5% CO2, and the solution was measured
spectrophotometrically at 450 nm (Varioskan Flash, Agilent
Technologies, Inc.). For evaluating the role of circRNA_0079201 on
the chondrocyte proliferation, the normal control group (empty
vector) and the blank group (neither overcircRNA_0079201 or empty
vector) were used as the control group in order to compare to
overcircRNA_0079201 group. All statistical analyses were performed
using GraphPad Prism (v5.0; GraphPad Software, Inc.) software.
Chondrocytes were incubated with EdU (Guangzhou
Ribobio Co., Ltd.) for 2 h according to the manufacturer's
instructions. The chondrocytes were then treated with 200 µl
1X Apollo reaction mixture for 25 min (Cell proliferation test kit;
Beijing Solarbio Science and Technology Co., Ltd.). Subsequently,
Hoechst 33342 (5 µg/ml) was added to each well to stain at
room temperature (22±3°C) for 30 min, following which the cells
were observed using a fluorescence microscope (×4 magnification)
and images were obtained.
Luciferase reporter assay
The sequence of hsa_ circ_0079201-wt
(5′-GCCCAAGAGCTCCCAGCTCCCACTCCCAGCAGCAGGCACTGCGAGCAAGACTGGCCGCCGGATTCCAGCGAGGAGGGGCTCCCGCGGCCCCGCTCCCCCTGCTCTGATGGGAGAAGAGACGCCGCGGCCAGAGTCCTGCAGGCCCAGTGGAAGGTGTACAAGCACAAGAAAAAAAAGGCTGTTCTGGATGAGGCGGCTGTGGTGCTTCAGGCAGCTTTCAGGGGACATCTCACGCGGACAAAGCTCTTAGCAAGCAAAGCACATGGCTCAGAGCCACCCAGCGTGCCAGGCCTCCCAGACCAG-3′)
and the sequence of hsa_circ_0079201-mu
(5′-GCCCAAGAGCTCCCAGCTCCCACTCCCAGCAGCAGGCACTGCGAGCAAGACTGGCCGCCGGATTCCAGCGAGGAGGGGCTCCCGCGGCCCCGCTCCCCCTGCTCTGATGGGAGAAGAGACGCCGCGGCCAGAGTCCTGCAGGCCCAGTGGAAGGTGTACAAGCACAAGAAAAAAAAGGCTGTTCTGGATGAGGCGGgTcTcGaGCTTCAGGCAGCTTTCAGGGGACATCTCACGCGGACAAAGCTCTTAGCAAGCAAAGCACATGGCTCAGAGCCACCCAGCGTGCCAGGCCTCCCAGACCAG-3′)
were added into the pSI-Check2 vector. The hsa_circRNA_0079201
plasmid or its mutated fragments (Fig. S1A) and miR-140-3p mimic were
co-transfected into the 293T cell line using Lipofiter (Hanbio
Biotechnology Co., Ltd.) according to the manufacturer′s
instructions. Similarly, the sequence of SMAD2-3UTR-wt was:
5′-AGCTTCACCAATCAAGTCCCATGAAAAGACTTAATGTAACAACTCTTCTGTCATAGCATTGTGTGTGGTCCCTATGGACTGTTTACTATCCAAAAGTTCAAGAGAGAAAACAGCACTTGAGGTCTCATCAATTAAAGCACCTTGTGGAATCTGTTTCCTATATTTGAATATTAGATGGGAAAATTAGTGTCTAGAAATACTCTCCCATTAAAGAGGAAGAGAAGATTTTAAAGACTTAATGATGTCTTATTGGGCATAAAACTGAGTGTCCCAAAGGTTTATTAATAACAGTAGTAGTTA-3′,
and the sequence of SMAD2-3UTR-mu was:
5′-AGCTTCACCAATCAAGTCCCATGAAAAGACTTAATGTAACAACTCTTCTGTCATAGCATTGTGaGaGcTCCCTATGGACTGTTTACTATCCAAAAGTTCAAGAGAGAAAACAGCACTTGAGGTCTCATCAATTAAAGCACCTTGTGGAATCTGTTTCCTATATTTGAATATTAGATGGGAAAATTAGTGTCTAGAAATACTCTCCCATTAAAGAGGAAGAGAAGATTTTAAAGACTTAATGATGTCTTATTGGGCATAAAACTGAGTGTCCCAAAGGTTTATTAATAACAGTAGTAGTTA-3′
were also added into the pSI-Check2 vector. SMAD2 plasmid
(h-SMAD2-3UTR, Hanbio Biotechnology Co., Ltd.) or its mutated
fragments (Fig. S1B) and
miR-140-3p were also co-transfected into the 293T cell line.
Lowercase letters repre-sent the mutation sites. After 48 h of
transfection, a dual-luciferase assay system (Promega Corporation)
was used to determine the firefly and Renilla luciferase
activities of the transfected cells.
Western blot analysis
After harvesting the chondrocytes from the
overcircRNA_0079201 and NC groups, the protein samples were
extracted using cell lysis (Tissue cell total protein extraction
kit; Applygen Technologies Inc.). The concentration of total
protein was evaluated using a BCA assay (Pierce; Thermo Fisher
Scientific, Inc.). Subsequently, the protein lysates were separated
using SDS-PAGE (Beijing Biosynthesis Biotechnology), transferred
onto PVDF membranes and then blocked using skimmed milk (Beijing
Solarbio science and Technology, Inc.) for 1 h at room temperature
(22±3°C). Following which, the membranes were incubated with
anti-Smad2 (1:2,000 dilution; cat. no. ab40855), anti-RUNX2
(1:2,000 dilution; cat. no. ab192256), anti-collagen type X
(COL10A1; 1:2,000 dilution; ab58632), and anti-Indian Hedgehog
(1:2,000 dilution; ab39634) (all from Abcam) primary antibodies,
overnight at 4°C. The membrane was then rinsed using 1X
TBS-Tween-20 (Beijing Solarbio science and Technology, Inc.) and
subsequently incubated for 1 h at room temperature with a
HRP-labeled rabbit anti-mouse (1:3,000 dilution; cat. no. ab6721;
Abcam) secondary antibody. Finally, the immunoreactive bands were
visualized using an enhanced chemiluminescent kit (Pierce; Thermo
Fisher Scientific, Inc.). GAPDH was used as the internal control
(1:3,000, cat. no. ab8245; Abcam). If necessary, more than one
protein was detected on the same western blot membrane using the WB
stripping buffer (cat. no. 21059; Thermo Fisher Scientific, Inc.)
and the membrane was probed again with a different primary
antibody. ImageJ software (v8.0; National Institutes of Health) was
used to measure gray scale values.
In situ hybridization
Human chondrocytes were incubated at 65°C for 48 h
with 500 ng/ml FAM-labeled probe and Cy3-labeled probe (single
stranded RNA oligonucleotide probe; hsa-circ-0079201 sequence;
5′-Cy3-GAG CTCT TGG GCC TGG TCT GGG AGG CCT GGC ACG CTG GGT GGC
TC-3′, length 45 bp; hsa-miR-140-3p sequence; 5′-FAM-CCG TGG TTC
TAC CCT GTG GTA-3′, length 21 bp) according to the manufacturer's
instructions (miRCURY LNA miRNA ISH kit; Thermo Fisher Scientific,
Inc.), to examine the distribution of hsa_circRNA_0079201 and
miR-140-3p expression.
A total of 6, 6-week-old C57 mice of either sex (two
females mice and four males), with a weight of 17.5-20.5 g were
used in the study. The mice was fed using mixed feed (Beijing Keao
Xieli Feed Co., Ltd.) at 23±1°C, with a light/dark cycle of 12/12
h. A total of 12 femur samples from the six mice were extracted
once the the six mice had been euthanized with an intraperitoneal
injection of 160 mg/kg sodium pentobarbital and stored in a 4%
formaldehyde solution. Subsequently, the samples were decalcified
in 10% EDTA solution for 4 weeks. Samples were then embedded in
paraffin and paraffin-embedded specimens were further cut into
4-µm thick sections for in situ hybridization
(miRCURY LNA miRNA ISH kit; Thermo Fisher Scientific, Inc.). The
present study was approved by the Animal Ethics Committee of
Nanchang University (Nanchang, China).
Von Kossa and alkaline phosphatase (ALP)
staining
Formation of mineralized nodules by chondrocytes
in vitro was analyzed using Von Kossa staining. The
chondrocytes (72 h post-transfection with the overcircRNA-0079201
vector) were rinsed twice with PBS and fixed in 95% ethanol
solution at room temperature for 10 min. Von Kossa silver solution
was used to stain the chondrocytes for 1 min followed by exposure
to ultraviolet light for 10 min at room temperature (22±3°C). The
chondrocytes were then mixed with 1 ml Hypo solution for 1 min at
room temperature, after rinsing with distilled water. Subsequently,
1 ml hematoxylin solution was used to stain the chondrocytes for 2
min, followed by 1 ml eosin for 1 min at room temperature. The
calcium nodules exhibited a black/brown or a dark black color and
the background presented red.
The cultured chondrocytes cells were washed with PBS
three times and fixed with 4% paraformaldehyde for 15 min at room
temperature. Then, the fixed chondrocytes cells were treated with a
5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium AP
color development kit (Beijing Solarbio Science and Technology,
Co., Ltd.) in the dark for 48 h. The chondrocyte cell lysates were
used for detection according to the manufacturer's instructions of
the AP assay kit.
Statistical analysis
As aforementioned, quantile normalization and data
processing were performed using the Quantile algorithm and 3.11
version of limma packages (https://www.bioconductor.org/packages/release/bioc/html/limma.html)
in R 3.6.0 software (https://www.R-project.org/). Statistical significance
was determined using one-way analysis of variance followed by
Tukey's post hoc test or with an unpaired t-test between two
groups. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS (v20.0; IBM Corp.) and GraphPad Prism (v5.0; GraphPad
Software, Inc.) software.
Results
Screening of differentially expressed
circRNAs and their miRNA interactions
A total of 152 patients with ISS and healthy
individuals were enrolled into the present study, and their
demographic and clinical information are summarized in Table II. Among all the individuals,
four pairs of patients with ISS and age-/sex-matched controls were
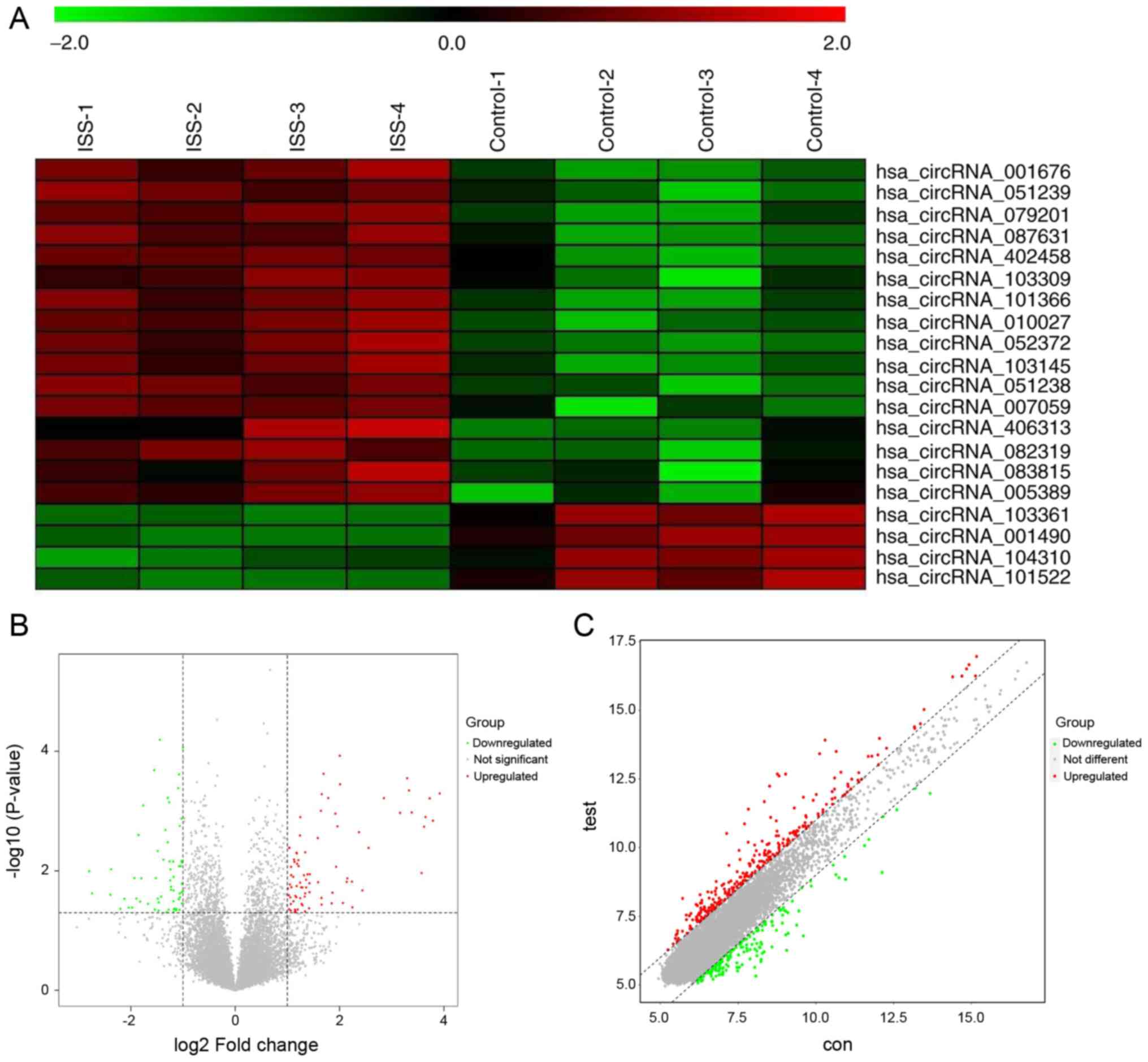
screened using circRNA microarray analysis (Fig. 1A). Among the patients with ISS,
145 circRNAs were significantly differentially expressed
(P<0.05; |logFC| >2.0). Compared with that in the normal
individuals, the expression levels of 83 and 62 circRNAs were up-
and downregulated, respectively, in patients with ISS.
Bioinformatics software was used to analyze the enrichment of the
145 differentially expressed circRNAs, as they play an essential
role in controlling the transcriptional levels of their parental
genes (20-22). The variations in circRNA
expression levels between patients with ISS and normal controls
were additionally identified using volcano plot filtering (Fig. 1B). The vertical lines correspond
to 2-fold up- and -downregulated expression, respectively, and the
horizontal line represent P=0.05. The red color represents the
upregulated circRNAs and the green color represents the
downregulated circRNAs with statistical significance. A scatter
plot of the circRNA expression profile was also used to evaluate
variations between the two groups (Fig. 1C). The x- and y-axis in the
scatter plot are the mean normalized circRNA signal values (log2
scaled). The black FC lines represent 2× FC, therefore the circRNAs
lying above and below these black lines displayed >2.0-fold up-
or downregulation, respectively. The red color represents the
upregulated circRNAs and the green color represents the
downregulated circRNAs with statistical significance.
 | Table IIClinical characteristics of patients
with ISS and healthy individuals. |
Table II
Clinical characteristics of patients
with ISS and healthy individuals.
| Variable | ISS | Healthy
controls |
|---|
| Sex | | |
| Male | 34 | 39 |
| Female | 42 | 37 |
| Mean age ± SD,
years | 9.03±2.75 | 8.38±2.22 |
| Age range,
years | 4.00-12.3 | 4.10-12.00 |
| Mean height ± SD,
cm | 120.39±14.05 | 132.24±12.47 |
| Height range,
cm | 87.10-142.2 | 103.90-151.20 |
circRNA_0079201, but not linear IQCE, is
upregulated in ISS and inhibits chondrocyte proliferation and
hyper- trophy, and reduces mineralization
The top five upregulated circRNAs
(hsa_circRNA_0079201, hsa_circRNA_0000423, hsa_circRNA_0051239,
hsa_circRNA_0087631 and hsa_ circRNA_0064644), with the highest
differential expression values were verified using RT-qPCR, with 76
samples from patients with ISS and normal controls.
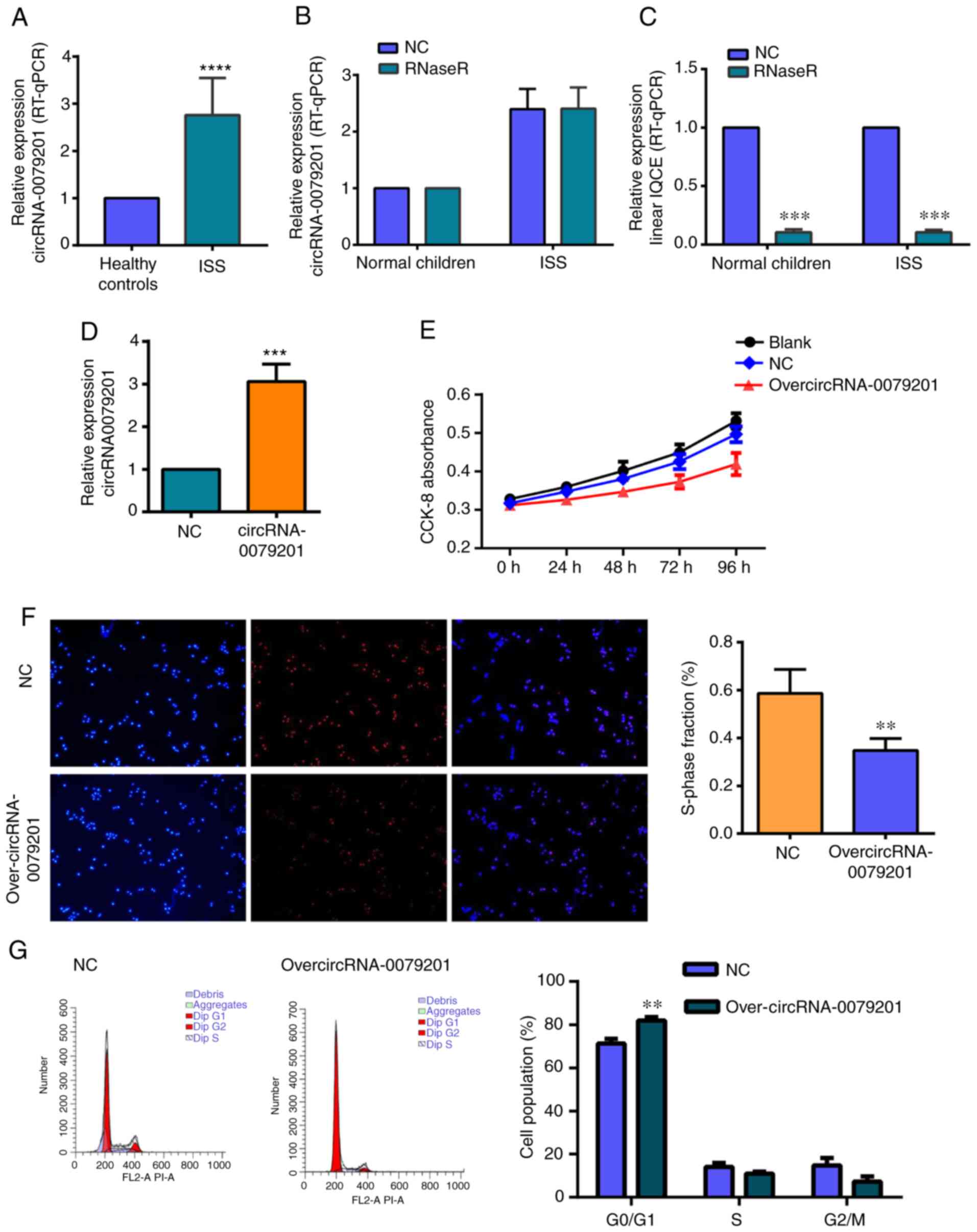
hsa_circRNA_0079201 expression level was significantly higher in
the ISS group compared with that in the control group (Fig. 2A; P<0.05), while no significant
difference was found in hsa_circRNA_0000423, hsa_circRNA_0051239,
hsa_circRNA_0087631 and hsa_circRNA_0064644 between the ISS group
and the normal control group (P>0.05). IQCE is the host gene of
hsa_circRNA_0079201. For verifying the upregulation of
hsa_circRNA_0079201, but not linear IQCE, RNase R was used to
degrade linear RNA. hsa_circRNA_0079201 expression was not
significantly different between the groups with RNase R treatment
and the normal control (Fig. 2B).
However, linear IQCE was significantly decreased following RNase R
treatment (Fig. 2C; P<0.05).
Furthermore, it was observed that the expression level of linear
IQCE did not exhibit a significant difference between the ISS and
the normal control groups (Fig.
2C).
Transfection efficiency was successfully performed,
as shown in cells transfected with either overcircRNA_0079201 or
the empty plasmid (Fig. S2) and
hsa_circRNA_0079201 expression was significantly increased
following transfection with overcircRNA_0079201 (Fig. 2D; P<0.05), whilst the CCK-8 and
EdU assays revealed that overcircRNA_0079201 significantly
inhibited the proliferation of human chondrocytes (Fig. 2E and F; P<0.05). Furthermore,
flow cytometry assays revealed that chondrocytes transfected with
overcir-cRNA_0079201 exhibited changes in the cell cycle. As shown
in Fig. 2G, the number of cells
arrested in the G0/G1 phase was significantly
higher in the pHBLV-hsa_circRNA_0079201 group compared with that in
the NC group (P<0.05). Furthermore, markedly fewer cells were
observed in the G2/M and S phases, indicating that
G1 cell cycle arrest was higher in the
overcircRNA_0079201 group compared with that in the negative
control group.
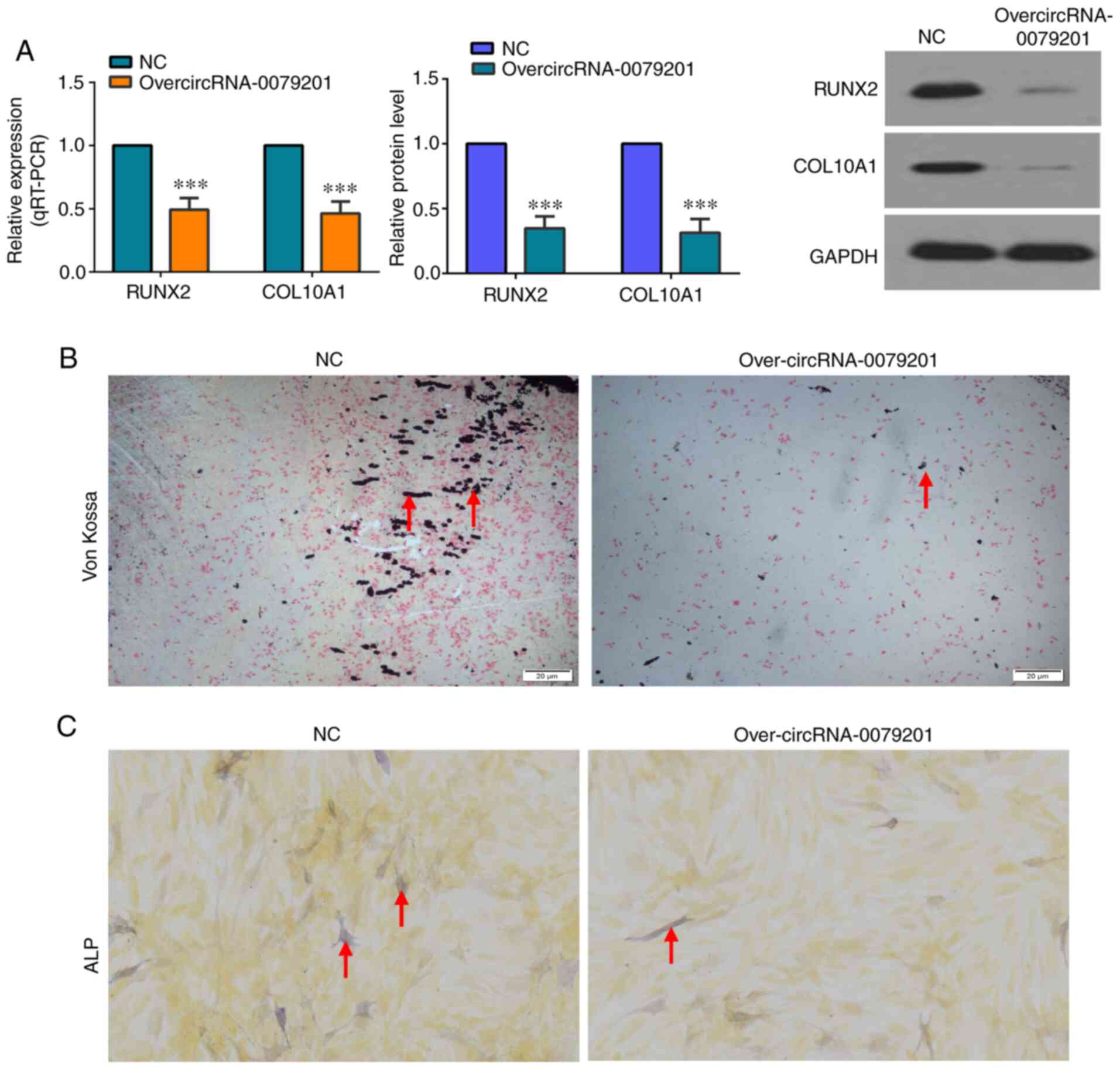
To analyze the effect of overcircRNA-0079201 on
chondrocyte hypertrophy and mineralization, the mRNA and protein
expression level of COL10A1 and RUNX2 was investigated, along with
Von Kossa and ALP staining. Significantly lower mRNA and protein
expression levels of COL10A1 and RUNX2 were found using RT-qPCR and
western blot analysis, respectively, in the overcircRNA-0079201
group (Fig. 3A; P<0.05). This
indicated that overcircRNA-0079201 inhibited chondrocyte
hypertrophy. Von Kossa staining was an intuitive method to observe
the degree of calcification in tissue and cells. As shown in
Fig. 3B, Von Kossa staining
revealed the reduced mineralization in the overcircRNA-0079201
group. In addition, ALP activity was also reduced in the
overcir-cRNA-0079201 group (Fig.
3C). These results support the hypothesis that
over-circRNA_0079201 inhibits chondrocyte proliferation and
hypertrophy and reduces mineralization.
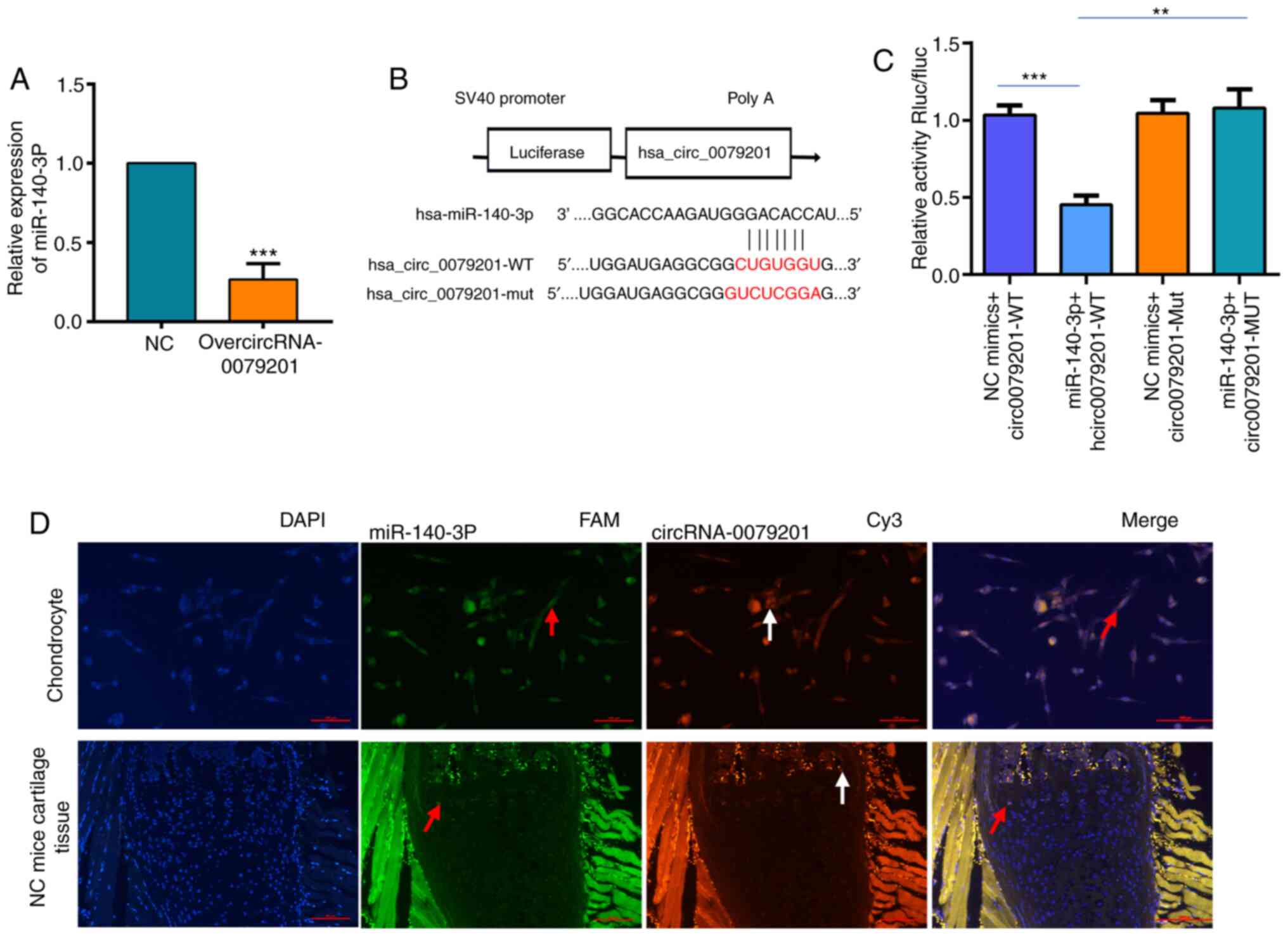
miR-140-3p is a hsa_circRNA_0079201
target gene
As some circRNAs can act as a miRNA sponge by
binding to target miRNAs and affecting gene expression levels
(20-22), the present study examined the
potential interactions between hsa_circRNA _0079201 and miRNAs. The
CircInteractome database revealed that hsa_circRNA_0079201 had 13
target genes (hsa-miR-140-3P, hsa-miR-1225-3P, hsa-miR-1236,
hsa-miR-1286, hsa-miR-1205, hsa-miR-331-3P, hsa-miR-370,
hsa-miR-431, hsa-miR-515-3P, hsa-miR-519e, hsa-miR-532-3p,
hsa-miR-564, hsa-miR-578, hsa-miR-581, hsa-miR-635, hsa-miR-636 and
hsa-miR-942). The expression level of three of these miRNAs
(hsa-miR-140-3P, hsa-miR-635 and hsa-miR-564) was decreased
following transfection with overcircRNA_0079201 (Fig. S3). miR-140-3P was selected as a
candidate target gene of circRNA_0079201, due to it exhibiting the
most significant difference (3.75-fold) (Fig. 4A; P<0.05). Subsequently, it was
found that luciferase activity was reduced in the human
chondrocytes co-transfected with wild-type (WT) circRNA_0079201 and
miR-140-3p mimic; however, this effect was restored in the cells
co-transfected with their mutants (Fig. 4B and C), suggesting that
miR-140-3p was a potential target of hsa_circRNA_0079201 in
patients with ISS. Notably, in situ hybridization confirmed
the co-localization of hsa_circRNA_0079201 and miR-140-3p in human
chondrocytes and the neonatal femur growth plate of C57 mice
(Fig. 4D).
circRNA_0079201 inhibits chondrocyte
proliferation and hypertrophy and reduces mineralization by
regulating miR-140-3p
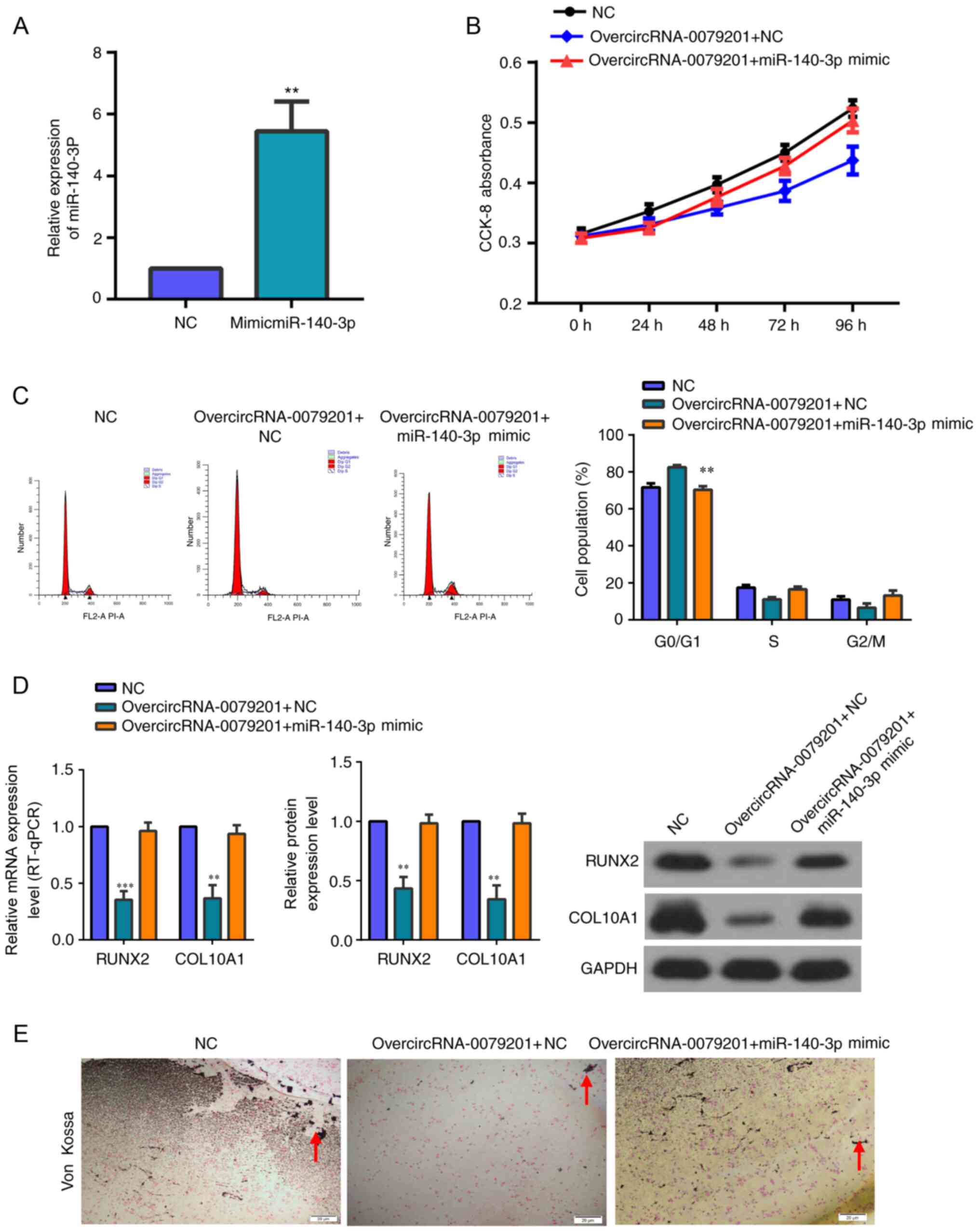
The transfection efficiency of miR-140-3p mimics is
shown in Fig. 5A. The expression
of miR-140-3p was upregulated by miRNA mimics 3 days after
circRNA_0079201 overexpression via overcircRNA_0079201. Rescue
experiments showed that miR-140-3p overexpression reversed the
inhibition of human chondrocyte proliferation, hypertrophy and
endochondral ossification caused by overcircRNA_0079201 (Fig. 5B-E).
hsa_circRNA_0079201 significantly
increases SMAD2 expression by suppressing miR-140-3p
The miR-140-3p target genes were predicted using
Starbase database and KEGG pathway analyses was performed on these
genes, 221 targeted signaling pathways were identified, 110 of
which were significantly different (P<0.05). A total of 4
[TGF-β, mTOR, Wnt, insulin-like growth factor (IGF) and cell cycle]
were associated with chondrocyte development and maturation
(Fig. 6A). Notably, SMAD2
participates in three of these pathways (TGF-β, Wnt and cell cycle)
(29-31), and SMAD2 and miR-140-3p were
predicted to have three binding sites with high scores by Starbase
(Fig. 6B). Therefore, SMAD2 was
considered to be a candidate gene in the present study. Luciferase
activity was restored in cells co-transfected with SMAD2-Mut and
miR-140-3p mimics, and reduced in the 293T cell line co-transfected
with SMAD2-WT and miR-140-3p mimics (Fig. 6C and D; P<0.05). Meanwhile,
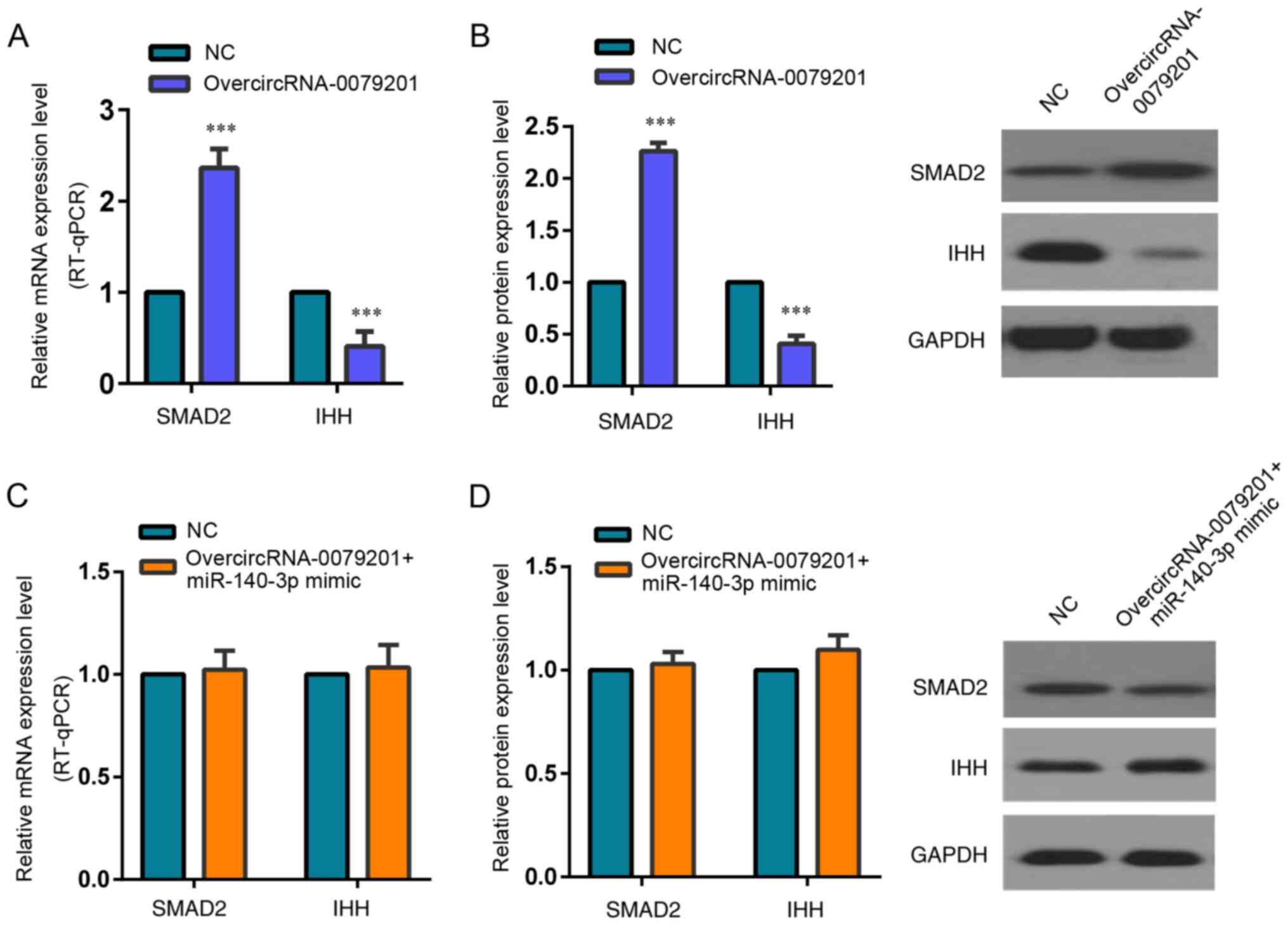
SMAD2 was significantly increased compared with that in the control
group (Fig. 7A and B;
P<0.05).
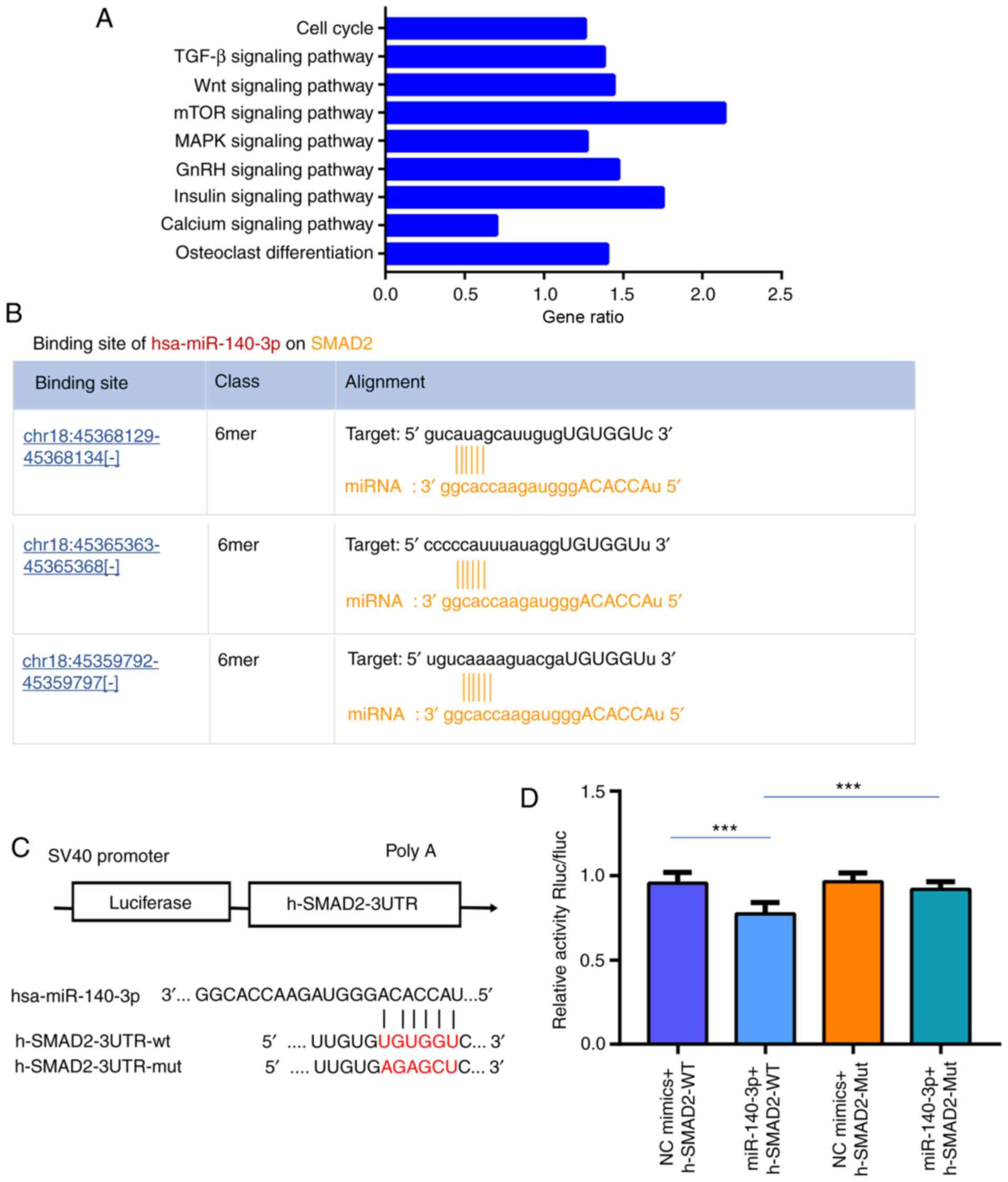 | Figure 6SMAD2 is a target gene of miR-140-3p.
(A) miR-140-3p target genes were predicted using Starbase and Kyoto
Encyclopedia of Genes and Genomes pathway analyses was performed on
these genes, 4 of which (TGF-β, mTOR, Wnt, and cell cycle) were
associated with chondrocyte development and matu-ration. (B) SMAD2
and miR-140-3p were predicted to have three binding sites with high
scores using Starbase. (C) The poteintial target binding site of
miR-140-3p and SMAD2. (D) Luciferase reporter assays confirmed that
SMAD2 was the target gene of miR-140-3p. The data are presented as
the mean ± SD. n=3. ***P<0.001 vs. control. SMAD2,
SMAD family member 2; TGF-β, transforming growth factor-β;
mTOR, mammalian target of rapamycin; Wnt, wingless/integrated;
circRNA, circular RNA; miR, microRNA; WT, wild-type; Mut, mutant;
UTR, untranslated region; chr, chromosome; GnRH, gonadotropin
releasing hormone. |
Wang et al (31) confirmed that SMAD2 could inhibit
IHH expression and significantly reduced chondrocyte proliferation
and hypertrophy in the neonatal growth plate. Therefore, we
detected the SMAD2 and IHH expression following transfection with
overcircRNA_0079201 vector in the present study. The protein and
mRNA expression of SMAD2 was upregulated in the overcircRNA_0079201
group (Fig. 7A and B; P<0.05).
Meanwhile, the expression of IHH, a SMAD2 target gene, presented
downregulation compared with that in the control group following
transfection with overcircRNA_0079201 vector (Fig. 7A and B; P<0.05). However, the
difference of SMAD2 and IHH expression was not observed between
over-circRNA_0079201+ miR-140-3p mimic group and the control group
(Fig. 7C and D; P>0.05).
Discussion
ISS is responsible for 50-80% of children with short
stature; however, its pathogenesis remains unclear (32). The GH-IGF-I axis is known to be a
vital regulator of skeletal growth during childhood; however, the
majority of patients with ISS exhibit no specific abnormalities in
this axis (33). Blair and Savage
(33) proposed that clinical and
molecular scientists should attempt to identify new pathogenetic
mechanisms to accurately categorize and treat ISS. Non-coding
circRNAs have received increasing attention as novel biomarkers for
the diagnosis and treatment of diseases, such as gastric cancer,
hepatocellular carcinoma, hypertension, diabetes and rheumatoid
arthritis, primarily due to their highly conserved sequences and
high stability in mammalian cells compared with that in other
non-coding miRNAs and lncRNAs (25,34,35). Xiong et al (36) analyzed differences in the circRNA
expression level in peripheral blood samples from 5 patients with
Hashimoto's thyroiditis and 5 healthy volunteers, and identified
627 differentially expressed circRNAs in the patients with
Hashimoto's thyroiditis. Upregulated hsa_circ_0089172 expression
was confirmed using RT-qPCR, whilst in vitro experiments
revealed that hsa_circ_0089172 could be a potential diagnostic
biomarker, as it plays a crucial role in the pathogenesis of
Hashimoto's thyroiditis by sponging miR-125a-3p. Zhao et al
(37), Qian et al
(38) and Wang et al
(39) assessed differences in the
circRNA expression level of peripheral blood samples from patients
with community-acquired pneumonia, active pulmonary tuberculosis,
and in patients who were small for their gestational age,
respectively, with their results suggesting that circRNAs could be
reliable biomarkers for diagnosing these conditions. Notably, to
the best of our knowledge, the effects of aberrant circRNA
expression on ISS has not been investigated, even though an
increasing number of studies have investigated the functions of
circRNAs in diseases (34-39).
The present study was the first to perform
microarray analysis to determine the circRNA expression profiles of
patients with ISS and hierarchical clustering to verify the
differentially expressed circRNAs. Of the 145 differentially
expressed circRNAs, 83 and 62 circRNAs were up- and down-regulated,
respectively in patients with ISS compared with that in the healthy
controls. As circRNAs have been found to regulate mRNAs or parental
genes (20-22), the biological roles of the
ISS-associated circRNAs and their target genes were investigated.
hsa_circRNA_0079201 was overexpressed in human chondrocytes to
elucidate its role in ISS pathogenesis. As a result, chondrocyte
proliferation, hypertrophy and endochondral ossification were
significantly suppressed following overcircRNA_0079201.
Bioinformatics analysis predicted that miR-140-3p could act as a
sponge for hsa_circRNA_0079201, with overcircRNA_0079201 in human
chondrocytes inhibiting miR-140-3p expression. Luciferase reporter
assays confirmed that miR-140-3p was a target gene of
hsa_circRNA_0079201. The co-localization of hsa_circRNA_0079201 and
miR-140-3p in the human chondrocyte and neonatal femur growth plate
of C57 mice was verified using in situ hybridization. Rescue
experiments demonstrated that miR-140-3p overexpression reversed
the inhibition of human chondrocyte proliferation, hypertrophy and
endochondral ossification caused by over-circRNA_0079201,
suggesting that hsa_circRNA_0079201 suppressed chondrocyte,
hypertrophy and endochondral ossification via miR-140-3p.
Miyaki et al (17) reported that miR-140-5p null mice,
via knockdown experiments, exhibited retarded post-natal growth and
short stature phenotypes and that Dnpep was a target gene of
miR-140; however, even though miR-140-5p-null mice clearly
exhibited a short stature phenotype, Dnpep only had a weak
antagonistic effect on bone morphogenetic protein signaling.
Therefore, miR-140 may regulate other target genes that inhibit
post-natal growth. The present study used Starbase to predict the
target genes of miR-140-3p, identifying 221 targeted signaling
pathways. A total of 4 pathways (TGF-β, mTOR, Wnt and cell cycle)
were associated with chondrocyte development and maturation
according to the literature (29-31). Notably, SMAD2, a member of the
SMAD protein family, participates in three of the signaling
pathways (TGF-β, Wnt and cell cycle) (29-31). SMAD proteins are not only signal
transducers, but also transcriptional regulators that mediate
multiple signaling pathways, such as TGF-β, BMP, and Wnt signaling
pathways (29-31). Wang et al (31) observed that SMAD2 could inhibit
IHH mRNA and protein expression levels and significantly reduced
chondrocyte proliferation and hypertrophy in the neonatal mouse
growth plate. Multiple studies have confirmed that downregulated
IHH mRNA and protein expression levels could inhibit chondrocyte
proliferation and impede longitudinal bone growth (18,40,41); therefore, the present study
investigated the role of SMAD2 in ISS. As expected, Starbase
identified that SMAD2 and miR-140-3p have three binding sites with
a high score, whilst luciferase assays verified that miR-140-3p
binds to SMAD2. Following hsa_circRNA_0079201 overexpression in
human chondrocytes, miR-140-3p mRNA expression level was found to
be decreased, while SMAD2 protein expression level was upregulated.
Furthermore, hsa_circRNA_0079201 overexpression decreased the
protein expression of IHH, a SMAD2 target gene, in human
chondrocytes.
The present study was limited by the fact that it
only observed the effects of hsa_circRNA_0079201 on chondrocyte
proliferation, hypertrophy and endochondral ossification via the
miR-140-3p/SMAD2/IHH pathway in vitro. Therefore, these
findings must be confirmed in vivo in WT animals, such as
mice or Rhesus monkey's and chondrocyte physiology should be
investigated in hsa_circRNA_0079201 overexpression or knockout
models. Taken together, these results suggested a novel axis
whereby hsa_circRNA_0079201 binds to miR-140-3p to regulate the
SMAD2/IHH signaling pathway, thus playing an important role in ISS
pathogenesis.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 81960392) and the
project of Science and Technology Department of Jiangxi Province
(grant nos. 20161BAB215245 and 20181BAB215019).
Availability of data and materials
The data used and/or analyzed in this study are
available from the corresponding author with reasonable
request.
Authors' contributions
XL conceived and designed the experiments. XL, CY
and XD performed the experiments. JJ analyzed the data and wrote
the paper. XL, CY and JJ revised the manuscript. All authors
approved the final version of the manuscript.
Ethics approval and consent to
participate
Human specimen collection was approved by the Human
Research Ethics Committee of the Second Affiliated Hospital of
Nanchang University. As all the participants were under the age of
12 years, written informed consent was provided by their parents or
legal guardians before their enrolment. In addition, this study was
also approved by the Animal Ethics Committee of Nanchang University
(Nanchang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cohen LE: Idiopathic short stature: A
clinical review. JAMA. 311:1787–1796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang MJ: Novel genetic cause of idiopathic
short stature. Ann Pediatr Endocrinol Metab. 22:153–157. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Montalbano A, Juergensen L, Fukami M,
Thiel CT, Hauer NH, Roeth R, Weiss B, Naiki Y, Ogata T, Hassel D
and Rappold GA: Functional missense and splicing variants in the
retinoic acid catabolizing enzyme CYP26C1 in idiopathic short
stature. Eur J Hum Genet. 26:1113–1120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dias C, Giordano M, Frechette R, Bellone
S, Polychronakos C, Legault L, Deal CL and Goodyer CG: Genetic
variations at the human growth hormone receptor (GHR) gene locus
are associated with idiopathic short stature. J Cell Mol Med.
21:2985–2999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hattori A, Katoh-Fukui Y, Nakamura A,
Matsubara K, Kamimaki T, Tanaka H, Dateki S, Adachi M, Muroya K,
Yoshida S, et al: Next generation sequencing-based mutation
screening of 86 patients with idiopathic short stature. Endocr J.
64:947–954. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim J, Suh BK, Ko CW, Lee KH, Shin CH,
Hwang JS, Kim HS, Chung WY, Kim CJ, Han HS, et al: Recombinant
growth hormone therapy for prepubertal children with idiopathic
short stature in Korea: A phase III randomized trial. J Endocrinol
Invest. 41:475–483. 2018. View Article : Google Scholar :
|
|
7
|
Heo SH, Choi JH, Kim YM, Jung CW, Lee J,
Jin HY, Kim GH, Lee BH, Shin CH and Yoo HW: Comparative proteomic
analysis in children with idiopathic short stature (ISS) before and
after short-term recombinant human growth hormone (rhGH) therapy.
Proteomics. 13:1211–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Gool SA, Kamp GA, Odink RJ, de Muinck
Keizer-Schrama SM, Delemarre-van de Waal HA, Oostdijk W and Wit JM:
High-dose GH treatment limited to the prepubertal period in young
children with idiopathic short stature does not increase adult
height. Eur J Endocrinol. 162:653–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cutfield WS and Albert BB: Growth hormone
treatment for idiopathic short stature. Pediatr Endocrinol Rev.
16(Suppl 1): S113–S122. 2018.
|
|
10
|
Ying YQ, Hou L, Liang Y, Wu W and Luo XP:
Efficacy and safety of recombinant human growth hormone in treating
Chinese children with idiopathic short stature. Growth Horm IGF
Res. 42-43:80–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michigami T: Regulatory mechanisms for the
development of growth plate cartilage. Cell Mol Life Sci.
70:4213–4221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DS, Roh SY, Choi H and Park JC: NFI-C
is required for epiphyseal chondrocyte proliferation during
postnatal cartilage development. Mol Cells. 43:739–748.
2020.PubMed/NCBI
|
|
13
|
Chen J and Long F: mTORC1 signaling
controls mammalian skeletal growth through stimulation of protein
synthesis. Development. 141:2848–2854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, She Y, Wu H, Zhong D and Zhang J:
Long non-coding RNA Gas5 regulates proliferation and apoptosis in
HCS-2/8 cells and growth plate chondrocytes by controlling FGF1
expression via miR-21 regulation. J Biomed Sci. 25:182018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jee YH, Wang J, Yue S, Jennings M, Clokie
SJ, Nilsson O, Lui JC and Baron J: mir-374-5p mir-379-5p and
mir-503-5p regulate proliferation and hypertrophic differentiation
of growth plate chondrocytes in male rats. Endocrinology.
159:1469–1478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyaki S, Sato T, Inoue A, Otsuki S, Ito
Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, et al:
MicroRNA-140 plays dual roles in both cartilage development and
homeostasis. Genes Dev. 24:1173–1185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun J, Wei X, Li S, Sun C, Wang C, Li P,
Wei DL and Wei L: The effects of Indian hedgehog deletion on
mesenchyme cells: Inducing intermediate cartilage scaffold
ossification to cause growth plate and phalange joint absence,
short limb, and dwarfish phenotypes. Stem Cells Dev. 27:1412–1425.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Li C, Tan C and Liu X: Circular
RNAs: A new frontier in the study of human diseases. J Med Genet.
53:359–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greene J, Baird AM, Brady L, Lim M, Gray
SG, McDermott R and Finn SP: Circular RNAs: Biogenesis, function
and role in human diseases. Front Mol Biosci. 4:382017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ebbesen KK, Kjems J and Hansen TB:
Circular RNAs: Identification, biogenesis and function. Biochim
Biophys Acta. 1859:163–168. 2016. View Article : Google Scholar
|
|
23
|
Haque S and Harries LW: Circular RNAs
(circRNAs) in health and disease. Genes (Basel). 8:3532017.
View Article : Google Scholar
|
|
24
|
Yu CX and Sun S: An emerging role for
circular RNAs in osteoarthritis. Yonsei Med J. 59:349–355. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Mo Y, Gong Z, Yang X, Yang M,
Zhang S, Xiong F, Xiang B, Zhou M, Liao Q, et al: Circular RNAs in
human cancer. Mol Cancer. 16:252017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aufiero S, Reckman YJ, Pinto YM and
Creemers EE: Circular RNAs open a new chapter in cardiovascular
biology. Nat Rev Cardiol. 16:503–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lui JC: Regulation of body growth by
microRNAs. Mol Cell Endocrinol. 456:2–8. 2017. View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Kozhemyakina E, Lassar AB and Zelzer E: A
pathway to bone: Signaling molecules and transcription factors
involved in chondrocyte development and maturation. Development.
142:817–831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van der Kraan PM, Goumans MJ, Blaney
Davidson E and ten Dijke P: Age-dependent alteration of TGF-β
signalling in osteoarthritis. Cell Tissue Res. 347:257–265. 2012.
View Article : Google Scholar
|
|
31
|
Wang W, Song B, Anbarchian T, Shirazyan A,
Sadik JE and Lyons KM: Smad2 and Smad3 regulate chondrocyte
proliferation and differentiation in the growth plate. PLoS Genet.
12:e10063522016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang L, Zhang C, Wang W, Wang J, Xiao Y,
Lu W, Ma X, Chen L, Ni J, Wang D, et al: Pathogenic gene screening
in 91 Chinese patients with short stature of unknown etiology with
a targeted next-generation sequencing panel. BMC Med Genet.
19:2122018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blair JC and Savage MO: The GH-IGF-I axis
in children with idiopathic short stature. Trends Endocrinol Metab.
13:325–330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Z, Yang T and Xiao J: Circular RNAs:
Promising biomarkers for human diseases. EBioMedicine. 34:267–274.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arnaiz E, Sole C, Manterola L,
Iparraguirre L, Otaegui D and Lawrie CH: CircRNAs and cancer:
Biomarkers and master regulators. Semin Cancer Biol. 58:90–99.
2019. View Article : Google Scholar
|
|
36
|
Xiong S, Peng H, Ding X, Wang X, Wang L,
Wu C, Wang S, Xu H and Liu Y: Circular RNA expression profiling and
the potential role of hsa_circ_0089172 in Hashimoto's thyroiditis
via sponging miR125a-3p. Mol Ther Nucleic Acids. 17:38–48. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao T, Zheng Y, Hao D, Jin X, Luo Q, Guo
Y, Li D, Xi W, Xu Y, Chen Y, et al: Blood circRNAs as biomarkers
for the diagnosis of community-acquired pneumonia. J Cell Biochem.
120:16483–16494. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qian Z, Liu H, Li M, Shi J, Li N, Zhang Y,
Zhang X, Lv J, Xie X, Bai Y, et al: Potential diagnostic power of
blood circular RNA expression in active pulmonary tuberculosis.
EBioMedicine. 27:18–26. 2018. View Article : Google Scholar :
|
|
39
|
Wang Y, Li SF, Dang YJ, Shi XM, Chen L,
Wang N, Cai Y and Zhao YY: Differentially expressed circular RNAs
in maternal and neonatal umbilical cord plasma from SGA compared
with AGA. J Cell Biochem. 121:713–722. 2020. View Article : Google Scholar
|
|
40
|
Deng A, Zhang H, Hu M, Liu S, Wang Y, Gao
Q and Guo C: The inhibitory roles of Ihh downregulation on
chondrocyte growth and differentiation. Exp Ther Med. 15:789–794.
2018.PubMed/NCBI
|
|
41
|
Karp SJ, Schipani E, St-Jacques B,
Hunzelman J, Kronenberg H and McMahon AP: Indian hedgehog
coordinates endochondral bone growth and morphogenesis via
parathyroid hormone related-protein-dependent and -independent
pathways. Development. 127:543–548. 2000.PubMed/NCBI
|