Introduction
Colorectal cancer (CRC) is the third most prevalent
malignant neoplasm and one of the leading causes of
cancer-associated mortality worldwide (1,2).
Despite great efforts dedicated to therapeutic improvements, the
prognosis of patients with CRC remains far from satisfactory
(3,4). Hypoxia is a hallmark of the
microenvironment and contributes to tumor progression and
metastasis in various types of cancer, including CRC. For example,
hypoxia has been shown to promote the metastasis of CRC by
upregulating GDF15 expression (5). Hypoxia also facilitates
epithelial-mesenchymal transition in CRC cells by regulating USP47
(6). Accordingly, it is
imperative to elucidate the mechanisms governing CRC and the
function of hypoxia in CRC.
Circular RNAs (circRNAs) are a category of
non-coding RNA transcripts with circular configuration, which
originate from exonic, intronic and intergenic regions (7,8).
There is ample evidence to indicate that circRNAs are involved in
the regulation of various biological activities of malignant
tumors. For example, circRNA_00580631 has been shown to promote
tumorigenesis of bladder cancer by sponging miR-486-3p and
targeting FOXP4 (9). circRNA MTO1
inhibits gastric carcinoma progression via targeting the
miR-3200-5p/PEBP1 axis (10).
circRNA_103809 functions as a competing endogenous RNA (ceRNA) to
promote the proliferation and migration of CRC cells by targeting
the miR-532-3p/FOXO4 axis (11).
circHIPK3 acts as a sponge of miR-7 to facilitate CRC growth and
metastasis (12). It has been
reported that circCCDC66 is upregulated in colon cancer and
promotes the growth and metastasis of colon cancer (13). In addition, several studies have
demonstrated that circRNAs are associated with hypoxia-mediated
progression of various cancers, such as bladder cancer and breast
cancer (14,15). However, the role and molecular
mechanisms of circCCDC66 in hypoxia-induced CRC remain largely
unknown.
MicroRNAs (miRNAs or miRs) are a type of short
non-coding RNA that comprise 18-25 nucleotides and are involved in
the development and evolution of multiple diseases (16,17). The dysregulation of miRNAs has
been justified in a wide range of cancers and miRNAs function as
oncogenes or tumor suppressors via the modulation of cellular
processes, such as cell viability, apoptosis, migration, invasion
and differentiation (18-20). miR-3140 has been reported to
inhibit tumor growth in vivo or in vitro (21). Nevertheless, whether miR-3140 is
involved in CRC remains to be further clarified.
In the present study, the specific function of
circCCDC66 in CRC under hypoxic conditions was investigated and it
was found that circCCDC66 promoted the malignant behaviors of
hypoxia-exposed CRC cells by regulating the miR-3140/autophagy
pathway. These findings may provide insight into the development of
more effective therapeutic strategies for patients with CRC.
Materials and methods
Clinical specimens
The CRC tissues were collected from 29 patients (19
males and 10 females; 13 stage I-II and 16 stage III-IV) with a
median age of 47 years (range, 31-79 years) between May 2015 and
August 2018 at the First People's Hospital of Changzhou. Written
informed consent was obtained from all patients prior to the study
start. All tissue samples were obtained and immediately stored at
−80°C prior to further experiments. The present study was approved
by the First People's Hospital of Changzhou.
Cell culture and treatment
The human CRC cell lines (HCT116 and SW620), human
colon mucosal cells (NCM460) and 293T cells were purchased from the
American Type Culture Collection (ATCC) and maintained in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 100 µg/ml streptomycin and 100
U/ml penicillin. Cells in the normoxia group were incubated at 37°C
with 95% atmospheric air and 5% CO2 at 6 l/min for 4 h.
Cells in the hypoxia group were incubated at 37°C in 94%
N2, 5% CO2 and 1% O2 at 6 l/min
for 4 h.
Cell transfection
The short hairpin RNAs (shRNAs) targeting circCCDC66
(sh-circCCDC66) with the negative control (sh-NC), miR-3140 mimic
with the negative control (NC mimic) and miR-3140 inhibitor with
the negative control (NC inhibitor) were synthesized by GenePharma
(Shanghai, China). Transfection was conducted with Lipofectamine
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions.
Cell counting Kit-8 (CCK-8) assay
Following transfection, 3×103 cells/well
were seeded into 96-well plates and then cultured at 37°C. At 0,
24, 48 and 72 h, each well was supplemented with 10 µl CCK-8
reagent (Dojindo Molecular Technologies, Inc.) and incubated for a
further 4 h. The absorbance was examined at 450 nm using a
microplate reader (Molecular Devices LLC).
Flow cytometric analysis
HCT116 and SW620 cells were collected with trypsin
and rinsed twice using cold PBS, stained with Annexin V for 10 min
in the dark and then treated with PI for 5 min, according to the
manufacturer's recommendations (BD Biosciences). Following the
addition of Annexin V-binding buffer, apoptotic cells were detected
with a FACSCalibur flow cytometer (BD Biosciences) and analyzed
using FlowJo software (version 7.5, TreeStar). The upper right and
low right segments of the flow cytometry dot plots were counted to
determine the level of apoptotic cells.
Wound healing assay
The migratory ability of the cells was evaluated by
a wound healing assay. Cells were seeded into 6-well plates and
grown until 100% confluent. Subsequently, a scratch wound was
produced with a sterile 200-µl pipette tip and the cell
debris was washed away using PBS. Cells were incubated in 1%
FBS-supplemented DMEM for an additional 24 h. The scratch wound was
observed at 0 and 24 h following incubation. Images were captured
in randomly selected view fields under an Olympus CX31 microscope
(Olympus Corporation) and analyzed using ImageJ software (version
1.48, National Institutes of Health).
Transwell assay
Cell invasion was assessed by a Transwell assay. A
total of 5×105 cells and 200 µl serum-free DMEM
were plated into the upper chamber of a Matrigelcoated 8-µm
pore membrane (BD Biosciences). The bottom chamber was supplemented
with 600 µl DMEM with 10% FBS. Following 24 h of incubation,
cells in the upper chamber were removed using a cotton swab. The
invaded cells were fixed by 70% ethanol, stained with 0.1% crystal
violet and counted in five random fields using a light microscope
(Nikon Corporation; magnification, ×200).
Quantitative real-time polymerase chain
reaction (RT-qPCR)
Total RNA from tissues and cell lines was extracted
by using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Reverse
transcription (RT) was conducted by using the Takara PrimeScript
Kit (Takara) at 37°C for 15 min. Subsequently, the RT-quantitative
(q)PCR assay was performed with the ViiATM 7 Real-Time PCR System
(Thermo Fisher Scientific, Inc.) to detect the gene expression
level. Relative gene expression was calculated using the
2−ΔΔCq method (22).
GAPDH and U6 were set as internal controls. The PCR amplification
reaction was carried out using cDNA as template with the following
conditions: 95°C for 10 min, 95°C for 15 sec, 62°C for 30 sec, and
72°C for 30 sec. Primers used for PCR are as follows: circCCDC66
forward, 5′-ACC TAC AAC CGG AAG CCA G-3′ and reverse, 5′-AGC AGT
ACT GTT TCC TGA TGC-3′; miR-3140 forward, 5′-CTT CCA CTC GAC GTG
CTG GAA GT-3′ and reverse, 5′-ACG GTC TCG TGC AGT CGT CAA CG-3′;
Beclin1 forward, 5′-ACC GTG TCA CCA TCC AGG AA-3′ and reverse,
5′-GAA GCT GTT GGC ACT TTC TGT-3′; p62 forward, 5′-GCA GAA TGC CAT
GGT TTC CC-3′ and reverse, 5′-GTG ATG GCT CCC CTT AC-3′; HIF1A
forward, 5′-TAT GAG CCA GAA GAA CTT TTA GGC-3′ and reverse, 5′-CAC
CTC TTT TGG CAA GCA TCC TG-3′; GAPDH forward, 5′-ACA ACT TTG GTA
TCG TGG AAG G-3′ and reverse, 5′-GCC ATC ACG CCA CAG TT TC-3′; U6
forward, 5′-CTC GCT TCG GCA GCA CAT A-3′ and reverse, 5′-AAC GAT
TCA CGA ATT TGC GT-3′.
Western blot analysis
Cell lysates were obtained with RIPA lysis buffer
containing 1% protease inhibitor and protein concen-trations were
determined using a BCA kit (Thermo Fisher Scientific, Inc.).
Subsequently, 10 µg protein/lane was detached by 10%
SDS-PAGE and transferred to PVDF membranes. After sealing in 5%
skim milk for 2 h, the membranes were probed with primary
antibodies against Beclin1 (dilution, 1:1,000; #3738, Cell
Signaling Technology, Inc.), p62 (dilution 1:1,000; #88588, Cell
Signaling Technology, Inc.), Bcl-2 (dilution 1:1,000; #15071, Cell
Signaling Technology, Inc.), Bax (dilution 1:1,000; #2774, Cell
Signaling Technology, Inc.), HIF1A (dilution 1:1,000; #3716, Cell
Signaling Technology, Inc.) and GAPDH (dilution 1:1,000; sc-47724;
Santa Cruz Biotechnology, Inc.) at 4°C overnight, followed by
incubation with horseradish peroxidase-conjugated secondary
antibodies (dilution 1:1,000; goat anti-mouse IgG, ab205719 and
goat anti-rabbit IgG, ab205718; Abcam) for 2 h at room temperature
and visualized with the enhanced chemiluminescence (ECL) kit
(Thermo Fisher Scientific, Inc.). Protein expression was quantified
using Image-Pro® Plus software (version 6.0; Media
Cybernetics, Inc.).
Bioinformatics analysis and luciferase
reporter assays
The starBase database (http://starbase.sysu.edu.cn/) was used to predict the
potential target genes of circCCDC66. The sequence of the 3′-UTR
(untranslated region) of miR-3140 was identified as a novel
potential target gene. To evaluate the association between miR-3140
and circCCDC66, a luciferase reporter assay was carried out. The
fragments of circCCDC66 containing the speculated miR-3140 binding
sites were inserted into the reporter vectors pmiRGLO (Shanghai
GenePharma Co., Ltd.) to construct circCCDC66-WT plasmids. The
circCCDC66-Mut vectors were synthesized by mutating the sequences
of the binding sites. Subsequently, 293T cells were co-transfected
with indicated reporter vectors and NC mimic, miR-3140 mimic, NC
inhibitor, and miR-3140 inhibitor. At 48 h post-transfection, the
Dual Luciferase assay system (Promega Corp.) was utilized to
measure the luciferase activity based on the manufacturer's
instructions.
Statistical analysis
Data are presented as the mean ± SD of three
independent assays. All statistical analyses were carried out with
SPSS 17.0 software (SPSS, Inc.). Comparisons of parameters between
two groups were analyzed by a paired Student's t-test. Comparisons
among multiple groups were performed using one-way ANOVA followed
by Tukey's test. All experiments were conducted for three
replicates. P<0.05 was considered to indicate a statistically
significant difference.
Results
Knockdown of circCCDC66 inhibits the
progression of CRC cells
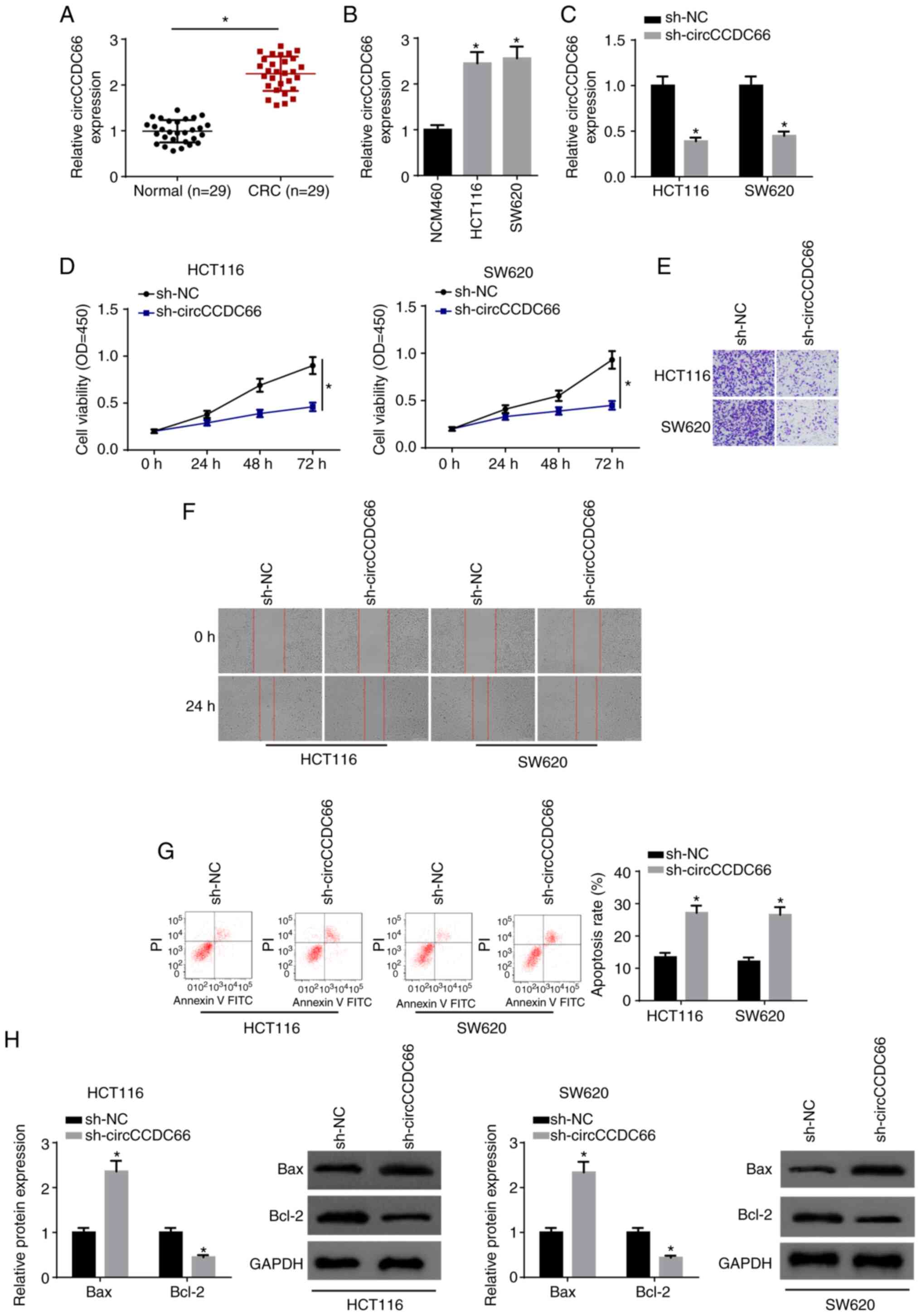
Firstly, we determined the expression of circCCDC66
in CRC tissues and cell lines (HCT116 and SW620). As demonstrated
by RT-qPCR, circCCDC66 expression was significantly increased in
CRC tissues compared with that in the corresponding normal tissues
(Fig. 1A). In addition,
circCCDC66 expression was higher in CRC cell lines than that of the
NCM460 control (Fig. 1B). To
investigate the biological role of circCCDC66 in CRC, HCT116 and
SW620 cells were transfected with sh-NC and sh-circCCDC66. The
transfection efficiency was confirmed by RT-qPCR (Fig. 1C). Moreover, knockdown of
circCCDC66 reduced viability, migration and invasion, and enhanced
the apoptosis of CRC cells (Fig.
1D-G). Western blot analysis indicated that circCCDC66
knockdown significantly increased the level of Bax and
significantly decreased Bcl-2 expression (Fig. 1H). Overall, the data indicated
that circCCDC66 promoted the tumorigenesis of CRC.
Hypoxia contributes to the progression of
CRC and upregulates circCCDC66 expression
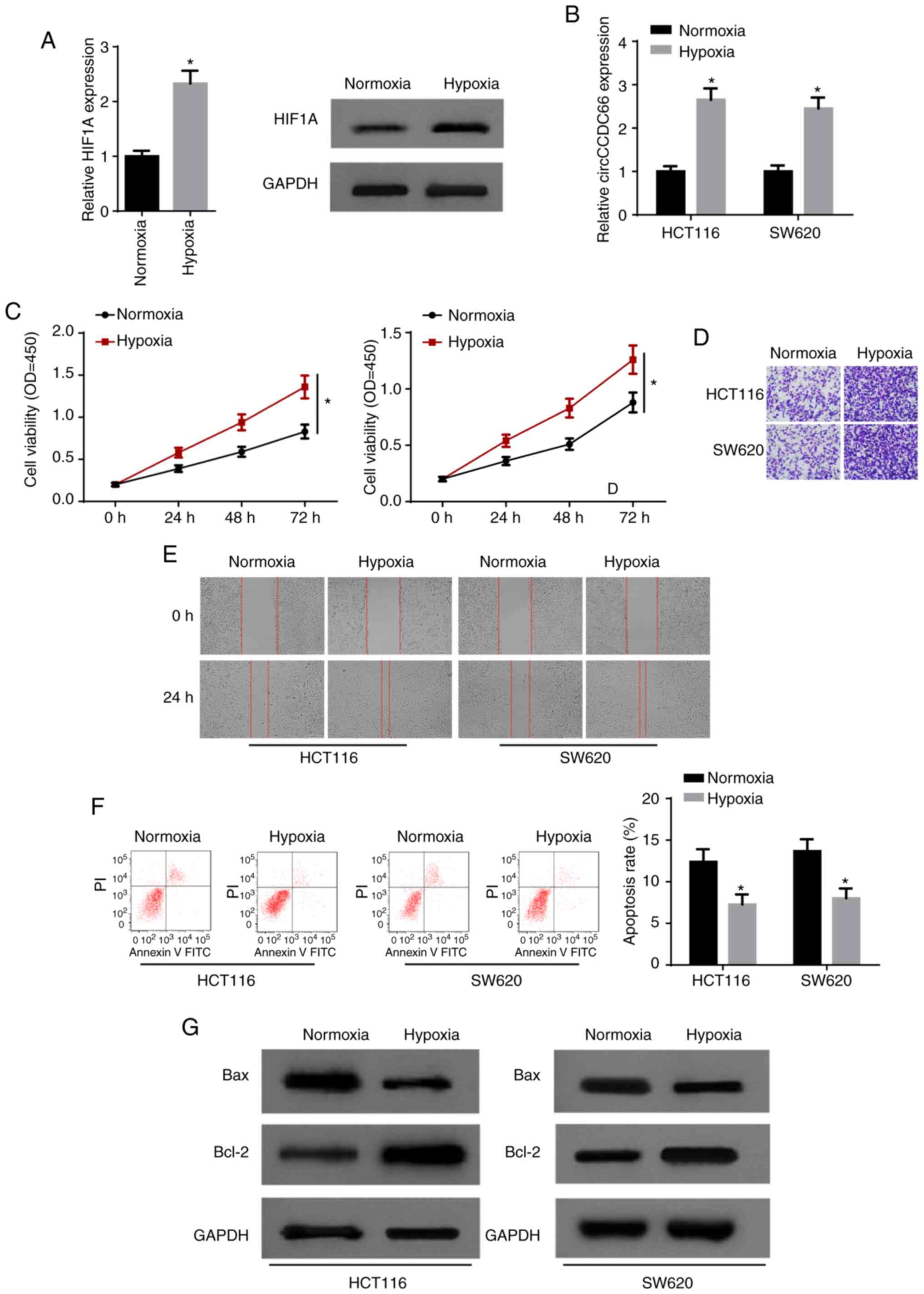
In order to investigate the role of hypoxia in CRC,
HCT116 and SW620 cells were exposed to hypoxia. As a factor closely
related to hypoxic changes, hypoxia inducible factor 1 subunit α
(HIF1A) mRNA and protein levels were both significantly promoted
following exposure to hypoxia (Fig.
2A). Furthermore, RT-qPCR indicated that hypoxia significantly
increased the expression of circCCDC66 in CRC cells (Fig. 2B). CCK-8 assay indicated that
hypoxia led to a significant increase in cell viability (Fig. 2C). Transwell and wound healing
assays demonstrated that exposure to hypoxia facilitated the
invasion and migration of HCT116 and SW620 cells (Fig. 2D and E). Consistently, it was
observed that the apoptosis of CRC cells was significantly
suppressed by hypoxia (Fig. 2F and
G). Taken together, these results indicated that hypoxia
promoted the progression of CRC and circCCDC66 was upregulated in
hypoxia-exposed CRC cells.
Silencing of circCCDC66 suppresses the
hypoxia-induced CRC cell phenotype
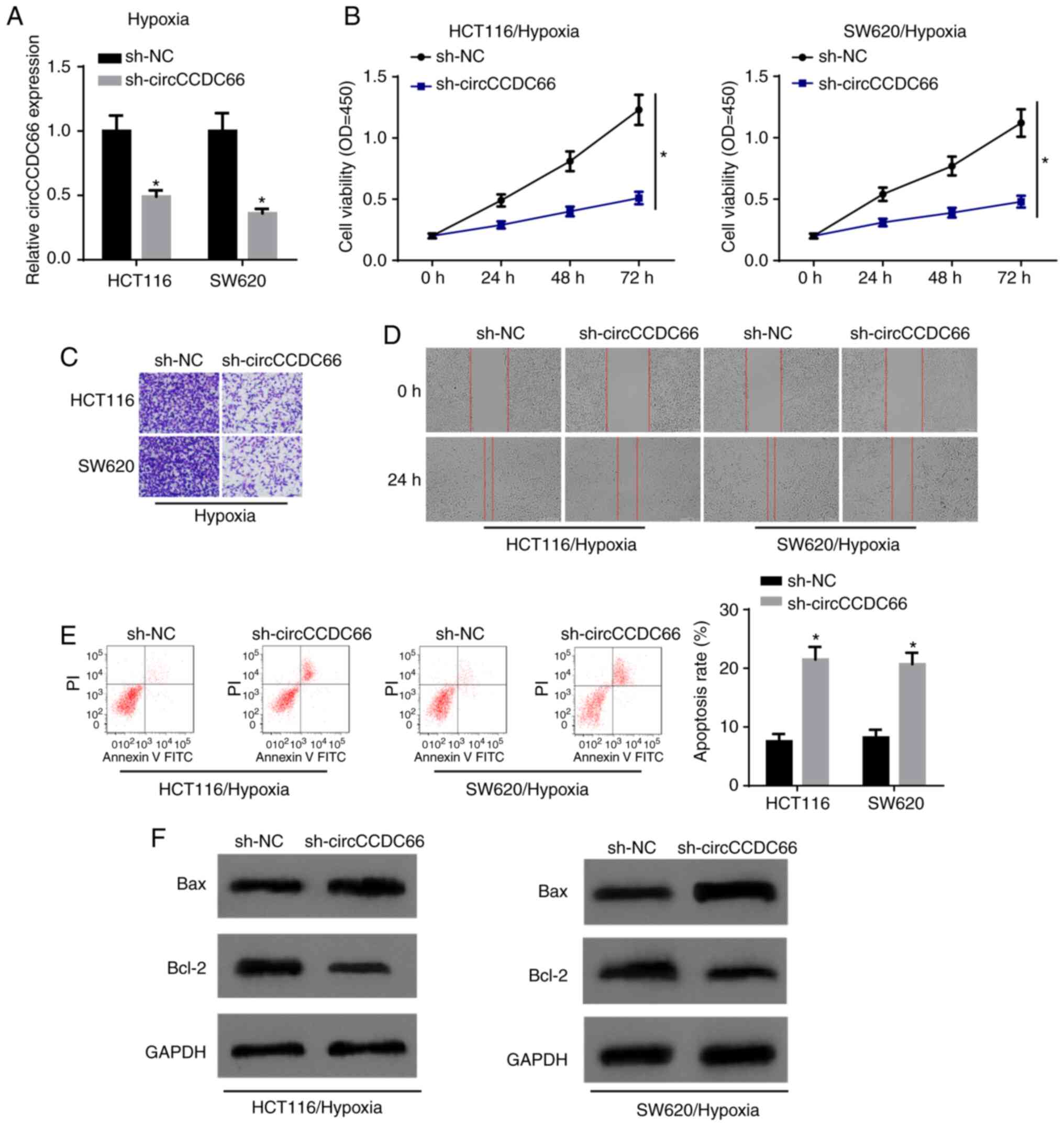
Subsequently, the present study aimed to investigate
the exact function of circCCDC66 in CRC. circCCDC66 expression was
knocked down in HCT116 and SW620 cells by transfection with
shcircCCDC66 (Fig. 3A). CCK-8
assay revealed that the depletion of circCCDC66 significantly
decreased cell viability following exposure to hypoxia (Fig. 3B). Transwell assay and wound
healing assay disclosed that the ability of invasion and migration
was suppressed by the downregulation of circCCDC66 in the
hypoxia-exposed CRC cells (Fig. 3C
and D). Consistently, flow cytometric analysis and western blot
analysis illustrated that circCCDC66 knockdown contributed to a
significant increase in the apoptosis of the hypoxia-exposed HCT116
and SW620 cells (Fig. 3E and F).
Taken together, these results provided strong evidence that the
inhibition of circCCDC66 suppresses the hypoxia-induced malignant
behaviors of CRC cells.
circCCDC66 functions as a sponge of
miR-3140
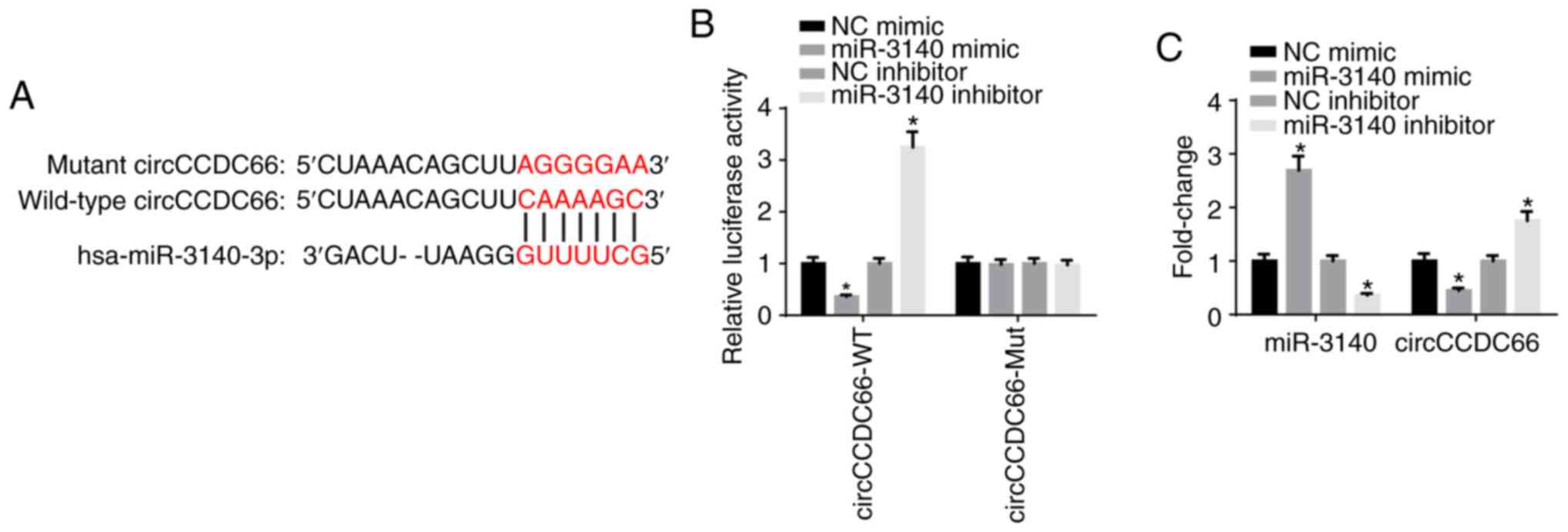
By using the starBase database, miR-3140 was
predicted as a potential downstream target gene of circCCDC66
(Fig. 4A). Luciferase reporter
assay delineated that the luciferase activity of wild-type
circCCDC66 was impaired by miR-3140 mimic and increased by miR-3140
inhibitor; however, the luciferase activity of mutant circCCDC66
exhibited no response to miR-3140 mimic or inhibitor, which
confirmed that circCCDC66 directly bound to miR-3140 (Fig. 4B). Furthermore, the results of
RT-qPCR analysis elucidated that the overexpression of miR-3140
significantly diminished the expression of circCCDC66, whereas the
depletion of miR-3140 significantly increased the circCCDC66 level
(Fig. 4C). In a word, these
findings affirmed that miR-3140 was sponged by circCCDC66.
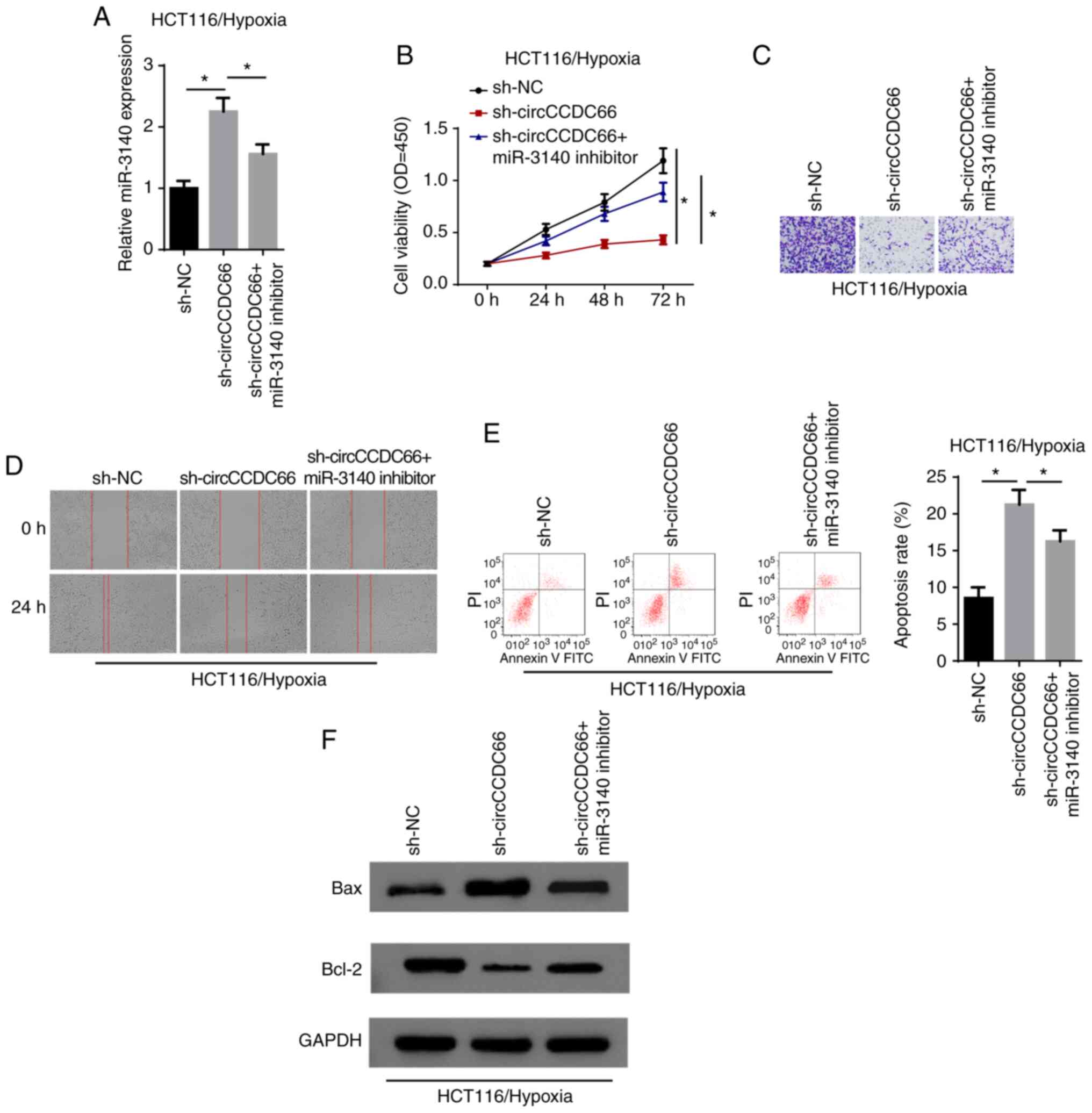
Inhibition of miR-3140 partially
abrogates the effects of circCCDC66 knockdown on hypoxia-exposed
CRC cells
Thereafter, the present study aimed to validate
whether circCCDC66 exerts its function through miR-3140. The
results of RT-qPCR revealed that miR-3140 expression was increased
by the knockdown of circCCDC66 and restoration of the miR-3140
level occurred following transfection with miR-3140 inhibitor
(Fig. 5A). CCK-8 assay revealed
that the viability of hypoxia-exposed HCT116 cells, which was
suppressed by circCCDC66 downregulation, was increased by the
inhibition of miR-3140 (Fig. 5B).
Moreover, the suppression of the cell invasive and migratory
abilities induced by the silencing of circCCDC66 was abolished by
transfection with miR-3140 inhibitor (Fig. 5C and D). In agreement with the
above-mentioned findings, flow cytometric analysis and western blot
analysis revealed that the increased apoptosis rate following
circCCDC66 depletion on cell apoptosis was partly abrogated by the
downregulation of miR-3140 in the hypoxia-exposed cells (Fig. 5E and F). Thus, circCCDC66 executed
its oncogenic role in hypoxia-exposed CRC cells by regulating
miR-3140.
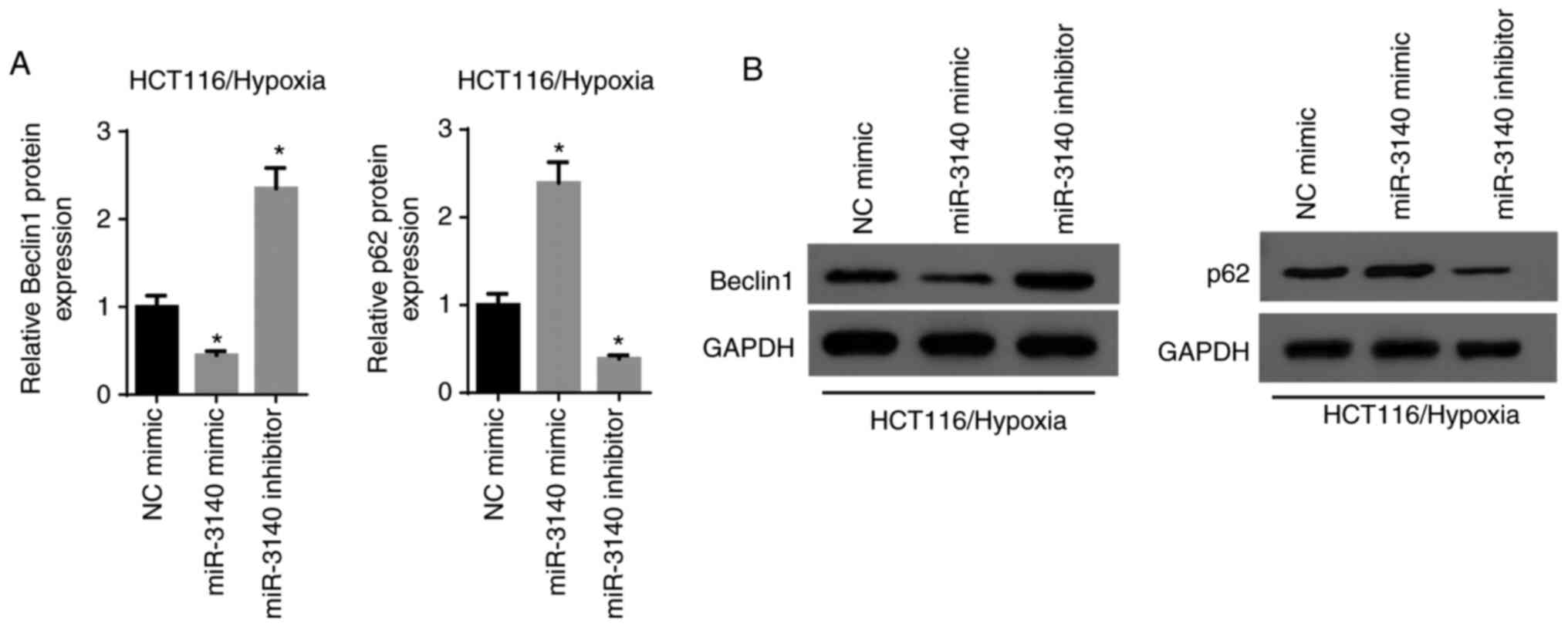
miR-3140 inhibits the autophagy
pathway
Considering that autophagy plays a vital role in the
development and evolution of malignancies, the association between
autophagy and miR-3140 was subsequently investigated. The results
of RT-qPCR and western blot analysis revealed that the elevated
expression of miR-3140 resulted in a significant reduction in
Beclin1 expression and a significant increase in the p62 level,
while the silencing of miR-3140 increased the level of Beclin1 and
significantly decreased p62 expression (Fig. 6A and B). Based on these results,
it was concluded that miR-3140 led to the suppression of
autophagy.
Activation of autophagy recovers the
miR-3140-regulated cell phenotype of hypoxia-exposed CRC cells
Rescue experiments were performed to verify the
specific function of autophagy in the circCCDC66/miR-3140 axis.
CCK-8 assay revealed that the enhanced expression of miR-3140
decreased cell viability and the autophagy inducer rapamycin (RAP)
significantly recovered the cell proliferative capability (Fig. 7A). Moreover, it was validated that
cell invasion and migration, which were suppressed by the
overexpression of miR-3140 were renewed owing to the activation of
autophagy (Fig. 7B and C).
Additionally, flow cytometric analysis and western blot revealed
that cell apoptosis was significantly promoted by miR-3140
upregulation; however, treatment with RAP abolished the effects of
miR-3140 ectopic expression on hypoxia-exposed HCT116 cells
(Fig. 7D and E). In summary, it
was concluded that miR-3140 suppressed the progression of CRC under
hypoxic conditions via autophagy.
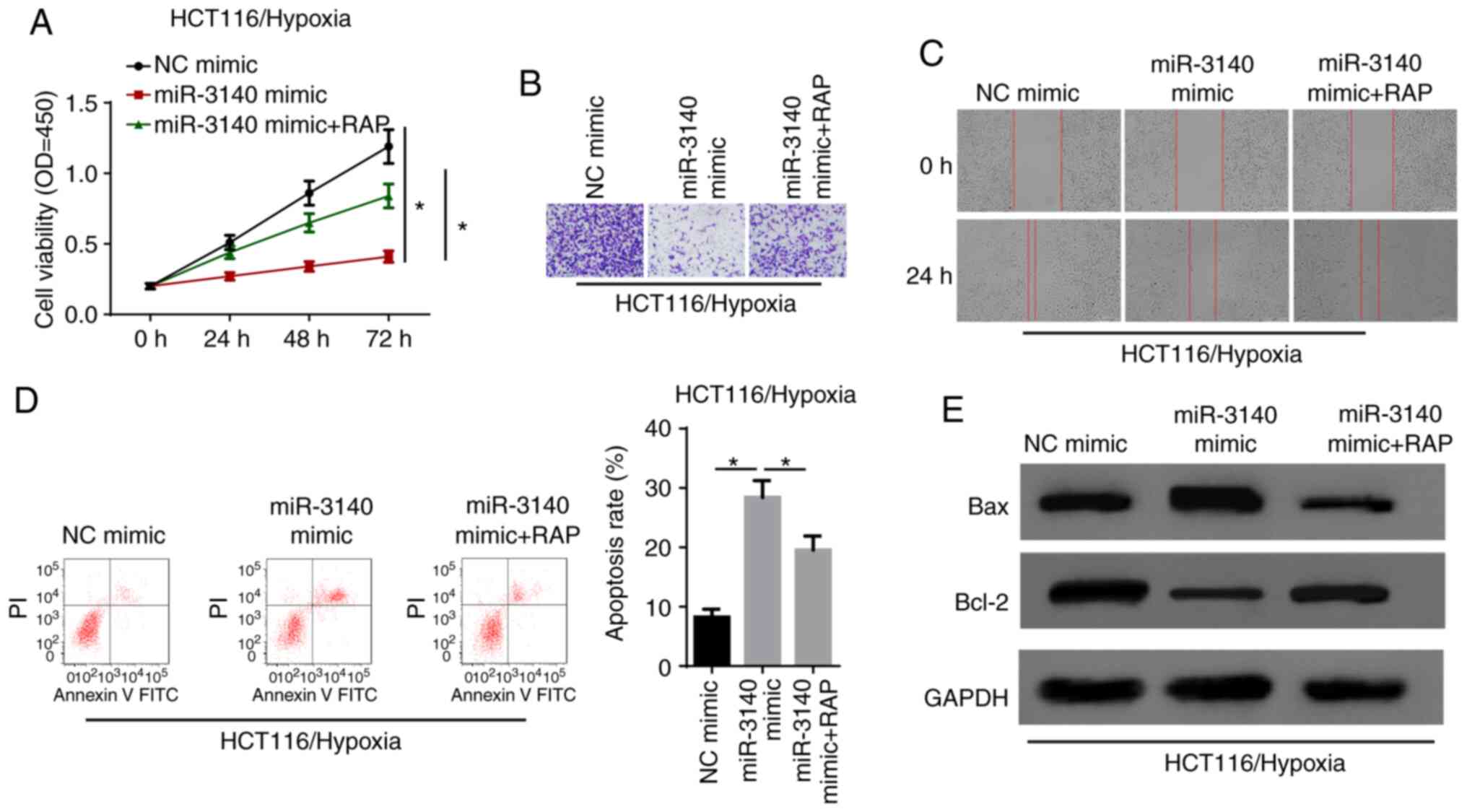 | Figure 7Activation of autophagy recovers the
miR-3140-regulated cell phenotype of hypoxia-exposed CRC cells. (A)
After hypoxia treatment, the cell viability were analyzed by CCK-8
assay in HCT116 cells transfected with NC mimic, miR-3140 mimic,
miR-3140 mimic + RAP. *P<0.05. (B and C) Transwell
assay and wound healing assay showed the migration and invasion of
HCT116 cells transfected with NC mimic, miR-3140 mimic, miR-3140
mimic + RAP after exposure to hypoxia condition. (D) Flow cytometry
assay showed the apoptosis rate of hypoxia-exposed HCT116 cells
transfected with NC mimic, miR-3140 mimic, miR-3140 mimic + RAP.
*P<0.05. (E) Western blot analysis showed the protein
levels of Bax and Bcl-2 in hypoxia-exposed HCT116 cells transfected
with NC mimic, miR-3140 mimic, miR-3140 mimic + RAP. CRC,
colorectal cancer; RAP, rapamycin. |
Discussion
Previous evidence has illuminated that circulating
RNAs (circRNAs) act as vital regulators in tumorigenesis and the
development of diverse malignancies by modulating different
cellular activities (23,24). For example, circRNA_0084043
facilitates malignant melanoma progression via miR-153-3p/Snail
axis (25). CircRNA CBL.11
inhibits cell proliferation of colorectal cancer by targeting
miR-6778-5p in (26). It has been
demonstrated that circCCDC66 plays an oncogenic role in colon
cancer (13). Consistent with the
previous study, we demonstrated that circCCDC66 expression was
upregulated in colorectal cancer (CRC) tissues and cell lines, and
knockdown of circCCDC66 inhibited the development and progression
of CRC.
Considering that intratumoral hypoxia is one of the
driving forces of tumor occurrence and development (27,28), the present study explored the
biological role of hypoxia in CRC. The findings revealed that
hypoxia induced the progression of CRC by promoting cell viability,
invasion and migration, whereas it inhibited cell apoptosis.
Furthermore, the exposure of CRC cells to hypoxic conditions
increased the expression of circCCDC66. To further explore the
involvement of circCCDC66 in hypoxia-induced CRC, loss-of-function
assays were performed under hypoxic conditions. The results
revealed that the depletion of circCCDC66 inhibited hypoxia-exposed
CRC cell growth and metastasis. Moreover, the competing endogenous
(ceRNA) network exhibits its regulatory function in human cancer,
and circRNAs can function as ceRNAs, playing a role in the
regulation of cancer (29-32).
For example, circ-0001313 promotes the development and progression
of colon cancer by acting as a ceRNA through sponging miR-510 and
regulating AKT2 (33).
circ_0007843 promotes colon cancer tumorigenesis by downregulating
miR-518c (34). In the present
study, it was found that circCCDC66 inhibited miR-3140 expression
via direct interaction. Additionally, the inhibition of miR-3140
partly abolished the effects of circCCDC66 downregulation on
hypoxia-exposed CRC cells.
Autophagy, a highly conservative process of cell
self-degradation, generally appears in a wide spectrum of cancer
cells (35). Previous studies
have demonstrated that autophagy is activated in response to
hypoxia and other types of stress, and the activation of autophagy
contributes to the tumorigenesis of numerous malignancies,
including CRC. For example, hypoxia-induced autophagy promotes the
occurrence and progression of CRC via the PRKC/PKC-EZR pathway
(36). Che et al reported
that miR-20a suppressed hypoxia-induced autophagy by targeting
ATG5/FIP200 in CRC (37).
Therefore, to gain a better under-standing of the molecular
regulatory mechanisms underlying circCCDC66, the present study
examined the association between miR-3140 and autophagy. The
experimental data indicated that miR-3140 suppressed autophagy and
the activation of autophagy by RAP renewed the miR-3140-regulated
cell phenotype of hypoxia-exposed CRC cells.
In conclusion, the present study, to the best of our
knowl-edge, is the first to demonstrate that circCCDC66 activates
autophagy to facilitate CRC progression under hypoxic conditions by
sponging miR-3140, which provides a novel therapeutic strategy for
CRC. However, some limitations remain to be further addressed:
First, the regulatory mechanisms of circCCDC66/miR-3140 in CRC
in vivo warrant further investigation. Secondly, the other
downstream effectors of circCCDC66 should be investigated in future
studies. Thirdly, the specific regulatory mechanisms of miR-3140 in
the modulation of autophagy remain to be clarified. In addition,
further studies are required to identify the target genes of
miR-3140 which are responsible for its association with
autophagy.
Acknowledgments
Not applicable.
Funding
This work was supported by the Applied Basic
Research Project of Changzhou Science and Technology Bureau (no.
CJ20180070) and Changzhou High-Level Medical Talents Training
Project (no. 2016-CZBJ046).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JF, ZL and XH designed the present study. LL and HX
performed the experiments. QL and JF analyzed the data and prepared
the figures. JF and XH drafted the initial manuscript. XH reviewed
and revised the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integ-rity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients prior to the study start. The present study was approved
by the First People's Hospital of Changzhou (Changzhou, Jiangsu,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar
|
|
3
|
Bruera G and Ricevuto E: Intensive
chemotherapy of metastatic colorectal cancer: Weighing between
safety and clinical efficacy: Evaluation of Masi G, Loupakis F,
Salvatore L, et al: Bevacizumab with FOLFOXIRI (irinotecan,
oxaliplatin, fluorouracil, and folinate) as first-line treatment
for metastatic colorectal cancer: A phase 2 trial. Lancet Oncol.
11:845–852. 2010. View Article : Google Scholar
Expert Opin Biol Ther. 11:821–814. 2011.
View Article : Google Scholar
|
|
4
|
Petrelli F and Barni S: Correlation of
progression-free and post-progression survival with overall
survival in advanced colorectal cancer. Ann Oncol. 24:186–192.
2013. View Article : Google Scholar
|
|
5
|
Zheng H, Wu Y, Guo T, Liu F, Xu Y and Cai
S: Hypoxia induces growth differentiation factor 15 to promote the
metastasis of colorectal cancer via PERK-eIF2α signaling. Biomed
Res Int. 2020:59582722020. View Article : Google Scholar
|
|
6
|
Choi BJ, Park SA, Lee SY, Cha YN and Surh
YJ: Hypoxia induces epithelial-mesenchymal transition in colorectal
cancer cells through ubiquitin-specific protease 47-mediated
stabilization of Snail: A potential role of Sox9. Sci Rep.
7:159182017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo JU, Agarwal V, Guo H and Bartel DP:
Expanded identification and characterization of mammalian circular
RNAs. Genome Biol. 15:4092014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barrett SP, Wang PL and Salzman J:
Circular RNA biogenesis can proceed through an exon-containing
lariat precursor. Elife. 4:e075402015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang H, Huang H, Li Y, Lu Y and Ye T:
CircRNA_0058063 functions as a ceRNA in bladder cancer progression
via targeting miR-486-3p/FOXP4 axis. Biosci Rep.
40:BSR201934842020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu K, Qin X, Shao Y, Zhou Y, Ye G and Xu
S: Circular RNA MTO1 suppresses tumorigenesis of gastric carcinoma
by sponging miR-200-5p and targeting PEBP1. Mol Cell Probes.
52:1015622020. View Article : Google Scholar
|
|
11
|
Bian L, Zhi X, Ma L, Zhang J, Chen P, Sun
S, Li J, Sun Y and Qin J: Hsa_circRNA_103809 regulated the cell
proliferation and migration in colorectal cancer via
miR-532-3p/FOXO4 axis. Biochem Biophys Res Commun. 505:346–352.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T,
Sun H, Pan Y, He B and Wang S: CircHIPK3 promotes colorectal cancer
growth and metastasis by sponging miR-7. Cell Death Dis. 9:4172018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsiao KY, Lin YC, Gupta SK, Chang N, Yen
L, Sun HS and Tsai SJ: Noncoding effects of circular RNA CCDC66
promote colon cancer growth and metastasis. Cancer Res.
77:2339–2350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei Y, Zhang Y, Meng Q, Cui L and Xu C:
Hypoxia-induced circular RNA has_circRNA_403658 promotes bladder
cancer cell growth through activation of LDHA. Am J Transl Res.
11:6838–6849. 2019.PubMed/NCBI
|
|
15
|
Ren S, Liu J, Feng Y, Li Z, He L, Li L,
Cao X, Wang Z and Zhang Y: Knockdown of circDENND4C inhibits
glycolysis, migration and invasion by up-regulating miR-200b/c in
breast cancer under hypoxia. J Exp Clin Cancer Res. 38:3882019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Turchinovich A, Weiz L and Burwinkel B:
Extracellular miRNAs: The mystery of their origin and function.
Trends Biochem Sci. 37:460–465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomou T, Mori MA, Dreyfuss JM, Konishi M,
Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R,
Grinspoon SK, et al: Adipose-derived circulating miRNAs regulate
gene expression in other tissues. Nature. 542:450–455. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun N, Zhang L, Zhang C and Yuan Y:
miR-144-3p inhibits cell proliferation of colorectal cancer cells
by targeting BCL6 via inhibition of Wnt/β-catenin signaling. Cell
Mol Biol Lett. 25:192020. View Article : Google Scholar
|
|
19
|
Weihua Z, Guorong Z, Xiaolong C and
Weizhan L: MiR-33a functions as a tumor suppressor in
triple-negative breast cancer by targeting EZH2. Cancer Cell Int.
20:852020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang QY, Deng QF, Luo J and Zhou CC:
MiRNA-20a-5p accelerates the proliferation and invasion of
non-small cell lung cancer by targeting and downregulating KLF9.
Eur Rev Med Pharmacol Sci. 24:2548–2556. 2020.PubMed/NCBI
|
|
21
|
Tonouchi E, Gen Y, Muramatsu T, Hiramoto
H, Tanimoto K, Inoue J and Inazawa J: miR-3140 suppresses tumor
cell growth by targeting BRD4 via its coding sequence and
downregulates the BRD4-NUT fusion oncoprotein. Sci Rep. 8:44822018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Dong Y, He D, Peng Z, Peng W, Shi W, Wang
J, Li B, Zhang C and Duan C: Circular RNAs in cancer: An emerging
key player. J Hematol Oncol. 10:22017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P
and Wu M: CircRNA: Functions and properties of a novel potential
biomarker for cancer. Mol Cancer. 16:942017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luan W, Shi Y, Zhou Z, Xia Y and Wang J:
CircRNA_0084043 promote malignant melanoma progression via
miR-153-3p/Snail axis. Biochem Biophys Res Commun. 502:22–29. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Jin X, Liu B, Zhang P, Chen W and Li
Q: CircRNA CBL.11 suppresses cell proliferation by sponging
miR-6778-5p in colorectal cancer. BMC Cancer. 19:8262019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim CW, Oh ET, Kim JM, Park JS, Lee DH,
Lee JS, Kim KK and Park HJ: Hypoxia-induced microRNA-590-5p
promotes colorectal cancer progression by modulating matrix
metalloproteinase activity. Cancer Lett. 416:31–41. 2018.
View Article : Google Scholar
|
|
28
|
Ullmann P, Nurmik M, Schmitz M, Rodriguez
F, Weiler J, Qureshi-Baig K, Felten P, Nazarov PV, Nicot N, Zuegel
N, et al: Tumor suppressor miR-215 counteracts hypoxia-induced
colon cancer stem cell activity. Cancer Lett. 450:32–41. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Song X, Chen X, Wang Q, Zheng X,
Wu C and Jiang J: Circular RNA CircCACTIN promotes gastric cancer
progression by sponging MiR-331-3p and regulating TGFBR1
expression. Int J Biol Sci. 15:1091–1103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei S, Zheng Y, Jiang Y, Li X, Geng J,
Shen Y, Li Q, Wang X, Zhao C, Chen Y, et al: The circRNA circPTPRA
suppresses epithelial-mesenchymal transitioning and metastasis of
NSCLC cells by sponging miR-96-5p. EBioMedicine. 44:182–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer
A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J
and Califano A: An extensive microRNA-mediated network of RNA-RNA
interactions regulates established oncogenic pathways in
glioblastoma. Cell. 147:370–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tu FL, Guo XQ, Wu HX, He ZY, Wang F, Sun
AJ and Dai XD: Circ-0001313/miRNA-510-5p/AKT2 axis promotes the
development and progression of colon cancer. Am J Transl Res.
12:281–291. 2020.PubMed/NCBI
|
|
34
|
He JH, Han ZP, Luo JG, Jiang JW, Zhou JB,
Chen WM, Lv YB, He ML, Zheng L, Li YG and Zuo JD: Hsa_Circ_0007843
acts as a mIR-518c-5p sponge to regulate the migration and invasion
of colon cancer SW480 cells. Front Genet. 11:92020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qureshi-Baig K, Kuhn D, Viry E, Pozdeev
VI, Schmitz M, Rodriguez F, Ullmann P, Koncina E, Nurmik M,
Frasquilho S, et al: Hypoxia-induced autophagy drives colorectal
cancer initiation and progression by activating the PRKC/PKC-EZR
(ezrin) pathway. Autophagy. Jan 17–2019.Epub ahead of print.
PubMed/NCBI
|
|
37
|
Che J, Wang W, Huang Y, Zhang L, Zhao J,
Zhang P and Yuan X: miR-20a inhibits hypoxia-induced autophagy by
targeting ATG5/FIP200 in colorectal cancer. Mol Carcinog.
58:1234–1247. 2019.PubMed/NCBI
|