Introduction
Bone implant surgery is required for a variety of
reasons, such as bone degenerative changes, trauma, tumors and
deformities. However, whether the implant can remain stably fixed
for a long period of time directly affects the surgical outcome and
post-operative rehabilitation of the patient. Some studies have
demonstrated that the poor bone integration of implants is the
major cause of implant loosening (1,2).
Ideal implant-bone integration involves a complex reaction between
the bone and the implant surface. Recent studies have also
indicated that the migration and adhesion of functional cells to
the surface of an implant plays a pivotal role in the process of
implant osseointegration (3,4).
Osteoblasts are considered to be derived from the osteoblastic
differentiation of multipotent bone marrow-derived stromal cells
(BMSCs), and the effects of BMSC osteoblastic differentiation on
the surface of the implant has been investigated in previous
studies (5-8).
Parathyroid hormone (PTH) is secreted by the
parathyroid gland and can regulate calcium and phosphorus
metabolism, promote osteoblast activity and accelerate bone
transformation. Recombinant human PTH(1-34) [rhPTH(1-34)], which
has been approved for the treatment of osteoporosis, can promote
bone formation, increase bone strength and reduce the risk of
fracture, unlike other drugs used in the treatment of osteoporosis
that suppress bone resorption (9,10).
The effects of PTH on bone anabolism and catabolism depend on the
dosage and duration of administration. Constant PTH stimulation can
activate protein cleavage and degradation pathways and improve bone
turnover. However, the intermittent use of PTH has been shown to
promote bone anabolism in some animal experiments (11).
Despite extensive studies, the mechanisms through
which PTH promotes bone anabolism remain unclear. A potential
mechanism is attributed to the increased proliferation of
osteoblasts via the expression of insulin insulin-like growth
factor-I (IGF-I) and the differentiation of osteoprogenitor cells
in the bone marrow. The results of a recent study suggested that
PTH can regulate the migration and osteogenic differentiation of
human periodontal ligament stem cells (PDLSCs). The results of that
study demonstrated that PTH can activate the adhesion of
multipotent bone marrow stromal precursors, and this observation
may represent an early stage of the PTH-induced osseointegration
response in bone (12).
Mammalian target of rapamycin (mTOR) is an atypical
serine/threonine protein kinase and a member of the
phosphatidylinositol kinase-related kinase (PIKK) protein family.
The mTOR pathway can regulate cell metabolism, migration,
self-renewal and autophagy (13,14). There are two different mTOR
complexes in mammalian cells: mTOR complex 1 (mTORC1) and mTOR
complex 2 (mTORC2). In recent years, the key role of mTORC2 in
regulating cell migration, adhesion and cytoskeletal reorganization
has been confirmed (15-17). In mammalian cells, mTORC2
regulates the orderly assembly of the actin cytoskeleton through
protein kinase Cα (PKCα) (18).
Sen et al (19) observed
that following physical stimulation intervention in mesenchymal
stem cells (MSCs), the expression of F-actin and vinculin increased
and involved the mTORC2 pathway. In addition, previous studies have
indicated that mTORC2 is involved in the migration of colorectal
cancer, osteosarcoma and bladder cancer cells (20-22).
Based on the above-mentioned findings, in the
present study, it was hypothesized that PTH(1-34) can stimulate the
migration and adhesion of BMSCs by activating the mTORC2 signaling
pathway. Therefore, in order to further elucidate the cellular and
molecular mechanisms through which PTH affects osteoimplant
integration, the effects of PTH(1-34) on BMSC migration and
adhesion and the potential role of mTORC2 signaling in mediating
these effects were investigated.
Materials and methods
Isolation and culturing of BMSCs
A total of 20 male Sprague-Dawley rats (4-6 weeks
old; 100-120 g) were purchased from the Experimental Animal Center
of Sun Yat-Sen University for use in the present study. The rats
were allowed to adapt to the housing conditions for 7 days, during
which the rats were maintained under a 12-h light/dark cycle at
22°C with free access to food and water (23,24). The present study was approved by
the Animal Care Committee of Sun Yat-Sen University [no. (2014)52]
and was performed in accordance with the guidelines for the use of
laboratory animals. In summary, BMSCs were harvested from the
femoral and tibial medullary cavities by flushing with a 5 ml
syringe and suspended in Dulbecco's modified Eagle medium F-12
(DMEM/F12) growth medium (GM). The cells were cultured in a
humidified incubator at 37°C under an atmosphere with 5%
CO2 and the third generation of BMSCs were used in the
experiments.
Wound healing assay
A wound healing assay was performed in 6-well
plates. BMSCs were seeded in 6-well plates at a density of
5×105 cells per well. The cells were deprived of serum
for 24 h and then scratched with a 100-µl pipette tip.
Subsequently, various treatments were applied, including PP242 (10
µM/l), PTH (1-34) (10 nM/l), PP242 and PTH(1-34), rapamycin
(20 nM), rapamycin and PTH(1-34), raptor(-), raptor(-) and
PTH(1-34), rictor(-), and rictor(-) and PTH (1-34). In the PTH
group, BMSCs were exposed to 10 nM PTH(1-34) in DMEM medium for the
first 4 h of each 8 h cycle and then washed with phosphate-buffered
saline (PBS) and the process was repeated for the subsequent 20 h.
The surface scratches were monitored using a microscope (Zeiss AG)
at 0 and 24 h.
Lentivirus production
siRNA targeting rat rictor and raptor lentivirus
were procured from Shanghai GeneChem Co., and the target sequences
were as follows: (sirictor, GTC CAG AGA ATC ACA GAG AAA; siraptor,
CGG GTC CTC TTC CAC TAT AA; siControl, CGC TTC CGC GGC CCG TTC AA).
The 400-position transfection mixture containing sirictor or
siraptor, VSVG, PAX2 and transfection reagent polyethyleneimine
(Polysciences) in DMEM, was incubated at room temperature for 15
min, and then added to BMSCs (5×105 cells per well).
Cells were cultured in a 37°C virus incubator for 48 h. The
transfected cells were selected and incubated in puromycin at 3
µg/ml for 24 h. The transfection efficiency was determined
by western blot analysis of the control group, siCtrl group,
siraptor groups and sirictor groups.
Transwell migration assay
Transwell migration assays were performed in 24-well
plates. BMSCs were serum-starved for 24 h before being seeded into
the upper chambers of a Transwell plate and cultured with
serum-free medium at a concentration of 1×105 cells per
well. In the lower chambers, 300 µl of medium with PP242 (10
µM/l), PTH (1-34) (10 nM/l), PP242 and PTH(1-34), rapamycin
(20 nM), rapamycin and PTH(1-34), raptor(-), raptor(-) and
PTH(1-34), rictor(-), and rictor(-) and PTH(1-34) were added. In
the containing PTH group, cells were exposed to 10 nM PTH(1-34) in
DMEM medium for the first 4 h of each 8-h cycle and the process was
repeated for the subsequent 20 h. The 24-well plates were then
placed in an incubator at 37°C under an atmosphere with 5%
CO2. Migrated cells were fixed in 4% paraformaldehyde
for 15 min and stained with crystal violet for 30 min at room
temperature. The migration of the BMSCs was monitored using a
microscope (DMI3000 B; Leica). Subsequently, 5 random microscopic
fields at a ×20 magnification were selected to quantify the
migrated cells. All experiments were repeated 3 times.
Adhesion assay
For this assay, 96-well plates were pre-coated with
10 µg/ml fibronectin (FN; 70 µl per well), washed 3
times in PBS at 4°C, blocked with 1% bovine serum albumin (BSA) for
1 h at 37°C, and then washed 3 times with PBS. The cells to be
examined were cultured to the logarithmic growth phase, digested
and suspended in serum-free medium, counted with a hemocytometer,
adjusted to a concentration of 5×105/ml, and then
inoculated into a fibronectincoated 96-well plate. Each well
contained 5,000 cells, and the experimental groups and control
groups included 3 duplicate wells. After incubating the plates for
1 h at 37°C, PBS was used to wash away unadhered cells.
Subsequently, 100 µl of methanol were added to each well,
and the cells were fixed in 4% paraformaldehyde for 15 min. Giemsa
dye solution (100 µl) was then added to each well for 15 min
at room temperature, and the cells were washed with PBS. A total of
5 random fields were selected to count the number of adherent
cells.
Western blot analysis
Western blot analysis was performed using standard
techniques. Following 2 days of the treatment with 2 ml various
concentrations (1, 10, 20, 50 and 100 nM) of PTH and 2 inhibitors
(20 nM rapamycin and 10 µM PP242), BMSCs were lysed in 50
µl of protein extraction reagent (M-PER) (BestBio) and
protease inhibitor cocktail. The protein samples were harvested
following centrifugation for 15 min (12,000 × g, 4°C) and boiled
for 5 min total protein concentration was determined using a
NanoDrop 2000 spec-trophotometer. Equal volumes (20 µl) of
the samples were separated via 10% SDS-PAGE and subsequently
transferred to polyvinylidene fluoride (PVDF) membranes. The
membranes were blocked with 5% non-fat dry milk and then incubated
overnight at 4°C with primary antibodies specific for rabbit
anti-phosphorylated S6 (p-S6) monoclonal antibody (mAb; 1:2,000;
cat. no. 4858, Cell Signaling Technology, Inc.), rabbit anti-S6
(mAb; 1:2,000; cat. no. 2217, Cell Signaling Technology, Inc.),
rabbit anti-CXCR4 (PcAb; 1:2,000; cat. no. PA1-22486, Invitrogen;
Thermo Fisher Scientific, Inc.), rabbit anti-CCR2 (mAb; 1:1,000;
cat. no. 12199, Cell Signaling Technology, Inc.), rabbit
anti-intercellular adhesion molecule 1 (ICAM-1) (mAb; 1:1,000; cat.
no. 67836, Cell Signaling Technology, Inc.), rabbit anti-FN (mAb;
1:2,000; cat. no. 45688, Abcam), rabbit anti-integrin β1 (mAb;
1:1,000; cat. no. 34971, Cell Signaling Technology, Inc.), rabbit
anti-β-actin (mAb; 1:1,000; cat. no. 4970) and anti-GAPDH (mAb;
1:1,000; cat. no. 5174) (Cell Signaling Technology, Inc.), followed
by incubation with a alkaline phosphatase-conjugated goat anti-IgG
(1:2,000, cat. no. 7054; Cell Signaling Technology, Inc.) for 1 h
at room temperature. The immunoreactive proteins were visualized
using a chemiluminescence kit (EMD Millipore), and band density
analysis was carried out using Photoshop CS5 (Adobe Systems
Incorporated, Inc.) and ImageJ 1.8.0 software (NIH).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells
following treatment with 2 ml of various concentrations (1, 10, 20,
50 and 100 nM) of PTH and 2 inhibitors (20 nM rapamycin and 10
µM PP242) for 1 day using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The RNA was then used for cDNA synthesis
with the PrimeScript RT-PCR kit (Takara Biotechnology Co., Ltd.) in
a 10 µl reaction volume containing 0.5 µl of
PrimeScript RT Enzyme Mix I, 0.5 µl of oligo dT primer, and
2.0 µl of PrimeScript Buffer. Subsequently, 1 µl of
cDNA was used for qPCR in a 10 µl reaction volume containing
0.3 µl of the forward primer, 0.3 µl of the reverse
primer, 5 µl of SYBR® Premix Ex Taq, and 3.4
µl of diethylpyrocarbonate (DEPC)-treated water. Each cDNA
sample was assayed in triplicate. Fluorescence data was analyzed
using a CFX96™ Real-Time PCR Detection System (Bio-Rad
Laboratories, Inc.). Gene expression levels were calculated using
the 2−ΔΔCq method (25), and the data are presented as the
fold change relative to control samples. The primer sequences used
for the RT-qPCR analyses of rat CXCR4, CCR2, ICAM-1, FN, integrin
β1 and β-actin are presented in Table
I.
 | Table IGene primers used for RT-qPCR. |
Table I
Gene primers used for RT-qPCR.
| Gene | Primer | Sequence |
|---|
| CXCR-4 | Forward |
5′-GGCTGACCTCTTTGT-3′ |
| Reverse |
5′-GTTTCCTTCGCCTTTGAC-3′ |
| CCR-2 | Forward |
5′-GGAATCTTCTTCATTATCCTCCTGAC-3′ |
| Reverse |
5′-TGACTACACTTGTTATTACCCCAAAGG-3′ |
| ICAM-1 | Forward |
5′-TTGGGCATAGAGACCCCGTT-3′ |
| Reverse |
5′-GCACATTGCTCAGTTCATACACC-3′ |
| Fibronectin | Forward |
5′-CGGTGGCTGTCAGTCAAAG-3′ |
| Reverse |
5′-AAACCTCGGCTTCCTCCATAA-3′ |
| Integrin β1 | Forward |
5′-TGAATGTGAATGCCAAAGCGA-3′ |
| Reverse |
5′-CAATGTCTACCAACACGCCC-3′ |
| β-actin | Forward |
5′-GTCTGCCTTGGTAGTGGATAATG-3′ |
| Reverse |
5′-TCGAGGACGCCCTATCATGG-3′ |
Statistical analysis
Statistical analyses were performed using SPSS 22.0
software (SPSS, Inc.). The data are presented as the means ±
standard deviation. Differences between the quantitative values
among multiple groups were analyzed with one-way analysis of
variance (ANOVA) with the Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
PTH(1-34) promotes the migration of
BMSCs
It was observed that PTH(1-34) promoted BMSC
migration, as shown by wound healing and Transwell migration
assays. The intermittent use of various concentrations of PTH(1-34)
(1, 10, 20, 50 and 100 nM) markedly enhanced the motility of the
BMSCs in a concentration-dependent manner. In Transwell migration
assays, the BMSCs treated with various concentrations of PTH(1-34)
migrated to the lower chamber significantly more frequently than
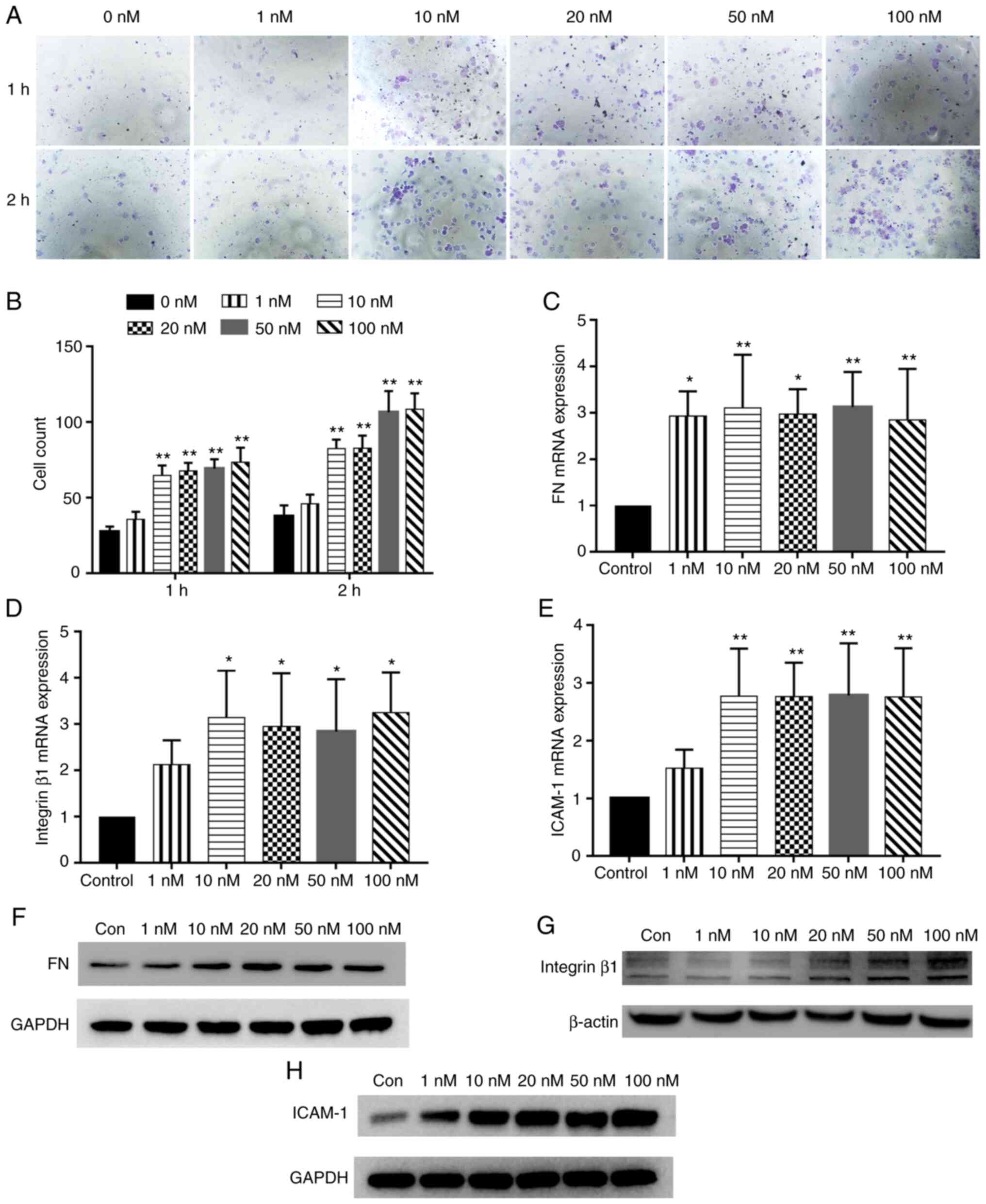
the BMSCs in the control group (P<0.01; Fig. 1A and B). In addition, the 10, 20,
50 and 100 nM concentrations of PTH(1-34) promoted BMSC migration
compared to that observed with 0 nM PTH(1-34), and no significant
differences between the 1 nM treatment and the control were
observed, while intermittent PTH(1-34) treatment at various
concentrations significantly increased the migration of the BMSCs
(P<0.01) (Fig. 1C and D). In
addition, PTH(1-34) promoted BMSC migration more effectively at the
concentrations of 10, 20, 50 and 100 nM than at 1 nM, and there
were no significant differences between these treatments
(P>0.05).
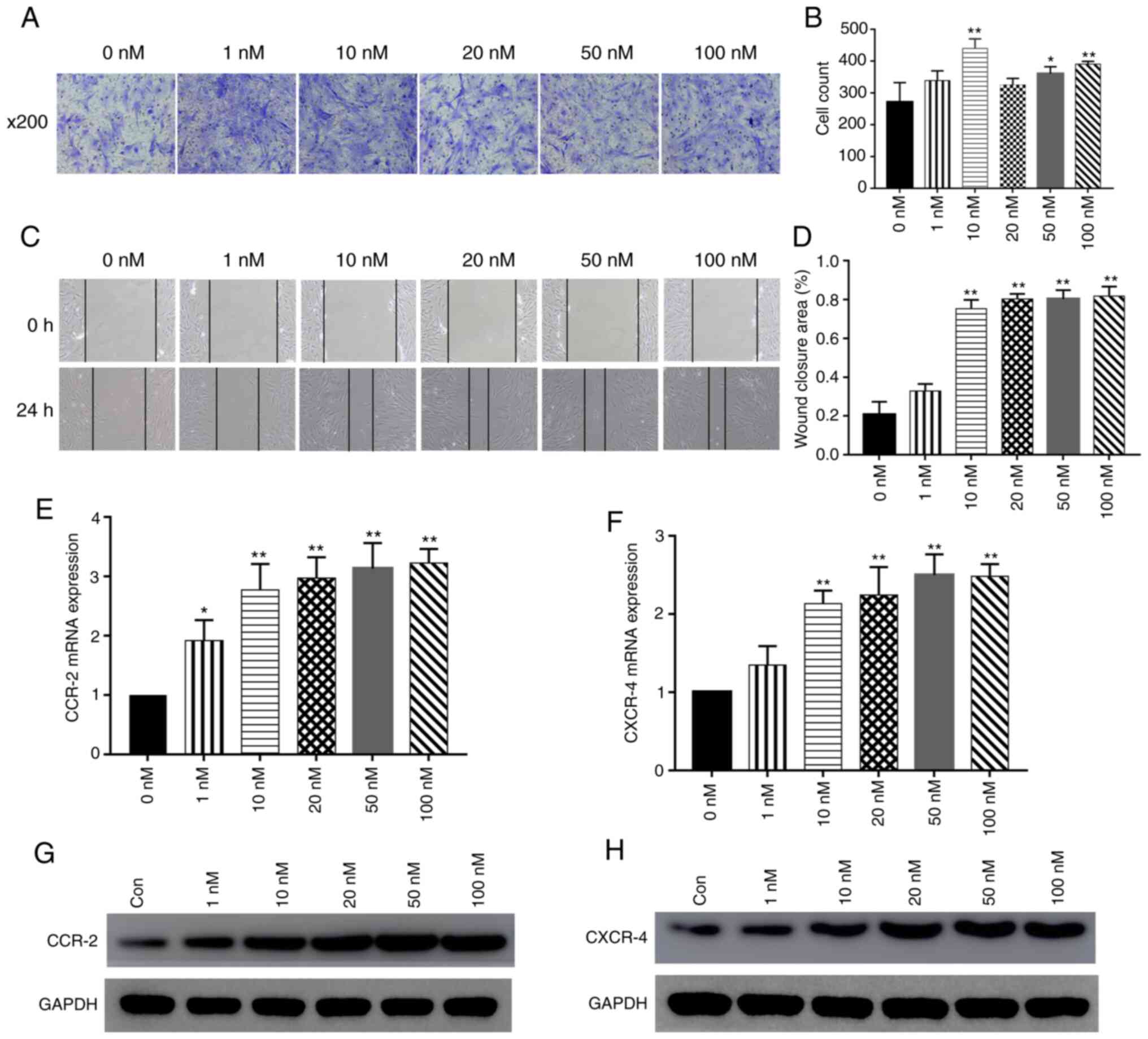 | Figure 1Effect of PTH(1-34) on BMSC
migration. (A and B) BMSCs were seeded in the upper chamber of
Transwell plates. The bottom wells were loaded with medium alone or
medium containing 1, 10, 20, 50 or 100 nM PTH (1-34) for 4 h. The
number of cells that migrated to the bottom wells was counted 24 h
later. (C and D) Intermittent use of PTH(1-34) (1, 10, 20, 50 and
100 nM) (for the first 4 h of each 8-h cycle). The cell layer was
scratched with a 200 µl sterile pipette tip, and the wound
surface area was measured at the 0 and 24 h points. Cells cultured
in medium were used as a blank control. The results are presented
as the percentage of the scratch closure area. Scale bars, 100
µm; data are the means ± SD; n=3; *P<0.05,
**P<0.01 vs. control group. (E and F) The mRNA
expression of chemokine receptors, including CXCR4 and CCR2, was
measured by RT-qPCR. data are the means ± SD; n=3;
*P<0.05, **P<0.01 vs. control group. (G
and H) Western blot analysis of CXCR4 and CCR2 expression in BMSCs
treated with various concentrations of PTH(1-34). BMSCs, bone
marrow-derived stromal cells; PTH, parathyroid hormone. |
The mRNA expression levels of chemokine receptors
were determined by RT-qPCR following the treatment of BMSCs with
various concentrations of PTH(1-34). The results revealed that the
expression of CXCR4 and CCR2 significantly increased by PTH(1-34)
treatment at various concentrations (P<0.05; Fig. 1E and F). In addition, PTH(1-34)
stimulation increased CXCR4 and CCR2 protein expression (Fig. 1G and H). In summary, these data
reveal that PTH(1-34) can promote the migration of BMSCs.
PTH (1-34) promotes the adhesion of
BMSCs
The effects of various concentrations of PTH(1-34)
on the adhesive ability of the BMSCs were examined. Compared with
the control treatment, PTH(1-34) significantly increased the number
of adherent cells (P<0.01); however, there were no significant
differences between the tested concentrations themselves
(P>0.05; Fig. 2A and B).
The mRNA expression levels of key adhesion factors
were detected by RT-qPCR after the BMSCs were treated with various
concentrations of PTH(1-34). The results revealed that the
expression levels of FN, ICAM-1 and integrin β1 were significantly
increased by PTH (1-34) treatment at various concentrations
(P<0.05; Fig. 2C-E). The
results of western blot analysis also demonstrated that compared
with that observed in the control group, the expression of adhesion
factor proteins, including FN, ICAM-1 and integrin β1, was markedly
increased in the PTH(1-34) stimulation group (Fig. 2F-H).
Activation of the mTORC pathway by
PTH(1-34) in BMSCs
However, whether PTH(1-34) can activate the
migration and adhesion of BMSCs through the mTORC2 pathway in
vitro remain unclear. Therefore, the effects of PTH(1-34) on
the activity of the mTORC1 and mTORC2 pathways were investigated
and the results were verified by detecting the phosphorylation of
S6 on its regulatory Ser235/236 site and the phosphorylation of Akt
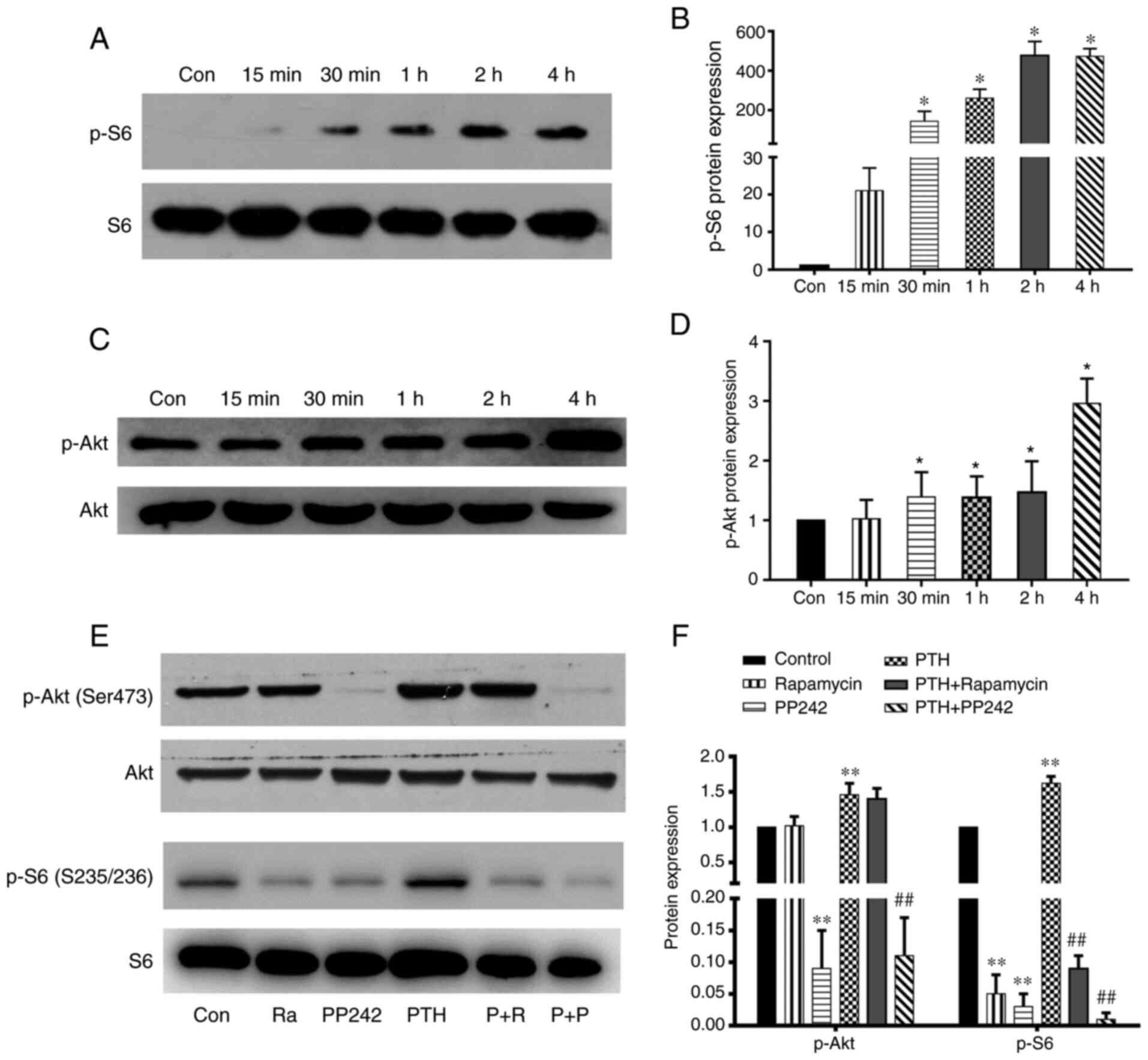
on its regulatory Ser-473 site. PTH(1-34) an induced increase in
Akt Ser-473 phosphorylation and S6 Ser235/236 phosphorylation in
the BMSCs. Time course experiments revealed that PTH(1-34) also
induced an increase in the phosphorylation of S6 Ser235/236 in the
BMSCs as early as 30 min (P<0.05) with the level of p-S6 peaking
at 2 and 4 h (P<0.05; Fig. 3A and
B). On the other hand, time course experiments revealed that
PTH(1-34) increased the phosphorylation of these sites as early as
30 min in the BMSCs (P<0.05), and the level of p-Akt was highest
at 4 h following treatment with PTH(1-34) (P<0.01; Fig. 3C and D). Although no marked
difference in the level of p-Akt was observed at 30 min, or at 1
and 2 h following PTH(1-34) treatment, the level was still
significantly higher compared to that of the control group
(P<0.05).
The effects of mTORC1 inhibitor, and mTORC1/2
inhibitor and PTH(1-34) on phosphorylation were then investigated.
The results of western blot analysis revealed that changes in the
phosphorylation of these proteins occurred following the
pre-treatment of BMSCs with an mTORc1 inhibitor (rapamycin) and an
mTORc1/2 inhibitor (PP242). As shown in Fig. 3E and F, compared to the control,
rapamycin and PP242 decreased the levels of p-S6 in the BMSCs, and
the inhibitory effect of PP242 was more potent than that of
rapamycin (P<0.01). Following stimulation with PTH(1-34), the
levels of p-S6 in the BMSCs were significantly increased. Following
the addition of the inhibitors however, the stimulatory effect of
PTH(1-34) was inhibited, and the inhibitory effect of PP242 was
more potent than that of the other inhibitors (P<0.01).
Compared to the control group, PP242 also suppressed
p-Akt expression, while rapamycin did not (P<0.01). Following
pre-treatment with PTH(1-34), the level of p-Akt in the BMSCs was
significantly higher; however, the expression of p-Akt was
significantly lower following the addition of PP242 (P<0.01).
Notably, in the rapamycin plus PTH(1-34) treatment group, the
expression of p-Akt did not differ from that of the PTH(1-34)
treatment group (P>0.05). These findings suggest that PTH (1-34)
is more likely to function through the mTORC2 pathway.
Involvement of the mTORC2 signaling
pathway in the PTH-dependent migration and adhesion of BMSCs
To investigate whether the mTORC2 signaling pathway
is involved in PTH-induced BMSC migration, wound healing and
Transwell migration assays were performed. Several groups,
including the blank group, were treated or not with 10 nM
PTH(1-34), with or without pre-treatment with mTORC1 inhibitor (20
nM rapamycin) or mTORC1/2 inhibitor (10 µM PP242). Treatment
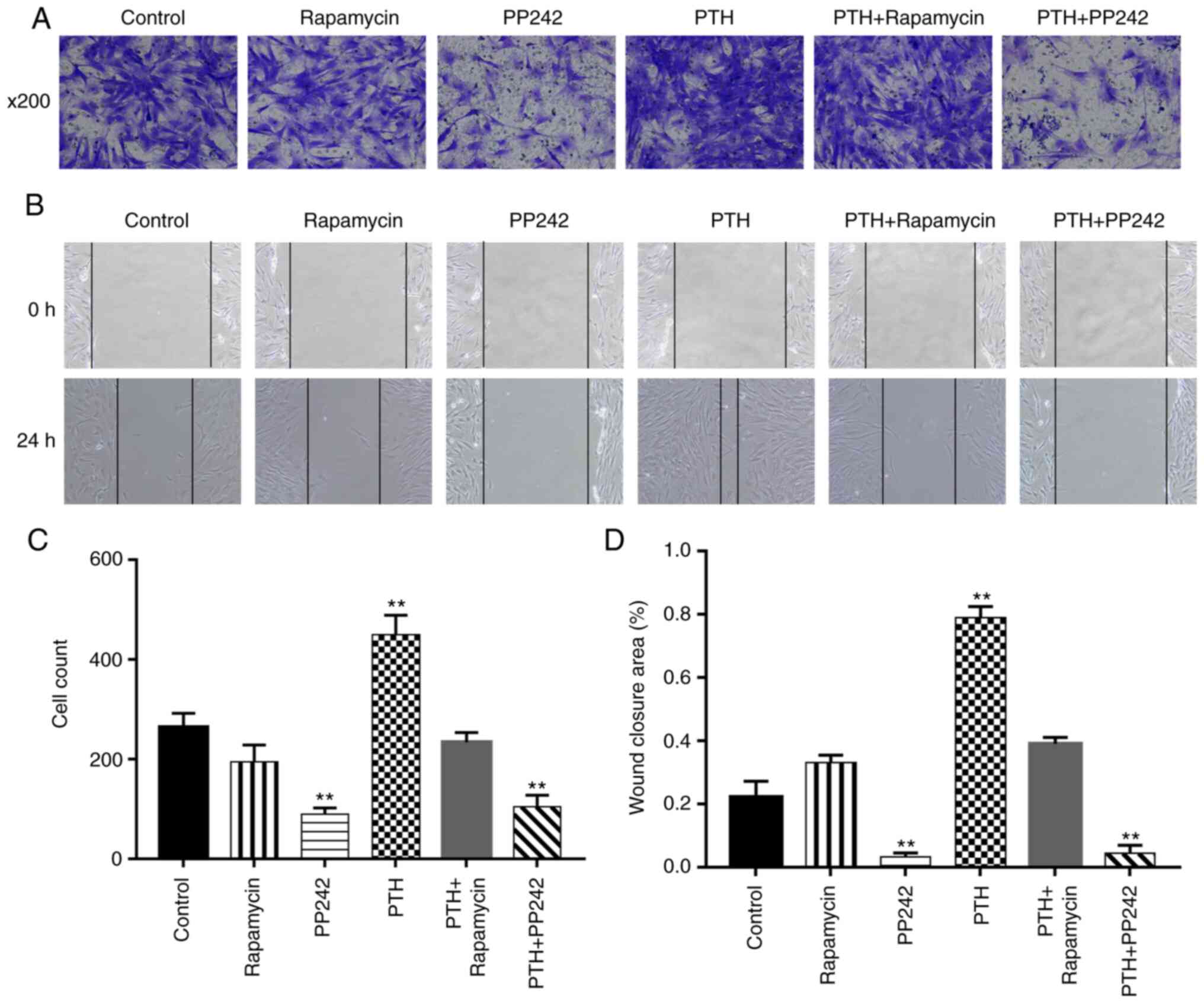
with PTH(1-34) markedly increased the mobility of the BMSCs
(P<0.01). The mTORC1/2 inhibitor (PP242) markedly suppressed
PTH-induced cell migration (P<0.01; Fig. 4), while the mTORC1 inhibitor
(rapamycin) did not exert any distinct inhibitory on PTH-dependent
BMSC migration (P>0.05). Similar results were observed in the
Transwell migration experiments. The PTH(1-34) treatment group
exhibited a notable increase in the number of cells migrating to
the lower chamber (P<0.01), while PP242 suppressed the effects
of PTH(1-34) (P<0.01). No significant difference was observed
between the control group and the rapamycin group (P>0.05).
The results of RT-qPCR and western blot analyses of
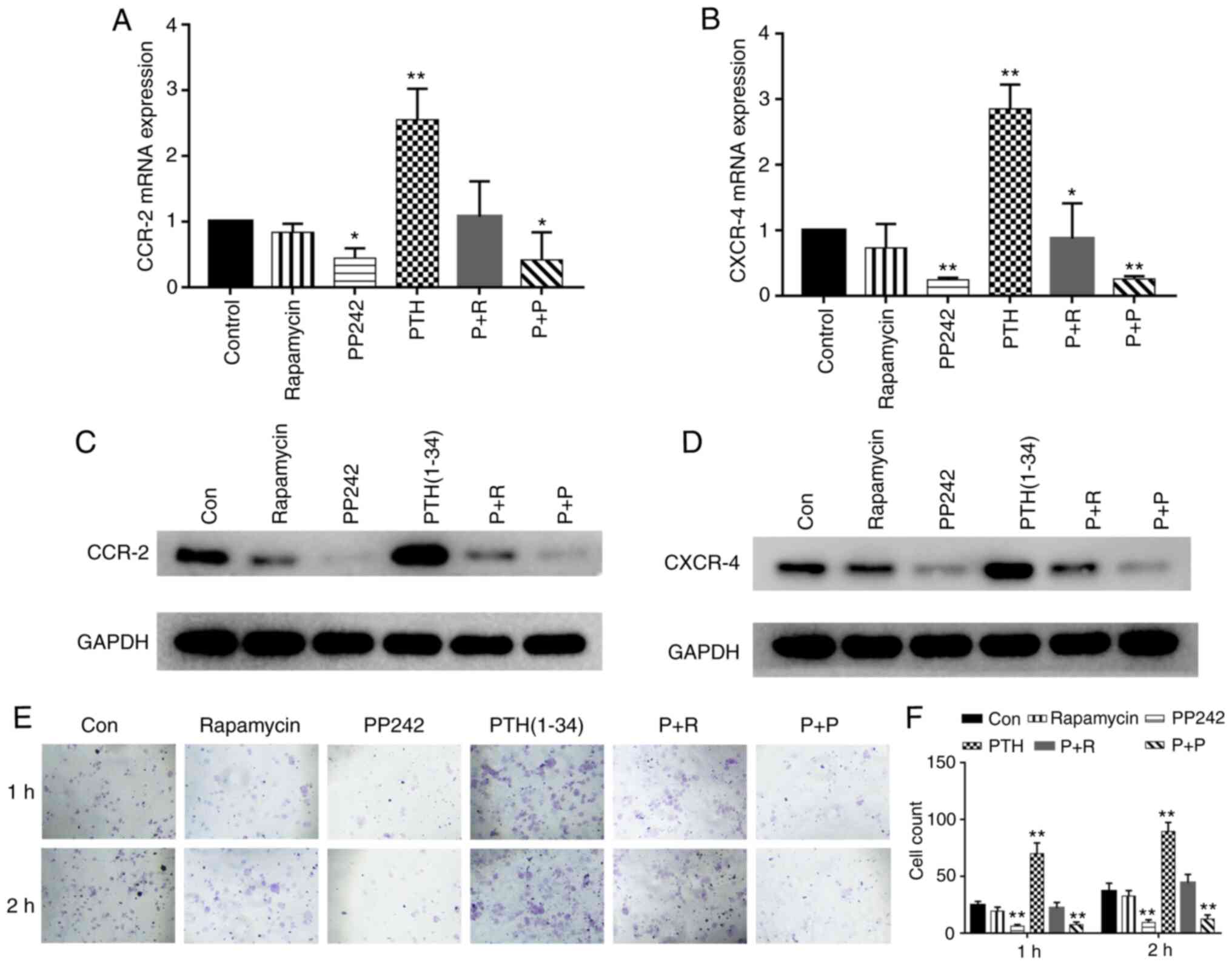
the expression of chemokine receptors are shown in Fig. 5A-D. The results revealed that
PTH(1-34) significantly increased the expression of CXCR4 and CCR2
(P<0.05), while PP242 significantly inhibited the PTH-induced
mRNA expression of CXCR4 and CCR2 (P<0.05). Accordingly, the
protein expression of CXCR4 and CCR2 was stimulated by PTH(1-34)
(P<0.01). PP242 decreased the expression of the chemokine
receptors (P<0.05), whereas following treatment with rapamycin,
the expression of the marker proteins was not significantly altered
compared to that of the control group (P>0.05). These findings
suggest that PTH (1-34) activates the migration of BMSCs by
increasing the expression of chemokine receptors through the mTORC2
pathway.
To confirm the role of mTORC2 signaling in
PTH-induced BMSC adhesion, an adhesion assay was performed to
examine the adhesive capacity of each group. Compared with the
control, stimulation with PTH(1-34) promoted the adhesion of the
BMSCs after 1 and 2 h; however, following the addition of PP242,
the number of adhered cells was significantly decreased compared
with that observed in the PTH group (1 and 2 h) (P<0.05;
Fig. 5E and F). RT-qPCR analysis
of the expression of adhesion factors is shown in Fig. 6A-C. The mRNA expression of key
adhesion factors in BMSCs was induced by the intermittent use of
PTH(1-34) (P<0.05). PP242 exerted a more potent inhibitory
effect on the expression of ICAM-1, FN and integrin β1 than
rapamycin (P<0.05) or the PTH(1-34) group (P<0.01). The
results of western blot analysis of ICAM-1, FN and integrin β1
expression in all groups are shown in Fig. 6D. Following stimulation with
PTH(1-34), the expression of adhesion factors in the BMSCs was
significantly increased, whereas it was significantly downregulated
following pre-treatment with PP242 (P<0.01). However, compared
to the control treatment, rapamycin exerted minimal effects on the
expression of adhesion factors (P>0.05). Therefore, the
above-mentioned results suggest that the mTORC2 signaling pathway
is involved in the PTH-induced adhesion of BMSCs.
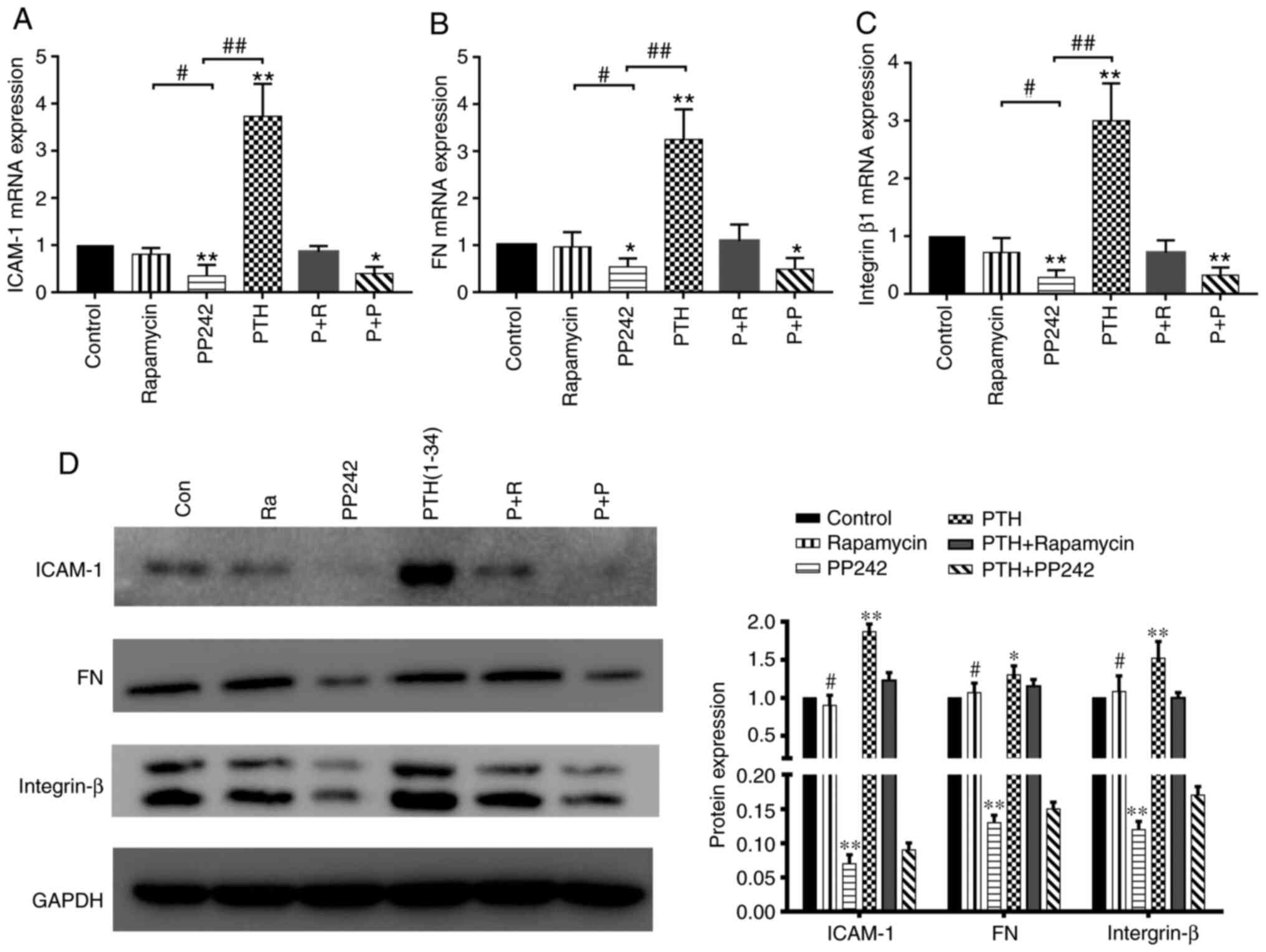 | Figure 6(A-C) mRNA expression of adhesion
factors, including ICAM-1, FN and integrin β1, in BMSCs treated
with inhibitors and PTH was measured by RT-qPCR (means ± SD; n=3).
*P<0.05, **P<0.01 vs. control group.
#P<0.05, PP242 vs. rapamycin group
##P<0.01, PP242 vs. PTH group. (D) The protein
expression of ICAM-1, FN, and integrin β in BMSCs treated with
inhibitors and PTH was verified by western blot analysis. BMSCs,
bone marrow-derived stromal cells; PTH, parathyroid hormone;
ICAM-1, intercellular adhesion molecule 1; FN, fibronectin. |
BMSCs lacking rictor rather than raptor
are insensitive to the effects of PTH(1-34)
Subsequently, the present study wished to determine
whether rictor, which is crucial for the kinase activity of mTORC2,
is essential for the PTH-induced BMSC migration and adhesion via
lentiviral silencing. As was expected, the lentiviruses
downregulated rictor/mTORC2 signaling and the phosphorylation of
Akt (S473) or raptor/mTORC1 pathway and the phosphorylation of S6
(Ser235/236) (Fig. 7A). In the
wound healing and Transwell assays (Fig. 7B and C), the intermittent use of
PTH(1-34) significantly increased the mobility of the BMSCs
compared to that observed in the negative control groups
(P<0.01). The results revealed that PP242 markedly suppressed
BMSC migration (P<0.01), similar to that observed in the
siRNA(rictor) group (P>0.05). Moreover, the migratory ability of
the siRNA(raptor)-treated BMSCs differed significantly from that
observed in the siRNA(rictor) group (P<0.05). For the gene
expression analysis, the levels of the chemokine receptors in the
siRNA(rictor) group were significantly lower than those in the
control group (P<0.01), but were similar to those observed in
the PP242 group (P>0.05) (Fig.
7D). By contrast, no significant differences were observed
between the siRNA(raptor) group and the control group (P>0.05).
Accordingly, the protein levels of CXCR4 and CCR2 in the PP242 and
siRNA(rictor) groups were significantly decreased compared to those
observed in the control group (P<0.01), whereas the levels of
these proteins in the siRNA(raptor) BMSC group were similar to
those detected in the control group (P>0.05; Fig. 7E). The above-mentioned results
suggest that rictor, the key component of mTORC2, is involved in
the PTH-induced migration of BMSCs.
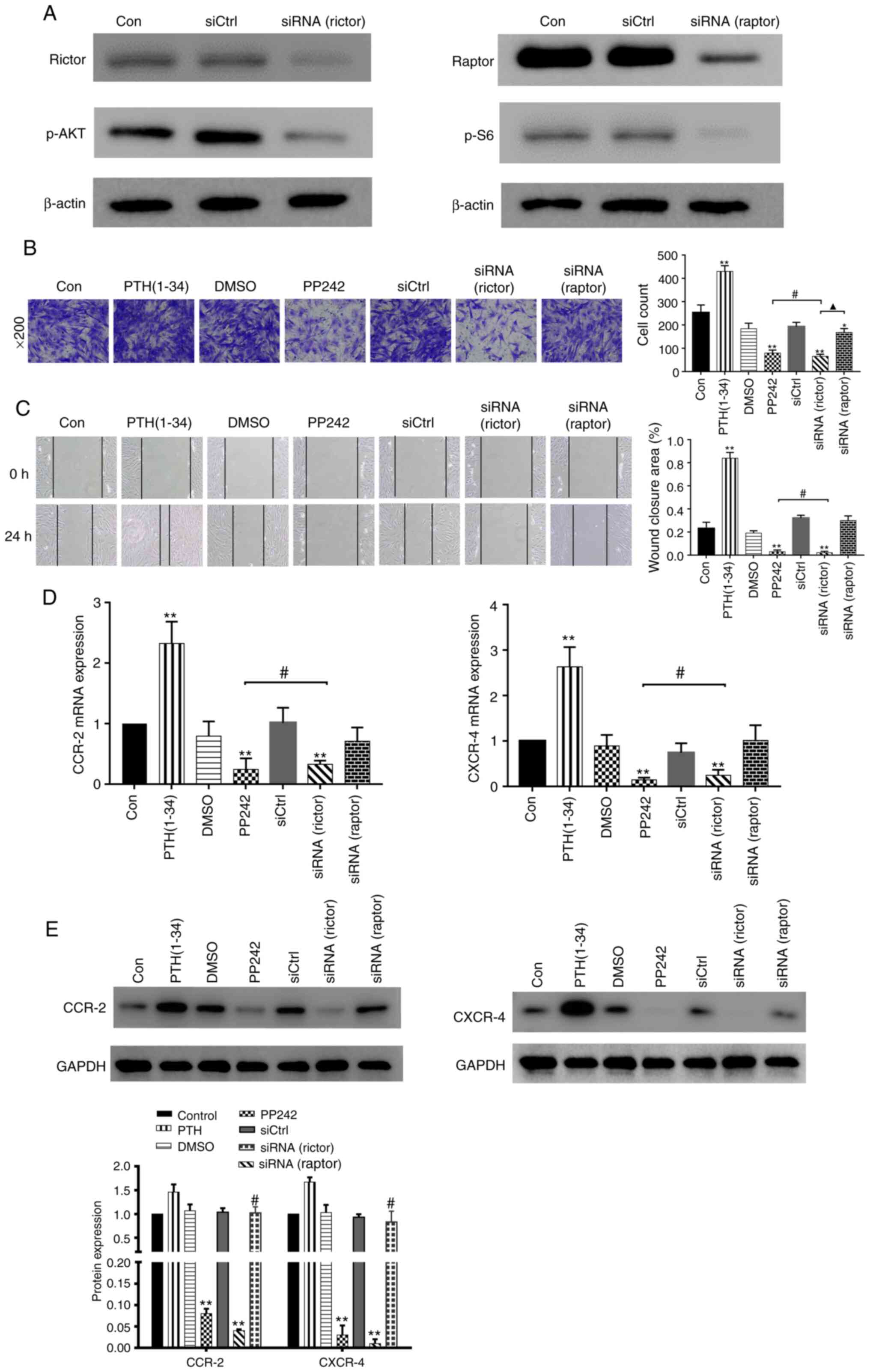 | Figure 7PTH(1-34) mediates BMSC migration and
adhesion by targeting rictor. (A) Western blot analysis of rictor
and raptor expression and phosphorylation in BMSCs from rictor(-)
and raptor(-) cells transfected with lentivirus. (B) Cells in the
different groups, including PTH(1-34), DMSO, PP242, siCtrl,
siRNA(rictor) and siRNA(raptor), were seeded in the upper chambers
of Transwell plates. The number of cells that migrated to the
bottom wells was counted 24 h later. *P<0.05,
**P<0.01 vs. control group. #P>0.05,
siRNA(rictor) vs. PP242 group. ▲P<0.05 siRNA(rictor)
vs. siRNA(raptor) group. (C) Results of scratch assays in the
different groups, including PTH (1-34), DMSO, PP242, siCtrl,
siRNA(rictor), and siRNA(raptor). **P<0.01 vs.
control group. #P>0.05, siRNA(rictor) vs. PP242 group
(D) mRNA expression of CXCR4 and CCR2 measured by RT-qPCR. (E)
Western blot analysis of CXCR4 and CCR2 expression in the different
groups. **P<0.01 vs. control group,
#P>0.05, siRNA(rictor) vs. PP242 group. BMSCs, bone
marrow-derived stromal cells; PTH, parathyroid hormone. |
An adhesion assay was also performed to examine the
adhesive capacity of each group. Compared with the control group,
the adhesive ability of the cells in the PP242 pre-treatment and
siRNA(rictor) groups was significantly decreased after 1 and 2 h
(P<0.01), while the numbers of adhered cells in the
siRNA(raptor) group were similar to those detected in the negative
control group (1 and 2 h) (P>0.05; Fig. 8A). The results of RT-qPCR analysis
revealed that the adhesion factor mRNA levels in the siRNA(rictor)
and the PP242 group were similar (P>0.05). By contrast, the mRNA
expression levels exhibited no significant differences between the
siRNA(raptor) group and the control group (P>0.05; Fig. 8B). The protein expression of key
adhesion factors in the BMSCs from the different treatment groups
was examined by western blot analysis. The protein levels of
ICAM-1, FN and integrin β1, in the PP242 and the siRNA(rictor)
group were similar (P>0.05), whereas those detected in the
siRNA(raptor) BMSC group were similar to those observed in the
control group (P>0.05; Fig.
8C). Taken together, these results suggest that PTH (1-34)
directly targets rictor to affect BMSC migration and adhesion.
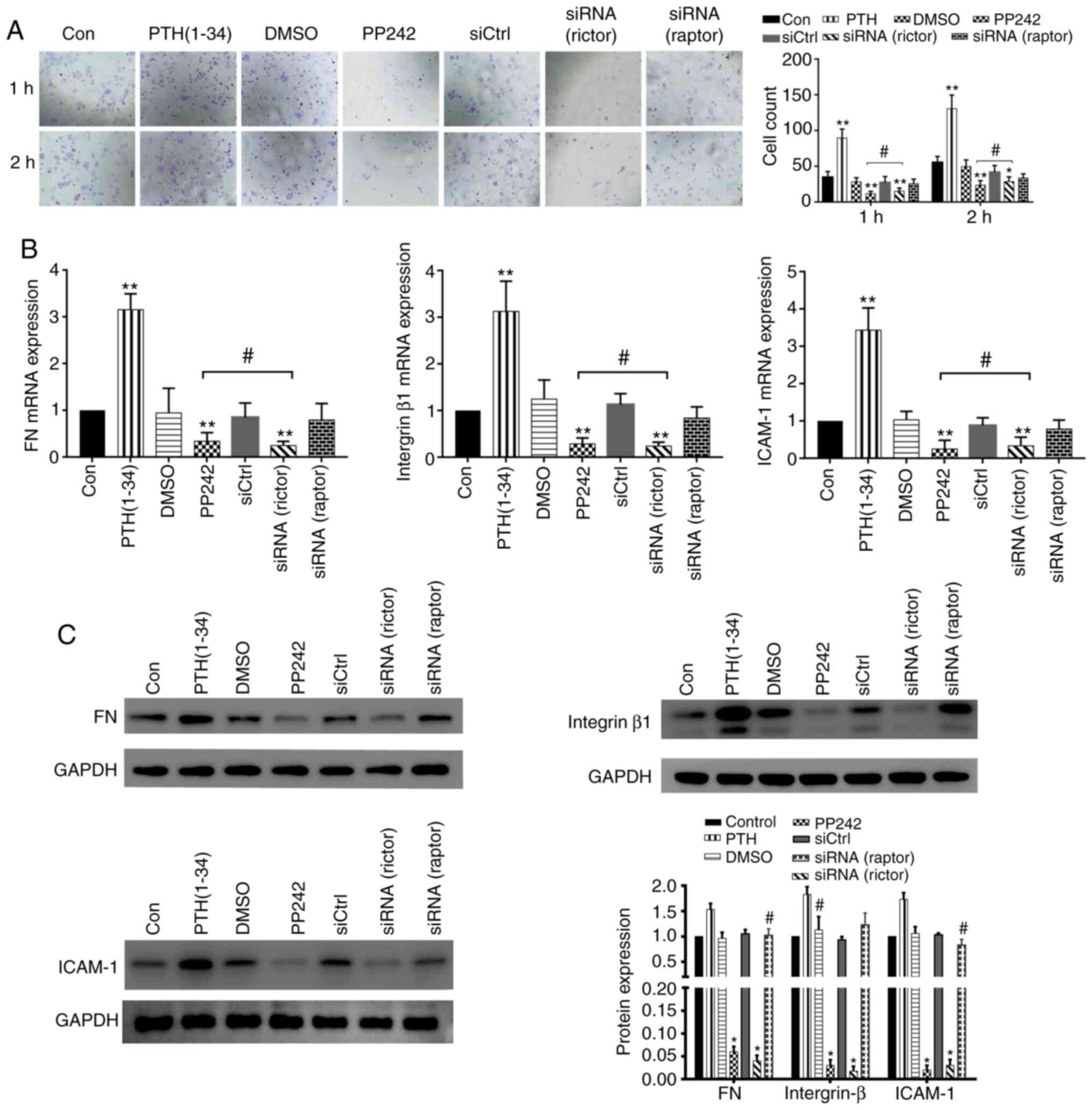 | Figure 8PTH(1-34) mediates BMSC migration and
adhesion by targeting rictor. (A) Adhesion analysis of BMSCs from
each group. *P<0.05, **P<0.01 vs.
control group, #P>0.05, siRNA(rictor) vs. PP242
group. (B) mRNA expression of adhesion factors, including ICAM-1,
FN and integrin β1 (means ± SD; n=3). **P<0.01 vs.
control group, #P>0.05 siRNA(rictor) vs. PP242 group.
(C) The protein expression of ICAM-1, FN, and integrin β1 was
verified by western blot analysis. (means ± SD; n=3).
*P>0.05 vs. siRNA(rictor) vs. PP242 group,
#P>0.05 vs. control group. BMSCs, bone marrow-derived
stromal cells; PTH, parathyroid hormone; ICAM-1, intercellular
adhesion molecule 1; FN, fibronectin. |
Discussion
Implant-bone integration refers to the structural
and functional connection between the bone surface and an implant
(26). A series of intracellular
and extracellular responses are involved in the formation and
remodeling phases of bone healing (27,28). The active ingrowth of the bone to
the implant surface is the most important aspect of bone
integration. The migration and adhesion of bone marrow MSCs and
osteoblasts to the surface are pivotal aspects of ingrowth
(3,6), as both of these processes are
required for osteogenic differentiation and bone mineralization.
Thus, successful integration depends on these biological
processes.
A series of studies have established that the
intermittent administration of PTH can improve osteogenic
differentiation and bone mineralization (10,29). Other studies have demonstrated
that PTH(1-34) can enhance bone regeneration around the implant and
improve the healing rate of the implant-bone interface in the bone
defect area (30). However, the
mechanistic aspects of this process, particularly the regulation of
BMSC migration and adhesion, are not yet fully understood. In the
present study, it was observed that PTH(1-34) promoted not only the
migratory, but also the adhesive ability of the BMSCs. It was also
demonstrated that the mTORC2 pathway can be activated by PTH(1-34),
and that rictor is an important contributor to this process. It is
considered that this new mechanism may aid in the elucidation of
the role of PTH in promoting bone-implant integration.
It has recently been demonstrated that PTH can
significantly enhance MSC migration to the lumbar region, where
MSCs differentiate into bone-forming cells (31). SDF-1 belongs to the CXC chemokine
family and exerts multiple biological functions. The previous study
by Du et al (12)
suggested that the combination of PTH and SDF-1α plays an essential
role in the migration and osteogenic differentiation of human
PDLSCs. In addition, the SDF-1/CXCR4 axis can mediate the
recruitment of MSCs to the periodontal defect (32). It has been reported that MCP-1 can
induce MSC migration in vitro, and that the application of
PTH can mobilize endogenous BMSCs/progenitors as an effective and
feasible regeneration treatment for patients with ischemic stroke
(33). In addition, it has been
demonstrated that MCP-1 migration is mediated by CCR2. Ryan et
al examined the effects of MCP-1 activation, and demonstrated
that it is important for stem cell migration (34). In the present study, scratch wound
healing and Transwell migration assays were performed to examine
the effects of PTH on the migration of BMSCs. It was observed that
PTH(1-34) upregulated the expression of CXCR4 and CCR2. The
specific elimination of rictor and the application of inhibitors
significantly suppressed the effects of PTH. These data indicate
that PTH(1-34) exerts a stimulatory effect on BMSC migration
through the rictor/mTORC2 signaling pathway.
The adhesibve ability is one factor in the first
stage of the cell-material interaction. MSCs are sensitive to
substrate properties, and adhesion determines the proliferation and
differentiation ability of cells after contact with implants. The
expression of adhesion factors, such as ICAM-1, FN and integrin β1,
is associated with the adhesive capacity of cells (35,36). Davies and Chambers (37) suggested that the adhesion of such
stromal precursors, termed colony-forming units-fibroblastic
(CFU-F) to bone may be an early event in the bone response to PTH.
Lee et al (38)
established a flexor digitorum longus (FDL) tendon repair model in
C57Bl/6J mice to evaluate adhesion formation. They observed that
PTH accelerated the deposition of reparative tissue and increased
cell adhesion. Furthermore, the ICAM-1-mediated cell-to-cell
adhesion of osteoblast and osteoclast precursors can be stimulated
by PTH (39). In the present
study, cell adhesion assays were performed to examine the effects
of PTH(1-34) on the adhesion of BMSCs, and the data revealed that
PTH(1-34) promoted BMSC adherence to the surface. In addition, the
adhesive ability of inhibitor-treated and rictor(-) cells was
significantly decreased. The expression of ICAM-1, FN and integrin
β1 in the different BMSC groups was assessed by western blot
analysis and mRNA expression analyses following treatment with
PTH(1-34), as our results showed. The results further demonstrated
that the expression of adhesion factors was significantly
upregulated by PTH(1-34), suggesting that PTH(1-34) enhances the
adhesion of BMSCs through the rictor/mTORC2 signaling pathway.
The rictor/mTORC2 pathway is involved in various
biological processes, such as cell migration, adhesion and
cytoskeletal reorganization. Wang et al (16) analyzed overall proteome changes in
cells in which rictor was knocked down and found that rictor was
highly involved in cell migration and adhesion. Subsequently,
assays were performed to determine whether the migratory and
adhesive ability of cells in which rictor was knocked down was
significantly reduced. PTH can induce insulin-like growth factor
(Igf) signaling, and the meta-bolic effects of Igf depend on the
activation of mTORC2 (40).
Additionally, Chantaravisoot et al (41) suggested that the mTORC1/2
inhibitor PP242, but not rapamycin attenuated focal adhesion
formation and cell migration in U87vIII glioblastoma cells. These
results were similar to those obtained in the present study. The
results suggested that rapamycin partially reversed the PTH-induced
migration and adhesion of BMSC cells. The mTORC2 complex was
originally considered to be rapamycin-insensitive; however, some
researchers have found that chronic treatment with rapamycin can
suppress mTORC2 activity and disrupt mTORC2 assembly (42). Rapamycin has been demonstrated to
mediate rictor dephosphorylation in a time- and
concentration-dependent manner, at physiologically relevant
rapamycin concentrations (43).
Wang et al (44) found
that prolonged rapamycin pre-treatment reduced the expression of
tumor necrosis factor (TNF)-induced vascular cell adhesion
molecule-1 by inhibiting mTORC2, thereby reducing the effect of
TNF-treated endothelial cells on capturing leukocytes. Lamming
et al (45) suggested that
chronic rapamycin treatment impaired the insulin-mediated
suppression of hepatic gluconeogenesis via the disruption of the
mTORC2 in vivo. However, the underlying mechanisms warrant
further investigation.
The findings of the present study suggested that
PTH(1-34) significantly increased the level of p-Akt, and that
mTORC1/2 inhibitors and rictor silencing decreased the p-Akt
levels. As rapamycin predominantly suppressed mTORC1, the
inhibition of mTORC2 appeared to account for the effects of PP242.
Furthermore, blocking the rictor/mTORC2 signaling pathway by PP242
and reducing the rictor levels clearly suppressed the migration and
adhesion of BMSCS. The above-mentioned data suggest that the
rictor/mTORC2 signaling pathway may play important roles in the
PTH-induced cell migration and adhesion of BMSCs.
In spite of the large amount of research performed
to demonstrate the original hypothesis, the present study has
several limitations. Rather than using more genetically applicable
mice or more clinically translatable human cellular models, BMSCs
from rats were used in the present study. Thus, additional in
vitro and in vivo studies are required to further
explore the molecular mechanisms of this pathway.
In conclusion, the results of the present study
demonstrate that PTH(1-34) promotes BMSC migration and adhesion
in vitro, and that the downregulation of the rictor/mTORC2
pathway is involved in mediating the effects of PTH(1-34). This
pathway may be a mechanism through which PTH promotes the bone
integration of implants and may provide a novel target for future
experimental and clinical studies for the prevention of implant
loosening.
Acknowledgments
Not applicable.
Abbreviations:
|
BMSCs
|
bone marrow-derived stromal cells
|
|
PTH
|
parathyroid hormone
|
|
rhPTH
|
recombinant human parathyroid
hormone
|
|
IGF-I
|
insulin-like growth factor-I
|
|
PDLSCs
|
periodontal ligament stem cells
|
|
mTOR
|
mammalian target of rapamycin
|
|
PIKK
|
phosphatidylinositol kinase-related
kinase
|
|
mTORC
|
mammalian target of rapamycin
complex
|
|
PKCα
|
protein kinase Cα
|
|
DMEM/F12
|
Dulbecco's modified Eagle's medium
F-12
|
|
GM
|
growth medium
|
|
BSA
|
bovine serum albumin
|
|
PVDF
|
polyvinylidene fluoride
|
|
ICAM-1
|
intercellular adhesion molecule 1
|
|
FN
|
fibronectin
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
DEPC
|
diethyl pyrocarbonate
|
|
one-way ANOVA
|
one-way analysis of variance
|
|
MSC
|
mesenchymal stem cell
|
|
CCR2
|
chemokine receptor 2
|
|
CFU-F
|
colony-forming units-fibroblastic
|
|
FDL
|
flexor digitorum longus
|
Funding
The present study was supported by grants from the
Natural Science Foundation of China (no. 31570976), and the Science
and Technology Program of Guangzhou, China (no. 201604020148). The
content is solely the responsibility of the authors and does not
necessarily represent the official views of the Natural Science
Foundation of China.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
BC conceived and designed the study. ZL, XB, XZ and
TL performed the experiments. AM and BC analyzed and interpreted
the data and wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
Committee of Sun Yat-Sen University [no. (2014)52] and was
performed in accordance with the guidelines for the use of
laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ponnusamy KE, Iyer S, Gupta G and Khanna
AJ: Instrumentation of the osteoporotic spine: Biomechanical and
clinical consider-ations. Spine J. 11:54–63. 2011. View Article : Google Scholar
|
|
2
|
Dvorak G, Arnhart S, Heuberer C, Huber G,
Watzek R and Gruber R: Peri-implantitis and late implant failures
in postmenopausal women: A cross-sectional study. J Clin
Periodontol. 38:950–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cameron P, Travers C, Chander T, Buckland
C, Campion B and Noble B: Directed osteogenic differentiation of
human mesen-chymal stem/precursor cells on silicate substituted
calcium phosphate. J Biomed Mater Res A. 101:13–22. 2013.
View Article : Google Scholar
|
|
4
|
Mavrogenis AF, Dimitriou R, Parvizi J and
Babis GC: Biology of implant osseointegration. J Musculoskelet
Neuronal Interact. 9:61–71. 2009.PubMed/NCBI
|
|
5
|
Davies JE: Understanding peri-implant
endosseous healing. J Dent Educ. 67:932–949. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olivares-Navarrete R, Hyzy SL, Hutton DL,
Erdman CP, Wieland M, Boyan BD and Schwartz Z: Direct and indirect
effects of microstructured titanium substrates on the induction of
mesenchymal stem cell differentiation towards the osteoblast
lineage. Biomaterials. 31:2728–2735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puleo DA and Nanci A: Understanding and
controlling the bone-implant interface. Biomaterials. 20:2311–2321.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Özdal-Kurt F, Tuğlu I, Vatansever HS, Tong
S, Şen BH and Deliloğlu-Gürhan SI: The effect of different implant
biomaterials on the behavior of canine bone marrow stromal cells
during their differentiation into osteoblasts. Biotech Histochem.
91:412–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaback LA, Soung DY, Naik A, Geneau G,
Schwarz EM, Rosier RN, O'Keefe RJ and Drissi H: Teriparatide (1-34
human PTH) regulation of osterix during fracture repair. J Cell
Biochem. 105:219–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang L, Zhang W, Wei L, Zhou Q, Yang G,
Qian N, Tang Y, Gao Y and Jiang X: Early effects of parathyroid
hormone on vascularized bone regeneration and implant
osseointegration in aged rats. Biomaterials. 179:15–28. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohkawa Y, Tokunaga K and Endo N:
Intermittent administration of human parathyroid hormone (1-34)
increases new bone formation on the interface of
hydroxyapatitecoated titanium rods implanted into ovariectomized
rat femora. J Orthop Sci. 13:533–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du L, Feng R and Ge S: PTH/SDF-1α
cotherapy promotes proliferation, migration and osteogenic
differentiation of human periodontal ligament stem cells. Cell
Prolif. 49:599–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sengupta S, Peterson TR and Sabatini DM:
Regulation of the mTOR complex 1 pathway by nutrients, growth
factors, and stress. Mol Cell. 40:310–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View Article : Google Scholar
|
|
15
|
Diz-Muñoz A, Thurley K, Chintamen S,
Altschuler SJ, Wu LF, Fletcher DA and Weiner OD: Membrane tension
acts through PLD2 and mTORC2 to limit actin network assembly during
neutrophil migration. PLoS Biol. 14:e10024742016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Shao X, He Q, Wang C, Xia L, Yue
D, Qin G, Jia C and Chen R: Quantitative proteomics implicates
rictor/mTORC2 in cell adhesion. J Proteome Res. 17:3360–3369. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang L, Zhang Y, Xu C, Gu X, Niu L, Wang
J, Sun X, Bai X, Xuan X, Li Q, et al: Rictor positively regulates B
cell receptor signaling by modulating actin reorganization via
ezrin. PLoS Biol. 15:e20017502017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarbassov DD, Ali SM, Kim DH, Guertin DA,
Latek RA, Erdjument-Bromage H, Tempst P and Sabatini DM: Rictor, a
novel binding partner of mTOR, defines a rapamycin-insensitive and
raptor-independent pathway that regulates the cytoskeleton. Curr
Biol. 14:1296–1302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sen B, Xie Z, Case N, Thompson WR, Uze G,
Styner M and Rubin J: mTORC2 regulates mechanically induced
cytoskeletal reorganization and lineage selection in marrow-derived
mesenchymal stem cells. J Bone Miner Res. 29:78–89. 2014.
View Article : Google Scholar
|
|
20
|
Gulhati P, Bowen KA, Liu J, Stevens PD,
Rychahou PG, Chen M, Lee EY, Weiss HL, O'Connor KL, Gao T and Evers
BM: mTORC1 and mTORC2 regulate EMT, motility, and metastasis of
colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res.
71:3246–3256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Lai P, Zhang Z, Huang M, Wang L,
Yin M, Jin D, Zhou R and Bai X: Targeted inhibition of mTORC2
prevents osteosarcoma cell migration and promotes apoptosis. Oncol
Rep. 32:382–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hau AM, Leivo MZ, Gilder AS, Hu JJ, Gonias
SL and Hansel DE: mTORC2 activation is regulated by the urokinase
receptor (uPAR) in bladder cancer. Cell Signal. 29:96–106. 2017.
View Article : Google Scholar
|
|
23
|
Maniatopoulos C, Sodek J and Melcher AH:
Bone formation in vitro by stromal cells obtained from bone marrow
of young adult rats. Cell Tissue Res. 254:317–330. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smajilagić A, Aljičević M, Redžić A,
Filipović S and Lagumdžija A: Rat bone marrow stem cells isolation
and culture as a bone formative experimental system. Bosn J Basic
Med Sci. 13:27–30. 2013. View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expres-sion data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Alghamdi HS, Bosco R, Both SK, Iafisco M,
Leeuwenburgh SC, Jansen JA and van den Beucken JJ: Synergistic
effects of bisphos-phonate and calcium phosphate nanoparticles on
peri-implant bone responses in osteoporotic rats. Biomaterials.
35:5482–5490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu D, Su Y, Young ML, Ma J, Zheng Y and
Tang L: Biological responses and mechanisms of human bone marrow
mesenchymal stem cells to Zn and Mg biomaterials. ACS Appl Mater
Interfaces. 9:27453–27461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Petrie TA, Raynor JE, Dumbauld DW, Lee TT,
Jagtap S, Templeman KL, Collard DM and Garcia AJ: Multivalent
integrin-specific ligands enhance tissue healing and biomaterial
integration. Sci Transl Med. 2:45r–60r. 2010. View Article : Google Scholar
|
|
29
|
Misof BM, Roschger P, Dempster DW, Zhou H,
Bilezikian JP, Klaushofer K and Rubin MR: PTH(1-84) administration
in hypo-parathyroidism transiently reduces bone matrix
mineralization. J Bone Miner Res. 31:180–189. 2016. View Article : Google Scholar
|
|
30
|
Morgan EF, Mason ZD, Bishop G, Davis AD,
Wigner NA, Gerstenfeld LC and Einhorn TA: Combined effects of
recombi-nant human BMP-7 (rhBMP-7) and parathyroid hormone (1-34)
in metaphyseal bone healing. Bone. 43:1031–1038. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sheyn D, Shapiro G, Tawackoli W, Jun DS,
Koh Y, Kang KB, Su S, Da X, Ben-David S, Bez M, et al: PTH induces
systemically administered mesenchymal stem cells to migrate to and
regenerate spine injuries. Mol Ther. 24:318–330. 2016. View Article : Google Scholar :
|
|
32
|
Kitaori T, Ito H, Schwarz ME, Tsutsumi R,
Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T and Nakamura T:
Stromal cell-derived factor 1/CXCR4 signaling is critical for the
recruitment of mesenchymal stem cells to the fracture site during
skeletal repair in a mouse model. Arthritis Rheum. 60:813–823.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang LL, Chen D, Lee J, Gu X, Alaaeddine
G, Li J, Wei L and Yu SP: Mobilization of endogenous bone marrow
derived endothelial progenitor cells and therapeutic potential of
parathyroid hormone after ischemic stroke in mice. PLoS One.
9:e872842014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ryan CM, Brown JA, Bourke E, Prendergast
M, Kavanagh C, Liu Z, Owens P, Shaw G, Kolch W, O'Brien T and Barry
FP: ROCK activity and the Gβg complex mediate chemotactic migration
of mouse bone marrow-derived stromal cells. Stem Cell Res Ther.
6:1362015. View Article : Google Scholar
|
|
35
|
Yang L, Froio RM, Sciuto TE, Dvorak AM,
Alon R and Luscinskas W: ICAM-1 regulates neutrophil adhesion and
trans-cellular migration of TNF-alpha-activated vascular
endothelium under flow. Blood. 106:584–592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pendegrass CJ, El-Husseiny M and Blunn GW:
The development of fibronectin-functionalised hydroxyapatite
coatings to improve dermal fibroblast attachment in vitro. J Bone
Joint Surg Br. 94:564–569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Davies T and Chambers TJ: Parathyroid
hormone activates adhesion in bone marrow stromal precursor cells.
J Endocrinol. 180:505–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee DJ, Southgate RD, Farhat YM, Loiselle
AE, Hammert WC, Awad HA and O'Keefe RJ: Parathyroid hormone 1-34
enhances extracellular matrix deposition and organization during
flexor tendon repair. J Orthop Res. 33:17–24. 2015. View Article : Google Scholar
|
|
39
|
Okada Y, Morimoto I, Ura K, Watanabe K,
Eto S, Kumegawa M, Raisz L, Pilbeam C and Tanaka Y: Cell-to-cell
adhesion via inter-cellular adhesion molecule-1 and leukocyte
function-associated antigen-1 pathway is involved in
1alpha,25(OH)2D3, PTH and IL-1alpha-induced osteoclast
differentiation and bone resorption. Endocr J. 49:483–495. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yin Y, Hua H, Li M, Liu S, Kong Q, Shao T,
Wang J, Luo Y, Wang Q, Luo T and Jiang Y: mTORC2 promotes type I
insulin-like growth factor receptor and insulin receptor activation
through the tyrosine kinase activity of mTOR. Cell Res. 26:46–65.
2016. View Article : Google Scholar :
|
|
41
|
Chantaravisoot N, Wongkongkathep P, Loo
JA, Mischel PS and Tamanoi F: Significance of filamin A in mTORC2
function in glioblastoma. Mol Cancer. 14:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rosner M and Hengstschläger M: Cytoplasmic
and nuclear distribution of the protein complexes mTORC1 and
mTORC2: Rapamycin triggers dephosphorylation and delocalization of
the mTORC2 components rictor and sin1. Hum Mol Genet. 17:2934–2948.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akcakanat A, Singh G, Hung MC and
Meric-Bernstam F: Rapamycin regulates the phosphorylation of
rictor. Biochem Biophys Res Commun. 362:330–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang C, Qin L, Manes TD, Kirkiles-Smith
NC, Tellides G and Pober JS: Rapamycin antagonizes TNF induction of
VCAM-1 on endothelial cells by inhibiting mTORC2. J Exp Med.
211:395–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lamming DW, Ye L, Katajisto P, Goncalves
MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima
R, et al: Rapamycin-induced insulin resistance is mediated by
mTORC2 loss and uncoupled from longevity. Science. 335:1638–1643.
2012. View Article : Google Scholar : PubMed/NCBI
|