Introduction
Lung cancer remains the leading cause of
cancer-associated mortality worldwide and non-small cell lung
cancer (NSCLC) accounts for ~85% of lung cancer cases (1). Significant improvements have been
made in the treatment of lung cancer; however, its 5-year survival
rate remains low, primarily due to treatment resistance, which may
be present before or develop during the course of treatment
(2).
Recently, circadian rhythm disruption by shiftwork
has been reported in tumorigenesis. Previous epidemiological
studies have revealed that individuals working night shifts are at
higher risk of developing cancer or exhibit poorer cancer prognosis
(3-5). Systemic disruption of the circadian
machinery may result in changes in cellular functions that are
highly associated with cancer (6-8).
An important role of the core circadian genes has been reported in
carcinogenesis (9,10). Under normal conditions, the key
circadian genes, such as Bmal1, CLOCK, period, cryptochrome and
casein kinase Iε, function in tightly regulated feedback loops
(11,12). For example, the circadian gene
CLOCK may contribute to glioma progression, which is directly
modulated by microRNA (miR)-124 (13). Chronic shift-lag may alter CLOCK
expression in natural killer cells, which notably induces lung
cancer growth in vivo (14). However, the detailed role of CLOCK
in lung cancer remains to be further investigated.
The cancer stem cell (CSC) hypothesis suggests that
a small population of cancer cells with self-renewing ability are
responsible for tumor relapse (14,15). The existence of CSCs has been
verified in various types of tumors (16,17). A recent report revealed that the
circadian dynamics of CSCs are modulated by the tumor
microenvironment and provide a principle for the treatment of
breast cancer (18). Disruption
of CLOCK may also affect glioblastoma stem cells (19).
The aim of the present study was to determine
whether CLOCK can regulate lung CSCs. CLOCK was induced and knocked
down in lung CSCs to determine its effects on CSC-like properties
and whether these were mediated by the Wnt signaling pathway.
Furthermore, it was investigated whether epigallocatechin-3-gallate
(EGCG) can inhibit the stemness of lung cancer cells by regulating
CLOCK expression, in order to determine whether CLOCK is a
potential target for suppressing CSC-like characteristics in lung
cancer cells.
Materials and methods
Cell culture and reagents
The A549 and H1299 cell lines were purchased from
the Chinese Academy of Sciences Committee on Type Culture
Collection Cell Bank (Shanghai, China) and cultured in RPMI-1640
medium supplemented with 10% heat-inactivated fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 mg/ml streptomycin. Cells were cultured at 37°C in a humidified
incubator with 5% CO2. EGCG (purity ≥95%) powder was
purchased from Sigma-Aldrich; Merck KGaA.
Western blot analysis
Total protein was extracted from the cell samples
following lysis using RIPA buffer (Beyotime Institute of
Biotechnology), supplemented with protease and phosphatase
inhibitors, and was quantified using a BCA protein assay. The
proteins were then separated on a 10% SDS-PAGE (Invitrogen; Thermo
Fisher Scientific, Inc.), transferred onto nitrocellulose filter
membranes (Cytiva Bioscience) and incubated with primary antibodies
overnight at 4°C. After 24 h, the membranes were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibodies for 1
h at room temperature. The following primary antibodies were used:
Anti-CLOCK (1:1,000; cat no. ab3517, Abcam), anti-CD133 (1:1,000;
cat no. ab216323, Abcam), anti-CD44 (1:1,000; cat no. ab189524,
Abcam), anti-sex determining region Y-box (Sox)2 (1:1,000; cat no.
ab92494, Abcam), anti-Nanog (1:1,000; cat no. ab109250, Abcam),
anti-octamer-binding transcription factor (Oct)4 (1:1,000; cat no.
ab181557, Abcam), anti-glycogen synthase kinase (GSK)3β (1:1,000;
cat no. ab32391, Abcam), anti-phosphorylated (p)-GSK3β (1:1,000;
ab131097, Abcam), anti-β-catenin (1:1,000; cat no. ab32572, Abcam),
anti-p-β-catenin (1:1,000; cat no. ab27798, Abcam), anti-β-actin
(1:1,000; cat no. ab179467, Abcam) and anti-GAPDH (1:1,000; cat no.
A00227-1, Boster Biological Technology, Ltd.). The following
secondary antibodies were used: HRP-conjugated AffiniPure goat
anti-rabbit IgG (1:2,000; cat no. TA130015, OriGene Technologies,
Inc.) and HRP-conjugated AffiniPure goat anti-mouse IgG (1:2,000;
cat no. TA130001, OriGene Technologies, Inc.). The immunoreactive
proteins were then detected using an enhanced chemiluminescence kit
(Cell Signaling Technology, Inc.).
Reverse transcription-quantitative PCR
analysis
Total RNA was extracted using a RNAiso Plus kit
(Takara Biotechnology Co., Ltd.) and the Prime Script™ RT Master
mix (Takara Biotechnology Co., Ltd.) was utilized to
reverse-transcribe RNA into cDNA according to the manufacturer's
instructions, while qPCR was performed using the SYBR Premix Ex Taq
II kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions. Reactions were carried out using the
following thermocycling conditions: Pre-denaturation at 95°C for 1
min; 40 cycles at 95°C for 5 sec, 60°C for 15 sec, and a final step
at 72°C for 15 sec. The RT-qPCR primers are provided in Table I. GAPDH was used as an internal
mRNA control. qPCR was performed using the Applied Biosystems 7,300
Real Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The fold change was calculated using the 2−∆∆Cq
method (20).
 | Table IPrimers used for quantitative PCR
analysis. |
Table I
Primers used for quantitative PCR
analysis.
| Genes | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| GAPDH |
CAAGGTCATCCATGACAACTTTG |
GTCCACCACCCTGTTGCTGTAG |
| CLOCK |
ATGGATTGGTGGAAGAAG |
ACCATCAAGAGCCTCTAAC |
Sphere formation assay
Cells were treated with 10 ng/ml of human
recombinant basic fibroblast growth factor (FGF; R&D Systems,
Inc.) and 20 ng/ml of epidermal growth factor (EGF; R&D
Systems, Inc.) in serum-free DMEM-F12 (Gibco; Thermo Fisher
Scientific, Inc.). The medium was changed every 48 h and the cells
were cultured for 7 days. Tumor spheres were observed using a MOTIC
inverted microscope (Olympus Corporation) at a magnification of
×100.
Flow cytometry analysis
For CD133+ cell analyses, the cells were
first washed, resuspended in RPMI-1640 medium with 10% FBS, and
then incubated at 4°C in the freezer for 30 min with
fluorescence-conjugated monoclonal antibodies against human CD133
PE (1:100; cat no. 566593, BD Biosciences) and its isotype IgG.
Short inhibiting (si)RNA and plasmid
transfection
siRNA targeting CLOCK at a concentration of 100 nmol
or a corresponding negative control (Guangzhou RiboBio, Co., Ltd.)
were transfected into NSCLC/CSCs using Lipofectamine™ 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions at room temperature. After transfection
for 48 h, cells were collected for the subsequent experiments.
Xenograft studies
A total of 12 female BALB/c nude mice, aged 5-6
weeks and weighing 18-20 g, were purchased from the Shanghai Animal
Laboratory Center and maintained at the Experimental Animal Center
at Nanjing Medical University, with a temperature and humidity of
22±1°C and 55±5%, respectively. The mice were daily observed for
abnormal behavior, including inability to eat or drink, or lack of
response upon stimulation or touch. All aspects of animal welfare
were considered, and measures were taken to minimize the suffering
and distress of the animals. Each mouse was subcutaneously injected
with exponentially growing A549 sphere cells (5×106) on
the back and the animals were randomly divided into EGCG and
control groups. After 2 weeks, 20 mg/kg EGCG was administered to
the mice by intraperitoneal injection weekly. The length and width
of the tumors were measured using a caliper, and the volumes were
calculated using the following formula: Volume
(mm3)=(length × width2)/2. The maximum
diameter of the tumor was 12 mm and the minimum 5 mm. The maximum
weight loss observed in mice from start to endpoint was 1.5 g and
the maximum percentage of weight loss was <10%. After receiving
treatment with EGCG for 4 weeks, the mice were euthanized using
cervical dislocation. Death was confirmed by observing the eyes
turn pale and by monitoring lack of heartbeat and breathing and
lack of response to external stimuli after cervical dislocation.
The study protocol was based on the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health.
Immunohistochemistry
Immunohistochemical staining for Ki67 was performed
on xenograft tumor tissues using antibodies against Ki67 (1:100;
cat. no. ab15580, Abcam) at the Department of Pathology of The
First Affiliated Hospital of Nanjing Medical University. Image-Pro
Plus software (version 6.0; Media Cybernetics, Inc.) was used to
analyze the staining results. The ratios of positively stained
tumor cells were classified into four groups with scores from 0 to
3 (<10, 0; >10, 1; >25, 2; and >50%, 3). The staining
intensities were scored as follows: No staining, 0; low, 1; medium,
2; and high, 3). A final IHC score was calculated by adding the two
scores; a score >3 was considered to indicate positive
expression, and a score ≤3 negative expression.
Histopathology
Histopathological examination of xenograft tissues
was performed using hematoxylin-eosin (HE) staining. The samples
were placed in 10% formaldehyde solution overnight, dehydrated
through a graded ethanol series every 5 min, embedded in paraffin
and then cut into 4-µm sections. Subsequently, the sections
were stained using HE (Beijing Solarbio Science & Technology
Co., Ltd.) at room temperature according to the manufacturer's
instructions and observed under a light microscope (Leica
Microsystems GmbH) at a magnification of ×100.
Statistical analysis
All data were recorded as the mean ± standard
deviation of at least three independent experiments. Comparisons
between quantitative variables were performed using a Student's
t-test and one-way ANOVA followed by Dunnett's post hoc test. For
comparisons among all groups, one-way ANOVA followed by Tukey's
post hoc test was used. P<0.05 was considered to indicate a
statistically significant difference. SPSS v17.0 (SPSS Inc.) and
GraphPad Prism v5.0 (GraphPad Software, Inc.) were used for
statistical analysis.
Results
Successfully enrichment of CSC-like cells
from parental lung cancer cells
Serum-free medium was used to form spheroid
populations to enrich CSCs (21).
In the present study, A549 and H1299 cells were cultured using a
serum-free suspension medium to induce CSCs for 7 days, and the
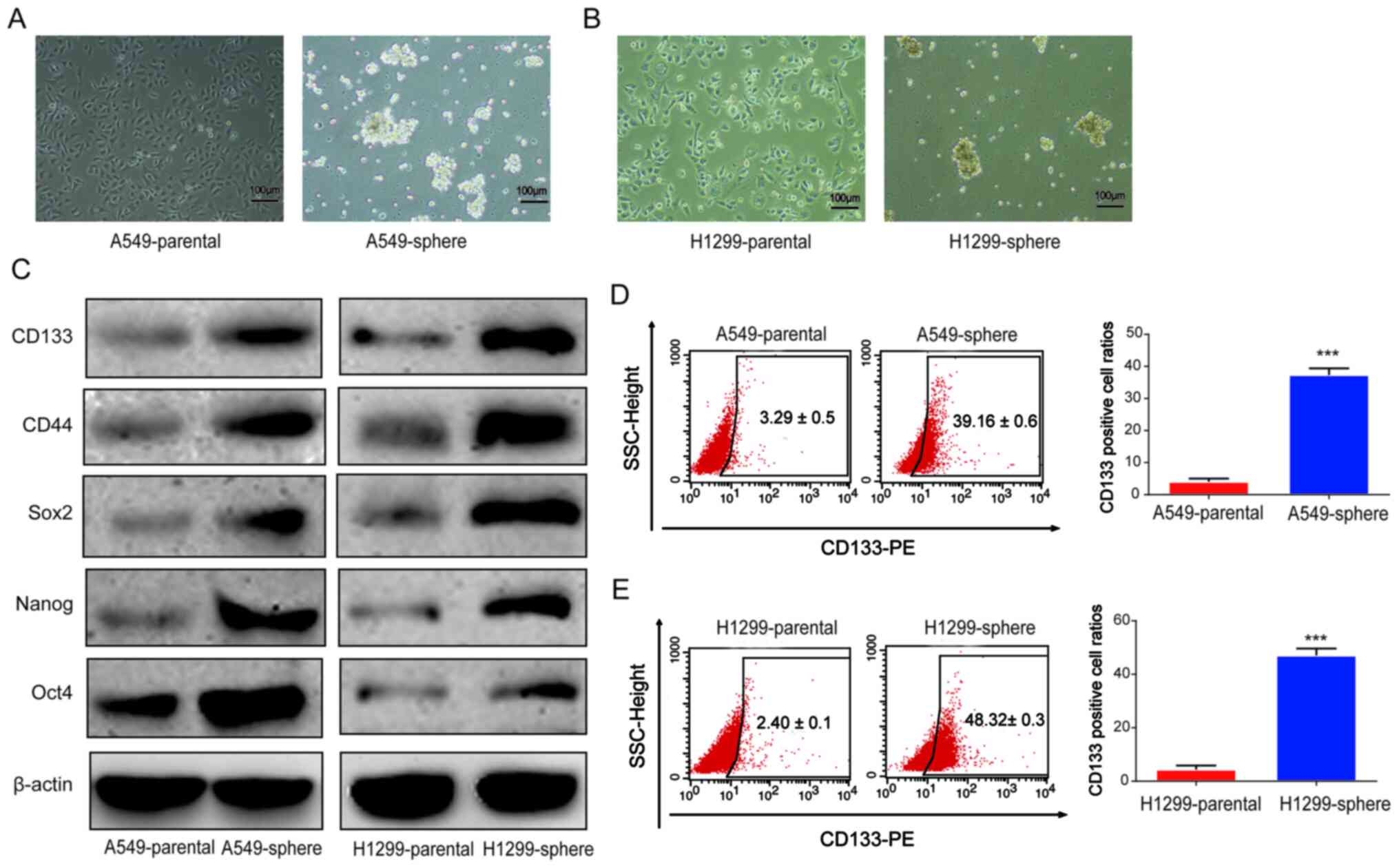
formation of tumor spheres was observed (Fig. 1A and B). Subsequently, to verify
the stemness of sphere-forming cells, the protein expression levels
of CD133, CD44, Sox2, Nanog and Oct4 were determined using western
blot analysis. A shown in Fig.
1C, the protein expression levels of these CSC markers were
notably upregulated in the A549 and H1299 sphere cells. Next, as
shown in Fig. 1D and E, the
percentage of CD133+ cells was found to be markedly
increased among the A549 and H1299 sphere cells compared with that
in the corresponding parental A549 and H1299 cells, as indicated by
flow cytometry analysis. These data suggested that CSC-like cells
were successfully obtained from parental lung cancer cells.
Circadian rhythm-related CLOCK gene is
upregulated in lung CSCs
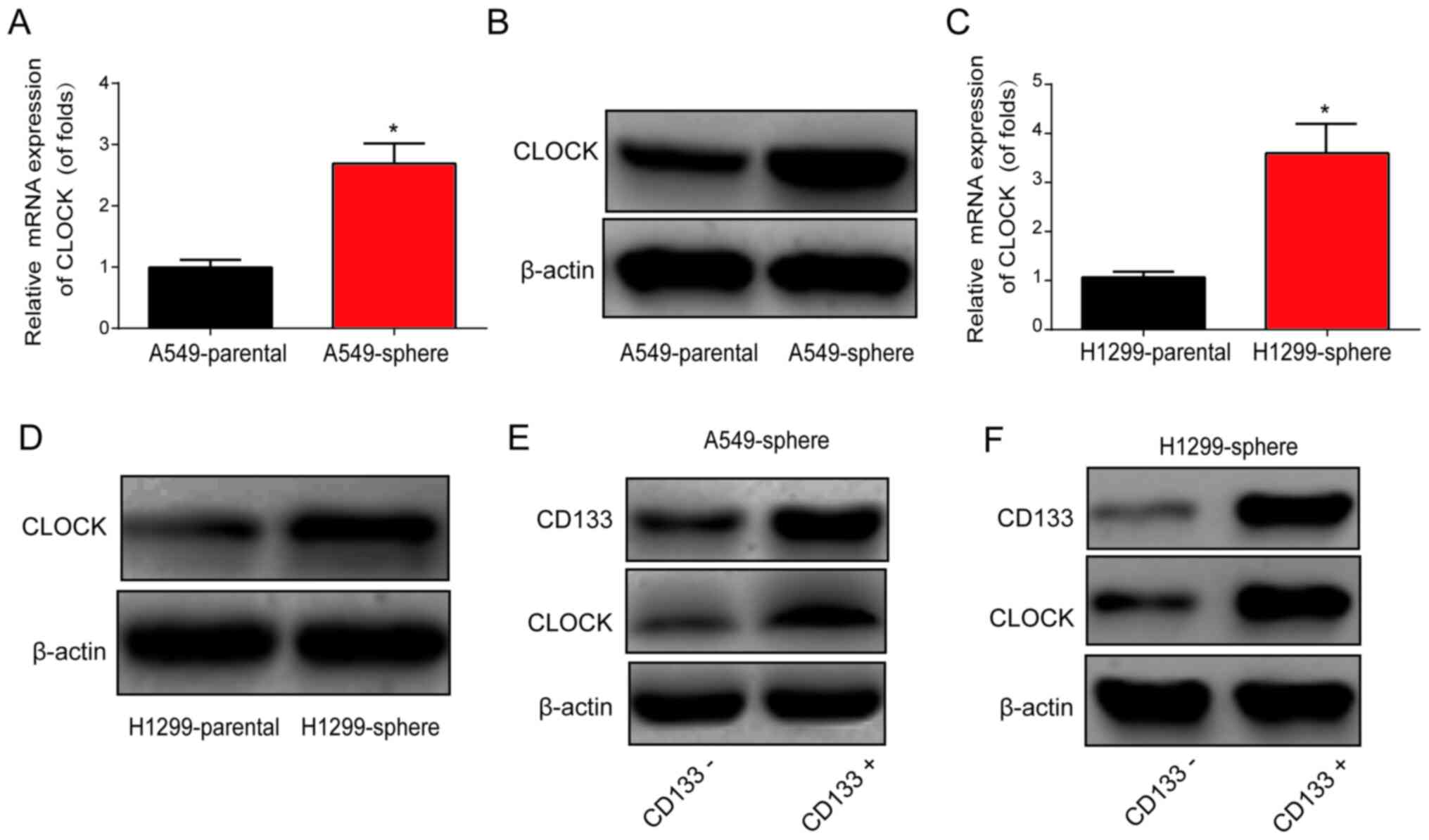
Subsequently, qPCR and western blot assays were
performed, and CLOCK mRNA and protein expression levels were found
to be markedly upregulated in A549 and H1299 sphere cells, as shown
in Fig. 2A-D. Furthermore, cell
sorting was performed using flow cytometry to isolate
CD133− and CD133+ cells from the A549 and
H1299 sphere cells. As shown in Fig.
2E and F, CD133+ cells exhibited an upregulation of
CLOCK protein expression in the A549 and H1299 sphere cells,
suggesting a role for CLOCK in lung CSCs.
Knockdown of CLOCK represses the stemness
of lung CSCs
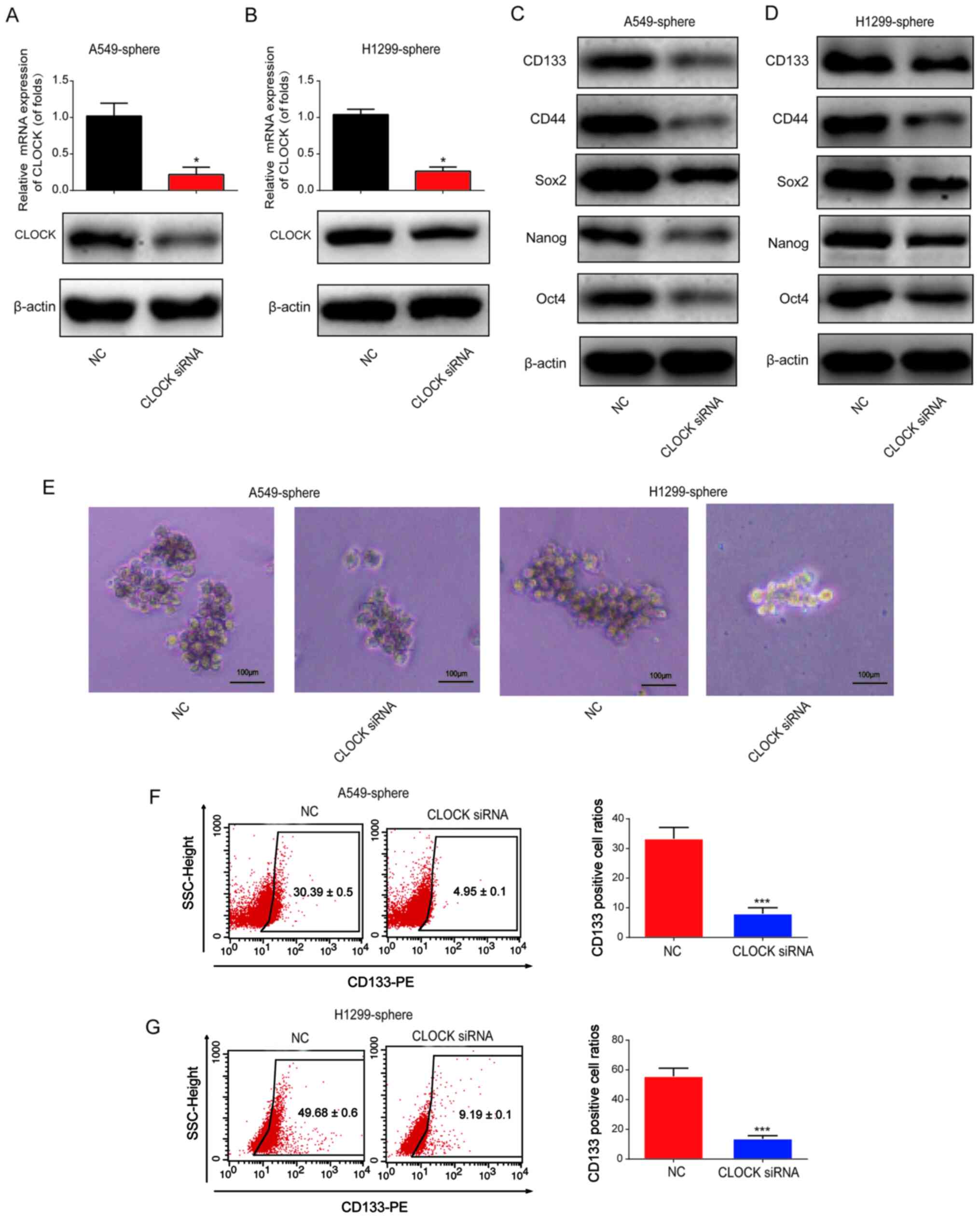
To determine the effect of CLOCK on the stemness of
lung CSCs, CLOCK siRNA or siRNA control was transfected into the
A549 and H1299 sphere cells for 24 h, after which time the mRNA and
protein expression level of CLOCK was found to be significantly
downregulated in the A549 and H1299 sphere cells (Fig. 3A and B). It was then observed that
the protein expression levels of CD133, CD44, Sox2, Nanog and Oct4
were notably reduced by CLOCK siRNA (Fig. 3C and D). As shown in Fig. 3E, the volume of the tumor spheres
was reduced following CLOCK knockdown, which indicated that CLOCK
siRNA reduced the number of lung CSCs. Subsequently, flow cytometry
was used to determine the ratio of CD133+ cells. As
shown in Fig. 3F and G, the
percentage of CD133+ cells was markedly decreased
following CLOCK knockdown in the A549 and H1299 sphere cells. These
data indicated that knockdown of CLOCK was able to inhibit the
stemness of lung CSCs.
EGCG represses the CSC-like properties of
lung CSCs by targeting CLOCK
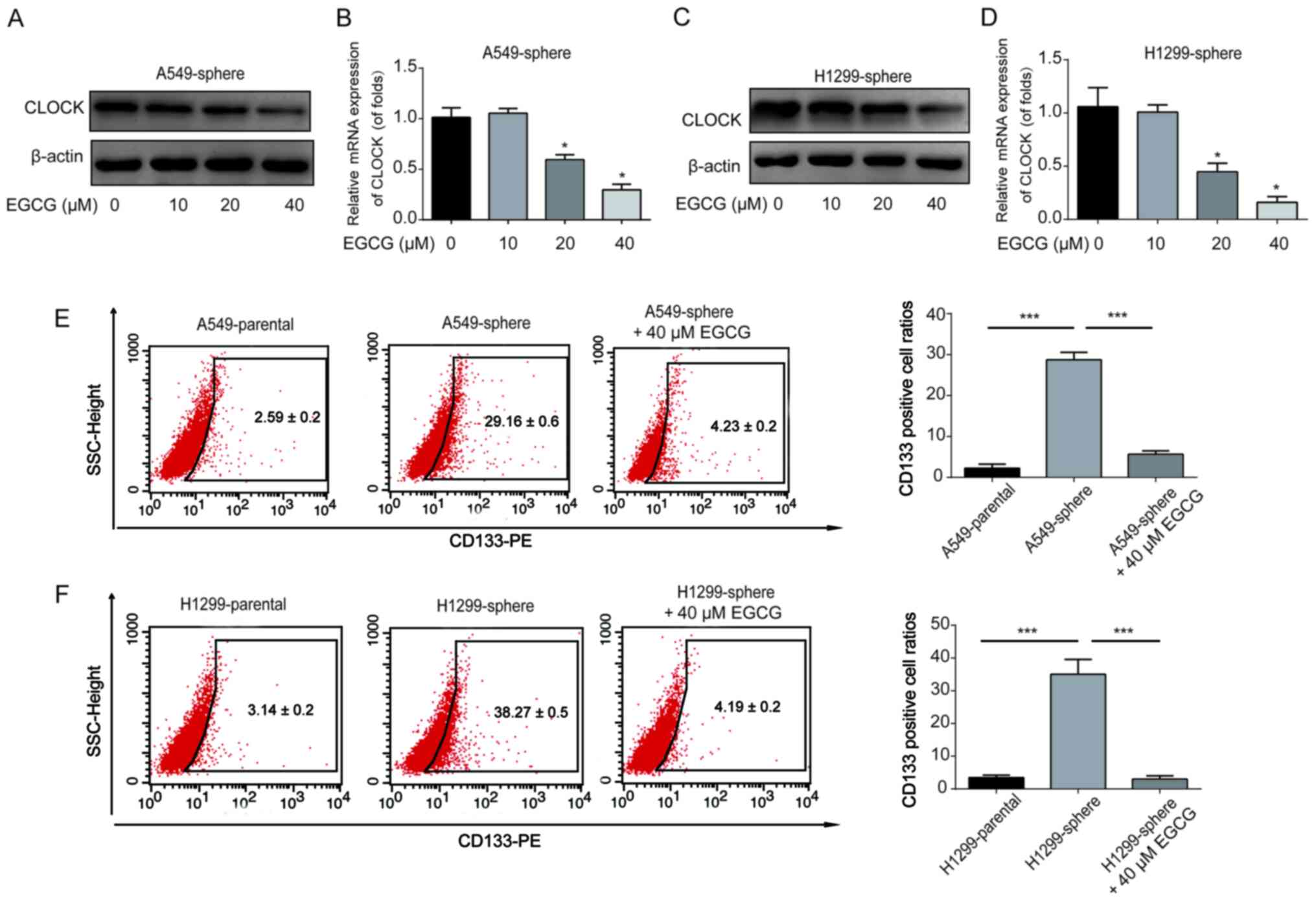
To investigate whether EGCG could regulate CLOCK
expression in lung CSCs, the A549 and H1299 sphere cells were
treated with the indicated doses of EGCG for 48 h. CLOCK protein
and mRNA expression in the A549 sphere cells was notably
downregulated by EGCG in a dose-dependent manner (Fig. 4A and B). Consistently, it was
observed that 40 µM EGCG could significantly reduce CLOCK
expression in H1299 sphere cells (Fig. 4C and D). In addition, the ratio of
CD133+ cells among A549 and H1299 sphere cells was
reduced by 40 µM EGCG (Fig. 4E
and F). These results demonstrated that EGCG was able to reduce
NSCLC/CSC stemness by reducing the expression of CLOCK.
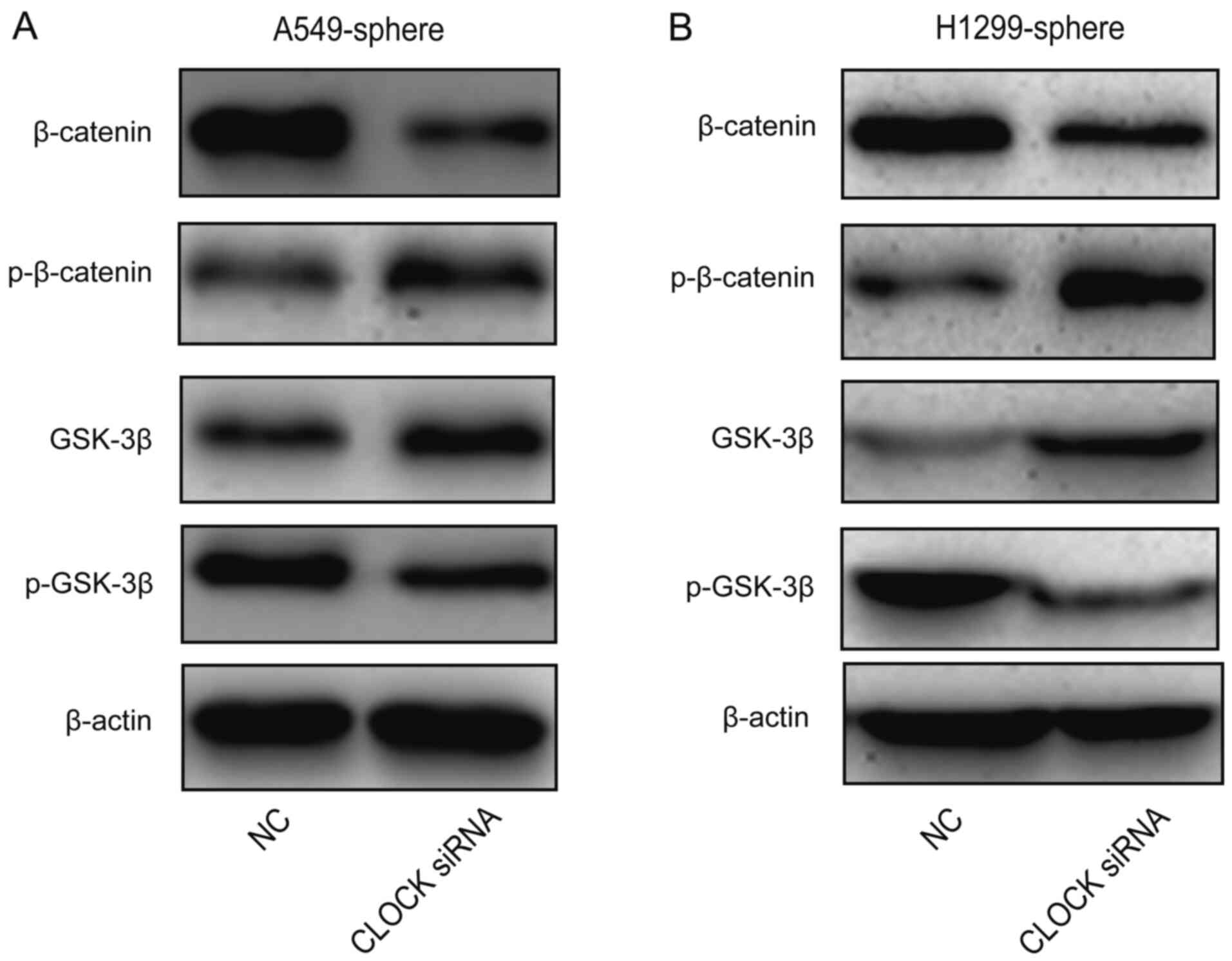
Wnt/β-catenin signaling is blocked by the
knockdown of CLOCK in lung CSCs
Next, the effect of CLOCK on Wnt/β-catenin activity
was investigated. As shown in Fig. 5A
and B, β-catenin and p-GSK-3β protein expression levels were
decreased, while p-β-catenin and GSK-3β levels were increased by
CLOCK siRNA in the A549 and H1299 sphere cells. These data
suggested that the Wnt signaling pathway was inhibited by the
knockdown of CLOCK in lung CSCs.
EGCG represses CSC-like characteristics
of lung cancer cells by targeting CLOCK in vivo
An A549/CSCs nude mouse xenograft model was
established to investigate whether EGCG reduced CSC-like phenotype
by targeting CLOCK in vivo. The mice were divided into
control and EGCG groups. The subcutaneous tumors were excised from
the nude mice and are presented in Fig. 6A. EGCG effectively reduced tumor
volume in a time-dependent manner, as shown in Fig. 6B. Body weight was not altered
after EGCG treatment, while tumor weight was obviously reduced by
EGCG (Fig. 6C and D). The HE and
immunohistochemistry staining results are shown in Fig. 6E and F. Subsequently, western blot
analysis revealed that EGCG decreased CD133, CD44, Sox2, Nanog, and
Oct4 protein expression levels by targeting CLOCK (Fig. 6G). These results suggested that
EGCG may target CSC-like properties of lung cancer cells by
modulating CLOCK in vivo.
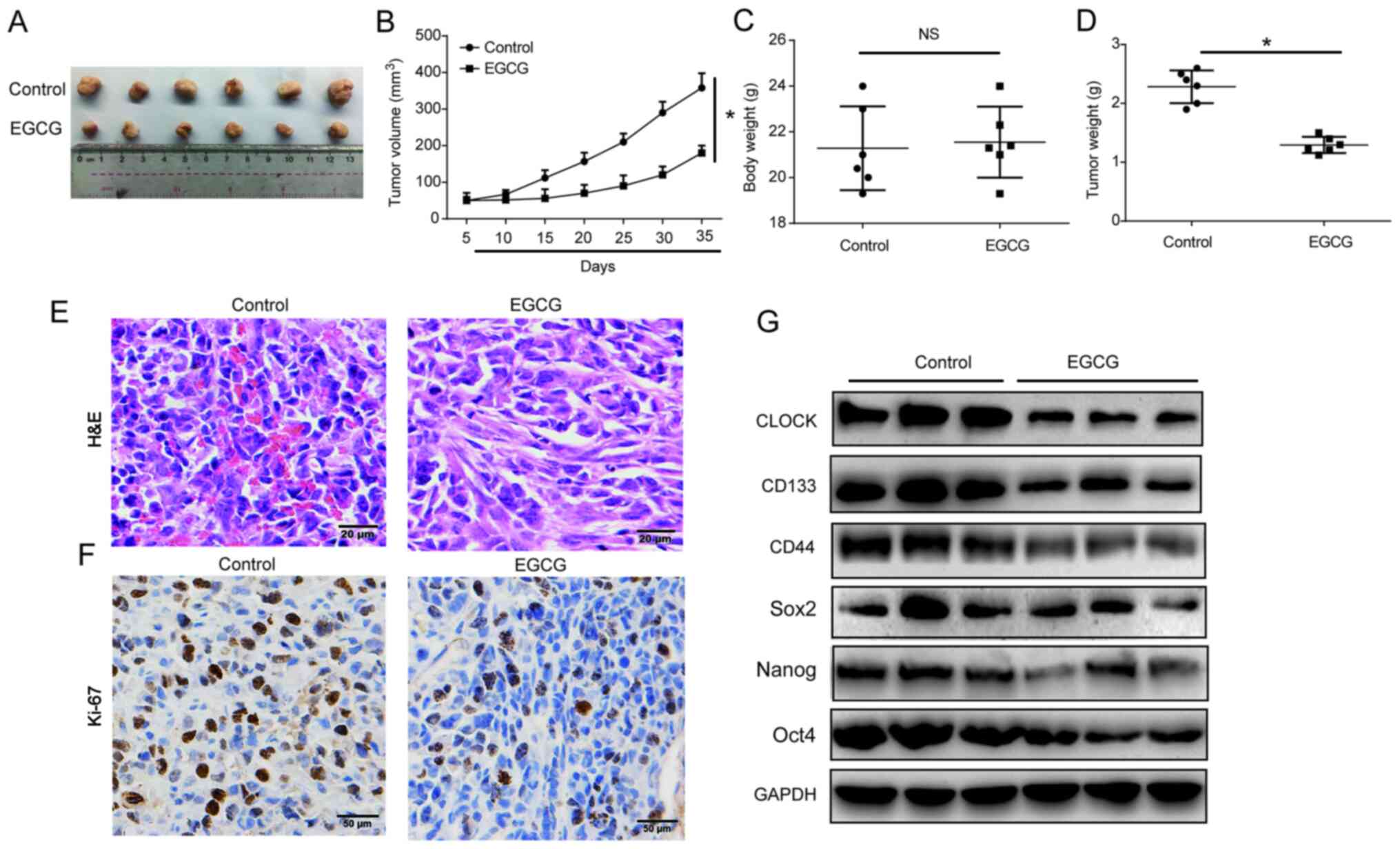 | Figure 6EGCG inhibited CSC-like
characteristics of lung cancer via targeting CLOCK in vivo.
A total of 12 5-week-old female BALB/c nude mice were injected with
5×106 A549 sphere cells. Two treatment groups were
established as control and EGCG, with 6 mice in each. (A) Solid
tumors were excised from the subcutaneous tissue. (B) Tumor volume.
(C) Body weight. (D) Tumor weight. (E) Hematoxylin and eosin
staining (bar, 20 µm) and (F) immunohis-tochemistry staining
(bar, 50 µm) of Ki-67 in the tumor tissues. (G) CLOCK,
CD133, CD44, Sox2, Nanog and Oct4 protein expression levels in the
tumor tissues. GAPDH was used as the loading control. Three
independent experiments were performed. Data are presented as the
mean ± SD from at least triplicate experiments.
*P<0.05. CSC, cancer stem cell; EGCG,
epigallocatechin-3-gallate; Sox2, sex determining region Y-box 2;
Oct4, octamer-binding transcription factor 4; NS, not
significant. |
Discussion
The role of CLOCK in lung CSCs was extensively
investigated in the present study. CLOCK was found to be markedly
increased in the A549 and H1299 sphere cells, while knockdown of
CLOCK markedly reduced the CSC-like properties of lung CSCs. In
addition, EGCG reduced stemness by targeting CLOCK in a
dose-dependent manner. Furthermore, it was observed that the Wnt
signaling pathway was inactivated by the knockdown of CLOCK, and
the in vitro data were confirmed using in vivo
assays. These findings revealed a novel mechanism involving
EGCG-mediated repression of CSC-like properties by CLOCK
regulation.
CSCs represent a small cell population that can
differentiate into cancerous cells (22). CSCs have been widely investigated,
due to their crucial role in cancer progression. Strategies
targeting CSCs are becoming increasingly significant for new
approaches to cancer therapy (23). Assessing the role of CSCs in tumor
recurrence relies heavily on the use of specific CSC markers,
including CD133, CD44, Sox2, Nanog and Oct4 (24). In the present study, lung CSCs
were enriched from the A549 and H1299 sphere cells. In addition,
the sphere cells exhibited higher levels of CD133, CD44, Sox2,
Nanog and Oct4, as well as an increased ratio of CD133+
cells, following culture for 7 days in a serum-free suspension
medium. It was previously confirmed that the proportion of
CD133+ cells reached 80% in a 6-week culture (25). Freshly dissociated lung cancer
cells were cultured at low density in serum-free medium with EGF
and FGF to determine whether lung cancer CD133+ cells
can expand long-term cultures in vitro. It was herein
demonstrated the proportion of CD133+ cells gradually
increased (3.29 vs. 39.16% in A549 cells, 2.4 vs. 48.32% in H1299
cells) over 7 days and a higher percentage of CD133+
cells may be enriched during if cultured for >7 days. Fresh lung
cancer tissues will be used to obtain lung CSCs and longer culture
time will be considered in future studies.
Circadian CLOCK is a conserved timekeeper, which can
adapt the body's physiology to diurnal cycles. Perturbation of
circadian CLOCK participates in the development of various
diseases, including cancer (26).
PER3 is a circadian CLOCK gene and its overexpression inhibited
colorectal cancer stem-like cells by inactivating the Notch and
β-catenin signaling pathways (27,28). Circadian CLOCK in colon tumor
tissues may promote tumor progression by regulating intracellular
iron levels (28), while the
circadian gene CLOCK may affect ovarian cancer drug-resistant genes
and cell proliferation through autophagy (29). The present study demonstrated that
CLOCK was increased in lung CSCs and knockdown of CLOCK notably
reduced the stemness of lung cancer cells. The detailed mechanism
of action of CLOCK in the proliferation of lung CSCs, involving
cell apoptosis or cell cycle arrest, will be investigated in our
future study.
EGCG has been widely investigated as a
chemopreventive agent with potential anticancer effects (30,31). Our previously study reported that
EGCG inhibited lung CSC-like properties by targeting miR-485 and
RXRα (32). In addition, EGCG was
found to suppress CSC-like characteristics by regulating the
miR-485 and CD44 axis in A549-cisplatin resistant cells (33). The aim of the present study was to
confirm the inhibitory effect of EGCG on lung CSCs and further
investigate the underlying mechanism. CLOCK was found to be notably
inhibited by EGCG in a dose-dependent manner. In addition, EGCG was
able to reduce CSC-like properties in A549 and H1299 sphere cells
via repressing CLOCK expression.
The canonical Wnt/β-catenin pathway is crucial for
maintaining CSCs. Dysregulation of this pathway has been identified
in various types of human cancer (34,35). The Wnt/β-catenin signaling pathway
in lung CSCs is a crucial target for developing novel anticancer
drugs (36) and may mediate the
inhibitory effects of EGCG on lung CSCs (37). Blocking the Wnt/β-catenin pathway
markedly inhibited the proliferation of lung CSCs (38). The present study uncovered that
CLOCK is the key regulatory molecule mediating EGCG inhibition of
lung cancer stem-like cells, and knockdown of CLOCK was shown to
markedly reduce the activity of the Wnt/β-catenin pathway. The
focus of future studies will be to investigate the intervention
effects of EGCG and the Wnt/β-catenin signaling pathway in a CLOCK
overexpression model in vitro and in vivo in order to
verify the data of the present study.
To summarize, we herein reported the role of CLOCK
in promoting CSC-like characteristics in lung cancer cells. CLOCK
is a significant regulator mediating the suppressive effects of
EGCG on the stemness characteristics of lung cancer stem-like
cells, and EGCG may suppress stemness by targeting CLOCK in A549
and H1299 sphere cells.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Priority
Academic Program Development of Jiangsu Higher Education
Institutions and The National Key Research and Development Program
of China (grant no. 2018YFC1313600).
Availability of materials and data
All data generated or analyzed during the present
study are included in this article.
Authors' contributions
QF and PJ conceived and designed the experiments.
PJ, CX, PZ, JR, FM, XW, LC and FZ conducted the experiments. QF, SL
and PJ analyzed the data and revised the manuscript. All the
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All animal experiments were performed according to
the requirements outlined in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health and all the
protocols were approved by the Committee on the Ethics of Animal
Experiments of Nanjing Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansen J: Increased breast cancer risk
among women who work predominantly at night. Epidemiology.
12:74–77. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kloog I, Haim A, Stevens RG and Portnov
BA: Global co-distribution of light at night (LAN) and cancers of
prostate, colon, and lung in men. Chronobiol Int. 26:108–125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Viswanathan AN, Hankinson SE and
Schernhammer ES: Night shift work and the risk of endometrial
cancer. Cancer Res. 67:10618–10622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bass J and Takahashi JS: Circadian
integration of metabolism and energetics. Science. 330:1349–1354.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masri S, Papagiannakopoulos T, Kinouchi K,
Liu Y, Cervantes M, Baldi P, Jacks T and Sassone-Corsi P: Lung
adenocarcinoma distally rewires hepatic circadian homeostasis.
Cell. 165:896–909. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puram RV, Kowalczyk MS, de Boer CG,
Schneider RK, Miller PG, McConkey M, Tothova Z, Tejero H, Heckl D,
Järås M, et al: Core circadian clock genes regulate leukemia stem
cells in AML. Cell. 165:303–316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Dycke KC, Rodenburg W, van Oostrom CT,
van Kerkhof LW, Pennings JL, Roenneberg T, van Steeg H and van der
Horst GT: Chronically alternating light cycles increase breast
cancer risk in mice. Curr Biol. 25:1932–1937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reppert SM and Weaver DR: Coordination of
circadian timing in mammals. Nature. 418:935–941. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schibler U and Sassone-Corsi P: A web of
circadian pacemakers. Cell. 111:919–922. 2002. View Article : Google Scholar
|
|
13
|
Li A, Lin X, Tan X, Yin B, Han W, Zhao J,
Yuan J, Qiang B and Peng X: Circadian gene Clock contributes to
cell proliferation and migration of glioma and is directly
regulated by tumor-suppressive miR-124. FEBS Lett. 587:2455–2460.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghotra VP, Puigvert JC and Danen EH: The
cancer stem cell microenvironment and anti-cancer therapy. Int J
Radiat Biol. 85:955–962. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kreso A and Dick JE: Evolution of the
cancer stem cell model. Cell Stem Cell. 14:275–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Florian S, Sonneck K, Hauswirth AW, Krauth
MT, Schernthaner GH, Sperr WR and Valent P: Detection of molecular
targets on the surface of CD34+/CD38-stem cells in various myeloid
malignancies. Leuk Lymphoma. 47:207–222. 2006. View Article : Google Scholar
|
|
17
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsunaga N, Ogino T, Hara Y, Tanaka T,
Koyanagi S and Ohdo S: Optimized dosing schedule based on circadian
dynamics of mouse breast cancer stem cells improves the antitumor
effects of aldehyde dehydrogenase inhibitor. Cancer Res.
78:3698–3708. 2018.PubMed/NCBI
|
|
19
|
Dong Z, Zhang G, Qu M, Gimple RC, Wu Q,
Qiu Z, Prager BC, Wang X, Kim LJY, Morton AR, et al: Targeting
glioblastoma stem cells through disruption of the circadian clock.
Cancer Discov. 9:1556–1573. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Zhang DG, Jiang AG, Lu HY, Zhang LX and
Gao XY: Isolation, cultivation and identification of human lung
adenocarcinoma stem cells. Oncol Lett. 9:47–54. 2015. View Article : Google Scholar
|
|
22
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: An evolving concept. Nat Rev Cancer. 12:133–13.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leon G, MacDonagh L, Finn SP, Cuffe S and
Barr MP: Cancer stem cells in drug resistant lung cancer: Targeting
cell surface markers and signaling pathways. Pharmacol Ther.
158:71–90. 2016. View Article : Google Scholar
|
|
24
|
Suresh R, Ali S, Ahmad A, Philip PA and
Sarkar FH: The role of cancer stem cells in recurrent and
drug-resistant lung cancer. Adv Exp Med Biol. 890:57–74. 2016.
View Article : Google Scholar
|
|
25
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–114. 2008.
View Article : Google Scholar
|
|
26
|
Dierickx P, Van Laake LW and Geijsen N:
Circadian clocks: From stem cells to tissue homeostasis and
regeneration. EMBO Rep. 19:18–28. 2018. View Article : Google Scholar :
|
|
27
|
Zhang F, Sun H, Zhang S, Yang X, Zhang G
and Su T: Overexpression of PER3 inhibits self-renewal capability
and chemoresistance of colorectal cancer stem-like cells via
inhibition of notch and β-catenin signaling. Oncol Res. 25:709–719.
2017. View Article : Google Scholar
|
|
28
|
Okazaki F, Matsunaga N, Okazaki H, Azuma
H, Hamamura K, Tsuruta A, Tsurudome Y, Ogino T, Hara Y, Suzuki T,
et al: Circadian clock in a mouse colon tumor regulates
intracellular iron levels to promote tumor progression. J Biol
Chem. 291:7017–1028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Y, Jin L, Sui YX, Han LL and Liu JH:
Circadian gene CLOCK affects drug-resistant gene expression and
cell proliferation in ovarian cancer SKOV3/DDP cell lines through
autophagy. Cancer Biother Radiopharm. 32:139–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaveri NT: Green tea and its polyphenolic
catechins: Medicinal uses in cancer and noncancer applications.
Life sci. 78:2073–2080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kweon MH, Adhami VM, Lee JS and Mukhtar H:
Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in
A549 cells contributes to resistance to apoptosis induced by
epigallocatechin 3-gallate. J Biol Chem. 281:33761–33772. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang P, Xu C, Chen L, Chen A, Wu X, Zhou
M, Haq IU, Mariyam Z and Feng Q: Epigallocatechin-3-gallate
inhibited cancer stem cell-like properties by targeting
hsa-mir-485-5p/RXRα in lung cancer. J Cell Biochem. 119:8623–8635.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang P, Xu C, Chen L, Chen A, Wu X, Zhou
M, Haq IU, Mariyam Z and Feng Q: EGCG inhibits CSC-like properties
through targeting miR-485/CD44 axis in A549-cisplatin resistant
cells. Mol Carcinog. 57:1835–1844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jang GB, Kim JY, Cho SD, Park KS, Jung JY,
Lee HY, Hong IS and Nam JS: Blockade of Wnt/β-catenin signaling
suppresses breast cancer metastasis by inhibiting CSC-like
phenotype. Sci Rep. 5:124652015. View Article : Google Scholar
|
|
35
|
Shapiro M, Akiri G, Chin C, Wisnivesky JP,
Beasley MB, Weiser TS, Swanson SJ and Aaronson SA: Wnt pathway
activation predicts increased risk of tumor recurrence in patients
with stage I nonsmall cell lung cancer. Ann Surg. 257:548–554.
2013. View Article : Google Scholar :
|
|
36
|
Jiang HL, Jiang LM and Han WD:
Wnt/β-catenin signaling pathway in lung cancer stem cells is a
potential target for the development of novel anticancer drugs. J
BUON. 20:1094–1100. 2015.PubMed/NCBI
|
|
37
|
Zhu J, Jiang Y, Yang X, Wang S, Xie C, Li
X, Li Y, Chen Y, Wang X, Meng Y, et al: Wnt/β-catenin pathway
mediates (-)-Epigallocatechin-3-gallate (EGCG) inhibition of lung
cancer stem cells. Biochem Biophys Res Commun. 482:15–21. 2017.
View Article : Google Scholar
|
|
38
|
Zhang X, Lou Y, Zheng X, Wang H, Sun J,
Dong Q and Han B: Wnt blockers inhibit the proliferation of lung
cancer stem cells. Drug Des Devel Ther. 9:2399–2407.
2015.PubMed/NCBI
|