Introduction
Thyroid cancer (TC) is one of the most common
malignancies worldwide (1). The
incidence of TC is steadily increasing in some regions and nations
including those in South and North America, Italy, Japan and the
Pacific Islands (2). An
estimation from the American Cancer Society stated that ~52,070 new
TC cases were diagnosed in 2019 in the USA, resulting in >2,170
TC-associated mortalities (3). A
study involving 255 patients reported that the incidence of TC is
~10.58/100,000 in China (4). TC
can be divided into the four following subtypes according to its
histopathological characteristics: i) Papillary thyroid cancer
(PTC); ii) follicular thyroid cancer (FTC); iii) poorly
differentiated thyroid cancer; and iv) anaplastic thyroid cancer
(ATC) (5-7). Among these subtypes, PTC is the most
frequently diagnosed type of TC, accounting for ~85% of all TC
cases, and ATC is the most aggressive type of TC, with a poor
prognosis (8). Therefore,
investigating the underlying molecular mechanisms of TC
pathogenesis for the development of novel effective diagnostic and
therapeutic targets is needed.
Long non-coding RNAs (lncRNAs) are a type of RNA
molecule with >200 nucleotides but which do not have
protein-encoding ability (9).
LncRNAs are located in the nucleus and are more tissue-specific
compared with mRNAs (10). TC is
closely associated with aberrant expression profiles of lncRNAs
(11). LncRNAs are implicated in
tumor occurrence and development (12). Certain lncRNAs have been
identified to play an important role in TC tumorigenesis, such as
taurine up-regulated gene 1, X-inactive specific transcript and
metastasis associated lung adenocarcinoma transcript 1 (11). LncRNAs regulate the expression and
function of their target genes through multiple mechanisms by
acting as miRNAs sponges, molecular scaffolds or protein decoys
(13).
LncRNA CCAT2 (CCAT2) has previously been reported to
be involved in the tumor progression of multiple human tumors, such
as colorectal cancer, osteosarcoma and pituitary adenomas (14-16). However, to the best of our
knowledge, the role of CCAT2 in TC has not been investigated. The
present study aimed to investigate whether CCAT2 plays a role in
TC, hoping to provide an improved understanding of the complex
pathogenesis of TC and promote the development of therapeutic
targets.
Materials and methods
Collection of TC tissue samples and cell
culture
TC tissue samples and adjacent normal tissue samples
(30 pairs containing 17 papillary, 9 follicular, and 4 anaplastic)
were collected from 30 patients who underwent thyroidectomy at The
Changzhou Second People's Hospital Affiliated to Nanjing Medical
University (Changzhou, China) between 2013 October and 2018
October, including 8 male patients and 22 female patients. The age
range of the patients was 37-60 years, with a mean age of 43 years.
The adjacent normal tissue was ~2 cm away from TC tissue samples.
All patients did not receive preoperative radiotherapy or
chemotherapy prior to the study. Benign or malignant thyroid
nodules were diagnosed based on pathological features by the
pathologists as aspiration of thyroid nodules was used for primary
diagnosis on TC, which was confirmed in a previous study (17). Patients with TC were divided into
the high-CCAT2 group and low-CCAT2 group according to the median
level of tissue CCAT2 (n=15 per group). Tumor stages were
determined based on the tumor-node-metastasis (TNM) criteria of the
American Joint Committee on Cancer classification (8th edition)
(18). Written informed consent
was obtained from all the patients, and this study was approved by
the Ethics Committee of the Changzhou Second People's Hospital
Affiliated to Nanjing Medical University (approval no.
NK2013100101).
The human thyroid follicular epithelial cell line
(Nthy-ori3-1) and the TC cell lines (TPC-1, HTH83, IHH4, FTC-133
and FTC-238) were all obtained from the Chinese Academy of Medical
Sciences (Shanghai, China), which included three PTC cell lines
(TPC-1, HTH83 and IHH4) and two FTC cell lines (FTC-133 and
FTC-238). The cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.), which was supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.),
and maintained in cell incubators at 37°C with 5%
CO2.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
RNA was extracted from TC tissues and cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNAs
were synthesized using a BestarTM qPCR RT kit (DBI Bioscience)
according to the manufacturer's protocol. qPCR was conducted in an
ABI 7500 system (ABI Biosystems) using a SYBR-Green kit (Bio-Rad
Laboratories, Inc.) and a 20-µl reaction mixture containing
0.3 µl cDNA and 0.4 µM primers. The amplification
conditions were as follows: 5 min at 95°C, 35 cycles of 35 sec at
95°C, 50 sec at 60°C, 20 sec at 72°C and 2 min at 72°C. Relative
gene expression was calculated using the 2−ΔΔCq method
(19), and GAPDH was used as the
reference gene. The sequences of the primers used in the study were
as follows: CCAT2 forward, 5′-CTT CCA GCT CCA CCT CTG AC-3′ and
reverse, 5′-GAG CTC AAA GGA CGA TGA GG-3′; and GAPDH forward,
5′-GCA CCG TCA AGG CTG AGA AC-3′ and reverse, 5′-TGG TGA AGA CGC
CAG TGG A-3′.
CCAT2 silencing in TC cells
Since TPC-1 and FTC-133 cells exhibited a higher
CCAT2 expression compared with the other TC cells, they were used
for subsequent studies. To silence CCAT2 expression in TC cells, a
specific small interfering (si)RNA against CCAT2 (si-CCAT2) and a
negative control (si-NC) were purchased from Sangon Biotechnology
Co., Ltd. After culturing the cells in 24-well plates at 37°C for
24 h, the TC cells (1×105 cells/well) were transfected
with si-CCAT2 (50 nmol) or si-NC (50 nmol) using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Following transfection for ≥24 h, the
CCAT2-silenced TC cells were used for further study. Knockdown
efficiency of si-CCAT2 in TC cells was detected by RT-qPCR. The
sequences used were as follows: si-CCAT2 sense, 5′-AAG UCC ACC UGA
UCA CCU CGG-3′ and antisense, 5′-GAG GUG AUC AGG UGG ACU UUC-3′;
and si-NC sense, 5′-AUA AGU CAC CUC CAC CUG CGG-3′ and antisense,
5′-GCU UGA GAA GGG UGA UCU GUC-3′.
CCK-8 assay
The TC cells transfected with si-NC or si-CCAT2 were
separately seeded into 96-well plates at a density of
1×105 cells/well and maintained at 37°C overnight. CCK-8
solution (10 µl) was then added into each well and incubated
with the cells for 10 min. The absorbance of each well at 450 nm
was detected using a microplate reader at the indicated time points
(24, 48 and 72 h post-cell culture).
Colony formation assay
The TC cells were collected and re-seeded into
6-well plates at a concentration of 3,000 cells/well following the
transfection with si-NC or si-CCAT2 for 48 h. After culture at 37°C
under 5% CO2 for 2 weeks, the cell colonies were stained
with Giemsa solution at room temperature for 30 min, and the visual
colonies were counted under a stereo microscope (SZX10; Olympus
Corporation) at ×10 magnification.
Cell cycle analysis
After transfection for 48 h, the TC cells were
harvested and washed using PBS three times, and then fixed in
pre-cold 70% ethanol at 4°C overnight. For analyzing the cell
cycle, the fixed TC cells were subjected to propidium iodide (PI)
staining at room temperature for 20 min in the dark. Cell cycle was
analyzed using a FACSCalibur cytometer (Becton-Dickinson and
Company) and FlowJo software (version 10; FlowJo, LLC).
Cell apoptosis analysis
For cell apoptosis analysis, the fixed TC cells were
stained with PI (5 µl; Sigma-Aldrich; Merck KGaA; cat. no.
81845) and Annexin V-FITC (5 µl; BD Biosciences; cat. no.
556420) at room temperature for 30 min, and the cell apoptosis was
analyzed using a FACSCalibur cytometer (Becton-Dickinson and
Company) and FlowJo software (version 10; FlowJo, LLC).
Western blot analysis
Whole cell extracts were collected using RIPA cell
lysis buffer (Beyotime Institute of Biotechnology) with 1 mM PMSF.
Total proteins of the treated TC cells were extracted, and the
protein concentration was determined using Bradford kit (Beijing
Solarbio Science & Technology, Inc.) and quantified using
densitometry. The NE-PER Nuclear and Cytoplasmic Extraction Reagent
kit (Pierce; Thermo Fisher Scientific, Inc.) was used for the
extraction of cytoplasmic and nuclear proteins from the treated TC
cells. Total proteins (50 µg/lane) were loaded and isolated
using 10% SDS-PAGE, and then the target proteins were transferred
to polyvinylidene difluoride (PVDF) membranes (EMD Millipore).
Next, the PVDF membranes were immersed in 5% non-fat milk at room
temperature for 2 h. The membranes were then probed with the
following specific primary antibodies overnight at 4°C: Bcl-2 (cat.
no. ab59348, 1:1,000), Bax (cat. no. ab32503, 1:1,000), cleaved
(C)-caspase-3 (cat. no. ab2302, 1:1,000), β-catenin (cat. no.
ab68183, 1:2,000), c-Myc (cat. no. ab39688, 1:1,000), Cyclin D1
(cat. no. ab134175, 1:10,000), Histon3 (cat. no. ab1791, 1:1,000)
and GAPDH (cat. no. ab8245, 1:2,000). Histon3 and GAPDH were used
as the internal controls. After washing the membranes with PBS
three times, the membranes were incubated with horse-radish
peroxidase-conjugated secondary antibodies, including goat
anti-rabbit IgG H&L (cat. no. ab205718; 1:2,000) and goat
anti-mouse IgG H&L (cat. no. ab205719; 1:2,000), at room
temperature for 1 h. All the primary and secondary antibodies were
purchased from Abcam. The signals were visualized using a ECL
detection reagent (Amersham Biosciences). The blots were analyzed
with an iBright CL750 Imaging System (cat. no. A44116l Thermo
Fisher Scientific, Inc.) and grey values were calculated using
ImageJ 5.0 (National Institute of Health).
Wound healing assay
The transfected TC cells were seeded into 6-well
plates and allowed to grow to 100% confluence at 37°C. Then, a 2-mm
wide wound was created on the cell surface using a sterile plastic
scriber. The cells were then cultured in serum-free medium. The
width of the wounds was observed using an inverted optical
microscope (DP27; Olympus Corporation; magnification, ×100) at 0
and 48 h, and then measured using Image-Pro Plus Analysis software
(version 6.0; Media Cybernetics, Inc.).
Transwell assay
Transwell chambers (Corning, Inc.) with 8-µm
pores were used to detect the effects of CCAT2 knockdown on TC cell
invasion. The upper chamber was pre-coated with Matrigel at 37°C
for 30 min. The TC cells stably transfected with si-NC or si-CCAT2
were harvested and re-suspended in serum-free culture medium at a
final concentration of 2×105 cells/ml. Subsequently, 200
µl TC cell suspension was added to the upper chamber, while
500 µl culture medium with 10% FBS was added to the lower
chamber. Following culture for 48 h, the non-invasive cells were
removed, while the invaded TC cells were fixed in 4%
paraformaldehyde and stained by crystal violet at room temperature
for 20 min. The invaded cells were counted under an inverted
optical micro-scope (DP27; Olympus Corporation) at ×200
magnification.
Wnt/β-catenin pathway activity
assessment
The effects of si-CCAT2 on the activity of the
Wnt/β-catenin cascade were assessed by a TCF/LEF Reporter kit (cat.
no. 60500, BPS Bioscience, Inc.). In brief, the TC cells were
seeded into 96-well plates at a concentration of 1×105
cells/well, cultured overnight and then transfected with 50 ng
LEF/TCF reporter plasmid. The LEF/TCF reporter-containing TC cells
were then treated with LiCl or LiCl + si-CCAT2 (the concentration
of LiCl was set at 10 mM) at 37°C for 48 h, and the Wnt pathway
activity was determined by a Dual-Luciferase Assay System (cat. no.
E1910; Promega Corporation).
Statistical analysis
The data in the present study are shown as the mean
± standard error of the mean. Statistical analysis for patients was
performed using a Student's paired t-test, while other analyses
were performed using one-way ANOVA followed by Tukey's post hoc
test. Associations between CCAT2 expression and patient
characteristics were analyzed using Fisher's exact test. GraphPad
(version 7; GraphPad Software, Inc.) was used for analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
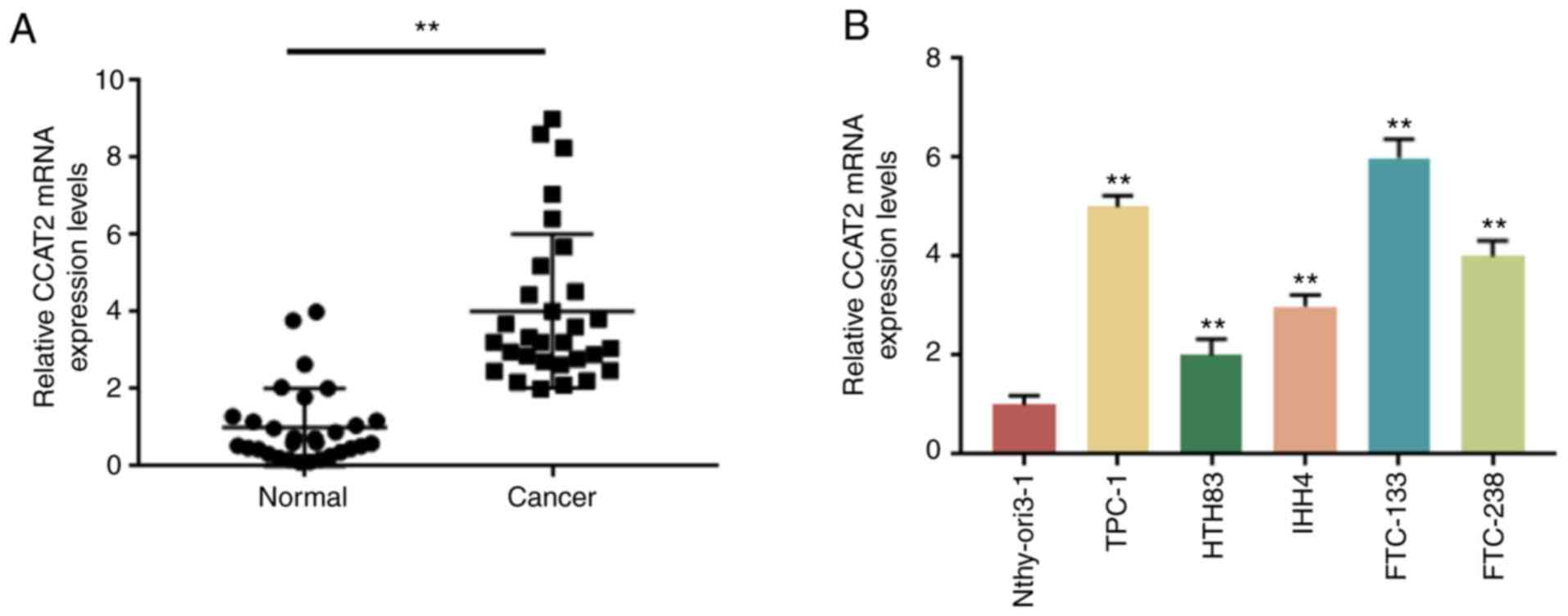
CCAT2 expression is increased in TC
To investigate whether CCAT2 is associated with TC,
its expression in 30 paired tissue specimens collected from
patients with TC was detected by RT-qPCR. Compared with the normal
tissue samples, the expression of CCAT2 was significantly higher in
the TC samples (Fig. 1A).
Consistently, a significantly higher level of CCAT2 was observed in
five TC cell lines (TPC-1, HTH83, IHH4, FTC-133 and FTC-238)
compared with the Nthy-ori3-1 cell line (Fig. 1B). In addition, the association
between tissue CCAT2 level and clinicopathological characteristics
of TC patients were analyzed by Fisher's exact test. Patients with
TC were divided into the high-CCAT2 group and low-CCAT2 group
according to the median level of tissue CCAT2. No significant
associations between tissue CCAT2 level and age, sex, tumor size, T
stage, extrathyoridal extension or lymph node metastasis were
observed. However, tissue CCAT2 level was significantly associated
with TNM stage (Table I). In
summary, CCAT2 may participate in the progression of TC.
 | Table IClinicopathological characteristics
of patients with thyroid cancer (n=30). |
Table I
Clinicopathological characteristics
of patients with thyroid cancer (n=30).
| Characteristic | Total, n | lncRNA CCAT2
expression
| P-value |
|---|
| Low, n (%) | High, n (%) |
|---|
| Age, years | | | | |
| <45 | 18 | 10 (55.56) | 8 (44.44) | 0.710 |
| ≥45 | 12 | 5 (41.67) | 7 (58.33) | |
| Sex | | | | |
| Male | 8 | 3 (37.50) | 5 (62.50) | 0.682 |
| Female | 22 | 12 (54.55) | 10 (45.45) | |
| Tumor size, cm | | | | |
| <2 | 17 | 10 (58.82) | 7 (41.18) | 0.462 |
| ≥2 | 13 | 5 (38.46) | 8 (61.54) | |
| T stage | | | | |
| T1-T2 | 21 | 13 (61.90) | 8 (38.10) | 0.109 |
| T3-T4 | 9 | 2 (22.22) | 7 (77.78) | |
| TNM stage | | | | |
| I/II | 22 | 14 (63.64) | 8 (36.36) | 0.035a |
| III/IV | 8 | 1 (12.50) | 7 (87.50) | |
| Extrathyroidal
extension | | | | |
| Yes | 16 | 7 (43.75) | 9 (56.25) | 0.715 |
| No | 14 | 8 (57.14) | 6 (42.86) | |
| Lymph node
metastasis | | | | |
| N0 | 18 | 11 (61.11) | 7 (38.89) | 0.264 |
| N1 | 12 | 4 (33.33) | 8 (66.67) | |
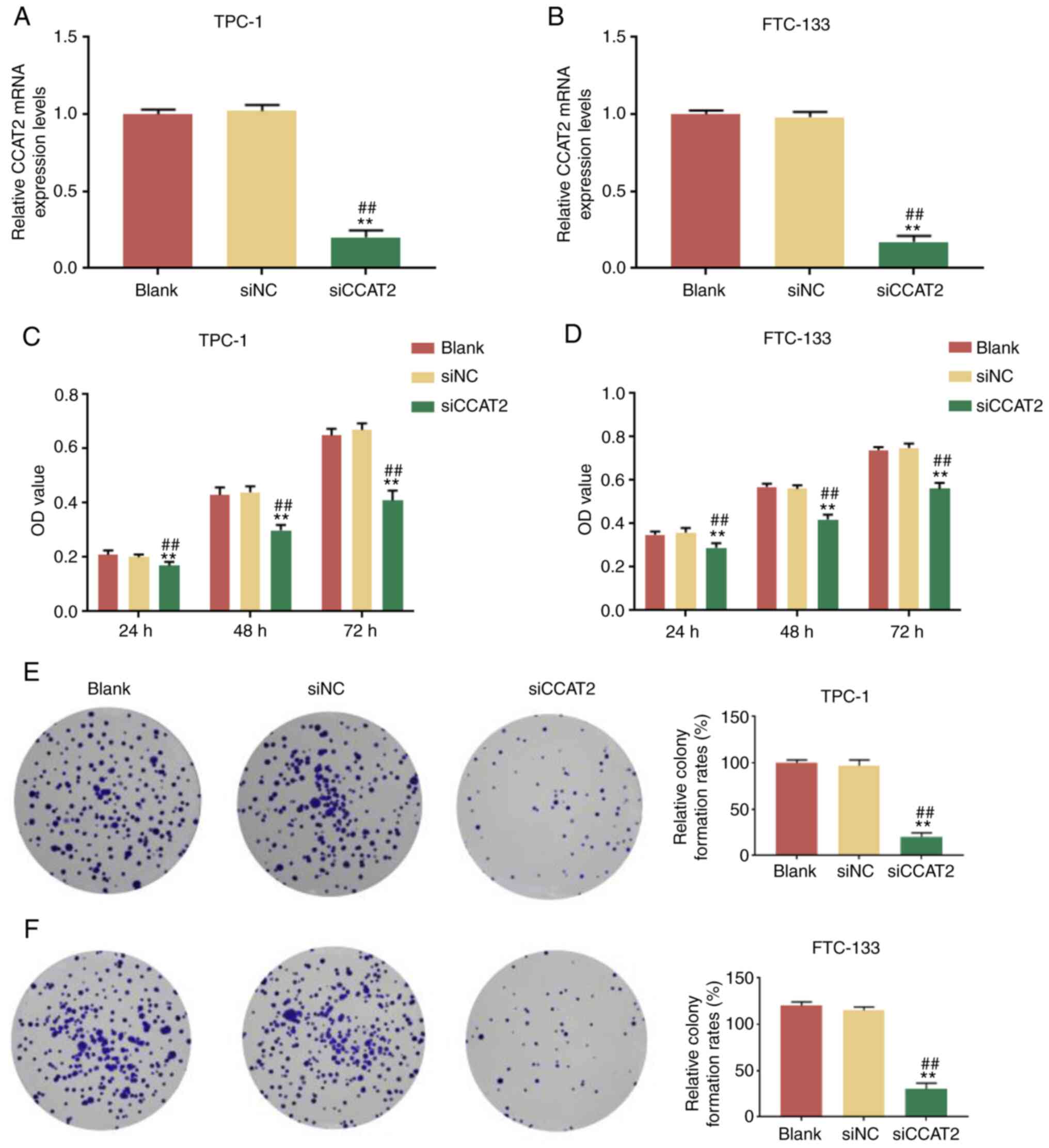
CCAT2 silencing suppresses TC cell
proliferation
Next, the expression of CCAT2 was silenced in TPC-1
and FTC-133 cells by a specific siRNA (si-CCAT2) and the cell
viability and colony forming ability were examined. Knockdown
efficiency of si-CCAT2 was evaluated by RT-qPCR. After transfection
for 48 h, the relative CCAT2 expression was significantly lower in
the si-CCAT2-treated TPC-1 and FTC-133 cells compared with the
blank and si-NC groups (Fig. 2A and
B). CCK-8 assay demonstrated that at 24, 48 and 72 h post-CCAT2
knockdown, the cell viabilities of TPC-1 and FTC-133 cells were
significantly reduced compared with the blank and si-NC groups
(Fig. 2C and D). To further
investigate the long-term inhibitory effects of CCAT2-knockdown on
TC cells, a colony formation assay was performed. The results
indicated that knockdown of CCAT2 significantly inhibited the
colony formation of TC cells compared with the blank and si-NC
groups (Fig. 2E and F).
Collectively, these findings suggested that CCAT2-knockdown
inhibited TC cell viability in vitro.
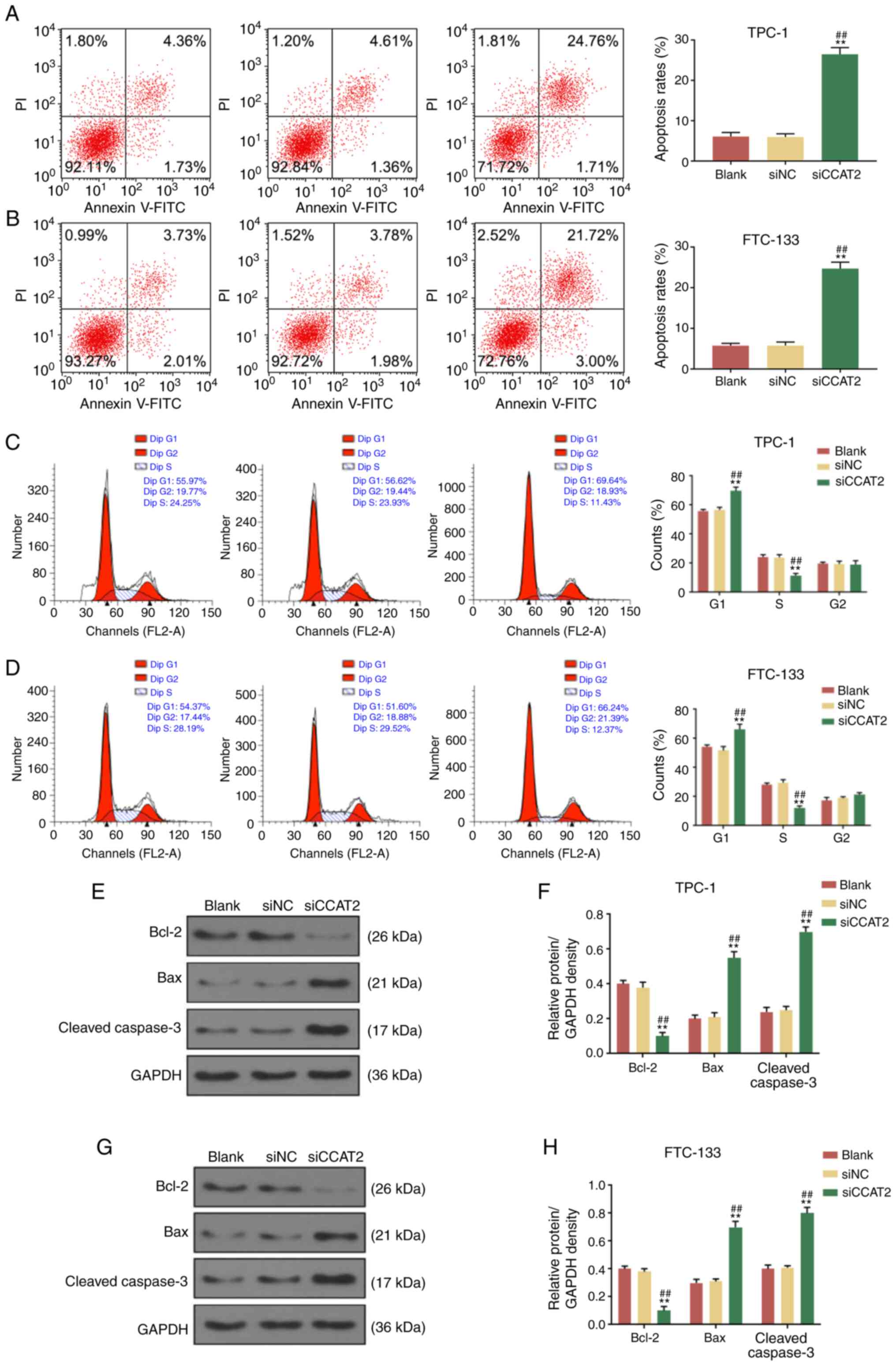
CCAT2 silencing promotes TC cell cycle
arrest and apoptosis
Subsequently, the effects of CCAT2 silencing on TC
cell cycle and apoptosis were analyzed by flow cytometry. It was
identified that CCAT2 suppression significantly increased the
apoptosis rate of TPC-1 and FTC-133 cells (Fig. 3A and B). Moreover, CCAT2 knockdown
in TPC-1 and FTC-133 cells significantly increased the percentage
of cells in the G1 phase and significantly reduced the percentage
of cells in the S phase (Fig. 3C and
D), indicating that the cell cycle of TC cells was arrested at
the G1 phase in the si-CACT2 group. In addition, the protein
expression levels of Bcl-2, Bax and C-caspase-3 in TPC-1 and
FTC-133 cells of the blank, si-NC and si-CCAT2 groups were analyzed
by western blotting. Compared with the blank and si-NC groups,
si-CCAT2 significantly reduced Bcl-2 expression and significantly
increased Bax and C-caspase-3 expression in both TPC-1 (Fig. 3E and F) and FTC-133 cells
(Fig. 3G and H). These findings
indicated that CCAT2 knock-down promoted cell apoptosis and cell
cycle arrest of the TC cells.
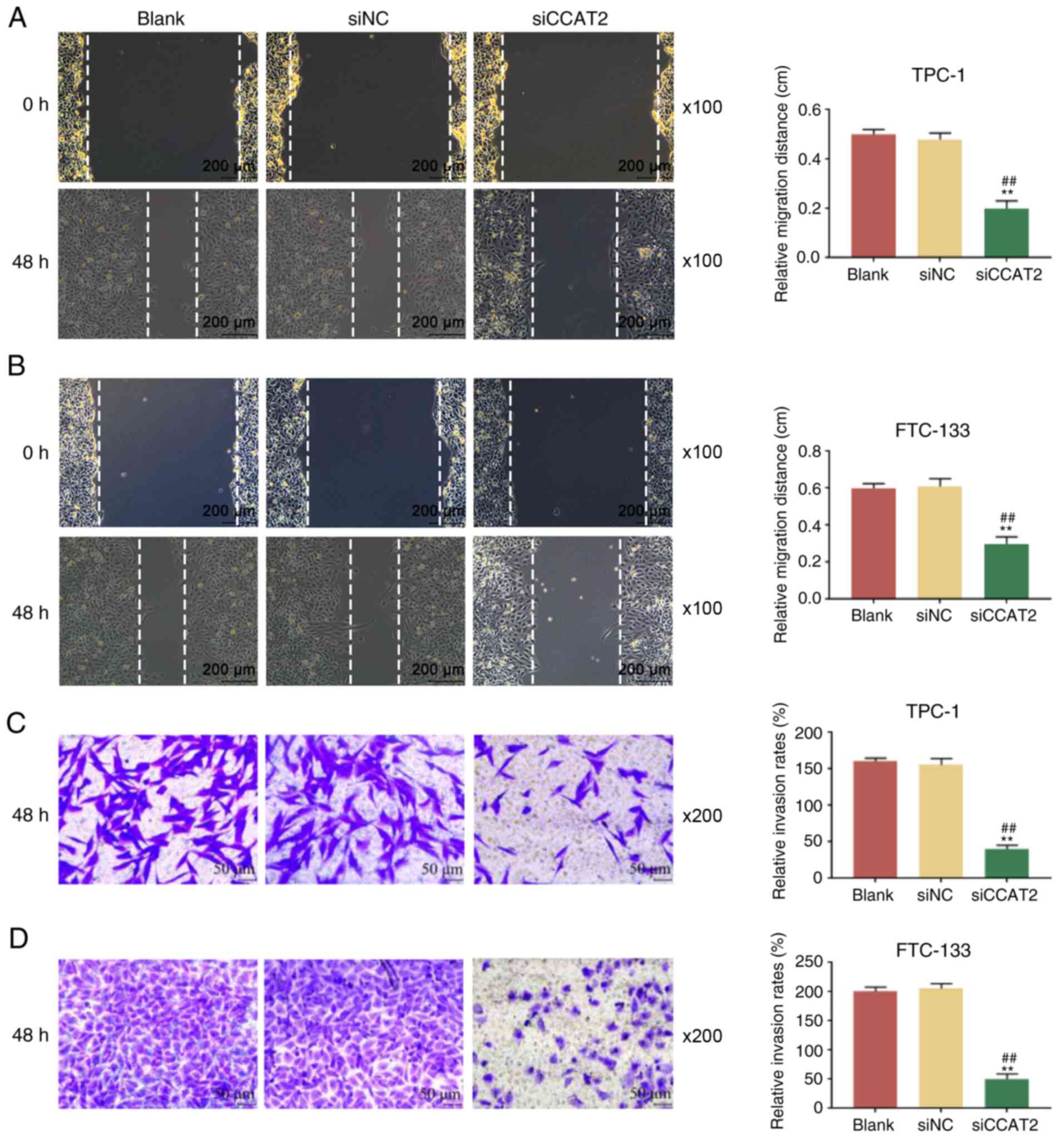
CCAT2 silencing suppresses TC cell
migration and invasion
The effects of CCAT2 silencing on the cell migration
and invasion of TPC-1 and FTC-133 cells were subsequently evaluated
by wound healing and Transwell assays. Wound healing assay
demonstrated that the migration of TPC-1 and FTC-133 cells
transfected with si-CCAT2 was significantly attenuated compared
with the cells in the blank and si-NC groups (Fig. 4A and B). Furthermore, Transwell
assay revealed that the invasion of TPC-1 and FTC-133 cells was
also significantly suppressed by si-CCAT2 compared with the si-NC
treatment and blank groups (Fig. 4C
and D). These results indicated that CCAT2 silencing suppressed
the migration and invasion of TC cells.
CCAT2 silencing inhibits Wnt/β-catenin
activity of TC cells
The Wnt/β-catenin cascade is implicated in the
proliferation, apoptosis, differentiation and invasion of tumor
cells (20). Previous studies
have also shown that CCAT2 is involved in the progression of
multiple human cancers through regulating the Wnt/β-catenin
signaling pathway (21,22). Thus, whether the Wnt/β-catenin
signaling pathway is involved in the biological functions of CCAT2
in TC was examined. Dual-luciferase reporter assay demonstrated
that the luciferase activities of the TCF/LEF reporter-TPC-1 and
-FTC-133 cells treated with LiCl (10 nM, agonist of Wnt/β-catenin
signaling pathway) were significantly increased compared with
untreated cells, but the effects could be partially reversed by the
transfection of si-CCAT2 (Fig. 5A and
B). In addition, the expression levels of several Wnt/β-catenin
cascade-associated proteins, including β-catenin, c-Myc and Cyclin
D1, were detected in the CCAT2-slicenced TPC-1 and FTC-133 cells.
No significant alteration of total β-catenin was observed in the
blank, si-NC and si-CCAT2 groups for TPC-1 (Fig. 5C and D) and FTC-133 cells
(Fig. 5E and F). Compared with
the blank group and si-NC group, si-CCAT2 transfection
significantly reduced the expression levels of c-Myc and Cyclin D1,
and significantly increased the expression of cytoplasmic β-catenin
in TPC-1 (Fig. 5C and D) and
FTC-133 cells (Fig. 5E and F). In
addition, it was identified that nuclear β-catenin expression was
significantly reduced in si-CCAT2 transfected TPC-1 (Fig. 5G and H) and FTC-133 cells
(Fig. 5I and J) compared with the
blank and si-NC groups. Histon3 was used as an internal control.
Taken together, CCAT2 knockdown significantly inhibited the
Wnt/β-catenin signaling pathway in TC cells.
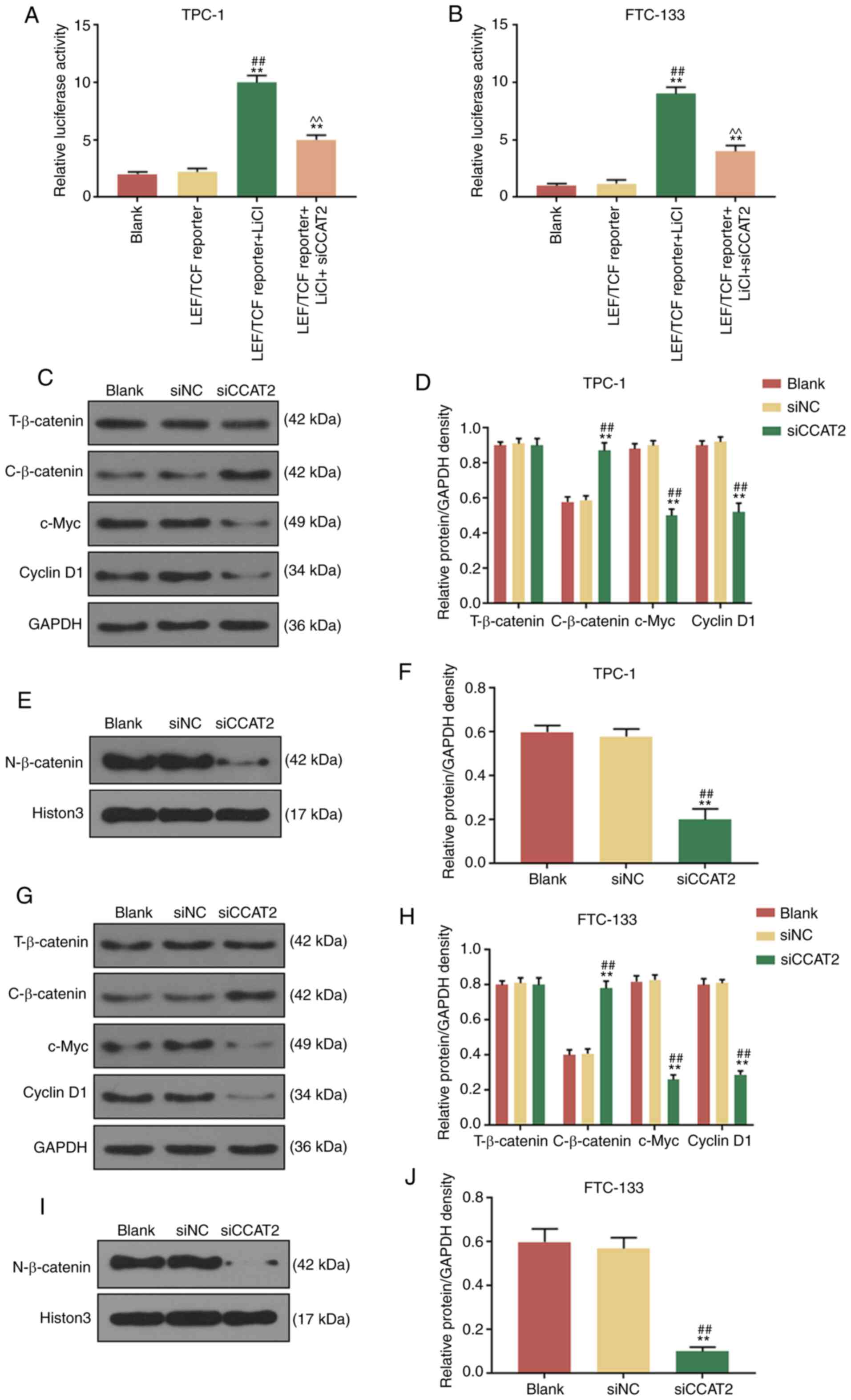 | Figure 5CCAT2 inhibits the Wnt/β-catenin
signaling pathway of thyroid cancer cells. Relative luciferase
activities of (A) TPC-1 and (B) FTC-133 cells treated with LiCI and
transfected with si-CCAT2 in the presence of LEF/TCF reporter were
determined. **P<0.01 vs. blank;
##P<0.01 vs. LEF/TCF reporter. (C-F) Effects of
si-CCAT2 on the protein relative density of T-β-catenin,
C-β-catenin, c-Myc, Cyclin D1 and N-β-catenin were assessed in
TPC-1 cells by western blotting. (G-J) Effects of si-CCAT2 on the
protein relative density of T-β-catenin, C-β-catenin, c-Myc, Cyclin
D1 and N-β-catenin were assessed in FTC-133 cells by western
blotting. **P<0.01 vs. blank; ##P<0.01
vs. si-NC. si, small interfering RNA; NC, negative control; T,
total; C, cytoplasmic; N, nuclear; LEF, lymphoid enhancer binding
factor; TCF, transcription factor. |
Discussion
In the present study, CCAT2 expression was increased
in the TC tissue samples and cell lines compared with the controls,
suggesting that it may be involved in the tumorigenesis of TC.
However, in the present study the samples size were relatively
small, the genotype of the tumors (i.e. the presence of BRAF or ras
mutations) and the radioiodine refractory tumors were not recorded,
which may be limitations. To determine the function of CCAT2 in TC,
the proliferation, apoptosis, cell cycle, migration and invasion of
the CCAT2-silenced TC cells in vitro were detected. The
results indicated that CCAT2 knockdown suppressed TC progression.
In addition, the Wnt/β-catenin cascade was inhibited in
CCAT2-silenced TC cells, suggesting that the Wnt/β-catenin
signaling pathway might act as a downstream pathway of CCAT2 in TC.
In conclusion, CCAT2 knockdown suppressed TC progression through
inhibiting Wnt/β-catenin signaling pathway.
Understanding of the molecular mechanisms of TC is
essential to the development of effective therapeutic treatments
for patients with TC. LncRNAs have increasingly been found to serve
a role in the regulation of tumor progression of TC in recent years
(23). Numerous lncRNAs are
dysregulated in TC, which allows them to serve as diagnostic and
therapeutic targets for TC (23,24). However, restricted by a number of
factors, there are very few lncRNAs that can be used for clinical
detection of TC. CCAT2 is located on chromosome 8q24.21 (25), and its genomic locus contains a
SNP rs6983267, which has been revealed to be closely associated
with the increased risks of developing malignancies such as colon
cancer, osteosarcoma and cholangiocarcinoma (26). Similarly, the present findings
indicated that CCAT2 acts as an oncogene of TC, because the
proliferation, cell cycle, migration and invasion of the TC cells
with CCAT2 silencing were inhibited and the cell apoptosis was
increased, indicating that CCAT2 plays an important role in tumor
progression.
Bcl-2, Bax, and Caspase-3 are apoptosis-related
genes, and c-Myc and cyclin D1 are involved in cell cycle
progres-sion (27). In the Bcl-2
family, Bax promotes apoptosis and Bcl-2 inhibits apoptosis
(28,29). Caspases will be activated before
exerting their effects on cell apoptosis (30). Cyclin D1 is an important
checkpoint regulator factor during the transition from the G1 to S
phase (31). The present study
found that the mechanism through which CCAT2 induces the inhibition
of TC cell apoptosis involved decreased activation of caspase
cascades and Bax, and increased activation of Bcl-2.
Wnt/β-catenin is a highly conserved signaling
pathway involved in the development of an embryo, maintenance of
homeostasis and certain human diseases (32). Abnormal activation of the
Wnt/β-catenin signaling pathway can not only result in a rapid
accumulation of β-catenin in the cell nucleus, thereby facilitating
the transcription of a wide range of onco-genes (32,33), but can also induce the
tumorigenesis of multiple human cancers, including TC (33,34). CircRNA_102171 silencing suppresses
the proliferation, migration and invasion of PTC cells and promotes
apoptosis via regulating the Wnt/β-catenin pathway (35). The association between different
lncRNAs and the Wnt/β-catenin signaling pathway in cancer cells is
widely acknowledged (21,22,36,37). Zhao et al (21) found that CCAT2 could facilitate
the occurrence of non-small lung cancer through activating the
Wnt/β-catenin cascade. Ma et al (22) revealed that knocking down CCAT2
suppresses the malignancy of oral squamous cell carcinoma through
inhibiting the Wnt pathway. The present study demonstrated that
LiCl (a Wnt pathway activator) enhanced the luciferase activity of
TC cells transfected with a LEF/TCF reporter plasmid, which was
partially reversed by si-CCAT2. Taken together, CCAT2 could promote
TC progression through activating the Wnt/β-catenin cascade.
It should be noted that there were certain
limitations of the current study. For example, we did examine other
molecular mechanisms including the MAPK pathway, and droplet
digital PCR was not performed to further support the results of
RT-qPCR, which will be studied in our future research.
In conclusion, the current study demonstrated that
CCAT2 acts as an oncogene of TC; knocking down CCAT2 suppressed the
proliferation, migration and invasion of TC cells partially by
inhibiting the Wnt/β-catenin cascade. Thus, CCAT2 has the potential
to be used as a promising therapeutic target of TC.
Acknowledgments
Not applicable.
Abbreviations:
|
TC
|
thyroid cancer
|
|
FTC
|
follicular thyroid cancer
|
|
PTC
|
papillary thyroid cancer
|
|
ATC
|
anaplastic thyroid cancer
|
|
lncRNA
|
long non-coding RNA
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
PI
|
propidium iodide
|
|
LEF
|
lymphoid enhancer binding factor
|
|
TCF
|
transcription factor
|
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX and XY substantially contributed to the
conception and design of the study, acquired, analyzed and
interpreted the data, and drafted the article and critically
revised it for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. Written informed consent was obtained from all the
patients and the experiment in the study was approved by the Ethics
Committee of the Changzhou Second People's Hospital Affiliated to
Nanjing Medical University (approval no. NK2013100101).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
La Vecchia C, Malvezzi M, Bosetti C,
Garavello W, Bertuccio P, Levi F and Negri E: Thyroid cancer
mortality and incidence: A global overview. Int J Cancer.
136:2187–2195. 2015. View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang L, Zheng RS, Wang N, Zeng HM, Yuan
YN, Zhang SW, Li HC, Liu S, Chen WQ and He J: Analysis of incidence
and mortality of thyroid cancer in China, 2013. Zhonghua Zhong Liu
Za Zhi. 39:862–867. 2017.In Chinese. PubMed/NCBI
|
|
5
|
Baloch ZW and LiVolsi VA: Special types of
thyroid carcinoma. Histopathology. 72:40–52. 2018. View Article : Google Scholar
|
|
6
|
Azar FK, Lee SL and Rosen JE: Medullary
thyroid cancer: An update for surgeons. Am Surg. 81:1–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Massimino M, Evans DB, Podda M, Spinelli
C, Collini P, Pizzi N and Bleyer A: Thyroid cancer in adolescents
and young adults. Pediatr Blood Cancer. 65:e270252018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raue F and Frank-Raue K: Thyroid cancer:
Risk-stratified management and individualized therapy. Clin Cancer
Res. 22:5012–5021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu S, Liu X, Li J, Zhou H, Carr MJ, Zhang
Z and Shi W: Long noncoding RNAs: Novel regulators of virus-host
interactions. Rev Med Virol. 29:e20462019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar
|
|
11
|
Sui F, Ji M and Hou P: Long non-coding
RNAs in thyroid cancer: Biological functions and clinical
significance. Mol Cell Endocrinol. 469:11–22. 2018. View Article : Google Scholar
|
|
12
|
Gugnoni M and Ciarrocchi A: Long noncoding
RNA and epithelial mesenchymal transition in cancer. Int J Mol Sci.
20:19242019. View Article : Google Scholar :
|
|
13
|
Lee KT, Gopalan V and Lam AK: Roles of
long-non-coding RNAs in cancer therapy through the PI3K/Akt
signalling pathway. Histol Histopathol. 34:593–609. 2019.PubMed/NCBI
|
|
14
|
Fu D, Zhang Y and Cui H: Long noncoding
RNA CCAT2 is activated by E2F1 and exerts oncogenic properties by
interacting with PTTG1 in pituitary adenomas. Am J Cancer Res.
8:245–255. 2018.PubMed/NCBI
|
|
15
|
Ruan R and Zhao XL: LncRNA CCAT2 enhances
cell proliferation via GSK3β/β-catenin signaling pathway in human
osteosarcoma. Eur Rev Med Pharmacol Sci. 22:2978–2984.
2018.PubMed/NCBI
|
|
16
|
Fosselteder J, Calin GA and Pichler M:
Long non-coding RNA CCAT2 as a therapeutic target in colorectal
cancer. Expert Opin Ther Targets. 22:973–976. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miao S, Jing M, Sheng R, Cui D, Lu S,
Zhang X, Jing S, Zhang X, Shan T, Shan H, et al: The analysis of
differential diagnosis of benign and malignant thyroid nodules
based on ultrasound reports. Gland surgery. 9:653–660. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee H, Lee M, Byun SS, Lee SE and Hong SK:
Evaluation of prostate cancer stage groups updated in the 8th
edition of the American Joint Committee on cancer
tumor-node-metastasis staging manual. Clin Genitourin Cancer.
17:e221–e226. 2019. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Srivastava NS and Srivastava RAK: Curcumin
and quercetin synergistically inhibit cancer cell proliferation in
multiple cancer cells and modulate Wnt/β-catenin signaling and
apoptotic path-ways in A375 cells. Phytomedicine. 52:117–128. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao C, Qiao C, Zong L and Chen Y: Long
non-coding RNA-CCAT2 promotes the occurrence of non-small cell lung
cancer by regulating the Wnt/β-catenin signaling pathway. Oncol
Lett. 16:4600–4606. 2018.PubMed/NCBI
|
|
22
|
Ma Y, Hu X, Shang C, Zhong M and Guo Y:
Silencing of long non-coding RNA CCAT2 depressed malignancy of oral
squamous cell carcinoma via Wnt/β-catenin pathway. Tumour Biol.
39:10104283177176702017. View Article : Google Scholar
|
|
23
|
Murugan AK, Munirajan AK and Alzahrani AS:
Long noncoding RNAs: Emerging players in thyroid cancer
pathogenesis. Endocr Relat Cancer. 25:R59–R82. 2018. View Article : Google Scholar
|
|
24
|
Zhang R, Hardin H, Chen J, Guo Z and Lloyd
RV: Non-coding RNAs in thyroid cancer. Endocr Pathol. 27:12–20.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xin Y, Li Z, Zheng H, Chan MTV and Ka Kei
Wu W: CCAT2: A novel oncogenic long non-coding RNA in human
cancers. Cell Prolif. 50:e123422017. View Article : Google Scholar
|
|
26
|
Ling H, Spizzo R, Atlasi Y, Nicoloso M,
Shimizu M, Redis RS, Nishida N, Gafà R, Song J, Guo Z, et al:
CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic
progression and chromosomal instability in colon cancer. Genome
Res. 23:1446–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jo S, Ha TK, Han SH, Kim ME, Jung I, Lee
HW, Bae SK and Lee JS: Myricetin induces apoptosis of human
anaplastic thyroid cancer cells via mitochondria dysfunction.
Anticancer Res. 37:1705–1710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tirapelli DPDC, Lustosa IL, Menezes SB,
Franco IM, Rodrigues AR, Peria FM, Marinho AMDN, Serafini LN,
Carlotti CG Jr and Tirapelli LF: High expression of XIAP and Bcl-2
may inhibit programmed cell death in glioblastomas. Arq
Neuropsiquiatr. 75:875–880. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Warren CFA, Wong-Brown MW and Bowden NA:
BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis.
10:1772019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamivand Z, Haddadi G and Fardid R:
Expression of Bax and Bcl2 genes in peripheral blood lymphocytes of
patients with differentiated thyroid cancer. J Med Phys. 43:41–45.
2018.PubMed/NCBI
|
|
31
|
Albasri AM, Elkablawy MA, Ansari IA and
Alhujaily AS: Prognostic significance of cyclin D1 Over-expression
in colorectal cancer: An experience from Madinah, Saudi Arabia.
Asian Pac J Cancer Prev. 20:2471–2476. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shang S, Hua F and Hu ZW: The regulation
of β-catenin activity and function in cancer: Therapeutic
opportunities. Oncotarget. 8:33972–33989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar
|
|
35
|
Bi W, Huang J, Nie C, Liu B, He G, Han J,
Pang R, Ding Z, Xu J and Zhang J: CircRNA circRNA_102171 promotes
papillary thyroid cancer progression through modulating
CTNNBIP1-dependent activation of β-catenin pathway. J Exp Clin
Cancer Res. 37:2752018. View Article : Google Scholar
|
|
36
|
Jing N, Huang T, Guo H, Yang J, Li M, Chen
Z and Zhang Y: LncRNA CASC15 promotes colon cancer cell
proliferation and metastasis by regulating the
miR4310/LGR5/Wnt/β-catenin signaling pathway. Mol Med Rep.
18:2269–2276. 2018.PubMed/NCBI
|
|
37
|
Zarkou V, Galaras A, Giakountis A and
Hatzis P: Crosstalk mechanisms between the WNT signaling pathway
and long non-coding RNAs. Noncoding RNA Res. 3:42–53. 2018.
View Article : Google Scholar : PubMed/NCBI
|