1. Introduction
PU.1 is a member of the erythroblast transformation
specific (ETS) transcription factor family. All ETS family members
have a common DNA binding sequence of approximately 85 amino acids,
that is the ETS domain. The sequences are highly conserved, and can
be recognized by these proteins that contain the core motif
5′-GGAA/T-3′ (1). However, the
binding of individual ETS proteins also has its characteristics
owing to the existence of the flanking sequences.
PU.1 is coded by the Spi-1 gene; Spi-1 was
discovered by Moreau-Gachelin et al in 1988. Spi-1 is a
putative oncogene that was isolated from a murine erythrocyte
leukemia induced by the acute leukemogenic retrovirus spleen
focus-forming virus (SFFV) (2).
PU.1 is a vital transcriptional regulator of
hematopoietic stem cell differentiation (3); granulocytes, macrophages and
lymphocytes are crucial immune cells, and they play important roles
in immune system. They are involved in a serious of diseases,
including inflammatory diseases, neoplastic diseases and immune
diseases, such as myeloma, leukemia, systemic lupus erythematosus
(SLE) and rheumatoid arthritis (RA) (4-8).
Therefore, it is particularly crucial to understand the mechanisms
of PU.1 in immune cells. The present study reviews the advancements
made in the elucidation of the mechanisms of the transcription
factor, PU.1, in immune cell differentiation.
2. Expression of PU.1 in immune cells
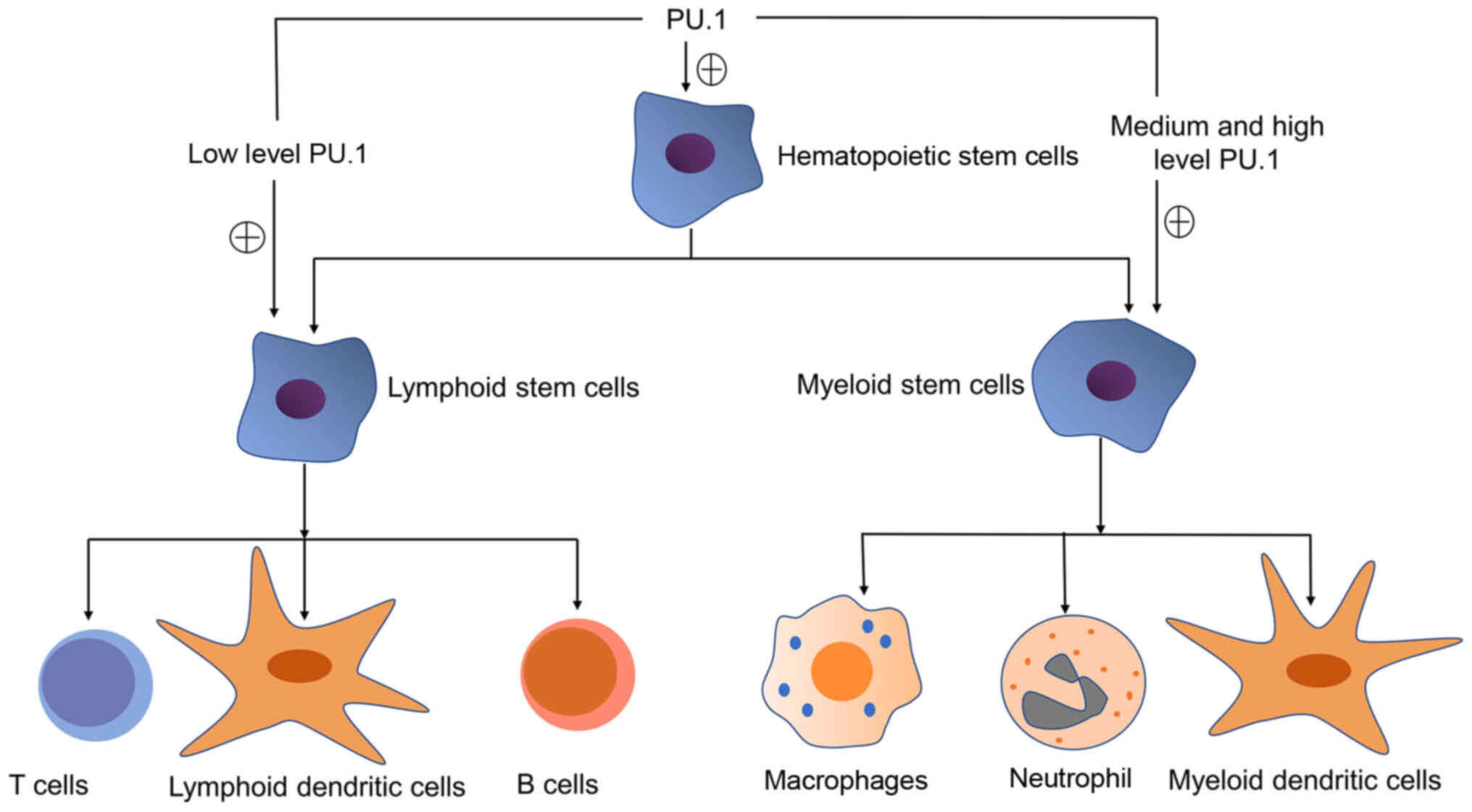
PU.1 is expressed in a tissue-specific manner and
the different levels of PU.1 expression determine the type of
hematopoietic stem cell differentiation (9). Thus, precise levels of PU.1 are
crucial for differentiation into distinct blood lineages, and even
modest decreases in its expression can lead to leukemogenesis. It
would be of interest to determine the exact mechanisms through
which target genes sense different PU.1 levels. Pospíšil et
al used myeloid progenitors encoding inducible PU.1 transgene.
They found that high levels of PU.1 produced macrophages, while
intermediate levels of PU.1 induced differentiation into
granulocyte-like cells (10).
Moreover, at intermediate concentrations, PU.1 bound to high
affinity binding sites in several enhancers of granulocyte genes,
resulting in their successive interaction, leading to
transcriptional activation (10).
Nutt et al found that the expression of PU.1 was silenced in
erythroid cells, but elevated in macrophages, with a sufficiently
lower level in committed B cells (11). In neutrophils, PU.1 expression was
found at moderately high levels. As for dendritic cells (DCs), PU.1
was expressed in all DC subsets, with a high amount of PU.1 in
myeloid DCs. In comparison, plasmacytoid DCs (pDCs)
characteristically expressed a low level (11). As regards T cells, some studies
have found that PU.1 is decreased during T cell lineage
differentiation (9,12,13). Thus, PU.1 expression is increased
during granulocyte and macrophage differentiation, while it is
decreased during erythrocyte, T lymphocyte and B lymphocyte
differentiation (9).
In cells of the immune system, PU.1 can activate a
number of factors, such as chemokines, cytokines and cytokine
receptors that are significant for immune cell differentiation and
function, including cytokine receptors, such as macrophage colony
stimulating factor receptor (M-CSFR), granulocyte colony
stimulating factor receptor (G-CSFR), granulocyte-macrophage colony
stimulating factor receptor (GM-CSFR), interleukin 3 receptor
(IL-3R), FcγR, cytokine CD11b and chemokines, such as CCL-22
(9,14). The expression of PU.1 in immune
cells is illustrated in Fig.
1.
3. Regulatory mechanisms of PU.1
PU.1 has several domains that are important for PU.1
to regulate immune cell differentiation. One domain is the ETS
domain of 85 amino acids, which is located near the C-terminus of
the protein. It is the highly conserved DNA-binding sequence that
recognizes the sequence 5′-GGAA/T-3′. Other domains within PU.1
include a glutamine-rich domain and acidic residues near the
N-terminal half of the protein, which are indispensable for
transactivation, and a central proline, glutamic acid, serine and
threonine (PEST) domain that is involved in the interaction between
PU.1 and other transcription factors (15).
Furthermore, PU.1 regulates gene expression through
binding to consensus sequences, not only as a monomer but also
through its interaction with other transcription factors, including
interferon regulatory factor (IRF)4, IRF8, C/enhanced binder
protein α and β (C/EBPα and C/EBPβ) and c-Jun (16-18). However, in addition to chaperone
molecules, PU.1 also has antagonistic molecules. For example, the
GATA family of transcription factors can antagonize the activity of
PU.1 by interacting with it. Initially, this antagonism was
proposed to occur through the prevention of DNA binding (19). However, some studies have revealed
that PU.1 and GATA-1 co-exist on DNA. The repression of PU.1
activity appears to involve altering the chromatin structure around
the binding site (20-22).
4. Role of PU.1 in macrophage
differentiation
In differentiated macrophages, two major phenotypes
have been identified, which are of significance to the occurrence
and progression of inflammation. One is a pro-inflammatory
phenotype that is activated with lipopolysaccharide (LPS), either
with or without interferon-γ (known as M1 or classically activated
macrophages). The other is an anti-inflammatory phenotype that is
activated with IL-4 and IL-13 (known as M2 or alternatively
activated macrophages). The two macrophage phenotypes have a very
different metabolic profile (23,24).
M1 macrophages are identified as CD64(+)CD80(+),
while M2 macrophage are identified as CD11b(+)CD209(+). Polarized
M1 macrophages secrete IP-10, IFN-γ, IL-8, TNF-α, IL-1β and RANTES,
whereas M2 macrophages secrete IL-13, CCL17 and CCL18 (20). Moreover, M2 macrophages are
characterized by an abundant expression of CD163, arginase, mannose
receptor (MR/CD206), chitinase-like molecules (Ym-1/2) and
resistin-like molecule α (RELMα/Fizz-1) (25).
Furthermore, apart from the peroxisome
proliferator-activated receptor (PPAR), Krüppel-like factor (KLF),
IRF, signal transducer and activator of transcription (STAT),
nuclear factor (NF)-κB and hypoxia-inducible factor (HIF) families,
and microRNAs (miRNAs or miRs) (26), PU.1 is also a critical molecule in
macrophage differentiation. PU.1 plays a key role not only in M1
macrophage differentiation, but also M2 macrophage differentiation
(27-30). Karpurapu et al identified a
direct link between PU.1 and inflammation by establishing a bone
marrow chimera model with functional PU.1 knockout phenotype in
mature macrophages (28). In
these mice, the expression of NF-κB activation, that mediates
inflammation, was markedly attenuated in different organs compared
with the wild-type (WT) mice. These findings indicated that PU.1 is
required for LPS-induced classical macrophage activation. After
macrophages are fully differentiated, PU.1 is also important for
the survival of mature macrophages (28). Qian et al identified that
PU.1 can promote alternative macrophage polarization and induce the
expression of the pro-allergic factors, Ym-1 and Fizz-1, which play
a role in the development of allergic inflammation (29).
The regulatory mechanisms of PU.1 in macrophage
differentiation involve the prevention of polycomb repressive
complex 2 (PRC2)-mediated heterochromatin formation at
macrophage-specific genes, the activation of pre-existing and de
novo myeloid enhancers and cell cycle arrest (30-32). E2F transcription factor 1 (E2F1)
has been described to regulate genes encoding enzymes involved in
lipid anabolism to promote cell cycle progression (33). However, the E2F1 mRNA transcript
levels can be repressed by miR-233, that is induced by PU.1. In
summary, the transcription factor, PU.1, is essential for the
coordination of macrophage differentiation with cell cycle arrest
(32).
Pu.1 regulates macrophage differentiation not only
as a monomer, but also through its interaction with other
transcription factors, such as IRF4 (34) and c-Jun (35). Nevertheless, the levels of PU.1
can be repressed; miR-150-5p, miR-150-5p directly interact and
suppresses PU.1 transcript sequences. The overexpression of
miR-150-5p can suppress the PU.1 levels which affects the
polarization of macrophages (36).
5. PU.1 acts as a safeguard to ensure an
appropriate neutrophil immune response
As the master immune cells in peripheral blood,
neutrophils are the first line of defense against fungal infections
and bacterial infections (37).
Neutrophils are also important regulators of the adaptive immune
system, as they can release immune-activating or -suppressing
cytokines (38).
PU.1 is recognized to be involved in neutrophil
differentiation. However, the exact regulatory mechanisms involved
remain unknown. Some studies have found that cell cycle arrest may
play a role (39,40). PU.1 can activate
microtubule-associated protein 1S (MAP1S) and death-associated
protein kinase 2 (DAPK2) transcription by binding to their
promoter. MAP1S with a link to autophagy (a cellular recycling
pathway) is induced during neutrophil differentiation (39). DAPK2 (also known as DRP-1) is
crucial for the cell cycle arrest in neutrophil terminal
differentiation and belongs to a family of proapoptotic
Ca2+/calmodulin-regulated serine/threonine kinases and
activates programmed cell death (40,41). The promotion of nuclear
segmentation is also a mechanism through which PU.1 regulates
neutrophil differentiation. PU.1 can control the transcription of
the gene encoding lamin B receptor (LBR), an inner nuclear membrane
protein, which is required for neutrophil nuclear segmentation
(42). Furthermore, the
PU.1/Zbtb11/Tp53 pathway is a regulator of neutrophil development
(43). PU.1 is involved in
neutrophil differentiation by directly activating the transcription
of HK3 and MIR29B (44,45). In summary, PU.1 is a master
transcription factor in neutrophil differentiation. However, when
inappropriately activated, a prolonged immune response of
neutrophils can lead to collateral tissue excessive damage and
contribute to autoimmunity. Therefore, a feedback inhibitory
response is required to weaken the activity of neutrophils. As an
essential transcription factor in neutrophil differentiation, PU.1
can also suppress neutrophil activation via the regulation of the
inflammatory epigenome of neutrophils. PU.1 inhibits enhancer
accessibility via the recruitment of histone deacetylases (HDACs),
thereby impeding the AP-1 transcription factor JUNB (46) that represents a key mediator of
the inflammatory activation from entering chromatin (47).
Taken together, PU.1 acts as a safeguard to ensure
an appropriate neutrophil immune response. It controls the
simultaneous activation or inhibition in neutrophils, promoting
microbial sensing and inhibiting mobilization. PU.1 exerts an
effect not only on the expression of neutrophil differentiation
genes, but also on genes controlling infection and inflammation
(47).
6. Expression of PU.1 in all dendritic
cells
DCs are able to recognize danger signals and undergo
major alterations in gene expression to produce mediators, such as
chemokines and cytokines. DCs also have the ability to degrade
proteins sampled from the environment, thereby presenting peptide
epitopes in the context of MHCI or MHCII, thereby stimulating T
cells to initiate adaptive immunity (23). Therefore, DCs are pivotal in
binding between innate and adaptive immunity. Steady-state DC
subsets are categorized into two major lineages: pDCs and
conventional DCs (cDCs). pDCs are characterized by the production
of high levels of type I IFN; cDCs have two phenotypically and
functionally distinct subsets: cDC1s and cDC2s (48,49).
PU.1 is required for the differentiation of all DCs.
cDCs express high levels of PU.1. By contrast, pDCs
characteristically express a low level of PU.1 (50). DC differentiation is related to
the demethylation (51) and
phosphorylation of PU.1. PU.1 activity can be induced through the
phosphorylation of its transactivation domain (TAD) by PKCδ to
promote DC differentiation (52).
PU.1 is required for DC identity. For example, the
leukocyte integrin, CD11c (encoded by ITGAX) is well known as a
specific hallmark of DCs, which is expressed by all DC subsets.
PU.1 is involved in the transcription and subsequent protein
expression of CD11c by transactivating the ITGAX promoter via
direct binding to the cis-element on the gene in DCs
(53,54).
PU.1 is required for DC function, and plays a key
role in the expression of several genes that are significant for
the function of DCs, including fms-like tyrosine kinase 3 (Flt3),
CD80, CD86, OX40L, retinaldehyde dehydrogenase 2 (RALDH2) and major
histocompatibility complex class II (MHCII) (55-58). PU.1 regulates the expression of
CD80 and CD86 through binding via the upper promoter and their
promoters (55). PU.1 regulates
the expression of OX40L through specifically binding to the
proximal region of the OX40L promoter and the PU.1-binding is
constitutive, which is not affected by the TLR ligand-dependent
maturation of DCs (56). PU.1
regulates the expression of RALDH2 that plays a crucial role in the
development of regulatory T cells in mesenteric lymph nodes through
binding to the RALDH2 promoter (57). The expression of MHCII is
regulated by a cofactor termed class II transactivator (CIITA) that
has three distinct promoters, pI, pIII and pIV. In cDCs, pI
promoter mainly functions. PU.1 regulates CIITA-pI as a monomer,
but not as a heterodimer. It not only functions as a transcription
factor, but also regulates histone acetylation in the promoter
region of CIITA-pI (58). In
pDCs, the pIII promoter mainly functions. PU.1 plays a role by
binding to Tts-motifs in the pIII in pDCs (59).
However, some studies have demonstrated that PU.1
may be recognized as a bifunctional regulator of DC functions
(60,61). PU.1 mainly acts as a
transcriptional activator for the expression of DC-characteristic
gene, while PU.1 acts partly as a transcriptional suppressor for
the expression of non-characteristic gene, such as Th2 cytokines
including IL-13, IL-5 in bone marrow-derived DCs. PU.1 represses
Th2 cytokines expression by suppressing the expression of GATA3,
that is a key regulator of Th2 differentiations (60).
7. Regulatory effects of PU.1 on gene
expression in early T cells
During T lymphocytes, cells undergo at least three
major transcription factor action stages before they are fully
programmed, including the earliest 'early T cell precursors' (or
Kit-high double-negative DN1), DN2 and DN3. The first
transformation, commitment, separates the DN1 and DN2a phases from
the DN2b and DN3 phases. This is when initially, multipotent
precursors lose access to other options and become fully committed
to the fate of the T cell. One factor that accounts for the
majority of all open regulatory sites in the pre-commitment (DN1
and DN2a) cell genome is PU.1 (62). However, in the majority of pro-T
cells, PU.1 collaborators, such as IRF4, IRF8 and C/EBPα are
lacking (63,64), with the exception of SATB homeobox
1 (Satb1) and Runt-related transcription factor 1 (Runx1). Satb1
and Runx1 interact with PU.1 in early T cells (65).
PU.1 regulates gene expression in early T cells
positively and negatively via two distinct mechanisms: It not only
opens chromatin and nucleates with transcriptional co-factors, such
as Satb1 and Runx1 at its binding sites to form cooperative
complexes, but also competitively redistributes its co-factors,
often removing them from sites where they can regulate different
genes (65,66). The function of triggering
chromatin opening occurs, particularly, at a major subgroup of its
non-promoter sites, in which PU.1 can rapidly induce transposase
accessibility and then recruit histone acetyltransferases (67). In summary, PU.1 regulates pro-T
cell expression through its effects on chromatin opening and the
deployment of other factors, and a number of its negative
regulations are mediated indirectly (68).
Helper T lymphocytes, including Th1, Th2, Th9 and
Th17, play an important role in the immune system, while the role
of PU.1 in Th9 cells has been extensively studied lately. Ramming
et al found that differentiation of Th9 cells was controlled
by the unique and dynamic epigenetic modifications in the promoter
region of its main transcription factor, PU.1 (69). The inhibition of repressive
histone methylation can induce Th9-specific PU.1 expression
(69). Goswami and Kaplan
demonstrated that PU.1 promoted IL-9 expression by recruiting the
Gcn5 histone acetyltransferase to the IL9 locus and promoting the
expression of additional genes enriched in Th9 cells (70). However, PU.1 is degraded through
selective autophagy to repress Th9 cell differentiation and reduce
Th9 cell-derived IL-9 production (71).
8. PU.1 is required for B cell
differentiation
Naïve B cells develop into germinal center (GC) B
cells and plasma cells (PC) following activation by cognate antigen
in combination with signals from T helper cells and DCs (72). Therefore, the receptors through
which B cells sense and respond to these signals are of particular
importance for B cell differentiation. In addition, immunoglobulin
class-switch recombination (CSR) is also required for early B cell
differentiation into antibody-secreting plasma cells (PCs)
(73).
PU.1 as a critical transcription factor of ETS
family and regulates the expression of several components of the B
cell receptor signaling pathway and the receptors for CD40L,
Toll-like receptor (TLR) ligands and B cell-activating factor
(BAFF) (74). Moreover, PU.1 also
regulates the expression of genes that are involved in
immunoglobulin gene rearrangement (75). Thus, PU.1 is required for gene
expression during B cell differentiation.
The regulatory effects of PU.1 on cell
differentiation are dependent on its DNA-binding ability and its
synergism with other transcription factors, such as IRF4. PU.1 can
bind to IRF4 and IRF8 at composite ETS-interferon consensus element
(EICE) sites to regulate the immunoglobulin class switch,
recombination and cell differentiation (76,77). The DNA-binding ability can be
enhanced by the histone acetyltransferases, CBP and EP300, by
mediating PU.1 acetylation (78).
However, SPIC weakens the DNA-binding ability of PU.1 by binding to
regulatory elements similar to PU.1 binding in pre-B cells,
resulting in PU.1 translocation from these regions;
Bcl-2-associated transcription factor1 (BCLAF1) is recruited to
gene-regulatory elements, thus inhibiting the expression of
important B developmental genes (79). In addition to SPIC, E2A also
weakens the DNA-binding ability of PU.1 (80). In summary, PU.1 is required for B
cell differentiation.
9. PU.1 serves as a bridge between immune
cells
Immune cells are important members of the immune
system, they are independent and related to each other, and PU.1
serves as a bridge between them. The concentration of the PU.1
transcription factor affects the development of myeloid/lymphoid
lineage. A high level of PU.1 promotes the acquisition of myeloid
cell fate at the expense of B cell differentiation. Myelopoiesis
may require high levels of PU.1 to overcome the inhibition of PU.1
activity by transcription factors that promote B cell development.
Rogers et al demonstrated that E2A can antagonize PU.1
activity to direct B cell development, but high levels PU.1 can
direct myeloid differentiation through over-coming this antagonism
(80). Furthermore, PU.1 can
regulate the differentiation of T lymphocytes by promoting the
function of antigen-presenting cells (APCs), including mature DCs,
B cells and macrophages. For example, PU.1 regulates the gene
expression of the chemokine, Ccl22, in DCs and macrophages, and
Ccl22 can mediate Th2 cell development by binding to the receptor,
CCR4, in Th2 cells, thus regulating the development of atopic
dermatitis (AD) and asthma (14).
PU.1 is transcriptional activator of the CD80, CD86 and OX40L genes
in APCs, which are the co-stimulatory molecules predominantly
involved in Th2 and Tfh cell differentiation (81). Taken together, different levels of
PU.1 tend to differentially affect immune cell differentiation, and
PU.1 in APCs is required for precise T cell stimulation and
differentiation. PU.1 serves as the bridge between immune cells.
The effects of the PU.1 transcription factor on immune cell
differentiation are illustrated in Fig. 2.
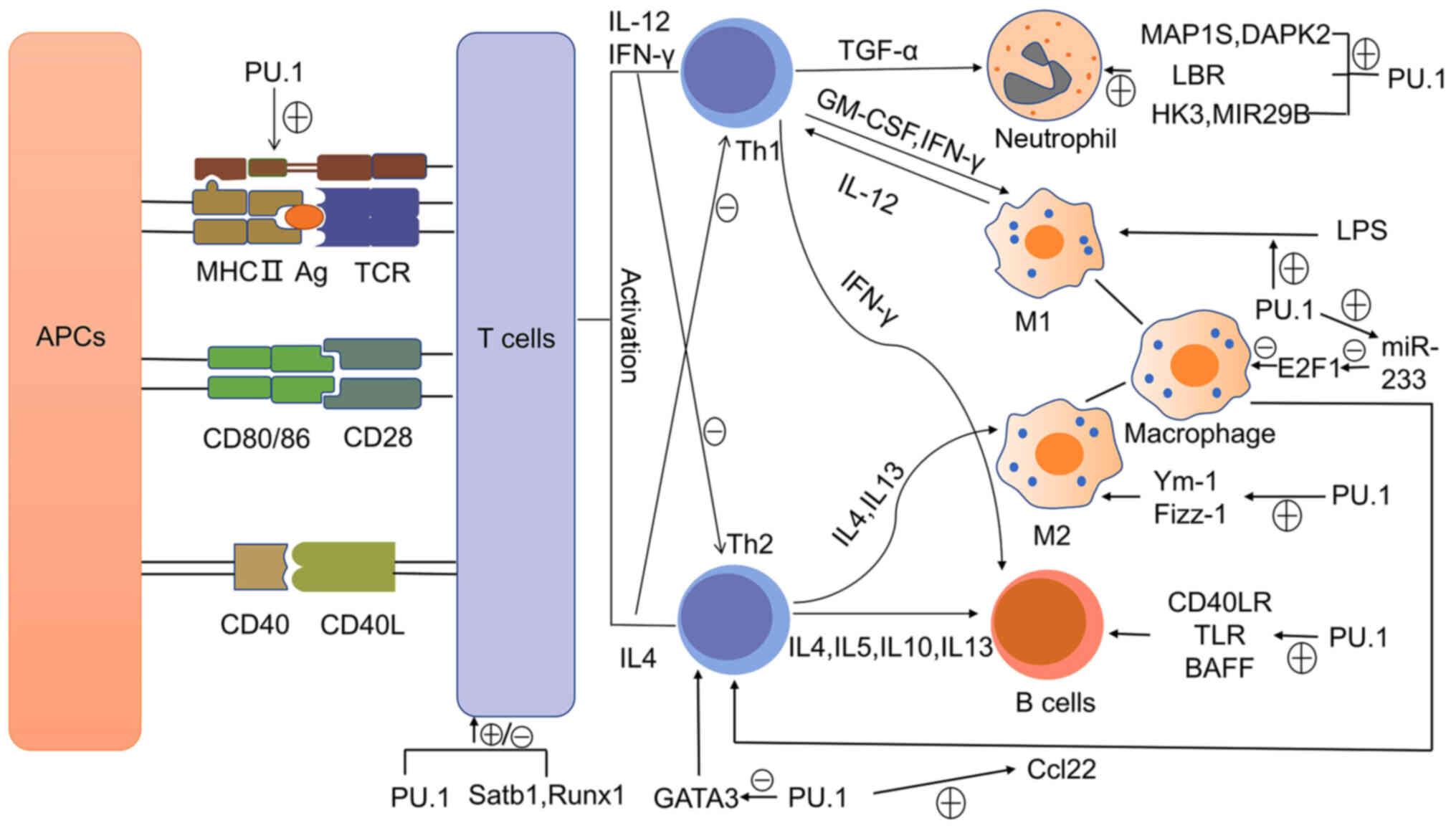 | Figure 2PU.1 regulates the differentiation of
immune cells. PU.1 activates MHCII and co-stimulating factors, such
as CD80/86 and CD40. APCs present the antigen to T cells through
MHCII and provide co-stimulating factors, so as to activate T cells
and themselves. Activated T cells induce differential immune cell
differentiation by secreting various cytokines. PU.1 regulates the
differentiation of immune cells through its involvement in signal
transduction. APCs, antigen-presenting cells; MHCII, major
histocompatibility complex class II; Satb1, SATB homeobox 1; Runx1,
Runt-related transcription factor 1; IL, interleukin; IFN,
interferon; TGF, transforming growth factor; GM-CSF,
granulocyte-macrophage colony stimulating factor; LBR, lamin B
receptor; LPS, lipopolysaccharide; E2F1, E2F transcription factor
1; Fizz-1, resistin-like molecule α (RELMα); TKR, Toll-like
receptor; BAFF, B cell-activating factor. |
10. Conclusion
Immune cells play an important role in the
occurrence and development of a serious of diseases, including
inflammatory diseases, neoplastic diseases and immune diseases. The
transcription factor, PU.1, is required in immune cell
differentiation. In view of the important position of PU.1 in the
regulation of the immune system, the elucidation of its regulatory
mechanisms in immune cells may provide new clues and may lead to
the development of novel strategies with which to reduce the damage
induced by inflammation to organs. This may thus lead to more
effective therapeutic strategies for the treatment of inflammatory
diseases and immune-mediated diseases mediated by various immune
cells.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81700670) and
Natural Science Foundation of Guangdong Province (no.
2019A1515011594).
Availability of data and materials
Not applicable.
Authors' contributions
GL, WHao and WHu contributed to the conception and
design of the present review. GL contributed to the writing and
drafting of the manuscript. WHao and WHu contributed to the
critical revision of the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karim FD, Urness LD, Thummel CS, Klemsz
MJ, McKercher SR, Celada A, Van Beveren C, Maki RA, Gunther CV, Nye
JA, et al: The ETS-domain: A new DNA-binding motif that recognizes
a purine-rich core DNA sequence. Genes Dev. 4:1451–1453. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moreau-Gachelin F, Tavitian A and
Tambourin P: Spi1 is a putative oncogene in virally induced murine
erythroleukaemias. Nature. 331:277–280. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta P, Gurudutta GU, Saluja D and
Tripathi RP: PU.1 and partners: Regulation of haematopoietic stem
cell fate in normal and malignant haematopoiesis. J Cell Mol Med.
13:4349–4363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueno N, Nishimura N, Ueno S, Endo S,
Tatetsu H, Hirata S, Hata H, Matsuoka M, Mitsuya H and Okuno Y:
PU.1 acts as tumor suppressor for myeloma cells through direct
transcriptional repression of IRF4. Oncogene. 36:4481–4497. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tenen DG, Hromas R, Licht JD and Zhang DE:
Transcription factors, normal myeloid development, and leukemia.
Blood. 90:489–519. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Batista CR, Lim M, Laramée AS,
Abu-Sardanah F, Xu LS, Hossain R, Bell GL, Hess DA and DeKoter RP:
Driver mutations in Janus kinases in a mouse model of B-cell
leukemia induced by deletion of PU.1 and Spi-B. Blood Adv.
2:2798–2810. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dozmorov MG, Wren JD and Alarcón-Riquelme
ME: Epigenomic elements enriched in the promoters of autoimmunity
susceptibility genes. Epigenetics. 9:276–285. 2014. View Article : Google Scholar :
|
|
8
|
Alivernini S, Kurowska-Stolarska M,
Tolusso B, Benvenuto R, Elmesmari A, Canestri S, Petricca L,
Mangoni A, Fedele AL, Di Mario C, et al: MicroRNA-155 influences
B-cell function through PU.1 in rheumatoid arthritis. Nat Commun.
7:129702016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turkistany SA and DeKoter RP: The
transcription factor PU.1 is a critical regulator of cellular
communication in the immune system. Arch Immunol Ther Exp (Warsz).
59:431–440. 2011. View Article : Google Scholar
|
|
10
|
Pospíšil V, Krsmanovic P, Chramostová K,
Vokurka K, Laslo P and Stopka T: Graded PU.1 levels activate
granulocyte vs macrophage genes via multiple(super) enhancer
elements. Exp Hematol. 76(Suppl): S822019. View Article : Google Scholar
|
|
11
|
Nutt SL, Metcalf D, D'Amico A, Polli M and
Wu L: Dynamic regulation of PU.1 expression in multipotent
hematopoietic progenitors. Exp Med. 201:221–231. 2005. View Article : Google Scholar
|
|
12
|
Yashiro T, Takeuchi H, Kasakura K and
Nishiyama C: PU.1 regulates Ccr7 gene expression by binding to its
promoter in naïve CD4+ T cells. FEBS Open Bio.
10:1115–1121. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anderson MK, Weiss AH, Hernandez-Hoyos G,
Dionne CJ and Rothenberg EV: Constitutive expression of PU.1 in
fetal hematopoietic progenitors blocks T cell development at the
pro-T cell stage. Immunity. 16:285–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yashiro T, Nakano S, Nomura K, Uchida Y,
Kasakura K and Nishiyama C: A transcription factor PU.1 is critical
for Ccl22 gene expression in dendritic cells and macrophages. Sci
Rep. 9:11612019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lloberas J, Soler C and Celada A: The key
role of PU.1/SPI-1 in B cells, myeloid cells and macrophages.
Immunol Today. 20:184–189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petrovick MS, Hiebert SW, Friedman AD,
Hetherington CJ, Tenen DG and Zhang DE: Multiple functional domains
of AML1: PU.1 and C/EBPalpha synergize with different regions of
AML1. Mol Cell Biol. 18:3915–3925. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marecki S and Fenton MJ: PU.1/interferon
regulatory factor interactions: Mechanisms of transcriptional
regulation. Cell Biochem Biophys. 33:127–148. 2000. View Article : Google Scholar
|
|
18
|
Behre G, Whitmarsh AJ, Coghlan MP, Hoang
T, Carpenter CL, Zhang DE, Davis RJ and Tenen DG: c-Jun is a
JNK-independent coactivator of the PU.1 transcription factor. J
Biol Chem. 274:4939–4946. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang P, Zhang X, Iwama A, Yu C, Smith KA,
Mueller BU, Narravula S, Torbett BE, Orkin SH and Tenen DG: PU.1
inhibits GATA-1 function and erythroid differentiation by blocking
GATA-1 DNA binding. Blood. 96:2641–2648. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rekhtman N, Choe KS, Matushansky I, Murray
S, Stopka T and Skoultchi AI: PU.1 and pRB interact and cooperate
to repress GATA-1 and block erythroid differentiation. Mol Cell
Biol. 23:7460–7474. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stopka T, Amanatullah DF, Papetti M and
Skoultchi AI: PU.1 inhibits the erythroid program by binding to
GATA-1 on DNA and creating a repressive chromatin structure. EMBO
J. 24:3712–3723. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burda P, Laslo P and Stopka T: The role of
PU.1 and GATA-1 transcription factors during normal and
leukemogenic hematopoiesis. Leukemia. 24:1249–1257. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Neill LA and Pearce EJ: Immunometabolism
governs dendritic cell and macrophage function. J Exp Med.
213:15–23. 2016. View Article : Google Scholar :
|
|
24
|
Tarique AA, Logan J, Thomas E, Holt PG,
Sly PD and Fantino E: Phenotypic, functional, and plasticity
features of classical and alternatively activated human
macrophages. Am J Respir Cell Mol Biol. 53:676–688. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nair MG, Gallagher IJ, Taylor MD, Loke P,
Coulson PS, Wilson RA, Maizels RM and Allen JE: Chitinase and Fizz
family members are a generalized feature of nematode infection with
selective upregulation of Ym1 and Fizz1 by antigen-presenting
cells. Infect Immun. 73:385–394. 2005. View Article : Google Scholar :
|
|
26
|
Wang N, Liang H and Zen K: Molecular
mechanisms that influence the macrophage m1-m2 polarization
balance. Front Immunol. 5:6142014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Juhas U, Ryba-Stanisławowska M, Szargiej P
and Myśliwska J: Different pathways of macrophage activation and
polarization. Postepy Hig Med Dosw (Online). 69:496–502. 2015.
View Article : Google Scholar
|
|
28
|
Karpurapu M, Wang X, Deng J, Park H, Xiao
L, Sadikot RT, Frey RS, Maus UA, Park GY, Scott EW and Christman
JW: Functional PU.1 in macrophages has a pivotal role in NF-κB
activation and neutrophilic lung inflammation during endotoxemia.
Blood. 118:5255–5266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian F, Deng J, Lee YG, Zhu J, Karpurapu
M, Chung S, Zheng JN, Xiao L, Park GY and Christman JW: The
transcription factor PU.1 promotes alternative macrophage
polarization and asthmatic airway inflammation. J Mol Cell Biol.
7:557–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tagore M, McAndrew MJ, Gjidoda A and Floer
M: The lineage-specific transcription factor PU.1 prevents
polycomb-mediated heterochromatin formation at macrophage-specific
genes. Mol Cell Biol. 35:2610–2625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Oevelen C, Collombet S, Vicent G,
Hoogenkamp M, Lepoivre C, Badeaux A, Bussmann L, Sardina JL,
Thieffry D, Beato M, et al: C/EBPα activates pre-existing and de
novo macrophage enhancers during induced Pre-B cell
transdifferentiation and myelopoiesis. Stem Cell Reports.
5:232–247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Solomon LA, Podder S, He J,
Jackson-Chornenki NL, Gibson K, Ziliotto RG, Rhee J and DeKoter RP:
Coordination of myeloid differentiation with reduced cell cycle
progression by PU.1 induction of MicroRNAs targeting cell cycle
regulators and lipid anabolism. Mol Cell Biol. 37:e00013–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Denechaud PD, Lopez-Mejia IC, Giralt A,
Lai Q, Blanchet E, Delacuisine B, Nicolay BN, Dyson NJ, Bonner C,
Pattou F, et al: E2F1 mediates sustained lipogenesis and
contributes to hepatic steatosis. J Clin Invest. 126:137–150. 2016.
View Article : Google Scholar :
|
|
34
|
Eguchi J, Kong X, Tenta M, Wang X, Kang S
and Rosen ED: Interferon regulatory factor 4 regulates
obesity-induced inflammation through regulation of adipose tissue
macrophage polarization. Diabetes. 62:3394–3403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen C, Chen MT, Zhang XH, Yin XL, Ning
HM, Su R, Lin HS, Song L, Wang F, Ma YN, et al: The PU.1-modulated
MicroRNA-22 is a regulator of monocyte/macrophage differentiation
and acute myeloid leukemia. PLoS Genet. 12:e10062592016. View Article : Google Scholar :
|
|
36
|
Shakerian L, Ghorbani S, Talebi F and
Noorbakhsh F: MicroRNA-150 targets PU.1 and regulates macrophage
differentiation and function in experimental autoimmune
encephalomyelitis. J Neuroimmunol. 323:167–174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kruger P, Saffarzadeh M, Weber AN, Rieber
N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J and Hartl
D: Neutrophils: Between host defence, immune modulation, and tissue
injury. PLoS Pathog. 11:e10046512015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mantovani A, Cassatella MA, Costantini C
and Jaillon S: Neutrophils in the activation and regulation of
innate and adaptive immunity. Nat Rev Immunol. 11:519–531. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haimovici A, Brigger D, Torbett BE, Fey MF
and Tschan MP: Induction of the autophagy-associated gene MAP1S via
PU.1 supports APL differentiation. Leuk Res. 38:1041–1047. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Humbert M, Federzoni EA, Britschgi A,
Schläfli AM, Valk PJ, Kaufmann T, Haferlach T, Behre G, Simon HU,
Torbett BE, et al: The tumor suppressor gene DAPK2 is induced by
the myeloid transcription factors PU.1 and C/EBPα during
granulocytic differentiation but repressed by PML-RARα in APL. J
Leukoc Biol. 95:83–93. 2014. View Article : Google Scholar :
|
|
41
|
Bialik S and Kimchi A: The
death-associated protein kinases: Structure, function, and beyond.
Annu Rev Biochem. 75:189–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Malu K, Garhwal R, Pelletier MG, Gotur D,
Halene S, Zwerger M, Yang ZF, Rosmarin AG and Gaines P: Cooperative
activity of GABP with PU.1 or C/EBPε regulates lamin B receptor
gene expression, implicating their roles in granulocyte nuclear
maturation. J Immunol. 197:910–922. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Keightley MC, Carradice DP, Layton JE,
Pase L, Bertrand JY, Wittig JG, Dakic A, Badrock AP, Cole NJ,
Traver D, et al: The Pu.1 target gene Zbtb11 regulates neutrophil
development through its integrase-like HHCC zinc finger. Nat
Commun. 8:149112017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Federzoni EA, Valk PJ, Torbett BE,
Haferlach T, Löwenberg B, Fey MF and Tschan MP: PU.1 is linking the
glycolytic enzyme HK3 in neutrophil differentiation and survival of
APL cells. Blood. 119:4963–4970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Batliner J, Buehrer E, Federzoni EA, Jenal
M, Tobler A, Torbett BE, Fey MF and Tschan MP: Transcriptional
regulation of MIR29B by PU.1 (SPI1) and MYC during neutrophil
differentiation of acute promyelocytic leukaemia cells. Br J
Haematol. 157:270–274. 2012. View Article : Google Scholar
|
|
46
|
Fontana MF, Baccarella A, Pancholi N,
Pufall MA, Herbert DR and Kim CC: JUNB is a key transcriptional
modulator of macrophage activation. J Immunol. 194:177–186. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fischer J, Walter C, Tönges A, Aleth H,
Jordão MJC, Leddin M, Gröning V, Erdmann T, Lenz G, Roth J, et al:
Safeguard function of PU.1 shapes the inflammatory epigenome of
neutrophils. Nat Immunol. 20:546–558. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guilliams M, Ginhoux F, Jakubzick C, Naik
SH, Onai N, Schraml BU, Segura E, Tussiwand R and Yona S: Dendritic
cells, monocytes and macrophages: A unified nomenclature based on
ontogeny. Nat Rev Immunol. 14:571–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Belz GT and Nutt SL: Transcriptional
programming of the dendritic cell network. Nat Rev Immunol.
12:101–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Carotta S, Dakic A, D'Amico A, Pang SH,
Greig KT, Nutt SL and Wu L: The transcription factor PU.1 controls
dendritic cell development and Flt3 cytokine receptor expression in
a dose-dependent manner. Immunity. 32:628–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lapko N, Zawadka M, Polosak J, Worthen GS,
Danet-Desnoyers G, Puzianowska-Kuźnicka M and Laudanski K:
Long-term monocyte dysfunction after sepsis in humanized mice is
related to persisted activation of macrophage-colony stimulation
factor (M-CSF) and demethylation of PU.1, and it can be reversed by
blocking M-CSF in vitro or by transplanting naïve autologous stem
cells in vivo. Front Immunol. 8:4012017. View Article : Google Scholar
|
|
52
|
Hamdorf M, Berger A, Schüle S, Reinhardt J
and Flory E: PKCδ-induced PU.1 phosphorylation promotes
hematopoietic stem cell differentiation to dendritic cells. Stem
Cells. 29:297–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yashiro T, Kasakura K, Oda Y, Kitamura N,
Inoue A, Nakamura S, Yokoyama H, Fukuyama K, Hara M, Ogawa H, et
al: The hematopoietic cell-specific transcription factor PU.1 is
critical for expression of CD11c. Int Immunol. 29:87–94. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhu XJ, Yang ZF, Chen Y, Wang J and
Rosmarin AG: PU.1 is essential for CD11c expression in
CD8(+)/CD8(-) lymphoid and monocyte-derived dendritic cells during
GM-CSF or FLT3L-induced differentiation. PLoS One. 7:e521412017.
View Article : Google Scholar
|
|
55
|
Kanada S, Nishiyama C, Nakano N, Suzuki R,
Maeda K, Hara M, Kitamura N, Ogawa H and Okumura K: Critical role
of transcription factor PU.1 in the expression of CD80 and CD86 on
dendritic cells. Blood. 117:2211–2222. 2011. View Article : Google Scholar
|
|
56
|
Yashiro T, Hara M, Ogawa H, Okumura K and
Nishiyama C: Critical role of transcription factor PU.1 in the
function of the OX40L/TNFSF4 promoter in dendritic cells. Sci Rep.
6:348252016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yashiro T, Yamaguchi M, Watanuki Y,
Kasakura K and Nishiyama C: The transcription factors PU.1 and IRF4
determine dendritic cell-specific expression of RALDH2. J Immunol.
201:3677–3682. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kitamura N, Yokoyama H, Yashiro T, Nakano
N, Nishiyama M, Kanada S, Fukai T, Hara M, Ikeda S, Ogawa H, et al:
Role of PU.1 in MHC class II expression through transcriptional
regulation of class II transactivator pI in dendritic cells. J
Allergy Clin Immunol. 129:814–824.e6. 2012. View Article : Google Scholar
|
|
59
|
Miura R, Kasakura K, Nakano N, Hara M,
Maeda K, Okumura K, Ogawa H, Yashiro T and Nishiyama C: Role of
PU.1 in MHC class II expression via CIITA transcription in
plasmacytoid dendritic cells. PLoS One. 11:e01540942016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yashiro T, Kubo M, Ogawa H, Okumura K and
Nishiyama C: PU.1 suppresses Th2 cytokine expression via silencing
of GATA3 transcription in dendritic cells. PLoS One.
10:e01376992015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nakano N, Nishiyama C, Kanada S, Niwa Y,
Shimokawa N, Ushio H, Nishiyama M, Okumura K and Ogawa H:
Involvement of mast cells in IL-12/23 p40 production is essential
for survival from polymicrobial infections. Blood. 109:4846–4855.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yui MA and Rothenberg EV: Developmental
gene networks: A triathlon on the course to T cell identity. Nat
Rev Immunol. 14:529–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Heinz S, Romanoski CE, Benner C, Allison
KA, Kaikkonen MU, Orozco LD and Glass CK: Effect of natural genetic
variation on enhancer selection and function. Nature. 503:487–492.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Natoli G, Ghisletti S and Barozzi I: The
genomic landscapes of inflammation. Genes Dev. 25:101–106. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hosokawa H, Ungerbäck J, Wang X, Matsumoto
M, Nakayama KI, Cohen SM, Tanaka T and Rothenberg EV: Transcription
factor PU.1 represses and activates gene expression in early T
cells by redirecting partner transcription factor binding.
Immunity. 49:7822018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rothenberg EV, Hosokawa H and Ungerbäck J:
Mechanisms of action of hematopoietic transcription factor PU.1 in
initiation of T-cell development. Front Immunol. 10:2282019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ungerbäck J, Hosokawa H, Wang X, Strid T,
Williams BA, Sigvardsson M and Rothenberg EV: Pioneering, chromatin
remodeling, and epigenetic constraint in early T-cell gene
regulation by SPI1 (PU.1). Genome Res. 28:1508–1519. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Champhekar A, Damle SS, Freedman G,
Carotta S, Nutt SL and Rothenberg EV: Regulation of early T-lineage
gene expression and developmental progression by the progenitor
cell transcription factor PU.1. Genes Dev. 29:832–848. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ramming A, Druzd D, Leipe J, Schulze-Koops
H and Skapenko A: Maturation-related histone modifications in the
PU.1 promoter regulate Th9-cell development. Blood. 119:4665–4674.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Goswami R and Kaplan MH: Gcn5 is required
for PU.1-dependent IL-9 induction in Th9 cells. J Immunol.
189:3026–3033. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rivera Vargas T, Cai Z, Shen Y, Dosset M,
Benoit-Lizon I, Martin T, Roussey A, Flavell RA, Ghiringhelli F and
Apetoh L: Selective degradation of PU.1 during autophagy represses
the differentiation and antitumour activity of TH9 cells. Nat
Commun. 8:5592017. View Article : Google Scholar :
|
|
72
|
Goodnow CC, Vinuesa CG, Randall KL, Mackay
F and Brink R: Control systems and decision making for antibody
production. Nat Immunol. 11:681–688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Carotta S, Willis SN, Hasbold J, Inouye M,
Pang SH, Emslie D, Light A, Chopin M, Shi W, Wang H, et al: The
transcription factors IRF8 and PU.1 negatively regulate plasma cell
differen-tiation. J Exp Med. 211:2169–2181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Willis SN, Tellier J, Liao Y, Trezise S,
Light A, O'Donnell K, Garrett- Sinha LA, Shi W, Tarlinton DM and
Nutt SL: Environmental sensing by mature B cells is controlled by
the transcription factors PU.1 and SpiB. Nat Commun. 8:14262017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Batista CR, Li SK, Xu LS, Solomon LA and
DeKoter RP: PU.1 regulates Ig light chain transcription and
rearrangement in Pre-B cells during B cell development. J Immunol.
198:1565–1574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ochiai K, Maienschein-Cline M, Simonetti
G, Chen J, Rosenthal R, Brink R, Chong AS, Klein U, Dinner AR,
Singh H and Sciammas R: Transcriptional regulation of germinal
center B and plasma cell fates by dynamical control of IRF4.
Immunity. 38:918–929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pang SH, Minnich M, Gangatirkar P, Zheng
Z, Ebert A, Song G, Dickins RA, Corcoran LM, Mullighan CG,
Busslinger M, et al: PU.1 cooperates with IRF4 and IRF8 to suppress
pre-B-cell leukemia. Leukemia. 30:1375–1387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Scialdone A, Khazaei S, Hasni MS,
Lennartsson A, Gullberg U and Drott K: Depletion of the
transcriptional coactivators CREB-binding protein or EP300
downregulates CD20 in diffuse large B-cell lymphoma cells and
impairs the cytotoxic effects of anti-CD20 antibodies. Exp Hematol.
79:35–46.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Soodgupta D, White LS, Yang W, Johnston R,
Andrews JM, Kohyama M, Murphy KM, Mosammaparast N, Payton JE and
Bednarski JJ: RAG-mediated DNA breaks attenuate PU.1 activity in
early B cells through activation of a SPIC-BCLAF1 complex. Cell
Rep. 29:829–843.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Rogers JH, Owens KS, Kurkewich J,
Klopfenstein N, Iyer SR, Simon MC and Dahl R: E2A antagonizes PU.1
activity through inhibition of DNA binding. Biomed Res Int.
2016:39836862016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Laslo P, Spooner CJ, Warmflash A, Lanck
DW, Lee HJ, Sciammas R, Gantner BN, Dinner AR and Singh H:
Multilineage transcriptional priming and determination of alternate
hematopoietic cell fates. Cell. 126:755–766. 2006. View Article : Google Scholar : PubMed/NCBI
|