Introduction
Bronchopulmonary dysplasia (BPD), a severe lung
dysfunction, is common in premature infants born at a gestational
age of <29 weeks, when lungs are in the cystic development stage
(1). The incidence of BPD can be
as high as 40%, particularly in infants that weigh <1,500 g
(2). Moreover, BPD affects the
survival and quality of life of premature infants (3). The majority of these infants require
long-term oxygen therapy to maintain lung function (4). Alveoli are gas-exchange sacs lined
by cuboidal shaped squamous alveolar epithelial type I cells
(AECIs) and alveolar epithelial type II cells (AECIIs), which
secrete active substances on their surface (5). AECIIs are widely referred to as
'alveolar stem cells' and contribute toward lung repair and
regeneration (5). Indeed, studies
have demonstrated that the proliferation and differentiation of
AECIIs into AECIs plays a key role in the repair of damaged alveoli
(6,7). Since arrested alveolar development
is the main pathological feature of neonatal BPD, a number of
studies have investigated alveolar epithelial cell damage and
repair to improve alveolarization (8-10)
and enhance the understanding of the mechanisms of alveolar
epithelial cell regeneration in order to develop treatment methods
for BPD.
Yes-associated protein (YAP) is a key downstream
effector of the Hippo pathway (11,12), and recent studies have
demon-strated that YAP exerts regulatory effects on a number of
stem cells (13-15). However, the role of YAP in the
proliferation and differentiation of AECIIs into AECIs remains
controversial (16-19). For instance, Sun et al
reported that although YAP is not involved in these processes, TAZ
plays a critical role in AECIIs to AECIs differentiation and
maintaining alveolar integrity following injury (20); however, Seo et al found
that YAP/TAZ contributed towards alveolar epithelial regeneration
(21). Thus, the regulatory role
of YAP in the proliferation and differentiation of AECIIs is likely
complex and requires further investigation.
Previously, the authors reported that Wnt signaling
plays key roles in alveolar injury and repair by regulating the
proliferation and differentiation of AECIIs (22,23). The Wnt signaling pathway consists
of both canonical and noncanonical pathways (24) involving Wnt proteins, receptors
and regulatory factors that control numerous biological processes,
including cell proliferation, development and differentiation
(25,26). Wnt3a signals via the canonical
pathway and is expressed in bronchial and alveolar epithelial
cells; however, it exhibits an increased expression in fibrotic
regions and the hypertrophic alveolar epithelium (27). Several studies have reported
conflicting associations between YAP and the Wnt/β-catenin
signaling pathway (19-21,28,29). For instance, Imajo et al
reported that the Hippo pathway effectors, YAP and TAZ, bound to
β-catenin and retained it in the cytoplasm to suppress
Wnt/β-catenin signaling from 293T cells (29), whereas Jia et al found that
YAP promoted the osteogenic differentiation of human periodontal
ligament stem cells in vitro and inhibited adipogenic
differentiation via low-density lipoprotein receptor-related
protein 6 (LRP6) and dishevelled segment polarity protein 3
(DVL3)-mediated Wnt/β-catenin signaling (28). Therefore, it remains unclear
whether YAP affects the proliferation and differentiation of AECIIs
via the Wnt/β-catenin pathway in BPD.
The present study thus aimed to determine the role
of YAP in the proliferation and differentiation of AECIIs and the
involvement of the Wnt/β-catenin pathway in this process in BPD. It
was demonstrated that YAP expression was decreased in experimental
BPD and played a key role in promoting the proliferation and
differentiation of AECIIs, consistent with a previous study by the
authors on Wnt3a (23). In
addition, it was found that Wnt3a overexpression could compensate
for YAP depletion and vice versa; however, neither altered the
expression of the other. Moreover, neither YAP nor Wnt3a
overexpression increased total β-catenin expression in AECIIs in
vitro, but both increased nuclear β-catenin levels in these
cells. Thus, a functional association was identified between YAP
and Wnt/β-catenin signaling. This indicates that Wnt3a and YAP
cooperatively regulate the proliferation and differentiation of
AECIIs in experimental BPD.
Materials and methods
Animal models
All animal experiments were examined and approved by
the Experimental Animal Ethics Committee of China Medical
University (2017PS086K). The BPD model in the present study has
been previously described (22,30). In brief, 20 adult Sprague-Dawley
(SD) rats were purchased from Liaoning Changsheng Biotechnology,
and females and males were mated at a ratio of 4:1. Feeding was
carried out in a temperature-controlled room during a 12-h
light/dark cycle, with a temperature of 23±2°C and a relative
humidity of 40-60%. Each pregnant rat was fed independently until
natural delivery on days 21-23. Within 12 h of delivery, the
neonatal and maternal rats were randomly divided into the BPD model
or control groups that were exposed to 80-85% oxygen in a sealed
plexiglas tank for 1-21 days or room air (21% oxygen),
respectively. The number of rats in each animal model preparation
was assigned as follows: In total, 3 cages (1 maternal and 12-13
newborn rats in each cage) were placed in an oxygen chamber as the
BPD model group, and another 3 cages (1 maternal and 12-13 newborn
rats in each cage) were placed in the room with normal air as the
control group. The oxygen concentration was monitored continuously
using an oxygen meter. The box was opened regularly for 30 min each
day and maternal rats were switched between the model and control
groups every 24 h to prevent oxygen toxicity.
Lung tissue specimens and lung
histology
On postnatal days 1, 3, 7, 14 and 21, 10 neonatal
rats from each group were randomly selected and all rats were
anesthetized by an intraperitoneal injection of 1% pentobarbital
sodium (35 mg/kg). Under aseptic conditions, the left auricle was
reduced, the right ventricle was per-fused with PBS and lung
tissues were collected. The lower lobe of the right lung was fixed
in 4% paraformaldehyde (PFA) and 3-µm-thick sections were
prepared for hematoxylin and eosin (H&E), immunohistochemical
(IHC) and immunofluorescence (IF) staining. The remaining lung
lobes were preserved at −80°C for use in subsequent analyses. Lung
tissue sections (3-µm-thick) were deparaffinized in xylene
for 20 min and rehydrated in 100, 95, 85 and 70% alcohol for 10 min
before H&E staining was performed. Briefly, specimens were
stained with hematoxylin for 2 min at 37°C, rinsed, and blued for
nuclear staining under running water for 15 min. Sections were then
stained with eosin for 1 min at 37°C, dehydrated using 70, 95 and
100% alcohol, made transparent using xylene, and embedded in
neutral resin. Under a microscope (Nikon Corporation), by drawing a
perpendicular line from the center of the most peripheral
bronchiole to the pleura or the nearest interlobular septum, the
number of alveoli transected by this line was defined as the radial
alveolar count (RAC), an important index used to evaluate the stage
of lung development (31). Image
J software version 1.51 was used to measure alveolar wall
thickness.
Isolation of AECIIs
AECIIs from the neonatal rats were isolated,
purified and cultured as previously described (22). Following cardiopulmonary lavage,
the cut lung tissue was digested in 0.25% trypsin-EDTA (25200056,
Gibco; Thermo Fisher Scientific, Inc.) for 30 min and then
digestion was terminated using the same volume of DMEM/F12 medium
(SH30023.01B, Gibco; Thermo Fisher Scientific, Inc.) containing 10%
fetal bovine serum (10099141, Gibco; Thermo Fisher Scientific,
Inc.). The disassociated cells were filtered through a 200-mesh
cell strainer, centrifuged (4°C, 5 min and 200 × g) and the cells
were then digested using 0.1% type I collagenase (MB2686,
Meilunbio) for 20 min. The cells were harvested by centrifugation
(4°C, 5 min and 200 × g), resuspended in DMEM/F12 medium containing
1% penicillin streptomycin double antibody (Gibco; Thermo Fisher
Scientific, Inc.) and 10% fetal bovine serum, and incubated in a 5%
CO2 incubator at 37°C. Fibroblasts were removed using
the differential attachment procedure (repeated 3 times, 1 h each)
and purified. Following 24 h of culture, the activity (>95%) and
purity (>90%) of AECIIs were measured using trypan blue staining
for 3 min at 37°C (C0011-1, Beyotime Institute of Biotechnology,
Inc.) and AECII-specific labeling of surfactant protein C (SPC)
immunostaining, respectively.
IHC staining
Prior to IHC staining, the sections were
deparaffinized, microwave-heated (98-100°C), and treated
sequentially with 3% H2O2 for 20 min and goat
serum for 1 h at 37°C. The sections were then incubated with the
following primary antibodies diluted in PBS at 4°C overnight:
Anti-YAP (#14074, 1:400, Cell Signaling Technology, Inc.) and
anti-β-catenin (WL0962a, 1:100, Wanleibio, Co., Ltd.). The sections
were then incubated with secondary antibodies (goat
anti-rabbit/mouse, kit9710, MXB Biotechnologies) at 37°C and
streptavidin-horseradish peroxidase for 20 min at 37°C, developed
with 3,3′-diaminobenzidine, and counterstained with hematoxylin for
2 min at 37°C. Finally, the sections were observed under a light
microscope (Nikon Corporation), where 6 fields of view were
randomly selected for each sample.
IF staining
Prior to IF staining, the cells were fixed with 4%
PFA for 15 min. After blocking with 10% goat serum (SL038, Beijing
Solarbio Science & Technology Co., Ltd.) at 37°C for 15 min and
incubated with 4 combinations of 2 primary antibodies overnight at
4°C as follows: i) Rabbit polyclonal anti-SPC (PA5-71680, 1:40,
Thermo Fisher Scientific, Inc.) and mouse monoclonal anti-YAP
(66900-1-lg, 1:50, Proteintech Group, Inc.); ii) Rabbit polyclonal
anti-SPC and mouse monoclonal anti-β-catenin (610154, 1:100, BD
Biosciences); iii) Rabbit polyclonal anti-aquaporin 5 (AQP5;
ab78486, 1:200, Abcam) and mouse monoclonal anti-YAP; iv) Rabbit
polyclonal anti-AQP5 (ab78486, 1:200, Abcam) and mouse monoclonal
anti-β-catenin. The sections were then incubated with goat
anti-rabbit IgG (SA00007-2, 1:200, Proteintech Group, Inc.) and
goat anti-mouse IgG (SA00003-1, 1:200, Proteintech Group, Inc.)
secondary antibodies coupled to fluorescein isothiocyanate in the
dark for 4 h at 37°C, followed by 4′,6-diamidino-2-phenylindole
(DAPI, D820010, Beijing Solarbio Science & Technology Co.,
Ltd.) to nuclear staining for 5 min at 37°C. Double IF images were
acquired using a confocal laser-scanning microscope (Nikon
Corporation) and 6 fields of view were randomly selected for each
sample.
Cell transfection
Primary cells obtained from BPD and control rats
were inoculated in 6-well plates (a total of 200,000 cells were
seeded into each well of the 6-well) and cultured to a cell density
of approximately 60-70%. While the cells in the control group were
left untreated, the cells in the BPD group were transfected with
either small interfering RNA (siRNA)-negative control (NC,
GenePharma), siRNA-YAP (GenePharma), siRNA-Wnt3a (GenePharma),
overexpression plasmid YAP (OEYAP, Genechem), overexpression
plasmid Wnt3a (OEWnt3a, Genechem), or overexpression plasmid NC
(OENC, Genechem). The siRNA sequences were as follows: siYAP sense,
5′-GAU UGA AAC AGC AGG AGU UTT-3′ and antisense, 5′-AAC UCC UGC UGU
UUC AAU CTT-3′; siWnt3a sense, 5′-GGU GGU CGC AGC CUG ACU UTT-3′
and antisense, 5′-AAG UCA GGC UGC GAC CAC CTT-3′; NC sense, 5′-UUC
UCC GAA CGU GUC ACG UTT-3′ and antisense, 5′-ACG UGA CAC GUU CGG
AGA ATT-3′. At transfection in the 6-well plate, the amount of
siRNA and plasmid was 7.5 and 2 µg respectively, and the
amount in the control group was the same. Transfection was
performed using Lipofectamine 3000 (L3000015, Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions and
was confirmed by PCR and western blot analysis. After 48-72 h,
cells were collected for further analysis.
Western blot analysis
Total proteins from the lung tissues and AECIIs were
lysed using radio immunoprecipitation assay (RIPA) buffer (P0013B,
Beyotime Institute of Biotechnology, Inc.) with 1%
phenylmethanesulfonyl fluoride (PMSF; ST506, Beyotime Institute of
Biotechnology, Inc.). Nuclear and cytoplasmic proteins were
extracted using a Nuclear and Cytoplasmic Protein Extraction kit
(20126ES50, Beyotime Institute of Biotechnology, Inc.) according to
the manufacturer's instructions. The protein concentration was
determined by the bicinchoninic acid (BCA; p0010, Beyotime
Institute of Biotechnology, Inc.) method. An equal amount of
protein (40 µg) of each sample was used for western
blot-ting. Proteins were then separated by 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene fluoride (PVDF) membranes. After
blocking with 5% non-fat milk for 1 h at 37°C, the membranes were
incubated with the following primary anti-bodies diluted in PBS
overnight at 4°C: Anti-YAP (66900-1-lg, 1:500, Proteintech Group,
Inc., 65 kDa), anti-Wnt3a (DF6113, 1:1,000, Affinity, 39 kDa),
anti-SPC (PA5-71680, 1:2,000, Thermo Fisher Scientific, Inc., 21
kDa), anti-AQP5 (ab78486, 1:5,000, Abcam, 24 kDa), anti-β-catenin
(610154, 1:2,000, BD Biosciences, 92 kDa), anti-GAPDH (10494-1-AP,
1:5,000, Proteintech Group, Inc., 36 kDa) and anti-LaminA (1:1,000,
ab26300, Abcam, 74 kDa). Following incubation with goat anti-mouse
IgG secondary antibodies (SA00001-1, 1:5,000, Proteintech Group,
Inc.) or goat anti-rabbit IgG secondary antibodies (SA00001-2,
1:5,000, Proteintech Group, Inc.) at 37°C for 2 h, signals were
detected by enhanced chemiluminescence (34577, Thermo Fisher
Scientific, Inc.). The gray values of the protein bands were
analyzed using Image J software version 1.51.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the lung tissues or
AECIIs using TRIzol reagent (9108, Takara Bio, Inc.) and the
samples were all treated with RNA DNase before the reverse
transcription process according to the official instruction, and 1
µg per sample was then reverse transcribed into cDNA using
the PrimeScriptRT reagent kit with gDNA Eraser (RR047A,
Takara Bio, Inc.). Each targeted cDNA (2 µl) was amplified
using the TB Green PCR Core kit (RR820A, Takara Bio, Inc.) via the
ABI 7500 system, and the following primers: SPC forward, 5′-GTG GTT
GTG GTG GTA GTC CTT GTC-3′ and reverse, 5′-CGA TGC CAG TGG AGC CAA
TAG AG-3′; AQP5 forward, 5′-CAA CAA CAC AAC GCC TGG CAA G-3′ and
reverse, 5′-AGA GTC GGT GGA GGA GAA GAT GC-3′; YAP forward, 5′-GCC
ATG AAC CAG AGG ATC ACT CAG-3′ and reverse, 5′-AGC CTC TCC TTC TCC
ATC TGT AGC-3′; β-catenin forward, 5′-GTT GCT CCA CTC CAG GAA TGA
AGG-3′ and reverse, 5′-GCA CCA ATG TCC AGT CCG AGA TC-3′; Wnt3a
forward, 5′-TGG TGG TGG TGG TGG CAG AG-3′ and reverse, 5′-CAC AGC
CAA GGA CCA GAG AAG AAC-3′; GAPDH forward, 5′-GAC ATG CCG CCT GGA
GAA AC-3′ and reverse, 5′-AGC CCA GGA TGC CCT TTA GT-3′. The
following cycling conditions were used: 95°C for 30 sec; 40 cycles
of 95°C for 5 sec and 60°C for 34 sec; 95°C for 15 sec; 60°C for 1
min; and 95°C for 15 sec. To determine relative gene expression,
target mRNA expression was calculated relative to GAPDH using the
2−ΔΔCq method (32).
Statistical analysis
Graphpad prism 5 was used for statistical analysis.
All experiments involved at least 6 rats and were performed in
triplicate. Results were expressed as the means ± standard
deviation (SD). All raw data were analyzed by one-way or two-way
analysis of variance (ANOVA) followed by the Bonferroni test for
post hoc comparisons. P-values of <0.05 were considered to
indicate statistically significant differences.
Results
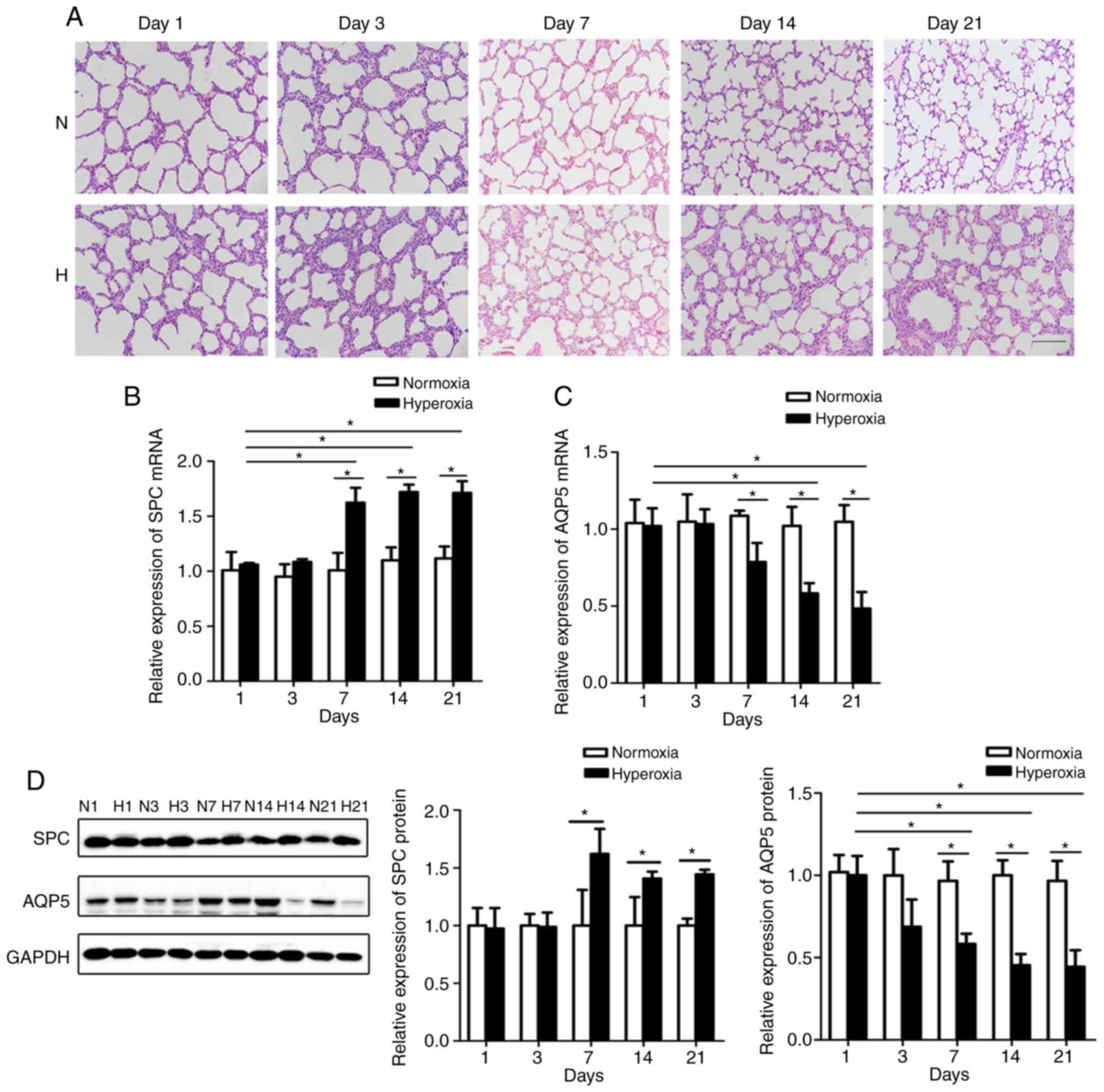
Experimental BPD is established by the
induction of hyperoxia in neonatal rats
The results of H&E staining revealed that the
alveolar space was thick and there were fewer alveoli at 1-3 days
following incubation with 21% oxygen in the control group rats;
however, the alveolar space was thinner after 7 days and after
14-21 days, the alveolar space became uniform, the pulmonary space
was thinner, a spinous process appeared and the number of alveoli
increased. Conversely, the hyperoxia-exposed neonatal rats
displayed enlarged alveoli from 7 days and after 14-21 days, the
number of alveoli significantly decreased, the alveolar septum
thickened and the alveolar structure was simplified. In the control
group, the thickness of the alveolar septum became thinner, while
in the model group the alveolar septum became thicker, which was
clearly different on days 7, 14 and 21 (Fig. 1A). The RAC was determined to
evaluate the stage of lung development (Fig. S1A); the model group exhibited a
significantly lower number of alveoli than the control group,
beginning from 7 days following exposure to hyperoxia (P<0.05.
In addition, the thickness of the alveolar wall was measured by
morphometry (Fig. S1B); the
alveolar septa significantly increased at 7 days following exposure
to hyperoxia in the model group (P<0.05).
Differentiation of AECIIs into AECIs is
inhibited in rats with BPD
To investigate the differentiation of AECIIs into
AECIs in the alveoli of rats with BPD, SPC (AECIIs marker) and AQP5
(AECIs marker) expression levels were detected by RT-qPCR and
western blot analysis in the lung tissues of neonatal rats after 1,
3, 7, 14 and 21 days of exposure to hyperoxia or normal air. The
upregulation of SPC mRNA expression was first detected on day 7 in
the hyperoxic lung tissues compared to the normoxic group (Fig. 1B), followed by an increased
expression over the subsequent days. In addition, AQP5 expression
was markedly diminished compared to that in the control rats
(Fig. 1C and D). Western blot
analysis and RT-qPCR yielded similar results (Fig. 1D), indicating that differentiation
was inhibited in the lungs of hyperoxia-exposed rats.
Expression of YAP, Wnt3a and nuclear
β-catenin expression decreases in lungs affected by BPD
To determine whether YAP expression is affected in
lungs of rats exposed to hyperoxia, YAP expression was verified in
lung tissue at 1, 3, 7, 14 and 21 days in the experimental and
control group neonatal rats by RT-qPCR, western blot analysis and
IHC. The YAP mRNA levels began to decline on day 7 following
exposure to hyperoxia compared with the normoxic group and
continued to decrease thereafter (Fig. 2A), with western blot analysis, IHC
and RT-qPCR yielding similar results (Fig. 2C and E). In addition, Wnt3a
expression (Fig. 2B and C) and
nuclear β-catenin accumulation (Fig.
2D and F) were consistent with the results obtained for YAP
expression.
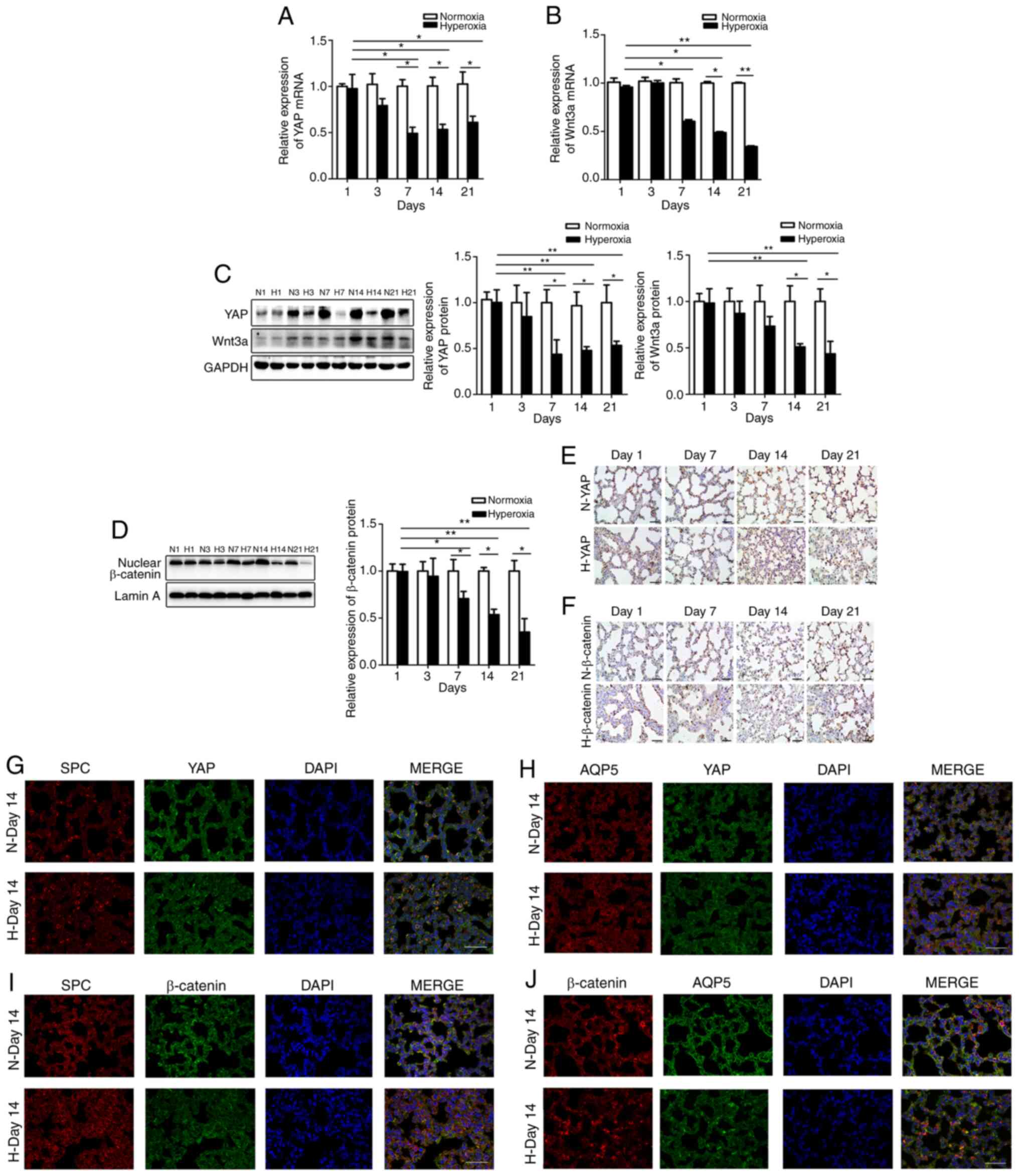 | Figure 2YAP, Wnt3a and β-catenin expression
is decreased in lung tissue affected by BPD. (A) YAP mRNA and (C)
protein expression in the lungs. (B) Wnt3a mRNA and (C) protein
expression in the lungs. (D) β-catenin protein expression in lung
nucleoli. Data are expressed as the means ± SD (n=6).
*P<0.05, **P<0.01. (E) YAP and (F)
β-catenin expression in IHC-stained lung sections. Scale bar, 40
µm. Expression of (G) SPC and (G) YAP, as well as (H) AQP5
and YAP detected by double IF staining. Scale bar, 40 µm. (I
and J) Expression of (I) SPC and β-catenin, as well as (J) AQP5 and
β-catenin detected by double IF staining. Scale bar, 40 µm.
N, normoxia-exposed rats; H, hyperoxia-exposed rats; SPC,
surfactant protein C; AQP5, aquaporin 5; BPD, bronchopulmonary
dysplasia. |
To determine whether YAP and β-catenin are expressed
on AECIIs or AECIs, double IF staining was performed for
YAP/β-catenin and SPC (AECII marker) or AQP5 (AECI marker).
Although both YAP and β-catenin were expressed in the AECIIs and
AECIs, their expression decreased in the AECIIs and AECIs exposed
to hyperoxia (Figs. 2G-J and
S2). Therefore, YAP and
β-catenin may be involved in the differentiation of AECIIs into
AECIs.
Effects of YAP on the proliferation of
AECIIs
The expression of SPC was detected in primary
cultured AECIIs by IF (Fig.
S3A), indicating successful cell isolation. Subsequently, YAP
was overexpressed and knocked down (KD) in these cells using OEYAP
plasmids and siRNAs, respectively. YAP mRNA and protein expression
levels were higher in the OEYAP group than in the OENC group
(Fig. S3B and D), and were
significantly lower in the KDYAP group than in the KDNC group
(Fig. S3C and D).
YAP overexpression upregulated SPC expression in the
OEYAP group compared with the OENC group (Fig. 3A), with stronger double IF
staining observed for SPC and YAP in the OEYAP group (Fig. 3C). Therefore, YAP overexpression
significantly promoted the proliferative capacity of the
AECIIs.
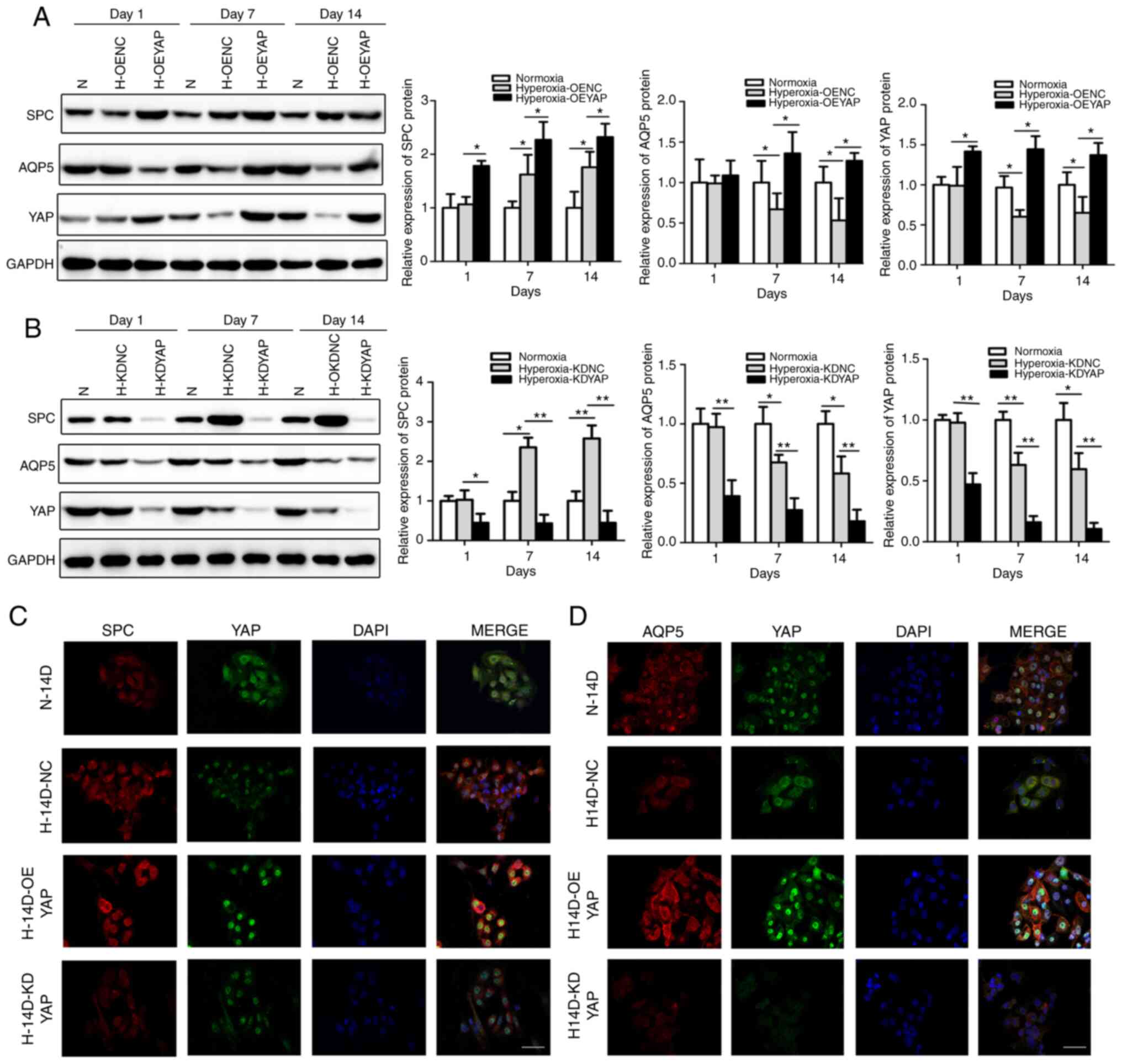 | Figure 3Effects of YAP on the proliferation
and differentiation of AECIIs into AECIs. (A and B) SPC and AQP5
expression in OEYAP, KDYAP and corre-sponding control (OENC, KDNC)
AECIIs detected by western blot analysis. Data are expressed as the
means ± SD (n=3). *P<0.05, **P<0.01. (C
and D) Double IF staining for (C) SPC and YAP or (D) AQP5 and YAP
in OEYAP, KDYAP and corresponding control (OENC, KDNC) AECIIs.
Scale bar, 40 µm. N, normoxia-exposed rats; H,
hyperoxia-exposed rats; AECIIs and AECIs, alveolar epithelial type
II and I cells; SPC, surfactant protein C; AQP5, aquaporin 5; OE,
overexpression; NC, negative control; KD, knockdown. |
Conversely, YAP knockdown (KDYAP) decreased SPC
expression compared to that in the KDNC group (Fig. 3B), with weaker double IF staining
observed for SPC and YAP in the KDYAP group (Fig. 3C). Thus, the genetic inhibition of
YAP with siRNA appeared to significantly suppress the proliferation
of AECIIs.
Effects of YAP on the differentiation of
AECIIs into AECIs
YAP overexpression increased AQP5 protein expression
compared to that in the OENC group (Fig. 3A) and produced stronger double IF
staining for AQP5 and YAP (Fig.
3D), indicating that YAP overexpression promoted the
differentiation of AECIIs into AECIs. Consistently, YAP knockdown
decreased AQP5 protein expression compared to that in the KDNC
group (Fig. 3B) and produced
weaker double IF staining signals for AQP5 and YAP (Fig. 3D). Thus, YAP knockdown using siRNA
against YAP may inhibit the differentiation of AECIIs into
AECIs.
YAP affects the Wnt/β-catenin signaling
pathway via β-catenin, but not via Wnt3a in AECIIs
To investigate the mechanisms through which YAP
affects the Wnt/β-catenin signaling pathway, β-catenin expression
was detected in AECIIs in which YAP was overexpressed or knocked
down. Total β-catenin expression differed slightly between the
different groups (Fig. 4A and B),
with higher nuclear β-catenin levels detected in the OEYAP group
than in the OENC group (Fig. 4A),
and lower β-catenin levels detected in the nuclei of the KDYAP
group than in the KDNC group (Fig.
4B). However, YAP overexpression or knockdown did not
significantly alter the Wnt3a levels, although it did activate
nuclear β-catenin in the Wnt/β-catenin signaling pathway (Fig. 4A and B). Taken together, these
data indicate that YAP positively regulates Wnt/β-catenin signaling
by increasing the nuclear transfer of β-catenin without affecting
Wnt3a expression in AECIIs.
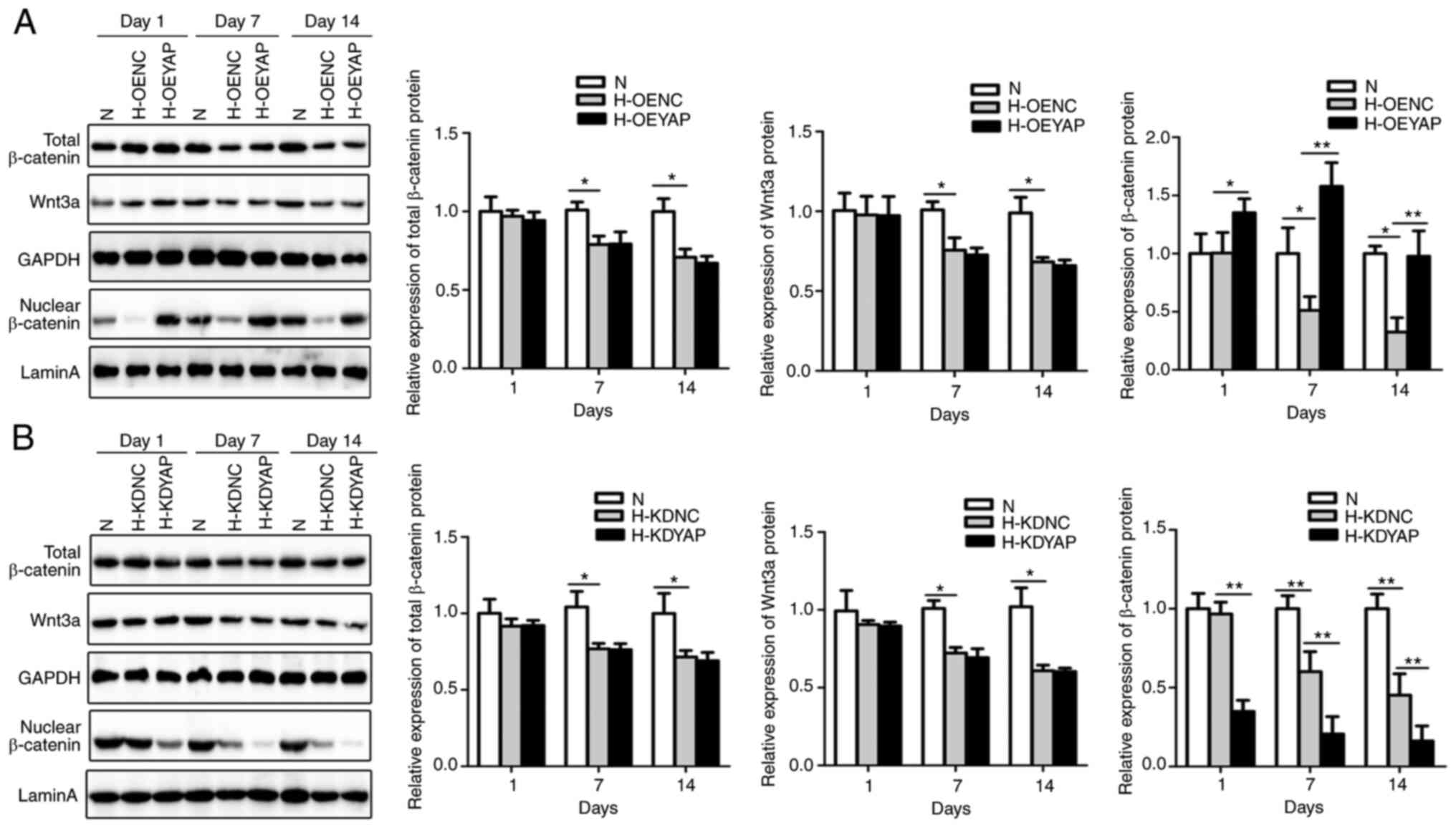 | Figure 4YAP overexpression and knockdown
affects nuclear β-catenin, but not Wnt3a expression. (A and B)
Expression of total and nuclear β-catenin in OEYAP, KDYAP and
corresponding control (OENC, KDNC) AECIIs from rats exposed to
hyperoxia for 1, 7 and 14 days, as determined by western blot
analysis. Wnt3a expression in OEYAP, KDYAP and corresponding
control (OENC, KDNC) AECIIs exposed to hyperoxia for 1, 7 and 14
days, as determined by western blot analysis. Data are expressed as
the means ± SD (n=3). *P<0.05,
**P<0.01. N, normoxia-exposed rats; H,
hyperoxia-exposed rats; AECIIs and AECIs, alveolar epithelial type
II and I cells; OE, overexpression; NC, negative control; KD,
knockdown. |
Wnt3a affects the Wnt/β-catenin signaling
pathway via β-catenin, but not YAP in AECIIs
Wnt3a protein expression was markedly elevated in
OEWnt3a AECIIs compared to that in the control AECIIs (Fig. S4A), while Wnt3a knock-down using
siRNA decreased the Wnt3a mRNA and protein levels considerably
compared to those in the KDNC group (Fig. S4B and C). Wnt3a overexpression
increased SPC and AQP5 expression in the AECIIs compared with the
OENC group (Fig. 5C), whereas
Wnt3a knockdown decreased SPC and AQP5 expression (Fig. 5B). These results suggest that
Wnt3a promotes the proliferation and differentiation of AECIIs into
AECIs.
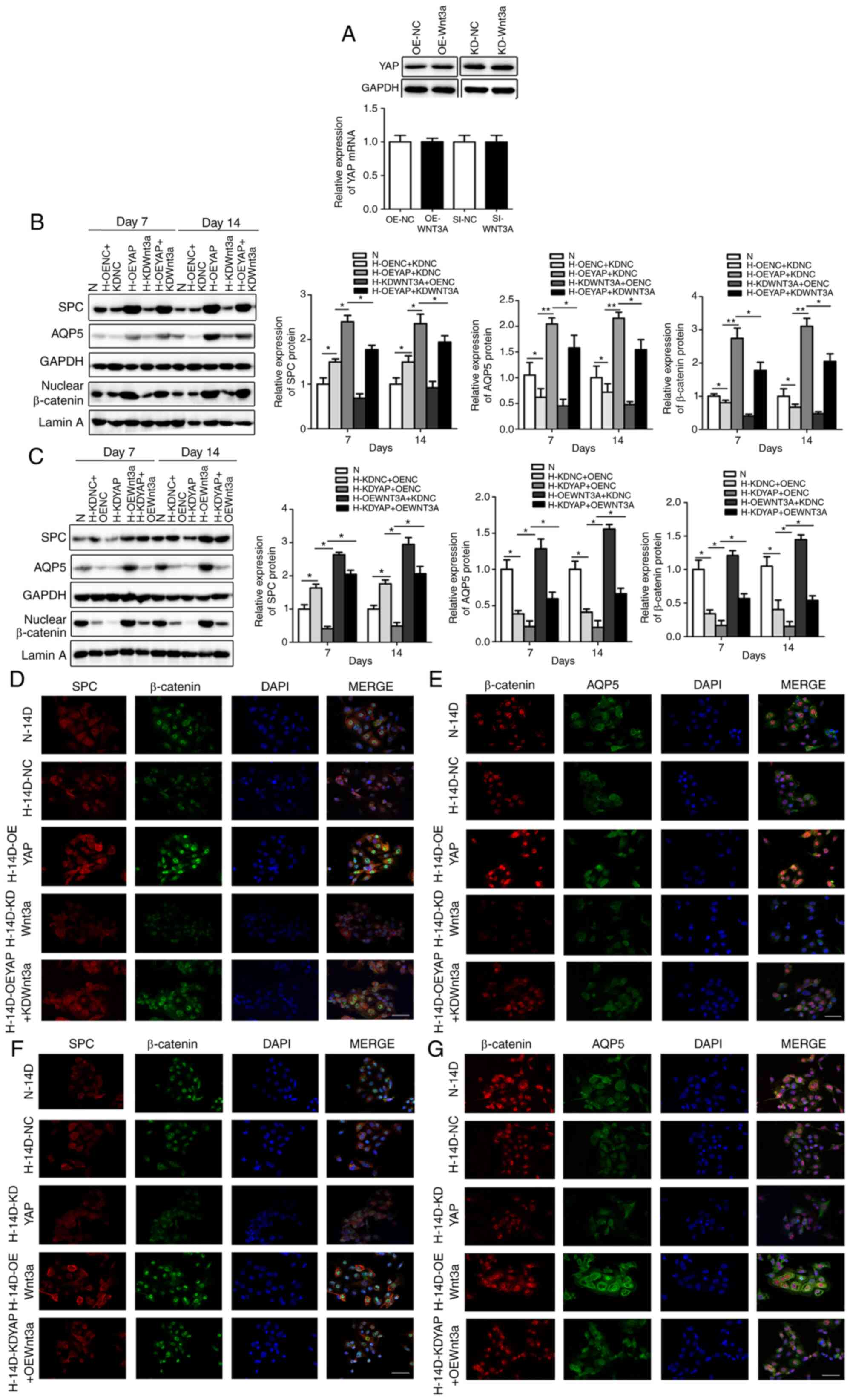 | Figure 5Wnt3a overexpression and knockdown
reversed the effects of YAP on AECIIs proliferation and
differentiation. (A) YAP expression in OEWnt3a, KDWnt3a and
corresponding control (OENC, KDNC) AECIIs determined by western
blot analysis. Data are expressed as the means ± SD (n=3). (B, D
and E) SPC, AQP5 and nuclear β-catenin expression detected by (B)
western blot analysis and double IF staining for (D) SPC and
β-catenin or (E) AQP5 and β-catenin in OEYAP, KDWnt3a, OEYAP +
KDWnt3a, and corresponding control (OENC, KDNC) AECIIs extracted
from hyperoxia-exposed rats after 14 days. Data are expressed as
the means ± SD (n=3). *P<0.05,
**P<0.01. Scale bar, 40 µm. (C, F and G) SPC,
AQP5 and nuclear β-catenin expression detected by (C) western blot
analysis and double IF staining for (F) SPC and β-catenin or (G)
AQP5 and β-catenin in KDYAP, OEWnt3a, KDYAP + OEWnt3a and
corresponding control (OENC, KDNC) AECIIs extracted from
hyperoxia-exposed rats after 14 days. Data are expressed as the
means ± SD (n=3). *P<0.05. Scale bar, 40 µm.
N, normoxia-exposed rats; H, hyperoxia-exposed rats; AECIIs and
AECIs, alveolar epithelial type II and I cells; OE, overexpression;
NC, negative control; KD, knockdown; SPC, surfactant protein C;
AQP5, aquaporin 5. |
To confirm the mechanisms through which Wnt3a
overexpression or knockdown affect nuclear β-catenin expression,
β-catenin expression was detected in the nucleus. Wnt3a
overexpression increased nuclear β-catenin levels compared to those
in the OENC group (Fig. 5C),
whereas Wnt3a knockdown decreased the β-catenin levels in the
nucleus compared to those in the KDNC group (Fig. 5B). However, Wnt3a overexpression
or knockdown did not affect YAP expression (Fig. 5A), suggesting that Wnt3a
positively regulates Wnt/β-catenin signaling by increasing the
nuclear transfer of β-catenin without affecting YAP expression in
AECIIs.
Wnt3a overexpression and knockdown
reverse the effects of YAP on the proliferation and differentiation
of AECIIs
Since significant differences were observed
following YAP overexpression or knockdown in the proliferation and
differentiation of AECIIs extracted from rats exposed to hyperoxia
for 7 and 14 days, these two time points for were selected
subsequent functional experiments.
It was found that nuclear β-catenin expression was
lower in the AECIIs in which YAP was overexpressed and Wnt3a was
knocked down than in the OEYAP group (Fig. 5B), but was increased in AECIIs in
which YAP was knocked down and Wnt3a was overexpressed compared
with the KDYAP group (Fig. 5C).
Thus, Wnt3a knockdown appears to reverse the effect of YAP
overexpression on nuclear β-catenin, while Wnt3a overexpression
reverses the suppressive effect of YAP knockdown on nuclear
β-catenin.
Subsequently, functional experiments were performed
to determine whether YAP regulates the proliferation and
differentiation of AECIIs via Wnt/β-catenin signaling. It was found
that SPC and nuclear β-catenin expression and double IF staining
were lower in the YAP-overexpressing AECIIs subjected to Wnt3a
knockdown than in the OEYAP group (Fig. 5B and D). Moreover, SPC and nuclear
β-catenin expression were higher in the AECIIs in which YAP was
knocked down and in which Wnt3a was overexpressed than in the KDYAP
group (Fig. 5C and F). The
differentiation assay revealed that AQP5 and β-catenin expression
and double IF staining were lower in the YAP-overexpressing AECIIs
subjected to Wnt3a knockdown than in the OEYAP group (Fig. 5B and E), whereas AQP5 expression
was higher in the AECIIs in which YAP was knocked down and
subjected to Wnt3a overexpression than in the KDYAP group (Fig. 5C and G). Taken together, these
results indicate that the effects of YAP overexpression or
knockdown on the proliferation or differentiation of AECIIs can be
partially reversed by inhibiting or activating the Wnt/β-catenin
signaling pathway.
Discussion
Recently, increasing attention has been paid to the
regulation of stem cell biology by the Hippo-YAP/TAZ pathway;
however, the effects of YAP on stem cell differentiation have not
yet been fully defined (33-35). In the present study, it was found
that YAP overexpression enhanced the number of AECIIs and AECIs,
whereas its knockdown suppressed this process. Moreover, it was
found that YAP regulated AECII proliferation and differentiation
into AECIs in experimental BPD. It is generally believed that
following lung injury, AECIIs can divide and restore the epithelium
by differentiating into AECIs and replenishing the AECIIs
population (36,37). Previous research has suggested
that YAP regulates the proliferation of alveolar epithelial
progenitors after acute lung injury (38); therefore, it was hypothesized that
YAP may be a potential regulatory target in AECIIs and that its
overexpression can facilitate lung regeneration or alveolar repair
following hyperoxia-induced injury. Thus, inducing AECII
proliferation and differentiation may serve as a novel therapeutic
strategy for alveolar repair in BPD.
The in vitro and in vivo experiments
performed in the present study demonstrated that the decreased YAP
expression in hyperoxia-exposed lungs upregulated SPC and
downregulated AQP5 expression; however, the number of AECIs
decreased alongside the increase in AECIIs. This phenomenon may be
explained by two factors: Firstly, other regulatory mechanisms may
be involved in enhancing AECIIs proliferation; and secondly, the
decreased differentiation of AECIIs into AECIs may lead to their
accumulation in vivo and inhibit epithelial repair. Hou
et al reported that the trans-differentiation of AECs was
not suppressed, but rather was increased following exposure to
hyperoxia by compensation; however, such repair during injury
cannot offset pulmonary epithelial air exchange and barrier
dysfunction caused by structural damage to AECs (39). In a word, the present study aimed
to elucidate the pathological process of BPD from different
perspectives and the possibility of different molecular expressions
under different conditions. Depending on the cellular environment
and stimuli, AECs respond to injury by undergoing different
pathways. Different perspectives may lead to different outcomes,
providing different insights for the treatment of BPD. In addition,
it was also observed that the hyperoxia-induced inhibition of
AECIIs differentiation into AECIs in vivo was accompanied by
the downregulation of YAP expression; however, YAP overexpression
contributed toward the proliferation and differentiation of AECIIs
into AECIs in vitro. The different mechanisms employed by
YAP in vivo and in vitro are likely due to
alternative pathways that enhance the proliferation of AECIIs and
inhibit YAP expression or involve decreased AP expression
inhibiting the differentiation of AECIIs to AECIs, resulting in
AECIIs aggregation in vivo.
YAP has also been shown to promote the proliferation
of AECIIs in other models and induce their complete differentiation
into AECIs (40). Moreover,
previous studies have demonstrated that YAP is critical for the
proliferation of AECIIs and differentiation into AECIs in response
to mechanical tension in the adult lung following pneumonectomy
(16). Another study reported
that Hippo/YAP signaling regulates Ajuba in respiratory epithelial
cells and controls progenitor cell proliferation and
differentiation in developing and mature lungs (17). Furthermore, recent research has
demonstrated that YAP adjusts AECII activities, including their
proliferation, differentiation into AECIs, and inflammatory
responses to balance both homeostasis and lung regeneration
following lung injury induced by bacterial pneumonia (19). Conversely, some studies have
suggested that LAS or MST deactivation leads to nuclear YAP
accumulation, promotes the state of progenitor cells, and inhibits
differentiation (41-45). Similarly, others have indicated
that TAZ is necessary for the proliferation and differentiation of
AECIIs into AECIs in vivo during alveolar repair after
bleomycin injury; however, YAP is not involved in this process
(20). The present study
suggested that YAP contributed toward the proliferation and
differentiation of AECIIs following hyperoxic lung injury. The
inconsistent conclusions of these studies may be the result of
factors, such as different stem cell sources, culture conditions,
and YAP intervention models; however, they also suggest that the
role of YAP in stem cell regulation is complicated and requires
further research to be fully characterized.
The Wnt protein family controls a variety of
developmental processes, including cell fate specification,
proliferation, and migration. Therefore, Wnt signaling disorders
during embryonic development can result in developmental defects
(46). Wnt signaling involves
both a canonical and noncanonical pathway, which are known to be
involved in lung development and diseases (25). In the present study, it was
demonstrated that Wnt3a expression was downregulated in the lungs
of rats exposed to hyperoxia and that Wnt3a overexpression and
knockdown promoted and suppressed both SPC and AQP5 expression,
respectively. In addition, it was found that Wnt3a regulated AECIIs
proliferation and differentiation into AECIs in hyperoxia-induced
BPD. Similarly, Wnt/β-catenin has previously been reported to
promote the differentiation of AECIIs into AECIs and an increased
Wnt3a expression may contribute toward the secretion of
pro-inflammatory factors in epithelial cells via the Wnt/β-catenin
pathway (47). However, the
regulatory mechanisms of Wnt3a in AECIIs warrant further
investigation.
Of note, the present study found that YAP
overexpression or knockdown had no significant effect on total
β-catenin and Wnt3a expression in vitro, but did affect
nuclear β-catenin protein levels. To investigate the regulatory
mechanisms of YAP in AECIIs, the present study examined the
association between YAP and the Wnt/β-catenin signaling pathway,
finding that nuclear β-catenin levels, such as YAP expression, were
lower in the lungs of rats exposed to hyperoxia. Therefore, it was
hypothesized that the mechanism of β-catenin in AECIIs was similar
to that of YAP. It was also found that YAP positively regulated the
nuclear transfer of β-catenin, which was unaffected by Wnt3a in
AECIIs, suggesting that YAP and Wnt signaling play essential roles
in alveolar regeneration in AECIIs by mediating the switch to an
AECIs fate after proliferation. Thus, the fine tuning of these two
signaling pathways may help AECIIs to proliferate and differentiate
sequentially, ultimately restoring the complex tissue architecture
of the alveolar epithelium. The results are consistent with those
of other research, in which YAP was reported to facilitate the
nuclear translocation of β-catenin by promoting GSK-3b
phosphorylation or reducing endogenous Dkk1 expression (48). Moreover, nuclear YAP/TAZ has been
suggested to promote β-catenin nuclear translocation by mediating T
cytokine-dependent transcription (49,50). However, other studies have
demonstrated that YAP inhibits Wnt signaling by inducing the
endogenous expression of Dkk1, an inhibitory ligand of the Wnt
pathway, thereby blocking Wnt/β-catenin signaling (21). In addition, cytoplasmic YAP/TAZ
can limit Wnt signaling activity by interacting with disheveled
(DVL) or β-catenin (14,29,51). In the absence of Wnt signaling,
β-catenin remains phosphorylated, resulting in its degradation and
the transcriptional inhibition of Wnt target genes (52). Therefore, nuclear YAP/TAZ can
enhance Wnt signal transduction, whereas cytoplasmic YAP/TAZ
inhibits Wnt signal transduction by activating the Hippo pathway
(53). Although these studies
were based on different cell models, their inconsistent conclusions
suggest that YAP may regulate β-catenin via a variety of
mechanisms.
To confirm whether Wnt3a regulates the proliferation
and differentiation of AECIIs via YAP, Wnt3a interference assays
were conducted. Wnt3a overexpression or knock-down reversed the
effects of YAP on the proliferation and differentiation of AECIIs
without affecting YAP expression, supporting the hypothesis that
YAP promotes the proliferation and differentiation of AECIIs via
nuclear β-catenin and not Wnt3a. Based on the effect of YAP on
Wnt/β-catenin signaling, it was confirmed that YAP partially
regulated the proliferation and differentiation of AECIIs by
activating the Wnt/β-catenin signaling pathway; however, YAP must
also be able to regulate this process via other pathways, which
should be investigated in future studies. For instance, a recent
study in a Pseudomonas aeruginosa acute infection model
suggested that Dlk-1 downregulated Notch signaling to promote
AECII-AECI differentiation (54).
Thus, multiple develop-mental pathways, including Wnt, Hippo and
Notch, likely work together to mediate tissue repair following
alveolar injury. In particular, it has been suggested that alveolar
epithelial cells from humans with pulmonary fibrosis display
increased YAP activity and crosstalk with the mTOR/PI3K/AKT
pathways (55). The current
observations, as well as those of other mechanistic analyses
(56), suggest that proper
balance, sequencing, and cell-type specificity between the activity
of the Wnt and Hippo pathways may be necessary to promote
appropriate alveolar epithelial regeneration. YAP/TAZ is a
transcriptional co-activating factor within and downstream of the
Hippo signaling pathway that regulates multidirectional stem cell
differentiation not only via Hippo signaling, but in coordination
with a variety of pathways related to differentiation that form an
interactive signaling network that jointly regulates the biological
functions of stem cells. However, it remains unclear whether these
reported mechanisms of YAP control the proliferation and
differentiation of AECIIs since the role of YAP differs among
organisms, making its regulatory mechanisms even more complex.
In conclusion, the present study demonstrated that
both Wnt3a and YAP promote the proliferation and differentiation of
AECIIs into AECIs during the pathogenesis of experimental BPD by
activating the nuclear transfer of β-catenin. In addition, it was
found that Wnt3a and YAP act independently without interacting with
each other, Wnt3a knockdown appears to reverse the effect of YAP
overexpression on nuclear β-catenin, while Wnt3a overexpression
reverses the suppressive effect of YAP knockdown on nuclear
β-catenin, and may play different roles in cell fate under
different situations. Thus, further studies are required to
elucidate their association in more detail. Taken together, the
findings of the present study indicate that Wnt3a or YAP may serve
as regulatory targets for improving the outcomes of BPD.
Supplementary Data
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81771621), the
Key Research and Development Guidance Plan of Liaoning Province
(no. 2019JH8/10300023), and the Natural Science Foundation of
Liaoning Province (no. 20170541023).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX and XJ designed the study. XJ wrote and revised
the manuscript. XJ performed the research. BW, JH, LF and MY helped
to design the overall study and analyze the data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were examined and approved by
the Experimental Animal Ethics Committee of China Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors are grateful to the Benxi Laboratory and
the teachers at the laboratory for their assistance.
References
|
1
|
Li W, Chen Y, Zhang J, Hong L, Yuan N,
Wang X and Lv H: IKBKE upregulation is positively associated with
squamous cell carcinoma of the lung in vivo and malignant
transformation of human bronchial epithelial cells in vitro. Med
Sci Monit. 21:1577–1586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Reilly M and Thébaud B: Animal models of
bronchopulmonary dysplasia. The term rat models Am J Physiol Lung
Cell Mol Physiol. 307:L948–L958. 2014. View Article : Google Scholar
|
|
3
|
Islam JY, Keller RL, Aschner JL, Hartert
TV and Moore PE: Understanding the short- and long-term respiratory
outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir
Crit Care Med. 192:134–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lodha A, Seshia M, McMillan DD, Barrington
K and Yang J: Association of early caffeine administration and
neonatal outcomes in very preterm neonates. JAMA Pediatr.
169:33–38. 2015. View Article : Google Scholar
|
|
5
|
Williams MC: Alveolar type I cells:
Molecular phenotype and development. Annu Rev Physiol. 65:669–695.
2003. View Article : Google Scholar
|
|
6
|
Miyake Y, Kaise H, Isono KI, Koseki H,
Kohno K and Tanaka M: Protective role of macrophages in
noninflammatory lung injury caused by selective ablation of
alveolar epithelial type II Cells. J Immunol. 178:5001–5009. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mason RJ: Biology of alveolar type II
cells. Respirology. 11:S12–S15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shafa M, Ionescu LI, Vadivel A, Collins
JJ, Xu L, Zhong S, Kang M, de Caen G, Daneshmand M, Shi J, et al:
Human induced pluripotent stem cell-derived lung progenitor and
alveolar epithelial cells attenuate hyperoxia-induced lung injury.
Cytotherapy. 20:108–125. 2018. View Article : Google Scholar
|
|
9
|
Vega MA, Chupin C, Pascariu M, Privé A,
Dagenais A, Berthiaume Y and Brochiero E: Dexamethasone fails to
improve bleomycin-induced acute lung injury in mice. Physiol Rep.
7:e142532019.
|
|
10
|
Quantius J, Schmoldt C, Vazquez-Armendariz
AI, Becker C, El Agha E, Wilhelm J, Morty RE, Vadász I, Mayer K,
Gattenloehner S, et al: Influenza virus infects epithelial
stem/progenitor cells of the distal lung: Impact on Fgfr2b-driven
epithelial repair. PLoS Pathog. 12:e10055442016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yagi R, Chen LF, Shigesada K, Murakami Y
and Ito Y: A WW domain-containing yes-associated protein (YAP) is a
novel transcriptional co-activator. EMBO J. 18:2551–2562. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng Z, Moroishi T and Guan KL: Mechanisms
of hippo pathway regulation. Genes Dev. 30:1–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan H, Xie Y, Zhang Z, Li K, Hu D, Zheng
X, Fan Q and Tang T: YAP-Mediated mechanotransduction regulates
osteogenic and adipogenic differentiation of BMSCs on hierarchical
structure. Mechanisms of hippo pathway regulation. Colloids Surf B
Biointerfaces. 152:344–353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barry ER, Morikawa T, Butler BL, Shrestha
K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et
al: Restriction of intestinal stem cell expansion and the
regenerative response by YAP. Nature. 493:106–110. 2013. View Article : Google Scholar :
|
|
15
|
Sun C, De Mello V, Mohamed A, Quiroga HP,
Garcia-Munoz A, Al Bloshi A, Tremblay AM, von Kriegsheim A,
Collie-Duguid E and Vargesson N: Common and distinctive functions
of the hippo effectors taz and yap in skeletal muscle stem cell
function. Stem Cells. 35:1958–1972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Wu H, Jiang K, Wang Y, Zhang W, Chu
Q, Li J, Huang H, Cai T, Ji H, et al: MAPK-Mediated YAP activation
controls mechanical-tension-induced pulmonary alveolar
regeneration. Cell Rep. 16:1810–1819. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lange AW, Sridharan A, Xu Y, Stripp BR,
Perl AK and Whitsett JA: Hippo/Yap signaling controls epithelial
progenitor cell proliferation and differentiation in the embryonic
and adult lung. J Mol Cell Biol. 7:35–47. 2015. View Article : Google Scholar :
|
|
18
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
LaCanna R, Liccardo D, Zhang P, Tragesser
L, Wang Y, Cao T, Chapman HA, Morrisey EE, Shen H, Koch WJ, et al:
Yap/Taz regulate alveolar regeneration and resolution of lung
inflammation. J Clin Invest. 129:2107–2122. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun T, Huang Z, Zhang H, Posner C, Jia G,
Ramalingam TR, Xu M, Brightbill H, Egen JG, Dey A and Arron JR: TAZ
is required for lung alveolar epithelial cell differentiation after
injury. JCI Insight. 5:e1286742019. View Article : Google Scholar
|
|
21
|
Seo E, Basu-Roy U, Gunaratne PH, Coarfa C,
Lim DS, Basilico C and Mansukhani A: SOX2 regulates YAP1 to
maintain stemness and determine cell fate in the osteoadipo
lineage. Cell Rep. 3:2075–2087. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu W, Xu B, Zhao Y, Yang N, Liu C, Wen G
and Zhang B: Wnt5a reverses the inhibitory effect of hyperoxia on
transdifferentiation of alveolar epithelial type II cells to type I
cells. J Physiol Biochem. 71:823–838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu W, Zhao Y, Zhang B, Xu B, Yang Y, Wang
Y and Liu C: Wnt3a mediates the inhibitory effect of hyperoxia on
the trans-differentiation of AECIIs to AECIs. J Histochem Cytochem.
63:879–891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cohen JC, Larson JE, Killeen E, Love D and
Takemaru K: CFTR and Wnt/beta-catenin signaling in lung
development. BMC Dev Biol. 8:702008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Freese JL, Pino D and Pleasure SJ: Wnt
signaling in development and disease. Neurobiol Dis. 38:148–153.
2010. View Article : Google Scholar :
|
|
26
|
Katoh M: WNT signaling in stem cell
biology and regenerative medicine. Curr Drug Targets. 9:565–570.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aumiller V, Balsara N, Wilhelm J, Günther
A and Königshoff M: WNT/β-Catenin signaling induces IL-1β
expression by alveolar epithelial cells in pulmonary fibrosis. WNT
signaling in stem cell biology and regenerative medicine. Am J
Respir Cell Mol Biol. 49:96–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia L, Zhang Y, Ji Y, Xiong Y, Zhang W,
Wen Y and Xu X: YAP balances the osteogenic and adipogenic
differentiation of hPDLSCs in vitro partly through the
Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun.
518:154–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Imajo M, Miyatake K, Iimura A, Miyamoto A
and Nishida E: A molecular mechanism that links Hippo signalling to
the inhibition of Wnt/β-catenin signalling. EMBO J. 31:1109–1122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun H, Choo-Wing R, Fan J, Leng L, Syed
MA, Hare AA, Jorgensen WL, Bucala R and Bhandari V: Small molecular
modulation of macrophage migration inhibitory factor in the
hyperoxia-induced mouse model of bronchopulmonary dysplasia. Respir
Res. 14:272013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jakkula M, Le Cras TD, Gebb S, Hirth KP,
Tuder RM, Voelkel NF and Abman SH: Inhibition of angiogenesis
decreases alveolarization in the developing rat lung. Am J Physiol
Lung Cell Mol Physiol. 279:L600–L607. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Wang Y, Yu A and Yu FX: The Hippo pathway
in tissue homeo-stasis and regeneration. Protein Cell. 8:349–359.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cai J, Zhang N, Zheng Y, de Wilde RF,
Maitra A and Pan D: The hippo signaling pathway restricts the
oncogenic potential of an intestinal regeneration program. Genes
Dev. 24:2383–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grijalva JL, Huizenga M, Mueller K,
Rodriguez S, Brazzo J, Camargo F, Sadri-Vakili G and Vakili K:
Dynamic alterations in hippo signaling pathway and YAP activation
during liver regeneration. Am J Physiol Gastrointest Liver Physiol.
307:G196–G204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leslie CC, McCormick-Shannon K, Mason RJ
and Shannon JM: Proliferation of rat alveolar epithelial cells in
low density primary culture. Am J Respir Cell Mol Biol. 9:64–72.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Evans MJ, Cabral LJ, Stephens RJ and
Freeman G: Renewal of alveolar epithelium in the rat following
exposure to NO2. Am J Pathol. 70:175–198. 1973.PubMed/NCBI
|
|
38
|
Hu C, Sun J, Du J, Wen D, Lu H, Zhang H,
Xue Y, Zhang A, Yang C, Zeng L and Jiang J: The hippo-YAP pathway
regulates the proliferation of alveolar epithelial progenitors
after acute lung injury. Cell Biol Int. 43:1174–1183. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hou A, Fu J, Yang H, Zhu Y, Pan Y, Xu S
and Xue X: Hyperoxia stimulates the transdifferentiation of type II
alveolar epithelial cells in newborn rats. Am J Physiol Lung Cell
Mol Physiol. 308:L861–L872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou B, Flodby P, Luo J, Castillo DR, Liu
Y, Yu FX, McConnell A, Varghese B, Li G, Chimge NO, et al:
Claudin-18-Mediated YAP activity regulates lung stem and progenitor
cell homeostasis and tumorigenesis. J Clin Invest. 128:970–984.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao R, Fallon TR, Saladi SV,
Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M,
Nunna N, Hariri LP, et al: Yap tunes airway epithelial size and
architecture by regulating the identity, maintenance, and
self-renewal of stem cells. Dev Cell. 30:151–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin C, Yao E and Chuang PT: A conserved
MST1/2-YAP axis mediates hippo signaling during lung growth. Dev
Biol. 403:101–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chung C, Kim T, Kim M, Kim M, Song H, Kim
TS, Seo E, Lee SH, Kim H, Kim SK, et al: Hippo-Foxa2 signaling
pathway plays a role in peripheral lung maturation and surfactant
homeostasis. Proc Natl Acad Sci USA. 110:7732–7737. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Szymaniak AD, Mahoney JE, Cardoso WV and
Varelas X: Crumbs3-Mediated polarity directs airway epithelial cell
fate through the hippo pathway effector yap. Dev Cell. 34:283–296.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Volckaert T, Yuan T, Chao CM, Bell H,
Sitaula A, Szimmtenings L, El Agha E, Chanda D, Majka S, Bellusci
S, et al: Fgf10-Hippo epithelial-mesenchymal crosstalk maintains
and recruits lung basal stem cells. Dev Cell. 43:48–59. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Huang C, Chintagari RN, Bhaskaran
M, Weng T, Guo Y, Xiao X and Liu L: MiR-375 regulates rat alveolar
epithelial cell trans-differentiation by inhibiting Wnt/β-catenin
pathway. Nucleic Acids Res. 41:3833–3844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang B, Sun H, Song F, Yu M, Wu Y and Wang
J: YAP1 negatively regulates chondrocyte differentiation partly by
activating the β-catenin signaling pathway. Int J Biochem Cell
Biol. 87:104–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takahashi A, Tsutsumi R, Kikuchi I, Obuse
C, Saito Y, Seidi A, Karisch R, Fernandez M, Cho T, Ohnishi N, et
al: SHP2 tyro-sine phosphatase converts parafibromin/Cdc73 from a
tumor suppressor to an oncogenic driver. Mol Cell. 43:45–56. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tsutsumi R, Masoudi M, Takahashi A, Fujii
Y, Hayashi T, Kikuchi I, Satou Y, Taira M and Hatakeyama M: YAP and
TAZ, Hippo signaling targets, act as a rheostat for nuclear SHP2
function. Dev Cell. 26:658–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Varelas X, Miller BW, Sopko R, Song S,
Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill
H, et al: The hippo pathway regulates Wnt/beta-catenin signaling.
Dev Cell. 18:579–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Clevers H, Loh KM and Nusse R: Stem cell
signaling. An integral program for tissue renewal and regeneration:
Wnt signaling and stem cell control. Science. 346:12480122014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Heallen T, Zhang M, Wang J,
Bonilla-Claudio M, Klysik E, Johnson RL and Martin JF: Hippo
pathway inhibits Wnt signaling to restrain cardiomyocyte
proliferation and heart size. Science. 332:458–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Finn J, Sottoriva K, Pajcini KV,
Kitajewski JK, Chen C, Zhang W, Malik AB and Liu Y: Dlk1-Mediated
temporal regulation of notch signaling is required for
differentiation of alveolar type II to type I cells during repair.
Cell Rep. 26:2942–2954. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gokey JJ, Sridharan A, Xu Y, Green J,
Carraro G, Stripp BR, Perl AK and Whitsett JA: Active epithelial
Hippo signaling in idiopathic pulmonary fibrosis. JCI Insight.
3:e987382018. View Article : Google Scholar :
|
|
56
|
Aspal M and Zemans RL: Mechanisms of
ATII-to-ATI Cell Differentiation during lung regeneration. Int J
Mol Sci. 21:31882020. View Article : Google Scholar :
|