1. Introduction
Since the beginning of the 21st century, the
emergence of severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2, previously known as 2019-nCoV) has marked the third
large-scale epidemic of a highly pathogenic coronavirus (CoV) in
the human population, following SARS-CoV in 2002 and the ongoing
Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012
(1,2). Coronavirus disease 2019 (COVID-19),
an acute respiratory disease, has rapidly spread beyond its Chinese
epicenter in Wuhan since late 2019, creating a public health burden
of international concern.
While the COVID-19 pandemic continues to advance
globally, the reports on clinical outcomes and risk factors for
morbidity and mortality are increasingly emerging as the virus
reaches new geographic areas. Despite the difficulties in
cross-country comparisons, COVID-19 cases and mortality rates
clearly vary around the globe (3,4).
Several factors may account for this discrepancy, including
differences in population composition, age distribution, genetic
predisposition, general health, healthcare access and socioeconomic
status (5,6). Although striking differences in
SARS-CoV-2 infection within and between populations indicate that
ethnicity may affect disease outcomes (7), little is known of the mechanisms
through which genetic disparities could interplay with viral
spreading and severity indices. As several vaccine prospects
against SARS-CoV-2 are currently awaiting validation (8-12),
the need to decrease COVID-19-related fatalities among vulnerable
populations remains urgent. It is thus important to elucidate host
factors that may influence susceptibility and/or response to
SARS-CoV-2.
In this regard, interest has currently focused on
the potential impact of vitamin D status on COVID-19 outcomes
(13-15). In fact, it is already evident that
the prevalence of vitamin D deficiency in Europe, particularly in
the northern mid-latitudes, seems to be closely aligned to
increased COVID-19 morbidity and mortality (16,17). Moreover, certain non-white ethnic
groups that are at higher risk of severe vitamin D deficiency
[Black, Asian, and Minority Ethnic (BAME)] appear to be
disproportionately affected by COVID-19 (18,19).
Indeed, recent research has indicated that vitamin D
signaling, mediating several antiviral and immune-enhancing
pathways, may exert beneficial effects at different stages of
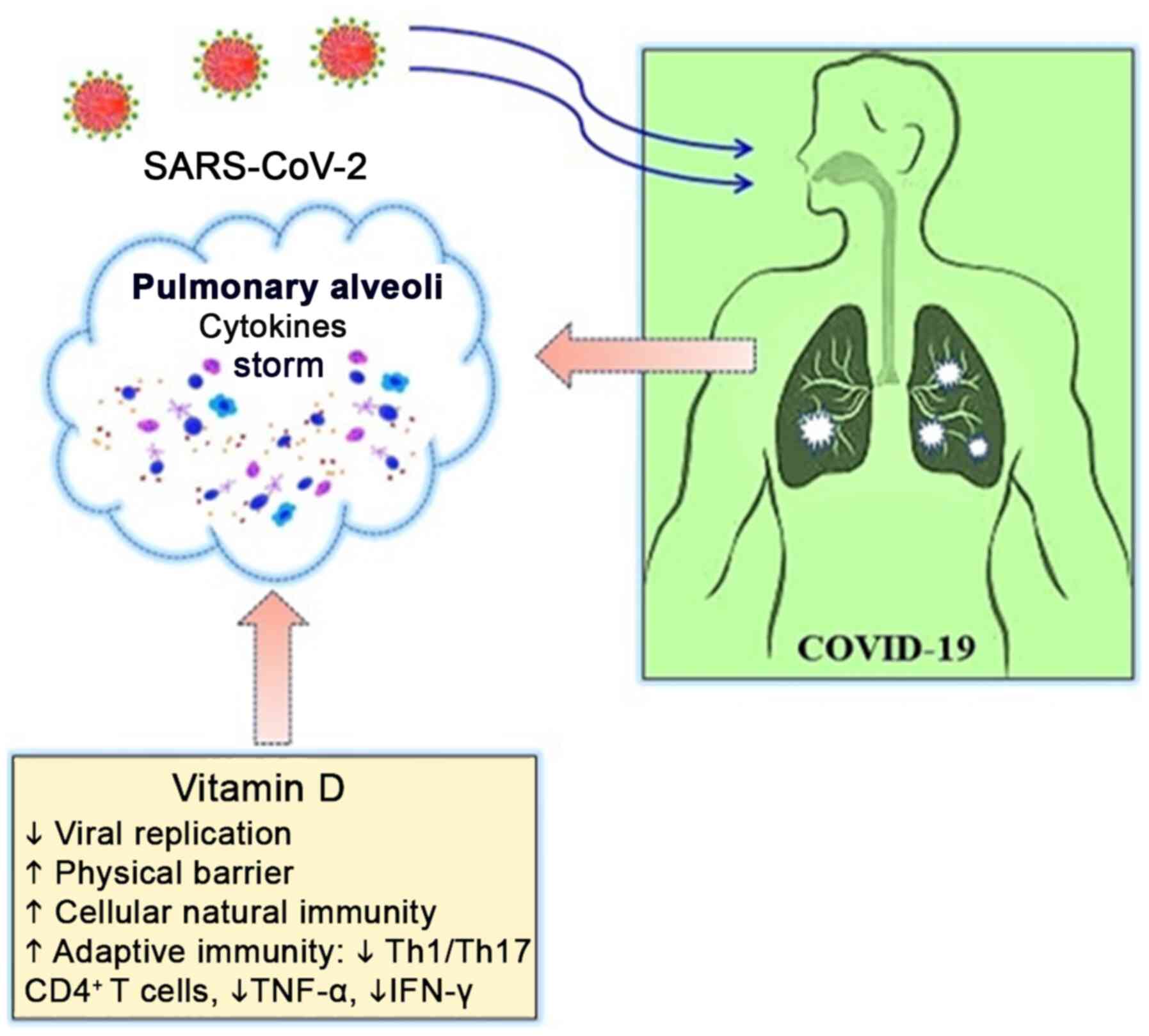
COVID-19. The regulatory role of vitamin D in immune cell function,
particularly by maintaining a dynamic balance between pro- and
anti-inflammatory cytokine signals through modulating effects on
the renin-angiotensin system (RAS), seems to be of particular
importance in the context of severe COVID-19. Thus, although
vitamin D deficiency may increase the risk of upper respiratory
viral infections, it is the impact of the vitamin D status on
cytokine profiles that is potentially much more relevant to the
pathogenesis of COVID-19, pointing to a higher inflammatory
response ('cytokine storm') in vitamin D-deficient individuals if
exposed to SARS-CoV-2 (13-15,17,20).
Given the supportive role of vitamin D in immune
responses against respiratory viruses (21), these observations require
particular attention. Since vitamin D mainly results from
endogenous skin production following exposure to ultraviolet (UV)
solar radiation (22), skin
pigmentation may thus play a role in non-white ethnic variations of
COVID-19, as melanin can reduce the capacity of the skin to
effectively absorb sunlight and synthesize vitamin D3. The present
review article aimed to explore the evidence related to vitamin D
status and melanin pigmentation that may have clinical implications
on the course and outcomes of COVID-19.
2. SARS-CoV-2 infection
SARS-CoV-2 is an enveloped, positive-sense, and
single- stranded RNA β-coronavirus that spreads mainly through the
respiratory tract by exploiting the angiotensin-converting enzyme 2
(ACE2) as an entry receptor to infect lung alveolar and intestinal
epithelial cells (1,23). The initial binding between
SARS-CoV-2 Spike (S) glycoprotein and ACE2 receptor on the surface
of target cells is thus a critical step for virus endocytosis,
determining the virus-host range and cellular tropism, as well as
the virus-cell membrane fusion. ACE2, found in abundance in the
human respiratory tract, has therefore taken center stage in the
COVID-19 outbreak, as it can regulate both the cross-species and
human-to-human transmission of SARS-CoV-2 (1).
The clinical spectrum of COVID-19 disease has been
well documented. Infection with SARS-CoV-2 seems to follow a
strikingly divergent course, ranging from non-symptomatic to
life-threatening: The majority (80-85%) of patients affected remain
asymptomatic or display mild flu-like symptoms, while the remaining
15% develop severe disease and 5% of cases progress to a critical
condition, with deaths mainly occurring in older and chronically
ill patients as a result of acute respiratory distress syndrome
(ARDS), sepsis and multiorgan failure (24,25).
Of note, recent observational data have demonstrated
an unusual risk factor pattern. While arterial hypertension and
diabetes mellitus are the most commonly reported risk factors for
more severe outcomes, patients with underlying lung conditions,
i.e., chronic obstructive pulmonary disease, seem to be relatively
protected from severe forms of COVID-19 (26). In addition, unlike influenza, a
mild clinical course in the pediatric population has already been
described. Curiously, the latter two groups are generally
considered more vulnerable to respiratory pathogens (15,26).
Although the immune response is vital for the
control and resolution of SARS-CoV-2 infection (1), virus-host interactions trigger a
diverse set of immune mediators against the invading virus, which
is followed by immune overreaction and induction of a cytokine
storm (excessive cytokine release) in at-risk individuals, as a
known pathogenic event of ARDS development (1,16).
In this context, the RAS cascade has emerged at the forefront of
COVID-19 pathophysiological mechanisms, although angiotensin II
(Ang II) has been more well known for its cardiovascular and renal
functions. Considering the ACE2 targeting by SARS-CoV-2, the RAS
imbalance has been proposed to play a pivotal role in the
pathogenesis of COVID-19. This hypothesis is mainly based on the
reduction of transmembrane ACE2 as a result of enzyme endocytosis
along with the S-glycoprotein of the virus. The consequent
over-stimulation of the classical ACE/Ang II/AT1 receptor (AT1R)
axis in line with the downregulation of the anti-inflammatory
ACE2/Ang-(1-7)/Mas Receptor (MasR) arm can compromise
the depressor/pressor balance of RAS, resulting in a
hyper-inflammatory state that may partially be responsible for the
severe complications associated with COVID-19 (26).
3. COVID-19 in skin of color
As the pandemic continues to spread worldwide, it
has become evident that individuals of different ethnic
backgrounds, but sharing a BAME origin appear to be more severely
affected by COVID-19 compared to Caucasians (19). Increasing numbers of reports have
demonstrated a pattern of higher risk for infection and adverse
clinical outcomes from COVID-19 in ethnic minority groups (7,27-30). Recent UK data have revealed that
over one-third of COVID-19 confirmed cases admitted to intensive
care are of a BAME background (7). In addition, based on the UK Biobank
data (2006-2010), both all-cause and COVID-19-related mortality
rates have been estimated to be higher among ethnic minorities
compared to the Caucasian British population during the first
pandemic wave (19). Similarly,
increased incidence and severity among African-American and
minority communities have also been documented in the United States
(31).
While the reason behind these disparities is
probably multifactorial, involving a lower socioeconomic or medical
comorbidity status, the disproportionate effect of COVID-19 on
certain ethnic groups requires special attention. Although
ethnicity may interplay with SARS-CoV-2 morbidity and mortality
through different cultural, behavioral and social traits (7), exploring the mechanisms underlying
the association between genetic variability and COVID-19 outcomes
is obviously warranted by the evidence (5,19).
Currently available data suggest that vitamin D
deficiency may represent a potential mediator for poor COVID-19
outcomes in people of color (18). Given that highly melanized skin
has long been shown to attenuate the cutaneous biosynthesis of
vitamin D (32), indeed, key
biological variables, including melanin, that may impact these
observations should be considered.
4. Potential role of melanin in the era of
COVID-19
Melanin pigments are considered the main drivers of
human pigmentary status (33).
Two types of melanin, i.e., eumelanin (brown/black) and pheomelanin
(red/yellow) polymer, are produced within specific organelles
(melanosomes) in epidermal melanocytes and are then transported
into the surrounding keratinocytes. Specialized melanocytic enzymes
and proteins are involved in melanin biosynthesis, with tyrosinase
being the key enzyme catalyzing the initial step of melanogenesis,
i.e., the oxidation and polymerization of the amino acid tyrosine
to form the intermediate dopaquinone (33-35). Skin color diversity across
individuals is mainly the result of differences in melanin content;
the amounts of melanin in epidermal cells vary depending on the
cutaneous phototype, being higher in dark and lower in light skin
phenotypes (34-36).
Although skin, eye, and hair coloration is largely
deter-mined by genetics (33,35), multiple intrinsic pathways (i.e.,
endocrine, immune, inflammatory signals) and extrinsic factors
(i.e., UV light intensity, environmental pollution) are also
involved in modulating the pigmentation patterns within and between
populations (33,35-38). Notably, the localization of Ang II
and its receptors in the skin, particularly the expression of
functional AT1R in melanocytes, has been suggested to play a role
in this regard. Apart from the well-established cardiovascular
effects, Ang II has also been indicated to play an additional role
in human skin pigmentation via the regulation of the melanogenic
pathway (39-41).
Liu et al, exploring the effects of
angiotensin on melanogenesis, recently demonstrated an increased
tyrosinase activity and melanin content in human cultured
melanocytes following AT1R stimulation in response to treatment
with Ang II (39). These findings
provide evidence to suggest an association between Ang II,
tyrosinase and AT1R activation, supporting a stimulatory
melanogenic effect of Ang II, which may be involved in cutaneous
pigmentation.
Apart from defining an important phenotypic trait,
melanin appears to play a major role in the natural photoprotection
of skin (42,43). In evolution, skin pigmentation in
the human lineage has developed via a process of natural selection
primarily to protect the skin from the damaging effects of solar UV
radiation (UVR) (44). Since UVR
can exert cytotoxic, mutagenic and immunosuppressive effects,
either by direct action on DNA or indirectly by generating reactive
oxygen species (ROS) and oxidative stress, the epidermis has been
armed with melanin to maintain and/or restore local homeostasis
against the UVR-driven insults (37,42,43,45). Melanosomes, by forming
supranuclear caps, protect keratinocytes from solar UV-induced DNA
damage, while eumelanin acts as a direct scavenger of ROS generated
upon UV exposure, reducing oxidative cellular damage (34,42,43).
However, along with the well-known radical
scavenging and antioxidant properties, an increased skin melanin
content has long been recognized to be inversely related to the
vitamin D status (32), which may
probably account for the observed ethnic differences in vitamin D
deficiency. Vitamin D is primarily obtained from
7-dehydrocholesterol (7-DHC) in the skin through solar exposure,
with the UVB spectrum (290-315 nm) mostly contributing to its
endogenous photosynthesis (22,46,47). In fair-skinned individuals, short
periods (20-30 min) of midday sun exposure 2-3 times per week is
sufficient to achieve and maintain an optimal 25-hydroxyvitamin D
[25(OH)D] status. However, this exposure pattern cannot be applied
to darker skin populations (skin types V-VI), as well as the
elderly who require higher weekly UVR doses to meet vitamin D
needs. The equivalent exposure time or frequency for these specific
groups has been estimated to be 2- to 10-fold higher compared to
white-skinned, young Caucasians (47-49).
In fact, melanin can act as an effective natural
filter by absorbing and scattering UVR, thereby impairing the solar
UVB-mediated conversion of 7-DHC to pre-vitamin D3
(32,45,47,50,51). As a result, the skin
photosynthesis of 25(OH)D3 can be reduced by as much as
99% (32). This places
dark-skinned individuals at higher risk for hypovitaminosis D than
light-skinned ones and is particularly important in northern
regions where pigmented skin (non-white ethnicity) is considered
the major risk factor for vitamin D insufficiency/deficiency across
all age groups (47,50). Previous studies have consistently
provided evidence to support the ethnic aspects of vitamin D
inadequacy, demonstrating a higher prevalence in people with
naturally darker skin (52-54). Of note, skin hyperpigmentation has
also been recognized as a key risk factor for hypovitaminosis D in
sunny lower latitudes, such as Australia (47).
Intriguingly, various biological functions affecting
human health and disease have only recently been attributed to
melanin pigments, but remain largely unexplored. In this respect,
less clear is the link between melanin and immunity (55). However, accumulating evidence from
several systems suggests that melanins are potent immunomodulators
with both pro- and anti-inflammatory properties, depending on the
type of melanin and host response (56). In humans, host melanin has long
been implicated in the setting of ocular and gingival inflammatory
disorders (57-59). Using a murine model, Kaya et
al demonstrated an enhanced intraocular inflammatory response
to uveitis in heavily pigmented eyes, possibly as a result of the
pro-inflammatory effects of melanin (59). Similarly, in the human gingiva, a
significant positive correlation between melanin distribution and
presence of gingival inflammation has also been reported (57).
It should also be emphasized that melanin is
considered to affect inflammatory responses directly and/or
indirectly by influencing the host cytokine/chemokine production
(56). Both in vitro and
ex vivo data have indicated that melanin can modulate
cytokine-mediated signaling cascades, increasing the release of
pro-inflammatory mediators, such as interleukin (IL)-1, IL-6,
interferon γ (IFN-γ), and tumor necrosis factor-α (TNF-α).
Additional evidence supporting a potential role of melanin in the
course of host immune responses during infection is provided by
in vitro findings demonstrating a melanin-induced activation
of the nuclear factor-κB (NF-κB) in monocytes through a Toll-like
receptor (TLR)-dependent process (55,60). A key question that remains to be
addressed is whether or not these pathways could elicit an
excessive immune response that may ultimately lead to tissue damage
through a vigorous inflammatory reaction (55,61).
Although the interactions between the pigmentary and
immune system have not yet been fully elucidated, melanocytes, as a
melanin source, have been reported to normally exist in oral and
nasopharyngeal mucosa (62-64). These findings could thus pave the
way for further consideration in different populations to translate
heterogeneous basic research into a clinical perspective relevant
to infectious disease, including SARS-CoV-2.
5. Vitamin D in respiratory antiviral
defense
Several lines of evidence suggest that the vitamin D
endocrine system is involved in multiple biologic processes and
pathways, affecting not only musculoskeletal health, but also a
variety of apparently different disease models, including
infectious disease (46,65-67). Apart from its classical role in
calcium and bone homeostasis, a modulatory role of vitamin D in
immunity, inflammation and epithelial repair has previously been
described (68,69). The active metabolite
1,25-dihydroxyvitamin D3
[1,25(OH)2D3] has long been recognized to
possess immune regulatory properties. Vitamin D receptors (VDRs)
are widely present in immunocompetent cells, such as
antigen-presenting cells, T- and B-cells. By binding to VDR,
1,25(OH)2D3 modulates and downregulates
adaptive, but enhances innate immunity and improves redox balance,
thus counterbalancing inflammation on multiple levels (70,71).
A growing number of studies have demonstrated that
vitamin D can contribute to the defense against viral infections;
notably, acute upper respiratory tract infections (21,72-80). Indeed,
1,25(OH)2D3 has been shown to exert antiviral
effects, either directly by inhibiting viral replication, or via an
anti-inflammatory and immunomodulatory mode of action (69,81). Although the underlying mechanisms
are very complex, vitamin D appears to support antiviral immunity
by targeting three distinct pathways: physical barrier, cellular
natural immunity, and adaptive immunity (79).
Vitamin D helps in preserving the epithelial
intercellular junction integrity, which improves host mucosal
defense against pathogen invasion (15,68,82). At a cellular level, vitamin D
metabolites have long been known to support innate antiviral
responses in part by up-regulating antimicrobial peptides, such as
human cathelicidin and defensins, to promote autophagy (68-70,83). The adaptive immune effects of
vitamin D include the inhibition of Th1/Th17 CD4+
T-cells and cytokines, such as TNF-α and IFN-γ, along with
stimulatory actions on Th2 and regulatory T-cells (Tregs) (69,70,84,85). By downregulating early
pro-inflammatory signaling in favor of an anti-inflammatory
Th2/Treg profile (69,70,85), 1,25(OH)2D3
can suppress the altered cytokine milieu induced by viral and
bacterial stimuli, as observed in COVID-19 patients, thus reducing
the risk of extensive tissue damage due to uncontrolled
inflammation (Fig. 1) (15).
Of note, vitamin D has also been shown to exert
beneficial effects on local 'respiratory homeostasis' (69). Although several mechanisms may be
involved in this regard, there are available data suggesting that
vitamin D/VDR signaling may exert lung-protective effects at least
partially by regulating the balance between key elements of the RAS
(86-88). In fact, an inverse correlation
between the vitamin D/VDR and RAS cascades has already been
described. As vitamin D may act as a potent negative endocrine
regulator of the RAS, vitamin D deficiency has been proposed as the
other face of RAS overstimulation (89,90). Considering that both systems have
evolved in a similar and parallel manner, participating in the
regulation of inflammatory and immunologic processes, as well as
the presence of vitamin D (VDR) and RAS (AT1R) receptors in almost
the same tissues, this link seems even more plausible (89).
Indeed, previous in vitro and in vivo
experimental studies have demonstrated that the vitamin D/VDR
pathway may trigger the ACE2/Ang-(1-7)/MasR axis, while inhibiting renin and
the classical ACE/Ang II/AT1R cascade (86,91,92). As ACE2 can directly exert
lung-protective effects, whereas ACE exhibits an opposing function
(93,94), such evidence further supports the
protective function of the vitamin D endocrine system in lung
tissue (86-88). This feedback relation is also
evident in other pathologies that are not discussed in this review
but have been well documented, such as hypertension and chronic
kidney disease (89).
6. Implications of vitamin D for
COVID-19
Since vitamin D deficiency and/or insufficiency has
emerged as a global pandemic linked to an increasing number of
non-skeletal disorders, the importance of vitamin D/VDR signaling
in overall health and well-being has attracted increasing attention
in recent years (65-67,95,96). Hypovitaminosis D has also been
recognized as an independent risk factor for total mortality in the
general population (95,97).
Although randomized controlled trials and
large-scale cohort studies investigating the links between the
vitamin D status and COVID-19 incidence and severity are currently
limited, evolving epidemiological evidence supports the hypothesis
that vitamin D inadequacy can negatively affect COVID-19 outcomes
(14,15,20,98).
The outbreak and peak of SARS-CoV-2 in wintertime,
when vitamin D levels drop to their lowest, as well as the pattern
of geographical spread of COVID-19 seem to reflect higher
population rates of vitamin D deficiency (15,20,98). Of note, COVID-19 associated
fatality rates appear to coincide with vitamin D deficiency rates,
with northern mid-latitude countries, where vitamin D deficiency is
still widely prevalent, bearing a greater burden of morbidity and
mortality (15,20,98).
Moreover, a striking overlap between the high-risk
groups for severe COVID-19 and vitamin D insufficiency has already
been reported. Indeed, severe COVID-19 infection and
hypo-vitaminosis D appear to share numerous risk factors, including
advanced age, male sex, obesity, darker skin pigmentation,
inadequate sunlight exposure, and chronic disease comorbidity,
particularly hypertension, cardiovascular disease, and diabetes
(14-16,98,99).
To further support this hypothesis, several clinical
and observational studies have thus far demonstrated an inverse
correlation between vitamin D status and COVID-19-associated
morbidity and mortality (15-17,20,100). Cross-sectional analyses in 20
European countries have reported a significant negative association
between the mean vitamin D levels and the number of COVID-19
cases/1 million population (16)
and between the average vitamin D levels and COVID-19-related
deaths/1 million population (100). A recent review comprising 188
studies (47 original human research studies) on the relation
between vitamin D and COVID-19 also provided biological
plausibility supporting the assertions that vitamin D deficiency
can explain every major risk factor, including the mystery of why
elderly males and individuals with naturally melanin-rich skin are
especially vulnerable, as well as every complication of COVID-19
(15).
From a biological perspective, there is compelling
evidence to indicate that the vitamin D/VDR pathway can favorably
modulate the host immunity to SARS-CoV-2, both in the early
viraemic and later hyperinflammatory stages of COVID-19. In fact,
vitamin D deficiency seems to compromise innate immune functions,
increasing the risk of viral infections in the respiratory
epithelium, including COVID-19 (14,15). Despite the sparse laboratory data
regarding the impact of vitamin D on host responses to SARS-CoV-2,
a recent in vitro study explored four compound libraries for
antiviral activity demonstrating a direct inhibitory effect of
calcitriol (the active form of vitamin D) on human nasal epithelial
cells infected with SARS-CoV-2 (101).
However, it is the impact of vitamin D on
unregulated cytokine production and, potentially, on the
severity/risk of ARDS that is of particular importance in COVID-19
(15,17,102). In this respect, the finding that
vitamin D deficiency may increase the potential for cytokine storm
by deregulating the X-chromosome-linked RAS appears to be much more
specific in the context of severe COVID-19, where overactivation of
the RAS has been associated with a poorer prognosis (14,15).
Although conclusive scientific data may eventually
be available, such correlational evidence might be of great
interest in darker-skinned individuals, who are more likely to be
vitamin D deficient, as it points toward an aberrant inflammatory
response if exposed to SARS-CoV-2, possibly indicating a higher
risk of COVID-19 adverse outcomes.
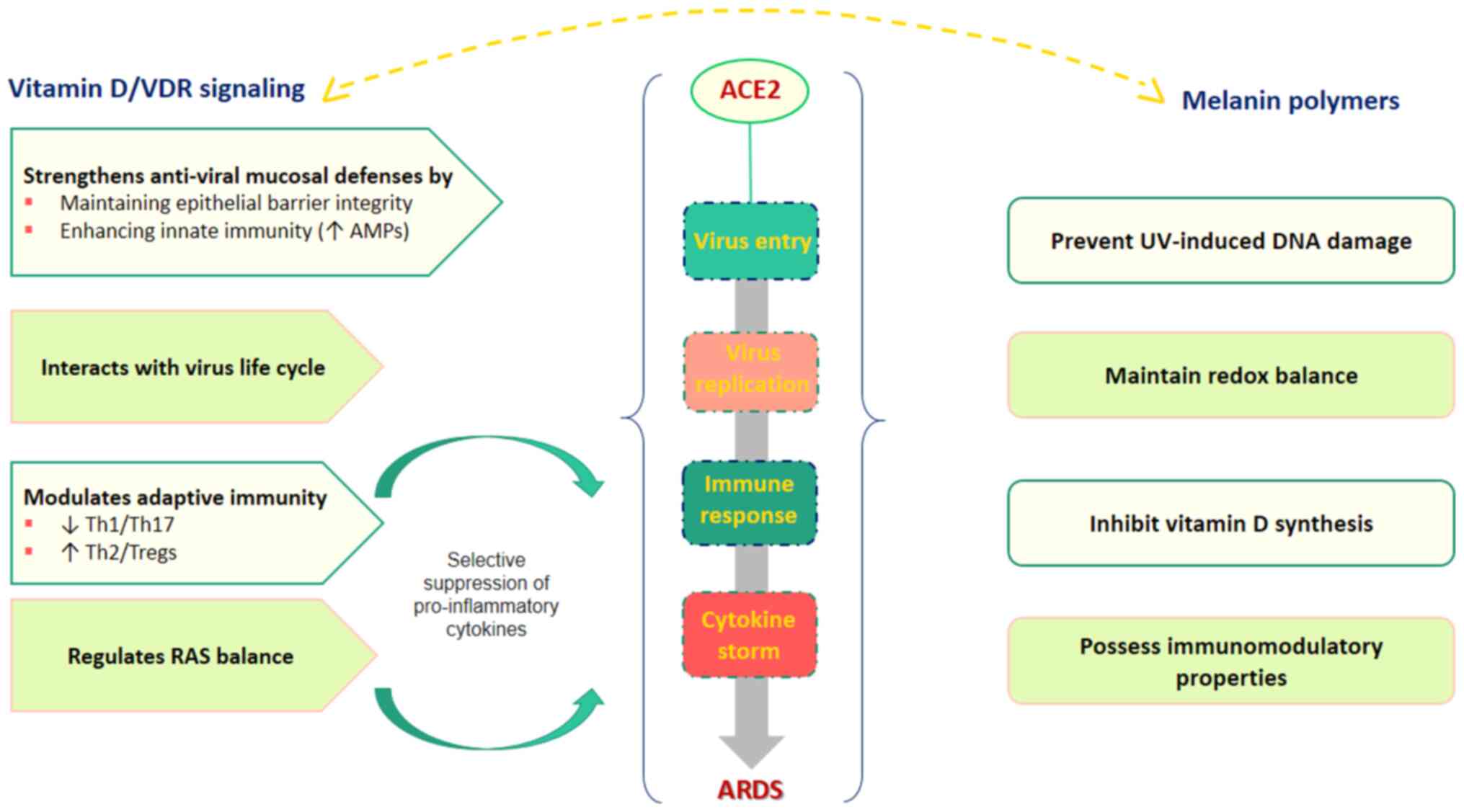
A schematic diagram of the vitamin D/VDR and melanin
related signaling pathways along with their potential implications
for COVID-19 is presented in Fig.
2.
7. Conclusions
In summary, the present review attempts to broaden
current knowledge of host biological factors, such as vitamin D
status and melanin polymers, possibly related to clinical outcomes
of COVID-19. Although contradictory data exist, vitamin D may turn
into an effective adjuvant to mitigate the impact of the current
pandemic, especially in populations where hypovitaminosis D is
prevalent. Notably, the concept of vitamin D regulation of cytokine
storm through the RAS opens up new perspectives on the functions of
vitamin D/VDR signaling, providing a basis for exploring the
potential use of vitamin D analogs in the prevention and/or
treatment of COVID-19.
Given that vitamin D is a safe, inexpensive, and
widely available agent, even in countries with limited resources,
vitamin D inadequacy is obviously an easily modifiable risk factor.
Therefore, from the literature reviewed here, prevention and/or
restoration of vitamin D deficiency/insufficiency through vitamin D
supplementation during the COVID-19 period seems to be highly
supported by the evidence.
For greater benefits, however, consideration of
basic biological variables, especially in different population
settings, is clearly warranted as the COVID-19 infection rates are
once again on the rise. At this point, our study might provide
early insights into a range of mostly overlooked host factors
throughout the disease pathway. Besides the well-known limiting
effects of melanin on the cutaneous biosynthesis of vitamin D,
which could negatively affect COVID-19 outcomes, the potential
interplay between the pigmentary and immune system may also require
special emphasis regarding the current pandemic. Further research
is needed to address these observations and elucidate whether any
of the implicated effects could be specific to SARS-CoV-2.
Abbreviations:
|
ACE2
|
angiotensin-converting enzyme 2
|
|
Ang II
|
angiotensin II
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
AT1R
|
AT1 receptor
|
|
BAME
|
Black, Asian and Minority Ethnic
|
|
CoV
|
coronavirus
|
|
COVID-19
|
coronavirus disease 2019
|
|
7-DHC
|
7-dehydrocholesterol
|
|
1,25(OH)2D3
|
1,25-dihydroxyvitamin
D3
|
|
25(OH)D
|
25-hydroxyvitamin D
|
|
IFN-γ
|
interferon-γ
|
|
MasR
|
Mas receptor
|
|
NF-κB
|
nuclear factor-κB
|
|
RAS
|
renin-angiotensin system
|
|
ROS
|
reactive oxygen species
|
|
S
|
spike
|
|
SARS-CoV-2
|
severe acute respiratory syndrome
coronavirus 2
|
|
Th
|
T helper
|
|
TLR
|
Toll-like receptor
|
|
TNF-α
|
tumor necrosis factor-α
|
|
Tregs
|
regulatory T cells
|
|
UV
|
ultraviolet
|
|
UVR
|
ultraviolet radiation
|
|
VDR
|
vitamin D receptor
|
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
DC conceived the presented idea, while PS retrieved
the data and wrote the manuscript under the supervision of ND. ND
aided in data extraction and revised the manuscript critically. PS
and AOD designed the figures. AOD, VN, MSK, DAS and AT contributed
to the editing and revision of the manuscript. All authors have
read and agreed to the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD,
Jin HJ, Tan KS, Wang DY and Yan Y: The origin, transmission and
clinical therapies on coronavirus disease 2019 (COVID-19) outbreak
- an update on the status. Mil Med Res. 7:112020.PubMed/NCBI
|
|
2
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re-emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI
|
|
3
|
Lai CC, Wang CY, Wang YH, Hsueh SC, Ko WC
and Hsueh PR: Global epidemiology of coronavirus disease 2019
(COVID-19): Disease incidence, daily cumulative index, mortality,
and their association with country healthcare resources and
economic status. Int J Antimicrob Agents. 55:1059462020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goumenou M, Sarigiannis D, Tsatsakis A,
Anesti O, Docea AO, Petrakis D, Tsoukalas D, Kostoff R, Rakitskii
V, Spandidos DA, et al: COVID-19 in Northern Italy: An integrative
overview of factors possibly influencing the sharp increase of the
outbreak (Review). Mol Med Rep. 22:20–32. 2020.PubMed/NCBI
|
|
5
|
Stopsack KH, Mucci LA, Antonarakis ES,
Nelson PS and Kantoff PW: TMPRSS2 and COVID-19: Serendipity or
Opportunity for Intervention? Cancer Discov. 10:779–782. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsatsakis A, Petrakis D, Nikolouzakis TK,
Docea AO, Calina D, Vinceti M, Goumenou M, Kostoff RN, Mamoulakis
C, Aschner M, et al: COVID-19, an opportunity to reevaluate the
correlation between long-term effects of anthropogenic pollutants
on viral epidemic/pandemic events and prevalence. Food Chem
Toxicol. 141:1114182020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pareek M, Bangash MN, Pareek N, Pan D, Sze
S, Minhas JS, Hanif W and Khunti K: Ethnicity and COVID-19: An
urgent public health research priority. Lancet. 395:1421–1422.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nitulescu GM, Paunescu H, Moschos SA,
Petrakis D, Nitulescu G, Ion GND, Spandidos DA, Nikolouzakis TK,
Drakoulis N and Tsatsakis A: Comprehensive analysis of drugs to
treat SARS-CoV-2 infection: Mechanistic insights into current
COVID-19 therapies (Review). Int J Mol Med. 46:467–488. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calina D, Sarkar C, Arsene AL, Salehi B,
Docea AO, Mondal M, Islam MT, Zali A and Sharifi-Rad J: Recent
advances, approaches and challenges in targeting pathways for
potential COVID-19 vaccines development. Immunol Res. 68:315–324.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kostoff RN, Kanduc D, Porter AL, Shoenfeld
Y, Calina D, Briggs MB, Spandidos DA and Tsatsakis A: Vaccine- and
natural infection-induced mechanisms that could modulate vaccine
safety. Toxicol Rep. 7:1448–1458. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calina D, Docea AO, Petrakis D, Egorov A
M, Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho
F, Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calina D, Hartung T, Docea AO, Spandidos
DA, Egorov AM, Shtilman MI, Carvalho F and Tsatsakis A: COVID-19
vaccines: Ethical framework concerning human challenge studies.
Daru. Aug 27–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arboleda JF, Urcuqui-Inchima S and Vitamin
D: Vitamin D Supplementation: A Potential Approach for
Coronavirus/COVID-19 Therapeutics? Front Immunol. 11:15232020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martineau AR and Forouhi NG: Vitamin D for
COVID-19: A case to answer? Lancet Diabetes Endocrinol. 8:735–736.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benskin LL: A Basic Review of the
Preliminary Evidence That COVID-19 Risk and Severity Is Increased
in Vitamin D Deficiency. Front Public Health. 8:5132020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ali N: Role of vitamin D in preventing of
COVID-19 infection, progression and severity. J Infect Public
Health. 13:1373–1380. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laird E, Rhodes J and Kenny RA: Vitamin D
and inflammation: potential implications for severity of Covid-19.
Ir Med J. 113:812020.PubMed/NCBI
|
|
18
|
Darling AL, Ahmadi KR, Ward KA, Harvey NC,
Alves AC, Dunn-Waters DK, Lanham-New SA, Cooper C and Blackbourn
DJ: Vitamin D status, body mass index, ethnicity and COVID-19:
Initial analysis of the first-reported UK Biobank COVID-19 positive
cases 580 compared with negative controls 723. MedRxiv. https://doi.org/10.1101/2020.04.29.20084277urisimplehttps://doi.org/10.1101/2020.04.29.20084277.
|
|
19
|
Patel P, Hiam L, Sowemimo A, Devakumar D
and McKee M: Ethnicity and covid-19. BMJ. 369:m22822020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grant WB, Lahore H, McDonnell SL, Baggerly
CA, French CB, Aliano JL and Bhattoa HP: Evidence that Vitamin D
Supplementation Could Reduce Risk of Influenza and COVID-19
Infections and Deaths. Nutrients. 12:9882020. View Article : Google Scholar :
|
|
21
|
Martineau AR, Jolliffe DA, Hooper RL,
Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa
D, Ginde AA, et al: Vitamin D supplementation to prevent acute
respiratory tract infections: systematic review and meta-analysis
of individual participant data. BMJ. 356:i65832017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pike JW and Christakos S: Biology and
mechanisms of action of the vitamin D hormone. Endocrinol Metab
Clin North Am. 46:815–843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: China Novel Coronavirus
Investigating and Research Team: A novel coronavirus from patients
with pneumonia in China, 2019. N Engl J Med. 382:727–733. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsatsakis A, Calina D, Falzone L, Petrakis
D, Mitrut R, Siokas V, Pennisi M, Lanza G, Libra M, Doukas SG, et
al: SARS-CoV-2 pathophysiology and its clinical implications: An
integrative overview of the pharmacotherapeutic management of
COVID-19. Food Chem Toxicol. 146:1117692020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lanza K, Perez LG, Costa LB, Cordeiro TM,
Palmeira VA and Ribeiro VT: Covid-19: The renin-angiotensin system
imbalance hypothesis. Clin Sci (Lond). 134:1259–1264. 2020.
View Article : Google Scholar
|
|
27
|
Aldridge RW, Lewer D, Katikireddi SV,
Mathur R, Pathak N, Burns R, Fragaszy EB, Johnson AM, Devakumar D,
Abubakar I, et al: Black, Asian and Minority Ethnic groups in
England are at increased risk of death from COVID-19: Indirect
standardisation of NHS mortality data. Wellcome Open Res. 5:882020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khunti K, Singh AK, Pareek M and Hanif W:
Is ethnicity linked to incidence or outcomes of covid-19? BMJ.
369:m15482020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan D, Sze S, Minhas JS, Bangash MN,
Pareek N, Divall P, Williams CM, Oggioni MR, Squire IB, Nellums LB,
et al: The impact of ethnicity on clinical outcomes in COVID-19: A
systematic review. EClinicalMedicine. 23:1004042020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sze S, Pan D, Gray LJ, Nevill CR, Martin
CA, Nazareth J, Minhas JS, Divall P, Khunti K, Abrams K, et al:
Ethnicity and clinical outcomes in COVID-19: A systematic review
and meta-analysis. EClinicalMedicine. Nov 12–2020.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vahidy FS, Nicolas JC, Meeks JR, Khan O,
Pan A, Jones SL, Masud F, Sostman HD, Phillips R, Andrieni JD, et
al: Racial and ethnic disparities in SARS-CoV-2 pandemic: Analysis
of a COVID-19 observational registry for a diverse US metropolitan
population. BMJ Open. 10:e0398492020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clemens TL, Adams JS, Henderson SL and
Holick MF: Increased skin pigment reduces the capacity of skin to
synthesise vitamin D3. Lancet. 1:74–76. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pavan WJ and Sturm RA: The Genetics of
Human Skin and Hair Pigmentation. Annu Rev Genomics Hum Genet.
20:41–72. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abdel-Malek ZA and Swope VB: Epidermal
melanocytes: regulation of their survival, proliferation, and
function in human skin. Melanoma Development. Springer; Vienna: pp.
7–33. 2011, View Article : Google Scholar
|
|
35
|
Rees JL: Genetics of hair and skin color.
Annu Rev Genet. 37:67–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Del Bino S, Duval C and Bernerd F:
Clinical and biological characterization of skin pigmentation
diversity and its consequences on UV impact. Int J Mol Sci.
19:26682018. View Article : Google Scholar :
|
|
37
|
Costin GE and Hearing VJ: Human skin
pigmentation: Melanocytes modulate skin color in response to
stress. FASEB J. 21:976–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Serre C, Busuttil V and Botto JM:
Intrinsic and extrinsic regulation of human skin melanogenesis and
pigmentation. Int J Cosmet Sci. 40:328–347. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu LH, Fan X, Li HT, An XX and Yang RY:
Angiotensin II promotes melanogenesis via angiotensin II type 1
receptors in human melanocytes. Mol Med Rep. 12:651–656. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu LH, Fan X, Xia ZK, An XX and Yang RY:
Angiotensin II stimulates melanogenesis via the protein kinase C
pathway. Exp Ther Med. 10:1528–1532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Steckelings UM, Wollschläger T, Peters J,
Henz BM, Hermes B and Artuc M: Human skin: Source of and target
organ for angiotensin II. Exp Dermatol. 13:148–154. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brenner M and Hearing VJ: The protective
role of melanin against UV damage in human skin. Photochem
Photobiol. 84:539–549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Solano F: Photoprotection and skin
pigmentation: melanin-related molecules and some other new agents
obtained from natural sources. Molecules. 25:15372020. View Article : Google Scholar :
|
|
44
|
Rocha J: The evolutionary history of human
skin pigmentation. J Mol Evol. 88:77–87. 2020. View Article : Google Scholar
|
|
45
|
Slominski A and Postlethwaite AE: Skin
under the sun: when melanin pigment meets vitamin D. Endocrinology.
156:1–4. 2015. View Article : Google Scholar :
|
|
46
|
Bikle DD: Vitamin D metabolism, mechanism
of action, and clinical applications. Chem Biol. 21:319–329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pearce SH and Cheetham TD: Diagnosis and
management of vitamin D deficiency. BMJ. 340:b56642010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Webb AR, Kazantzidis A, Kift RC, Farrar
MD, Wilkinson J and Rhodes LE: Meeting vitamin D requirements in
White Caucasians at UK latitudes: Providing a choice. Nutrients.
10:4972018. View Article : Google Scholar :
|
|
49
|
Farrar MD, Kift R, Felton SJ, Berry JL,
Durkin MT, Allan D, Vail A, Webb AR and Rhodes LE: Recommended
summer sunlight exposure amounts fail to produce sufficient vitamin
D status in UK adults of South Asian origin. Am J Clin Nutr.
94:1219–1224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bonilla C, Ness AR, Wills AK, Lawlor DA,
Lewis SJ and Davey Smith G: Skin pigmentation, sun exposure and
vitamin D levels in children of the Avon Longitudinal Study of
Parents and Children. BMC Public Health. 14:5972014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hameed A and Akhtar N: The skin melanin:
an inhibitor of vitamin-D3 biosynthesis: with special emphasis with
structure of skin. A mini review Dermatol Case Rep. 4:12019.
|
|
52
|
Richard A, Rohrmann S and Quack Lötscher
KC: Prevalence of vitamin D deficiency and its associations with
skin color in pregnant women in the first trimester in a sample
from Switzerland. Nutrients. 9:2602017. View Article : Google Scholar :
|
|
53
|
Alzaman NS, Dawson-Hughes B, Nelson J,
D'Alessio D and Pittas AG: Vitamin D status of black and white
Americans and changes in vitamin D metabolites after varied doses
of vitamin D supplementation. Am J Clin Nutr. 104:205–214. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Harris SS: Vitamin D and African
Americans. J Nutr. 136:1126–1129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
ElObeid AS, Kamal-Eldin A, Abdelhalim MAK
and Haseeb AM: Pharmacological properties of melanin and its
function in health. Basic Clin Pharmacol Toxicol. 120:515–522.
2017. View Article : Google Scholar
|
|
56
|
Mednick AJ, Nosanchuk JD and Casadevall A:
Melanization of Cryptococcus neoformans affects lung inflammatory
responses during cryptococcal infection. Infect Immun.
73:2012–2019. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Patsakas A, Demetriou N and Angelopoulos
A: Melanin pigmentation and inflammation in human gingiva. J
Periodontol. 52:701–704. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Smith JR, Rosenbaum JT and Williams KA:
Experimental melanin-induced uveitis: Experimental model of human
acute anterior uveitis. Ophthalmic Res. 40:136–140. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kaya M, Edward DP, Tessler H and Hendricks
RL: Augmentation of intraocular inflammation by melanin. Invest
Ophthalmol Vis Sci. 33:522–531. 1992.PubMed/NCBI
|
|
60
|
Pugh ND, Balachandran P, Lata H, Dayan FE,
Joshi V, Bedir E, Makino T, Moraes R, Khan I and Pasco DS: Melanin:
Dietary mucosal immune modulator from Echinacea and other botanical
supplements. Int Immunopharmacol. 5:637–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wilms H, Rosenstiel P, Sievers J, Deuschl
G, Zecca L and Lucius R: Activation of microglia by human
neuromelanin is NF-kappaB dependent and involves p38
mitogen-activated protein kinase: Implications for Parkinson's
disease. FASEB J. 17:500–502. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Feller L, Masilana A, Khammissa RA, Altini
M, Jadwat Y and Lemmer J: Melanin: the biophysiology of oral
melanocytes and physiological oral pigmentation. Head Face Med.
10:82014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fritz MA, Roehm PC, Bannan MA and Lalwani
AK: Extracellular and intracellular melanin in inflammatory middle
ear disease. Laryngoscope. 124:E241–E244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Na JY, Kim YH, Choi YD and Lee JS:
Melanotic oncocytic metaplasia of the nasopharynx: A report of
three cases and review of the literature. Korean J Pathol.
46:201–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hossein-nezhad A and Holick MF: Vitamin D
for health: A global perspective. Mayo Clin Proc. 88:720–755. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wacker M and Holick MF: Sunlight and
vitamin D: A global perspective for health. Dermatoendocrinol.
5:51–108. 2013. View Article : Google Scholar
|
|
67
|
Holick MF: Vitamin D: Extraskeletal
health. Endocrinol Metab Clin North Am. 39:381–400. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Schwalfenberg GK: A review of the critical
role of vitamin D in the functioning of the immune system and the
clinical implications of vitamin D deficiency. Mol Nutr Food Res.
55:96–108. 2011. View Article : Google Scholar
|
|
69
|
Zdrenghea MT, Makrinioti H, Bagacean C,
Bush A, Johnston SL and Stanciu LA: Vitamin D modulation of innate
immune responses to respiratory viral infections. Rev Med Virol.
27:e19092017. View Article : Google Scholar
|
|
70
|
Prietl B, Treiber G, Pieber TR and Amrein
K: Vitamin D and immune function. Nutrients. 5:2502–2521. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hoeck AD and Pall ML: Will vitamin D
supplementation ameliorate diseases characterized by chronic
inflammation and fatigue? Med Hypotheses. 76:208–213. 2011.
View Article : Google Scholar
|
|
72
|
Jolliffe D, Camargo CA, Sluyter J, Aglipay
M, Aloia J, Bergman P, Damsgaard C, Dubnov-Raz G, Esposito S,
Ganmaa D, et al: Vitamin D supplementation to prevent acute
respiratory infections: systematic review and meta-analysis of
aggregate data from randomised controlled trials. medRxiv.
https://doi.org/10.1101/2020.07.14.20152728urisimplehttps://doi.org/10.1101/2020.07.14.20152728.
|
|
73
|
Azmi H, Najwa H and Ennaji MM: Vitamin D
Immunomodulatory Role in Chronic and Acute Viral Diseases. Emerging
and Reemerging Viral Pathogens. Academic Press; 489. pp.
5062020
|
|
74
|
Beard JA, Bearden A and Striker R: Vitamin
D and the anti-viral state. J Clin Virol. 50:194–200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Greiller CL and Martineau AR: Modulation
of the immune response to respiratory viruses by vitamin D.
Nutrients. 7:4240–4270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Abhimanyu and Coussens AK: The role of UV
radiation and vitamin D in the seasonality and outcomes of
infectious disease. Photochem Photobiol Sci. 16:314–338. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lang PO, Aspinall R and Vitamin D: Vitamin
D status and the host resistance to infections: What it is
currently (not) understood. Clin Ther. 39:930–945. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gruber-Bzura BM: Vitamin D and
influenza-prevention or therapy? Int J Mol Sci. 19:24192018.
View Article : Google Scholar
|
|
79
|
Rondanelli M, Miccono A, Lamburghini S,
Avanzato I, Riva A, Allegrini P, Faliva MA, Peroni G, Nichetti M
and Perna S: Self-Care for Common Colds: The pivotal role of
Vitamin D, Vitamin C, Zinc, and Echinacea in three main immune
inter-active clusters (physical barriers, innate and adaptive
immunity) involved during an episode of common colds-practical
advice on dosages and on the time to take these
nutrients/botanicals in order to prevent or treat common colds.
Evid Based Complement Alternat Med. 2018:58130952018. View Article : Google Scholar
|
|
80
|
Gombart AF, Pierre A and Maggini S: A
review of micronutrients and the immune system-working in harmony
to reduce the risk of infection. Nutrients. 12:2362020. View Article : Google Scholar
|
|
81
|
Teymoori-Rad M, Shokri F, Salimi V and
Marashi SM: The interplay between vitamin D and viral infections.
Rev Med Virol. 29:e20322019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shi YY, Liu TJ, Fu JH, Xu W, Wu LL, Hou AN
and Xue XD: Vitamin D/VDR signaling attenuates
lipopolysaccharide-induced acute lung injury by maintaining the
integrity of the pulmonary epithelial barrier. Mol Med Rep.
13:1186–1194. 2016. View Article : Google Scholar :
|
|
83
|
White JH: Vitamin D as an inducer of
cathelicidin antimicrobial peptide expression: Past, present and
future. J Steroid Biochem Mol Biol. 121:234–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cantorna MT, Snyder L, Lin YD and Yang L:
Vitamin D and 1,25(OH)2D regulation of T cells.
Nutrients. 7:3011–3021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wu D, Lewis ED, Pae M and Meydani SN:
Nutritional modulation of immune function: analysis of evidence,
mechanisms, and clinical relevance. Front Immunol. 9:31602019.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xu S, Chen YH, Tan ZX, Xie DD, Zhang C,
Xia MZ, Wang H, Zhao H, Xu DX and Yu DX: Vitamin D3 pretreatment
alleviates renal oxidative stress in lipopolysaccharide-induced
acute kidney injury. J Steroid Biochem Mol Biol. 152:133–141. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shi Y, Liu T, Yao L, Xing Y, Zhao X, Fu J
and Xue X: Chronic vitamin D deficiency induces lung fibrosis
through activation of the renin-angiotensin system. Sci Rep.
7:33122017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kong J, Zhu X, Shi Y, Liu T, Chen Y, Bhan
I, Zhao Q, Thadhani R and Li YC: VDR attenuates acute lung injury
by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol
Endocrinol. 27:2116–2125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ferder M, Inserra F, Manucha W and Ferder
L: The world pandemic of vitamin D deficiency could possibly be
explained by cellular inflammatory response activity induced by the
renin-angiotensin system. Am J Physiol Cell Physiol.
304:C1027–C1039. 2013. View Article : Google Scholar
|
|
90
|
Ajabshir S, Asif A and Nayer A: The
effects of vitamin D on the renin-angiotensin system. J
Nephropathol. 3:41–43. 2014.PubMed/NCBI
|
|
91
|
Li YC: Vitamin D regulation of the
renin-angiotensin system. J Cell Biochem. 88:327–331. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li YC, Kong J, Wei M, Chen ZF, Liu SQ and
Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine
regulator of the renin-angiotensin system. J Clin Invest.
110:229–238. 2002. View Article : Google Scholar
|
|
93
|
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan
B, Yang P, Sarao R, Wada T, Leong-Poi H, et al:
Angiotensin-converting enzyme 2 protects from severe acute lung
failure. Nature. 436:112–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Treml B, Neu N, Kleinsasser A, Gritsch C,
Finsterwalder T, Geiger R, Schuster M, Janzek E, Loibner H,
Penninger J, et al: Recombinant angiotensin-converting enzyme 2
improves pulmonary blood flow and oxygenation in
lipopolysaccharide-induced lung injury in piglets. Crit Care Med.
38:596–601. 2010. View Article : Google Scholar
|
|
95
|
Nair R, Maseeh A and Vitamin D: Vitamin D:
The 'sunshine' vitamin. J Pharmacol Pharmacother. 3:118–126.
2012.
|
|
96
|
Holick MF: The vitamin D deficiency
pandemic: Approaches for diagnosis, treatment and prevention. Rev
Endocr Metab Disord. 18:153–165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Melamed ML, Michos ED, Post W and Astor B:
25-hydroxyvitamin D levels and the risk of mortality in the general
population. Arch Intern Med. 168:1629–1637. 2008. View Article : Google Scholar
|
|
98
|
Mitchell F: Vitamin-D and COVID-19: Do
deficient risk a poorer outcome? Lancet Diabetes Endocrinol.
8:5702020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
House N, Holborn H and Wc L: ICNARC report
on COVID-19 in critical care. ICNARC. 17:1–26. 2020.
|
|
100
|
Ilie PC, Stefanescu S and Smith L: The
role of vitamin D in the prevention of coronavirus disease 2019
infection and mortality. Aging Clin Exp Res. 32:1195–1198. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mok CK, Ng YL, Ahidjo BA, Lee RC, Loe MW,
Liu J, Tan KS, Kaur P, Chng WJ, Wong JE, et al: Calcitriol, the
active form of vitamin D, is a promising candidate for COVID-19
prophylaxis. bioRxiv. https://doi.org/10.1101/2020.06.21.162396urisimplehttps://doi.org/10.1101/2020.06.21.162396.
|
|
102
|
Daneshkhah A, Agrawal V, Eshein A,
Subramanian H, Roy HK and Backman V: Evidence for possible
association of vitamin D status with cytokine storm and unregulated
inflammation in COVID-19 patients. Aging Clin Exp Res.
32:2141–2158. 2020. View Article : Google Scholar : PubMed/NCBI
|