Introduction
Acute kidney injury (AKI) is characterized by an
abrupt deterioration of renal function and dysregulation of the
release of fluids, electrolytes and waste into urine (1), and it will cause life-threatening
complications (2). Due to a lack
of available therapeutic options, AKI is prevented and the majority
of patients with AKI receive only dialysis to replace kidney
function (3).
Multiple pathological conditions, such as
ischemia-reperfusion, sepsis, trauma and release of nephrotoxic
agents, as well as side-effects caused by therapeutic drugs are all
responsible for the occurrence of AKI (4). Nephrotoxic AKI is caused by
nephrotoxic agents, including drugs with therapeutic uses (5). Nephrotoxic AKI accounts for
approximately 33% of all AKI cases (6). As an effective chemotherapeutic
agent, cisplatin (CIS) is commonly used for treating hematological
and solid tumor malignancies (7).
Nephrotoxicity is a principal side effect of CIS treatment
(8). Proximal tubule cells can
absorb CIS accumulated in all segments of the nephron, which causes
severe injury (9). Thus, the
efficacy of CIS is limited by its nephrotoxicity, and effective
strategies or original drugs to protect patients against the
nephrotoxic effect of CIS are urgently required.
Multiple natural products have been identified for
the prevention and therapy of renal diseases (10). Formononetin (FOR) is a natural and
bioactive isoflavone isolated from herbal medicines, such as
Trifolium pretense, Astragalus membranaceus and
Pueraria lobate (11). It
has recently been reported that FOR prevents CIS-induced AKI
(12); however, to the best of
our knowledge, the underlying molecular mechanism of FOR in
preventing CIS-induced AKI is not clearly understood. Evidence has
increasingly demonstrated that CIS-mediated nephrotoxicity is
caused by a number of molecular pathways, such as oxidative stress,
apoptosis and inflammatory reactions (8,13).
Malondialdehyde (MDA) is an indicator of lipid peroxidation,
catalase (CAT) catalyzes the decomposition of hydrogen peroxide
into oxygen and water, and myeloperoxidase (MPO) serves as an
oxidative marker and an indicator of neutrophil infiltration into
kidneys (14). A previous study
reported that FOR has various potential pharmacological and
biological effects, such as anti-inflammatory (15), antioxidative (16) and anti-apoptotic effects (17). Thus, the present study
investigated the effects and protective mechanism of FOR by
examining oxidative stress, apoptosis and inflammatory pathways in
CIS-induced AKI rats and cells.
It has been demonstrated that FOR protects against
rhabdomyolysis-induced AKI (18).
FOR prevents against CIS-induced AKI by targeting organic cation
transporter 2 and P53; however, to the best of our knowledge, the
exact effect and potential mechanism underlying the effects of FOR
in CIS-induced AKI remain unclear. Thus, the present study
determined the target gene of FOR in CIS-induced AKI. Peroxisome
proliferator-activated receptor α (PPARα) is a member of the
steroid hormone receptor superfamily (18). Gonzalez-Manan et al
(19) reported that in C57BL/6J
mice fed a high-fat diet, the activation of PPARα is involved in
the inhibition of oxidative stress and inflammatory reaction. Wang
et al (20) demonstrated
that activation of PPARα inhibits apoptosis of vascular adventitial
fibroblasts. Furthermore, FOR is involved in the regulation of
PPARα in ameliorating cholestasis (21).
Previously, it has been revealed that activation of
nuclear factor erythroid 2-related factor 2 (Nrf2) improves renal
function (22). Upregulation of
the Nrf2-mediated signaling pathway protects against CIS-induced
renal injury (23). In addition,
when cells undergo chemical/oxidative stress, Nrf2 accumulates
within the nuclei, in which Nrf2 activates the expressions of
antioxidant response element (ARE)-driven genes, such as heme
oxygenase-1 (HO-1) and NAD(P)H quinone dehydrogenase 1 (NQO1)
(24). The Nrf2/HO-1 signaling
pathway protects cells from CIS-induced nephrotoxicity (25).
The current study investigated the protective
effects of FOR on CIS-induced AKI and the potential mechanisms,
such as via the Nrf2 pathway. It was identified that FOR could
serve as a preventative drug in treating CIS-induced AKI.
Materials and methods
Ethics statement
Animal experiments were performed in accordance with
the guidelines of the China Council on Animal Care and Use. The use
of the animals was approved by the Ethical Committee of
Experimental Animals of Zigong First People's Hospital (approval
no. ZFPH201904223), and the animal experiments were performed at
Zigong First People's Hospital (Zigong, China). All possible
efforts were made to minimize pain and discomfort caused to the
animals.
Animals and drug treatments
A total of 24 male Wistar rats (body weight, 220-250
g; age, 9-10 weeks) were purchased from the Experimental Animal
Center of Zigong First People's Hospital (Zigong, China). All rats
were housed in standard cages with a room temperature of 22°C and
humidity of 55%, under a normal 12/12 h light/dark cycle, and
provided food and water ad libitum. The rats were divided
into four different treatment groups, as follows: i) Control group;
ii) CIS group; iii) FOR group; and iv) CIS + FOR group, with 6 rats
in each group. FOR was purchased from Dalian Meilun Biotech Co.,
Ltd.
In the control group, vehicle (75 mg/kg 10%
hydroxypropyl β-cyclodextrin (Sigma-Aldrich; Merck KGaA) in 500 mM
phosphate buffer, pH 7.0) was administrated into the rats by oral
gavage once a day for 5 days. On days 3 and 4, the rats were also
intraperitoneally injected with normal saline 4 h after vehicle
treatment.
The CIS group was treated as the control group,
except that on days 3 and 4, the rats were intraperitoneally
injected with CIS (12 mg/kg; Sigma-Aldrich; Merck KGaA) 4 h after
vehicle treatment instead of with phosphate buffer. The
concentration of CIS to use was determined as previously described
(12).
In the FOR group, FOR (75 mg/kg), which has been
reported to induce the strongest inhibition of CIS-induced
nephrotoxicity previously (12),
was administrated into the rats by oral gavage once a day for 5
days. On days 3 and 4, the rats were intraperitoneally injected
with physiological saline 4 h after FOR treatment.
In the CIS + FOR group, FOR (75 mg/kg) was
administrated into the rats by oral gavage once a day for 5 days.
On days 3 and 4, the rats were intraperitoneally injected with CIS
(12 mg/kg) 4 h after FOR treatment.
On day 5, 4 h after treatment, all the rats were
sacrificed with pentobarbital sodium (165 mg/kg, i.p.) and the
heartbeat was checked. The both kidneys and blood (1 ml from the
tail vein) were collected, and plasma was separated from the blood.
The renal tissue samples were immediately fixed with 10% formalin
at room temperature for 24 h, paraffin-embedded, sectioned into 5
µm-thick slides, and then stained with hematoxylin and eosin
(H&E). In addition, the renal tissue homogenate was centrifuged
at 3,500 × g for 10 min at 4°C, and MDA content, and CAT and MPO
activities were detected using the supernatant. Levels of blood
urea nitrogen (BUN), creatinine, TNF-α and IL-1β were also detected
in the rat plasma.
Renal histological studies
Paraffin-embedded kidney sections were stained with
H&E. Histological changes, such as degree of tubular injury,
were visualized and photographs were obtained using a light
microscope (magnification, ×200). In detail, following dewaxing,
the section was stained with hematoxylin solution (cat. no. HHS16;
Sigma-Aldrich; Merck KGaA) at room temperature for 10 min.
Subsequently, the section were washed and stained with eosin
solution (cat. no. 318906; Sigma-Aldrich; Merck KGaA) at room
temperature for 5 min. A semi-quantitative pathological grading
method was used to assess the degree of tubular injury, including
tubular dilation, necrosis and cell membrane bleb formation
(26). Two pathologists, who were
blinded to the treatment, independently evaluated the
histopathological changes of each image using a light microscope
(magnification, ×200). A total of 24 images of one section were
used to calculate the pathological changes (%) according to the
injury score (0 for 0%, 1 for <5%, 2 for 5-24%, 3 for 25-74%, 4
for 75-100%), which was based on a system previously reported
(26).
Biochemical measurements
The levels of BUN and creatinine in rat plasma were
determined using a Urea assay kit (cat. no. C013-2-1) and
Creatinine assay kit (cat. no. C011-1-1), respectively. Kidney
tissues were rinsed in 100 mg/ml normal saline, then weighed and
homogenized. MDA content and activities of CAT and MPO in the
homogenate supernatant were determined. MDA in the supernatant of
homogenized renal tissues from rats was measured using a MDA assay
kit (cat. no. A003-1-2). The CAT enzymatic activity was measured
using the CAT assay kit (cat. no. A007-1-1). The activity of MPO
was measured by MPO assay kit (cat. no. A044-1-1). All kits were
purchased from Nanjing Bioengineering Institute Co., Ltd and used
according to the manufacturer's protocols. The levels of TNF-α and
IL-1β in the rat plasma were measured using a Rat TNF-α ELISA kit
(cat. no. SEKR-0009; Beijing Solarbio Science & Technology Co.,
Ltd.) and a Rat IL-1β ELISA kit (cat. no. SEKR-0002; Beijing
Solarbio Science & Technology Co., Ltd.).
Western blotting
Lysates from renal tissues of the Wistar rats and
HK-2 cells were lysed with RIPA lysis buffer (Cell Signaling
Technology, Inc.) to isolate proteins, and PPARα, Nrf2, HO-1 and
NQO1 proteins were analyzed. Briefly, total proteins of renal
tissues were extracted using RIPA buffer (cat. no. R0010; Beijing
Solarbio Science & Technology Co., Ltd.) with a complete
protease inhibitor cocktail (cat. no. 539133; Merck KGaA) on ice
for 30 min. Subsequently, the supernatant was collected, and the
protein concentration was determined using a BCA protein assay kit
(cat. no. PC0020; Beijing Solarbio Science & Technology Co.,
Ltd.). Proteins (30 µg/lane) were then separated by 12%
SDS-PAGE (cat. no. P0012A; Beyotime Institute of Biotechnology) and
transferred onto PVDF membranes (cat. no. IPVH00010; EMD
Millipore). The membranes were blocked with 5% (w/v) non-fat milk
in Tris-buffered saline containing 0.5% Tween-20 (w/v) at 37°C for
1 h. Next, the membranes were probed with the specific primary
anti-bodies listed in Table I at
4°C overnight, and then washed with TBS containing 0.1% Tween-20.
β-actin was used as the reference gene. The membranes were then
incubated with HRP-conjugated goat anti-mouse secondary antibody
(1:2,000; cat. no. ab205719; Abcam) and HRP-conjugated goat
anti-rabbit secondary antibody (1:2,000; cat. no. ab205718; Abcam)
at 37°C for 1 h. After washing the membranes three times at an
interval of 10 min, the signals were visualized using ECL detection
kit (Promega Corporation) and Protein expression levels were
quantified using ImageJ software (version 1.8.0; National
Institutes of Health) and normalized to that of β-actin.
 | Table IPrimary antibodies used for western
blotting. |
Table I
Primary antibodies used for western
blotting.
| Target protein | Antibody | Cat. no. | Supplier | Working
dilution |
|---|
| PPARα | Rabbit anti-PPARα
antibody | ab24509 | Abcam | 1:100 |
| Nrf2 | Mouse anti-Nrf2
antibody | ab89443 | Abcam | 1:1,000 |
| HO-1 | Rabbit anti-HO-1
antibody | ab13243 | Abcam | 1:2,000 |
| NQO1 | Mouse anti-NQO1
antibody | ab28947 | Abcam | 1:1,000 |
| β-actin | Mouse anti-β-actin
antibody | ab8226 | Abcam | 1:1,000 |
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from tissue and cells using
TRIzol reagent (cat. no. 15596018, Invitrogen; Thermo Fisher
Scientific, Inc.), and the mRNA expression levels of PPARα, Nrf2,
HO-1 and NQO1 were determined. PrimeScript™ RT Master mix was used
for RT (cat. no. RR036B; Takara Bio, Inc.) and incubated at 37°C
for 15 min, followed by incubation at 85°C for 5 sec. qPCR was
performed with a 7300 real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using TB Green® Premix
Ex Taq™ II (cat. no. RR820Q; Takara Bio, Inc.). The thermocycling
conditions were as follows: Incubation at 95°C for 30 min, followed
by amplification at 95°C for 5 sec and 60°C for 34 sec for a total
of 40 cycles, 72°C for 30 sec, with a final extension at 72°C for
90 sec. The expression levels of genes were normalized to that of
β-actin using the 2−ΔΔCq method (27). The sequences of primers used are
presented in Table II and
III.
 | Table IIPrimer sequences used for reverse
transcription-quantitative PCR analysis of rat samples. |
Table II
Primer sequences used for reverse
transcription-quantitative PCR analysis of rat samples.
| Gene | | Primer sequence
(5′-3′) |
|---|
| PPARα | Forward |
AGAGCCCCATCTGTCCTCTC |
| Reverse |
ACTGGTAGTCTGCAAAACCAAA |
| Nrf2 | Forward |
TCTTGGAGTAAGTCGAGAAGTGT |
| Reverse |
GTTGAAACTGAGCGAAAAAGGC |
| HO-1 | Forward |
AGGTACACATCCAAGCCGAGA |
| Reverse C |
ATCACCAGCTTAAAGCCTTCT |
| NQO1 | Forward |
AGGATGGGAGGTACTCGAATC |
| Reverse |
AGGCGTCCTTCCTTATATGCTA |
| β-actin | Forward |
GGCTGTATTCCCCTCCATCG |
| Reverse CC |
AGTTGGTAACAATGCCATGT |
 | Table IIIPrimer sequences used for reverse
transcription-quantitative PCR analysis of HK-2 cells. |
Table III
Primer sequences used for reverse
transcription-quantitative PCR analysis of HK-2 cells.
| Gene | | Primer sequence
(5′-3′) |
|---|
| PPARα | Forward |
ATGGTGGACACGGAAAGCC |
| Reverse |
CGATGGATTGCGAAATCTCTTGG |
| Nrf2 | Forward |
TCAGCGACGGAAAGAGTATGA |
| Reverse |
CCACTGGTTTCTGACTGGATGT |
| HO-1 | Forward |
AAGACTGCGTTCCTGCTCAAC |
| Reverse |
AAAGCCCTACAGCAACTGTCG |
| NQO1 | Forward |
GAAGAGCACTGATCGTACTGGC |
| Reverse |
GGATACTGAAAGTTCGCAGGG |
| β-actin | Forward |
CATGTACGTTGCTATCCAGGC |
| Reverse |
CTCCTTAATGTCACGCACGAT |
Cell culture
HK-2 (human kidney proximal tubule) cells were
purchased from the American Type Culture Collection and incubated
in DMEM/F12 (cat. no. BNCC342221; BeNa Culture Collection)
containing 10% fetal bovine serum (cat. no. 11011-8611; Beijing
Solarbio Science & Technology Co., Ltd.) and 1%
Penicillin-Streptomycin Liquid (cat. no. P1400; Beijing Solarbio
Science & Technology Co., Ltd.) with 5% CO2 at
37°C.
To investigate the role of PPARα in FOR preventing
CIS-induced injury of HK-2 cells, GW6471 (cat. no. G5045;
Sigma-Aldrich; Merck KGaA), which is a selective PPARα antagonist,
was used to inhibit PPARα in HK-2 cells. The cells were pre-treated
with normal saline or 25 µM GW6471 for 30 min, and then
cultured with 10 or 25 µM FOR for 2 h at room temperature.
Next, the cells were treated with 25 µM CIS for 24 h. The
treatments for different cell groups were as follows: i) Control
group, cells treated with PBS; ii) CIS group, cells treated with 25
µM CIS; iii) CIS + FOR-L group, cells treated with 25
µM CIS and 10 µM FOR; iv) CIS + FOR-H group, cells
treated with 25 µM CIS and 25 µM FOR; and v) CIS +
FOR-H + GW6471 group, cells treated with 25 µM CIS, 25
µM FOR and 5 µM GW6471.
Transfection
The expression of Nrf2 was inhibited using Nrf2
small interfering RNA (siRNA; siNrf2) to further investigate the
potential mechanism of PPARα in FOR prevention of HK-2 cells
treated with CIS. The siNrf2 (5′-GAC ATG GAT TTG ATT GAC ATA CT-3′)
and siRNA negative control (siNC; 5′-CAC TTG AAT CCG ACG GAT TTG
CA-3′) were purchased from Guangzhou RiboBio Co., Ltd. HK-2 cells
transfected with siNC or siNrf2 served as the negative control
group or siNrf2 group, respectively. The cells, cultured in 6-well
culture plates in serum-free OPTI-MEM medium (Gibco; Thermo Fisher
Scientific, Inc.), were transfected with 100 nM siNC or 100 nM
siNrf2 using Lipofectamine (Invitrogen; Thermo Fisher Scientific,
Inc.) for 24 h. The next day, the cells were incubated in fresh
medium for a further 48 h, and then subjected to the following
treatments.
Following cell transfection with siNrf2, the cells
were pre-treated with 50 µg/ml eupatilin (PPARα agonist;
cat. no. HY-N0783; MedChemExpress) for 24 h, and then cultured with
25 µM FOR for 2 h at room temperature. Eupatilin was used to
treat the cells for the purpose of comparing its effect with that
of FOX on CIS injury. Subsequently, the cells were cultured with 25
µM CIS for 24 h. The treatments for different cell groups
were as follows: i) siNC, transfection with siNC only; ii) siNC +
CIS, siNC transfection and CIS (25 µM) treatment; iii) CIS +
eupatillin + siNC, siNC transfection with CIS (25 µM) and
eupatillin (50 µg/ml) treatment; iv) CIS + eupatillin +
siNrf2, siNrf2 transfection with CIS (25 µM) and eupatillin
(50 µg/ml) treatment; v) CIS + FOR-H + siNC, siNC
transfection with CIS (25 µM) and FOR (25 µM)
treatment; and vi) CIS + FOR-H + siNrf2, siNrf2 transfection with
CIS (25 µM) and FOR (25 µM) treatment. The
concentrations of FOR and CIS to be used were selected as
previously described (8).
MTT assay
The cells (2×104 cells/well) were plated
in 96-well plates, and cell proliferation was determined using a
MTT kit (cat. no. IM0280; Beijing Solarbio Science & Technology
Co., Ltd.). Briefly, following the corresponding treatments, MTT
solution (20 µl; 5 mM) was added to each well of the 96-well
plates and incubated for a further 4 h. The absorbance was measured
at 570 nm using a microplate reader.
Flow cytometry assay
Apoptosis of the treated cells was measured by flow
cytometry using an Annexin V-FITC Apoptosis Detection kit (cat. no.
CA1020; Beijing Solarbio Science & Technology Co., Ltd.). The
collected cells were washed with cold sterile PBS, counted and
resuspended in 1 X binding buffer, then incubated with Annexin
V-FITC and propidium iodide in the dark at room temperature for 15
min. The cells were then further diluted using PBS to 500
µl, and cell apoptosis was evaluated using a flow cytometer
and FlowJo software (version 7.6.1; FlowJo LLC).
Biochemical measurements of the
cells
MDA, CAT enzymatic activity and the activity of MPO
in HK-2 cells were measured using the same methods as described for
tissues. The levels of TNF-α and IL-1β in HK-2 cells were measured
by Human TNF-α ELISA kit (cat. no. SEKH-0047; Beijing Solarbio
Science & Technology Co., Ltd.) and Human IL-1β ELISA kit (cat.
no. SEKH-0002; Beijing Solarbio Science & Technology Co., Ltd.)
according to the manufacturer's instructions.
Statistical analysis
The data are presented as the mean ± standard
deviation. All experiments were performed in triplicate.
Statistical significance in the study was analyzed by one-way ANOVA
followed by Tukey's post hoc test and Bonferroni's correction.
P<0.05 was considered to indicate a statistically significant
difference. The analyses were performed using SPSS 17.0 software
(SPSS, Inc.).
Results
FOR protects against CIS-induced
nephrotoxicity in the rat model
As presented in Fig.
1A, epithelial cell swelling, vacuolar degeneration and massive
necrosis in the proximal tubules were observed in rats treated by
CIS, and these effects were not observed in the CIS + FOR treatment
group. Furthermore, FOR treatment significantly reduced the
CIS-increased injury score in renal tissues compared with the CIS
group (P<0.00; Fig. 1A).
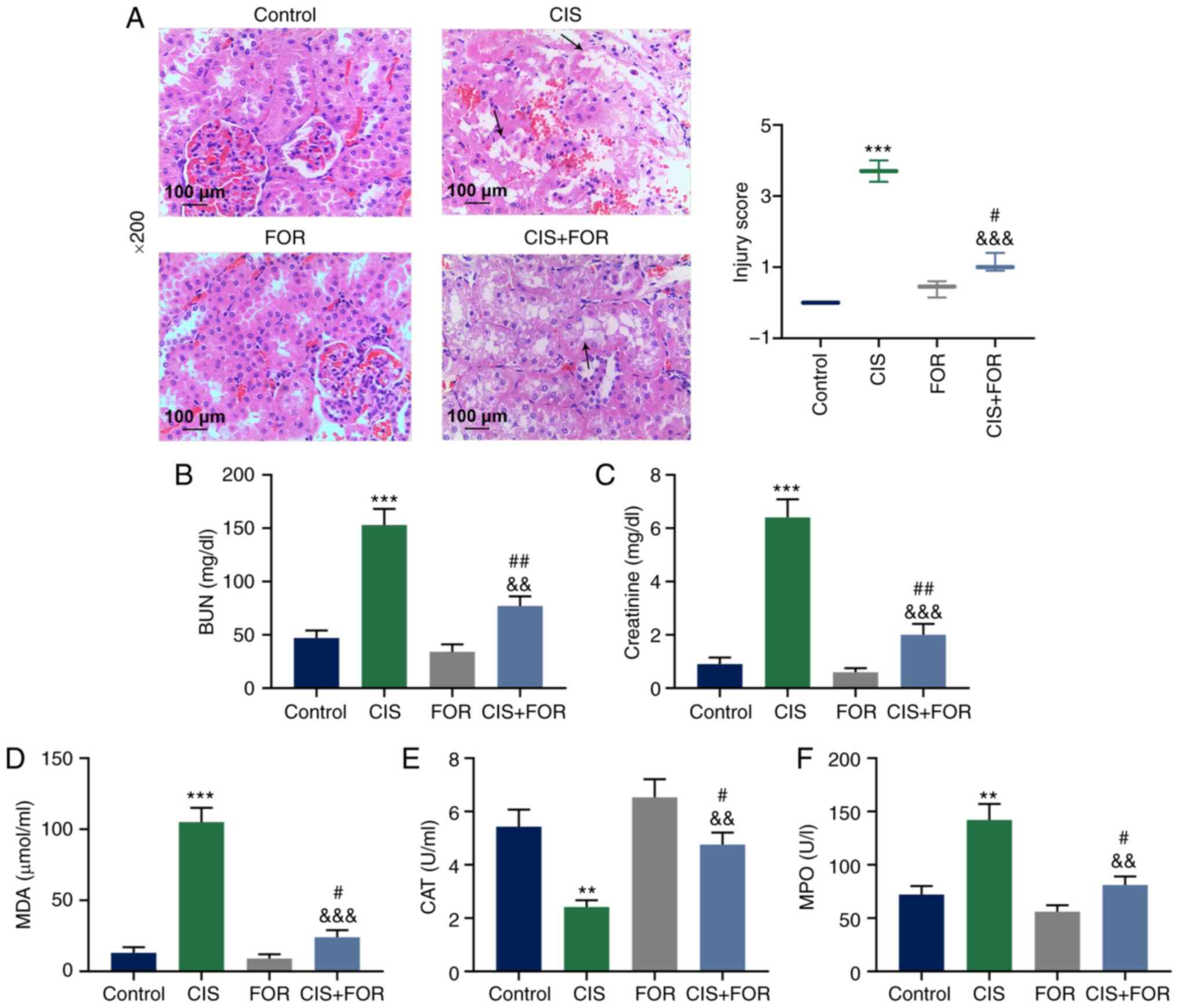 | Figure 1FOR ameliorates CIS-mediated acute
kidney injury in a rat model. (A) Kidney histopathological
examination was performed by hematoxylin and eosin staining in the
rat model (magnification, ×200). Arrows indicate areas of severe
renal necrosis. The changes in (B) BUN and (C) creatinine levels in
plasma, (D) MDA level, and (E) CAT and (F) MPO activities in kidney
were ameliorated by FOR following treatment with CIS. Data are
presented as mean ± standard deviation. **P<0.01,
***P<0.001 vs. Control.
&&P<0.01,
&&&P<0.001 vs. CIS.
#P<0.05, ##P<0.01 vs. FOR. FOR,
formononetin; CIS, cisplatin; BUN, blood urea nitrogen; MDA,
malondialdehyde; MPO, myeloperoxidase; CAT, catalase. |
The levels of BUN and creatinine in the plasma were
significantly increased in the CIS group compared with the control
group (P<0.001), but significantly reduced in the CIS + FOR
group compared with the CIS group (P<0.001; Fig. 1B and C).
The level of MDA in the rats was greatly increased
by CIS treatment, but was significantly reduced in the CIS + FOR
group (P<0.001; Fig. 1D).
Furthermore, the CAT activity was significantly reduced by CIS
treatment, but significantly increased in the CIS + FOR group
(P<0.01; Fig. 1E). The MPO
activity of the rats was significantly increased by CIS treatment,
and was significantly reduced by FOR treatment (P<0.01; Fig. 1F). The levels of TNF-α (Fig. 2A; P <0.001) and IL-1β (Fig. 2B, P<0.001) were significantly
increased by treatment of rats with CIS (P<0.001), and were
significantly decreased in the CIS + FOR group (P<0.001;
Fig. 2A and B).
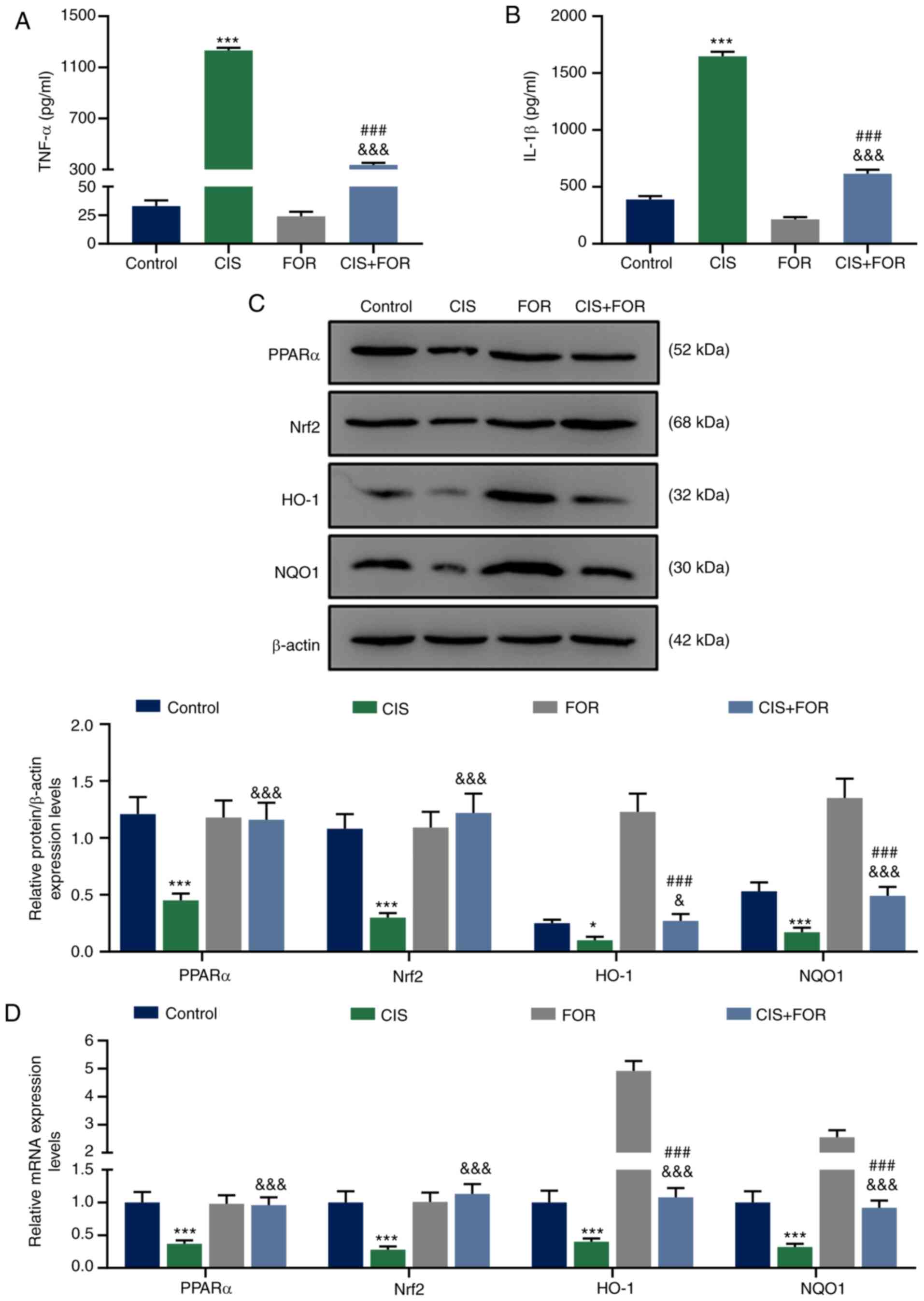 | Figure 2FOR increases CIS-reduced TNF-α and
IL-1β levels and expressions of PPARα, Nrf2, HO-1 and NQO1 in the
rat model. FOR increased CIS-reduced (A) TNF-α and (B) IL-1β
levels. FOR increased the CIS-reduced (C) protein and (D) mRNA
expression levels of PPARα, Nrf2, HO-1 and NQO1. Data are presented
as mean ± standard deviation. *P<0.05,
***P<0.001 vs. Control. &P<0.05,
&&&P<0.001 vs. CIS.
###P<0.001 vs. FOR. FOR, formononetin; CIS,
cisplatin; PPARα, peroxisome proliferator-activated receptor α;
Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme
oxygenase-1; NQO1, NAD(P)H quinone dehydrogenase 1. |
FOR increases the expression levels of
PPARα, Nrf2, HO-1 and NQO1, which are reduced by CIS in the rat
models
As presented in Fig.
2C and D, compared with the control group, CIS treatment
significantly suppressed the mRNA and proteins levels of PPARα,
Nrf2, HO-1 and NQO1 in the rats (P<0.05), and these levels were
significantly reversed by FOR treatment (P<0.001).
FOR protects HK-2 cells against
CIS-induced injury in a PPARα-dependent manner
As presented in Fig.
3A, CIS treatment significantly suppressed proliferation of
HK-2 cells at 12 (P<0.05), 24 (P<0.01) and 48 h (P<0.001)
compared with the control group; however, this suppression was
significantly reversed by FOR-L treatment at 48 h (P<0.05) and
by FOR-H treatment at 24 (P<0.05) and 48 h (P<0.001).
Notably, the promotive effect of FOR-H at 24 and 48 h on cell
proliferation was significantly prevented by GW6471 treatment at 48
h (P<0.001).
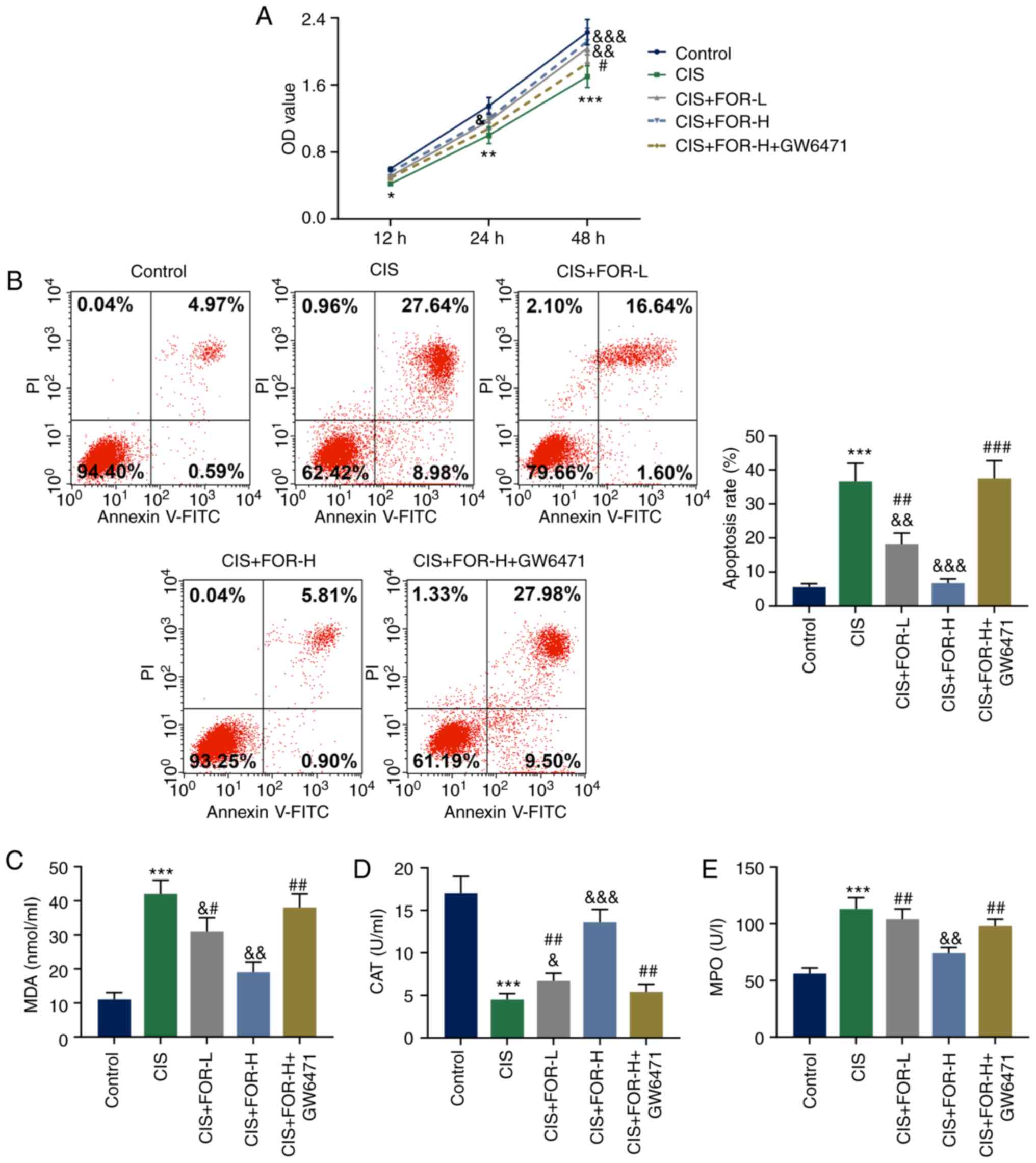 | Figure 3FOR protects against CIS-induced
injury of HK-2 cells in a PPARα-dependent manner. (A) FOR increased
the CIS-reduced proliferation in a PPARα-dependent manner. (B) FOR
reduced the CIS-increased apoptosis in a PPARα-dependent manner.
(C) FOR reduced the CIS-increased MDA level in a PPARα-dependent
manner. FOR increased the CIS-reduced (D) CAT and (E) MPO
activities in a PPARα-dependent manner. Data are presented as mean
± standard deviation. *P<0.05,
**P<0.01, ***P<0.001 vs. Control.
&P<0.05, &&P<0.01,
&&&P<0.001 vs. CIS.
#P<0.05, ##P<0.01,
###P<0.001 vs. CIS + FOR-H. FOR, formononetin; CIS,
cisplatin; PPARα, peroxisome proliferator-activated receptor α;
MDA, malondialdehyde; MPO, myeloperoxidase; CAT, catalase; PI,
propidium iodide; OD, optical density; L, low dose; H, high
dose. |
As presented in Fig.
3B, the cell apoptosis rate of HK-2 cells was significantly
increased in the CIS group (P<0.001), but was reduced by FOR-L
(P<0.01) and FOR-H (P<0.001) in a dose-dependent manner.
However, these effects of FOR-H were reversed by GW6471 treatment
(P<0.001).
As presented in Fig.
3C, the MDA level in HK-2 cells was significantly increased in
the CIS group (P<0.001), but was reduced by FOR-L (P<0.05)
and FOR-H (P<0.01) in a dose-dependent manner. However, the
effect of FOR-H on MDA level was significantly reversed by GW6471
treatment (P<0.01). As shown in Fig. 3D, CIS treatment significantly
suppressed CAT activity in HK-2 cells (P<0.001), which was
significantly reversed by FOR-L (P<0.05) and FOR-H (P<0.01)
treatment in a dose-dependent manner, and this effect of FOR-H was
reversed by GW6471 treatment (P<0.01). As presented in Fig. 3E, CIS treatment significantly
enhanced the MPO activity in HK-2 cells (P<0.001), which was
significantly reduced by FOR-H treatment (P<0.01), and GW6471
treatment significantly reversed this effect of FOR-H
(P<0.01).
As shown in Fig. 4A
and B, CIS treatment significantly increased the levels of
TNF-α (P<0.001) and IL-1β (P<0.001) in HK-2 cells, which were
reversed by FOR-L (P<0.01) and FOR-H (P<0.001). However,
GW6471 treatment significantly reversed this effect of FOR-H
(P<0.001).
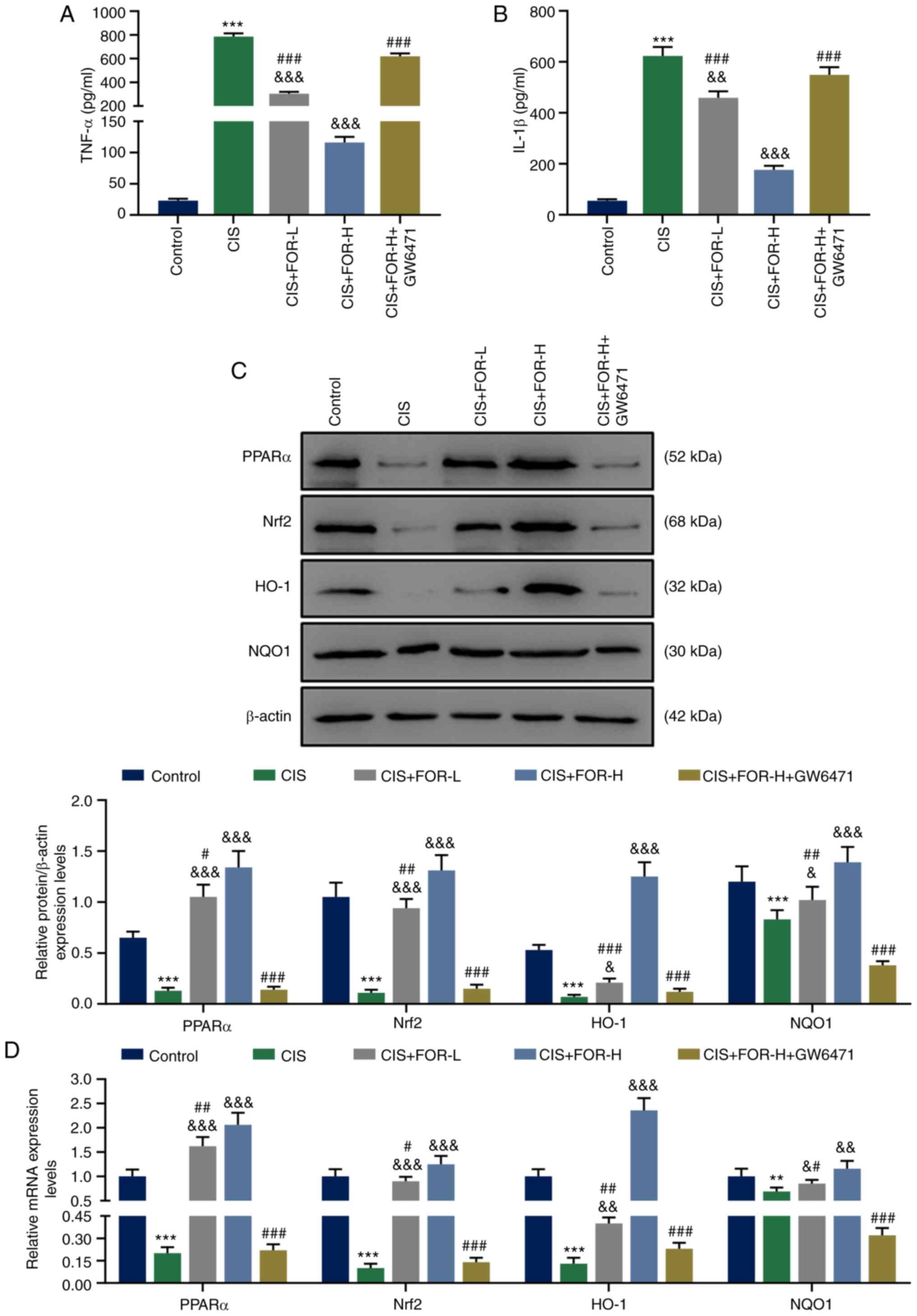 | Figure 4FOR protects against CIS-induced
injury and expression levels of PPARα, Nrf2, HO-1 and NQO1 in HK-2
cells in a PPARα-dependent manner. FOR reduced the CIS-increased
(A) TNF-α and (B) IL-1β levels in a PPARα-dependent manner. FOR
increased the CIS-reduced (C) protein and (D) mRNA levels of PPARα,
Nrf2, HO-1 and NQO1 in a PPARα-dependent manner. Data are presented
as mean ± standard deviation. **P<0.01,
***P<0.001 vs. Control. &P<0.05,
&&P<0.01,
&&&P<0.001 vs. CIS.
#P<0.05, ##P<0.01,
###P<0.001 vs. CIS + FOR-H. FOR, formononetin; CIS,
cisplatin; PPARα, peroxisome proliferator-activated receptor α;
Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme
oxygenase-1; NQO1, NAD(P)H quinone dehydrogenase 1; L, low dose; H,
high dose. |
FOR increases the expression levels of
PPARα, Nrf2, HO-1 and NQO1, which are inhibited by CIS, in
PPARα-dependent manner
As presented in Fig.
4C and D, CIS treatment significantly reduced the mRNA and
proteins levels of PPARα (P<0.001), Nrf2 (P<0.001), HO-1
(P<0.001) and NQO1 (P<0.001) in HK-2 cells, which was
reversed by FOR-L (P<0.05) and FOR-H treatment (P<0.01).
Furthermore, GW6471 treatment significantly reversed the effect of
FOR-H of these expression levels (P<0.001).
Nrf2 silencing effectively reverses the
protective effect of eupatilin or FOR on CIS-induced injury of HK-2
cells
Following transfection with siNrf2, the Nrf2 mRNA
expression was significantly downregulated in siNrf2 cells
(P<0.001; Fig. 5A). As shown
in Fig. 5B and C, the
CIS-inhibited decrease in viability and increase in apoptosis of
HK-2 cells were greatly attenuated by eupatilin (P<0.01) or FOR
treatment (P<0.01), but significantly reversed by Nrf2 silencing
(P<0.05). As presented in Fig.
5D-F, eupatilin (P<0.01) or FOR treatment (P<0.01)
significantly attenuated the CIS-promoted MDA level and MPO
activity, and the CIS-suppressed CAT activity. However, these
effects were reversed by Nrf2 silencing (P<0.05). As shown in
Fig. 6A and B, eupatilin
(P<0.001) or FOR treatment (P<0.001) significantly attenuated
the CIS-increased TNF-α and IL-1β levels, which were reversed by
Nrf2 silencing (P<0.001).
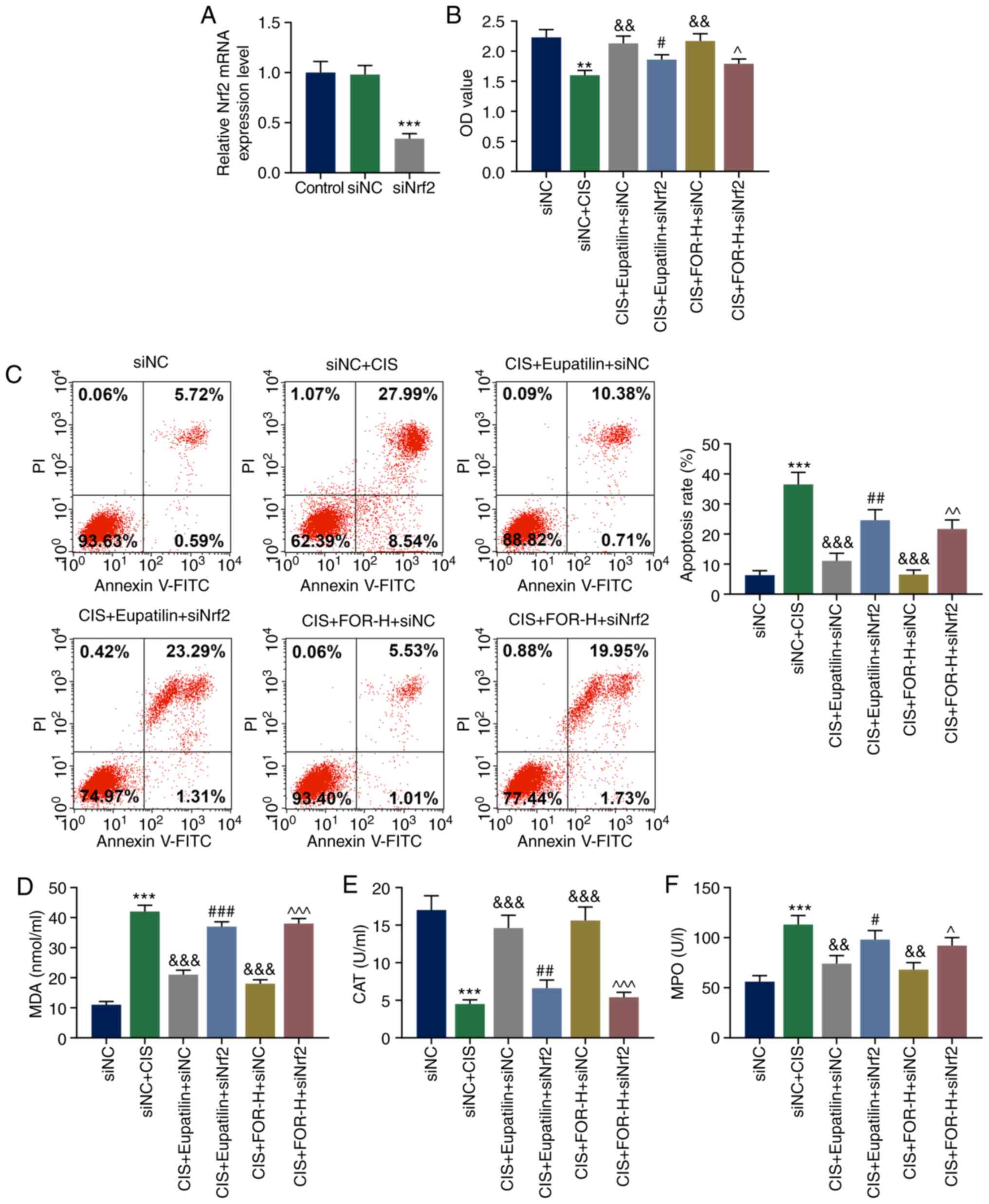 | Figure 5Nrf2 silencing effectively reverses
the protective effect of eupatilin or FOR on CIS-induced injury of
HK-2 cells. (A) Nrf2 mRNA expression was significantly
downregulated in HK-2 cells by transfection with siNrf2. (B)
Eupatilin or FOR increased the CIS-reduced proliferation in a
Nrf2-dependent manner. (C) Eupatilin or FOR reduced the
CIS-increased apoptosis in a Nrf2-dependent manner. (D) Eupatilin
or FOR reduced the CIS-increased MDA level in a Nrf2-dependent
manner. Eupatilin or FOR increased the CIS-reduced (E) CAT and (F)
MPO activities in a Nrf2-dependent manner. Data are presented as
mean ± standard deviation. **P<0.01,
***P<0.001 vs. siNC. &&P<0.01,
&&&P<0.001 vs. siNC + CIS.
#P<0.05, ##P<0.01,
###P<0.001 vs. CIS + Eupatilin + siNC. ^P<0.05,
^^P<0.01, ^^^P<0.001 vs. CIS + FOR-H +
siNC. Nrf2, nuclear factor erythroid 2-related factor 2; FOR,
formononetin; CIS, cisplatin; MDA, malondi-aldehyde; MPO,
myeloperoxidase; CAT, catalase; PI, propidium iodide; OD, optical
density; H, high dose; si, small interfering RNA; NC, negative
control. |
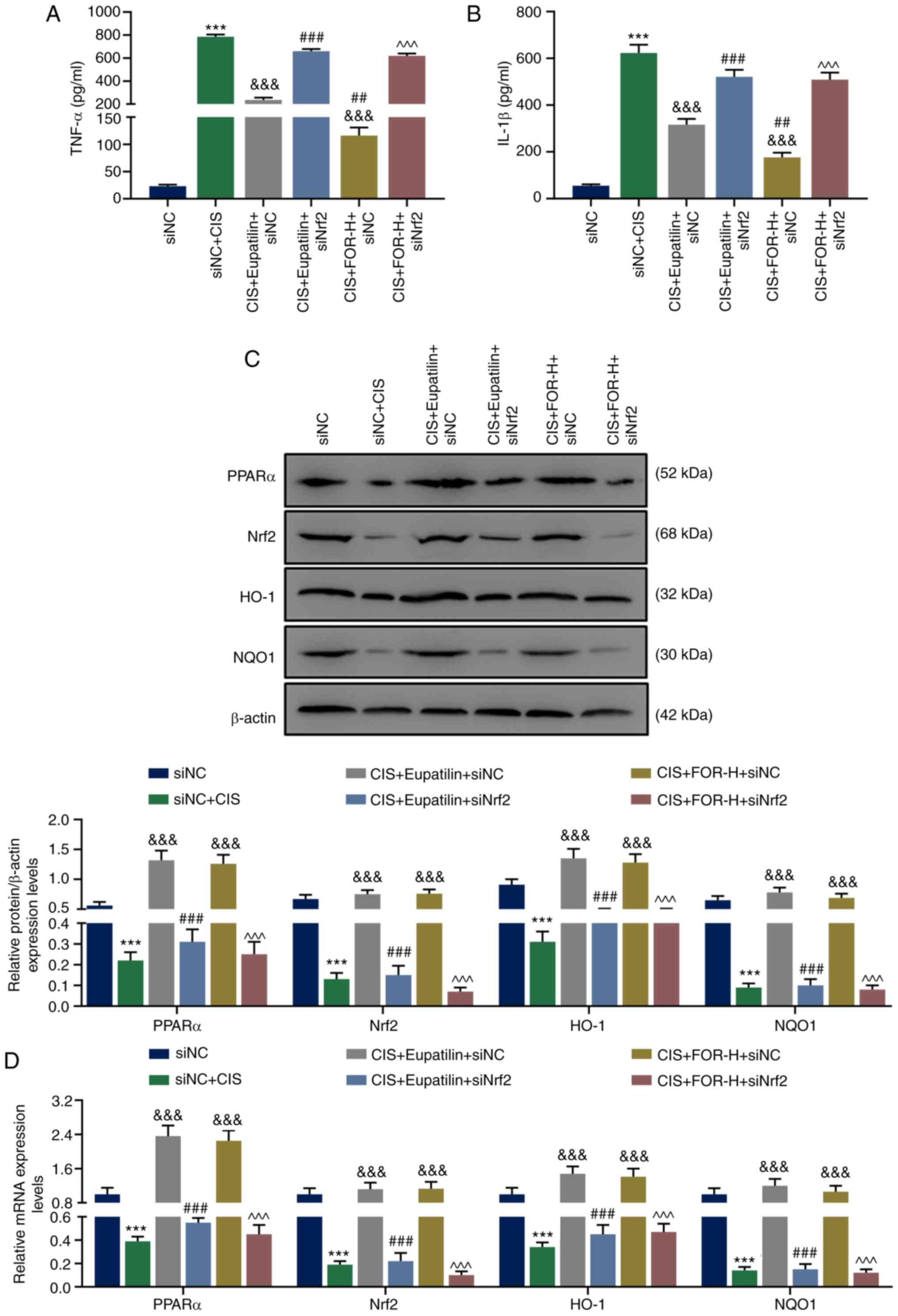 | Figure 6Nrf2 silencing effectively reverses
the protective effect of eupatilin or FOR CIS-induced injury of
HK-2 cells, and reverses the stimulative effect of eupatilin or FOR
on the CIS-inhibited PPARα/Nrf2/HO-1/NQO1 pathway in HK-2 cells.
Eupatilin or FOR reduced the CIS-induced increases of (A) TNF-α and
(B) IL-1β levels in a Nrf2-dependent manner. Eupatilin or FOR
increased the CIS-induced reduced (C) protein and (D) mRNA
expression levels of PPARα, Nrf2, HO-1 and NQO1 in a Nrf2-dependent
manner. Data are presented as mean ± standard deviation.
***P<0.001 vs. siNC.
&&&P<0.001 vs. siNC + CIS.
##P<0.01, ###P<0.001 vs. CIS +
Eupatilin + siNC. ^^^P<0.001 vs. CIS + FOR-H + siNC.
Nrf2, nuclear factor erythroid 2-related factor 2; FOR,
formononetin; CIS, cisplatin; H, high dose; si, small interfering
RNA; NC, negative control; PPARα, peroxisome proliferator-activated
receptor α; Nrf2, nuclear factor erythroid 2-related factor 2;
HO-1, heme oxygenase-1; NQO1, NAD(P)H quinone dehydrogenase 1. |
Nrf2 silencing effectively reverses the
stimulatory effect of eupatilin or FOR on the CIS-inhibited
PPARα/Nrf2/HO-1/NQO1 pathway in HK-2 cells
As presented in Fig.
6C and D, eupatilin (P<0.001) and FOR treatment (P<0.001)
signifi-cantly increased the mRNA and proteins levels of PPARα,
Nrf2, HO-1 and NQO1 of HK-2 cells, which were previously reduced by
CIS; however, this was reversed by Nrf2 silencing (P<0.001).
Discussion
The level of CIS in the kidney is the highest in the
proximal tubule (28). Proximal
tubular cell injury and death are responsible for CIS-induced AKI
(29), and proximal tubular cell
death is associated with high morbidity and mortality (30). Therefore, in order to relieve
kidney injury of patients with AKI and inves-tigate the protective
effect of FOR on CIS-induced AKI, the present study examined kidney
and proximal tubular cells under CIS condition. The present study
found that FOR protected against CIS-induced nephrotoxicity, as
supported by improved renal function, attenuated histopathological
changes of the rat model, and improved cell viability, reduced
apoptosis, oxidative stress, and inflammatory reaction of renal
proximal tubular cells.
PPARα protects against metabolic and inflammatory
derangements associated with AKI (31). Therefore, GW6471 (a selective
PPARα antagonist) was used to treat renal proximal tubular cells to
investigate the effect of PPARα on FOR in preventing HK-2 cells
from CIS-induced injury. The results demonstrated that FOR
protected against CIS-induced injury of HK-2 cells and increased
the expression levels of PPARα, Nrf2, HO-1 and NQO1, which were
previously reduced by CIS, in a PPAR-α-dependent manner.
In response to natural Nrf2 activators, oxidative
stress, inflammation and kidney disease are induced in animal
models, while Nrf2 silencing amplifies these pathogenic pathways
(32). Zoja et al
(33) also reported that blocking
Nrf2-activation promotes oxidative stress, inflammation and the
development of tissue damage in the kidney. Targeting Nrf2
ameliorates oxidative stress and inflammatory reactions in kidney
disease (34). Furthermore, FOR
upregulates Nrf2 in vivo and in vitro to protect
against AKI (18). The role of
Nrf2 in FOR protecting HK-2 cells from CIS-induced injury was
further investigated, and it was identified that FOR treatment or
upregulated PPAR-α protected HK-2 cells against CIS-induced injury
in an Nrf2-dependent manner, indicating that FOR treatment protects
HK-2 cells against renal injury induced by CIS through activation
of the PPARα/Nrf2 pathway.
Aladaileh et al (35) reported that FOR upregulates
Nrf2/HO-1 signaling to prevent oxidative stress, inflammatory
reaction and kidney impairment in methotrexate-induced rats.
Upregulated Nrf2/ARE/HO-1 signaling suppresses oxidative stress,
inflammatory reactions and apoptosis in rat kidneys exposed to
cyclophosphamide (36). Gang
et al (37) indicated that
NQO1 protects cells against renal failure induced by CIS. The
present study demonstrated that the PPARα, Nrf2, HO-1 and NQO1
expression levels previously reduced by CIS were reversed by FOR or
upregulated PPAR-α in an Nrf2-dependent manner. Therefore, FOR
treatment protected HK-2 cells against CIS-induced renal injury
through activation the of α/Nrf2/HO-1/NQO1 pathway.
The current findings demonstrated that the
protective effects of FOR on CIS-induced AKI were associated with
activation of the PPARα-Nrf2-HO-1/NQO1 pathway. However, whether
FOR could also clinically reduce the tumoricidal efficacy of CIS
remains unclear; therefore, more experiments are needed to
investigate the combined effects of FOR and CIS on patients with
AKI and the associated molecular mechanisms. Additionally, other
better alternative products, such as PPAR agonists, may be applied
as first line therapy for AKI in future study. The present study
focused both on cells and animals, supporting the results of each
other. Certainly, studies could be conducted more deeply both in
cells and animals in the future.
Taken together, the protective effect of FOR against
CIS-induced AKI, and improvement of renal function and alleviation
of histopathological changes were associated with the reduced
oxidative stress, inflammatory reaction, and activation of the
PPARα-Nrf2-HO-1/NQO1 pathway. The current research demonstrated
that FOR is a promising drug for preventing CIS-injured AKI.
Abbreviations:
|
FOR
|
formononetin
|
|
CIS
|
cisplatin
|
|
AKI
|
acute kidney injury
|
|
BUN
|
blood urea nitrogen
|
|
PPARα
|
peroxisome proliferator-activated
receptor α
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
HO-1
|
heme oxygenase-1
|
|
NQO1
|
NAD(P)H quinone dehydrogenase 1
|
|
MDA
|
malondialdehyde
|
|
MPO
|
myeloperoxidase
|
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and JM substantially contributed to the
conception and design of the study. WL, LP, YC and QZ acquired,
analyzed and interpreted the data. YH and JM drafted the article
and critically revised it for important intellectual content. YH
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of the work are
appropriately investigated and resolved. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethical Committee of
Experimental Animals of Zigong First People's Hospital, Zigong,
China (approval no. ZFPH201904223).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Lee YJ, Chan JP, Hsu WL, Lin KW and Chang
CC: Prognostic factors and a prognostic index for cats with acute
kidney injury. J Vet Intern Med. 26:500–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farrar A: Acute kidney injury. Nurs Clin
North Am. 53:499–510. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brix S and Stahl R: Acute kidney injury.
Dtsch Med Wochenschr. 142:290–300. 2017.In German. PubMed/NCBI
|
|
4
|
Ni J, Hou X, Wang X, Shi Y, Xu L, Zheng X,
Liu N, Qiu A and Zhuang S: 3-deazaneplanocin A protects against
cisplatin-induced renal tubular cell apoptosis and acute kidney
injury by restoration of E-cadherin expression. Cell Death Dis.
10:3552019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Almeida CDC, Simões E, Silva AC, de
Queiroz Oliveira JA, Batista ISF, Pereira FH, Gonçalves JE, Nobre V
and Martins MAP: Vancomycin-associated nephrotoxicity in
non-critically ill patients admitted in a Brazilian public
hospital: A prospective cohort study. PLoS One. 14:e02220952019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lameire NH, Bagga A, Cruz D, De Maeseneer
J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W and
Vanholder R: Acute kidney injury: An increasing global concern.
Lancet. 382:170–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rashid S, Nafees S, Siddiqi A, Vafa A,
Afzal SM, Parveen R, Ali N, Hasan SK, Barnwal P, Shahid A and
Sultana S: Partial protection by 18β glycrrhetinic acid against
cisplatin induced oxidative intestinal damage in wistar rats:
Possible role of NFkB and caspases. Pharmacol Rep. 69:1007–1013.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee H, Lee D, Kang KS, Song JH and Choi
YK: Inhibition of intracellular ROS accumulation by formononetin
attenuates cisplatin-mediated apoptosis in LLC-PK1 cells. Int J Mol
Sci. 19:8132018. View Article : Google Scholar :
|
|
9
|
Li J, Jiang K, Qiu X, Li M, Hao Q, Wei L,
Zhang W, Chen B and Xin X: Overexpression of CXCR4 is significantly
associated with cisplatin-based chemotherapy resistance and can be
a prognostic factor in epithelial ovarian cancer. BMB Rep.
47:33–38. 2014. View Article : Google Scholar :
|
|
10
|
Park JY, Lee D, Jang HJ, Jang DS, Kwon HC,
Kim KH, Kim SN, Hwang GS, Kang KS and Eom DW: Protective effect of
artemisia asiatica extract and its active compound eupatilin
against cisplatin-induced renal damage. Evid Based Complement
Alternat Med. 2015:4839802015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang YZ, Zhang J, Tan L, Xia Z, Wang CZ,
Zhou LD, Zhang Q and Yuan CS: Preparation and evaluation of
temperature and magnetic dual-responsive molecularly imprinted
polymers for the specific enrichment of formononetin. J Sep Sci.
41:3060–3068. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang D, Wang C, Duan Y, Meng Q, Liu Z,
Huo X, Sun H, Ma X and Liu K: Targeting Oct2 and P53: Formononetin
prevents cisplatin-induced acute kidney injury. Toxicol Appl
Pharmacol. 326:15–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chtourou Y, Aouey B, Aroui S, Kebieche M
and Fetoui H: Anti-apoptotic and anti-inflammatory effects of
naringin on cisplatin-induced renal injury in the rat. Chem Biol
Interact. 243:1–9. 2016. View Article : Google Scholar
|
|
14
|
Ning C, Gao X, Wang C, Huo X, Liu Z, Sun
H, Yang X, Sun P, Ma X, Meng Q and Liu K: Protective effects of
ginsenoside Rg1 against lipopolysaccharide/d-galactosamine-induced
acute liver injury in mice through inhibiting toll-like receptor 4
signaling pathway. Int Immunopharmacol. 61:266–276. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang JS, Kang ES, Han SG, Lim DS, Paek
KS, Lee CH and Seo HG: Formononetin inhibits
lipopolysaccharide-induced release of high mobility group box 1 by
upregulating SIRT1 in a PPARδ-dependent manner. Peer J.
6:e42082018. View Article : Google Scholar
|
|
16
|
Mu H, Bai YH, Wang ST, Zhu ZM and Zhang
YW: Research on antioxidant effects and estrogenic effect of
formononetin from Trifolium pratense (red clover). Phytomedicine.
16:314–319. 2009. View Article : Google Scholar
|
|
17
|
Nguyen LTH, Nguyen UT, Kim YH, Shin HM and
Yang IJ: Astragali Radix and its compound formononetin ameliorate
diesel particulate matter-induced skin barrier disruption by
regulation of keratinocyte proliferation and apoptosis. J
Ethnopharmacol. 228:132–141. 2019. View Article : Google Scholar
|
|
18
|
Huang D, Wang C, Meng Q, Liu Z, Huo X, Sun
H, Yang S, Ma X, Peng J and Liu K: Protective effects of
formononetin against rhabdomyolysis-induced acute kidney injury by
upregulating NRF2 in vivo and in vitro. RSC Adv. 6:110874–110883.
2016. View Article : Google Scholar
|
|
19
|
Gonzalez-Manan D, D'Espessailles A, Dossi
CG, San Martin M, Mancilla RA and Tapia GS: Rosa mosqueta oil
prevents oxidative stress and inflammation through the upregulation
of PPAR-α and NRF2 in C57BL/6J mice fed a high-fat diet. J Nutr.
147:579–588. 2017. View Article : Google Scholar
|
|
20
|
Wang WR, Liu EQ, Zhang JY, Li YX, Yang XF,
He YH, Zhang W, Jing T and Lin R: Activation of PPAR alpha by
fenofibrate inhibits apoptosis in vascular adventitial fibroblasts
partly through SIRT1-mediated deacetylation of FoxO1. Exp Cell Res.
338:54–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang S, Wei L, Xia R, Liu L, Chen Y, Zhang
W, Li Q, Feng K, Yu M, Zhang W, et al: Formononetin ameliorates
cholestasis by regulating hepatic SIRT1 and PPARα. Biochem Biophys
Res Commun. 512:770–778. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen J, Rasmussen M, Dong QR, Tepel M and
Scholze A: Expression of the NRF2 target gene NQO1 is enhanced in
mono-nuclear cells in human chronic kidney disease. Oxid Med Cell
Longev. 2017:90918792017. View Article : Google Scholar
|
|
23
|
Cao SS, Yan M, Hou ZY, Chen Y, Jiang YS,
Fan XR, Fang PF and Zhang BK: Danshen modulates Nrf2-mediated
signaling pathway in cisplatin-induced renal injury. J Huazhong
Univ Sci Technolog Med Sci. 37:761–765. 2017.PubMed/NCBI
|
|
24
|
Shelton LM, Park BK and Copple IM: Role of
Nrf2 in protection against acute kidney injury. Kidney Int.
84:1090–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ansari MA: Sinapic acid modulates
Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in
rats. Biomed Pharmacother. 93:646–653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen Y, Qiu T, Liu XH, Zhang L, Wang ZS
and Zhou JQ: Renal ischemia-reperfusion injury attenuated by
splenic ischemic preconditioning. Eur Rev Med Pharmacol Sci.
22:2134–2142. 2018.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Camano S, Lazaro A, Moreno-Gordaliza E,
Torres AM, de Lucas C, Humanes B, Lazaro JA, Milagros Gomez-Gomez
M, Bosca L and Tejedor A: Cilastatin attenuates cisplatin-induced
proximal tubular cell damage. J Pharmacol Exp Ther. 334:419–429.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: Mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coelho S, Cabral G, Lopes JA and Jacinto
A: Renal regeneration after acute kidney injury. Nephrology
(Carlton). 23:805–814. 2018. View Article : Google Scholar
|
|
31
|
Iwaki T, Bennion BG, Stenson EK, Lynn JC,
Otinga C, Djukovic D, Raftery D, Fei L, Wong HR, Liles WC and
Standage SW: PPARα contributes to protection against metabolic and
inflammatory derangements associated with acute kidney injury in
experimental sepsis. Physiol Rep. 7:e140782019. View Article : Google Scholar
|
|
32
|
Kong W, Fu J, Liu N, Jiao C, Guo G, Luan
J, Wang H, Yao L, Wang L, Yamamoto M, et al: Nrf2 deficiency
promotes the progression from acute tubular damage to chronic renal
fibrosis following unilateral ureteral obstruction. Nephrol Dial
Transplant. 33:771–783. 2018. View Article : Google Scholar
|
|
33
|
Zoja C, Benigni A and Remuzzi G: The Nrf2
pathway in the progression of renal disease. Nephrol Dial
Transplant. 29(Suppl 1): i19–i24. 2014. View Article : Google Scholar
|
|
34
|
Ruiz S, Pergola PE, Zager RA and Vaziri
ND: Targeting the transcription factor Nrf2 to ameliorate oxidative
stress and inflammation in chronic kidney disease. Kidney Int.
83:1029–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aladaileh SH, Hussein OE, Abukhalil MH,
Saghir SAM, Bin-Jumah M, Alfwuaires MA, Germoush MO, Almaiman AA
and Mahmoud AM: Formononetin upregulates Nrf2/HO-1 signaling and
prevents oxidative stress, inflammation, and kidney injury in
methotrexate-induced rats. Antioxidants (Basel). 8:4302019.
View Article : Google Scholar
|
|
36
|
AL Haithloul HAS, Alotaibi MF, Bin-Jumah
M, Elgebaly H and Mahmoud AM: Olea europaea leaf extract
up-regulates Nrf2/ARE/HO-1 signaling and attenuates
cyclophos-phamide-induced oxidative stress, inflammation and
apoptosis in rat kidney. Biomed Pharmacother. 111:676–685. 2019.
View Article : Google Scholar
|
|
37
|
Gang GT, Kim YH, Noh JR, Kim KS, Jung JY,
Shong M, Hwang JH and Lee CH: Protective role of NAD(P)H:quinone
oxidoreductase 1 (NQO1) in cisplatin-induced nephrotoxicity.
Toxicol Lett. 221:165–175. 2013. View Article : Google Scholar : PubMed/NCBI
|




















