1. Introduction
The number of people diagnosed with cancer worldwide
has consistently increased in recent years. There were >18
million new patients diagnosed with cancer and 9 million deaths
caused by cancer according to 2018 GLOBOCAN (1).
Interactions between stromal, epithelial and
extracellular matrix (ECM) components are increasingly recognized
as being important in cancer development and progression (2). Stromal cells, together with ECM
components, make up the tumour microenvironment (TME), which is
vital for cancer cell proliferation, invasion and metastatic
progression (2). Stromal
cell-derived factors (SDFs) comprise a group of proteins derived
from stromal cells (3). The
family mainly includes SDF-1, SDF-2, SDF2-like 1 (SDF2L1), SDF-3,
SDF-4 and SDF-5. These factors have all been identified in human
cancer tissues except for SDF-3, which has been found to be present
only in murine samples (3)
(Table I). Although they are
named similarly and belong to the same group, they exhibit
different structures and functions in cancer (3). SDF-1 has been the most studied
factor in this group, while less is known about the other factors
and their association with cancer.
 | Table ISDFs expression in different types of
tumour. |
Table I
SDFs expression in different types of
tumour.
| SDFs | Types of tumour
(Refs.) |
|---|
| SDF-1 | Oesophago-gastric,
pancreatic, lung, breast, colorectal and ovarian cancer (7) |
| SDF-2 | Breast cancer
(3), colorectal carcinoma
(34), hormone-independent tumour
from a medroxyprogesterone acetate mouse breast cancer model
(41) and oxaliplatin-resistant
gastric cancer cells (42) |
| SDF2L1 | Breast cancer
(3) and ovarian serous carcinoma
(40) |
| SDF-4 | Pancreatic cancer
(48,49), cervical cancer HeLa cell line
(44) and breast cancer (44) |
| SFRP2 | Lung cancer cells
(66), choriocarcinoma (67), gastric cancer (68), prostate cancer (69), melanoma (71,79), colorectal cancer (74), liver cancer (73) and breast cancer (78) |
2. Role of SDF-1 in promoting cancer
SDF-1, a 68-amino acid protein, belongs to the
chemokine family (4). Chemokines
are a large family that can regulate stem or progenitor cell
proliferation and movement (5).
Chemokines have been classified into four main subfamilies: CXC,
CC, C3XC and XC, depending on the presence and number of amino
acids between N-terminal cysteine residues (6). SDF-1 belongs to the CXC chemokine
family and it is also known as CXC motif ligand 12 (CXCL12). It is
expressed in various types of cancer, including oesophago-gastric,
pancreatic, lung, breast, colorectal and ovarian cancer (7). As a receptor of CXCL12, CXC motif
chemokine receptor 4 (CXCR4) has been found to be overexpressed in
>30 types of malignant tumours (7), such as lung (4), breast (8) and intestinal cancer (9). There is increasing evidence
demonstrating that CXCL12, as a secreted factor in the TME,
enhances tumour growth and survival (10,11), adhesion (12,13), chemoresistance (4), immunotherapy resistance (14-16), migration and invasion (9,17-19), angiogenesis and metastasis
(20) via the CXCL12/CXCR4 axis
(20,21), mainly in lung cancer, breast
cancer, gastric cancer, colon cancer, pancreatic cancer, ovarian
carcinoma, cervical carcinoma, endometrial cancer and leukaemia
(8,13,22-25). The JAK2/STAT3, MAPK, PI3K/AKT and
PI3K/Pyk2 signalling pathways have been identified as acting
downstream of the CXCL12/CXCR4 axis (4,17).
As aforementioned, the connection between CXCL12 and CXCR4
contributes to malignant behaviour. Therefore, targeting the
CXCL12/CXCR4 axis may be a viable target for tumour treatment.
CTCE-9908, a CXCL12 analogue, has been proven to inhibit
osteosarcoma growth, adhesion and metastasis (26), as well as breast cancer cell
proliferation (27). Plerixafor
(AMD3100), as a CXCR4 inhibitor, competitively inhibits prostate
tumorigenesis in the bone (28),
decreases ovarian cancer growth and metastasis (29), prevents brain-specific metastasis
(30) and protects the
blood-brain barrier in patients with lung cancer (30).
It is well known that genetic polymorphisms serve a
vital role in cancer pathogenesis. A meta-analysis including 17,876
participants concluded the association between SDF-1 rs1801157
polymorphism and cancer risk, demonstrating that the SDF-1
rs1801157 polymorphism may serve as a risk factor for lung and
urologic cancer (31).
The function of SDF-1 has been widely investigated
in cancer; however, the functions of the other stromal derived
factors, SDF-2, SDF2L1, SDF-4 and SDF-5, are less known in cancer.
Although they are named similarly and belong to the same family,
they exhibit distinct structures and functions.
3. Role of SDF-2 and SDF2L1 in endoplasmic
reticulum (ER) stress and cancer
SDF-2 is a small protein of 211 amino acids,
consisting of protein (O-mannosyltransferase, inositol 1, 4,
5-triphosphate receptor and ryanodine receptor) domains, which are
known as MIR motifs (32). Since
SDF-2 lacks a hydrophobic region in addition to that at the N
terminus, it was initially thought that SDF-2 may be a secretory,
but not membranous protein (33).
The SDF-2 gene is located on human chromosome 17 at qll.2, as
identified using in situ hybridization, and on chromosome 11
in mice (33). SDF-2 is
ubiquitously expressed in lung, breast, colon, liver and kidney
(3,33,34). SDF-2 in humans and mice contains a
tetrapeptide at its C-terminus similar to the KDEL motif (a
C-terminal sequence consisting of Lys-Asp-Glu-Leu), which
characterises ER resident proteins (35). SDF2L1 is a homologue of SDF-2; it
is an ER stress-inducible gene and a member of the
O-mannosyltransferase protein family (35). Mouse SDF2L1 and SDF-2 sequences
are 78% similar and 68% identical (36). SDF2L1 contains a C-terminal HDEL
sequence, which is an ER retention-like motif that acts as an ER
localisation signal (35). Both
proteins are ER residents, but SDF-2 is constitutively expressed,
whereas SDF2L1 expression is induced by ER stress (36). Since the ER is the appropriate
environment for protein folding, secretion and quality control, ER
impairment can lead to the accumulation of unfolded proteins, a
phenomenon known as ER stress, activating the unfolded protein
response (UPR) pathway (37).
Chronic ER stress can be triggered by a number of human diseases,
such as cancer, diabetes and neurodegenerative disorders (38). Similarly, an adverse cellular
environment, including low nutrient levels, low pH, oxygen
deprivation and gene mutation, can also lead to ER stress (36). The UPR process acts to maintain
cellular functions and sustain homeostasis, or to activate
apoptosis, acting as quality control (36). The UPR process, together with
other homeostatic regulatory systems, helps to minimise the
disturbances in the environment (36). Schott et al (39) demonstrated that SDF2-like proteins
are induced by ER-stress causing the accumulation of unfolded
proteins and that they served a functional role in ER-stress, as
well as in the UPR pathway. Additionally, it has been observed that
following ER stress and the activation of the UPR pathway,
SDF2-like proteins were increased, while silencing SDF2-like
proteins led to an imbalance in the cellular environment (35). Therefore, SDF2L1 is suggested to
be a critical protein in ER stress. A further understanding of the
mechanisms of SDF2L1 on ER stress may be helpful in identifying its
possible down-stream targets in cancer (37).
There is some direct evidence that has given insight
into the impact of SDF-2 and SDF2L1 in cancer. Kang et al
(3) demonstrated that SDF-2 and
SDF2L1 displayed differ-ential expression patterns in patients with
breast cancer. Upregulation of these factors was associated with a
better clinical outcome, which is markedly different from SDF-1
(3). SDF-2 transcript levels were
significantly lower in patients with metastasis (0.0014±0.0001) and
in those who died of the disease (0.049±0.037) (3). Furthermore, using the Nottingham
Prognostic Index as a prognostic factor led to the same outcome
that lower transcript levels were associated with a poor prognosis
(3). Similar to SDF-2, the
transcript levels of SDF2L1 were also lower in patients with a poor
prognosis (0.012±0.008) than in patients with a good prognosis
(0.529±0.50) (3). Moreover, the
levels of SDF2L1 in patients with metastatic disease and in those
who had died from cancer were markedly lower than in patients who
were disease-free, and the group with the highest tumour grade
exhibited the lowest transcript levels of SDF2L1 (3). Overall, this suggested that lower
levels of SDF-2 and SDF2L1 indicated a poor prognosis (3). Considering the various 'omic'
technologies that have expanded the expectations for biomarkers in
the cancer field, Vendrell et al (34) analysed genomics and
transcriptomics data in a series of R0 Dukes B and Dukes C
colorectal carcinomas to evaluate the identification of outcome
predictors; the multivariate Cox model was used to identify 68
genes associated with disease-free survival. Consequently, 74% of
these genes were upregulated (34). In the transcriptomic analyses,
downregulation of SDF-2 expression was associated with a poor
prognosis (P<0.01) (34).
Lower SDF-2 expression was observed to be an indicator of shorter
disease-free survival in colorectal cancer (34). In order to discover potential
predictive biomarkers, Willis et al (40) performed a meta-analysis; the
results of the meta-analysis were statistically significant with a
false discovery rate <0.05. The results revealed that low SDF2L1
mRNA expression was associated with a poor prognosis in patients
with ovarian serous carcinoma (40).
However, other studies have demonstrated the
opposite results. For example, Giulianelli et al (41) demonstrated that SDF-2 was highly
expressed in the hormone-independent tumour stroma compared with in
the hormone-dependent tumour stroma from a medroxyprogesterone
acetate-induced mouse breast cancer model. The aforementioned study
supported the hypothesis that SDF-2 may be involved with
hormone-independent tumour growth (41). Furthermore, Takahashi et al
(42) revealed that SDF-2
expression was upregulated in oxaliplatin (OXA)-resistant gastric
cancer cells and identified SDF-2 as a heat shock protein 72
(hsp72) client protein, which is unique to OCUM-2M/OXA cells. Hsp72
is a well-known stress-induced molecule that assists in the folding
of nascent polypeptides and the refolding of denatured proteins;
its constitutive overexpression enhances cancer cell stress
tolerance and enables the cancer cell to adapt to harsh conditions
(42). Hsp72 binds to the SDF-2
protein to promote refolding and prevents SDF-2 degradation
(42). The data from the
aforementioned study demonstrated that suppression of SDF-2 results
in enhanced OXA-induced anti-proliferative effects and apoptosis
(42). The data suggested that
SDF-2 may be a novel therapeutic target in the treatment of
OXA-resistant gastric cancer cells (42). Nevertheless, to the best of our
knowledge there is currently no molecule that has been developed to
target SDF-2 for cancer treatment. SDF-2 may potentially provide an
interesting target for tumour therapies and as a prognostic factor
in the future, but further studies are required.
4. Role of SDF-4 in cancer cell mobility,
proliferation and migration
The SDF-4 protein is a Ca2+-binding
protein of 45 kDa (Cab45), and a member of the
Cab45/reticulocalbin/ERC45/calumenin protein family (43). It is associated with
Ca2+-dependent secretory pathways and involved in
multiple diseases, including cancer (44). It consists of 361 amino acids and
the corresponding gene has seven exons (45). It is located on the 1p36.33
chromosome (44). Cab45 has a
signal sequence, six EF-hands and a HEEF motif at the C-terminal
(43,46). Alternative splicing produces three
isoforms with different physiological and pathophysiological
characters, namely the Golgilocalised variant (Cab45-G), the
cytosolic variant (Cab45-C) and the secreted variant (Cab45-S)
(47). Cab45-G is named according
to its main localisation to the Golgi complex (44). Cab45-C is nearly identical to the
130 amino acids of Cab45-G, except that Cab45-C lacks the signal
sequence and is localised in the cytosol (48). Cab45-C has neither a sixth-EF-hand
nor a HEEF sequence, and it participates in the exocytosis of
zymogen granules (48). Cab45-S
is located in the ER, is secreted and differs from Cab45-G at the
C-terminal sequences with no sixth EF-hand or HEEF motif (44).
Cab45 has been implicated to serve a role in
numerous diseases, including cancer, where it is involved in cell
migration and proliferation through a number of molecular
mechanisms (44). Cab45
upregulation has been suggested in Panc1 pancreatic cancer cells
(49), LIM1215 colon cancer cells
(50), HeLa cervical cancer cells
(44) and oesophageal cancer
cells (51), suggesting that
Cab45 may be involved in cancer progression. Cancer cell mobility
is a vital feature in the process of invasion and migration
(52). In order to promote
migration and invasion, factors are secreted into the environment
to provide proteins to the leading edge of a cell, which can pull
the cell forward (53). The
polarized orientation of the Golgi complex towards the leading edge
of a migrating cell is important to ensure that factors required
for persistent migratory activity are secreted at the cell's
leading edge (54). It has been
reported that after inhibiting vesicle formation at the trans-Golgi
Network (TGN), the resultant blocking of secretion disturbs
membrane delivery to the leading edge and directional cell
migration (55). Cab45 has been
proposed to serve a pivotal role in secretion at the Golgi complex
(44). Hence, Cab45 may serve a
potential role in tumour cell mobility (56).
Cab45 has also been implicated in cancer cell
proliferation. A previous study revealed that Cab45 expression was
significantly higher in pancreatic cancer cells compared with in
non-neoplastic ductal cells using the stable isotope labelling with
amino acids method (48).
Additionally, Grønborg et al (49) confirmed the upregulation of Cab45
in pancreatic cancer cells, in contrast to non-neoplastic ductal
cells, by immunohistochemical labelling, and indicated that Cab45
may be a potential biomarker in pancreatic tumours. Luo et
al (44) arrived to a similar
conclusion, revealing that there was high Cab45-G expression in the
HeLa cervical cancer cell line. The aforementioned findings are in
accordance with the finding that Cab45-S regulates the
Ca2+ level of the ER by binding to sarco/ER
Ca2+-ATPase 2b and acts as a crucial regulator of
proliferation in cervical cancer cell lines (47). It is well known that
Ca2+ serves a vital role in cellular proliferation.
Cab45-S can lead to nuclear translocation of the nuclear factor of
activated T cells (NFAT) by increasing Ca2+ levels that
result in cell proliferation (47) (Fig.
1). Intracellular levels of Ca2+ are limited, with
prolonged bouts of signalling dependent on the influx of external
Ca2+ via the store operated Ca2+ channels
(SOCs) in the plasma membrane (50). Weiss et al (57) demonstrated that the
anti-proliferative effect of non-steroidal anti-inflammatory drugs
(NSAIDs) in colorectal cancer (CRC) cells was enhanced when the
entry of SOCs was inhibited. Additionally, Ji et al
(50) revealed that, using
difference gel electrophoresis analysis, when the CRC LIM1215 cell
line was treated with sulindac, a type of NSAID, Cab45 was
secreted. A similar conclusion was confirmed by western blot
analysis in other CRC cell lines, such as HCT116, SW480 and SW1463
(50). Therefore, it may be
hypothesised that Cab45 may participate in SOCs entry to regulate
the effect of NSAIDs on CRC cells and that Cab45 upregulation may
be associated with tumour cell proliferation.
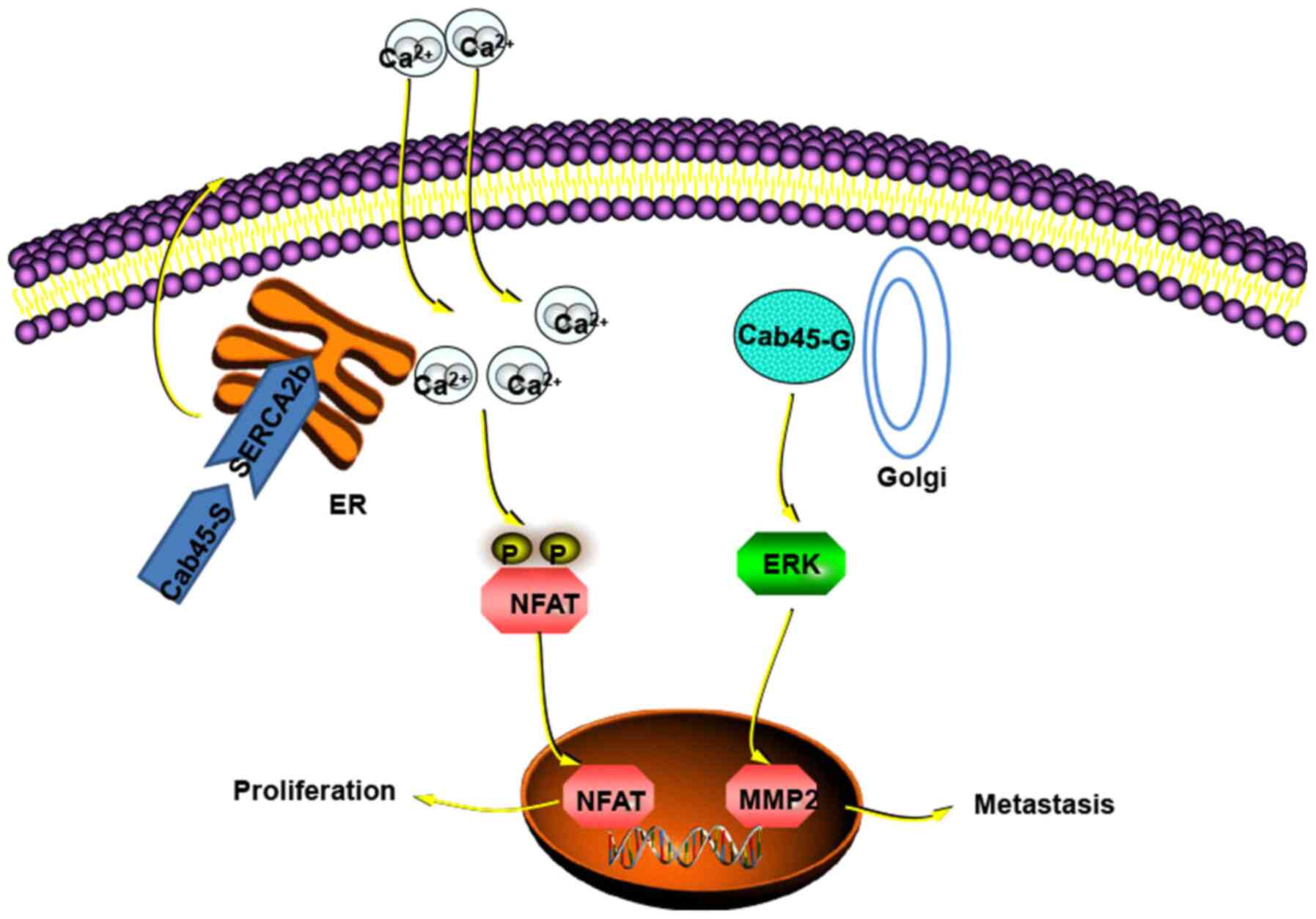 | Figure 1Function of Cab45-S in the ER and
Cab45-G in the Golgi apparatus. In the ER, Cab45-S binding to
SERCA2b leads to extracellular Ca2+ entry, which
activates Ca2+-NFAT signalling. This causes NFAT
translocation to the nucleus, resulting in cell proliferation.
Cab45-G enhances cancer metastasis by increasing MMP2 expression
via the ERK signalling pathway (44,47). The figure was prepared utilising
templates obtained from www.proteinlounge.com. ER, endoplasmic reticulum;
SERCA2b, sarco/ER Ca2+-ATPase 2b; NFAT, nuclear factor
of activated T cells; Cab, Ca2+-binding protein; S,
secreted variant; G, Golgilocalised variant; MMP2, matrix
metalloproteinase 2. |
Finally, Cab45-G was found to promote cell migration
and metastasis. The process of tumour migration and metastasis is
complex and involves numerous biological aspects, including the
effect of epithelial-mesenchymal transition (EMT), matrix
metalloproteinases (MMPs) and ECM proteins. EMT is a critical
process in epithelial cells, allowing them to attain migratory and
diffusive abilities (58). During
EMT, epithelial cells lose cell-to-cell junctions and apicobasal
polarity, and develop an enhanced migratory capacity and upregulate
N-Cadherin and β-catenin expression, which then leads to tumour
cell migration (53). Firstly,
Cab45 contributes to the enhanced expression levels of
EMT-associated proteins, including N-Cadherin, β-catenin and
vimentin, in breast cancer, while the levels of E-Cadherin are
decreased (44). In addition,
Cab45-G has been associated with MMPs in human cervical cancer
tissues (44). MMPs contribute to
the cleavage of the matrisome (global composition of the ECM
proteome) and of proteins that are responsible for ECM remodelling,
causing ECM degradation (59).
Additionally, MMPs activate some bioactive molecules, including
cytokines, growth factors and receptors, which have been proved to
promote tumour progression and metastasis (60). Cab45-G was demonstrated to be
significantly associated with MMP2 expression, which has been
implicated in tumour metastasis by activating the ERK signalling
pathway in HeLa cells (44)
(Fig. 1). Similar findings have
revealed that Cab45 is responsible for successful sorting of a
subset of proteins at the TGN for cell migration, such as the
ECM-associated proteins matrix Gla protein, thrombospondins 1 and
3, and MMP2 (56). Therefore, the
aforementioned studies suggest that Cab45-G may regulate cell
migration through ECM-associated proteins, MMPs and molecular
mediators of EMT (44,56,58,60).
Overall, Cab45 may serve as a therapeutic target for
cancer treatment and a potential predictor. However, to the best of
our knowledge, no studies have been performed to explore the
corresponding targeted therapy to Cab45.
5. Controversial roles of SFRP2 in the
Wnt/β-catenin signalling pathway and methylation of SFRP2 in
cancer
SDF-5 was initially discovered from a cDNA library
from a murine bone marrow stromal cell line (61). Kang et al (3) reported that SDF-5 transcripts were
decreased in breast cancer, but little knowledge is available with
regard to SDF-5 function. SDF-5 belongs to the secreted frizzled
proteins and it is similar to secreted frizzled-related protein 2
(SFRP2) (36). SFRP2 was
identified as a member of the SFRP family (62). SDF-5 is homologous to Frizzled,
which is the extracellular portion of the Wnt receptor (62), and so is able to compete with
Frizzled receptors in interactions with Wnt proteins (63).
Wnt/β-catenin signalling serves a key role in
various biological processes including carcinogenesis, embryonic
development and neurodegenerative diseases (62). It is highly recognised that
Wnt/β-catenin signalling is activated in various types of human
cancer, including non-small cell lung cancer (NSCLC), melanoma and
colorectal cancer. When the Wnt ligand binds to the
Frizzled/lipoprotein receptor-related protein 5/6, the
Wnt/β-catenin signalling pathway is activated and dishevelled 3
simultaneously starts to accumulate, which inhibits the combination
of β-catenin, Axin, adenomatosis polyposis coli and glycogen
synthase kinase-3β; this in turn suppresses phosphorylation and
degradation of β-catenin (63).
Consequently, the increased accumulation of β-catenin in the
cytoplasm results in its translocation to the nucleus, where it
interacts with members of the T-cell factor/lymphoid enhancer
factor family of transcription factors to stimulate the expression
of genes involved in cell survival, proliferation and osteoblastic
differentiation (63,64) (Fig.
2A).
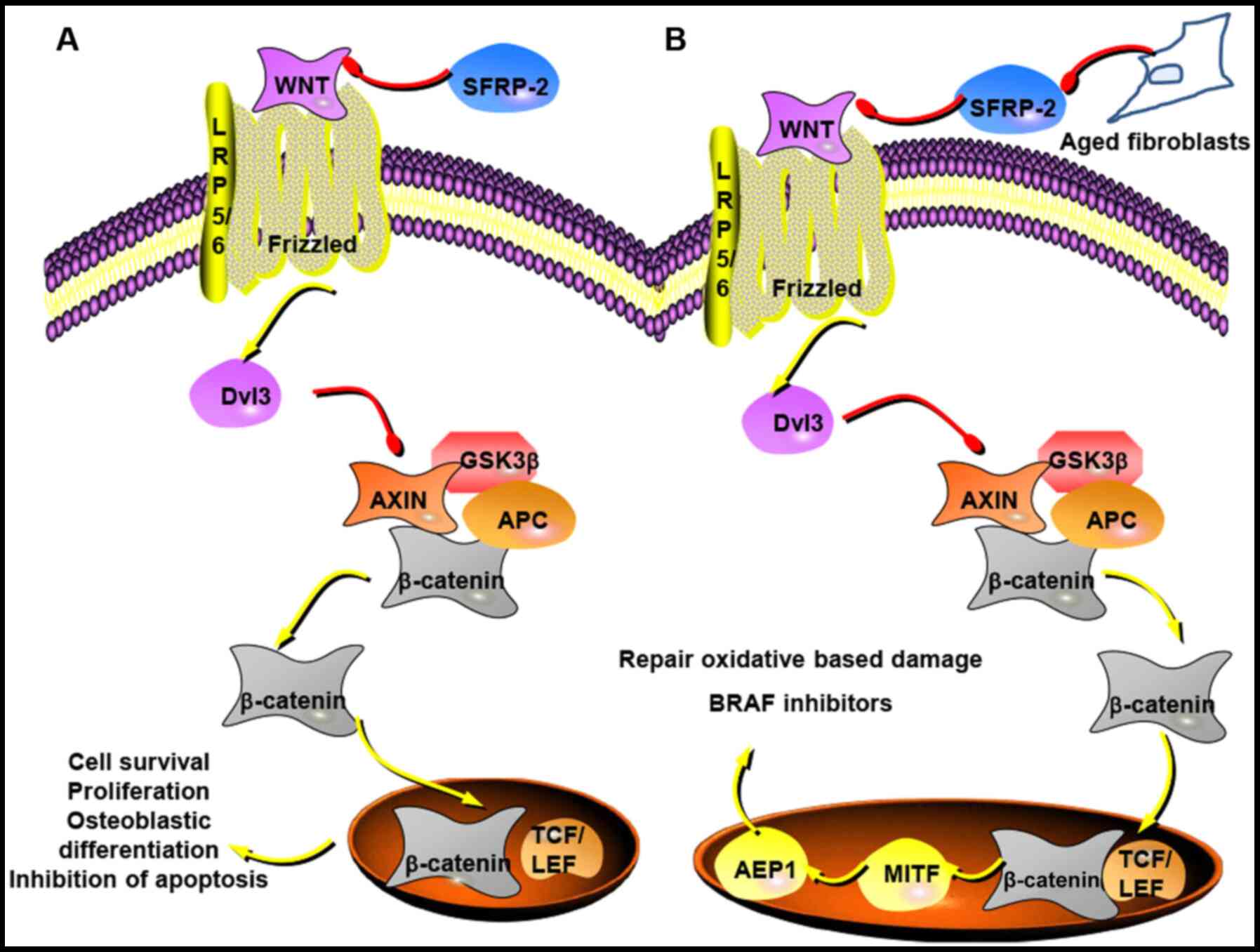 | Figure 2SFRP-2 in the Wnt/β-catenin
signalling pathway in cancer. (A) Wnt binding to Frizzled/LRP5/6
induces Dvl3 accumulation, which inhibits the complex of β-catenin,
Axin, APC and GSK-3β. This leads to an increase of cytoplasmic
β-catenin, which then translocates to the nucleus to interact with
TCF/LEF family of transcription factors that contribute to cell
survival, proliferation and osteoblastic differentiation. SFRP-2 as
a competitive receptor of Wnt may regulate the Wnt/β-catenin
signalling pathway to inhibit cell proliferation and survival, and
lead to apoptosis (63,64). (B) SFRP-2 derived from aged
fibroblasts may inhibit β-catenin and MITF signalling, resulting in
a decrease of AEP1 that makes melanoma cells more sensitive to
oxidative stress and resistant to BRAF inhibitors (74). The figure was prepared utilising
templates obtained from www.proteinlounge.com. SFRP-2, secreted
frizzled-related protein 2; LRP5/6, lipoprotein receptor-related
protein 5/6; Dvl3, dishevelled 3; APC, adenomatosis polyposis coli;
GSK-3β, glycogen synthase kinase-3β; TCF/LEF, T-cell
factor/lymphoid enhancer factor; MITF, microphthalmia-associated
transcription factor; AEP1, apurinic/apyrimidinic endonuclease
1. |
Increasing evidence has demonstrated that SFRP2 can
regulate Wnt/β-catenin signalling in tumours, acting as an
antagonist and contributing to the inhibition of tumour malignancy
(65). SFRP2 expression is
downregulated in the NSCLC A549 cell line compared with in a normal
pulmonary epithelial cell line (65). Furthermore, SFRP2 may inhibit the
survival and metastasis of NSCLC cells via the Wnt/β-catenin
signalling pathway (66). Zeng
et al (67) reported that
SFRP2 expression was downregulated in choriocarcinoma, and low
SFRP2 expression induced tumour migration and invasion via
Wnt/β-catenin signalling. Cheng et al observed the same
phenomena in gastric cancer, in which SFRP2 expression was
decreased in tumour tissues compared with in adjacent non-cancer
tissues (68). Additionally,
Perry et al (69) revealed
that SFRP2 expression was lower in prostate cancer tissues compared
with in normal tissues (P<0.01). Moreover, it has been
demonstrated that increasing the expression levels of SFRP2 can
inhibit cancer cell proliferation and lead to cell apoptosis in
vivo (68).
The methylation of SFRP2 serves an important role in
tumour invasion and metastasis. Methylation of gene promoter
regions, mainly located in CpG islands, is a common feature in
human cancer, where it typically leads to epigenetic silencing of
tumour suppressor genes (70).
SFRP2 is often methylated in several types of human cancer, such as
melanoma, gastric carcinoma, colorectal, liver and lung cancer
(63,71-73). In an analysis of SFRP2, which
included 10 studies, 6 studies compared 936 patients with CRC and
794 normal colonic mucosa, and 763 patients with CRC and 487 benign
mucosal lesions. The data demonstrated that SFRP2 methylation in
CRC was higher than in normal colonic mucosa and benign mucosal
lesions [odds ratio (OR)=31.38 and P<0.001, and OR=4.83 and
P<0.001, respectively] (74),
indicating that SFRP2 methylation may be used as a non-invasive
biomarker for the diagnosis of CRC. Zhang et al (65) indicated that, compared with in
normal lung tissues, SFRP2 mRNA expression was significantly
decreased in NSCLC tissues, while the methylation of the SFRP2 gene
displayed the opposite trend. In NSCLC cell lines, the
demethylation of the SFRP2 gene aided in the restoration of SFRP2
expression at the RNA and protein levels (65). Therefore, tumour cell invasion may
be inhibited by the stimulation of SFRP2 (65). It is reported that the
demethylation of the SFRP2 gene appeared to inhibit two key
factors, zinc finger E-box binding homeobox 1 (ZEB1) and MMP9
(65). ZEB1 is a promoter
involved in EMT, while MMP9 is an important MMP family member that
leads to the degradation of the basement membrane, which results in
tumour metastasis (65). SFRP2
may therefore suppress NSCLC invasion by inhibiting ZEB1 and MMP9,
and SFRP2 methylation may promote NSCLC invasion (65).
Numerous studies have indicated that SFRP2 acts as
an anti-oncogene (65,66,68-70); however, other studies have
suggested the opposite and that it serves as an oncogene to promote
carcinoma development and progression (63,75-77). In breast cancer, SFRP2 expression
is upregulated in canine mammary gland tumours compared with in
normal tissues (78). Lee et
al (77) indicated that SFRP2
inhibited canine mammary gland tumour UV- induced apoptosis via
activation of the NF-κB signalling pathway or by suppressing the
JNK signal-ling pathway. Additionally, SFRP2 serves a role in
leading cell adhesion and anti-apoptotic functions by integrating
with the fibronectin-integrin protein complex in mammary tumours
(75). Furthermore, SFRP2 was
proposed to lead to tumour growth in glioma and renal cancer via
activation of canonical Wnt signal-ling, or to promote angiogenesis
through calcineurin/NFAT in breast cancer (76). Data suggests that as dermal
fibroblasts grow old, SFRP2 expression increases as an antagonist
of the Wnt signalling pathway to drive melanoma cell metastasis
(79). SFRP2 inhibits β-catenin
and microphthalmia-associated transcription factor signalling,
which is a main regulator of melanoma metabolism; this in turn
decreases the redox regulator apurinic/apyrimidinic endonuclease 1,
which is involved in repairing oxidative-based damage, makes
melanoma cells more sensitive to oxidative stress and drives
resistance to BRAF inhibitors, which are an effective treatment of
metastatic melanoma (79)
(Fig. 2B). In elderly patients,
the TME is more likely to activate signalling pathways that drive
more aggressive melanoma cell behaviour (79). It has previously been reported
that downregulating SFRP2 results in a decrease of breast cancer
tumour volume to 46% compared with control tissues in mice
(80). A similar trend was
observed in lung cancer, where overexpression of SFRP2 in the NSCLC
A549 cell line promoted lung cancer cell proliferation (81). After downregulating SFRP2, both
CDK4 and cyclin D1 mRNA and protein expression was downregulated,
and cell proliferation was suppressed at the G1 phase
(81). Considering that when
cyclin D1 binds to its activator CDK4, it enables the cell cycle
G1 checkpoint to continue, previous data suggests that
SFRP2 may serve a role in enhancing lung cancer cell proliferation,
invasion and migration (81).
Additionally, Liu et al (63) revealed that overexpression of
SFRP2 in lung cancer may promote tumour diffusion and availability
of Wnt proteins, resulting in activation of canonical Wnt
signalling and tumorigenesis.
Accumulating studies have demonstrated that SFRP2
regulation is associated with Wnt signalling activity and tumour
progression, including lung cancer, choriocarcinoma, gastric
cancer, prostate cancer, colorectal cancer and melanoma (66-69,71,74). SFRP2 has the potential to act as a
methylation biomarker for the diagnosis of NSCLC (72). It functions as an oncogene or
anti-oncogene in numerous types of cancer, and whether it acts as
an antagonist or agonist in Wnt signalling remains controversial.
Regarding the therapeutic aspects, due to the inhibitory functions
of SFRP2 observed in the majority of studies (65,66,68-70), SFRP2-like molecules or drug
inhibitors should be explored to target tumour cells and inhibit
Wnt signalling. XAV939, a Wnt inhibitor, has been demonstrated to
reverse the EMT phenotype and stemness markers, resulting in the
inhibition of migratory and invasive abilities in choriocarcinoma
(67). Moreover, due to the role
of SFRP2 methylation in promoting tumour progression, drugs that
reverse or prevent this methylation may be helpful for tumour
therapy. For example, 5-Aza-2'-deoxycytidine is able to cause
promoter demethylation of SFRP2 and has been demonstrated to
inhibit tumour migration and invasion in choriocarcinoma (67). Similarly, in addition to agents
that inhibit Wnt signalling and activation of the SFRP2 gene,
therapies that activate silenced genes, such as SFRP2,
epigenetically may be of interest (82). DNA methyltransferases (DNMTs)
contribute to the epigenetic regulation, and novel drugs targeting
DNMTs are the subjects of scientific research in ongoing clinical
trials in different types of cancer (63).
6. Outlook and future perspectives
For several decades, it was considered that the
process of tumour development was associated with genetic
alterations. There is now growing recognition that the TME serves
an essential role in the process of cancer development. SDFs, which
are derived from stromal cells, have an impact in tumour-stroma
networks in cancer. SDF-1 has been the subject of most scientific
research and is involved in the progression of various types of
cancer and stages, including tumorigenesis, metastasis and
survival. SDF-2 and SDF2L1 are ER-resident proteins associated with
ER stress. SDF2L1 expression is upregulated by ER stress and SDF-2
is constitutively expressed. They may function as negative
regula-tors in cancer through activation of the ER stress to
balance the cellular environment. It has been demonstrated that
cancer cells may overcome this mechanism, preventing ER
stress-induced apoptosis (83).
Additionally, it has been demonstrated that SDF-2 can promote
acquisition of OXA resistance via enhancing the ER stress to avoid
OXA-induced stress (42). These
results make it difficult to understand the role of SDF-2/SDF2L1 in
cancer and therefore future work is required to identify and fully
establish the function of SDF-2/SDF2L1 in tumour development, ER
stress and drug resistance. This elucidation may lead to potential
therapeutic targets for novel cancer treatments. However, no drug
or SDF-2-like therapeutics have currently been developed to target
SDF-2 for cancer treatment. The splicing isoforms of SDF-4 (Cab45)
may regulate cancer cell mobility, proliferation and migration
through various mechanisms, including by regulating EMT, MMPs and
ECM, but the underlying molecular mechanisms surrounding this
regulation remain unclear. SDF-4 appears to have promise as a
therapeutic target for the treatment of cancer, but further studies
are required to fully explore the associated mechanisms. Based on
the majority of previous studies (65,66,68-70), SFRP2 functions as a negative
regulator in tumour growth and metastasis in a number of types of
cancer; however, there are contrasting results. Overall, the
specific role of SFRP2 in cancer remains unclear. The function of
SFRP2 may be associated with different signalling pathways,
different stages of tumour progression, human age and different
types of tumour. Defining the exact function of SFRP2 in different
types of cancers requires to be further elucidated. If this can be
clarified, SFRP2 may serve as a potential target for cancer
therapy. A number of studies have demonstrated that SFRP2 serves a
pivotal role in the Wnt signalling pathway and cancer (65-67,76,79). SFRP2-like therapeutics or drug
inhibitors warrant scientific attention for their capacity to
target tumour cells and inhibit Wnt signalling. Although there is
currently no Wnt inhibitor used in the clinical setting, there have
been increasing therapeutic approaches under research, such as
promoters of SFRP2 activation, Wnt inhibitors and DNA
methyltransferases. Further studies regarding the role of SFRP2 in
cancer are required to highlight its potential for tumour
treatment.
7. Conclusion
Overall, SDFs serve an important role in
tumorigenesis and tumour progression. SDF-1 is widely known to
enhance tumour malignant behaviour. SDF-1 analogues or CXCR4
inhibitors have been demonstrated to inhibit tumour growth and
metastasis. SDF-2 has some potential as a predictive biomarker in
tumours, but little progress has been made on the development of
treatments targeting SDF-2. SDF-4 (Cab45) has been observed to
enhance tumour cell mobility, proliferation and metastasis, and may
therefore serve as a potential target for tumour treatment.
However, further studies are required to explore the therapeutic
potential of targeting Cab45. SFRP2 has been observed to serve a
controversial role in cancer. The majority of previous studies
suggest that it functions as an antagonist, and there have been
some molecular therapeutic interventions targeting SFRP2 or Wnt
signalling under research, which may provide new tumour treatments
in the future.
Funding
This study was supported by the Cardiff University
China Medical Scholarship and the Outstanding Young Scholarship
from Yantai Yuhuangding Hospital (grant no. YDH050719).
Availability of data and materials
Not applicable.
Authors' contributions
WG wrote the manuscript. TAM, AJS and WGJ
contributed to manuscript preparation. PS, WGJ and AJ were involved
in the conception of the study. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Micke P and Ostman A: Tumour-stroma
interaction: Cancer-associated fibroblasts as novel targets in
anti-cancer therapy? Lung Cancer. 45(Suppl 2): S163–S175. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang H, Escudero-Esparza A, Douglas-Jones
A, Mansel RE and Jiang WG: Transcript analyses of stromal cell
derived factors (SDFs): SDF-2, SDF-4 and SDF-5 reveal a different
pattern of expression and prognostic association in human breast
cancer. Int J Oncol. 35:205–211. 2009.PubMed/NCBI
|
|
4
|
Wang M, Lin T, Wang Y, Gao S, Yang Z, Hong
X and Chen G: CXCL12 suppresses cisplatin-induced apoptosis through
activation of JAK2/STAT3 signaling in human non-small-cell lung
cancer cells. Onco Targets Ther. 10:3215–3224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller MC and Mayo KH: Chemokines from a
structural perspective. Int J Mol Sci. 18:20882017. View Article : Google Scholar :
|
|
6
|
Mélik-Parsadaniantz S and Rostène W:
Chemokines and neuro-modulation. J Neuroimmunol. 198:62–68. 2008.
View Article : Google Scholar
|
|
7
|
Samarendra H, Jones K, Petrinic T, Silva
MA, Reddy S, Soonawalla Z and Gordon-Weeks A: A meta-analysis of
CXCL12 expression for cancer prognosis. Br J Cancer. 117:124–135.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ling X, Spaeth E, Chen Y, Shi Y, Zhang W,
Schober W, Hail N Jr, Konopleva M and Andreeff M: The CXCR4
antagonist AMD3465 regulates oncogenic signaling and invasiveness
in vitro and prevents breast cancer growth and metastasis in vivo.
PLoS One. 8:e584262013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kollmar O, Rupertus K, Scheuer C, Junker
B, Tilton B, Schilling MK and Menger MD: Stromal cell-derived
factor-1 promotes cell migration and tumor growth of colorectal
metastasis. Neoplasia. 9:862–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kryczek I, Wei S, Keller E, Liu R and Zou
W: Stroma-derived factor (SDF-1/CXCL12) and human tumor
pathogenesis. Am J Physiol Cell Physiol. 292:C987–C995. 2007.
View Article : Google Scholar
|
|
11
|
Wu M, Chen Q, Li D, Li X, Li X, Huang C,
Tang Y, Zhou Y, Wang D, Tang K, et al: LRRC4 inhibits human
glioblastoma cells proliferation, invasion, and proMMP-2 activation
by reducing SDF-1 alpha/CXCR4-mediated ERK1/2 and Akt signaling
pathways. J Cell Biochem. 103:245–255. 2008. View Article : Google Scholar
|
|
12
|
Dehghani M, Kianpour S, Zangeneh A and
Mostafavi-Pour Z: CXCL12 modulates prostate cancer cell adhesion by
altering the levels or activities of β1-containing integrins. Int J
Cell Biol. 2014:9817502014. View Article : Google Scholar
|
|
13
|
Shen X, Wang S, Wang H, Liang M, Xiao L
and Wang Z: The role of SDF-1/CXCR4 axis in ovarian cancer
metastasis. J Huazhong Univ Sci Technolog Med Sci. 29:363–367.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou W, Guo S, Liu M, Burow ME and Wang G:
Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr Med Chem.
26:3026–3041. 2019. View Article : Google Scholar
|
|
15
|
Chen Y, Ramjiawan RR, Reiberger T, Ng MR,
Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP, et al:
CXCR4 inhibition in tumor microenvironment facilitates
anti-programmed death receptor-1 immunotherapy in sorafenib-treated
hepatocellular carcinoma in mice. Hepatology. 61:1591–1602. 2015.
View Article : Google Scholar :
|
|
16
|
Wald O: CXCR4 based therapeutics for
non-small cell lung cancer (NSCLC). J Clin Med. 7:3032018.
View Article : Google Scholar :
|
|
17
|
Otsuka S and Bebb G: The CXCR4/SDF-1
chemokine receptor axis: A new target therapeutic for non-small
cell lung cancer. J Thorac Oncol. 3:1379–1383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang B, Wang W, Niu W, Liu E, Liu X, Wang
J, Peng C, Liu S, Xu L, Wang L and Niu J: SDF-1/CXCR4 axis promotes
directional migration of colorectal cancer cells through
upregulation of integrin alphavbeta6. Carcinogenesis. 35:282–291.
2014. View Article : Google Scholar
|
|
19
|
Walentowicz-Sadlecka M, Sadlecki P, Bodnar
M, Marszalek A, Walentowicz P, Sokup A, Wilińska-Jankowska A and
Grabiec M: Stromal derived factor-1 (SDF-1) and its receptors CXCR4
and CXCR7 in endometrial cancer patients. PLoS One. 9:e846292014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mao W, Yi X, Qin J, Tian M and Jin G:
CXCL12/CXCR4 axis improves migration of neuroblasts along corpus
callosum by stimulating MMP-2 secretion after traumatic brain
injury in rats. Neurochem Res. 41:1315–1322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahirwar DK, Nasser MW, Ouseph MM, Elbaz M,
Cuitiño MC, Kladney RD, Varikuti S, Kaul K, Satoskar AR, Ramaswamy
B, et al: Fibroblast-derived CXCL12 promotes breast cancer
metastasis by facilitating tumor cell intravasation. Oncogene.
37:4428–4442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lang J, Zhao X, Qi Y, Zhang Y, Han X, Ding
Y, Guan J, Ji T, Zhao Y and Nie G: Reshaping prostate tumor
microenvironment To suppress metastasis via cancer-associated
fibroblast inactivation with peptide-assembly-based nanosystem. ACS
Nano. 13:12357–12371. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mahadevan D and Von Hoff DD: Tumor-stroma
interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther.
6:1186–1197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling
X, Frolova O, Levis M, Rubin JB, Negrin RR, Estey EH, et al:
Targeting the leukemia microenvironment by CXCR4 inhibition
overcomes resistance to kinase inhibitors and chemotherapy in AML.
Blood. 113:6215–6224. 2009. View Article : Google Scholar :
|
|
25
|
Kong L, Guo S, Liu C, Zhao Y, Feng C, Liu
Y, Wang T and Li C: Overexpression of SDF-1 activates the NF-kappaB
pathway to induce epithelial to mesenchymal transition and cancer
stem cell-like phenotypes of breast cancer cells. Int J Oncol.
48:1085–1094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SY, Lee CH, Midura BV, Yeung C,
Mendoza A, Hong SH, Ren L, Wong D, Korz W, Merzouk A, et al:
Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the
development of murine pulmonary metastases. Clin Exp Metastasis.
25:201–211. 2008. View Article : Google Scholar
|
|
27
|
Meng W, Xue S and Chen Y: The role of
CXCL12 in tumor microenvironment. Gene. 641:105–110. 2018.
View Article : Google Scholar
|
|
28
|
Conley-LaComb MK, Semaan L, Singareddy R,
Li Y, Heath EI, Kim S, Cher ML and Chinni SR: Pharmacological
targeting of CXCL12/CXCR4 signaling in prostate cancer bone
metastasis. Mol Cancer. 15:682016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ray P, Lewin SA, Mihalko LA, Schmidt BT,
Luker KE and Luker GD: Noninvasive imaging reveals inhibition of
ovarian cancer by targeting CXCL12-CXCR4. Neoplasia. 13:1152–1161.
2011. View Article : Google Scholar
|
|
30
|
Li H, Chen Y, Xu N, Yu M, Tu X, Chen Z,
Lin M, Xie B, Fu J and Han L: AMD3100 inhibits brain-specific
metastasis in lung cancer via suppressing the SDF-1/CXCR4 axis and
protecting blood-brain barrier. Am J Transl Res. 9:5259–5274.
2017.
|
|
31
|
Tong X, Ma Y, Deng H, Wang X, Liu S, Yan
Z, Peng S and Fan H: The SDF-1 rs1801157 polymorphism is associated
with cancer risk: An update pooled analysis and FPRP test of 17,876
participants. Sci Rep. 6:274662016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ponting CP: Novel repeats in ryanodine and
IP3 receptors and protein O-mannosyltransferases. Trends Biochem
Sci. 25:48–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamada T, Tashiro K, Tada H, Inazawa J,
Shirozu M, Shibahara K, Nakamura T, Martina N, Nakano T and Honjo
T: Isolation and characterization of a novel secretory protein,
stromal cell-derived factor-2 (SDF-2) using the signal sequence
trap method. Gene. 176:211–214. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vendrell E, Ribas M, Valls J, Solé X, Grau
M, Moreno V, Capellà G and Peinado MA: Genomic and transcriptomic
prognostic factors in R0 Dukes B and C colorectal cancer patients.
Int J Oncol. 30:1099–1107. 2007.PubMed/NCBI
|
|
35
|
Fukuda S, Sumii M, Masuda Y, Takahashi M,
Koike N, Teishima J, Yasumoto H, Itamoto T, Asahara T, Dohi K and
Kamiya K: Murine and human SDF2L1 is an endoplasmic reticulum
stress-inducible gene and encodes a new member of the Pmt/rt
protein family. Biochem Biophys Res Commun. 280:407–414. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lorenzon-Ojea AR, Caldeira W, Ribeiro AF,
Fisher SJ, Guzzo CR and Bevilacqua E: Stromal cell derived factor-2
(Sdf2): A novel protein expressed in mouse. Int J Biochem Cell
Biol. 53:262–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lorenzon-Ojea AR, Yung HW, Burton GJ and
Bevilacqua E: The potential contribution of stromal cell-derived
factor 2 (SDF2) in endoplasmic reticulum stress response in severe
preeclampsia and labor-onset. Biochim Biophys Acta Mol Basis Dis.
1866:1653862020. View Article : Google Scholar
|
|
38
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schott A, Ravaud S, Keller S,
Radzimanowski J, Viotti C, Hillmer S, Sinning I and Strahl S:
Arabidopsis stromal-derived Factor2 (SDF2) is a crucial target of
the unfolded protein response in the endoplasmic reticulum. J Biol
Chem. 285:18113–18121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Willis S, Villalobos VM, Gevaert O,
Abramovitz M, Williams C, Sikic BI and Leyland-Jones B: Single gene
prognostic biomarkers in ovarian cancer: A meta-analysis. PLoS One.
11:e01491832016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Giulianelli S, Herschkowitz JI, Patel V,
Lamb CA, Gutkind JS, Molinolo A, Perou CM and Lanari C: MPA-induced
gene expression and stromal and parenchymal gene expression
profiles in luminal murine mammary carcinomas with different
hormonal requirements. Breast Cancer Res Treat. 129:49–67. 2011.
View Article : Google Scholar
|
|
42
|
Takahashi K, Tanaka M, Yashiro M,
Matsumoto M, Ohtsuka A, Nakayama KI, Izumi Y, Nagayama K, Miura K,
Iwao H and Shiota M: Protection of stromal cell-derived factor 2 by
heat shock protein 72 prevents oxaliplatin-induced cell death in
oxaliplatin-resistant human gastric cancer cells. Cancer Lett.
378:8–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Honoré B: The rapidly expanding CREC
protein family: Members, localization, function, and role in
disease. Bioessays. 31:262–277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luo J, Li Z, Zhu H, Wang C, Zheng W, He Y,
Song J, Wang W, Zhou X, Lu X, et al: A novel role of Cab45-G in
mediating cell migration in cancer cells. Int J Biol Sci.
12:677–687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Honoré B and Vorum H: The CREC family, a
novel family of multiple EF-hand, low-affinity Ca(2+)-binding
proteins localised to the secretory pathway of mammalian cells.
FEBS Lett. 466:11–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Scherer PE, Lederkremer GZ, Williams S,
Fogliano M, Baldini G and Lodish HF: Cab45, a novel (Ca2+)-binding
protein localized to the Golgi lumen. J Cell Biol. 133:257–268.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen L, Xu S, Xu Y, Lu W, Liu L, Yue D,
Teng J and Chen J: Cab45S promotes cell proliferation through
SERCA2b inhibition and Ca2+ signaling. Oncogene. 35:35–46. 2016.
View Article : Google Scholar
|
|
48
|
Lam PP, Hyvärinen K, Kauppi M,
Cosen-Binker L, Laitinen S, Keränen S, Gaisano HY and Olkkonen VM:
A cytosolic splice variant of Cab45 interacts with Munc18b and
impacts on amylase secretion by pancreatic acini. Mol Biol Cell.
18:2473–2480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Grønborg M, Kristiansen TZ, Iwahori A,
Chang R, Reddy R, Sato N, Molina H, Jensen ON, Hruban RH, Goggins
MG, et al: Biomarker discovery from pancreatic cancer secretome
using a differential proteomic approach. Mol Cell Proteomics.
5:157–171. 2006. View Article : Google Scholar
|
|
50
|
Ji H, Greening DW, Kapp EA, Moritz RL and
Simpson RJ: Secretome-based proteomics reveals sulindac-modulated
proteins released from colon cancer cells. Proteomics Clin Appl.
3:433–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Blank B and von Blume J: Cab45-Unraveling
key features of a novel secretory cargo sorter at the trans-Golgi
network. Eur J Cell Biol. 96:383–390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
54
|
Bisel B, Wang Y, Wei JH, Xiang Y, Tang D,
Miron-Mendoza M, Yoshimura S, Nakamura N and Seemann J: ERK
regulates Golgi and centrosome orientation towards the leading edge
through GRASP65. J Cell Biol. 182:837–843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Prigozhina NL and Waterman-Storer CM:
Protein kinase D-mediated anterograde membrane trafficking is
required for fibroblast motility. Curr Biol. 14:88–98. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kienzle C and von Blume J: Secretory cargo
sorting at the trans-Golgi network. Trends Cell Biol. 24:584–593.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Weiss H, Amberger A, Widschwendter M,
Margreiter R, Ofner D and Dietl P: Inhibition of store-operated
calcium entry contributes to the anti-proliferative effect of
non-steroidal anti-inflammatory drugs in human colon cancer cells.
Int J Cancer. 92:877–882. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Merchant N, Nagaraju GP, Rajitha B,
Lammata S, Jella KK, Buchwald ZS, Lakka SS and Ali AN: Matrix
metalloproteinases: Their functional role in lung cancer.
Carcinogenesis. 38:766–780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shirozu M, Tada H, Tashiro K, Nakamura T,
Lopez ND, Nazarea M, Hamada T, Sato T, Nakano T and Honjo T:
Characterization of novel secreted and membrane proteins isolated
by the signal sequence trap method. Genomics. 37:273–280. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shi Y, He B, You L and Jablons DM: Roles
of secreted frizzled-related proteins in cancer. Acta Pharmacol
Sin. 28:1499–1504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu Y, Zhou Q, Zhou D, Huang C, Meng X and
Li J: Secreted frizzled-related protein 2-mediated cancer events:
Friend or foe? Pharmacol Rep. 69:403–408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Arce L, Yokoyama NN and Waterman ML:
Diversity of LEF/TCF action in development and disease. Oncogene.
25:7492–7504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang X, Rong X, Chen Y and Su L:
Methylation-mediated loss of SFRP2 enhances invasiveness of
non-small cell lung cancer cells. Hum Exp Toxicol. 37:155–162.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li P, Zhao S and Hu Y: SFRP2 modulates
nonsmall cell lung cancer A549 cell apoptosis and metastasis by
regulating mitochondrial fission via Wnt pathways. Mol Med Rep.
20:1925–1932. 2019.PubMed/NCBI
|
|
67
|
Zeng X, Zhang Y, Xu H, Zhang T, Xue Y and
An R: Secreted frizzled related protein 2modulates
epithelial-mesenchymal transition and stemness via Wnt/β-catenin
signaling in chorio-carcinoma. Cell Physiol Biochem. 50:1815–1831.
2018. View Article : Google Scholar
|
|
68
|
Cheng YY, Yu J, Wong YP, Man EP, To KF,
Jin VX, Li J, Tao Q, Sung JJ, Chan FK and Leung WK: Frequent
epigenetic inactivation of secreted frizzled-related protein
2(SFRP2) by promoter methylation in human gastric cancer. Br J
Cancer. 97:895–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Perry AS, O'Hurley G, Raheem OA, Brennan
K, Wong S, O'Grady A, Kennedy AM, Marignol L, Murphy TM, Sullivan
L, et al: Gene expression and epigenetic discovery screen reveal
methylation of SFRP2 in prostate cancer. Int J Cancer.
132:1771–1780. 2013. View Article : Google Scholar
|
|
70
|
Bhangu JS, Beer A, Mittlböck M, Tamandl D,
Pulverer W, Schönthaler S, Taghizadeh H, Stremitzer S, Kaczirek K,
Gruenberger T, et al: Circulating free methylated tumor DNA markers
for sensitive assessment of tumor burden and early response
monitoring in patients receiving systemic chemotherapy for
colorectal cancer liver metastasis. Ann Surg. 268:894–902. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Luo X, Wei B, Chen A, Zhao H, Huang K and
Chen J: Methylation-mediated loss of SFRP2 enhances melanoma cell
invasion via Wnt signaling. Am J Transl Res. 8:1502–1509.
2016.PubMed/NCBI
|
|
72
|
Liu S, Chen X, Chen R, Wang J, Zhu G,
Jiang J, Wang H, Duan S and Huang J: Diagnostic role of Wnt pathway
gene promoter methylation in non small cell lung cancer.
Oncotarget. 8:36354–36367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shih YL, Hsieh CB, Yan MD, Tsao CM, Hsieh
TY, Liu CH and Lin YW: Frequent concomitant epigenetic silencing of
SOX1 and secreted frizzled-related proteins (SFRPs) in human
hepatocel-lular carcinoma. J Gastroenterol Hepatol. 28:551–559.
2013. View Article : Google Scholar
|
|
74
|
Yang Q, Huang T, Ye G, Wang B and Zhang X:
Methylation of SFRP2 gene as a promising noninvasive biomarker
using feces in colorectal cancer diagnosis: A systematic
meta-analysis. Sci Rep. 6:333392016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lee JL, Lin CT, Chueh LL and Chang CJ:
Autocrine/paracrine secreted Frizzled-related protein 2induces
cellular resistance to apoptosis: A possible mechanism of mammary
tumorigenesis. J Biol Chem. 279:14602–14609. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Courtwright A, Siamakpour-Reihani S,
Arbiser JL, Banet N, Hilliard E, Fried L, Livasy C, Ketelsen D,
Nepal DB, Perou CM, et al: Secreted frizzle-related protein 2
stimulates angiogenesis via a calcineurin/NFAT signaling pathway.
Cancer Res. 69:4621–4628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lee JL, Chang CJ, Chueh LL and Lin CT:
Secreted frizzled related protein 2(sFRP2) decreases susceptibility
to UV-induced apoptosis in primary culture of canine mammary gland
tumors by NF-kappaB activation or JNK suppression. Breast Cancer
Res Treat. 100:49–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lee JL, Chang CJ, Wu SY, Sargan DR and Lin
CT: Secreted frizzled-related protein 2(SFRP2) is highly expressed
in canine mammary gland tumors but not in normal mammary glands.
Breast Cancer Res Treat. 84:139–149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Viros A, Girotti MR and Marais R: So you
can teach old fibro-blasts new tricks. Cancer Discov. 6:581–583.
2016. View Article : Google Scholar
|
|
80
|
Fontenot E, Rossi E, Mumper R, Snyder S,
Siamakpour-Reihani S, Ma P, Hilliard E, Bone B, Ketelsen D, Santos
C, et al: A novel monoclonal antibody to secreted frizzled-related
protein 2 inhibits tumor growth. Mol Cancer Ther. 12:685–695. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xiao X, Xiao Y, Wen R, Zhang Y, Li X, Wang
H, Huang J, Liu J, Long T and Tang J: Promoting roles of the
secreted frizzled-related protein 2as a Wnt agonist in lung cancer
cells. Oncol Rep. 34:2259–2266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang Y, Li Q and Chen H: DNA methylation
and histone modifications of Wnt genes by genistein during colon
cancer development. Carcinogenesis. 34:1756–1763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang WA, Groenendyk J and Michalak M:
Endoplasmic reticulum stress associated responses in cancer.
Biochim Biophys Acta. 1843:2143–2149. 2014. View Article : Google Scholar : PubMed/NCBI
|
















