Introduction
Parkinson's disease (PD) is a common
neurodegenerative disorder. It is characterized by the loss of
dopaminergic neurons in the substantia nigra pars compacta with
Lewy bodies in the brain (1,2).
Patients with PD are clinically characterized by tremors, rigidity,
bradykinesia and postural instability (3,4).
Several studies have indicated that oxidative stress and
environmental factors are highly associated with PD (5-7).
It has been reported that oxidative stress can induce a high level
of toxic aldehydes, such as 3,4-Dihydroxyphenylacetaldehyde (DOPAL)
in brains of PD patients. Such aldehydes can then trigger
dopaminergic neuronal cell death (5,6,8).
Neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydro pyridine (MPTP) can
cause PD-like symptoms. It is metabolized to
1-methyl-4-phenylpyridinium (MPP+) which can then lead
to dopaminergic neuronal cell death (9,10).
Cytosolic enzyme aldose reductase (AR) is widely
expressed in human tissues. It catalyzes the reduction of toxic
aldehydes, including 4-hydroxy-2-nonenal (4HNE) produced by lipid
peroxidation. 4HNE is known as a biomarker of oxidative stress
(11-13). AR can also reduce toxic aldehyde
DOPAL and induce dopaminergic neuronal cell death when this
aldehyde is increased in the brain under conditions of oxidative
stress (14,15). Several studies have demonstrated
that AR can protect cells against oxidative stress by decreasing
reactive oxygen species (ROS) levels in various cells, such as
smooth muscle and lens epithelial cells (16-18).
It is known that macromolecule protein fused with
protein transduction domains (PTDs) consisting of 10-16 amino acids
can be transduced into cells (19). A number of studies have reported
that PTD fused protein can be efficiently transduced into cells as
a tool for protein therapy (20-26). In the presents study, a Tat-AR
protein expression vector was constructed based on the pET-15b
vector. The Tat-AR expression vector contained a cDNA sequence
encoding human AR, Tat PTD and an amino-terminal tag consisting of
6 histidine residues. Tat PTD was cloned into the NdeI and
XhoI sites, and human AR cDNA was cloned into the
XhoI and BamHI sites of pET-15b vector. Tat-AR
protein or control AR expression vector consist of a His tag
consisting of 6 histidine residues. A recent study demonstrated
that a deficiency of AR can lead to dopaminergic neuronal loss
(27). Thus, the objective of the
present study was to investigate whether transduced Tat-AR protein
can protect dopaminergic neuron cells against oxidative stress both
in vitro and in vivo.
Materials and methods
Cell lines and reagents
Human neuroblastoma SH-SY5Y cells were obtained from
the American Type Culture Collection (ATCC) and maintained in
Dulbecco's modified Eagle's medium (Lonza/BioWhittaker) containing
15% FBS, 4 mM glutamine, 100 U/ml penicillin, and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
5% CO2 incubator.
Dichlorofluorescein diacetate (DCF-DA),
methyl-4-phenyl-pyridinium (MPP+) and
1-methyl-4-phenyl-1,2,3,6-tetrahydro pyridine (MPTP) were obtained
from Sigma-Aldrich; Merck KGaA. Tat peptide was synthesized from
Peptron, Inc. Enhanced chemiluminescence agent was purchased from
Amersham; Cytiva. Antibodies were obtained from Cell Signaling
Technology, Inc. Tyrosine Hydroxylase (TH) was purchased from Santa
Cruz Biotechnology, Inc. All other chemicals and reagents, unless
otherwise stated, were of the highest analytical grade
available.
Purification and transduction of Tat-AR
proteins into SH-SY5Y cells
Tat-AR protein was prepared as described in a
previous study (28). To develop
a therapeutic protein, a human AR gene fused with a Tat PTD to
produce a cell permeable Tat-AR expression vector which contains 6X
His, Tat PTD (RKKRRQRRR) and the AR gene. Bovine serum albumin was
used as a standard and the purified Tat-AR protein concentration
was measured by Bradford assay (29).
To examine the Tat-AR protein transduction
efficiency, SH-SY5Y cells were exposed to various concentrations of
Tat-AR and AR protein (0.5-3 µM) for 1 h. The SH-SY5Y cells
were exposed of Tat-AR and AR protein (3 µM) for various
periods of time (15-60 min). The cells were then washed with PBS
and treated with trypsin-EDTA (Gibco; Thermo Fisher Scientific,
Inc.). The intracellular stability of Tat-AR protein was also
determined. To confirm the stability of Tat-AR protein, the cells
were further cultured (1-30 h) following transduction. The levels
of transduced proteins were measured by western blot analysis using
anti-histidine antibody.
Western blot analysis
Cell lysates were prepared with RIPA lysis buffer
containing a cocktail of protease inhibitors (Elpis-Biotech, Inc.).
The protein concentration was measured using the Bradford method.
Equal amounts of each protein (30 µg) were loaded into 12%
SDS-PAGE and electrotransferred to a polyvinylidene difluoride
(PVDF) membrane (Thermo Fisher Scientific, Inc.). The membrane was
blocked with TBS-T (25 mM Tris-HCl, 140 mM NaCl, 0.1% Tween-20, pH
7.5) buffer containing 5% non-fat dry milk for 1 h at room
temperature. After washing with TBST, the membrane was incubated
with the indicated primary antibodies overnight at 4°C and followed
by incubation with horseradish peroxidase-conjugated secondary
antibodies for 1 h at 37°C. His (1:5,000; sc-804; Santa Cruz
Biotechnology, Inc.), JNK (1:1,000; #9258), p-JNK (1:1,000; #9251),
ERK (1:2,000; #9102), p-ERK (1:2,000; #4376), p38 (1:2,000; #9212),
p-p38 (1:2,000; #4631), Bcl-2 (1:1,000; #2876), Bax (1:1,000;
#2772), caspase-3 (1:1,000; #9662), cleaved caspase-3 (1:1,000;
#9661), β-actin (1:5,000; #4967), and appropriate secondary
antibodies (1:10,000; #7074). All of the above-mentioned antibodies
were purchased from the Cell Signaling Technology, Inc. The
membrane was then washed with TBST buffer 3 times and the protein
bands were identified using chemiluminescent reagents as
recommended by the manufacturer (Amersham; Cytiva). Bands were
quantified using ImageJ software (version 1.48; National Institutes
of Health) (25,30).
Confocal fluorescence microscopy
analysis
To determine the intracellular distribution of
transduced Tat-AR protein in SH-SY5Y cells, confocal fluorescence
microscopy was performed, as previously described (24). SH-SY5Y cells were placed on
coverslips and treated with 3 µM Tat-AR protein 1 h. The
cells were washed with PBS twice and fixed with 4% paraformaldehyde
for 5 min. The cells were treated in PBS containing 3% bovine serum
albumin, 0.1% Triton X-100 (PBS-BT) at room temperature for 30 min
and washed with PBS-BT. Histidine primary antibody (sc-804; Santa
Cruz Biotechnology, Inc.) was diluted 1:1,500 and incubated at room
temperature for 3 h. Alexa Fluor 488-conjugated secondary antibody
(#32723; Invitrogen; Thermo Fisher Scientific, Inc.) was diluted
1:1,500 and incubated in the dark for 1 h at room temperature.
Nuclei were stained with 1 µg/ml DAPI (Roche Applied
Science, Mannheim, Germany) for 2 min at room temperature. The
stained cells were analyzed by confocal fluorescence microscopy
using a confocal laser-scanning system (Bio-Rad MRC-1024ES, Bio-Rad
Laboratories, Inc.).
MTT assay
Cell viability was measured by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, as previously described (24). The SH-SY5Y cells were pre-treated
with Tat-AR (0.5-3 µM), AR (0.5-3 µM) and Tat peptide
(0.5-3 µM) for 1 h and MPP+ (5 mM) was then added
to the culture medium for 13 h. The absorbance was measured at 570
nm using an ELISA microplate reader (Labsystems Multiskan MCC/340;
Thermo Fisher Scientific, Inc.) and the cell viability was defined
as the percentage of untreated control cells.
Terminal deoxynucleotidyl
transferase-mediated biotinylated dUTP nick-end labelling (TUNEL)
staining
To examine whether transduced Tat-AR proteins
protect against MPP+-induced DNA damage in cells, the
SH-SY5Y cells were pre-treated with Tat-AR (3 µM), AR (3
µM) and Tat peptide (3 µM) for 1 h and
MPP+ (5 mM) was added to the culture medium for 16 h.
TUNEL staining was performed using a Cell Death Detection kit
(Roche Applied Science). Nuclei were stained with DAPI (1
µg/ml) for 2 min at room temperature. Fluorescent images
were obtained using a fluorescence microscope (Nikon eclipse 80i,
Nikon Corporation) and cells exhibiting fluorescence were counted
under a phase contrast microscope (×200 magnification; Nikon
Corporation), as previously described (24,25).
Measurement of ROS production
Intracellular ROS levels were determined by
2′,7′-dichlorofluorescein diacetate (DCF-DA) staining as previously
described (28,31). To deter-mine the effects of Tat-AR
protein against MPP+-induced intracellular ROS
production in SH-SY5Y cells, the cells were placed on coverslips in
24-well plates, incubated for 12 h, and washed twice with PBS.
After pretreated with Tat-AR, AR, and Tat peptide (3 µM) for
1 h, MPP+ (5 mM) was added to the culture medium for 1
h. The cells were washed with PBS and incubated for 30 min with
DCF-DA (10 µM). Protein was separately processed as
mentioned above to obtain the fluorescent image and fluorescence
intensity. One was used to obtain a fluorescent image. To obtain
fluorescent images for each well, the cells were washed with PBS,
mounted, and the cell images were obtained using a fluorescence
microscope (Nikon eclipse 80i, Nikon Corporation).
The other was used to obtain the fluorescence
intensity. To detect the fluorescence intensity for each well, the
cells were collected and washed with PBS. After added 300 µl
of PBS buffer and resuspended the cells, the cells (100 µl)
were transferred into 96-well plate reader. The fluorescence
intensity of the samples was measured using a Fluoroskan ELISA
plate reader (Labsystems Diagnostics Oy) at an excitation
wavelength of 485 nm and an emission wavelength of 538 nm.
Animal experiments and
immunohistochemistry
Male C57BL/6 mice (total, n=35), 8 weeks old
(weighing 16-20 g), were acquired from the Hallym University
Experimental Animal Center. They were housed at 23°C and a humidity
of 60%. They were exposed to regulated 12 h cycles of light and
dark and were provided with ad libitum access to food and
water. The procedures for the care of animals conformed to the
Guide for the Care and Use of Laboratory Animals of the National
Veterinary Research and Quarantine Service of Korea and approved by
the Institutional Animal Care and Use Committee of Soonchunhyang
University (SCH16-0051).
To determine whether transduced Tat-AR protein
protects against PD, the mice were divided into 5 groups (n=7/each
group) as follows: The normal control, MPTP-treated,
Tat-AR-treated, control AR-treated and Tat peptide-treated groups.
The mice received 4 injections of MPTP (20 mg/kg) at 2-h intervals.
Mice were intraperitoneally (i.p.) injected with Tat-AR (2 mg/kg)
12 h prior to MPTP treatment. At 1 week after the final injection,
the animals were deeply anesthetized with a mixture of 3%
isoflurane (Baxter Healthcare Corporation) in 33% oxygen and 67%
nitrous oxide. The brains of these animals were then harvested for
immunohistochemistry.
Immunohistochemistry was performed as described in a
previous study (31). The frozen
and sectioned midbrains were prepared and fixed with 4%
paraformaldehyde for 10 min. For removal of non-specific
immunoreactivity, free-floating sections were first incubated with
0.3% Triton X-100 and 10% normal goat serum in PBS for 1 h at room
temperature. They were then incubated with a rabbit anti-tyrosine
hydroxylase (TH) monoclonal antibody (diluted 1:200; sc-14007;
Santa Cruz Biotechnology, Inc.) for 48 h at 4°C and sequentially
incubated with a biotinylated goat anti-rabbit IgG (diluted 1:250;
BA-1000; Vector Laboratories, Inc.) for 2 h at room temperature.
The sections were then visualized with 3,3-diaminobenzidine (DAB)
(40 mg DAB, 0.045% H2O2 in 100 ml PBS)
mounted on gelatin-coated slides. To detect viable cells, cresyl
violet (0.1%, Sigma-Aldrich; Merck KGaA) counterstaining for Nissl
bodies was conducted for 20 min at room temperature following TH
immunostaining. The sections were visualized with
3,3′-diaminobenzidine in 0.1 M Tris buffer and mounted on
gelatin-coated slides. Images were captured and analyzed using an
Olympus DP72 digital camera and a DP2-BSW microscope digital camera
software. Figures were prepared using Adobe Photoshop 7.0. The
manipulation of images was restricted to threshold and brightness
adjustments applied to the entire image. The images shown are
representatives from each group and the sections were processed and
analyzed by a blinded observer. To establish the specificity of the
immunostaining, a negative control test was carried out with
pre-immune serum instead of the primary antibody. The negative
control resulted in the absence of immunoreactivity in any
structures.
For the quantification of TH immunostaining, a cell
count was performed. TH immunostaining images (10 sections/mouse)
were captured in the same region. Images were sampled from at least
5 different points within each SN section. Thereafter, the number
of TH-positive cells was actu-ally counted within the sampled
images. All immunoreactive cells were counted regardless the
intensity of labeling. Cell counts were performed by 2 different
investigators who were blind to the classification of the
tissues.
Statistical analysis
Data are expressed as the means ± SEM of 3
experiments. Differences between groups were analyzed by ANOVA
followed by a Bonferroni's post-hoc test (using GraphPad Prism 8;
GraphPad Software, Inc.). Statistical significance was considered
at P<0.05.
Results
Transduction of Tat-AR protein into
SH-SY5Y cells
To produce cell-permeable Tat-AR protein, the Tat-AR
protein expression vector was constructed by subcloning the cDNA
encoding human AR into a pET-15b plasmid containing a Tat PTD.
Tat-AR protein expression vector contained a continuous cDNA
sequence encoding human AR, a Tat PTD and 6 histidines. In
addition, a control AR expression vector containing no Tat PTD was
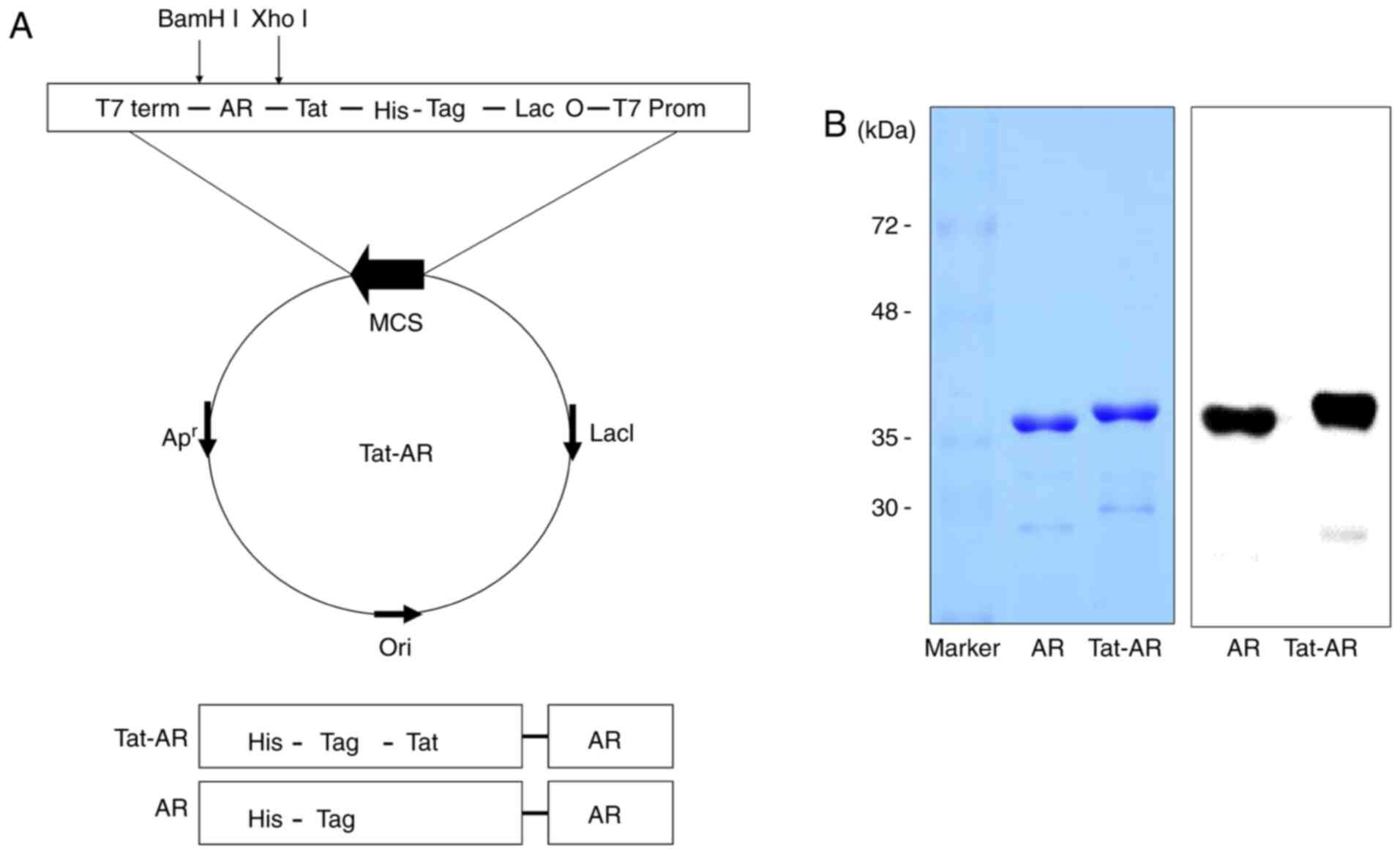
constructed (Fig. 1A). The
purified Tat-AR and AR proteins are presented in Fig. 1B. To investigate the transduction
efficiency of Tat-AR protein, SH-SY5Y neuro-blastoma cells were
treated with Tat-AR protein (0.5-3 µM) for 1 h or with
Tat-AR protein at 3 µM for 15-60 min. Tat-AR protein was
transduced into the cells in a concentration- and time-dependent
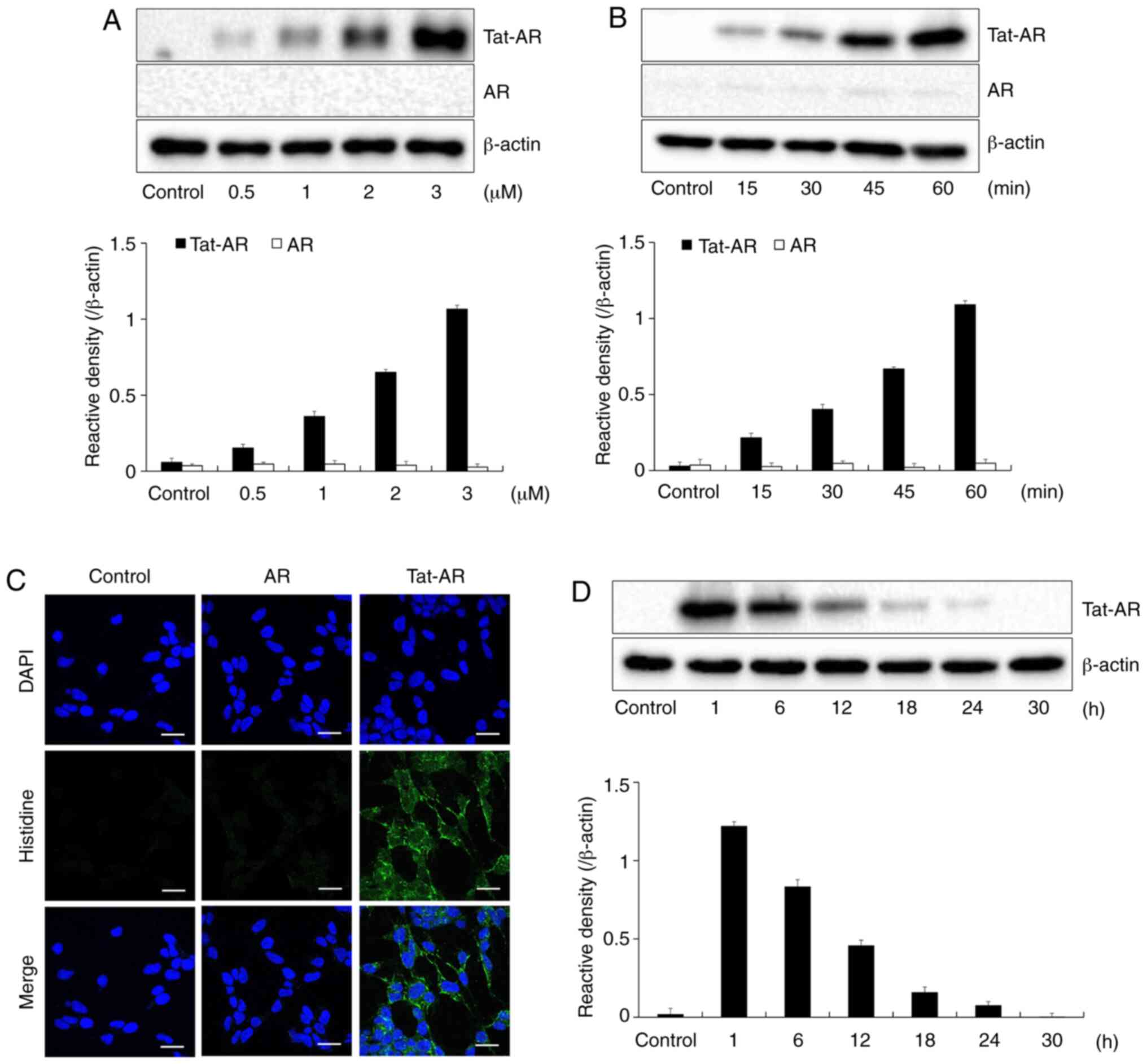
manner (Fig. 2A and B). Tat-AR
protein was also transduced into the cytosol and nucleus of the
cells (Fig. 2C). Since stability
is one of the major factors in protein therapy, the stability of
the transduced Tat-AR protein was examined. Transduced Tat-AR
protein persisted until 12 h in the cells (Fig. 2D). These results indicate that
Tat-AR protein can be efficiently transduced into the SH-SY5Y cells
and can exist for at least 12 h in the cells.
Effects of Tat-AR protein against
MPP+-induced SH-SY5Y cells
It is known that MPP+ can induce ROS
production in dopaminergic neuronal cells, and can cause DNA damage
and cell death (32). Thus, the
protective effects of transduced Tat-AR protein against
MPP+-induced cell death were examined. Cell viability
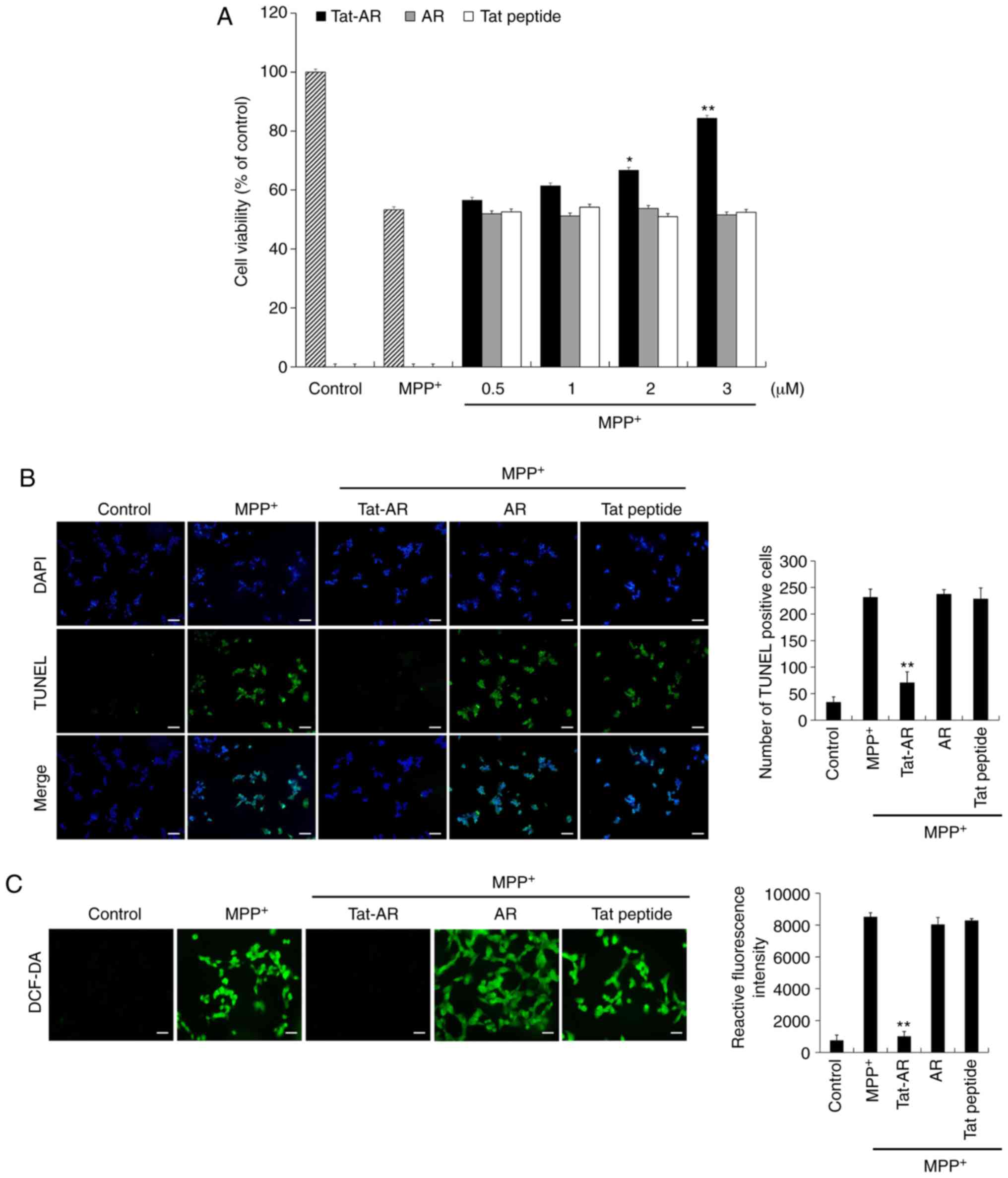
was 53% in the cells exposed only to MPP+. However, cell
viability was markedly increased up to 84% in the cells treated
with Tat-AR protein following exposure to MPP+. By
contrast, AR protein and Tat peptide failed to prevent cell death
under the same experimental conditions (Fig. 3A).
The protective effects of Tat-AR protein against
MPP+-induced DNA damage and intracellular ROS production
were also determined by TUNEL and DCF-DA staining, respectively
(Fig. 3B and C). DNA damage and
intracellular ROS production levels were increased in the cells
exposed only to MPP+. However, DNA damage and
intracellular ROS production levels were markedly inhibited in the
Tat-AR protein-treated cells following MPP+ exposure.
However, AR protein and Tat peptide failed to inhibit DNA damage
and intracellular ROS production. These results indicate that
Tat-AR protein can inhibit SH-SY5Y cell death by decreasing DNA
damage and intra-cellular ROS production, functioning as an
antioxidant in the cells.
Effects of Tat-AR protein on
MPP+-induced signaling pathways
MPP+ can trigger mitogen-activated
protein kinase (MAPK) signaling pathway activation (9,33,34). Thus, the effects of Tat-AR
proteins on MPP+ induced MAPKs signaling pathways were
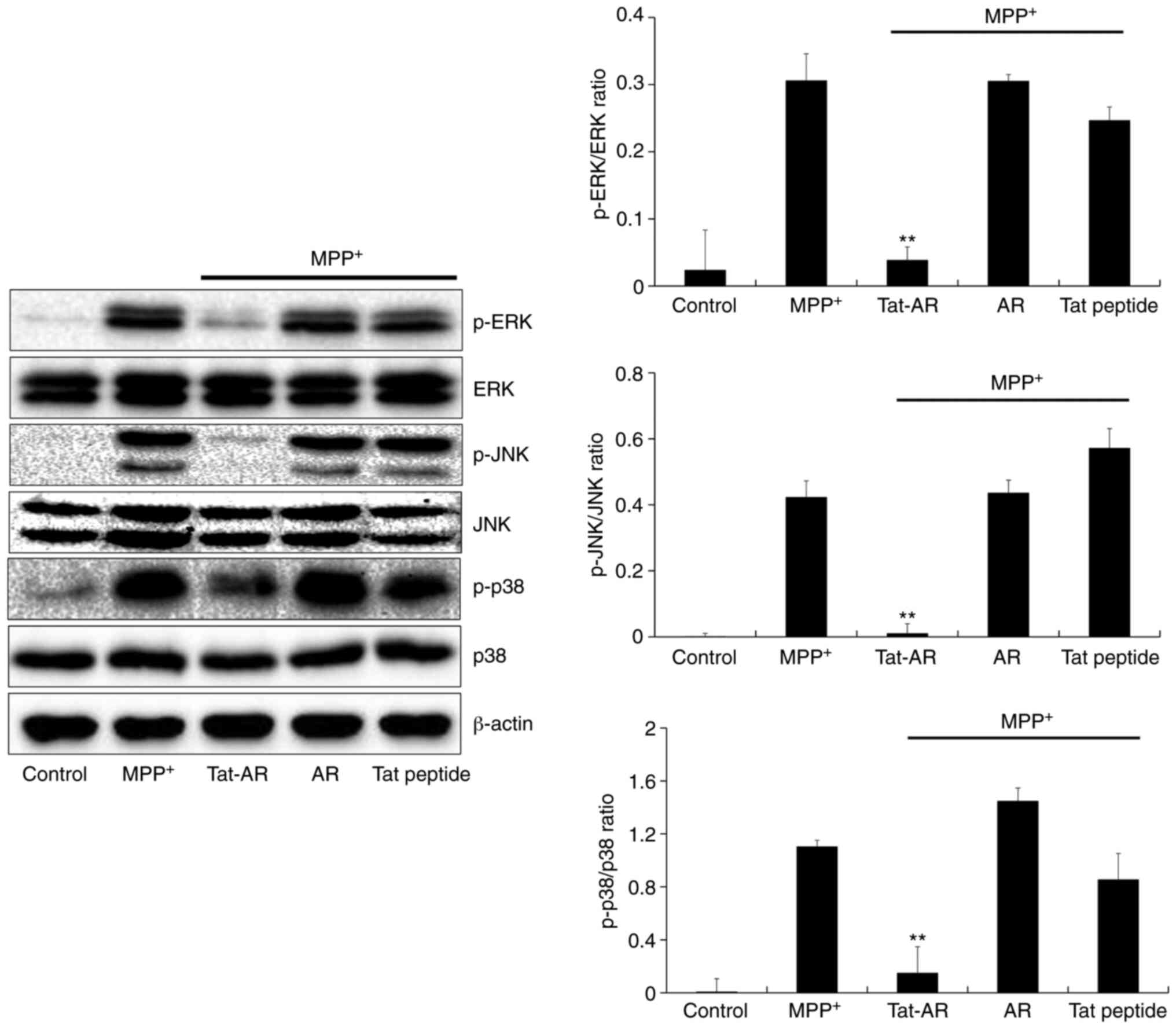
determined in the present study. In the SH-SY5Y cells exposed to
MPP+, the expression levels of phosphorylated MAPKs were
higher than those in the control cells. By contrast, Tat-AR protein
significantly decreased expression the levels of phosphorylated
MAPKs in the cells exposed to MPP+. However, the
expression levels of phosphorylated MAPKs in the cells treated with
AR protein or Tat peptide were similar to those in the untreated
cells exposed to MPP+ (Fig. 4).
It is well known that the neurotoxin,
MPP+, can cause the overproduction of ROS in cells and
activate apoptosis signaling pathways (32). Thus, effects of Tat-AR proteins on
the MPP+-induced expression levels of Bax, Bcl-2 and
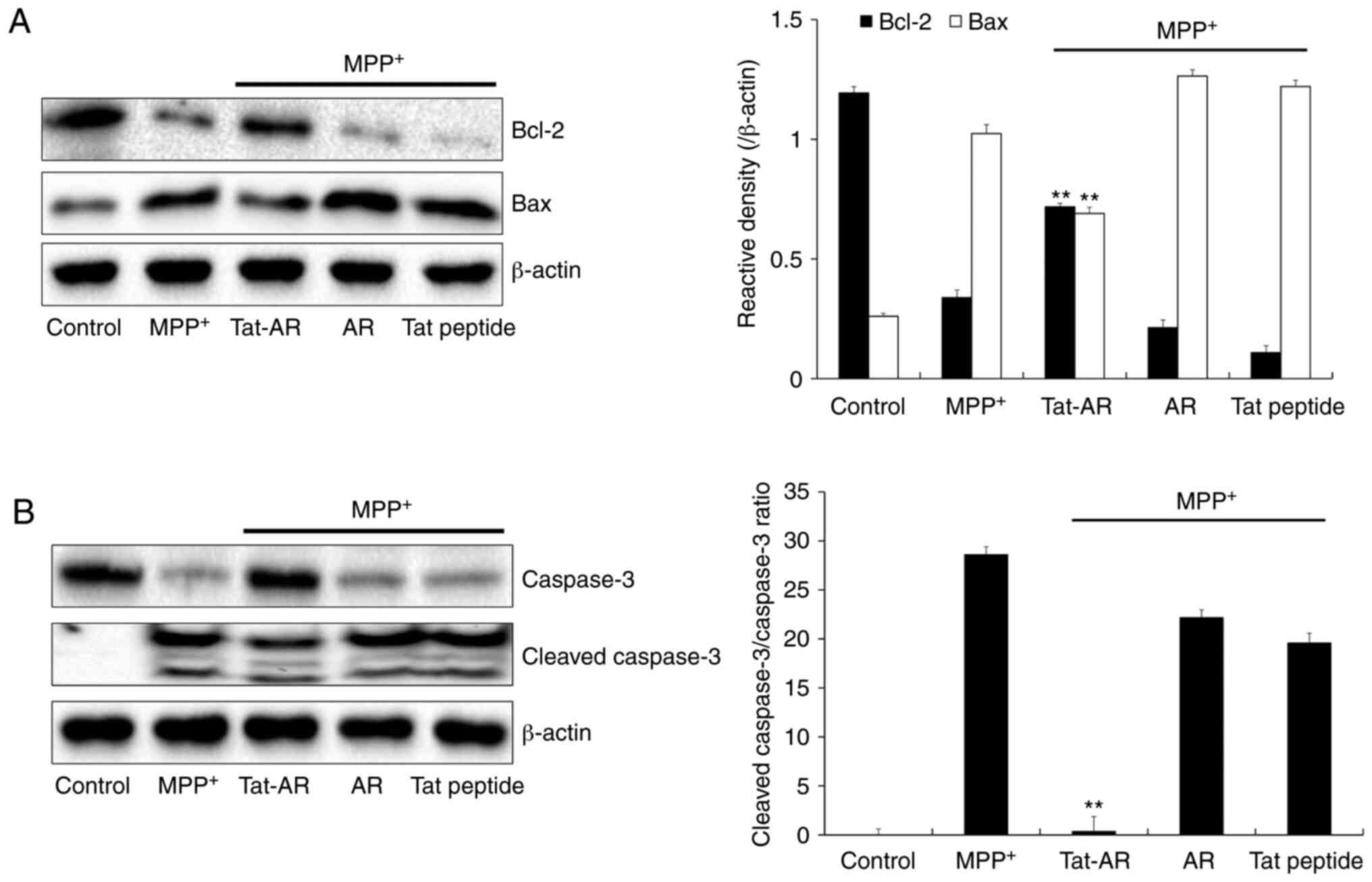
caspase 3 were investigated. As shown in Fig. 5A, the Bcl-2 expression levels were
decreased in the MPP+-exposed SH-SY5Y cells. By
contrast, Tat-AR protein significantly increased the Bcl-2
expression levels in cells the exposed to MPP+. However,
the expression levels of Bax exhibited opposite results from those
of Bcl-2. Tat-AR protein also markedly increased the expression of
caspase-3 in the MPP+-exposed cells. The cleaved
caspase-3 expression levels were significantly decreased in the
cells exposed only to MPP+. In addition, Tat-AR protein
reduced the cleaved Caspase-3/Caspase-3 ratio in MPP+
treated cells. AR protein and Tat peptide failed to affect the
expression levels of caspase-3 and cleaved caspase-3 proteins
induced by MPP+ (Fig.
5B). These results indicate that transduced Tat-AR protein can
prevent SH-SY5Y cell death from MPP+ by regulating
phosphorylation levels of MAPKs and apoptosis-related protein
expression.
Protective effects of Tat-AR protein
against MPTP-induced cell death in an animal model of PD
After Tat-AR protein (2 mg/kg) was i.p. injected
into the mice, immunohistochemistry was performed using histidine
antibody to determine whether Tat-AR protein was transduced into
the mouse brains (Fig. 6A).
Tat-AR protein was highly expressed in the SN region of the
midbrain. However, AR protein was not detected in the SN region of
the midbrain. These results indicate that Tat-AR protein can be
transduced into mouse brains by crossing the blood-brain barrier
(BBB).
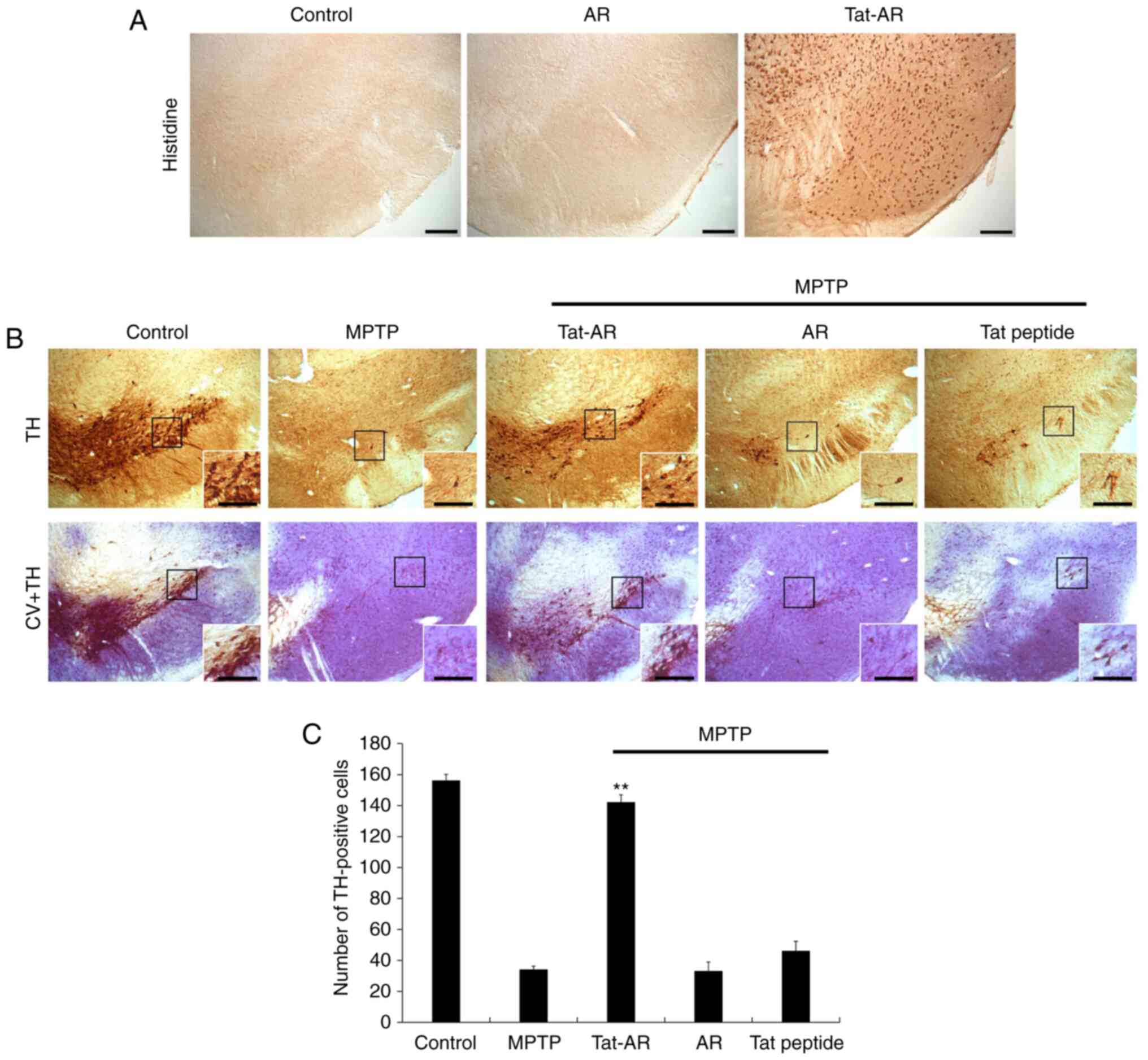 | Figure 6Transduced Tat-AR protein inhibits
dopaminergic neuronal cell death in an animal model of PD.
Transduction of Tat-AR protein into the SN. Tat-AR protein (2
mg/kg) was injected i.p. into mice, followed by collecting the
brains 12 h later. (A) Brain tissues were immunostained with an
anti-histidine antibody. Scale bars, 100 µm. (B) Protective
effects of transduced Tat-AR protein in the animal model of PD.
Tat-AR protein (2 mg/kg) was injected i.p. into mice, followed by
collecting the brains 1 weeks. Brain sections showing TH
immunoreactivity and double staining with cresyl violet (CV) and TH
immunoreactivity. Scale bars, 100 and 50 µm. (C) Number of
TH-positive neurons. Quantification of the number of positive
dopaminergic neurons in 250×250 µm2 is shown in
the graph. **P<0.01, statistically significant
difference between MPTP and other groups. Tat-AR, Tat-aldose
reductase; PD, Parkinson's disease; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TH, tyrosine
hydroxylase. |
Dopaminergic neuronal cell death is major indicator
of PD. Thus, the protective effects of Tat-AR protein against
MPTP-induced dopaminergic neuronal cell death were determined by
immunohistochemistry using a TH antibody and cresyl violet
staining. As shown in Fig. 6B and
C, the TH-positive cell numbers were increased in the SN of the
Tat-AR protein-treated group. In addition, neuronal cell survival
was markedly increased in the Tat-AR protein-treated group. By
contrast, the AR protein- and Tat peptide-treated groups did not
exhibit such protective effects. These results indicate that Tat-AR
protein can markedly prevent dopaminergic neuronal cell death.
Discussion
PD is a progressive neurodegenerative disorder,
characterized by the loss of dopamine neurons in the SN pars
compacta and the presence of Lewy bodies in the brain (1-3).
Since oxidative stress is highly associated with the pathogenesis
of various diseases, including PD, the regulation of oxidative
stress is crucial to preventing these diseases (5-7,35-39). The definitive cause of dopamine
neuron cell death in PD and precise etiological mechanism remain
unclear.
AR can reduce levels of toxic aldehydes, including
4HNE. It can increase cell survival by decreasing oxidative stress
and toxic aldehydes (11-15). Although several studies have
suggested that AR plays a crucial role in cellular responses to
oxidative stress by detoxifying ROS and decreasing reactive
aldehydes in keratinocytes (40)
and by neutralizing the toxicity of lipid peroxidation in arterial
wall injuries (41), the precise
protective role of AR in dopaminergic cells against oxidative
stress remains poorly understood. Therefore, the present study
investigated whether Tat-AR protein can protect SH-SY5Y cells
against MPP+-induced dopaminergic neuronal cell death
whether it can protect animals against MPTP-induced PD. The results
revealed that purified Tat-AR protein could be transduced into
SH-SY5Y cells in a time- and dose-dependent manner, and that such a
transduced protein could significantly enhance cell survival and
inhibit DNA damage in MPP+-exposed SH-SY5Y cells.
It is well known that neurotoxin MPP+ can
induce oxidative stress and lead to dopaminergic neuronal death via
mitochondrial damage (9,10,32). Other studies have demonstrated
that the overexpression of AR protein can protect cells against
methylglyoxal (MG)-induced aortic smooth muscle cell damage
(42) and prevent
aldehyde-induced human lens epithelial cell death (18). AR is also involved in cell
survival as a detoxification enzyme (43). The results of the present study
demonstrated the same protective pattern. Further studies are
required to confirm such results.
MPP+ can trigger cellular signaling
pathways, including MAPKs and apoptosis signaling pathways
(32). Thus, the effects of
Tat-AR protein on MAPK signaling pathways, and the expression
levels of Bax, Bcl-2 and caspase-3 were determined in the present
study. In the MPP+-exposed SH-SY5Y cells, Tat-AR protein
markedly inhibited Bax and cleaved caspase-3 expression levels, but
significantly increased the Bcl-2 expression levels. Other studies
have also demonstrated that the overexpression of AR can increase
the Bcl-2 level, but can decrease Bax expression levels and the
phosphorylation level of JNK and p38 in aldehyde-induced human lens
epithelial cells (18).
In addition, AR can reduce ultraviolet-B
(UVB)-induced JNK and p38 phosphorylation in HaCaT cells (40). The phosphorylation levels of
EKR1/2 have been shown to be significantly increased in
MPTP-exposed AR−/− mouse brains (27). These reports suggest that
dopaminergic neuronal loss is associated with the phosphorylation
of EKR1/2 and that AR plays an important role in protecting
dopaminergic neurons in PD (27,40). The results of the present study
also demonstrated that Tat-AR protein reduced the activation of
MAPKs in MPP+-exposed SH-SY5Y cells, suggesting that
Tat-AR protein plays a protective role against
MPP+-induced dopaminergic neuronal cell death via the
modulation of MAPKs and apoptosis signaling pathways.
The MPTP-induced mouse model of PD has been
generally used to examine the pathological mechanisms of PD as this
model exhibits a similar pathophysiology to human PD (44,45). In animal models of MPTP-induced
PD, Tat-AR protein was shown to significantly protect dopaminergic
neuronal cell death in the present study. Goldstein et al
previously demonstrated that the overexpression of AR protein
significantly reduced cell death in patients with PD (8). In addition, a number of studies have
reported that the levels of toxic aldehyde DOPAL are markedly
increased in patients with PD and that AR can decrease DOPAL levels
(5,6,8),
suggesting that AR plays a pivotal role in detoxifying DOPAL in PD
(14,15,46). A recent study demonstrated that AR
deficiency in MPTP-exposed mice led to increases in the
characteristics of PD and that AR protected dopaminergic neurons
against neurotoxic metabolites (27).
In conclusion, the presents study demonstrated that
Tat-AR protein could be transduced into SH-SY5Y cells and SN in
mouse brains, and that Tat-AR protein could significantly protect
against MPP+- and MPTP-induced dopaminergic neuronal
cell death both in vitro and in vivo. Although
further studies are required to elucidate the precise protective
mechanisms, the present results suggest that Tat-AR protein may be
useful as a therapeutic agent for PD.
Funding
The present study was supported by the National
Research Foundation of Korea (NRF) grant funded the Korea
government (MIST) (NRF-2018R1A2B6001941) and in part by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education
(2019R1A6A1A11036849).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SBC, WSE, MJS, DWK and SYC were involved in the
conceptualization of the study. SBC, WSE, MJS, HJK and DSK were
involved in the study methodology. HJY, EJY and YJC were involved
in data validation. SWC, JP, KHH, KWL, JKP and SYC were involved in
data curation. DWK and SYC were involved in the writing and editing
of the manuscript and provided final approval of the version to be
published. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The procedures for the care of animals conformed to
the Guide for the Care and Use of Laboratory Animals of the
National Veterinary Research and Quarantine Service of Korea and
approved by the Institutional Animal Care and Use Committee of
Soonchunhyang University (SCH16-0051).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
de Rijk MC, Launer LJ, Berger K, Breteler
MM, Dartigues JF, Baldereschi M, Fratiglioni L, Lobo A,
Martinez-Lage J, Trenkwalder C and Hofman A: Prevalence of
Parkinson's disease in Europe: A collaborative study of
population-based cohorts. Neurology. 54(11 Suppl 5): S21–S23.
2000.PubMed/NCBI
|
|
2
|
Braak H, Del Tredici K, Rüb U, de Vos RA,
Jansen Steur EN and Braak E: Staging of brain pathology related to
sporadic Parkinson's disease. Neurobiol Aging. 24:197–211. 2003.
View Article : Google Scholar
|
|
3
|
Calne D: A definition of Parkinson's
disease. Parkinsonism Relat Disord. 11(Suppl 1): S39–S40. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hughes AJ, Daniel SE and Lees AJ: Improved
accuracy of clinical diagnosis of Lewy body Parkinson's disease.
Neurology. 57:1497–1499. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fitzmaurice AG, Rhodes SL, Lulla A, Murphy
NP, Lam HA, O'Donnell KC, Barnhill L, Casida JE, Cockburn M,
Sagasti A, et al: Aldehyde dehydrogenase inhibition as a pathogenic
mechanism in Parkinson disease. Proc Natl Acad Sci USA.
110:636–641. 2013. View Article : Google Scholar
|
|
6
|
Koutsilieri E, Scheller C, Grunblatt E,
Nara K, Li J and Riederer P: Free radicals in Parkinson's disease.
J Neurol. 249(Suppl 2): II1–II5. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drechsel DA and Patel M: Role of reactive
oxygen species in the neurotoxicity of environmental agents
implicated in Parkinson's disease. Free Radic Biol Med.
44:1873–1886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goldstein DS, Sullivan P, Holmes C, Kopin
IJ, Basile MJ and Mash DC: Catechols in post-mortem brain of
patients with Parkinson disease. Eur J Neurol. 18:703–710. 2011.
View Article : Google Scholar
|
|
9
|
Singer TP and Ramsay RR: Mechanism of the
neurotoxicity of MPTP: An update. FEBS Lett. 274:1–8. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goping G, Pollard HB, Adeyemo OM and
Kuijpers GA: Effect of MPTP on dopaminergic neurons in the goldfish
brain: A light and electron microscope study. Brain Res. 687:35–52.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Connor T, Ireland LS, Harrison DJ and
Hayes JD: Major differences exist in the function and
tissue-specific expression of human B1 aldehyde reductase and the
principal human aldoketo reductase AKR1 family members. Biochem J.
343:487–504. 1999. View Article : Google Scholar
|
|
12
|
Esterbauer H, Schaur RJ and Zollner H:
Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and
related aldehydes. Free Radic Biol Med. 11:81–128. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doorn JA and Petersen DR:
4-Hydroxynonenal-mediated inhibition of enzyme catalyzed oxidation
of the reactive electrophile 3,4-dihydroxyphenylacetaldehyde.
Toxicol Sci. 78:9802004.
|
|
14
|
Burke WJ, Li SW, Chung HD, Ruggiero DA,
Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA
and Zahm DS: Neurotoxicity of MAO metabolites of catechol-amine
neurotransmitters: Role in neurodegenerative diseases.
Neurotoxicol. 25:101–115. 2004. View Article : Google Scholar
|
|
15
|
Burke WJ, Li SW, Williams EA, Nonneman R
and Zahm DS: 3,4-Dihydroxyphenyl-acetaldehyde is the toxic dopamine
metabolite in vivo: Implications for Parkinson's disease
pathogenesis. Brain Res. 989:205–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srivastava SK, Yadav UC, Reddy AB, Saxena
A, Tammali R, Shoeb M, Ansari NH, Bhatnagar A, Petrash MJ,
Srivastava S and Ramana KV: Aldose reductase inhibition suppresses
oxidative stress-induced inflammatory disorders. Chem Biol
Interact. 191:330–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lyon RC, Li D, McGarvie G and Ellis EM:
Aldo-keto reductases mediate constitutive and inducible protection
against aldehyde toxicity in human neuroblastoma SH-SY5Y cells.
Neurochem Int. 62:113–121. 2013. View Article : Google Scholar
|
|
18
|
Pladzyk A, Ramana KV, Ansari NH and
Srivastava SK: Aldose reductase prevents aldehyde toxicity in
cultured human lens epithelial cells. Exp Eye Res. 83:408–416.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moon JI, Han MJ, Yu SH, Lee EH, Kim SM,
Han K, Park CH and Kim CH: Enhanced delivery of protein fused to
cell penetrating peptides to mammalian cells. BMB Rep. 52:324–329.
2019. View Article : Google Scholar :
|
|
20
|
van den Berg A and Dowdy SF: Protein
transduction domain delivery of therapeutic macromolecules. Curr
Opin Biotechnol. 22:888–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dietz GP: Cell-penetrating peptide
technology to deliver chaper-ones and associated factors in
diseases and basic research. Curr Pharm Biotechnol. 11:167–174.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gump JM and Dowdy SF: TAT transduction:
The molecular mechanism and therapeutic prospects. Trends Mol Med.
13:443–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim DW, Shin MJ, Choi YJ, Kwon HJ, Lee SH,
Lee S, Park J, Han KH, Eum WS and Choi SY: Tat-ATOX1 inhibits
inflamma-tory responses via regulation of MAPK and NF-κB pathways.
BMB Rep. 51:654–659. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin MJ, Kim DW, Lee YP, Ahn EH, Jo HS,
Kim DS, Kwon OS, Kang TC, Cho YJ, Park J, et al: Tat-glyoxalase
protein inhibits against ischemic neuronal cell damage and
ameliorates ischemic injury. Free Radic Biol Med. 67:195–210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin MJ, Kim DW, Jo HS, Cho SB, Park JH,
Lee CH, Yeo EJ, Choi YJ, Kim JA, Hwang JS, et al: Tat-PRAS40
prevent hippo-campal HT-22 cell death and oxidative stress induced
animal brain ischemic insults. Free Radic Biol Med. 97:250–262.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeo HJ, Shin MJ, You JH, Kim JS, Kim MY,
Kim DW, Kim DS, Eum WS and Choi SY: Transduced Tat-CIAPIN1 reduces
the inflammatory response on LPS- and TPA-induced damages. BMB Rep.
52:695–699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yeung PKK, Lai AKW, Son HJ, Zhang X, Hwang
O, Chung SSM and Chung SK: Aldose reductase deficiency leads to
oxidative stress-induced dopaminergic neuronal loss and autophagic
abnormality in an animal model of Parkinson's disease. Neurobiol
Aging. 50:119–133. 2017. View Article : Google Scholar
|
|
28
|
Cho SB, Eum WS, Shin MJ, Kwon HJ, Park JH,
Choi YJ, Park J, Han KH, Kang JH, Kim DS, et al: Transduced
Tat-aldose reductase protects hippocampal neuronal cells against
oxidative stress-induced damage. Exp Neurobiol. 28:612–627. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bradford M: A rapid and sensitive method
for the quantification of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han AR, Yang JW, Na JM, Choi SY and Cho
SW: Protective effects of N,4,5-trimethylthiazol-2-amine
hydrochloride on hypoxia-induced β-amyloid production in SH-SY5Y
cells. BMB Rep. 52:439–444. 2019. View Article : Google Scholar :
|
|
31
|
Ahn EH, Kim DW, Shin MJ, Kim YN, Kim HR,
Woo SJ, Kim SM, Kim DS, Kim J, Park J, et al: PEP-1-ribosomal
protein S3 protects dopaminergic neurons in an MPTP-induced
Parkinson's disease mouse model. Free Radic Biol Med. 55:36–45.
2013. View Article : Google Scholar
|
|
32
|
Kalivendi SV, Kotamraju S, Cunningham S,
Shang T, Hillard CJ and Kalyanaraman B: 1-methyl-4-phenylpyridinium
(MPP+)-induced apoptosis and mitochondrial oxidant
generation: Role of transferrin-receptor-dependent iron and
hydrogen peroxide. Biochem J. 371:151–164. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chongthammakun V, Sanvarinda Y and
Chongthammakun S: Reactive oxygen species production and MAPK
activation are implicated in tetrahydrobiopterin induced SH-SY5Y
cell death. Neurosci Lett. 449:178–182. 2009. View Article : Google Scholar
|
|
34
|
Zhu JH, Kulich SM, Oury TD and Chu CT:
Cytoplasmic aggre-gates of phosphorylated extracellular
signal-regulated protein kinases in Lewy body diseases. Am J
Pathol. 161:2087–2098. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Romuk EB, Szczurek W, Oles M, Gabrysiak A,
Skowron M, Nowak P and Birkner E: The evaluation of the changes in
enzy-matic antioxidant reserves and lipid peroxidation in chosen
parts of the brain in an animal model of Parkinson disease. Adv
Clin Exp Med. 26:953–959. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang YL, Ju B, Zhang YZ, Yin HL, Liu YJ,
Wang SS, Zeng ZL, Yang XP, Wang HT and Li JF: Protective effect of
curcumin against oxidative stress-induced injury in rat with
Parkinson's disease through the Wnt/β-catenin signaling pathway.
Cell Physiol Biochem. 43:2226–2241. 2017. View Article : Google Scholar
|
|
37
|
Madamanchi NR, Vendrov A and Runge MS:
Oxidative stress and vascular disease. Arterioscler Thromb Vasc
Biol. 25:29–38. 2005. View Article : Google Scholar
|
|
38
|
Andersen JK: Oxidative stress in
neurodegeneration: Cause or consequence? Nat Med. 10:S18–S25. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cooke MS, Evans MD, Dizdaroglu M and Lunec
J: Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB
J. 17:1195–1214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang ES, Iwata K, Ikami K, Ham SA, Kim HJ,
Chang KC, Lee JH, Kim JH, Park SB, Km JH, et al: Aldose reductase
in kera-tinocytes attenuates cellular apoptosis and senescence
induced by UV radiation. Free Radic Biol Med. 50:680–688. 2011.
View Article : Google Scholar
|
|
41
|
Rittner HL, Hafner V, Klimiuk PA, Szweda
LI, Goronzy JJ and Weyand CM: Aldose reductase functions as a
detoxification system for lipid peroxidation products in
vasculitis. J Clin Invest. 103:1007–1013. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yabe-Nishimura C, Nishinaka T, Iwata K and
Seo HG: Up-regulation of aldose reductase by the substrate,
methyglyoxal. Chem Biol Interact. 143-144:317–323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kang ES, Woo IS, Kim HJ, Eun SY, Paek KS,
Kim HJ, Chang KC, Lee JH, Lee HT, Kim JH, et al: Up-regulation of
aldose reductase expression mediated by phosphatidylinositol
3-kinase/Akt and Nrf2 is involved in the protective effect of
curcumin against oxidative stress. Free Radic Biol Med. 43:535–545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Camicioli R, Grossmann SJ, Spencer PS,
Hudnell K and Anger WK: Discriminating mild parkinsonism: Methods
for epidemiological research. Mov Disord. 16:33–40. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park SW, Kim SH, Park KH, Kim SD, Kim JY,
Baek SY, Chung BS and Kang CD: Protective effect of antioxidants in
MPTP-induced mouse model of Parkinson's disease. Neurosci Lett.
363:243–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Goldstein DS, Sullivan P, Cooney A,
Jinsmaa Y, Sullivan R, Gross DJ, Holmes C, Kopin IJ and Sharabi Y:
Vesicular uptake blockade generates the toxic dopamine metabolite
3,4- dihydroxyphenylacetaldehyde in PC12 cells: Relevance to the
pathogenesis of Parkinson's disease. J Neurochem. 123:932–943.
2012. View Article : Google Scholar : PubMed/NCBI
|