Introduction
EpCAM, the epithelial cell adhesion molecule, is a
hemophilic Ca2+-independent cell-cell adhesion molecule,
and it is expressed in different kinds of epithelial tissues
(1-3). In addition to its function in cell
adhesion, EpCAM has been reported to contribute to various
biological processes, such as signaling, migration and
proliferation (2,4). EpCAM has been considered to be a
cell surface marker for many kinds of stem cells (5,6).
Moreover, EpCAM is also highly expressed in epithelial tumor
tissues (7). However, the
molecular mechanisms of these processes remain to be
elucidated.
EpCAM mutations in humans can cause congenital
tufting enteropathy (CTE), a rare diarrheal disease that can cause
neonatal death (8). Several EpCAM
knockout mouse models have been reported to generate a CTE
phenotype (9-11). From these reports, it can be
concluded that EpCAM has important physiological functions in the
intestines of human and mammalian animals. EpCAM was determined to
be highly expressed in developing adult intestinal epithelium
(2,10). In addition, EpCAM is also highly
expressed in many kinds of stem cells, neoplastic tissues, and
other normal epithelial tissues, such as embryonic stem cells,
hepatocyte progenitor cells, and labyrinthine layer of placentas
(1,12-14). EpCAM recruits proteins of the
claudin family to maintain functional tight junctions in the
intestinal epithelium (10,15,16). Loss of functional tight junctions
in the intestinal epithelium may be one of the important causes of
diarrheal disease in humans and mice with mutated EpCAM. EpCAM is
not only localized to tight junctions but is also enriched at the
basolateral membrane of the intestinal epithelium (2). This suggests that EpCAM may have
some interactions with other types of cell junctions. However, the
conclusions regarding the function of EpCAM in regulating adherens
junctions are contradictory. Previous findings have shown that
EpCAM may suppress E-cadherin-mediated cell aggregation by
disrupting the association of E-cadherin with the cytoskeleton in
cultured murine fibroblast L cells (17,18). It has also been reported that
E-cadherin could exert opposing effects on EpCAM, especially in
tumor tissues (2). In addition,
E-cadherin protein was decreased (19) or mislocalized (20) in EpCAM-depleted human colorectal
adenocarcinoma Caco-2 cell line. However, Wu et al found
that the expression and localization of E-cadherin and β-catenin
were still normal after EpCAM knockdown in human colorectal
adenocarcinoma T84 cell line (16). Results from in vivo studies
were also different. In biopsy specimens from children with CTE,
the expression of E-cadherin was normal (21). Lei et al also found that
the expression level and localization of E-cadherin and β-catenin
were normal in the embryonic intestines of EpCAM knockout mice
(10). However, it has been
reported that E-cadherin and β-catenin proteins progressively lose
cell membrane localization and accumulate in the cytoplasm in the
intestinal epithelium of EpCAM knockout mice after birth (9). In EpCAM mutant zebrafish, the
expression of E-cadherin, β-catenin and α-catenin was reduced in
the enveloping layer, but the localization of these proteins was
still normal (4). Moreover,
global depletion of EpCAM in Xenopus embryos caused a significant
decrease in C-cadherin at the protein level (19). The molecular roles of EpCAM in
regulating adherens junctions, especially in mammalian models, are
still unclear.
In the present study, to investigate the functions
of EpCAM in regulating adherens junctions in the mammalian
intestinal epithelium, the expression of proteins that compose
adherens junctions was assessed at both the mRNA and protein levels
in different sections of the intestinal epithelium from
EpCAM−/− and wild-type (WT) mice at E18.5 to P3,
providing new evidence for the effects of EpCAM on adherens
junctions in the intestinal epithelium.
Materials and methods
Animal treatment
All mouse experiments were approved by the Committee
on Laboratory Animal Care and Use of Guangdong Pharmaceutical
University [Guangzhou, China (gdpu2016073)].
EpCAM−/− mice were previously generated
by CRISPR/Cas9 technology (22).
Mice were housed in the specific pathogen-free (SPF) animal
facility at 25°C with a 12-h light/dark cycle and 50-55% humidity.
The animals had free access to water and food. Heterozygous males
and females were mated and a total of 47 pairs of WT and
EpCAM−/− embryos and pups were used for the current
study. E18.5 embryos were harvested from pregnant females. E18.5
embryos and P0 and P3 pups were sacrificed, after which their
intestines were collected for experiments. Pregnant mice were
sacrificed by cervical dislocation. E18.5 embryos were obtained by
dissecting pregnant females. E18.5 Embryos, P0 and P3 pups were
sacrificed humanely by decapitation. The information of the number
and genotype of mice used in the present work was summarized in
Table SI.
Histological analysis
For histological analysis, duodenum, jejunum, ileum
and colon tissues from WT and EpCAM−/− mice were fixed
overnight with 4% paraformaldehyde in PBS at 4°C before being
dehydrated and embedded in paraffin. Then, hematoxylin and eosin
(H&E) staining was performed on 4-µm paraffin sections;
the sections were incubated in hematoxylin for 2 min and eosin for
30 sec at room temperature. Images were captured using the
PerkinElmer Automated Quantitative Pathology System.
Immunofluorescence staining
For immunofluorescence staining, duodenum, jejunum,
ileum and colon tissues from WT and EpCAM−/− mice were
fixed overnight with 4% para-formaldehyde in PBS at 4°C before
being dehydrated and embedded in optimal cutting temperature
compound (OCT) (Sakura Finetek). Then, 7-µm frozen sections
were boiled in 10 mM citric acid (Merck) at pH 6.0 for 5 min. After
exposure to goat serum blocking buffer (ZSGB-BIO, ZLI-9056) at room
temperature for 1 h, the sections were incubated overnight at 4°C
with primary antibodies and then with secondary antibodies at room
temperature for 1 h. Primary antibodies were used as follows:
Rabbit anti-E-cadherin (Cell Signaling Technology, Inc.; cat. no.
14472, 1:200), rabbit anti-p120-catenin (Santa Cruz Biotechnology,
Inc.; cat. no. 15D2, 1:200), and rabbit anti-β-catenin (BD
Biosciences; cat. no. 610154, 1:200). Immunofluorescence analysis
was performed with Alexa Fluor 488-labeled donkey anti-rabbit IgG
(H+L) secondary antibodies (Thermo Fisher Scientific, Inc.; cat.
no. A21206, 1:1,000), and DAPI (Sigma-Aldrich; Merck KGaA; cat. no.
D9564, 1:10,000) was used to stain the nuclei of tissues.
Immunofluorescence images were observed using a PerkinElmer
Automated Quantitative Pathology System.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from mouse small intestines was extracted
using an RNAiso Plus kit (Takara; cat. no. 9109), which was
subjected to reverse transcription using a PrimeScript™ RT Reagent
Kit with gDNA Eraser (Takara; cat. no. RR047A) at 37°C for 15 min
and 85°C for 5 sec. The sequences of primers for RT-qPCR are listed
in Table SII and they were
produced by Sangon Biotech Co., Ltd. RT-qPCR was conducted using a
TB Green® Premix Ex Taq™ II Kit (Takara; cat. no.
RR820A) and the PikoReal PCR system (Thermo Fisher Scientific,
Inc.). The thermocycling program was 95°C for 30 sec, followed by
40 cycles of 95°C for 5 sec, 60°C for 20 sec and 65°C for 15 sec.
Mouse GAPDH was used as an internal reference gene.
Western blot analysis
The small and large intestines of mice were
collected and washed in cold PBS (HyClone). Then, they were lysed
in radioimmunoprecipitation assay lysis buffer containing 1% PMSF
as well as 1% protease inhibitor cocktail (Dalian Meilun
Biotechnology Co., Ltd.), and centrifuged at 13,680 × g at 4°C for
30 min, and the supernatant was frozen at -80°C. Protein
concentration was determined by BCA assay (Beyotime), and equal
amounts of protein (20 µg) were subjected to SDS-PAGE on a
10 or 12% gel. The separated proteins were transferred
electrophoretically to a PVDF membrane, after which the membrane
was blocked with 5% non-fat milk at room temperature for 1 h and
then incubated with primary antibodies including E-cadherin (Cell
Signaling Technology, Inc.; cat. no. 14472, 1:1,000), p120-catenin
(Santa Cruz Biotechnology, Inc.; cat. no. 15D2, 1:1,000), β-catenin
(BD Biosciences; cat. no. 610154, 1:1,000), nectin 1 (Abcam; cat.
no. ab66985), and α-catenin (Sigma-Aldrich; Merck KGaA; cat. no.
C2081) at 4°C overnight. Subsequently, the membrane was incubated
with horseradish peroxidase-labeled antibodies [goat anti-rabbit
IgG H&L (HRP) (Abcam; cat. no. ab6721, 1:2,000) and goat
anti-mouse IgG H&L (HRP) (Abcam; cat. no. ab6789, 1:5,000)] at
room temperature for 1 h. The resultant signals were detected using
enhanced chemiluminescence reagent (Bio-Rad Laboratories, Inc.;
cat. no. 170-5060). Western blotting bands were quantitatively
analyzed using Lane 1d software (version 5.1.0.0;
SageCreation).
Statistical analysis
Statistical differences were determined using SPSS
software (23.0). Unpaired two sample t-test was used to determine
the difference among groups. The data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
The architecture of intestines from
postnatal EpCAM mutant mice was gradually broken down during
development
To study the functions of EpCAM in vivo,
EpCAM−/− mice that we previously generated through
CRISPR-Cas9 technology were used software used for densitometry
(22). The phenotype of
EpCAM−/− mice is similar to that of EpCAM mutant mice,
which were generated using traditional gene targeting methods
(9,10,15). The newborn EpCAM−/−
mice were indistinguishable from their WT and heterozygous
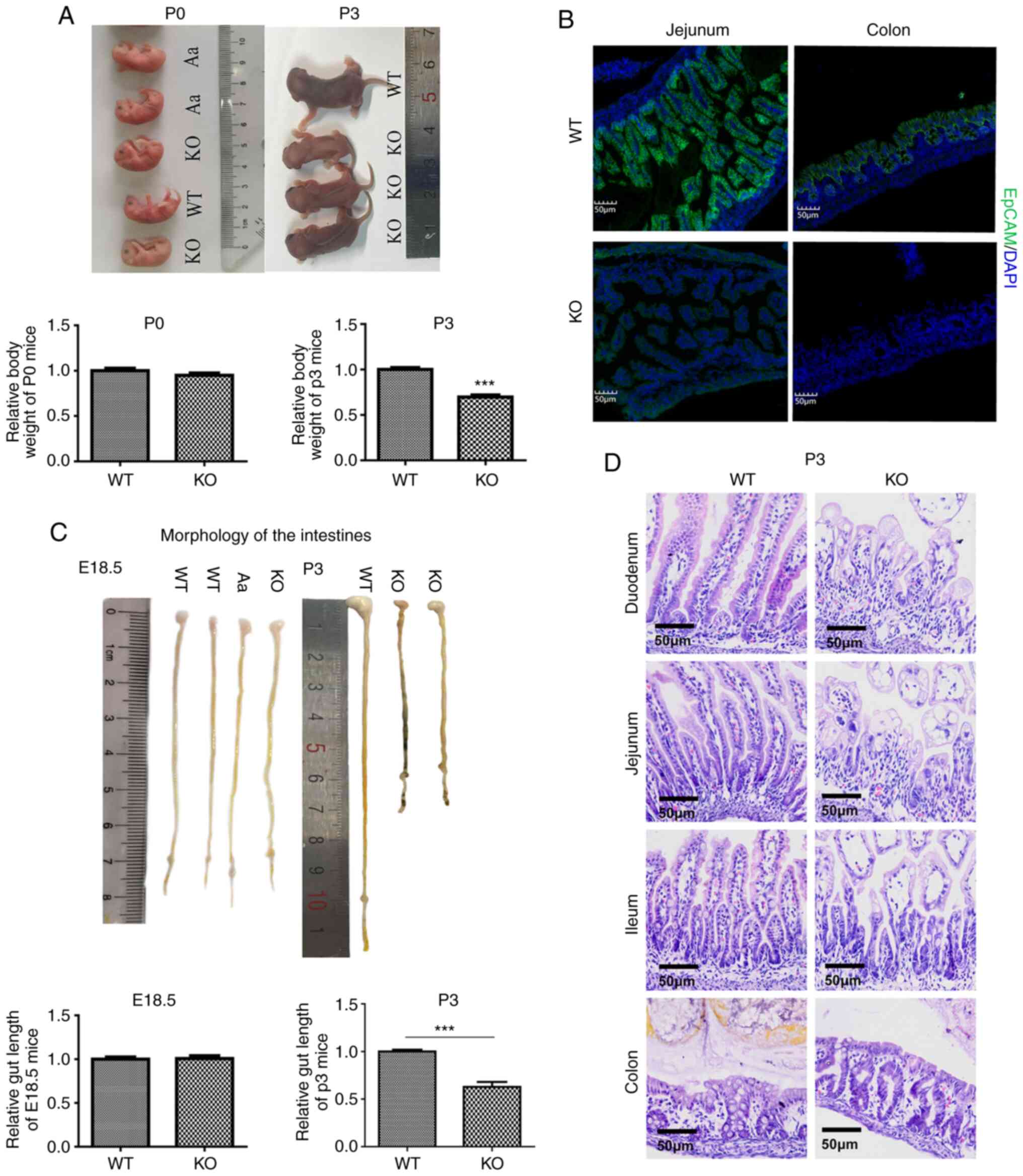
littermates at P0. However, there was no increase in the body size
of EpCAM−/− pups during development, and the body weight
of P3 EpCAM−/− pups was significantly lower than that of
their littermates (Fig. 1A).
Since a loss-of-function mutation in EpCAM can cause
CTE in patients (8) and the
intestines were seriously affected in previously reported EpCAM
mutant mouse models (9), the
morphological and histological changes of intestines of
EpCAM−/− mice from E18.5 to P3 were investigated in this
study. It was first confirmed that the EpCAM protein was completely
lost in the intestines of EpCAM−/− mice (Fig. 1B). The morphology of the
intestines from EpCAM−/− mice looked normal for both
E18.5 (Fig. 1C) and P0 (data not
shown). Then, it became abnormal at P3. The length of the
intestines of mutant mice at P3 was significantly shorter than that
for WT pups at P3. The diameter of the ileum from the WT pups was
thinner than that of the duodenum and jejunum, but in
EpCAM−/− pups, the diameter of the ileum became larger
than that of the duodenum and jejunum. Blood could be observed in
the intestinal lumen of P3 EpCAM−/− mice (Fig. 1C). Tufts of villi could be
detected in the small intestines of EpCAM−/− mice from
E18.5 to P3, as shown by H&E staining (Figs. 1D and S1). There was no significant damage to
villi from EpCAM−/− mice at E18.5 and P0 (Fig. S1). However, the intestinal
epithelium was gradually broken down during development of the
mutant mice after birth, and the size of intestinal epithelial
cells in mutant mice at P3 became very large (Fig. 1D).
Proteins that compose adherens junctions
gradually mislocalize in the epithelial cells of the small
intestine in postnatal EpCAM mutant mice during development
To investigate whether the composition and functions
of adherens junctions (AJs) in the intestinal epithelium were
affected by the loss of EpCAM, the proteins that compose the AJs
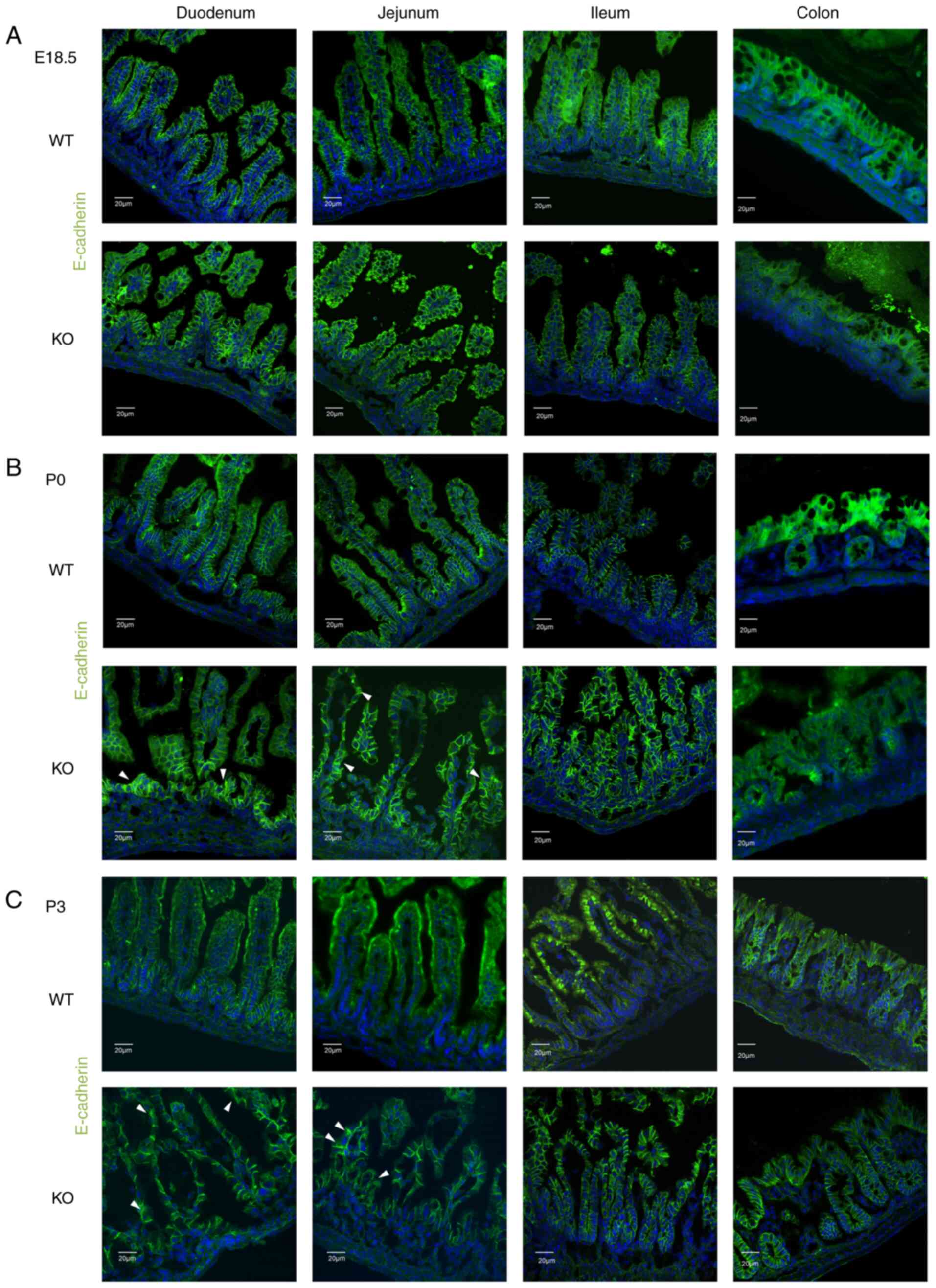
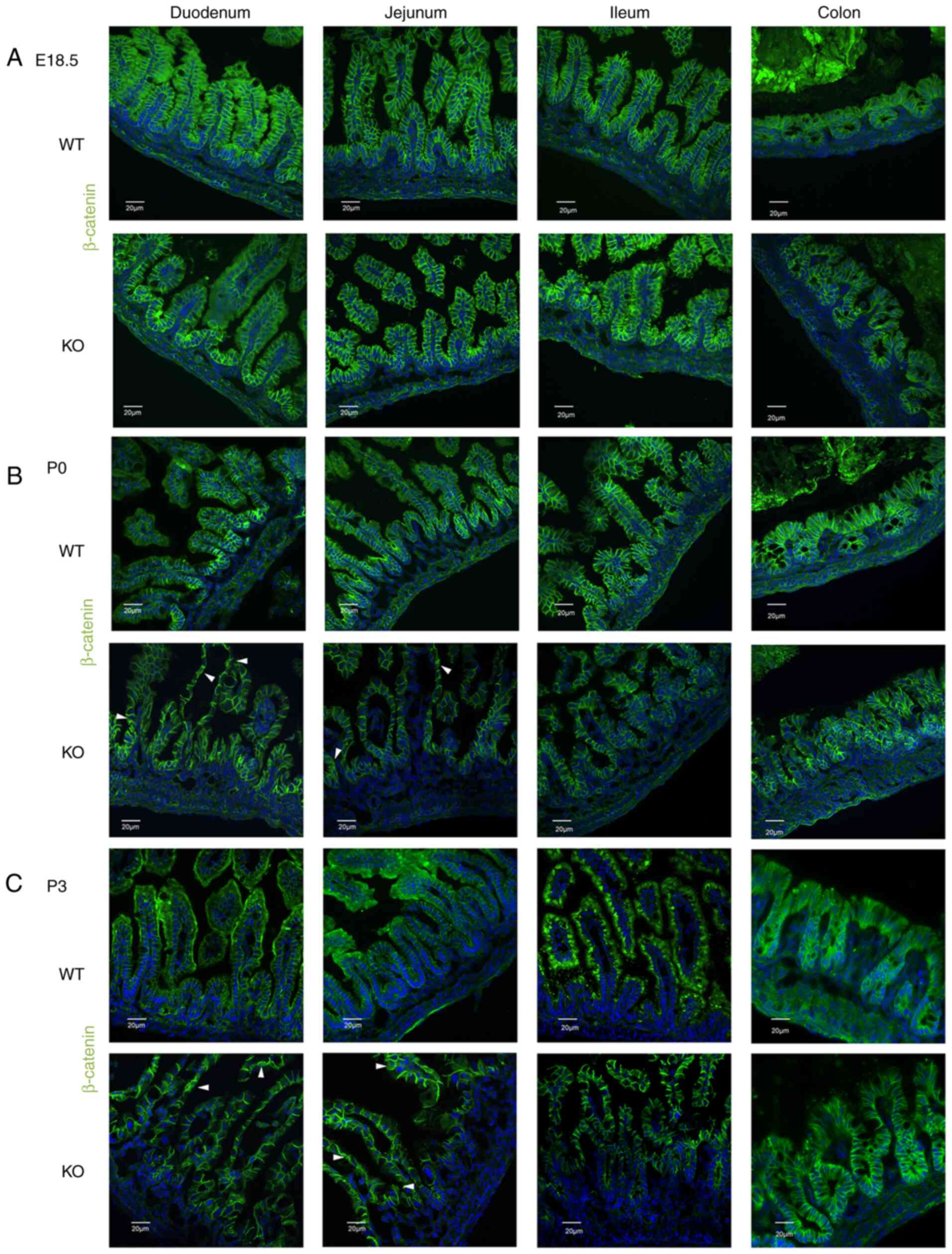
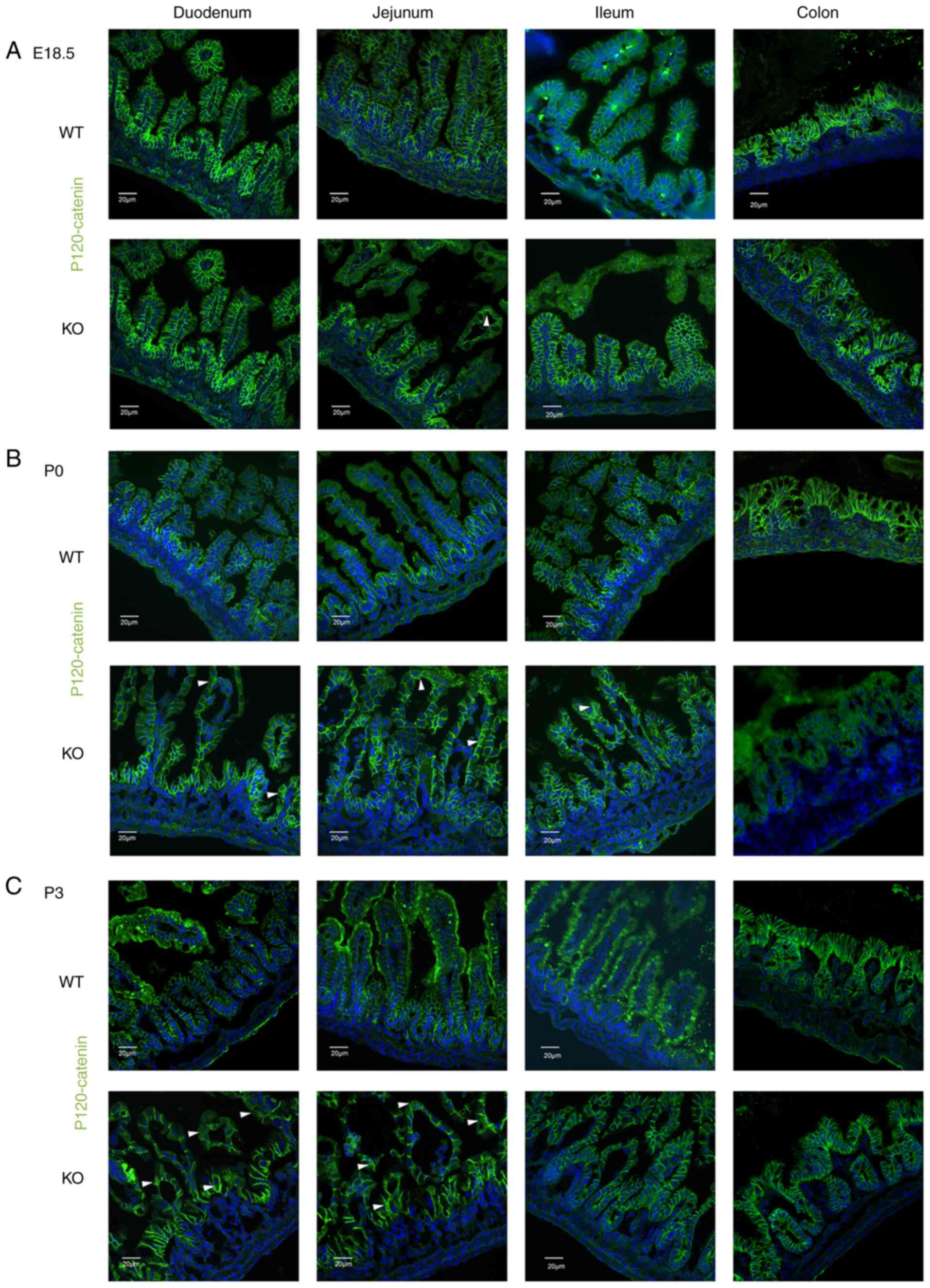
were analyzed via immunofluorescence (IF) staining. E-cadherin,
β-catenin and p120 were only found to be localized to the membrane
between epithelial cells in the intestines of WT mice from E18.5 to
P3 (Figs. 2Figure 3-4). The localization of these proteins
was normal in the intestines of EpCAM−/− mice at E18.5,
and the IF signal of these proteins was even higher in the duodenum
and jejunum of EpCAM−/− mice than that in the WT mice at
this stage (Figs. 2A, 3A, and 4A, and S2A, S3A, and S4A). Since the intestinal
tight junctions of EpCAM−/− mice were impaired at the
E18.5 stage (10), the intestinal
epithelium was affected, although it was still not clear at the
morphology level. The higher IF signal was induced by the affected
tissues of the EpCAM−/− mice. However, some of these
proteins began to be detected in the cytoplasm of epithelial cells
in the duodenum and jejunum of EpCAM−/− pups at P0
(Figs. 2B, 3B, and 4B, and S2B, S3B, and S4B). At P3, the
signals of these proteins clearly localized to the cytoplasm of
epithelial cells in the duodenum and jejunum of EpCAM−/−
pups. The localization of E-cadherin, β-catenin and p120 in the
epithelium of the ileum and colon in EpCAM−/− mice was
still normal from E18.5 to P3. The signal of these proteins was not
detected in the nucleus of the epithelial cells of the intestines
of either WT or EpCAM−/− mice from E18.5 to P3 (Figs. 2C, 3C, and 4C, and S2C, S3C, and S4C).
Proteins that compose adherens junctions
are gradually reduced in the intestinal epithelial cells of
postnatal EpCAM mutant mice during development
The expression levels of proteins that compose
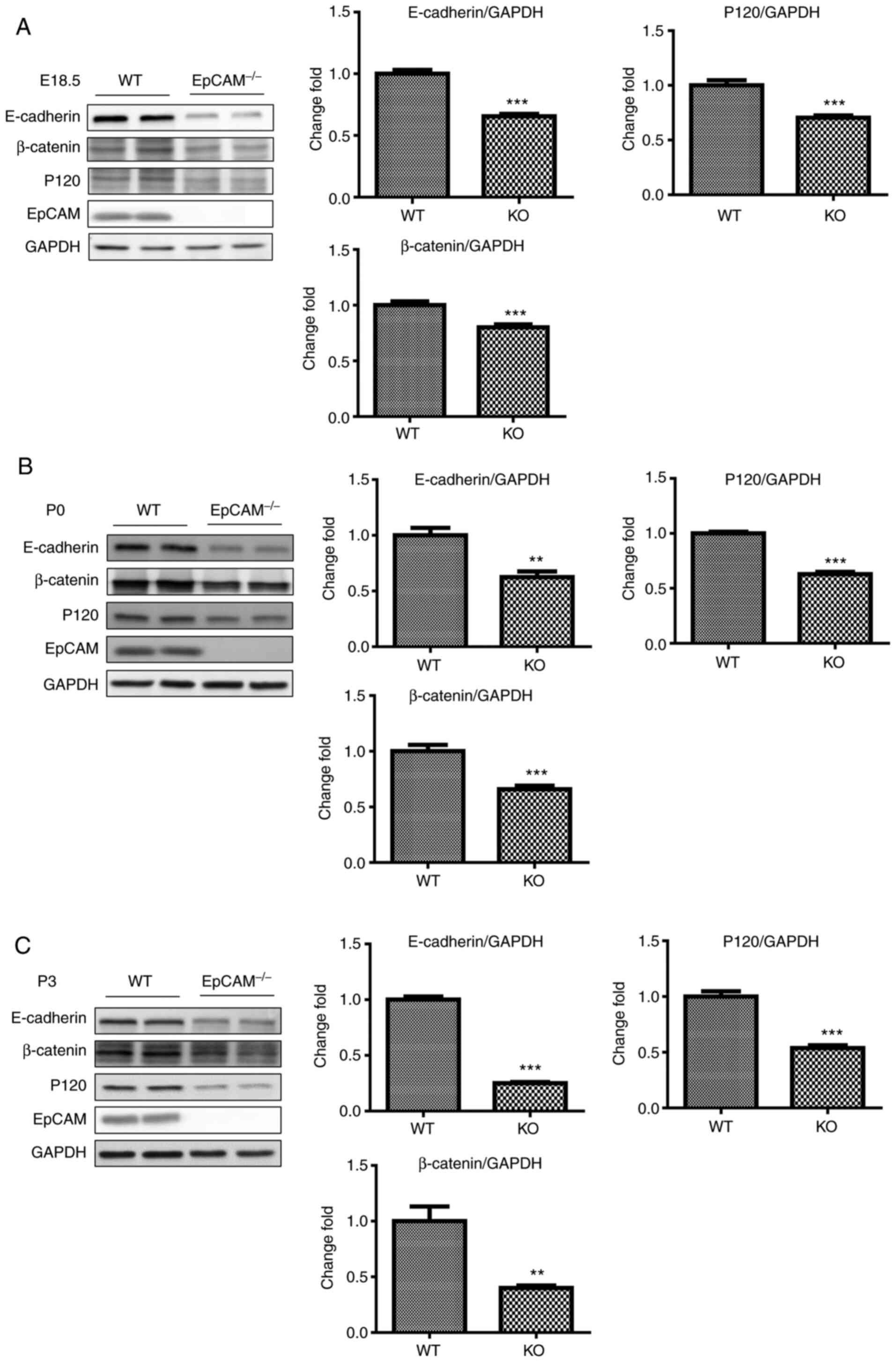
adherens junctions in the small intestines were further analyzed by
western blot analysis. The protein levels of E-cadherin, β-catenin
and p120 in the small intestines of EpCAM−/− mice at
E18.5 was slightly lower than that of WT mice, but their reduction
was significant (Fig. 5A). At P0,
the reduction in the protein levels of E-cadherin, β-catenin and
p120 in the small intestines of EpCAM−/− mice was more
apparent (Fig. 5B). At P3, only
approximately half as much p120 protein was observed in the small
intestines of EpCAM−/− pups, and the protein levels of
E-cadherin and β-catenin in the small intestines of
EpCAM−/− pups were reduced by more than half compared
with the levels in WT pups (Fig.
5C).
mRNA expression of proteins that compose
adherens junctions was normal in the intestines of postnatal EpCAM
mutant mice during development
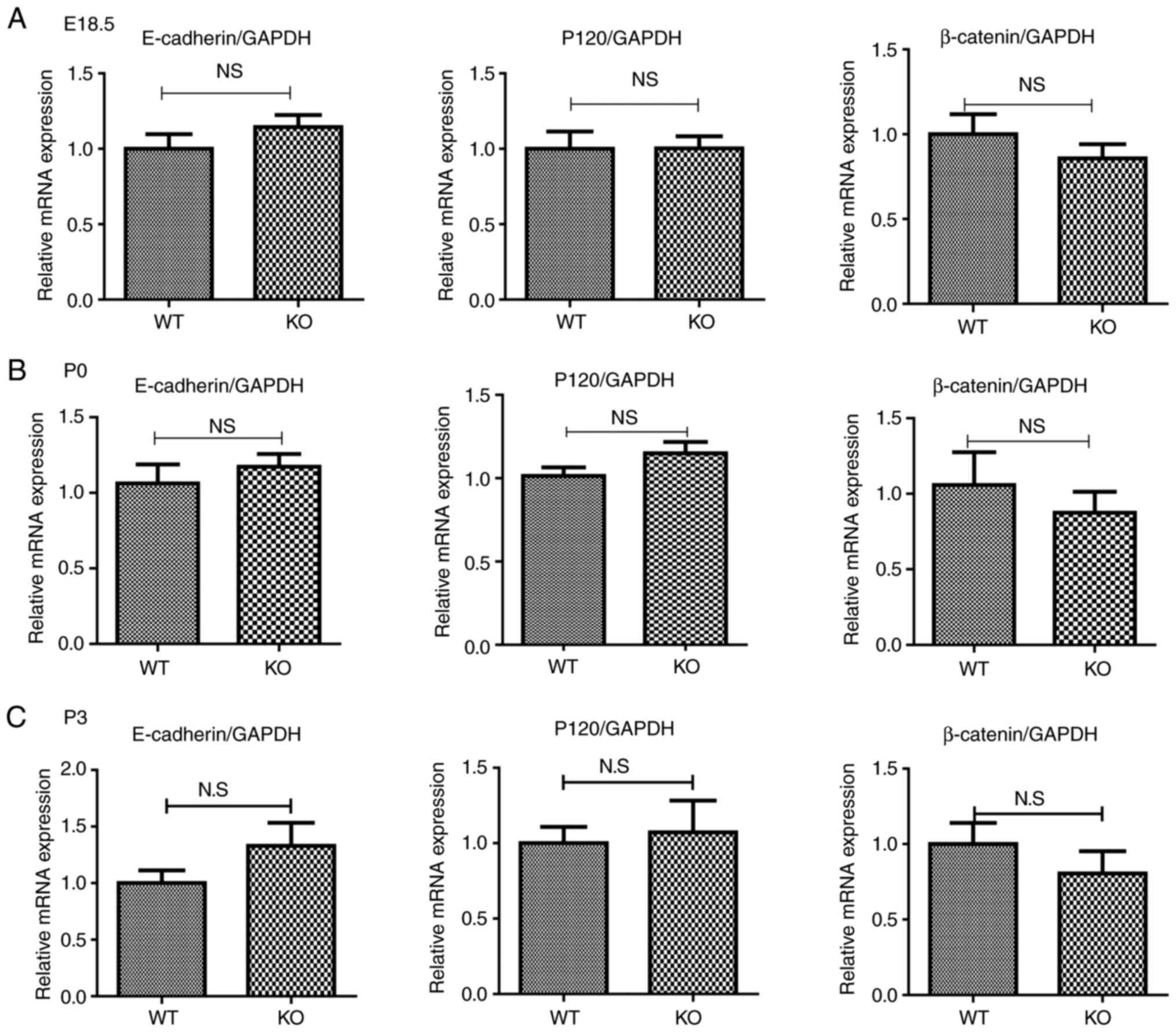
To examine how EpCAM regulated the localization and
expression of proteins that compose adherens junctions, their gene
expression was assessed at the mRNA level by qPCR. At E18.5 and P3,
the expression of E-cadherin, β-catenin and p120 mRNA in the small
intestines was similar between WT and EpCAM−/− mice
(Fig. 6). The expression of
E-cadherin mRNA in the small intestines of EpCAM−/− pups
was increased compared with that of WT pups at the same stage, and
the expression of β-catenin mRNA in the small intestines of
EpCAM−/− mice was reduced, but these changes were not
significant (Fig. 6).
Expression of nectin 1 became gradually
reduced in the intestines of postnatal EpCAM mutant mice during
development
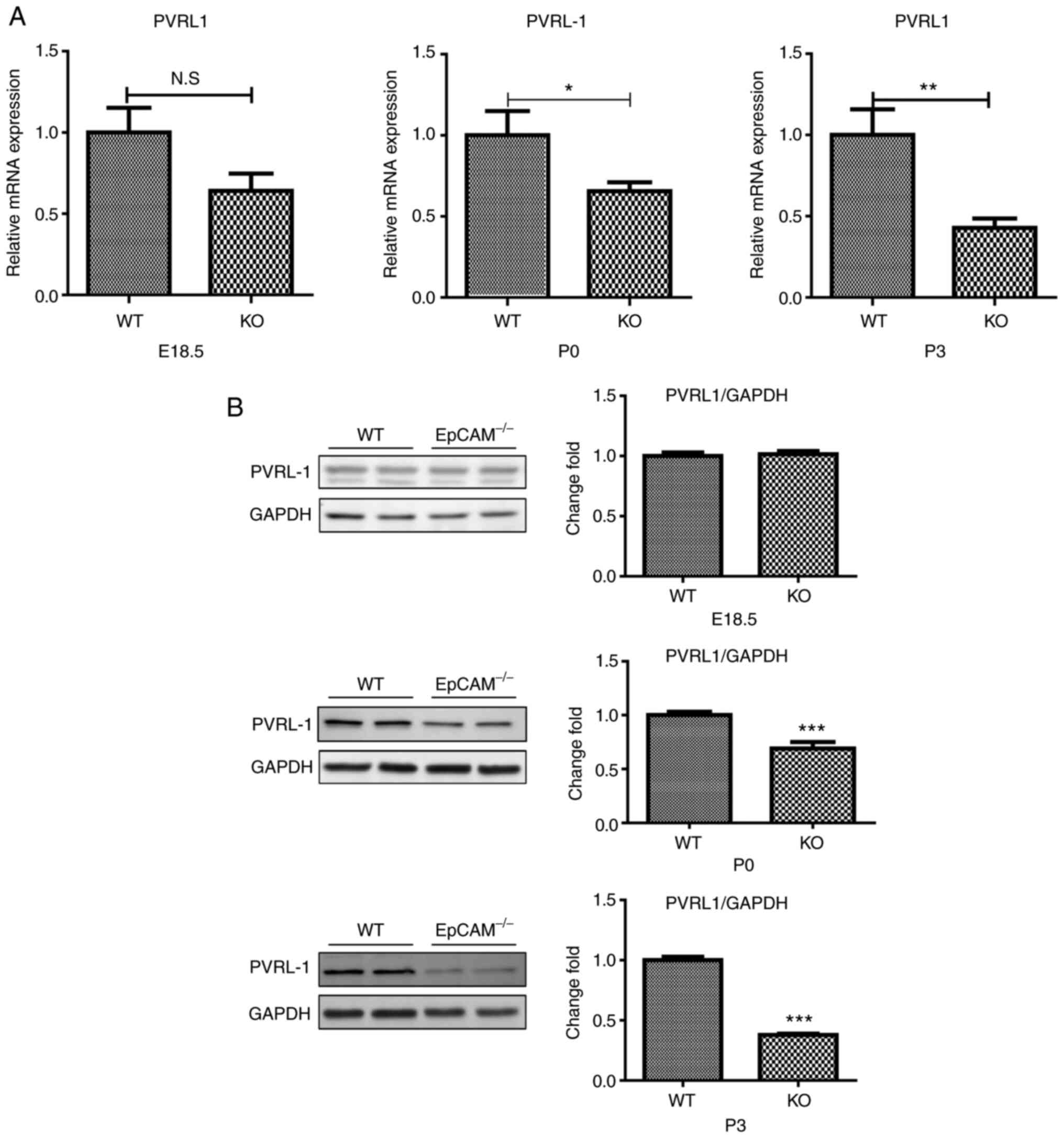
The expression of genes that encoded proteins that
compose adherens junctions was further assessed. Nectin 1, also
known as PVRL1, which can regulate the assembly and adhesion
activity of E-cadherin (23), was
found to be significantly changed in the intestines of
EpCAM−/− mice (Fig. 7A and
B). The qPCR results showed that the expression of nectin 1
mRNA in the small intestines of EpCAM−/− mice was
significantly reduced at P0 and P3, although it was still normal at
E18.5 (Fig. 7A). The expression
of nectin 1 protein in the small intestines of EpCAM−/−
mice was normal at E18.5 and P0, but compared to control mice,
there was only half as much nectin 1 protein in the intestines of
P3 EpCAM−/− mice (Fig.
7B).
Discussion
In the present study, we confirmed that the adherens
junctions in the upper part of the small intestinal epithelium of
postnatal EpCAM−/− mice were gradually affected, but
they were still normal in the lower part of the intestinal
epithelium in EpCAM−/− mice. The results of H&E
staining showed that the architecture of the duodenum and jejunum
of EpCAM−/− mice at P3 was broken down, but the
architecture of the ileum and colon was not affected even at P3.
Moreover, the expression level and localization of E-cadherin,
β-catenin and p120 were also gradually affected in the duodenum and
jejunum of EpCAM−/− mice from E18.5 to P3 stages, but
they were not affected in the ileum and colon of
EpCAM−/− mice even at P3 stage. Finally, the expression
of nectin 1 was reduced at both the mRNA and protein levels, which
may be one of the important reasons for the breakdown of adherens
junctions in the intestinal epithelium of postnatal
EpCAM−/− mice. These results first showed the detailed
process and mechanism of the adherens junctions breakdown in the
intestinal epithelium of EpCAM−/− mice.
The intestine was the most severely damaged organ in
the EpCAM mutant mice, and the mutation of EpCAM could also cause
CTE in human. Therefore, we only assessed the AJs in the intestines
at the current study. The assumption was that the AJs of some other
organs in the EpCAM mutant mice may also be affected slightly, and
we aim to test AJs in other tissues of EpCAM−/− mice in
future studies. The function of EpCAM in maintaining the
architecture of the intestinal epithelium has been confirmed by
several studies, since it was found that the mutation of EpCAM
could cause CTE (8,9-11,24). Total or partial villus atrophy and
crypt hyperplasia could be found in the epithelium of small
intestines from CTE patients (8,25).
Lei et al found that the intestines of EpCAM knockout mice
were relatively normal at the neonatal stage, as revealed by both
macroscopic and histological data, but the disruption of mucosal
architecture and sloughing of epithelial cells could be detected in
EpCAM knockout mice at P5, especially in the duodenum and jejunum
(10). Guerra et al also
reported the increasing severity of villous atrophy and an increase
in epithelial tufts in EpCAM mutant intestines (9). In the present study, significant
changes in the intestinal epithelium of EpCAM−/− mice
also occurred after birth. The upper part of the intestinal
epithelium was broken down, but the lower part was normal even at
P3. These results demonstrated that the intestinal architecture
breakdown occurred earlier in the upper parts of the intestines
than it did in the lower parts. Since the EpCAM mutation could
impair tight junction formation in the intestinal epithelium, Lei
et al suggested that the abundant proteases in the upper
parts of the small intestines might be the cause of this phenotype
(10). In the present study, we
hypothesize that these proteases could easily penetrate the upper
parts of the intestinal epithelium in the EpCAM mutant and cause
damage. Therefore, many swollen cells appeared in the upper parts
of the small intestines of EpCAM−/− mice, which could
exacerbate the architectural breakdown of the upper parts of the
small intestines in the EpCAM mutant. Therefore, the compositions
of adherens junctions in the duodenum and jejunum of EpCAM mutant
mice may be affected by proteases.
Several proteins that compose adherens junctions
have been demonstrated to be essential for maintaining the
homeostasis and morphogenesis of intestines. Specific knocking out
E-cadherin in the intestinal epithelium of adult mice can cause
hemorrhagic diarrhea because of the abnormal intestinal epithelial
architecture (26), and deletion
of E-cadherin from the developing mouse intestinal epithelium
causes death shortly after birth because of the impairment of
intestinal morphogenesis (27,28). Transgenic expression of a
dominant-negative N-cadherin (DN-cadherin) in the mouse small
intestine can cause loss of endogenous E-cadherin protein and
induce Crohn-like inflammatory bowel disease at the age of 3
months, with most of these mice developing adenomas within 6 months
(29). Hermiston et al
also found that the forced expression of E-cadherin in the mouse
intestinal epithelium resulted in slower cellular migration from
crypts to villi (30). Knocking
out p120 in the mouse intestinal epithelium resulted in mucosal
damage and inflammation, leading to bleeding and death within 3
weeks of birth (31).
Smalley-Freed et al found that limited ablation of
p120-catenin in the adult mouse intestinal epithelium could promote
adenoma formation by an indirect non-cell autonomous mechanism
(32), and Short et al
confirmed that p120 was an obligate haploinsufficient tumor
suppressor in intestinal neoplasia, as shown by a conditional p120
knockout in Apc-sensitized mouse models (33). β-catenin is the key molecule in
the Wnt signaling pathway (34),
and it is essential for many biological processes in the
intestines, such as maintenance of intestinal stem cells,
inflammation and carcinogenesis (15,35-38). Therefore, any factor that could
affect the expression or localization of proteins that compose
adherens junctions in the intestines would affect the homeostasis
of the intestinal epithelium. Since EpCAM is essential for
maintaining the homeostasis of the intestinal epithelium, previous
studies have tried to elucidate the changes in proteins that
compose adherens junctions in the EpCAM mutant intestines (9,10,21), but their conclusions were based on
different developmental stages and different parts of the
intestines. Furthermore, intestinal tissues from CTE patients are
very difficult to obtain. In the present study, we compared the
whole intestines of WT and EpCAM−/− mice at E18.5, P0
and P3, and we found that the E-cadherin, p120 and β-catenin
proteins in the duodenum and jejunum of P0 EpCAM−/− mice
started to decrease and mislocalize at P0, and they were seriously
affected at P3, but these proteins in the ileum and colon were not
affected even at P3. These results demonstrated that EpCAM could
protect the architecture of the duodenum and jejunum by maintaining
the expression and localization of proteins that compose adherens
junctions in these areas. The mRNA levels of these adherens
junction-associated genes were not decreased, so we concluded that
EpCAM may regulate the expression of these genes at the
post-transcriptional level.
E-cadherin, β-catenin, and p120 are three important
proteins which composed adherens junctions, and the inter-actions
between them have been clearly studied. There is a multiple complex
at adherens junctions, and the binding sides of β-catenin and p120
on E-cadherin are very clear now (39). If EpCAM could directly interact
with one of these proteins in the multiple complex of the adherens
junctions, it could be detected by Co-IP experiments. However, Wu
et al have found that EpCAM and E-cadherin were not tightly
associated in T84 cells through Co-IP experiments (16). It means that EpCAM is not directly
associated with one of the proteins in the multiple complex of the
adherens junctions.
Nectins are a type of immunogroblin (Ig)-like cell
adhesion molecule, and they can regulate the formation of adherens
junctions and tight junctions (40,41). We found that the mRNA level of
nectin 1 was significantly reduced at the P0 and P3 stages, while
its protein level was also significantly reduced at the P3 stage in
EpCAM−/− mice. Sato et al reported that nectin 1
could regulate the assembly and adhesion activity of E-cadherin in
MDCK cells (23). Martinez-Rico
et al found that nectin 1 had important roles in the
regulation of E-cadherin-based adhesion (42). We hypothesize that the reduction
of nectin 1 may be one of the important reasons for the decrease
and mislocalization of proteins that compose adherens junctions. It
has been reported that EpCAM could control at least four
independent signaling pathways (1-3),
and the expression of nectin 1 may control one or more of these
signaling pathways. Furthermore, the impaired tight junctions of
the intestinal epithelium from EpCAM−/− mice may disrupt
intracellular signaling pathways which are essential to regulate
the nectin 1 expression. Our previous study had already shown that
claudin proteins were downregulated in the intestines of EpCAM
mutant mice (10).
In summary, we examined the expression of claudin-7
at both mRNA and protein levels after obtaining the current
EpCAM−/− model through CRISPR/Cas9 technology. The
results yielded were similar to those of our previous studies.
Since the current study focused on adherens junctions of the
intestinal epithelium, the claudin-7 results were not included.
Nevertheless, further investigations are needed to examine the
manner in which EpCAM regulates the expression of nectin 1.
Supplementary Data
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81830113, 81803912,
31671520, and 81530102), the Major Basic and Applied Basic Research
Projects of Guangdong Province of China (grant no. 2019B030302005),
the National Key R&D plan 'Research on modernization of
traditional Chinese medicine' (grant no. 2018YFC1704200), the
Opening Foundation of the Key Laboratory of Regenerative Biology,
the Guangzhou Institutes of Biomedicine and Health, the Chinese
Academy of Sciences (grant no. KLRB201807), the Science and
Technology Planning Project of Guangzhou City (grant no.
201803010069), the Scientific Research Project of the
Administration of Traditional Chinese Medicine of Guangdong
Province (grant no. 20182079), the Characteristic Innovation
Project (Natural Science) of the Education Department of Guangdong
Province and the 'Innovation Strong School Project' of Guangdong
Pharmaceutical University (grant no. 2017KTSCX102), and the Science
and Technology Project of Yue-Xiu District of Guangzhou (grant no.
2018-WS-011).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JG and ZL designed the study and conceived its
execution. ZL and YY analyzed and interpreted the results, and
wrote and revised the manuscript. GC, WL and LH maintained the
mouse model and performed all qPCR and immunostaining experiments.
YY, LY, YL, and HW performed the H&E staining and western
blots. All authors read the final manuscript and approved it.
Ethics approval and consent to
participate
All mouse experiments were approved by the Committee
on Laboratory Animal Care and Use of Guangdong Pharmaceutical
University [Guangzhou, China (gdpu2016073)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Balzar M, Winter MJ, de Boer CJ and
Litvinov SV: The biology of the 17-1A antigen (Ep-CAM). J Mol Med
(Berl). 77:699–712. 1999. View Article : Google Scholar
|
|
2
|
Huang L, Yang Y, Yang F, Liu S, Zhu Z, Lei
Z and Guo J: Functions of EpCAM in physiological processes and
diseases (Review). Int J Mol Med. 42:1771–1785. 2018.PubMed/NCBI
|
|
3
|
Schnell U, Cirulli V and Giepmans BN:
EpCAM: Structure and function in health and disease. Biochim
Biophys Acta. 1828:1989–2001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slanchev K, Carney TJ, Stemmler MP,
Koschorz B, Amsterdam A, Schwarz H and Hammerschmidt M: The
epithelial cell adhesion molecule EpCAM is required for epithelial
morphogenesis and integrity during zebrafish epiboly and skin
development. PLoS Genet. 5:e10005632009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamimoto K, Kaneko K, Kok CY, Okada H,
Miyajima A and Itoh T: Heterogeneity and stochastic growth
regulation of biliary epithelial cells dictate dynamic epithelial
tissue remodeling. Elife. 5:e150342016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmelzer E, Zhang L, Bruce A, Wauthier E,
Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, et al:
Human hepatic stem cells from fetal and postnatal donors. J Exp
Med. 204:1973–1987. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carneiro FP, Muniz-Junqueira MI, De
Vasconcelos Carneiro M, De Araújo Oliveira Í, Soares AC, De Vargas
Haar N, Takano GHS, De Sousa Vianna LM, De Carvalho Caldas G,
Vieira DLM, et al: Anti-EpCAM antibodies for detection of
metastatic carcinoma in effusions and peritoneal wash. Oncol Lett.
18:2019–2024. 2019.PubMed/NCBI
|
|
8
|
Sivagnanam M, Mueller JL, Lee H, Chen Z,
Nelson SF, Turner D, Zlotkin SH, Pencharz PB, Ngan BY, Libiger O,
et al: Identification of EpCAM as the gene for congenital tufting
enteropathy. Gastroenterology. 135:429–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guerra E, Lattanzio R, La Sorda R, Dini F,
Tiboni GM, Piantelli M and Alberti S: mTrop1/Epcam knockout mice
develop congenital tufting enteropathy through dysregulation of
intestinal E-cadherin/β-catenin. PLoS One. 7:e493022012. View Article : Google Scholar
|
|
10
|
Lei Z, Maeda T, Tamura A, Nakamura T,
Yamazaki Y, Shiratori H, Yashiro K, Tsukita S and Hamada H: EpCAM
contributes to formation of functional tight junction in the
intestinal epithelium by recruiting claudin proteins. Dev Biol.
371:136–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mueller JL, McGeough MD, Peña CA and
Sivagnanam M: Functional consequences of EpCam mutation in mice and
men. Am J Physiol Gastrointest Liver Physiol. 306:G278–G288. 2014.
View Article : Google Scholar :
|
|
12
|
Ng V, Ang S, Chan J and Choo A:
Characterization of epithelial cell adhesion molecule as a surface
marker on undifferentiated human embryonic stem cells. Stem Cells.
28:29–35. 2010. View Article : Google Scholar
|
|
13
|
Gerlach J, Foka H, Thompson R, Gridelli B
and Schmelzer E: Epithelial cell adhesion molecule fragments and
signaling in primary human liver cells. J Cell Physiol.
233:4841–4851. 2018. View Article : Google Scholar
|
|
14
|
Nagao K, Zhu J, Heneghan M, Hanson J,
Morasso M, Tessarollo L, Mackem S and Udey MC: Abnormal placental
development and early embryonic lethality in EpCAM-null mice. PLoS
One. 4:e85432009. View Article : Google Scholar :
|
|
15
|
Das S, Yu S, Sakamori R, Vedula P, Feng Q,
Flores J, Hoffman A, Fu J, Stypulkowski E, Rodriguez A, et al:
Rab8a vesicles regulate Wnt ligand delivery and Paneth cell
maturation at the intestinal stem cell niche. Development.
142:2147–2162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu CJ, Mannan P, Lu M and Udey MC:
Epithelial cell adhesion molecule (EpCAM) regulates claudin
dynamics and tight junctions. J Biol Chem. 288:12253–12268. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Litvinov SV, Balzar M, Winter MJ, Bakker
HA, Briaire-de Bruijn IH, Prins F, Fleuren GJ and Warnaar SO:
Epithelial cell adhesion molecule (Ep-CAM) modulates cell-cell
interactions mediated by classic cadherins. J Cell Biol.
139:1337–1348. 1997. View Article : Google Scholar
|
|
18
|
Winter MJ, Nagelkerken B, Mertens AE,
Rees-Bakker HA, Briaire-de Bruijn IH and Litvinov SV: Expression of
Ep-CAM shifts the state of cadherin-mediated adhesions from strong
to weak. Exp Cell Res. 285:50–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maghzal N, Kayali HA, Rohani N, Kajava AV
and Fagotto F: EpCAM controls actomyosin contractility and cell
adhesion by direct inhibition of PKC. Dev Cell. 27:263–277. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salomon J, Gaston C, Magescas J,
Duvauchelle B, Canioni D, Sengmanivong L, Mayeux A, Michaux G,
Campeotto F, Lemale J, et al: Contractile forces at tricellular
contacts modulate epithelial organization and monolayer integrity.
Nat Commun. 8:139982017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patey N, Scoazec JY, Cuenod-Jabri B,
Canioni D, Kedinger M, Goulet O and Brousse N: Distribution of cell
adhesion molecules in infants with intestinal epithelial dysplasia
(tufting enter-opathy). Gastroenterology. 113:833–843. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, Liu S, Lei Z, Chen G, Huang L,
Yang F, Lei Y, Liu Y, Yang L, Liu W, et al: Circular RNA profile in
liver tissue of EpCAM knockout mice. Int J Mol Med. 44:1063–1077.
2019.PubMed/NCBI
|
|
23
|
Sato T, Fujita N, Yamada A, Ooshio T,
Okamoto R, Irie K and Takai Y: Regulation of the assembly and
adhesion activity of E-cadherin by nectin and afadin for the
formation of adherens junctions in Madin-Darby canine kidney cells.
J Biol Chem. 281:5288–5299. 2006. View Article : Google Scholar
|
|
24
|
Pathak SJ, Mueller JL, Okamoto K, Das B,
Hertecant J, Greenhalgh L, Cole T, Pinsk V, Yerushalmi B, Gurkan
OE, et al: EPCAM mutation update: Variants associated with
congenital tufting enteropathy and Lynch syndrome. Hum Mutat.
40:142–161. 2019. View Article : Google Scholar :
|
|
25
|
Sherman PM, Mitchell DJ and Cutz E:
Neonatal enteropathies: Defining the causes of protracted diarrhea
of infancy. J Pediatr Gastroenterol Nutr. 38:16–26. 2004.
View Article : Google Scholar
|
|
26
|
Schneider MR, Dahlhoff M, Horst D, Hirschi
B, Trülzsch K, Müller-Höcker J, Vogelmann R, Allgäuer M, Gerhard M,
Steininger S, et al: A key role for E-cadherin in intestinal
homeo-stasis and Paneth cell maturation. PLoS One. 5:e143252010.
View Article : Google Scholar
|
|
27
|
Bondow BJ, Faber ML, Wojta KJ, Walker EM
and Battle MA: E-cadherin is required for intestinal morphogenesis
in the mouse. Dev Biol. 371:1–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gloushankova NA, Rubtsova SN and Zhitnyak
IY: Cadherin-mediated cell-cell interactions in normal and cancer
cells. Tissue Barriers. 5:e13569002017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hermiston ML and Gordon JI: Inflammatory
bowel disease and adenomas in mice expressing a dominant negative
N-cadherin. Science. 270:1203–1207. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hermiston ML, Wong MH and Gordon JI:
Forced expression of E-cadherin in the mouse intestinal epithelium
slows cell migration and provides evidence for nonautonomous
regulation of cell fate in a self-renewing system. Genes Dev.
10:985–996. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smalley-Freed WG, Efimov A, Short SP, Jia
P, Zhao Z, Washington MK, Robine S, Coffey RJ and Reynolds AB:
Adenoma formation following limited ablation of p120-catenin in the
mouse intestine. PLoS One. 6:e198802011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smalley-Freed WG, Efimov A, Burnett PE,
Short SP, Davis MA, Gumucio DL, Washington MK, Coffey RJ and
Reynolds AB: p120-catenin is essential for maintenance of barrier
function and intestinal homeostasis in mice. J Clin Invest.
120:1824–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Short SP, Kondo J, Smalley-Freed WG,
Takeda H, Dohn MR, Powell AE, Carnahan RH, Washington MK, Tripathi
M, Payne DM, et al: p120-Catenin is an obligate haploinsufficient
tumor suppressor in intestinal neoplasia. J Clin Invest.
127:4462–4476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chiacchiera F, Rossi A, Jammula S, Piunti
A, Scelfo A, Ordóñez-Morán P, Huelsken J, Koseki H and Pasini D:
Polycomb complex PRC1 preserves intestinal stem cell identity by
sustaining Wnt/β-catenin transcriptional activity. Cell Stem Cell.
18:91–103. 2016. View Article : Google Scholar
|
|
36
|
Ahmad R, Kumar B, Chen Z, Chen X, Müller
D, Lele SM, Washington MK, Batra SK, Dhawan P and Singh AB: Loss of
claudin-3 expression induces IL6/gp130/Stat3 signaling to promote
colon cancer malignancy by hyperactivating Wnt/β-catenin signaling.
Oncogene. 36:6592–6604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Janeckova L, Fafilek B, Krausova M,
Horazna M, Vojtechova M, Alberich-Jorda M, Sloncova E, Galuskova K,
Sedlacek R, Anderova M and Korinek V: Wnt signaling inhibition
deprives small intestinal stem cells of clonogenic capacity.
Genesis. 54:101–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun X, Yao L, Liang H, Wang D, He Y, Wei
Y, Ye L, Wang K, Li L, Chen J, et al: Intestinal epithelial PKM2
serves as a safeguard against experimental colitis via activating
β-catenin signaling. Mucosal Immunol. 12:1280–1290. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kobielak A and Fuchs E: Alpha-catenin: At
the junction of inter-cellular adhesion and actin dynamics. Nat Rev
Mol Cell Biol. 5:614–625. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shimono Y, Rikitake Y, Mandai K, Mori M
and Takai Y: Immunoglobulin superfamily receptors and adherens
junctions. Subcell Biochem. 60:137–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takai Y, Ikeda W, Ogita H and Rikitake Y:
The immunoglobulin-like cell adhesion molecule nectin and its
associated protein afadin. Annu Rev Cell Dev Biol. 24:309–342.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martinez-Rico C, Pincet F, Perez E, Thiery
JP, Shimizu K, Takai Y and Dufour S: Separation force measurements
reveal different types of modulation of E-cadherin-based adhesion
by nectin-1 and -3. J Biol Chem. 280:4753–4760. 2005. View Article : Google Scholar
|