Introduction
Bone is a unique type of tissue that undergoes
continuous modelling and remodeling, a process that is accomplished
by 2 major cell types: Bone-forming osteoblasts and bone-resorbing
osteoclasts (1). Osteoblasts and
osteoclasts are involved in the complex and precise network of
signaling pathways that regulate this process (2-4).
However, the mechanisms underlying osteoblastogenesis, which is a
complex process, remain to be elucidated. However, several
molecules and signaling pathways, such as the Wnt signaling
pathway, BMP-2/Smad/Runx2 pathway and AMPK pathway (5-7),
have been found to regulate the maturation, differentiation and
function of pre-osteoblasts and osteoblasts. The differentiation
and function of osteoclasts are mainly regulated by the
RANKL/RANK/OPG pathway (8-11).
Various skeletal diseases occur when the balance between bone
resorption and formation is lost.
Inflammation is one of the major reasons for this
imbalance and is manifested as impaired bone formation, as well as
excessive bone degradation (1,12).
Inflammatory cytokines and other messenger molecules produced by
activated cells are delivered to target cells of bone tissue.
Lipopolysaccharide (LPS), a main component of Gram-negative
bacterial membranes, is commonly used as a stimulator of the
inflammatory response in cell experiments and has been demonstrated
to suppress osteoblastic differentiation (13-20), increase osteoclast activity
(21) and induce bone loss
(22,23). Recent studies have demonstrated
that LPS-activated autophagy negatively regulates Wnt signaling via
the autophagic degradation of dishevelled 2 (Dvl2), which plays an
important role in osteoclastogenesis from pre-osteoclasts (24). Accordingly, exploration at the
genetic level seems necessary for elucidating the mechanisms that
mediate the inflammatory process in skeletal disease.
Long non-coding RNAs (lncRNAs) are non-coding RNAs
(ncRNAs) of >200 bp in length, which have been reported to be
involved in a variety of biological functions, such as the
regulation of gene expression through epigenetic regulation,
transcriptional regulation and post-transcriptional regulation
(25,26). Recent research has indicated that
several lncRNAs expressed in macrophages and monocytes mediate
pro-inflammatory and anti-inflammatory processes, cell
differentiation and survival (27). Previous studies have demonstrated
that lncRNAs may regulate osteogenic differentiation by interacting
with miRNAs or specific pathways (28-30). Additionally, several lncRNAs
involved in bone mineral homeostasis and osteoclastogenesis have
been found in monocytes of osteoporotic patients (31).
Nuclear enriched abundant transcript 1 (NEAT1) is a
classic lncRNA that is specifically located in paraspeckles and
functions as an essential structural determinant by interacting
with members of the Drosophila behavior human splicing
(DBHS) family of proteins (32-35). Recently, emerging evidence has
suggested that NEAT1 is intricately associated with inflammation.
First, NEAT1 has been identified as an inflammatory regulator in
human lupus, sepsis and atherosclerosis (36-40). The expression levels of several
chemokines and cytokines are significantly linked with the status
of NEAT1. Additionally, NEAT1 has been found to promote the
formation of Nod-like receptor protein 3 (NLRP3), which leads to
enhanced pro-caspase-1 processing, caspase-1 activation and
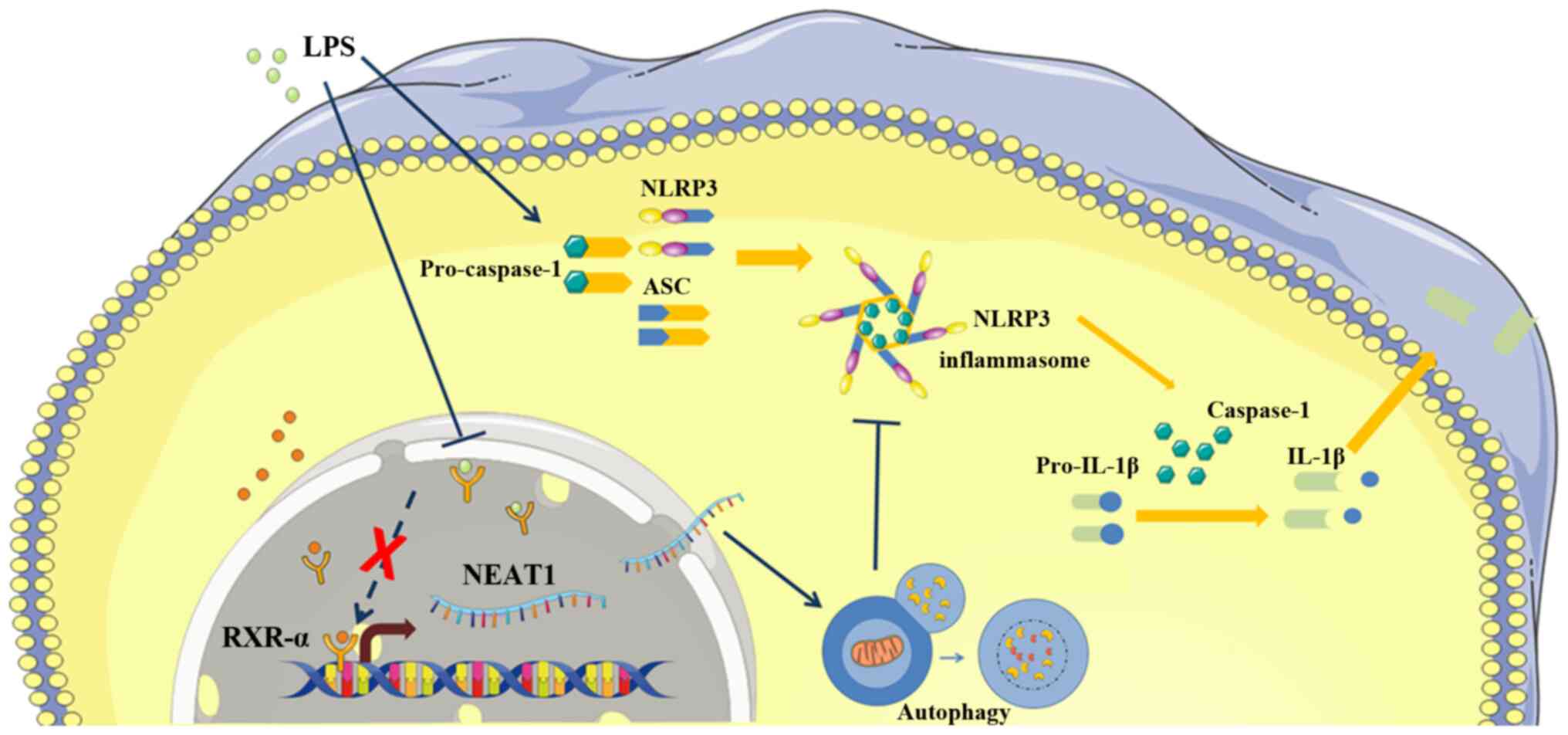
interleukin (IL)-1β maturation (41). In the present study, novel
mechanisms of NEAT1 were explored in LPS-induced inflammation in
osteoblasts via activating autophagy and suppressing the NLRP3
inflammasome, which may provide novel insight for the therapeutic
application of lncRNAs in inflammatory diseases.
Materials and methods
Cells and cell culture
The human osteosarcoma cell line, MG63, donated by
the State Key Laboratory of Oral Disease, Sichuan University was
used to establish the osteoblast model. A total of 1×106
cells were seeded in each 10-cm dish and cultured with 8 ml α-MEM
medium containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a 5% CO2 incubator.
Passaging was achieved using 2 ml trypsin containing 0.25% EDTA
(HyClone; Cytiva) when cells were fused to 80%.
Cell stimulation
To construct an optimal inflammatory model, MG63
cells were separately stimulated with 0.5, 1 and 2 µg/ml
Porphyromonas gingivalis-derived LPS (LPS-PG; Invitrogen;
Thermo Fisher Scientific, Inc.) for 3 h and then examined by
reverse transcription-quantitative PCR (RT-qPCR). The MG63 were
then stimulated with LPS-PG at the optimal drug concentration for
2, 3, 4, 5 and 6 h separately and examined by RT-qPCR to identify
the optimal treatment time.
To determine the optimal drug concentration of
retinoid X receptor (RXR)-α agonist, bexarotene, the MG63 cells
were separately treated with 0, 0.1, 0.3, 0.9 and 2.7 µg/ml
bexarotene (Cell Signaling Technology, Inc.) for 24 h and then
examined by RT-qPCR. Bafilomycin A1 (Baf A1) and MCC950 were
obtained from Selleck Chem. Co. Ltd. To inhibit autophagy, cells
were incubated with 200 nM Baf A1 for 4 h. The cells were also
incubated with 1 µM MCC950 for 3 h to selectively inhibit
NLRP3.
NEAT1 overexpression
The NEAT1 overexpression plasmid was constructed by
GeneCopoeia, and the null vector was used as a control. The heat
shock method was used for the transformation of the plasmid DNA
into E. coli. In brief, following a 30 min of incubation on
ice, a mixture of 10 µl of competent bacteria and 2
µl of plasmid were placed at 42°C for 90 sec and placed back
on ice for 2 min. A total of 2 ml of Luria-Bertani (LB) medium
(Thermo Fisher Scientific, Inc.) was added to an agar plate
containing ampicillin and 200 µl of the mixture was added
onto the LB. The plates were incubated at 37°C overnight.
Recombinant plasmid DNA was isolated using a E.Z.N.A.®
Endo-Free Plasmid Midi kit (Omega Bio-tek) according to the
manufacturer's instructions. Agarose gel electrophoresis was
conducted for the selection of the plasmid DNA. Sequencing of the
DNA was performed as follows by TSINGKE Biological Technology. The
MG63 cells were seeded at a density of 1×105 cells each
well into a 6-well plate for transfection. After 24 h, the cells
were 70 to 90% confluent and the medium were replaced with an
antibiotic-free and serum-free medium. A total of 300 µl of
mixture of the plasmid DNA and Lipo2000™ transfection reagent
(Beyotime Institute of Biotechnology) were added to each well and
the cells were incubated for 6 h at 37°C in a 5% CO2
incubator. The medium was then replaced with α-MEM medium
containing 10% FBS. After 24 to 48 h, the success of transfection
was determined by RT-qPCR and using a fluorescence microscope
(Olympus Corporation).
Western blot analysis
Total protein was extracted using a whole cell lysis
assay (Nanjing KeyGen Biotech. Co., Ltd.) according to the
manufacturer's protocols. The total protein concentration was
determined by BCA protein assay (Nanjing KeyGen Biotech. Co.,
Ltd.). A total of 4 V of protein sample were mixed with 1 V of 5X
protein loading buffer (Sigma-Aldrich; Merck KGaA), incubated for 5
min in boiling water and stored at -20°C. Western blot analysis was
performed following a standard protocol. A total of 20 µg of
protein lysate was subjected to 7.5% or 10% sodium dodecyl sulfate
polyacrylamide gels (Epizyme, Inc.) and transferred onto
polyvinylidene difluoride (PVDF) membranes (KeyGen BioTech). The
membranes were blocked with 5% non-fat dried milk in TBST (Kelong
Chemical Co.) for 1 h at room temperature, and subsequently probed
with specific primary antibodies [anti-GAPDH (mouse, 60004-1-Ig),
anti-β-actin (rabbit, 66009-1-Ig), anti-Unc-51 like autophagy
activating kinase (ULK1; rabbit, 20986-1-AP), anti-p62 (rabbit,
18420-1-AP) (all from Proteintech Group, Inc. and diluted at
1:500), anti-apoptosis-associated speck-like protein containing a
CARD (ASC; rabbit, ab151700; Abcam), anti-caspase-1 (rabbit,
ab179515; Abcam), anti-NLRP3 (rabbit, ab270449; Abcam) diluted at
1:1,000, anti-p-ULK-1 (rabbit, #14202; Cell Signaling Technology,
Inc.) diluted at 1:500, anti-interleukin (IL)-1β (rabbit; Abcam,
ab234437 for full-length IL-1β and ab9722 for cleaved IL-1β) at
1:200), caspase-3 (rabbit, #9662; Cell Signaling Technology, Inc.)
diluted at 1:500, cytochrome c (cyto c, rabbit,
10993-1-AP) diluted at 1:200, LC3 (rabbit, 14600-1-AP) at 1:200,
osteopontin (OPN; rabbit, 22952-1-AP) diluted at 1:500,
poly(ADP-ribose) polymerase (PARP; rabbit, 13371-1-AP) diluted at
1:500, Bax (rabbit, 50599-2-Ig) diluted at 1:200, collagen type I
(Col-I; rabbit, 14695-1-AP) diluted at 1:500 (all from Proteintech
Group, Inc.) at 4°C overnight]. Goat anti-mouse IgG antibody or
goat anti-rabbit IgG antibody or donkey anti-goat IgG antibody
(AS09-602 and AS10-1427; AmyJet Scientific, Inc.) were used as the
secondary antibodies with a 1:200 dilution at room temperature for
1-2 h. The signal was detected using an ECL kit (EMD Millipore)
with a Bio-Rad Geldoc EZ instrument (Bio-Rad Laboraroties, Inc.),
according to the manufacturer's instructions. ImageJ 1.7 soft-ware
was used to quantify the density and size of the blots, and
statistical analysis was performed using the Graphpad Prism 7.0
package.
Agarose gel electrophoresis
A total of 500 ml of 50X TAE buffer was prepared
with 121 g of Tris base (Sigma-Aldrich; Merck KGaA), 28.55 ml of
acetic acid and 50 ml of 0.5 ml/l EDTA. Subsequently, 50 ml of 50X
TAE buffer was diluted to 500 ml of 5X TAE buffer. Agarose (0.4 g)
was then added into 40 ml of 5X TAE buffer, and the mixture was
then heated in a microwave oven followed by the addition of 4 drops
of Genecolor (Bio-Gene Technology Ltd.) to prepare the agarose gel.
A total of 3 µl of DNA samples were loaded into each well
with 1 µl of loading buffer. Electrophoresis was run at 120
V and 50 mA for 20 min. The fragments of DNA were visualized under
UV light.
RT-qPCR
According to the manufacturer's instructions, RNA
was extracted from the cultured cells using an RNA-Quick
Purification kit (Yishan Biotechnology, Co. Ltd.). Reverse
transcription was accomplished using the PrimeScriptTM RT reagent
kit with a gDNA eraser (Takara Bio, Inc.), and samples were
immediately stored at −80°C. Sequences of the primers of GAPDH,
IL-1β, IL-6, NEAT1-1, NEAT1-2, Runx2, Col-I, and osteocalcin in the
MG63 cells were designed and synthesized by TSINGKE Biological
Technology as shown in Table I.
The thermocycling was set following the manufacturer's
instructions: The cDNA synthesis reaction mix was incubated at 37°C
for 15 min, and followed by 85°C for 5 sec. Then the reaction was
terminated at 4°C. The qPCR reaction mix was incubated at 95°C for
30 sec (stage 1 for 1 cycle), at 95°C for 5 sec and at 60°C for 30
sec (stage 2 for 40 cycles), followed by dissociation stage.
Quantitative PCR was performed on a CFX96 Real-Time PCR Detection
System (Bio-Rad Laboratories, Inc.) with 25 µl reaction mix.
The relative expression of a gene of interest was calculated using
the 2−ΔΔCq method (42) and normalized against an internal
control (GAPDH).
 | Table ISequences of primers used in RT-qPCR
analysis. |
Table I
Sequences of primers used in RT-qPCR
analysis.
| Gene | Primer |
|---|
| GAPDH | |
| F |
5′-ACAACTTTGGTATCGTGGAAGG-3 |
| R |
5′-GCCATCACGCCACAGTTTC-3′ |
| IL-1β | |
| F |
5′-TGTGAAATGCCACCTTTTGA-3′ |
| R |
5′-TGAGTGATACTGCCTGCCTG-3′ |
| IL-6 | |
| F |
5′-AGCCACTCACCTCTTCAGAAC-3′ |
| R |
5′-GCCTCTTTGCTGCTTTCACAC-3′ |
| NEAT1-1 | |
| F |
5′-AGCTGCGTCTATTGAATTGGTAAAGTAA-3′ |
| R |
5′-GACAGAAAGATCCCAACGATAAAAATAA-3′ |
| NEAT1-2 | |
| F |
5′-GTCTTTCCATCCACTCACGTCTATTT-3′ |
| R |
5′-GTACTCTGTGATGGGGTAGTCAGTCAG-3′ |
| Runx2 | |
| F |
5′-TGGTTACTGTCATGGCGGGTA-3′ |
| R |
5′-TCTCAGATCGTTGAACCTTGCTA-3′ |
| Col-I | |
| F |
5′-AGGGACACAGAGGTTTCAGT-3′ |
| R |
5′-AGCACCATCATTTCCACGAG-3′ |
| Osteocalcin | |
| F |
5′-CTCACACTCCTCGCCCTATTG-3′ |
| R |
5-GCTTGGACACAAAGGCTGCAC-3 |
RNA sequencing and bioinformatics
analysis
The purity, quantity and integrity of RNA was
measured using a NanoDrop™One/OneC spectrophotometer (Thermo Fisher
Scientific, Inc.), a Qubit™ RNA HSAssay kit (Thermo Fisher
Scientific, Inc.) and an Agilent 4200 TapeStation (Agilent
Technologies, Inc.) separately. Libraries were generated by PCR
amplification, purified by AmPure XP magnetic beads, and quantified
using a Kapa qPCR and Agilent 4200 TapeStation. The PCR
amplification mix was incubated in the following progress: 1 cycle
98°C for 30 sec; 15 cycles 98°C for 10 sec, 60°C for 30 sec, 72°C
for 30 sec; 1 cycle 72°C for 10 min; hold at 4°C. Libraries were
pooled and sequenced on an Illumina HiSeq4000 platform. The
expression profiles of each lncRNAs and coding-genes were
quantified and normalized by the DESeq method, and the differential
expressed genes among each group were calculated by FDR (false
discovery rate) and a Student's t-test. The significant
differentially expressed genes (DEGs) were recognized with an FDR
<0.01 (or adjusted P-value) and a ≥2-fold change in expression.
The DEGs were enriched by their Gene Ontology (GO) or Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathways with the GSEA
method, as well as the lncRNA annotation, Encyclopedia of DNA
Elements (ENCODE) database, and mapping to Reactome database
(43) using the clusterProfiler
package (44).
Immunofluorescence
Cells in 6-well plates were selected to be
transfected with GFP-LC3 plasmid (Addgene, #21073) based on group
design. MG63 cells were grown in α-MEM medium containing 10% FBS,
cells at sub-confluency were transfected with the indicated cDNAs
using Lipo3000 reagent (Thermal Fisher Scientific, Inc.). Cells
were analyzed at 24-48 h following transfection. Mock transfection
was performed using the empty vector. First, the medium was
discarded. The cells were then washed twice with PBS for 5 min each
time and fixed using 4% paraformaldehyde for 15 min. After washing
in cold PBS twice, the cells were permeabilized using 0.5% Triton
X-100 in PBS at room temperature for 20 min. After rinsing with PBS
3 times, the cells were blocked with 5% goat serum (Sigma-Aldrich;
Merck KGaA) in PBS for 30 min at room temperature and incubated
with 30 µl of the indicated primary antibodies
[anti-caspase-1 (rabbit, 22915-1-AP; Proteintech Group, Inc.),
anti-ASC (rabbit, ab155970; Abcam), anti-NLRP3 (rabbit, ab270449;
Abcam) and LC3-II (rabbit, 18725-1-AP; Proteintech Group, Inc.)] in
a wet box at 4°C overnight. After reheating at room temperature for
30 min and washing 3 times with PBS, the cells were incubated with
goat anti-rabbit IgG antibody conjugated with Alexa Fluor 488,
Alexa Fluor 594 or Cy3 (AS09-608, AS11-1814 and AS11-1815; AmyJet
Scientific, Inc.) at a dilution of 1:100 at 37°C for 1 h, and 100
µl of Hoechst stain solution or DAPI were added for 5 min,
and the cells were again rinsed with PBS 3 times. A blocking
solution containing an anti-fluorescent quencher was used for
mounting. Images of the cells were captured under a fluorescence
microscope (Olympus Corporation).
Fluorescent in situ hybridization
(FISH)
A fluorescence in situ hybridization kit
(Guangzhou RiboBio Co., Ltd.) was used, according to the
manufacturer's instructions. The methods used for fixation and
permeabilization were the same as those used for immunofluorescence
(described above). The cells were incubated with 200 µl of
prehybridization solution at 37°C for 30 min and were then
incubated with 20 µl of DNA probe/hybridization buffer at
37°C overnight. The following steps were performed in the dark:
Each well was washed sequentially with washing solution I, II, III
and PBS, while blocking, staining and observation of the cells was
performed using the same methods as those described above for
immunofluorescence.
Transmission electron microscopy
(TEM)
The cells were collected using a cell scraper,
centrifugated at 100 × g for 5 min at room temperature and washed
twice with PBS. The cells were fixed using 2.5% glutaraldehyde at
4°C overnight and seeded to a formvarstabilized carbon support
films, negatively stained with phosphotungstic acid solution (2%
w/v), and then dried naturally for TEM analysis. The intracellular
morphology of MG63 cells was analyzed using a transmission electron
microscopy (TEM, H-600; Hitachi, Ltd.).
Cell cycle analysis and apoptosis
assay
The cells were dissociated using trypsin without
EDTA and the suspension was centrifuged at 1,000 × g for 5 min at
room temperature. The cells were gently washed with PBS twice and
again centrifugated at 1,000 × g for 5 min at room temperature. The
supernatant was discarded, and the cells were collected and
detected on a BD FACSCanto-II flowcytometry instrument (BD
Biosciences). For the cell cycle assay, the cells were resus-pended
and fixed using cold 70% (v/v) ethanol at 4°C for at least 30 min.
The ethanol used was discarded through centrifugation at 1,000 × g
for 5 min at 4°C. After washing with PBS twice, the cells were
incubated with 1 ml of PI staining solution in the dark at room
temperature for 20 min and the positive cells were then detected.
For the cell apoptosis assay, the cells were resuspended using a 1X
binding buffer. The mixture was incubated with 10 µl of PI
staining solution and 5 µl of Annexin V-FITC solution
(C1062L, Annexin V-FITC/PI dual staining kit; Beyotime Institute of
Biotechnology, Inc.) in the dark at room temperature for 5 min.
Subsequently, 400 µl of 1X of a binding buffer was added to
each tube and the positive cells were then detected.
Statistical analysis
All experiments were performed at least 3 times and
all results are reported as the means ± standard deviation (SD).
Statistical analyses were performed using an independent Student's
t-test, one-way ANOVA with the Bonferroni post hoc test and
Pearson's correlation analysis on GraphPad Prism 7.0 software or R
packages. P-values of <0.05 were considered to indicate a
statistically significant difference.
Results
Expression of NEAT1 is decreased
following LPS stimulation
In order to construct an optimal inflammatory model,
MG63 cells were stimulated with various concentrations of LPS-PG
for different periods of time and the results of RT-qPCR revealed
that following stimulation with 0.5, 1 and 2 µg/ml LPS-PG
for 3 h, the expression levels of IL-6 and IL-1β were upregulated
in the MG63 cells, as shown in Fig.
S1A. The levels of IL-6 and IL-1β were significantly increased
in the 1 and 2 µg/ml groups compared with the control group,
although the difference was not significant between these 2 groups.
Thereafter, the levels of inflammatory factors were detected at
different time points. The results revealed that the levels of IL-6
and IL-1β were significantly upregulated within a short time
duration (2 and 3 h); however, they decreased along with the
increase in treatment duration (5 and 6 h) (Fig. S1B). Therefore, stimulation with 1
µg/ml LPS-PG for 2 h was confirmed to produce the optimal
MG63 inflammatory model (Fig.
S1C), and these conditions were used to treat the cells used in
further RNA-seq experiments.
In order to assess LPS-induced changes in RNA
expression in MG63 cells, high-throughput sequencing of RNAs from
MG63 cells with and without LPS stimulation was conducted. Over
14,000 genes were identified (Table
II) and 427 genes were found to be differentially expressed in
the MG63 cells following LPS stimulation, compared with that of
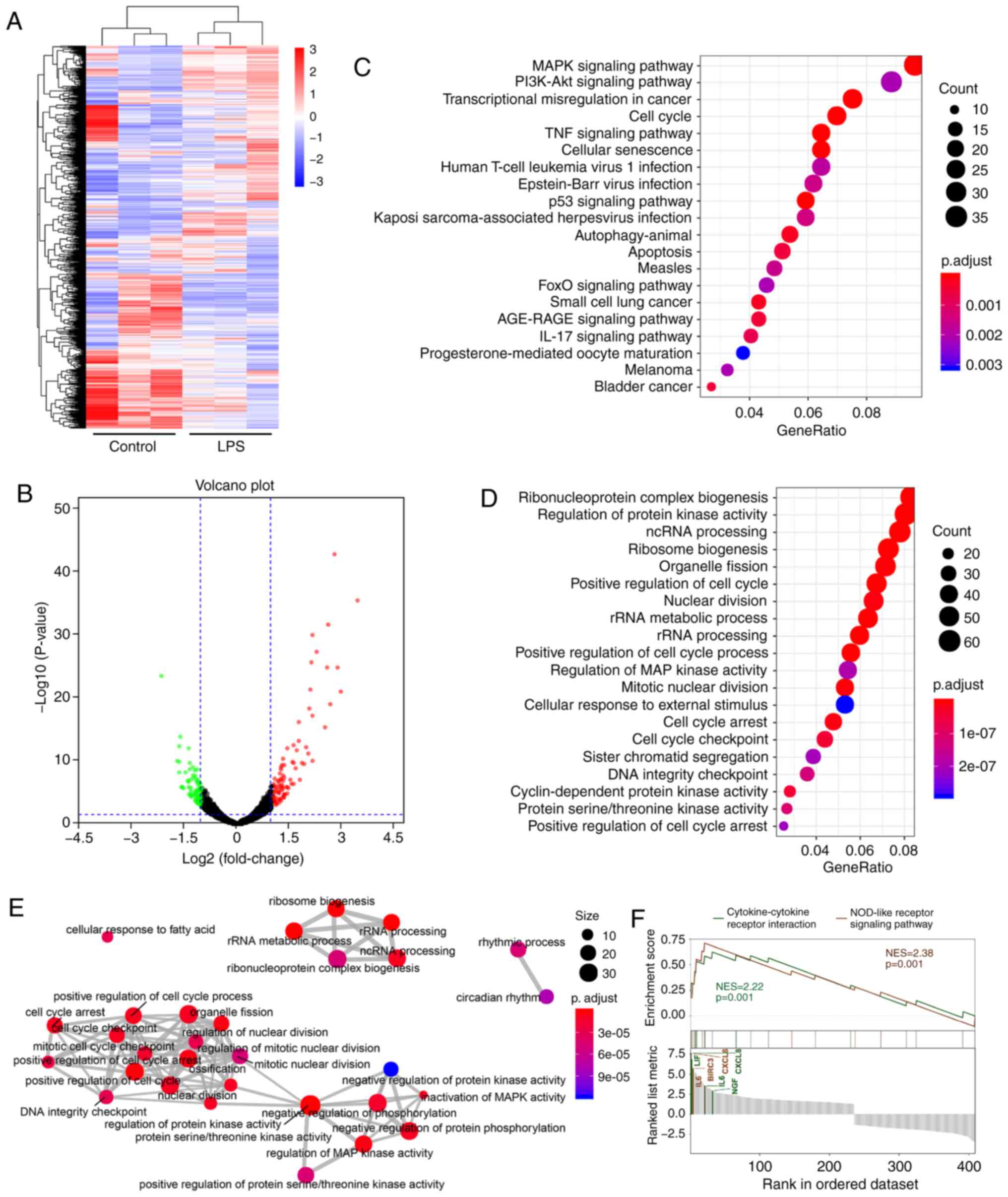
MG63 cells without stimulation (Fig.
1A and B). Among these genes, 241 genes were upregulated
(Log2 fold change >0), while 186 were downregulated
(Log2 fold change <0). The top 10 genes with the most
significant differences in abundance (upregulated and
downregulated) between the MG63 cells with or without LPS
stimulation are presented in Table
III.
 | Table IIResults of comparison of reads to
reference genomes. |
Table II
Results of comparison of reads to
reference genomes.
| Sample | Total clean
reads | Total mapped
reads | Total mapping ratio
(%) | Uniq mapping ratio
(%) | Total gene
number |
|---|
| Control-1 | 41,449,747 | 30,129,821 | 72.69 | 48.49 | 14029 |
| Control-2 | 41,153,924 | 30,375,711 | 73.81 | 49.21 | 14068 |
| Control-3 | 40,884,000 | 30,556,702 | 74.74 | 49.93 | 14034 |
| LPS-1 | 41,325,764 | 30,386,834 | 73.53 | 48.91 | 14205 |
| LPS-2 | 40,855,494 | 30,800,957 | 75.39 | 50.22 | 14430 |
| LPS-3 | 41,469,689 | 31,060,797 | 74.90 | 49.91 | 14444 |
 | Table IIITop 10 differential expressed up- and
downregulated genes. |
Table III
Top 10 differential expressed up- and
downregulated genes.
| Gene name | Gene
description | Log2
fold change |
|---|
| Upregulated
genees | | |
| CXCL8 | C-X-C motif
chemokine ligand 8 | 3.831031313 |
| LIF | LIF, interleukin 6
family cytokine | 3.434091582 |
| EGR1 | Early growth
response 1 | 2.949483692 |
| BIRC3 | Baculoviral IAP
repeat containing 3 | 2.869020403 |
| SERPINE1 | Serpin family E
member 1 | 2.767710691 |
| KRTAP1-5 | Keratin associated
protein 1-5 | 2.641349766 |
| CCL2 | C-C motif chemokine
ligand 2 | 2.582271496 |
| EDN1 | Endothelin 1 | 2.571977567 |
| CXCL3 | C-X-C motif
chemokine ligand 3 | 2.512770455 |
| GADD45B | Growth arrest and
DNA damage inducible beta | 2.263219387 |
| Downregulated
genes | | |
| TXNIP | Thioredoxin
interacting protein | −2.166372864 |
| TEF | TEF, PAR bZIP
transcription factor | −1.727267478 |
| SESN3 | Sestrin 3 | −1.679757426 |
| SNAI2 | Snail family
transcriptional repressor 2 | −1.672595539 |
| PPP1R3C | Protein phosphatase
1 regulatory subunit 3C | −1.659475556 |
| ARRDC3 | Arrestin domain
containing 3 | −1.620649654 |
| C10orf10 | Chromosome 10 open
reading frame 10 | −1.617743297 |
| HIST1H3G | Histone cluster 1
H3 family member g | −1.563950632 |
| NEURL1B | Neuralized E3
ubiquitin protein ligase 1B | −1.554898668 |
| MN1 | MN1 proto-oncogene,
transcriptional regulator | −1.497164046 |
The results of the GO enrichment analysis and
Reactome Pathway Database analysis indicated that the cell cycle,
MAPK pathway and ncRNA processing may play a key role in
inflammation induced by LPS (Fig.
1C-E). Further results obtained through the GO analysis are
shown in Fig. S2.
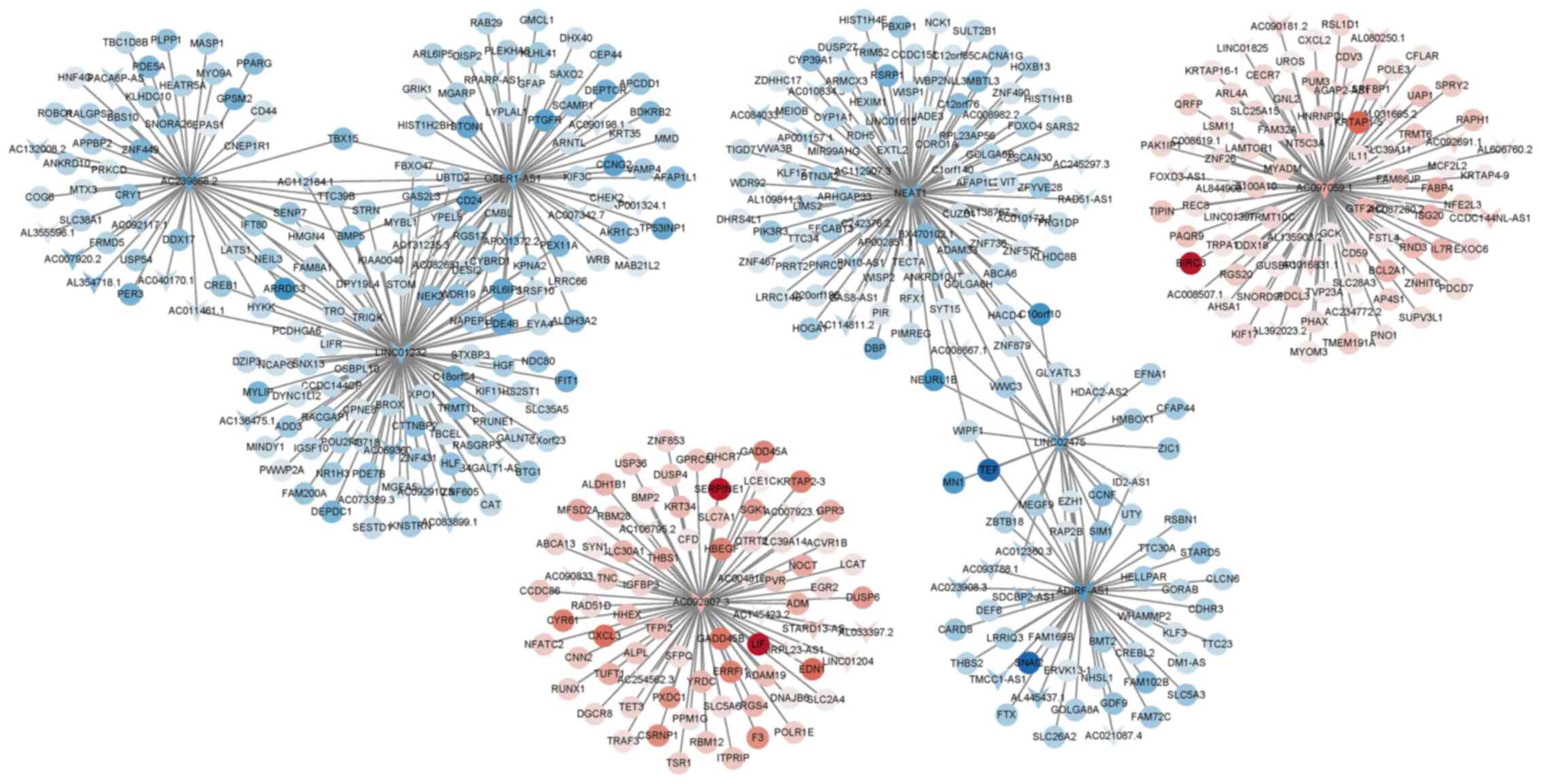
After analysis using the ENCODE database, 10
differentially expressed lncRNAs were identified and these are
listed in Table IV, among which
NEAT1, LINC02475, OSER-AS1, AC097059.1 and AC092807.3 exhibited a
high co-expression association with multiple mRNAs (Fig. 2). Consistent with these results,
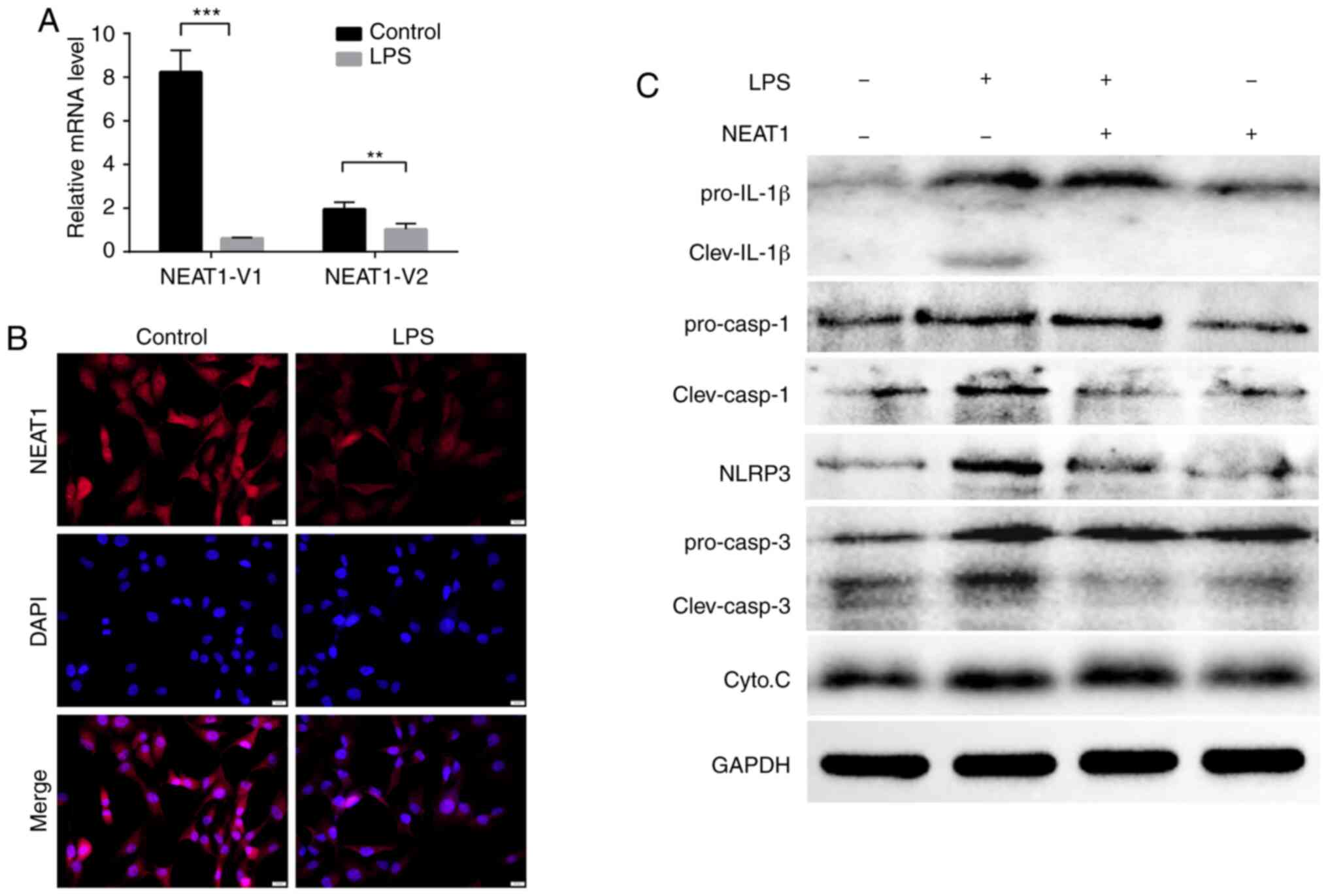
the RNA expression levels of the NEAT1 transcripts (NEAT1-1 and
NEAT1-2) decreased significantly in the LPS-stimulated MG63 cells,
as shown through immunofluorescence analysis and RT-qPCR (Fig. 3A and B).
 | Table IVDifferential expressed lncRNAs. |
Table IV
Differential expressed lncRNAs.
| lncRNA | Gene
description | Log2
fold change |
|---|
| NEAT1 | Nuclear paraspeckle
assembly transcript 1 | −1.217166677 |
| LINC02475 | Long intergenic
non-protein coding RNA 2475 | −1.216221753 |
| OSER1-AS1 | OSER1 antisense RNA
1 (head to head) | −1.164111533 |
| PSMB8-AS1 | PSMB8 antisense RNA
1 (head to head) | −1.12672866 |
| AC048341.3 | | −1.092688339 |
| AP001372.2 | | −1.022974571 |
| AC083843.2 | | −0.924327576 |
| AC097059.1 | | 1.415095278 |
| AC092807.3 | | 1.131243985 |
| SNHG15 | Small nucleolar RNA
host gene 15 | 0.982590385 |
Furthermore, combining the results of the KEGG
pathway analysis and gene set enrichment analysis (GESA), revealed
that the NOD-like receptor signaling pathway was significantly
activated, indicating that it may be a potential key pathway for
the mediation of inflammatory responses in the current inflammatory
model (Fig. 1F).
NEAT1 suppresses LPS-induced inflammatory
responses via the NOD-like pathway
In order to examine the effects of NEAT1 expression
on the activation of the NOD-like pathway, MG63 cells were
successfully transfected with NEAT1 overexpression plasmid
(Fig. S3). The expression levels
of IL-1β precursors and splicers, caspase-1 precursors and
splicers, caspase-3 precursors and splicers, and NLRP3 were found
to be upregulated following LPS stimulation and were downregulated
by NEAT1 overexpression (Fig.
3C), although the expression levels of cytochrome c were
not markedly altered, the above-mentioned results still suggest
that NEAT1 can inhibit the expression of NLRP3 and the maturation
of IL-1β and caspase-1.
Overexpression of NEAT1 promotes
autophagy and further reduces the activation of the NLPR3
inflammasome in MG63 cells
Previous studies have theoretically found that
autophagy may be involved in the control of IL-1β secretion by
targeting pro-IL-1β for lysosomal degradation or by regulating the
activation of the NLRP3 inflammasome or by other potential
mechanisms (45,46). The present study explored
autophagy induction in MG63 cells in response to LPS stimulation or
NEAT1 overexpression. Following transfection with NEAT1
overexpression plasmid, the LPS-stimulated MG63 cells secreted
increased levels of ULK1, p-ULK1 and LC3, and a decreased level of
p62, indicating that autophagy was inhibited in response to LPS,
but was promoted when the cells overexpressed NEAT1 (Fig. 4B). Furthermore, the increased
expression of GFP-labeled-LC3 was observed on the autophagosome
membrane in NEAT1-overexpressing MG63 cells, while decreased levels
were observed in LPS-stimulated MG63 cells (Fig. 4A). In addition, an increased
number of autophagosomes in NEAT1-overexpressing cells was observed
through TEM (Fig. 4D).
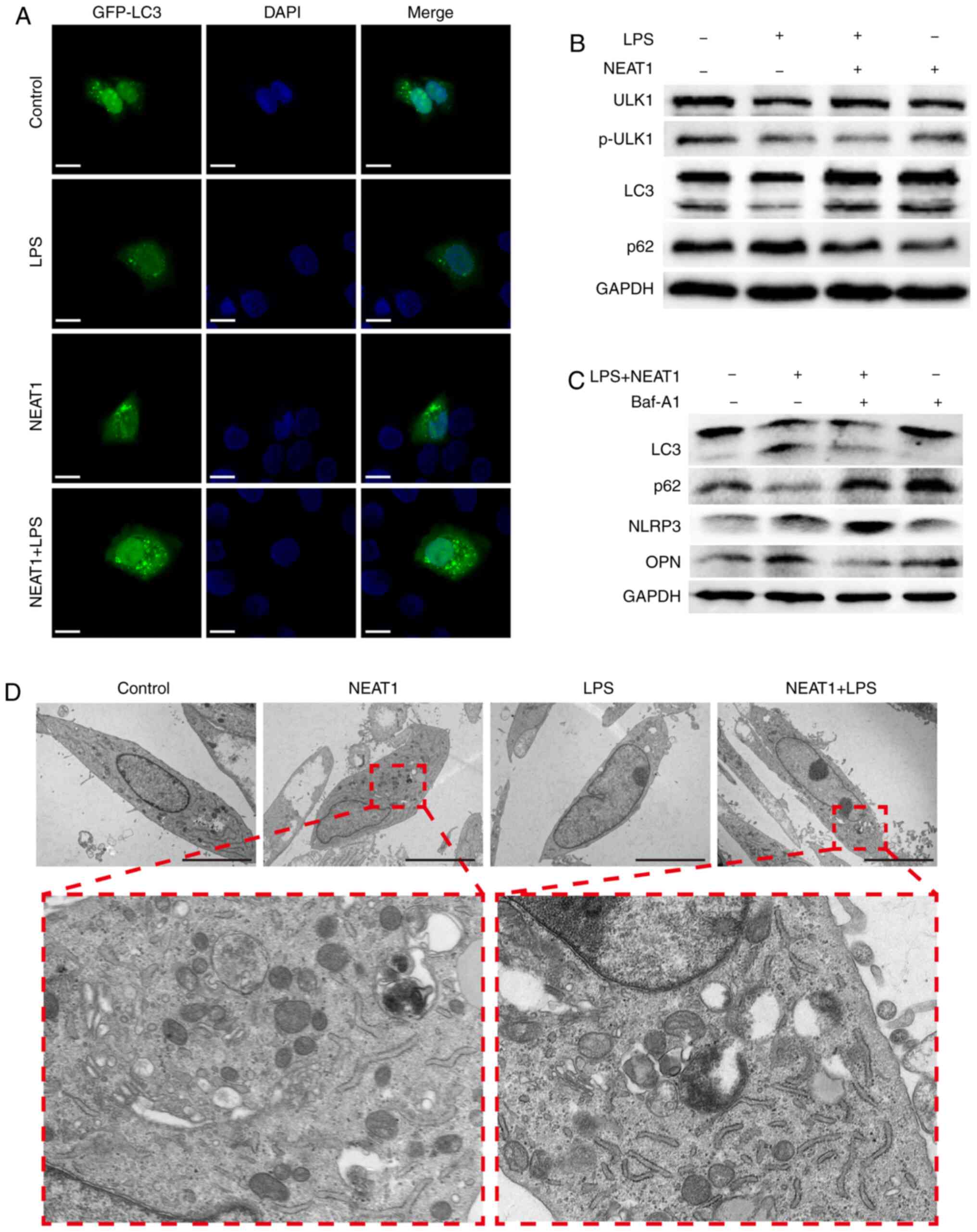 | Figure 4NEAT1 inhibits the activation of the
NLRP3 inflammasome through autophagy. (A) GFP-labeled LC3 (green)
was shown by immunofluorescence in cells subjected to different
treatments. The position of the nucleus is shown in blue color.
Scale bar, 6 µM. (B and C) Expression levels of ULK1,
p-ULK1, LC3, p62, NLRP3, OPN and GAPDH were measured in each group
by western blot analysis. (D) The autophagosome vacuoles are shown
by TEM, and the number of autophagosomes increased in MG63 cells
overexpressing NEAT1. Scale bar, 5 µM. LPS,
lipopolysaccharide; NEAT1, nuclear enriched abundant transcript 1;
NLRP3, Nod-like receptor protein 3; ULK1, Unc-51 like autophagy
activating kinase; LC3, light chain 3; OPN, osteopontin. |
The assembly of an inflammasome complex has been
shown to be required for the activation of caspase-1 and the
processing of pro-IL-1β (47).
Combining previous results that demonstrated that the expression of
NLRP3 was upregulated by LPS and downregulated by NEAT1, in the
present study, the autophagy inhibitor, Baf A1 (200 nM for 4 h of
incubation), was applied on the cells in the different groups, and
western blot analysis of LC3, p62, NLRP3 and OPN expression was
then performed. As shown in Fig.
4C, BafA1 markedly decreased the expression of LC3-II and
increased the expression of p62, demonstrating that autophagy may
be inhibited by Baf A1. The expression of NLRP3 increased following
the application of Baf A1 on NEAT1-overexpressing cells stimulated
with LPS, and the changes in the expression of OPN in each group
were in contrast to those of NLRP3. These results suggested that
the inhibitory effect of NEAT1 on NLRP3 was dependent on the
suppression of the cellular autophagy process.
Overexpression of NEAT1 affects the cell
cycle and impairs the osteogenic function of MG63 cells
Considering the potential of cell cycle involvement
in inflammation, based on the RNA-seq results and the association
between inflammasomes, autophagy and cell death pathways involved
in the inflammatory process, flow cytometry was performed on the
MG63 cells following the different treatments to compare the
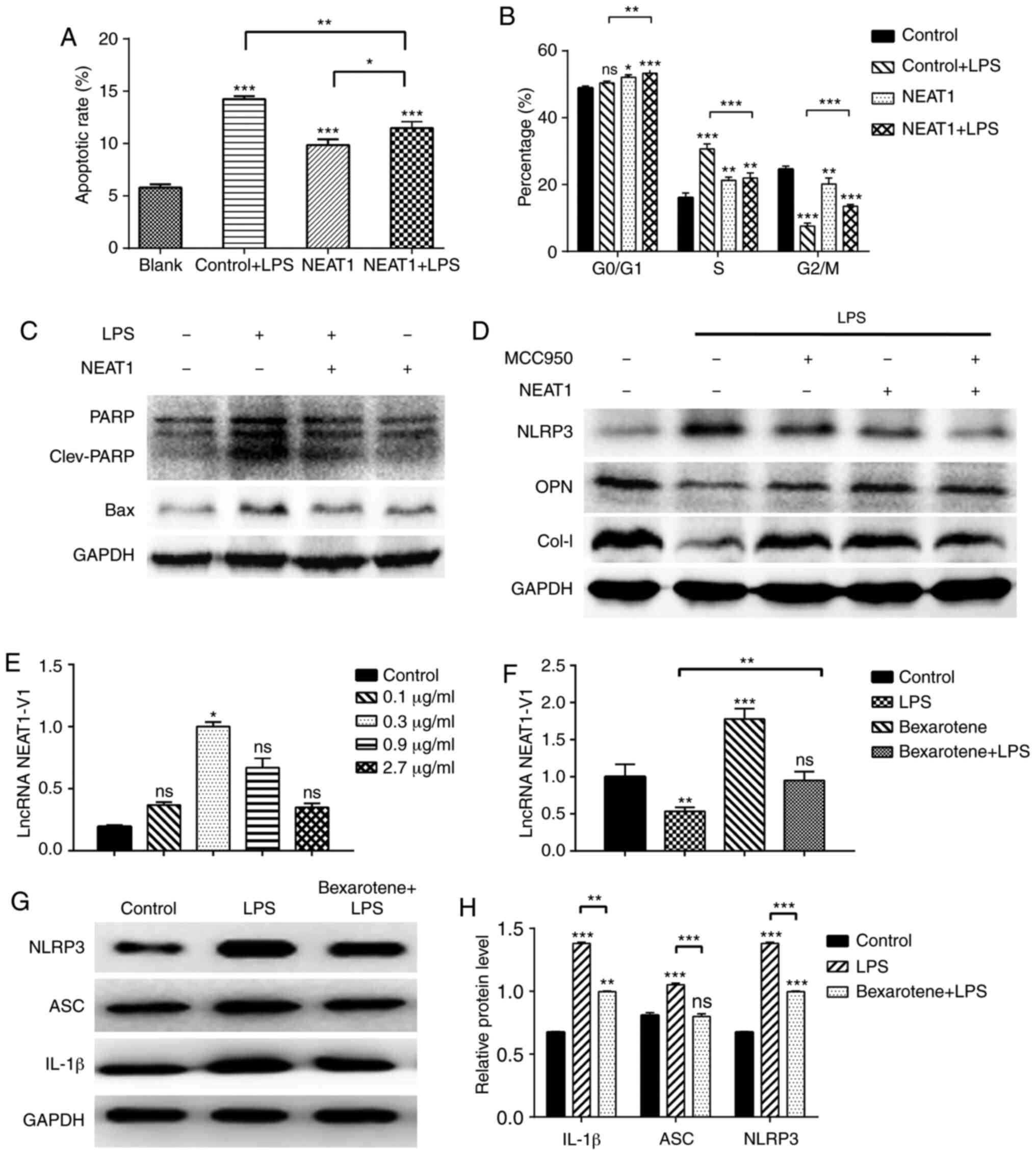
apoptotic ratio and phases of the cell cycle. The results revealed
that the apoptotic ratio increased significantly in the
LPS-stimulated cells, but was attenuated in the
NEAT1-overexpressing cells (Figs.
5A and S4). Additionally,
NEAT1 decreased the levels of the apoptosis-related proteins, PARP
and Bax, as shown through the results of western blot analysis
(Fig. 5C). The changes in the
cell cycle of the LPS-stimulated MG63 cells were mainly reflected
by the increase in the proportion of cells at the S phase and the
decrease in the proportion of cells at the G2/M phase, compared
with the untreated group. The overexpression of NEAT1 increased the
proportion of cells at the G0/G1 phase to varying degrees. Compared
with the LPS group, the proportion of cells at the S phase
decreased, and the proportion of cells at the G2/M phase increased
in the NEAT1 + LPS group, indicating that the overexpression of
NEAT1 inhibited the cell cycle progression of MG63 cells that were
in an inflammatory state (Figs.
5B and S5).
In addition, the levels of osteogenesis-related
proteins were detected in the MG63 cells. The LPS-stimulated cells
secreted lower levels of OPN and Col-I, and the overexpression of
NEAT1 significantly reversed this effect. The novel selective NLRP3
inhibitor, MCC950 (1 µM for 3 h of incubation), exerted the
same effect on the expression of OPN and Col-I, as that exerted by
the NEAT1 overexpression plasmid; however, no synergistic effect
was observed (Fig. 5D). It was
hypothesized that NEAT1 and MCC950 may function in a similar
manner.
RXR-α agonist reverses the LPS-induced
loss of osteogenic factors by regulating NEAT1 expression
In order to determine whether RXR-α is an upstream
transcription factor of NEAT1 that can regulate NEAT1 expression,
the response of MG63 cells to various concentrations of bexarotene
(0.1-2.7 µg/ml for 24 h of incubation), an RXR-α agonist,
was observed. All 4 concentrations of bexarotene used increased the
expression of NEAT1 and the concentration of 0.3 µg/ml was
found to be optimal (Fig. 5E). As
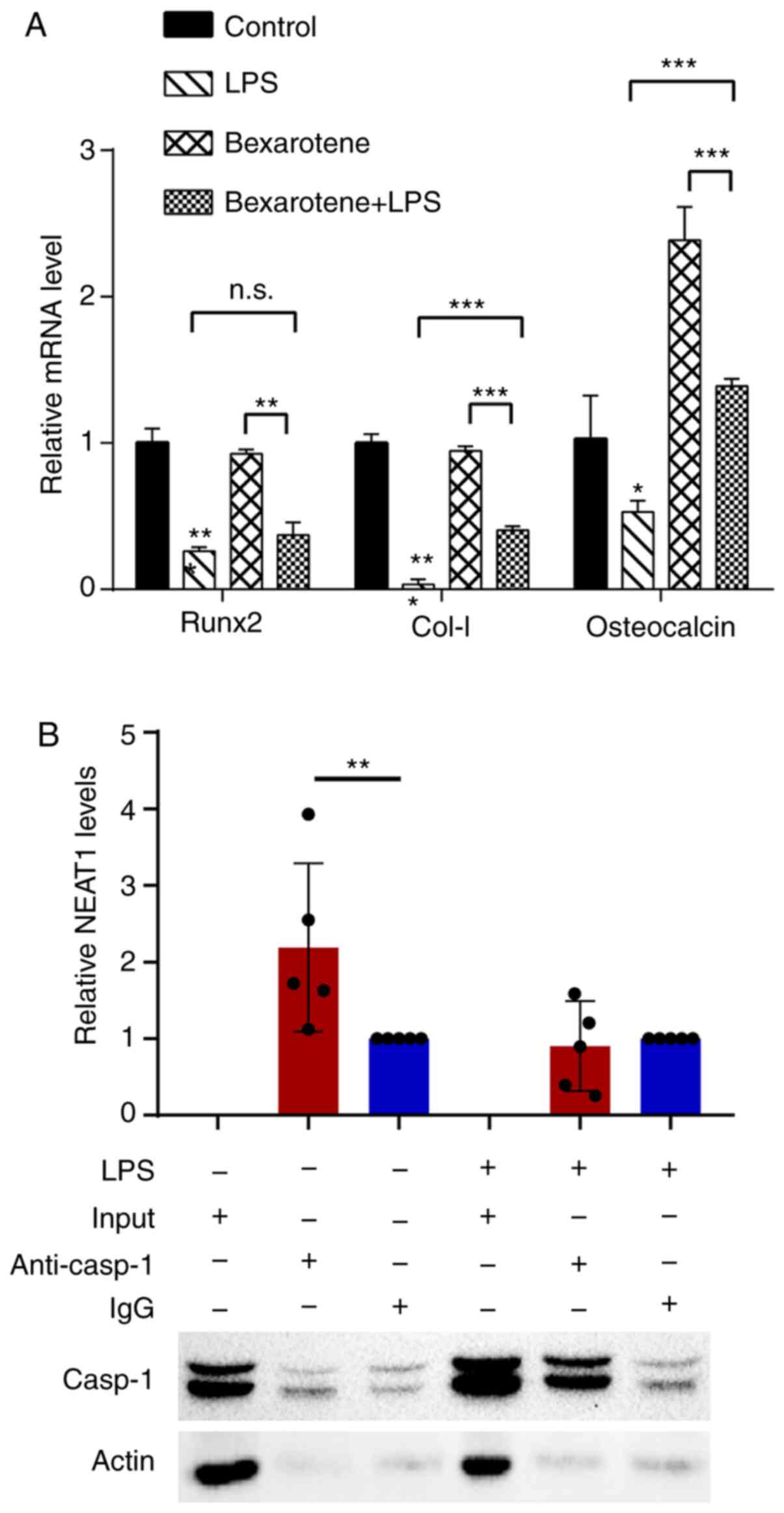
shown in Fig. 5F, bexarotene also
effectively increased the expression of NEAT1 in MG63 cells under
the inflammatory condition. In addition, the protein expression
levels of IL-1β, ASC and NLRP3 increased (Fig. 5G and H), and the mRNA expression
levels of osteocalcin, COL-I and Runx2 decreased (Fig. 6A) in response to bexarotene. These
results suggested that bexarotene reversed the inflammatory process
and osteogenesis-related gene expression induced by NEAT1 in MG63
cells. Additionally, it was found that RXR-α may be located
upstream of NEAT1 and could positively regulate NEAT1 expression,
further abrogating the biological functional damage of osteoblasts
induced by inflammation.
Given that there was a recent report indicating that
lncRNA Neat1 (murine form) interacted with the NLRP3 inflamma-some
in murine BMDMs and functioned as an inflammation stimulator
(41), the present study
determined the interactions between NEAT1 and caspase-1 by using
RIP (RNA immuno-precipitated) from the MG63 cellular lysates with
or without LPS stimulation (Fig.
6B). The results suggested that NEAT1 was significantly
enriched in caspase-1 immunoprecipitants in the control group;
however, the NEAT1 levels did not exhibit any difference in the
immunoprecipitants of caspase-1 and IgG in the LPS-stimulated group
(Fig. 6B).
To explore the mechanisms through which bexarotene
may regulate NEAT1, immunofluorescence and FISH techniques were
further combined to observe the levels of NEAT1, caspase-1, ASC,
LC3-II and NLRP3 expression in MG63 cells. The results revealed
that NEAT1 was widely distributed in the nucleus and cytoplasm.
Following stimulation with LPS for 2 h, NEAT1 accumulated in the
nucleus, while there was also an evident increase in the size of
the paraspeckle. Moreover, the level of caspase-1 tended to
increase in the nucleus (Fig.
7A), and the increased expression of ASC was observed (Fig. 7B) in the LPS-stimulated MG63
cells. However, treatment with 0.3 µg/ml bexarotene
decreased the LPS-induced activation of the NLRP3 inflammasome, and
promoted NEAT1 expression and autophagosome accumulation (Fig. 7C). Taken together, it was
hypothesized that the regulation of inflammasomes by NEAT1 may be
associated with the cytoplasmic transport of related proteins and
the post-transcriptional regulation of proteins, for which the
mechanisms of action remain to be proven through further
experiments.
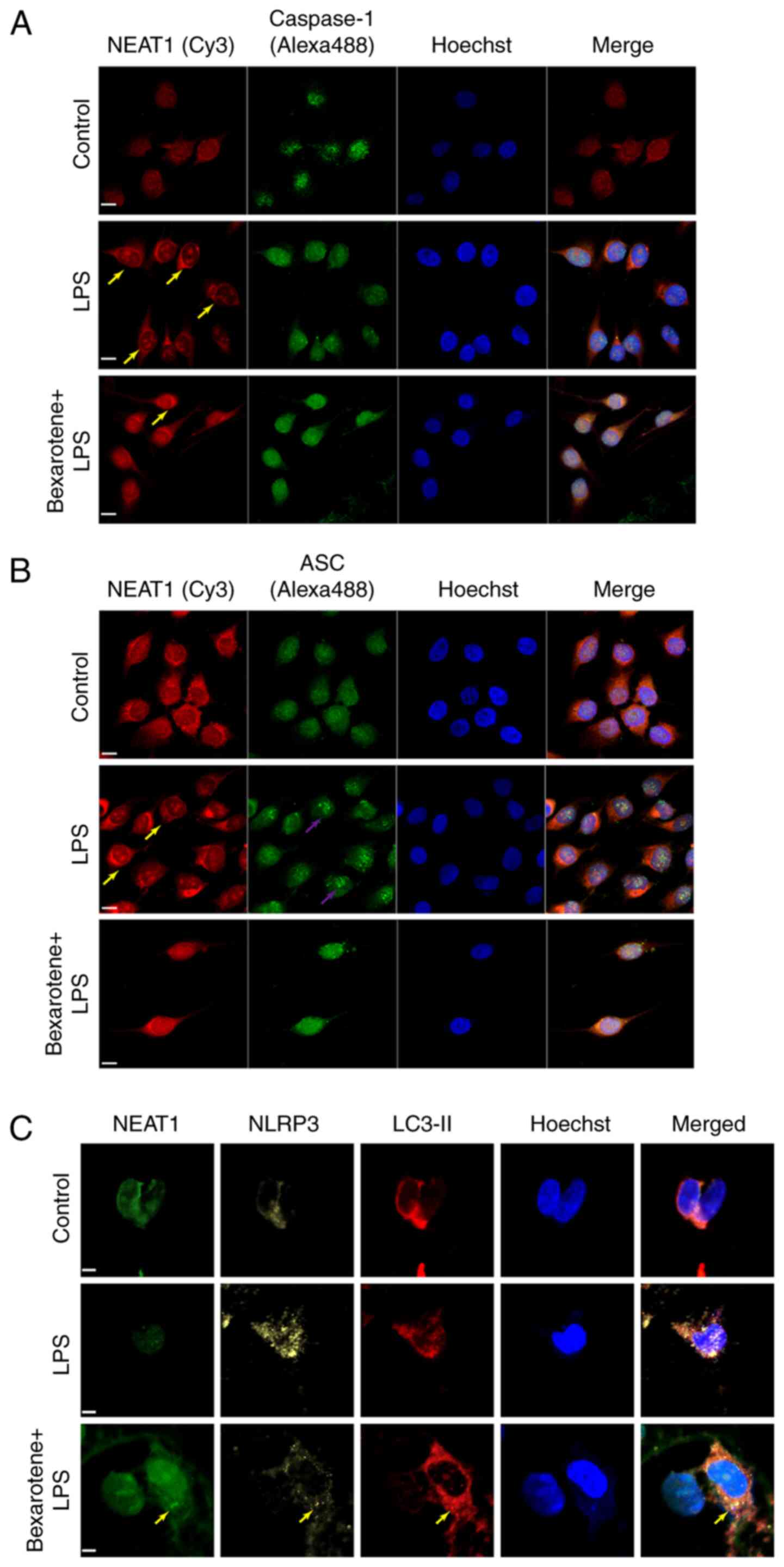 | Figure 7Bexarotene upregulates the expression
of NEAT1 and inhibits the activation of the inflammasome. The
fluorescence laser confocal microscopic images of NEAT1 and
co-stained with (A) caspase-1 and (B) ASC and NLRP3 (yellow) by
using immunofluorescence and FISH. Scale bar, 6 µM. Yellow
arrows indicate the position of paraspeckles, purple arrows
indicate the potential position of the inflammasome. Cy3, red;
Alexa Fluor 488, green. (C) Co-staining of NEAT1, NLRP3 and LC3-II
by immunofluorescence and FISH. Yellow arrows indicate the fusion
of NLRP3 inflammasome and autophagosomes. LPS, lipopolysaccharide;
NEAT1, nuclear enriched abundant transcript 1; ASC,
apoptosis-associated speck-like protein containing a CARD; NLRP3,
Nod-like receptor protein 3; LC3, light chain 3. |
Discussion
Previous studies have suggested that the metabolism
of bone tissue and inflammation are regulated by various lncRNAs
(47,48). In the present study, RNA-seq
analysis was performed on MG63 cells and it was found that NEAT1, a
key lncRNA, was downregulated in response to LPS. NEAT1 suppressed
the downstream inflammatory process and impaired osteoblastic
function by promoting autophagy, which inhibited the activation of
NLRP3 and downregulated the expression levels of caspase-1 and
IL-1β. In addition, the finding that bexarotene increased NEAT1
expression, decreased the expression of inflammatory cytokines and
restored osteogenic activity in LPS-stimulated MG63 cells indicated
that RXR-α may be a positive regulator of NEAT1. As far as is
known, this is the first study to reveal the role of NEAT1 in the
inflammation process of bone tissue.
In the present study, it was first found that the
expression of NEAT1 was downregulated by LPS. However, the
mechanism of NEAT1 expression regulation is complex, which includes
gene mutations, copy number alterations, transcription factors, DNA
methylation, miRNA and RNA-binding proteins. Several research
studies have found that the expression of NEAT1 is increased in
prostate cancer, gastric cancer, hepatocellular carcinoma,
papillary renal-cell carcinoma and clear cell renal cell carcinoma
(49-52), suggesting that NEAT1 may
potentially be involved in the process of carcinogenesis in certain
tumors. However, the expression of NEAT1 has been found to be
decreased in multiple myeloma and leukemia (53-55), indicating that NEAT1 may also
function as a tumor suppressor gene in certain other tumors.
Therefore, it is currently considered that NEAT1 plays differential
roles in different cells. NEAT1 downregulation is generally
considered to be regulated by the p53-dependent DNA damage response
mechanism. p53 is a cell pressure sensor that responds to signals,
such as DNA damage and oncogene expression, as well as mediates
cell cycle regulation and apoptosis by regulating hundreds of
target genes. As a transcription factor of NEAT1, p53 induces
paraspeckle formation by regulating the expression of NEAT1,
thereby decreasing DNA damage-induced cell death under
oncogene-induced replication stress. In turn, paraspeckles inhibit
replication-related DNA damage and p53 activation, which is another
mechanism through which p53 maintains genomic integrity (56,57). Apart from p53, NEAT1 expression
can also be upregulated by Oct4, HIF-2α, RXR-α and Runx1, and
downregulated by BRCA1 (58-62).
RXRs, a family of nuclear receptors, are the primary
receptors and mediators of retinoid effects (63). There are 3 isotypes: α, β and γ.
The role RXRs in bone metabolism and inflammation remains
controversial due to multiple combinations of its isotypes and
experimental treatment conditions. The 3 RXR isotypes and their
heterodimer partners are widely expressed in the osteoblast
lineage, while only RXR-α and RXR-β are expressed in bone marrow
myeloid cells (osteoclast progenitors) (64,65). It is also widely accepted that
RXRs can modulate osteoclast and osteoblast formation, and function
at several levels of cell differentiation and activation. For
example, a previous study demonstrated that bexarotene, a selective
agonist of RXRs, increased bone turnover in rats (66). On the other hand, Wang et
al found that LPS inhibited RXR function and the reduction of
its pathway-associated proteins (67). Of note, bexarotene upregulates
NEAT1, which inhibits apoptosis and inflammation, thereby resulting
in better functional recovery in mice following traumatic brain
injury (60). Combined with the
results of the present study, RXR-α has been found to be the most
probable upstream regulator of NEAT1.
Apart from upstream of NEAT1, downstream is also
crucial to understanding its mechanisms, such as the noteworthy
targets: Inflammasomes, particularly the NLRP3 inflammasome, which
are vital members of the innate immune system (68-71). NLRP3 receives extracellular
stimulation and then interacts with ASC, which recruits and
activates pro-caspase-1, inducing the maturation, activation and
secretion of IL-1β and IL-18, which improve the inflammatory
process (72). Several diseases
have been proven to be associated with NLRP3 inflammasome
activation dysfunction and NLRP3 agonists are a feasible target
option for the alleviation of these diseases. However, none of the
NLRP3 agonists, which are structurally and functionally diverse,
can directly bind to NLRP3. Afonina et al(73) promoted the hypothesis that all
NLRP3 agonists may act via a common intermediate, with which they
communicate through the induction of membrane damage, potassium
efflux and elevation of intracellular calcium. Interestingly, NEAT1
has been demonstrated to enhance the activation of NLRP3 and
caspase-1, the processing of pro-IL-1β and the secretion of mature
IL-1β in mouse macrophages, and these results are different from
the present results (41). It is
hypothesized that different cell types or different transcription
factors produce differential effects, and that macrophages can
differentiate into osteoclasts, which exert different biological
effects from that of osteoblasts (Fig. 8).
The present study also wished to determine the
mechanisms through which NEAT1 regulates inflammasomes. The results
in the literature published on this topic were summarized and it
was found that autophagy can inhibit inflammasomes, and it was then
conjectured that activated autophagosomes may act as the mediator
between NEAT1 and NLRP3. This has been demonstrated in a number of
studies, indicating that NEAT1 promotes autophagy in various cell
types (74,75). Moreover, Cao et al(76) discussed the interactions between
autophagy and the NLRP3 inflammasome in detail. The mechanism of
autophagy inhibition in NLRP3 inflammasomes, which has been
observed mostly in macrophages, may be related to the reduction in
ASC activity, the phosphorylation of NLRP3 and the clearance of
mitochondrial ROS. On the contrary, autophagy may also positively
regulate the activation of the NLRP3 inflammasome by enhancing the
activation of caspase-1 through a Atg5-dependent non-classical
pathway in yeast under starvation conditions.
In order to further understand the function of
NEAT1, researchers can perform experiments in vivo or
investigate the role of NEAT1 in inflammation induced by hypoxia. A
previous study demonstrated that NEAT1-induced para-speckle
formation was dependent on HIF-2α expression. Moreover, osteoclasts
are another important cell type for bone metabolism, and the KEGG
analysis performed in the present study revealed the involvement of
osteoclast-differentiation-related-genes, indicating that
osteoclasts may be a potential cell target.
In conclusion, the present study clearly
demonstrated that NEAT1 suppressed the downstream inflammatory
process and impaired osteoblastic function by promoting autophagy
and downregulating the expression levels of caspase 1 and IL-1β via
the RXR-α/NEAT1/NLRP3 axis. It would be of great interest to
further verify the exact mechanism of NEAT1 function in the current
inflammatory model and investigate potential inflammasome
inhibitors in order to identify potential therapeutic targets.
Supplementary Data
Funding
The authors are grateful for the financial support
from the National Natural Science Foundation of China (grant nos.
81870804 and 81571009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WD, MW, RS and DB conceived and designed the study.
MW, PW and JWe performed the experiments. WD, MW, JWa, SC, XX and
YH analyzed the data and prepared the figures. WD drafted and wrote
the initial manuscript. WD and RS reviewed and revised the
manuscript. All authors discussed the results and commented on the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Redlich K and Smolen JS: Inflammatory bone
loss: Pathogenesis and therapeutic intervention. Nat Rev Drug
Discov. 11:234–250. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zaidi M: Skeletal remodeling in health and
disease. Nat Med. 13:791–801. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuo K and Irie N: Osteoclast-osteoblast
communication. Arch Biochem Biophys. 473:201–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karner CM and Long F: Wnt signaling and
cellular metabolism in osteoblasts. Cell Mol Life Sci.
74:1649–1657. 2017. View Article : Google Scholar :
|
|
6
|
Komori T: Regulation of proliferation,
differentiation and Functions of osteoblasts by Runx2. Histochem
Cell Biol. 20:16942019.
|
|
7
|
Abdallah BM, Jafari A, Zaher W, Qiu W and
Kassem M: Skeletal (stromal) stem cells: An update on intracellular
signaling path-ways controlling osteoblast differentiation. Bone.
70:28–36. 2015. View Article : Google Scholar
|
|
8
|
Nakagawa N, Kinosaki M, Yamaguchi K, Shima
N, Yasuda H, Yano K, Morinaga T and Higashio K: RANK is the
essential signaling receptor for osteoclast differentiation factor
in osteoclastogenesis. Biochem Biophys Res Commun. 253:395–400.
1998. View Article : Google Scholar
|
|
9
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar
|
|
10
|
Kobayashi Y, Udagawa N and Takahashi N:
Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot
Gene Expr. 19:61–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Walter TS, Huang P, Zhang S, Zhu X,
Wu Y, Wedderburn LR, Tang P, Owens RJ, Stuart DI, et al: Structural
and functional insights of RANKL-RANK interaction and signaling. J
Immunol. 184:6910–6919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mundy GR: Osteoporosis and inflammation.
Nutr Rev. 65:S147–151. 2007. View Article : Google Scholar
|
|
13
|
Kadono H, Kido J, Kataoka M, Yamauchi N
and Nagata T: Inhibition of osteoblastic cell differentiation by
lipopolysaccha-ride extract from Porphyromonas gingivalis. Infect
Immun. 67:2841–2846. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bandow K, Maeda A, Kakimoto K, Kusuyama J,
Shamoto M, Ohnishi T and Matsuguchi T: Molecular mechanisms of the
inhibitory effect of lipopolysaccharide (LPS) on osteoblast
differ-entiation. Biochem Biophys Res Commun. 402:755–761. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Daigang L, Jining Q, Jinlai L, Pengfei W,
Chuan S, Liangku H, Ding T, Zhe S, Wei W, Zhong L and Kun Z:
LPS-stimulated inflammation inhibits BMP-9-induced osteoblastic
differentiation through crosstalk between BMP/MAPK and Smad
signaling. Exp Cell Res. 341:54–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang RL, Yuan Y, Zou GM, Liu G, Tu J and
Li Q: LPS-stimulated inflammatory environment inhibits
BMP-2-induced osteoblastic differentiation through crosstalk
between TLR4/MyD88/NF-κB and BMP/Smad signaling. Stem Cells Dev.
23:277–289. 2014. View Article : Google Scholar
|
|
17
|
Yu X, Quan J, Long W, Chen H, Wang R, Guo
J, Lin X and Mai S: LL-37 inhibits LPS-induced inflammation and
stimulates the osteogenic differentiation of BMSCs via P2X7
receptor and MAPK signaling pathway. Exp Cell Res. 372:178–187.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao Z, Yang Z, Chen F, Jiang Y, Fu C, Wang
Y, Lu R and Wu H: Autophagy is essential for the endothelial
differentiation of breast cancer stemlike cells. Int J Mol Med.
45:255–264. 2020.
|
|
19
|
Zhang X, Yang Y, Li X, Zhang H, Gang Y and
Bai L: Alterations of autophagy in knee cartilage by treatment with
treadmill exercise in a rat osteoarthritis model. Int J Mol Med.
43:336–344. 2019.
|
|
20
|
Yang M, Feng C, Zhang Y, Liu C, Li B, Zhu
Q, Huang B and Zhou Y: Autophagy protects nucleus pulposus cells
from cyclic mechanical tensioninduced apoptosis. Int J Mol Med.
44:750–758. 2019.PubMed/NCBI
|
|
21
|
Liu J, Wang S, Zhang P, Said-Al-Naief N,
Michalek SM and Feng X: Molecular mechanism of the bifunctional
role of lipopolysaccharide in osteoclastogenesis. J Biol Chem.
284:12512–12523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Orcel P, Feuga M, Bielakoff J and De
Vernejoul MC: Local bone injections of LPS and M-CSF increase bone
resorption by different pathways in vivo in rats. Am J Physiol.
264:E391–E397. 1993.PubMed/NCBI
|
|
23
|
Chiang CY, Kyritsis G, Graves DT and Amar
S: Interleukin-1 and tumor necrosis factor activities partially
account for calvarial bone resorption induced by local injection of
lipopolysaccharide. Infect Immun. 67:4231–4236. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Yang Y, Bao J, Wang Z, Xia M, Dai
A, Tan J, Zhou L, Wu Y and Sun W: Autophagy negative-regulating Wnt
signaling enhanced inflammatory osteoclastogenesis from Pre-OCs in
vitro. Biomed Pharmacother. 126:1100932020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar
|
|
27
|
Chen YG, Satpathy AT and Chang HY: Gene
regulation in the immune system by long noncoding RNAs. Nat
Immunol. 18:962–972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Wu F, Song Y, Li X, Wu Q, Duan Y
and Jin Z: Long noncoding RNA related to periodontitis interacts
with miR-182 to upregulate osteogenic differentiation in
periodontal mesenchymal stem cells of periodontitis patients. Cell
Death Dis. 7:e23272016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin C, Jia L, Huang Y, Zheng Y, Du N, Liu
Y and Zhou Y: Inhibition of lncRNA MIR31HG promotes osteogenic
differentiation of human adipose-derived stem cells. Stem Cells.
34:2707–2720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He S, Yang S, Zhang Y, Li X, Gao D, Zhong
Y, Cao L, Ma H, Liu Y, Li G, et al: LncRNA ODIR1 inhibits
osteogenic differentiation of hUC-MSCs through the
FBXO25/H2BK120ub/H3K4me3/OSX axis. Cell Death Dis. 10:9472019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Wang XQ, Liu XT, Guo R and Zhang RD:
Integrative analysis reveals key mRNAs and lncRNAs in monocytes of
osteoporotic patients. Math Biosci Eng. 16:5947–5971. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clemson CM, Hutchinson JN, Sara SA,
Ensminger AW, Fox AH, Chess A and Lawrence JB: An architectural
role for a nuclear noncoding RNA: NEAT1 RNA is essential for the
structure of paraspeckles. Mol Cell. 33:717–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naganuma T, Nakagawa S, Tanigawa A, Sasaki
YF, Goshima N and Hirose T: Alternative 3′-end processing of long
noncoding RNA initiates construction of nuclear paraspeckles. EMBO
J. 31:4020–4034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo Y, Hao T, Zhang J, Zhang M, Sun P and
Wu L: MicroRNA-592 suppresses the malignant phenotypes of thyroid
cancer by regulating lncRNA NEAT1 and downregulating NOVA1. Int J
Mol Med. 44:1172–1182. 2019.PubMed/NCBI
|
|
35
|
Liu R, Tang A, Wang X, Chen X, Zhao L,
Xiao Z and Shen S: Inhibition of lncRNA NEAT1 suppresses the
inflammatory response in IBD by modulating the intestinal
epithelial barrier and by exosome-mediated polarization of
macrophages. Int J Mol Med. 42:2903–2913. 2018.PubMed/NCBI
|
|
36
|
Zhang F, Wu L, Qian J, Qu B, Xia S, La T,
Wu Y, Ma J, Zeng J, Guo Q, et al: Identification of the long
noncoding RNA NEAT1 as a novel inflammatory regulator acting
through MAPK pathway in human lupus. J Autoimmun. 75:96–104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He F, Zhang C and Huang Q: Long noncoding
RNA nuclear enriched abundant transcript 1/miRNA-124 axis
correlates with increased disease risk, elevated inflammation,
deteriorative disease condition, and predicts decreased survival of
sepsis. Medicine (Baltimore). 98:e164702019. View Article : Google Scholar
|
|
38
|
Huang Q, Huang C, Luo Y, He F and Zhang R:
Circulating lncRNA NEAT1 correlates with increased risk, elevated
severity and unfavorable prognosis in sepsis patients. Am J Emerg
Med. 36:1659–1663. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen DD, Hui LL, Zhang XC and Chang Q:
NEAT1 contributes to ox-LDL-induced inflammation and oxidative
stress in macro-phages through inhibiting miR-128. J Cell Biochem.
Sep 11–2018.Epub ahead of print. View Article : Google Scholar
|
|
40
|
Huang-Fu N, Cheng JS, Wang Y, Li ZW and
Wang SH: Neat1 regulates oxidized low-density lipoprotein-induced
inflammation and lipid uptake in macrophages via paraspeckle
formation. Mol Med Rep. 17:3092–3098. 2018.
|
|
41
|
Zhang P, Cao L, Zhou R, Yang X and Wu M:
The lncRNA Neat1 promotes activation of inflammasomes in
macrophages. Nat Commun. 10:14952019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
43
|
Joshi-Tope G, Gillespie M, Vastrik I,
D'Eustachio P, Schmidt E, de Bono B, Jassal B, Gopinath GR, Wu GR,
Matthews L, et al: Reactome: A knowledgebase of biological
pathways. Nucleic Acids Res. 33:D428–D432. 2005. View Article : Google Scholar :
|
|
44
|
Yu G, Wang LG, Han Y and He QY:
ClusterProfiler: An R package for comparing biological themes among
gene clusters. Omics. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Claude-Taupin A, Bissa B, Jia J, Gu Y and
Deretic V: Role of autophagy in IL-1β export and release from
cells. Seminars in cell & developmental biology. 83:36–41.
2018. View Article : Google Scholar
|
|
46
|
Harris J, Hartman M, Roche C, Zeng SG,
O'Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J,
et al: Autophagy controls IL-1beta secretion by targeting
pro-IL-1beta for degradation. J Biol Chem. 286:9587–9597. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Krakauer T: Inflammasomes, autophagy, and
cell death: The trinity of innate host defense against
intracellular bacteria. Mediators Inflamm. 2019:24712152019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gao C, Chen J, Fan F, Long Y, Tang S,
Jiang C, Wang J and Xu Y and Xu Y: RIPK2-mediated autophagy and
negatively regulated ROS-NLRP3 inflammasome signaling in GMCs
stimulated with high glucose. Mediators Inflamm. 2019:62075632019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chakravarty D, Sboner A, Nair SS,
Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K,
Kossai M, et al: The oestrogen receptor alpha-regulated lncRNA
NEAT1 is a critical modulator of prostate cancer. Nat Commun.
5:53832014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fu JW, Kong Y and Sun X: Long noncoding
RNA NEAT1 is an unfavorable prognostic factor and regulates
migration and invasion in gastric cancer. J Cancer Res Clin Oncol.
142:1571–1579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li S, Shuch BM and Gerstein MB:
Whole-genome analysis of papillary kidney cancer finds significant
noncoding alterations. PLoS Genetics. 13:e10066852017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ning L, Li Z, Wei D, Chen H and Yang C:
LncRNA, NEAT1 is a prognosis biomarker and regulates cancer
progression via epithelial-mesenchymal transition in clear cell
renal cell carcinoma. Cancer Biomark. 19:75–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Taiana E, Favasuli V, Ronchetti D,
Todoerti K, Pelizzoni F, Manzoni M, Barbieri M, Fabris S,
Silvestris I, Gallo Cantafio ME, et al: Long non-coding RNA NEAT1
targeting impairs the DNA repair machinery and triggers anti-tumor
activity in multiple myeloma. Leukemia. 34:234–244. 2020.
View Article : Google Scholar
|
|
54
|
Zeng C, Liu S, Lu S, Yu X, Lai J, Wu Y,
Chen S, Wang L, Yu Z, Luo G and Li Y: The c-Myc-regulated lncRNA
NEAT1 and paraspeckles modulate imatinib-induced apoptosis in CML
cells. Mol Cancer. 17:1302018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L,
Chen S and Li Y: Inhibition of long non-coding RNA NEAT1 impairs
myeloid differentiation in acute promyelocytic leukemia cells. BMC
Cancer. 14:6932014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Adriaens C, Standaert L, Barra J, Latil M,
Verfaillie A, Kalev P, Boeckx B, Wijnhoven PW, Radaelli E, Vermi W,
et al: p53 induces formation of NEAT1 lncRNA-containing
paraspeckles that modulate replication stress response and
chemosensitivity. Nat Med. 22:861–868. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mello SS, Sinow C, Raj N and Attardi LD:
Neat1 is a p53-inducible lincRNA essential for transformation
suppression. Genes Dev. 31:1095–1108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jen J, Tang YA, Lu YH, Lin CC, Lai WW and
Wang YC: Oct4 transcriptionally regulates the expression of long
non-coding RNAs NEAT1 and MALAT1 to promote lung cancer
progression. Mol Cancer. 16:1042017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zheng X, Zhang Y, Liu Y, Fang L, Li L, Sun
J, Pan Z, Xin W and Huang P: HIF-2α activated lncRNA NEAT1 promotes
hepa-tocellular carcinoma cell invasion and metastasis by affecting
the epithelial-mesenchymal transition. J Cell Biochem.
119:3247–3256. 2018. View Article : Google Scholar
|
|
60
|
Zhong J, Jiang L, Huang Z, Zhang H, Cheng
C, Liu H, He J, Wu J, Darwazeh R, Wu Y and Sun X: The long
non-coding RNA Neat1 is an important mediator of the therapeutic
effect of bexarotene on traumatic brain injury in mice. Brain Behav
Immun. 65:183–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Barutcu AR, Hong D, Lajoie BR, McCord RP,
van Wijnen AJ, Lian JB, Stein JL, Dekker J, Imbalzano AN and Stein
GS: RUNX1 contributes to higher-order chromatin organization and
gene regulation in breast cancer cells. Biochim Biophys Acta.
1859:1389–1397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lo PK, Zhang Y, Wolfson B, Gernapudi R,
Yao Y, Duru N and Zhou Q: Dysregulation of the BRCA1/long
non-coding RNA NEAT1 signaling axis contributes to breast
tumorigenesis. Oncotarget. 7:65067–65089. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Henning P, Conaway HH and Lerner UH:
Retinoid receptors in bone and their role in bone remodeling. Front
Endocrinol (Lausanne). 6:312015. View Article : Google Scholar
|
|
64
|
Menéndez-Gutiérrez MP and Ricote M: The
multi-faceted role of retinoid X receptor in bone remodeling. Cell
Mol Life Sci. 74:2135–2149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Menéndez-Gutiérrez MP, Rőszer T, Fuentes
L, Núñez V, Escolano A, Redondo JM, De Clerck N, Metzger D,
Valledor AF and Ricote M: Retinoid X receptors orchestrate
osteoclast differentiation and postnatal bone remodeling. J Clin
Invest. 125:809–823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nowak B, Matuszewska A, Filipiak J,
Nikodem A, Merwid-Ląd A, Pieśniewska M, Fereniec-Gołębiewska L,
Kwiatkowska J and Szeląg A: The influence of bexarotene, a
selective agonist of the retinoid receptor X (RXR), and tazarotene,
a selective agonist of the retinoid acid receptor (RAR), on bone
metabolism in rats. Adv Med Sci. 61:85–89. 2016. View Article : Google Scholar
|
|
67
|
Wang Y, Moser AH, Shigenaga JK, Grunfeld C
and Feingold KR: Downregulation of liver X receptor-alpha in mouse
kidney and HK-2 proximal tubular cells by LPS and cytokines. J
Lipid Res. 46:2377–2387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang Y, Wang H, Hao Y, Lin H, Dong M, Ye
J, Song L, Wang Y, Li Q, Shan B, et al: Myeloid PTEN promotes
chemotherapy-induced NLRP3-inflammasome activation and antitumour
immunity. Nat Cell Biol. 22:716–727. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
He H, Jiang H, Chen Y, Ye J, Wang A, Wang
C, Liu Q, Liang G, Deng X, Jiang W and Zhou R: Oridonin is a
covalent NLRP3 inhibitor with strong anti-inflammasome activity.
Nat Commun. 9:25502018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yao X, Zhang C, Xing Y, Xue G, Zhang Q,
Pan F, Wu G, Hu Y, Guo Q, Lu A, et al: Remodelling of the gut
microbiota by hyperactive NLRP3 induces regulatory T cells to
maintain homeostasis. Nat Commun. 8:18962017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang X, Jiang W, Yan Y, Gong T, Han J,
Tian Z and Zhou R: RNA viruses promote activation of the NLRP3
inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat
Immunol. 15:1126–1133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hoss F, Rodriguez-Alcazar JF and Latz E:
Assembly and regulation of ASC specks. Cell Mol Life Sci.
74:1211–1229. 2017. View Article : Google Scholar
|
|
73
|
Afonina IS, Zhong Z, Karin M and Beyaert
R: Limiting inflammation-the negative regulation of NF-kappaB and
the NLRP3 inflammasome. Nat Immunol. 18:861–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kong Y, Huang T, Zhang H, Zhang Q, Ren J,
Guo X, Fan H and Liu L: The lncRNA NEAT1/miR-29b/Atg9a axis
regulates IGFBPrP1-induced autophagy and activation of mouse
hepatic stellate cells. Life Sci. 237:1169022019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu F, Ai FY, Zhang DC, Tian L, Yang ZY
and Liu SJ: LncRNA NEAT1 knockdown attenuates autophagy to elevate
5-FU sensitivity in colorectal cancer via targeting miR-34a. Cancer
Med. 9:1079–1091. 2020. View Article : Google Scholar
|
|
76
|
Cao Z, Wang Y, Long Z and He G:
Interaction between autophagy and the NLRP3 inflammasome. Acta
Biochim Biophys Sin (Shanghai). 51:1087–1095. 2019. View Article : Google Scholar
|