Introduction
Myocardial infarction (MI) results from oxygen
deprivation after a block of one of the coronary artery branches
together with sudden halt of blood flow and myocardial anoxia
(1). MI is one of the primary
causes of death worldwide and is characterized by vascular
endothelial injury induced by inflammation, apoptosis, cardiac
fibrosis and pathological remodeling (2), which contributes to high morbidity
and mortality globally (3). Acute
MI (AMI) can contribute to the enhancement of reactive oxygen along
with activation of deleterious cellular signals, thereby elevating
inflammatory cytokine expression in endothelial cells and inducing
the infiltration of inflammatory cells to the infarct zone
(4). Risk stratification and
management of patients with AMI remains challenging, although great
efforts have been made by numerous clinicians and researchers in
the last decades (5). Therefore,
a novel specific and highly-sensitive biomarker is urgently needed
in order to provide early diagnosis of AMI and to identify
potential regulatory targets of AMI.
Bone marrow mesenchymal stem cells (BMSCs) are a
widely studied cellular therapy that can improve cardiac function
after MI (6). An increasing
number of regimens have been found for gene delivery to the heart,
such as intrapericardial, intracoronary and direct myocardial
injection (7). Novel gene
therapies, especially non-invasive methods that are able to
stimulate stem cell homing to the injured heart, can improve
function by inducing regional perfusion along with tissue
regeneration in the infarcted myocardium (8,9).
Recently, ultrasound-targeted microbubble destruction (UTMD) has
been found to act as a novel approach of tissue-specific gene
delivery that transfers nucleic acid drugs through the cavitation
effect in a range of the microvasculature of target tissues, which
is useful for local delivery and to evade biological barriers in
tissues (10). It has been
revealed that UTMD-mediated gene therapy alleviates myocardial
perfusion and cardiac function in mice with MI (11). Although the capability of UTMD to
induce histologically definable microlesions has been demonstrated
in small animals, the effects of such microlesions on the heart
have not yet been fully examined (12).
Galectin is a novel target associated with
unfavorable prognosis in patients with heart failure. Elevated
galectin levels are related to heart failure, and may be a primary
regulator of cardiac remodeling with myocardial fibrosis (13). Galectin-7, as a member of galectin
family, plays a role in various events related to the development
of the pluristratified epithelium (14). It is also correlated with
epithelial cell migration and plays a part in the
re-epithelialization process of corneal and epidermal wounds
(15). A previous study
discovered that galectin-7 knockdown contributed to declined
differentiation and elevated proliferation of keratinocytes
(16). However, the role of
galectin-7 in AMI remains unknown. Galectin-1 and galectin-3 are
proposed to be involved in MI (17,18). The role of galectin-7 has not been
reported in MI, but galectin-7 can promote CD4+ T cell
proliferation and T helper cell (Th) 1/2 polarization by inhibiting
the TGFβ/Smad3 pathway (19), and
galectin-7 plays a role in apoptosis by binding to Bcl-2 (20). Moreover, the TGFβ/Smad3 pathway
and apoptosis are involved in the process of MI (21). Hence, it is speculated that
galectin-7 may play a role in MI. Therefore, the present study
aimed to elucidate the synergistic effect of UTMD-mediated
galectin-7-small interfering (si)RNA with the homing of BMSCs for
AMI, along with its underlying mechanism, which could provide
useful findings in the discovery of novel therapeutic targets of
AMI.
Materials and methods
Ethics statement
Animal experiments were implemented in accordance
with the Guide to the Management and Use of Laboratory Animals
(22). The animal experiment
protocols were approved by the Institutional Animal Care and Use
Committee of People's Hospital of Deyang City (approval no.
20190075; Deyang, China). The rats were housed in a controlled
environment, at a temperature of 22-24°C and 12/12 h light/dark
cycles, with free access to standard rat food and water.
Experimental animals
A total of 84 healthy male Sprague-Dawley (SD) rats
(250±10 g, 6 weeks old) and four SD rats (two male and two female,
100±5 g, 3 weeks old) were acquired from The Sixth Affiliated
Hospital of Sun Yat-sen University [license no. SYXK (Yue)
2018-0190; Guangzhou, China] and were fed under normal
conditions.
Isolation, culture, identification and
labeling of rat BMSCs
A total of four 3-week-old rats were anesthetized
(intraperitoneal injection of 100 mg/kg pentobarbital) and
sacrificed by cervical dislocation. The femur was separated under
aseptic conditions. After washing, the bone marrow cavity was
exposed after removal of both ends of the epiphysis. The bone
marrow cavity was rinsed repeatedly with DMEM/F12 medium solution
(Sigma Aldrich; Merck KGaA) containing 10% fetal bovine serum (FBS;
Beijing Solarbio Science & Technology Co., Ltd.). Bone marrow
cells were collected and placed in Percoll separation solution
(Beijing Solarbio Science & Technology Co., Ltd.). BMSCs were
separated and purified by density gradient equilibrium
centrifugation and adherent screening method (23). BMSCs were observed using an IX-70
inverted phase contrast microscope (Olympus Corporation).
BMSCs in the 3rd passage were detached with 0.25%
trypsin and fluorescein isothiocyanate (FITC)-conjugated antibodies
against CD29 (cat. no. ab21845; 1:10), CD34 (cat. no. ab78165;
1:10), CD44 (cat. no. ab30405; 1:10) and CD45 (cat. no. ab27287;
1:10) (all purchased from Abcam) were added. The corresponding
negative control (NC) was set for each tube and incubated for 45
min in the dark at 4°C. After washing with phosphate buffered
saline (PBS) three times, the BMSCs were centrifuged at 400 × g for
5 min at 4°C and suspended with 50 µl PBS, following which
they were analyzed with a BD FACSCanto™ II Flow Cytometer (BD
Biosciences) and BD CellQuest™ Pro software (BD Biosciences).
An enhanced green fluorescence protein (EGFP)
plasmid (3.0 µg per 1×104 cells/well)
(Sigma-Aldrich; Merck KGaA) was transfected into BMSCs using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) 2 days before cell transplantation. After
transfection for 24 h, the liquid was replaced and observed under a
fluorescence microscope (Olympus Corporation). After detaching the
cells with 0.25% trypsin, DMEM-F12 medium was added. A cell
suspension of 5×106 cells/ml was prepared and
transplanted for use.
Osteogenic and adipogenic induction and
identification of rat BMSCs
BMSCs were incubated at 37°C with 5% CO2,
and 1 µmol/l dexamethasone, 10 µmol/l
β-glycerophosphate, 50 mg/l vitamin, and L-DMEM (Beijing Solarbio
Science & Technology Co., Ltd.) containing 10% FBS were added
48 h later to induce osteogenesis, and the medium was renewed once
every 2 to 3 days. The control group was cultured in L-DMEM without
the aforementioned inducers. The method of adipogenic induction was
the same, but the culture was supplemented with 1 µmol/l
dexamethasone, 0.5 mmol/l 3-isobutyl-1 methylxanthine (IBMX), 10
mg/l insulin and H-DMEM (Beijing Solarbio Science & Technology
Co., Ltd.) with 10% FBS. The control group was cultured in H-DMEM
without the aforementioned inducers.
The coverslips of the cells were immersed in PBS for
1 min three times, fixed with 4% formaldehyde for 15 min at 37°C,
immersed in PBS for 3 min three times, stained with 0.2% Alizarin
red (Beyotime Institute of Biotechnology) for 5-30 min at 37°C,
followed by washing with PBS and microscopic examination under a
light microscope (BX51; Olympus Corporation).
The coverslips were fixed with formaldehyde-calcium
for 10 min at 37°C, washed with distilled water; rinsed with 60%
isopropanol, stained with oil red O (Beyotime Institute of
Biotechnology) at 37°C for 10 min, differentiated with 60%
isopropanol, followed by washing with distilled water, hematoxylin
counterstaining at 37°C for 10 min, rinsing with water and
microscopic examination under a light microscope.
Establishment of AMI rat models and
identification
The rats were anesthetized with an intraperitoneal
injection of 2% pentobarbital sodium (40 mg/kg; Sigma-Aldrich;
Merck KGaA) and laid down on the operating table. The AMI rat model
was established by ligating the left anterior descending branch of
the coronary artery (24). The
electrocardiogram (Chengdu Medical Instrument Factory) was detected
before the coronary artery ligation and 7 days after the model was
established.
Animal grouping
On the 7th day after the establishment of the AMI
model, rats were transplanted with BMSCs and divided into five
groups (n=15/group) according to different treatments: i) Sham
group, rats with thoracotomy and without ligation of the left
anterior descending branch of the coronary artery; ii) AMI group,
AMI rats with no treatments; iii) BMSC group, AMI rats injected
with 5×106 labeled BMSCs via the caudal vein; iv) BUTMD
+ siRNA NC group, BMSC transplantation, UTMD and siRNA NC; v) BUTMD
+ Galectin-7-siRNA group, BMSC transplantation, UTMD and
Galectin-7-siRNA. For the BUTMD + siRNA NC group, siRNA NC (100
µM; Sigma-Aldrich; Merck KGaA) was mixed with sulphur
hexafluoride microbubbles (Bracco Suisse SA) and incubated for 30
min at 25°C to construct siRNA NC encapsulated in sulphur
hexafluoride microbubbles; then the siRNA NC encapsulated in SF6
microbubbles was added to EGFP-labeled BMSCs and the mixture was
injected into rats via the tail vein. Ultrasound (frequency 1 M Hz,
power 2 W/cm2) was immediately irradiated to the
anterior wall of the left ventricle of rats for 120 sec. For the
BUTMD + Galectin-7-siRNA group, after incubation of
Galectin-7-siRNA (100 µM; Sigma-Aldrich; Merck KGaA) with
microvesicles at 25°C for 30 min, the subsequent steps were the
same as the BUTMD + siRNA NC group. Galectin-7-siRNA sequences were
sense, 5′-CAU CCG AGG UUG UCU UCA ACA-3′ and antisense, 5′-UUG AAG
ACA ACC UCG GAU GUG-3′.
Echocardiographic evaluation and
hemodynamic monitoring
Four weeks after transplantation of the BMSCs, rats
were anesthetized with 30 mg/kg pentobarbital sodium, and the left
ventricular end diastolic diameter (LVEDd) and left ventricular end
systolic diameter (LVESd) were detected using a Vivid-7 color
Doppler ultrasound scanner (GE Healthcare), and the left
ventricular ejection fraction (EF) was calculated using the
following formula: EF = stroke volume/end-diastolic volume ×100%.
After the cardiac color Doppler ultrasound, a catheter filled with
heparin saline was placed in the right common carotid artery to the
left ventricle, and the left ventricular systolic pressure (LVSP),
left ventricular end-diastolic pressure (LVEDP) and left
ventricular maximal rate of rise and fall (± dp/dtmax) were
recorded using a multi-conductive physiological recorder (Chengdu
Medical Instrument Factory).
Tissue specimen collection
The heart of the rats was examined using ultrasound,
and then the rats were euthanized by an intraperitoneal injection
of 800 mg/kg pentobarbital sodium (25). The myocardial tissues around the
infarct area were collected. The myocardial tissues of six rats in
each group were fixed with 4% paraformaldehyde at 25°C for 24 h,
and then the paraffin-embedded tissues were sliced (5 µm)
for histological staining. The myocardial tissues of six rats in
each group were made into tissue homogenate. The myocardial tissue
sections (5 µm) of the remaining three rats in each group
were frozen at -20°C.
H&E staining
Paraffin-embedded sections were routinely dewaxed
and dehydrated, stained for 3 min with hematoxylin at 25°C (Beijing
Solarbio Science & Technology Co., Ltd.), differentiated for 15
sec with 1% hydrochloric acid alcohol, stained with eosin (Beijing
Solarbio Science & Technology Co., Ltd.) at 25°C for 2 min and
observed under an optical microscope (Olympus Corporation).
Masson staining
Paraffin-embedded sections were routinely dewaxed,
stained with the Masson's Trichrome Stain kit (Beijing Solarbio
Science & Technology Co., Ltd.), according to the
manufacturer's protocols, and finally, myocardial infarct size was
calculated using Image ProPlus 6.0 (Media Cybernetics, Inc.).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) staining
Paraffin-embedded sections were routinely dewaxed,
and then stained with the TUNEL Apoptosis Detection kit (Shanghai
Yeasen Biotechnology Co., Ltd.), according to the instructions of
the manufacturer. Finally, a few drops of 100 ng/ml
4′,6-Diamidino-2-phenylindole (DAPI; Beyotime Institute of
Biotechnology) was added for nuclear staining at room temperature
for 10 min, and TUNEL-positive cells were captured under a
fluorescence microscope (BX51; Olympus Corporation). At least three
sections were observed in each tissue, and three visual fields were
randomly selected for statistical analysis.
Frozen sections to observe the homing of
BMSCs
Frozen sections were sealed in a sealing liquid
containing DAPI (Beyotime Institute of Biotechnology) and imaged
under a laser confocal fluorescence microscope (TCS SP5; Leica
Microsystems GmbH).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the tissue homogenate
of each group using a TRIzol® kit (Beijing Dingguo
Changsheng Biotechnology Co., Ltd.). RNA was reverse transcribed
into cDNA at 42°C for 60 min using the ReverTra Ace™ qPCR RT Master
Mix kit (Toyobo Life Science). PCR was conducted using
SYBR® Premix Ex Taq™ II (Takara Bio, Inc.).
Thermocycling conditions were as follows: 95°C for 10 min; 35
cycles of 94°C for 60 sec, Tm 56°C, 72°C for 60 sec; and then, 72°C
for 10 min. β-actin was used as the loading control. The primer
sequences used are shown in Table
I, and the mRNA expression was analyzed using the
2−ΔΔCq method (26).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primer sequences
(5′→3′) |
|---|
| SDF-1 | F:
TTGCCAGCACAAAGACACTCC |
| R:
CTCCAAAGCAAACCGAATACAG |
| VEGF | F:
GTGAGCCTTGTTGTTCAGCG |
| R:
GACGGTGACGATGGTGG |
| VCAM-1 | F:
GTCGTGGTCAGCCCCTCCTC |
| R:
CAGCCTGGTTAATCCCTTCACAC |
| IL-1β | F:
CTTCGGGCTCTCCACCTCA |
| R:
AATCGCTTTTCCATCTTCCTCTT |
| TGF-β1 | F:
AGGGCTACCATGCCAACTTC |
| R:
CCACGTAGTAGACGATGGGC |
| Smad3 | F:
AGACACCAGTGATACCTCCA |
| R:
CCAGCGGGGAAGTTAGTGTT |
| Smad7 | F:
CAGGCTCCAGAAGAAGTTGG |
| R:
AGTTCAACGGCACAGTCAAG |
| β-actin | F:
TGGCTGGCCGGGACCTGACTGA |
| R:
CGCGCCGTGGCCATCTCCTG |
Western blot analysis
The protein was extracted using RIPA buffer (Thermo
Fisher Scientific, Inc.). Total protein concentration of tissue
homogenate was detected using a bicinchoninic acid detection kit
(Pierce; Thermo Fisher Scientific, Inc.). The proteins (30
µg/well) were separated via sodium dodecyl sulfate
polyacrylamide gel electrophoresis (Beyotime Institute of
Biotechnology) on a 10% gel, and then transferred onto
polyvinylidene difluoride membranes. Next, the membranes were
blocked for 1 h with 5% skimmed milk at 37°C, and then incubated at
4°C overnight with primary antibodies against: Galectin-7 (cat. no.
ab206435; 1:1,000), Bcl-2 (cat. no. ab182858; 1:2,000), Bax (cat.
no. ab32503; 1:1,000), stromal cell-derived factor 1 (SDF-1; cat.
no. ab25117; 1:1,000), vascular endothelial growth factor (VEGF;
cat. no. ab32152; 1:1,000), vascular cell adhesion molecule-1
(VCAM-1; cat. no. ab134047; 1:2,000), interleukin (IL)-1β (cat. no.
ab2105; 1:1,000), cardiac troponin I (cTnI; cat. no. ab47003;
1:1,000), α-myosin heavy chain (α-MHC; cat. no. ab180779; 1:2,000),
collagen I (cat. no. ab6308; 1 µg/ml), transforming growth
factor-β1 (TGF-β1; cat. no. ab179695; 1:1,000), Smad3 (cat. no.
ab40854; 1:1,000), Smad7 (cat. no. ab216428; 1:1,000) and
glyceraldehyde phosphate dehydrogenase (GAPDH; cat. no. ab8245;
1:1,000) (all purchased from Abcam). Following which, membranes
were incubated with a horseradish peroxidase-labeled anti-rabbit
IgG secondary antibody (cat. no. ab205718; 1:2,000; Abcam) and
incubated for 1 h at 25°C. Then, membranes were developed with a
chemiluminescence reagent (Thermo Fisher Scientific, Inc.). GAPDH
was used as the internal reference. Relative protein expression was
expressed as the ratio of the gray value of the target protein band
to that of the internal reference. The gray value of the target
band was analyzed using ImageJ version 1.8.0 software (National
Institutes of Health).
Enzyme-linked immunosorbent assay
(ELISA)
Following centrifugation of the tissue homogenate at
4°C and 1,200 × g for 5 min, the levels of TGF-β1 (cat. no.
ml002856) and cTnI (cat. no. ml059111) in the supernatant were
measured using ELISA kits (Shanghai Enzyme-linked Biotechnology
Co., Ltd.), according to the manufacturer's instructions.
Statistical analysis
The independent experiments were repeated three
times. Data analyses were performed using SPSS 21.0 software (IBM
Corp.). The measurement data conforming to the normal distribution
after verification using a Kolmogorov-Smirnov test were depicted as
mean ± standard deviation. One-way or two-way analysis of variance
(ANOVA) was performed for comparisons among multiple groups, and
Tukey's multiple comparisons test was applied for pairwise
comparisons following ANOVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
Rat AMI model and BMSC
identification
Compared with the sham-operated rats, the
electrocardiogram of the rats with AMI showed an upward ST-segment
elevation and merged with a T wave into a one-way curve (Fig. 1A). H&E staining showed that
the myocardial fibrous tissue of the rats with AMI was disordered
and broken, and the nucleus was ruptured compared with the
sham-operated rats (Fig. 1B).
These results indicated that the rat model of AMI was successfully
established.
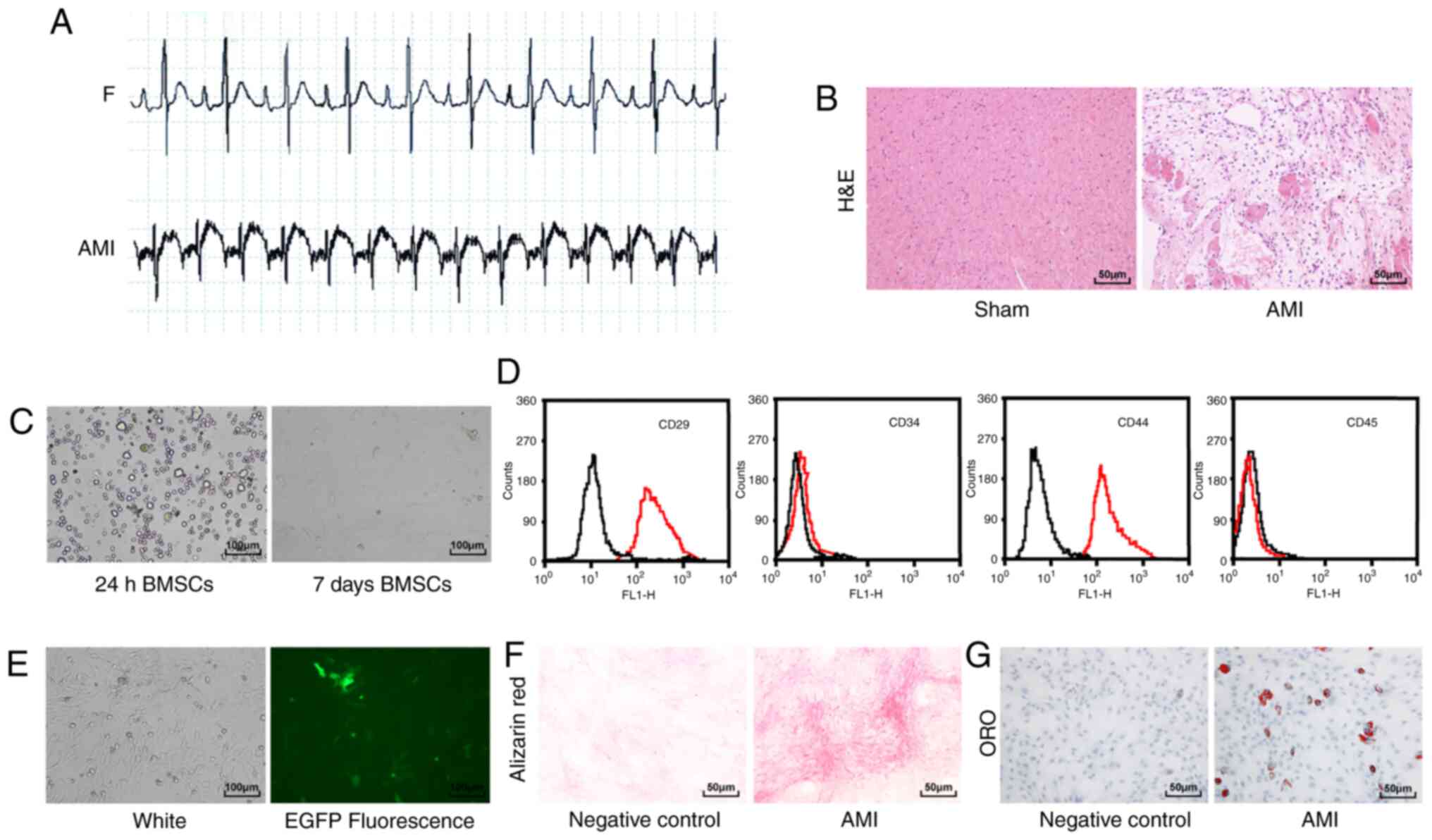 | Figure 1Rat AMI model and BMSC
identification. (A) Electrocardiogram was used to detect changes in
the heart's electrical activity before and after AMI in rats
(n=12/group). (B) Detection of myocardial tissue injury in rats
(n=6/group) using H&E staining (magnification, ×200; scale bar,
50 µm). (C) Observation of BMSC morphology using an inverted
phase contrast microscope (magnification, ×100; scale bar, 100
µm). (D) Flow cytometry analysis of BMSC surface markers.
The black line in the figure represents the control IgG, and red
line represents the surface markers. (E) Observation of
EGFP-labeled BMSCs under a fluorescence microscope (magnification,
×100; scale bar, 100 µm). (F) Alizarin red staining of BMSCs
(magnification, ×200; scale bar, 50 µm). (G) ORO staining of
BMSCs (magnification, ×200; scale bar, 50 µm). AMI, acute
myocardial infarction; BMSC, bone marrow mesenchymal stem cell;
H&E, hematoxylin and eosin; EGFP, enhanced green fluorescence
protein; ORO, oil red O. |
The BMSCs cultured for 24 h were spherical under a
microscope, and BMSCs cultured for 7 days were long fusiform
(Fig. 1C). Flow cytometry results
showed that CD29 and CD44 were positively expressed on the surface
of BMSCs, whereas CD34 and CD45 showed negative expression
(Fig. 1D). BMSCs transfected with
EGFP showed green fluorescence under a fluorescence microscope,
confirming the successful transfection of EGFP (Fig. 1E). Finally, the BMSCs were
cultured under osteogenic and adipogenic inductive environments and
the results revealed that BMSCs had multi-lineage differentiation
ability (Fig. 1F and G). These
results indicated that BMSCs were successfully cultured.
BMSC transplantation improves AMI in
rats
After BMSC transplantation, the cardiac function and
hemodynamics of rats in the BMSC group were significantly improved
compared with that of the AMI group (P<0.01; Fig. 2A and B). The results of
pathological examination indicated that the myocardial fiber injury
of rats was reduced (P<0.01; Fig.
2C and D) and cardiomyocyte apoptosis rate was decreased after
BMSC transplantation, but there was still a notable difference
between the BMSCs group and the sham-operated group (P<0.01;
Fig. 2E and F). The green
fluorescence in the myocardial tissues of rats in the BMSC group
increased significantly (P<0.01; Fig. 2G). These results indicated that
BMSCs notably improved AMI injury in rats.
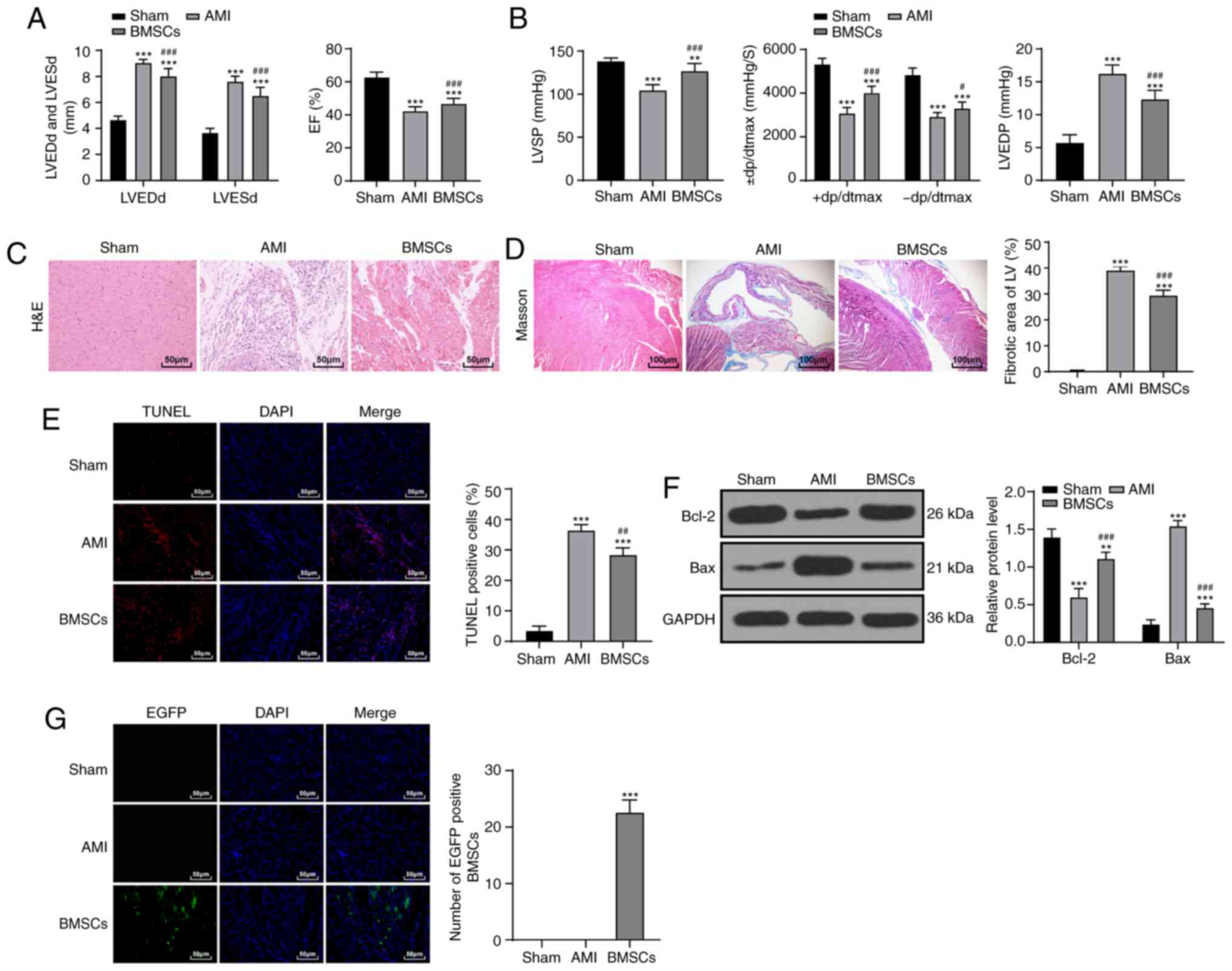 | Figure 2BMSC transplantation improves AMI in
rats. (A) Detection of cardiac function indices in rats
(n=15/group). (B) Hemodynamic determination in rats (n=15/group).
(C) The morphology of myocardial tissue in each group (n=6/group)
was observed with H&E staining (magnification, ×200; scale bar,
50 µm). (D) Observation of myocardial fibers in each group
(n=6/group) via Masson staining (magnification, ×400; scale bar,
100 µm). (E) Detection of apoptosis in rats in each group
(n=6/group) via TUNEL staining (magnification, ×400; scale bar, 50
µm). (F) Detection of Bcl-2 and Bax protein expression by
western blot analysis (n=6/group). (G) Observation of BMSC homing
ability in rats (n=3/group) using fluorescence microscopy
(magnification, ×400; scale bar, 50 µm). Data in panels A
(right), B (right/left), D, E and G were analyzed using one-way
ANOVA, and data in panels A (left), B (middle) and F were analyzed
using two-way ANOVA, followed by Tukey's multiple comparisons test.
**P<0.01 and ***P<0.001 vs. Sham group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. AMI group. AMI, acute myocardial
infarction; BMSC, bone marrow mesenchymal stem cell; H&E,
hematoxylin and eosin; TUNEL, transferase-mediated deoxyuridine
triphosphate-biotin nick end labeling; LVEDd, left ventricular end
diastolic diameter; LVESd, left ventricular end systolic diameter;
EF, ejection fraction; LVSP, left ventricular systolic pressure;
LVEDP, left ventricular end-diastolic pressure; ± dp/dtmax, left
ventricular maximal rate of rise and fall; LV, left ventricle;
EGFP, enhanced green fluorescence protein. |
UTMD-mediated Galectin-7-siRNA promotes
the homing of BMSCs to alleviate AMI in rats
It has been reported that Galectin-7 promotes
CD4+ T cell proliferation and Th1/2 cell polarization by
inhibiting the TGFβ/Smad3 pathway (19), and Galectin-7 can play a role in
apoptosis via binding to Bcl-2 (20). The TGFβ/Smad3 pathway and
apoptosis are involved in the process of MI (21). Myocardial cells were transfected
with Galectin-7-siRNA to decrease Galectin-7 expression. To verify
the specificity of Galectin-7-siRNA, the mRNA levels of Galectin-1,
Galectin-3 and Galectin-7 were detected using RT-qPCR. The results
showed that the expression levels of Galectin-3 and Galectin-1 were
not affected by Galectin-7-siRNA, and Galectin-7 expression was
significantly reduced (P<0.01; Fig. 3A). Galectin-7 expression of rats
in the BUTMD+Galectin-7-siRNA group was significantly decreased
compared with that of the BMSC group (P<0.01; Fig. 3B). Subsequently, it was found that
UTMD could promote the promoting effect of BMSCs on MI (BUTMD +
siRNA NC), and UTMD + Galectin-7-siRNA group showed the most
obvious effect (P<0.01; Fig. 3C
and D). Compared with the BMSCs group, rats in the
BUTMD+Galectin-7-siRNA group alleviated the degree of myocardial
injury (P<0.01; Fig. 3E and
F), decreased the number of myocardial apoptotic cells
(P<0.01; Fig. 3G), enhanced
Bcl-2 and reduced Bax expression (P<0.01; Fig. 3H), and increased the number of
homing BMSCs in the myocardial tissues (P<0.01; Fig. 3I). These results suggested that
UTMD-mediated Galectin-7-siRNA had improved efficacy compared with
BMSCs or UTMD treatment.
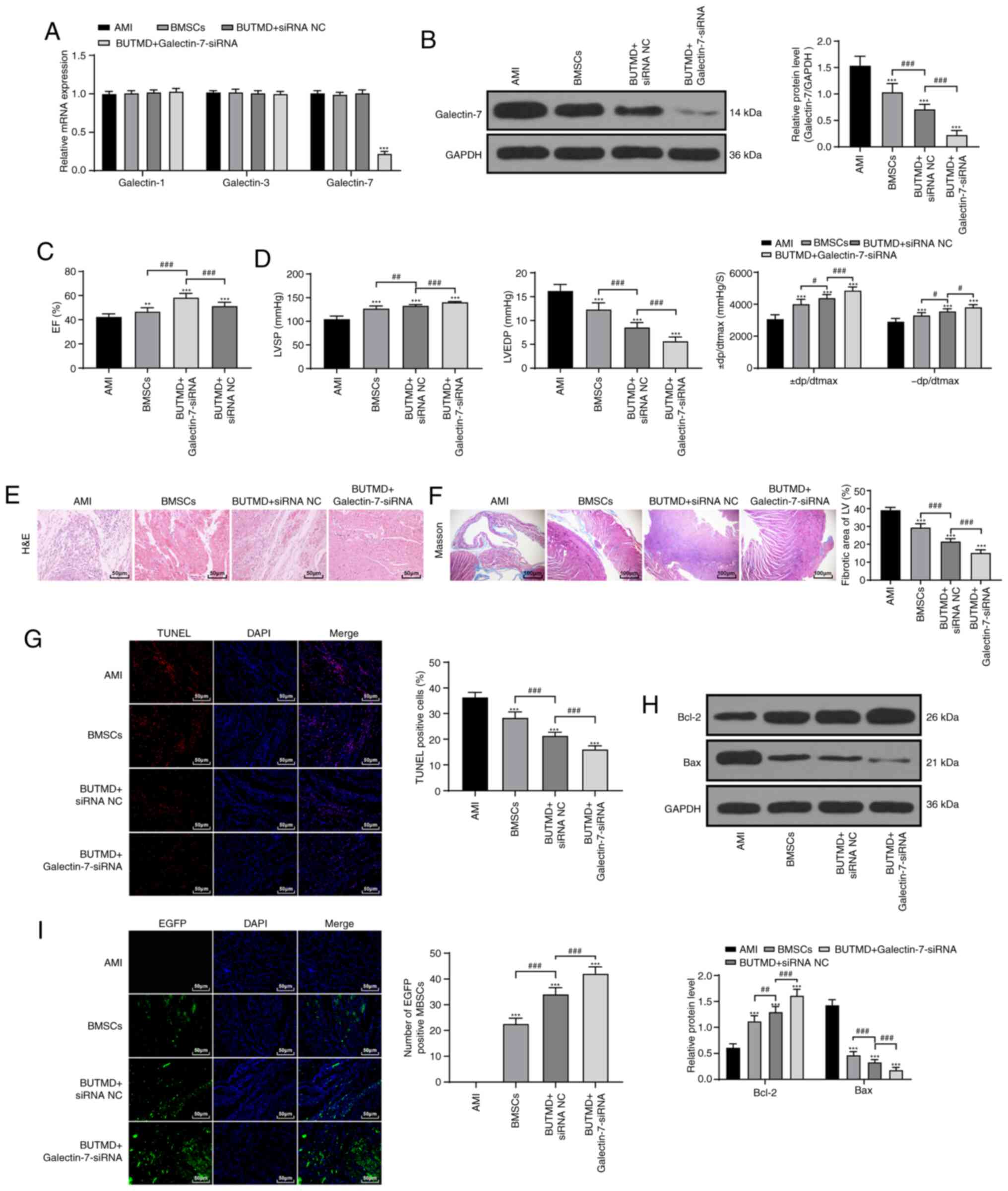 | Figure 3UTMD-mediated Galectin-7-siRNA
promotes the homing of BMSCs to alleviate AMI in rats. (A) siRNA
transfection efficiency was verified by reverse
transcription-quantitative PCR (n=6/group). (B) Galectin-7 protein
expression was detected by western blotting (n=6/group). (C)
Detection of EF (n=15/group). (D) Hemodynamic examination
(n=15/group). (E) Observation of myocardium histomorphology using
H&E staining (n=6/group; magnification, ×200; scale bar, 50
µm). (F) Observation of myocardial fibers via Masson
staining (n=6/group; magnification, ×400; scale bar, 100
µm). (G) Detection of apoptosis in rats of each group
(n=6/group) via TUNEL staining (magnification, ×400; scale bar, 50
µm). (H) Detection of Bcl-2 and Bax protein expression via
western blotting, (n=6/group). (I) Observation of BMSC homing in
rats (n=3/group) using fluorescence microscopy (magnification,
×400; scale bar, 50 µm). Data in panels B, C, D
(left/middle), F, G and I were analyzed using one-way ANOVA, and
data in panels A, D (right) and H were analyzed using two-way
ANOVA, followed by Tukey's multiple comparisons test.
**P<0.01 and ***P<0.001 vs. AMI group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. indicated groups. UTMD,
ultrasound-targeted microbubble destruction; siRNA, small
interfering RNA; AMI, acute myocardial infarction; BMSC, bone
marrow mesenchymal stem cell; H&E, hematoxylin and eosin;
TUNEL, transferase-mediated deoxyuridine triphosphate-biotin nick
end labeling; EF, ejection fraction; LVSP, left ventricular
systolic pressure; LVEDP, left ventricular end-diastolic pressure;
± dp/dtmax, left ventricular maximal rate of rise and fall; NC,
negative control; BUTMD, BMSCs + UTMD; LV, left ventricle; EGFP,
enhanced green fluorescence protein. |
UTMD-mediated Galectin-7-siRNA improves
the myocardial microenvironment
The related indexes of myocardial microenvironment
in rats were detected. Relative to the sham-operated rats, the AMI
rats presented elevated expression levels of SDF-1, VEGF, VCAM-1
and IL-1β, increased cTnI and collagen I protein, and decreased
α-MHC protein. Compared with the AMI group, UTMD-mediated
Galectin-7-siRNA notably promoted the expression levels of SDF-1,
VEGF, VCAM-1, IL-1β and α-MHC, and decreased levels of cTnI and
collagen I in rat myocardial tissues (all P<0.05; Fig. 4A-D). These results suggested that
the combined treatment with UTMD-mediated Galectin-7-siRNA can
improve the myocardial microenvironment of rats.
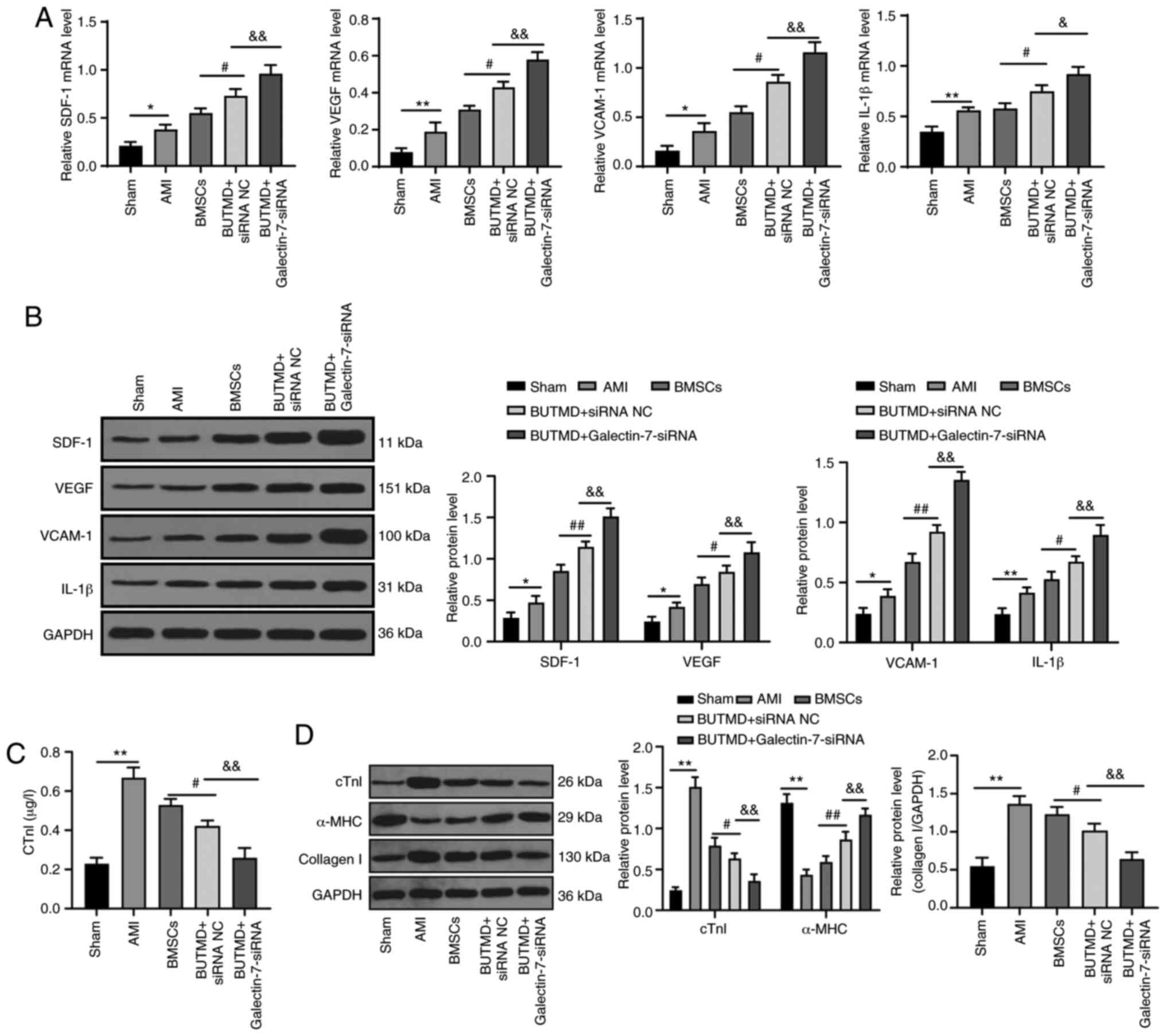 | Figure 4UTMD-mediated Galectin-7-siRNA
improves the myocardial microenvironment. (A) Detection of mRNA
expression levels of SDF-1, VEGF, VCAM-1 and IL-1β via reverse
transcription-quantitative PCR. (B) Detection of protein expression
levels of SDF-1, VEGF, VCAM-1 and IL-1β by western blot analysis.
(C) cTnI protein expression was measured using ELISA. (D) cTnI,
α-MHC and collagen I protein expression levels were detected using
western blotting (n=6/group). Data in panels A, C and D (right)
were analyzed using one-way ANOVA, and data in panels B and D
(left) were analyzed using two-way ANOVA, followed by Tukey's
multiple comparisons test. *P<0.05 and
**P<0.01; #P<0.05 and
##P<0.01; &P<0.05 and
&&P<0.01. UTMD, ultrasound-targeted
microbubble destruction; siRNA, small interfering RNA; SDF-1,
stromal cell-derived factor 1; VEGF, vascular endothelial growth
factor; VCAM-1, vascular cell adhesion molecule-1; IL-1β,
interleukin-1β; cTnI, cardiac troponin I; α-MHC, α-myosin heavy
chain; ELISA, enzyme-linked immunosorbent assay; AMI, acute
myocardial infarction; BMSC, bone marrow mesenchymal stem cell; NC,
negative control; BUTMD, BMSCs + UTMD. |
UTMD-mediated Galectin-7-siRNA suppresses
the TGF-β/Smad signaling pathway
To further explore the downstream mechanism of the
combined treatment group in MI, the expression levels of
TGF-β/Smads pathway-related proteins were detected in the rat
myocardium. The mRNA and protein levels of TGF-β1 and Smad3 in rats
with AMI significantly increased, whereas the mRNA and protein
levels of Smad7 decreased compared with the sham-operated rats. In
rats treated with UTMD-mediated Galectin-7-siRNA, the levels of
TGF-β1 and Smad3 in myocardial tissue homogenate were significantly
reduced, and the mRNA and protein levels of Smad7 significantly
increased, compared with AMI rats (all P<0.05; Fig. 5A-C). Overall, these results
suggested that the combined treatment group relieved AMI via
inhibiting the TGF-β/Smads pathway.
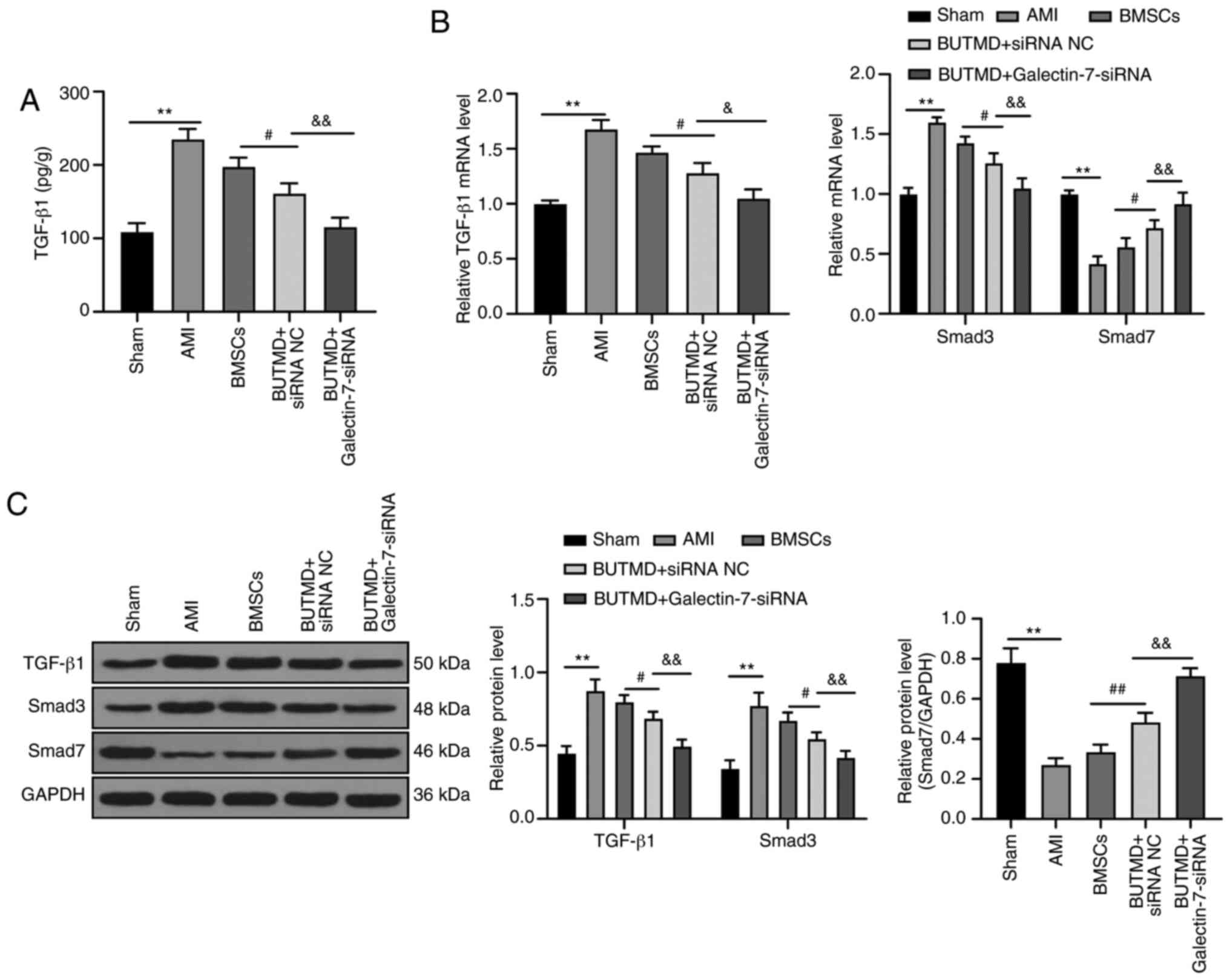 | Figure 5UTMD-mediated Galectin-7-siRNA
suppresses the TGF-β/Smads signaling pathway. (A) Detection of
TGF-β1 in myocardial tissue homogenate using ELISA. (B) Reverse
transcription-quantitative PCR was performed to detect mRNA
expression levels of TGF-β1, Smad3 and Smad7. (C) Western blot-ting
was conducted to detect the protein expression levels of TGF-β1,
Smad3 and Smad7 (n=6/group). Data in panels A, B (left) and C
(right) were analyzed using one-way ANOVA, and data in panels B
(right) and C (left) were analyzed using two-way ANOVA, followed by
Tukey's multiple comparisons test. **P<0.01;
#P<0.05 and ##P<0.01;
&P<0.05 and &&P<0.01. UTMD,
ultrasound-targeted microbubble destruction; TGF-β1, transforming
growth factor-β1; siRNA, small interfering RNA; ELISA,
enzyme-linked immunosorbent assay; AMI, acute myocardial
infarction; BMSC, bone marrow mesenchymal stem cell; NC, negative
control; BUTMD, BMSCs + UTMD. |
Discussion
Stem cell transplantation is an effective method of
treating MI (27). However,
several limitations have been found in previous methods of in
vitro transplantation of stem cells (28). Besides, the homing of exogenous
BMSCs does not reach the infarcted areas efficiently, which limits
their ability to improve cardiac function (29). Therefore, the present study was
conducted to elucidate the synergistic effect of UTMD-mediated
Galectin-7-siRNA with the homing of BMSCs for AMI.
One of the most significant findings of the present
study was that following BMSC transplantation, AMI rats exhibited
improved cardiac function and left ventricular hemodynamics-related
indices, and reduced myocardial fiber damage, myocardial infarct
size and apoptotic cells. BMSCs are useful in the treatment of
various diseases and injuries, including intervertebral disc
degeneration and diabetic nephropathy, due to their multipotent
differentiation and immunomodulatory functions (30,31). SDF-1 and its specific receptor
C-X-C chemokine receptor type 4 (CXCR-4) have been reported to play
significant roles in adhesion, migration and survival of BMSCs to
targeted tissues (32,33). Strategies to increase the homing
of infused stem cells consist of modifying SDF-1 levels and CXCR-4
receptor levels in host tissues (34). The homing process of BMSCs
involves attachment to vascular endothelial cells, chemotactic
response by interactions between the chemokine-chemokine receptor,
as well as transendothelial migration into the parenchyma (35,36). Meanwhile, it has been revealed
that inflammatory cytokines and certain adhesion molecules,
including P-selecin and VCAM-1, are also essential components
implicated in the migration of stem cells to the inflamed sites
(37-39).
To the best of our knowledge, relatively little is
known concerning the role of Galectin-7 in AMI at present, and the
present study was the first to show that AMI rats treated with
UTMD-mediated Galectin-7-siRNA exhibited improved cardiac function
and left ventricular hemodynamics-related indices, as well as
decreased myocardial fiber damage, myocardial infarct size and
apoptotic cells. UTMD could be a potential tailored therapy used to
induce cardiac regeneration following an extensive infarct, which
is able to deliver repeated regimens until ventricular function and
myocardial perfusion have been restored (40). A previous study demonstrated that
systemic administration of UTMD can improve the targeted gene
transfer to the heart (41).
Furthermore, increasing evidence has indicated that UTMD increases
the migration of BMSCs to the injured kidney and infarcted
myocardium (42,43). In addition, UTMD has also been
found to result in microvascular rupture in target organs, leading
to the enhancement of certain cytokines, and elevated BMSC
migration into the heart and kidney (44-46). It has been reported that
overexpression of CXCR-4 combined with UTMD could significantly
augment the homing ability of BMSCs (43). Another study revealed that UTMD
combined with other non-viral vectors could improve gene
transfection efficiency and protect the multi-directional
differentiation and reproductive ability of the transfected BMSCs
(47). Some articles have
elucidated that SDF-1 and VCAM-1 expression is implicated in the
liver homing of BMSCs (48-50). Recently, it has been revealed that
increased VCAM-1 expression indicates that UTMD may induce BMSC
migration by enhancing interstitial and intercellular capillary
permeability (51). Furthermore,
when the UTMD procedure is performed following a pre-injection of
BMSCs, or when UTMD is combined with BMSC transplantation, there is
a beneficial effect on the engraftment of cells (52,53). Ultrasound microbubbles can enhance
the therapeutic effect of BMSCs in diseases and promote BMSC homing
(51,54,55). In the present study, the BUTMD +
siRNA NC group had an intermediate therapeutic effect. The present
study contributes towards our knowledge of the ability of
Galectin-7 in AMI or the combination of Galectin-7 with UTMD in MI,
however this topic deserves further in-depth exploration in order
to be applied to the clinic. Galectin-3, another member of Galectin
family, is mainly secreted by activated macrophages, which has been
established as a prognostic target in various heart failure cohorts
(56). Galectin-3 has been found
to be implicated in heart failure and predicts enhanced mortality
and morbidity in heart failure. Heart failure often develops after
MI, and contributes to worse outcomes (56). TGF-β/Smad is a critical pathway
that modulates damage-induced and programmed senescence (57). The TGF-β/Smads pathway may be
implicated in the reconstruction of MI scars through continuous
stimulation of matrix deposition (58). Xinfuli Granule has been
demonstrated to ameliorate ventricular remodeling and myocardial
fibrosis after MI in rats via modulation of the TGF-β/Smad pathway
(59). Moreover, a previous study
revealed that Galectin-7 can inhibit the TGF-β/Smad3 pathway to
promote the proliferation of activated CD4+ T cells
(19). BMSCs can improve spinal
cord function in rats with spinal cord injury via the TGF-β/Smads
pathway (60). The present study
showed that the levels of TGF-β1 and Smad3 in myocardial tissue
homogenate were reduced in rats treated with UTMD-mediated
Galectin-7-siRNA. Thus, these results suggested that UTMD-mediated
Galectin-7-siRNA treatment could improve AMI in rat via inhibiting
the TGF-β/Smads pathway.
In summary, the present study suggested that
UTMD-mediated Galectin-7-siRNA treatment enhanced the homing
ability of BMSCs, improved myocardial microenvironment, and
inhibited the TGF-β/Smads signaling pathway to alleviate AMI in
rats. The advancement of BMSC homing ability is vital for effective
clinical application, however further studies are needed to
evaluate its safety and efficacy. It is often difficult to achieve
the desired therapeutic effect with siRNA treatment alone as siRNA
is prone to degrade in vivo, so microbubbles are used to
protect the structure of siRNA. UTMD can promote siRNA targeting
into pathological tissues and thus play a role in gene
intervention. Therefore, there was not an siRNA group used in this
study. In the future, relevant research will be performed to verify
the effect of UTMD Galectin-7 siRNA treatment alone in order to
improve the reliability of these conclusions.
Funding
This study was supported by the Youth Fund in
Guizhou Provincial People's Hospital [grant no. GZSYQN2018(02)] and
the Clinical Research Center Project of Department of Science and
Technology of Guizhou Province [grant no. (2017)5405].
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XW, JT, HZ are the guarantors of integrity of the
entire study. JT and HZ contributed to the conception and design of
the present study, and definition of intellectual content. XW and
WZ contributed to the literature research. XW, YZ and WZ performed
experimental studies and data acquisition. XW and YZ contributed to
the analysis of data and prepared the manu-script. XW performed the
statistical analyses. XW, YZ and JT contributed to the manuscript
editing. JT and HZ contributed to the manuscript review. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were performed in accordance with
the Guide to the Management and Use of Laboratory Animals (22). The animal experiments were
approved by the Institutional Animal Care and Use Committee of
People's Hospital of Deyang City (approval no. 20190075; Deyang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ghartavol MM, Gholizadeh-Ghaleh S, Babaei
G, Farjah GH and Ansari MH: The protective impact of betaine on the
tissue structure and renal function in isoproterenol-induced
myocardial infarction in rat. Mol Genet Genomic Med. 7:e005792019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang S, Fan T, Hu Q, Xu W, Yang J, Xu C,
Zhang B, Chen J and Jiang H: Downregulation of microRNA-17-5p
improves cardiac function after myocardial infarction via
attenuation of apop-tosis in endothelial cells. Mol Genet Genomics.
293:883–894. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang J, Brown ME, Zhang H, Martinez M,
Zhao Z, Bhutani S, Yin S, Trac D, Xi JJ and Davis ME:
High-Throughput screening identifies microRNAs that target nox2 and
improve function after acute myocardial infarction. Am J Physiol
Heart Circ Physiol. 312:H1002–H1012. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu C, Wang X, Ha T, Hu Y, Liu L, Zhang X,
Yu H, Miao J, Kao R, Kalbfleisch J, et al: Attenuation of cardiac
dysfunction and remodeling of myocardial infarction by
microRNA-130a are mediated by suppression of PTEN and activation of
PI3K dependent signaling. J Mol Cell Cardiol. 89:87–97. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vignoli A, Tenori L, Giusti B, Takis PG,
Valente S, Carrabba N, Balzi D, Barchielli A, Marchionni N, Gensini
GF, et al: NMR-Based metabolomics identifies patients at high risk
of death within two years after acute myocardial infarction in the
AMI-Florence II cohort. BMC Med. 17:32019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He JG, Li HR, Li BB, Xie QL, Yan D and
Wang XJ: Bone marrow mesenchymal stem cells overexpressing GATA-4
improve cardiac function following myocardial infarction.
Perfusion. 34:696–704. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S and Grayburn PA:
Ultrasound-Targeted microbubble destruction for cardiac gene
delivery. Methods Mol Biol. 1521:205–218. 2017. View Article : Google Scholar
|
|
8
|
Jayasankar V, Woo YJ, Bish LT, Pirolli TJ,
Chatterjee S, Berry MF, Burdick J, Gardner TJ and Sweeney HL: Gene
transfer of hepatocyte growth factor attenuates postinfarction
heart failure. Circulation. 108:II230–II236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yau TM, Fung K, Weisel RD, Fujii T, Mickle
DA and Li RK: Enhanced myocardial angiogenesis by gene transfer
with trans-planted cells. Circulation. 104:I218–I222. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun W, Li Z, Zhou X, Yang G and Yuan L:
Efficient exosome delivery in refractory tissues assisted by
ultrasound-targeted microbubble destruction. Drug Deliv. 26:45–50.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujii H, Sun Z, Li SH, Wu J, Fazel S,
Weisel RD, Rakowski H, Lindner J and Li RK: Ultrasound-Targeted
gene delivery induces angiogenesis after a myocardial infarction in
mice. JACC Cardiovasc Imaging. 2:869–879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hernot S, Cosyns B, Droogmans S, Garbar C,
Couck P, Vanhove C, Caveliers V, Van Camp G, Bossuyt A and Lahoutte
T: Effect of high-intensity ultrasound-targeted micro-bubble
destruction on perfusion and function of the rat heart assessed by
pinhole-gated SPECT. Ultrasound Med Biol. 36:158–165. 2010.
View Article : Google Scholar
|
|
13
|
Karetnikova V, Osokina A, Gruzdeva O,
Uchasova E, Zykov M, Kalaeva V, Kashtalap V, Shafranskaya K,
Hryachkova O and Barbarash O: Serum galectin and renal dysfunction
in ST-segment elevation myocardial infarction. Dis Markers.
2016:15490632016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Advedissian T, Deshayes F and Viguier M:
Galectin-7 in epithelial homeostasis and carcinomas. Int J Mol Sci.
18:2017.PubMed/NCBI
|
|
15
|
Saussez S and Kiss R: Galectin-7. Cell Mol
Life Sci. 63:686–697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen HL, Chiang PC, Lo CH, Lo YH, Hsu DK,
Chen HY and Liu FT: Galectin-7 regulates keratinocyte proliferation
and differentiation through JNK-miR-203-p63 signaling. J Invest
Dermatol. 136:182–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-Salam S and Hashmi S: Galectin-1 in
early acute myocardial infarction. PLoS One. 9:e869942014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shirakawa K, Endo J, Kataoka M, Katsumata
Y, Yoshida N, Yamamoto T, Isobe S, Moriyama H, Goto S, Kitakata H,
et al: IL (Interleukin)-10-STAT3-Galectin-3 axis is essential for
osteopontin-producing reparative macrophage polarization after
myocardial infarction. Circulation. 138:2021–2035. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo Z, Ji Y, Tian D, Zhang Y, Chang S,
Yang C, Zhou H and Chen ZK: Galectin-7 promotes proliferation and
Th1/2 cells polarization toward Th1 in activated CD4+ T cells by
inhib-iting The TGFβ/Smad3 pathway. Mol Immunol. 101:80–85. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Villeneuve C, Baricault L, Canelle L,
Barboule N, Racca C, Monsarrat B, Magnaldo T and Larminat F:
Mitochondrial proteomic approach reveals galectin-7 as a novel
BCL-2 binding protein in human cells. Mol Biol Cell. 22:999–1013.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tran BH, Yu Y, Chang L, Tan B, Jia W,
Xiong Y, Dai T, Zhong R, Zhang W, Le VM, et al: A novel liposomal
S-propargyl-cysteine: A sustained release of hydrogen sulfide
reducing myocardial fibrosis via TGF-beta1/smad Pathway. Int J
Nanomedicine. 14:10061–10077. 2019. View Article : Google Scholar
|
|
22
|
Guide for the Care and use of Laboratory
Animals: National Research Council (US) Committee for the Update of
the Guide for the Care and Use of Laboratory Animals. 8th edition.
National Academies Press; Washington, DC: 2011
|
|
23
|
Lu D, Liao Y, Zhu SH, Chen QC, Xie DM,
Liao JJ, Feng X, Jiang MH and He W: Bone-Derived nestin-positive
mesenchymal stem cells improve cardiac function via recruiting
cardiac endothelial cells after myocardial infarction. Stem Cell
Res Ther. 10:1272019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olivetti G, Capasso JM, Meggs LG,
Sonnenblick EH and Anversa P: Cellular basis of chronic ventricular
remodeling after myocardial infarction in rats. Circ Res.
68:856–869. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zatroch KK, Knight CG, Reimer JN and Pang
DS: Refinement of intraperitoneal injection of sodium pentobarbital
for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet Res.
13:602017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Nguyen PK, Rhee JW and Wu JC: Adult stem
cell therapy and heart failure, 2000 to 2016: A systematic review.
JAMA Cardiol. 1:831–841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Z, Zeng C and Wang WE: Progress of
stem cell transplantation for treating myocardial infarction. Curr
Stem Cell Res Ther. 12:624–636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mangi AA, Noiseux N, Kong D, He H, Rezvani
M, Ingwall JS and Dzau VJ: Mesenchymal stem cells modified with akt
prevent remodeling and restore performance of infarcted hearts. Nat
Med. 9:1195–1201. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leung VY, Aladin DM, Lv F, Tam V, Sun Y,
Lau RY, Hung SC, Ngan AH, Tang B, Lim CT, et al: Mesenchymal stem
cells reduce intervertebral disc fibrosis and facilitate repair.
Stem Cells. 32:2164–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li D, Wang N, Zhang L, Hanyu Z, Xueyuan B,
Fu B, Shaoyuan C, Zhang W, Xuefeng S, Li R and Chen X: Mesenchymal
stem cells protect podocytes from apoptosis induced by high glucose
via secretion of epithelial growth factor. Stem Cell Res Ther.
4:1032013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Liu S, Li Y, Wang X, Xue W, Ge G
and Luo X: The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic
effects of hypoxia-preconditioned mesenchymal stem cells for renal
ischemia/reperfusion injury. PLoS One. 7:e346082012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ryu CH, Park SA, Kim SM, Lim JY, Jeong CH,
Jun JA, Oh JH, Park SH, Oh WI and Jeun SS: Migration of human
umbilical cord blood mesenchymal stem cells mediated by stromal
cell-derived factor-1/CXCR4 axis via akt, ERK, and p38 signal
transduction pathways. Biochem Biophys Res Commun. 398:105–110.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gul-Uludag H, Xu P, Marquez-Curtis LA,
Xing J, Janowska-Wieczorek A and Chen J: Cationic liposome-mediated
CXCR4 gene delivery into hematopoietic stem/progenitor cells:
Implications for clinical transplantation and gene therapy. Stem
Cells Dev. 21:1587–1596. 2012. View Article : Google Scholar :
|
|
35
|
Deak E, Seifried E and Henschler R: Homing
pathways of mesenchymal stromal cells (MSCs) and their role in
clinical applications. Int Rev Immunol. 29:514–529. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karp JM and Teo GS: Mesenchymal stem cell
homing: The devil is in the details. Cell Stem Cell. 4:206–216.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chamberlain G, Wright K, Rot A, Ashton B
and Middleton J: Murine mesenchymal stem cells exhibit a restricted
repertoire of functional chemokine receptors: Comparison with
human. PLoS One. 3:e29342008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li L and Jiang J: Regulatory factors of
mesenchymal stem cell migration into injured tissues and their
signal transduction mechanisms. Front Med. 5:33–39. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lotfinegad P, Shamsasenjan K,
Movassaghpour A, Majidi J and Baradaran B: Immunomodulatory nature
and site specific affinity of mesenchymal stem cells: A hope in
cell therapy. Adv Pharm Bull. 4:5–13. 2014.PubMed/NCBI
|
|
40
|
Fujii H, Li SH, Wu J, Miyagi Y, Yau TM,
Rakowski H, Egashira K, Guo J, Weisel RD and Li RK: Repeated and
targeted transfer of angiogenic plasmids into the infarcted rat
heart via ultrasound targeted microbubble destruction enhances
cardiac repair. Eur Heart J. 32:2075–2084. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang SL, Mu YM, Tang KQ, Jiang XK, Bai WK,
Shen E and Hu B: Enhancement of recombinant adeno-associated virus
mediated transgene expression by targeted echo-contrast agent.
Genet Mol Res. 12:1318–1326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Imada T, Tatsumi T, Mori Y, Nishiue T,
Yoshida M, Masaki H, Okigaki M, Kojima H, Nozawa Y, Nishiwaki Y, et
al: Targeted delivery of bone marrow mononuclear cells by
ultrasound destruction of microbubbles induces both angiogenesis
and arteriogenesis response. Arterioscler Thromb Vasc Biol.
25:2128–2134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang G, Zhang Q, Zhuo Z, Wu S, Xu Y, Zou
L, Gan L, Tan K, Xia H, Liu Z and Gao Y: Enhanced homing of CXCR-4
modified bone marrow-derived mesenchymal stem cells to acute kidney
injury tissues by micro-bubble-mediated ultrasound exposure.
Ultrasound Med Biol. 42:539–548. 2016. View Article : Google Scholar
|
|
44
|
Enomoto S, Yoshiyama M, Omura T, Matsumoto
R, Kusuyama T, Nishiya D, Izumi Y, Akioka K, Iwao H, Takeuchi K and
Yoshikawa J: Microbubble destruction with ultrasound augments
neovascularisation by bone marrow cell transplantation in rat hind
limb ischaemia. Heart. 92:515–520. 2006. View Article : Google Scholar
|
|
45
|
Wu S, Li L, Wang G, Shen W, Xu Y, Liu Z,
Zhuo Z, Xia H, Gao Y and Tan K: Ultrasound-Targeted stromal
cell-derived factor-1-loaded microbubble destruction promotes
mesenchymal stem cell homing to kidneys in diabetic nephropathy
rats. Int J Nanomedicine. 9:5639–5651. 2014.PubMed/NCBI
|
|
46
|
Zhong S, Shu S, Wang Z, Luo J, Zhong W,
Ran H, Zheng Y, Yin Y and Ling Z: Enhanced homing of mesenchymal
stem cells to the ischemic myocardium by ultrasound-targeted
microbubble destruction. Ultrasonics. 52:281–286. 2012. View Article : Google Scholar
|
|
47
|
Li P, Gao Y, Liu Z, Tan K, Zuo Z, Xia H,
Yang D, Zhang Y and Lu D: DNA transfection of bone marrow stromal
cells using microbubble-mediated ultrasound and polyethylenimine:
An in vitro study. Cell Biochem Biophys. 66:775–786. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Belema-Bedada F, Uchida S, Martire A,
Kostin S and Braun T: Efficient homing of multipotent adult
mesenchymal stem cells depends on FROUNT-mediated clustering of
CCR2. Cell Stem Cell. 2:566–575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kuo TK, Hung SP, Chuang CH, Chen CT, Shih
YR, Fang SC, Yang VW and Lee OK: Stem cell therapy for liver
disease: Parameters governing the success of using bone marrow
mesen-chymal stem cells. Gastroenterology. 134:2111–2121. 2008.
View Article : Google Scholar
|
|
50
|
Togel FE and Westenfelder C: Role of SDF-1
as a regulatory chemokine in renal regeneration after acute kidney
injury. Kidney Int Suppl. 2011:87–89. 2011. View Article : Google Scholar
|
|
51
|
Sun T, Gao F, Li X, Cai Y, Bai M, Li F and
Du L: A combination of ultrasound-targeted microbubble destruction
with transplantation of bone marrow mesenchymal stem cells promotes
recovery of acute liver injury. Stem Cell Res Ther. 9:3562018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li L, Wu S, Li P, Zhuo L, Gao Y and Xu Y:
Hypoxic precondi-tioning combined with microbubble-mediated
ultrasound effect on MSCs promote SDF-1/CXCR4 expression and its
migration ability: An in vitro study. Cell Biochem Biophys.
73:749–757. 2015. View Article : Google Scholar
|
|
53
|
Zhang Y, Ye C, Wang G, Gao Y, Tan K, Zhuo
Z, Liu Z, Xia H, Yang D and Li P: Kidney-targeted transplantation
of mesenchymal stem cells by ultrasound-targeted microbubble
destruction promotes kidney repair in diabetic nephropathy rats.
Biomed Res Int. 2013:5263672013.PubMed/NCBI
|
|
54
|
Wang G, Zhang Q, Zhuo Z, Wu S, Liu Z, Xia
H, Tan K, Zou L, Gan L and Gao Y: Effects of diagnostic
ultrasound-targeted microbubble destruction on the homing ability
of bone marrow stromal cells to the kidney parenchyma. Eur Radiol.
26:3006–3016. 2016. View Article : Google Scholar
|
|
55
|
Qian J, Wang L, Li Q, Sha D, Wang J, Zhang
J, Xu P and Fan G: Ultrasound-Targeted microbubble enhances
migration and therapeutic efficacy of marrow mesenchymal stem cell
on rat middle cerebral artery occlusion stroke model. J Cell
Biochem. 120:3315–3322. 2019. View Article : Google Scholar
|
|
56
|
Szadkowska I, Wlazeł RN, Migała M,
Szadkowski K, Zielińska M, Paradowski M and Pawlicki L: The
association between galectin-3 and clinical parameters in patients
with first acute myocardial infarction treated with primary
percutaneous coronary angioplasty. Cardiol J. 20:577–582. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lyu G, Guan Y, Zhang C, Zong L, Sun L,
Huang X, Huang L, Zhang L, Tian XL, Zhou Z and Tao W: TGF-Beta
signaling alters H4K20me3 status via miR-29 and contributes to
cellular senescence and cardiac aging. Nat Commun. 9:25602018.
View Article : Google Scholar
|
|
58
|
Hao J, Ju H, Zhao S, Junaid A, Scammell-La
Fleur T and Dixon IM: Elevation of expression of smads 2, 3, and 4,
decorin and TGF-beta in the chronic phase of myocardial infarct
scar healing. J Mol Cell Cardiol. 31:667–678. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ma J, Li ZY, Liang XP, Guo CX, Lu PP and
Ma LH: Xinfuli granule improves post-myocardial infarction
ventricular remodeling and myocardial fibrosis in rats by
regulating TGF-β/smads signaling pathway. J Geriatr Cardiol.
14:301–307. 2017.PubMed/NCBI
|
|
60
|
Lv C, Zhang T, Li K and Gao K: Bone marrow
mesenchymal stem cells improve spinal function of spinal cord
injury in rats via TGF-β/smads signaling pathway. Exp Ther Med.
19:3657–3663. 2020.PubMed/NCBI
|



















