Osteoporosis is a systemic skeletal disorder with a
globally high incidence that is exacerbated by the problem of an
aging population. Osteoporosis has three types, which are termed
primary osteoporosis, secondary osteoporosis and idiopathic
osteoporosis (5). The most common
type in older women is postmenopausal osteoporosis, which is a form
of primary osteoporosis. Subsequent fractures, particularly hip
fractures, seriously affect the survival prospects and life quality
of the elderly. Based on predictions of the Asian Federation of
Osteoporosis Societies, the total number of hip fractures are due
to reach 2.56 million by 2050 in the studied Asian countries
(6). Calcium supplements are the
most well-known non-prescription therapy for strengthening bone
mineral density and preventing osteoporosis. However, the calcium
paradox, a consequence of damaged calcium metabolism, is identified
as the loss of calcium in the bones parallel with the formation of
calcification in the arteries in the elderly (7), and exists as a potential risk of
calcium supplements. Evidence has accumulated that vitamin K can be
of benefit in avoiding the calcium paradox. In addition, VKDPs,
such as osteocalcin (OC), indicate a beneficial effect on bone
strength loss.
Cardiovascular diseases (CVDs), such as acute
myocardial infarction, atherosclerosis and heart failure, are the
main cause of human deaths worldwide. These diseases, not only pose
a great threat to patients' health, but also disturb their families
and even society. An epidemiological study of 709 multiethnic
adults, with follow-up at an average of 11.0 years, showed VKDP
activity is associated with the incidence of ischemic
cardiovascular events (8). The
relationship between vascular calcification and disease has become
a research focus due to the increasing rates of morbidity and
mortality of CVDs. An epidemiological study of 116,309 individuals,
with follow-up at an average of 28 years, indicated an aortic arch
calcification exhibited a positive correlation with an increased
risk of coronary heart disease (9). Moreover, another epidemiological
study showed coronary artery calcium was independently associated
with cardiac events (10). The
matrix Gla protein (MGP), a kind of VKDP, synthesized by vascular
smooth muscle cells (VSMCs) is widely expressed in soft tissues,
such as cartilages and blood vessels (11), especially in calcified tissues. It
has been suggested that MGP regulates vascular calcification and
various important pathological processes. In fact, many emerging
proteins related to vitamin K are involved in the fight against
vascular calcification and are described in more detail below.
Kidney disease poses a great threat to health.
According to the course duration of the disease, the disease can be
classified as acute kidney disease or chronic kidney disease (CKD).
Various causes have been aligned closely with CKD. To be specific,
diabetes and hypertension are the two main contributing factors of
CKD in developed countries. However, glomerular diseases still
occupy an important position in developing countries. Sub-clinical
vitamin K deficiency exists in most CKD patients, with the
characteristic of low circulating vitamin K level and high inactive
VKDP level (12-14). The factors that contribute to this
situation include low vitamin K intake and reduction in the
carboxylation process of VKDPs (15). In addition, cardiovascular
complications are the main reason for the mortality of CKD patients
(16). The protective effect of
some VKDPs, such as MGP, on both the kidney and cardiovascular
system, has been widely explored.
Numerous studies are available on OC and MGP. The
aim of the current review is to focus on three emerging VKDPs that
are increasingly being studied: Growth arrest-specific protein 6
(Gas6), Gla-rich protein (GRP), and periostin and their roles in
various physiological and pathological processes.
At present, scholars have identified 17 types of
VKDPs in humans. Seven of them are dependent on vitamin
K1 to play their roles in the liver (coagulation factor
II, VII, IX, X and anticoagulant proteins C, S, Z). Six of them
were modified by vitamin K after transcription and were involved in
various physiological and pathological processes in extrahepatic
tissues. They are OC, MGP, Gas6, GRP, periostin and
periostin-like-factor. The remaining four proteins need further
study (proline-rich Gla protein 1, proline-rich Gla protein 2,
transmembrane Gla protein 3 and transmembrane Gla protein 4)
(Table I).
OC was the first VKDP to be identified that is
synthesized and secreted by bones. Originally, researchers found
osteocalcin has the ability to attract calcium ions. Vitamin K
lowers serum undercarboxylated OC (ucOC) concentrations and
increases carboxylated OC (cOC). Furthermore, cOC can bind with
hydroxyapatite crystals, the material of the bone matrix, while
simultaneously promoting bone mineral density (41,73). Moreover, it has been suggested
during the past decade that OC shows functions of regulating
systemic glucose and energy metabolism (42).
MGP plays a beneficial role in vascular
calcification and various pathological processes. MGP regulates
vascular calcification by eliminating the calcification effect of
bone morphogenetic protein (BMP)-2 and BMP-4 (46,47). Additionally, the MGP-fetal-A
complex inhibits ectopic mineralization by binding to alkaline
calcium phosphate crystals (47).
Based on these mechanisms, MGP is related to the prevention of
cardiovascular and chronic kidney disease. In a previous review, we
presented a new viewpoint, namely, that osteophyma may be caused by
the accumulation of uncarboxylated MGP, in which vitamin K is
required by the carboxylation process (43).
In recent years, a number of emerging VKDPs, such as
Gas6, GRP, and periostin, have been considered to participate in
multifarious physiological and pathological processes.
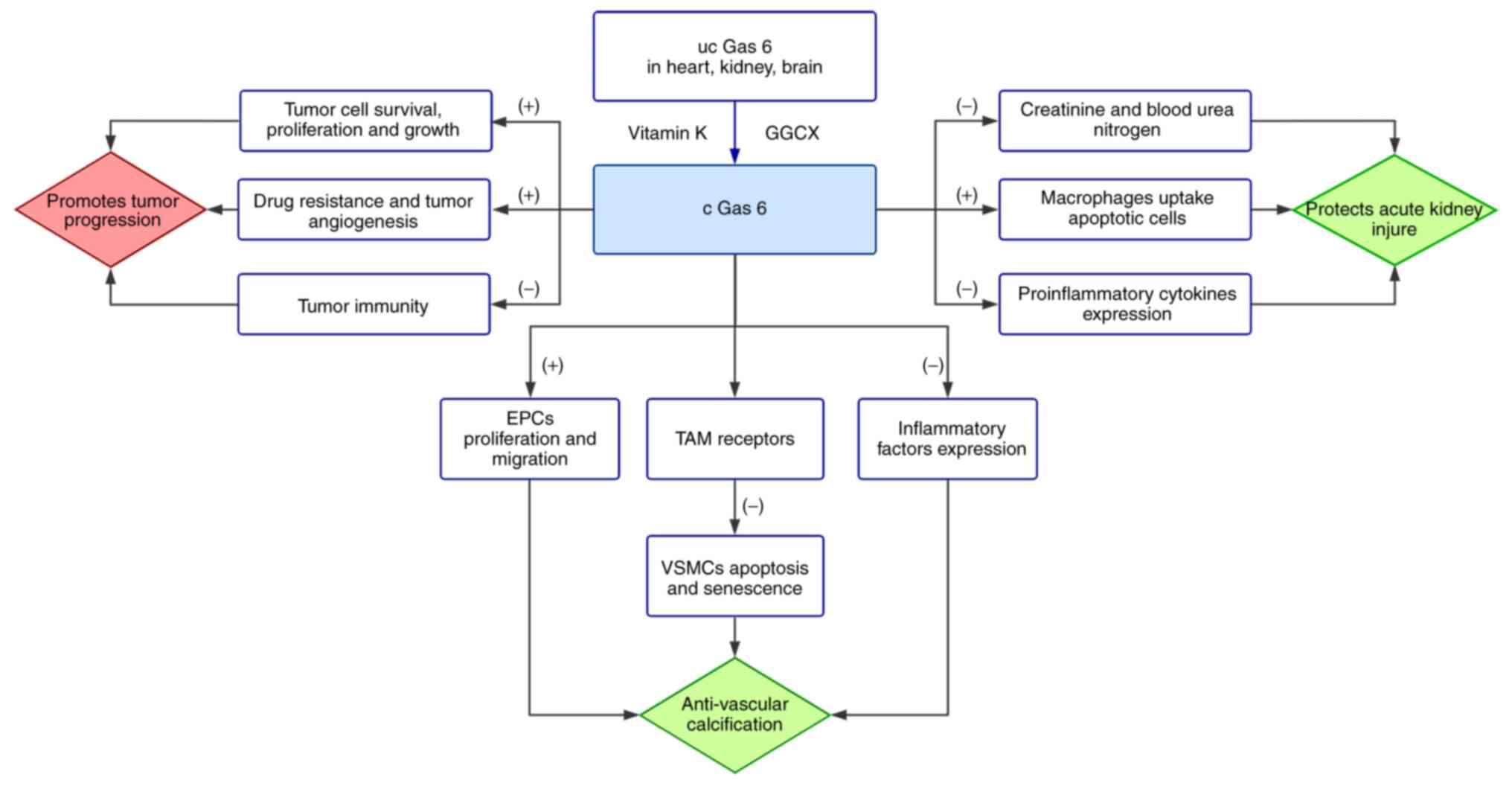
Gas 6, weighing 75 kDa, is a relatively large member
of the VKDP family. The concentration of plasma Gas 6 ranges from
approximately 2.5 to 18.8 µg/l in healthy adults (74). Gas 6 is highly homologous with
Protein S and carries an N-terminal Gla domain after vitamin K
carboxylation. Gas 6 is widely expressed in brain, heart, lung,
kidney and other tissues, with the exception of the liver (43). In 1995, Gas 6 was reported as the
endogenous ligand for the TAM family for the first time (75). TAM is the acronym for three
receptors: Tyro3, Axl and Mer. Among these, Axl has the highest
affinity to Gas 6 (76). It has
been reported that the laminin-like globular domain of Gas 6 at
C-terminus appears to be the binding site of TAM receptors
(75). However, after warfarin
inhibits vitamin K-dependent carboxylation, inactivated Gas6, not
only completely inhibits the autophosphorylation of the Axl
receptor, but also fails to bind to the Axl receptor in
vitro (77,78). Therefore, vitamin K-dependent
carboxylation is the key to the interaction between Gas 6 and TAM
receptor. In light of numerous previous studies, the binding of Gas
6 and its receptors activated downstream signaling, such as of
phosphatidylinositol 3-kinase (PI3K), extracellular signal
regulated kinase (ERK) and nuclear factor
kappa-light-chain-enhancer of activated B cells (NF-κB) pathways,
to adjust the processes of apoptosis, survival, proliferation,
migration and adhesion (48,79-82).
There is an inseparable relationship between Gas 6
and the cardiovascular system. The binding of Gas 6 and Axl limits
the apoptosis of VSMCs by activating Akt and PI3K (80). It is worth mentioning that vitamin
K2 can inhibit VSMC calcification and apoptosis by
restoring Gas6 expression and activating downstream signaling by
Axl, Akt and Bcl2 (49,50). Endothelial progenitor cells (EPCs)
are involved in the saving response to ischemic tissue through
forming new blood vessels or proliferation of pre-existing
vasculature. Autologous EPC transplantation therapy has been
indicated as safe and practical in chronic myocardial ischemia
(51). It has been identified
that Gas6 has the ability to stimulate EPC proliferation and
migration in vitro by activating the Akt signaling pathway
(48). The finding provides a
basis for the further therapy of vascular re-endothelialization.
Vascular aging, a risk factor of CVDs, is characterized by vascular
stiffness, vascular remodeling and endothelial dysfunction
(83). Aging vessels provide a
good environment for CVDs. Gas6/Axl can delay cell cycle arrest,
which is a key cause in the development of VSMC senescence and
promotes their transition from the G1 to the S phase. The
PI3K/Akt/Forkhead box O (FoxO) signaling pathway is considered the
major target of Gas6/Axl signaling in VSMC senescence, with FoxO
being the key factor (84).
Furthermore, clinical investigation has demonstrated that Gas 6
plasma levels at admission reflect the existence of potential
cardiovascular risks and can prognosticate cardiovascular events
(52).
Accumulating evidence has indicated that Gas 6 is
significantly secreted by VSMCs in human atherosclerotic plaques,
but there is no secretion in healthy blood vessels. The
anti-inflammatory cytokine transforming growth factor β (TGF-β)
induces the secretion of Gas 6 in VSMCs, and then, stimulated by
Gas 6, the VSMCs suppress the expression of inflammatory factors,
such as tumor necrosis factor (TNF) α and intracellular adhesion
molecule (ICAM)-1 (85). Thus,
Gas 6 acts as a protective factor in human atherosclerosis. Of
note, Gas6 levels inversely related to complexity and stability in
patients with carotid atherosclerotic plaques (85,86). In particular, it should be noted
that Gas6-deficient mice show more stable atherosclerotic lesions
than normal mice, and inhibition of Gas 6 is considered to be
beneficial to plaque stabilization (87). The contradictory feature between
humans and mice is associated with obvious species physiological
differences (88).
Of note, overexpression of Gas 6 has a detrimental
effect on some pathological processes. The
renin-angiotensin-aldosterone system is closely connected with
cardiovascular and renal inflammation and fibrosis. It has been
emphasized that Gas 6 deficiency prevents the damage of aldosterone
on target organs (89). In
addition, cardiomyocyte-specific Gas 6 overexpression hastens the
deterioration of pathological cardiac hypertrophy, mainly due to
the activation of mitogen-activated protein kinase (MAPK) kinase
1/2-ERK 1/2 signaling (90).
The contribution of Gas 6 to acute kidney injury is
closely related to its biological functions, such as
anti-inflammation and immunoregulation (91,92). The kidney, despite being a rich
blood supplying organ, is susceptible to hypoxic injury due to the
complex balance of renal blood flow, glomerular filtration rate,
oxygen consumption and arteriovenous oxygen shunting (93). Previous findings suggested that
Gas 6 protected against renal ischemia-reperfusion injury in a
mouse model (94). To be
specific, with the assistance of Gas 6 treatment, creatinine and
blood urea nitrogen decreased by 29 and 27%, respectively. Cell
apoptosis was significantly decreased, attributable to Gas 6
enhancing macrophages to uptake apoptotic cells (95). Furthermore, the expression of
pro-inflammatory cytokines, such as interleukin (IL)-1β and TNF-α,
was markedly reduced by another Gas 6 function, dampening the
inflammatory responses (11,91,94). Similarly, concentration of Gas 6
rose in sepsis-induced acute kidney injury mice, and improved the
survival rate by reducing serum urea nitrogen, creatinine and renal
tissue apoptosis (53). In
addition, several reports demonstrated that Gas6 levels were
significantly increased in CKD patients and chronic hemodialysis
patients (96,97). Opinions regarding the potential
mechanisms vary. Researchers tend to associate the elevation with
endothelial function (Gas6 is expressed by endothelial cells) and
inflammation because pro-inflammatory cytokines are abundant in the
blood of these patients (96,98). It is reported that endothelial
cells in CKD are subjected to specific stress overtime which leads
to accelerated cardiovascular disease and high mortality (99). Disruption and inflammation of
glomerular capillaries influence the evolution of CKD, and,
consequently, elevated Gas 6 levels (100). It is worth noting that Gas 6 is
upregulated in many forms of inflammatory nephropathy, for example,
lupus nephritis and IgA nephropathy (101,102).
Diabetic nephropathy is a common complication of
diabetes that can further develop into end-stage renal disease.
There are opposing conclusions on the tendency of plasma Gas 6 in
diabetes and diabetic nephropathy. Nagai et al first
reported that the expression of both Gas6 and Axl was distinctly
increased in diabetic rats and proved Gas 6 can induce mesangial
cell hypertrophy, which further leads to glomerular hypertrophy in
the early stage of diabetic nephropathy (103). Furthermore, a reliable mechanism
was proposed in which high glucose stimulates mesangial cells,
followed by activating Gas6/Axl and the Akt/mTOR pathway, which
results in mesangial and glomerular hypertrophy (104). By contrast, Hung et al
indicated that plasma Gas6 levels in impaired glucose tolerance
patients and type 2 diabetes were significantly decreased (105). A study based on individuals with
different degrees of albuminuria offers some insight into this
controversy, and showed the blood level of Gas 6 decreased with the
deterioration of proteinuria (106). Silaghi et al formulated a
hypothesis that the interaction between molecular charge and weight
may participate in glomerular filtration of Gas 6 (100). More specifically, Gas 6 and
albumin (approximately 66 kDa) have a similar molecular weight and
a net negative charge repelled the glomerular membrane. Complex
interactions eventually lead Gas 6 to filter through the glomerular
membrane and be excreted from the body (100). Therefore, the concentration of
plasma Gas 6 changes in different stages of diabetes.
The contribution of Gas6 to cancer has been reported
for a large number of cancer types. For example, Gas 6 is
upregulated in breast cancer, melanoma and ovarian cancer (107-109). Tumor cells lack the competence
to produce Gas 6, but can educate infiltrating macrophages to
promote the production of Gas6 by producing IL-10 and macrophage
colony-stimulating factor (M-CSF) (110). Previous findings have shown the
pro-tumor effects of Gas6/TAM signaling. In the case of Gas 6
overexpression, the survival of myeloma cells was significantly
increased in vitro and, conversely, the deficiency of Gas 6
led to rapid cell death of myeloma (111). In addition, the autocrine Gas 6
assists the resistance of myeloma cells to bortezomib (111). Recently, the pro-tumor effects
of Gas 6 was also reported in lung cancer cells (54). In addition, blocking Gas 6/Mer
signaling with Mer receptor inhibitors significantly limits the
proliferation and growth of lung cancer cells (54). Interestingly, a high level
expression of Axl and its ligand Gas 6 were recognized in non-small
cell lung cancer patients, who acquired resistance with epidermal
growth factor receptor tyrosine kinase inhibitors (112). It has been reported that Gas 6
negatively regulates the proliferation and interferon-γ production
of natural killer cells to inhibit tumor immunity through binding
with Casitas B cell lymphoma-b/TAM receptors (113). In addition, Gas 6 prolongs VSMC
survival in the tumor microenvironment, which is requisite to tumor
angiogenesis (79). Several
investigations have indicated the roles of Gas 6 in predicting the
prognostic risk of cancer. Gas 6 protein as an independent
predictor always indicates a poor prognosis (107,109) (Fig. 1).
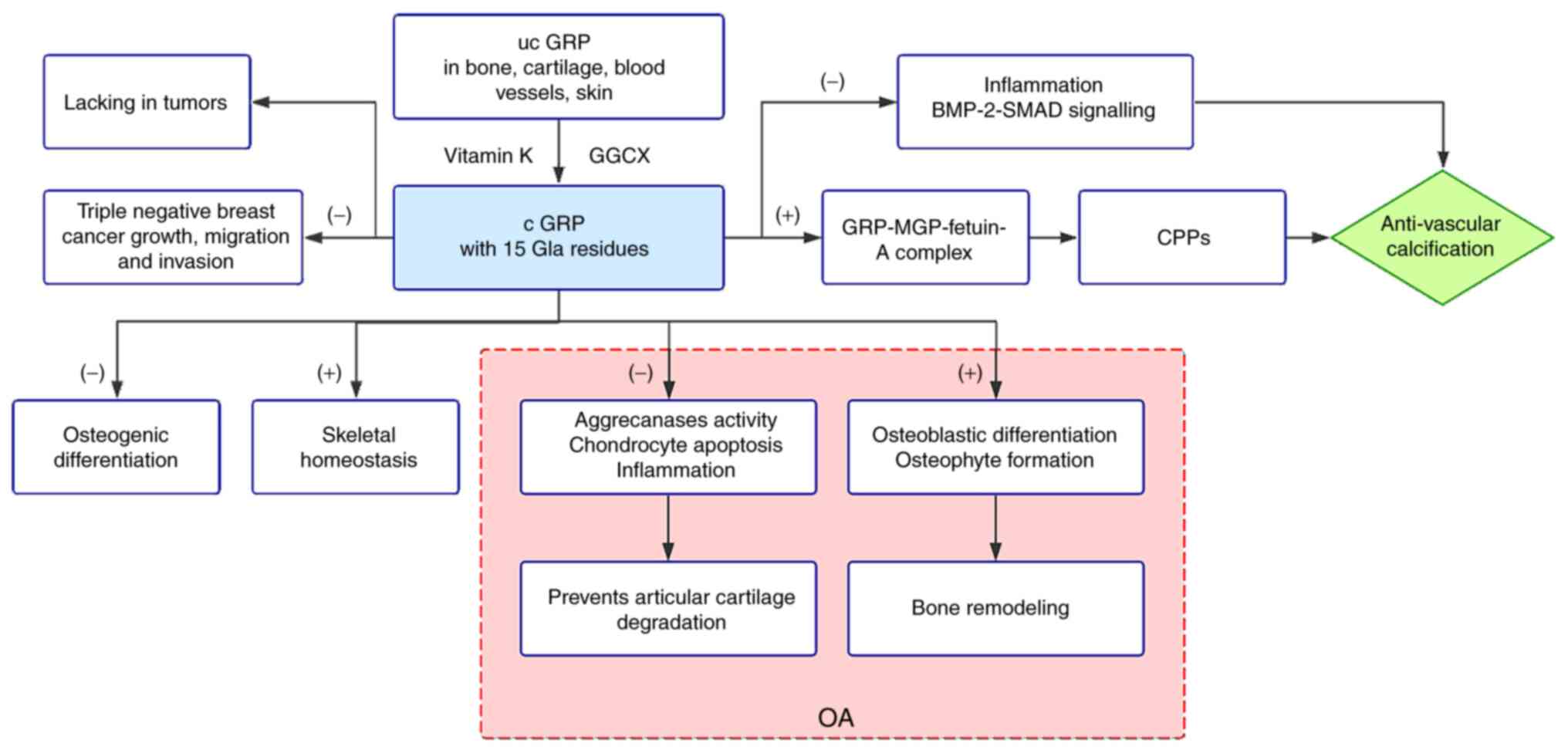
As its name suggests, GRP, which was first
identified in sturgeon cartilage, has abundant Gla residues (15 Gla
residues in human) (114,115).
With unusually high capacity to bind calcium through Gla resdues,
GRP accumulates in bone, cartilage and ectopic calcification, such
as blood vessels and skin (112). During physiological conditions,
GRP participates in the stabilization of cartilage matrix,
chondrogenesis and inhibition of osteogenesis (116-118). Recently, GRP has attracted
attention due to its crucial performance in combating ectopic
calcification.
The growth of long bones is inseparable from the
process of endochondral ossification. First, chondrocytes
participate through a combination of proliferation, extracellular
matrix secretion and hypertrophy. Then, hydroxyapatite crystals are
deposited in the extracellular matrix surrounding late hypertrophic
chondrocytes, known as mineralization. Next, chondrocyte death,
matrix degradation and contents invasion occur. Finally, the growth
plates close and the bones mature (119). Surmann-Schmitt et al
reported GRP in the upper zone of the growth plate, termed unique
cartilage matrix-associated protein, which exhibits a negative
correlation with osteogenic differentiation (116). Both GRP knockdown zebrafish and
warfarin-exposed zebrafish show irreversible growth retardation and
altered skeletal development; therefore Gla residues are necessary
for the function of GRP (117).
It is worth mentioning that a similar feature is found in human
warfarin embryopathy, which results in pregnant women from warfarin
therapy (120,121). Surprisingly, GRP is not
essential for mouse skeletal development (55). However, the fact that GRP is still
expressed in adult mouse cartilage indicates that GRP may
contribute to skeletal homeostasis and other
calcification-associated pathological processes after infancy.
Osteoarthritis (OA), a painful joint disease, is
characterized by articular cartilage degradation, bone remodeling,
tissue inflammation and abnormal extracellular matrix
mineralization. In fact, GRP plays a dual role in OA. GRP prevents
articular cartilage degradation in two practical ways. On the one
hand, GRP blocks the aggrecanase activity of A disintegrin and
metalloproteinase with thrombospondin motifs (ADAMTS)-4 and
ADAMTS-5 by physical interaction (56,57). Aggrecanolysis is considered the
main process of cartilage degradation, thus GRP protects cartilage
by increasing its resistance to aggrecan cleavage in OA. By
contrast, enhanced chondrocyte apoptosis accelerates the cartilage
damage in OA. It is reported that chondrocyte cell death is
markedly increased in GRP-deficient mice; thus, GRP protects
articular cartilage by reducing chondrocyte apoptosis (56). However, GRP has also been
implicated in bone remodeling, which is mediated with the altered
function and metabolism of osteoblasts and osteoclasts in OA
(122). Previous findings have
shown osteoblasts contribute to phenotypic changes and osteoclasts
are associated with cartilage destruction in OA (56,123). Additionally, GRP, as a
downstream gene of runt-related transcription factor 2 and Osterix,
stimulates osteoblast differentiation in OA (60). Similar results have been found in
mice, in which osteoblasts and osteoclasts decreased during
experimental OA in GRP-deficient mice, while there was no
fluctuation in normal mice (56).
The obvious conflict regarding the effect of GRP on osteoblastic
differentiation may be explained by post-translational modification
of GRP (56,60,116). Moreover, certain data have
indicated GRP promotes osteophyte formation in OA and the effect
occurs via bone remodeling rather than cartilage maturation
(57,60). In addition, inflammation presents
before the joint structure changes in OA joints (124). It has been demonstrated that GRP
has a similar inhibitory effect on calcification and inflammation
processes (58). Furthermore,
synovial fluid GRP levels in OA patients exhibit a positive
correlation with radiographic findings and symptomatic severity of
OA (125). However, it is
noteworthy that studies have shown that vitamin K deficiency is a
potential risk factor for knee OA (126). Due to the lack of effective
treatment and prevention methods currently, fully carboxylated GRP
by vitamin K supplementation is a convenient and inexpensive
candidate for the treatment of OA.
Vascular calcification is a pathological process
characterized by the deposition of calcium phosphate crystals in
vessel walls (127,128). According to the location of
calcification, it can be classified into intimal calcification
(related to plaque burden and luminal narrowing) and medial
calcification (associated with vessel stiffness and vascular
compliance decline) (128).
VSMCs are a contractile phenotype in the physiological state that
can regulate vascular tension. However, they lose expression of
contractility-related genes when vascular injury exists and are
further transformed into osteoblast-like cells (129). In addition, bone matrix
regulatory proteins, such as BMP-2, BMP-4, osteopontin, MGP and OC,
are expressed in calcifying vessels (130). In response to the high level of
extracellular calcium and the lack of calcification inhibitors,
VSMCs release extracellular vesicles (EVs) into circulation with
good mineralization capability and form a nucleation site for
hydroxyapatite (131,132). Fetuin-A, a 48 kDa protein, is
synthesized in the liver and secreted into circulation as a
powerful calcification inhibitor. Interestingly, fetuin-A is too
large to enter the collagen fibril where the mineral grows
(133). It is reported that the
mineral grows only inside the fibril when fetuin-A exists, whereas
it grows beyond the fibril without fetuin-A (134). Therefore, fetuin-A is the key
factor in determining the location of mineral growth. Moreover,
inflammatory activity takes part in early calcification. Many
studies have indicated a synergistic interaction between macrophage
and VSMC calcification. Activated macrophages produce a large
number of proteases to enhance the degradation of elastin and
collagen (124,135). Macrophages markedly increased
BMP-2 expression in VSMCs and also released EVs with calcification
capacity (136,137). In addition, many other factors
influence the process of vascular calcification, for instance, VSMC
apoptosis, oxidative stress and endothelial dysfunction (138,139).
GRP, a VKDP, has been identified as a powerful
inhibitor of vascular calcification. GRP, MGP and fetuin-A form a
large complex that is loaded in noncalcifying EVs but distinctly
lowered in high calcium-loaded vesicles, thus recommending GRP as
an important mineralization inhibitor (59,61). Furthermore, calciprotein particle
(CPP), a fetuin-mineral complex, principally contains mineral,
fetuin-A, MGP and GRP, and contributes greatly to the stabilization
of minerals. Research has demonstrated that CKD patients possess
CPPs with a lower content of fetuin-A and GRP compared with healthy
individuals (138). Fetuin-A is
predominant in healthy CPPs and retards the deterioration toward
calcifying CPPs through collaboration with GRP (140,141). Moreover, GRP shows the ability
to counteract inflammation and is found in macrophage-derived EVs
(142). In vitro studies
found calcification in both GRP-deficient and normal VSMCs in
response to osteogenic medium after 6 days, yet GRP-deficient VSMCs
calcified about twice as much as normal VSMCs 9 days later
(143). Of note, there is an
apparent increase in the expression of BMP-2 and its downstream
marker (small mother against decapentaplegic, SMAD) and, finally,
after comparing GRP with two different carboxylation states, the
direct interaction between the carboxylated GRP and BMP-2 was
confirmed (143). Therefore, GRP
disturbs the BMP-2-SMAD signaling in calcifying VSMCs, playing a
central role in VSMC calcification (Fig. 2).
Microcalcification, a small deposit of calcium with
a diameter less than 1 mm in mammographic images, is vital for the
diagnosis and prognosis of breast cancer (61). Ductal carcinoma in situ can
be as high as 20-25% in women with asymptomatic breast cancer
(144). Furthermore, 70% of
ductal carcinoma in situ can be diagnosed only by
microcalcification in mammography (145). Recent findings have shown that
linear branching microcalcifications in mammography indicate the
aggressive of tumor tissue (146). A differential accumulation
pattern of carboxylated GRP (cGRP) and under-carboxylated GRP
(ucGRP) by vitamin K has been recently emphasized in human breast
cancer (147). In healthy
mammary gland tissues, cGRP was predominant, while ucGRP was found
to be either co-localized or undetectable. By contrast, ucGRP was
widely detected in tumor cytoplasm, while cGRP was only
intermittently found in certain tumor cells. There are many
explanations for the large quantity of ucGRP in tumors. It has been
observed that the decreased level of vitamin K in tumor areas is in
contrast to non-tumorous areas (148). Patients with tumor
complications, such as venous thromboembolism, have received
long-term therapy with vitamin K antagonists and the potential
detrimental effects to GRP should be noted (149). In addition, prolonged
subclinical vitamin K deficiency has been identified in cancer
patients. Furthermore, vitamin K preferentially supports the
coagulation factor synthetic process in the liver, and only after
the vitamin K supply has met the liver's need is the excess vitamin
K transported to extra-hepatic tissues (177,150). Thus, ucGRP is widespread in
tumor tissues. Furthermore, the formation mechanism of
microcalcification in breast tumor tissue is similar to
physiological bone mineralization and pathological vascular
mineralization (151). Both cGRP
and ucGRP showed an advanced affinity to calcium mineral deposits
in breast cancer tissue. Thus, with the capability of resisting
ectopic calcification, GRP is considered a novel effective
antagonist against cancer. It is worth noting that triple-negative
breast cancer is a subtype with low expression of estrogen
receptor, progesterone receptor and human epidermal growth factor
receptor 2 receptor (62).
Therefore, there is a lack of effective targeted therapy drugs for
triple-negative breast cancer. However, recent research may be
useful in resolving this issue. GRP inhibits the growth, migration
and invasion of triple-negative breast cancer tissues in
vitro and in vivo (62). Moreover, according to survival
analysis in the open database, the relapse-free survival rate of
patients with triple-negative breast cancer was significantly
correlated with high GRP expression (62).
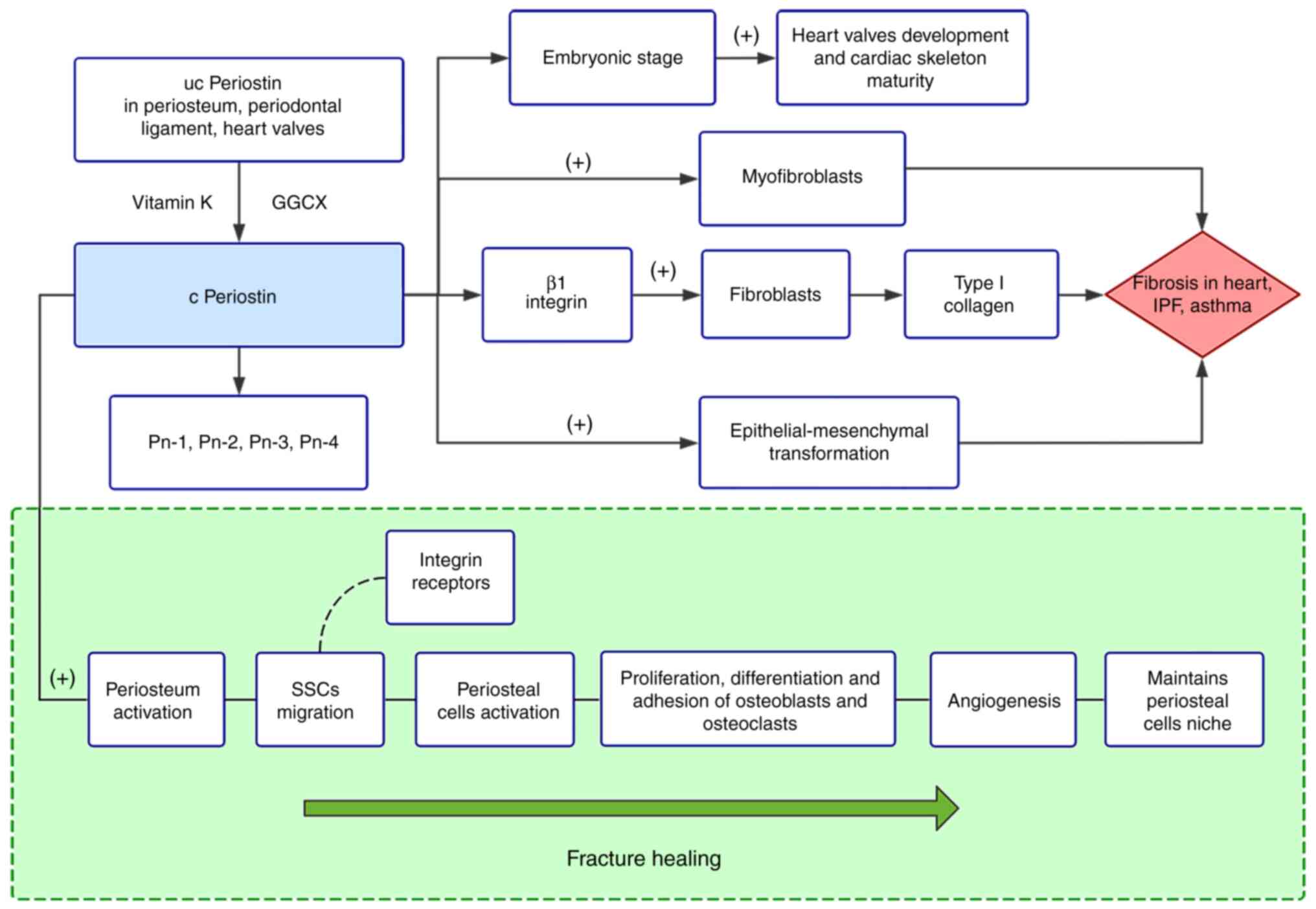
Periostin, initially known as osteoblast-specific
factor 2, was first cloned from a cDNA library of the mouse
osteoblastic cell line MC3T3-E1 in Japan (152). Over a decade later, the
Gla-containing protein, periostin, was determined to require
vitamin K-dependent carboxylation and became the 13th member of the
VKDP family (153).
Characterized by fasciclin domains, periostin is particularly
expressed in connective tissues submitted to constant mechanical
stresses (153). For example,
periosteum, the periodontal ligament, heart valves and skin.
Periostin has also been implicated in fibrosis, inflammation, tumor
metastasis and the fracture healing process (67,154-156).
Fractures are one of the most common traumatic
injuries to humans. Most fractures can be repaired to their
pre-injury state through a process similar to embryonic skeletal
development. According to the characteristics of fracture healing,
the process is divided into four partially overlapping phases: The
inflammation phase, the soft callus phase, the hard callus phase
and the remodeling phase (157).
The inflammation phase is marked by acute inflammation, hematoma
formation and skeletal stem cell recruitment. During the soft
callus phase, cartilaginous callus and nascent blood vessels form.
During the hard callus phase, the most active phase of
osteogenesis, the cartilage is reabsorbed and bone is deposited by
osteoblasts (158). Angiogenesis
also continues during this phase. During the last phase, primary
bone is eventually replaced by lamellar bone, which supports normal
skeletal functions, and vascular remodeling is finally completed
(158,159). There is a vital association
between periosteum and fracture repair. In a mouse model in which
graft femoral bone was segmentally transplanted, the periosteum
showed positive osteogenic and angiogenic activity, leading to
superior healing and repair of live isografts (160). However, absence of the
periosteum led to poor cartilaginous callus formation, and even
fracture non-union (160,161).
The periosteum is anatomically comprised of an outer fibrous layer
and an inner cambium layer. The fibrous layer contains fibroblasts,
collagen, and elastin fibers, along with a nerve and microvascular
network (162). The cambium
layer is directly closed to the bone surface and contains
high-quality mesenchymal progenitor cells, osteoblasts,
fibroblasts, microvessels and sympathetic nerves (162,163). In human bones, periostin is
highly expressed in the cambium layer, where it is highly active
during bone remodeling (164).
In a mouse model of fracture, rapid periostin gene expression
occurred during the inchoate phase of fracture healing (155). In the first 1-2 weeks after
fracture, human serum periostin is decreased initially, prior to a
progressive elevation that peaks at 8 weeks, and is present for
about 26 weeks (165).
Periostin participates in almost all phases of
fracture healing. In the early inflammation phase, as a result of
the inflammatory response or paracrine effects of the periosteum,
periostin is present at a low level in serum (165). Transplantation of the periosteum
of periostin-deficient mice to the fracture site of wild-type mice
induced negative fracture repair, indicating that periostin
regulates periosteum activation (66). Skeletal stem cells (SSCs), with
local osteogenic potential, are recruited in the early stage of
bone regeneration and periosteum and is considered one of its major
sources (166). As an
extracellular matrix protein, periostin promotes the migration of
SSCs by binding integrin receptors on the cell surface (162,167). Notably, periosteal cells,
another form of convened cells that shares a common embryonic
origin with SSCs, have been revealed to have greater regenerative
potential than SSCs (63).
Moreover, periosteal cell functions are impaired in mice lacking
periostin, suggesting that periostin contributes to periosteal cell
activation (63). During the
callus phases, induced by BMP-2, periostin is upregulated in soft
callus and osteoblasts (168).
Accumulating evidence has indicated that abundant periostin
facilitates the proliferation, differentiation and adhesion of
osteoblasts in bone formation (64,65). In addition, periostin may
interfere with osteoclasts in a similar way (65). It is reported that periostin
markedly increases arterioles in a calvarial defects model, proving
periostin promotes angiogenesis (66). Periostin has a crucial mission in
the last phase of fracture healing, that is, to recover the
periosteum niche of periosteal cells. In a periosteum
transplantation model, periosteal cells may still be re-activated
to contribute to cartilage within the callus after three injury
cycles (63). By contrast, when
periostin-deficient grafts were transplanted into wild-type hosts,
the contribution of periosteal cells to repairing of the second
fracture injury disappeared, leading to defective callus formation
and fibrosis, and furthermore this was not due to deficient
proliferation (63). Therefore,
periostin plays a crucial role in maintaining periosteal cell niche
and supporting bone remodeling.
Long-term anticoagulant therapy with vitamin K
antagonists, such as warfarin, reduces bone density and increases
the risk of osteoporosis (169,170). Previous findings have shown that
warfarin significantly inhibits osteoblastic differentiation
(171). Warfarin interferes in
the carboxylation of periostin by antagonizing the function of
vitamin K, and the decrease of carboxylated periostin is one of the
main causes of bone density reduction (172). By contrast, vitamin
K2 promotes mineralization of osteoblasts (173). In recent years, periostin has
been recommended as a potential predictive marker of bone events.
Osteoporotic fracture is a major cause of disability in the
elderly, while the ability of current predicting methods is
limited. In a cohort of 607 postmenopausal women from France that
were followed up for 7 years, a positive correlation between serum
periostin and fracture risk was observed (174). Furthermore, the association was
independent of bone mineral density and prior fractures, indicating
that periostin is an independent predictive marker of fracture
risk. This hypothesis was confirmed in another case control study
of Korean postmenopausal women (175). Interestingly, high plasma
periostin levels prefer non-vertebral fractures to vertebral
fractures, such as limb fractures (175). These clinical outcomes seem
contrary to the popular view of periostin. The specific mechanisms
for these conclusions need further study as they may be related to
the carboxylation state of periostin or to the distribution of
periostin in the body. Specifically, periostin in bone are induced
to circulation. Notably, it has been demonstrated that lower serum
periostin concentrations were related to prevalence of knee OA in
women (176). This provides a
new idea for the application of periostin in bone event
prediction.
During embryogenesis, periostin supports normal
valve leaflet morphogenesis and cardiac skeleton maturity (177). Periostin is implicated in CVDs,
such as myocardial infarction, atherosclerosis and cardiac
fibrosis-related diseases (67).
Cardiac fibrosis is a prominent feature of cardiac remodeling that
can further lead to heart failure and impaired cardiac function.
Fibroblasts, the most abundant cell population in the heart except
cardiomyocytes, rapidly differentiate into myofibroblasts in the
cardiac fibrosis process (67).
Abundant differentiated myofibroblasts found in hearts suffering
failure also support the transformation (179). Emerging evidence suggests that
the myofibroblast phenotype still has latent reversibility in
end-stage heart failure (67,178). Of note, periostin, as the most
specific product, is expressed in essentially all myofibroblasts
(67,68). Certain data have indicated
targeted ablated periostin-expressing myofibroblasts led to a
diminished fibrotic area and improved the ejection fraction in
hearts in AngII-induced fibrosis mice (68). In addition, not only was cardiac
fibrosis reduced, but treatment also did not affect scar stability
in myocardial infarction mice (68). Moreover, periostin antibody
treatment visibly restricted cell viability of myofibroblasts in
vitro (69). Therefore,
periostin is a novel central factor contributing to the function of
myofibroblasts during cardiac fibrosis. Research has demonstrated
that ginsenoside Rb1, the bioactive component of ginseng, reduced
the expression levels of periostin and protected rats against
myocardial fibrosis (179).
According to whether exons 17 and 21 exist or not,
periostin can be divided into four isoforms, i.e., Pn-1 to Pn-4. In
detail, Pn-1 is a full-length form, Pn-2 is short of exon 17, Pn-3
is short of exon 21, and Pn-4 is short of exons 17 and 21 (69). Using an antibody that specifically
inhibits exon 17, the dispute regarding the functions of different
periostin isoforms has been settled. It is not surprising that the
expression of periostin increased in the border zone on day 5 after
myocardial infarction (69).
However, total infarction and fibrosis size were notably reduced in
an adult mouse model by selectively neutralizing an antibody
against exon 17 (69). In
addition, cardiac dysfunctions were improved. Moreover, Pn-2
contributes to angiogenesis in in vitro experiments, while
Pn-1 does not (69). Low
expression of TGF-β, a fibrosis-related gene, was associated with
the inhibition of fibrosis. Thus, Pn-1 contributes to fibrosis and
heart remodeling after myocardial infarction, and there is
potential to improve the prognosis of myocardial infarction via
selectively inhibited Pn-1 treatment. Nevertheless, neonatal mice
were capable of regenerating myocardium after myocardial
infarction. On day 21 after myocardial infarction, the infarcted
areas of neonatal mice almost disappeared (180). However, myocardial regeneration
was inhibited in periostin-deficient neonatal mice, presenting with
a larger infarcted area, which was attributed to the inhibition of
PI3K/glycogen synthase kinase 3β/cyclin D1 signaling pathway
(180). Therefore, periostin is
pivotal for myocardial regeneration at the early stage of
myocardial infarction, and is involved in fibrillogenesis and scar
generation in the later chronic stage.
Previous findings have shown that periostin is
abundantly expressed in patients with atherosclerosis (181-183). In the 'Pathobiological
Determinants of Atherosclerosis in Youth' study, the variant
encoding periostin gene was connected with atherosclerotic lesion
traits (181). Matrix
metalloproteinases, enzymes implicated in atherosclerosis and
vascular remodeling, which were induced by periostin, led to valve
thickening in mice fed high-fat diets (182). Additionally, periostin
stimulates angiogenesis both in vitro and ex vivo (179). In response to injury, periostin
was markedly upregulated in neointimal SMCs and adventitial
myofibroblasts, and promoted cell migration (183). By contrast, the plaques of
periostin-deleted mice, not only had a smaller necrotic core and
fibrous cap, but also possessed more cholesterol clefts (184). The deficiency of periostin also
reduced the infiltration of macrophages into the plaque (184). Thus, periostin plays a
considerable role in atherosclerosis, and targeted periostin
treatment may delay progression of diseases associated with
atherosclerosis (Fig. 3).
In the last decade, the role of periostin in airway
development and diseases has been widely emphasized. For instance,
periostin was reduced in tracheal aspirate fluid of
bronchopulmonary dysplasia during the window period (185). Then, TGF-β upregulated the
expression of periostin in the interstitial fibrosis region
(185,186). Thus, periostin is recognized as
a potential biomarker that predicts the risk of bronchopulmonary
dysplasia and the need for preventative therapies in preterm
infants. Periostin has been involved in many respiratory disorders,
such as idiopathic pulmonary fibrosis (IPF), asthma, chronic
rhinosinusitis, idiopathic eosinophilic pneumonia and allergic
bronchopulmonary aspergillosis (156,187-190). The most notable of these are IPF
and asthma.
IPF, a common pulmonary fibrotic conditions, is a
chronic progressive parenchymal lung disease of unclear cause that
is limited to the lungs (191,192). Patients are predominantly older
individuals and typically have progressive worsening lung function,
leading to a grave prognosis (193). It has been indicated that
periostin was elevated in IPF patients' circulation (190). Furthermore, more periostin was
found in the lungs of IPF patients and concentrated in areas of
active fibrosis (187).
Interestingly, the exon 21 of the periostin gene is more likely to
be spliced out in IPF lung samples than in the control (194). Injury factors activate alveolar
epithelial cells disrupting the homeostatic balance between
epithelial and mesenchymal cells, thus fibrotic response is driven.
As an extracellular matrix protein, periostin and TGF-β regulate
each other in fibroblasts (195); specifically, TGF-β increases the
expression of periostin. In return, periostin significantly
upregulates the production of TGF-β in fibroblasts and increases
type I collagen production (70,195). However, periostin activates
fibroblasts to produce type I collagen via β1 integrin, rather than
the TGF-β signal (195). Similar
to heart fibrosis, periostin promotes differentiation of
fibroblasts to myofibroblasts. By mediating epithelial-mesenchymal
transformation, periostin induces alveolar epithelial cells to take
on the characteristics of mesenchymal cells, which leads to the
aggravation of fibrosis (70).
Emerging evidence suggests that periostin silencing drives the
fibroblasts into G1 arrest of the cell cycle and retards the
proliferation in IPF (196).
Thus, periostin plays a pivotal role in lung fibroblast
proliferation. Currently, early lung transplantation is a
beneficial therapeutic option for IPF patients, and another two
available drugs (Pirfenidone and Nintedanib) are able to limit IPF
progress (197). Recently, a
compound known as CP4715 was found to prohibit the interaction
between TGF-β and periostin (197). CP4715, not only lessened
bleomycin-induced pulmonary fibrosis, but also disturbed TGF-β
signals in fibroblasts from IPF (195). Therefore, CP4715 may become a
latent drug therapy to provide more therapeutic possibilities for
IPF. It is worth mentioning that vitamin K antagonists are related
to the rising mortality of IPF (198). The carboxylation status of
periostin in IPF patients deserves further study. Some scholars
have proposed that the use of vitamin K instead of vitamin K
antagonists may help reduce the progression of IPF, but this idea
needs further verification (198).
Asthma, as a heterogeneous disease, has been
defined as several phenotypes according to different clinical
features and physiological indexes. Nevertheless, type-2 airway
inflammation is one of the main causes of asthma, which is
supported by activity of type 2 cytokines, such as IL-4 and IL-13.
As a result of chronic airflow limitation, airway remodeling
develops in chronic severe asthma. Many studies have shown
periostin is deeply involved in the process of asthma, from airway
inflammation to remodeling. The periostin gene is highly induced in
asthmatic airway epithelial cells with a 4.4-fold increase compared
to healthy controls (199). In a
cohort of asthmatics from Sweden, a negative correlation between
serum periostin and lung function was observed (71). Type-2 inflammation attracts large
numbers of immune cells to release cytokines, such as IL-4, IL-13
and TGF-β. These cytokines stimulate the production of periostin
from fibroblasts, epithelial cells and endothelial cells, which are
known as the main sources of periostin in asthma (200), and some researchers have
hypothesized that eosinophils also secrete periostin (201). As an integrin ligand, periostin
binds to integrin αMβ2 and α4β1 on eosinophil, guiding recruitment
of eosinophils and increasing eosinophil adhesion to fibronectin
(156,202). In addition, through its
fibrogenic function, periostin participates in the process of
subepithelial fibrosis, which is feature of airway remodeling in
asthma (185). Periostin,
secreted from airway epithelial cells, activates TGF-β and
upregulates type I collagen via autocrine effects (70,203). Similarly, periostin activates
TGF-β-mediated fibroblasts to increase the production of type I
collagen (70,203). Clinical studies from Japan have
reported that vitamin K2 therapy has an effective rate of up to
90.9% in patients with mild asthma (204). The effective rate was 86.7 and
72.7% in moderate and severe patients, respectively (204). In addition, vitamin K2 has a
powerful ability to inhibit the release of inflammatory cytokines
(205). It has also been shown
that vitamin D, also a fat-soluble vitamin, can regulate
inflammatory chemokines in asthma and significantly inhibit airway
smooth muscle cell proliferation (205). Therefore, whether vitamin K2 can
regulate the release of inflammatory factors in asthma and thus
inhibit the production of large amounts of periostin remains to be
further studied. Additionally, periostin increases gel elasticity
formed by type 1 collagen, thus mediating the biomechanical
capabilities of the airway and leading to airway remodeling
(206). Accumulated evidence has
indicated that high serum periostin concentrations were implicated
in certain characteristics of asthma. It is reported that serum
periostin concentrations were not combined with atopic status or
treatment status of asthma, while high level serum periostin was
related to older patients at the onset of asthma, aspirin
intolerance or nasal disorders (207-209). As serum biomarkers are more
convenient than lung function tests in some special cases of
asthma, periostin has become one of the practical biomarkers of
asthma. For instance, periostin rises significantly in severe
asthma and acute asthma exacerbation of children, which is an
important serum biomarker in assessing the severity of asthma
(206). Of note, periostin is a
helpful biomarker to detect long-term bronchial obstruction in
severe asthmatic patients, as well as the sensitivity of sputum
periostin beyond the serum periostin (210).
In this review, we highlighted three emerging VKDPs
(Gas 6, GRP and periostin) that need vitamin K to conduct
carboxylation and then perform various biological functions in the
human body, such as bone homeostasis, heart development and
anti-vascular calcification. In combination with previous studies,
we believe that a high intake of vitamin K, especially vitamin
K2, is beneficial for the cardiovascular system and
bones. However, some questions about the relationship between
vitamin K and cancer remain unsolved. Many studies have shown
vitamin K2 has anticancer effects. Ishizuka et al
reported that vitamin K2 has a moderately suppressive
effect on hepatocellular carcinoma recurrence (217). Zhong et al indicated that
vitamin K2 reduces the hepatocellular carcinoma
recurrence rate after 1 year (218). Similarly, vitamin K2
exerts anti-cancer effects in cancer cell lines, such as
cholangiocellular carcinoma, ovarian cancer and pancreatic cancer
(219-221). Accumulating evidence has
indicated that vitamin K2 not only inhibits the
proliferation and differentiation of tumor cells, but also induces
the apoptosis and autophagy of tumor cells (222). In addition, however, some VKDPs
represented by Gas6 have been indicated to facilitate the survival
and metastasis of cancer cells. Moreover, as mentioned above, GRP
carboxylation status in breast cancer tissues is significantly
different from those in normal tissues, but there are few studies
measuring this in other diseases. Thus, the relationship between
measurement of VKDP carboxylation status and disease progression
remains to be further investigated. Furthermore, periostin is a
newly identified VKDP that has been extensively studied in the
heart and respiratory system. However, the role of periostin as a
VKDP has been rarely studied. A large number of studies have shown
that the Gla domain after vitamin K carboxylation is an important
structure for VKDPs to play a role; thus, this review provides a
new idea for the further exploration of periostin. Overall, the
process of γ-carboxylation modification has a significant effect on
biological functions, although the functional results of
γ-carboxylation for these proteins are not yet clear. These three
emerging proteins act in different directions, so their specific
roles with vitamin K2 need further study.
In conclusion, Gas6, GRP and periostin are involved
in a variety of physiological and pathological processes in the
body. Vitamin K is essential for their function, and thus may be a
potential preventive and therapeutic agent for many diseases.
Additionally, VKDPs are expected to be biomarkers for many
diseases.
This study was funded by the National Nature
Science Foundation of China (grant no. 30971065), the Science and
Technology Plan of Dalian (grant no. 2012E12SF074) and the
Education Fund Item of Liaoning province (grant no. 2009 A
194).
Not applicable.
SL supervised the writing of the present review as
well as directing its structure, and provided the final approval of
the version to be published. HX designed the concept of the review
and its structure, wrote and revised the manuscript. JC and LD were
involved in the writing of the review. All authors agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Dam H: The antihaemorrhagic vitamin of the
chick. Biochem J. 29:1273–1285. 1935. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palmer CR, Blekkenhorst LC, Lewis JR, Ward
NC, Schultz CJ, Hodgson JM, Croft KD and Sim M: Quantifying dietary
vitamin K and its link to cardiovascular health: A narrative
review. Food Funct. 11:2826–2837. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirota Y, Tsugawa N, Nakagawa K, Suhara Y,
Tanaka K, Uchino Y, Takeuchi A, Sawada N, Kamao M, Wada A, et al:
Menadione (vitamin K3) is a catabolic product of oral phylloquinone
(vitamin K1) in the intestine and a circulating precursor of tissue
menaquinone-4 (vitamin K2) in rats. J Biol Chem. 288:33071–33080.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simes DC, Viegas CSB, Araújo N and
Marreiros C: Vitamin K as a diet supplement with impact in human
health: Current evidence in age-related diseases. Nutrients.
12:1382020. View Article : Google Scholar :
|
|
5
|
Mirza F and Canalis E: Management of
endocrine disease: Secondary osteoporosis: Pathophysiology and
management. Eur J Endocrinol. 173:R131–R151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheung CL, Ang SB, Chadha M, Chow ES,
Chung YS, Hew FL, Jaisamrarn U, Ng H, Takeuchi Y, Wu CH, et al: An
updated hip fracture projection in Asia: The Asian federation of
osteoporosis societies study. Osteoporos Sarcopenia. 4:16–21. 2018.
View Article : Google Scholar
|
|
7
|
Wasilewski GB, Vervloet MG and Schurgers
LJ: The bone-vasculature axis: Calcium supplementation and the role
of vitamin K. Front Cardiovasc Med. 6:62019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Danziger J, Young RL, Shea MK, Tracy RP,
Ix JH, Jenny NS and Mukamal KJ: Vitamin K-dependent protein
activity and incident ischemic cardiovascular disease: The
multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc
Biol. 36:1037–1042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iribarren C, Sidney S, Sternfeld B and
Browner WS: Calcification of the aortic arch: Risk factors and
association with coronary heart disease, stroke, and peripheral
vascular disease. JAMA. 283:2810–2815. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondos GT, Hoff JA, Sevrukov A, Daviglus
ML, Garside DB, Devries SS, Chomka EV and Liu K: Electron-beam
tomography coronary artery calcium and cardiac events: A 37-month
follow-up of 5635 initially asymptomatic low- to intermediate-risk
adults. Circulation. 107:2571–2576. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferland G: The discovery of vitamin K and
its clinical applications. Ann Nutr Metab. 61:213–218. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung CL, Sahni S, Cheung BM, Sing CW and
Wong IC: Vitamin K intake and mortality in people with chronic
kidney disease from NHANES III. Clin Nutr. 34:235–240. 2015.
View Article : Google Scholar
|
|
13
|
Fusaro M, Plebani M, Iervasi G and
Gallieni M: Vitamin K deficiency in chronic kidney disease:
Evidence is building up. Am J Nephrol. 45:1–3. 2017. View Article : Google Scholar
|
|
14
|
Turner ME, Adams MA and Holden RM: The
vitamin K metabolome in chronic kidney disease. Nutrients.
10:10762018. View Article : Google Scholar :
|
|
15
|
Kaesler N, Magdeleyns E, Herfs M,
Schettgen T, Brandenburg V, Fliser D, Vermeer C, Floege J,
Schlieper G and Krüger T: Impaired vitamin K recycling in uremia is
rescued by vitamin K supplementation. Kidney Int. 86:286–293. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Lullo L, House A, Gorini A, Santoboni
A, Russo D and Ronco C: Chronic kidney disease and cardiovascular
complications. Heart Fail Rev. 20:259–272. 2015. View Article : Google Scholar
|
|
17
|
Shearer MJ, Mallinson CN, Webster GR and
Barkhan P: Clearance from plasma and excretion in urine, faeces and
bile of an intravenous dose of tritiated vitamin K 1 in man. Br J
Haematol. 22:579–588. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schurgers LJ, Teunissen KJF, Hamulyák K,
Knapen MH, Vik H and Vermeer C: Vitamin K-containing dietary
supplements: Comparison of synthetic vitamin K1 and natto-derived
menaquinone-7. Blood. 109:3279–3283. 2007. View Article : Google Scholar
|
|
19
|
Halder M, Petsophonsakul P, Akbulut AC,
Pavlic A, Bohan F, Anderson E, Maresz K, Kramann R and Schurgers L:
Vitamin K: Double bonds beyond coagulation insights into
differences between vitamin K1 and K2 in health and disease. Int J
Mol Sci. 20:8962019. View Article : Google Scholar :
|
|
20
|
Willems BAG, Vermeer C, Reutelingsperger
CP and Schurgers LJ: The realm of vitamin K dependent proteins:
Shifting from coagulation toward calcification. Mol Nutr Food Res.
58:1620–1635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kearon C, Akl EA, Comerota AJ, Prandoni P,
Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali
F, et al: Antithrombotic therapy for VTE disease: Antithrombotic
therapy and prevention of thrombosis, 9th ed: American college of
chest physicians evidence-based clinical practice guidelines.
Chest. 141(2 Suppl): e419S–e496S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tie JK and Stafford DW: Structural and
functional insights into enzymes of the vitamin K cycle. J Thromb
Haemost. 14:236–247. 2016. View Article : Google Scholar :
|
|
23
|
Huang M, Rigby AC, Morelli X, Grant MA,
Huang G, Furie B, Seaton B and Furie BC: Structural basis of
membrane binding by Gla domains of vitamin K-dependent proteins.
Nat Struct Biol. 10:751–756. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Girolami A, Ferrari S, Cosi E, Santarossa
C and Randi ML: Vitamin K-dependent coagulation factors that may be
responsible for both bleeding and thrombosis (FII, FVII, and FIX).
Clin Appl Thromb Hemost. 24(9 Suppl): 42S–47S. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mahdi AJ, Obaji SG and Collins PW: Role of
enhanced half-life factor VIII and IX in the treatment of
haemophilia. Br J Haematol. 169:768–776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muller MP, Wang Y, Morrissey JH and
Tajkhorshid E: Lipid specificity of the membrane binding domain of
coagulation factor X. J Thromb Haemost. 15:2005–2016. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rezaie AR: Regulation of the protein C
anticoagulant and antiinflammatory pathways. Curr Med Chem.
17:2059–2069. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mosnier LO, Zlokovic BV and Griffin JH:
The cytoprotective protein C pathway. Blood. 109:3161–3172. 2007.
View Article : Google Scholar
|
|
29
|
Mosnier LO and Griffin JH: Protein C
anticoagulant activity in relation to anti-inflammatory and
anti-apoptotic activities. Front Biosci. 11:2381–2399. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Majid Z, Tahir F, Ahmed J, Bin Arif T and
Haq A: Protein C deficiency as a risk factor for stroke in young
adults: A review. Cureus. 12:e74722020.PubMed/NCBI
|
|
31
|
Bernard GR, Vincent JL, Laterre PF, LaRosa
SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE,
Helterbrand JD, Ely EW, et al: Efficacy and safety of recombinant
human activated protein C for severe sepsis. N Engl J Med.
344:699–709. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dahlbäck B: Vitamin K-dependent protein S:
Beyond the protein C pathway. Semin Thromb Hemost. 44:176–184.
2018. View Article : Google Scholar
|
|
33
|
Suleiman L, Négrier C and Boukerche H:
Protein S: A multi-functional anticoagulant vitamin K-dependent
protein at the crossroads of coagulation, inflammation,
angiogenesis, and cancer. Crit Rev Oncol Hematol. 88:637–654. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fricke DR, Chatterjee S and Majumder R:
Protein S in preventing thrombosis. Aging (Albany NY). 11:847–848.
2019. View Article : Google Scholar
|
|
35
|
Yasuma T, Yano Y, D'Alessandro-Gabazza CN,
Toda M, Gil-Bernabe P, Kobayashi T, Nishihama K, Hinneh JA,
Mifuji-Moroka R, Roeen Z, et al: Amelioration of diabetes by
protein S. Diabetes. 65:1940–1951. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Topalidou M, Effraimidou S, Farmakiotis D,
Papadakis E, Papaioannou G, Korantzis I and Garipidou V: Low
protein Z levels, but not the intron F G79A polymorphism, are
associated with unexplained pregnancy loss. Thromb Res. 124:24–27.
2009. View Article : Google Scholar
|
|
37
|
Ghozlan MF, Mohamed AAE, Eissa DS and
Eldawy HS: Low protein Z level: A thrombophilic risk biomarker for
acute coronary syndrome. Indian J Hematol Blood Transfus.
35:339–346. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kulman JD, Harris JE, Haldeman BA and
Davie EW: Primary structure and tissue distribution of two novel
proline-rich gamma-carboxyglutamic acid proteins. Proc Natl Acad
Sci USA. 94:9058–9062. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kulman JD, Harris JE, Xie L and Davie EW:
Proline-rich Gla protein 2 is a cell-surface vitamin K-dependent
protein that binds to the transcriptional coactivator
Yes-associated protein. Proc Natl Acad Sci USA. 104:8767–8772.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kulman JD, Harris JE, Xie L and Davie EW:
Identification of two novel transmembrane gamma-carboxyglutamic
acid proteins expressed broadly in fetal and adult tissues. Proc
Natl Acad Sci USA. 98:1370–1375. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Iwamoto J: Vitamin K2 therapy
for postmenopausal osteoporosis. Nutrients. 6:1971–1980. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mizokami A, Kawakubo-Yasukochi T and
Hirata M: Osteocalcin and its endocrine functions. Biochem
Pharmacol. 132:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wen L, Chen J, Duan L and Li S: Vitamin
K-dependent proteins involved in bone and cardiovascular health
(Review). Mol Med Rep. 18:3–15. 2018.PubMed/NCBI
|
|
44
|
Naito K, Watari T, Obayashi O, Katsube S,
Nagaoka I and Kaneko K: Relationship between serum
undercarboxylated osteocalcin and hyaluronan levels in patients
with bilateral knee osteoarthritis. Int J Mol Med. 29:756–760.
2012.PubMed/NCBI
|
|
45
|
Sweatt A, Sane DC, Hutson SM and Wallin R:
Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic
calcified lesions of aging rats. J Thromb Haemost. 1:178–185. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yao Y, Zebboudj AF, Shao E, Perez M and
Boström K: Regulation of bone morphogenetic protein-4 by matrix GLA
protein in vascular endothelial cells involves activin-like kinase
receptor 1. J Biol Chem. 281:33921–33930. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roy ME and Nishimoto SK: Matrix Gla
protein binding to hydroxyapatite is dependent on the ionic
environment: Calcium enhances binding affinity but phosphate and
magnesium decrease affinity. Bone. 31:296–302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zuo PY, Chen XL, Lei YH, Liu CY and Liu
YW: Growth arrest-specific gene 6 protein promotes the
proliferation and migration of endothelial progenitor cells through
the PI3K/AKT signaling pathway. Int J Mol Med. 34:299–306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qiu C, Zheng H, Tao H, Yu W, Jiang X, Li
A, Jin H, Lv A and Li H: Vitamin K2 inhibits rat vascular smooth
muscle cell calcification by restoring the Gas6/Axl/Akt
anti-apoptotic pathway. Mol Cell Biochem. 433:149–159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang X, Tao H, Qiu C, Ma X, Li S, Guo X,
Lv A and Li H: Vitamin K2 regression aortic calcification induced
by warfarin via Gas6/Axl survival pathway in rats. Eur J Pharmacol.
786:10–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kawamoto A, Tkebuchava T, Yamaguchi J,
Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma
H, et al: Intramyocardial transplantation of autologous endothelial
progenitor cells for therapeutic neovascularization of myocardial
ischemia. Circulation. 107:461–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiang L, Liu CY, Yang QF, Wang P and Zhang
W: Plasma level of growth arrest-specific 6 (GAS6) protein and
genetic variations in the GAS6 gene in patients with acute coronary
syndrome. Am J Clin Pathol. 131:738–743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen LW, Chen W, Hu ZQ, Bian JL, Ying L,
Hong GL, Qiu QM, Zhao GJ and Lu ZQ: Protective effects of growth
arrest-specific protein 6 (Gas6) on sepsis-induced acute kidney
injury. Inflammation. 39:575–582. 2016. View Article : Google Scholar
|
|
54
|
Novitskiy SV, Zaynagetdinov R, Vasiukov G,
Gutor S, Han W, Serezani A, Matafonov A, Gleaves LA, Sherrill TP,
Polosukhin VV and Blackwell TS: Gas6/MerTK signaling is negatively
regulated by NF-κB and supports lung carcinogenesis. Oncotarget.
10:7031–7042. 2019. View Article : Google Scholar
|
|
55
|
Eitzinger N, Surmann-Schmitt C, Bösl M,
Schett G, Engelke K, Hess A, von der Mark K and Stock M: Ucma is
not necessary for normal development of the mouse skeleton. Bone.
50:670–680. 2012. View Article : Google Scholar
|
|
56
|
Stock M, Menges S, Eitzinger N, Geßlein M,
Botschner R, Wormser L, Distler A, Schlötzer-Schrehardt U, Dietel
K, Distler J, et al: A dual role of upper zone of growth plate and
cartilage matrix-associated protein in human and mouse
osteoarthritic cartilage: Inhibition of aggrecanases and promotion
of bone turnover. Arthritis Rheumatol. 69:1233–1245. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Seuffert F, Weidner D, Baum W, Schett G
and Stock M: Upper zone of growth plate and cartilage matrix
associated protein protects cartilage during inflammatory
arthritis. Arthritis Res Ther. 20:882018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cavaco S, Viegas CS, Rafael MS, Ramos A,
Magalhães J, Blanco FJ, Vermeer C and Simes DC: Gla-rich protein is
involved in the cross-talk between calcification and inflammation
in osteoarthritis. Cell Mol Life Sci. 73:1051–1065. 2016.
View Article : Google Scholar
|
|
59
|
Viegas CS, Cavaco S, Neves PL, Ferreira A,
João A, Williamson MK, Price PA, Cancela ML and Simes DC: Gla-rich
protein is a novel vitamin K-dependent protein present in serum
that accumulates at sites of pathological calcifications. Am J
Pathol. 175:2288–2298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee YJ, Park SY, Lee SJ, Boo YC, Choi JY
and Kim JE: Ucma, a direct transcriptional target of Runx2 and
Osterix, promotes osteoblast differentiation and nodule formation.
Osteoarthritis Cartilage. 23:1421–1431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
O'Grady S and Morgan MP:
Microcalcifications in breast cancer: From pathophysiology to
diagnosis and prognosis. Biochim Biophys Acta Rev Cancer.
1869:310–320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lee SH, Lee YJ, Park SI and Kim JE: Unique
cartilage matrix-associated protein inhibits the migratory and
invasive potential of triple-negative breast cancer. Biochem
Biophys Res Commun. 530:680–685. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Duchamp de Lageneste O, Julien A,
Abou-Khalil R, Frangi G, Carvalho C, Cagnard N, Cordier C, Conway
SJ and Colnot C: Periosteum contains skeletal stem cells with high
bone regenerative potential controlled by Periostin. Nat Commun.
9:7732018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhu S, Barbe MF, Liu C, Hadjiargyrou M,
Popoff SN, Rani S, Safadi FF and Litvin J: Periostin-like-factor in
osteogenesis. J Cell Physiol. 218:584–592. 2009. View Article : Google Scholar
|
|
65
|
Cobo T, Viloria CG, Solares L, Fontanil T,
González-Chamorro E, De Carlos F, Cobo J, Cal S and Obaya AJ: Role
of periostin in adhesion and migration of bone remodeling cells.
PLoS One. 11:e01478372016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Heo SC, Shin WC, Lee MJ, Kim BR, Jang IH,
Choi EJ, Lee JS and Kim JH: Periostin accelerates bone healing
mediated by human mesenchymal stem cell-embedded
hydroxyapatite/tricalcium phosphate scaffold. PLoS One.
10:e01166982015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kanisicak O, Khalil H, Ivey MJ, Karch J,
Maliken BD, Correll RN, Brody MJ, J Lin SC, Aronow BJ and Tallquist
MD: Genetic lineage tracing defines myofibroblast origin and
function in the injured heart. Nat Commun. 7:122602016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kaur H, Takefuji M, Ngai CY, Carvalho J,
Bayer J, Wietelmann A, Poetsch A, Hoelper S, Conway SJ, Möllmann H,
et al: Targeted ablation of periostin-expressing activated
fibroblasts prevents adverse cardiac remodeling in mice. Circ Res.
118:1906–1917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Taniyama Y, Katsuragi N, Sanada F, Azuma
J, Iekushi K, Koibuchi N, Okayama K, Ikeda-Iwabu Y, Muratsu J, Otsu
R, et al: Selective blockade of periostin exon 17 preserves cardiac
performance in acute myocardial infarction. Hypertension.
67:356–361. 2016. View Article : Google Scholar
|
|
70
|
Izuhara K, Conway SJ, Moore BB, Matsumoto
H, Holweg CT, Matthews JG and Arron JR: Roles of periostin in
respiratory disorders. Am J Respir Crit Care Med. 193:949–956.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
James A, Janson C, Malinovschi A, Holweg
C, Alving K, Ono J, Ohta S, Ek A, Middelveld R, Dahlén B, et al:
Serum periostin relates to type-2 inflammation and lung function in
asthma: Data from the large population-based cohort Swedish
GA(2)LEN. Allergy. 72:1753–1760. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Litvin J, Blagg A, Mu A, Matiwala S,
Montgomery M, Berretta R, Houser S and Margulies K: Periostin and
periostin-like factor in the human heart: Possible therapeutic
targets. Cardiovasc Pathol. 15:24–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zoch ML, Clemens TL and Riddle RC: New
insights into the biology of osteocalcin. Bone. 82:42–49. 2016.
View Article : Google Scholar
|
|
74
|
Cagman Z, Bingol Ozakpinar O, Cirakli Z,
Gedikbasi A, Ay P, Colantonio D, Uras AR, Adeli K and Uras F:
Reference intervals for growth arrest-specific 6 protein in adults.
Scand J Clin Lab Invest. 77:109–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Varnum BC, Young C, Elliott G, Garcia A,
Bartley TD, Fridell YW, Hunt RW, Trail G, Clogston C, Toso RJ, et
al: Axl receptor tyrosine kinase stimulated by the vitamin
K-dependent protein encoded by growth-arrest-specific gene 6.
Nature. 373:623–626. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li M, Ye J, Zhao G, Hong G, Hu X, Cao K,
Wu Y and Lu Z: Gas6 attenuates lipopolysaccharide-induced TNF-α
expression and apoptosis in H9C2 cells through NF-κB and MAPK
inhibition via the Axl/PI3K/Akt pathway. Int J Mol Med. 44:982–994.
2019.PubMed/NCBI
|
|
77
|
Tanabe K, Nagata K, Ohashi K, Nakano T,
Arita H and Mizuno K: Roles of gamma-carboxylation and a sex
hormone-binding globulin-like domain in receptor-binding and in
biological activities of Gas6. FEBS Lett. 408:306–310. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bellido-Martín L and de Frutos PG: Vitamin
K-dependent actions of Gas6. Vitam Horm. 78:185–209. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wu G, Ma Z, Cheng Y, Hu W, Deng C, Jiang
S, Li T, Chen F and Yang Y: Targeting Gas6/TAM in cancer cells and
tumor microenvironment. Mol Cancer. 17:202018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Melaragno MG, Cavet ME, Yan C, Tai LK, Jin
ZG, Haendeler J and Berk BC: Gas6 inhibits apoptosis in vascular
smooth muscle: Role of Axl kinase and Akt. J Mol Cell Cardiol.
37:881–887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
McCloskey P, Fridell YW, Attar E, Villa J,
Jin Y, Varnum B and Liu ET: GAS6 mediates adhesion of cells
expressing the receptor tyrosine kinase Axl. J Biol Chem.
272:23285–23291. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Stenhoff J, Dahlbäck B and Hafizi S:
Vitamin K-dependent Gas6 activates ERK kinase and stimulates growth
of cardiac fibroblasts. Biochem Biophys Res Commun. 319:871–878.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rizzoni D, Rizzoni M, Nardin M, Chiarini
G, Agabiti-Rosei C, Aggiusti C, Paini A, Salvetti M and Muiesan ML:
Vascular aging and disease of the small vessels. High Blood Press
Cardiovasc Prev. 26:183–189. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jin CW, Wang H, Chen YQ, Tang MX, Fan GQ,
Wang ZH, Li L, Zhang Y, Zhang W and Zhong M: Gas6 delays senescence
in vascular smooth muscle cells through the PI3K/Akt/FoxO signaling
pathway. Cell Physiol Biochem. 35:1151–1166. 2015. View Article : Google Scholar
|
|
85
|
Clauser S, Meilhac O, Bièche I, Raynal P,
Bruneval P, Michel JB and Borgel D: Increased secretion of Gas6 by
smooth muscle cells in human atherosclerotic carotid plaques.
Thromb Haemost. 107:140–149. 2012. View Article : Google Scholar
|
|
86
|
Holden RM, Hétu MF, Li TY, Ward EC,
Couture LE, Herr JE, Christilaw E, Adams MA and Johri AM:
Circulating Gas6 is associated with reduced human carotid
atherosclerotic plaque burden in high risk cardiac patients. Clin
Biochem. 64:6–11. 2019. View Article : Google Scholar
|
|
87
|
Tjwa M, Moons L and Lutgens E: Pleiotropic
role of growth arrest-specific gene 6 in atherosclerosis. Curr Opin
Lipidol. 20:386–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Meir KS and Leitersdorf E: Atherosclerosis
in the apolipoprotein-E-deficient mouse: A decade of progress.
Arterioscler Thromb Vasc Biol. 24:1006–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Park JK, Theuer S, Kirsch T, Lindschau C,
Klinge U, Heuser A, Plehm R, Todiras M, Carmeliet P, Haller H, et
al: Growth arrest specific protein 6 participates in DOCA-induced
target-organ damage. Hypertension. 54:359–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhao YF, Xu DC, Zhu GF, Zhu M, Tang K, Li
WM and Xu YW: Growth arrest-specific 6 exacerbates pressure
overload-induced cardiac hypertrophy. Hypertension. 67:118–129.
2016. View Article : Google Scholar
|
|
91
|
van der Meer JH, van der Poll T and van 't
Veer C: TAM receptors, Gas6, and protein S: Roles in inflammation
and hemostasis. Blood. 123:2460–2469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao GJ, Zheng JY, Bian JL, Chen LW, Dong
N, Yu Y, Hong GL, Chandoo A, Yao YM and Lu ZQ: Growth
arrest-specific 6 enhances the suppressive function of
CD4+CD25+ regulatory T cells mainly through
axl receptor. Mediators Inflamm. 2017:68484302017.
|
|
93
|
Haase VH: Mechanisms of hypoxia responses
in renal tissue. J Am Soc Nephrol. 24:537–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Giangola MD, Yang WL, Rajayer SR,
Kuncewitch M, Molmenti E, Nicastro J, Coppa GF and Wang P: Growth
arrest-specific protein 6 protects against renal
ischemia-reperfusion injury. J Surg Res. 199:572–579. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ishimoto Y, Ohashi K, Mizuno K and Nakano
T: Promotion of the uptake of PS liposomes and apoptotic cells by a
product of growth arrest-specific gene, gas6. J Biochem.
127:411–417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lee IJ, Hilliard B, Swami A, Madara JC,
Rao S, Patel T, Gaughan JP, Lee J, Gadegbeku CA, Choi ET and Cohen
PL: Growth arrest-specific gene 6 (Gas6) levels are elevated in
patients with chronic renal failure. Nephrol Dial Transplant.
27:4166–4172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hallajzadeh J, Ghorbanihaghjo A, Argani H,
Dastmalchi S and Rashtchizadeh N: Growth arrest-specific 6 protein
and matrix Gla protein in hemodialysis patients. Iran J Kidney Dis.
9:249–255. 2015.PubMed/NCBI
|
|
98
|
Panichi V, Migliori M, De Pietro S,
Taccola D, Bianchi AM, Giovannini L, Norpoth M, Metelli MR,
Cristofani R, Bertelli AAE, et al: C-reactive protein and
interleukin-6 levels are related to renal function in predialytic
chronic renal failure. Nephron. 91:594–600. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Weiner DE, Tabatabai S, Tighiouart H,
Elsayed E, Bansal N, Griffith J, Salem DN, Levey AS and Sarnak MJ:
Cardiovascular outcomes and all-cause mortality: Exploring the
interaction between CKD and cardiovascular disease. Am J Kidney
Dis. 48:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Silaghi CN, Ilyés T, Filip VP, Farca M,
van Ballegooijen AJ and Crăciun AM: Vitamin K dependent proteins in
kidney disease. Int J Mol Sci. 20:15712019. View Article : Google Scholar :
|
|
101
|
Wu CS, Hu CY, Tsai HF, Chyuan IT, Chan CJ,
Chang SK and Hsu PN: Elevated serum level of growth arrest-specific
protein 6 (Gas6) in systemic lupus erythematosus patients is
associated with nephritis and cutaneous vasculitis. Rheumatol Int.
34:625–629. 2014. View Article : Google Scholar
|
|
102
|
Nagai K, Miyoshi M, Kake T, Fukushima N,
Matsuura M, Shibata E, Yamada S, Yoshikawa K, Kanayama HO, Fukawa
T, et al: Dual involvement of growth arrest-specific gene 6 in the
early phase of human IgA nephropathy. PLoS One. 8:e667592013.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Nagai K, Arai H, Yanagita M, Matsubara T,
Kanamori H, Nakano T, Iehara N, Fukatsu A, Kita T and Doi T: Growth
arrest-specific gene 6 is involved in glomerular hypertrophy in the
early stage of diabetic nephropathy. J Biol Chem. 278:18229–18234.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Nagai K, Matsubara T, Mima A, Sumi E,
Kanamori H, Iehara N, Fukatsu A, Yanagita M, Nakano T, Ishimoto Y,
et al: Gas6 induces Akt/mTOR-mediated mesangial hypertrophy in
diabetic nephropathy. Kidney Int. 68:552–561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hung YJ, Lee CH, Chu NF and Shieh YS:
Plasma protein growth arrest-specific 6 levels are associated with
altered glucose tolerance, inflammation, and endothelial
dysfunction. Diabetes Care. 33:1840–1844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Li W, Wang J, Ge L, Shan J, Zhang C and
Liu J: Growth arrest-specific protein 6 (Gas6) as a noninvasive
biomarker for early detection of diabetic nephropathy. Clin Exp
Hypertens. 39:382–387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tian W, Wang L, Yuan L, Duan W, Zhao W,
Wang S and Zhang Q: A prognostic risk model for patients with
triple negative breast cancer based on stromal natural killer
cells, tumor-associated macrophages and growth-arrest specific
protein 6. Cancer Sci. 107:882–889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ammoun S, Provenzano L, Zhou L, Barczyk M,
Evans K, Hilton DA, Hafizi S and Hanemann CO: Axl/Gas6/NFκB
signalling in schwannoma pathological proliferation, adhesion and
survival. Oncogene. 33:336–346. 2014. View Article : Google Scholar
|
|
109
|
Buehler M, Tse B, Leboucq A, Jacob F,
Caduff R, Fink D, Goldstein DR and Heinzelmann-Schwarz V:
Meta-analysis of microarray data identifies GAS6 expression as an
independent predictor of poor survival in ovarian cancer. Biomed
Res Int. 2013:2382842013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Loges S, Schmidt T, Tjwa M, van Geyte K,
Lievens D, Lutgens E, Vanhoutte D, Borgel D, Plaisance S, Hoylaerts
M, et al: Malignant cells fuel tumor growth by educating
infiltrating leukocytes to produce the mitogen Gas6. Blood.
115:2264–2273. 2010. View Article : Google Scholar
|
|
111
|
Waizenegger JS, Ben-Batalla I, Weinhold N,
Meissner T, Wroblewski M, Janning M, Riecken K, Binder M,
Atanackovic D, Taipaleenmaeki H, et al: Role of growth
arrest-specific gene 6-Mer axis in multiple myeloma. Leukemia.
29:696–704. 2015. View Article : Google Scholar
|
|
112
|
Husain H, Scur M, Murtuza A, Bui N,
Woodward B and Kurzrock R: Strategies to overcome bypass mechanisms
mediating clinical resistance to EGFR tyrosine kinase inhibition in
lung cancer. Mol Cancer Ther. 16:265–272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Paolino M, Choidas A, Wallner S, Pranjic
B, Uribesalgo I, Loeser S, Jamieson AM, Langdon WY, Ikeda F, Fededa
JP, et al: The E3 ligase Cbl-b and TAM receptors regulate cancer
metastasis via natural killer cells. Nature. 507:508–512. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Viegas CS, Simes DC, Laizé V, Williamson
MK, Price PA and Cancela ML: Gla-rich protein (GRP), a new vitamin
K-dependent protein identified from sturgeon cartilage and highly
conserved in vertebrates. J Biol Chem. 283:36655–36664. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Viegas CS, Rafael MS, Enriquez JL,
Teixeira A, Vitorino R, Luís IM, Costa RM, Santos S, Cavaco S,
Neves J, et al: Gla-rich protein acts as a calcification inhibitor
in the human cardiovascular system. Arterioscler Thromb Vasc Biol.
35:399–408. 2015. View Article : Google Scholar
|
|
116
|
Surmann-Schmitt C, Dietz U, Kireva T, Adam
N, Park J, Tagariello A, Onnerfjord P, Heinegård D,
Schlötzer-Schrehardt U, Deutzmann R, et al: Ucma, a novel secreted
cartilage-specific protein with implications in osteogenesis. J
Biol Chem. 283:7082–7093. 2008. View Article : Google Scholar
|
|
117
|
Neacsu CD, Grosch M, Tejada M, Winterpacht
A, Paulsson M, Wagener R and Tagariello A: Ucmaa (Grp-2) is
required for zebrafish skeletal development. Evidence for a
functional role of its glutamate γ-carboxylation. Matrix Biol.
30:369–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cancela ML, Conceição N and Laizé V:
Gla-rich protein, a new player in tissue calcification? Adv Nutr.
3:174–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Mackie EJ, Tatarczuch L and Mirams M: The
skeleton: A multi-functional complex organ: The growth plate
chondrocyte and endochondral ossification. J Endocrinol.
211:109–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Granadeiro L, Dirks RP, Ortiz-Delgado JB,
Gavaia PJ, Sarasquete C, Laizé V, Cancela ML and Fernández I:
Warfarin-exposed zebrafish embryos resembles human warfarin
embryopathy in a dose and developmental-time dependent manner-from
molecular mechanisms to environmental concerns. Ecotoxicol Environ
Saf. 181:559–571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
R Sousa A, Barreira R and Santos E:
Low-dose warfarin maternal anticoagulation and fetal warfarin
syndrome. BMJ Case Rep. 2018:bcr20172231592018. View Article : Google Scholar
|
|
122
|
Maruotti N, Corrado A and Cantatore FP:
Osteoblast role in osteoarthritis pathogenesis. J Cell Physiol.
232:2957–2963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Sanchez C, Deberg MA, Piccardi N, Msika P,
Reginster JYL and Henrotin YE: Subchondral bone osteoblasts induce
phenotypic changes in human osteoarthritic chondrocytes.
Osteoarthritis Cartilage. 13:988–997. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Sokolove J and Lepus CM: Role of
inflammation in the pathogenesis of osteoarthritis: Latest findings
and interpretations. Ther Adv Musculoskelet Dis. 5:77–94. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Okuyan HM, Terzi MY, Ozcan O and Kalaci A:
Association of UCMA levels in serum and synovial fluid with
severity of knee osteoarthritis. Int J Rheum Dis. 22:1884–1890.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Misra D, Booth SL, Tolstykh I, Felson DT,
Nevitt MC, Lewis CE, Torner J and Neogi T: Vitamin K deficiency is
associated with incident knee osteoarthritis. Am J Med.
126:243–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hunt JL, Fairman R, Mitchell ME, Carpenter
JP, Golden M, Khalapyan T, Wolfe M, Neschis D, Milner R, Scoll B,
et al: Bone formation in carotid plaques: A clinicopathological
study. Stroke. 33:1214–1219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Cozzolino M, Fusaro M, Ciceri P, Gasperoni
L and Cianciolo G: The role of vitamin K in vascular calcification.
Adv Chronic Kidney Dis. 26:437–444. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Hruska KA: Vascular smooth muscle cells in
the pathogenesis of vascular calcification. Circ Res. 104:710–711.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Dhore CR, Cleutjens JP, Lutgens E,
Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM,
Vermeer C and Daemen MJ: Differential expression of bone matrix
regulatory proteins in human atherosclerotic plaques. Arterioscler
Thromb Vasc Biol. 21:1998–2003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kapustin AN and Shanahan CM: Calcium
regulation of vascular smooth muscle cell-derived matrix vesicles.
Trends Cardiovasc Med. 22:133–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kapustin AN, Davies JD, Reynolds JL,
McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot
D, Mayr M and Shanahan CM: Calcium regulates key components of
vascular smooth muscle cell-derived matrix vesicles to enhance
mineralization. Circ Res. 109:e1–e12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Toroian D, Lim JE and Price PA: The size
exclusion characteristics of type I collagen: Implications for the
role of noncollagenous bone constituents in mineralization. J Biol
Chem. 282:22437–22447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Price PA, Toroian D and Lim JE:
Mineralization by inhibitor exclusion: The calcification of
collagen with fetuin. J Biol Chem. 284:17092–17101. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
New SEP and Aikawa E: Molecular imaging
insights into early inflammatory stages of arterial and aortic
valve calcification. Circ Res. 108:1381–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ikeda K, Souma Y, Akakabe Y, Kitamura Y,
Matsuo K, Shimoda Y, Ueyama T, Matoba S, Yamada H, Okigaki M and
Matsubara H: Macrophages play a unique role in the plaque
calcification by enhancing the osteogenic signals exerted by
vascular smooth muscle cells. Biochem Biophys Res Commun.
425:39–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
New SEP, Goettsch C, Aikawa M, Marchini
JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K and
Aikawa E: Macrophage-derived matrix vesicles: An alternative novel
mechanism for microcalcification in atherosclerotic plaques. Circ
Res. 113:72–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Evrard S, Delanaye P, Kamel S, Cristol JP
and Cavalier E: SFBC/SN joined working group on vascular
calcifications: Vascular calcification: From pathophysiology to
biomarkers. Clin Chim Acta. 438:401–414. 2015. View Article : Google Scholar
|
|
139
|
Tesfamariam B: Involvement of vitamin
K-dependent proteins in vascular calcification. J Cardiovasc
Pharmacol Ther. 24:323–333. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Viegas CSB, Santos L, Macedo AL, Matos AA,
Silva AP, Neves PL, Staes A, Gevaert K, Morais R, Vermeer C, et al:
Chronic kidney disease circulating calciprotein particles and
extracellular vesicles promote vascular calcification: A role for
GRP (Gla-Rich Protein). Arterioscler Thromb Vasc Biol. 38:575–587.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Pasch A, Farese S, Gräber S, Wald J,
Richtering W, Floege J and Jahnen-Dechent W: Nanoparticle-based
test measures overall propensity for calcification in serum. J Am
Soc Nephrol. 23:1744–1752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Viegas CSB, Costa RM, Santos L, Videira
PA, Silva Z, Araújo N, Macedo AL, Matos AP, Vermeer C and Simes DC:
Gla-rich protein function as an anti-inflammatory agent in
monocytes/macrophages: Implications for calcification-related
chronic inflammatory diseases. PLoS One. 12:e01778292017.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Willems BA, Furmanik M, Caron MMJ, Chatrou
MLL, Kusters DHM, Welting TJM, Stock M, Rafael MS, Viegas CSB,
Simes DC, et al: Ucma/GRP inhibits phosphate-induced vascular
smooth muscle cell calcification via SMAD-dependent BMP signalling.
Sci Rep. 8:49612018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Karamouzis MV, Likaki-Karatza E, Ravazoula
P, Badra FA, Koukouras D, Tzorakoleftherakis E, Papavassiliou AG
and Kalofonos HP: Non-palpable breast carcinomas: Correlation of
mammographically detected malignant-appearing microcalcifications
and molecular prognostic factors. Int J Cancer. 102:86–90. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Kim JH, Ko ES, Kim DY, Han H, Sohn JH and
Choe DH: Noncalcified ductal carcinoma in situ: Imaging and
histologic findings in 36 tumors. J Ultrasound Med. 28:903–910.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Avdan Aslan A, Gültekin S, Esendağli
Yilmaz G and Kurukahvecioğlu O: Is there any association between
mammographic features of microcalcifications and breast cancer
subtypes in ductal carcinoma in situ? Acad Radiol. Jun
30–2020.Online ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Viegas CS, Herfs M, Rafael MS, Enriquez
JL, Teixeira A, Luís IM, van 't Hoofd CM, João A, Maria VL, Cavaco
S, et al: Gla-rich protein is a potential new vitamin K target in
cancer: Evidences for a direct GRP-mineral interaction. Biomed Res
Int. 2014:3402162014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Huisse MG, Leclercq M, Belghiti J, Flejou
JF, Suttie JW, Bezeaud A, Stafford DW and Guillin MC: Mechanism of
the abnormal vitamin K-dependent gamma-carboxylation process in
human hepatocellular carcinomas. Cancer. 74:1533–1541. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Pasierski T: Vitamin K antagonists in
anticoagulant therapy of patients with cancer. Pol Arch Med Wewn.
122:60–64. 2012.PubMed/NCBI
|
|
150
|
Vermeer C: Vitamin K: The effect on health
beyond coagulation-an overview. Food Nutr Res. 56:2012. View Article : Google Scholar
|
|
151
|
Cox RF, Hernandez-Santana A, Ramdass S,
McMahon G, Harmey JH and Morgan MP: Microcalcifications in breast
cancer: Novel insights into the molecular mechanism and functional
consequence of mammary mineralisation. Br J Cancer. 106:525–537.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Takeshita S, Kikuno R, Tezuka K and Amann
E: Osteoblast-specific factor 2: Cloning of a putative bone
adhesion protein with homology with the insect protein fasciclin I.
Biochem J. 294:271–278. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Coutu DL, Wu JH, Monette A, Rivard GE,
Blostein MD and Galipeau J: Periostin, a member of a novel family
of vitamin K-dependent proteins, is expressed by mesenchymal
stromal cells. J Biol Chem. 283:17991–8001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zhong H, Li X, Zhang J and Wu X:
Overexpression of periostin is positively associated with gastric
cancer metastasis through promoting tumor metastasis and invasion.
J Cell Biochem. 120:9927–9935. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Nakazawa T, Nakajima A, Seki N, Okawa A,
Kato M, Moriya H, Amizuka N, Einhorn TA and Yamazaki M: Gene
expression of periostin in the early stage of fracture healing
detected by cDNA microarray analysis. J Orthop Res. 22:520–525.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Li W, Gao P, Zhi Y, Xu W, Wu Y, Yin J and
Zhang J: Periostin: Its role in asthma and its potential as a
diagnostic or therapeutic target. Respir Res. 16:572015. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Duchamp de Lageneste O and Colnot C:
Periostin in bone regeneration. Adv Exp Med Biol. 1132:49–61. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Ai-Aql ZS, Alagl AS, Graves DT,
Gerstenfeld LC and Einhorn TA: Molecular mechanisms controlling
bone formation during fracture healing and distraction
osteogenesis. J Dent Res. 87:107–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Einhorn TA and Gerstenfeld LC: Fracture
healing: Mechanisms and interventions. Nat Rev Rheumatol. 11:45–54.
2015. View Article : Google Scholar :
|
|
160
|
Zhang X, Xie C, Lin AS, Ito H, Awad H,
Lieberman JR, Rubery PT, Schwarz EM, O'Keefe RJ and Guldberg RE:
Periosteal progenitor cell fate in segmental cortical bone graft
transplantations: Implications for functional tissue engineering. J
Bone Miner Res. 20:2124–2137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Neagu TP, Ţigliş M, Cocoloş I and Jecan
CR: The relationship between periosteum and fracture healing. Rom J
Morphol Embryol. 57:1215–1220. 2016.
|
|
162
|
Kudo A: Periostin in bone biology. Adv Exp
Med Biol. 1132:43–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Allen MR, Hock JM and Burr DB: Periosteum:
Biology, regulation, and response to osteoporosis therapies. Bone.
35:1003–1012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Kashima TG, Nishiyama T, Shimazu K,
Shimazaki M, Kii I, Grigoriadis AE, Fukayama M and Kudo A:
Periostin, a novel marker of intramembranous ossification, is
expressed in fibrous dysplasia and in c-Fos-overexpressing bone
lesions. Hum Pathol. 40:226–237. 2009. View Article : Google Scholar
|
|
165
|
Varughese R, Semprini R, Munro C,
Fingleton J, Holweg C, Weatherall M, Beasley R and Braithwaite I:
Serum periostin levels following small bone fractures, long bone
fractures and joint replacements: An observational study. Allergy
Asthma Clin Immunol. 14:302018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Roberts SJ, van Gastel N, Carmeliet G,
Carmeliet G and Luyten FP: Uncovering the periosteum for skeletal
regeneration: The stem cell that lies beneath. Bone. 70:10–18.
2015. View Article : Google Scholar
|
|
167
|
Matsuzawa M, Arai C, Nomura Y, Murata T,
Yamakoshi Y, Oida S, Hanada N and Nakamura Y: Periostin of human
periodontal ligament fibroblasts promotes migration of human
mesenchymal stem cell through the αvβ3 integrin/FAK/PI3K/Akt
pathway. J Periodont Res. 50:855–863. 2015. View Article : Google Scholar
|
|
168
|
Hwang EY, Jeong MS, Park EK, Kim JH and
Jang SB: Structural characterization and interaction of periostin
and bone morphogenetic protein for regulation of collagen
cross-linking. Biochem Biophys Res Commun. 449:425–431. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Rezaieyazdi Z, Falsoleiman H, Khajehdaluee
M, Saghafi M and Mokhtari-Amirmajdi E: Reduced bone density in
patients on long-term warfarin. Int J Rheum Dis. 12:130–135. 2009.
View Article : Google Scholar
|
|
170
|
Tufano A, Coppola A, Contaldi P, Franchini
M and Minno GD: Oral anticoagulant drugs and the risk of
osteoporosis: New anticoagulants better than old? Semin Thromb
Hemost. 41:382–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Jeong HM, Cho DH, Jin YH, Chung JO, Chung
MY, Chung DJ and Lee KY: Inhibition of osteoblastic differentiation
by warfarin and 18-α-glycyrrhetinic acid. Arch Pharm Res.
34:1381–1387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Verma D, Kumar R, Pereira RS, Karantanou
C, Zanetti C, Minciacchi VR, Fulzele K, Kunz K, Hoelper S,
Zia-Chahabi S, et al: Vitamin K antagonism impairs the bone marrow
microenvironment and hematopoiesis. Blood. 134:227–238. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Sugimoto I, Hirakawa K, Ishino T, Takeno S
and Yajin K: Vitamin D3, vitamin K2, and warfarin regulate bone
metabolism in human paranasal sinus bones. Rhinology. 45:208–213.
2007.PubMed/NCBI
|
|
174
|
Rousseau JC, Sornay-Rendu E, Bertholon C,
Chapurlat R and Garnero P: Serum periostin is associated with
fracture risk in postmenopausal women: A 7-year prospective
analysis of the OFELY study. J Clin Endocrinol Metab. 99:2533–2539.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Kim BJ, Rhee Y, Kim CH, Baek KH, Min YK,
Kim DY, Ahn SH, Kim H, Lee SH, Lee SY, et al: Plasma periostin
associates significantly with non-vertebral but not vertebral
fractures in post-menopausal women: Clinical evidence for the
different effects of periostin depending on the skeletal site.
Bone. 81:435–441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Rousseau JC, Sornay-Rendu E, Bertholon C,
Garnero P and Chapurlat R: Serum periostin is associated with
prevalent knee osteoarthritis and disease incidence/progression in
women: The OFELY study. Osteoarthr Cartil. 23:1736–1742. 2015.
View Article : Google Scholar
|
|
177
|
Snider P, Hinton RB, Moreno-Rodriguez RA,
Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L,
et al: Periostin is required for maturation and extracellular
matrix stabilization of noncardiomyocyte lineages of the heart.
Circ Res. 102:752–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Nagaraju CK, Robinson EL, Abdesselem M,
Trenson S, Dries E, Gilbert G, Janssens S, Van Cleemput J, Rega F,
Meyns B, et al: Myofibroblast phenotype and reversibility of
fibrosis in patients with end-stage heart failure. J Am Coll
Cardiol. 73:2267–2282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Zheng X, Wang S, Zou X, Jing Y, Yang R, Li
S and Wang F: Ginsenoside Rb1 improves cardiac function and
remodeling in heart failure. Exp Anim. 66:217–228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Chen Z, Xie J, Hao H, Lin H, Wang L, Zhang
Y, Chen L, Cao S, Huang X, Liao W, et al: Ablation of periostin
inhibits post-infarction myocardial regeneration in neonatal mice
mediated by the phosphatidylinositol 3 kinase/glycogen synthase
kinase 3β/cyclin D1 signalling pathway. Cardiovasc Res.
113:620–632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Hixson JE, Shimmin LC, Montasser ME, Kim
DK, Zhong Y, Ibarguen H, Follis J, Malcom G, Strong J, Howard T, et
al: Common variants in the periostin gene influence development of
atherosclerosis in young persons. Arterioscler Thromb Vasc Biol.
31:1661–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Hakuno D, Kimura N, Yoshioka M, Mukai M,
Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, et al:
Periostin advances atherosclerotic and rheumatic cardiac valve
degeneration by inducing angiogenesis and MMP production in humans
and rodents. J Clin Invest. 120:2292–2306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Lindner V, Wang Q, Conley BA, Friesel RE
and Vary CP: Vascular injury induces expression of periostin:
Implications for vascular cell differentiation and migration.
Arterioscler Thromb Vasc Biol. 25:77–83. 2005. View Article : Google Scholar
|
|
184
|
Schwanekamp JA, Lorts A, Vagnozzi RJ,
Vanhoutte D and Molkentin JD: Deletion of periostin protects
against atherosclerosis in mice by altering inflammation and
extracellular matrix remodeling. Arterioscler Thromb Vasc Biol.
36:60–68. 2016. View Article : Google Scholar
|
|
185
|
Ahlfeld SK, Gao Y, Wang J, Horgusluoglu E,
Bolanis E, Clapp DW and Conway SJ: Periostin downregulation is an
early marker of inhibited neonatal murine lung alveolar septation.
Birth Defects Res A Clin Mol Teratol. 97:373–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Bozyk PD, Bentley JK, Popova AP, Anyanwu
AC, Linn MD, Goldsmith AM, Pryhuber GS, Moore BB and Hershenson MB:
Neonatal periostin knockout mice are protected from
hyperoxia-induced alveolar simplication. PLoS One. 7:e313362012.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Naik PK, Bozyk PD, Bentley JK, Popova AP,
Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, et al:
Periostin promotes fibrosis and predicts progression in patients
with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol
Physiol. 303:L1046–L1056. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Kanemitsu Y, Suzuki M, Fukumitsu K, Asano
T, Takeda N, Nakamura Y, Ozawa Y, Masaki A, Ono J, Kurokawa R, et
al: A novel pathophysiologic link between upper and lower airways
in patients with chronic rhinosinusitis: Association of sputum
periostin levels with upper airway inflammation and olfactory
function. World Allergy Organ J. 13:1000942020. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Katoh S, Matsumoto N, Tanaka H, Yasokawa
N, Kittaka M, Kurose K, Abe M, Yoshioka D, Shirai R, Nakazato M and
Kobashi Y: Elevated levels of periostin and TGF-β1 in the
bronchoalveolar lavage fluid of patients with idiopathic
eosinophilic pneumonia. Asian Pac J Allergy Immunol. 38:208–213.
2020.
|
|
190
|
Tanaka J, Hebisawa A, Oguma T, Tomomatsu
K, Suzuki J, Shimizu H, Kawabata Y, Ishiguro T, Takayanagi N, Ueda
S, et al: Evaluating serum periostin levels in allergic
bronchopulmonary aspergillosis. Allergy. 75:974–977. 2020.
View Article : Google Scholar
|
|
191
|
Wilson MS and Wynn TA: Pulmonary fibrosis:
Pathogenesis, etiology and regulation. Mucosal Immunol. 2:103–121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Behr J, Kreuter M, Hoeper MM, Wirtz H,
Klotsche J, Koschel D, Andreas S, Claussen M, Grohé C, Wilkens H,
et al: Management of patients with idiopathic pulmonary fibrosis in
clinical practice: The INSIGHTS-IPF registry. Eur Respir J.
46:186–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Okamoto M, Hoshino T, Kitasato Y, Sakazaki
Y, Kawayama T, Fujimoto K, Ohshima K, Shiraishi H, Uchida M, Ono J,
et al: Periostin, a matrix protein, is a novel biomarker for
idiopathic interstitial pneumonias. Eur Respir J. 37:1119–1127.
2011. View Article : Google Scholar
|
|
194
|
Nance T, Smith KS, Anaya V, Richardson R,
Ho L, Pala M, Mostafavi S, Battle A, Feghali-Bostwick C, Rosen G
and Montgomery SB: Transcriptome analysis reveals differential
splicing events in IPF lung tissue. PLoS One. 9:e975502014.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Ashley SL, Wilke CA, Kim KK and Moore BB:
Periostin regulates fibrocyte function to promote myofibroblast
differentiation and lung fibrosis. Mucosal Immunol. 10:341–351.
2017. View Article : Google Scholar :
|
|
196
|
Yoshihara T, Nanri Y, Nunomura S,
Yamaguchi Y, Feghali-Bostwick C, Ajito K, Murakami S, Mawatari M
and Izuhara K: Periostin plays a critical role in the cell cycle in
lung fibroblasts. Respir Res. 21:382020. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Nanri Y, Nunomura S, Terasaki Y, Yoshihara
T, Hirano Y, Yokosaki Y, Yamaguchi Y, Feghali-Bostwick C, Ajito K,
Murakami S, et al: Cross-talk between transforming growth
factor-beta and periostin can be targeted for pulmonary fibrosis.
Am J Respir Cell Mol Biol. 62:204–216. 2020. View Article : Google Scholar
|
|
198
|
De Brouwer B, Piscaer I, Von Der Thusen
JH, Grutters JC, Schutgens RE, Wouters EF and Janssen R: Should
vitamin K be supplemented instead of antagonised in patients with
idiopathic pulmonary fibrosis? Expert Rev Respir Med. 12:169–175.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Woodruff PG, Boushey HA, Dolganov GM,
Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP,
Pantoja C, et al: Genome-wide profiling identifies epithelial cell
genes associated with asthma and with treatment response to
corticosteroids. Proc Natl Acad Sci USA. 104:15858–15863. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Izuhara K, Nunomura S, Nanri Y, Ogawa M,
Ono J, Mitamura Y and Yoshihara T: Periostin in inflammation and
allergy. Cell Mol Life Sci. 74:4293–4303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Tartibi HM and Bahna SL: Clinical and
biological markers of asthma control. Expert Rev Clin Immunol.
10:1453–1461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Johansson MW, Annis DS and Mosher DF:
α(M)β(2) integrin-mediated adhesion and motility of IL-5-stimulated
eosinophils on periostin. Am J Respir Cell Mol Biol. 48:503–510.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Sidhu SS, Yuan S, Innes AL, Kerr S,
Woodruff PG, Hou L, Muller SJ and Fahy JV: Roles of epithelial
cell-derived periostin in TGF-beta activation, collagen production,
and collagen gel elasticity in asthma. Proc Natl Acad Sci USA.
107:14170–14175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Kimur I, Tanizaki Y, Sato S, Saito K and
Takahashi K: Menaquinone (vitamin K2) therapy for bronchial asthma.
II. Clinical effect of menaquinone on bronchial asthma. Acta medica
Okayama. 29:127–135. 1975.PubMed/NCBI
|
|
205
|
Litonjua AA: Fat-soluble vitamins and
atopic disease: What is the evidence? Proc Nutr Soc. 71:67–74.
2012. View Article : Google Scholar :
|
|
206
|
El Basha NR, Osman HM, Abdelaal AA, Saed
SM and Shaaban HH: Increased expression of serum periostin and
YKL40 in children with severe asthma and asthma exacerbation. J
Investig Med. 66:1102–1108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Matsusaka M, Kabata H, Fukunaga K, Suzuki
Y, Masaki K, Mochimaru T, Sakamaki F, Oyamada Y, Inoue T, Oguma T,
et al: Phenotype of asthma related with high serum periostin
levels. Allergol Int. 64:175–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Kim MA, Izuhara K, Ohta S, Ono J, Yoon MK,
Ban GY, Yoo HS, Shin YS, Ye YM, Nahm DH and Park HS: Association of
serum periostin with aspirin-exacerbated respiratory disease. Ann
Allergy Asthma Immunol. 113:314–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Asano T, Kanemitsu Y, Takemura M, Yokota
M, Fukumitsu K, Takeda N, Ichikawa H, Uemura T, Takakuwa O, Ohkubo
H, et al: Serum periostin as a biomarker for comorbid chronic
rhinosinusitis in patients with asthma. Ann Am Thorac Soc.
14:667–675. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Cianchetti S, Cardini C, Puxeddu I,
Latorre M, Bartoli ML, Bradicich M, Dente F, Bacci E, Celi A and
Paggiaro P: Distinct profile of inflammatory and remodelling
biomarkers in sputum of severe asthmatic patients with or without
persistent airway obstruction. World Allergy Organ J.
12:1000782019. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Li Y, Chen JP, Duan L and Li S: Effect of
vitamin K2 on type 2 diabetes mellitus: A review. Diabetes Res Clin
Pract. 136:39–51. 2018. View Article : Google Scholar
|
|
212
|
Mukai K, Morimoto H, Kikuchi S and Nagaoka
S: Kinetic study of free-radical-scavenging action of biological
hydroquinones (reduced forms of ubiquinone, vitamin K and
tocopherol quinone) in solution. Biochim Biophys Acta.
1157:313–317. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Westhofen P, Watzka M, Marinova M, Hass M,
Kirfel G, Müller J, Bevans CG, Müller CR and Oldenburg J: Human
vitamin K 2,3-epoxide reductase complex subunit 1-like 1 (VKORC1L1)
mediates vitamin K-dependent intracellular anti-oxidant function. J
Biol Chem. 286:15085–15094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Vos M, Esposito G, Edirisinghe JN, Vilain
S, Haddad DM, Slabbaert JR, Van Meensel S, Schaap O, De Strooper B,
Meganathan R, et al: Vitamin K2 is a mitochondrial electron carrier
that rescues pink1 deficiency. Science. 336:1306–1310. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Fujii S, Shimizu A, Takeda N, Oguchi K,
Katsurai T, Shirakawa H, Komai M and Kagechika H: Systematic
synthesis and anti-inflammatory activity of ω-carboxylated
menaquinone derivatives-investigations on identified and putative
vitamin K2 metabolites. Bioorg Med Chem. 23:2344–2352. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Myneni VD and Mezey E: Immunomodulatory
effect of vitamin K2: Implications for bone health. Oral Dis.
24:67–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Ishizuka M, Kubota K, Shimoda M, Kita J,
Kato M, Park KH and Shiraki T: Effect of menatetrenone, a vitamin
k2 analog, on recurrence of hepatocellular carcinoma after surgical
resection: A prospective randomized controlled trial. Anticancer
Res. 32:5415–5420. 2012.PubMed/NCBI
|
|
218
|
Zhong JH, Mo XS, Xiang BD, Yuan WP, Jiang
JF, Xie GS and Li LQ: Postoperative use of the chemopreventive
vitamin K2 analog in patients with hepatocellular carcinoma. PLoS
One. 8:e580822013. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Enomoto M, Tsuchida A, Miyazawa K,
Yokoyama T, Kawakita H, Tokita H, Naito M, Itoh M, Ohyashiki K and
Aoki T: Vitamin K2-induced cell growth inhibition via autophagy
formation in cholangiocellular carcinoma cell lines. Int J Mol Med.
20:801–808. 2007.PubMed/NCBI
|
|
220
|
Sibayama-Imazu T, Fujisawa Y, Masuda Y,
Aiuchi T, Nakajo S, Itabe H and Nakaya K: Induction of apoptosis in
PA-1 ovarian cancer cells by vitamin K2 is associated with an
increase in the level of TR3/Nur77 and its accumulation in
mitochondria and nuclei. J Cancer Res Clin Oncol. 134:803–812.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Showalter SL, Wang Z, Costantino CL,
Witkiewicz AK, Yeo CJ, Brody JR and Carr BI: Naturally occurring K
vitamins inhibit pancreatic cancer cell survival through a
caspase-dependent pathway. J Gastroenterol Hepatol. 25:738–744.
2010. View Article : Google Scholar
|
|
222
|
Xv F, Chen J, Duan L and Li S: Research
progress on the anticancer effects of vitamin K2. Oncol Lett.
15:8926–8934. 2018.PubMed/NCBI
|