Introduction
Diabetic nephropathy (DN) is a major microvascular
complication of diabetes that can lead to end-stage renal disease
(1,2). The pathogenesis of DN is remarkably
complex, involving hyperglycemia-mediated intracellular metabolism
disorders, autophagy, oxidative stress and endoplasmic reticulum
stress, thereby resulting in increased mesangial matrix, thickened
basement membrane and extensive, persistent proteinuria (3,4).
However, the underlying mechanisms have not yet been elucidated to
date, and there are currently no effective prevention or treatment
strategies. Podocytes are highly differentiated epithelial cells
that enclose glomerular capillaries and are involved in maintaining
the glomerular filtration barrier (5,6).
The structural integrity of podocytes is crucial for preventing the
leakage of albumin and microalbuminuria (7,8).
At present, the mechanism of podocyte dysfunction in chronic kidney
disease has been attracting increasing attention.
Polyamines (spermine, spermidine and putrescine) in
humans, which are absorbed by the small intestine, are mainly
produced by food intake, cell synthesis and intestinal
microorganisms (5,6). Polyamines have a variety of
biological functions, including regulating cell proliferation,
differentiation and apoptosis (7,8).
Polyamine metabolism, the abnormalities of which affect the
progression of cardiovascular and kidney diseases, is mainly
regulated by ornithine decarboxylase (ODC) and spermidine/spermine
N1-acetyltransferase (SSAT) (9).
Among them, spermine has the strongest biological activity, which
can play a protective role in the progression of diabetic
cardiomyopathy, myocardial hypertrophy and renal
ischemia/reperfusion (I/R) injury by promoting autophagy,
inhibiting inflammatory reactions, oxidative stress and endoplasmic
reticulum stress, and regulating calcium homeostasis (10-12). However, whether DN and podocyte
damage are implicated in abnormal polyamine metabolism in the
kidney is unclear, and no relevant studies have been conducted thus
far.
In the present study, type 1 diabetic (T1D) rats and
high glucose (HG)-induced podocyte injury were investigated; the
association between polyamine homeostasis and autophagy and the
related signaling pathways were examined. In addition, exogenous
polyamine treatment was used to observe the central role of
abnormal polyamine metabolism in the pathogenesis of DN. These
results may help elucidate the pathogenesis of DN from a novel
perspective and suggest future strategies for the prevention and
treatment of this condition.
Materials and methods
Animal and model
Male Wistar rats (age, 8 weeks old; weight, 200-250
g) were purchased from the animal care facility at the Second
Affiliated Hospital of Harbin Medical University (Harbin, China).
All animal experimental protocols complied with the Guide for the
Care and Use of Laboratory Animals published by the National
Institutes of Health (13). The
present study was approved by the Institutional Animal Research
Committee of Harbin Medical University. All animals were housed at
the animal care facility of Harbin Medical University at 25°C with
a 12/12 h light/dark cycle in a vivarium with humidified airflow,
and were allowed free access to normal rat chow and water
throughout the study period.
Diabetes can be induced by an intraperitoneal of
streptozotocin (STZ). A total of 45 rats were randomly divided into
three groups: i) Control (n=15), 0.1 M sterile citrate buffer (pH
4.5) was injected intraperitoneally as a control at the same volume
as that in the model group; ii) T1D (n=15), a single
intraperitoneal injection of STZ (60 mg/kg, dissolved in 0.1 M, pH
4.5 citric acid-citrate sodium buffer) was used to establish the
T1D model. When the concentration of serum glucose was >16.7
mmol/l, the T1D model was considered to be established successfully
(14); and iii) T1D + Sp (n=15),
spermine solution (2.5 mg/kg/day, dissolved in normal saline) was
injected intraperitoneally every day for 2 weeks before STZ
injection, followed by an injection of spermine (2.5 mg/kg/day)
every other day for 12 weeks. Rats in each group were euthanized at
week 12 of modeling. Under anesthesia with intraperitoneal 2%
pentobarbital (50 mg/kg, dissolved in normal sodium), the kidneys
were removed by opening the abdomen and 0.1% lidocaine (2 mg/kg,
dissolved in normal saline) was used to infiltrate the incision
wound for analgesia. The rats were sacrificed after surgery with an
overdose of 2% pentobarbital (100 mg/kg injected intravenously)
(15).
Measurement of biochemical
parameters
Blood glucose and body weight were measured at weeks
0, 4, 8 and 12 in each group. Urine samples were collected for 24 h
on week 12, while blood was collected from the retro-orbital vein
by removing the eyeballs. Subsequently, the rats were euthanized,
and the kidneys were then removed and weighed. The levels of
urinary albumin excretion (UAE; cat. no. ZK-H1678), serum creatine
(Scr; cat. no. GMS70021.7) and urea (cat. no. GMS70022.1) were
measured using appropriate ELISA kits (Shenzhen Ziker Biological
Technology Co., Ltd.).
Kidney histology and
immunohistochemistry
Sections of kidney tissues were fixed with 4%
paraformaldehyde at room temperature for 48 h, embedded in paraffin
and sectioned into 4-µm sections. Renal sections were
stained at room temperature with hematoxylin and eosin (H&E)
for 3 min, and with periodic acid-Schiff staining (PAS) for 30 sec
for 5 min, and then sections were assessed by light microscopy.
Images of 20 glomeruli per rat were obtained, and the PAS-positive
(purple) area per glomeruli was quantified using ImageJ v1.8.0
software (National Institutes of Health).
For immunohistochemistry, paraffin-embedded sections
were blocked with 5% BSA (cat. no. SW3015; Beijing Solarbio Science
& Technology Co., Ltd.) for 30 min at room temperature and
stained with an antibody against podocin (1:50; cat. no. ab50339;
Abcam) at 4°C overnight, and then washed with PBS. Next, the
mixture was incubated with the secondary antibody (goat anti-rabbit
IgG; 1:500; cat. no. ab6721; Abcam) for 1 h at room temperature and
washed with PBS. Finally, the sections were stained with a solution
of diaminobenzidine for 2 min at room temperature, washed with PBS,
dehydrated and permeabilized with xylene. Stained images (10
glomeruli per kidney) were visualized by light microscopy and all
the images were analyzed with ImageJ software.
Cell culture and treatment
Rat podocytes (cat. no. BNCC338697; BeiNa Culture
Collection; Beijing Beina Chunglian Biotechnology Research
Institute) were cultured at 37°C in a 5% CO2 humidified
incubator in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin. Podocytes were cultured for
6-12 h in DMEM containing 5.5 mM D-glucose (Sigma-Aldrich; Merck
KGaA) without FBS before exposure to various experimental
conditions. The cells were randomly divided into six groups: i)
Normal-glucose group (NG), where the podocytes were incubated in
DMEM containing 5.5 mM glucose for 48 h; ii) mannitol group (M),
where the podocytes were incubated in DMEM containing 40 mM
mannitol and 5.5 mM glucose for 48 h; iii) high-glucose group (HG),
where the podocytes were incubated in DMEM containing 40 mM glucose
for 48 h; iv) spermine-treated group (HG + Sp), where the podocytes
were pretreated with 1, 5, 10, 20 and 50 µM spermine
(Sigma-Aldrich; Merck KGaA) for 2 h before being subjected to HG
conditions; v) rapamycin-treated group (HG + Rap), where the
podocytes were pretreated with 10 mM rapamycin (MCE) for 2 h before
being subjected to HG conditions; and vi) Compound C treatment
group (HG + Sp + Compound C), where 10 mM Compound C (Selleck
Chemicals), an AMPK inhibitor, was added to the medium 1 h before
HG and spermine addition.
Cell viability assay
Quantitative evaluation of cell viability was
performed using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) assay according to the manufacturer's
instructions. Cells were seeded in 96-well plates at a
concentration of 1×103 cells/well. After 24 h of
treatment in each group of cells, 10 µl CCK-8 was added to
each well and incubated for 2 h at 37°C. Next, the absorbance at
570 nm was determined using a microplate spectrophotometer
reader.
Apoptosis assay by Hoechst 33342
staining
Hoechst 33342 is a blue, fluorescent dye that can
penetrate the cell membrane and is used for apoptosis detection.
Podocytes were plated into 24-well dishes (2×105
cells/well) and were cultured under the aforementioned conditions.
Then, cells were rinsed with PBS and incubated for 10 min at room
temperature using 5 mg/ml Hoechst 33342. Staining was observed by
fluorescence microscopy (Nikon Corporation) at ×400 magnification.
The excitation wavelength was maintained at 380 nm. When apoptosis
occurred, the nuclei appeared as dense or fragmented.
Electron microscopy
The ultrastructure of the kidney was detected by
electron microscopy. Samples of renal sections were cut into pieces
(<1 mm) and fixed in 2.5% glutaraldehyde for 4 h at room
temperature. Tissues were post-fixed in 1% osmium tetroxide with
0.1 M sodium cacodylate, dehydrated through graded concentrations
of ethanol and propylene oxide, and subsequently embedded in Epon
812 for 24 h at 60°C. Ultrathin sections were cut from blocks and
mounted on copper grids for counterstaining with lead citrate and
uranium acetate for 7 min each at 75°C. The sections were observed
under a Philips CM 120 electron microscope (Philips Medical
Systems, Inc.).
Immunoblotting
Rat renal tissues and rat podocytes were homogenized
in 0.5 ml RIPA buffer prior to being transferred to small tubes and
rotated 20 sec per 5 min six times. Solubilized proteins were
collected after centrifugation at 10,000 × g at 4°C for 30 min. The
supernatant was collected and stored at −80°C. The protein
concentration of each sample was quantified using an enhanced BCA
protein assay kit. For detection of protein levels, protein lysates
(40 µg protein loaded per lane) from each group of cells and
tissues were separated via SDS-PAGE on a 10% gel, and subsequently
separated proteins were electrotransferred onto a PVDF membrane
(EMD Millipore). After blocking with TBS with 0.1% Tween-20 (TBST)
containing 5% non-fat dry milk for 1 h at 4°C, the membrane was
incubated overnight at 4°C with antibodies against ODC (1:1,000;
cat. no. ab97395; Abcam), SSAT (1:1,000; cat. no. ab105220; Abcam),
nephrin (1:400; cat. no. BA1669; Boster Biological Technology),
podocin (1:400; cat. no. BA3416; Boster Biological Technology),
CD2-associated protein (CD-2AP; 1:1,000; cat. no. bs-0512R; BIOSS),
microtube-associated proteins 1A/1B light chain 3 (LC3; 1:1,000;
cat. no. 3868T; Cell Signaling Technology, Inc.), Beclin 1
(1:1,000; cat. no. 3495T; Cell Signaling Technology, Inc.), P62
(1:1,000; cat. no. 8025T; Cell Signaling Technology, Inc.),
autophagy protein 5 (Atg5; 1:1,000; cat. no. 12994T; Cell Signaling
Technology, Inc.), cleaved caspase-3 (1:1,000; cat. no. 9661T; Cell
Signaling Technology, Inc.), total (t)-mTOR (1:1,000; cat. no.
A00003-2; Boster Biological Technology), phosphorylated (p)-mTOR
(1:600; cat. no. BM4840; Boster Biological Technology), t-AMPK
(1:1,000; cat. no. A00994-6; Boster Biological Technology), and
p-AMPK (1:1,000; cat. no. P00994; Boster Biological Technology).
The membrane blots were washed and incubated at 4°C with
anti-rabbit (cat. no. ab6721) or anti-mouse (cat. no. ab6728) IgG
antibodies (1:10,000; Abcam) for 1.5 h. The signals were detected
by an enhanced chemiluminescence kit (Thermo Fisher Scientific,
Inc.) and the Multiplex Fluorescence Imaging System
(ProteinSimple). The intensities of the protein bands were
semi-quantified by AlphaView SA 3.0 software (ProteinSimple) and
normalized to β-actin.
Immunofluorescence staining
Podocytes were plated into 24-well dishes
(2×105 cells/well) and cultured under the indicated
conditions. Cells were fixed with 4% paraformaldehyde at room
temperature for 24 h, permeabilized in 0.5% Triton X-100 for 30 min
and blocked with 1% BSA for 1 h at 4°C. Next, cells were washed and
incubated with anti-nephrin antibodies (1:1,000; cat. no. ab216341;
Abcam) overnight at 4°C. Finally, the cells were incubated with a
secondary anti-body (goat anti-rabbit IgG; 1:1,000; cat. no.
ab6721; Abcam) for 2 h at room temperature, and observed with a
fluorescence microscope at ×400 magnification (Nikon
Corporation).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Each measurement was obtained by performing ≥3
independent experiments. Statistical comparisons were conducted
using paired or unpaired t-tests or one-way ANOVA followed by
Bonferroni post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Spermine improves the general condition
and renal function parameters of T1D rats
The present study established a rat model of
intraperitoneally injected STZ-induced diabetes. The results showed
that, compared with those of the control group, the blood glucose
and the kidney weight/body weight ratio of the T1D rats were
increased (Fig. 1A and C), while
their body weight was mildly decreased (Fig. 1B). The animals also developed
common signs of T1D, including polydipsia, polyuria, and noticeable
hypoactivity and weakness (data not shown). Renal function
parameters were evaluated, and it was found that Scr, serum urea
and UAE in the T1D group were significantly increased (Fig. 1D-F).
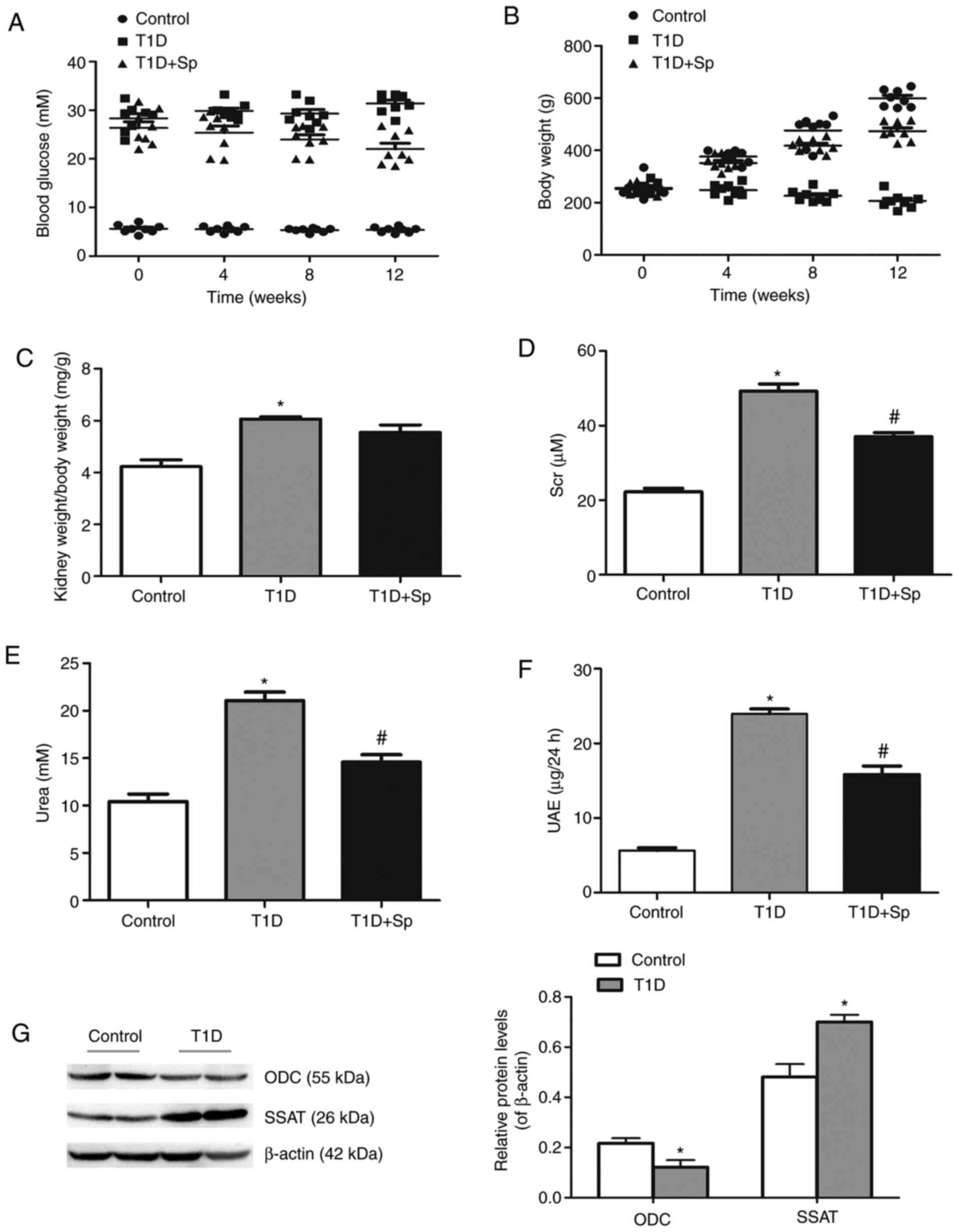 | Figure 1Spermine improves the general
condition and renal function parameters of T1D rats. (A) Blood
glucose levels and (B) body weight were measured at 0, 4, 8 and 12
weeks after the STZ-induced diabetic rat model was established. (C)
Kidney weight/body weight ratio, (D) Scr, (E) urea and (F) UAE were
measured at 12 weeks in STZ-induced diabetic rats and
spermine-pretreated rats. (G) Representative immunoblotting
analysis of the protein levels of ODC and SSAT in STZ-induced rats.
Data are expressed as the mean ± standard error of the mean (n=6).
*P<0.05 vs. the control group; #P<0.05
vs. the T1D group. T1D, type 1 diabetic; STZ, streptozotocin; Scr,
serum creatinine; UAE, urinary albumin excretion; ODC, ornithine
decarboxylase; SSAT, spermidine/spermine N1-acetyltransferase; Sp,
spermine-treated group. |
Next, the expression of ODC and SSAT was detected by
immunoblotting. The results showed that the expression of ODC was
decreased, while that of SSAT was increased, in the T1D group
(Fig. 1G), indicating that the
metabolism of polyamines was altered and the content of endogenous
spermine was decreased. Therefore, exogenous spermine pretreatment
was added in subsequent experiments. The results revealed that
exogenous spermine could improve the general condition and renal
function-related indicators in T1D rats, which suggested that
spermine may exert a protective effect on diabetic renal
function.
Spermine improves the morphological and
ultrastructural changes of the kidney in T1D rats
PAS and H&E staining were used to observe the
morphological changes of the kidney. The results revealed moderate
mesangial proliferation, matrix accumulation and parietal
endothelial cell proliferation in the T1D group, which further
verified renal injury. These pathological changes were attenuated
in the presence of spermine (Fig.
2A, B and D). Electron microscopic examination revealed that
podocytes were disorganized with obvious loss and effacement of
foot processes (FPs) and thickening of the glomerular basement
membrane (GBM) in the T1D group, but these abnormalities were
alleviated in the T1D + Sp group (Fig. 2C and E). Taken together, these
results suggested that spermine may protect against diabetic kidney
damage.
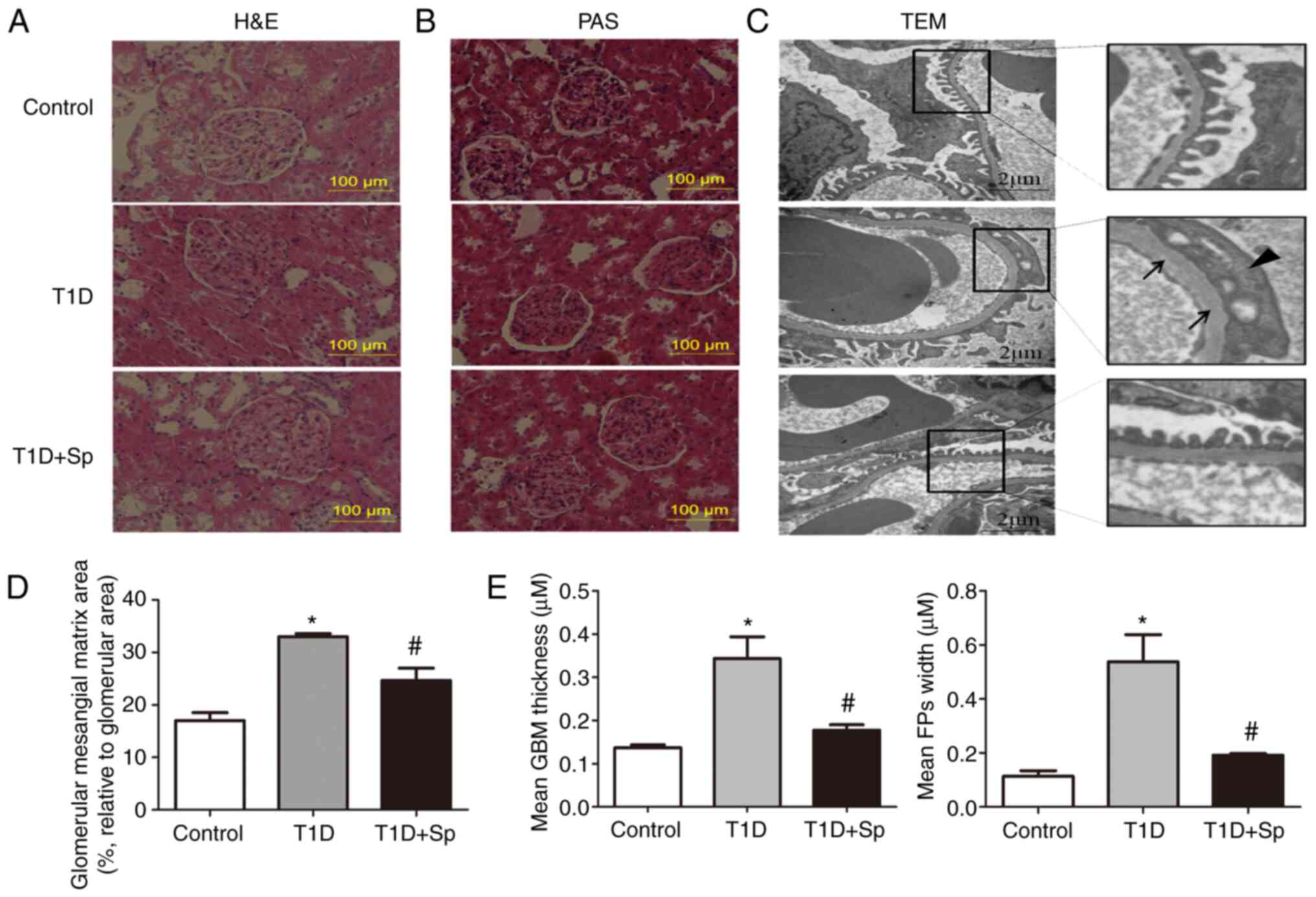 | Figure 2Spermine improves the morphological
and ultrastructural changes of the kidney in T1D rats. (A)
Representative images of H&E staining (scale bar, 100
µm). (B) Representative images of PAS staining (Scale bar,
100 µm). (C) Representative TEM images (scale bar, 2
µm). (D) Quantification of the glomerular mesangial matrix
area (percentage relative to the glomerular area) of the kidney
sections. (E) Quantitative assessment of the GBM thickness (arrows)
and FPs width (triangle). Data are expressed as the mean ± standard
error of the mean (n=6). *P<0.05 vs. the control
group; #P<0.05 vs. the T1D group. T1D, type 1
diabetic; H&E, hematoxylin and eosin; PAS, periodic
acid-Schiff; GBM, glomerular basement membrane; FPs, foot
processes; TEM, transmission electron microscopy; Sp,
spermine-treated group. |
Spermine attenuates podocyte injury and
promotes autophagy in T1D rats
In vivo, the expression levels of
podocyte-specific proteins, including nephrin, CD-2AP and podocin,
were detected by immunoblotting and immunohistochemistry. The
expression levels of podocyte-related proteins were significantly
reduced in the T1D group (Fig.
3A). Immunohistochemical staining of podocin further supported
the aforementioned results (Fig.
3B).
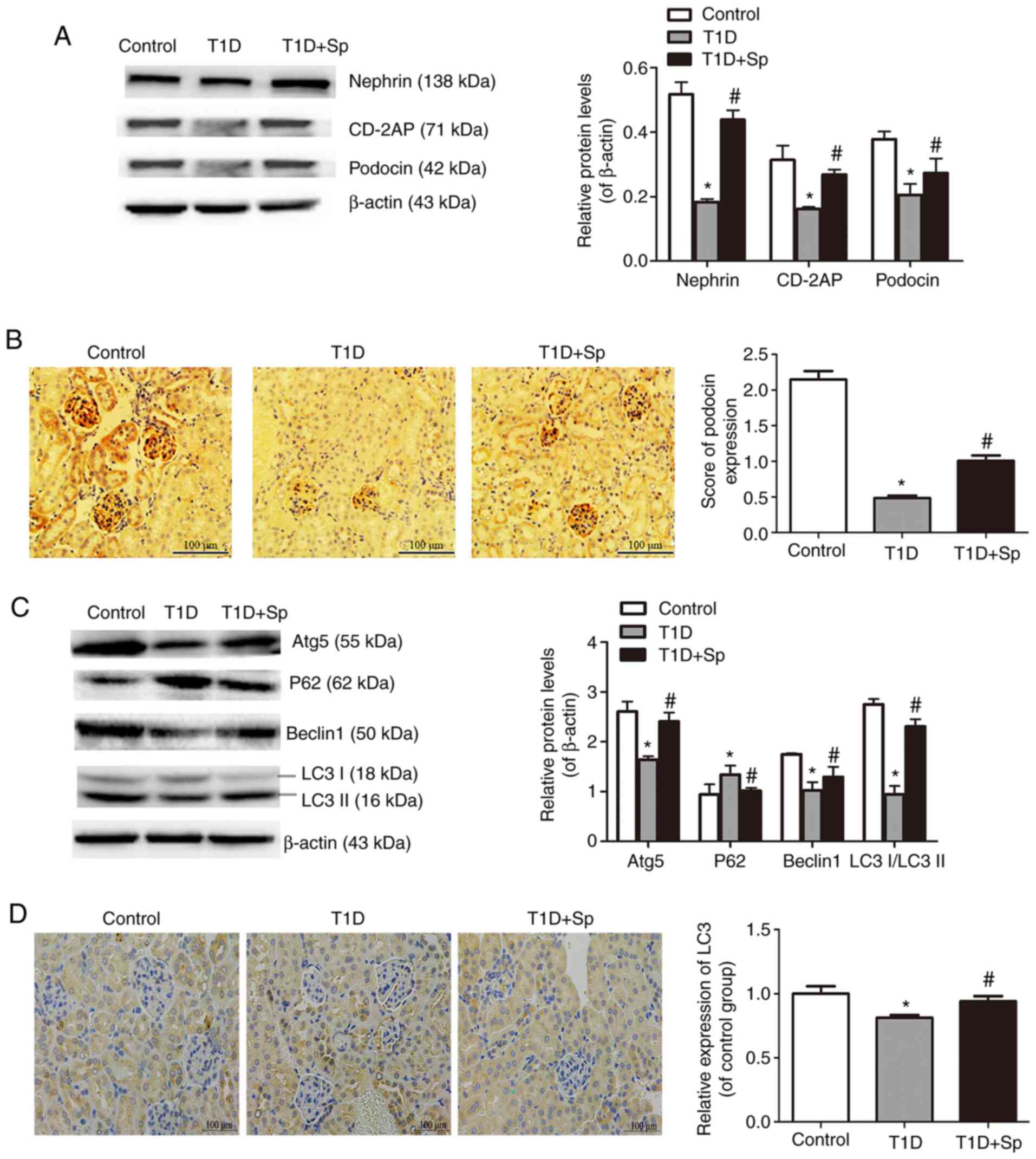 | Figure 3Spermine attenuates podocyte injury
and promotes autophagy in T1D rats. (A) Representative
immunoblotting of nephrin, CD2-AP and podocin protein levels in
kidney sections. (B) Representative immunohistochemical staining
for podocin in kidney sections (scale bar, 100 µm) and its
quantification. (C) Representative immunoblotting for Atg5, P62,
Beclin1 and LC3II/LCI in kidney sections. (D) Representative
immunohistochemical staining for LC3 in kidney sections (scale bar,
100 µm) and its quantification. Data are expressed as the
mean ± standard error of the mean (n=6). *P<0.05 vs.
the control group; #P<0.05 vs. the T1D group. T1D,
type 1 diabetic; CD-2AP, CD2-associated protein; Atg5, autophagy
protein 5; LC3, microtube-associated proteins 1A/1B light chain 3;
Sp, spermine-treated group. |
Next, the expression of autophagy-related proteins
were examined. In comparison with the control group, the expression
levels of Atg5, Beclin 1 and LC3II/LC3I were decreased and P62 was
increased in the T1D group (Fig.
3C). Immunohistochemical staining revealed a mildly decreased
expression of LC3 localized to glomeruli in T1D rats (Fig. 3D), which confirmed autophagy
insufficiency in DN rats. These changes were obviously improved by
exogenous spermine pretreatment. Collectively, these results
indicated that the effect of spermine in DN was associated with
activation of autophagy.
Spermine mitigates HG-induced podocyte
injury
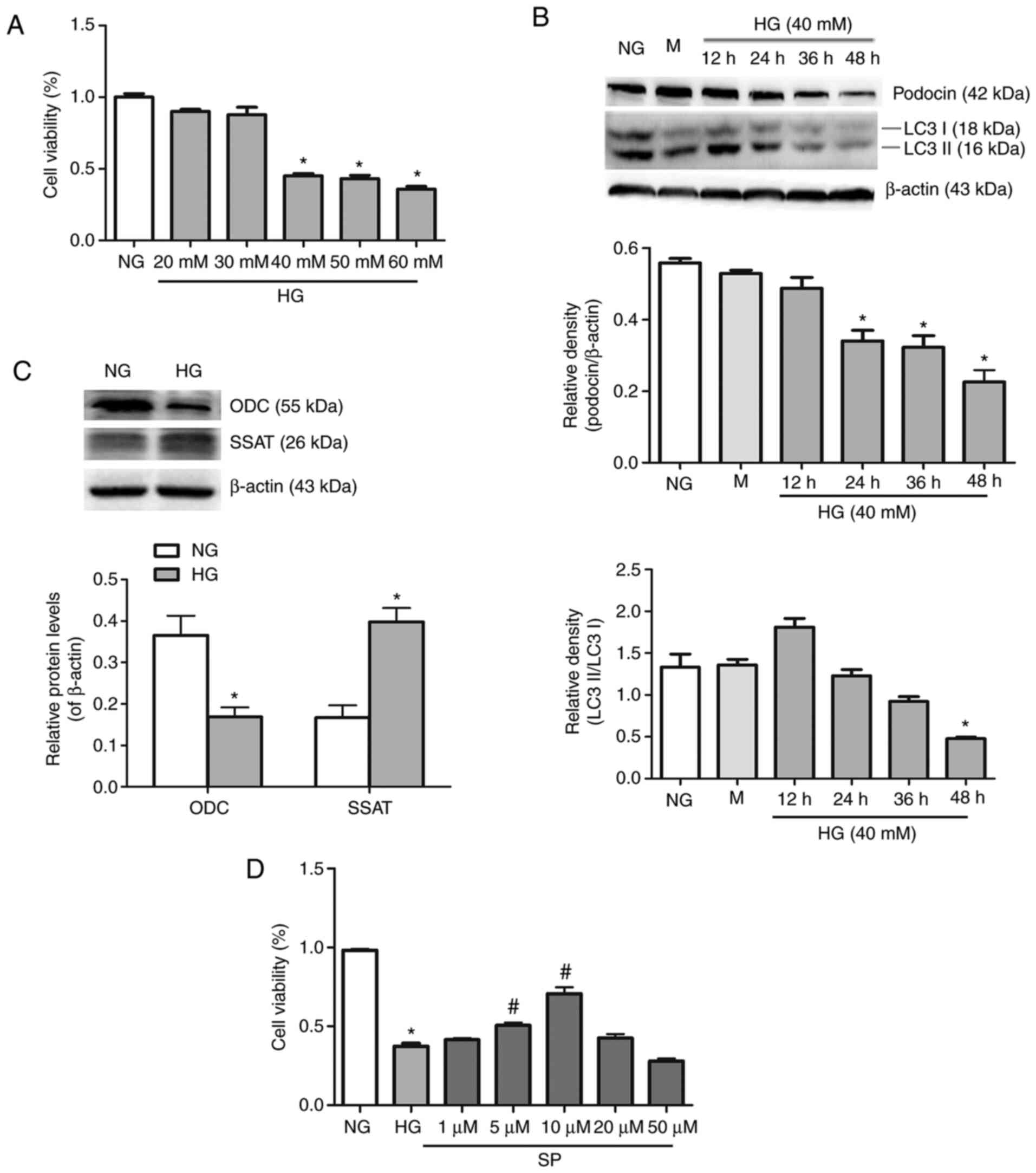
A CCK-8 assay was used in vitro to detect the
activity of podocytes subjected to different glucose
concentrations. Previous studies reported that podocytes exhibited
lower activity under HG stimulation for 48 h (16). The present study found that, when
podocytes were treated with glucose at a concentration of >40 mM
for 48 h, cell viability was decreased significantly (Fig. 4A). LC3 is a soluble protein,
which can be hydrolyzed to LC3I to form LC3II, thus reflecting the
change in autophagy flow (17).
Immunoblotting was used to observe the protein expression of
podocin and LC3II/LC3I at different time points in order to
determine the association between podocyte injury and autophagy. As
shown in Fig. 4B, under HG
conditions, podocin expression was decreased in a time-dependent
manner, while LC3II/LC3I expression was decreased gradually after
12 h, which indicated that podocyte injury may be associated with a
reduction of autophagy.
To observe whether podocyte injury was associated
with polyamine metabolism, the present study detected changes in
polyamine metabolizing enzymes. As shown in Fig. 4C, immunoblotting revealed
decreased ODC expression and increased SSAT expression in
HG-induced podocytes. Podocytes were treated with different
concentrations of spermine (1, 5, 10, 20 and 50 µM) in HG
medium. The results indicated that 5 and 10 µM spermine
treatment of HG-induced podocytes increased cell viability.
However, when the concentration of spermine was >10 µM,
cell activity was decreased (Fig.
4D). The aforementioned results validated the conditions used
in subsequent experiments.
Spermine reduces HG-induced apoptosis by
activating autophagy in podocytes
To determine the role of autophagy during spermine
treatment of podocytes, the effect of rapamycin, which is known to
induce autophagy, on podocyte injury was examined. The podocytes
were pretreated with rapamycin for 2 h before HG treatment. In
accordance with the results of spermine pretreatment, rapamycin
also partially reversed the effects of HG on cell viability
(Fig. 5A), indicating that
spermine can also act as an autophagic agonist.
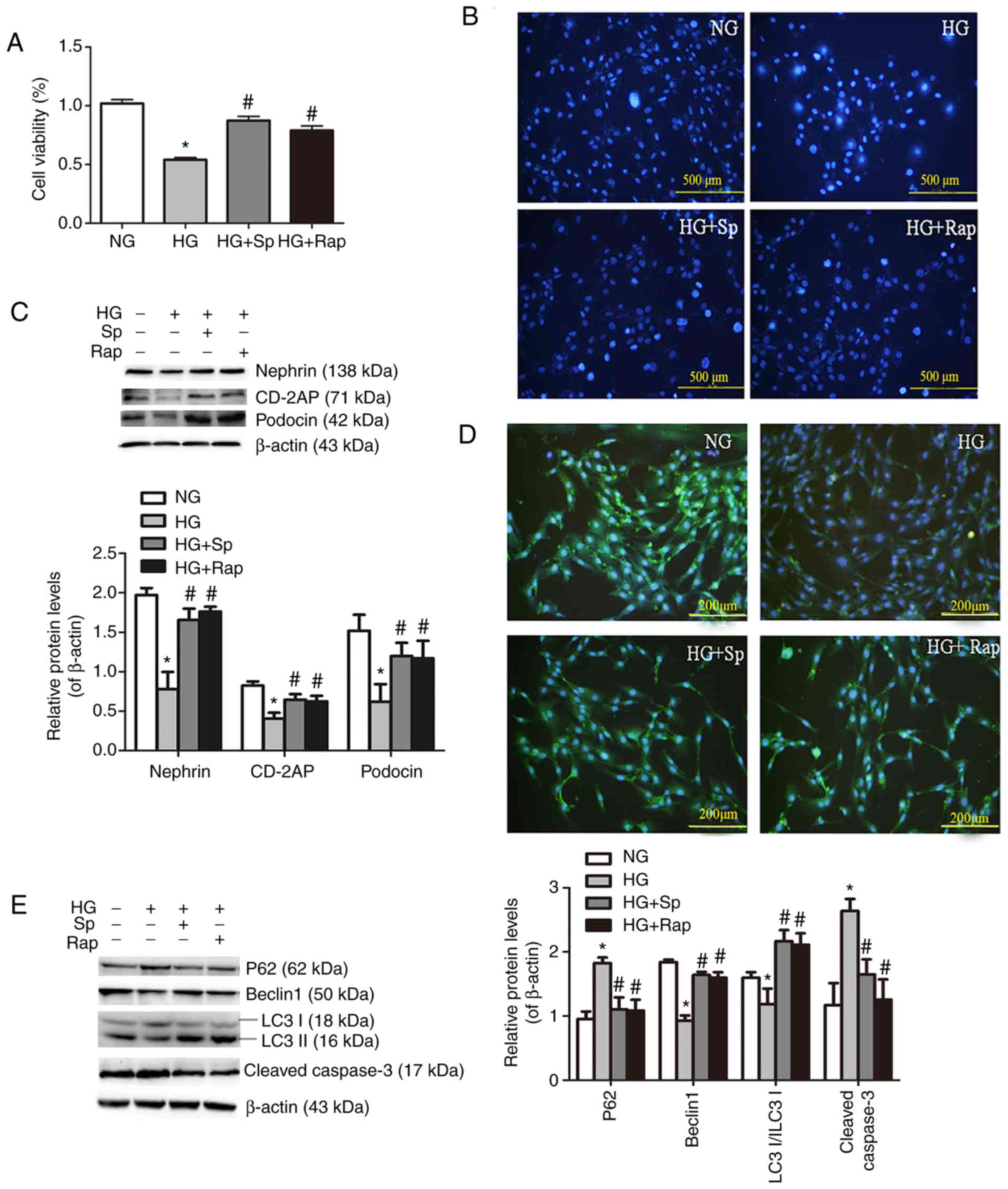 | Figure 5Spermine reduces HG-induced apoptosis
via activating autophagy in podocytes. (A) Cell Counting Kit-8
assays were performed using podocytes incubated under HG conditions
in the presence of spermine or rapamycin. (B) Hoechst 33342
staining (scale bar, 500 µm). (C) Representative
immunoblotting analyses of the proteins levels of nephrin, CD-2AP
and podocin protein levels. (D) Representative images of
immunofluorescence staining for nephrin under different culture
conditions (scale bar, 200 µm). (E) Representative
immunoblotting analyses for P62, Beclin1, LC3II/LC3I and cleaved
caspase-3. Data are expressed as the mean ± standard error of the
mean (n=6-8). *P<0.05 vs. the NG group;
#P<0.05 vs. the HG group. HG, high glucose; CD-2AP,
CD2-associated protein; LC3, microtube-associated proteins 1A/1B
light chain 3; Sp, spermine-treated group; NG, normal glucose. |
Cell apoptosis was quantified by Hoechst 33342
staining and immunoblotting. It was observed that HG-induced
podocytes showed markedly increased nuclear aggregation, DNA
fragmentation and caspase-3 cleavage activity (Fig. 5B and E), indicating that HG
treatment induced podocytes apoptosis. Next, podocyte injury was
evaluated by immunoblotting and immunofluorescence with
podocyte-specific markers. There was a significant reduction in
nephrin, podocin and CD-2AP expression in the HG group (Fig. 5C). In agreement with the results
of immunoblotting, the data were confirmed by measuring the
nephrin-positive area by immunofluorescence staining (Fig. 5D). Finally, to further observe
the autophagy of podocytes in vitro, autophagy-related
proteins were detected. Immunoblotting showed that Beclin 1 and
LC3II/LC3I were decreased, while P62 was increased (Fig. 5E), which was consistent with the
in vivo results. Taken together, the aforementioned findings
indicated that DN changes were improved by spermine and rapamycin
treatment, which suggested that the protective effect of spermine
on podocyte injury may be due to a reduction in autophagy.
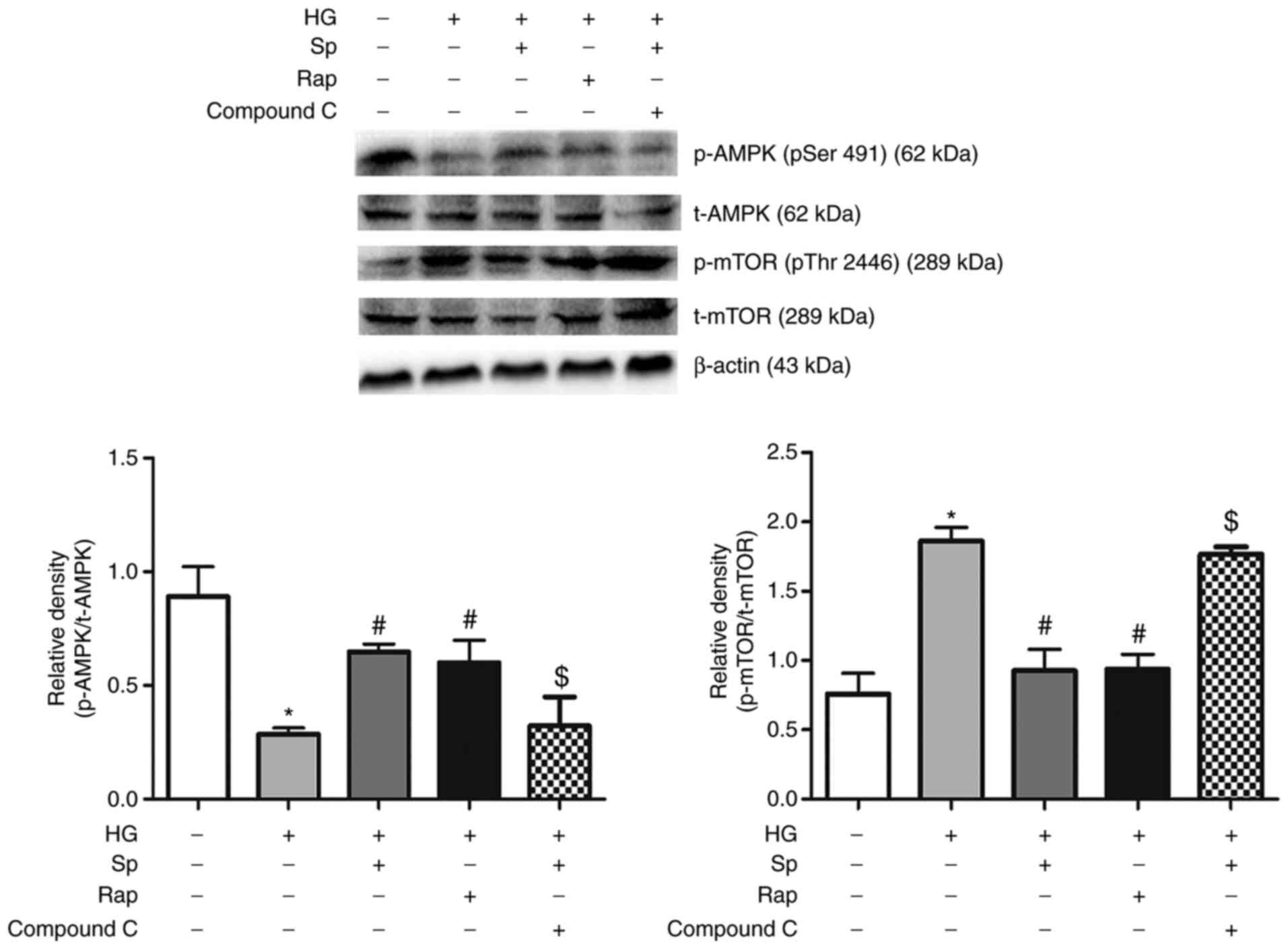
Spermine protects against HG-induced
podocyte injury by inhibiting AMPK/mTOR signaling
mTOR kinase is a key molecule in autophagy induction
that can be inhibited by activated AMPK. In the present study,
p-AMPK and p-mTOR expression were determined by immunoblotting. The
results revealed that p-AMPK was decreased and p-mTOR was increased
in the HG group. However, spermine and rapamycin reversed the
inhibitory effect of HG on AMPK and mTOR activity. The addition of
Compound C, an AMPK inhibitor, further confirmed the
autophagy-promoting effect of spermine by increasing AMPK
phosphorylation and decreasing mTOR phosphorylation (Fig. 6).
Discussion
The present study mainly demonstrated that
hyperglycemia may cause abnormal polyamine metabolism in rat kidney
tissue, and further induce podocyte apoptosis and reduction of
autophagy, which may be one of the important mechanisms of DN.
Exogenous spermine pretreatment was shown to exert its
renoprotective effect by regulating the AMPK/mTOR signaling
pathway.
Intraperitoneal injection of STZ was used to
establish a diabetic rat model. The blood glucose increased and
body weight of the model rats decreased compared with those of the
normal control group, indicating that the T1D rat model was
successfully established. To explore whether diabetes affected the
kidneys, renal function tests and morphological analyses were
carried out. The results demonstrated that the Scr and urea
content, kidney weight/body weight ratio and UAE were increased.
Creatinine and urea are both non-protein nitrogen molecules, and an
increase in their levels indicates glomerular filtration
dysfunction (18). Under normal
conditions, the filtration membrane, which is composed of
glomerular capillary endothelial cells, the basement membrane and
podocytes, prevents proteins with negative charge and
macromolecules from passing through the filtration membrane via
mechanical and chemical barriers (19). Increased microalbuminuria
indicated that glomerular filtration function was damaged in T1D
rats. H&E and PAS staining further confirmed renal injury
(mesangial hyperplasia and matrix accumulation) in the T1D group.
Therefore, DN was successfully established in T1D rats, thus
providing an experimental platform for further experiments.
Abnormal polyamine metabolism affects gene
transcription, translation, expression regulation, autophagy and
stress resistance in numerous cellular processes, which has been
associated with the occurrence and development of asthma,
Parkinson's disease, acute liver injury, acute kidney injury and
cancer (20-23). There are two key enzymes in
polyamine metabolism: ODC, which is the key enzyme of polyamine
synthesis, and SSAT, which is the key enzyme of polyamine
degradation (9,20,23). The present results revealed that
the expression of ODC decreased, while the expression of SSAT
increased, in the renal tissue of DN rats, suggesting that
disruption of polyamine metabolism may be implicated in the
pathogenesis of DN. Spermine is a multivalent cation of four amino
acids with strong activity (21,25). Our previous study demonstrated
that spermine preconditioning may improve myocardial infarction and
myocardial I/R injury, and plays an important protective role in
numerous cardiovascular diseases (26). Therefore, the present study also
tried to improve DN by applying exogenous spermine
pretreatment.
Since the aforementioned results suggested that
hyperglycemia reduces endogenous spermine in T1D rat kidney tissue,
exogenous spermine treatment was applied in subsequent experiments.
The present results demonstrated that spermine significantly
inhibited renal dysfunction, improved morphological damage, reduced
the thickness of the GBM and the fusion of the FPs. These results
suggested a preliminary protective effect of spermine against
DN.
Podocytes are the main components of the glomerular
barrier, and FPs are normally arranged on the outside of the GBM
and connected laterally via the glomerular slit diaphragm (27). The slit diaphragm constitutes a
signaling platform that contains a protein complex of nephrin and
podocin, which plays a major role in maintaining the structural and
functional integrity of the glomerular filtration barrier (28). The slit diaphragm is linked to
the actin-based cytoskeleton located in particular parts of
podocytes through CD-2AP in FPs (29,30). Therefore, podocyte damage is the
main cause of proteinuria. The present study found that nephrin,
CD-2AP and podocin levels were markedly reduced in diabetic kidney
and HG-treated podocytes, while spermine pretreatment strongly
mitigated this reduction. These results suggested that podocyte
injury caused by abnormal polyamine metabolism plays a key role in
DN, but its underlying mechanism must be further investigated.
Autophagy is involved in the regulation of cell
differentiation, development, nutritional metabolism and
maintenance of the cell environment by transferring damaged
organelles and misfolded proteins to lysosomes for degradation
(31,32). The damage caused by autophagy
leads to the loss of podocytes and extensive proteinuria in DN
(33). In the present study,
changes in the corresponding autophagy indices were observed in
diabetic kidney and HG-induced podocytes. The results demonstrated
that spermine pretreatment could increase autophagy activation by
increasing the levels of Atg5, LC3-II/LC3-I ratio and Beclin 1, and
by reducing the accumulation of P62, which indicated that reduced
autophagy caused by abnormal polyamine metabolism is an intrinsic
cause of diabetic podocyte dysfunction. Exogenous spermine may play
a protective role by restoring autophagy.
Autophagy in podocytes is a complex process that can
be controlled by several signaling pathways, with AMPK/mTOR
signaling being one of the major pathways that regulates autophagy.
mTOR and AMPK belong to the serine/threonine protein kinase family,
which plays a major role in maintaining blood glucose, body energy
levels and homeostasis (34).
mTOR is an upstream regulatory protein of the autophagy pathway,
and increased activity of mTOR can inhibit downstream autophagy
signaling proteins (35,36). Excessive mTOR activity in
podocytes leads to dysregulation of autophagy, resulting in
autophagic cell death, which plays a major role in the development
of DN (37,38). Podocyte maintenance is
safeguarded by the mTOR pathway, which is known to inhibit
autophagy (39). Early findings
in the glomeruli included depletion of energy equivalents, which
resulted in increased AMPK signaling and changes in the proteins
that regulate the metabolites (40). The present experiments also
revealed that, in HG-induced podocytes, p-AMPK decreased and p-mTOR
increased, which suggested the inhibition of autophagy. Spermine
and rapamycin (an autophagy agonist) alleviated the inhibition of
autophagy, whereas these effects were reversed by the effect of
Compound C (an AMPK inhibitor). That is to say, spermine can
protect against HG-induced podocyte injury by activating autophagy
through regulation of the AMPK/mTOR signaling pathway.
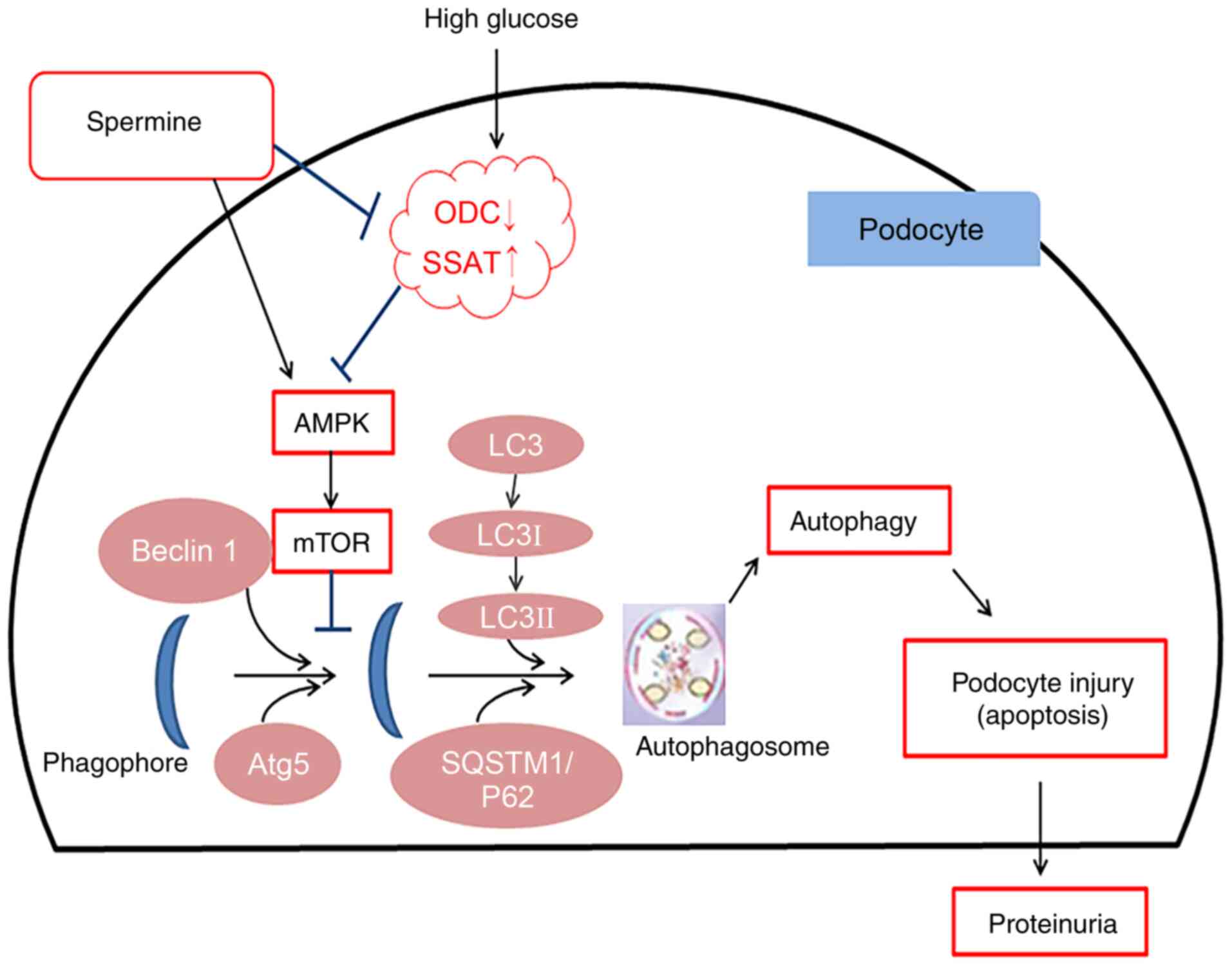
In conclusion, in the present study, abnormal
polyamine metabolism disorders were observed in both T1D rats and
HG-treated podocytes, which in turn triggered apoptosis and reduced
autophagy, resulting in podocyte injury and extensive albuminuria.
Exogenous spermine played a protective role by regulating AMPK/mTOR
signaling to restore autophagy (Fig.
7). These findings may provide theoretical and experimental
grounds for the prevention and treatment of DN, and support the
clinical application of spermine.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW, CX, XZ and LZ designed the research and drafted
the manuscript. ZC, SL, BC, NW and JC completed the experiments and
data analysis. CX and CW confirmed the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experimental protocols complied with the
Guide for the Care and Use of Laboratory Animals published by the
National Institutes of Health. The present study was approved by
the Institutional Animal Research Committee of Harbin Medical
University (Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Saran R, Li Y, Robinson B, Ayanian J,
Balkrishnan R, Bragg-Gresham J, Chen JT, Cope E, Gipson D, He K, et
al: US renal data system 2014 annual data report: Epidemiology of
kidney disease in the united states. Am J Kidney Dis. 66(Suppl 1):
SviiS1–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao L, Wang M, Yang S, Liu F and Sun L: A
glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling
in diabetic nephropathy. Biomed Res Int. 2013:9870642013.
View Article : Google Scholar
|
|
3
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bae DH, Lane DJR, Jansson PJ and
Richardson DR: The old and new biochemistry of polyamines. Biochim
Biophys Acta Gen Subj. 1862:2053–2068. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wallace HM, Fraser AV and Hughes ZA: A
perspective of polyamine metabolism. Biochem J. 376:1–14. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michael AJ: Polyamines in eukaryotes,
bacteria, and archaea. J Biol Chem. 291:14896–14903. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalac P: Health effects and occurrence of
dietary polyamines: A review for the period 2005-mid 2013. Food
Chem. 161:27–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lane DJR, Bae DH, Siafakas AR, Suryo
Rahmanto Y, Al-Akra L, Jansson PJ, Casero RA Jr and Richardson DR:
Coupling of the polyamine and iron metabolism pathways in the
regulation of proliferation: Mechanistic links to alterations in
key polyamine biosynthetic and catabolic enzymes. Biochim Biophys
Acta Mol Basis Dis. 1864:2793–2813. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zahedi K, Barone S, Wang Y, Murray-Stewart
T, Roy-Chaudhury P, Smith RD, Casero RA Jr and Soleimani M:
Proximal tubule epithelial cell specific ablation of the
spermidine/spermine N1-acetyltransferase gene reduces the severity
of renal ischemia/reperfusion injury. PLoS One. 9:e1101612014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Igarashi K and Kashiwagi K: Modulation of
cellular function by polyamines. Int J Biochem Cell Biol. 42:39–51.
2010. View Article : Google Scholar
|
|
12
|
Wang J, Li S, Wang J, Wu F, Chen Y, Zhang
H, Guo Y, Lin Y, Li L, Yu X, et al: Spermidine alleviates cardiac
aging by improving mitochondrial biogenesis and function. Aging
(Albany NY). 12:650–671. 2020. View Article : Google Scholar
|
|
13
|
Mason TJ and Matthews M: Aquatic
environment, housing, and management in the eighth edition of the
Guide for the care and use of laboratory animals: Additional
considerations and recommendations. J Am Assoc Lab Anim Sci.
51:329–32. 2012.PubMed/NCBI
|
|
14
|
Wang Y, Chen J, Li S, Zhang X, Guo Z, Hu
J, Shao X, Song N, Zhao Y, Li H, et al: Exogenous spermine
attenuates rat diabetic cardiomyopathy via suppressing ROS-p53
mediated downregulation of calcium-sensitive receptor. Redox Biol.
32:1015142020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsai HJ, Liao MH, Shih CC, Ka SM, Tsao CM
and Wu CC: Angiotensin-(1-7) attenuates organ injury and mortality
in rats with polymicrobial sepsis. Crit Care. 22:2692018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Liu Q, Shan Z, Mi W, Zhao Y, Li M,
Wang B, Zheng X and Feng W: Catalpol ameliorates podocyte injury by
stabilizing cytoskeleton and enhancing autophagy in diabetic
nephropathy. Front Pharmacol. 10:14772019. View Article : Google Scholar
|
|
17
|
Choi ME: Autophagy in kidney disease. Annu
Rev Physiol. 82:287–322. 2020. View Article : Google Scholar
|
|
18
|
Medler S and Harrington F: Measuring
dynamic kidney function in an undergraduate physiology laboratory.
Adv Physiol Educ. 37:384–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reiser J and Sever S: Podocyte biology and
pathogenesis of kidney disease. Annu Rev Med. 64:357–366. 2013.
View Article : Google Scholar :
|
|
20
|
Wang W, Zhang H, Xue G, Zhang L, Zhang W,
Wang L, Lu F, Li H, Bai S, Lin Y, et al: Exercise training
preserves ischemic preconditioning in aged rat hearts by restoring
the myocardial polyamine pool. Oxid Med Cell Longev.
2014:4574292014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jain V, Raina S, Gheware AP, Singh R,
Rehman R, Negi V, Murray Stewart T, Mabalirajan U and Mishra AK:
Reduction in polyamine catabolism leads to spermine-mediated airway
epithelial injury and induces asthma features. Allergy.
73:2033–2045. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Green DR: Polyamines and aging: A CLEAR
connection? Mol Cell. 76:5–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zahedi K, Barone S and Soleimani M:
Polyamine catabolism in acute kidney injury. Int J Mol Sci.
20:47902019. View Article : Google Scholar :
|
|
24
|
Mollet G, Ratelade J, Boyer O, Muda AO,
Morisset L, Lavin TA, Kitzis D, Dallman MJ, Bugeon L, Hubner N, et
al: Podocin inactivation in mature kidneys causes focal segmental
glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol.
20:2181–2189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Russell DH: Clinical relevance of
polyamines. Crit Rev Clin Lab Sci. 18:261–311. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei C, Li H, Wang Y, Peng X, Shao H, Li H,
Bai S and Xu C: Exogenous spermine inhibits
hypoxia/ischemia-induced myocardial apoptosis via regulation of
mitochondrial permeability transition pore and associated pathways.
Exp Biol Med (Maywood). 241:1505–1515. 2016. View Article : Google Scholar
|
|
27
|
Ying Q and Wu G: Molecular mechanisms
involved in podocyte EMT and concomitant diabetic kidney diseases:
An update. Ren Fail. 39:474–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asanuma K and Mundel P: The role of
podocytes in glomerular pathobiology. Clin Exp Nephrol. 7:255–259.
2003. View Article : Google Scholar
|
|
29
|
Tolvanen TA, Dash SN, Polianskyte-Prause
Z, Dumont V and Lehtonen S: Lack of CD2AP disrupts Glut4
trafficking and attenuates glucose uptake in podocytes. J Cell Sci.
128:4588–4600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ristola M and Lehtonen S: Functions of the
podocyte proteins nephrin and Neph3 and the transcriptional
regulation of their genes. Clin Sci (Lond). 126:315–328. 2014.
View Article : Google Scholar
|
|
31
|
Turkmen K: Inflammation, oxidative stress,
apoptosis, and autophagy in diabetes mellitus and diabetic kidney
disease: The four horsemen of the apocalypse. Int Urol Nephrol.
49:837–844. 2017. View Article : Google Scholar
|
|
32
|
Yang D, Livingston MJ, Liu Z, Dong G,
Zhang M, Chen JK and Dong Z: Autophagy in diabetic kidney disease:
Regulation, pathological role and therapeutic potential. Cell Mol
Life Sci. 75:669–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yasuda-Yamahara M, Kume S, Tagawa A,
Maegawa H and Uzu T: Emerging role of podocyte autophagy in the
progression of diabetic nephropathy. Autophagy. 11:2385–2386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szrejder M and Piwkowska A: AMPK
signalling: Implications for podocyte biology in diabetic
nephropathy. Biol Cell. 111:109–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li W, Zhu L, Ruan ZB, Wang MX, Ren Y and
Lu W: Nicotinamide protects chronic hypoxic myocardial cells
through regulating mTOR pathway and inducing autophagy. Eur Rev Med
Pharmacol Sci. 23:5503–5511. 2019.PubMed/NCBI
|
|
36
|
Miao H, Qiu F, Huang B, Liu X, Zhang H,
Liu Z, Yuan Y, Zhao Q, Zhang H, Dong H and Zhang Z: PKCα replaces
AMPK to regulate mitophagy: Another PEDF role on ischaemic
cardio-protection. J Cell Mol Med. 22:5732–5742. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Li Q, Lv W, Jiang L, Geng C, Yao X,
Shi X, Liu Y and Cao J: The interaction of Atg4B and Bcl-2 plays an
important role in Cd-induced crosstalk between apoptosis and
autophagy through disassociation of Bcl-2-Beclin1 in A549 cells.
Free Radic Biol Med. 130:576–591. 2019. View Article : Google Scholar
|
|
38
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bork T, Liang W, Yamahara K, Lee P, Tian
Z, Liu S, Schell C, Thedieck K, Hartleben B, Patel K, et al:
Podocytes maintain high basal levels of autophagy independent of
mtor signaling. Autophagy. 16:1932–1948. 2020. View Article : Google Scholar :
|
|
40
|
Rinschen MM, Palygin O, Guijas C, Palermo
A, Palacio-Escat N, Domingo-Almenara X, Montenegro-Burke R,
Saez-Rodriguez J, Staruschenko A and Siuzdak G: Metabolic rewiring
of the hypertensive kidney. Sci Signal. 12:eaax97602019. View Article : Google Scholar : PubMed/NCBI
|