Introduction
Esophageal carcinoma (EC) is a common malignant
tumor in the upper digestive tract system that occurs worldwide;
the incidence of EC ranked seventh and the mortality rate ranked
sixth in the world in 2018 according to the GLOBOCAN 2018 estimates
of cancer (1). There are two
types of esophageal cancer: Esophageal adenocarcinoma and
esophageal squamous cell carcinoma (ESCC), whereby the incidence of
ESCC accounts for more than esophageal adenocarcinoma in China as
of 2015 (2). Based on various
factors, such as diet and the environment, China is one of the
countries that has a high incidence of EC (3). The overall 5-year survival rate of
patients with esophageal cancer is between 15-25% (3). Early diagnosis of the disease can
result in better prognosis. Furthermore, the early clinical
manifestations of EC are not obvious, and thus most patients are
diagnosed at the advanced stage and have a poor prognosis (4,5).
Therefore, it is important to understand the molecular mechanisms
of EC development and to identify novel biomarkers for the early
diagnosis and treatment of EC.
With the rapid development of genome-wide and
transcriptome sequencing technologies, the function of long
non-coding (lnc)RNAs, which were previously thought to have a
'noise' role in the transcription process, has received increasing
attention (6). lncRNAs are a
group of RNA molecules with a length of >200 nucleotides, which
have no protein coding function due to a lack of complete open
reading frame (7). However,
previous studies have reported that lncRNAs play an important role
in regulating chromatin status, gene activity, gene expression at
transcriptional and translational levels, and regulating
tumorigenesis (8,9). Moreover, it has been revealed that
several lncRNAs, such as metastasis associated lung adenocarcinoma
transcript 1 (10), HOX
transcript antisense RNA (11)
and H19 (12), are abnormally
expressed in ESCC and are involved in its development. However, the
functional and molecular mechanisms of the majority of lncRNAs
remain unknown in ESCC.
Long intergenic non-protein coding RNA 491
(LINC00491) is a recently studied lncRNA, which is transcribed by
chromosome 5 (13). Furthermore,
the differential expression of LINC00491 is significantly
associated with the prognosis of human endometrial cancer (14), colon cancer (15,16) and breast cancer (17). Moreover, LINC00491 acts as a
novel molecular biomarker to promote proliferation, migration and
invasion of colonic adenocarcinoma cells via sponging microRNA
(miR)-145 (13). However, to the
best of our knowledge, the expression and function of LINC00491 in
ESCC have not been previously reported. The present study
identified a large number of differentially expressed lncRNAs in
ESCC using high-throughput sequencing, including LINC00491. In
addition, the present study examined the expression of LINC00491 in
ESCC tissues and cell lines, and then analyzed the effects of
LINC00491 on cell proliferation, migration, invasion and apoptosis.
It was demonstrated that LINC00491 plays an important role in the
development of ESCC and may provide an experimental basis for
further clinical research.
Materials and methods
Clinical samples and RNA sequencing
assay
In total, two separate samples were collected (20
cases in total). The first group included 4 paired cases of ESCC
and normal tissues (53-75 years; 3 males; 1 female), which were
collected between May 2018 and July 2018 during resection of cancer
via open surgery from the Department of Thoracic Surgery at Zhongda
Hospital Affiliated to Southeast University (Nanjing, China). The
second group included 16 pairs of ESCC tissues and corresponding
normal tissue samples (50-75 years; 10 males; 6 females), which
were collected between February 2019 and September 2019 after
surgical operation and endoscopic submucosal dissection surgery at
the Zhongda Hospital Affiliated to Southeast University (Nanjing,
China). Healthy or normal esophageal epithelial tissue was defined
as tissues that were ≥5 cm apart from the margin of the cancer
tissues. All patients with ESCC had not received local or systemic
treatment before operation. Excised ESCC tissue samples and
corresponding healthy tissue samples were immediately frozen with
liquid nitrogen and stored at 80°C. The study was approved by the
Research Ethics Committee of the Southeast University (Nanjing,
China; approval no. 2019ZDSYLL022-P01). All patients provided
written informed consent.
Next-generation RNA sequencing assay was performed
at Kangchen Biotechnology Co., Ltd. using Illumina HiSeq 4000
(Illumina, Inc.) to detect the mRNA and ncRNA expression profiles.
Image processing and base recognition were performed using Solexa
pipeline version 1.8 software (Off-Line Base Caller software).
Then, the reference genome was compared using Hisat2 software
(18) (version 2.1.0; http://daehwankimlab.github.io/hisat2/),
and a FPKM calculation at the gene and transcript levels was
performed using R software 'Ballgown'. The differentially expressed
lncRNAs and mRNAs were selected by fold change (FC)/P-value/false
discovery rate (FDR) filtration (multiple ≥1.5, P<0.05 and FDR
<0.05).
Functional enrichment analyses and
coding-non-coding gene co-expression (CNC) network
The Gene Ontology (GO) project (19,20) provides a controlled vocabulary to
describe gene and gene product attributes in any organism
(http://www.geneontology.org). GO covers
three domains: Biological Process, Cellular Component and Molecular
Function. Pathway analysis is a functional analysis mapping genes
to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The
P-value (EASE-score, Fisher-P-value or Hypergeometric-P-value)
denotes the significance of the KEGG pathway associated with the
conditions. A CNC network of LINC00491 and coding genes (mRNAs) was
constructed, and P<0.05 was considered to indicate a significant
co-expression association between the two genes. The network was
drawn using Cytoscape 2.8.3 (https://cytoscape.org) (21).
Data availability
Gene Expression Omnibus (GEO) is the National Center
for Biotechnology Information GEO, from which GSE45670 (22) was downloaded, which contains
surgical samples from 28 patients with ESCC and 10 samples from
healthy esophageal epithelium. Differential genes were analyzed
using R language.
Cell lines and cell culture
The human ESCC cell lines KYSE-410 and KYSE-30 were
purchased from Cell Cook Company and the normal esophageal
epithelial cell line (HEEC) was purchased from BeNa Culture
Collection; Beijing Beina Chunglian Biotechnology Research
Institute. Cells were cultured using RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS(Gibco;
Thermo Scientific, Inc.) and cells were maintained at 37°C in an
incubator containing 5% CO2.
Cell transfection
The short hairpin (sh)RNAs specifically targeting
LINC00491 and a negative control shRNA (sh-NC) were designed and
synthesized by Shanghai GenePharma Co., Ltd. KYSE30 and KYSE410
cells in the logarithmic growth phase were seeded in a petri dish.
Cell transfection with shRNA was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to manufacturer's protocol; the ratio
of shRNA (500 µg/µl): Lipofectamine® 2000
(µl) was 1:3. The sequences of shRNAs were as follows:
sh-LINC00491#1, 5′-GGT GTA TTC CAC ATT GTC TCT-3′; sh-LINC00491#2,
5′-GGC CAA AGG TCT GAT AAT TGC-3′; sh-LINC00491#3, 5′-GGA TAT GTG
CAG GGA GTC TAG-3′; and sh-NC, 5′-TTC TCC GAA CGT GTC ACG T-3′.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from ESCC tissues, adjacent
healthy tissues and HEEC, KYSE-30 and KYSE-410 cells using the
Hipure Universal RNA kit (Magen) according to the manufacturer's
instructions. Then, the extracted total RNA samples were quantified
using a Colibri spectrometer (Titertek Berthold). A total of 1
µg RNA was used for RT, which was performed using the
HiScript Q RT SuperMix for qPCR (Including 4X g DNA wiper Mix;
Vazyme Biotech Co., Ltd.), following the manufacturer's
instructions. qPCR was performed using SYBR Green Master Mixture
(Low ROX Premixed; Vazyme Biotech Co., Ltd.) with the Applied
Biosystems QuantStudio 3 RT PCR system (Thermo Fisher Scientific,
Inc.). The reaction system was as follows: 10 µl qPCR SYBR
Green Master mix (Low Rox Premixed), 0.4 µl forward primer
(10 µM), 0.4 µl reverse primer (10 µM), 8.2
µl RNase-free water and 1 µl cDNA. The thermocycling
conditions were as follows: Initial denaturation at 95°C for 5 min,
followed by 40 cycles at 95°C for 10 sec and 60°C for 30 sec, and
melting curve stage at 95°C for 15 sec, 60°C for 60 sec and 95°C
for 15 sec. GAPDH was used as a standardized internal control. The
primers were synthesized by Sangon Biotech Co., Ltd, and the primer
sequences were as follows: LINC00491 forward, 5′-CCC CTT AAC CAA
CTG GAA-3′ and reverse, 5′-GTG GAC CTT CTC CCA GCA AT-3′; and GAPDH
forward, 5′-TGC ACC ACC AAC TGC TTA GC-3′ and reverse, 5′-GGC ATG
GAC TGT GGT CAT GAG-3′. The results were quantified by the
2−ΔΔCq method (23).
Each test was performed in triplicate.
Cell Counting Kit-8 (CCK-8) assay
KYSE30 and KYSE410 cells in the logarithmic growth
phase were adjusted to a cell density of 3×104 cells/ml,
and seeded into 96-well cell culture plates, with 100 µl per
well and three replicates per group. Until the cells were attached,
20 µl serum-free RPMI-1640 medium was used to dilute shRNA
(0.15 µg) and Lipofectamine® 2000 (0.45
µl) for transient transfection. After 48 h of transfection
of ESCC cells, 10 µl CCK-8 reagent was added to each well
(110 µl/well) of a 96-well plate and incubated for 3 h in a
5% CO2 incubator. Then, the absorbance of 450 nm water
soluble tetrazolium salt was measured at 0, 24, 48 and 72 h to
quantify cell proliferation.
Wound healing assay
KYSE-30 and KYSE-410 cells in the logarithmic growth
phase were seeded into a 6-well plate, and the cell density was
adjusted to 1×105 cells/ml, with 3 ml per well and three
replicates in each group. Until the cells were attached, transient
transfection was performed with 500 µl serum-free RPMI-1640
medium diluted shRNA (2.5 µg) and Lipofectamine®
2000 (7.5 µl). After 48 h of transfection, scratch wounds
were created with the tip of a 200-µl pipette to scratch the
cells when the cell density reached >90%. The cells were then
washed twice with 0.01% PBS and serum-free medium was added. Then,
cells were maintained at 37°C in an incubator containing 5%
CO2. Random images were taken at 0, 24 and 48 h using a
microscope (×50 magnification; Leica Microsystems GmbH) to assess
the ability of cells to migrate. The independent experiments were
repeated three times.
Cell migration and invasion assay
KYSE-30 and KYSE-410 cells in the logarithmic growth
phase were seeded into a 12-well plate. The cell density was
adjusted to 1×104 cells/ml, with 3 ml per well and three
replicates in each group. After the cells adhered to the wall,
shRNA (1.5 µg) and Lipofectamine® 2000 (4.5
µl) were diluted with 200 µl serum-free RPMI-1640
medium for transient transfection. Migration and invasion assays
were performed using a Transwell chamber. For the migration assay,
1×104 cells were seeded into the upper chamber of
Transwell (Corning, Inc.). For the invasion assay, 1×104
cells were added to the upper chamber (previously coated with
Matrigel) and placed in the incubator for 1 h at 37°C. In both
assays, cells were maintained in serum-free medium in the upper
chamber and medium containing 10% FBS was added as a
chemoattractant to the lower chamber. After 24 h of incubation,
cells that did not migrate or invade the membrane were removed. The
membrane was then fixed with methanol (15 min at room temperature)
and stained with 0.1% crystal violet (15 min at room temperature).
Each chamber was counted in three random fields using an inverted
microscope (×100 magnification; Carl Zeiss AG) and each experiment
was repeated three times.
Apoptosis assays
The cell culture and transfection methods for the
apoptosis assay were same as that of the wound healing assay, which
have been described above. Cells were transfected and incubated for
48 h. The cells were digested with EDTA-free trypsin and collected,
and then 300 µl 1X Binding Buffer (Fcmacs Biotech Co., Ltd.)
suspended cells were added. Subsequently, 100 µl cell
suspension (total number of cells, 1×105) were pipetted
into a new tube. Then, 10 µl Annexin V-APC and 5 µl
PI (Fcmacs Biotech Co., Ltd.) were added, and after mixing, the
cells were incubated for 15 min at room temperature in the dark.
Apoptotic cells were detected using flow cytometry (BD FACSVerse;
BD Biosciences) and analyzed using FlowJo 7.6.1 (TreeStar,
Inc.).
Statistical analysis
All data were analyzed using SPSS 20 (IBM Corp.) and
GraphPad Prism 5.0 (GraphPad Software, Inc.). Data are presented as
the mean ± standard deviation. Comparisons between the two groups
were performed using unpaired Student's t-test, and comparisons
between ≥3 groups were performed using one-way ANOVA and post hoc
analysis with Tukey's test. Each experiment was repeated three
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
Differentially expressed lncRNAs in ESCC
compared with healthy tissue
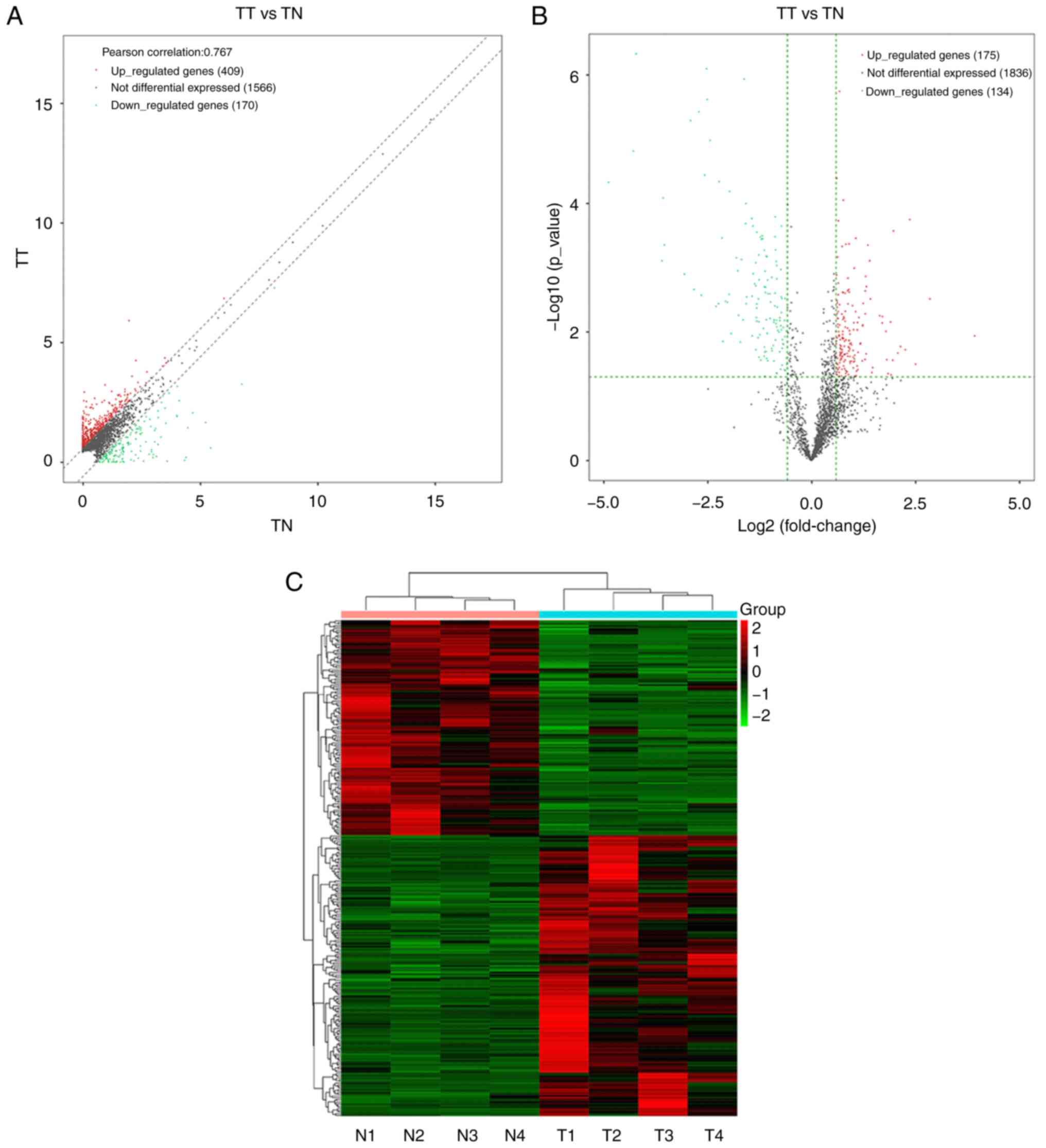
RNA sequences were used to detect 2,145 different
lncRNAs from four pairs of human ESCC and healthy esophageal
tissue. lncRNAs with FC ≥1.5 and P<0.05 were considered to have
significant differences. Among them, 409 were found to be
upregulated and 170 were downregulated with FC ≥1.5 (Fig. 1A). Further expressional analysis
demonstrated that a series of lncRNAs were differentially expressed
in ESCC compared with the control group; of the 309 differentially
expressed lncRNAs, 175 were upregulated in ESCC and 134 were
downregulated with FC ≥1.5 and P<0.05 (Fig. 1B and C). Moreover, LINC00491 was
included in the genes that were significantly differentially
expressed.
GO and pathway analysis
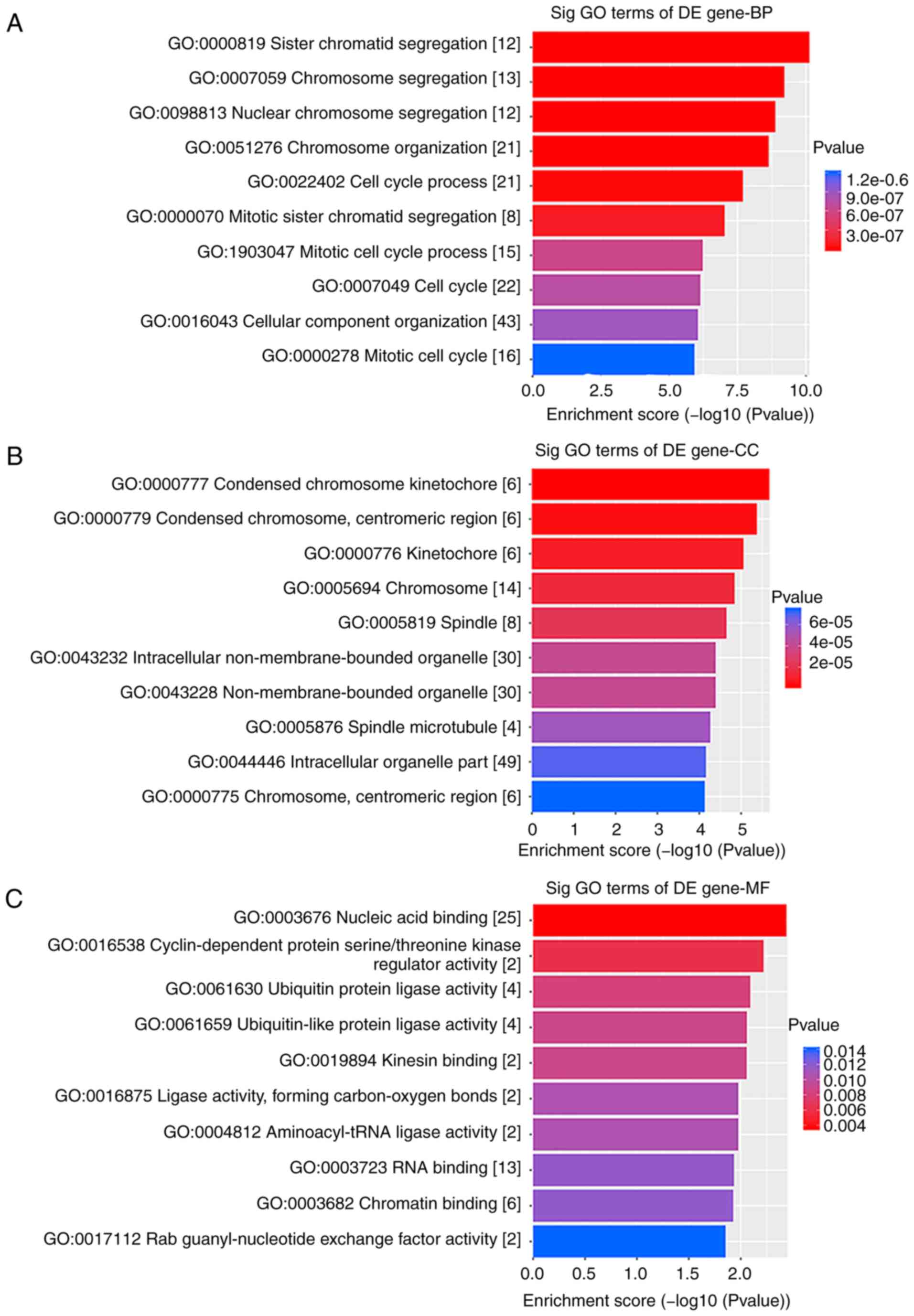
GO analysis results indicated that the
differentially expressed genes between ESCC and the matched control
group were mainly involved in 'chromosome segregation', 'cell cycle
process' and 'mitotic cell cycle process', which affect cell growth
and proliferation (Fig. 2).
Furthermore, pathway analysis identified that the related genes
were also primarily involved in 'cell cycle' and 'oocyte meiosis'
(Fig. 3A).
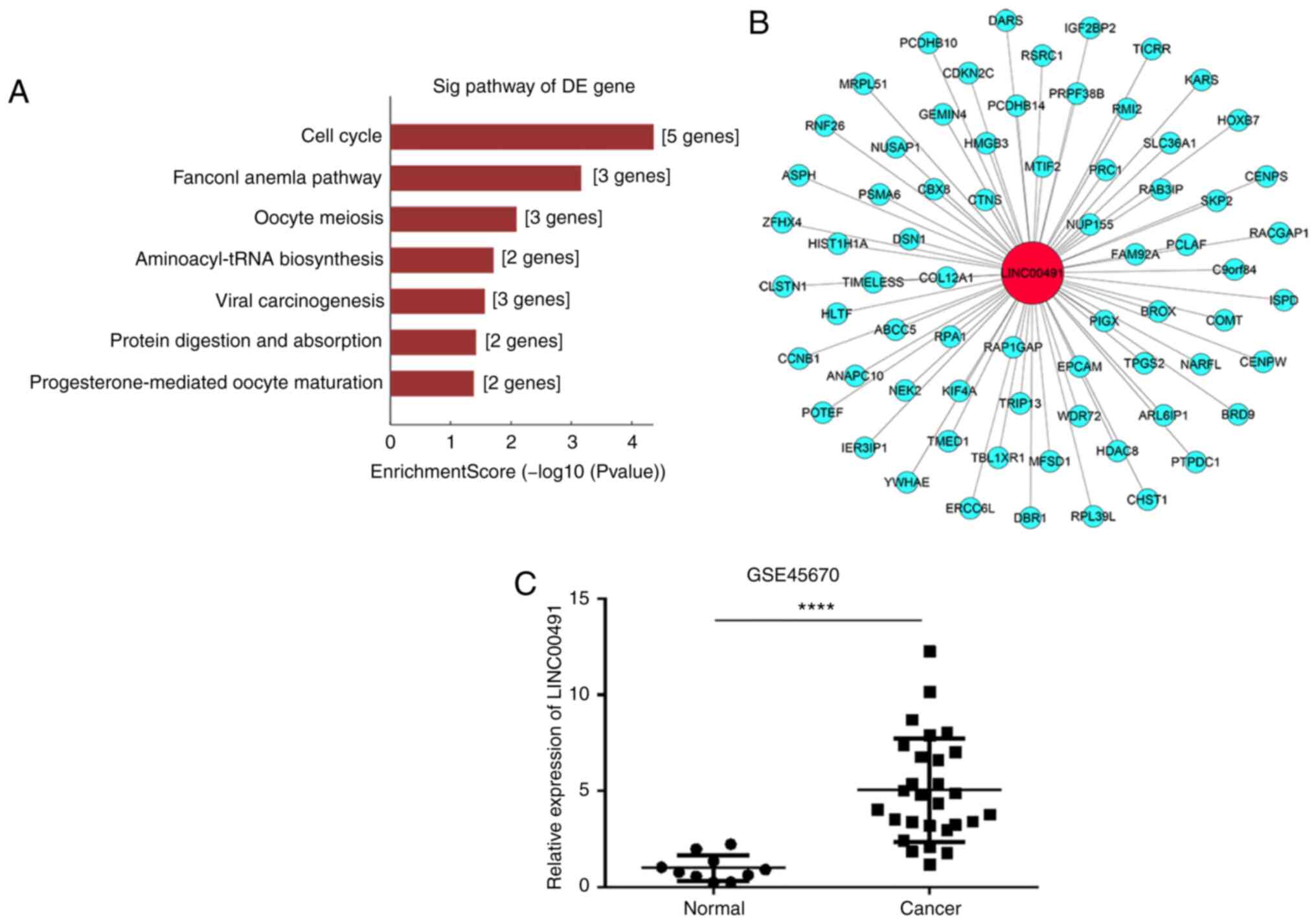
CNC network and GSE45670 analysis
CNC network analysis calculates the correlation
co-efficient by comparing the FPKM data of LINC00491 with the
differential expression transcripts of all the mRNAs in the
transcript, and selecting mRNAs with Pearson's correlation
coefficient ≥0.9, P<0.05 and FDR=1. GEO data were obtained from
the GEO database. The results of CNC analysis demonstrated that 69
mRNAs were co-expressed with LINC00491, including the oncogenes
thyroid hormone receptor interactor 13 (TRIP13) (24) and homeobox B7 (HOXB7) (25), which are associated with ESCC
(Fig. 3B). In addition, analysis
of the GSE45670 dataset found that compared with the control group,
the expression of LINC00491 in EC tissue was significantly higher
compared with normal tissue (Fig.
3C).
Increased expression of LINC00491 in ESCC
tissues and cells
RT-qPCR results demonstrated that LINC00491
expression was significantly higher in 13/16 cases of ESCC compared
with the controls (Fig. 4A).
Moreover, the expression of LINC00491 was significantly higher in
two ESCC cell lines (KYSE-30 and KYSE-410) compared with human
normal esophageal epithelial cells (HEEC; Fig. 4B). When sh-LINC00491#1,
sh-LINC00491#2, sh-LINC00491#3 or sh-NC were transfected into
KYSE-30 and KYSE-410 cells, the expression of LINC00491 was
significantly reduced, with the highest reduction in
sh-LINC00491#2, compared with the control group (Fig. 4C and D). In summary, the results
demonstrated that LINC00491 was highly expressed in ESCC tissues
and cell lines, and sh-LINC00491#2 had the highest inhibition
efficiency and thus was used for subsequent experiments.
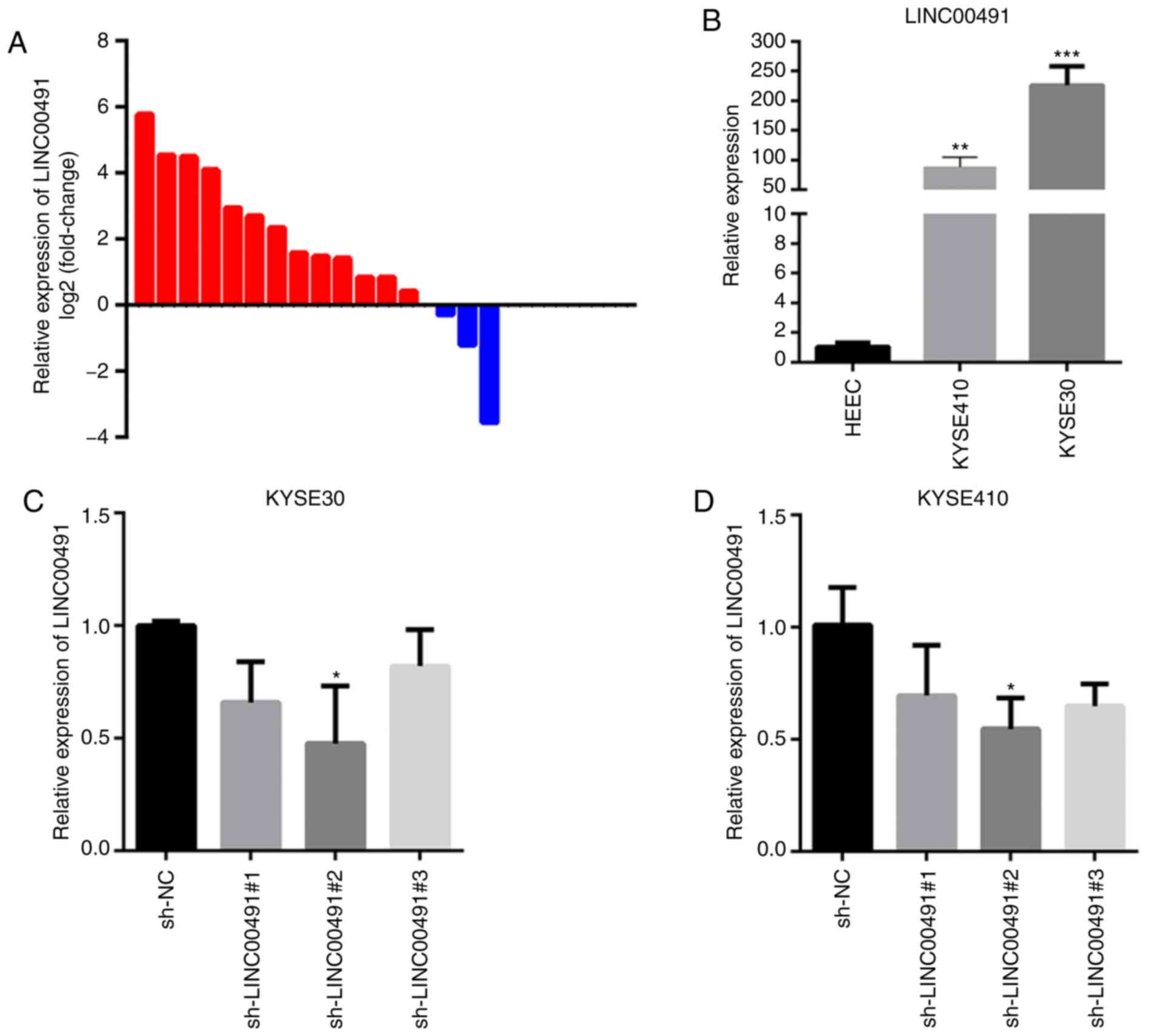 | Figure 4Expression of LINC00491 in ESCC
tissues and cells. (A) RT-qPCR was used to detect the relative
expression of LINC00491 in 16 cases of ESCC, among which the
expression of LINC00491 was upregulated in 13 cases. Expression of
lncRNA was normalized to GAPDH. (B) RT-qPCR was used to detect the
relative expression of LINC00491 in ESCC cell lines and human
normal esophageal epithelial cells. Data are presented as the mean
± SD, the expressions of LINC00491 in KYSE-410, KYSE-30 and HEEC
cells were 88.12±16.59, 225.92±31.79 and 1.03±0.28, respectively.
**P<0.01, ***P<0.001 vs. HEEC. (C and
D) Three specific sh-LINC00491 were transfected into esophageal
squamous cells. After 48 h, RT-qPCR was used to detect the
expression of LINC00491 in KYSE-30 cells and KYSE-410 cells. The
expressions of LINC00491 in sh-LINC00491#1, sh-LINC00491#2,
sh-LINC00491#3 and sh-NC were 0.66±0.18, 0.47±0.25, 0.82±0.16 and
1.00±0.02, respectively. In KYSE-410 cells, the expressions of
LINC00491 in sh-LINC00491#1, sh-LINC00491#2, sh-LINC00491#3 and
sh-NC were 0.69±0.22, 0.54±0.13, 0.64±0.09 and 1.01±0.16,
respectively. *P<0.05 vs. sh-NC. NC, negative
control; sh, short hairpin RNA; LINC00491, long intergenic
non-protein coding RNA 491; ESCC, esophageal squamous cell
carcinoma; RT-qPCR, reverse transcription-quantitative PCR. |
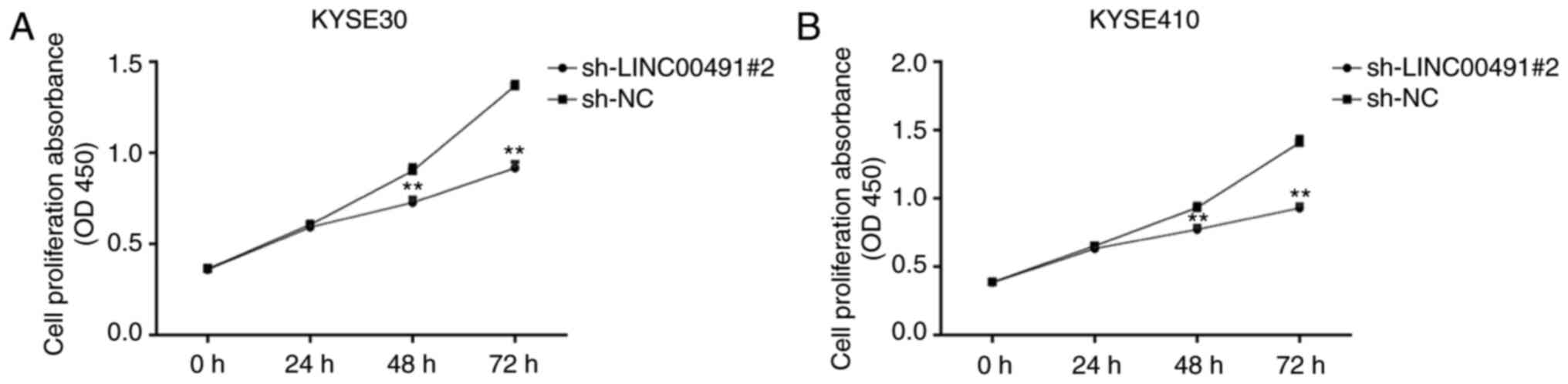
Inhibition of the expression of LINC00491
reduces the proliferation of EC cells
Using the CCK-8 experiment, the effect of knocking
down the expression of LINC00491 on cell proliferation was examined
in ESCC cells, and the optical density values were measured at 0,
24, 48 and 72 h. It was identified that in KYSE30 and KYSE410
cells, compared with the sh-NC group, the proliferation ability of
the sh-LINC00491#2 transfection group was significantly lower after
48 h of culture (P<0.01; Fig. 5A
and B).
Decreased expression of LINC00491
inhibits migration of ESCC cells
The wound healing assay results indicated that
interference of the expression of LINC00491 significantly inhibited
the migration of KYSE-30 and KYSE-410 cells (Fig. 6).
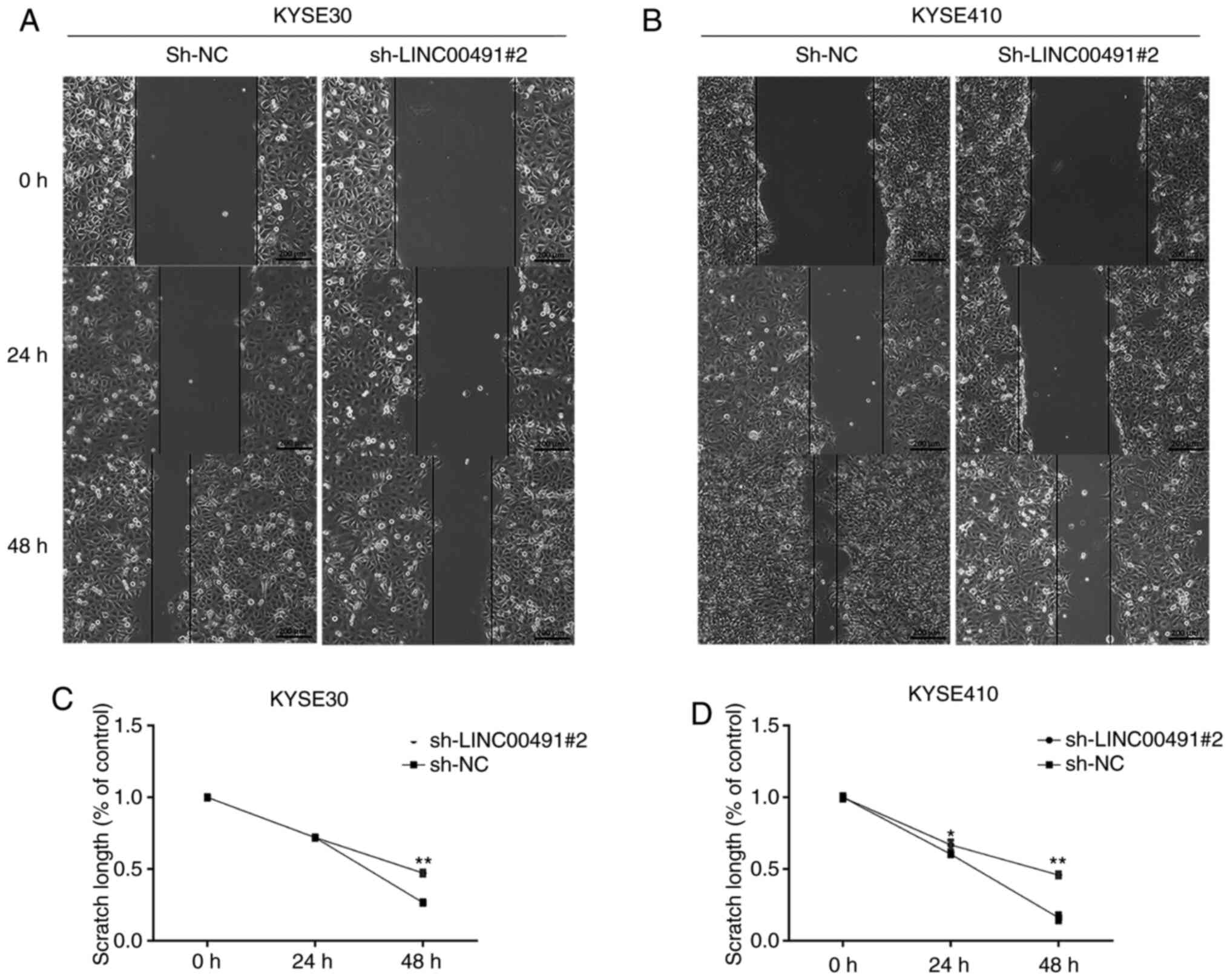 | Figure 6Wound healing assay. After 48 h of
transfection, the effect of LINC00491 knockdown on the migration of
KYSE30 and KYSE410 cells was detected by wound healing assay. Cell
migration at 0, 24 and 48 h of (A) KYSE30 and (B) KYSE 410 cells.
Cells transfected with sh-LINC00491#2 showed less migration
compared with sh-NC. Graphs illustrate the scratch width based on
time intervals in which a larger scratch width was observed in
sh-LINC00491#2 compared with sh-NC transfected (C) KYSE30 and (D)
KYSE410 cells. sh, short hairpin RNA; NC, negative control;
LINC00491, long intergenic non-protein coding RNA 491. The y-axis
refers to the width, which is the distance between the margins of
area of cell scratch and x-axis is the time interval. Data are
shown as the mean ± SD of a representative experiment performed in
triplicate. *P<0.05, **P<0.01 vs. sh-NC
group. NC, negative control; sh, short hairpin RNA; LINC00491, long
intergenic non-protein coding RNA 491. |
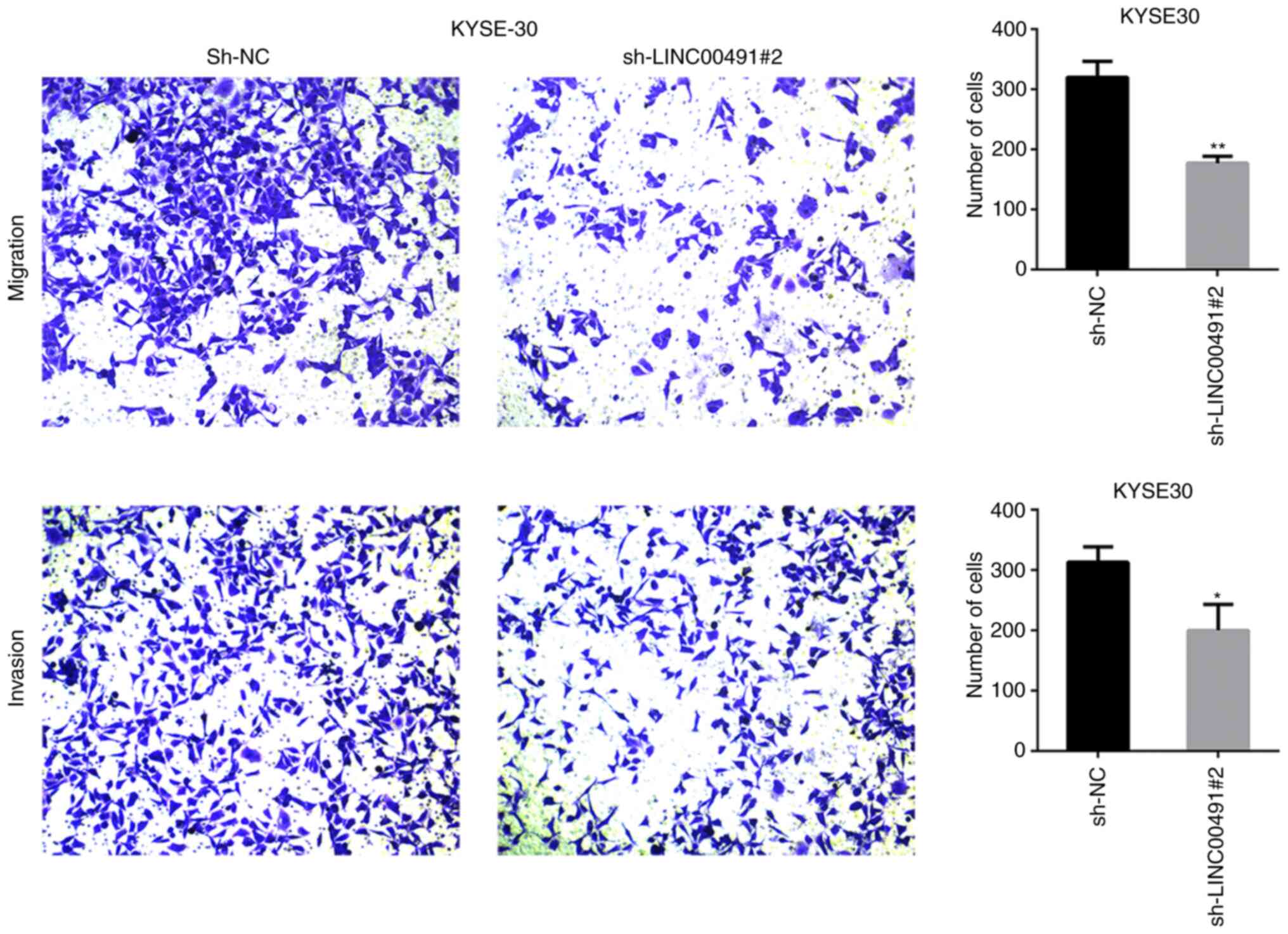
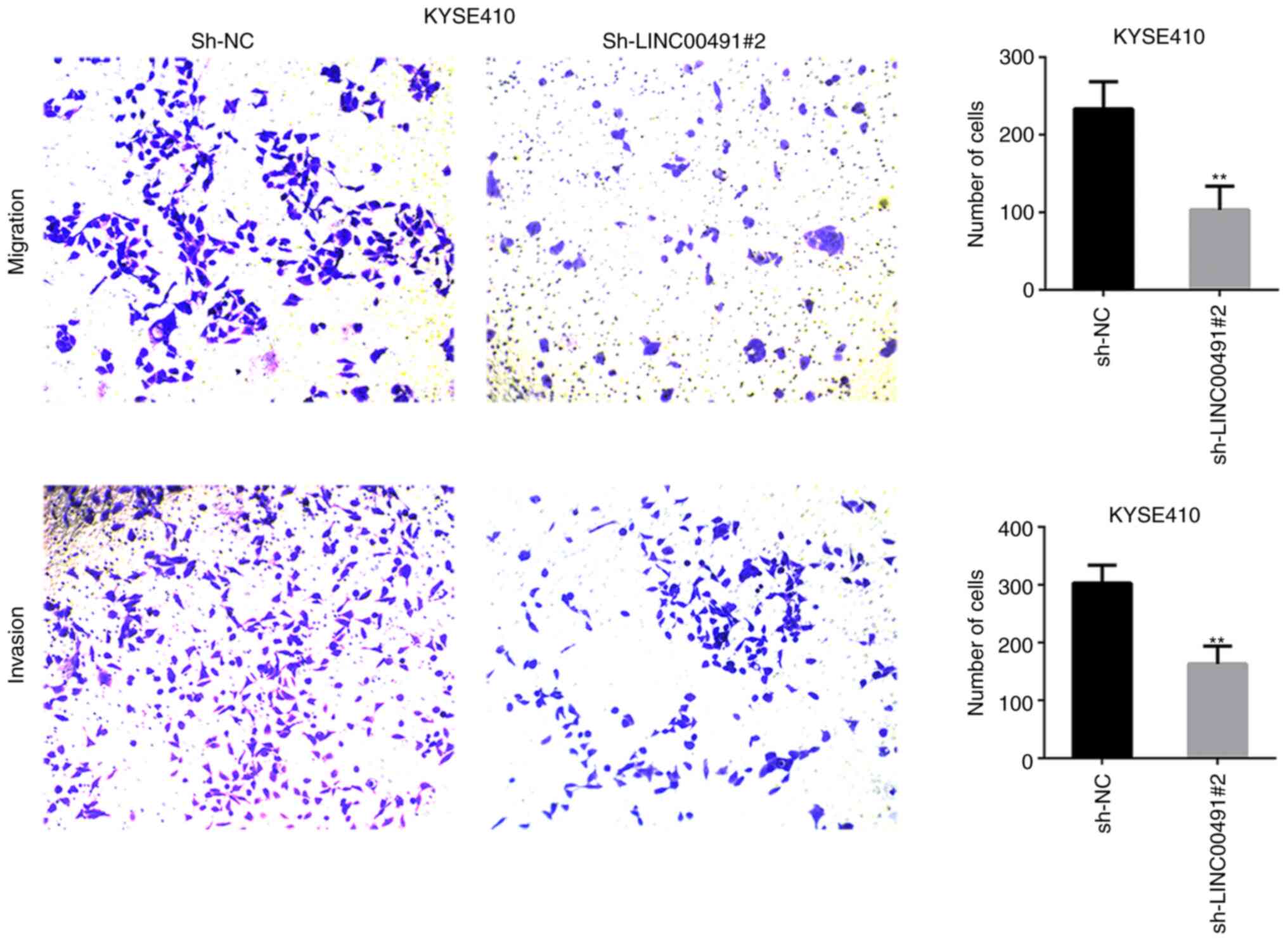
LINC00491 silencing suppresses ESCC cell
migration and invasion
Transwell chamber experiments were performed on ESCC
cells to investigate the function of LINC00491 during cell
migration and invasion. It was found that silencing LINC00491
expression in KYSE30 and KYSE410 cells significantly inhibited cell
invasion and migration compared with the NC group (Figs. 7 and 8). Thus, it was speculated that
upregulation of LINC00491 expression in ESCC could accelerate tumor
cell migration and invasion.
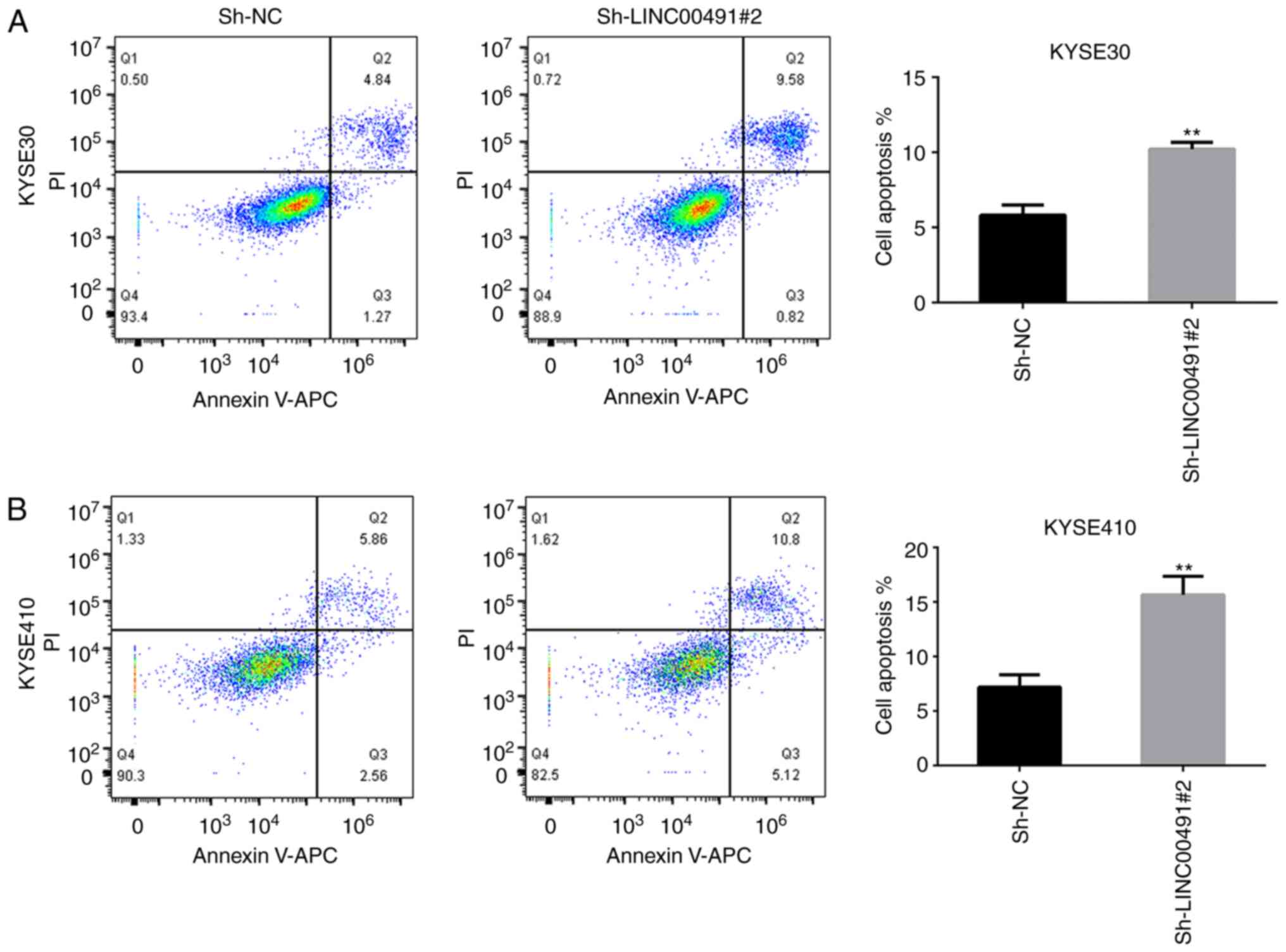
Inhibition of LINC00491 expression
promotes apoptosis of ESCC cells
Flow cytometry results demonstrated that the
apoptosis rate of KYSE-30 and KYSE-410 ESCC cells transfected with
sh-LINC00491#2 was significantly increased compared with the
control group (Fig. 9).
Discussion
Cancer is a complex disease associated with a
combination of genetic and environmental factors. Currently,
although there are various methods for the treatment of malignant
tumors, the results of these are not satisfactory, and the main
limitations are that the long-term survival rate of patients with
cancer have not significantly improved, and relapse is common
(26). EC is a common malignant
tumor of the digestive system, and is associated with poor
prognosis, and high recurrence and invasive rates (27-29). In recent years, although progress
has been made in the research of EC, its pathogenesis remains
unknown, and in terms of diagnosis and prognosis, there remains a
lack of highly specific and sensitive biomarkers.
lncRNA was originally thought to only be a
by-product of RNA polymerase II transcription as it does not encode
a protein, and thus it was considered to have no biological
function (30). However,
previous studies have reported that lncRNA is a key regulator of
gene transcription and translation (31). Moreover, abnormal expression of
lncRNA promotes tumor development by affecting the expression of
cancer-related genes at the pre-transcriptional, transcriptional
and post-transcriptional levels (32). Furthermore, tumor cell
proliferation, invasion, apoptosis, metastasis and drug resistance
are all associated with the imbalance of lncRNA expression
(33). Therefore, lncRNAs play a
key role in tumorigenesis and progression, and can be used as
potential biomarkers for various cancer types (34,35). For example, Chen et al
(36) revealed that LINC00472
inhibits proliferation, migration and invasion of liver cancer
cells via the miR-93-5p/programmed cell death 4 pathway. In
addition, Gong et al (37) reported that urothelial cancer
associated 1 acts as a competing endogenous lncRNA, which
competitively binds to miR-203 and subsequently increases the
expression level of the transcription factor zinc finger
E-box-binding homeobox 2 to promote the metastasis of gastric
cancer. Furthermore, downregulation of lncRNA colon cancer
associated transcript 1 enhances the sensitivity of human colon
cancer cells to 5-fluorouracil (38). Based on the present RNA
sequencing and GEO database analysis results, it was speculated
that the expression of LINC00491 in ESCC was upregulated compared
with healthy esophageal tissue. The expression of LINC00491 in the
healthy tissue is considered as normal expression and the
upregulated expression was validated by RT-qPCR. Moreover,
LINC00491 CNC network analysis demonstrated that LINC00491 is
co-expressed with a variety of oncogenes, such as TRIP13 and HOXB7,
and further GO and KEGG enrichment analysis identified that the
differential expression of LINC00491 in ESCC may be involved in
'chromosome segregation', 'ubiquitin protein ligase activity' and
'cell cycle process'.
Rapid proliferation is an important characteristic
of tumor cells. A normal cell cycle is a key process to ensure the
orderly proliferation of cells, and cell cycle disorders may cause
normal cells to transform into infinitely increasing tumor cells
(39). Thus, LINC00491 may
promote the occurrence and development of ESCC by affecting the
expression of tumor-related genes.
In the present study, the sample size was expanded
to detect the expression of LINC00491 in ESCC and to study the
effect of knocking down the expression of LINC00491 on the
biological features of ESCC cell lines, such as proliferation,
migration, invasion and apoptosis. It was demonstrated that the
expression of LINC00491 in 13/16 ESCC tissues was higher compared
with healthy tissues. Furthermore, RT-qPCR detection of LINC00491
in KYSE30 and KYSE410 cells revealed it to be highly expressed.
Subsequent interference with the expression of LINC00491 in ESCC
cell lines revealed that compared with the control group,
sh-LINC00491#2 significantly reduced the proliferation, migration
and invasion capabilities, and significantly increased the rate of
apoptosis. Thus, it was indicated that knockdown of LINC00491 may
have an inhibitory effect on ESCC disease progression. Due to time
constrains, the main limitation of the present study was that the
number of ESCC tissue samples collected was small; however, the
experimental tissue sample size will be expanded in future
research. In addition, the current study only included in
vitro functional experiments, and the potential mechanism of
action of LINC00491 and biological functions in vivo require
further research. However, the present study provided an
experimental basis for future clinical research and may have
potentially beneficial effects on the treatment of diseases.
In conclusion, it was demonstrated that the
expression of LINC00491 was significantly upregulated in ESCC
tissues and cells. Furthermore, in ESCC cells, knockdown of
LINC00491 inhibited proliferation and migration, and promoted
apoptosis.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 6590000161), the Science and
Technology Project of Jiangsu Province (grant no. 7790000102) and
the Jiangsu Health Commission Fund (grant no. 2017ZXK7QW08).
Availability of data and materials
The datasets used and/or analyzed during the current
study can be obtained from the corresponding author on reasonable
request.
Authors' contributions
RS designed and supervised the study. HY designed
and conducted experiments and performed data analysis. SMS helped
in collecting specimens, performed some experiments and guided the
experiments. HY and SMS wrote the manuscript. JZ helped in data
analysis. YD designed the study, provided technical assistance in
operating the equipments and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This research was approved by the Ethics Committee
for Clinical Research of Zhongda Hospital affiliated to Southeast
University (Nanjing, China; approval no. 2019ZDSYLL022-P01). All
patients signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to express their gratitude to
Professor Daqing Gao (Immunology Department, School of Medicine,
Southeast University, Nanjing, China) for providing laboratory
assistance. The authors would also like to thank Dr Qinghua Ji
(School of Medicine, Southeast University, Nanjing, China) for his
assistance in the laboratory work.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han LC and Chen Y: Small and long
non-coding RNAs: Novel targets in perspective cancer therapy. Curr
Genomics. 16:319–326. 2015. View Article : Google Scholar
|
|
5
|
Carninci P and Hayashizaki Y: Noncoding
RNA transcription beyond annotated genes. Curr Opin Genet Dev.
17:139–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: Past, present, and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andrey G and Duboule D: SnapShot: Hox gene
regulation. Cell. 156:8562014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gomez-Maldonado L, Tiana M, Roche O,
Prado-Cabrero A, Jensen L, Fernandez-Barral A, Guijarro-Muñoz I,
Favaro E, Moreno-Bueno G, Sanz L, et al: EFNA3 long noncoding RNAs
induced by hypoxia promote metastatic dissemination. Oncogene.
34:2609–2620. 2015. View Article : Google Scholar
|
|
9
|
Yu WD, Wang H, He QF, Xu Y and Wang XC:
Long noncoding RNAs in cancer-immunity cycle. J Cell Physiol.
233:6518–6523. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y
and Yang K: Up-regulation of long noncoding RNA MALAT1 contributes
to proliferation and metastasis in esophageal squamous cell
carcinoma. J Exp Clin Cancer Res. 34:72015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Wu Z, Mei Q, Li X, Guo M, Fu X and
Han W: Long non-coding RNA HOTAIR, a driver of malignancy, predicts
negative prognosis and exhibits oncogenic activity in oesophageal
squamous cell carcinoma. Br J Cancer. 109:2266–2278. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan D, Wu Y, Hu L, He P, Xiong G, Bai Y
and Yang K: Long noncoding RNA H19 is up-regulated in esophageal
squamous cell carcinoma and promotes cell proliferation and
metastasis. Dis Esophagus. 30:1–9. 2017.
|
|
13
|
Wan J, Deng D, Wang X, Wang X, Jiang S and
Cui R: LINC00491 as a new molecular marker can promote the
proliferation, migration and invasion of colon adenocarcinoma
cells. Onco Targets Ther. 12:6471–6480. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia L, Wang Y, Meng Q, Su X, Shen J, Wang
J, He H, Wen B, Zhang C and Xu M: Integrated bioinformatic analysis
of a competing endogenous RNA network reveals a prognostic
signature in endometrial cancer. Front Oncol. 9:4482019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Z, Fu P, Yu Z, Zhen F and Gu Y:
Comprehensive analysis of lncRNA-miRNA-mRNA network ascertains
prognostic factors in patients with colon cancer. Technol Cancer
Res Treat. 18:15330338198532372019. View Article : Google Scholar
|
|
16
|
Wang WJ, Li HT, Yu JP, Han XP, Xu ZP, Li
YM, Jiao ZY and Liu HB: A competing endogenous RNA network reveals
novel potential lncRNA, miRNA, and mRNA biomarkers in the prognosis
of human colon adenocarcinoma. J Surg Res. 235:22–33. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan CN, Ma L and Liu N: Systematic
analysis of lncRNA-miRNA-mRNA competing endogenous RNA network
identifies four-lncRNA signature as a prognostic biomarker for
breast cancer. J Transl Med. 16:2642018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim D, Langmead B and Salzberg SL: HISAT:
A fast spliced aligner with low memory requirements. Nat Methods.
12:357–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
The Gene Ontology Consortium: The gene
ontology resource: 20 Years and still going strong. Nucleic Acids
Res. 47:D330–D338. 2019. View Article : Google Scholar
|
|
21
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wen J, Yang H, Liu MZ, Luo KJ, Liu H, Hu
Y, Zhang X, Lai RC, Lin T, Wang HY and Fu JH: Gene expression
analysis of pretreatment biopsies predicts the pathological
response of esophageal squamous cell carcinomas to
neo-chemoradiotherapy. Ann Oncol. 25:1769–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Di S, Li M, Ma Z, Guo K, Li X and Yan X:
TRIP13 upregulation is correlated with poor prognosis and tumor
progression in esophageal squamous cell carcinoma. Pathol Res
Pract. 215:1524152019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou T, Fu H, Dong B, Dai L, Yang Y, Yan W
and Shen L: HOXB7 mediates cisplatin resistance in esophageal
squamous cell carcinoma through involvement of DNA damage repair.
Thorac Cancer. 11:3071–3085. 2020. View Article : Google Scholar :
|
|
26
|
Tiffon C: The impact of nutrition and
environmental epigenetics on human health and disease. Int J Mol
Sci. 19:34252018. View Article : Google Scholar :
|
|
27
|
Zhan XH, Jiao JW, Zhang HF, Li CQ, Zhao
JM, Liao LD, Wu JY, Wu BL, Wu ZY, Wang SH, et al: A three-gene
signature from protein-protein interaction network of LOXL2- and
actin-related proteins for esophageal squamous cell carcinoma
prognosis. Cancer Med. 6:1707–1719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu L, Qin YR, Ming XY, Zuo XB, Diao YW,
Zhang LY, Ai J, Liu BL, Huang TX, Cao TT, et al: RNA editing of
SLC22A3 drives early tumor invasion and metastasis in familial
esophageal cancer. Proc Natl Acad Sci USA. 114:E4631–E4640. 2017.
View Article : Google Scholar
|
|
29
|
Liu SY, Chen W, Chughtai EA, Qiao Z, Jiang
JT, Li SM, Zhang W and Zhang J: PIK3CA gene mutations in Northwest
Chinese esophageal squamous cell carcinoma. World J Gastroenterol.
23:2585–2591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deveson IW, Hardwick SA, Mercer TR and
Mattick JS: The dimensions, dynamics, and relevance of the
mammalian noncoding transcriptome. Trends Genet. 33:464–478. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao
SD, Zhang Y, Yang L, Shan W, He Q, et al: Comprehensive genomic
characterization of long non-coding RNAs across human cancers.
Cancer Cell. 28:529–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Washietl S, Kellis M and Garber M:
Evolutionary dynamics and tissue specificity of human long
noncoding RNAs in six mammals. Genome Res. 24:616–628. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ning L, Li Z, Wei D, Chen H and Yang C:
LncRNA, NEAT1 is a prognosis biomarker and regulates cancer
progression via epithelial-mesenchymal transition in clear cell
renal cell carcinoma. Cancer Biomark. 19:75–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sugihara H, Ishimoto T, Miyake K, Izumi D,
Baba Y, Yoshida N, Watanabe M and Baba H: Noncoding RNA expression
aberration is associated with cancer progression and is a potential
biomarker in esophageal squamous cell carcinoma. Int J Mol Sci.
16:27824–27834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu M, Zou C, Pan L, Liang W, Qian H, Xu W,
Jiang P and Zhang X: Long noncoding RNAs in digestive system
cancers: Functional roles, molecular mechanisms, and clinical
implications (Review). Oncol Rep. 36:1207–1218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen C, Zheng Q, Kang W and Yu C: Long
non-coding RNA LINC00472 suppresses hepatocellular carcinoma cell
proliferation, migration and invasion through miR-93-5p/PDCD4
pathway. Clin Res Hepatol Gastroenterol. 43:436–445. 2019.
View Article : Google Scholar
|
|
37
|
Gong P, Qiao F, Wu H, Cui H, Li Y, Zheng
Y, Zhou M and Fan H: LncRNA UCA1 promotes tumor metastasis by
inducing miR-203/ZEB2 axis in gastric cancer. Cell Death Dis.
9:11582018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang C, Pan Y and Deng SP: Downregulation
of lncRNA CCAT1 enhances 5-fluorouracil sensitivity in human colon
cancer cells. BMC Mol Cell Biol. 20:92019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|