Introduction
Cardiovascular complications are the major causes of
mortality for patients with type 2 diabetes mellitus (1). Endothelial cell injury and
endothelial dysfunction play an important role in the development
and progression of diabetic vascular disease (2). There is emerging evidence to
indicate that chronic hyperglycemia is an independent risk factor
for diabetic vascular disease in patients with type 1 diabetes and
in those with type 2 diabetes mellitus (3). High glucose (HG) is well-known to
exert multiple pathological effects on cardiovascular cells,
including endothelial cells (ECs), smooth muscle cells and
cardiomyocytes (4-6). In ECs, persistent exposure to HG
induces the inflammatory response, oxidative stress, and cellular
senescence and apoptosis, resulting in endothelial dysfunction in
patients with diabetes (7-9).
Thus, the pathogenesis of diabetic vascular complications is
complex and multifactorial, and involves regulatory interactions
between some important biological molecules. Despite advancements
being made in the understanding of the toxic effects of HG on the
cardiovascular system, the role of HG and the mechanisms of action
of HG in mediating endothelial dysfunction are not yet fully
understood.
AKT2 (GenBank Accession no. NM_001243027.3) is one
of 3 closely related serine/threonine-protein kinases (AKT1, AKT2
and AKT3) and plays critical roles in cell survival, growth,
proliferation, angiogenesis and cell metabolism (10,11). The aberrant loss or gain of AKT
activation underlies the pathophysiological properties of a variety
of complex diseases, including type 2 diabetes and cancer (10). The 3 AKT isoforms also play
complex, yet critical roles in vascular health and vascular
abnormalities (12). The
activation of AKT1 by vascular endothelial growth factor (VEGF)
promotes EC proliferation, migration and survival (13), and the loss of AKT1 in mouse ECs
results in reduced nitric oxide (NO) release and impaired
angiogenesis (14). In addition,
AKT2 knockout mice exhibit insulin resistance and glucose
intolerance and have type 2 diabetes (15,16), and dominant-negative mutations in
AKT2 lead to the genetic development of severe diabetes in humans
(17). Taken together, these
findings suggest that AKT signaling plays a major role in normal EC
physiological functions and the pathogenesis of type 2 diabetes.
However, the mechanisms underlying the regulation of AKT2 by HG in
ECs have not yet been completely elucidated.
Long non-coding RNAs (lncRNAs) regulate gene
expression at the transcription, epigenetic or translation level,
thereby altering cellular response to various stimuli (18). They have been implicated in the
regulation of the angiogenic response of endothelial cells in
vitro and vascularization in vivo (19). Aberrant lncRNA expression is
associated with a number of human disorders, particularly with
proliferative diseases (19,20). A previous study revealed that
lncRNA-MIAT knockdown evidently ameliorated diabetes-induced
retinal microvascular dysfunction in vivo, and inhibited
endothelial cell proliferation, migration and tube formation in
vitro (21). There is also
evidence to indicate that lncRNA-ATB promotes the viability,
migration and angiogenesis of human microvascular endothelial cells
by sponging miR-195 (22).
Conversely, lncRNA MEG3 has been shown to negatively regulate the
proliferation and angiogenesis of vascular endothelial cells by
sponging miR-9 (23). Although
lncRNAs are shown to regulate endothelial cell function, there is
currently little information to describe the function of lncRNAs
induced by HG in vascular endothelial cells.
In the present study, the expression of lncRNAs in
HG-exposed endothelial cells was characterized and the functional
role of lncRNA MIR181A2HG in the regulation of the proliferation
and migration of human umbilical vein endothelial cells (HUVECs) in
response to HG was investigated.
Materials and methods
Cell culture and treatment
HUVECs (cat. no. 8000) were purchased from ScienCell
Research Laboratories, and routinely cultured in ECM (cat. no.
1001; ScienCell Research Laboratories) supplemented with glucose
(5.5 mM), 1% penicillin/streptomycin (PS, cat. no. 0503; ScienCell
Research Laboratories), 5% fetal bovine serum (FBS, cat. no. 0025;
ScienCell Research Laboratories) and 1% endothelial growth factor
(EGF, cat. no. 1052; ScienCell Research Laboratories), in a
humidified incubator at 37°C with 5% CO2. The growth
medium was replaced every 2 days, and the cells were sub-cultured
every 3 days at a ratio of 1 to 4 upon reaching 80% confluence.
Human aortic smooth muscle cells (HASMCs, cat. no. 6110) were
purchased from ScienCell Research Laboratories, and routinely
cultured in smooth muscle cell medium (cat. no. 1101; ScienCell
Research Laboratories) in a humidified incubator at 37°C with 5%
CO2. D-glucose (Sigma-Aldrich; Merck KGaA) was added to
the medium which contained 2% FBS, 1% PS and 1% EGF to generate
hyperglycemic conditions. D-mannitol (30 mM; Sigma-Aldrich; Merck
KGaA) was added to ensure a similar osmotic pressure. 293 cells
stored in The Key Laboratory of Neural and Vascular Biology were
maintained in high-glucose Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS. All cells were transfected using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Briefly, in 24-well plates, 1 μg
plasmid or 20 pmol shRNA, miRNA mimcs or control and 1 μl
Lipofectamine 2000 reagent were mixed gently in Opti-MEM™ (Thermo
Fisher Scientific, Inc.) at room temperature. After 15 min, the
mixture was incubated with the cells for 4 h and replaced with ECM
medium. For luciferase reporter assay, 0.5 μg plasmid and 10
pmol miRNA mimics for miR-6832-5p, miR-6842-5p or miR-8056, shRNA
or control were mixed to co-transfect cells with 1 μl
Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Inc.)
together in a 24-well plate. AKT inhibitor (1 μM; MK2206;
Beyotime Institute of Biotechnology) was used to treat the HUVECs
for 72 h to block the AKT signaling pathway.
Microarray analysis
HUVECs were exposed to 30 or 5.5 mM D-glucose for 24
h. Quantile normalization and subsequent data processing were
performed using the GeneSpring GX v12.1 software package (Agilent
Technologies, Inc.). A NanoDrop ND-1000 spectrophotometer (Thermo
Fisher Scientific, Inc.) was used to measure the quantity and
quality of the RNA. Arraystar Human lncRNA Microarray V3.0 was
designed for the global profiling of human lncRNAs. Following the
isolation of rRNA (using the mRNA-ONLY™ Eukaryotic mRNA Isolation
kit, Epicentre), mRNA was purified from total RNA. The RNA was
amplified and transcribed into fluorescent cRNA by utilizing a
random priming method (Arraystar Flash RNA Labeling kit,
Arraystar). Each labeled cRNA was fragmented by the addition of
Blocking Agent and Fragmentation Buffer (Agilent Technologies,
Inc.), and the mixture was then heated at 60°C for 30 min. The
labeled cRNA was diluted by 2xGE Hybridization buffer (Agilent
Technologies, Inc.). The hybridization solution was then dispensed
into the gasket slide and assembled to the lncRNA expression
microarray slide for 17 h at 65°C in Hybridization Oven (Agilent
Technologies, Inc.). Finally, the hybridized arrays were washed,
fixed and scanned with using the Agilent DNA Microarray Scanner
(no. G2505C; Agilent Technologies, Inc.).
Isolation of RNA and RT-qPCR
Total RNA was extracted using the
E.Z.N.A.® Total RNA kit I (Omega Bio-tek) according to
the manufacturer's instructions. The quality of the RNA was
measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). For lncRNA and AKT2 mRNA, cDNA was synthesized
using a M-MLV First-Strand cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.). RT-PCR was performed at 65°C (10 min), on
ice (2 min), 40°C (30 min) and 70°C (10 min). The amplification
program was 50°C (2 min), 95°C (2 min), followed by 40 cycles of
95°C (15 sec) and 60°C (30 sec). For microRNAs (miRNAs or miRs),
RT-PCR and RT-qPCR were performed using the microRNA reverse
transcription and RT-qPCR kit (GenePharma, Inc.). qPCR was carried
on a Bio-Rad CFX Manager (Bio-Rad Laboratories, Inc.). The reverse
transcription program was 25°C (30 min), 42°C (30 min) and 85°C (30
min). The amplification program was 95°C (3 min), followed by 40
cycles of 95°C (12 sec) and 62°C (40 sec). A relative amount of
transcripts was normalized using the 2−ΔΔCq method with
β-actin for large mRNAs and U6 for miRNAs. The specific primers
used are listed in Table I.
 | Table ISequences of primes used in the
present study. |
Table I
Sequences of primes used in the
present study.
| Primer name | Sequence |
|---|
|
AKT2-qPCR-Forward |
TGGTCGCCAACAGCCTCA |
| AKT2-qPCR-Reverse
CC |
GCCACTTCCATCTCCTCA |
|
MIR181A2HG-qPCR-Forward C |
GCGGTTCAATACCTCGTCT |
|
MIR181A2HG-qPCR-Reverse |
TGCTGTGGCTAGAGGACAAC |
|
β-actin-qPCR-Forward |
ACTCTTCCAGCCTTCCTTCC |
|
β-actin-qPCR-Reverse C |
GTACAGGTCTTTGCGGATG |
|
shRNA-MIR181A2HG-Forward |
GGGAUAGUAGAAAGUAACAGGCUCUTT |
|
shRNA-MIR181A2HG-Reverse |
AGAGCCUGUUACUUUCUACUAUCCCTT |
| MIR181A2HG probe 1
human-392 |
CTTTCCACAGGACAGTTCGC |
| MIR181A2HG probe 2
human-377 |
TTCGCCTTTCCTTCCTTTCCTCTGT |
| MIR181A2HG probe 3
human-19 |
CCAGATTTCCTTGCAGCCC |
| miR-6832-5p mimics
sense |
AGUAGAGAGGAAAAGUUAGGGUC |
| miR-6832-5p mimics
antisense |
CCCUAACUUUUCCUCUCUACUUU |
| miR-6842-5p mimics
sense |
UGGGGGUGGUCUCUAGCCAAGG |
| miR-6842-5p mimics
antisense |
UUGGCUAGAGACCACCCCCAUU |
| miR-7110-5p mimics
sense |
UGGGGGUGUGGGGAGAGAGAG |
| miR-7110-5p mimics
antisense |
CUCUCUCCCCACACCCCCAUU |
| miR-6752-5p mimics
sense |
GGGGGGUGUGGAGCCAGGGGGC |
| miR-6752-5p mimics
antisense |
CCCCUGGCUCCACACCCCCCUU |
| miR-8056 mimics
sense |
CGUGGAUUGUCUGGAUGCAU |
| miR-8056 mimics
antisense |
GCAUCCAGACAAUCCACGUU |
| miR-181-3p mimics
sense |
ACCACTGACCGTTGACTGTAC |
| miR-181-3p mimics
antisense |
UACAGUCAACGGUCAGUGGUUU |
| miR-181-5p mimics
sense |
AACATTCAACGCTGTCGGTGAGT |
| miR-181-5p mimics
antisense |
UCACCGACAGCGUUGAAUGUUU |
| mimics Ctl
sense |
UUCUCCGAACGUGUCACGUTT |
| mimics Ctl
antisense |
ACGUGACACGUUCGGAGAATT |
| shRNA-AKT2-Forward
C |
GUGGUGAAUACAUCAAGATT |
|
shRNA-AKT2-Reverse |
UCUUGAUGUAUUCACCACGTT |
|
miR-6832-5p-qPCR-Forward C |
GCTGCGAGTAGAGAGGAAAAG |
|
miR-6832-5p-qPCR-Reverse |
TATGGTTCTTCACGACTGGTTCAC |
|
miR7110-5p-qPCR-Forward C |
TTTATTATGGGGGTGTGGGG |
|
miR-7110-5p-qPCR-Reverse |
TATGGTTGTTCACGAGTCCTTGTC |
|
miR-8056-5p-qPCR-Forward CC |
TCGCTCGTGGATTGTCT |
|
miR-8056-5p-qPCR-Reverse |
TATGGTTGTTCACCTCTCCTTCAC |
|
miR-181-3p-qPCR-Forward C |
TTTATTAACCACTGACCGT |
|
miR-181-3p-qPCR-Reverse |
TATGGTTCTTCACGACTGGTTCAC |
|
miR-181-5p-qPCR-Forward C |
TTTATTAAACATTCAACGC |
|
miR-181-5p-qPCR-Reverse |
TATGGTTCTTCACGACTGGTTCAC |
| U6-qPCR-Forward
C |
GCTTCGGCAGCACATATAC |
|
U6-qPCR-Reverse |
TTCACDAATTTGCGTGTCATC |
Plasmid construction and shRNA
Recombinant plasmids expressing lncRNA-MIR181A2HG
and AKT2 were constructed by GENEWIZ. The MIR181A2HG sequences and
the 3′UTR sequences of AKT2 mRNA containing wild-type (WT) and
mutant binding sites for miR-6832-5p, miR-6842-5p or miR-8056
(synthesized by GENEWIZ) were inserted into pmirGLO Dual-Luciferase
vector (Promega Corporation). shRNAs against the lncRNA-MIR181A2HG
and AKT2 were designed and synthesized by GENEWIZ. The detailed
sequences are listed in Table
I.
Target prediction and luciferase reporter
assay
The TargetScan prediction algorithm (http://www.targetscan.org/) to predict the binding
between MIR181A2HG, miRNAs and AKT2. Luciferase reporter assay was
performed as previously described (22). In brief, 293 cells were seeded
into a 24-well plate and pmirGLO reporter plasmids (WT or mutant)
or their empty plasmid were co-transfected with the miRNA mimic
(GenePharma, Inc.) or miR-Ctl (GenePharma, Inc.) and pRL-TK.
Following 24 h of transfection, luciferase activity was measured
using a Dual-Glo Luciferase Assay System (Promega Corporation) with
a Flash and Glow reader. The luciferase activity was expressed as
the relative activity ratio of Firefly luciferase to Renilla
luciferase. The sequences of MIR181A2HG are presented in Table SI.
RNA pulldown assay
Biotinylated lncRNA MIR181A2HG was used to perform
RNA pull-down. HUVECs were cross-linked with 1% formaldehyde in PBS
and quenched with 0.125 M glycine. HUVECs were dissolved in lysis
buffer on ice for 10 min and then sonicated with a microtip
ultrasonicator on ice. A total of 100 pmol biotinylated probes were
added to the cell lysate diluted with hybridization buffer.
Subsequently, 100 μl washed/blocked Streptavidin Dynabeads
(Thermo Fisher Scientific, Inc.) blocked in lysis buffer containing
yeast tRNA and BSA were added per 100 pmol of probes, and the whole
mix was then rotated for 30 min at 37°C. Dynabeads were captured by
magnets (Thermo Fisher Scientific, Inc.), washed with wash buffer
and then eluted with elution buffer.
Fluorescence in situ hybridization
(FISH)
In situ hybridization was performed using
fluorescence Cy3-labeled lncRNA-MIR181A2HG probes (50 nM) in
hybridization buffer (Exiqon) by incubation at 55°C for 1 h.
Following stringent washing with SSC buffer, non-specific binding
sites were blocked with 10% normal goat serum (KPL). Images were
acquired using a Leica microscope (Leica Microsystems GmbH) and
digitized with a software of LAS AF software. The sequences of
MIR181A2HG probes are presented in Table I.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde and
pre-incubated with 10% normal goat serum (KPL) at room temperature
and then incubated with primary antibody anti-AKT2 (mouse
monoclonal, 1:100, ab175354, Abcam) at 4°C overnight. Secondary
antibody was FITC-labeled antibody to mouse IgG (cat. no.
5230-0307, KPL, 1:50, US) and incubated at room temperature for 2
h. Antifading mounting medium (with DAPI) (Beijing Solarbio Science
& Technology Co., Ltd.) was used for nuclear staining at room
temperature and stored at 4°C. Images were captured using a
confocal microscope (Leica Microsystems GmbH) and digitized using
LAS AF software (v. 2.6.0, Leica Microsystems GmbH).
MTS assay
After the HUVECs were treated with plasmids and 5.5
or 30 mM glucose, the viability of the HUVECs cultured in 96-well
plates was measured by MTS assay, as previously described (24). Following the addition of glucose
1-5 days later or transfection 24 later, the ECM medium of cultured
HUVECs was replaced with 100 μl ECM medium containing 10
μl of CellTiter 96 AQueous One Solution (Promega
Corporation). The plates were then incubated at 37°C for 4 h. The
96-well plate was then examined at 490 nm using a Multiskan
spectrum spectrophotometer (Thermo Fisher Scientific, Inc.).
Scratch test
HUVECs were treated with plasmids and 5.5 or 30 mM
glucose. when the cells were 90-95% confluent, a sterile 200
μl pipette tip was used to draw straight lines in the middle
of the well, and serum-free ECM medium was used to wash the cells
twice, and this was then replaced with ECM medium containing 2%
FBS, 1% PS and 1% EGF, as previously described (25). Images were collected (Canon EOS
600d) at this time as a 0 h control. The culture was continued for
a further 24 h at 37°C. The image data were collected after 24
h.
Transwell migration assay
A Transwell chamber was placed in a 24-well plate,
and 800 μl of ECM medium containing 10% FBS was added to
each well. A total of 2×104 cells were diluted in 200
μl of serum-free ECM medium and were added to the upper
chamber. Following incubation at 37°C for 24 h, the upper surface
of the membrane was wiped with a cotton-tipped applicator to remove
non-migratory cells and the migratory cells on the under surface
were fixed with 10% formaldehyde and stained with 2% crystal violet
(Beijing Solarbio Science & Technology Co., Ltd.) solution for
10 min at room temperature. The membranes were mounted on glass
slides and the number of migrated cells from 10 random microscopic
fields were counted by manual counting after obtaining images. The
image data were collected using a Canon EOS 600d camera (Canon EOS
600d).
3D-culture
Matrigel gel was placed in a 24-well plate at 40
μl per well in a 37°C incubator for 2 h. After the cells
were properly treated, HUVECs were digested and the cell density
was adjusted to 3×105/ml. The cell suspension was added
to the surface of Matrigel gel and placed in a 37°C incubator. The
degree of tube formation was evaluated using a microscope after 24
h incubation at 37°C and 5% CO2. The full capillary-like
structures in the field were counted and collected using a Canon
EOS 600d camera.
Measurement of total adenosine
triphosphate (ATP) concentration
The total ATP level was detected using the ATP Assay
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. A total of 100 μl working
solution was added to a 10 μl diluted sample, and the
luciferase activity was immediately evaluated by Modulus™ II
Microplate multifunction tester. The ATP content was determined by
comparison to a concurrent standard curve and was then normalized
by cell number and expressed as μmol/ml.
Glucose uptake assay and glycogen
synthesis assay
Before and after the cells were transfected with
pGFPN1, pGFPN1/MIR181A2HG, pGFPN1/AKT2 or miRNA mimics,
glucose in the cell culture medium was measured using
Glucose Uptake Colorimetric assay kit (Sigma-Aldrich; MerckKGaA).
Glucose uptake was calculated by the difference before and after
the cells were treated. After the cells were treated, the glycogen
level was detected using a glycogen assay kit (Nanjing Jiancheng
Bioengineering Inc.), and the results were normalized to a standard
curve and expressed as μg of glycogen, according to the
manufacturer's instructions.
Western blot analysis
Cells were lysed in RIPA lysis buffer (Beyotime
Institute of Biotechnology). Equal amounts of protein (20 μg
for total protein and for 60 μg for p-GSK3β) were run on 10%
SDS-PAGE, and electro-transferred to a polyvinylidene fluoride
(PVDF) membranes (EMD Millipore). PVDF membranes were blocked with
5% milk in TTBS at room temperature for 2 h and then incubated with
primary anti-bodies, overnight at 4°C. The antibodies used were as
follows: Anti-AKT2 (mouse monoclonal, 1:500, ab175354),
anti-proliferating cell nuclear antigen (PCNA; rabbit monoclonal,
1:500, ab92552), anti-matrix metalloproteinase 2 (MMP2; rabbit
polyclonal, 1:1,000, ab97779), anti-VE-cadherin (rabbit polyclonal,
1:500, ab33168), anti-GLUT1 (rabbit monoclonal, 1:1,000, ab150299),
anti-glycogen synthase kinase 3β (GSK3β; mouse monoclonal, 1:500,
ab93926), anti-p-GSK3β (rabbit polyclonal, 1:1,000, ab75745) or
anti-β-actin (rabbit polyclonal, 1:1,000, ab8227 and mouse
polyclonal, 1:1,000, ab8226) (all from Abcam). PVDF membranes were
then incubated with the HRP-conjugated secondary antibody (goat
anti-rabbit cat. no. 074-1506 or goat anti-mouse, cat. no. 074-1806
1:8,000, KPL) for 1 h at room temperature. The blots were treated
using a high-sensitivity ECL chemiluminescence detection kit
(Nanjing Vazyme Biotech Co., Ltd.) and exposed by films. The
densitometry of blots was measured using ImageJ software (v.1.51j8,
National Institutes of Health).
Statistical analysis
All statistical analyses were performed using the
GraphPad Prism software (version 5.0; GraphPad Software, Inc.).
Data are expressed as the means ± standard deviation. All data were
tested for normality and equal variance and were proved to be
consistent with variance with R studio software. The Student's
t-test was used to compare the statistical differences between 2
groups. One-way ANOVA or two-way ANOVA followed by Tukey's post hoc
test was used for comparisons among >2 groups. All experiments
were repeated independently at least 3 times. A value of P≤0.05 was
considered to indicate a statistically significant difference.
Results
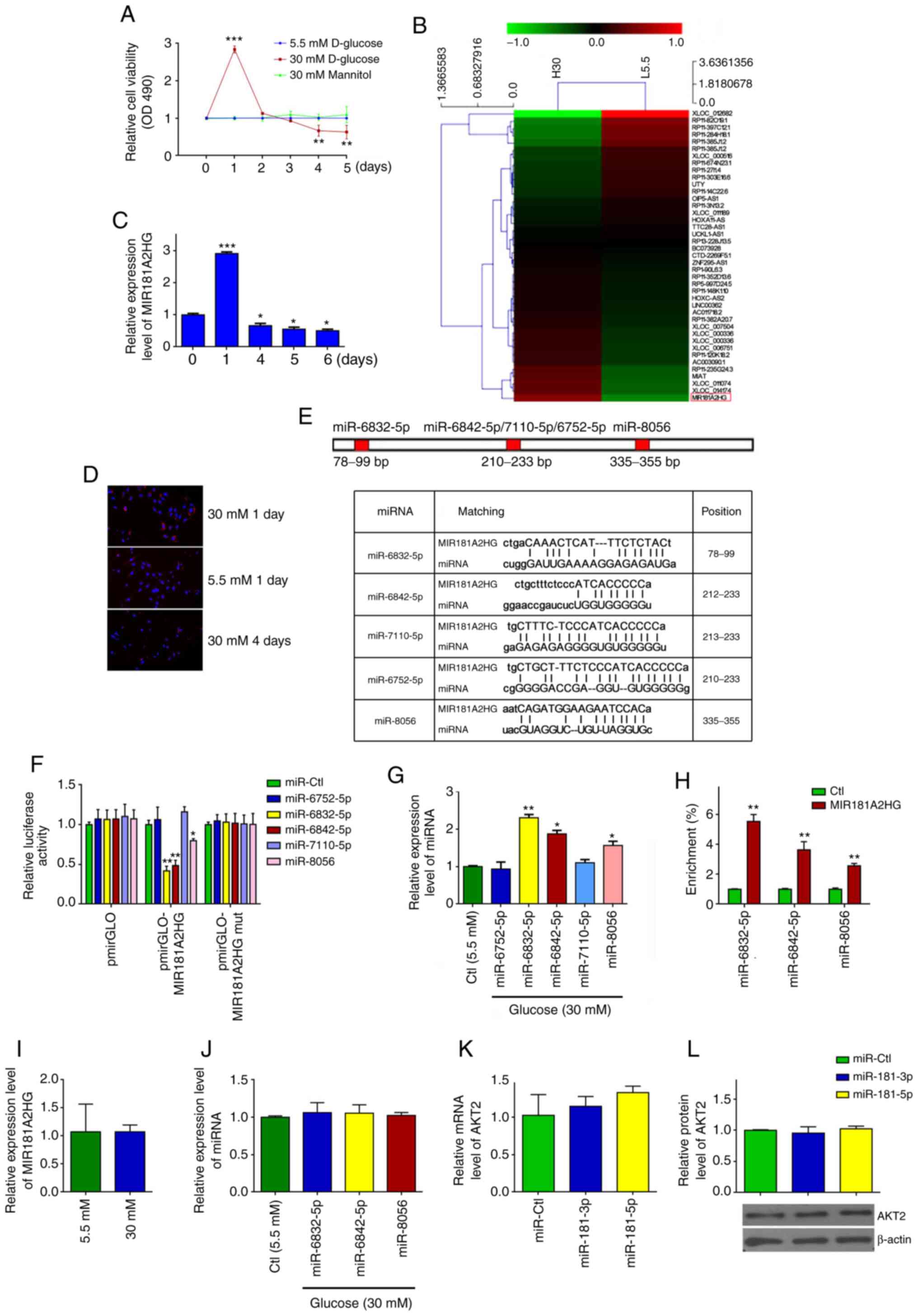
Persistent exposure of HUVECs to HG
increases miR-6832-5p, miR-6842-5p and miR-8056 expression levels
by downregulating MIR181A2HG
As lncRNAs are known to be involved in diabetes
mellitus-induced vascular dysfunction (21), the present study determined the
effects of the persistent exposure of HUVECs to HG on lncRNA
expression. HUVECs were exposed to 5.5 mM (normal glucose) and 30
mM glucose (HG) for different periods of time. It was found that HG
significantly increased cell viability during the first 2 days,
whereas after 3 days of exposure to HG, the viability of the
HG-exposed HUVECs decreased significantly compared with the cells
treated with normal glucose or 30 mM mannitol, which was used to
eliminate the possible effects of osmotic pressure (Fig. 1A). Subsequently, lncRNA
microarray analysis was performed in the HUVECs exposed to 5.5 or
30 mM glucose for 24 h. The results revealed that 709 or 457
lncRNAs were upregulated or downregulated, respectively, in the
HUVECs exposed to HG for 24 h compared to those treated with 5.5 mM
glucose (Fig. 1B and Table SII). As the expression level of
MIR181A2HG was highest in the HUVECs exposed to HG for 24 h
compared with those of the other lncRNAs, lncRNA MIR181A2HG
(GenBank Accession no. NR_038975.1) was selected to validate the
microarray data by RT-qPCR. The results revealed that MIR181A2HG
expression was markedly increased after 1 day of stimulation with
HG, but significantly decreased 4 days later (Fig. 1C). Similar results were obtained
by FISH using probes against MIR181A2HG (Fig. 1D). These results suggest that the
expression of MIR181A2HG may be associated with the viability of
HUVECs exposed to HG.
As a number of lncRNAs have been known to act as
competing endogenous RNAs (ceRNA) by interacting with miRNAs
(26,27), the present study investigated
whether MIR181A2HG could interact with miRNAs in HUVECs. Using the
TargetScan prediction algorithm (http://www.targetscan.org/), 5 miRNAs were predicted
(miR-6832-5p, miR-6842-5p, miR-7110-5p, miR-6752-5p and miR-8056)
targeting sites on MIR181A2HG (Fig.
1E). Subsequently, MIR181A2HG was cloned, which contains WT or
the mutated binding sites for the predicted 5 miRNAs, into pm i r
GLO plasm id (pm i r GLO -MI R181A2HG or pmirGLO-MIR181A2HG-mut)
and examined the effects of the predicted 5 miRNAs on
MIR181A2HG-directed luciferase activity. After the successful
transfection of these 5 miRNA mimics into HUVECs was confirmed by
RT-qPCR (Fig. S1A), it was found
that co-transfection of miR-6832-5p, miR-6842-5p or miR-8056, but
not miR-6752-5p and miR-7110-5p, with pmirGLO-MIR181A2HG
significantly reduced the luciferase activities directed by the WT
reporter vector. The mutation of binding sites for miR-6832-5p,
miR-6842-5p or miR-8056 on MIR181A2HG (pmirGLO-MIR181A2HG-mut)
abolished this inhibition (Fig.
1F). In parallel with these findings, RT-qPCR revealed that the
levels of miR-6832-5p, miR-6842-5p and miR-8056 expression were
significantly increased in the HUVECs exposed to HG for 4 days
compared with that of the cells exposed to normal glucose (Fig. 1G). To further validate the direct
association of MIR181A2HG with these 3 miRNAs, RNA pull-down assay
was performed, demonstrating that miR-6832-5p, miR-6842-5p and
miR-8056 were enriched in the precipitates of 3′-biotinylated
MIR181A2HG (Fig. 1H). These
results suggested that MIR181A2HG specifically associated with
these 3 miRNAs, and that the prolonged exposure of HUVECs to HG
downregulated MIR181A2HG expression, abolishing the interaction
between MIR181A2HG and miR-6832-5p, miR-6842-5p or miR-8056 in
HUVECs.
The present study also examined that the effects of
HG on the expression of MIR181A2HG and the 3 miRNAs in HASMCs. The
results revealed that the exposure of HASMCs to HG did not
significantly affect the expression of MIR181A2HG (Fig. 1I) and the 3 miRNAs (Fig. 1J), suggesting that the effect and
regulatory mechanisms of HG on MIR181A2HG/miRNAs in HUVECs was
specific, which is different from the HASMCs. Furthermore,
considering that lncRNA MIR181A2HG is the MIR181A2 (including
miR-181-3p and miR-181-5p) host gene, the present study
investigated whether miR-181-3p and miR-181-5p directly target
AKT2. After the successful transfection of miR-181-3p and
miR-181-5p into HUVECs was verified (Fig. S1B), RT-qPCR and western blot
analysis were performed and it was revealed that the transfection
of HUVECs with these 2 miRNAs did not affect the expression of AKT2
mRNA and protein (Fig. 1K and
L), indicating that lncRNA MIR181A2HG-derived miR181-3p and
miR181-5p do not directly target the AKT2 gene in HUVECs.
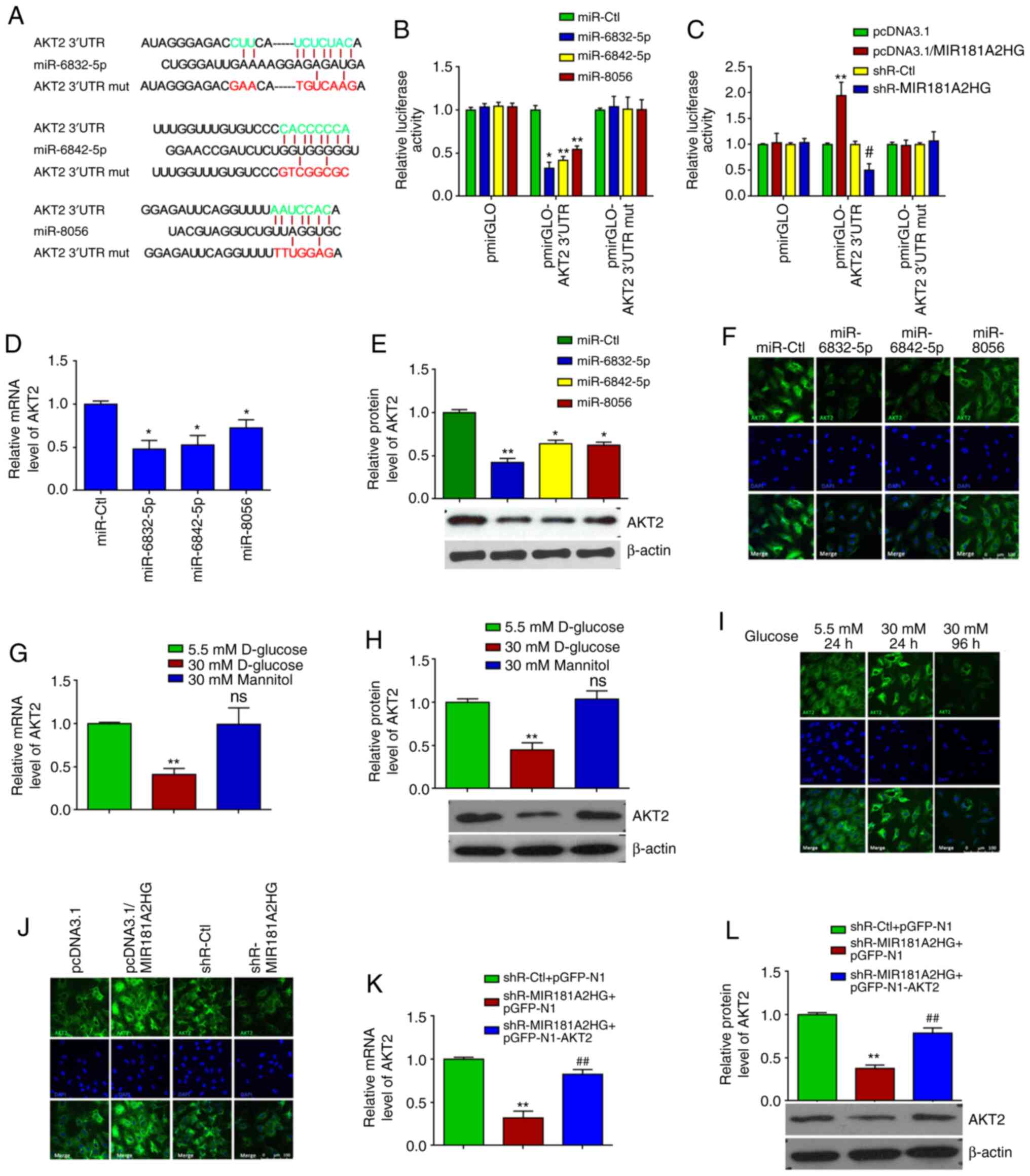
miR-6832-5p, miR-6842-5p and miR-8056 target the AKT2 3′UTR and
regulate its expression in HUVECs. As the downregulation of
lncRNA MIR181A2HG induced by HG increased the miR-6832-5p,
miR-6842-5p and miR-8056 levels, the bioinformatics database,
TargetScan, was used to predict the targets of miR-6832-5p,
miR-6842-5p and miR-8056. It was found that the AKT2 3′UTR
contained the potential binding sites of these 3 miRNAs (Fig. 2A). The AKT downstream substrates
GSK3β and GLUT1 contained no binding sites for these miRNAs within
their 3′UTR. Subsequently, luciferase reporter plasmids which
contained the WT (pmirGLO-AKT2 3′UTR) or mutated (pmirGLO-AKT2
3′UTR-mut) binding sites for these miRNAs in the AKT2 3′UTR were
constructed. Luciferase reporter assay revealed that
co-transfection with miR-6832-5p, miR-6842-5p or miR-8056 and
pmirGLO-AKT2 3′UTR reduced the luciferase activity, respectively,
by 67.59, 58.27 and 45.58%, while no obvious changes were observed
when these 3 miRNAs were co-transfected with pmirGLO-AKT2 3′UTR-mut
(Fig. 2B). After the successful
MIR181A2HG knockdown and overexpression were confirmed via RT-qPCR
(Fig. S1C), it was found that
the knockdown of MIR181A2HG decreased, whereas the overexpression
of MIR181A2HG increased the luciferase activity directed by
pmirGLO-AKT2 3′UTR, but not pmirGLO-AKT2 3′UTR-mut (Fig. 2C).
To provide further evidence that MIR181A2HG
increased the expression of AKT2 by sponging miR-6832-5p,
miR-6842-5p and miR-8056, HUVECs were transfected with these
miRNAs, respectively. RT-qPCR and western blot analysis revealed
that miR-6832-5p, miR-6842-5p and miR-8056 overexpression
significantly decreased AKT2 expression at both the mRNA and
protein level (Fig. 2D and E). A
similar result was also obtained by the immunofluorescence staining
of AKT2, demonstrating that these 3 miRNAs evidently reduced AKT2
expression compared to the miR-Ctl (Fig. 2F). Simultaneously, the expression
of AKT2 mRNA and protein was significantly suppressed in the HUVECs
exposed to HG, but not in those exposed to normal glucose or
mannitol, for 4 days, as shown by RT-qPCR, western blot and
immunofluorescence staining (Fig.
2G-I). In addition, immunofluorescence staining revealed that
MIR181A2HG overexpression enhanced, whereas its knockdown
attenuated the protein expression of AKT2 in the HUVECs relative to
their corresponding control (Fig.
2J). After the successful overexpression of AKT2 was confirmed
in HUVECs (Fig. S1D), rescue
experiments showed that the inhibition of AKT2 mRNA and
protein expression induced by MIR181A2HG knockdown could be rescued
by overexpression of AKT2 (Fig. 2K
and L). These results suggest that the prolonged exposure of
HUVECs to HG resulted in MIR181A2HG downregulation and thus in its
reduced association with miR-6832-5p, miR-6842-5p and miR-8056,
subsequently leading to an inhibition of AKT2 expression.
Knockdown of MIR181A2HG inhibits the
proliferation, migration and capillary-like structures of
HUVECs
The above-mentioned results led us to investigate
the biological functions of MIR181A2HG in HUVECs. Using loss- and
gain-of function approaches, it was found that overexpression of
MIR181A2HG promoted, whereas the knockdown of MIR181A2HG inhibited
the proliferation of HUVECs, as shown by MTS assay (Fig. 3A). A scratch test and Transwell
migration assay were then performed to determine the effects of
MIR181A2HG on the migration of HUVECs. The overexpression or
knockdown of MIR181A2HG promoted or inhibited the migration of
HUVECs, respectively, compared with their corresponding control
(Fig. 3B and C). Subsequently,
3D-culture was performed to examine the ability of MIR181A2HG to
form capillary-like structures. The results revealed that the
knockdown of MIR181A2HG significantly attenuated the formation of
capillary-like structures, while the overexpression of MIR181A2HG
increased this capacity (Fig.
3D). Taken together, these results indicated that MIR181A2HG
facilitated the proliferation, migration and capillary-like
structure formation in HUVECs.
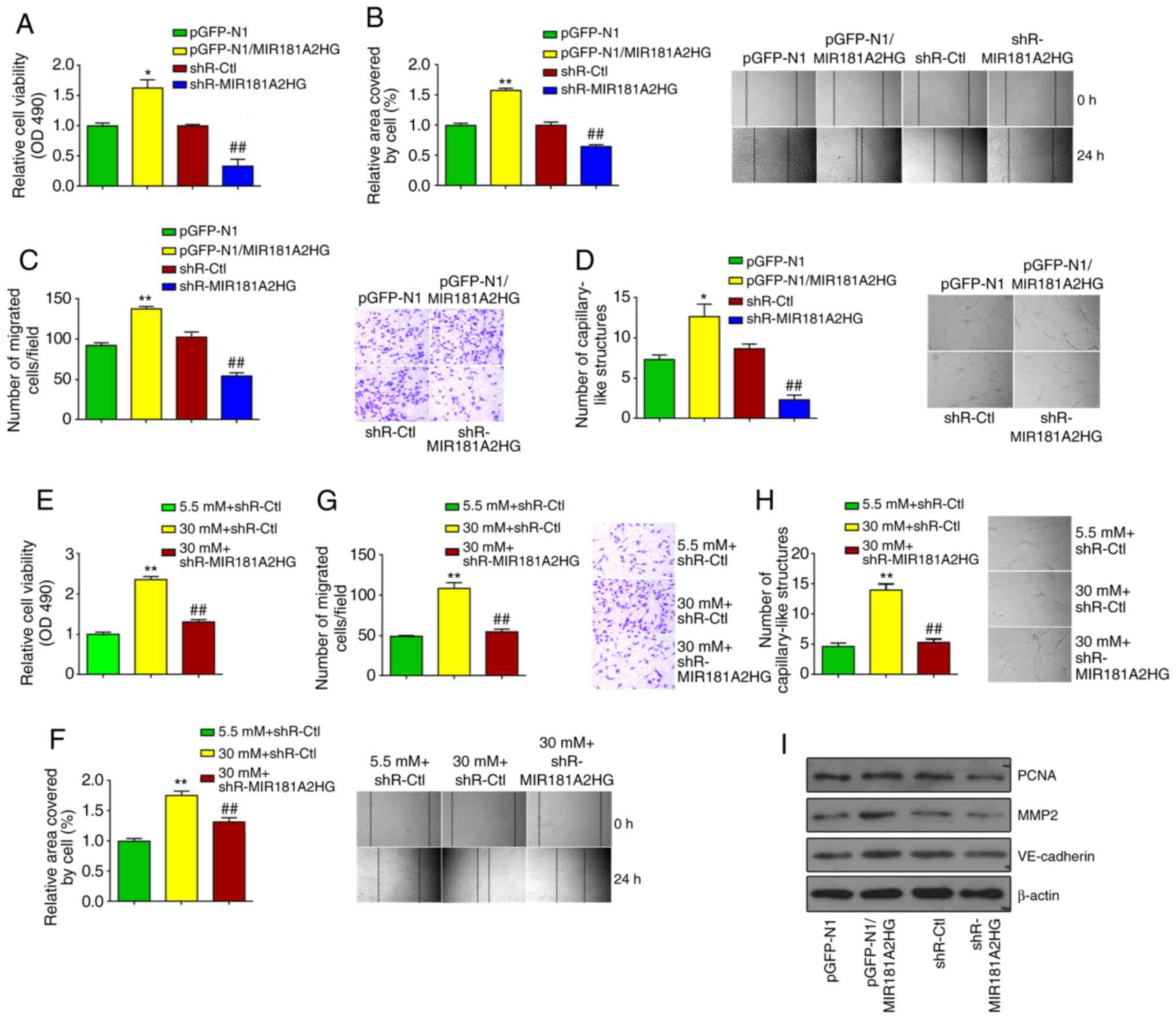 | Figure 3Knockdown of MIR181A2HG inhibits
proliferation, migration and capillary-like structures of HUVECs.
(A-C) HUVECs were transfected with pGFP-N1-MIR181A2HG,
shR-MIR181A2HG or their corresponding control. (A) MTS assay, (B)
scratch test, and (C) Transwell migration assay were used to detect
the cell viability and migration. *P<0.05 and
**P<0.01 vs. pGFP-N1; ##P<0.01 vs.
shR-Ctl. (D) 3D-culture was used to detect the formation of
capillary-like structures. *P<0.05 vs. pGFP-N1;
##P<0.01 vs. shR-Ctl. (E-H) HUVECs were transfected
with shR-MIR181A2HG or shR-Ctl and exposed to 5.5 or 30 mM
D-glucose. (E) MTS assay, (F) scratch test, (G) Transwell
migration, and (H) 3D-culture were used to detect the cell
viability, migration and the formation of capillary-like
structures. **P<0.01 vs. shR-Ctl+5.5 mM D-glucose;
##P<0.01 vs. shR-Ctl+30 mM D-glucose. (I) Western
blot analysis was used to detect PCNA, MMP2 and VE-cadherin
expression in HUVECs transfected with pGFP-N1-MIR181A2HG,
shR-MIR181A2HG or their corresponding control. HUVECs, human
umbilical vein endothelial cells. |
To further corroborate the causal role of MIR181A2HG
in HUVEC proliferation and migration, HUVECs were exposed to HG for
24 h to stimulate HUVEC proliferation and migration, and the
effects of MIR181A2HG depletion on HUVEC biological behavior were
observed. As was anticipated, the exposure of HUVECs to HG for 24 h
significantly enhanced cell proliferation, whereas the knockdown of
MIR181A2HG greatly abolished this effect (Fig. 3E). Similarly, exposure to HG for
24 h combined with MIR181A2HG knockdown also abrogated the inducing
effects of HG on HUVEC migration, as shown by scratch wound healing
assay and Transwell migration assay (Fig. 3F and G). Moreover, MIR181A2HG
depletion markedly reduced the formation of capillary-like
structures induced by exposure to HG for 24 h (Fig. 3H). To further verify the
functions of MIR181A2HG, cell proliferation and migration-related
markers, including PCNA, MMP2 and VE-cadherin were detected. It was
found that MIR181A2HG promoted the expression of PCNA, MMP2 and
VE-cadherin in HUVECs (Fig.
3I).
MIR181A2HG/miRNAs/AKT2 axis regulates
proliferation, migration and capillary-like structure formation in
HUVECs
Subsequently, the present study investigated whether
these 3 miRNAs and AKT2 could exert effects on HUVEC proliferation,
migration and capillary-like structure formation. The transfection
of miR-6832-5p, miR-6842-5p or miR-8056 significantly inhibited the
proliferation and migration of HUVECs, compared with the control
group, as examined by MTS assay, scratch test and Transwell
migration assay (Fig. 4A-C).
Correspondingly, 3D-culture revealed that these miRNAs
significantly decreased the formation of the capillary-like
structures in HUVECs (Fig. 4D).
Consistent with the above-mentioned results, miR-6832-5p,
miR-6842-5p and miR-8056 markedly suppressed the expression levels
of PCNA, MMP2 and VE-cadherin (Fig.
4E). Moreover, western blot analysis revealed that the
expression of PCNA, MMP2 and VE-cadherin was markedly inhibited by
the AKT inhibitor, MK2206, in the HUVECs (Fig. 4F).
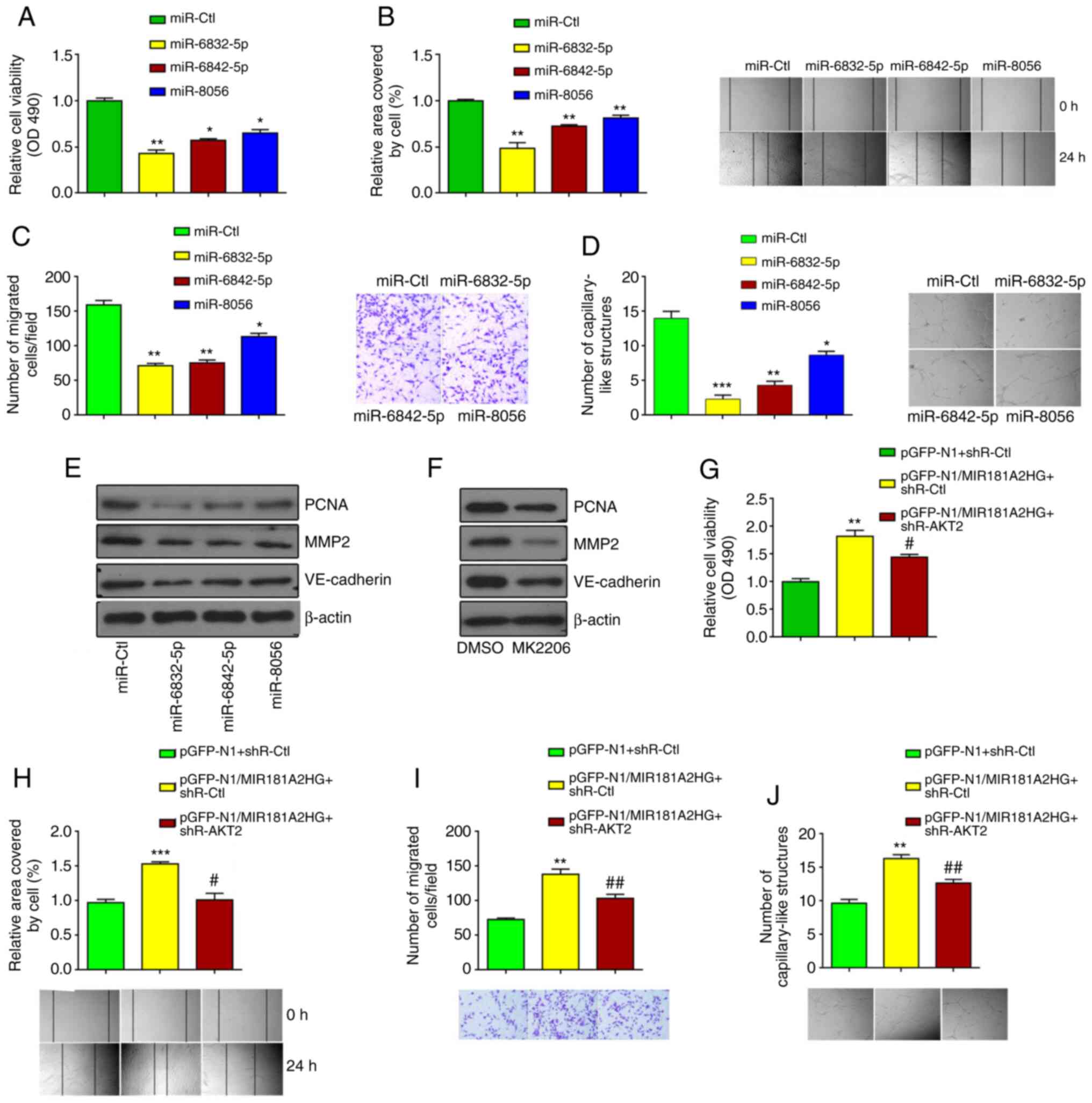 | Figure 4MIR181A2HG/miRNAs/AKT2 axis regulates
proliferation, migration and capillary-like structures of HUVECs.
(A-D) MTS assay, scratch test, Transwell migration, and 3D-culture
were used to detect the cell viability, migration, and the
formation of capillary-like structures. *P<0.05,
**P<0.01 and ***P<0.001 vs. miR-Ctl.
(E) Western blot analysis detected the expression of PCNA, MMP2 and
VE-cadherin in HUVECs transfected with the different miRNAs. (F)
Western blot analysis detected the expression of PCNA, MMP2 and
VE-cadherin in HUVECs treated with the AKT2 inhibitor, MK2206. (G)
MTS assay, (H) scratch test, (I) Transwell migration, and (J)
3D-culture were used to detect the cell viability, migration and
the formation of capillary-like structures in HUVECs transfected
with pGFP-N1-MIR181A2HG alone or together with shR-AKT2.
**P<0.01 and ***P<0.001 vs.
pGFP-N1+shR-Ctl; #P<0.05 and ##P<0.01
vs. pGFP-N1/MIR181A2HG+shR-Ctl. HUVECs, human umbilical vein
endothelial cells. |
To further determine whether AKT2 is required for
the MIR181A2HG-mediated effects, AKT2 was knocked down using shRNA
and MIR181A2HG was overexpressed by plasmid vector in HUVECs
(Fig. S1C and D). As shown in
Fig. 4G-J, the knockdown of AKT2
in HUVECs significantly abrogated the inducing effects of
MIR181A2HG on proliferation, migration and capillary-like structure
formation. Taken together, these results indicated that the
prolonged exposure of HUVECs to HG impaired cell functions by
dysregulating the MIR181A2HG/miRNAs/AKT2 axis.
The promotion of HUVEC proliferation and
migration by MIR181A2HG is accompanied by changes in glucose
metabolism
As AKT2 is known to be a central protein in many
cellular processes, such as cell survival, proliferation, glucose
uptake and metabolism and angiogenesis (28,29), the present study then sought to
examine whether MIR181A2HG, which upregulates the expression of
AKT2, also affects glucose metabolism in HUVECs. It was found that
the overexpression or knockdown of MIR181A2HG increased or
decreased, respectively, the ATP content, glucose uptake and
glycogen synthesis compared with their corresponding control in
HUVECs (Fig. 5A). The ATP
content, glucose uptake and glycogen synthesis were also
significantly decreased in the HUVECs transfected with miR-6832-5p,
miR-6842-5p or miR-8056 (Fig.
5B). Likewise, AKT2 also enhanced the ATP content, glucose
uptake and glycogen synthesis (Fig.
5C).
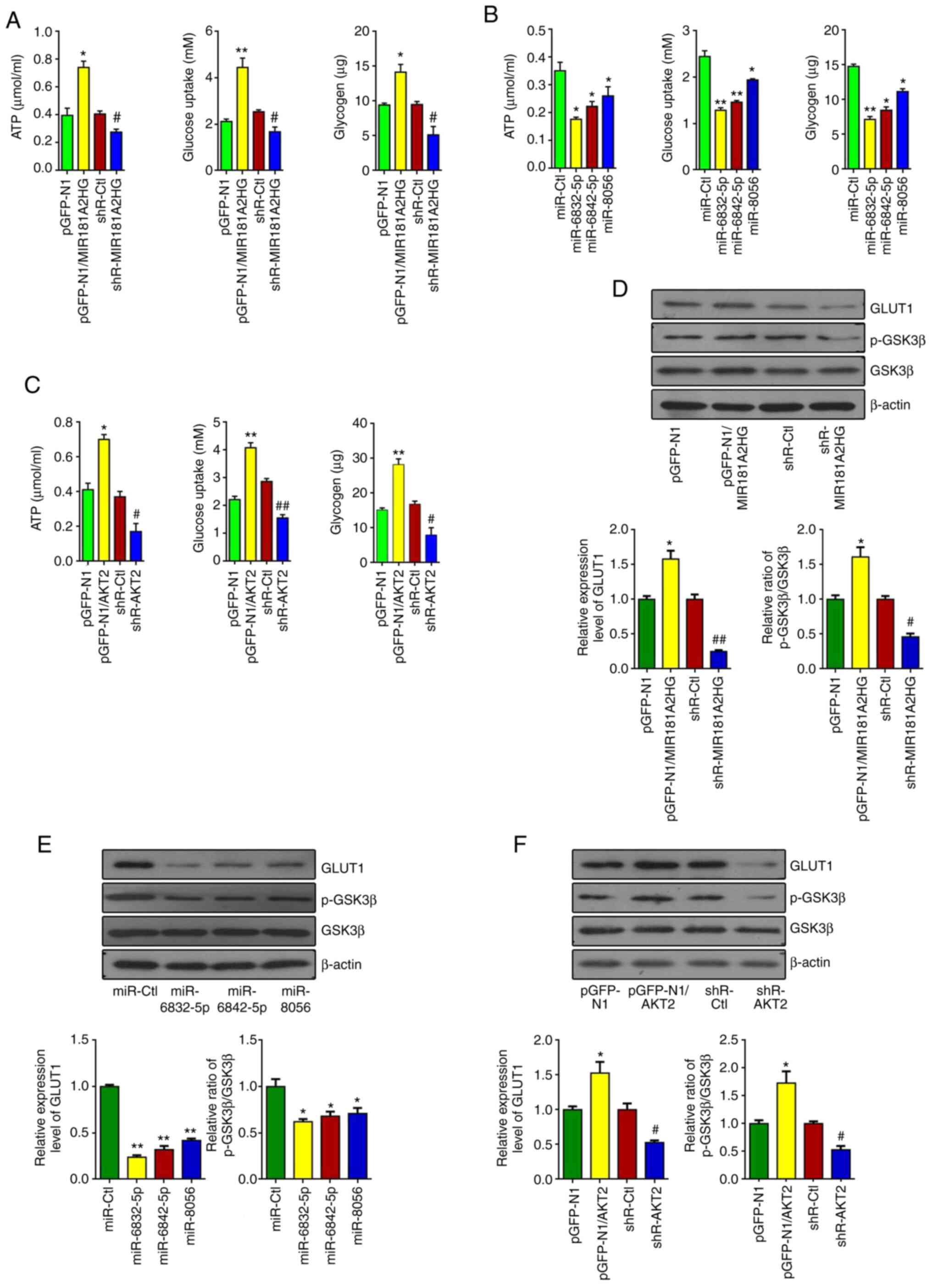 | Figure 5The promotion of proliferation and
migration by MIR181A2HG is accompanied by changes of glucose
metabolism. (A) ATP content, glucose uptake and glycogen synthesis
were detected by luminescence assay, the glucose oxidase (GOD)
method and glycogen colorimetric assay in HUVECs transfected with
pGFP-N1-MIR181A2HG, shR-MIR181A2HG or their corresponding control.
*P<0.05 and **P<0.01 vs. pGFP-N1,
#P<0.05 vs. shR-Ctl. (B) ATP content, glucose uptake
and glycogen were measured in HUVECs transfected with miR-6832-5p,
miR-6842-5p and miR-8056. *P<0.05 and
**P<0.01 vs. miR-Ctl. (C) ATP content, glucose uptake
and glycogen were measured in HUVECs transfected with pGFP-N1-AKT2,
shR-AKT2 and their corresponding control. *P<0.05 and
**P<0.01 vs. pGFP-N1; #P<0.05 and
##P<0.01 vs. shR-Ctl. (D-F) Western blot analyiss was
used to detect the expression of GLUT1, p-GSK3β and GSK3β in HUVECs
treated as in (A-C). *P<0.05 and
**P<0.01 vs. pGFP-N1 or miR-Ctl,
#P<0.05 and ##P<0.01 vs. shR-Ctl.
HUVECs, human umbilical vein endothelial cells. |
Considering the influence of MIR181A2HG
downregulation on glucose metabolism, the present study then
examined whether MIR181A2HG-miRNAs-AKT2 can regulate the expression
of glucose metabolism-related genes, such as GLUT1 and GSK3β. The
results revealed that the overexpression of MIR181A2HG enhanced
GLUT1 expression and the phosphorylation of GSK3β. By contrast, the
knockdown of MIR181A2HG reduced GLUT1 expression and GSK3β
phosphorylation in HUVECs (Fig.
5D). Moreover, the transfection of miR-6832-5p, miR-6842-5p and
miR-8056 inhibited GLUT1 expression and GSK3β phosphorylation
(Fig. 5E). Furthermore, AKT2
promoted GLUT1 expression and GSK3β phosphorylation in HUVECs
(Fig. 5F). Taken together, these
results indicated that the injury of vascular endothelial cells
induced by HG may be attributed to the downregulation of
MIR181A2HG, which leads to the energy scarcity and the disruption
of glucose metabolism.
Discussion
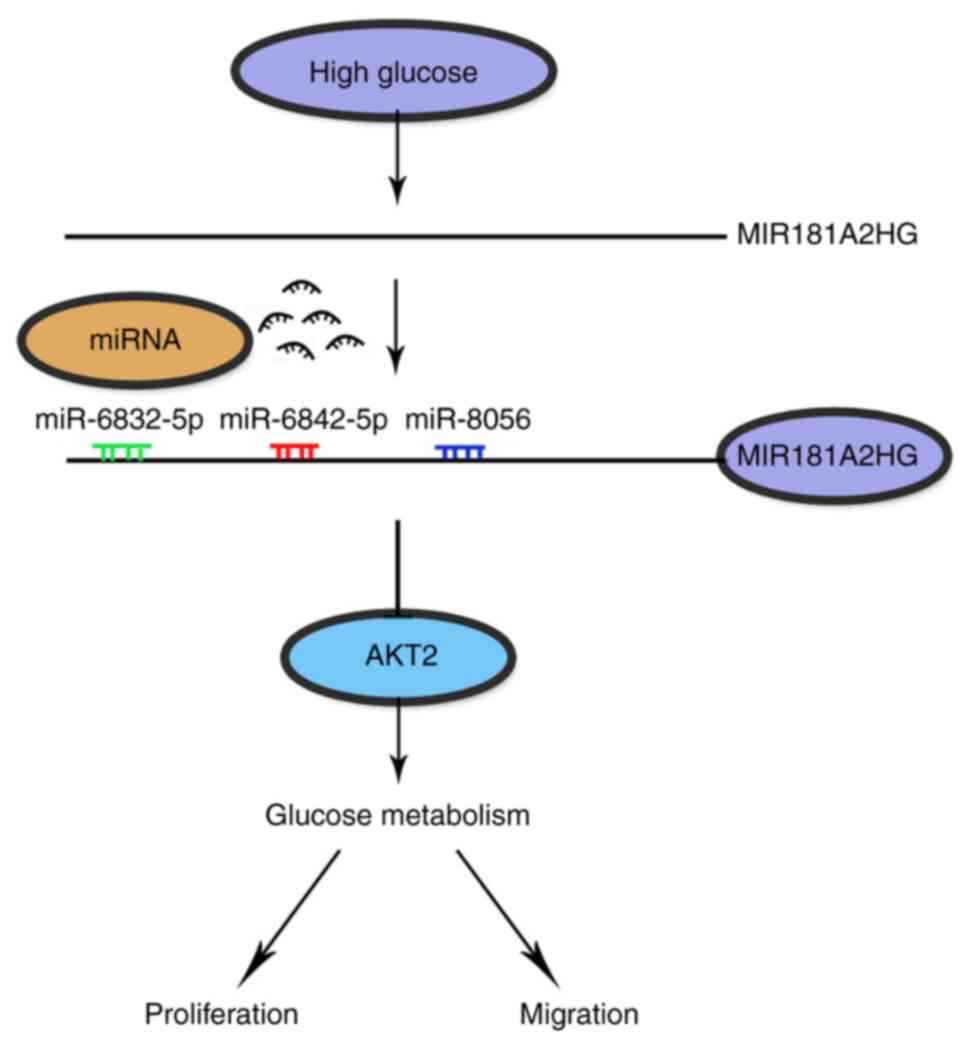
Previous studies have demonstrated that HG can
damage vascular ECs by inducing inflammation (30), oxidative stress (31), apoptosis and cellular senescence
(9). lncRNAs regulate EC
function and vessel growth (19). However, it remains largely
unclear whether and how HG-induced lncRNAs impair EC function. The
main findings of the present study are the following: i) The
persistent exposure of HUVECs to HG downregulated lncRNA MIR181A2HG
expression; ii) MIR181A2HG downregulation significantly suppressed
the proliferation, migration and angiogenesis of HUVECs by
decreasing its sponge for miR-6832-5p, miR-6842-5p and miR-8056;
iii) miR-6832-5p, miR-6842-5p and miR-8056 target the 3′UTR of
AKT2, and their increase elicited by MIR181A2HG downregulation
attenuated expression of AKT2, and thus suppressed HUVEC
proliferation, migration and the formation of the capillary-like
structures.
It is well known that the endothelial cell
monolayers located in the inner lining of blood vessels play a
protective role by blocking direct contact between blood cells and
the underlying tissue. Small defined areas of endothelial damage
are rapidly repaired by the migration and proliferation of ECs in
the vicinity (32). Thus, EC
proliferation and migration are a key process in wound repair
occurring on the luminal surface of blood vessels. Endothelial
dysfunction may alter EC proliferation and migration, resulting in
delayed endothelium repair with an increased risk of thrombosis
(33). Moreover, several studies
have demonstrated that the prevention of restenosis may be achieved
by promoting endothelial regeneration through the use of growth
factors (34), EC seeding
(35), and vessel reconstruction
with autologous EC/fibrin matrix (36). In the present study, it was
demonstrated that the viability of HUVECs was significantly
decreased in response to HG and that the toxic effects of HG on
HUVECs were increased with the duration of HG stimulation.
Specifically, the persistent exposure of HUVECs to HG resulted in
an obvious downregulation of lncRNA MIR181A2HG. Although MIR181A2HG
has been registered in the GenBank under the Accession no.
100379345, its function had not yet been reported. As the function
of lncRNAs, as competitive endogenous RNAs (ceRNA) to regulate gene
expression by sponging some miRNAs, is well established, we
identified putative miRNAs associated with MIR181A2HG by using
bioinformatics analyses. Luciferase reporter assays and the
affinity pull-down of miRNAs by MIR181A2HG revealed that
miR-6832-5p, miR-6842-5p and miR-8056 could be sponged by
MIR181A2HG. Furthermore, opposite to the changes in MIR181A2HG
expression in HUVECs exposed to HG for 4 days, the expression of
miR-6832-5p, miR-6842-5p and miR-8056 was significantly enhanced in
chronic HG-stimulated cells. These findings suggest that there is a
specific interaction between these 3 miRNAs and MIR181A2HG, and the
HG-induced downregulation of MIR181A2HG results in increased level
of miR-6832-5p, miR-6842-5p and miR-8056 by reducing the
association between MIR181A2HG and the 3 miRNAs.
Recently, some lncRNAs have been identified in ECs
treated with different stimuli, including TNF-α (37), serum deprivation (38), hypoxia (39) and rapamycin (40). These lncRNAs regulate the cell
viability, proliferation, migration and angiogenesis of ECs by
sponging miRNA-21 and miR-199a-5p, as well as by modulating VEGFR2,
ERK1/2, respectively. The present study then wished to determine
the mechanisms through which MIR181A2HG-regulated miR-6832-5p,
miR-6842-5p and miR-8056 affects HUVEC proliferation, migration and
the formation of the capillary-like structures. First, the targets
of miR-6832-5p, miR-6842-5p and miR-8056 we predicted by
bioinformatics databases, and it was identified that AKT2 contains
the potential binding sites of these 3 miRNAs in the 3′UTR of its
mRNA. Luciferase reporter assays, RT-qPCR and western blot analysis
confirmed that the overexpression of miR-6832-5p, miR-6842-5p and
miR-8056 enhanced, whereas their knockdown attenuated the
expression of AKT2 protein. Moreover, the persistent exposure of
HUVECs to HG significantly attenuated the expression of AKT2 mRNA
and protein, consistent with the above-mentioned observations that
HG downregulated the MIR181A2HG expression level, and thus
decreased its sponging for miR-6832-5p, miR-6842-5p and miR-8056,
subsequently facilitating the suppression of AKT2 expression by
miRNAs.
The serine/threonine kinase AKT, also known as
protein kinase B (PKB), is a central node in cell signaling
downstream of growth factors, cytokines, and other cellular stimuli
(10). Mouse and human genetic
studies have revealed physiological roles for the AKT network in
nearly every organ system, and AKT also has particularly important
roles in diverse pathological processes, including cardiovascular
disease, insulin resistance and type 2 diabetes (12). Accumulating evidence has
indicated shown that AKT expression is regulated at the
post-transcriptional level by miRNAs. For instance, miR-373-3p
targets the 3′UTR of AKT1 and inhibits its expression, and its
downregulation promotes the proliferation of prostate cancer cells
(41). miR-124 suppresses
proliferation, glycolysis and energy metabolism in non-small cell
lung cancer cells by targeting AKT1/2 (42). miR-16 inhibits cell proliferation
and induces apoptosis in oral squamous cell carcinoma through
decreasing AKT3 (43). However,
little is known about whether AKT is regulated in ECs by miRNAs. In
the present study, it was found that miR-6832-5p, miR-6842-5p and
miR-8056 targeted, respectively, their corresponding sites at the
3′UTR of AKT2 mRNA and regulated AKT2 expression by enhancing its
mRNA degradation and inhibiting translation. Among these 3 miRNAs,
the inhibitory effect of miR-6832-5p on AKT2 expression was much
more potent than the other 2 miRNAs. Thus, miR-6832-5p may have a
greater impact on AKT2-mediated HUVEC proliferation and migration.
These findings provide novel insight into the regulation of AKT2 at
the post-transcriptional level by miRNAs.
Considering that AKT-mediated phosphorylation of its
substrates, such as FOXO, GSK3, p27, eNOS, BAD and TSC2,
contributes to activation of the various cellular processes,
including survival, growth, proliferation, angiogenesis, glucose
uptake and metabolism (10), it
is reasonable to conclude that increased miR-6832-5p, miR-6842-5p
and miR-8056 by MIR181A2HG downregulation attenuated the expression
of AKT2 and thus impaired HUVEC proliferation, migration and
angiogenesis (Fig. 6). These
findings are consistent with previous evidence demonstrating that
AKT signaling is attenuated in metabolic tissues in the
insulin-resistant state that underlies type 2 diabetes (44). This newly identified
MIR181A2HG-miRNAs-AKT2 regulatory axis may be a potential
therapeutic target for HG-induced endothelial dysfunction.
Supplementary Data
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 31871152 and
8177085) and the Graduate Innovation Program of Hebei Province (no.
2015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW performed most of the experiments and wrote the
original draft of the manuscript. HZ performed cell cultures and
Transwell migration assay. XZ and YL performed the 3D-culture and
contributed to the acquisition of data and statistical analyses. JW
and BZ were involved in the study design, supervised the study,
obtained funding for the study and revised the manuscript. All
authors reviewed and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Gregg EW, Gu Q, Cheng YJ, Narayan KM and
Cowie CC: Mortality trends in men and women with diabetes 1971 to
2000. Ann Intern Med. 147:149–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joussen AM, Doehmen S, Le ML, Koizumi K,
Radetzky S, Krohne TU, Poulaki V, Semkova I and Kociok N: TNF-alpha
mediated apoptosis plays an important role in the development of
early diabetic retinopathy and long-term histopathological
alterations. Mol Vis. 15:1418–1428. 2009.PubMed/NCBI
|
|
3
|
Madonna R and De Caterina R: Cellular and
molecular mechanisms of vascular injury in diabetes-part I:
Pathways of vascular disease in diabetes. Vascul Pharmacol.
54:68–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pei-Yuan Z, Yu-Wei L, Xiang-Nan Z, Song T,
Rong Z, Xiao-Xiao H, Sheng-Shuai S, Kun W and Cheng-Yun L:
Overexpression of Axl reverses endothelial cells dysfunction in
high glucose and hypoxia. J Cell Biochem. Mar 8–2019.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang ML, Zheng B, Tong F, Yang Z, Wang
ZB, Yang BM, Sun Y, Zhang XH, Zhao YL and Wen JK: iNOS-derived
peroxynitrite mediates high glucose-induced inflammatory gene
expression in vascular smooth muscle cells through promoting KLF5
expression and nitration. Biochim Biophys Acta Mol Basis Dis.
1863:2821–2834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang P, Zhang D, Qiu H, Yi X, Zhang Y,
Cao Y, Zhao B, Xia Z and Wang C: Tiron ameliorates high
glucose-induced cardiac myocyte apoptosis by PKCδ-dependent
inhibition of osteopontin. Clin Exp Pharmacol Physiol. 44:760–770.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Zhou Q, Pei C, Liu B, Li M, Fang L,
Sun Y, Li Y and Meng S: Hyperglycemia and advanced glycation end
products regulate miR-126 expression in endothelial progenitor
cells. J Vasc Res. 53:94–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Stefano V, Cencioni C, Zaccagnini G,
Magenta A, Capogrossi MC and Martelli F: p66ShcA modulates
oxidative stress and survival of endothelial progenitor cells in
response to high glucose. Cardiovasc Res. 82:421–429. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi T, Matsui-Hirai H, Miyazaki-Akita
A, Fukatsu A, Funami J, Ding QF, Kamalanathan S, Hattori Y, Ignarro
LJ and Iguchi A: Endothelial cellular senescence is inhibited by
nitric oxide: Implications in atherosclerosis associated with
menopause and diabetes. Proc Natl Acad Sci USA. 103:17018–17023.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manning BD and Toker A: AKT/PKB Signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hemmings BA and Restuccia DF: The
PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol.
7:a0266092015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Somanath PR, Razorenova O, Chen
WS, Hay N, Bornstein P and Byzova TV: Akt1 regulates pathological
angiogenesis, vascular maturation and permeability in vivo. Nat
Med. 11:1188–1196. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ackah E, Yu J, Zoellner S, Iwakiri Y,
Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, et
al: Akt1/protein kinase Balpha is critical for ischemic and
VEGF-mediated angiogenesis. J Clin Invest. 115:2119–2127. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho H, Thorvaldsen JL, Chu Q, Feng F and
Birnbaum MJ: Akt1/PKBalpha is required for normal growth but
dispensable for maintenance of glucose homeostasis in mice. J Biol
Chem. 276:38349–38352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garofalo RS, Orena SJ, Rafidi K, Torchia
AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks
JR, et al: Severe diabetes, age-dependent loss of adipose tissue,
and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin
Invest. 112:197–208. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
George S, Rochford JJ, Wolfrum C, Gray SL,
Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini
CL, et al: A family with severe insulin resistance and diabetes due
to a mutation in AKT2. Science. 304:1325–1328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Wei G, Luo H, Wu W, Skogerbø G, Luo
J and Chen R: The long noncoding RNA SNHG1 promotes tumor growth
through regulating transcription of both local and distal genes.
Oncogene. 36:6774–6783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Michalik KM, You X, Manavski Y,
Doddaballapur A, Zörnig M, Braun T, John D, Ponomareva Y, Chen W,
Uchida S, et al: Long noncoding RNA MALAT1 regulates endothelial
cell function and vessel growth. Circ Res. 114:1389–1397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan SX, Yang F, Yang Y, Tao QF, Zhang J,
Huang G, Yang Y, Wang RY, Yang S, Huo XS, et al: Long noncoding RNA
associated with microvascular invasion in hepatocellular carcinoma
promotes angiogenesis and serves as a predictor for hepatocellular
carcinoma patients' poor recurrence-free survival after
hepatectomy. Hepatology. 56:2231–2241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li
YJ, Tao ZF, Song YC, Chen Q and Jiang Q: lncRNA-MIAT regulates
microvascular dysfunction by functioning as a competing endogenous
RNA. Circ Res. 116:1143–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu AD, Sun YY, Ma QJ and Xu F: lncRNA-ATB
promotes viability, migration, and angiogenesis in human
microvascular endothelial cells by sponging microRNA-195. J Cell
Biochem. 120:14360–14371. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He C, Yang W and Yang J, Ding J, Li S, Wu
H, Zhou F, Jiang Y, Teng L and Yang J: Long noncoding RNA MEG3
negatively regulates proliferation and angiogenesis in vascular
endothelial cells. DNA Cell Biol. 36:475–481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang LJ, Chen L, Lu Y, Wu JM, Xu B, Sun
ZG, Zheng SZ and Wang AY: Danshensu has anti-tumor activity in
B16F10 melanoma by inhibiting angiogenesis and tumor cell invasion.
Eur J Pharmacol. 643:195–201. 2010. View Article : Google Scholar
|
|
25
|
Wei N, Yu SP, Gu XH, Taylor TM, Song D,
Liu XF and Wei L: Delayed intranasal delivery of
hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced
cell homing and therapeutic benefits after ischemic stroke in mice.
Cell Transplant. 22:977–991. 2013. View Article : Google Scholar
|
|
26
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cesana M, Cacchiarelli D, Legnini I,
Santini T, Sthandier O, Chinappi M, Tramontano A and Bozzoni I: A
long noncoding RNA controls muscle differentiation by functioning
as a competing endogenous RNA. Cell. 147:358–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Häggblad Sahlberg S, Mortensen AC, Haglöf
J, Engskog MK, Arvidsson T, Pettersson C, Glimelius B, Stenerlow B
and Nestor M: Different functions of AKT1 and AKT2 in molecular
pathways, cell migration and metabolism in colon cancer cells. Int
J Oncol. 50:5–14. 2017. View Article : Google Scholar
|
|
29
|
Muslin AJ: Akt2: A critical regulator of
cardiomyocyte survival and metabolism. Pediatr Cardiol. 32:317–322.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu DD, Tang RN, Lv LL, Wen Y, Liu H,
Zhang XL, Ma KL and Liu BC: Interleukin-1β mediates high glucose
induced phenotypic transition in human aortic endothelial cells.
Cardiovasc Diabetol. 15:422016. View Article : Google Scholar
|
|
31
|
Lin Y, Berg AH, Iyengar P, Lam TK, Giacca
A, Combs TP, Rajala MW, Du X, Rollman B, Li W, et al: The
hyperglycemia-induced inflammatory response in adipocytes: The role
of reactive oxygen species. J Biol Chem. 280:4617–4626. 2005.
View Article : Google Scholar
|
|
32
|
Kishimoto T, Oguri T, Abe M, Kajitani H
and Tada M: Inhibitory effect of methylmercury on migration and
tube formation by cultured human vascular endothelial cells. Arch
Toxicol. 69:357–361. 1995. View Article : Google Scholar
|
|
33
|
Tang R, Zhang G and Chen SY: Smooth muscle
cell proangiogenic phenotype induced by cyclopentenyl cytosine
promotes endothelial cell proliferation and migration. J Biol Chem.
291:26913–26921. 2016. View Article : Google Scholar
|
|
34
|
Kipshidze N, Dangas G, Tsapenko M, Moses
J, Leon MB, Kutryk M and Serruys P: Role of the endothelium in
modulating neointimal formation: vasculoprotective approaches to
attenuate restenosis after percutaneous coronary interventions. J
Am Coll Cardiol. 44:733–9. 2004.PubMed/NCBI
|
|
35
|
Kipshidze N, Ferguson JJ III, Keelan MH
Jr, Sahota H, Komorowski R, Shankar LR, Chawla PS, Haudenschild CC,
Nikolaychik V and Moses JW: Endoluminal reconstruction of the
arterial wall with endothelial cell/glue matrix reduces restenosis
in an atherosclerotic rabbit. J Am Coll Cardiol. 36:1396–1403.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kipshidze N, Dangas G, Tsapenko M, Moses
J, Leon MB, Kutryk M and Serruys P: Role of the endothelium in
modulating neointimal formation: Vasculoprotective approaches to
attenuate restenosis after percutaneous coronary interventions. J
Am Coll Cardiol. 44:733–739. 2004.PubMed/NCBI
|
|
37
|
Halimulati M, Duman B, Nijiati J and
Aizezi A: Long noncoding RNA TCONS_00024652 regulates vascular
endothelial cell proliferation and angiogenesis via microRNA-21.
Exp Ther Med. 16:3309–3316. 2018.PubMed/NCBI
|
|
38
|
Hou J, Wang L, Wu Q, Zheng G, Long H, Wu
H, Zhou C, Guo T, Zhong T, Wang L, et al: Long noncoding RNA H19
upregulates vascular endothelial growth factor A to enhance
mesenchymal stem cells survival and angiogenic capacity by
inhibiting miR-199a-5p. Stem Cell Res Ther. 9:1092018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ruan W, Zhao F, Zhao S, Zhang L, Shi L and
Pang T: Knockdown of long noncoding RNA MEG3 impairs
VEGF-stimulated endothelial sprouting angiogenesis via modulating
VEGFR2 expression in human umbilical vein endothelial cells. Gene.
649:32–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun H, Wang S and Song M: Long non-coding
RNA SENCR alleviates the inhibitory effects of rapamycin on human
umbilical vein endothelial cells. Mol Med Rep. 18:1405–1414.
2018.PubMed/NCBI
|
|
41
|
Qu HW, Jin Y, Cui ZL and Jin XB:
MicroRNA-373-3p inhibits prostate cancer progression by targeting
AKT1. Eur Rev Med Pharmacol Sci. 22:6252–6259. 2018.PubMed/NCBI
|
|
42
|
Zhao X, Lu C, Chu W, Zhang B, Zhen Q, Wang
R, Zhang Y, Li Z, Lv B, Li H and Liu J: MicroRNA-124 suppresses
proliferation and glycolysis in non-small cell lung cancer cells by
targeting AKT-GLUT1/HKII. Tumour Biol. 39:10104283177062152017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang X and Li GH: MicroRNA-16 functions as
a tumor-suppressor gene in oral squamous cell carcinoma by
targeting AKT3 and BCL2L2. J Cell Physiol. 233:9447–9457. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang X, Liu G, Guo J and Su Z: The
PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci.
14:1483–1496. 2018. View Article : Google Scholar : PubMed/NCBI
|