1. Introduction
Myocardial infarction (MI) is one of the main causes
of morbidity and mortality in the United States, where
approximately every 40 sec, an individual will suffer from MI
(1). Recovering reperfusion
using thrombolytic agents and percutaneous coronary intervention
may rapidly reverse myocardial ischemia and preserve cardiac
systolic function (2). However,
the recanalization of blood flow can also result in irreversible
detrimental effects known as myocardial ischemia/reperfusion injury
(MIRI), which may cause myocardial stunning, reperfusion
arrhythmia, or the no-reflow phenomenon and lethal reperfusion
injury. Over the past two decades, a growing number of studies have
demonstrated that apoptosis, innate inflammation, oxidative stress,
calcium overload and autophagy are involved in the pathogenesis of
MIRI (3-5). However, there is still no effective
strategy for limiting or preventing MIRI. Thus, developing novel
therapies for MIRI is crucial and remains an opportunity that will
provide significant clinical benefits.
The human genome contains approximately 20,000
protein-coding genes. Only 2% of the human genome is transcribed
into RNA transcripts, which are translated into protein, with the
vast majority of transcripts comprising non-coding RNAs (ncRNAs)
(6,7). There are numerous types of ncRNAs,
which are generally divided into two categories according to their
nucleotide length: Long ncRNAs (lncRNAs), which are ≥200
nucleotides in length, and small ncRNAs, which are ≤200 nucleotides
in length, such as microRNAs (miRNAs/miRs) (8,9).
The newly defined classes of lncRNAs and miRNAs have been shown to
regulate gene expression at the transcription, post-transcription
and epigenetic levels. In addition, circRNAs have been identified
as part of the lncRNA family, which also play important roles in
the regulation of gene expression (10,11). These RNAs are covalently closed,
endogenous biomolecules with no 5′ end caps or 3′ poly(A) tails,
are not easily degraded by ribonuclease R and are more stable than
other lncRNAs (10). The
biogenesis of ncRNAs is complex, and the specific process of each
ncRNA is shown in Fig. 1. Recent
findings have shown that several novel ncRNAs can participate in a
variety of important biological control processes and the
development of multiple cardiovascular diseases, including MIRI
(9,12-14). Such leads, not only enhance the
understanding of disease pathogenesis, but also identify non-coding
transcripts that potentially serve as MIRI diagnosis biomarkers or
therapeutic targets.
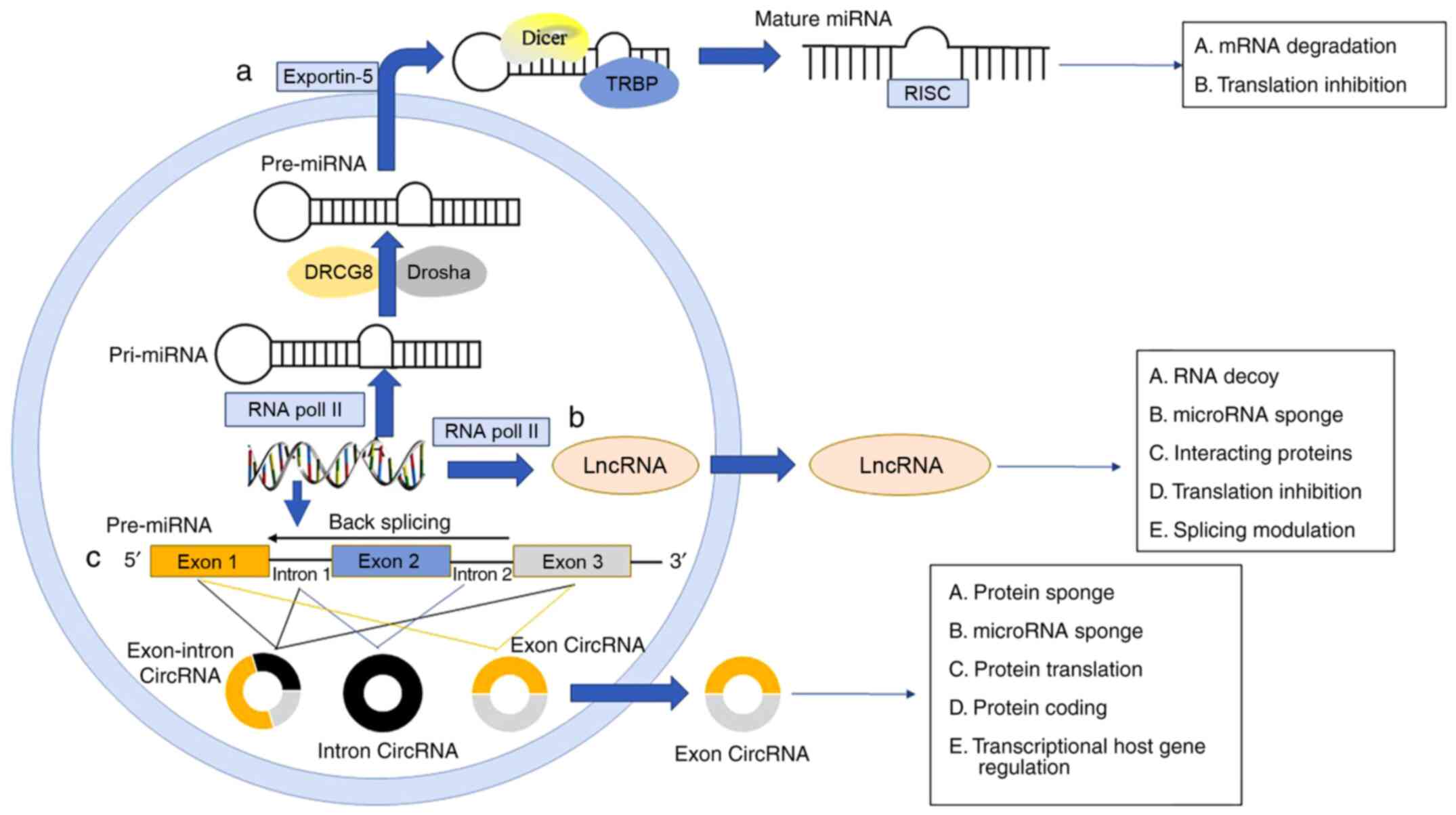 | Figure 1The biogenesis of miRNA, lncRNA and
ciRNA. (a) miRNA genes are transcribed as pri-miRNA by RNA Pol II
after which they are mediated by DRCG8 and RNAse III endonuclease
Drosha, and pre-miRNA translocates from the nucleus to the
cytoplasm mediated by exportin 5. Then, the RNase III endonuclease
Dicer interacting with TRBP cleaves the pre-miRNA and mature miRNAs
are incorporated into the RISC. (b) LncRNA genes are transcribed
mostly by Pol II, and its biogenesis process is similar to miRNA.
(c) circRNAs are generated by back-splicing events on maturing
pre-mRNA that join together an exon at the upstream 3′ splice site
to an exon at the downstream 5′ splice site resulting in a circular
product, including exon, intron and exon-intron circRNA. circRNAs
that are generated from exons only are mostly found in the
cytoplasm, which suggests a role in post-transcriptional gene
regulation. pri-miRNA, primary miRNA; Pol II, polymerase II; DRCG8,
DiGeorge syndrome criticial region 8; pre-miRNA, precursor miRNA;
TRBP, TAR RNA-binding protein; RISC, RNA-induced silencing
complex. |
The present review summarizes the characteristics
and biological roles of miRNAs, lncRNAs and circRNAs, with
particular emphasis on their role in MIRI. A more comprehensive
understanding of these ncRNAs may bridge a major gap in the
knowledge of the molecular mechanisms in the pathogenesis of
MIRI.
2. Biological functions of miRNA
miRNAs, which are typically 20-22 nucleotides in
length, are highly conserved and single-stranded ncRNAs that are
encoded by the genome of several DNA viruses and all eukaryotic
organisms (15). Their primary
function is to negatively modulate gene expression by binding to
the target mRNA and subsequently inhibiting its translation or
promoting degradation in a sequence-dependent manner (16). Only mature miRNAs are
incorporated into the RNA-induced silencing complex to direct mRNA
target silencing. Canonically, this complex binds a target gene via
partial sequence complementarity between the miRNA and a conserved
site within the 3′-untranslated region of their target mRNAs. There
are differences in the complementary degree between miRNAs: A
single gene can be modulated by several miRNAs, whereas mRNA
duplexes enable a single miRNA to target multiple mRNAs (17). miRNAs can regulate the
translation of >60% of protein-coding genes. Furthermore, miRNAs
can be sequestered by pseudogenes or other lncRNAs, introducing a
new layer of regulatory complexity (18).
miRNAs that are present in cardiac tissue have
important functional implications in cardiovascular biology, such
as proliferation, smooth muscle maturation, cardiogenesis and
endothelial function (19-22). All metabolic activities of an
organism in the cardiovascular system are considered to be
influenced by miRNA regulatory processes. Increasing evidence has
shown that miRNAs are involved in the pathogenesis of MIRI,
including inflammation, oxidative stress and apoptosis. Table I shows a summary of some
important miRNAs (23-25).
 | Table ISummary of miRNAs in the pathogenesis
of MIRI. |
Table I
Summary of miRNAs in the pathogenesis
of MIRI.
| miRNA | Expression with
MIRI | Targeted genes | Pathological
mechanism | (Refs.) |
|---|
| miR-1 | Increase | MAPK3 | Pro-apoptotic | (13) |
| miR-15b-5p | Increase | KCNJ2 | Pro-apoptotic | (31) |
| miR-22 | Decrease | CBP |
Anti-inflammatory | (38) |
| miR-29a | Increase | SIRT1 | pro-oxidative
stress | (41) |
| miR-30e | Decrease | Notch1 |
Anti-apoptotic
Anti-oxidative stress | (4) |
| miR-128-1-5p | Decrease | Gadd45g | Anti-apoptotic | (28) |
| miR-129-5p | Decrease | HMGB1 | Anti-apoptotic | (29) |
| miR-135a | Decrease | PTP1B | Anti-apoptotic | (26) |
| miR-138 | Decrease | HIF1-α | Anti-apoptotic | (27) |
| miR-140 | Decrease | YES1 | Anti-apoptotic | (30) |
| miR-145 | Decrease | CaMKII
NF-κB |
Anti-apoptotic
Anti-inflammatory | (31) |
| miR-181a | Decrease | c-Fos |
Anti-inflammatory | (24) |
| miR-193b | Decrease | MAML1 | Anti-apoptotic | (23) |
| miR-202-3p | Decrease | TRPM6 |
Anti-inflammatory
Anti-oxidative stress | (36) |
| miR-327 | Increase | ARC | Pro-apoptotic | (34) |
| | RP105 |
Pro-inflammatory | (39) |
| miR-330 | Increase | SRY | Ventricular
r;emodeling | (42) |
| miR-421 | Increase | Sirt3 |
Pro-apoptotic
Pro-oxidative stress | (25) |
| miR-483-3p | Increase | MDM4 | Anti-apoptotic | (35) |
| miR-489 | Increase | SPIN1 | Pro-apoptotic | (33) |
| miR-590-3p | Decrease | RIPK1 |
Anti-apoptotic
Anti-inflammatory
Anti-oxidative stress | (40) |
3. Role of miRNAs in MIRI
miRNAs as mediators of cardiomyocyte
apoptosis in MIRI
Cardiomyocyte apoptosis has been proven to be the
main cause responsible for the secondary injury of the myocardium
during MIRI. In addition, several miRNAs have been identified to be
involved in the pathogenesis of MIRI through regulating
cardiomyocyte apoptosis. It has been documented that the increased
expression of miR-30e, miR-193b, miR-135a, miR-138, miR-140,
miR-145, miR-15b-5p, miR-483 and miR-590-3p could suppress
cardiomyocyte apoptosis induced by MIRI. Similar findings were
observed following the decreased expression of miR-1, miR-327,
miR-128-1-5p, miR-129-5p, miR-489 and miR-421 (4,13,23,25-35). Zhang et al (23) indicated that miR-193b
overexpression may reduce apoptosis after MIRI and alleviate
IR-induced myocardial injury by targeting mastermind-like 1, which
is a transcriptional coactivator in the Notch signaling pathway.
Wang et al (26) revealed
that miR-135a targeted and negatively regulated protein tyrosine
phosphatase 1B (PTP1B), and the expression of
proapoptotic-related genes was reduced in association with
PTP1B downregulation. In addition, miR-135a improved MIRI by
reducing cardiomyocyte apoptosis both in vitro and in
vivo. Liu et al (27)
found that miR-138 upregulation inhibited the expression of
proteins related to mitochondrial morphology and apoptosis by
targeting hypoxia inducible factor-α (HIF1-α), thus exerting
protective functions against MIRI.
Additionally, miR-128-1-5p was found to play a
cardioprotective role in both H9c2 cardiomyocytes after
hypoxia/reoxygenation (H/R) and H2O2-treated
neonatal rat cardiomyocytes. This may have occurred by the
regulation of miR-128-1-5p on growth arrest DNA damage-inducible
gene 45 γ-mediated cardiomyocyte apoptosis in MIRI (28). Moreover, miR-129-5p
overexpression could also attenuate cardiomyocyte apoptosis both in
H9c2 cardiomyocytes after H/R and rats after I/R injury by
targeting high mobility group box-1 (HMGB1) (29). Yang et al (30) demonstrated that miR-140
overexpression attenuated myocardial infarct size and myocardial
enzymes via inhibition of mitochondrial-mediated apoptosis by
targeting Yes-associated protein 1 (YES-1) in the
progression of MIRI. Liu et al (31) reported that miR-145 exerted
protective effects against MIRI by reducing cardiomyocyte apoptosis
and the protective effects of miR-145 were possibly attributed to
the inhibition of the CaMKII-mediated apoptosis signal-regulating
kinase 1 anti-apoptotic signaling pathway.
Furthermore, Hao et al (13) found that inhibition of miR-1
alleviated MIRI by inhibiting MAPK3 via negatively regulating the
PI3K/Akt signaling pathway, which is associated with reduced
apoptosis. Niu et al (32) reported that miR-15b-5p was
upregulated in MIRI rat models and could promote myocardial
apoptosis via suppressing potassium voltage-gated channel subfamily
J member 2 expression. Similarly, inhibition of miR-489 was found
to promote activation of the PI3K/Akt signaling pathway by
negatively targeting spindlin-1 and inhibiting apoptosis in H/R
H9c2 cells (33). Our latest
study reported that miR-327 downregulation improved I/R-induced
myocardial injury and suppressed both extrinsic and intrinsic
apoptotic cascades via targeting apoptosis repressor with caspase
recruitment domain both in vitro and in vivo
(34). Recently, inhibition of
miR-483-3p was also demonstrated to ameliorate H/R injury and
inhibit apoptosis in H9c2 cells via targeting murine double minute
4 via the p53 signaling pathway (35).
miRNAs as mediators of inflammation in
MIRI
Innate inflammation is an important pathological
characteristic of MIRI, which can lead to the activation of diverse
inflammatory factors. This effect can be regulated by miR-145,
miR-181a, miR-202-3p, miR-22, miR-327 and miR-590-3p (24,29,32,34-36). Wei et al (24) found that miR-181a could
efficiently attenuate MIRI in rats by inhibiting the inflammatory
response and increasing the regulatory T-cell ratio. The mechanism
of action may involve the inhibition of c-Fos protein, which is
considered a key immunoactivator that contributes to dendritic
cell-related immune functions from all links and processions. Liu
et al (31) also
demonstrated that upregulated miR-145 alleviated I/R-induced
myocardial electrophysiological instability and inflammatory
response, which may predominantly be due to inhibition of the NF-κB
p65 anti-inflammatory signaling pathway. At the same time, Wu et
al (36) indicated that
miR-202-3p upregulation alleviated inflammatory response and
oxidative stress by activating the TGF-β1/Smads signaling pathway
by targeting transient receptor potential cation channel, subfamily
M, member 6 (TRPM6) expression. The TRPM6 gene is a
member of the transient receptor potential channel gene family and
has been found to play an important role in the regulation of
extracellular divalent cations in cardiomyocytes (37). Previous findings showed that
miR-22 had a protective effect on MIRI and this protective
mechanism, at least in part, was due to its anti-inflammatory
function via the suppression of the p38 MAPK/CBP/c-Jun-activator
protein-1 (AP-1) signaling pathway (38). In addition to this, our latest
findings suggested that inhibition of miR-327 improved MIRI by
negatively targeting radioprotective 105 kDa protein by suppressing
inflammation in rat models (39).
miRNAs regulate other pathological
characteristics of MIRI
In addition to cardiomyocyte apoptosis and innate
inflammation, miRNAs can also actively participate in the
modulation of oxidative stress and other pathological
characteristics of MIRI such as autophagy, pyroptosis and
ventricular remodeling. Liu et al (25) reported that miR-421 promotes
JNK/AP-1 pathway activation by directly targeting sirtuin-3 and
further promotes H/R-induced oxidative stress and
caspase-9/3-dependent cardiomyocyte apoptosis. Zheng et al
(4) suggested that miR-30e
serves a significant role in suppressing MIRI-induced oxidative
stress and apoptosis via the Notch1/Hes1/Akt signaling pathway. In
H/R H9c2 cells, overexpression of miR-590-3p inhibited activation
of the NF-κB signaling pathway by targeting the inhibition of the
receptor-interacting protein kinase 1 (RIPK1) gene, thereby
alleviating oxidative stress, apoptosis and inflammatory response
(40). In another H/R model and
MIRI rat models, downregulated miR-29a could improve myocardial
injury by negatively targeting sirtuin-1 by suppressing oxidative
stress and NOD-like receptor pyrin domain-containing-3
(NLRP3)-mediated pyroptosis (41). Additionally, our unpublished data
show that miR-327 knockdown can improve cardiac function after
MIRI, partly through the suppression of oxidative stress. Recently,
Liu et al (42)
demonstrated that inhibition of miR-330 inhibited TGF-β1/Smad3
signaling pathway activation by negatively targeting SRY and
thus prevented left ventricular remodeling after MIRI.
Moreover, recent findings indicated that vitamin C
attenuated the inflammation, apoptosis and oxidative damage of
H2O2-induced human umbilical vein endothelial
cells via miRNA signaling networks, including miR-3928-5p and
miR-323a-5p (43). Given the
important role of inflammation, apoptosis and oxidative stress in
MIRI, miR-3928-5p and miR-323a-5p constitute potential therapeutic
targets for treating MIRI. Future studies are needed to determine
whether these miRNAs that are modified by anti-inflammation,
anti-apoptotic and antioxidant effects affect MIRI. Generally, the
critical contribution of miRNAs in apoptosis, inflammation,
oxidative stress as well as other pathological characteristics via
different signaling pathways has opened up new avenues to exploit
their role in the pathogenesis of MIRI. Future research should pay
attention to the interaction between miRNAs with the regulatory
network in view of the contribution to the potential molecular
mechanisms of MIRI.
4. Biological functions of lncRNAs
lncRNAs, a class of transcripts without
protein-coding capacity, are expressed at low levels with 10-fold
lower median expression abundance than their protein-coding
counterparts (44). Although
most lncRNAs are localized in the nucleus, several lncRNAs also
exert functions in the cytoplasm (45). lncRNAs manifest specific and
diverse subcellular localizations in various cell types depending
on their molecular function. In the nucleus, lncRNAs can interact
with DNA to form RNA-DNA complexes to regulate gene expression and
act as molecular scaffolds to suppress or activate transcription.
In addition, other lncRNAs are enriched in the cytoplasm, where
they can also interact with proteins and modulate mRNA translation
(46,47). In fact, it was shown that lncRNAs
may participate in a wide range of pathophysiological processes and
biological events. They regulate target gene expression mainly
through cis- or trans-regulation (48).
Increasing evidence has suggested that lncRNAs act
as regulators of almost every cellular process, and the expression
of these molecules seems to be strictly regulated in physiological
conditions and several cardiovascular diseases, including MIRI
(14,49). Since the discovery of lncRNAs,
their functional characterization makes these molecules a potential
biomarker and therapeutic target for MIRI as shown in Table II, which provides a summary of
the major lncRNAs.
 | Table IISummary of lncRNAs in the
pathogenesis of MIRI. |
Table II
Summary of lncRNAs in the
pathogenesis of MIRI.
| lncRNA | Expression with
MIRI | Targeted genes | Pathological
mechanism | (Refs.) |
|---|
| FOXD3-AS1 | Increase | NFκB/iNOS/COX2 |
Pro-apoptotic
Pro-autophagy | (48) |
| LINC00652 | Increase | GLP-1R | Pro-apoptotic | (49) |
| AK139128 | Increase | miR-499 |
Pro-apoptotic
Pro-autophagy | (56) |
| H19 | Decrease |
miR-877-3p
Bcl-2
miR-103 |
Anti-apoptotic
Anti-apoptotic
Anti-apoptotic | (57) |
| miR-107 | Anti-necrosis | (71) |
| Oprm1 | Decrease | miR-30b-5p | Anti-apoptotic | (54) |
| TUG1 | Increase | miR-142-3p |
Pro-apoptotic
Pro-autophagy | (58) |
| Neat1 | Increase | miR-193a | Pro-apoptotic | (59) |
| AK088388 | Increase | miR-30a |
Pro-apoptotic
Pro-autophagy | (60) |
| MEG3 | Increase | miR-7-5p | Pro-apoptotic | (61) |
| KCNQ1OT1 | Increase | miR-204-5p | Pro-apoptotic | (62) |
| HIF1A-AS1 | Increase | miR-204 |
Pro-apoptotic
ventricular remodeling | (63) |
| NRF | Increase | miR-873 | Pro-necrosis | (67) |
| ROR | Increase | p38/MAPK | Pro-oxidative
stress | (73) |
| Gpr19 | Increase | miR-324-5p |
Pro-apoptotic
Pro-oxidative stress | (74) |
| GAS5 | Increase | miR-532-5p | Pro-apoptotic | (64) |
HULC
| Decrease
|
miR-377-5p
|
Anti-apoptotic
Anti-inflammatory | (70) |
| Gm4419 | Increase | miR-682 |
Pro-inflammatory
Pro-apoptotic | (71) |
| MALAT1 | Increase | miR-144-3p | Pro-apoptotic | (77) |
| miR-26b | | (78) |
5. Role of lncRNAs in MIRI
LncRNAs as mediators of cardiomyocyte
autophagy and apoptosis in MIRI
To date, a variety of lncRNAs have been revealed to
participate in the regulation of cardiomyocyte autophagy and
apoptosis during the process of MIRI. Ma et al (14) showed that lncRNA nuclear enriched
abundant transcript 1 (Neat1) expression was increased after MIRI
in diabetic rats, and increased Neat1 expression aggravated
autophagy and apoptosis response via regulating forkhead box
protein O1 expression during MIRI. LncRNA FOXD3 antisense RNA 1
(FOXD3-AS1) was also found to be upregulated during MIRI.
Furthermore, FOXD3-AS1 overexpression promoted cardiomyocyte
autophagy and apoptosis via activating the NF-κB/inducible nitric
oxide synthase/cyclooxygenase 2 signaling pathway, which led to
aggravated MIRI (49). During
sevoflurane-induced cardioprotection against MIRI, lncRNA LINC00652
overexpression was identified to reduce this protective effect and
increase infarct size and cell apoptosis via inactivating the
cAMP/protein kinase A pathway by negatively targeting glucagon-like
peptide-1 receptor (GLP-1R) (50). GLP-1R is a gut incretin
hormone that exerts an anti-oxidative protective effect on various
tissues. It has been identified as a vital physiological regulator
of insulin secretion and a major therapeutic target for the
treatment of ischemic stroke and diabetes (51,52). As a signaling molecule, hydrogen
sulfide (H2S) has been recognized as an endogenous
critical gas that participates in the physiological and
pathological progression of the cardiovascular system (53). Endogenous H2S is
produced by cystathionine-γ-lyase (CSE), which can be
inhibited by the miR-30 family (54). Hu et al (55) suggested that lncRNA Mu-type
opioid receptor (Oprm1) was competitively combined with miR-30b-5p,
which inhibited CSE expression. In-depth investigation
showed that Oprm1 overexpression increased endogenous
H2S and reduced I/R-induced myocardial injury and
apoptosis via activating the PI3K/Akt pathway by targeting the
miR-30b-5p/CSE axis (55).
Competing endogenous RNA (ceRNA) is a novel pattern
of lncRNA expression, which can indirectly modulate the expression
of target genes by competing for binding with miRNAs (56). Zhu and Zhao (57) revealed that lncRNA AK139128 could
be used as a ceRNA to adsorb miR-499 to suppress miR-499
expression, thereby promoting cardiomyocyte apoptosis and autophagy
in H/R injury. H19, a well-known conserved lncRNA, is also
abundantly expressed in murine hearts and can function as a ceRNA
to regulate the availability of miR-877-3p. Upregulated H19
attenuated mitochondrial apoptosis and cardiomyocyte injury induced
by MIRI via suppressing the miR-877-3p/Bcl-2 pathway (58). Su et al (59) reported that lncRNA
taurine-upregulated gene 1 increased the expression of HMGB1
and Rac1 by sponging miR-142-3p, which critically
contributed to autophagy and apoptosis under H/R conditions. A
recent study by Ren et al (60) also suggested that lncRNA Neat1
played a promotive role in MIRI, and inhibition of lncRNA Neat1
enhanced cell proliferation and inhibited cell apoptosis, which may
be the mechanisms through which lncRNA Neat1 negatively targets
miR-193a in H/R injury H9c2 cells. Similarly, Wang et al
(61) demonstrated that lncRNA
AK088388 could increase the expression of LC3-II and Beclin-1 and
eventually promote cardiomyocyte apoptosis and autophagy. The
mechanism of action was attributed to competitive binding of
miR-30a, which derepresses the miR-30a to regulate cardiomyocyte
injury in an H/R model. Subsequently, Zou et al (62) reported that lncRNA maternally
expressed gene 3 (MEG3) inhibition protected cardiomyocytes
against I/R-induced apoptosis both in vitro and in
vivo. The potential mechanisms were investigated, and it was
found that lncRNA MEG3 negatively targeted miR-7-5p and its
downstream target gene poly (ADP-ribose) polymerase (PARP)1
as well as the caspase-3 signaling pathway. In the same year,
another study by Rong et al (63) revealed that lncRNA KCNQ1OT1 could
regulate galectin-3 (LGALS3) expression by binding to miR-204-5p,
which promoted cardiomyocyte apoptosis both in H/R and MIRI mouse
models. Thus, the KCNQ1OT1/miR-204-5p/LGALS3 axis may provide a new
therapeutic target for MIRI treatment.
It was also shown that lncRNA HIF1A-AS1 could
function as a ceRNA of miR-204 to regulate the expression of
suppressors of cytokine signaling 2, thus playing a key role in
promoting ventricular remodeling and aggravating cardiac function
after MIRI in mice (64). Han
et al (65) revealed that
lncRNA growth arrest specific 5 (GAS5) served a function in MIRI
via the PI3K/Akt pathway by sponging miR-532-5p, and silencing
lncRNA GAS5 could improve cell survival, decrease the occurrence of
apoptosis and ultimately attenuate MIRI. Interestingly, lncRNA GAS5
was also found to function as a ceRNA of miR-137 to suppress
miR-137 expression (66).
Furthermore, previous findings have shown that miR-137 negatively
regulated H2O2-induced cardiomyocyte
apoptosis by targeting CDC42 (67). This indicated that the lncRNA
GAS5/miR-137/CDC42 axis may also be involved in the regulation of
I/R-induced myocardial injury and apoptosis. However, this
speculation requires further verification by experimental
studies.
LncRNAs as mediators of cell necrosis and
inflammation in MIRI
In addition to apoptosis, several lncRNAs can also
modulate cell necrosis and inflammation, which is one of the
pathological mechanisms of MIRI. Wang et al (68) showed that lncRNA necrosis-related
factor contributed to H2O2-induced
cardiomyocyte necrosis and I/R-induced myocardial injury by
targeting miR-873 and regulating RIPK1/RIPK3. It is well known that
RIPK1 is necessary in TRAIL, TNF-α and CD95 ligand-induced necrotic
cell death and RIPK3 phosphorylates the mixed lineage kinase
domain-like protein, which is also a key determinant in mediating
the RIPK1 necrotic signaling pathway (69,70). Liang et al (71) reported that lncRNA HULC was
downregulated and miR-377-5p was upregulated during MIRI, and HULC
overexpression inhibited the inflammation and apoptosis of
H/R-induced H9c2 cells. Mechanistic experiments revealed that
lncRNA HULC sponged miR-377-5p to inactivate the
NLRP3/caspase-1/IL-1β pathway to exert a protective effect.
Furthermore, lncRNA Gm4419 was demonstrated to regulate MIRI by
targeting the miR-682/TNF receptor-associated factor 3 axis, and
Gm4419 knockdown reduced the myocardial infarction area,
inflammatory cytokine levels and apoptosis (72). Another study by Wang et al
(73) also revealed a novel
function of H19 in modulating H9c2 cell necrosis induced by
H2O2 and demonstrated that H19 acts as a
ceRNA to repress miR-103/107, thereby regulating the expression of
FADD, which acted as a negative regulator of necrosis by preventing
the formation of the RIPK1/RIPK3 complex.
lncRNAs regulate other pathological
characteristics of MIRI
Blood reflow during MIRI can result in reactive
oxygen species (ROS) production, which causes cytotoxic and
oxidative stress damage. LncRNA regulator of reprogramming was
found to promote ROS production, NADPH oxidase (NOX) activity and
NOX2 protein levels by regulating the p38/MAPK signaling pathway,
thereby increasing oxidative damage and cardiomyocyte apoptosis as
well as aggravating MIRI (74).
Huang et al (75) showed
that lncRNA Gpr19 was upregulated in an oxygen glucose
deprivation/recovery system of neonatal rat ventricular
cardiomyocytes or MIRI in mice, and inhibition of Gpr19 attenuated
oxidative stress and apoptosis by regulation of the
miR-324-5p/mitochondrial fission regulator 1 axis. Additionally,
another lncRNA, metastasis associated lung adenocarcinoma
transcript 1 (MALAT1), which also acts as a ceRNA for numerous
miRNAs, was upregulated in cardiac tissue, and its inhibition
reduced cardiomyocyte apoptosis and improved left ventricular
function in diabetic rats (76).
Wang et al (77) inferred
that MALAT1 may modulate cardiomyocyte inflammation and myocardial
injury induced by I/R via negatively targeting miR-203 expression.
Interestingly, Gong et al (78) recently demonstrated that MALAT1
promoted cardiomyocyte apoptosis following MIRI via targeting
miR-144-3p, but not miR-203. However, the effects of MALAT1 in
regulating MIRI-induced inflammatory response have not been
completely elucidated. It is known that MALAT1 can also function as
a ceRNA to suppress miR-26b expression (79). In addition, Ge et al
(80) showed that miR-26b
reduced the inflammatory response and improved myocardial
remodeling during myocardial infarction via binding to
prostaglandin-endoperoxide synthase 2 (PTGS2) and
inactivating the MAPK pathway. Based on these data, we speculated
that the MALAT1-miR-26b-PTGs2 axis may participate in MIRI and
aggravate the inflammatory response induced by MIRI.
The exact molecular mechanism of lncRNAs in the
pathogenesis of inflammatory response remains to be determined.
Overall, the lncRNA-miRNA modulatory network is a potential axis
that affects the progression of MIRI by modulating apoptosis,
autophagy and oxidative stress. Although our understanding of
lncRNAs is still developing, these examples provided valuable
insights on how lncRNAs may ultimately be used in the clinic as new
therapeutic targets for MIRI in the future.
6. Biological functions of circRNAs
circRNAs, a novel type of large endogenous
transcripts, are typically produced by back-splicing events on
maturing pre-mRNAs that join a donor site with an upstream acceptor
site (81). These circRNAs
differ from linear RNAs in that they are circularized RNA molecules
with covalently closed loop structures without 5′-3′ polarity or a
polyadenylated tail. Most of them are stable, abundant, endogenous
and exhibit cell type-, tissue- and developmental stage-specific
expression patterns in eukaryotic cells (82). Compared to miRNAs and lncRNAs,
the understanding of circRNA functions are still limited. At
present, four main roles have been described and are shared by a
subset of circRNAs. CircRNAs can function as miRNA sponges to
inhibit the translation of mRNAs and interact with RNA-binding
proteins to regulate protein levels. In addition, they can also act
as regulators of splicing and transcription to alter gene
expression and dynamic protein scaffolds to facilitate contact and
assembly of proteins (11,83,84).
While the main function of circRNAs is exerted
through their activity as miRNA sponges, their second most
important function is exerted via circRNA-protein interactions,
including as protein sponges, scaffolds, decoys and recruiters
(84). In addition, several
circRNAs were also reported to encode proteins through the
translational machinery in a cap-independent manner (85). Thus, circRNA-protein interactions
can also influence protein expression, biogenesis and
pathophysiological processes, which requires further in-depth
studies. Deep sequencing of cardiac tissues revealed the abundance,
conservation, evolution and developmental stage-specific
differentiation of circRNAs expressed in the heart (86). Since this has been identified in
a number of other investigation areas, circRNA regulation and
function in the cardiovascular system have also attracted
widespread attention. Increasing evidence has demonstrated that
circRNAs are involved in a wide variety of biological processes,
such as cell proliferation, differentiation and apoptosis, and may
play significant roles in MIRI, as shown in Table III which includes a summary of
the major circRNAs (12).
 | Table IIISummary of circRNAs in the
pathogenesis of MIRI. |
Table III
Summary of circRNAs in the
pathogenesis of MIRI.
| circRNA | Expression with
MIRI | Targeted genes | Pathological
mechanism | (Refs.) |
|---|
| circNCX1 | Increase | miR-133a-3p | Pro-apoptotic | (87) |
| ACR | Decrease | Dnmt3B | Anti-autophagy | (93) |
| CircDLGAP4 | Decrease | miR-143 | Anti-ER stress | (96) |
| Anti-apoptotic | (98) |
| CircANXA2 | Increase | miRNA-133 | Pro-apoptotic | (88) |
| circTLK1 | Increase | miR-214 | Pro-apoptotic | (89) |
|
circRNA-0068566 | Decrease | miR-6322 |
Anti-apoptotic
Anti-oxidative stress | (99) |
| Circ-100338 | Decrease | miRNA-200a-3p | Pro-angiogenic | (100) |
7. Role of circRNAs in MIRI
CircRNAs as mediators of MIRI
More recently, deep sequencing studies revealed that
>15,318 and 3,017 cardiac circRNAs were identified in human and
mouse heart, respectively, some of which are correlated with
apoptosis, autophagy and endoplasmic reticulum (ER) stress during
MIRI (12,87). CircNCX1, a circRNA transcribed
from the 2nd exon of the sodium/calcium exchanger 1 (ncx1)
gene, is widely expressed in cardiac tissue and showed differential
expression levels during cardiomyopathy. Li et al (88) revealed that circNCX1 was
upregulated in response to MIRI. Furthermore, suppression of
circNCX1 reduced apoptosis and attenuated I/R-induced myocardial
injury by binding to miR-133a-3p and inhibiting miR-133a-3p
regulation on pro-apoptotic gene cell death-inducing protein
(CDIP1) activity. Thus, the circNCX1/miR-133a-3p/CDIP1 axis
plays a critical regulatory role in regulating cardiomyocyte
apoptosis, thus contributing to MIRI. Similarly, Zong and Wang
(89) showed that circANXA2
promoted myocardial apoptosis during MIRI via suppressing miRNA-133
expression and aggravated myocardial injury.
Moreover, circTLK1 is an upregulated circRNA found
in MIRI mouse models and could exacerbate myocardial injury and
cardiomyocyte apoptosis during MIRI by targeting the
miR-214/RIPK1-mediated TNF signaling pathway (90). As a conserved mitochondrial
serine/threonine protein kinase, PTEN-induced putative kinase 1
(PINK1) can be imported into the mitochondrial matrix and
targets the outer membrane of the mitochondria, which ensures
quality control of the mitochondria (91,92). Previous findings suggested that
PINK1 is downregulated during human end-stage heart failure,
which showed that PINK1 is essential for normal heart
function (93). Recently, Zhou
et al (94) reported that
a circRNA known as autophagy-related circular RNA (ACR) attenuated
cardiomyocyte autophagy and protected the heart from I/R injury by
positively targeting Pink1. Moreover, they identified ACR-activated
PINK1 expression by directly binding to Dnmt3B and blocking
Dnmt3B-mediated DNA methylation of Pink1 promoter and regulated its
downstream gene FAM65B, which inhibits autophagy and cell
death in the heart.
Vascular endothelial cell dysfunction plays a
significant role in the progression of MIRI, which is involved in
angiogenesis via various mechanisms, such as ER stress, oxidative
stress, autophagy and ubiquitination (95,96). CircDLGAP4 was found to be
decreased in endothelial cells after MIRI. Moreover, miR-143 was
the main sponge target of circDLGAP4, which was involved in
endothelial cell dysfunction caused by I/R exposure via regulating
miR-143 expression (97). One of
the potential targets of miR-143 is E3 ubiquitin-protein ligase
HECTD1 (HECTD1), a novel protein that participates in cell
migration and apoptosis in endothelial cells (98). It was suggested that circDLGAP4
can regulate HECTD1 expression by sponging miR-143 and
mediate I/R-induced myocardial injury in endothelial cells through
ER stress (97). Another
interesting phenomenon was that miR-143 could also act as a sponge
for Bcl-2, thereby inhibiting the apoptotic response (99). Consequently, the
circDLGAP4/miR-143/Bcl-2 pathway may also play a critical
regulatory role in cardiomyocyte apoptosis, thereby attenuating
MIRI. Zhou et al (100)
reported that circRNA-0068566 had a protective effect on MIRI and
may significantly suppress I/R-induced cardiac dysfunction,
apoptosis and oxidative stress by activating the miR-6322/PARP2
signaling pathway. In addition, Chang et al (101) revealed that Circ-100338 induced
angiogenesis and regulated MIRI metastasis by combining with
miRNA-200a-3p.
Other circRNAs identified in MIRI
At present, the understanding of circRNAs in the
molecular mechanisms of MIRI remains limited. It is important to
further investigate the pathological roles of circRNAs in the
development of MIRI or other cardiovascular diseases. Numerous
other circRNAs have been implicated in I/R injury. Ge et al
(102) recently showed that 119
circRNAs were downregulated and 66 circRNAs were upregulated during
MIRI, and 42 of them were newly identified circRNAs. Furthermore,
they constructed a circRNA-miRNA network with five circRNAs,
including mmu_circ_001007, mmu_circ_008351, mmu_circ_0001336,
mmu_circ_008228 and mmu-circ007845, which could modulate miRNA
expression. Moreover, circRNA-miRNA network analysis revealed that
one miRNA could combine with multiple circRNAs and one circRNA can
also function on different miRNAs. The identified circRNAs may
participate in the pathology of MIRI. Compared with lncRNAs and
miRNAs, the understanding of circRNAs in the molecular mechanisms
of diabetic cardiomyopathy is still limited. Future studies are
required to verify the exact mechanisms through which circRNAs
interact with proteins and other ncRNAs to mediate the pathogenesis
of MIRI.
8. Conclusion
ncRNAs, first discovered in 1993 by Lee and
colleagues, modulate cellular processes such as signal
transduction, transcription, chromatin remodeling and
post-transcriptional modification (103). The effects of ncRNAs on
cellular biology are more considerable than initially expected and
thus makes ncRNAs the focus of modern medical research.
Dysregulation of ncRNAs, including miRNAs, lncRNAs and circRNAs,
can have a significant impact on myocardial function, thereby
aggravating, maintaining and promoting MIRI processes. Another
class of ncRNAs known as PIWI-interacting RNAs (piRNAs) was
identified, and their regulatory roles in gene expression is
increasingly studied (104).
Evidence has demonstrated that piRNAs influence the Akt signaling
pathway, a crucial network in cardiac physiopathology (105). Moreover, it was recently
highlighted that anti-inflammatory treatment with vitamin C to
attenuate H2O2-mediated endothelial cell
senescence may be associated with changes in the expression of
piRNAs that are linked to the cell cycle (106). Thus, piRNAs could play a
functional role in inflammation and endothelial dysfunction-related
cardiovascular regulatory pathways. Future mechanistic studies are
required to determine whether the expression of piRNAs is crucial
to MIRI.
The present review summarized the recent progress in
the involvement of ncRNAs in the pathogenesis of MIRI, such as
apoptosis, autophagy, inflammation and oxidative stress. In fact, a
number of miRNAs, more recently circRNAs and lncRNAs, have been
shown to be potential therapeutic targets in the treatment of MIRI.
The main function of ncRNAs is to regulate the expression of target
genes by binding to the target mRNA or sponging miRNAs. The
therapeutic targeting of ncRNAs in MIRI has only been recently
identified and more information remains to be studied, particularly
on lncRNAs and circRNAs. In order to keep up the pace and identify
more novel ncRNAs, a highly organized network of ncRNA research
institutes is essential. Finally, further studies are required to
clarify the molecular mechanisms of ncRNAs in the development of
MIRI and determine whether there are other novel molecules that
regulate lncRNAs and circRNAs, thus forming novel therapeutic
targets for MIRI and other cardiovascular diseases.
In conclusion, the pathological roles of ncRNAs in
the progression of MIRI shows the complexity of the ischemic heart
and may provide valuable insights into the pathogenesis of ischemic
heart disease.
Funding
This study was supported by research grants from the
National Natural Science Foundation of China (grant no.
81970303).
Availability of data and materials
Not applicable.
Authors' contributions
QL, ZL, ZF, YY and CL conceived and designed the
article. QL and YY prepared the figures. QL and ZL drafted the
manuscript. CL and ZF critically revised the article for important
intellectual content. All authors read and approved the final
manuscript, and agree to be accountable for all aspects of the
work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Benjamin EJ, Muntner P, Alonso A,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Das SR, et al: Heart Disease and Stroke Statistics-2019
Update: A report from the American Heart Association. Circulation.
139:e56–e528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ibanez B, Heusch G, Ovize M and Van de
Werf F: Evolving therapies for myocardial ischemia/reperfusion
injury. J Am Coll Cardiol. 65:1454–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang EW, Han YY and Jia XS:
PAFR-deficiency alleviates myocardial ischemia/reperfusion injury
in mice via suppressing inflammation, oxidative stress and
apoptosis. Biochem Biophys Res Commun. 495:2475–2481. 2018.
View Article : Google Scholar
|
|
4
|
Zheng J, Li J, Kou B, Yi Q and Shi T:
MicroRNA-30e protects the heart against ischemia and reperfusion
injury through autophagy and the Notch1/Hes1/Akt signaling pathway.
Int J Mol Med. 41:3221–3230. 2018.
|
|
5
|
Xu T, Ding W, Ao X, Chu X, Wan Q, Wang Y,
Xiao D, Yu W, Li M, Yu F and Wang J: ARC regulates programmed
necrosis and myocardial ischemia/reperfusion injury through the
inhibition of mPTP opening. Redox Biol. 20:414–426. 2019.
View Article : Google Scholar
|
|
6
|
Stein LD: Human genome: End of the
beginning. Nature. 431:915–916. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorenzen JM and Thum T: Long noncoding
RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol.
12:360–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eddy SR: Non-coding RNA genes and the
modern RNA world. Nat Rev Genet. 2:919–929. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Altesha MA, Ni T, Khan A, Liu K and Zheng
X: Circular RNA in cardiovascular disease. J Cell Physiol.
234:5588–5600. 2019. View Article : Google Scholar
|
|
13
|
Hao YL, Fang HC, Zhao HL, Li XL, Luo Y, Wu
BQ, Fu MJ, Liu W, Liang JJ and Chen XH: The role of microRNA-1
targeting of MAPK3 in myocardial ischemia-reperfusion injury in
rats undergoing sevoflurane preconditioning via the PI3K/Akt
pathway. Am J Physiol Cell Physiol. 315:C380–C388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma M, Hui J, Zhang QY, Zhu Y, He Y and Liu
XJ: Long non-coding RNA nuclear-enriched abundant transcript 1
inhibition blunts myocardial ischemia reperfusion injury via
autophagic flux arrest and apoptosis in streptozotocin-induced
diabetic rats. Atherosclerosis. 277:113–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ebert MS, Neilson JR and Sharp PA:
MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian
cells. Nat Methods. 4:721–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu P, Vernooy SY, Guo M and Hay BA: The
Drosophila microRNA Mir-14 suppresses cell death and is required
for normal fat metabolism. Curr Biol. 13:790–795. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwon C, Han Z, Olson EN and Srivastava D:
MicroRNA1 influences cardiac differentiation in Drosophila and
regulates Notch signaling. Proc Natl Acad Sci USA. 102:18986–18991.
2005. View Article : Google Scholar
|
|
21
|
Katz MG, Fargnoli AS, Kendle AP, Hajjar RJ
and Bridges CR: The role of microRNAs in cardiac development and
regenerative capacity. Am J Physiol Heart Circ Physiol.
310:H528–H541. 2016. View Article : Google Scholar :
|
|
22
|
Cai W, Zhang J and Yang J, Fan Z, Liu X,
Gao W, Zeng P, Xiong M, Ma C and Yang J: MicroRNA-24 attenuates
vascular remodeling in diabetic rats through PI3K/Akt signaling
pathway. Nutr Metab Cardiovasc Dis. 29:621–632. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Niu J, Tian B and Zhao M:
MicroRNA-193b protects against myocardial ischemia-reperfusion
injury in mouse by targeting mastermind-like 1. J Cell Biochem.
120:14088–14094. 2019. View Article : Google Scholar
|
|
24
|
Wei Z, Qiao S, Zhao J, Liu Y, Li Q, Wei Z,
Dai Q, Kang L and Xu B: MiRNA-181a over-expression in mesenchymal
stem cell-derived exosomes influenced inflammatory response after
myocardial ischemia-reperfusion injury. Life Sci. 232:1166322019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Qian XM, He QC and Weng JK: MiR-421
inhibition protects H9c2 cells against
hypoxia/reoxygenation-induced oxidative stress and apoptosis by
targeting Sirt3. Perfusion. 35:255–262. 2020. View Article : Google Scholar
|
|
26
|
Wang S, Cheng Z, Chen X and Xue H:
MicroRNA-135a protects against myocardial ischemia-reperfusion
injury in rats by targeting protein tyrosine phosphatase 1B. J Cell
Biochem. 120:10421–10433. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Zou J, Liu X and Zhang Q:
MicroRNA-138 attenuates myocardial ischemia reperfusion injury
through inhibiting mitochondria-mediated apoptosis by targeting
HIF1-α. Exp Ther Med. 18:3325–3332. 2019.PubMed/NCBI
|
|
28
|
Wan X, Yao B, Ma Y, Liu Y, Tang Y, Hu J,
Li M, Fu S, Zheng X and Yin D: MicroRNA-128-1-5p attenuates
myocardial ischemia/reperfusion injury by suppressing
Gadd45g-mediated apoptotic signaling. Biochem Biophys Res Commun.
530:314–321. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen ZX, He D, Mo QW, Xie LP, Liang JR,
Liu L and Fu WJ: MiR-129-5p protects against myocardial
ischemia-reperfusion injury via targeting HMGB1. Eur Rev Med
Pharmacol Sci. 24:4440–4450. 2020.PubMed/NCBI
|
|
30
|
Yang S, Li H and Chen L: MicroRNA-140
attenuates myocardial ischemia-reperfusion injury through
suppressing mitochondria-mediated apoptosis by targeting YES1. J
Cell Biochem. 120:3813–3821. 2019. View Article : Google Scholar
|
|
31
|
Liu Z, Tao B, Fan S, Pu Y, Xia H and Xu L:
MicroRNA-145 protects against myocardial ischemia reperfusion
injury via CaMKII-Mediated antiapoptotic and anti-inflammatory
pathways. Oxid Med Cell Longev. 2019:89486572019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Niu S, Xu L, Yuan Y, Yang S, Ning H, Qin
X, Xin P, Yuan D, Jiao J and Zhao Y: Effect of down-regulated
miR-15b-5p expression on arrhythmia and myocardial apoptosis after
myocardial ischemia reperfusion injury in mice. Biochem Biophys Res
Commun. 530:54–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Wu S, Zhang XS, Wang DM and Qian
CY: MicroRNA-489 promotes cardiomyocyte apoptosis induced by
myocardial ischemia-reperfusion injury through inhibiting SPIN1.
Eur Rev Med Pharmacol Sci. 23:6683–6690. 2019.PubMed/NCBI
|
|
34
|
Li Q and Yang J, Zhang J, Liu XW, Yang CJ,
Fan ZX, Wang HB, Yang Y, Zheng T and Yang J: Inhibition of
microRNA-327 ameliorates ischemia/reperfusion injury-induced
cardiomyocytes apoptosis through targeting apoptosis repressor with
caspase recruitment domain. J Cell Physiol. 235:3753–3767. 2020.
View Article : Google Scholar
|
|
35
|
Zhang H, Wang J, Du A and Li Y: MiR-483-3p
inhibition ameliorates myocardial ischemia/reperfusion injury by
targeting the MDM4/p53 pathway. Mol Immunol. 125:9–14. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu HY, Wu JL and Ni ZL: Overexpression of
microRNA-202-3p protects against myocardial ischemia-reperfusion
injury through activation of TGF-β1/Smads signaling pathway by
targeting TRPM6. Cell Cycle. 18:621–637. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gwanyanya A, Amuzescu B, Zakharov SI,
Macianskiene R, Sipido KR, Bolotina VM, Vereecke J and Mubagwa K:
Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes:
Permeation of divalent cations and pH-mediated regulation. J
Physiol. 559:761–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang J, Fan Z, Yang J, Ding J, Yang C and
Chen L: MicroRNA-22 attenuates myocardial ischemia-reperfusion
injury via an anti-inflammatory mechanism in rats. Exp Ther Med.
12:3249–3255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y, Yang J, Liu XW, Ding JW, Li S, Guo
X, Yang CJ, Fan ZX, Wang HB, Li Q, et al: Down-regulation of
miR-327 alleviates ischemia/reperfusion-induced myocardial damage
by targeting RP105. Cell Physiol Biochem. 49:1049–1063. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao C, Jiang J, Wang YL and Wu YQ:
Overexpression of microRNA-590-3p promotes the proliferation of and
inhibits the apoptosis of myocardial cells through inhibition of
the NF-κB signaling pathway by binding to RIPK1. J Cell Biochem.
120:3559–3573. 2019. View Article : Google Scholar
|
|
41
|
Ding S, Liu D, Wang L, Wang G and Zhu Y:
Inhibiting microRNA-29a protects myocardial ischemia-reperfusion
injury by targeting SIRT1 and suppressing oxidative stress and
NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther.
372:128–135. 2020. View Article : Google Scholar
|
|
42
|
Liu ZY, Pan HW, Cao Y, Zheng J, Zhang Y,
Tang Y, He J, Hu YJ, Wang CL, Zou QC, et al: Downregulated
microRNA-330 suppresses left ventricular remodeling via the
TGF-β1/Smad3 signaling pathway by targeting SRY in mice with
myocardial ischemia-reperfusion injury. J Cell Physiol.
234:11440–11450. 2019. View Article : Google Scholar
|
|
43
|
Wu J, Liang J, Li M, Lin M, Mai L, Huang
X, Liang J, Hu Y and Huang Y: Modulation of miRNAs by vitamin C in
H2O2-exposed human umbilical vein endothelial cells. Int J Mol Med.
46:2150–2160. 2020. View Article : Google Scholar :
|
|
44
|
Zhang Y, Du W and Yang B: Long non-coding
RNAs as new regulators of cardiac electrophysiology and
arrhythmias: Molecular mechanisms, therapeutic implications and
challenges. Pharmacol Ther. 203:1073892019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cabili MN, Dunagin MC, McClanahan PD,
Biaesch A, Padovan-Merhar O, Regev A, Rinn JL and Raj A:
Localization and abundance analysis of human lncRNAs at single-cell
and single-molecule resolution. Genome Biol. 16:202015. View Article : Google Scholar :
|
|
46
|
Kuo CC, Hanzelmann S, Sentürk Cetin N,
Frank S, Zajzon B, Derks JP, Akhade VS, Ahuja G, Kanduri C, Grummt
I, et al: Detection of RNA-DNA binding sites in long noncoding
RNAs. Nucleic Acids Res. 47:e322019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tong G, Wang Y, Xu C, Xu Y, Ye X, Zhou L,
Zhu G, Zhou Z and Huang J: Long non-coding RNA FOXD3-AS1 aggravates
ischemia/reperfusion injury of cardiomyocytes through promoting
autophagy. Am J Transl Res. 11:5634–5644. 2019.PubMed/NCBI
|
|
50
|
Zhang SB, Liu TJ, Pu GH, Li BY, Gao XZ and
Han XL: Suppression of Long Non-Coding RNA LINC00652 restores
sevoflurane-induced cardioprotection against myocardial
ischemia-reperfusion injury by targeting GLP-1R through the
cAMP/PKA pathway in mice. Cell Physiol Biochem. 49:1476–1491. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fujita H, Morii T, Fujishima H, Sato T,
Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker
DJ, et al: The protective roles of GLP-1R signaling in diabetic
nephropathy: Possible mechanism and therapeutic potential. Kidney
Int. 85:579–589. 2014. View Article : Google Scholar
|
|
52
|
Zhang H, Liu Y, Guan S, Qu D, Wang L, Wang
X, Li X, Zhou S, Zhou Y, Wang N, et al: An orally active allosteric
GLP-1 receptor agonist is neuroprotective in cellular and rodent
models of stroke. PLoS One. 11:e01488272016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
van Goor H, van den Born JC, Hillebrands
JL and Joles JA: Hydrogen sulfide in hypertension. Curr Opin
Nephrol Hypertens. 25:107–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shen Y, Shen Z, Miao L, Xin X, Lin S, Zhu
Y, Guo W and Zhu YZ: MiRNA-30 family inhibition protects against
cardiac ischemic injury by regulating cystathionine-Ƴ-lyase
expression. Antioxid Redox Signal. 22:224–240. 2015. View Article : Google Scholar :
|
|
55
|
Hu X, Liu B, Wu P, Lang Y and Li T: LncRNA
Oprm1 overexpression attenuates myocardial ischemia/reperfusion
injury by increasing endogenous hydrogen sulfide via
Oprm1/miR-30b-5p/CSE axis. Life Sci. 254:1176992020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhu Z and Zhao C: LncRNA AK 139128
promotes cardiomyocyte autophagy and apoptosis in myocardial
hypoxia-reoxygenation injury. Life Sci. 1167052019.Epub ahead of
print. View Article : Google Scholar
|
|
58
|
Li X, Luo S, Zhang J, Yuan Y, Jiang W, Zhu
H, Ding X, Zhan L, Wu H, Xie Y, et al: lncRNA H19 alleviated
myocardial I/RI via suppressing miR-877-3p/Bcl-2-mediated
mitochondrial apoptosis. Mol Ther Nucleic Acids. 17:297–309. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Su Q, Liu Y, Lv XW, Ye ZL, Sun YH, Kong BH
and Qin ZB: Inhibition of lncRNA TUG1 upregulates miR-142-3p to
ameliorate myocardial injury during ischemia and reperfusion via
targeting HMGB1- and Rac1-induced autophagy. J Mol Cell Cardiol.
133:12–25. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ren L, Chen S, Liu W, Hou P, Sun W and Yan
H: Downregulation of long non-coding RNA nuclear enriched abundant
transcript 1 promotes cell proliferation and inhibits cell
apoptosis by targeting miR-193a in myocardial ischemia/reperfusion
injury. BMC Cardiovasc Disord. 19:1922019. View Article : Google Scholar
|
|
61
|
Wang JJ, Bie ZD and Sun CF: Long noncoding
RNA AK088388 regulates autophagy through miR-30a to affect
cardiomyocyte injury. J Cell Biochem. 120:10155–10163. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zou L, Ma X, Lin S, Wu B, Chen Y and Peng
C: Long noncoding RNA-MEG3 contributes to myocardial
ischemia-reperfusion injury through suppression of miR-7-5p
expression. Biosci Rep. 39:BSR201902102019. View Article : Google Scholar
|
|
63
|
Rong J, Pan H, He J, Zhang Y, Hu Y, Wang
C, Fu Q, Fan W, Zou Q, Zhang L, et al: Long non-coding RNA
KCNQ1OT1/microRNA-204-5p/LGALS3 axis regulates myocardial
ischemia/reperfusion injury in mice. Cell Signal. 66:1094412020.
View Article : Google Scholar
|
|
64
|
Xue X and Luo L: LncRNA HIF1A-AS1
contributes to ventricular remodeling after myocardial
ischemia/reperfusion injury by adsorption of microRNA-204 to
regulating SOCS2 expression. Cell Cycle. 18:2465–2480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Han Y, Wu N, Xia F, Liu S and Jia D: Long
non-coding RNA GAS5 regulates myocardial ischemia-reperfusion
injury through the PI3K/AKT apoptosis pathway by sponging
miR-532-5p. Int J Mol Med. 45:858–872. 2020.
|
|
66
|
Chen F, Zhang L, Wang E, Zhang C and Li X:
LncRNA GAS5 regulates ischemic stroke as a competing endogenous RNA
for miR-137 to regulate the Notch1 signaling pathway. Biochem
Biophys Res Commun. 496:184–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang J, Xu R, Wu J and Li Z: MicroRNA-137
negatively regulates H2O2-induced
cardiomyocyte apoptosis through CDC42. Med Sci Monit. 21:3498–3504.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang K, Liu F, Liu CY, An T, Zhang J, Zhou
LY, Wang M, Dong YH, Li N, Gao JN, et al: The long noncoding RNA
NRF regulates programmed necrosis and myocardial injury during
ischemia and reperfusion by targeting miR-873. Cell Death Differ.
23:1394–1405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cho YS, Challa S, Moquin D, Genga R, Ray
TD, Guildford M and Chan FK: Phosphorylation-driven assembly of the
RIP1-RIP3 complex regulates programmed necrosis and virus-induced
inflammation. Cell. 137:1112–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sun L, Wang H, Wang Z, He S, Chen S, Liao
D, Wang L, Yan J, Liu W, Lei X and Wang X: Mixed lineage kinase
domain-like protein mediates necrosis signaling downstream of RIP3
kinase. Cell. 148:213–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liang H, Li F, Li H, Wang R and Du M:
Overexpression of lncRNA HULC attenuates myocardial
Ischemia/reperfusion injury in rat models and apoptosis of
Hypoxia/reoxygenation cardiomyocytes via targeting miR-377-5p
through NLRP3/Caspase1/IL1β signaling pathway inhibition. Immunol
Invest. Jul 17–2020.Epub ahead of print. View Article : Google Scholar
|
|
72
|
Zhao G, Hailati J, Ma X, Bao Z, Bakeyi M
and Liu Z: LncRNA Gm4419 regulates myocardial ischemia/reperfusion
injury through targeting the miR-682/TRAF3 Axis. J Cardiovasc
Pharmacol. 76:305–312. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang JX, Zhang XJ, Li Q, Wang K, Wang Y,
Jiao JQ, Feng C, Teng S, Zhou LY, Gong Y, et al: MicroRNA-103/107
regulate programmed necrosis and myocardial ischemia/reperfusion
injury through targeting FADD. Circ Res. 117:352–363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang W, Li Y and Wang P: Long non-coding
RNA-ROR aggravates myocardial ischemia/reperfusion injury. Braz J
Med Biol Res. 51:e65552018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Huang L, Guo B, Liu S, Miao C and Li Y:
Inhibition of the LncRNA Gpr19 attenuates ischemia-reperfusion
injury after acute myocardial infarction by inhibiting apoptosis
and oxidative stress via the miR-324-5p/Mtfr1 axis. IUBMB Life.
72:373–383. 2020. View Article : Google Scholar
|
|
76
|
Zhang M, Gu H, Xu W and Zhou X:
Down-regulation of lncRNA MALAT1 reduces cardiomyocyte apoptosis
and improves left ventricular function in diabetic rats. Int J
Cardiol. 203:214–216. 2016. View Article : Google Scholar
|
|
77
|
Wang S, Yu W, Chen J, Yao T and Deng F:
LncRNA MALAT1 sponges miR-203 to promote inflammation in myocardial
ischemia-reperfusion injury. Int J Cardiol. 268:2452018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gong X, Zhu Y, Chang H, Li Y and Ma F:
Long noncoding RNA MALAT1 promotes cardiomyocyte apoptosis after
myocardial infarction via targeting miR-144-3p. Biosci Rep.
39:BSR201911032019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li Z, Li J and Tang N: Long noncoding RNA
Malat1 is a potent autophagy inducer protecting brain microvascular
endothelial cells against oxygen-glucose
deprivation/reoxygenation-induced injury by sponging miR-26b and
upregulating ULK2 expression. Neuroscience. 354:1–10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ge ZW, Zhu XL, Wang BC, Hu JL, Sun JJ,
Wang S, Chen XJ, Meng SP, Liu L and Cheng ZY: MicroRNA-26b relieves
inflammatory response and myocardial remodeling of mice with
myocardial infarction by suppression of MAPK pathway through
binding to PTGS2. Int J Cardiol. 280:152–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar :
|
|
83
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: CircRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Huang A, Zheng H, Wu Z, Chen M and Huang
Y: Circular RNA-protein interactions: Functions, mechanisms, and
identification. Theranostics. 10:3503–3517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Legnini I, Di Timoteo G, Rossi F, Morlando
M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade
M, et al: Circ-ZNF609 is a circular RNA that can be translated and
functions in Myogenesis. Mol Cell. 66:22–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Werfel S, Nothjunge S, Schwarzmayr T,
Strom TM, Meitinger T and Engelhardt S: Characterization of
circular RNAs in human, mouse and rat hearts. J Mol Cell Cardiol.
98:103–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tan WL, Lim BT, Anene-Nzelu CG,
Ackers-Johnson M, Dashi A, See K, Tiang Z, Lee DP, Chua WW, Luu TD,
et al: A landscape of circular RNA expression in the human heart.
Cardiovasc Res. 113:298–309. 2017.PubMed/NCBI
|
|
88
|
Li M, Ding W, Tariq MA, Chang W, Zhang X,
Xu W, Hou L, Wang Y and Wang J: A circular transcript of ncx1 gene
mediates ischemic myocardial injury by targeting miR-133a-3p.
Theranostics. 8:5855–5869. 2018. View Article : Google Scholar
|
|
89
|
Zong L and Wang W: CircANXA2 promotes
myocardial apoptosis in myocardial ischemia-reperfusion injury via
inhibiting miRNA-133 expression. Biomed Res Int. 2020:85908612020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Song YF, Zhao L, Wang BC, Sun JJ, Hu JL,
Zhu XL, Zhao J, Zheng DK and Ge ZW: The circular RNA TLK1
exacerbates myocardial ischemia/reperfusion injury via targeting
miR-214/RIPK1 through TNF signaling pathway. Free Radic Biol Med.
155:69–80. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cherra SR III, Dagda RK, Tandon A and Chu
CT: Mitochondrial autophagy as a compensatory response to PINK1
deficiency. Autophagy. 5:1213–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Clark IE, Dodson MW, Jiang C, Cao JH, Huh
JR, Seol JH, Yoo SJ, Hay BA and Guo M: Drosophila pink1 is required
for mitochondrial function and interacts genetically with parkin.
Nature. 441:1162–1166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Billia F, Hauck L, Konecny F, Rao V, Shen
J and Mak TW: PTEN-inducible kinase 1 (PINK1)/Park6 is
indispensable for normal heart function. Proc Natl Acad Sci USA.
108:9572–9577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhou LY, Zhai M, Huang Y, Xu S, An T, Wang
YH, Zhang RC, Liu CY, Dong YH, Wang M, et al: The circular RNA ACR
attenuates myocardial ischemia/reperfusion injury by suppressing
autophagy via modulation of the Pink1/FAM65B pathway. Cell Death
Differ. 26:1299–1315. 2019. View Article : Google Scholar
|
|
95
|
Zweier JL and Talukder MA: The role of
oxidants and free radicals in reperfusion injury. Cardiovasc Res.
70:181–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhu T, Yao Q, Wang W, Yao H and Chao J:
iNOS Induces vascular endothelial cell migration and apoptosis via
autophagy in Ischemia/Reperfusion injury. Cell Physiol Biochem.
38:1575–1588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen L, Luo W, Zhang W, Chu H, Wang J, Dai
X, Cheng Y, Zhu T and Chao J: circDLPAG4/HECTD1 mediates
ischaemia/reperfusion injury in endothelial cells via ER stress.
RNA Biol. 17:240–253. 2020. View Article : Google Scholar :
|
|
98
|
Fang S, Guo H, Cheng Y, Zhou Z, Zhang W,
Han B, Luo W, Wang J, Xie W and Chao J: circHECTD1 promotes the
silica-induced pulmonary endothelial-mesenchymal transition via
HECTD1. Cell Death Dis. 9:3962018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu M, Jia J, Wang X, Liu Y, Wang C and
Fan R: Long non-coding RNA HOTAIR promotes cervical cancer
progression through regulating BCL2 via targeting miR-143-3p.
Cancer Biol Ther. 19:391–399. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhou HF, Xu LL, Xie B, Ding HG, Fang F and
Fang Q: Hsa-circ-0068566 inhibited the development of myocardial
ischemia reperfusion injury by regulating hsa-miR-6322/PARP2 signal
pathway. Eur Rev Med Pharmacol Sci. 24:6980–6993. 2020.PubMed/NCBI
|
|
101
|
Chang H, Li ZB, Wu JY and Zhang L:
Circ-100338 induces angiogenesis after myocardial
ischemia-reperfusion injury by sponging miR-200a-3p. Eur Rev Med
Pharmacol Sci. 24:6323–6332. 2020.PubMed/NCBI
|
|
102
|
Ge X, Meng Q, Zhuang R, Yuan D, Liu J, Lin
F, Fan H and Zhou X: Circular RNA expression alterations in
extracellular vesicles isolated from murine heart post
ischemia/reperfusion injury. Int J Cardiol. 296:136–140. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Iwasaki YW, Siomi MC and Siomi H:
PIWI-Interacting RNA: Its biogenesis and functions. Annu Rev
Biochem. 84:405–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Vella S, Gallo A, Lo Nigro A, Galvagno D,
Raffa GM, Pilato M and Conaldi PG: PIWI-interacting RNA (piRNA)
signatures in human cardiac progenitor cells. Int J Biochem Cell
Biol. 76:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zheng S, Zheng H, Huang A, Mai L, Huang X,
Hu Y and Huang Y: Piwi-interacting RNAs play a role in vitamin
C-mediated effects on endothelial aging. Int J Med Sci. 17:946–952.
2020. View Article : Google Scholar : PubMed/NCBI
|















