1. Introduction
At the end of 2019, the first confirmed case of
Coronavirus disease 2019 (COVID-19) was reported, and the virus
rapidly spread to hundreds of countries worldwide (1). High-throughput sequencing
identified the COVID-19 pathogen as a novel β coronavirus (2), and the International Committee for
Classification of Viruses named it severe acute respiratory
syndrome coronavirus type 2 (SARS-CoV-2). Epidemiological studies
have demonstrated that COVID-19 infects individuals of all ages
(3), with the elderly and
patients with underlying comorbidities being at a higher risk of
adverse clinical outcomes and a poor prognosis (3,4).
Sequence analysis has identified that SARS-CoV-2 and 2003 SARS-CoV
share approximately 80% nucleotide identity (5). However, compared with SARS-CoV,
SARS-CoV-2 is more infectious than SARS-CoV, which has caused
>120 infections worldwide; since however, only some patients
exhibit a severity of symptoms similar to those of SARS-CoV
infection, its virulence seems lower. Similar to SARS-CoV,
according to reported cases, SARS-CoV-2 is primarily transmitted
through respiratory droplets and direct contact (3,6),
both of which can cause acute and highly fatal pneumonia (7). Unlike SARS-CoV, SARS-CoV-2-infected
patients rarely exhibit prominent upper respiratory signs and
symptoms. At the time of consultation, the majority of infected
patients present with a dry cough (83-99%) and dyspnea (59.4-82%),
and X-rays have revealed bilateral ground-glass shadows (1,8).
In the most severe cases, the characteristic symptom is respiratory
distress (~55%). In addition, SARS-CoV-2 needs to bind to its
receptor through the spike protein (S protein) on the surface of
the virus to enter the host cell (2).
The renin-angiotensin system (RAS) maintains blood
pressure homeostasis and water-salt balance (9). The dynamic balance of RAS is
essential for the physiological and pathological regulation of
various organs, including the heart, kidneys and lungs (9). Angiotensin-converting enzyme 2
(ACE2) is a key factor for RAS to negatively regulate blood
pressure and is essential for maintaining the dynamic balance of
RAS (10,11). A previous study found that ACE2
is a functional receptor for invasion by the novel coronavirus
(SARS-CoV-2); it interacts with the viral spike glycoprotein (S
protein) receptor binding domain to mediate viral invasion of host
cells (12). Therefore, during
the SARS coronavirus infection process, ACE2 is essential for the
virus to enter host cells. Using HeLa cells expressing human
(2), Chinese horseshoe bat,
civet cat and pig ACE2 protein for computational modeling and viral
infection experiments (13), the
experimental results demonstrated that the affinity of SARS-CoV-2
for ACE2 was 10-fold higher than that of SARS-CoV, which is
consistent with the high infection efficiency of SARS-CoV-2
(14). In addition, current
research results have indicated that when the ACE2 content is very
high, it can attenuate viral invasion and reduce acute lung injury
damage, while at the same time enhancing viral replication ability
and susceptibility. By contrast, when the ACE2 content is low, it
hinders the ability of the virus to replicate, but to a certain
extent, when the ACE2 content is too low, it increases the levels
of angiotensin II (AngII), which plays a role in promoting
inflammation and fibrosis, inducing multiple organ damage. These
findings indicate that ACE2 may be critical to the progression and
prognosis of human infection with SARS-CoV-2.
Since the emergence of this novel virus, there are
currently no clinically specific treatments available for this
virus, and symptomatic treatment is the main focus. Therefore, the
rapid search and development of specific drugs to inhibit
SARS-CoV-2 infection has become a priority. As a key receptor in
the process of viral invasion, existing research results suggest
that there is great potential for treating new coronary pneumonia
by adjusting the levels of ACE2. Therefore, the present review
article begins by discussing the physiological function of ACE2 and
summarizing the impact of the ACE2 content on viral susceptibility
and acute lung injury. Subsequently, from the perspective of drug
mechanisms, combined with the results of clinical trials, several
ACE2-centric treatments are specifically elaborated and analyzed.
The drug efficacy and areas that need improvement are reviewed. The
research discussed herein, to a certain extent, may aid medical
workers to correctly understand the role of ACE2 in the disease
process, understand the complex effects of the substance in viral
infections and acute lung injury, and use ACE2-centered drug
therapy in a prudent and standard manner. At the same time, several
therapies and mechanisms related to ACE2 listed in this review
article can also provide researchers with certain insight for the
development of novel drugs.
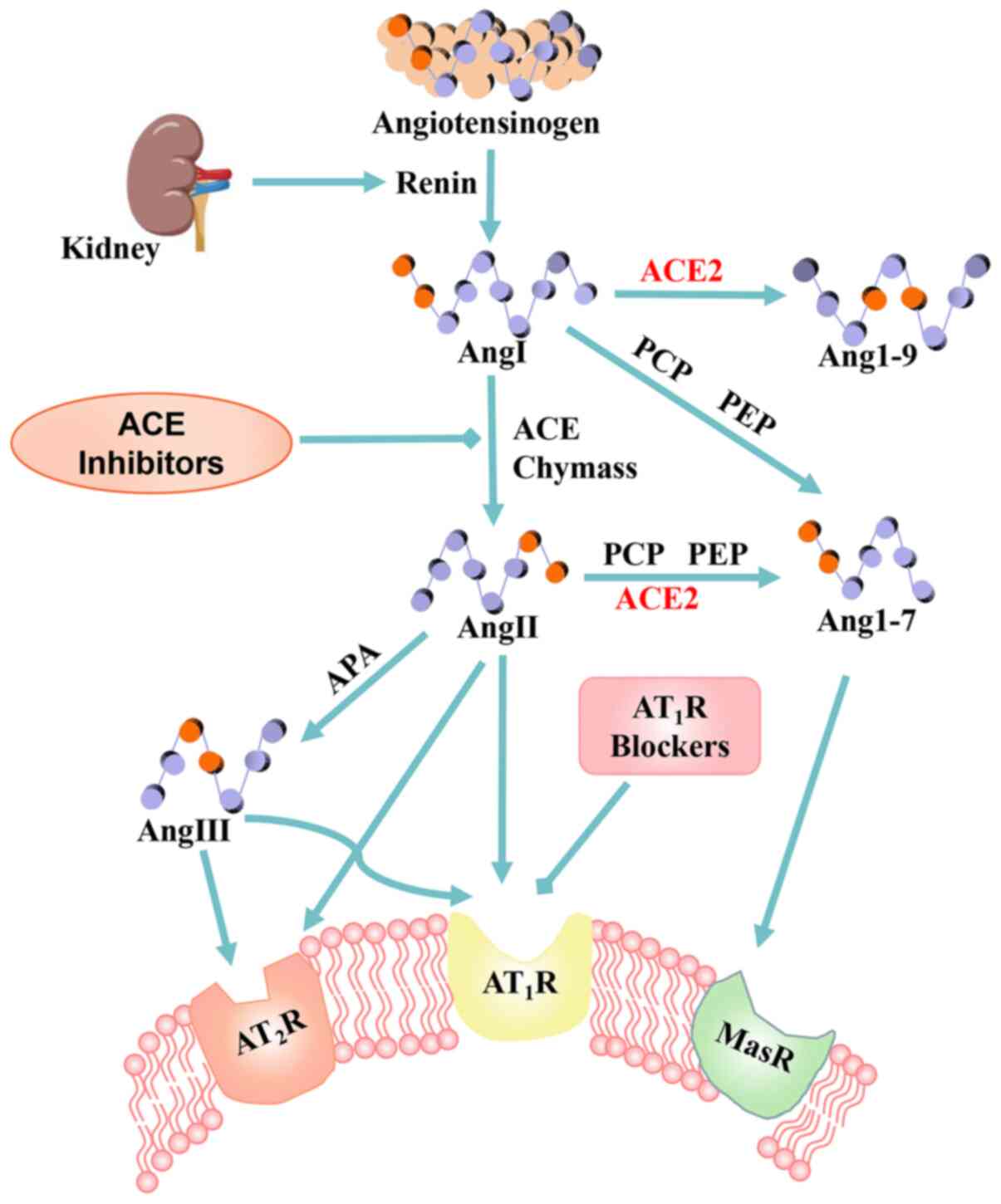
2. Angiotensin-converting enzyme 2
ACE is a type I transmembrane glycoprotein with a
single extracellular catalytic domain. It plays an important
regulatory role in the RAS and converting inactive angiotensin I
(AngI) into AngII, which regulates vasoconstriction. AngII is the
core effector molecule of the RAS system and mediates a number of
biological responses through angiotensin receptors (AT1 and
AT2).
ACE2 was the first human angiotensin converting
enzyme homolog discovered in 2000 (15,16) and is a zinc metalloproteinase.
Its coding gene is located on the X chromosome and belongs to the
family of type 1 transmembrane proteins. Its structure includes a
signal peptide, a transmembrane domain and a metalloproteinase
active site, containing a zinc binding domain of HEXXH. As a
monocarboxy peptidase, unlike its homolog ACE, which is a
dipeptidase, ACE2 is not antagonized by ACE inhibitors (ACEIs)
(17). ACE contains two active
catalytic domains, while ACE2 has a single catalytic domain with
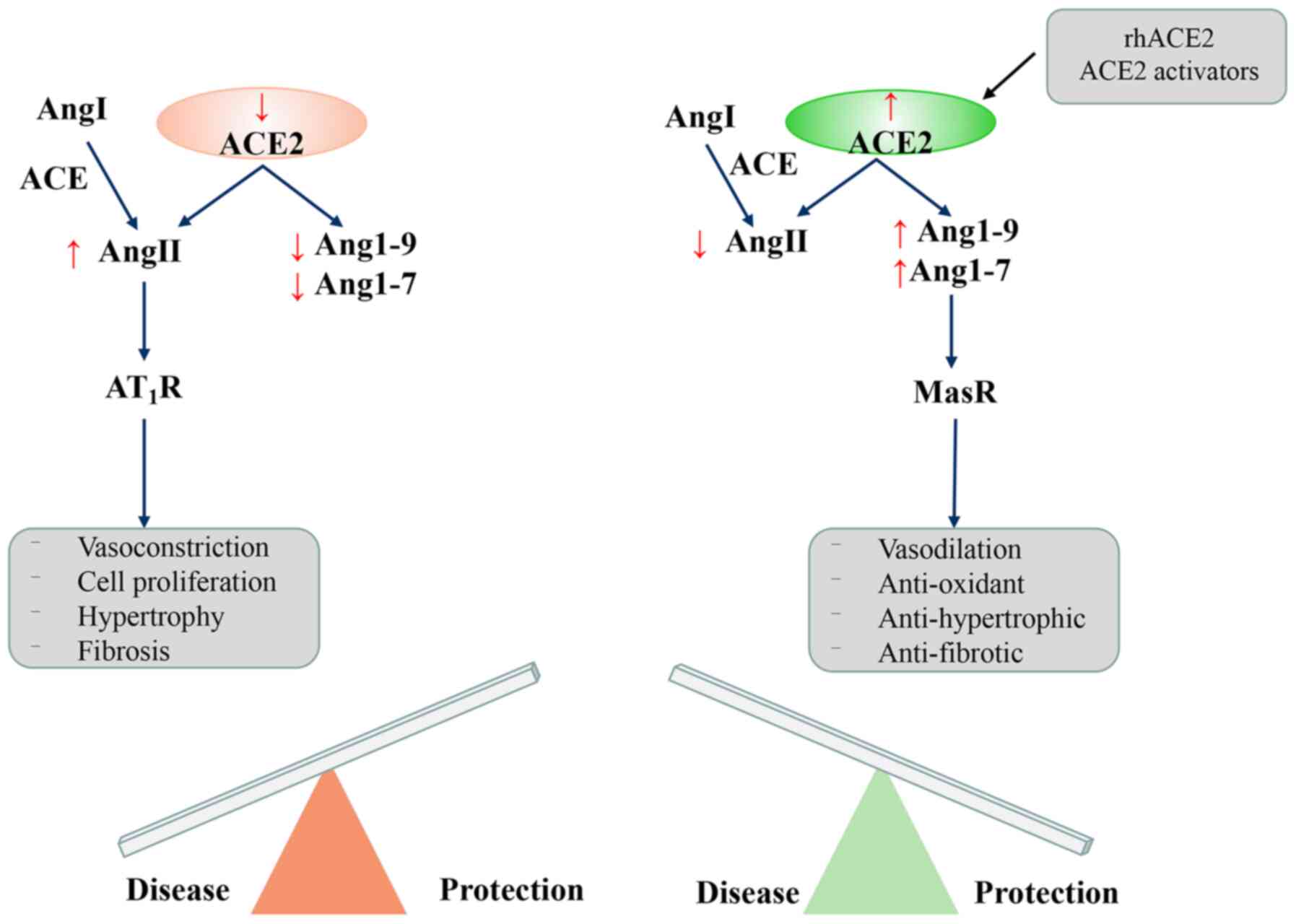
42% of the same residues (18,19) that not only degrades AngI to
produce nonapeptide Ang1-9, but also cleaves AngII into Ang1-7
polypeptides (20) (Fig. 1). While protecting the heart,
relaxing blood vessels, and exerting anti-growth and
anti-proliferative effects, it also enhances the activity of
bradykinin. ACE2/ACE and AngII/Ang1-7 in the human body exist in a
dynamic balance that regulates several important physiological
functions (21).
 | Figure 1Enzymatic cascade of the
renin-angiotensin system (RAS) and the key receptor systems. The
RAS cascade showing the angiotensin peptide metabolic pathway.
Angiotensinogen, as the starting substrate, is cleaved by renin to
AngI. AngI is cleaved by ACE to AngII, which is cleaved by ACE2 to
Ang(1-7). AngII acts on AT1 and AT2 receptors.
Ang(1-7) acts on Mas receptors and
counterbalances the AngII/AngII AT1R actions. RAS,
renin-angiotensin system; AngI, angiotensin I; AngII, angiotensin
II; ACE, angiotensin-converting enzyme; AT1R, angiotensin II type 1
receptor; APA, aminopeptidase A; PCP, prolyl carboxypeptidase; PEP,
proline endopeptidase. |
The expression of ACE2 exhibits high tissue
specificity (22). The primary
organs expressing ACE2 include the heart, brain, oral cavity and
nasal mucosa, nasopharynx, kidney, stomach, small intestine, colon,
skin, lymph nodes, thymus, bone marrow, spleen, liver and blood
vessels, which are targets of the SARS-CoV-2 virus (23,24). In addition, alveolar epithelial
cells have been regarded as the most important cell type for ACE2
expression (25). Accordingly,
it is hypothesized that SARS-CoV-2 may bind to RAS through
ACE2.
Yan et al (25) at West Lake University, examined
the composite structure of the new coronavirus spike S protein and
ACE2 receptor, observing that the novel coronavirus binds to the
human cell receptor ACE2 through the S protein. Coronavirus binding
to the ACE2 receptor induces a decrease in ACE2 levels, and the RAS
system becomes activated, leading to disease. Based on this
consistent pathogenesis, improving ACE2 and inhibiting the RAS
system may represent important options for the treatment of
pneumonia caused by the novel coronavirus (14).
3. ACE2 has potential for use in the
treatment of COVID-19
ACE2 is a key receptor in the invasion of
host cells by SARS-CoV2
Studies have confirmed that the key functional
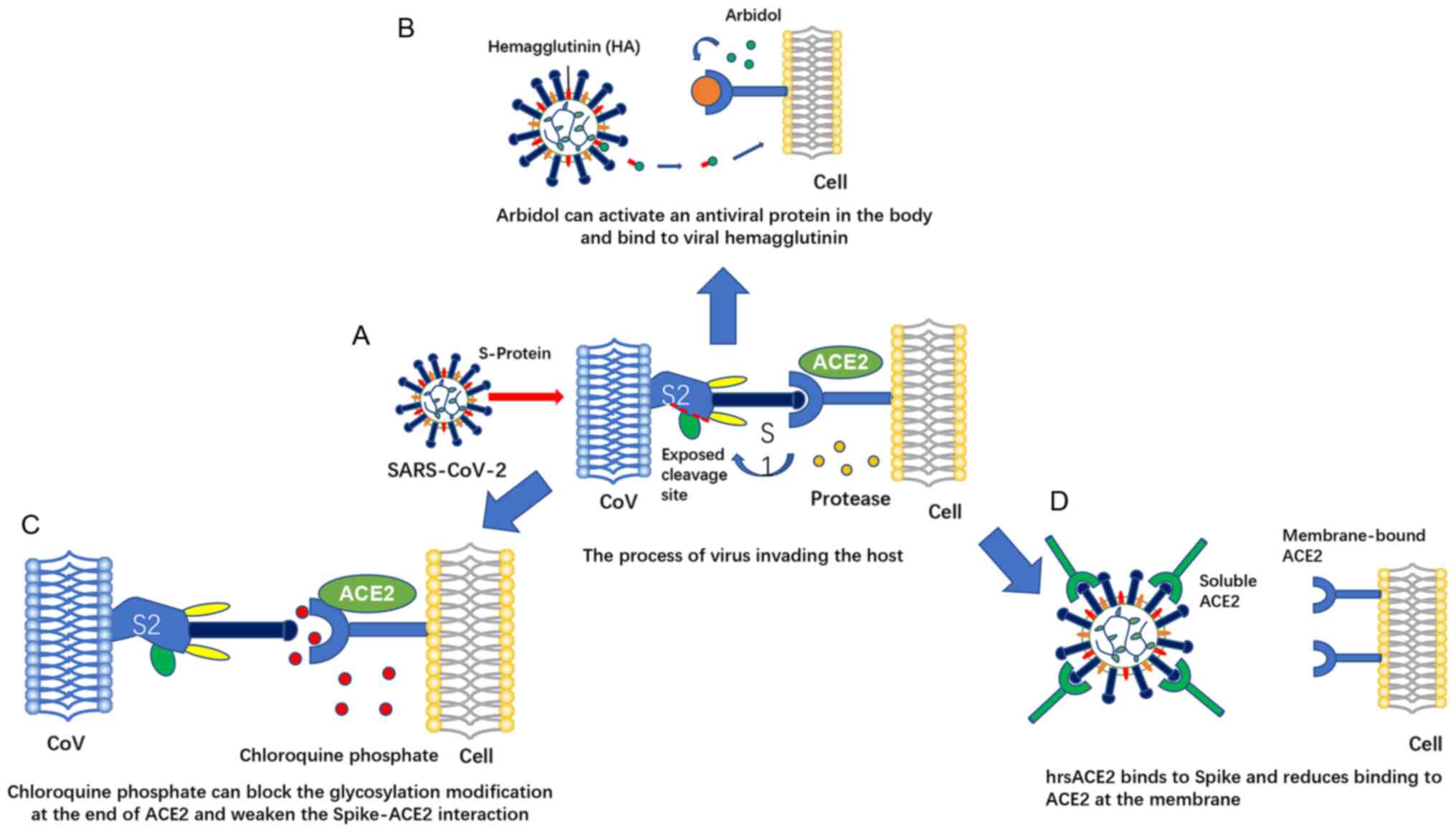
receptor for SARS-CoV2 to enter cells is ACE2 (15,16). The process occurs as follows: The
S protein on the surface of the virus that is responsible for
mediating receptor recognition and cell membrane fusion is cleaved
into the S1 and S2 subunits during development. The S1 subunit
contains a receptor binding domain that interacts with ACE2. When
bound, another cleavage site on S2 is exposed and cut by the host's
protease (17). This process is
of vital importance to the successful invasion of the virus.
Following the entry of the virus, the ACE2 protein is
downregulated, dysregulating the ACE-AngII/angiotensin II type 1
receptor (AT1R) axis and the ACE2-Ang1-7/Mas axis, and AngII levels
are relatively/absolutely increased, which overstimulate AT1R,
increase lung capillary permeability and induce acute lung failure.
This provides a molecular explanation for acute respiratory
distress syndrome (ARDS), the death mechanism of the coronavirus
infection (26). In response to
this mechanism, potential treatment strategies include blocking the
receptor binding domain (RBD) of the viral S protein or the
functional receptor of ACE2 to prevent the binding of human ACE2
and SARS-CoV-2. In addition to this blocking strategy, other
possible treatment options may include increasing the ACE2 levels
and the topical use of ACE2-derived peptides, small molecule
inhibitors, ACE2 antibodies, or single-chain antibody fragments
against ACE2 (Fig. 2).
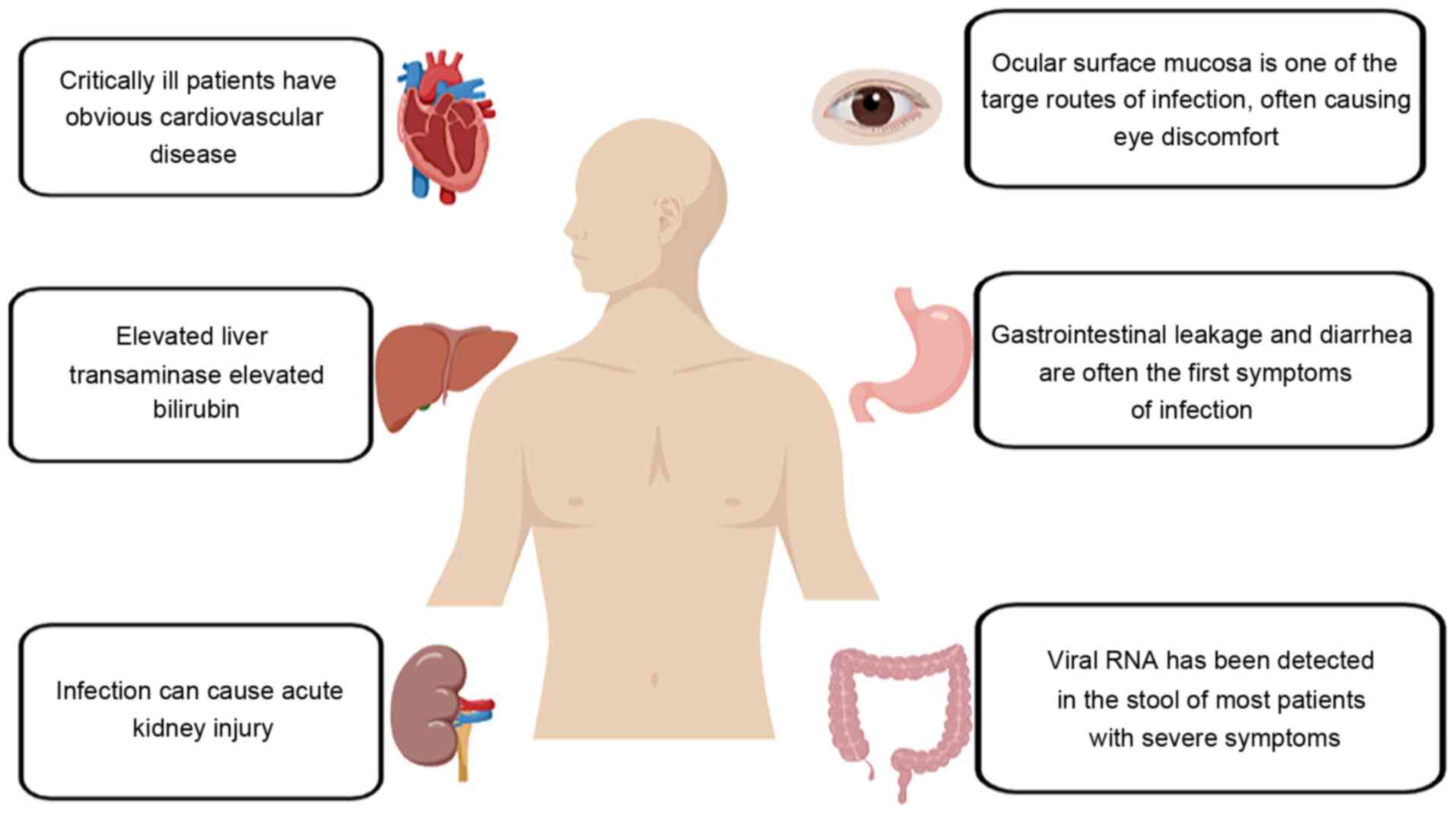
ACE2 and COVID-19
According to clinical diagnosis and treatment, the
main symptoms of patients infected with COVID-19 include high blood
pressure, diarrhea and damage to multiple organs, including the
heart, kidneys and testes (27).
These diffuse COVID-19 manifestations may be related to infection
and RAS overreaction. Therefore, it was hypothesized that, as an
important negative feedback regulator of RAS, ACE2 represents a
reasonable target for the prevention and treatment of COVID-19.
Fortunately, current research indicates that ACE2 has good
potential for use in the treatment of patients with ARDS,
cardiovascular disease, gastrointestinal malnutrition and other
viral-related symptoms (28).
This is discussed below.
i) ACE2 and acute respiratory distress
syndrome
ARDS is characterized by severe hypoxemia and
extreme difficulty in breathing, which represents the main cause of
mortality in patients with COVID-19. As mentioned above, imbalance
in the ACE-AngII/AT1R axis in the classic RAS pathway increases the
permeability of lung capillaries and the influx of calcium ions,
which indirectly promotes the occurrence of acute lung failure.
ACE2 can protect patients from lung injury by regulating this
process.
Previous studies performing animal experiments have
demonstrated that compared with normal wild-type (WT) control mice,
ACE2 knockout (KO) mice exhibit a very severe ARDS pathology,
including enhanced vascular permeability, severe pulmonary edema
and moderate accumulation of sex-specific granulocytes,
deterioration of lung function, etc. (9,29). These observations suggest that
the balance in the ACE2/ACE content is the key to lung
injury/protection during these inflammatory factor storms. Further
analyses have indicated that the artificial injection of the AT1R
blocker, angiotensin II receptor blocker (ARB), induces the
expression of ACE2, Ang 1-7 and Mas, leading to a decrease in
pro-inflammatory cytokine levels and an increase in the levels of
the anti-inflammatory cytokine, interleukin (IL)-10 (29). This protective effect of ACE2 may
reduce AGII by decomposing it into AG (1-7).
In another study using mice, SARS-CoV infection and its S protein
downregulated the expression of ACE2 (30). Clinical observations and
experiments at this stage have also yielded similar results. A
retrospective study found that in 300 patients with
COVID-19-induced acute pneumonia, multiple organ failure caused by
an inflammatory cytokine storm was the main cause of mortality
(31). Researchers speculate
that when the immune system is activated in response to SARS-CoV2
infection, the existing T helper cell 17 (Th17)/Treg functional
cells and immune cells secrete a large number of pro-inflammatory
cytokines, and this hyperactivity leads to an imbalance in cytokine
content. This imbalance, coupled with the loss of ACE2 due to the
invasion of COVID-19, ultimately leads to tissue and systemic
inflammation (32). Following
the injection of recombinant human ACE2 (rhACE2), an artificially
created ACE2 protein, pathological damage and inflammation in the
lung were improved (18). A
series of studies in Austria have proven that in acute lung injury
caused by SARS and certain influenza viruses, ACE2 buffers the lung
fibrosis and lung injury caused by the excessive activation of RAS
to a certain extent (33).
Moreover, levels of plasma AngII in patients with COVID-19 are
significantly increased, which exhibits a linear correlation with
viral titer and the degree of lung injury, suggesting that
SARS-CoV2 may cause acute lung injury by reducing the expression
levels of ACE2 and disrupting the ACE/ACE2 balance.
Based on the above-mentioned studies, it can be
concluded that in patients with COVID-19, ACE2 represents a
potential reasonable target for the prevention and treatment of
acute respiratory distress syndrome. Relying on the human body's
own RAS mechanisms to increase the ACE2 content is expected to
provide positive results for the treatment of patients with
ARDS.
ii) ACE2 and cardiovascular disease in
patients with COVID-19
In addition to acute pneumonia, the critical
illnesses caused by COVID-19 also includes cardiovascular disease.
A previous study demonstrated that in autopsy heart tissues from 20
patients who had succumbed to SARS-CoV, 7 heart samples exhibited
obvious cardiovascular lesions characterized by increased
myocardial fibrosis, inflammation, and decreased myocardial ACE2
expression (34). These patients
were also more severely ill and exhibited a higher mortality rate.
A similar phenomenon is observed in SARS-CoV2 patients: Individuals
with pre-existing diseases, such as hypertension and heart disease,
have an increased specific risk of COVID-19 infection, and in
patients with COVID-19, acute myocardial injury is frequent. Other
abnormal phenomena have also been observed in the laboratory,
including increased D-dimer and continuously increased inflammatory
cytokine levels throughout the clinical course (35,36). These phenomena all indicate that
the cardiovascular system is continuously experiencing inflammation
in patients with COVID-19.
As a regulator of the RAS system, ACE2 effectively
relieves a number of cardiovascular diseases, including
hypertension and coronary heart disease. As early as the end of the
last century, drugs for the treatment of cardiovascular disease
appeared that were developed based on the ACE2-Ang(1-7)/Mas axis, and they have currently
become classic prescription drugs. It has been demonstrated that in
a rat model of myocardial infarction, compared with the myocardial
tissue that survived 3 days following infarction, the infarct and
the peri-infarct area exhibited an increased expression of ACE2
(37). As ACE2 exerts a
protective effect on lung and heart tissue damage, it is
hypothesized that hypertensive patients with COVID-19 are more
likely to develop complications and severe cases. A large number of
observations have also confirmed this point. The severity of
COVID-19 infection in patients with hypertension is indeed greater.
In addition, compared with non-severe cases, severe cases of
COVID-19 exhibit relatively higher systolic blood pressure (145
mmHg vs. 122 mmHg) (38). This
phenomenon indicates that SARS-CoV-2 deprives tissues of ACE2,
leading to a marked decrease in ACE2 levels that indirectly lead to
the occurrence of hypertension.
At present, studies have suggested that the use of
ARB drugs in patients with COVID-19 may help to improve the
patient's condition or even decrease their risk of mortality. A
study completed in 2017 revealed that ARB effectively blocked AT1R,
antagonizing the main AngII actions and exhibiting protective
pleiotropic effects against hypertension and cardiovascular
inflammation, fibrosis and thrombosis (39). The data available from >20
clinical trials currently in progress (e.g., NCT04312009,
NCT04311177, NCT04318418) indicate that ARB decreases the viral
load, prevents peripheral T cell depletion and reduces plasma IL-6
levels, C-reactive protein (CRp) and procalcitonin levels (40). However, this therapy has been
mainly shown to be effective in subjects with hyperinflammatory
periods or previous hypertension. The European Society of
Cardiology and the American Heart Association advise against
terminating these maintenance treatments for patients with
COVID-19, particularly in the presence of high blood pressure or
heart failure (41). However, at
present, due to the lack of clinical statistics with a larger
sample size, a large controversy remains as to whether ARB drugs
should be used in the treatment of patients with COVID-19. Further
research is required to determine whether the long-term use of
these therapies will cause different consequences in specific
tissues.
iii) ACE2, enteric malnutrition and
disease progression in patients with COVID-19
As regards the clinical symptoms of the novel
coronary pneumonia, gastrointestinal symptoms are often the first
observed or accompanying symptoms. ACE2 is most commonly expressed
in intestinal epithelial cells, spreading over the surface of the
entire gastrointestinal lumen, making the intestine a secondary
site of SARS-CoV-2 infection. Gastrointestinal leakage in
experimental models of human diseases are improved and worsened by
the increased and decreased ACE2 levels, respectively (42,43). A study on 811 patients with
COVID-19 with gastrointestinal discomfort and diarrhea found that
gastrointestinal symptoms may appear earlier than symptoms of lung
infection (44). In addition,
the majority of the currently known coronaviruses affect the
integrity of the gastrointestinal blood barrier and cause
intestinal dysbiosis, bacteremia and systemic inflammation. The
development of gastrointestinal leakage and enteral malnutrition is
closely related to the excessive activation of the ACE/AngII/AT1R
axis caused by the loss of ACE2 (45,46). Fecal viral RNA has been detected
in up to 70% of patients with gastrointestinal virus shedding and a
more aggressive clinical course (47,48).
In addition to the direct impact of the virus on the
microbiome, diseases such as diabetes and lung diseases, also have
an adverse effect on the gut microbiome, and SARS-CoV-2 infection
may increase disease severity (49-51). In animal models and humans,
AngII-dependent hypertension is associated with enteral
malnutrition, increased intestinal leakage and pathological changes
in the intestinal wall (52,53). The destruction of ACE2 in the
biomedical model indicates that intestinal malnutrition is very
common and that this change in the microbial profile can alter the
systemic pathway and exacerbate diabetes and hypertension (26).
Therefore, it can be concluded that the decrease in
intestinal ACE2 expression caused by SARS-CoV-2 infection may
similarly reduce circulating angiogenic cells and damage the
integrity of endothelium and intestinal epithelium, leading to
malnutrition. However, further investigations are warranted to
verify whether this phenomenon is a direct or indirect effect of
viral infection. If further research confirms the existence of
viruses through intestinal infections, fecal bacteria
transplantation, probiotic therapy, etc., can regulate the
intestinal microecology to indirectly adjust the ACE/ACE2 balance,
which has potential for the treatment of patients with
COVID-19.
Potential risks
It has to be acknowledged that ACE2 has great
potential for the treatment of patients with COVID-19. However,
since the current considerations basically stem from theoretical
derivation and small basic experiments, there is still a lack of
convincing clinical data. Thus, during the pandemic of the novel
coronary pneumonia, the use of ACE2 and its related inhibitors for
the treatment of patients with COVID-19 still conveys potential
risks to a certain extent. This primarily manifests in two aspects.
First, in terms of dose, the levels of ACE2 have complexity in
novel coronary infection (Fig.
3). Second, in terms of therapeutic efficacy, the use of ACE2
and its related inhibitors may promote infection in patients with
complications, such as hypertension and cardiovascular disease
(54-56). This is discussed below.
i) The content of ACE2 is complex in
SARS-CoV-2 infections
The interaction between the SARS-CoV-2 virus and
ACE2 is considered a potential feature of its infectivity (26); thus, there may be approaches
which may be used to intervene in this process to resist SARS-CoV-2
infection, such as delivering excess soluble ACE2 or inhibiting
SARS-CoV-2 virus interaction with angiotensin-converting enzyme
2.
Generally, it is considered that excessive ACE2
levels, particularly soluble forms of ACE2, may slow viral entry
and spread. In addition, this may not only prevent lung injury by
neutralizing the virus, but also release cellular ACE2 and enhance
its activity. As a protective factor that inhibits lung injury and
multiple organ failure of the RAS classic axis, its expression
should be increased, but ACE2 is also a receptor for viral
infection. It has been demonstrated that ACE2 promotes the
replication of SARS-CoV (57).
The expression levels of ACE2 in cells are positively associated
with susceptibility to the SARS-CoV S protein. After the host cells
are infected with ACE2, viral replication capacity increases
(58). Moreover, the affinity of
SARS-CoV-2 for ACE2 is 10- to 20-fold greater than that of SARS-CoV
(13); thus, excessive levels of
ACE2 may increase the chance of infection with SARS-CoV-2.
A lack of ACE2 has both advantages and
disadvantages. For patients who have been infected, a lack of ACE2
leads to increased levels of AngII, which acts more on the target
organ AT1R to play a pro-inflammatory, pro-fibrotic and other
roles, causing target organ lesions, which in turn induces multiple
organ damage (24). In addition,
ACE2 deficiency also exerts a positive effect. Following SARS-CoV
infection in mice, the levels of ACE2 in the lungs of mice
significantly decrease, and the levels of SARS virus infection in
the lungs also significantly decreased (26). ACE2 KO mice are more resistant to
SARS-CoV infection, and ACE2 inhibitors also prevent SARS-CoV
replication (26,58,59).
However, it has been demonstrated that the
expression of ACE2 in intestinal epithelial cells is positively
associated with viral entry, release and cellular immunity genes,
but negatively associated with viral transcription, protein
translation, humoral immunity, phagocytosis and complement
activation (60). This suggests
that ACE2 may play a dual role in mediating susceptibility and
immunity to SARS-CoV-2 infection; thus, it needs to be used with
caution.
ii) ACE2 may promote infection in
patients with complications, such as hypertension and
cardiovascular disease
Recent evidence has indicated that patients with
diseases, such as diabetes, hypertension and obesity (61), have the highest prevalence of
novel coronary pneumonia infections and are at a risk of mortality
(62). While ACE2 inhibitors and
angiotensin receptor blockers are basic drugs for the treatment of
hypertension, heart disease and chronic kidney disease, ACE2 is an
important functional receptor for SARS-CoV-2 infected hosts. A
number of patients and physicians are therefore concerned that the
use of renin-angiotensin-aldosterone system (RAAS) inhibitors will
increase the risk of viral infection in these patients.
de Abajo et al compared the current use of
RAAS inhibitors with other anti-hypertensive drugs for some
confirmed admissions (63). The
results demonstrated that compared with the use of other
anti-hypertensive drugs, the use of RAAS inhibitors was not
associated with an increased risk of infection requiring admission,
but was associated with an increased risk of severe complications
requiring intensive care.
In contrast to the above-mentioned findings, de
Abajo et al found an interesting and potentially clinically
significant result in their study. They observed that the
administration of RAAS inhibitors reduced the risk of adverse
outcomes in diabetic patients with new-onset coronary pneumonia by
almost half compared with other anti-hypertensive drugs (63). Other studies have also suggested
that compared with other anti-hypertensive drugs, the use of RAAS
inhibitors may exert a protective effect on complications and
mortality in patients with the novel coronary pneumonia, although
these studies were not limited to diabetic patients (64,65).
Several studies with mixed conclusions (63-67) have demonstrated that there is
currently no clear evidence that RAAS inhibitors increase the risk
of infection in patients with the new-onset coronary pneumonia.
However, there is also no strong evidence to support that once
infected, the use of RAAS inhibitors increases the risk of
infection or the severity of complications compared to treatment
with other antihypertensive agents. The results of some studies
even obtained opposite findings. However, this potentially
important finding needs to be confirmed in large randomized
controlled trials (65,65).
In summary, although ACE2 has great potential for
use in the treatment of the novel coronavirus pneumonia, there are
indeed some potential risks surrounding the use of ACE2 and its
related inhibitors for treatment. In line with the humanitarian
principle of the supremacy of patient interests, when conducting
clinical trials and formulating diagnostic and treatment
strategies, it is necessary to understand the mechanisms of action
of the drugs in detail and their therapeutic effects as much as
possible in order to select the diagnostic and treatment strategy
with the optimal efficacy by synthesizing various factors. Thus, a
general understanding of the existing diagnostic and treatment
strategies is required.
4. ACE2-centered drug therapy
The above-mentioned findings suggest that ACE2 has
great potential for use in the treatment of COVID-19. As a whole,
although there are some potential treatment risks. However, if used
reasonably, ACE2 may become a reasonable target for the prevention
and treatment of COVID-19. At present, there are a number of
diagnostic and treatment programs available for ACE2, which can be
divided into 2 categories. One is based on the process of virus S
protein that needs to bind ACE2 to invade cells, targeting S
protein-ACE2 interactions to inhibit viral invasion and including
drugs, such as arbidol, chloroquine, etc. The mechanism of action
of another class of drugs is to regulate the ACE2 content for the
prevention and treatment of acute lung injury (Fig. 4). Below, the drug mechanisms are
discussed and the results of clinical trials are combined to
explain and analyze the therapeutic efficacy of these drugs and
areas for improvement. The relevant pharmacological data of several
current popular drugs are summarized in Table I.
 | Table IPharmacological data of several
current popular drugs for ACE2-centered therapy (69,70,73,75,78,84,85). |
Table I
Pharmacological data of several
current popular drugs for ACE2-centered therapy (69,70,73,75,78,84,85).
| Name | Arbidol (ARB) | Chloroquine
phosphate (CQ) | Clinical-grade
soluble human ACE2 (hrsACE2) |
|---|
| Mechanism | Spike-ACE2 and
Affect the virus life cycle | Inhibit the process
of virus replication and block terminal glycosylation modification
of ACE2 | Inhibit lung damage
and prevent virus invasion |
| Pharmacokinetic and
toxicological data | EC50=10
μM CC50=20-100 μM |
EC50=1.13 μM
CC50=100 μM | Not reported
(currently undergoing Phase 1 and 2 clinical trials; NCT00886353,
NCT01597635) |
| Applicable
stage | Early stage of
viral infection | Pneumonia worsening
stage | Prevent infection
stage; late lung injury stage |
| Suggested dose | 200 mg/day <10
days for adults | 1,000 mg/day
(twice) for 7 days | 25-200 μg/day |
| Combination
medication | With IFN-α or other
antiviral drug | High CQ doses are
not recommended when with azithromycin or oseltamivir | Not reported |
| Possible adverse
reactions | Not reported | May increase the
risk of fatal ventricular arrhythmia | May increase the
risk of virus infection |
| Problems to be
solved | Standardized animal
studies and controlled clinical trials | The gap between
in vitro antiviral activity and clinicaleffect; standardized
diagnosis and treatment plan yet to be formulated | The impact of
hrsACE2 in the later stages of the disease process |
Therapeutic drugs targeting the
spike-ACE2 interaction
Certain currently available therapeutic drugs
targeting ACE2 are discussed below.
i) Arbidol
Arbidol is a recommended anti-influenza drug. As a
hemagglutinin inhibitor, the mechanism of action for COVID-19 is
primarily through the activation of 2,5-oligoadenylate synthase in
the human body, which is an antiviral protein that specifically
inhibits the interaction between the S protein on the viral lipid
envelope and ACE2 on the host cell membrane by interrupting
adhesion and other interactions, blocking the process of viral gene
penetration into the nucleus (DAA) (24). At the same time, arbidol can also
act as a host targeting agent (HTA) by affecting the life cycle of
the virus. Results from preclinical animal toxicology experiments
have demonstrated that arbidol has good toxicological safety. The
lethal dose demonstrated in experiments using mice has been shown
to be approximately 40-fold the dose used in humans (68); clinical use records have reported
arbidol to be extremely well tolerated and to have a high
therapeutic index. In a retrospective study, the total effective
rate of arbidol antiviral therapy was 75% (69), and no severe adverse reactions
were observed (70), with its
resistance primarily arising from mutations in the HA2 fusion
protein. Although the drug has been used as an antiviral drug for
influenza for a number of years, it has not yet produced
significant viral resistance. The main part of arbidol metabolism
occurs in the liver, but rapidly spreads to various tissues. Within
48 h, approximately 40% of arbidol intake is excreted in feces.
Through searching past antiviral randomized
controlled studies of arbidol and clinical studies in which it was
used in patients with COVID-19, arbidol appears to have a positive
antiviral effect. On February 4, 2020, the research team of Lanjuan
announced that in vitro cell experiments revealed that very
low concentrations of arbidol effectively inhibited SARS-CoV-2 by
60-fold compared with the control group without drug treatment,
significantly inhibiting the pathological effect of the virus on
the cells. However, the team has not yet published the study, and
thus this is not conclusive evidence. At present, arbidol has been
used as an antiviral drug in COVID-19 patients and applied during
the early stages of viral infection. Previous studies have also
demonstrated that, including various coronaviruses and influenza
viruses, arbidol exhibits broad-spectrum antiviral activity
(71,72). In the 'New Coronavirus Infection
Pneumonia Diagnosis and Treatment Plan (Trial Version 6)', arbidol
was recommended for the first time for the antiviral treatment of
patients with COVID-19 (http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml).
According to the guidelines, the use of interferon (IFN)-α is
recommended in combination with arbidol or other antiviral drugs
for <10 days as an anti-COVID-19 therapy; however, there are
currently no large-scale clinical studies available on
arbidol/IFN-α in COVID-19 information or research. In a recent
study, arbidol combined with IFN-α2b was no more effective than
IFN-α2b as a single agent in COVID-19 with respect to RNA clearance
and hospitalization, raising questions as to the use of arbidol
(73).
Based on a large amount of literature, arbidol has
great potential for use in the treatment of patients with COVID-19;
however, at present, the efficacy and toxicity of the drug for
COVID-19 remains uncertain, and the combination of IFN-α and
arbidol used to obtain novel drug categories to improve efficacy
should be further confirmed in larger prospective randomized
studies. The relative lack of standardized animal studies and
controlled clinical trials for healthy and infected subjects is
also an issue that needs to be resolved. In view of the fact that
patients with COVID-19 are administered several drugs and their
clinical course is complex, it is necessary to perform
pharmaceutical monitoring following the administration of these
drugs to patients. Moreover, both in vivo and in
vitro studies have demonstrated that arbidol can be used in
combination with other antiviral drugs for the treatment of
patients with COVID-19 to obtain improved therapeutic effects;
however, the antiviral effects and mechanisms of action of arbidol
alone require more in-depth investigation in further clinical
studies.
ii) Chloroquine phosphate (CQ)
As a basic compound, chloroquine reduces the
interaction of spike-ACE2 by blocking the glycosylation
modification at the end of ACE2, inhibiting the virus from invading
Vero E6 cells (74). Chloroquine
also increases the pH of vesicles and blocks the replication
process of pH-dependent viruses, such as coronaviruses. Due to the
lack of reliable information on the target concentration or dose in
COVID-19, the 'loading dose' for adults (30 mg/kg within 48 h) and
children (70 mg/kg within 5 days) is currently used as a clinical
reference. Early toxicological test results have suggested that the
safety of the drug needs to be investigated. For mice, the
injection of CQ at 60 mg/kg per day for 4 consecutive weeks leads
to a 30-40% fatality rate. It has not yet been determined whether
the use of much lower doses that are currently recommended will
ensure the safety of this combination of drugs, and prescribers
should exercise caution. However, it was clinically observed that
the high-dose group (600 mg CQ) compared with the low-dose group
(300 mg CQ) experienced a reduced fatality rate of at least 50%
(75).
CQ has been used in the treatment of malaria and
autoimmune diseases for >70 years, and it has also been proven
to have positive effects against a variety of coronaviruses
(76). Chloroquine compounds
were first demonstrated in in vitro tests at the end of 2019
to effectively inhibit the invasion of SARS-CoV-2 and were
subsequently considered to be effective in attenuating the
progression of pneumonia and lung damage. As a result of imaging,
the viral load is reduced, and therefore, the course of the disease
is shortened, increasing the survival rate (77). The National Health Commission
released the 'New Coronavirus Pneumonia Diagnosis and Treatment
Plan (Trial Sixth Edition)' on February 18, 2020, adding CQ as an
antiviral trial drug (http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml).
An in vitro antiviral study at the Wuhan Institute of
Virology has demonstrated that the median effective concentration
(EC50) of chloroquine is 1.13 μmol/l (0.36 mg/l), and it
exerts antiviral effects during and after the viral entry stage
(77). At present, there are
>30 clinical studies of CQ in the treatment of COVID-19 at home
and abroad. According to published briefings, clinical treatment
observations from >100 patients have indicated that CQ inhibits
the deterioration of pneumonia, improves lung imaging results and
inhibits viruses. In terms of decreasing the disease course, CQ is
superior to the control treatment, and there were no severe adverse
reactions to CQ in the above-mentioned patients (78).
Although CQ has been tested clinically and has
acceptable safety, there are also some potential safety issues and
prolonged use risks, including a prolonged QT interval, ventricular
tachycardia and retinopathy, etc., which may increase the risk of
fatal ventricular arrhythmia (79). Therefore, safety is a key concern
in the current application of chloroquine. According to previous
findings, the therapeutic dose of chloroquine is very close to the
toxic and lethal doses, and the plasma drug concentration at a dose
of 500 mg/day will exceed EC90 (6.90 μmol/l) (80). Therefore, overdosing should be
avoided in clinical practice. Previous research has indicated that
the ACE2 receptor used by ARS-CoV-2 to enter cells is highly
expressed in cells, such as the lungs, gastrointestinal tract,
kidneys and heart, facilitating SARS-coronavirus 2 entry into these
organs (81). According to
previous research, CQ highly and slowly accumulates in these organs
(79). Therefore, the
distribution of CQ in these organs may be highly associated with
its potential efficacy against SARS-CoV-2 and adverse events.
However, according to the currently recommended dosing regimen for
the treatment of malaria or rheumatoid arthritis, the concentration
of the drug at the site of action may be much higher than the
effective concentration (EC50) required to inhibit
SARS-CoV-2 in vitro, and a higher accumulation of tissue CQ
may lead to adverse events. At the same time, due to the potential
safety hazards of COVID-19 in critically ill patients, particularly
when taking azithromycin and oseltamivir at the same time, higher
CQ doses have proven to be dangerous.
Therefore, objectively speaking, although the drug
achieves good efficacy in the treatment of COVID-19 patients, its
safety may be a major obstacle to its application in the future.
Among infected patients, approximately 30% are elderly and
approximately 2% are pregnant women and children, and the
probability of complications in these populations is higher
(21). At the same time, in
obese patients, if total body weight is used instead of calculating
the dose by ideal weight, obese patients may be administered an
overdose, as these drugs are not retained in adipose tissue.
Therefore, there is an urgent need for the development of a
personalized drug delivery strategy for each vulnerable group to
safely and effectively use CQ against SARS-CoV-2. Recent research
has found that the in vitro antiviral activity of CQ does
not necessarily translate into clinical efficacy in vivo
(82). In view of the fact that
the mechanisms of drug metabolism in the body remain unclear, this
is also an urgent issue to be solved. Therefore, considering the
exposure-efficacy and exposure-safety association of CQ to optimize
its dosage in each special population is a direction for future
development, and when medical conditions permit, it is recommended
that in the treatment of novel coronary pneumonia, whole blood
concentration of the drug after taking CQ should be closely
monitored to adjust the dosage of CQ according to the blood
concentration to formulate a more precise individualized treatment
plan. An electrocardiogram (ECG) should be closely monitored as
well to prevent adverse reactions.
Targeted regulation of ACE2 content and
treatment methods
The content of ACE2 as regards various treatment
methods is discussed below.
i) Clinical-grade soluble human
ACE2
A previous study demonstrated that human recombinant
soluble ACE2 (hrsACE2) reduced the recovery ability of SARS-CoV-2
in Vero cells by 1000-5,000-fold. There have since been phase 1 and
2 clinical trials conducted, both of which have proven that in the
early stage of infection, hrsACE2 significantly inhibits the
infection of human organs and organoids, hindering the growth of
the virus (83,84). Its coefficient for Vero E6 cells
is 1,000-5,000. Moreover, SARSCoV-2 directly infects engineered
human vascular organs and renal organs, and hrsACE2 inhibits its
infection (85). Recombinant
ACE2 may also effectively be used for the treatment of acute lung
failure in mice and eliminates the lung injury effect caused by
SARS protein by regulating the ACE2 pathway (81). In other lung injury models, such
as bleomycin-induced pulmonary fibrosis and monocrotaline-induced
pulmonary hypertension, recombinant ACE2 has recently been shown to
prevent chronic lung injury, fibrosis, and pulmonary
vasoconstriction (85). These
results suggest that recombinant ACE2 can be used clinically as a
novel treatment for chronic organ failure and acute lung
injury.
However, it must also be considered that compared
with endosomal ACE, endogenous circulating levels of soluble ACE2
are usually very low or even undetectable, and SARS-CoV-2 cannot be
sufficiently isolated in the circulation to prevent spread of the
virus. The degree to which artificial recombinant ACE2 competes
with SARS-CoV-2 to reduce viremia infection and tissue damage is
still unclear. At present, only the effect of hrsACE2 on early
infection by the virus is understood; however, the effect of
hrsACE2 in later stages of the disease process is not clear, and
its effects on the lungs are also poorly understood (86). A clinical trial on the infusion
of ACE2 protein in the stress group was also withdrawn. Although
increasing ACE2 protein content to increase the ratio of
Ang-(1-7): AngII may have a certain improvement
effect on organ damage induced by SARS-CoV-2, this method may also
be beneficial for viral invasion through the respiratory or
digestive system. When patients experience ARDS, it may alleviate
subsequent viral infections in other tissues; however, ACE2
infusion may reduce circulating AngII levels and increase
Ang(1-7) levels to promote infectious or
cardiogenic effects in later stages of the disease, including
shock. Patients with COVID-19 may experience blood pressure
imbalance, leading to hypotension.
In brief, it can be concluded that the research and
development of artificial hrsACE2 is still in a relatively immature
stage, and the relevant research results are still inconclusive. In
view of the complex effects of ACE2 levels on viral infections,
every step of the research in the future must be taken
cautiously.
ii) RAS inhibition
ACE2 is a component of RAS and participates in the
occurrence and development of hypertension together with ACE. RAS
inhibitors are drugs that need to be chronically taken by
hypertensive patients. Although it seems that there are different
responses to ARBs and ACEIs, as well as tissue-related responses,
the notion that RAAS block stimulates ACE2 expression and activity
has typically been supported by experimental studies (87-89). Recently, it was discovered that
in the hearts of mice with aortic stenosis, various ARBs
(olmesartan, losartan, valsartan, candesartan, telmisartan and
irbesartan) are all present to a similar degree (~2 times) and
increase ACE2 protein levels (90,91). Among patients with chronic kidney
disease (CKD), urinary ACE2 levels (tubular expression index) in
patients treated with ACEIs or ARBs are similar to those in
untreated patients. In addition, Kocks et al found that ACEI
treatment had no effect on the expression of ACE2 protein in kidney
biopsy samples of patients with various kidney diseases and kidney
transplant recipients (92). By
contrast, only patients treated with ACEI had higher intestinal
ACE2 mRNA levels than ARB (93).
However, ACE2 protein or activity was not evaluated to verify the
mRNA results. In addition to RAAS inhibitors, experimental studies
have indicated that statins enhance the expression of ACE2. Tikoo
et al reported increased ACE2 protein levels in the heart
and kidney of atherosclerotic rabbits treated with atorvastatin
(approximately 2-fold), which was related to epigenetic
modification of the ACE2 gene (94). Fluvastatin treatment has been
shown to significantly enhance the effect of insulin on inducing
the expression of ACE2 protein in the heart of diabetic rats
(95). As far as is known, the
effect of ARB or ACEI therapy combined with statins on ACE2
expression has not yet been determined. Finally, peroxisome
proliferator-activated receptor-γ (PPAR-γ) may also affect
expression of ACE2. After the aorta is narrowed, the PPAR-γ agonist
rosiglitazone causes the aorta of hypertensive rats to increase
ACE2 content (96). Researchers
in Austrian found that in acute lung injury caused by SARS and
certain influenza viruses, ACE2 buffers pulmonary fibrosis and lung
injury caused by excessive activation of RAS to a certain extent
(97). Studies using
experimental animal lung injury models have demonstrated that
RAS-induced upregulation of ACE2 by inhibitors can reduce lung
injury (98). Various ACE
inhibitors, such as captopril and lisinopril, do not affect the
activity of ACE2, and ACE2 activity can be inhibited by the
dipeptide pro-phe. Specific ACE2 inhibitors have been developed
accordingly, such as the peptide analog DX600 and MLN 4760
{(S,S)-2-[1-carboxy-2-[3-(3,5-dichlorobenzyl)-3H-imidazol4-yl]-ethylamino]-4-methyl
Valeric acid}. MLN 4760 is the first rationally designed ACE2
inhibitor based on the carbon-terminal dipeptide of AngI (His-Leu)
with high potency (Ki=0.44 nM) and specificity (99).
The current understanding of the cardiovascular
consequences in patients with SARS-CoV-2 infection at this early
stage is very limited. Lo et al reported that circulating
levels of AngII in patients with COVID-19 were significantly higher
than those in healthy controls, which is consistent with the lower
activity of ACE2. However, compared with experimental data,
particularly regarding the impact of ACEI and ARB, the current
ACE2-Ang(1-7) clinical data on the pathway is very
limited (99). In addition,
although the existing evidence is novel and insightful, it usually
comes from smaller cross-sectional observational studies.
Therefore, RAAS measurement is incomplete and cannot fully explain
the potential bias and confusion.
In summary, it is still controversial whether RAS
inhibitors are a 'nemesis' or 'accomplice' of COVID-19, and there
is no definite clinical evidence on whether RAS inhibitors
aggravate the condition of COVID-19 (100). The test results of different
RAS inhibitors affecting ACE2 expression and enzyme activity are
inconsistent, and related research is still advancing. In view of
the fact that the biological effects of basic research are not
equivalent to clinical effects, RAS inhibitors should not be rashly
activated or stopped in response to ambiguous research results.
New insight based on the SARS-CoV
epidemic experience
Research indicated that serum from SARS patients
during the recovery period prevents the invasion of SARS-CoV-2,
revealing the important commonality between SARS-CoV-2 and SARS-CoV
infection (89). Therefore, when
there are no specific drugs available for the treatment of
COVID-19, it is a fruitful practice to refer to the epidemic
experience in 2003 for the new use of old drugs. This is discussed
below.
i) Griffithsin (GRFT)
GRFT is a lectin extracted from red algae. This
lectin has a domain-swapped dimeric structure in which 2β-strands
of one monomer combine with 10 β-strands of the other monomer to
form a β-prism of 3 4-stranded sheets (101). Each GRFT monomer (mGRFT)
contains 3 binding sites with high affinity for mannose residues,
and this compound is one of the most potent microbicides isolated
to date. Mori et al confirmed the antiviral activity of GRFT
in in vivo and in vitro experiments in SARS-CoV
studies (102). In
vitro, GRFT specifically binds to the recombinant SARS-CoV S
protein in a dose-dependent manner, although it cannot
significantly inhibit the subsequent binding of ACE2 to the spike
protein. Interestingly, Mori et al found that GRFT
significantly ameliorated pulmonary edema lesions and alleviated
necrotizing bronchiolitis in mice following SARS-CoV infection, as
well as significantly downregulating the production of
pro-inflammatory cytokines, such as cytokines IL-1α, IL-1β, IL-6,
G-CSF, MCP-1 and IL-12 in lung tissue (102). This reminds us that the
subsequent development of GRFT is likely to be performed in the
setting of cytokine storms.
ii) Antibodies or peptide drugs that
strongly block spike-ACE2 binding
Passive immunization may be an effective treatment
for COVID-19, which requires the use of agents that can neutralize
the virus, either sera from persons recovering from SARS-CoV-2
infection or purified antibodies, in patients.
In a study on SARS-CoV, Hu et al found that a
peptide fragment S of viral RBD specifically blocked the binding of
spike RBD-ACE2 and inhibited infection in in vitro
experiments (103). Han et
al used an alanine scanning mutagenesis method and found that
the peptide composed of 2 ACE2 modules linked by glycine exhibited
potent antiviral activity (IC50=0.1 μmol/l) (104). Therefore, an effective method
to block the RBD-ACE2 interaction of the Newcomb virus is to search
for RBD domain-based peptides or their combination cocktails.
However, it must be noted that although antibodies to SARS-CoV have
been shown to achieve good efficacy, SARS-CoV2 and SARS-CoV spike
RBD amino acid sequence identity is only 72%; thus, therapeutic
antibodies and peptides targeting SARS-CoV RBD interact weakly with
the new coronary virus RBD, and many do not even cross-react with
SARS-CoV-2. Therefore, some degree of modification of existing
drugs is necessary to obtain better efficacy.
Recently, investigators from Vanderbilt University
Medical Center and other institutions have discovered a number of
monoclonal antibodies (mAbs) with effective neutralizing activity
that completely block the interaction between S protein
receptor-binding region (SRBD) and human ACE2 receptor (hACE2) from
a large number of human mAbs against spike glycoproteins.
Researchers found that these neutralizing monoclonal antibodies
effectively recognized nonoverlapping sites while binding to the S
protein and synergistically neutralized the authentic SARS-CoV-2
virus. At the same time, it was also demonstrated in 2 mouse
SARS-CoV-2 infection models that passive infusion of COV2-2196,
COV2-2130, or the combination of these 2 mAbs protected mice from
weight loss and reduced viral burden and lung inflammation. In
addition, researchers found that passive infusion of the 2 most
potent ACE2-blocking monoclonal antibodies (COV2-2196 or COV2-2381)
as a single treatment protected rhesus monkeys from SARS-CoV-2
infection. Collectively, these results identify protective epitopes
for SRBD and provide a structure-based framework for rational
vaccine design and selection of potent immunotherapies (105).
Coincidentally, Huo et al reported 2 closely
related nanobodies (H11-H4 and H11-D4) that could block SARS-CoV-2
spike binding to ACE2 in cell culture (106). One protein region targeted by
these nanobodies is closely adjacent to the ACE2 binding region and
has a small amount of overlap. Both nanobodies exhibited the
ability to neutralize live SARS-CoV-2, with H11-H4 being
particularly potent and enhanced in combination with human-derived
antibodies. The authors suggested that these nanobodies could be
used alone or in combination with other antibodies to help achieve
passive immunization in critically ill patients with COVID-19.
Since camelid animal-derived antibodies are highly conserved with
human-derived antibodies, they may only produce a low immune
response in humans, but a well-developed humanization strategy can
be exploited.
An important line of defense against SARS-CoV-2 is
the formation of neutralizing antibodies that can eliminate
invaders and have great potential in preventing and treating viral
infections. Therefore, from the perspective of humoral immunity,
the development of antibodies or peptides that can potently block
the binding of SARS-CoV-2 spikes to ACE2 will be an important
method for the prevention and treatment of COVID-19 at present.
Identification of other components of RAS
as targets for intervention
Studies have demonstrated that ACE2 expression is
positively associated with genes for viral entry, release and
cellular immunity, but negatively associated with viral
transcription, protein translation, humoral immunity, phagocytosis
and complement activation. This suggests that ACE2 may play a dual
role in mediating susceptibility and immunity to SARS-CoV-2
infection, making the idea of directly using ACE2 as a target for
drug intervention a dilemma. Therefore, the identification of other
intervention targets related to ACE2 may become a future research
hotspot.
Considering that risk factors associated with
hospitalization and mortality in patients with metabolic diseases,
including obesity, arterial hypertension, cardiovascular disease
and diabetes, may reflect global activation of the RAS system,
modulation of RAS homeostasis through the ACE2/(Ang1-7)/MAS pathway
should be considered to improve patient symptoms. The Mas gene is
an intrinsic receptor for Ang1-7 and is highly conserved. Ang1-7
plays an important regulatory role in neuroplasticity, memory and
anxiety by acting on Mas receptors to exert anti-angiogenic,
vasodilatory, anti-proliferative, anti-fibrotic and antithrombotic
effects.
In addition, clinical observational studies have
found that in the majority of cases, respiratory distress occurs
following a period of infection (usually approximately 14 days),
suggesting that this phenomenon may not be a direct effect of the
initial viral infection, but rather a host response to loss of ACE2
function, dysregulation of the AngII/ACE2 pathway, and activation
of autoproteases. The current central hypothesis is that the
binding of viral spike proteins to ACE2 leads to the shedding of
ACE2 receptors by various proteases, which in turn leads to the
loss of protective function of the ACE2/MAS axis in the lung and
other organs. In addition to the loss of this protective function,
tissue-specific proteases (e.g., cathepsins, chymotrypsin-like)
activate the classical pathway (ACE/RAS/AngII) and the alternative
pathway, also leading to AngII overproduction at the tissue level.
This process may further change the balance of Ang1-7/MAS and ACE2
protective function, thereby alleviating the adverse effects of
AngII elevation on pulmonary epithelial and intravascular
injury.
Thus, the induction of ACE2 downstream pathways
through the activation of the ACE2/ang1-7/MAS axis may be an
effective strategy to prevent pulmonary and cardiovascular injury
due to SARS-CoV-2 infection. Due to decreased ACE2/MAS activity and
enhanced AngII/AT1R activity, the risk of pulmonary vascular
endothelial/epithelial cell injury and lung histopathology is
increased. The inhibition of protease activity, the necessary
cleavage of viral spike proteins to prevent virus interaction with
receptors and their entry into cells, such as ADAM17 and
transmembrane serine protease 2 (TMPRSS2), which inhibit enzyme
activity, may be exploited as a novel therapeutic target. In
addition, it is feasible to use the protective effects of Ang1-7 or
its analogs, such as AVE0991 (107), to counteract the deleterious
effects of increased AngII and may be effective in the symptomatic
treatment of these patients.
5. Conclusions and future perspectives:
Potentials and pitfalls
The present review began by discussing the
physiological function of ACE2 and summarizing the impact of ACE2
content on viral susceptibility and acute lung injury.
Subsequently, from the perspective of drug mechanisms, combined
with the results of clinical trials, several ACE2-centric
treatments are specifically elaborated and analyzed. The drug
efficacy and areas that need improvement are also reviewed. The
research presented herein, to a certain extent, may assist medical
workers to correctly understand the role of ACE2 in the disease
process, understand the complex effects of the substance in viral
infections and acute lung injury, and use ACE2 centered drug
therapy in a prudent and standard manner. At the same time, several
therapies and mechanisms related to ACE2 listed in the present
review article can also provide researchers with certain ideas for
developing new drugs.
The effects of ACE2 on sensitivity to the novel
coronaviruses and acute lung injury must be investigated. The data
presented herein suggest that when the ACE2 content is too high, it
can attenuate the rate of viral invasion and reduce the degree of
destruction in acute lung injury; however, it also enhances viral
replication capacity and sensitivity. When the ACE2 content is low,
it hinders the replication ability of the virus, but at the same
time, it leads to increased AngII levels, plays a role in promoting
inflammation and fibrosis, and induces multiple organ damage.
Despite the limitations and risks of existing diagnostic and
therapeutic regimens for ACE2 and the fact that most drugs have not
yet undergone large-scale clinical trials, we still cannot ignore
the therapeutic potential of ACE2 as a key receptor for viral
invasion.
As regards future directions, a strict evaluation of
existing drugs and regimens needs to be conducted, to standardize
the use of doses, rationally combine drugs, and establish a sound
regulatory mechanism. Lessons can also be learnt from the
experience of SARS-CoV with respect to anti-pandemic and innovative
research ideas and begin to explore the directions of 'other
components of RAS as intervention targets' and 'clinical-grade
soluble human ACE2'. In this manner, the therapeutic potential of
ACE2 as a key receptor for viral invasion can be fully taken
advantage of to obtain the best therapeutic effect.
However, the present review article also has certain
limitations. For example, a number of the studies cited in the
article are still in progress, and no definite and comprehensive
conclusions have been drawn yet. The present review only focused on
the existing results. Moreover, there are numerous ACE2-centered
drug therapies, and only the 4 most common therapies were discussed
herein. Finally, large-scale clinical retrospective studies on
patients with COVID-19 have not yet been performed, at least to the
best of our knowledge. Thus, the conclusions drawn may not be
accurate or universal enough.
Abbreviations:
|
ACE2
|
angiotensin converting enzyme 2
|
|
ACEIs
|
ACE inhibitors
|
|
AngI
|
angiotensin I
|
|
AngII
|
angiotensin II
|
|
ARB
|
angiotensin II receptor blocker
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
AT1R
|
angiotensin II type 1 receptor
|
|
CKD
|
chronic kidney disease
|
|
COVID-19
|
Coronavirus disease 2019
|
|
CQ
|
chloroquine phosphate
|
|
CRP
|
C-reactive protein
|
|
ECG
|
electrocardiogram
|
|
GRFT
|
griffithsin
|
|
hrsACE2
|
human recombinant soluble ACE2
|
|
HTA
|
host targeting agent
|
|
KO
|
knockout
|
|
mAbs
|
monoclonal antibodies
|
|
mGRFT
|
GRFT monomer
|
|
PPAR-γ
|
peroxisome proliferator-activated
receptor-γ
|
|
RAAS
|
renin-angiotensin-aldosterone
system
|
|
RAS
|
renin-angiotensin system
|
|
RBD
|
receptor binding domain
|
|
rhACE2
|
recombinant human angiotensin
converting enzyme 2
|
|
S protein
|
spike protein
|
|
SARS-CoV-2
|
severe acute respiratory syndrome
coronavirus type 2
|
|
SRBD
|
S protein receptor-binding region
|
|
Th17
|
T helper cell 17
|
|
TMPRSS2
|
transmembrane serine protease 2
|
|
WT
|
wild-type
|
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
GY and MF conceived the study subject, and
critically reviewed the intellectual content of the manuscript and
made substantive revisions to the important contents of the
manuscript. LC and XG were the major contributors to the writing of
the manuscript, and assisted in the literature search. YC, YZ, pY
and LH provided suggestions and technical support and revised
important sections of the manuscript, and assisted in the
literature search. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H,
Wang W, Song H, Huang B, Zhu N, et al: Genomic characterisation and
epidemiology of 2019 novel coronavirus: Implications for virus
origins and receptor binding. Lancet. 395:565–574. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H,
Wu Y, Zhang L, Yu Z, Fang M, et al: Clinical course and outcomes of
critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:
A single-centered, retrospective, observational study. Lancet
Respir Med. 8:475–481. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gralinski LE and Menachery VD: Return of
the Coronavirus: 2019-nCoV. Viruses. 12:1352020. View Article : Google Scholar :
|
|
5
|
Wang G and Jin X: The progress of 2019
novel coronavirus event in China. J Med Virol. 92:468–472. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fouchier RA, Hartwig NG, Bestebroer TM,
Niemeyer B, de Jong JC, Simon JH and Osterhaus AD: A previously
undescribed coronavirus associated with respiratory disease in
humans. Proc Natl Acad Sci USA. 101:6212–6216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD,
Jin HJ, Tan KS, Wang DY and Yan Y: The origin, transmission and
clinical therapies on coronavirus disease 2019 (COVID-19)
outbreak-an update on the status. Mil Med Res. 7:112020.
|
|
8
|
Patel S, Rauf A, Khan H and Abu-Izneid T:
Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for
homeostasis and pathologies. Biomed Pharmacother. 94:317–325. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuba K, Imai Y, Ohto-Nakanishi T and
Penninger JM: Trilogy of ACE2: A peptidase in the renin-angiotensin
system, a SARS receptor, and a partner for amino acid transporters.
Pharmacol Ther. 128:119–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamming I, Cooper ME, Haagmans BL, Hooper
NM, Korstanje R, Osterhaus AD, Timens W, Turner AJ, Navis G and van
Goor H: The emerging role of ACE2 in physiology and disease. J
Pathol. 212:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuhn JH, Li W, Choe H and Farzan M:
Angiotensin-converting enzyme 2: A functional receptor for SARS
coronavirus. Cell Mol Life Sci. 61:2738–2743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu X, Chen P, Wang J, Feng J, Zhou H, Li
X, Zhong W and Hao P: Evolution of the novel coronavirus from the
ongoing Wuhan outbreak and modeling of its spike protein for risk
of human transmission. Sci China Life Sci. 63:457–460. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wrapp D, Wang N, Corbett KS, Goldsmith JA,
Hsieh CL, Abiona O, Graham BS and McLellan JS: Cryo-EM structure of
the 2019-nCoV spike in the prefusion conformation. Science.
367:1260–1263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tipnis SR, Hooper NM, Hyde R, Karran E,
Christie G and Turner AJ: A human homolog of angiotensin-converting
enzyme. Cloning and functional expression as a
captopril-insensitive carboxypeptidase. J Biol Chem.
275:33238–33243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donoghue M, Hsieh F, Baronas E, Godbout K,
Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan
R, et al: A novel angiotensin-converting enzyme-related
carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9.
Circ Res. 87:E1–E9. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rice GI, Thomas DA, Grant PJ, Turner AJ
and Hooper NM: Evaluation of angiotensin-converting enzyme (ACE),
its homologue ACE2 and neprilysin in angiotensin peptide
metabolism. Biochem J. 383:45–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soubrier F, Alhenc-Gelas F, Hubert C,
Allegrini J, John M, Tregear G and Corvol P: Two putative active
centers in human angiotensin I-converting enzyme revealed by
molecular cloning. Proc Natl Acad Sci USA. 85:9386–9390. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ehlers MR and Riordan JF:
Angiotensin-converting enzyme: Zinc- and inhibitor-binding
stoichiometries of the somatic and testis isozymes. Biochemistry.
30:7118–7126. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel VB, Zhong JC, Grant MB and Oudit GY:
Role of the ACE2/Angiotensin 1-7 axis of the renin-angiotensin
system in heart failure. Circ Res. 118:1313–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Te Riet L, van Esch JH, Roks AJ, van den
Meiracker AH and Danser AH: Hypertension:
Renin-angiotensin-aldosterone system alterations. Circ Res.
116:960–975. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baig AM, Khaleeq A, Ali U and Syeda H:
Evidence of the COVID-19 virus targeting the CNS: Tissue
distribution, host-virus interaction, and proposed neurotropic
mechanisms. ACS Chem Neurosci. 11:995–998. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li JW, Han TW, Woodward M, Anderson CS,
Zhou H, Chen YD and Neal B: The impact of 2019 novel coronavirus on
heart injury: A systematic review and meta-analysis. Prog
Cardiovasc Dis. 63:518–524. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamming I, Timens W, Bulthuis ML, Lely AT,
Navis G and van Goor H: Tissue distribution of ACE2 protein, the
functional receptor for SARS coronavirus. A first step in
understanding SARS pathogenesis. J Pathol. 203:631–637. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan R, Zhang Y, Li Y, Xia L, Guo Y and
Zhou Q: Structural basis for the recognition of SARS-CoV-2 by
full-length human ACE2. Science. 367:1444–1448. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan
B, Huan Y, Yang P, Zhang Y, Deng W, et al: A crucial role of
angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced
lung injury. Nat Med. 11:875–879. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang F, Deng L, Zhang L, Cai Y, Cheung CW
and Xia Z: Review of the clinical characteristics of coronavirus
disease 2019 (COVID-19). J Gen Intern Med. 35:1545–1549. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bourgonje AR, Abdulle AE, Timens W,
Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors
AA, Osterhaus AD, et al: Angiotensin-converting enzyme 2 (ACE2),
SARS-CoV-2 and the pathophysiology of coronavirus disease 2019
(COVID-19). J Pathol. 251:228–248. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
da Silva JS, Gabriel-Costa D, Wang H,
Ahmad S, Sun X, Varagic J, Sudo RT, Ferrario CM, Dell Italia LJ,
Sudo GZ and Groban L: Blunting of cardioprotective actions of
estrogen in female rodent heart linked to altered expression of
cardiac tissue chymase and ACE2. J Renin Angiotensin Aldosterone
Syst. 18:14703203177222702017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou P, Yang XL, Wang XG, Hu B, Zhang L,
Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al: A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature.
579:270–273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walls AC, Park YJ, Tortorici MA, Wall A,
McGuire AT and Veesler D: Structure, function, and antigenicity of
the SARS-CoV-2 Spike Glycoprotein. Cell. 181:281–292.e6. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye R and Liu Z: ACE2 exhibits protective
effects against LPS-induced acute lung injury in mice by inhibiting
the LPS-TLR4 pathway. Exp Mol Pathol. 113:1043502020. View Article : Google Scholar
|
|
33
|
Oudit GY, Kassiri Z, Jiang C, Liu PP,
Poutanen SM, Penninger JM and Butany J: SARS-coronavirus modulation
of myocardial ACE2 expression and inflammation in patients with
SARS. Eur J Clin Invest. 39:618–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Inciardi RM, Lupi L, Zaccone G, Italia L,
Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, et al:
Cardiac involvement in a patient with coronavirus disease 2019
(COVID-19). JAMA Cardiol. 5:819–824. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burrell LM, Risvanis J, Kubota E, Dean RG,
MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, et al:
Myocardial infarction increases ACE2 expression in rat and humans.
Eur Heart J. 26:369–375. 322–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qi YF, Zhang J, Wang L, Shenoy V, Krause
E, Oh SP, Pepine CJ, Katovich MJ and Raizada MK:
Angiotensin-converting enzyme 2 inhibits high-mobility group box 1
and attenuates cardiac dysfunction post-myocardial ischemia. J Mol
Med (Berl). 94:37–49. 2016. View Article : Google Scholar
|
|
38
|
Hashimoto T, Perlot T, Rehman A,
Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R,
Lipinski S, et al: ACE2 links amino acid malnutrition to microbial
ecology and intestinal inflammation. Nature. 487:477–481. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferrario CM and Mullick AE: Renin
angiotensin aldosterone inhibition in the treatment of
cardiovascular disease. Pharmacol Res. 125:57–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meng J, Xiao G, Zhang J, He X, Ou M, Bi J,
Yang R, Di W, Wang Z, Li Z, et al: Renin-angiotensin system
inhibitors improve the clinical outcomes of COVID-19 patients with
hypertension. Emerg Microbes Infect. 9:757–760. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Anker SD, Butler J, Khan MS, Abraham WT,
Bauersachs J, Bocchi E, Bozkurt B, Braunwald E, Chopra VK, Cleland
JG, et al: Conducting clinical trials in heart failure during (and
after) the COVID-19 pandemic: An expert consensus position paper
from the heart failure association (HFA) of the European society of
cardiology (ESC). Eur Heart J. 41:2109–2117. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim S, Rigatto K, Gazzana MB, Knorst MM,
Richards EM, Pepine CJ and Raizada MK: Altered gut microbiome
profile in patients with pulmonary arterial hypertension.
Hypertension. 75:1063–1071. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Santisteban MM, Kim S, Pepine CJ and
Raizada MK: Brain-gut-bone marrow axis: Implications for
hypertension and related therapeutics. Circ Res. 118:1327–1336.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cheng PK, Wong DA, Tong LK, Ip SM, Lo AC,
Lau CS, Yeung EY and Lim WW: Viral shedding patterns of coronavirus
in patients with probable severe acute respiratory syndrome.
Lancet. 363:1699–1700. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leung WK, To KF, Chan PK, Chan HL, Wu AK,
Lee N, Yuen KY and Sung JJ: Enteric involvement of severe acute
respiratory syndrome-associated coronavirus infection.
Gastroenterology. 125:1011–1017. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Beli E, Yan Y, Moldovan L, Vieira CP, Gao
R, Duan Y, Prasad R, Bhatwadekar A, White FA, Townsend SD, et al:
Restructuring of the gut microbiome by intermittent fasting
prevents retinopathy and prolongs survival in db/db mice. Diabetes.
67:1867–1879. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vallianou NG, Stratigou T and Tsagarakis
S: Microbiome and diabetes: Where are we now? Diabetes Res Clin
Pract. 146:111–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Budden KF, Gellatly SL, Wood DL, Cooper
MA, Morrison M, Hugenholtz P and Hansbro PM: Emerging pathogenic
links between microbiota and the gut-lung axis. Nat Rev Microbiol.
15:55–63. 2017. View Article : Google Scholar
|
|
51
|
Iyer SN, Lu D, Katovich MJ and Raizada MK:
Chronic control of high blood pressure in the spontaneously
hypertensive rat by delivery of angiotensin type 1 receptor
antisense. Proc Natl Acad Sci USA. 93:9960–9965. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sharma RK, Yang T, Oliveira AC, Lobaton
GO, Aquino V, Kim S, Richards EM, Pepine CJ, Sumners C and Raizada
MK: Microglial cells impact gut microbiota and gut pathology in
angiotensin II-induced hypertension. Circ Res. 124:727–736. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bron PA and Kleerebezem M: Lactic acid
bacteria for delivery of endogenous or engineered therapeutic
molecules. Front microbiol. 9:18212018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guzik TJ, Mohiddin SA, Dimarco A, Patel V,
Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P,
D'Acquisto F, et al: COVID-19 and the cardiovascular system:
Implications for risk assessment, diagnosis, and treatment options.
Cardiovasc Res. 116:1666–1687. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
South AM, Diz DI and Chappell MC:
COVID-19, ACE2, and the cardiovascular consequences. Am J physiol
Heart Circ physiol. 318:H1084–H1090. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Devaux CA, Rolain JM and Raoult D: ACE2
receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension,
multi-organ failure, and COVID-19 disease outcome. J Microbiol
Immunol Infect. 53:425–435. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li W, Moore MJ, Vasilieva N, Sui J, Wong
SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough
TC, et al: Angiotensin-converting enzyme 2 is a functional receptor
for the SARS coronavirus. Nature. 426:450–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ge XY, Li JL, Yang XL, Chmura AA, Z hu G,
E pstein JH, Mazet JK, Hu B, Zhang W, Peng C, et al: Isolation and
characterization of a bat SARS-like coronavirus that uses the ACE2
receptor. Nature. 503:535–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jia HP, Look DC, Shi L, Hickey M, Pewe L,
Netland J, Farzan M, Wohlford-Lenane C, Perlman S and McCray PB Jr:
ACE2 receptor expression and severe acute respiratory syndrome
coronavirus infection depend on differentiation of human airway
epithelia. J Virol. 79:14614–14621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang J, Zhao S, Liu M, Zhao Z, Xu Y, Wang
P, Lin M, Xu Y, Huang B, Zuo X, et al: ACE2 expression by colonic
epithelial cells is associated with viral infection, immunity and
energy metabolism. medRxiv. 2020–2022. 2020.
|
|
61
|
Cure E and Cumhur Cure M:
Angiotensin-converting enzyme inhibitors and angiotensin receptor
blockers may be harmful in patients with diabetes during COVID-19
pandemic. Diabetes Metab Syndr. 14:349–350. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zeng Y, Zhang B, Zhang X and Yi C:
Clinical characteristics of 9 cancer patients with SARS-CoV-2
infection. Chin Med. 15:472020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
de Abajo FJ, Rodríguez-Martín S, Lerma V,
Mejía-Abril G, Aguilar M, García-Luque A, Laredo L, Laosa O,
Centeno-Soto GA, Ángeles Gálvez M, et al: Use of
renin-angiotensin-aldosterone system inhibitors and risk of
COVID-19 requiring admission to hospital: A case-population study.
Lancet. 395:1705–1714. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie
J, Liu YM, Zhao YC, Huang X, Lin L, et al: Association of inpatient
use of angiotensin converting enzyme inhibitors and angiotensin II
receptor blockers with mortality among patients with hypertension
hospitalized with COVID-19. Circ Res. 126:1671–1681. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Daniel M, Bean ZK, Thomas S, Rebecca BA,
Amos Folarin LR, Kevin OG and Rosita Zakeri AM: Treatment with
ACE-inhibitors is associated with less severe disease with
SARS-Covid-19 infection in a multi-site UK acute hospital trust.
NewsRX LLC; pp. 6122020
|
|
66
|
Li J, Wang X, Chen J, Zhang H and Deng A:
Association of renin-angiotensin system inhibitors with severity or
risk of death in patients with hypertension hospitalized for
coronavirus disease 2019 (Covid-19) infection in Wuhan, China. JAMA
Cardiol. 5:825–830. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Alexandre J, Cracowski JL, Richard V and
Bouhanick B: Drugs, COVID-19' working group of the French Society
Of Pharmacology, Therapeutics: Renin-angiotensin-aldosterone system
and COVID-19 infection. Ann Endocrinol (Paris). 81:63–67. 2020.
View Article : Google Scholar
|
|
68
|
Blaising J, Polyak SJ and Pécheur EI:
Arbidol as a broad-spectrum antiviral: An update. Antiviral Res.
107:84–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T,
Lu J and Xue Y: Arbidol monotherapy is superior to
lopinavir/ritonavir in treating COVID-19. J Infect. 81:e21–e23.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang Z, Yang B, Li Q, Wen L and Zhang R:
Clinical features of 69 cases with coronavirus disease 2019 in
Wuhan, China. Clin Infect Dis. 71:769–777. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pécheur EI, Borisevich V, Halfmann P,
Morrey JD, Smee DF, Prichard M, Mire CE, Kawaoka Y, Geisbert TW and
Polyak SJ: The synthetic antiviral drug arbidol inhibits globally
prevalent pathogenic viruses. J Virol. 90:3086–3092. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hulseberg CE, Fénéant L, Szymańska-de Wijs
KM, Kessler Np, Nelson EA, Shoemaker CJ, Schmaljohn CS, Polyak SJ
and White JM: Arbidol and other low-molecular-weight drugs that
inhibit lassa and ebola viruses. J Virol. 93. pp. e02185–18. 2019,
View Article : Google Scholar
|
|
73
|
Xu P, Huang J, Fan Z, Huang W, Qi M, Lin
X, Song W and Yi L: Arbidol/IFN-α2b therapy for patients with
corona virus disease 2019: A retrospective multicenter cohort
study. Microbes Infect. 22:200–205. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Vincent MJ, Bergeron E, Benjannet S,
Erickson BR, Rollin PE, Ksiazek TG, Seidah NG and Nichol ST:
Chloroquine is a potent inhibitor of SARS coronavirus infection and
spread. Virol J. 2:692005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu
P, Liu X, Zhao L, Dong E, Song C, et al: In vitro antiviral
activity and projection of optimized dosing design of
hydroxychloroquine for the treatment of severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 71:732–739.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Multicenter collaboration group of
Department of Science and Technology of Guangdong Province and
Health Commission of Guangdong Province for chloroquine in the
treatment of novel coronavirus pneumonia: Expert consensus on
chloroquine phosphate for the treatment of novel coronavirus
pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 43:185–188. 2020.In
Chinese.
|
|
77
|
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu
M, Shi Z, Hu Z, Zhong W and Xiao G: Remdesivir and chloroquine
effectively inhibit the recently emerged novel coronavirus
(2019-nCoV) in vitro. Cell Res. 30:269–271. 2020. View Article : Google Scholar :
|
|
78
|
Gautret P, Lagier JC, Parola P, Hoang VT,
Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE,
et al: Hydroxychloroquine and azithromycin as a treatment of
COVID-19: Results of an open-label non-randomized clinical trial.
Int J Antimicrob Agents. 56:1059492020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Looareesuwan S, White NJ, Chanthavanich P,
Edwards G, Nicholl DD, Bunch C and Warrell DA: Cardiovascular
toxicity and distribution kinetics of intravenous chloroquine. Br J
Clin Pharmacol. 22:31–36. 1986. View Article : Google Scholar
|
|
80
|
Mackenzie AH: Dose refinements in
long-term therapy of rheumatoid arthritis with antimalarials. Am J
Med. 75:40–45. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang H, Penninger JM, Li Y, Zhong N and
Slutsky AS: Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2
receptor: Molecular mechanisms and potential therapeutic target.
Intensive Care Med. 46:586–590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cortegiani A, Ingoglia G, Ippolito M,
Giarratano A and Einav S: A systematic review on the efficacy and
safety of chloroquine for the treatment of COVID-19. J Crit Care.
57:279–283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Haschke M, Schuster M, Poglitsch M,
Loibner H, Salzberg M, Bruggisser M, Penninger J and Krähenbühl S:
Pharmacokinetics and pharmacodynamics of recombinant human
angiotensin-converting enzyme 2 in healthy human subjects. Clin
Pharmacokinet 5. 2:783–792. 2013. View Article : Google Scholar
|
|
84
|
Khan A, Benthin C, Zeno B, Albertson TE,
Boyd J, Christie JD, Hall R, Poirier G, Ronco JJ, Tidswell M, et
al: A pilot clinical trial of recombinant human
angiotensin-converting enzyme 2 in acute respiratory distress
syndrome. Crit Care. 21:2342017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Monteil V, Kwon H, Prado P, Hagelkrüys A,
Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del pozo C,
Prosper F, et al: Inhibition of SARS-CoV-2 infections in engineered
human tissues using clinical-grade soluble human ACE2. Cell.
181:905–913.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Patel VB, Clarke N, Wang Z, Fan D,
Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ and Oudit GY:
Angiotensin II induced proteolytic cleavage of myocardial ACE2 is
mediated by TACE/ADAM-17: A positive feedback mechanism in the RAS.
J Mol Cell Cardiol. 66:167–176. 2014. View Article : Google Scholar
|
|
87
|
Agata J, Ura N, Yoshida H, Shinshi Y,
Sasaki H, Hyakkoku M, Taniguchi S and Shimamoto K: Olmesartan is an
angiotensin II receptor blocker with an inhibitory effect on
angiotensin-converting enzyme. Hypertens Res. 29:865–874. 2006.
View Article : Google Scholar
|
|
88
|
Igase M, Strawn WB, Gallagher PE, Geary RL
and Ferrario CM: Angiotensin II AT1 receptors regulate ACE2 and
angiotensin-(1-7) expression in the aorta of spontaneously
hypertensive rats. Am J physiol Heart Circ physiol.
289:H1013–H1019. 2005. View Article : Google Scholar
|
|
89
|
Ferrario CM, Jessup J, Chappell MC,
Averill DB, Brosnihan KB, Tallant EA, Diz DI and Gallagher PE:
Effect of angiotensin-converting enzyme inhibition and angiotensin
II receptor blockers on cardiac angiotensin-converting enzyme 2.
Circulation. 111:2605–2610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sukumaran V, Tsuchimochi H, Tatsumi E,
Shirai M and Pearson JT: Azilsartan ameliorates diabetic
cardiomyopathy in young db/db mice through the modulation of
ACE-2/ANG 1-7/Mas receptor cascade. Biochem Pharmacol. 144:90–99.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sukumaran V, Veeraveedu PT, Lakshmanan AP,
Gurusamy N, Yamaguchi K, Ma M, Suzuki K, Kodama M and Watanabe K:
Olmesartan medoxomil treatment potently improves cardiac
myosin-induced dilated cardiomyopathy via the modulation of ACE-2
and ANG 1-7 mas receptor. Free Radic Res. 46:850–860. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kocks MJ, Lely AT, Boomsma F, de Jong PE
and Navis G: Sodium status and angiotensin-converting enzyme
inhibition: Effects on plasma angiotensin-(1-7) in healthy man. J
Hypertens. 23:597–602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ishiyama Y, Gallagher PE, Averill DB,
Tallant EA, Brosnihan KB and Ferrario CM: Upregulation of
angiotensin-converting enzyme 2 after myocardial infarction by
blockade of angiotensin II receptors. Hypertension. 43:970–976.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tikoo K, Patel G, Kumar S, Karpe PA,
Sanghavi M, Malek V and Srinivasan K: Tissue specific up regulation
of ACE2 in rabbit model of atherosclerosis by atorvastatin: Role of
epigenetic histone modifications. Biochem pharmacol. 93:343–351.
2015. View Article : Google Scholar
|
|
95
|
Shin YH, Min JJ, Lee JH, Kim EH, Kim GE,
Kim MH, Lee JJ and Ahn HJ: The effect of fluvastatin on cardiac
fibrosis and angiotensin-converting enzyme-2 expression in
glucose-controlled diabetic rat hearts. Heart Vessels. 32:618–627.
2017. View Article : Google Scholar
|
|
96
|
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan
B, Yang P, Sarao R, Wada T, Leong-poi H, et al:
Angiotensin-converting enzyme 2 protects from severe acute lung
failure. Nature. 436:112–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kuba K, Imai Y, Rao S, Jiang C and
Penninger JM: Lessons from SARS: Control of acute lung failure by
the SARS receptor ACE2. J Mol Med (Berl). 84:814–820. 2006.
View Article : Google Scholar
|
|
98
|
Huentelman MJ, Zubcevic J, Hernández prada
JA, Xiao X, Dimitrov DS, Raizada MK and Ostrov DA: Structure-based
discovery of a novel angiotensin-converting enzyme 2 inhibitor.
Hypertension. 44:903–906. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lo CS, Liu F, Shi Y, Maachi H, Chenier I,
Godin N, Filep JG, Ingelfinger JR, Zhang SL and Chan JS: Dual RAS
blockade normalizes angiotensin-converting enzyme-2 expression and
prevents hypertension and tubular apoptosis in Akita
angiotensinogen-transgenic mice. Am J Physiol Renal Physiol.
302:F840–F852. 2012. View Article : Google Scholar :
|
|
100
|
Lusvarghi S and Bewley CA: Griffithsin: An
antiviral lectin with outstanding therapeutic potential. Viruses.
8:2962016. View Article : Google Scholar :
|
|
101
|
O'Keefe BR, Giomarelli B, Barnard DL,
Shenoy SR, Chan PK, McMahon JB, Palmer KE, Barnett BW, Meyerholz
DK, Wohlford-Lenane CL and McCray PB Jr: Broad-spectrum in vitro
activity and in vivo efficacy of the antiviral protein griffithsin
against emerging viruses of the family Coronaviridae. J Virol.
84:2511–2521. 2010. View Article : Google Scholar :
|
|
102
|
Mori T, O'Keefe BR, Sowder RC II, Bringans
S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW Jr,
McMahon JB and Boyd MR: Isolation and characterization of
griffithsin, a novel HIV-inactivating protein, from the red alga
Griffithsia sp. J Biol Chem. 280:9345–9353. 2005. View Article : Google Scholar
|
|
103
|
Hu H, Li L, Kao RY, Kou B, Wang Z, Zhang
L, Zhang H, Hao Z, Tsui WH, Ni A, et al: Screening and
identification of linear B-cell epitopes and entry-blocking peptide
of severe acute respiratory syndrome (SARS)-associated coronavirus
using synthetic overlapping peptide library. J Comb Chem.
7:648–656. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Han Dp, Penn-Nicholson A and Cho MW:
Identification of critical determinants on ACE2 for SARS-CoV entry
and development of a potent entry inhibitor. Virology. 350:15–25.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zost SJ, Gilchuk P, Case JB, Binshtein E,
Chen RE, Nkolola JP, Schäfer A, Reidy JX, Trivette A, Nargi RS, et
al: Potently neutralizing and protective human antibodies against
SARS-CoV-2. Nature. 584:443–449. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Huo J, Le Bas A, Ruza RR, Duyvesteyn HME,
Mikolajek H, Malinauskas T, Tan TK, Rijal P, Dumoux M, Ward PN, et
al: Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block
interaction with ACE2. Nat Struct Mol Biol. 27:846–854. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pinheiro SV, Simões ESA, Sampaio WO, de
Paula RD, Mendes EP, Bontempo ED, Pesquero JB, Walther T, Alenina
N, Bader M, et al: Nonpeptide AVE 0991 is an angiotensin-(1-7)
receptor Mas agonist in the mouse kidney. Hypertension. 44:490–496.
2004. View Article : Google Scholar : PubMed/NCBI
|