Introduction
Inflammatory cytokine-induced chronic inflammation
is a high risk factor for the development of numerous malignancies
(1,2). One of the most important
inflammatory cytokines is tumor necrosis factor-α (TNF-α). TNF-α
can induce cancer malignancy as well as induce apoptosis of cancer
cells (3). To promote apoptosis,
TNF-α receptor 1 (TNFR1) serves a crucial role (4). TNFR1 contains a death domain in the
membrane region and once TNF-α binds to TNFR1, the Fas-associated
death domain adaptor protein and caspase-8 associate with
trimerized TNFR1 to direct activation of caspase-3 (5). Subsequent cleavage of
poly-(ADP-ribose)-polymerase (PARP) finally results in apoptosis
(6).
TNF-α induction of cancer malignancy often depends
on nuclear factor-κB (NF-κB) (7). Transforming growth factor-β
activated kinase (TAK1) phosphorylates downstream IκB kinase-α/β
(IKK-α/β) (8). Subsequently,
IKKs phosphorylate IκBα at Ser-32/36 to promote IκBα degradation by
the ubiquitin-proteasome pathway to release NF-κB from IκBα
(9). At the same time, they
activate NF-κB p65 by phosphorylation at Ser-536 to induce NF-κB
transcriptional activity (10).
NF-κB p65 regulates inflammatory factors as well as anti-apoptotic
genes (7,11,12). Therefore, the suppression of
NF-κB activation may be an effective strategy to inhibit cancer
malignancy.
Epidermal growth factor receptor (EGFR), a receptor
tyrosine kinase, is also involved in TNF-α-induced anti-apoptotic
signaling (13). TAK1 induces
p38 MAPK activation, leading to EGFR phosphorylation at Ser-1046/7
to block apoptosis (14).
Therefore, TNF-α acts as a double-edged sword in the tumor
microenvironment (15).
Helichrysetin, 2′,4,4′-trihydroxy-6′-methoxy
chalcone, is commonly found in the Alpinia species (16). The structure of helichrysetin is
shown in Fig. 1A. Helichrysetin
has anti-inflammatory (17),
apoptosis-inducing (18,19), anti-platelet aggregation
(20) and antioxidant (21) effects. Regarding inflammatory
signaling, helichrysetin decreases the transcriptional activity of
NF-κB by inhibiting NF-κB nuclear translocation in mouse pancreatic
β MIN-6 cells (17).
Additionally, helichrysetin inhibits the cell viability in
pancreatic cancer, fibrosarcoma (22), cervical adenocarcinoma (19,21), liver cancer, breast cancer
(16), colon cancer (23) and lung cancer (18) cell lines. To the best of our
knowledge, no molecular studies of helichrysetin have been
performed. Therefore, the present study aimed to identify the
effect of helichrysetin on activation of the NF-κB and EGFR
signaling pathways induced by TNF-α, and the synergistic effect of
helichrysetin and TNF-α on the apoptosis of HeLa and T98G
cells.
Materials and methods
Reagents
Helichrysetin was supplied by Professor Jingshan
Shen (Shanghai Institute of Material Medica, Chinese Academy of
Sciences, Shanghai, China), and high-performance liquid
chromatography analysis was performed as previously described
(16) to confirm that it had a
purity of >95%. Recombinant human TNF-α (cat. no. 210-TA) was
purchased from R&D Systems, Inc. Primary antibodies against
caspase-3 (cat. no. 9665s), PARP (cat. no. 9532s), TAK1 (cat. no.
5206s), pTAK1 (cat. no. 4536s), TAK1 binding protein 1 (TAB1) (cat.
no. 3226s), TAB2 (cat. no. 3745s), IKKβ (cat. no. 8943s),
phosphorylated (p)IKKα/β (cat. no. 2697s), EGFR (cat. no. 4267s),
pEGFR-S1046/7 (cat. no. 2238s), pNF-κB p65-S536 (cat. no. 3033s)
and β-actin (cat. no. 4970s) were purchased from Cell Signaling
Technology, Inc. Primary antibodies against IKKα (cat. no. c0514)
and NF-κB p65 (cat. no. k0515) were obtained from Santa Cruz
Biotechnology, Inc. Cell Counting Kit-8 (CCK-8) and Annexin V-FITC
Apoptosis Detection kit (cat. no. AD10) were purchased from Dojindo
Molecular Technologies, Inc. The Hoechst 33258 staining kit (cat.
no. MA0160) was purchased from Beyotime Institute of
Biotechnology.
Cell culture
Human cervical cancer (HeLa) and human glioma (T98G)
cells were purchased from The Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences and cultured in DMEM (high
glucose) containing 10% FBS, 100 U/ml penicillin and 100
μg/ml streptomycin (all Gibco; Thermo Fisher Scientific,
Inc.), in a humidified 5% CO2 atmosphere at 37°C.
Luciferase assay
HeLa cells were transfected with a luciferase
reporter plasmid p65 NF-κB under the control of 4× κB sites; the
luciferase reporter plasmid was provided by Professor Hiroaki
Sakurai (University of Toyama, Toyama, Japan) and also contained a
neo resistance gene. A stable clone (HeLa-κB) was isolated
in medium containing 500 μg/ml G418. Cells
(1.6×104) were seeded in a 96-well plate at 37°C for 48
h. After pretreatment with 50 μM helichrysetin at 37°C for
30 min, cells were stimulated with TNF-α (20 ng/ml) at 37°C for
another 6 h. Luciferase activity was detected using the ONE-Glo™
Luciferase Assay System (Promega Corporation) and measured using a
microplate reader.
CCK-8 assay
HeLa cells were seeded at 1.6×104
cells/well, and T98G cells were seeded at 2×104
cells/well in 96-well plates and cultured overnight to adhere at
37°C. After pretreatment with helichrysetin at 37°C for 30 min,
cells were stimulated with TNF-α (20 ng/ml) at 37°C for another 24
h. Subsequently, cells were incubated with CCK-8 solution (10
μl CCK-8 added to 90 μl medium per well) at 37°C for
1 h. Absorbance was detected at 450 nm on a Varioskan Flash
microplate reader (Thermo Fisher Scientific, Inc.). The cell
viability rate (%) was calculated as follows: (absorbance of
drug-treated sample-blank)/(absorbance of control sample-blank)
×100.
Hoechst 33258 staining
HeLa or T98G cells were seeded in 96-well culture
plates and cultured overnight to adhere at 37°C. After pretreatment
with helichrysetin at 37°C for 30 min, cells were stimulated with
TNF-α (20 ng/ml) for 24 h at 37°C. Subsequently, cells were fixed
with 4% paraformaldehyde at room temperature for 10 min and washed
with PBS. Cells were incubated with 50 μl Hoechst 33258
staining solution at room temperature for 5 min and then washed
twice with PBS. Cell morphology was observed and captured under a
fluorescence microscope (magnification, ×200).
Annexin V/PI staining
An Annexin V-FITC Apoptosis Detection kit was used
for apoptosis assays. HeLa cells were plated at 3.2×105
cells/well and T98G cells were plated at 4×105
cells/well in 35-mm cell culture dishes. Cells were pretreated with
helichrysetin at 37°C for 30 min, followed by stimulation with
TNF-α at 37°C for 24 h. Subsequently, the cells were harvested and
washed with PBS, and the cell number was adjusted to
1×106 cells/well. After collection by centrifugation at
300 × g at 4°C for 5 min, the cells were resuspended in 1× Binding
Buffer, and stained with Annexin V for 15 min and PI for 5 min at
room temperature in the dark. Apoptosis was analyzed on a CytoFlex
S flow cytometer (Beckman Coulter, Inc.) using the CytExpert v2.3
software (Beckman Coulter, Inc.).
Western blot analysis
HeLa and T98G cells were cultured in 35-mm dishes at
37°C overnight, then incubated with fresh culture medium containing
0.5% FBS at 37°C for another 24 h. Cells were then pretreated with
helichrysetin at 37°C for 30 min, followed by stimulation with
TNF-α at 37°C for 5 min, 10 min, 6 h or 12 h. Cells were harvested
and lysed in CelLytic™ MT Cell Lysis Reagent (cat. no. C3228;
Sigma-Aldrich; Merck KGaA) containing protease and phosphatase
inhibitors (cat. nos. 04693116001 and 04906837001; Roche
Diagnostics). The protein concentration was determined by BCA
assay. A total of 20 μg protein from each sample was
separated by standard SDS-PAGE (7.5, 10 or 12.5%) and transferred
to Immobilon-P membranes (EMD Millipore) by semi-dry transfer. The
membranes were incubated with SuperBlock™ (PBS) Blocking Buffer
(Thermo Fisher Scientific, Inc.) at room temperature for 2 h, and
then rinsed twice with 1× PBS-Tween (PBST) containing 1% Tween-20.
Membranes were incubated with primary antibodies (1:1,000) against
caspase-3, PARP, TAK1, pTAK1, TAB1, TAB2, pIKKα/β, EGFR,
pEGFR-s1046/7, pNF-κB p65-s536, β-actin, IKKα, IKKβ and NF-κB p65
overnight at 4°C. After washing twice with PBST, membranes were
incubated with HRP-conjugated anti-rabbit secondary antibody
(1:5,000; cat. no. 122107; Jackson ImmunoResearch Laboratories,
Inc.) for 1 h at room temperature. The target protein bands were
visualized with Immobilon Western Chemiluminescent HRP Substrate
(EMD Millipore) using a Tanon-5200 chemiluminescent imaging system
(cat. no. 20182351; Tanon Science and Technology Co., Ltd.). For
quantification, target proteins were normalized to β-actin within
the same sample using ImageJ v1.52a (National Institutes of
Health).
Reverse transcription-quantitative PCR
assay
HeLa and T98G cells cultured in 24-well plates were
pretreated with helichrysetin at 37°C for 30 min and then
stimulated with TNF-α at 37°C for 4 h. Total RNA was isolated from
the harvested cells using an RNA Faster 2000 kit (cat. no. 220011;
Fastagen) according to the manufacturer's protocol. cDNA was
reverse transcribed from RNA (1 μg) using PrimeScript™ RT
Master Mix (Perfect Real Time) (cat. no. RRD36A; Takara Bio, Inc.)
according to the manufacturer's protocol. Quantitative PCR was
performed using TB Green® Premix Ex Taq™ II (Tli RNaseH
Plus), ROX plus (Takara Bio, Inc.) on a Quant Studio 6 Flex System
(Thermo Fisher Scientific, Inc.) under the following conditions:
95°C for 30 sec; 40 cycles at 95°C for 5 sec and 60°C for 30 sec;
95°C for 15 sec; 60°C for 1 min; and 95°C for 15 sec.
Quantification of target genes was determined using the
2−ΔΔCq method (24).
The relative expression of individual target genes was normalized
to that of GAPDH in the same sample. The sequences of the primers
(Generay Biotech Co., Ltd.) used are listed in Table I.
 | Table IQuantitative PCR primers. |
Table I
Quantitative PCR primers.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| GAPDH |
GGGAAGGTGAAGGTCGGAGT |
GGGGTCATTGATGGCAACA |
| TNF-α |
GCTGCACTTTGGAGTGATCG C |
TTGTCACTCGGGGTTCGAG |
| IL-1β |
GGGACTGATGCTGGTGACAA |
ACAGGTCTGTTGGGAGTGGT |
| CCL2 |
AATCAATGCCCCAGTCACCT |
GGGTCAGCACAGATCTCCTT |
| CCL5 |
TGTGTGCCAACCCAGAGAAG |
GAAGCCTCCCAAGCTAGGAC |
| CXCL10 |
GCTGCCTTATCTTTCTG |
CTCTTCTCACCCTTCTT |
Thermal shift assay
HeLa and T98G cells were cultured in 100-mm dishes
at 37°C overnight and then incubated with fresh culture medium
containing 0.5% FBS at 37°C for another 24 h. After treatment with
helichrysetin at 37°C for 30 min, cells were collected and washed
with PBS, then resuspended in PBS with protease inhibitors. The
cell suspension was evenly distributed into PCR tubes and heated at
4, 40, 43, 46, 49 and 52°C for 3 min, and then cooled for another 3
min at room temperature. Subsequently, cells were lysed by rapid
freeze-thawing. The lysates were centrifuged at 20,000 × g for 20
min at 4°C. Soluble fractions were transferred to new tubes and
samples were prepared for western blot analysis as
aforementioned.
Statistical analysis
All data are presented as the mean ± SD. Differences
between 2 groups were analyzed via Student's unpaired t-test, and
differences among ≥3 groups were analyzed via one-way ANOVA with
Tukey's post-hoc test using GraphPad 7.0 software (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
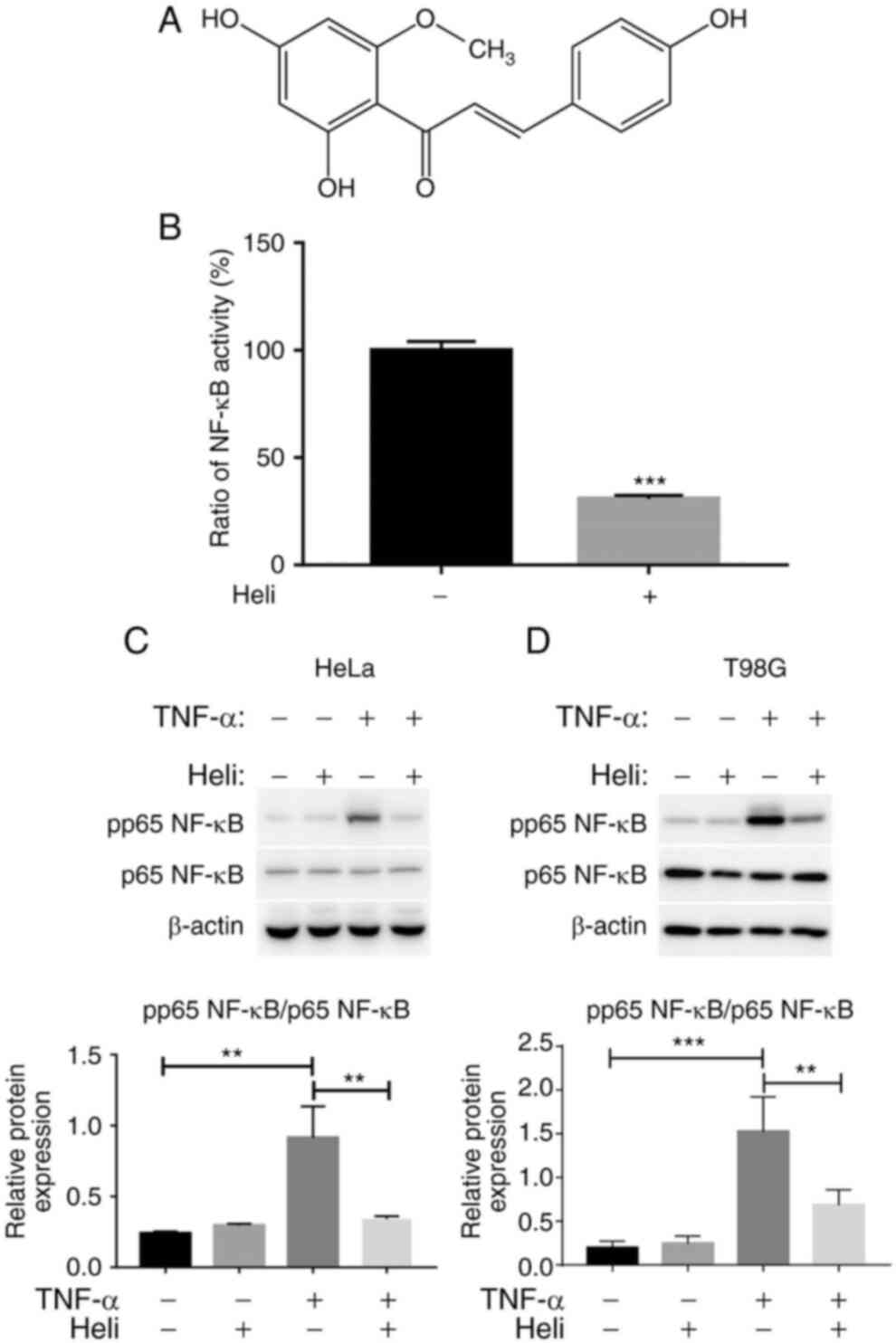
Helichrysetin inhibits the activation of
NF-κB
Helichrysetin inhibits NF-κB nuclear translocation
in mouse pancreatic β cells (17). However, the effect of
helichrysetin on NF-κB activation in cancer cells has not been
clarified. Therefore, the present study measured the effect of
helichrysetin on the transcriptional activity of NF-κB and the
phosphorylation of NF-κB in human cancer cells. HeLa-κB cells were
firstly used to measure the inhibitory effect of helichrysetin on
the transcriptional activity of NF-κB induced by TNF-α. HeLa-κB
cells were pretreated with 50 μM helichrysetin for 30 min
and then stimulated with TNF-α for 6 h. Helichrysetin significantly
inhibited the transcriptional activity of NF-κB induced by TNF-α
(Fig. 1B). Phosphorylation of
NF-κB at Ser-536 is crucial for its transcriptional activity. Thus,
the phosphorylation of NF-κB p65 at Ser-536 was analyzed. In order
to detect the effect of helichrysetin on the NF-κB and EGFR
signaling pathways, T98G and HeLa cells were used for further
experiments, since in the present study, both HeLa and T98G cells
had a good response upon TNF-α stimulation, which strongly induces
NF-κB activation, and both of them express wild-type EGFR. As
expected, although helichrysetin exhibited no effect on the protein
levels of p65 NF-κB, it inhibited the phosphorylation of p65 NF-κB
induced by TNF-α stimulation in both HeLa and T98G cells (Fig. 1C and D). Overall, helichrysetin
inhibited NF-κB activation in HeLa and T98G cells.
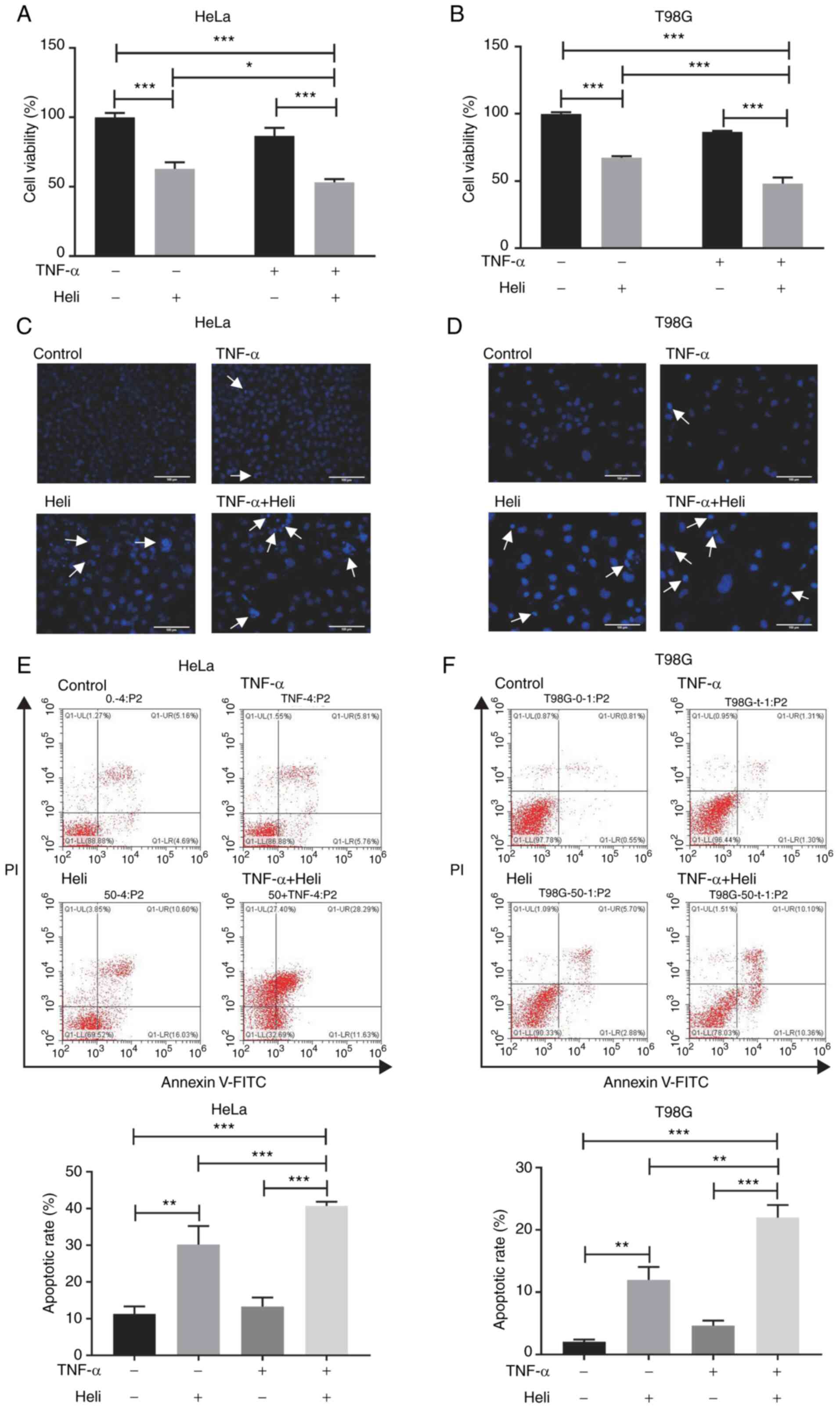
Helichrysetin and TNF-α synergistically
promote apoptosis of HeLa and T98G cells
To mimic the tumor microenvironment, HeLa and T98G
cells were treated with TNF-α and the synergistic effect of
helichrysetin and TNF-α on the apoptosis of cancer cells was
measured. Cells were pretreated with 50 μM helichrysetin for
30 min and then stimulated with TNF-α for 24 h. The results
revealed that helichrysetin, but not TNF-α, had an inhibitory
effect on cell viability in both cell lines; additionally, the
combination of helichrysetin and TNF-α synergistically decreased
cell viability (Fig. 2A and
B).
Consistent with the CCK-8 assay results, Hoechst
33258 staining demonstrated that the combination of helichrysetin
and TNF-α synergistically increased the number of T98G and HeLa
cells with dense stained nuclei, compared with cells treated with
helichrysetin alone (Fig. 2C and
D).
For apoptosis analysis, cells were pretreated with
50 μM helichrysetin for 30 min and then stimulated with
TNF-α for 8 (for HeLa cells) or 24 h (for T98G cells). Annexin V/PI
staining detected by flow cytometry demonstrated that
helichrysetin, but not TNF-α, significantly enhanced apoptosis, and
that the combination of helichrysetin and TNF-α synergistically
increased the ratio of apoptotic cells in both cell lines (Fig. 2E and F).
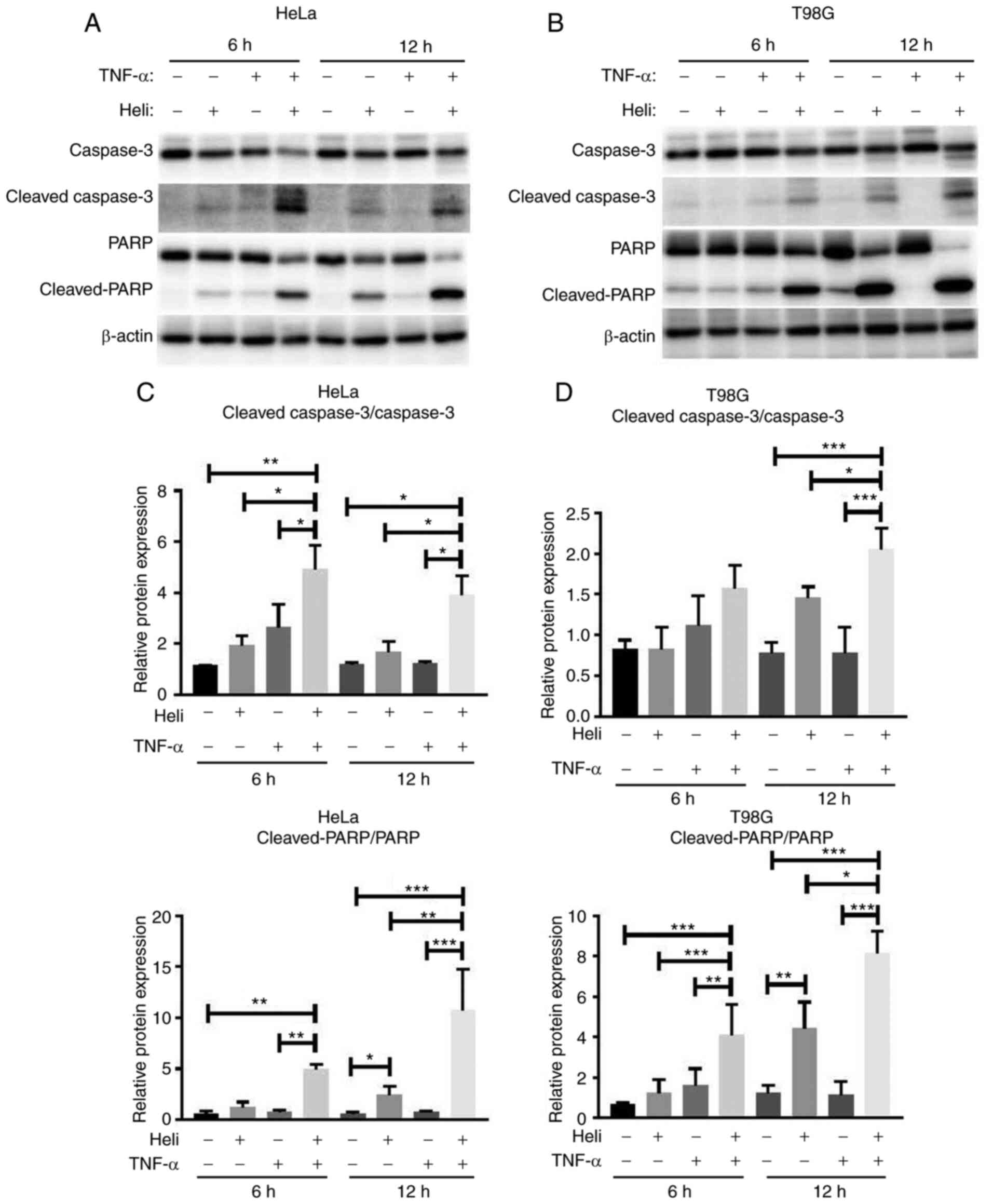
Helichrysetin and TNF-α synergistically
enhance the activity of apoptosis-associated proteins in HeLa and
T98G cells
Next, the activity of apoptosis-associated proteins
was determined. As demonstrated in Fig. 3, HeLa and T98G cells were
pretreated with 50 μM helichrysetin for 30 min and then
stimulated with TNF-α for 6 or 12 h. After stimulation with TNF-α
for 6 h, compared with the control group, helichrysetin, but not
TNF-α, significantly increased the protein expression levels of
cleaved PARP in both cell lines. The combination of helichrysetin
and TNF-α synergistically enhanced this increase. Additionally,
helichrysetin significantly increased the protein expression of
cleaved caspase-3 in HeLa but not T98G cells. After stimulation
with TNF-α for 12 h, helichrysetin, but not TNF-α, increased the
protein expression levels of cleaved PARP, and the combination of
helichrysetin and TNF-α synergistically enhanced the protein levels
of cleaved PARP and cleaved caspase-3 in both cell lines (Fig. 3). Overall, these results
demonstrated that the combination of helichrysetin and TNF-α had a
synergistic promoting effect on apoptosis.
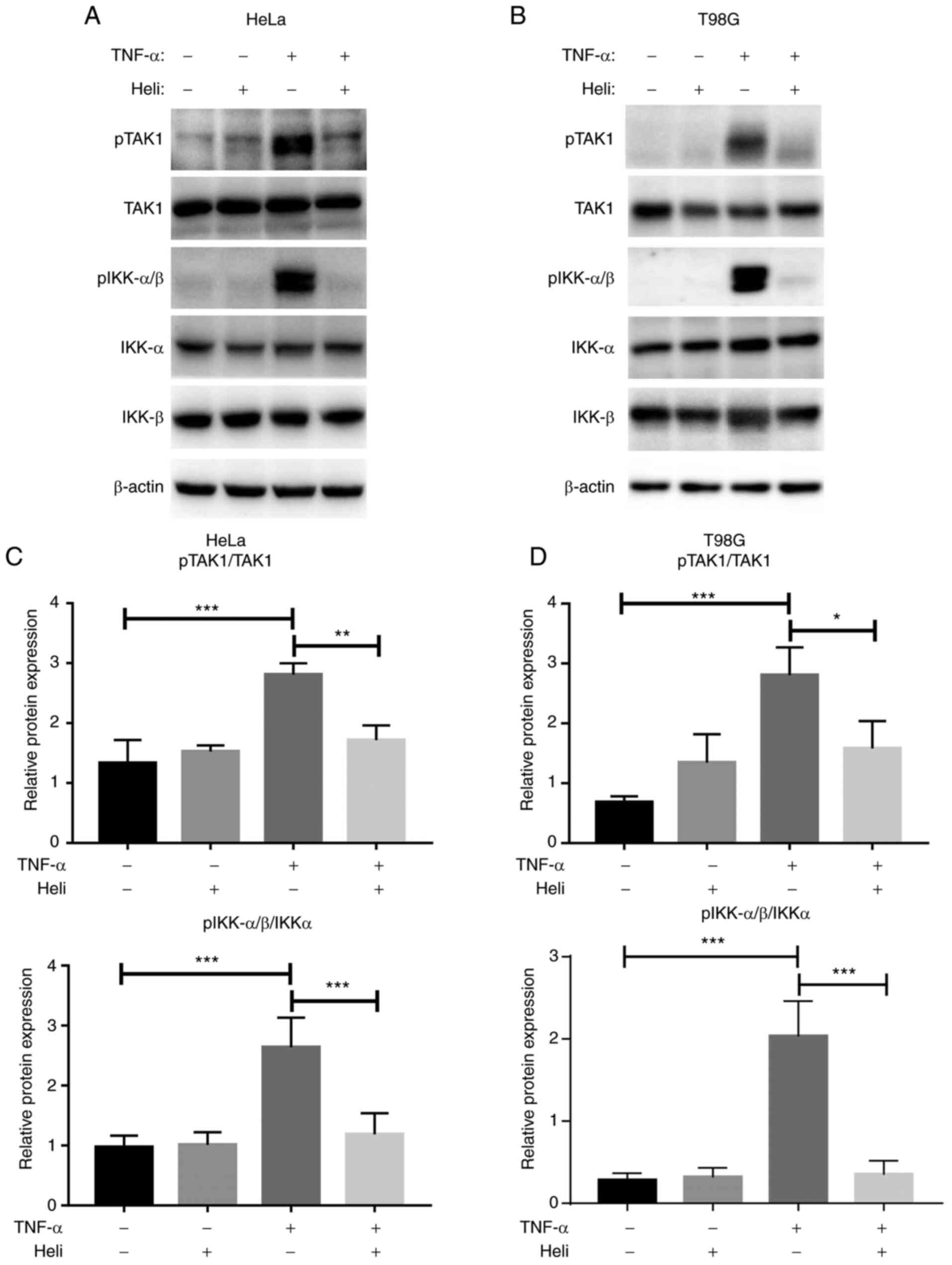
Helichrysetin inhibits TAK1/IKK/NF-kB
signaling induced by TNF-α in HeLa and T98G cells
To elucidate the detailed molecular mechanisms for
the observed effects, the phosphorylation of TAK1 and IKKs was
analyzed. As shown in Fig. 4,
TNF-α stimulation significantly upregulated the phosphorylation of
TAK1 and IKKα/β in both cell lines. Although helichrysetin alone
had no effect on the phosphorylation of these molecules, it
significantly counteracted the phosphorylation induced by TNF-α in
both cell lines (Fig. 4).
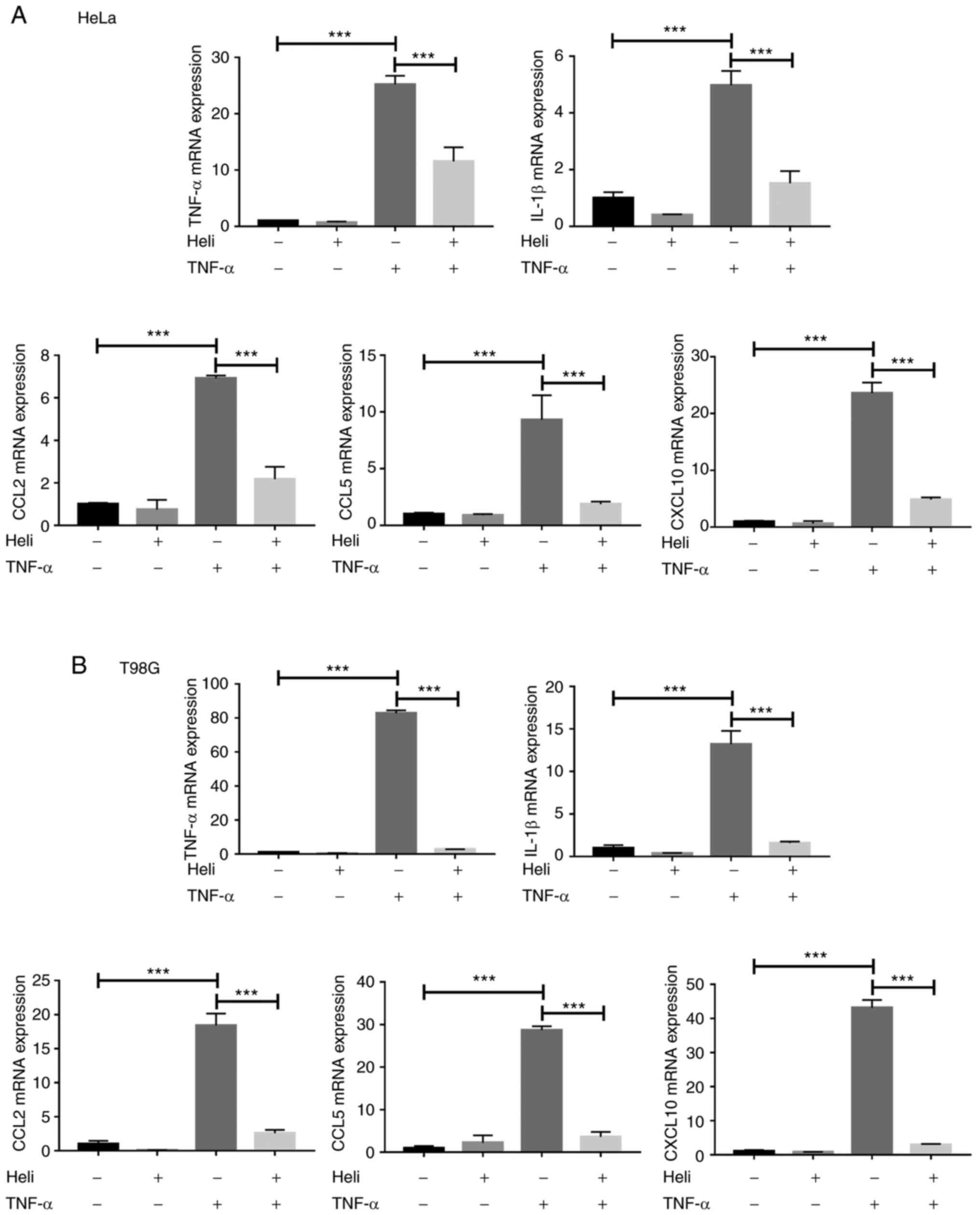
Activation of NF-κB promotes the expression levels
of many proinflammatory factors, such as TNF-α, IL1β, CCL2, CCL5
and CXCL10 (25-27). To further confirm the inhibitory
effect of helichrysetin on NF-κB activation induced by TNF-α, HeLa
and T98G cells were pretreated with 50 μM helichrysetin for
30 min and then stimulated with TNF-α for 4 h. After TNF-α
stimulation, the mRNA expression levels of TNF-α, IL1β, CCL2, CCL5
and CXCL10 were significantly increased in both cell lines; this
was completely reversed by helichrysetin treatment (Fig. 5). Overall, these results
indicated that helichrysetin blocked TAK1/IKK/NF-κB signaling
pathway.
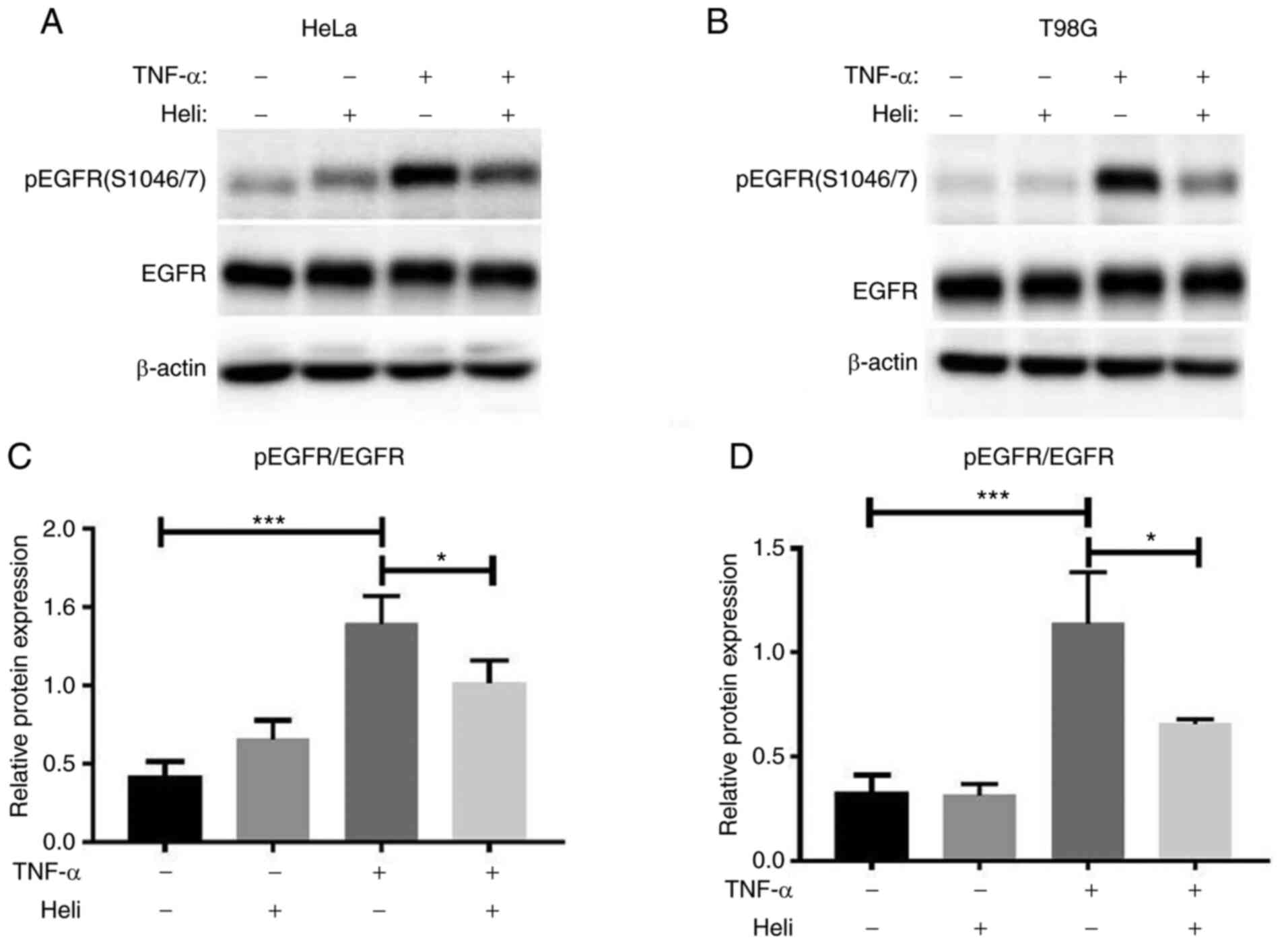
Helichrysetin inhibits the TNF-α-induced
phosphorylation of EGFR at Ser-1046/7
Finally, whether helichrysetin affected EGFR
phosphorylation at Ser-1046/7 was analyzed. As shown in Fig. 6, helichrysetin alone had no
effect on the phosphorylation of EGFR Ser-1046/7, but it
significantly inhibited TNF-α-induced EGFR phosphorylation.
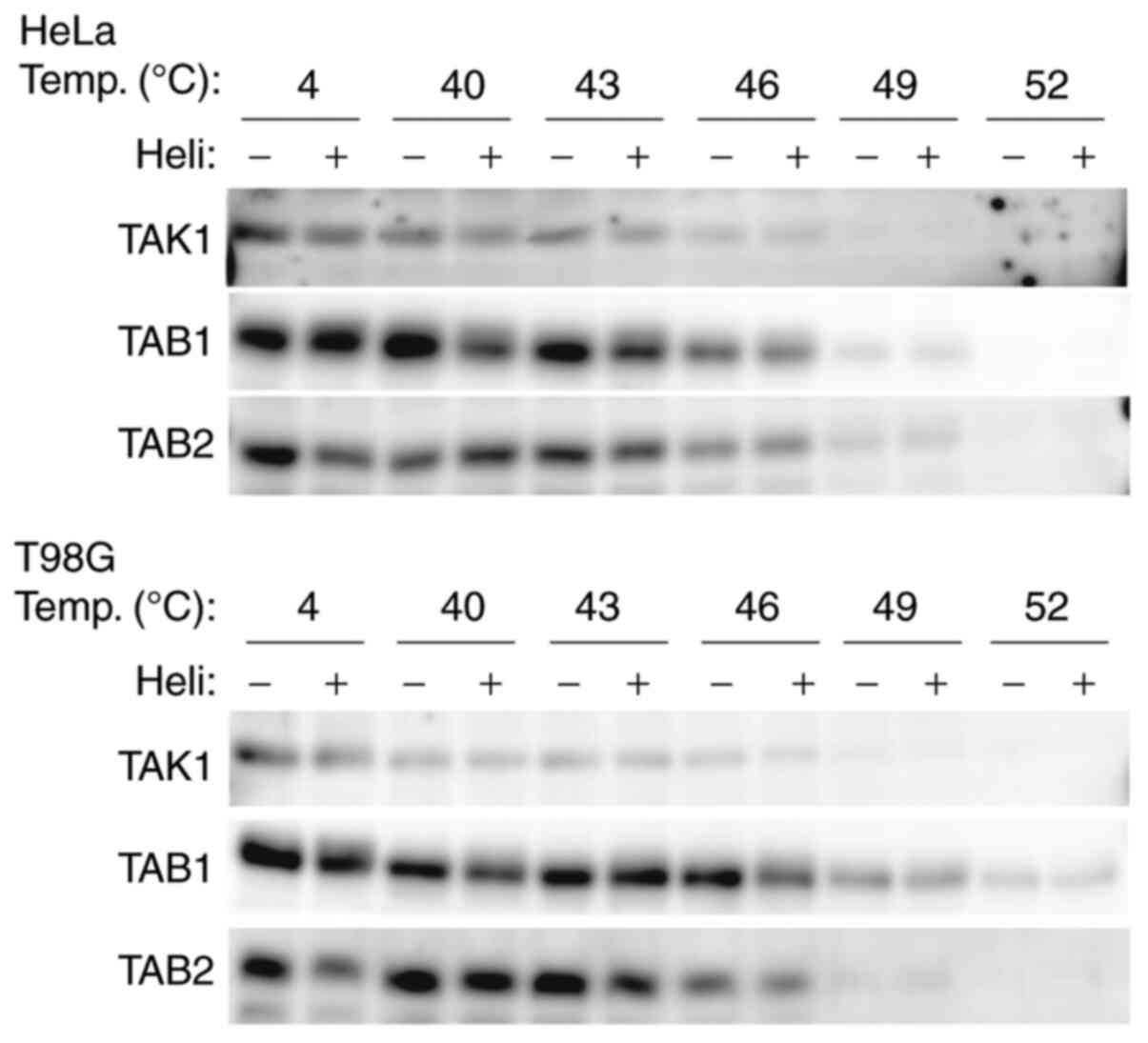
Helichr ysetin does not directly bin d to
the TAK1/TAB1/TAB2 complex
Whether helichrysetin could directly bind to the
TAK1/TAB1/TAB2 complex to inhibit TAK1 activity was further
analyzed using a thermal shift assay. When compound-protein
interactions exist, the stability of the complex will be increased
compared with that of a single protein at certain temperatures. In
other words, the expression of the complex will be higher than that
of a single protein in a thermal shift assay. As shown in Fig. 7, TAK1, TAB1 and TAB2 expression
was not increased in helichrysetin-treated cells compared with that
in helichrysetin-untreated cells. Therefore, it was demonstrated
that helichrysetin did not directly bind to the complex.
Discussion
Pro-inflammatory factors serve an important role in
cancer (28). TNF-α is a
double-edged sword for apoptosis; TNF-α has an anti-apoptotic
effect that depends on NF-κB activation and a pro-apoptotic effect
when the NF-κB signaling pathway is inhibited (29). The imbalance between
proliferation and apoptosis results in cancer growth (30-32). Helichrysetin inhibits NF-κB
activation in mouse pancreatic β cells (17). However, the effect of
helichrysetin on NF-κB activity in cancer cells has not been
previously investigated. Chemotherapeutic drugs fight cancer by
enhancing the apoptosis of cancer cells (33) and some flavonoids induce
apoptosis (34,35). Although helichrysetin has an
antitumor activity in several types of human cancer cells,
including pancreatic cancer, fibrosarcoma (22), cervical adenocarcinoma (19,21), liver cancer, breast cancer
(16), colon cancer (23) and lung cancer (18) cell lines, the detailed molecular
mechanisms for these effects are unclear. Therefore, the present
study aimed to elucidate the molecular targets of helichrysetin. It
was revealed that helichrysetin and TNF-α synergistically enhanced
the apoptosis of cancer cells by inhibiting TAK1 activation. PARP
is the main substrate of cleaved caspase-3, which is a key executor
of apoptosis, and cleaved PARP is an important indicator of
apoptosis (36-38). In the present study, the
combination of helichrysetin and TNF-α synergistically enhanced the
cleavage of caspase-3 and PARP, indicating that helichrysetin and
TNF-α synergistically promoted the apoptosis of cancer cells in a
caspase-3-dependent manner.
To elucidate the detailed molecular mechanism of the
synergistic effect of helichrysetin and TNF-α on the apoptosis of
cancer cells, NF-κB and EGFR phosphorylation was analyzed. TNF-α
induced NF-κB activation by phosphorylating NF-κB p65 at Ser-536
mediated by TAK1. TAK1 also activates the phosphorylation of EGFR
to promote TNF-α-induced anti-apoptotic signaling (14,39). The present findings revealed that
helichrysetin inhibited TNF-α-promoted NF-κB activation by blocking
the phosphorylation of TAK1, IKKα/β and NF-κB p65, resulting in
attenuated expression levels of NF-κB targeted genes. This
indicated that helichrysetin and TNF-α synergistically enhanced
apoptosis by repressing TAK1-mediated NF-κB activation.
Furthermore, the present results demonstrated that helichrysetin
did not directly bind to the TAK1/TAB1/TAB2 complex. Therefore,
helichrysetin may affect other molecules that lead to TAK1
inactivation. When TNF-α binds to TNFR1, numerous adaptor molecules
associate with TNFR1 to activate downstream molecules, including
TAK1 (1,40). The hypothesis of the present
study is that helichrysetin may bind to TNFR1 itself or its adaptor
molecules to inhibit TAK1 activation. The detailed mechanism by
which helichrysetin blocks the activation of TAK1 requires further
study.
Depending on its tyrosine phosphorylation, EGFR
participates in the regulation of genes that regulate cell
proliferation, survival, differentiation, autophagy and metabolism
(41-43). Additionally, TNF-α controls
TAK1-dependent phosphorylation of EGFR at Ser-1046/7, which blocks
TNF-α-induced apoptosis (14).
The current study demonstrated that helichrysetin inhibited the
phosphorylation of EGFR Ser-1046/7 in HeLa and T98G cells. Hence,
helichrysetin and TNF-α may synergistically promote apoptosis by
blocking the phosphorylation of EGFR. Similar results were obtained
in both HeLa and T98G cells. Therefore, the present findings may be
adapted to the cancer cell lines that express wild-type EGFR and
have a good response upon TNF-α stimulation.
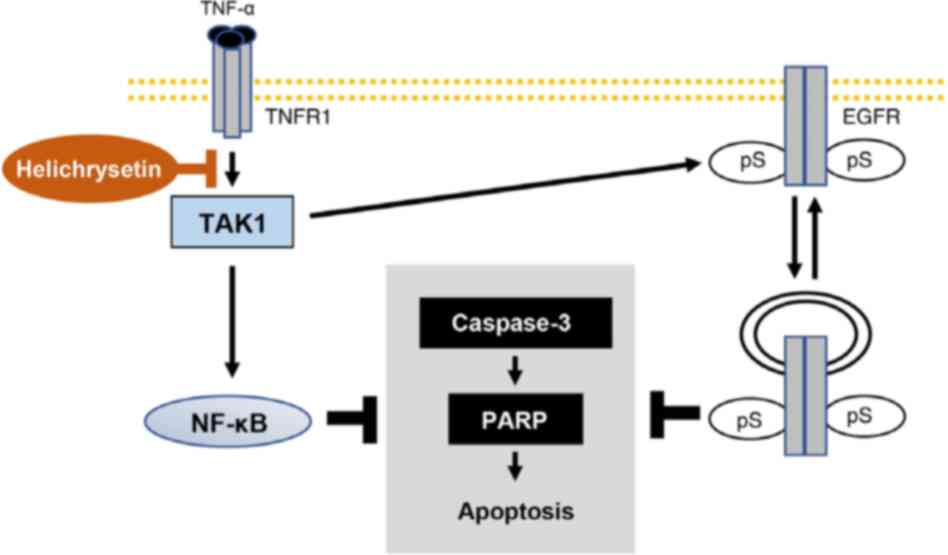
Overall, as shown in Fig. 8, helichrysetin and TNF-α may
synergistically promote the apoptosis of cancer cells by inhibiting
TNF-α-induced TAK1/IKK/NF-κB and TAK1/EGFR signaling pathways in
HeLa and T98G cells. This may indicate a potential therapeutic
strategy for human cervical cancer and glioblastoma.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81603156), the Educational
Commission of Shanghai of China (grant no. 2020LK014), the Young
Eastern Scholar Program (grant no. QD2016038), the Chenguang
Program (grant no. 16CG49) and the Graduate Student Innovation
Ability Project of Shanghai University of Traditional Chinese
Medicine (grant no. Y2020030).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZhiW and XL performed most of the experiments, and
wrote the original draft. WL and LD performed flow cytometry and
analyzed the data. AX and LY contributed to data analysis and
interpretation. XW and ZheW contributed to the conception and
design of the study. YZ and HS designed the experiments, wrote and
revised the manuscript. ZhiW, HS and YZ confirmed the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Sakurai H: Targeting of TAK1 in
inflammatory disorders and cancer. Trends Pharmacol Sci.
33:522–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crusz SM and Balkwill FR: Inflammation and
cancer: Advances and new agents. Nat Rev Clin Oncol. 12:584–596.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wajant H, Pfizenmaier K and Scheurich P:
Tumor necrosis factor signaling. Cell Death Differ. 10:45–65. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bodmer JL, Holler N, Reynard S,
Vinciguerra P, Schneider P, Juo P, Blenis J and Tschopp J: TRAIL
receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell
Biol. 2:241–243. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morris G, Walker AJ, Berk M, Maes M and
Puri BK: Cell death pathways: A novel therapeutic approach for
neuroscientists. Mol Neurobiol. 55:5767–5786. 2018. View Article : Google Scholar :
|
|
7
|
Varfolomeev E and Vucic D: Intracellular
regulation of TNF activity in health and disease. Cytokine.
101:26–32. 2018. View Article : Google Scholar
|
|
8
|
Camara-Clayette V, Lecluse Y, Schrader C,
Klapper W, Vainchenker W, Hermine O and Ribrag V: The NF-κB pathway
is rarely spontaneously activated in mantle cell lymphoma (MCL)
cell lines and patient's samples. Eur J Cancer. 50:159–169. 2014.
View Article : Google Scholar
|
|
9
|
He A, Ji R, Shao J, He C, Jin M and Xu Y:
TLR4-MyD88= TRAF6-TAK1 complex-mediated NF-κB activation contribute
to the anti-inflammatory effect of V8 in LPS-induced human cervical
cancer SiHa cells. Inflammation. 39:172–181. 2016. View Article : Google Scholar
|
|
10
|
Nighot M, Rawat M, Al-Sadi R, Castillo EF,
Nighot P and Ma TY: Lipopolysaccharide-Induced increase in
intestinal permeability is mediated by TAK-1 activation of IKK and
MLCK/MYLK gene. Am J Pathol. 189:797–812. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Capece D, Verzella D, Tessitore A, Alesse
E, Capalbo C and Zazzeroni F: Cancer secretome and inflammation:
The bright and the dark sides of NF-κB. Semin Cell Dev Biol.
78:51–61. 2018. View Article : Google Scholar
|
|
13
|
Morandell S, Stasyk T, Skvortsov S, Ascher
S and Huber LA: Quantitative proteomics and phosphoproteomics
reveal novel insights into complexity and dynamics of the EGFR
signaling network. Proteomics. 8:4383–4401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishimura M, Shin MS, Singhirunnusorn P,
Suzuki S, Kawanishi M, Koizumi K, Saiki I and Sakurai H:
TAK1-Mediated serine/threonine phosphorylation of epidermal growth
factor receptor via p38/extracellular signal-regulated kinase:
NF-{kappa}B-independent survival pathways in tumor necrosis factor
alpha signaling. Mol Cell Biol. 29:5529–5539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shin MS, Shinghirunnusorn P, Sugishima Y,
Nishimura M, Suzuki S, Koizumi K, Saiki I and Sakurai H: Cross
interference with TNF-alpha-induced TAK1 activation via
EGFR-mediated p38 phosphorylation of TAK1-binding protein 1.
Biochim Biophys Acta. 1793:1156–1164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao C, Han Q, Song J, Wang Z, Xu L and Xu
H: Analysis of eight bioactive compounds in alpinia species by
HPLC-DAD. Nat Prod Res Dev. 20:422–426. 2008.
|
|
17
|
Jaidee W, Andersen RJ, Chavez MAG, Wang
YA, Patrick BO, Pyne SG, Muanprasat C, Borwornpinyo S and
Laphookhieo S: Amides and flavonoids from the fruit and leaf
extracts of melodorum siamensis. J Nat Prod. 82:283–292. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ho YF, Karsani SA, Yong WK and Abd Malek
SN: Induction of apoptosis and cell cycle blockade by helichrysetin
in a549 human lung adenocarcinoma cells. Evid Based Complement
Alternat Med. 2013:8572572013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fong HY, Abd Malek SN, Yee HS and Karsani
SA: Helichrysetin induces DNA damage that triggers JNK-mediated
apoptosis in ca ski cells. Pharmacogn Mag. 13:607–612. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doug H, Chen SX, Xu HX, Kadota S and Namba
T: A new antiplatelet diarylheptanoid from alpinia blepharocalyx. J
Nat Prod. 61:142–144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vogel S, Ohmayer S, Brunner G and Heilmann
J: Natural and non-natural prenylated chalcones: Synthesis,
cytotoxicity and anti-oxidative activity. Bioorg Med Chem.
16:4286–4293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ali MS, Tezuka Y, Awale S, Banskota AH and
Kadota S: Six new diarylheptanoids from the seeds of alpinia
blepharocalyx. J Nat Prod. 64:289–293. 2001. View Article : Google Scholar
|
|
23
|
Gewali MB, Tezuka Y, Banskota AH, Ali MS,
Saiki I, Dong H and Kadota S: Epicalyxin F and calyxin I: Two novel
antiproliferative diarylheptanoids from the seeds of alpinia
blepharocalyx. Org Lett. 1:1733–1736. 1999. View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Antonelli A, Ferrari SM, Giuggioli D,
Ferrannini E, Ferri C and Fallahi P: Chemokine (C-X-C motif) ligand
(CXCL)10 in autoimmune diseases. Autoimmun Rev. 13:272–280. 2014.
View Article : Google Scholar
|
|
26
|
Somade OT, Ajayi BO, Safiriyu OA, Oyabunmi
OS and Akamo AJ: Renal and testicular up-regulation of
pro-inflammatory chemokines (RANTES and CCL2) and cytokines (TNF-α,
IL-1β, IL-6) following acute edible camphor administration is
through activation of NF-kB in rats. Toxicol Rep. 6:759–767. 2019.
View Article : Google Scholar :
|
|
27
|
Barruet E, Morales BM, Cain CJ, Ton AN,
Wentworth KL, Chan TV, Moody TA, Haks MC, Ottenhoff TH, Hellman J,
et al: NF-κB/MAPK activation underlies ACVR1-mediated inflammation
in human heterotopic ossification. JCI Insight. 3:2018. View Article : Google Scholar
|
|
28
|
Babapour N, Mehramiz M, Moghadam AR,
Behboodi N, Yousefi Z, Maftouh M, Talebian S, Khazaei M, Jafarian
A, Sharifi-Sistani N, et al: Association of TNF-308 G>A
polymorphism located in tumor necrosis factor a with the risk of
developing cervical cancer and results of pap smear. J Cell
Biochem. 120:5444–5448. 2019. View Article : Google Scholar
|
|
29
|
Borghi A, Verstrepen L and Beyaert R:
TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP
kinases and cell death. Biochem Pharmacol. 116:1–10. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fernald K and Kurokawa M: Evading
apoptosis in cancer. Trends Cell Biol. 23:620–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Annibaldi A and Meier P: Checkpoints in
TNF-induced cell death: Implications in inflammation and cancer.
Trends Mol Med. 24:49–65. 2018. View Article : Google Scholar
|
|
33
|
Sun LR, Zhou W, Zhang HM, Guo QS, Yang W,
Li BJ, Sun ZH, Gao SH and Cui RJ: Modulation of multiple signaling
pathways of the plant-derived natural products in cancer. Front
Oncol. 9:11532019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jandial DD, Blair CA, Zhang S, Krill LS,
Zhang YB and Zi X: Molecular targeted approaches to cancer therapy
and prevention using chalcones. Curr Cancer Drug Targets.
14:181–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu M, Hansen PE, Wang G, Qiu L, Dong J,
Yin H, Qian Z, Yang M and Miao J: Pharmacological profile of
xanthohumol, a prenylated flavonoid from hops (Humulus lupulus).
Molecules. 20:754–779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao W, Li H, Hou Y, Jin Y and Zhang L:
Combined administration of poly-ADP-ribose polymerase-1 and
caspase-3 inhibitors alleviates neuronal apoptosis after spinal
cord injury in rats. World Neurosurg. 127:e346–e352. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Malojirao VH, Vigneshwaran V, Thirusangu
P, Mahmood R and Prabhakar BT: The tumor antagonistic steroidal
alkaloid solanidine prompts the intrinsic suicidal signal mediated
DFF-40 nuclear import and nucleosomal disruption. Life Sci.
199:139–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma Y, Wang Y and Song B:
Griffipavixanthone induces apoptosis of human breast cancer MCF-7
cells in vitro. Breast Cancer. 26:190–197. 2019. View Article : Google Scholar
|
|
39
|
McElroy SJ, Frey MR, Yan F, Edelblum KL,
Goettel JA, John S and Polk DB: Tumor necrosis factor inhibits
ligand-stimulated EGF receptor activation through a TNF receptor
1-dependent mechanism. Am J Physiol Gastrointest Liver Physiol.
295:G285–G293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chariot A: The NF-kappaB-independent
functions of IKK subunits in immunity and cancer. Trends Cell Biol.
19:404–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schlessinger J: Receptor tyrosine kinases:
Legacy of the first two decades. Cold Spring Harb Perspect Biol.
6:a0089122014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tan X, Lambert PF, Rapraeger AC and
Anderson RA: Stress-Induced EGFR trafficking: Mechanisms,
functions, and therapeutic implications. Trends Cell Biol.
26:352–366. 2016. View Article : Google Scholar : PubMed/NCBI
|