Introduction
Colorectal cancer represented almost 10% of the
global cancer incidence burden in 2012, causing a mortality rate of
694,000 per year worldwide (1).
Despite recent advances in medicine, approximately 50% of patients
with colorectal cancer exhibit tumor recurrence (2), and the overall mortality rate
associated with the disease is approximately 40% (3). Colorectal cancer stem-like cells
(CCSCs) are considered to be one of the primary causes of tumor
recurrence.
Cancer stem-like cells (CSCs) are defined as 'cells
within a tumor that possess the capacity for self-renewal and that
can cause the heterogeneous lineages of cancer cells that
constitute the tumor' (4). At
present, CSCs have been proven to exist in a variety of solid
tumors, including colon cancer (5-7).
It is considered that only CSCs can drive tumor initiation,
proliferation and spreading (8).
In addition, CSCs are not affected by chemotherapy or radiation
(9). CSCs that survive
chemoradiotherapy will initiate and maintain the regrowth of the
tumor. A number of currently used protocols for cancer therapy are
now understood to fail, as marked by the reappearance of disease,
due to the inability to eradicate CSCs. Therefore, the requirement
and the challenge in fighting cancer is to develop novel therapies
targeting CSCs. To counteract the challenges, researchers have
turned to natural products. Some natural agents, including morusin
(10), have been proposed as
candidates for the targeting of CSCs alone or in combination with
chemoradiotherapy (11,12).
Morusin
(2-(2,4-dihydroxyphenyl)-5-hydroxy-8,8-di-methyl-3-(3-methylbut-2-enyl)pyranol[2,3-h]chromen-4-one,
C25H24O6) (NCBI PubChem Compound database, CID:5281671)
is a naturally occurring agent isolated from the traditional
Chinese medicinal (TCM) herb, Morus alba L. (Sang Bai Pi),
which is used as an antiphlogistic, an antipyretic, an expectorant,
an antitussive, a diuretic and an antidiabetic medication (13,14). Researchers have demonstrated that
morusin exerts antitumor effects in several types of neoplasms,
including hepatocellular carcinoma (15,16), gastric cancer (16), cervical cancer (10) and neuroblastoma (17). To the best of our knowledge, the
last and only study on the antitumor activity of morusin against
colorectal cancer was published in 2008 (14), in which it was reported that
morusin induced the nuclear factor (NF)-κB-mediated apoptosis of
HT-29 colorectal cancer cells. Based on previous research, it was
hypothesized that morusin may exert antitumor effects on CCSCs. To
confirm this hypothesis, in the present study, the effects of
morusin treatment on CCSCs were examined and the potential
underlying mechanisms were investigated.
Materials and methods
Drugs
Morusin (purity 99%) was purchased from the Shanghai
Research Center of Traditional Chinese Medicine Standardization.
Morusin was dissolved in dimethyl sulfoxide (DMSO) to a
concentration of 4,800 μM. The stock solution was stored at
−20°C and diluted in the medium before the experiment. The final
DMSO concentration did not exceed 1% throughout the study.
Reagents and antibodies
McCoy's 5A medium, Dulbecco's modified Eagle's
medium (DMEM)/F12 medium, fetal bovine serum and B27 supplement
(50×) were obtained from Gibco; Thermo Fisher Scientific, Inc.
Penicillin G, streptomycin and Heparin Na salt were purchased from
Sigma-Aldrich; Merck KGaA. Epidermal growth factor (EGF) and basic
fibroblast growth factor (bFGF) were purchased from PeproTech, Inc.
The primary antibodies [octamer-binding transcription factor 4
(Oct4) (cat. no. sc-5279), Sox2 (cat. no. sc-365823) and Nanog
(cat. no. sc-293121)] used for immunofluoresence assay were
purchased from Santa Cruz Biotechnology, Inc. The primary
antibodies used for western blot analysis were purchased from the
following companies: Cell Signaling Technology, Inc. [Oct4 (cat.
no. 2750), Sox2 (cat. no. 3579), Nanog (cat. no. 4903), β-catenin
(cat. no. 8480), transcription factor 4 (TCF4) (cat. no. 2569),
c-Myc (cat. no. 18583) and survivin (cat. no. 2808)],
Elabscience® [Akt (cat. no. E-AB-30471), phospho-Akt-473
(cat. no. E-AB-21135), glycogen synthase kinase (GSK)-3β (cat. no.
E-AB-20885) ], American Research Products, Inc. [CDK2 (cat. no.
A2439), cyclin D1 (cat. no. 10-M1033) and p21Cip1/WAF1 (cat. no.
24-1026-MSM3)] and ProteinTech Group, Inc. [(p27Kip1 (cat. no.
25614-1-AP), cyclin A2 (cat. no. 27242-1-AP), GAPDH (cat. no.
60004-1-Ig) and β-actin (cat. no. 20536-1-AP)].
Cells and cell culture
The HCT116 human colorectal cancer cell line was
purchased from the Shanghai Type Culture Collection of the Chinese
Academy of Sciences. HCT116 cells were cultured in McCoy's 5A
medium supplemented with 10% fetal bovine serum, 100 U/ml
penicillin G and 100 μg/ml streptomycin. The cells were
cultured in a humidified 5% CO2 atmosphere at 37°C. The
human fetal colon (FHC) cells (a gift from Dr Hu of Shanghai Funeng
Biotechnology Co., Ltd.) were cultured in a 1:1 mixture of Ham's
F12 and DMEM (Corning, Inc.) containing HEPES (25 mM), cholera
toxin (10 ng/ml; Sigma-Aldrich; Merck KGaA), insulin (5 lg/ml),
transferrin (5 lg/ml) and hydrocortisone (100 ng/ml; Sigma-Aldrich;
Merck KGaA), supplemented with 10% fetal bovine serum (FBS)
(Invitrogen; Thermo Fisher Scientific, Inc.).
Spheroid formation
Spheroid formation was enriched using a serum-free
and non-adhesive floating culture system. Prior to spheroid
formation, viable cells were counted by trypan blue staining and
seeded in 24-well ultra-low attachment plates (Corning Inc.) at a
density of 2,000 cells per well in 500 μl 1X serum-free
medium (SFM) (DMEM/F12 medium supplemented with 20 ng/ml EGF, 20
ng/ml bFGF, 4 μg/ml Heparin Na salt and 1X B27 supplement)
for 8 days; subsequently, 50 μl of 10X SFM medium per well
was added on days 3, 5 and 7. Tumor spheroids were dissociated,
digested and harvested on day 8, and the viable cells were stained
with 0.4% trypan blue staining solution for 5 min at room
temperature. Single-sphere cell suspensions were cultured in
McCoy's 5A medium, then treated with the morusin (0, 9.1, 18.2 and
36.4 μM). Spheroids were observed under an inverted
fluorescent microscope (Olympus Corporation).
Cell proliferation assay/Cell Counting
Kit (CCK)-8 assay
The proliferation of the HCT116 cells and FHC cells
was measured using a CCK-8 assay (Dojindo Molecular Technologies,
Inc.). There were 3 general groups: The blank group (McCoy's 5A
medium without cells or drugs), the control group (cells were
cultured in McCoy's 5A medium without the drug) and the
experimental groups (cells were exposed to 2.5, 5, 10, 15, 20, 25,
or 50 μM morusin for 72 h, or to 9.1, 18.2 or 36.4 μM
morusin for 24, 48 or 72 h). Following treatment, the culture
medium was discarded and 100 μl of fresh medium including 10
μl of CCK-8 was added to each well. Following 4 h of
incubation in a humidified 5% CO2 atmosphere at 37°C,
the absorbance was measured at 450 nm using a Multiskan Spectrum
Microplate Reader (Bio-Rad Laboratories, Inc.). The experiments
were performed in triplicate. The percentage inhibitory rate (IR%)
of the treated cells was calculated using the following formula:
IR%=1-[(EXP group OD450 nm-blank group OD450 nm)/(CTRL group OD450
nm-Blank group OD450 nm)] x100%.
5-Ethynyl-2'-deoxyuridine (EdU)
assay
Cells were seeded in Millicell EZ 8-well glass
slides (EMD Millipore) at a density of 1×104 cells/well,
and the cells were exposed to 9.1, 18.2 or 36.4 μM Morusin
for 24 h. Following treatment, the culture medium was discarded,
and an EdU incorporation assay was performed using a Cell-Light™
EdU Apollo® 567 In Vitro Imaging kit (Ribobio
Co.) according to the manufacturer's instructions. The stained
cells were observed under a fluorescence microscope (Olympus
Corporation), images were captured and analyzed using ImageJ
software (https://imagej.nih.gov/ij/download.html).
Immunofluorescence assay
Cells were seeded in Millicell EZ 8-well glass
slides (EMD Millipore) at a density of 1×104 cells/well,
washed with phosphate-buffered saline (PBS), fixed and permeated
using a BD Cytofix/Cytoperm™ kit (BD Biosciences). The cells were
then incubated with Oct4, Sox2 and Nanog antibodies at a dilution
of 1:50 in 1% bovine serum albumin (BSA)-PBS at 4°C overnight
followed by incubation with the secondary antibody [goat anti-mouse
IgG H&L (Alexa Fluor® 647) (Abcam, 1:200, cat. no.
ab150115) and donkey anti-goat IgG H&L (Cy5®)
(Abcam, 1:400, cat. no. ab97117)] in 1% BSA-PBS at room temperature
in the dark for 1 h. The cells were then washed with PBS and
stained with 10 μg/ml Hoechst 33342 (Abcam, cat. no.
ab228551) at room temperature in the dark for 5 min. The stained
cells were observed under a fluorescence microscope, and images
were captured and analyzed using ImageJ software.
Detection of cell cycle distribution by
flow cytometry
Following treatment with 9.1, 18.2 or 36.4 μM
morusin for 24 h, the cells were harvested, washed with 4°C PBS and
stored in cold 70% ethanol at -20°C overnight. The cells were
centrifuged (200 × g) for 4 min at room temperature, then stained
with propidium iodide (PI) treated with RNase A (100 U/ml) and
detected by flow cytometry (FACScan; BD Biosciences) for cell cycle
analysis.
Detection of apoptotic cells by flow
cytometry
Cell apoptosis was detected using an Annexin
V-Fluorescein Isothiocyanate (FITC) Apoptosis Detection kit I (BD
Biosciences) according to the following protocol: The cells were
seeded in 6-well plates at a density of 2×105 cells/well
and exposed to 9.1, 18.2 or 36.4 μM morusin for 24 h. The
cells were digested, incubated with FITC-Annexin buffer at room
temperature for 10 min, stained with PI and detected by flow
cytometry (FACScan; BD Biosciences).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the cells using the
RNeasy Micro kit (Qiagen GmbH). Total RNA (0.5 μg) was used
as a template to prepare cDNA (Reverse Transcription System,
Promega Corporation; cat. no. A3500) according to the
manufacturers' instructions. The amplification conditions were as
follows: Stage 1 (holding 95°C for 10 min); stage 2 (40 cycles of
denaturing at 95°C for 15 sec, annealing at 60°C for 45 sec and
extending at 72°C for 30 sec); stage 3 (extension at 72°C for 7
min). The primer sequences are listed in Table I. The housekeeping gene GAPDH was
used as an internal reference to normalize the results. All
experiments were performed in triplicate. Finally, the
2−ΔΔCq method was performed to calculate the relative
expression (18).
 | Table IPrimer sequences used for RT-PCR. |
Table I
Primer sequences used for RT-PCR.
| Gene | Primer sequences
(5′-3′) | Product length
(bp) |
|---|
| Oct4 | F: GGG TGG AGA GCA
ACT CCG A | 150 |
| R: GCT TGG CAA ATT
GCT CGA G |
| Sox2 | F: GTT CTA GTG GTA
CGG TAG GAG CTT TG | 150 |
| R: TTT GAT TGC CAT
GTT TAT CTC GAT |
| Nanog | F: CCA GCT GTG TGT
ACT CAA TGA ATT T | 150 |
| R: TTC TGC CAC CTC
TTA GAT TTC ATT C |
| 18s rRNA | F: TCG GAG GTT CGA
AGA CGA TC | 150 |
| R: CAG CTT TGC AAC
CAT ACT CCC |
Semi-quantitative RT-PCR
Similarly, total RNA was extracted from the cells
and the cDNAs were prepared as described above for RT-PCR.
Subsequently, 1% of the cDNA samples were used for GAPDH, Oct4,
Sox2, Nanog PCR. Sense primers were 32P-radiolabeled
with T4 polynucleotide kinase. The PCR reactions were performed
with the following profile: 30 sec denaturation at 94°C, 15 sec
annealing, 25 s extension at 72°C. An initial denaturation step at
94°C for 2.5 min and a final extension step at 72°C for 7 min were
also performed. The PCR products were analyzed by 2% agarose gel
electrophoresis and stained with ethidium bromide.
Western blot analysis
HCT116 sphere cells were seeded in 10-mm dishes at a
density of 2×106 cells per dish and were exposed to 9.1,
18.2, or 36.4 μM morusin for 24 h. Following treatment, the
cells were washed with cold PBS at 4°C, lysed in RIPA lysis buffer
including PhosSTOP (Roche Diagnostics) and 1 μM PMSF at 4°C
for 30 min. Cell lysates were homogenized and centrifuged of 14,000
× g at 4°C for 10 min. The protein concentration was detected by
Enhanced BCA Protein Assay kit (Beyotime Institute of
Biotechnology). Equal amounts of total protein (40 μg) were
boiled, loaded onto an 8% SDS-PAGE gel and electrotransferred to
polyvinylidene fluoride (PVDF) membranes (EMD Millipore). After
blocking with 5% non-fat milk in Tris-buffered saline (TBST) for 2
h at room temperature, the membranes were incubated with the
primary antibodies at 4°C overnight and incubated with 1:4,000
dilutions of secondary antibodies labeled with horseradish
peroxidase [rabbit anti-mouse IgG H&L (HRP), Abcam, cat. no.
ab6728)] at room temperature for 1 h. After being washed with TBST
3 times, the target proteins were detected using an Enhanced Chemi
Luminescence (ECL) kit (Thermo Fisher Scientific, Inc.). Finally,
images were captured by ChemiDoc™ XRS+ (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All data were analyzed using the Statistical Package
Social Science (SPSS) 19.0 software and are expressed as the means
± SD. Statistical analysis was performed among multiple groups by
one-way analysis of variance (ANOVA) followed by Tukey's post hoc
test. Values of P<0.05 and P<0.01 were considered to indicate
statistically significant and highly statistically significant
differences, respectively.
Results
Enrichment and biological
characterization of CCSCs
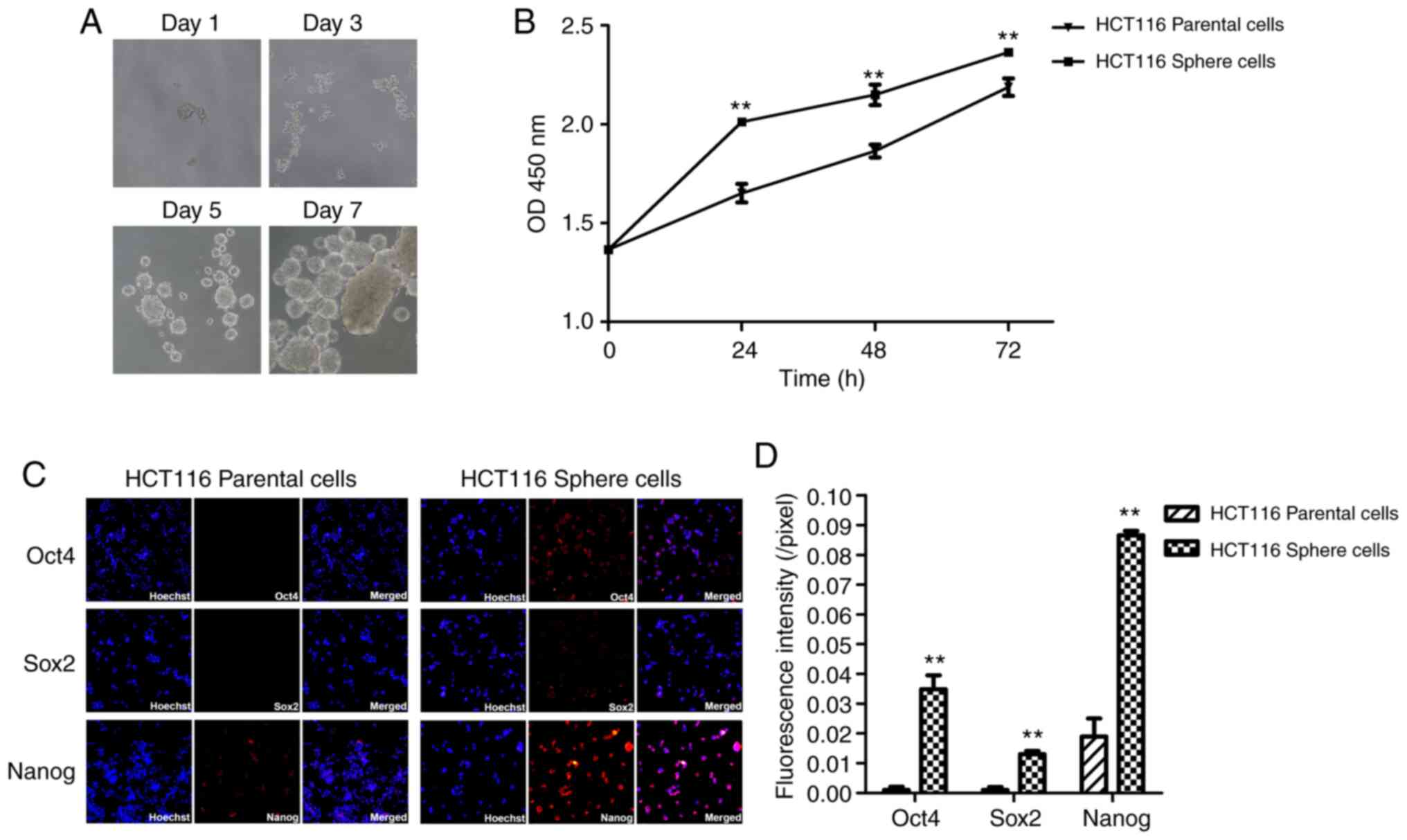
Spheroid formation was induced in a serum-free and
non-adhesive floating culture system (SFM) enriched with CCSCs
(19), as it has been
demonstrated that sphere cells are enriched with CSCs (20,21). Visible tumor spheroids were
observed under an optical microscope on day 3 and were then
gradually grown for the following 5 days (Fig. 1A). Following spheroid formation,
the number of viable cells in each well increased from 2,000 to
65,500±18,358. To evaluate the proliferative capacity of the HCT116
sphere cells, HCT116 parental and sphere cells were seeded in
96-well plates at a density of 5,000 cells per well in McCoy's 5A
medium, and CCK-8 assay was performed at 24, 48 and 72 h. The
sphere cells exhibited an increased proliferative capacity compared
with the parental cells (P<0.01; Fig. 1B). Subsequently, the expression
levels of stemness markers (Oct4, Sox2 and Nanog) was detected by
immunofluorescence staining. It was observed that Nanog mRNA and
protein expression was detectable in the HCT116 parental cells,
whereas Oct4 and Sox2 expression was undetectable. By contrast, the
Oct4, Sox2 and Nanog expression levels were significantly increased
in the HCT116 sphere cells, with a particular increase noted in
Nanog mRNA and protein expression (Fig. 1C). As shown in Fig. 1D, the fluorescence intensity of
Oct4, Sox2 and Nanog expression was significantly upregulated in
the HCT116 sphere cells (each P<0.01). Taken together, the
results indicated that there was an upregulation in the expression
of stemness-related transcription factors (Oct4, Sox2 and Nanog) in
HCT116 sphere cells.
Inhibitory effects of morusin on the
growth of HCT116 sphere cells (results of CCK-8 and EdU
assays)
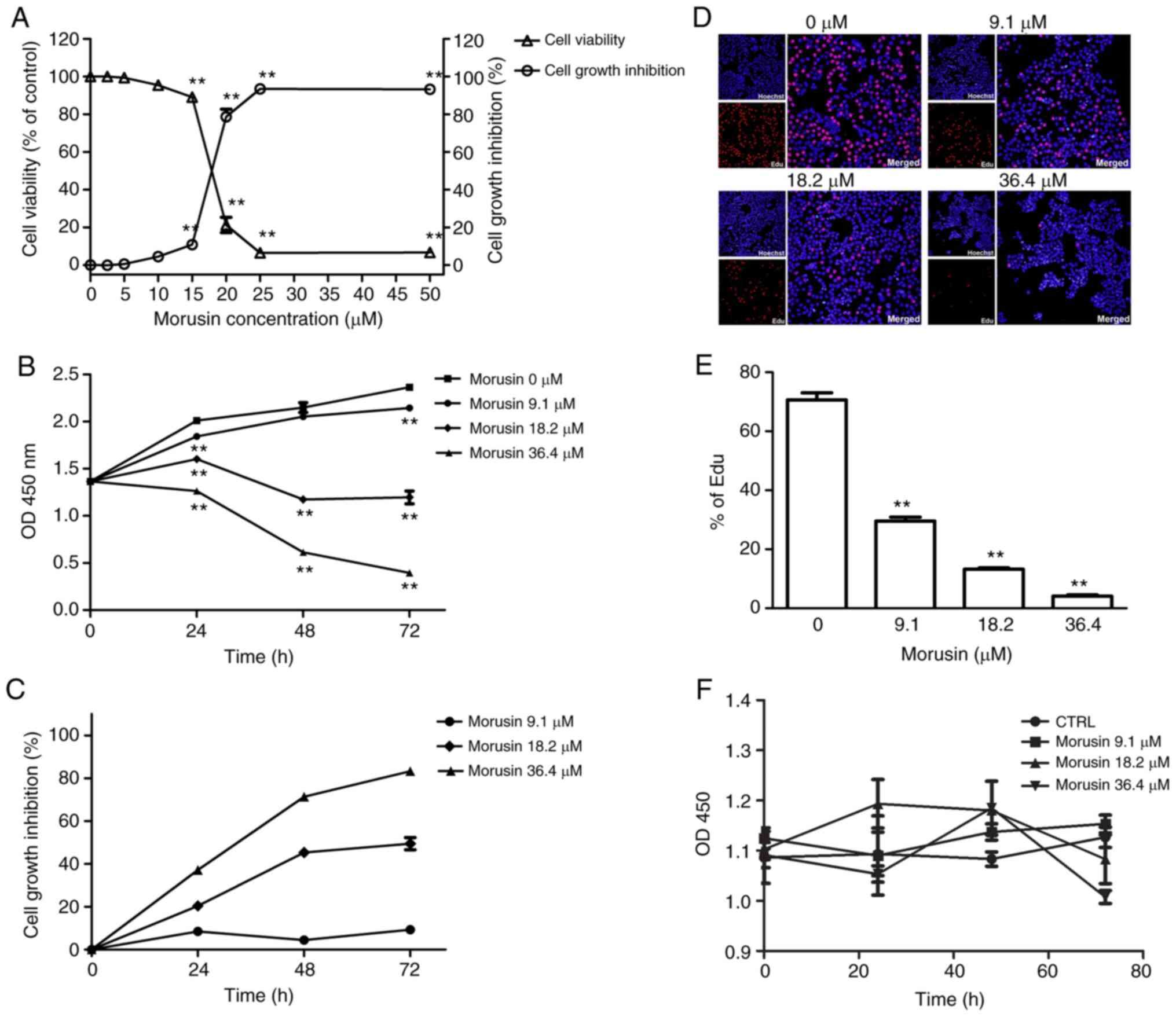
The proliferation of HCT116 sphere cells was
assessed by CCK-8 assay. The HCT116 sphere cells were treated with
various concentrations of morusin (2.5, 5.0, 10.0, 15.0, 20.0, 25.0
and 50.0 μM) for 72 h. When the concentration was ≥5.0
μM, morusin significantly inhibited the proliferation of
HCT116 sphere cells in a concentration-dependent manner
(P<0.01); an approximately 50% growth inhibition at 72 h was
achieved at the concentration of 18.2 μM (IC50)
(Fig. 2A). Subsequently, the
HCT116 sphere cells were treated with 9.1, 18.2, or 36.4 μM
morusin for 24, 48 and 72 h. Morusin significantly decreased cell
viability at each time point (P<0.01; Fig. 2B and C). These results indicated
that treatment with morusin led to a significant inhibition of cell
growth in a concentration- and time-dependent manner.
To further evaluate the proliferative activity of
HCT116 sphere cells following treatment with morusin, an EdU
incorporation assay was also performed (Fig. 2D). Following treatment with 9.1,
18.2, or 36.4 μM morusin for 24 h, cell viability was
decreased from 70.65±5.33 to 29.54±3.33, 13.23±1.19 and 4.12±1.13%,
respectively. There was a significant decrease in EdU incorporation
when the cells were incubated with morusin (P<0.01; Fig. 2E), suggesting that morusin
significantly inhibited the proliferative activity of the HCT116
sphere cells. In addition, the proliferation of normal FHC cells
treated with morusin (9.1, 18.2 and 36.4 μM) demonstrated
that morusin did not affect normal colonic mucosa cell viability
(Fig. 2F).
Morusin inhibits the initiation of
colorectal cancer spheroid formation
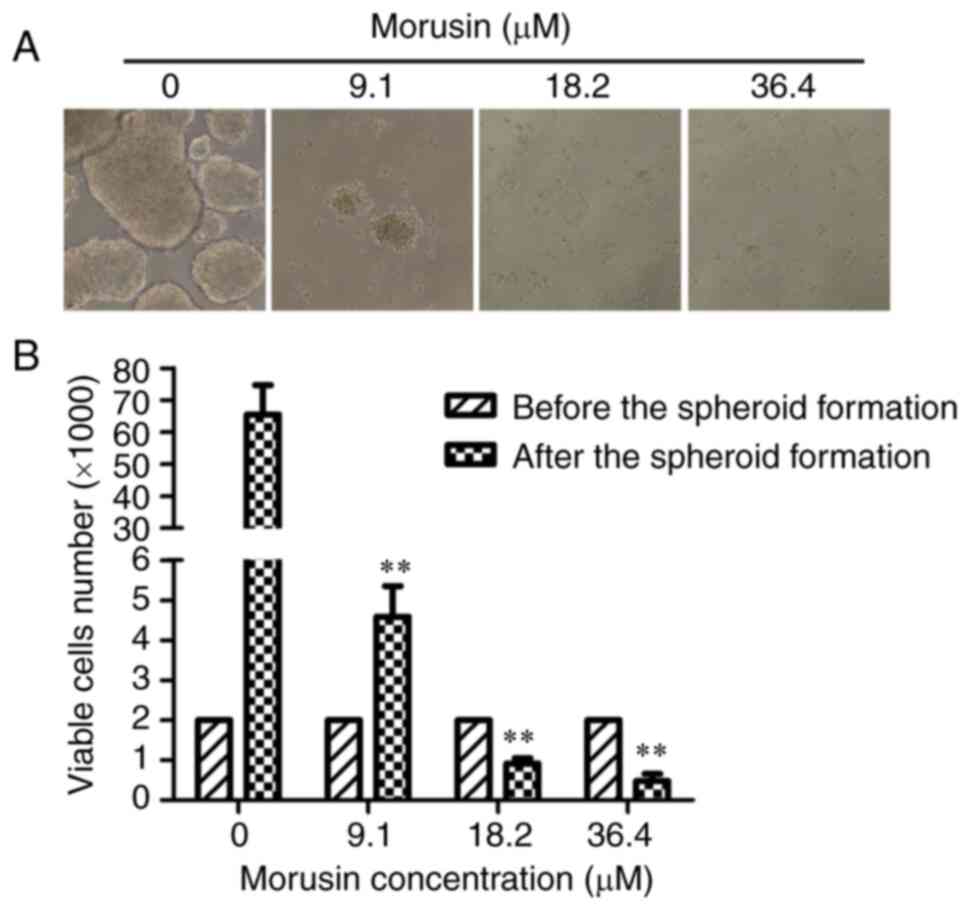
To determine whether morusin inhibits the initiation
of cancer spheroids formation, HCT116 cells were pre-treated with
9.1, 18.2, or 36.4 μM morusin for 24 h. The culture medium
was then removed and the cells were washed with PBS and collected;
viable cells were seeded at a density of 2,000 viable cells per
well. Following culture in CSC medium for 7 days, images of each
cell group were obtained and the viable cells were counted by
trypan blue staining. As shown in Fig. 3A, the HCT116 parental cells that
were pre-treated with 9.1 μM morusin could only rarely form
spheroids; in the higher concentration groups (18.2 and 36.4
μM), the HCT116 parental cells lost the ability to initiate
tumor spheroid formation entirely. Compared with the control group,
the number of viable cells in the experimental groups (morusin at
9.1, 18.2 and 36.4 μM) was significantly decreased
(P<0.01; Fig. 3B). These
results indicated that the spheroid-forming capacity of the HCT116
cells was suppressed by morusin.
Effect of Morusin on the cell cycle and
apoptosis of HCT116 cells
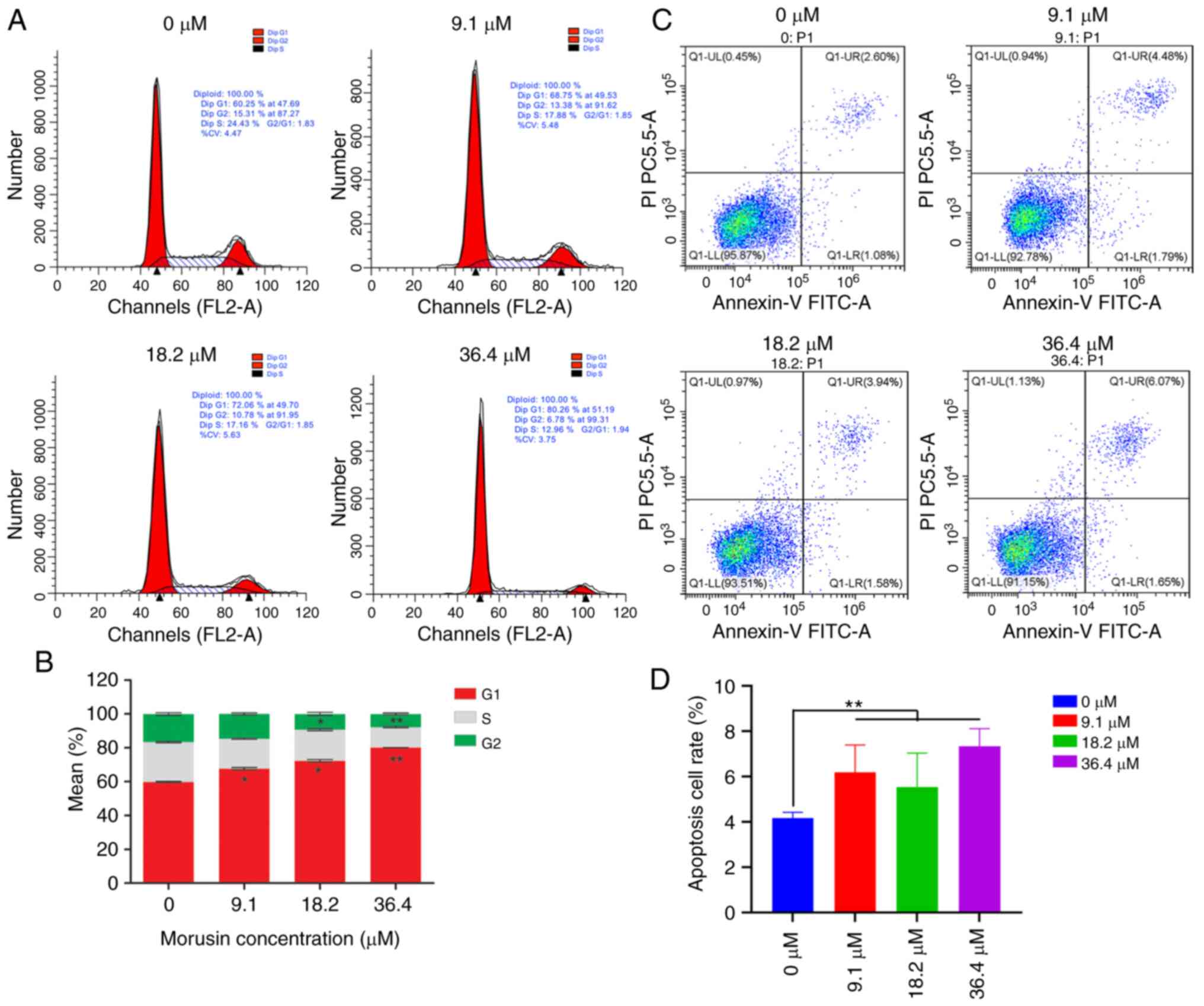
After being incubated with morusin for 24 h, the
cell cycle status and apoptosis were detected by flow cytometry.
Compared with the control group, morusin (9.1, 18.2 and 36.4
μM) significantly increased the percentage of cells in the S
phase (P<0.01) and decreased the percentage of cells in the
G0/G1 phase (P<0.01); the higher concentrations of morusin (18.2
and 36.4 μM) decreased the percentage of cells in the G2/M
phase (P<0.05; Fig. 4A and
B). These results indicated that morusin induced cell cycle
arrest at the S phase in the HCT116 sphere cells. The percentages
of apoptotic cells in each group are shown in Fig. 4C and D. It was found that morusin
induced the apoptosis of HCT116 sphere cells in a dose depended
manner. The apoptotic rate of the HCT116 sphere cells increased
with the increased concentration of morusin.
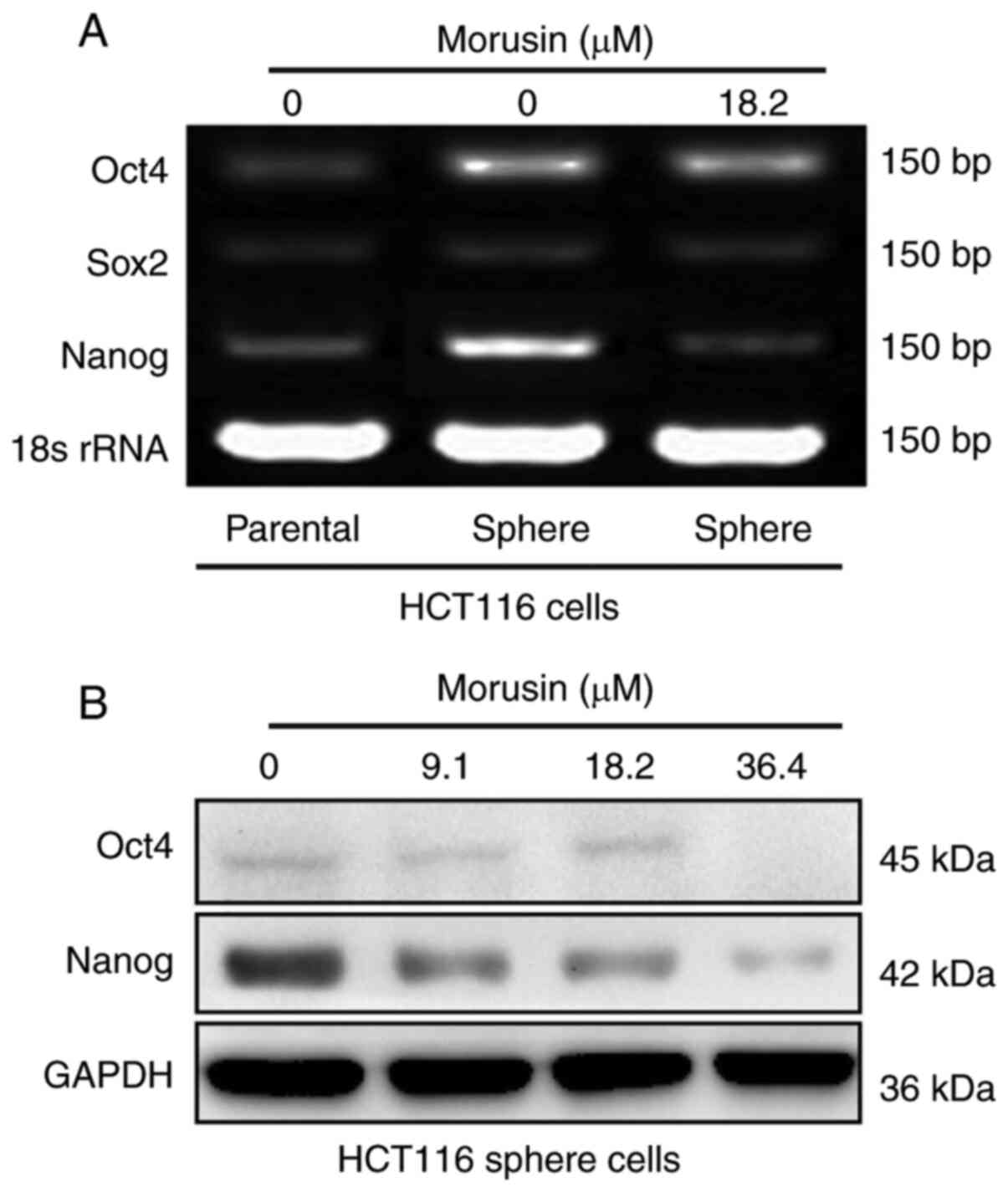
Effect of Morusin on the expression of
stemness markers
The expression levels of stemness markers (Oct4,
Sox2 and Nanog) were detected by RT-PCR and western blot assay.
There was a decreasing trend in Nanog mRNA expression following
morusin treatment. In addition, the results of western blot
analysis further confirmed that morusin inhibited the protein
expression of Nanog in a concentration-dependent manner.
Furthermore, the Oct4 protein expression level was decreased in the
group treated with the higher concentration of morusin (36.4
μM); however, the Sox2 protein expression level was too low
to be detectable (Fig. 5).
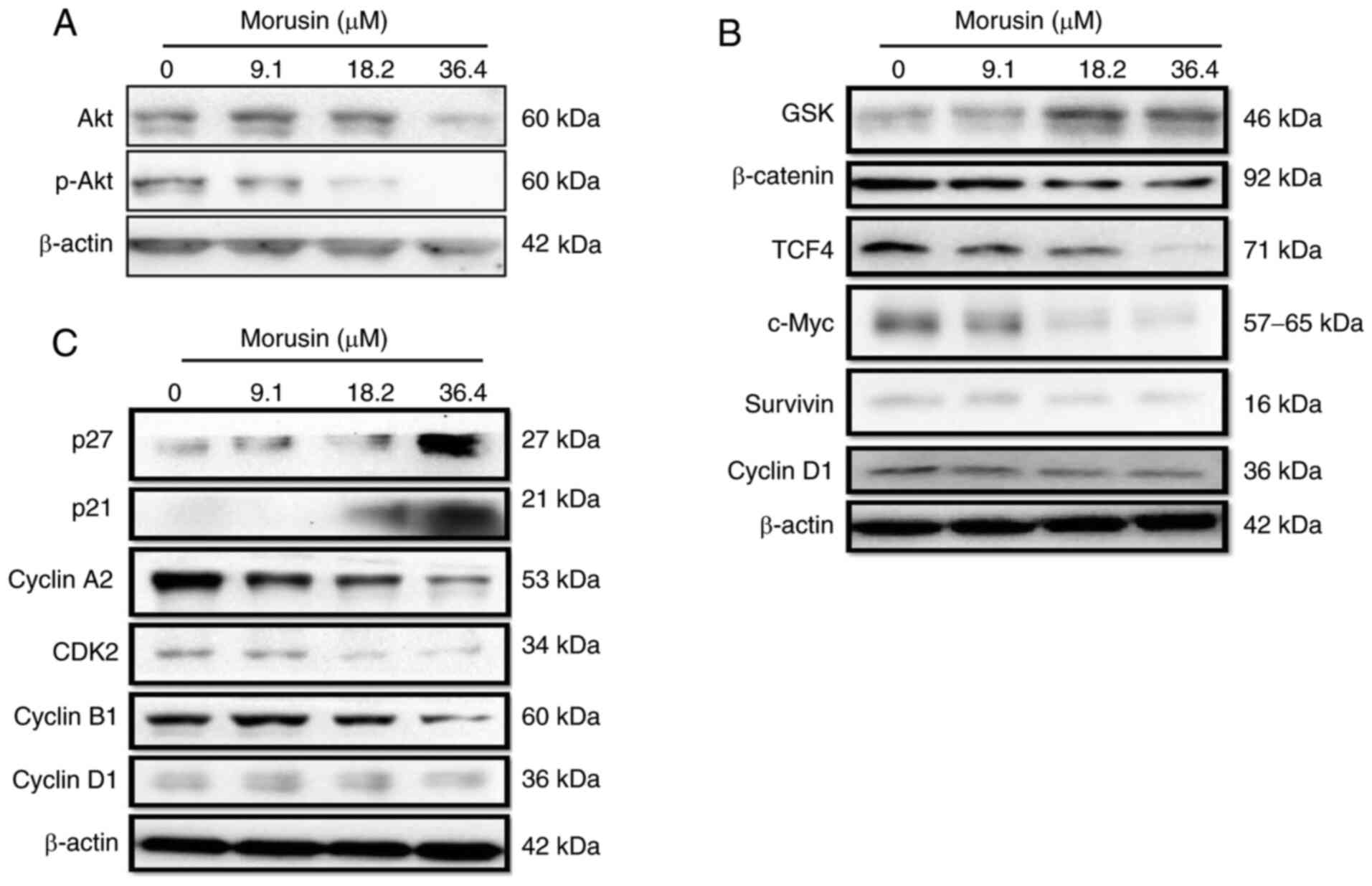
Morusin suppresses the activity of the
Akt/β-catenin pathway
Subsequently, the mechanisms involved in the
morusin-induced growth inhibition of HCT116 sphere cells were
investigated. Following incubation with 9.1, 18.2 and 36.4
μM morusin for 24 h, the activity of Akt/protein kinase B
and β-catenin signaling was detected by western blot analysis. The
expression levels of total Akt and phosphorylated Akt at Ser473 are
required for Akt activation (22). In the present study, morusin
inhibited the expression of total Akt at the higher concentration
(36.4 μM) and suppressed the phosphorylation of Akt in a
concentration-dependent manner (Fig.
6A). As shown in Fig. 6B,
the morusin-induced inactivation of Akt increased the expression of
Gsk-3β, which is a common target of the Akt and Wnt/β-catenin
pathway (23). Activated Gsk-3β
decreased the expression of β-catenin, followed by a reduction in
TCF4 expression. Furthermore, morusin suppressed downstream targets
of the Wnt/β-catenin pathway (c-Myc and survivin) in a
concentration-dependent manner, which decreased the expression of
cyclin D1 at the higher concentration of morusin (36.4
μM).
Morusin modulates the expression of cell
cycle-related proteins via the activation of p21Cip1/WAF1 and
p27Kip1
Inactivated Akt can also increase the expression of
p21Cip1/WAF1 and p27Kip1 (24).
Morusin upregulated the expression of p21Cip1/WAF1 and p27Kip1 via
the inactivation of Akt and then suppressed a number of cell
cycle-related proteins, including cyclin A and CDK2 (Fig. 6C), that participate in the
progression through the S and G2 phases cooperatively (25). The reduced cyclin A-CDK2 complex
expression induced cell cycle arrest at the S phase, which was
consistent with the results of the cell cycle distribution assay
(Fig. 4).
Discussion
Colorectal cancer causes approximately 694,000
deaths per year worldwide (1).
Despite recent advancements being made in therapies, the response
rate to current systemic therapies is ~50%, resistance develops in
nearly all patients (26).
Although the mechanisms invovled remain unclear, chemoresistant
colorectal CSCs are an important cause. Conventional antitumor
therapies that can eliminate the bulk of tumor cells, cannot target
CSCs that may lead to a reduction of the tumor mass, but not the
regression of the tumor (27).
Natural products are proposed as candidates for targeting CSCs
(28,29). The present study demonstrated
that morusin exerts antitumor effects on CCSCs.
In the present study, spheroid formation was induced
in a serum-free and non-adhesive floating culture system (SFM)
enriched with CCSCs (19), as it
has been demonstrated that sphere cells are enriched in CCSCs
(20,21). Compared with parental HCT116
cells, sphere cells exhibited an increased proliferative capacity
and a higher expression of pluripotent transcriptional factors
(Oct4, Sox2 and Nanog), core components of the pluripotency
regulation network that also maintain the stem-like properties of
CSCs (20,30,31) and are considered to be colorectal
CSCs markers (32). It was
observed that morusin not only inhibited the growth of colorectal
sphere cells, but also suppressed the initiation of colorectal
cancer spheroid formation. Similarly, morusin decreased the
expression of the stemness markers, Oct4 and Nanog. Subsequently,
further experiments were performed to examine the mechanisms
responsible for the effects of morusin on HCT116 sphere cells.
The potential molecular mechanisms of the effects of
morusin on CCSCs were demonstrated in the present study. First,
activated Akt downregulates the activity of GSK-3β (33), which is also a signaling target
of the canonical Wnt/β-catenin pathway. It is recognized that the
Wnt/β-catenin pathway carries activating mutations in virtually all
colon cancers (34) and plays an
important role in CCSCs (2). The
constitutive activation of the Wnt/β-catenin pathway triggers the
tumor-initiating potential (35,36) and maintains the growth of CSCs
(tumorspheres in vitro) (20). The Akt-mediated inactivation of
GSK-3β results in the stabilization and accumulation of β-catenin
in the cytoplasm, followed by the nuclear translocation of
β-catenin, which leads to the activation of the canonical Wnt
pathway. In the cell nucleus, β-catenin binds to members of the
T-cell factor (TCF)/lymphoid enhancer factor (LEF) family to
modulate the expression of target genes. In addition, Nanog and
Oct4 are also downstream targets of β-catenin pathway (37,38), which may partly explain why
Wnt/β-catenin signaling plays a role in sustaining stemness.
In the present study, the expression levels of total
Akt and phosphorylated Akt at Ser473, which is required for Akt
activation (22), were assayed
by western blot analysis. The morusin-induced inactivation of Akt
significantly increased the expression of GSK and decreased the
activity of β-catenin. In turn, the expression of TCF4 expression,
which is a critical factor in response to β-catenin (39), was decreased, and the
β-catenin/TCF4 complex is an important effector of Wnt/β-catenin
signaling. Morusin reduced the expression of the downstream
proteins, c-Myc [a known promoter of cell proliferation and growth
(40), survivin [a member of the
inhibitor of apoptosis protein (IAP) family that inhibits caspases
and blocks cell death (41), and
cyclin D1 (which facilitates cell-cycle progression through the G1
phase (42). In particular,
Nanog and Oct4 are directly regulated by the β-catenin/TCF complex
(37,38). Nanog not only is a CSC marker,
but also promotes cell proliferation (43,44); the results indicated that morusin
downregulated Oct4 and Nanog expression via the suppression of the
β-catenin/TCF4 complex. Taken together, the results demonstrated
that Morusin inhibited the growth of HCT116 sphere cells and
decreased stemness marker expression via the Akt/GSK/β-catenin
pathway.
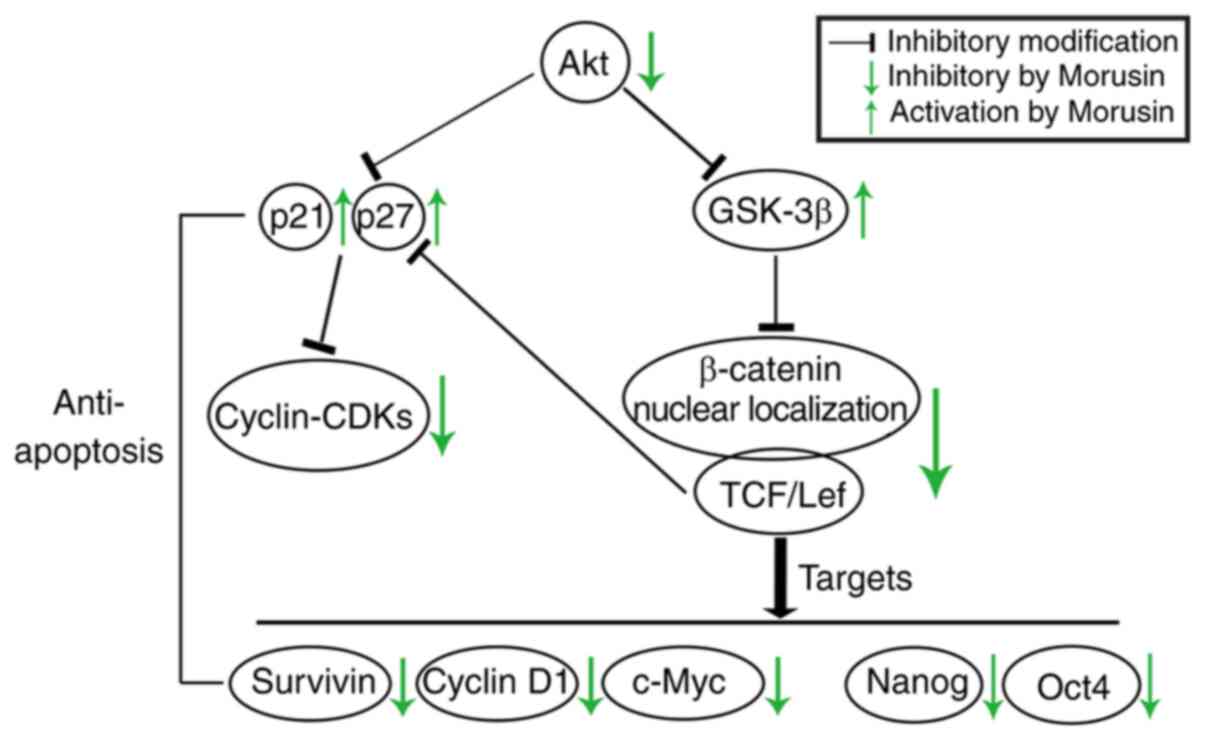
According to the second pathway shown in Fig. 7, activated Akt directly or
indirectly decreased the expression of p21Cip1/WAF1 and p27Kip1
(45,46), which belong to the CDK
interacting protein/kinase inhibitory protein (CIP/KIP) family, a
CDK inhibitor that can halt the cell cycle by interacting with a
variety of cyclin-CDK complexes (47). The morusin-induced inactivation
of Akt attenuated its suppression of the growth inhibitory activity
of p21 and p27, which led to a reduction in cyclin A-CDK2 complex
formation, which is required for cell cycle progression through the
S and G2 phases (25).
Furthermore, the morusin-induced reduction in TCF4 expression can
also contribute to the increase in p27 expression cooperatively
(48). As a result of the
inactivity of cyclin A-CDK2, morusin induced cell cycle arrest at
the S phase (Fig. 4), and the
EdU incorporation assay further demonstrated that the proliferative
ability was inhibited (Fig. 2C
and D). According to the above, morusin blocked cell cycle
progression in CCSCs via the Akt/p21Cip1/WAF1 p27Kip1 pathway to
inhibit cell proliferation.
It has been reported that morusin induced cancer
cell apoptosis (10,14). The results of the present study
were consistent with those of these other studies. It was found
that morusin promoted the apoptosis of HCT116 sphere cells in a
concentration-dependent manner. In addition, it was found that
morusin was able to increase the expression of p21Cip1/WAF1 and
p27Kip1, resulting in inducing cell cycle arrest. Although the
mechanism involved is unclear, it was hypothesized that it was
associated with the upregulation of p21Cip1/WAF1 expression
(49).
In conclusion, the present study demonstrated that
Morusin inhibited the growth of colorectal cancer sphere cells,
which were enriched with CCSCs. Morusin induced the inactivation of
Akt followed by the suppression of β-catenin signaling, which
resulted in a reduction in stemness marker expression and cell
growth inhibition. Morusin also increased the expression of
p21Cip1/WAF1 and p27Kip1 and induced cell cycle arrest. Morusin may
thus be considered a novel antitumor agent targeting CSCs.
Funding
The present study was supported by a grant from the
National Nature Science Foundation Project (no. 81703967).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
YZ performed the experiments and wrote the
manuscript. XL assisted in performing the experiments and processed
all the figures. MY designed of the study, provided funding support
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roy S and Majumdar AP: Signaling in colon
cancer stem cells. J Mol Signal. 7:112012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions.
Cancer Res. 166:9339–9344. 2006. View Article : Google Scholar
|
|
5
|
Ponti D, Zaffaroni N, Capelli C and
Daidone MG: Breast cancer stem cells: An overview. Eur J Cancer.
42:1219–1224. 2006. View Article : Google Scholar
|
|
6
|
Fan CW, Chen T, Shang YN, Gu YZ, Zhang SL,
Lu R, OuYang SR, Zhou X, Li Y, Meng WT, et al: Cancer-initiating
cells derived from human rectal adenocarcinoma tissues carry
mesenchymal phenotypes and resist drug therapies. Cell Death Dis.
4:e8282013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Jiao M, Li L, Wu D, Wu K, Li X,
Zhu G, Dang Q, Wang X, Hsieh JT and He D: Tumorspheres derived from
prostate cancer cells possess chemoresistant and cancer stem cell
properties. J Cancer Res Clin Oncol. 138:675–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puglisi MA, Barba M, Corbi M, Errico MF,
Giorda E, Saulnier N, Boninsegna A, Piscaglia AC, Carsetti R,
Cittadini A, et al: Identification of Endothelin-1 and NR4A2 as
CD133-regulated genes in colon cancer cells. J Pathol. 225:305–314.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones RJ, Matsui WH and Smith BD: Cancer
stem cells: Are we missing the target? J Natl Cancer Inst.
96:583–585. 2004. View Article : Google Scholar
|
|
10
|
Wang L, Guo H, Yang L, Dong L, Lin C,
Zhang J, Lin P and Wang X: Morusin inhibits human cervical cancer
stem cell growth and migration through attenuation of NF-κB
activity and apoptosis induction. Mol Cell Biochem. 379:7–18. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou
Y, Zhu J and Mi M: Resveratrol inhibits breast cancer stem-like
cells and induces autophagy via suppressing Wnt/β-catenin signaling
pathway. PLoS One. 9:e1025352014. View Article : Google Scholar
|
|
12
|
Li Y and Zhang T: Targeting cancer stem
cells by curcumin and clinical applications. Cancer Lett.
346:197–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee YJ, Chang CF, Lin CW, Huang YC, Hu CC,
Tsheng YM and Tseng TH: The first total synthesis of morusin and
himanimide D as arachidonate 5-lipoxygenase inhibitor in automated
docking. Biophys J. 96(Suppl 1): 86A2009. View Article : Google Scholar
|
|
14
|
Lee JC, Won SJ, Chao CL, Wu FL, Liu HS,
Ling P, Lin CN and Su CL: Morusin induces apoptosis and suppresses
NF-κB activity in human colorectal cancer HT-29 cells. Biochem
Biophys Res Commun. 372:236–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan LZ, Ma B and Zhang YQ: Preparation of
morusin from Ramulus mori and its effects on mice with transplanted
H22 hepatocarcinoma. Biofactors. 40:636–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma JP, Qiao X, Pan S, Shen H, Zhu GF and
Hou AJ: New isoprenylated flavonoids and cytotoxic constituents
from Artocarpus tonkinensis. J Asian Nat Prod Res. 12:586–592.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee HJ, Lyu da H, Koo U, Nam KW, Hong SS,
Kim KO, Kim KH, Lee D and Mar W: Protection of prenylated
flavonoids from Mori Cortex Radicis (Moraceae) against nitric
oxide-induced cell death in neuroblastoma SH-SY5Y cells. Arch Pharm
Res. 35:163–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vermeulen L, De Sousa E, Melo F, van der
Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M,
Merz C, Rodermond H, et al: Wnt activity defines colon cancer stem
cells and is regulated by the microenvironment. Nat Cell Biol.
12:468–476. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanwar SS, Yu Y, Nautiyal J, Patel BB and
Majumdar AP: The Wnt/beta-catenin pathway regulates growth and
maintenance of colonospheres. Mol Cancer. 9:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YF, Xiao B, Tu SF, Wang YY and Zhang
XL: Cultivation and identification of colon cancer stem
cell-derived spheres from the Colo205 cell line. Braz J Med Biol
Res. 45:197–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohigashi T, Mizuno R, Nakashima J, Marumo
K and Murai M: Inhibition of Wnt signaling downregulates Akt
activity and induces chemosensitivity in PTEN-mutated prostate
cancer cells. Prostate. 62:61–68. 2005. View Article : Google Scholar
|
|
23
|
Sutton LP and Rushlow WJ: Regulation of
Akt and Wnt signaling by the group II metabotropic glutamate
receptor antagonist LY341495 and agonist LY379268. J Neurochem.
117:973–983. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jain MV, Jangamreddy JR, Grabarek J,
Schweizer F, Klonisch T, Cieślar-Pobuda A and Łos MJ: Nuclear
localized Akt enhances breast cancer stem-like cells through
counter-regulation of p21(Waf1/Cip1) and p27(kip1). Cell Cycle.
14:2109–2120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chowdhury R, Chatterjee R, Giri AK, Mandal
C and Chaudhuri K: Arsenic-induced cell proliferation is associated
with enhanced ROS generation, Erk signaling and CyclinA expression.
Toxicol Lett. 198:263–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dallas NA, Xia L, Fan F, Gray MJ, Gaur P,
van Buren G II, Samuel S, Kim MP, Lim SJ and Ellis LM:
Chemoresistant colorectal cancer cells, the cancer stem cell
phenotype, and increased sensitivity to insulin-like growth
factor-I receptor inhibition. Cancer Res. 69:1951–1957. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paldino E, Tesori V, Casalbore P,
Gasbarrini A and Puglisi MA: Tumor initiating cells and
chemoresistance: Which is the best strategy to target colon cancer
stem cells? Biomed Res Int. 2014:8598712014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burnett J, Newman B and Sun D: Targeting
cancer stem cells with natural products. Curr Drug Targets.
13:1054–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bao B, Li Y, Ahmad A, Azmi AS, Bao G, Ali
S, Banerjee S, Kong D and Sarkar FH: Targeting CSC-related miRNAs
for cancer therapy by natural agents. Curr Drug Targets.
13:1858–1868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Levings PP, McGarry SV, Currie TP,
Nickerson DM, McClellan S, Ghivizzani SC, Steindler DA and Gibbs
CP: Expression of an exogenous human Oct-4 promoter identifies
tumor-initiating cells in osteosarcoma. Cancer Res. 69:5648–5655.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shan J, Shen J, Liu L, Xia F, Xu C, Duan
G, Xu Y, Ma Q, Yang Z, Zhang Q, et al: Nanog regulates self-renewal
of cancer stem cells through the insulin-like growth factor pathway
in human hepatocellular carcinoma. Hepatology. 56:1004–1014. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Merlos-Suárez A, Barriga FM, Jung P,
Iglesias M, Céspedes MV, Rossell D, Sevillano M, Hernando-Momblona
X, da Silva-Diz V, Muñoz P, et al: The intestinal stem cell
signature identifies colorectal cancer stem cells and predicts
disease relapse. Cell Stem Cell. 8:511–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen RH, Ding WV and McCormick F: Wnt
signaling to beta-catenin involves two interactive components
glycogen synthase kinase-3beta inhibition and activation of protein
kinase C. J Biol Chem. 275:17894–17899. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prasetyanti PR, Zimberlin CD, Bots M,
Vermeulen L, Melo Fde S and Medema JP: Regulation of stem cell
self-renewal and differentiation by Wnt and Notch are conserved
throughout the adenoma-carcinoma sequence in the colon. Mol Cancer.
12:1262013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shenoy AK, Fisher RC, Butterworth EA, Pi
L, Chang LJ, Appelman HD, Chang M, Scott EW and Huang EH:
Transition from colitis to cancer: High Wnt activity sustains the
tumor-initiating potential of colon cancer stem cell precursors.
Cancer Res. 72:5091–5100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Welm B, Podsypanina K, Huang S,
Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al:
Evidence that transgenes encoding components of the Wnt signaling
pathway preferentially induce mammary cancers from progenitor
cells. Proc Natl Acad Sci USA. 100:15853–15858. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ibrahim EE, Babaei-Jadidi R, Saadeddin A,
Spencer-Dene B, Hossaini S, Abuzinadah M, Li N, Fadhil W, Ilyas M,
Bonnet D and Nateri AS: Embryonic NANOG activity defines colorectal
cancer stem cells and modulates through AP1-and TCF-dependent
mechanisms. Stem cells. 30:2076–2087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Li J and Chen B: Oct4 was a novel
target of Wnt signaling pathway. Mol Cell Biochem. 362:233–240.
2012. View Article : Google Scholar
|
|
39
|
Zhang Y, Liu C, Duan X, Ren F, Li S, Jin
Z, Wang Y, Feng Y, Liu Z and Chang Z: CREPT/RPRD1B, a recently
identified novel protein highly expressed in tumors, enhances the
β-catenin·TCF4 transcriptional activity in response to Wnt
signaling. J Biol Chem. 289:22589–22599. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kelly RJ, Lopez-Chavez A, Citrin D, Janik
JE and Morris JC: Impacting tumor cell-fate by targeting the
inhibitor of apoptosis protein survivin. Mol Cancer. 10:352011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Diaz-Moralli S, Tarrado-Castellarnau M,
Miranda A and Cascante M: Targeting cell cycle regulation in cancer
therapy. Pharmacol Ther. 138:255–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang ML, Chiou SH and Wu CW: Targeting
cancer stem cells: Emerging role of Nanog transcription factor.
Onco Targets Ther. 6:1207–1220. 2013.PubMed/NCBI
|
|
44
|
Choi SC, Choi JH, Park CY, Ahn CM, Hong SJ
and Lim DS: Nanog regulates molecules involved in stemness and cell
cycle-signaling pathway for maintenance of pluripotency of P19
embryonal carcinoma stem cells. J Cell Physiol. 227:3678–3692.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH
and Hung MC: Cytoplasmic localization of p21 Cip1/WAF1 by
Akt-induced phosphorylation in HER-2/neu-overexpressing cells.
Nature Cell Biol. 3:245–252. 2001. View Article : Google Scholar
|
|
46
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zheng X, Wang Y, Liu B, Liu C, Liu D, Zhu
J, Yang C, Yan J, Liao X, Meng X and Yang H: Bmi-1-shRNA inhibits
the proliferation of lung adenocarcinoma cells by blocking the G1/S
phase through decreasing cyclin D1 and increasing p21/p27 levels.
Nucleic Acid Ther. 24:210–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xie J, Xiang DB, Wang H, Zhao C, Chen J,
Xiong F, Li TY and Wang XL: Inhibition of Tcf-4 induces apoptosis
and enhances chemosensitivity of colon cancer cells. PLoS One.
7:e456172012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Asada M, Yamada T, Ichijo H, Delia D,
Miyazono K, Fukumuro K and Mizutani S: Apoptosis inhibitory
activity of cytoplasmic p21(Cip1/WAF1) in monocytic
differentiation. EMBO J. 18:1223–1234. 1999. View Article : Google Scholar : PubMed/NCBI
|