Introduction
Ischemic stroke, which accounts for 85% of all
stroke cases, is the leading cause of destructive cerebrovascular
disease, and displays high mortality and morbidity rates (1). To date, pharmacological
thrombolysis is the most effective treatment, but displays a golden
treatment time, meaning only 3-8% of patients are eligible for this
therapy. Therefore, novel therapies against ischemic stroke are
required (2). Previous studies
have indicated that inflammation and ferroptosis serve significant
roles in the development of ischemic stroke (3,4).
The NLR family pyrin domain containing 3 (NLRP3)
inflammasome, which consists of NLRP3, apoptosis-associated
speck-like protein (ASC) and caspase-1, is an important component
of the innate immune system (5).
The NLRP3 inflammasome triggers the production of the
proinflammatory cytokine, interleukin (IL)-1β, which facilitates
the secretion of inflammatory mediators, including tumor necrosis
factor (TNF)-α and IL-6 (6).
NF-κB is a transcription factor that alters the formation of the
NLRP3 inflammasome, and the NF-κB/NLRP3 inflammasome signaling is a
key mechanism of ischemic stroke (7). Hippocampal NF-κB mRNA expression,
and serum TNF-α and IL-1β levels were increased in response to
ischemia stroke (8). Cerebral
ischemia-reperfusion injury activated the NLRP3 inflammasome
proteins in rats, which could be suppressed by electroacupuncture,
leading to improvement of neurological deficit scores and infarct
sizes in middle cerebral artery occlusion (MCAO) model rats
(9).
Ferroptosis is a novel type of cell death driven by
iron-dependent accumulation of lipid-based reactive oxygen species
(ROS), which is regulated by the inactivation of glutathione
peroxidase 4 (GPX4) that can reduce lipid peroxides at the expense
of glutathione (GSH) (10).
Numerous proteins are involved in ferroptosis, including acyl-CoA
synthetase long-chain family member 4 (ACSL4), transferrin receptor
1 (TFR1) and ferritin heavy chain 1 (FTH1) (11,12). ACSL4 is a lipid metabolism enzyme
that is required for ferroptosis, contributing to lipid
peroxidation and ferroptosis (13). TFR1 transports iron from the
extracellular environment into cells, facilitating the cellular
iron pool essential for ferroptosis (14). FTH1 is a major iron storage
protein responsible for maintaining the iron balance in cells
(15). Ferroptosis is implicated
in ischemic stroke (4,16). Increased ROS and iron levels were
observed in the brain of ischemic stroke model rodents (17).
Carthamin yellow (CY) is a flavonoid compound
isolated from safflower. In Traditional Chinese medicine, it is
considered that CY improves blood circulation and alleviates pain;
thus, CY is used for the treatment of coronary heart disease,
cerebrovascular disease and angiitis in China (18). Antioxidant and anti-inflammatory
properties of CY have been reported. CY inhibited
lipopolysaccharide-induced activation of TNF-α (19). In vivo and in vitro
studies demonstrated that CY protected against cardiac ischemia and
reperfusion injury via decreasing ROS release and NLRP3
inflammasome-related inflammatory responses (18). The aforementioned studies
indicated that whether CY could ameliorate ischemic stroke by
attenuating inflammation and ferroptosis required further
investigation. Therefore, the present study investigated the
protective effect of CY in ischemic stroke by using MCAO model
rats, and explored the involvement of inflammation and ferroptosis
in CY-mediated effects to explore the possible underlying
mechanisms.
Materials and methods
Animals
A total of 32 male Sprague-Dawley rats (aged 6-8
weeks; 250-280 g) were purchased from Shanghai Sipper-BK Lab Animal
Co., Ltd. Animals were housed in an animal center at a controlled
temperature (23±1°C) and humidity (60±2%) with 12-h light/dark
cycles, and free access to food and water. All experiments were
conducted in compliance with the Provision and General
Recommendation of Chinese Experimental Animals Administration
Legislation and approved by the Institutional Animal Care and Use
Ethics Committee at Nanjing University of Chinese Medicine
(approval no. 201907A544).
Materials
CY was obtained from Sigma-Aldrich (Merck KGaA;
Fig. 1). All antibodies were
purchased from Cell Signaling Technology, Inc., ProteinTech Group,
ABclonal Technology, Inc. or Abcam.
Experimental protocol
Animals were randomly divided into the following
four groups (n=8 per group): i) Sham; ii) MCAO; iii) CY (20 mg/kg);
and iv) CY (40 mg/kg). CY was administered intragastrically to rats
once daily for 2 weeks. At 60 min after the last administration,
MCAO surgery was performed as previously described (20). At 24 h post-reperfusion,
neurological scores, brain water content and infarct volume were
determined. Immunofluorescence staining, western blotting and flow
cytometry were performed to investigate the potential mechanisms.
After neurological scoring, rats were euthanized with 400 mg/kg
pentobarbital sodium. The brains were immediately removed, and the
cortex and the serum were collected and stored at −80°C until
further use.
Neurological scoring
For the evaluation of functional recovery,
neurological scores were assessed at 24 h post-MCAO induction by an
observer blinded to the treatments as previously reported (20). The neurological scoring ranged
from 0 to 4 (normal score, 0; maximal deficit score, 4).
Infarct area assessment
Rats were sacrificed and the brains were rapidly
removed. Coronal sections were cut into 2-mm thick slices and
stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC;
Sigma-Aldrich; Merck KGaA) for 30 min at 37°C followed by fixation
with 10% paraformaldehyde for 10 min. Infarction size was assessed
using ImageJ software (version 1.52; National Institutes of
Health). The size of infarct regions was calculated using the
following equation: Infarct rate (%) = Infarct volume/total volume
×100.
Brain water content determination
The wet-dry method was applied to determine brain
edema. The brains were immediately removed and weighed to obtain
the wet weight. Following drying in an oven at 100°C for >24 h,
the brains were weighed to obtain the dry weight. The percentage of
brain water content was calculated using the following formula:
Brain water content (%) = [(wet weight)−(dry weight)]/(wet weight)
×100.
Immunofluorescence staining
Immunofluorescence staining was performed to detect
microtubule-associated protein 2 (MAP-2) and NF-κB expression as
previously described (21).
Briefly, brain tissues were obtained, fixed with 4%
paraformaldehyde for 24 h at room temperature and transferred to
30% sucrose solution. Subsequently, the tissues were frozen in a
cryostat machine and cut into frozen sections (10-µm thick)
at -20°C. Slices were washed with 0.01 M PBS for 5 min, blocked
with 5% goat serum (cat. no. SL038; Solarbio Life Sciences, Inc.)
for 1 h at room temperature, and then incubated with rabbit
anti-mouse primary antibodies targeted against MAP-2 (1:50; product
no. 4542) and phosphorylated (p)-NF-κB p65 (1:500; product no.
3039; both from Cell Signaling Technology, Inc.) at 4°C overnight.
After washing, samples were incubated with a goat anti-rabbit
Alexa-Fluor IgG secondary antibody (1:100; cat. no. SA00003-2;
ProteinTech Group, Inc.) for 1 h at room temperature. Nuclei were
stained with DAPI for 10 min at room temperature. Images of the
injured cortex were observed under high-power fields
(magnification, ×200; Olympus BX53). The percentage of MAP-2- or
p-NF-κB p65-positive staining was determined and analyzed using
ImageJ software (version 1.52; National Institutes of Health).
Western blotting
Western blotting was performed as previously
described (22). Total protein
was isolated from samples using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.). Nuclear proteins were extracted
using Nuclear and Cytoplasmic Protein Extraction reagents (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Protein concentrations were measured using the BCA Protein Assay
kit (Thermo Fisher Scientific, Inc.). Equal amounts of protein (50
µg) were separated via 10% or 12% SDS-PAGE and transferred
to PVDF membranes. After blocking with 5% BSA (cat. no. A8020;
Solarbio Life Sciences, Inc.) for 1 h at room temperature, the
membranes were incubated at 4°C overnight with antibodies targeted
against: p-NF-κB p65 (1:1,000; product no. 3039), NF-κB p65
(1:1,000; product no. 8242), p-IκBα (1:1,000; product no. 5209),
IκBα (1:1,000; cat. no. 4812; all from Cell Signaling Technology,
Inc.), NLRP3 (1:1,000; product code ab263899; Abcam), caspase-1
(1:1,000; cat. no. 22915-1-AP; ProteinTech Group, Inc.), IL-1β
(1:1,000; cat. no. A1112; ABclonal Technology, Inc.), ACSL4
(1:1,000; cat. no. A14439; ABclonal Technology, Inc.), FTH1
(1:1,000; product no. 4393; Cell Signaling Technology, Inc.), GPX4
(1:1,000; cat. no. A1933; ABclonal Technology, Inc.), TFR1 (TFRC;
1:1,000; cat. no. A5865; ABclonal Technology, Inc.), β-tubulin
(1:5,000; cat. no. 10068-1-AP; ProteinTech Group, Inc.) and Lamin A
(1:1,000; cat. no. ab8980; Abcam). After washing with TBST (0.1%
Tween-20), the membranes were incubated with appropriate secondary
antibody (1:5,000; cat. no. SA00001-2; ProteinTech Group, Inc.) for
1 h at room temperature. Protein bands were visualized using an ECL
kit (cat. no. 180-501; Tanon Science & Technology Co., Ltd.).
Protein expression levels were semi-quantified using ImageJ
software (version 1.52; National Institutes of Health) with
β-tubulin or Lamin A as the loading control.
Determination of TNF-α, IL-1β, IL-6,
malondialdehyde (MDA), GSH and superoxide dismutase (SOD)
concentrations in serum
The levels of TNF-α (TNF-α ELISA Kit; cat. no.
PT516), IL-1β (IL-1β ELISA Kit; cat. no. PI303), IL-6 (IL-6 ELISA
Kit; cat. no. PI328), MDA (MDA detection kit; cat. no. S0131), GSH
(GSH detection kit; cat. no. S0052) and SOD (SOD detection kit;
cat. no. S0101) in serum were detected using different detection
kits (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol.
ROS measurement
ROS content was assayed using 2,7-dichlorofluorescin
diacetate (DCF), a ROS detection kit (cat. no. H131224; Shanghai
Aladdin Bio-Chem Technology Co., Ltd.) according to the
manufacturer's protocol.
Iron assay
Iron concentration was assessed using an Iron Assay
kit (cat. no. MAK025-1KT; Sigma-Aldrich; Merck KGaA) according to
the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 6; GraphPad Software, Inc.). Data are
presented as the mean ± SEM. Comparisons among multiple groups were
analyzed using one-way ANOVA followed by Bonferroni's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
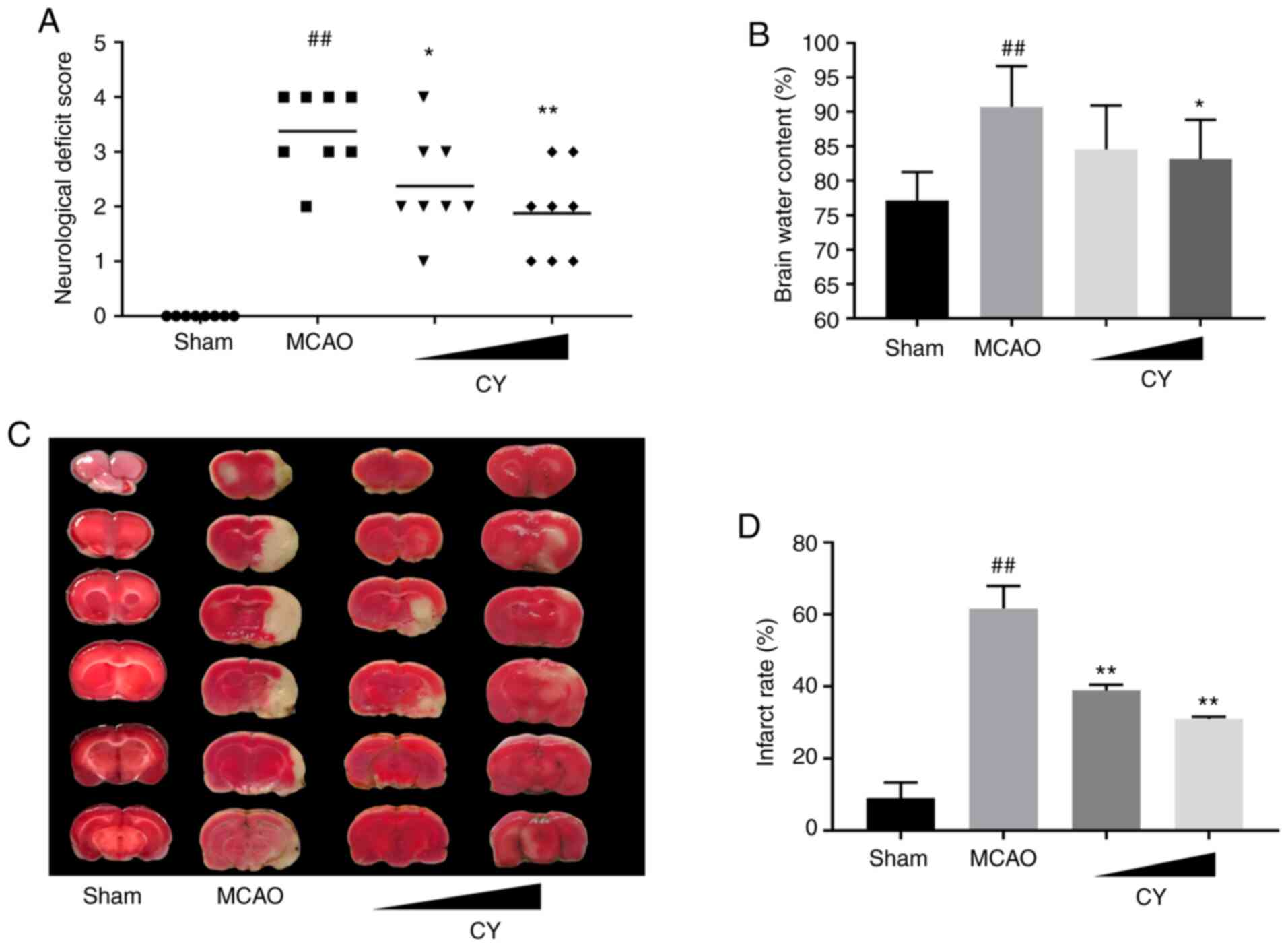
CY improves neurological scores
To determine whether CY induced neurological
function recovery in MCAO model rats, neurological scores were
evaluated at 24 h post-reperfusion (Fig. 2A). MCAO model rats displayed
increased neurological deficit scores compared with the Sham group,
whereas MCAO model-induced neurological deficits were significantly
relieved following treatment with CY. The results suggested that CY
improved neurological performance in MCAO model rats.
CY decreases infarction volume and brain
water content
MCAO induced a noticeable increase in brain water
content in the ischemic hemisphere at 24 h post-reperfusion, which
was reduced following CY treatment (Fig. 2B). The infarct volume at 24 h
post-reperfusion was determined by performing TTC staining. The
results revealed that the infarct area percentage of the brain
tissue in MCAO model rats was significantly higher compared with
the Sham group, and lower in the CY administration groups compared
with MCAO model rats (Fig. 2C and
D). The results indicated that CY effectively attenuated the
cerebral infarction area and brain water content in MCAO model
rats.
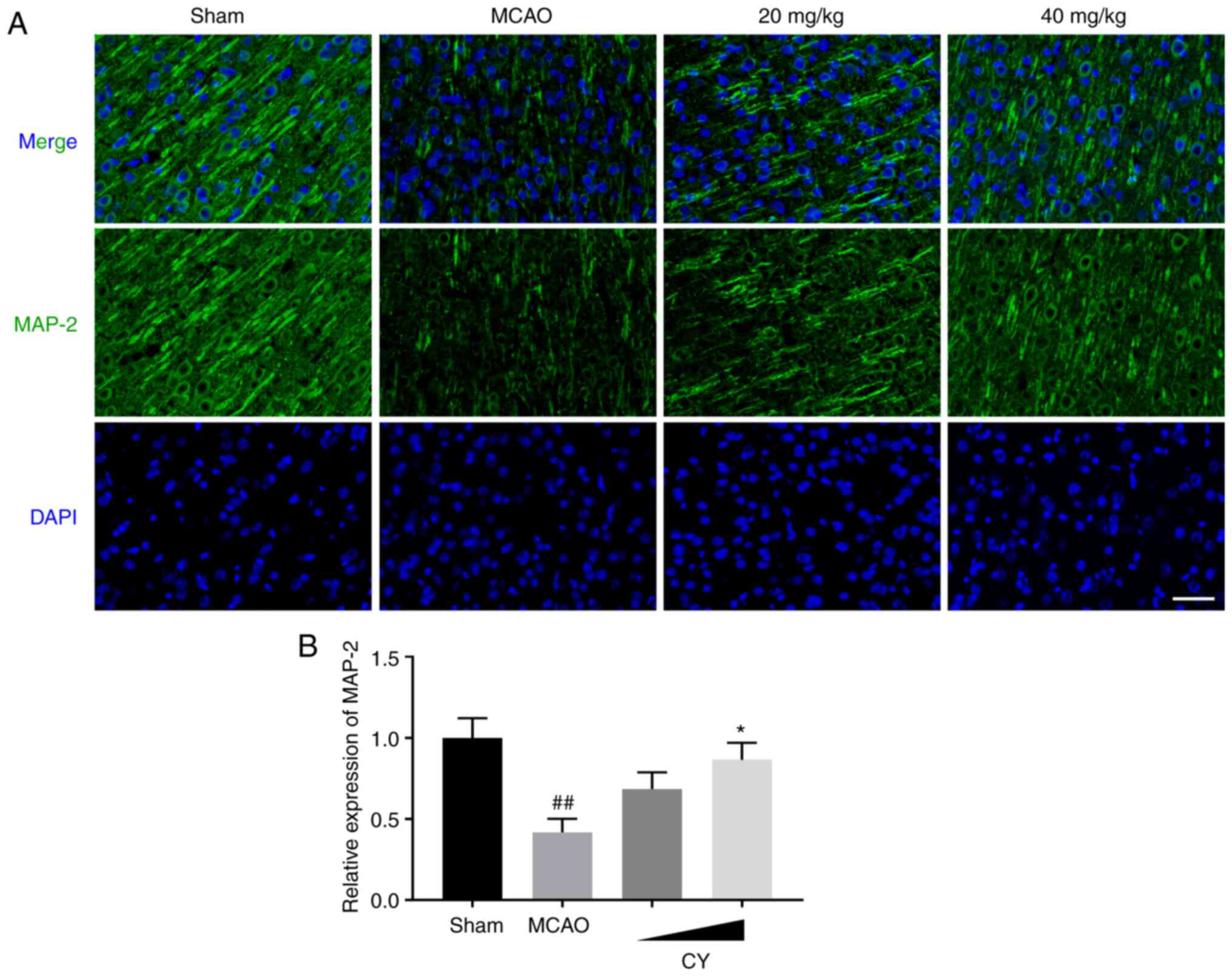
CY increases MAP-2 expression in the
cortex
To examine the protective effects of CY on neurons,
immunofluorescence staining for MAP2 was performed. Obvious MAP-2
immunostaining was observed in the cortex of Sham rats (Fig. 3). However, MAP-2 expression in
MCAO model rats at 24 h post-reperfusion was decreased compared
with the Sham group. Interestingly, CY successfully enhanced MAP-2
expression following administration for 14 days. The results
suggested that CY prevented neuronal damage in MCAO model rats.
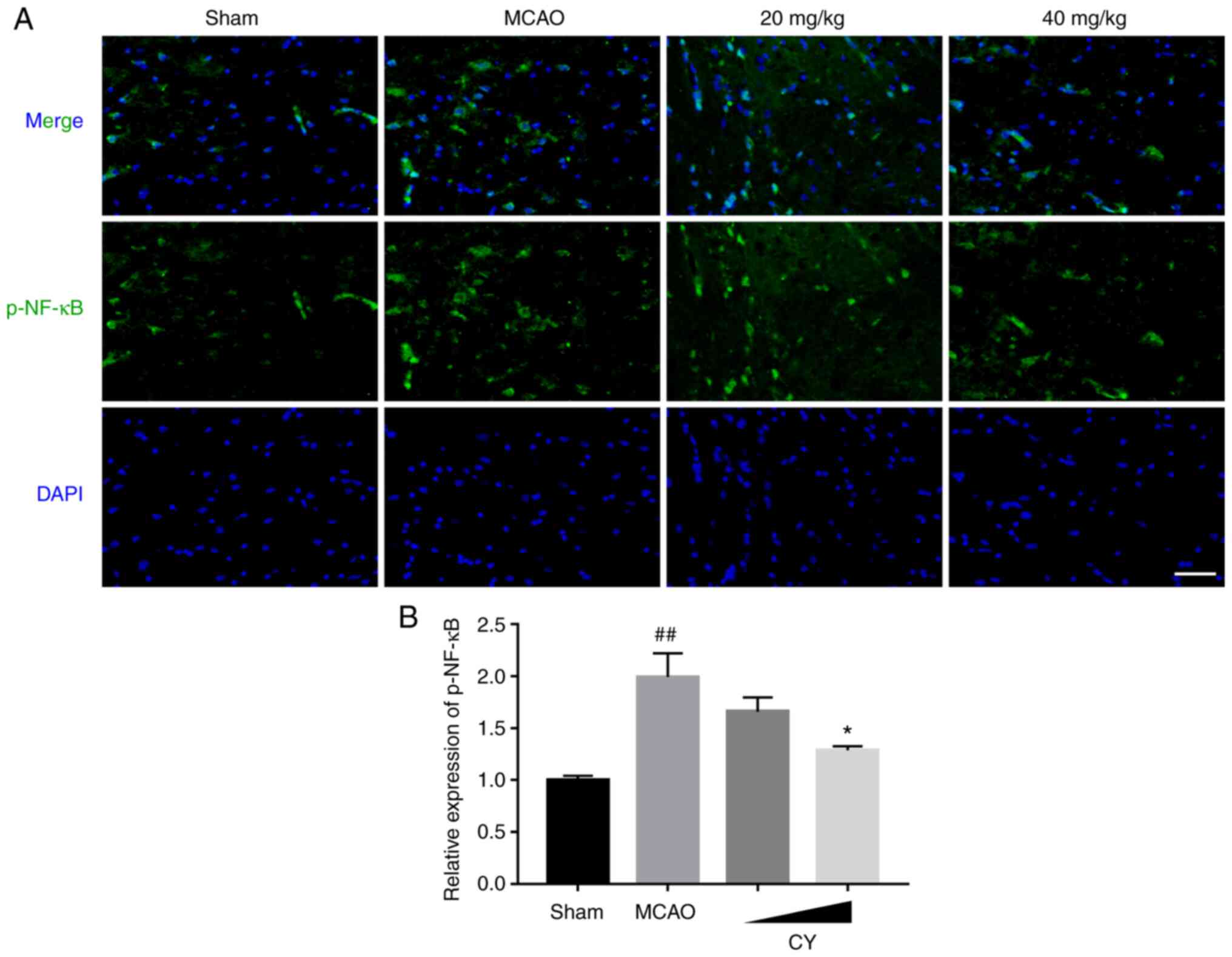
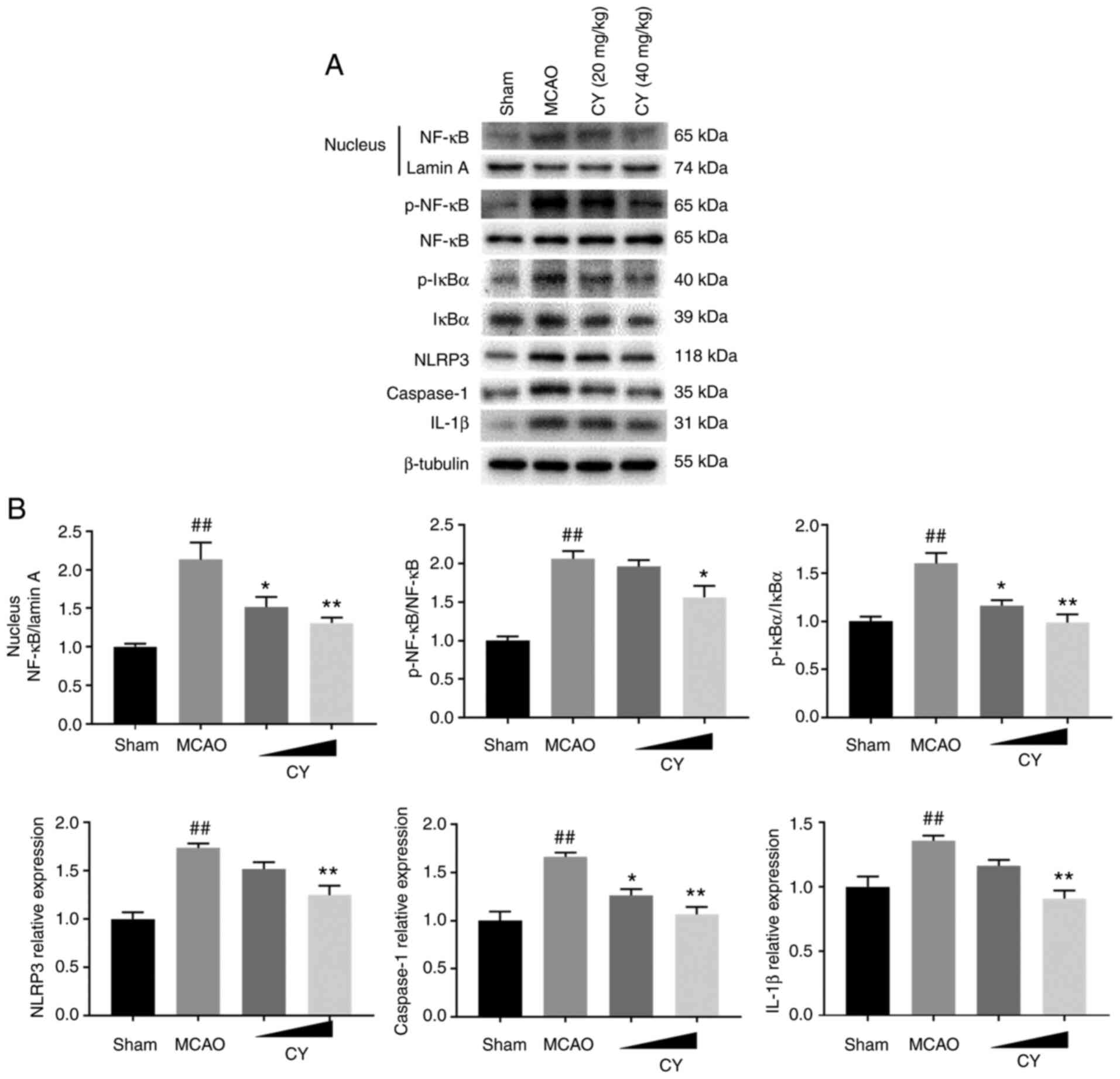
CY inhibits inflammation in the
cortex
Subsequently, immunofluorescence staining, western
blotting and ELISAs were performed to investigate the effects of CY
on inflammation. The results demonstrated that in the cortex of
MCAO model rats, p-NF-κB p65 expression detected via
immunofluorescence staining, as well as the protein expression
levels of p-NF-κB p65, p-IκBα, NLRP3, caspase-1 and IL-1β detected
via western blotting were significantly increased compared with the
Sham group (Figs. 4 and 5). Moreover, NF-κB p65 expression in
the nucleus was significantly increased compared with the Sham
group. Nonetheless, CY administration successfully reversed MCAO
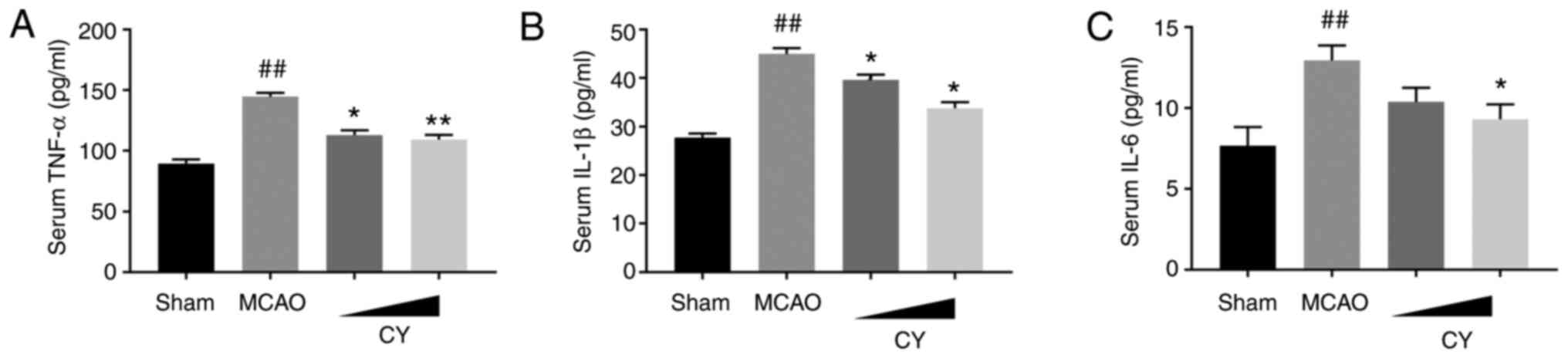
model-induced effects. In addition, the MCAO group displayed
evidently higher serum levels of TNF-α, IL-1β and IL-6 (Fig. 6). Notably, following CY
treatment, a marked decrease in TNF-α, IL-1β and IL-6 serum levels
was observed. Collectively, the results demonstrated that CY
treatment inhibited the inflammatory response in the cortex of MCAO
model rats.
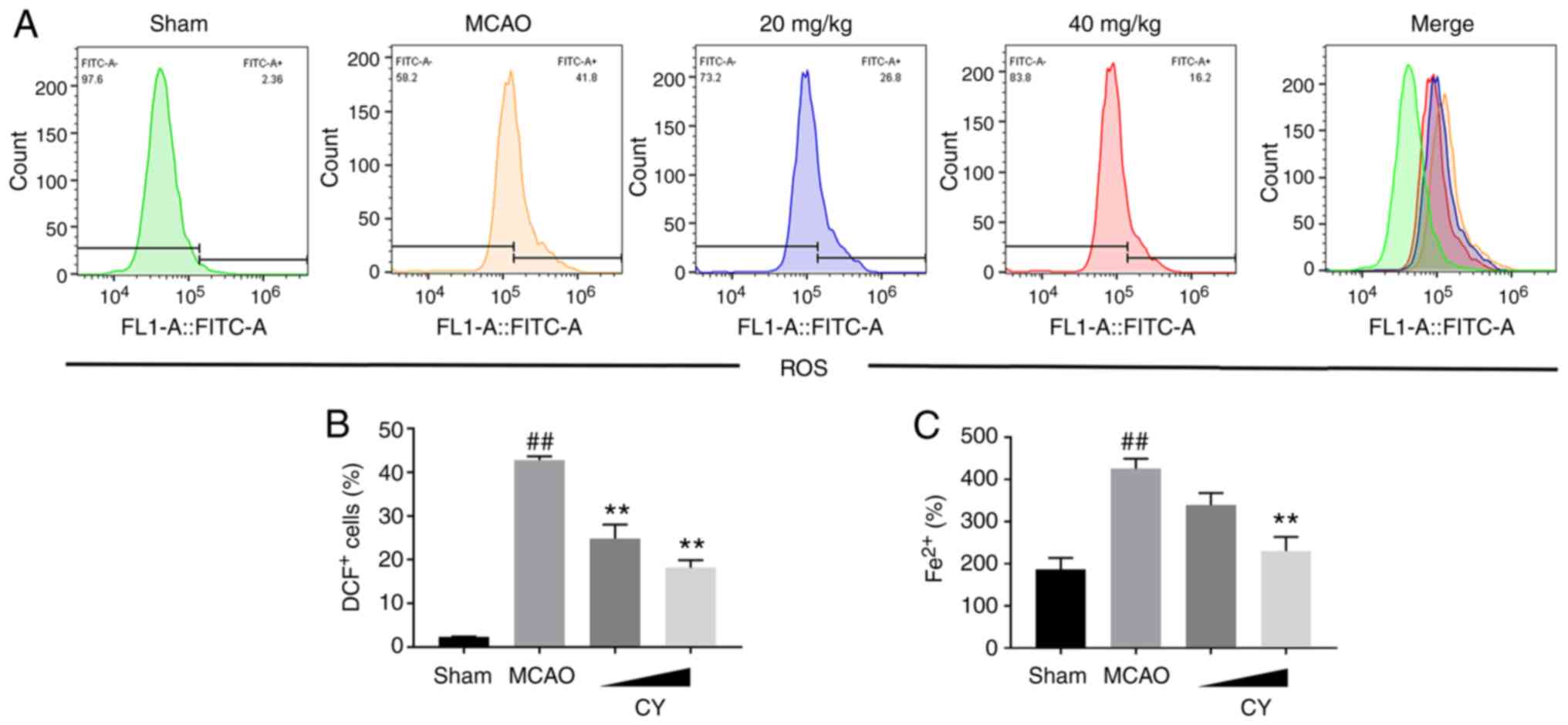
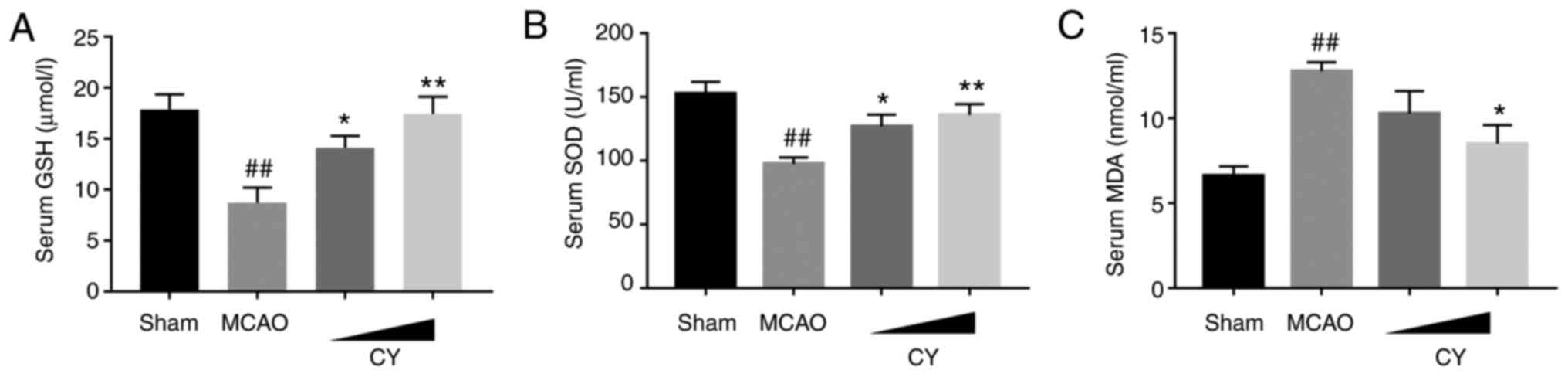
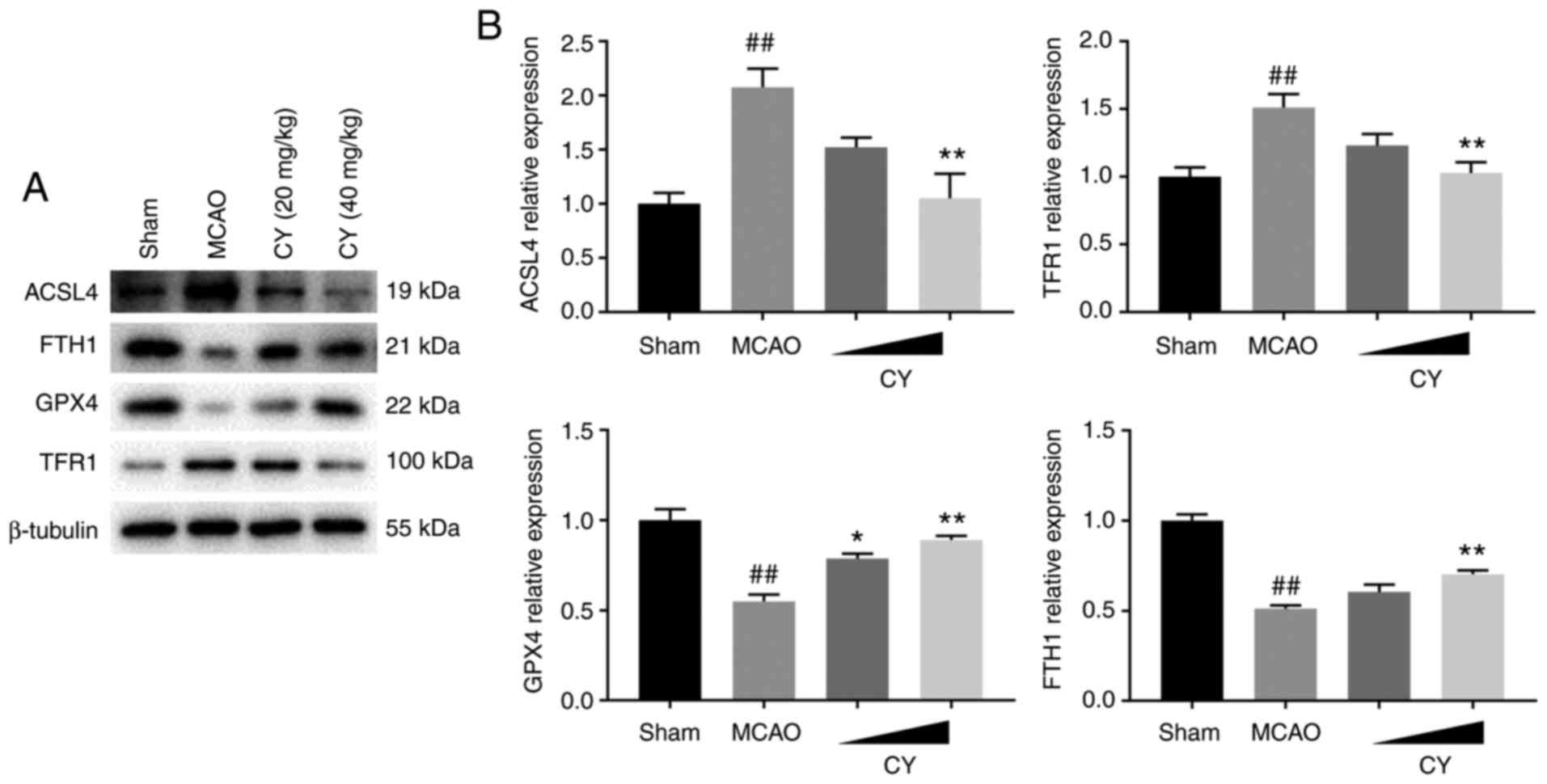
CY attenuates ferroptosis
To determine the involvement of ferroptosis in MCAO
model rats, iron and ROS accumulation, lipid peroxidation levels
and the expression levels of ferroptosis-related proteins were
assessed. MCAO model rats displayed obvious ROS and iron
accumulation in the cortex compared with Sham rats (Fig. 7). In addition, increased MDA
generation, but reduced GSH levels and SOD activities in the serum
were observed in MCAO model rats (Fig. 8). The western blotting results
indicated that MCAO challenge significantly increased the protein
expression levels of ACSL4 and TFR1 in the cortex, but decreased
the protein expression levels of FTH1 and GPX4 (Fig. 9). The aforementioned effects
induced by MCAO modeling were significantly ameliorated by
treatment with CY, indicating the antiferroptosis efficacy of CY in
MCAO model rats.
Discussion
In the present study, CY-mediated effects on
experimental ischemic stroke were investigated. CY improved
neurological deficit scores, brain water content, infarct area and
MAP-2 expression in MCAO model rats. CY also deactivated the
NF-κB/NLRP3 inflammasome signaling pathway in the cortex. In
addition, CY treatment decreased the serum concentrations of TNF-α,
IL-1β and IL-6. CY also alleviated MCAO-induced ferroptosis, as
demonstrated by reduced iron and ROS accumulation, reduced lipid
peroxidation level and restored ferroptosis-related protein
expression levels.
The MCAO model is one of the models that most
closely simulates human ischemic stroke (23). Animals that under-went cerebral
ischemia and reperfusion displayed neurological movement disorders
and pathological alterations, including brain edema and infarct
area (24). In the present
study, increased neurological deficit scores, brain water content
and cerebra infarct areas were detected in MCAO model rats,
suggesting the successful induction of ischemic stroke. However, CY
alleviated MCAO-induced neurological deficit, brain edema and
infarct area, indicating that CY pretreatment improved neurological
function and ischemic brain injury in MCAO model rats.
Ischemic stroke has been reported to damage neurons
(25). MAP-2, a protein
expressed primarily in neuronal dendrites, is critical for numerous
microtubule-related processes, including microtubule assembly,
stabilization and cross-linking (26). MAP-2 deficiency following
ischemic stroke has been documented in previous studies (27,28). In the present study, decreased
MAP-2 immunofluorescence activity was observed in MCAO model rats,
indicating MAP-2 defect in ischemic stroke. CY inhibited
MCAO-induced reductions in MAP-2, suggesting that CY protected rats
against MCAO-induced neuron injury.
Evidence suggests that the post-ischemic
inflammatory responses alter the outcome of ischemic stroke
(29). NF-κB is a pivotal
regulator of inflammation-related gene transcription (30). Studies have revealed that NF-κB
could serve as an upstream activator of the NLRP3 inflammasome,
which consists of NLRP3, ASC and caspase-1, and contribute to the
production of TNF-α, IL-1β and IL-6 (31,32). The NF-κB/NLRP3 inflammasome
signaling pathway has been implicated in ischemic stroke (33). Terai et al (25) examined the distribution of NF-κB
in brain samples obtained from patients who died following a
stroke, and discovered that the immunoreactivity of NF-κB was
enhanced in glial cells of infarcted areas compared with control
cases. Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan
derived from Arctium lappa L, has been reported to inhibit
MCAO-induced neuronal deterioration, cerebral infarct and brain
water content by inhibiting NLRP3 inflammasome activation and IL-1β
secretion (34). In the present
study, NF-κB/NLRP3 inflammasome signaling expression levels were
upregulated in the cortex of MCAO model rats, which was accompanied
by increased levels of TNF-α, IL-1β and IL-6 in the serum.
Following CY administration, the aforementioned MCAO-induced
effects were reversed, suggesting the involvement of NF-κB/NLRP3
inflammasome signaling in the pathology and treatment of ischemic
stroke.
Ferroptosis is a newly defined cell death process
that is characterized by iron-dependent accumulation of lipid
peroxides to lethal levels (35). Increasing evidence has
highlighted the role of ferroptosis in ischemic stroke (16). The content of free iron and MDA
in brain tissues were significantly augmented, whereas levels of
GPX4 and GSH were greatly decreased in MCAO model animals (36). Tau ablation protected mice
against from MCAO-induced iron accumulation in the brain and
inhibited ferroptosis, leading to the attenuation of the
ischemia-reperfusion injury including neurological defects, infarct
area, motor and cognitive dysfunctions (16). In the present study, MCAO
challenge promoted cortex iron and ROS accumulation and serum lipid
peroxidation levels, resulting in aberrant expression levels of
ferroptosis-related proteins in the brain, whereas treatment with
CY inhibited MCAO challenge-induced alterations. The aforementioned
results suggested that ferroptosis may partially account for
experimental ischemic stroke progression and CY-related treatment
effects in MCAO model rats.
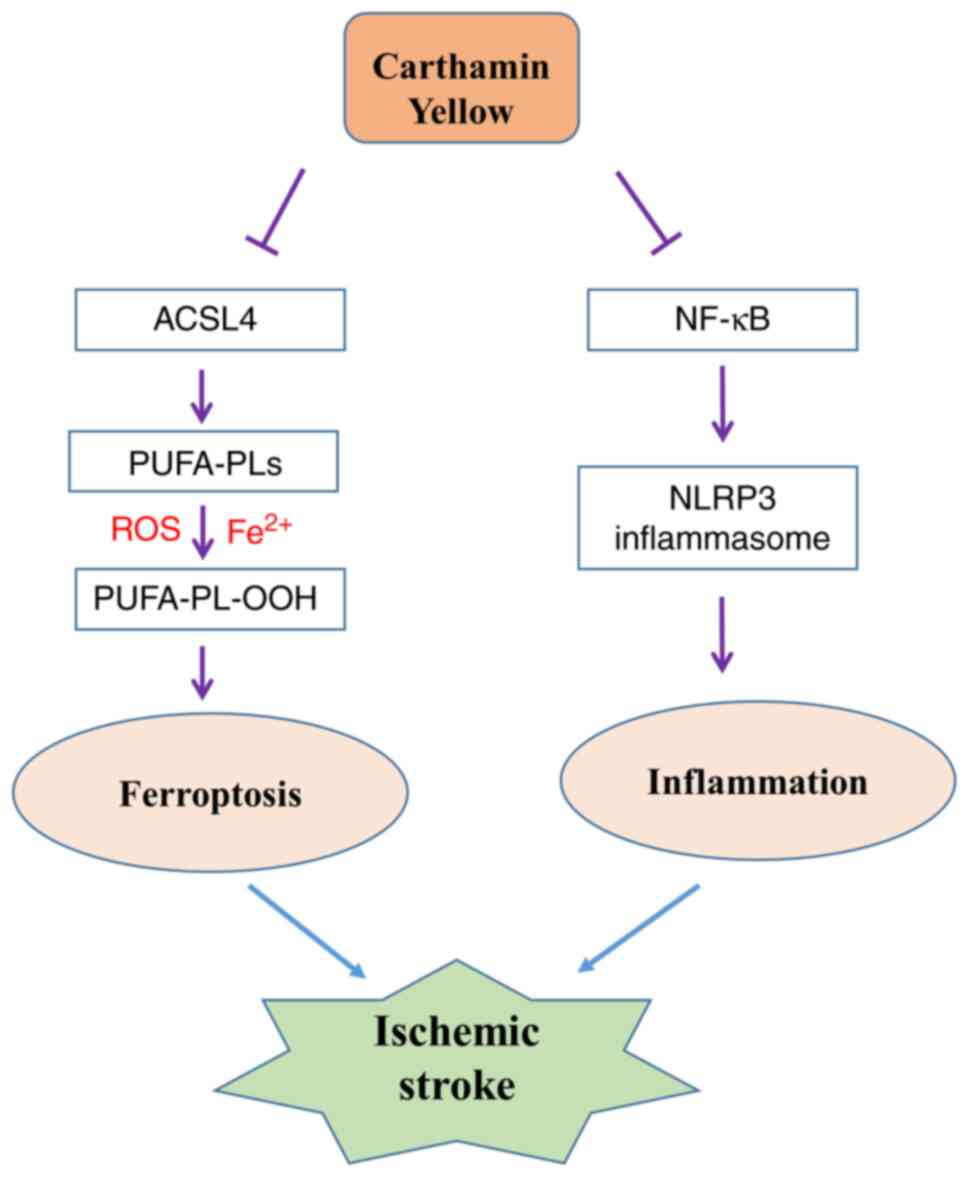
Collectively, the results of the present study
suggested that CY treatment protected rats against
ischemia-reperfusion injury by alleviating inflammation and
ferroptosis (Fig. 10). The
results indicated that CY may serve as a potential therapeutic
agent for ischemic stroke.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HG, CB and JL were involved in the design of the
study. HG, LZ and PT performed the experiments. DC analyzed the
data. YL interpretated the data and drafted the manuscript with the
support of all authors. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Animal Care and Use
Ethics Committee of Nanjing University of Chinese Medicine
(Nanjing, China) (approval no. 201907A544).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Cao G, Jiang N, Hu Y, Zhang Y, Wang G, Yin
M, Ma X, Zhou K, Qi J, Yu B and Kou J: Ruscogenin attenuates
cerebral ischemia-induced blood-brain barrier dysfunction by
suppressing TXNIP/NLRP3 inflammasome activation and the MAPK
pathway. Int J Mol Sci. 17:14182016. View Article : Google Scholar :
|
|
2
|
So PW, Ekonomou A, Galley K, Brody L,
Sahuri-Arisoylu M, Rattray I, Cash D and Bell JD: Intraperitoneal
delivery of acetate-encapsulated liposomal nanoparticles for
neuroprotection of the penumbra in a rat model of ischemic stroke.
Int J Nanomedicine. 14:1979–1991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anrather J and Iadecola C: Inflammation
and stroke: An overview. Neurotherapeutics. 13:661–670. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alim I, Caulfield JT, Chen Y, Swarup V,
Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie
EJ, et al: Selenium drives a transcriptional adaptive program to
block ferroptosis and treat stroke. Cell. 177:1262–1279.e25. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zahid A, Li B, Kombe AJK, Jin T and Tao J:
Pharmacological inhibitors of the NLRP3 inflammasome. Front
Immunol. 10:25382019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Negash AA, Olson RM, Griffin S and Gale M
Jr: Modulation of calcium signaling pathway by hepatitis C virus
core protein stimulates NLRP3 inflammasome activation. PLoS Pathog.
15:e10075932019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fann DY, Lim YA, Cheng YL, Lok KZ,
Chunduri P, Baik SH, Drummond GR, Dheen ST, Sobey CG, Jo DG, et al:
Evidence that NF-κB and MAPK signaling promotes NLRP inflammasome
activation in neurons following ischemic stroke. Mol Neurobiol.
55:1082–1096. 2018. View Article : Google Scholar
|
|
8
|
Wu H, Tang C, Tai LW, Yao W, Guo P, Hong
J, Yang X, Li X, Jin Z, Ke J and Wang Y: Flurbiprofen axetil
attenuates cerebral ischemia/reperfusion injury by reducing
inflammation in a rat model of transient global cerebral
ischemia/reperfusion. Biosci Rep. 38:BSR201715622018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang T, Wu M, Zhang Z, Yan C, Ma Z, He S,
Yuan W, Pu K and Wang Q: Electroacupuncture attenuated cerebral
ischemic injury and neuroinflammation through α7nAChR-mediated
inhibition of NLRP3 inflammasome in stroke rats. Mol Med.
25:222019. View Article : Google Scholar
|
|
10
|
Wang L, Liu Y, Du T, Yang H, Lei L, Guo M,
Ding HF, Zhang J, Wang H, Chen X and Yan C: ATF3 promotes
erastin-induced ferroptosis by suppressing system Xc. Cell Death
Differ. 27:662–675. 2020. View Article : Google Scholar
|
|
11
|
Bai T, Lei P, Zhou H, Liang R, Zhu R, Wang
W, Zhou L and Sun Y: Sigma-1 receptor protects against ferroptosis
in hepatocellular carcinoma cells. J Cell Mol Med. 23:7349–7359.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar :
|
|
13
|
Lei G, Zhang Y, Koppula P, Liu X, Zhang J,
Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H and Gan B: The role of
ferroptosis in ionizing radiation-induced cell death and tumor
suppression. Cell Res. 30:146–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng H, Schorpp K, Jin J, Yozwiak CE,
Hoffstrom BG, Decker AM, Rajbhandari P, Stokes ME, Bender HG, Csuka
JM, et al: Transferrin receptor is a specific ferroptosis marker.
Cell Rep. 30:3411–3423.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu J, Liu X, Tian Y, Li H, Ren Z, Liang S,
Zhang G, Zhao C, Li X, Wang T, et al: Moxibustion exerts a
neuroprotective effect through antiferroptosis in Parkinson's
disease. Evid Based Complement Alternat Med. 2019:27354922019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H,
Li XL, Liuyang ZY, Roisman L, Zhang ST, Ayton S, et al:
Tau-mediated iron export prevents ferroptotic damage after ischemic
stroke. Mol Psychiatry. 22:1520–1530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guan X, Li X, Yang X, Yan J, Shi P, Ba L,
Cao Y and Wang P: The neuroprotective effects of carvacrol on
ischemia/reperfusion-induced hippocampal neuronal impairment by
ferroptosis mitigation. Life Sci. 235:1167952019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu QY, Ma JQ, Duan YY, Sun Y, Yu S, Li B
and Zhang GM: Carthamin yellow protects the heart against
ischemia/reperfusion injury with reduced reactive oxygen species
release and inflammatory response. J Cardiovasc Pharmacol.
74:228–234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen B, Wang HT, Yu B, Zhang JD and Feng
Y: Carthamin yellow inhibits matrix degradation and inflammation
induced by LPS in the intervertebral disc via suppression of MAPK
pathway activation. Exp Ther Med. 14:1614–1620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun R, Song Y, Li S, Ma Z, Deng X, Fu Q,
Qu R and Ma S: Levo-tetrahydropalmatine attenuates neuron apoptosis
induced by cerebral ischemia-reperfusion injury: Involvement of
c-Abl activation. J Mol Neurosci. 65:391–399. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y, Hu Z, Du X, Davies H, Huo X and
Fang M: miR-16 and fluoxetine both reverse autophagic and apoptotic
change in chronic unpredictable mild stress model rats. Front
Neurosci. 11:4282017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen S, Dong Z, Cheng M, Zhao Y, Wang M,
Sai N, Wang X, Liu H, Huang G and Zhang X: Homocysteine exaggerates
microglia activation and neuroinflammation through microglia
localized STAT3 overactivation following ischemic stroke. J
Neuroinflammation. 14:1872017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fluri F, Schuhmann MK and Kleinschnitz C:
Animal models of ischemic stroke and their application in clinical
research. Drug Des Devel Ther. 9:3445–3454. 2015.PubMed/NCBI
|
|
24
|
Guo P, Jin Z, Wu H, Li X, Ke J, Zhang Z
and Zhao Q: Effects of irisin on the dysfunction of blood-brain
barrier in rats after focal cerebral ischemia/reperfusion. Brain
Behav. 9:e014252019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Terai K, Matsuo A, McGeer EG and McGeer
PL: Enhancement of immunoreactivity for NF-kappa B in human
cerebral infarctions. Brain Res. 739:343–349. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gumy LF, Katrukha EA, Grigoriev I, Jaarsma
D, Kapitein LC, Akhmanova A and Hoogenraad CC: MAP2 defines a
pre-axonal filtering zone to regulate KIF1-versus KIF5-dependent
cargo transport in sensory neurons. Neuron. 94:347–362.e7. 2017.
View Article : Google Scholar
|
|
27
|
He F, Dai R, Zhou X, Li X, Song X, Yan H,
Meng Q, Yang C and Lin Q: Protective effect of 4-Methoxy benzyl
alcohol on the neurovascular unit after cerebral ischemia
reperfusion injury. Biomed Pharmacother. 118:1092602019. View Article : Google Scholar
|
|
28
|
Xu SY, Hu YF, Li WP, Wu YM, Ji Z, Wang SN,
Li K and Pan SY: Intermittent hypothermia is neuroprotective in an
in vitro model of ischemic stroke. Int J Biol Sci. 10:873–881.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lambertsen KL, Finsen B and Clausen BH:
Post-stroke inflammation-target or tool for therapy? Acta
Neuropathol. 137:693–714. 2019. View Article : Google Scholar
|
|
30
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
31
|
Yu X, Lan P, Hou X, Han Q, Lu N, Li T,
Jiao C, Zhang J, Zhang C and Tian Z: HBV inhibits LPS-induced NLRP3
inflammasome activation and IL-1β production via suppressing the
NF-κB pathway and ROS production. J Hepatol. 66:693–702. 2017.
View Article : Google Scholar
|
|
32
|
Zeng J, Chen Y, Ding R, Feng L, Fu Z, Yang
S, Deng X, Xie Z and Zheng S: Isoliquiritigenin alleviates early
brain injury after experimental intracerebral hemorrhage via
suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome
activation by promoting Nrf2 antioxidant pathway. J
Neuroinflammation. 14:1192017. View Article : Google Scholar
|
|
33
|
Ye Y, Jin T, Zhang X, Zeng Z, Ye B, Wang
J, Zhong Y, Xiong X and Gu L: Meisoindigo protects against focal
cerebral ischemia-reperfusion injury by inhibiting NLRP3
inflammasome activation and regulating microglia/macrophage
polarization via TLR4/NF-κB signaling pathway. Front Cell Neurosci.
13:5532019. View Article : Google Scholar
|
|
34
|
Zhang S, Jiang L, Che F, Lu Y, Xie Z and
Wang H: Arctigenin attenuates ischemic stroke via SIRT1-dependent
inhibition of NLRP3 inflammasome. Biochem Biophys Res Commun.
493:821–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weiland A, Wang Y, Wu W, Lan X, Han X, Li
Q and Wang J: Ferroptosis and its role in diverse brain diseases.
Mol Neurobiol. 56:4880–4893. 2019. View Article : Google Scholar :
|
|
36
|
Lu J, Xu F and Lu H: LncRNA PVT1 regulates
ferroptosis through miR-214-mediated TFR1 and p53. Life Sci.
260:1183052020. View Article : Google Scholar : PubMed/NCBI
|