Transforming growth factor-β (TGF-β) is a
complicated polypeptide that exerts essential effects on cell cycle
regulation, growth and development, differentiation, extracellular
matrix (ECM) synthesis, hematopoiesis, chemotaxis and the immune
response (1-3). TGF-β1 and TGF-β receptor 1
(TGF-βR1) serve important roles in the TGF-β family, and have
irreplaceable effects on cell reproductive capacity, growth, wound
regeneration and immunological reactions (4,5).
Almost all cells in the body, not only the epithelium and
lymphocytes, but also the stroma, cellular immunity and
endotheliocytes, are associated with tumor occurrence and
development (6,7). Furthermore, most tumor cells
express TGF-β1 and TGF-βR1 (8-10). Previous studies have found that
cancer cells create an environment that hinders the immune response
by producing factors, such as TGF-β1, to evade T-cell surveillance
(11,12). Other studies have revealed that
TGF-β1 can cause epithelial-mesenchymal transformation (EMT),
resulting in increased migration of cancer cells (13,14). TGF-βR1 is an irreplaceable
downstream molecule of TGF-β1 that participates in the entire life
cycle of cells, including cell movement, differentiation,
adsorption, fission and death (4,15). Mutant cells that lack TGF-βR1 do
not respond to TGF-β1, which further affects the transduction of
the TGF-β signaling pathway (16,17). Additionally, the activation or
overexpression of TGF-βR1 is observed in different types of tumor
and can serve an important role in tumor cell proliferation and
migration to other tissues by taking part in EMT (18), such as in colon (19) and gastric cancer (20). Previous studies (20-22) have revealed that TGF-βR1 is
observed in different types of tumor and serves an important role
in tumor metastasis by participating in cancer development, cell
migration and blood vessel regeneration, leading to unsatisfactory
responses to treatment.
The present review primarily discusses the impact of
TGF-β1 and TGF-βR1 on malignant tumors, to offer different
strategies to restrain and cure these tumors.
The TGF-β superfamily is a class of structural and
functional polypeptide growth factor subfamilies, including bone
morphogenetic protein, growth differentiation factor,
anti-Mullerian tube hormone, activin Nodal and TGF-β (4,23). Since TGF-β was first isolated
from serum-free culture medium of mouse sarcoma virus-transformed
embryonic fibroblasts in 1978, five subtypes of TGF-β have been
identified, namely TGF-β1-5. However, only TGF-β1/2/3 exist in
mammals (24). These three
growth factors have 70-82% homology at the amino acid level, but
their functions are distinct, with TGF-β1 being the most important
(25). TGF-βR exists on the cell
surface and has high affinity for TGF-β (26). According to the features and
roles of the receptor, it can be divided into TGF-βR1 (or ALK-5),
TGF-βR2 and TGF-βR3 (27,28).
At present, seven types of TGF-βR1 and five types of TGF-βR2 have
been identified in humans (29).
TGF-β signaling positively influences early embryonic growth and
tissue and organ formation, immune supervision, tissue repair and
adult cell homeostasis (30).
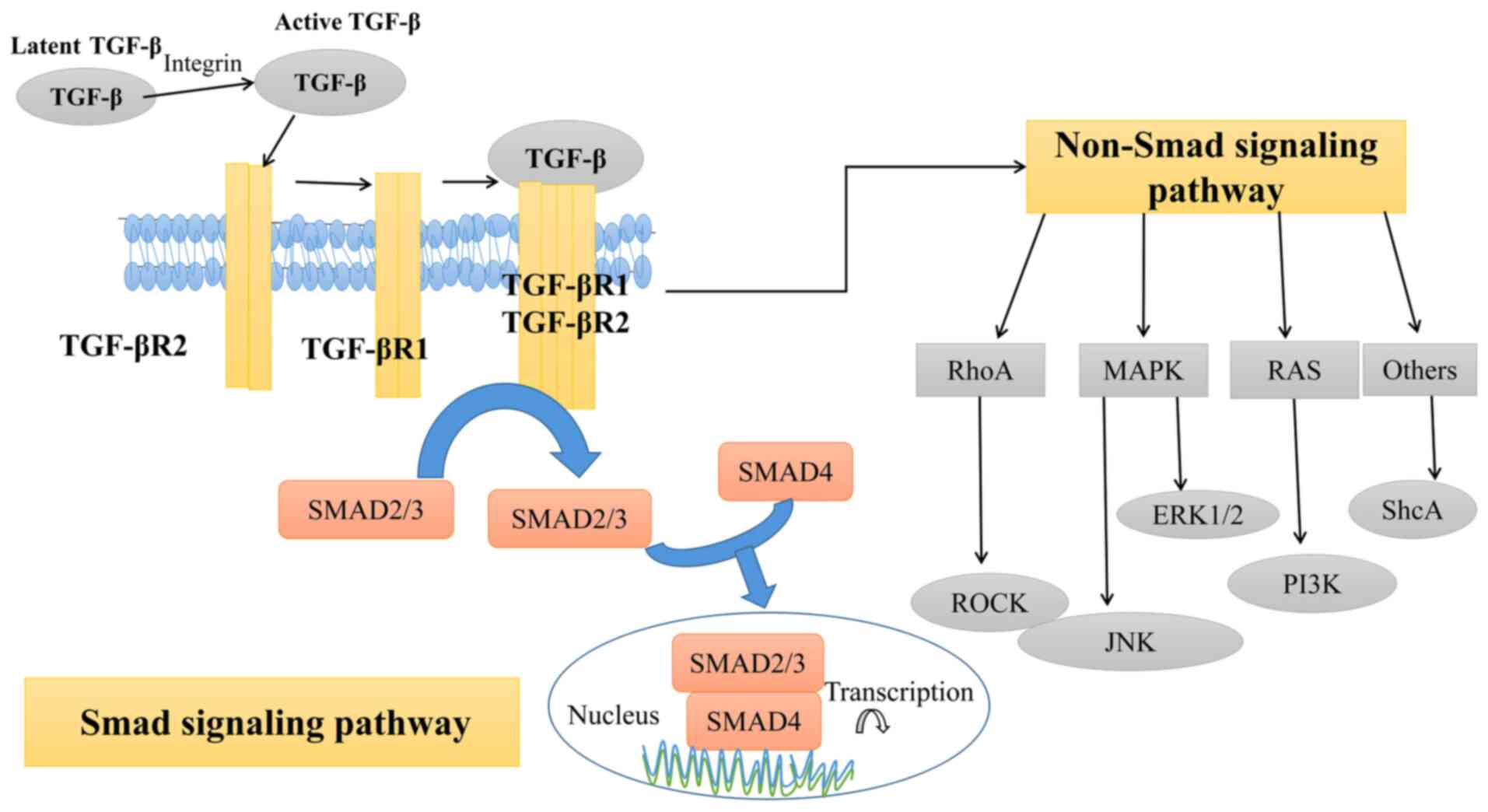
Abnormal TGF-β cell signaling transduction pathways are finely
regulated at different levels, including ligands, receptors, Smad
and nuclear transcription. In the classical Smad signaling pathway
(Fig. 1), TGF-β family cytokines
first induce serine/threonine kinase receptors on the cell membrane
to form functional complexes: two TGF-βR2 and two TGF-βR1 (31,32). Subsequently, TGF-βR2
phosphorylates the domains of glycine and serine in TGF-βR1,
activating the kinase activity of TGF-βR1, which then
phosphorylates Smad2 and Smad3, binding them to Smad4 and resulting
in the synthesis of Smad compounds, nuclear transport and Smad-DNA
binding (30). Next, Smad
mediates the transcription of target gene DNA to RNA, together with
the general transcription factor, other transcription factors or
helper proteins (2,33). Additionally, TGF-β can exert
signal transduction through non-Smad pathways (Fig. 1). To date, these pathways
primarily include the RhoA-Rock1, RAS, ShcA, ERK1/2 and MAPK
signaling pathways (34,35).
In mammals, the TGF-β family uses 33 genes to encode
a polypeptide, a predomain of 250 residues and a structural domain
of growth factors composed of 110 residues (24). TGF-β1 is an important member of
the cytokine TGF-β superfamily and is located on chromosome 19q3
(30). Mature TGF-β1 is composed
of 112 amino acids and contains nine highly conserved cysteine
residues at the C-terminus, which form the rigid structure of
cysteine through disulfide linkages (36). TGF-β1 exerts a critical effect on
cellular development with respect to cell proliferation,
differentiation, adsorption and programmed cell death (37).
The generation and secretion of TGF-β1 is based on
latency-associated peptide (LAP), which is a potentially inactive
compound with a large predomain and a dimeric non-covalent binding
TGF-β1 growth factor domain (36). Latent TGF-β1 is activated through
coordination with its binding protein. Latent TGF-β binding
proteins (LTBPs) consist of four subtypes (LTBP-1, LTBP-2, LTBP-3
and LTBP-4), which covalently bind to LAP through disulfide bonds
to form a potential complex with pre-TGF-β1 (38). Once hydrolyzed by proteases or
LAP interactions with integrin αvβ3, αvβ5 or αvβ8, TGF-β1 can
combine with downstream receptors (15).
At present, seven types of TGF-β1 receptors and five
types of TGF-β2 receptors have been identified in humans (29). The TGF-β1 receptor contains seven
protein activin receptor-like kinases (ALK1-ALK7), and ALK5 is also
known as TGF-βR1 (39). TGF-βR1,
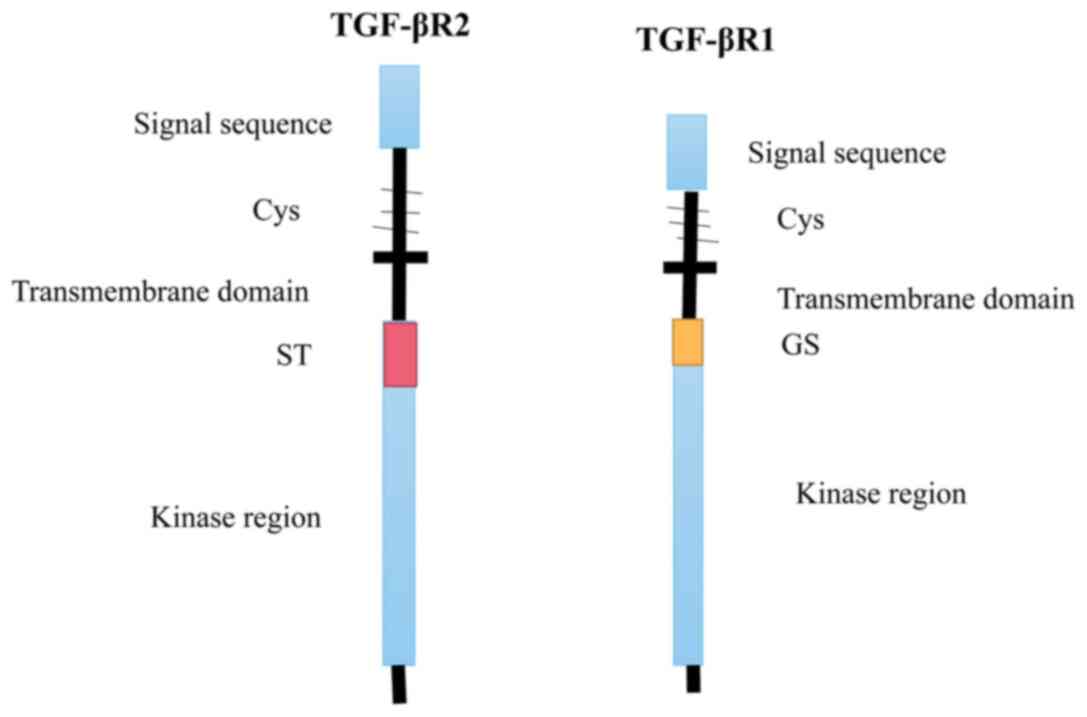
an essential molecule in the TGF-β signaling pathway with a weight
of 53 kDa, phosphorylates serine or threonine in downstream
signaling proteins; it consists of a signal peptide, a hydrophilic
extracellular region, a transmembrane domain and an intracellular
region (27,30). The extracellular region contains
multiple cysteines and has a glycosyl slip site; the intracellular
region near the membrane contains a region rich in glycine and
serine that is associated with its autophosphorylation (40). On the other hand, there is a
segment rich in serine/threonine in the intracellular region of
TGF-βR2 that can phosphorylate TGF-βR1 during signal transduction,
activating the TGF-βR1 kinase region, further phosphorylating
downstream substrates and transferring the TGF-β signal into cells
(2) (Fig. 2).
Some studies have demonstrated that TGF-β1
expression is increased in prostate cancer (46), ovarian cancer (47), hepatocellular carcinoma (12), bladder cancer (48), breast cancer (49) and cholangiocarcinoma (50), suggesting that abnormal TGF-β1
expression can influence tumor invasiveness and result in a poor
prognosis. Regarding TGF-βR1, a previous study has found that it
promotes tumor angiogenesis by upregulating matrix
metalloproteinase 9 in metastatic human melanoma cells (51) Moreover, changes in TGF-βR have
been observed in numerous types of human tumors, such as breast
(52,53), colon (54) and gastric cancer (20), and are characterized by gene
mutations, decreased levels or inactivation of TGF-βR. TGF-βR1
mutations have been reported in malignant tumors of the ovary,
breast and pancreas, as well as in colon cancer (16,52-56). These findings all suggest that
TGF-βR mutations serve an important role in the genesis and
progression of tumors (57). The
functions of TGF-β and TGF-βR1 can be either direct or indirect in
the pathogenesis of some tumors (Table I).
Worldwide, BC is one of the most common types of
cancer in women, with high mortality and recurrence rates (58,59). Although numerous efforts have
been made to increase the quality of treatment for BC, the 5-year
survival rate of patients after metastasis is 27% (60).
TGF-β1 is well known to regulate the development,
differentiation, carcinogenesis and tumor progression of breast
epithelial cells. TGF-β1 was first identified as a regulatory
factor of BC >20 years ago (49). Previous studies have revealed
that TGF-β1 promotes BC metastasis by promoting EMT in tumor cells
(61,62). These cells lose epithelial
characteristics during EMT, as well as cell polarity and adhesion,
developing migratory and invasive capacities (63). It has been demonstrated that
microRNAs (miRNAs/miRs) are key factors in the growth and
metastasis of numerous invasive tumors (64), and TGF-β1 signaling is associated
with miRNAs (65-67). Compared with benign proliferative
breast diseases, TGF-β1 upregulates miR-21 expression, but it
downregulates miR-196A-3p expression (66,68). miR-21 expression is significantly
upregulated in BC (66). A
series of steps to promote tumor development through miR-21 occur
via mutual interaction with tumor suppressor genes, such as PTEN
(66). Thus, the process of
TGF-β1 upregulating miR-21 expression and miR-21 interacting with
tumor suppressor genes promotes the progression and therapy
resistance of BC (66). The
downregulation of miR-196A-3p by TGF-β1 is associated with the
progression of BC and is a biomarker for predicting BC metastasis
and patient survival (68).
However, Wang et al (63)
revealed that overexpression of miR-133b markedly restrained the
function of TGF-βR1 in TGF-β1/Smad signal transduction and
inhibited TGF-β induced endometrial stromal transformation and BC
cell invasion in vitro.
In addition, a previous study has demonstrated that
TGF-β1 can regulate the expression of C-X-C motif chemokine
receptor 4 (CXCR4) in MCF-7 BC cells, which has a critical effect
on the metastasis of BC (69).
Moreover, the upstream regulator of the TGF-β1/Smad3 signaling
pathway in BC is hypoxia-inducible factor-1 (HIF-1), which
regulates the proliferation and apoptosis of BC cells (70). Furthermore, another study has
revealed that leptin mediates the metastatic invasiveness and
cancer stem cell behavior of BC cells via binding TGF-β1 and its
receptor (71), which may
explain why women with BC who are obese or overweight have a poor
prognosis according to epidemiological studies (72,73).
BAG-1 is a multifunctional protein associated with
the heat shock response, cell signal transduction, cell survival
and apoptosis (86). A previous
study has found that BAG-1 expression is upregulated during the
relatively early stages of colorectal tumorigenesis (87). Notably, BAG-1 is thought to
promote the progression of colorectal tumors by inhibiting TGF-β1
to allow more tumor cells to avoid death (87). Neuropilins were originally
thought to be neuron receptors and were later found to be
co-receptors for cancer-associated growth factors. The neuropilin
family consists of two genes, neuropilin-1 and neuropilin-2
(88). Grandclement et al
(19) revealed that neuropilin-2
was the receptor of TGF-β1 through surface isomer resonance
experiments. It was demonstrated that the synergistic action of
neuropilin-2 and TGF-βR1 facilitated EMT in colorectal cancer cells
(19). However, Huang et
al (89) revealed that
TGF-β1 induced glutathione peroxidase-1 (GPx-1), which is an
antioxidant enzyme (90),
expression and enzyme activity by activating
TGF-βR1/Smad2/ERK1/2/HIF-1α signaling cascades, and this GPx-1
upregulation protects human colon adenocarcinoma DLD-1 cells or
colorectal cancer cells from oxidative damage. TGF-β1 and TNF-α
also induce EMT in CC cells through the NF-κB signaling pathway
(91).
GC is a malignant neoplasm of the alimentary canal
that derives from the gastric mucosal epithelium (94). GC accounted for 5.7% of global
cancer cases, and its death rate (8.2%) ranked second among all
cancer cases according to the GLOBOCAN 2018 estimates of cancer
incidence and mortality produced by the International Agency for
Research on Cancer (IARC) (95).
At present, the pathogenesis of GC has not been entirely
elucidated, and previous studies have demonstrated that multiple
genes and regulatory factors are associated with the occurrence and
progression of solid tumors, such as GC (96,97). Abnormalities in any part of the
TGF-β/Smad signaling pathway may lead to signal transduction
disorders, which lead to the development and progression of GC
(98). Some studies have shown
that TGF-β1 and TGF-βR1 are highly expressed in GC and are
associated with the initiation, development and metastasis of GC
(20,99,100). Yanagihara and Tsumuraya
(101) have demonstrated that
TGF-β1 restrains proliferation and leads to apoptosis of the GC
cell lines HSC-39 and HSC-43 in vitro. It has been
speculated that TGF-β1 may regulate the metastatic ability of GC
cells by facilitating the destruction and penetration of the
basement membrane barrier, and the adhesion and activity of GC
cells (102). Therefore,
blocking the TGF-β1 signaling pathway may inhibit the invasion and
migration of GC cells. Furthermore, immunosuppression mediated by
regulatory T cells (Tregs) is an important mechanism of tumor
immune escape, as well as the primary obstacle to the success of
tumor immunotherapy (103). A
previous study has suggested that GC may gain strength by inducing
Tregs under hypoxic conditions through the TGF-β signaling pathway,
allowing tumor cells to escape immunosurveillance (104). In addition, increased Tregs in
the tumor are critically associated with a poor prognosis in
patients with GC (105). A
previous study has demonstrated that TGF-β1 can downregulate
miR-193b expression in GC cell lines, and that miR-193b can
downregulate urokinase-type plasminogen activator protein
expression in GC cells to promote the invasion and peritoneal
metastasis of GC cells (106).
In addition, some studies have revealed that the enhanced motility
of tumor cells, tumor development and metastasis are associated
with the ERK signaling pathway (107-109). TGF-β1 mediates the ERK
signaling pathway in GC with the participation of CD133 (107).
In addition to the aforementioned studies, an
increasing number of studies have confirmed a significant impact of
TGF-β1 and TGF-βR1 expression on the biological behavior of
malignant tumors, which is closely associated with prognosis
(56,75,78).
HCC is associated with more than half of the cases
of primary liver cancer, ranking sixth among the most frequent
types of cancer worldwide and third in cancer-associated deaths
according to the GLOBOCAN 2018 estimates of cancer incidence and
mortality produced by the IARC (95). The occurrence of liver cancer,
like other malignant tumors, is a complex process of multistep,
multifactorial and multilink interactions. In recent years, some
studies have begun to focus their attention on the TGF-β signaling
pathway in HCC (110-112). Numerous studies have
demonstrated that TGF-β1 and TGF-βR1 expression has critical
impacts on the growth, metastasis, invasion and prognosis of liver
cancer (32,111-114). Peng et al (12) analyzed the association between
TGF-β1 expression and clinicopathological characteristics in
patients with HCC using The Cancer Genome Atlas, and assessed the
impact of TGF-β1 expression on the ability to recover after
treatment. The results demonstrated that increased expression
levels of TGF-β1 promoted a poor prognosis in patients with HCC
(12). TGF-β1 expression is
significantly upregulated in HCC tissues and regulates the tumor
microenvironment by stimulating angiogenesis, increasing tumor cell
adhesion and immunosuppression, or inducing Treg production to
promote tumor invasion and metastasis (115). EMT has a critical effect on the
development and metastasis of human cancer. TGF-β1 induces EMT and
promotes HepG2 cells to metastasize and invade other tissues
through JAK/STAT3/Twist signal transduction (111). Moreover, the TGF-β1/miR-140-5p
axis promotes EMT in liver cell carcinoma by regulating the Flap
endonuclease 1 (67). In
addition, TGF-β1 affects the interaction between HCC-derived
stromal cells and liver-derived microvascular endothelial cells by
downregulating the expression levels of neural cell adhesion
molecule, in this way promoting vascular changes induced by HCC
(116). Another study has
reported that TGF-β1 activates miR-135a-5p to downregulate
Krüppel-like factor 4 (KLF4) to promote proliferation and
metastasis of HCC cells (117).
KLF4, a zinc finger transcription factor, can regulate the cell
cycle, proliferation and apoptosis (118), and inhibit tumor growth in HCC
(119). In addition, Zhang
et al (120) have
demonstrated that TGF-β1 targets the Hippo signaling pathway by
regulating a series of key proteins, such as large tumor suppressor
1 and Yes-association protein 1; this process inhibits the
proliferation of hepatoma cells.
TC represents a group of malignant tumors that
primarily originate from follicular cells, which are the main
components of the thyroid unicellular epithelium. Anaplastic TC
(ATC) is the main cause of death among all malignant thyroid
tumors, and the median survival time of patients is ~6 months
(122). The tolerance of ATC to
routine treatment of TC, including surgery and radioiodine and
thyrotropin inhibition, results in a very unsatisfactory
therapeutic effect (123). At
present, effective means to treat ATC have not been identified, and
therefore the survival rate of patients has not improved for >60
years (124). In TC, it has
been demonstrated that high expression levels of TGF-β1 closely
affect TC development (125,126). TGF-β1 promotes apoptosis of ATC
cells via TGF-β/ERK1/2/NF-κB/PUMA signaling (127). Additionally, TGF-β1 upregulates
the expression levels of high mobility group A1 (128), which belongs to the superfamily
of non-histone chromatin-binding proteins, serves an important role
in multiple cellular biology processes through the PI3K/Akt
signaling pathway and upregulates lncRNA-ATB expression to promote
TC cell proliferation, migration and invasion (129). miRNAs are also critical factors
in the occurrence and growth of numerous tumors. For example, Zhang
et al (126) found that
miR-483-3p targeting par-3 family cell polarity regulator induced
TGF-β1 to promote ATC cell migration, invasion and EMT. Notably, Li
et al (130) found that
epigallocatechin-3-gallate significantly inhibited the invasion and
migration of ATC8505C cells in vitro by mediating EMT and
the TGF-β/Smad signaling pathway.
For TGF-βR1, it has been found that solute carrier
family 35 member F2 activates the MAPK signaling pathway by
targeting the phosphorylation of TGF-βR1 and apoptosis
signal-regulating kinase 1, accelerating the proliferation and
migration of thyroid papillary carcinoma cells (131).
In the early stage of leukemia, megakaryocytes can
produce excessive TGF-β1 and directly upregulate early growth
response 3 expression to interfere with the development of normal
hematopoietic stem cells in patients with AML (142). This process may provide an
effective therapeutic target for improving normal hematopoiesis in
AML (142). Ma et al
(5) found that leucine-rich
repeat containing protein 33, a cell membrane-associated protein,
formed complexes with pro-TGF-β1 and regulated the function of
TGF-β1 in AML cells and other myeloid malignancies. However, to the
best of our knowledge, no studies have investigated the mechanism
of TGF-βR1 in leukemia.
Lung cancer, including non-small cell lung cancer
(NSCLC) and small cell lung cancer, is the dominant cause of
cancer-associated death worldwide according to the GLOBOCAN 2018
estimates of cancer incidence and mortality produced by the IARC
(95). Modern treatment
primarily depends on radiotherapy and chemotherapy (143). NSCLC is the major type of lung
cancer and is a severe public health issue in China and in numerous
developing countries (144).
More than half of patients with NSCLC experience tumor recurrence
after surgical resection, and the survival rate of these patients
is low (145). TGF-β1 is
closely associated with EMT in epithelial cancers, including NSCLC.
Li et al (146) found
that the interaction between heterogeneous ribonucleoprotein K
(HnRNP K) and microtubule-associated protein 1B-light chain 1
promoted the transformation of lung cancer cells from epithelial to
mesenchymal cells mediated by TGF-β1. Shi et al (147) investigated the function of
hematopoietic pre-B-cell leukemia transcription factor-interacting
protein (HPIP) in the transformation of A549 lung cancer cells
induced by TGF-β1 in vitro, revealing that HPIP silencing
significantly decreased the transformation, migration or invasion
of A549 cells mediated by TGF-β1, which makes HPIP a new potential
target for lung cancer treatment.
Previous studies have demonstrated that miRNAs have
critical effects on the early diagnosis and treatment of NSCLC. It
has been demonstrated that overexpression of miR-29c inhibits the
Sp1/TGF-β axis, which induces lung cancer endothelial cells to
metastasize (148). miR-144-3p
suppresses the metastasis and adhesion of lung carcinoma cells
induced by TGF-β1 by meditating the Src-Akt-Erk signaling pathway
(149). AWPPH is a recently
discovered lncRNA with carcinogenic effects in HCC and bladder
cancer (150,151). Tang et al (152) revealed that AWPPH upregulated
TGF-β1 expression, promoting long-term recurrence after NSCLC.
In recent years, with the improvements in screening
and diagnostic techniques and the development of new vaccines, both
the prevalence and mortality rates of cervical cancer have
decreased; however, cervical cancer ranked fourth in morbidity
(6.6%) and mortality (7.5%) rates among all female cancer cases
according to the GLOBOCAN 2018 estimates of cancer incidence and
mortality produced by the IARC (95). The pathogenesis of cervical
cancer is complex and human papilloma virus (HPV) is considered one
of the main risk factors (154). Development of EMT is a critical
reason for the progression of primary cervical cancer, increased
invasiveness and insensitivity to chemotherapy (155). TGF-β1 can regulate the
development of EMT and is considered to be the driving force of EMT
in cervical cancer (156). Yang
et al (157) reported
that semaphorin 4C (Sema4C) downregulation inhibited cervical
cancer cell EMT, invasion and metastasis, possibly by inhibiting
TGF-β1-induced activation of p38 MAPK in HeLa cells. However, Li
et al (156) found that
p68 promoted EMT in cervical cancer cells through transcriptional
activation of the TGF-β1 signaling pathway. Cheng et al
(158) revealed that miR-106b
was highly expressed in human cervical cancer tissues, and miR-106b
targeting disabled-2 (DAB2) genes enhanced TGF-β1-induced HeLa cell
migration and promoted cervical cancer progression. DAB2 is a
multimodular scaffold protein with signaling roles in cell
proliferation and differentiation (159). Some studies have shown that
TGF-β1 promotes the development and metastasis of cervical cancer
by regulating its role in the tumor microenvironment (160-162). Moreover, TGF-β1 facilitates
maspin expression in cervical cancer cells through Smad and
non-Smad signaling pathways (163). Recently, miRNAs involved in
cancer progression have come into focus. Studies have demonstrated
that carcinogenic HPV infection influences the levels of multiple
miRNAs in cervical cancer and cervical intraepithelial neoplasia
(154,164,165). Cheng et al (158) demonstrated that high levels of
miR-106b promoted cervical carcinoma cell metastasis by inducing
TGF-β1. Notably, it has been demonstrated that interference with
the lncRNA CDKN2B-AS1 upregulates the miR-181a-5p/TGF-β1 axis,
inhibiting metastasis of cervical carcinoma cells and accelerating
apoptosis and senescence (166). Additionally, let-7a restrains
cell proliferation in cervical carcinoma through the TGF-β/Smad
signaling pathway (167).
TGF-β1 and TGF-βR1 in the TGF-β signaling pathway
exert multiple functions in regulating tumorigenesis, tumor growth
and metastasis. Different inhibitors have been developed for
potential anticancer treatments. Numerous inhibitors have been
developed against TGF-βR1 or TGF-β1 (Table II), such as LY2382770 (176), LY2157299 (galunisertib)
(177-182), TEW-7197 (183,184) and LY3200882 (185), which have entered experimental
clinical research.
LY2382770 is a TGF-β1 inhibitor for the treatment
of diabetic nephropathy and diabetic glomerulosclerosis currently
in phase II clinical research (176). LY2157299 is a TGF-βR1 inhibitor
currently in development as a drug for the treatment of
myelodysplastic syndromes (MDS) in phase II/III (NCT0008318), HCC
in phase II (NCT012246986), pancreatic cancer in phase I
(NCT02154646) and NSCLC in phase I/II clinical studies
(NCT02423343). LY2157299 is the only small molecule inhibitor of
TGF-βR currently in a phase III clinical trial (177-182). TEW-7197 (vactosertib), an ALK5
kinase inhibitor developed by MedPacto, is currently undergoing
phase II clinical trials for MDS and phase I clinical trials for
advanced solid tumors, such as melanoma, BC, HCC and prostate
cancer (183,184). LY3200882 is another highly
selective small molecule ALK5 inhibitor developed by Eli Lilly and
Company that competitively binds to the ATP-binding site of the
ALK5 kinase domain; a phase I clinical trial for healthy
participants has been completed in 2019 (NCT03792139) and
participants for a phase I clinical trial for solid tumors are
currently being recruited (185).
However, some inhibitors are in the preclinical
phase of experimental research, such as SB-431542 (186-188), LY2109761 (189,190), SD208 (191), SB505154 (192), GW6604 (193), EW-7203 (194), Ki26894 (195) and SM16 (196) (Table II). LY2109761 completely
inhibits the phosphorylation of Smad2 mediated by TGF-β and has
indicated antitumor effects in pancreatic cancer models (189,190). SM16 is a new oral bioavailable
kinase inhibitor that combines with the ATP-binding pocket of ALK5,
inhibiting its activation (196). SD-208 suppresses the
proliferation and migration of mouse and human glioma cells, and
enhances their immunogenicity by suppressing ALK-5
autophosphorylation (191).
EW-7203 inhibits TGF-βR1 kinase activity, efficiently inhibiting
TGF-β1-induced Smad signaling, EMT and BC metastasis to the lung
in vivo (194). Further
inhibitors should be developed for the clinical treatment of
malignant tumors in the future.
The TGF-β signaling pathway serves an important
role in cell cycle regulation, growth and development,
differentiation, ECM synthesis, hematopoiesis, chemotaxis and
immune response (1-3). In recent years, studies on
malignant tumors have revealed that TGF-β1 and TGF-βR1 may serve
important roles in tumor occurrence and development, including in
promoting tumor angiogenesis, invasion, EMT and immune escape
(4,5,197). Increased expression levels of
miR-331-3p (22), HnRNP K
(146), Sema4C (157) and p68 (156), and the activation of the
JAK/STAT3/Twist (111), NF-κB
(127) and TGF-β signaling
pathways in tumor cells can promote proliferation, migration and
EMT through the action of TGF-β1 or TGF-βR1. Increased levels of
some molecules, such as miR-133b (63), miR-4458 and miR-27a (168), inhibit the progression of
tumors by acting on TGF-β1 or TGF-βR1. The increased levels of
TGF-β1 in the tumor itself lead to increases in miR-21, CXCR4, SMA
and ECM remodeling, activation of ERK, TGF-β/Smad and NF-κB
signaling pathways, and a decrease of growth factors, miR-196A-3p,
miR-193b and KLF4 expression, which promote tumor progression
(66,68,69,117,198). On the other hand, self-mutation
of TGF-βR1 is considered to promote tumor development through the
MAPK signaling pathway (93).
Some inhibitors have been developed for both TGF-β1 and TGF-βR1,
including LY2157299, TEW-7197 and LY3200882 (177-185). LY2157299 specifically
downregulates phosphorylation of Smad2 protein induced by TGF-β1,
and significantly inhibits the proliferation and migration of
cancer cells (177-182). TEW-7197 (183,184) and LY3200882 (185) competitively bind to the
ATP-binding site of the intracellular kinase domain of ALK5 to
produce kinase inhibitory activity. These inhibitors are currently
in clinical trials. Additionally, there are some inhibitors that
can block the activity of ALK5, which are currently in preclinical
research, such as SB-431542, LY2109761 and SD208 (186-191).
Therefore, TGF-β1 and TGF-βR1 seem to have dual
effects on tumors. With the development of molecular biology, the
dual mechanism of TGF-β1 inhibition and promotion in tumors is
becoming increasingly clear, but the mechanism of TGF-βR1 in tumors
remains unclear. At the same time, it has been difficult to clarify
the mechanism of TGF-β1 from tumor suppressor to tumor promoter.
However, most studies have indicated that malignant tumors
proliferate, metastasize, invade, undergo EMT and escape immune
surveillance by acting on TGF-β1 or TGF-βR1. With the development
of clinical trials in the future, the understanding of TGF-β1 and
TGF-βR1 will become more comprehensive. Further exploration of the
association between TGF-β1 and TGF-βR1, and the mechanism of the
occurrence and development of malignant tumors will provide useful
information for the discovery of new therapeutic targets.
Not applicable.
JW wrote the manuscript. JW, YL, HX and TW
investigated the roles of TGF-β1 and TGF-βR1 in tumors. JW and TW
are responsible for confirming the authenticity of the data. TW
supervised and revised the manuscript. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Massagué J: TGF-β signaling in development
and disease. FEBS Lett. 586:18332012. View Article : Google Scholar
|
|
2
|
Hata A and Chen YG: TGF-beta signaling
from receptors to smads. Cold Spring Harb Perspect Biol.
8:a0220612016. View Article : Google Scholar
|
|
3
|
Zhang Y, Alexander PB and Wang XF:
TGF-beta family signaling in the control of cell proliferation and
survival. Cold Spring Harb Perspect Biol. 9:a0221452017. View Article : Google Scholar
|
|
4
|
Wu MY and Hill CS: Tgf-beta superfamily
signaling in embryonic development and homeostasis. Dev Cell.
16:329–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma W, Qin Y, Chapuy B and Lu C: LRRC33 is
a novel binding and potential regulating protein of TGF-β1 function
in human acute myeloid leukemia cells. PLoS One. 14:e02134822019.
View Article : Google Scholar
|
|
6
|
Maishi N and Hida K: Tumor endothelial
cells accelerate tumor metastasis. Cancer Sci. 108:1921–1926. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Selleri S, Rumio C, Sabatino M, Marincola
FM and Wang E: Tumor microenvironment and the immune response. Surg
Oncol Clin N Am. 16:737–753. vii–viii. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto T, Akisue T, Marui T, Fujita I,
Matsumoto K, Hitora T, Kawamoto T, Nagira K, Nakatani T and
Kurosaka M: Expression of transforming growth factor beta isoforms
and their receptors in malignant fibrous histiocytoma of soft
tissues. Clin Cancer Res. 10:5804–5807. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dropmann A, Dediulia T, Breitkopf-Heinlein
K, Korhonen H, Janicot M, Weber SN, Thomas M, Piiper A, Bertran E,
Fabregat I, et al: TGF-β1 and TGF-β2 abundance in liver diseases of
mice and men. Oncotarget. 7:19499–19518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ebert MP, Yu J, Miehlke S, Fei G,
Lendeckel U, Ridwelski K, Stolte M, Bayerdörffer E and
Malfertheiner P: Expression of transforming growth factor beta-1 in
gastric cancer and in the gastric mucosa of first-degree relatives
of patients with gastric cancer. Br J Cancer. 82:1795–1800. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersson J, Tran DQ, Pesu M, Davidson TS,
Ramsey H, O'Shea JJ and Shevach EM: CD4+
FoxP3+ regulatory T cells confer infectious tolerance in
a TGF-beta-dependent manner. J Exp Med. 205:1975–1981. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng L, Yuan XQ, Zhang CY, Ye F, Zhou HF,
Li WL, Liu ZY, Zhang YQ, Pan X and Li GC: High TGF-beta1 expression
predicts poor disease prognosis in hepatocellular carcinoma
patients. Oncotarget. 8:34387–34397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neuzillet C, de Gramont A,
Tijeras-Raballand A, de Mestier L, Cros J, Faivre S and Raymond E:
Perspectives of TGF-β inhibition in pancreatic and hepatocellular
carcinomas. Oncotarget. 5:78–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Papageorgis P: TGFbeta signaling in tumor
initiation, epithelial-to-mesenchymal transition, and metastasis. J
Oncol. 2015:5871932015. View Article : Google Scholar
|
|
15
|
Vander Ark A, Cao J and Li X: TGF-β
receptors: In and beyond TGF-β signaling. Cell Signal. 52:112–120.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baxter SW, Choong DY, Eccles DM and
Campbell IG: Transforming growth factor beta receptor 1 polyalanine
polymorphism and exon 5 mutation analysis in breast and ovarian
cancer. Cancer Epidemiol Biomarkers Prev. 11:211–214.
2002.PubMed/NCBI
|
|
17
|
Liu J, Johnson K, Li J, Piamonte V, Steffy
BM, Hsieh MH, Ng N, Zhang J, Walker JR, Ding S, et al: Regenerative
phenotype in mice with a point mutation in transforming growth
factor beta type I receptor (TGFBR1). Proc Natl Acad Sci USA.
108:14560–14565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang F, Yu Q, Chu Y, Zhu X, Lu W, Liu Q
and Wang Q: MicroRNA-98-5p inhibits proliferation and metastasis in
non-small cell lung cancer by targeting TGFBR1. Int J Oncol.
54:128–138. 2019.
|
|
19
|
Grandclement C, Pallandre JR, Valmary
Degano S, Viel E, Bouard A, Balland J, Rémy-Martin JP, Simon B,
Rouleau A, Boireau W, et al: Neuropilin-2 expression promotes
TGF-β1-mediated epithelial to mesenchymal transition in colorectal
cancer cells. PLoS One. 6:e204442011. View Article : Google Scholar
|
|
20
|
He B, Xu T, Pan B, Pan Y, Wang X, Dong J,
Sun H, Xu X, Liu X and Wang S: Polymorphisms of TGFBR1, TLR4 are
associated with prognosis of gastric cancer in a Chinese
population. Cancer Cell Int. 18:1912018. View Article : Google Scholar :
|
|
21
|
Kim W, Kim E, Lee S, Kim D, Chun J, Park
KH, Youn H and Youn B: TFAP2C-mediated upregulation of TGFBR1
promotes lung tumorigenesis and epithelial-mesenchymal transition.
Exp Mol Med. 48:e2732016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Song X, Chen X, Wang Q, Zheng X,
Wu C and Jiang J: Circular RNA CircCACTIN promotes gastric cancer
progression by sponging MiR-331-3p and regulating TGFBR1
expression. Int J Biol Sci. 15:1091–1103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knight PG and Glister C: TGF-beta
superfamily members and ovarian follicle development. Reproduction.
132:191–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hinck AP, Mueller TD and Springer TA:
Structural biology and evolution of the TGF-β family. Cold Spring
Harb Perspect Biol. 8. pp. a0221032016, View Article : Google Scholar
|
|
25
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang T, David L, Mendoza V, Yang Y,
Villarreal M, De K, Sun L, Fang X, López-Casillas F, Wrana JL and
Hinck AP: TGF-β signalling is mediated by two autonomously
functioning TβRI:TβRII pairs. EMBO J. 30:1263–1276. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng XH and Derynck R: A kinase subdomain
of transforming growth factor-beta (TGF-beta) type I receptor
determines the TGF-beta intracellular signaling specificity. EMBO
J. 16:3912–3923. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lo RS, Chen YG, Shi Y, Pavletich NP and
Massagué J: The L3 loop: A structural motif determining specific
interactions between SMAD proteins and TGF-beta receptors. EMBO J.
17:996–1005. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Itman C, Mendis S, Barakat B and Loveland
KL: All in the family: TGF-beta family action in testis
development. Reproduction. 132:233–246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Attisano L and Wrana JL: Signal
transduction by the TGF-beta superfamily. Science. 296:1646–1647.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huynh LK, Hipolito CJ and Ten Dijke P: A
perspective on the development of TGF-beta inhibitors for cancer
treatment. Biomolecules. 9:7432019. View Article : Google Scholar
|
|
32
|
Wu Y, Tran T, Dwabe S, Sarkissyan M, Kim
J, Nava M, Clayton S, Pietras R, Farias-Eisner R and Vadgama JV:
A83-01 inhibits TGF-β-induced upregulation of Wnt3 and epithelial
to mesenchymal transition in HER2-overexpressing breast cancer
cells. Breast Cancer Res Treat. 163:449–460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katagiri T and Watabe T: Bone
morphogenetic proteins. Cold Spring Harb Perspect Biol.
8:a0218992016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katz LH, Li Y, Chen JS, Muñoz NM, Majumdar
A, Chen J and Mishra L: Targeting TGF-β signaling in cancer. Expert
Opin Ther Targets. 17:743–760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krenning G, Barauna VG, Krieger JE,
Harmsen MC and Moonen JR: Endothelial plasticity: Shifting
phenotypes through force feedback. Stem Cells Int.
2016:97629592016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T
and Springer TA: Latent TGF-β structure and activation. Nature.
474:343–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Li H, Yi D, Lai C, Wang H, Zou W
and Cao B: Knockdown of vascular cell adhesion molecule 1 impedes
transforming growth factor beta 1-mediated proliferation,
migration, and invasion of endometriotic cyst stromal cells. Reprod
Biol Endocrinol. 17:692019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robertson IB, Horiguchi M, Zilberberg L,
Dabovic B, Hadjiolova K and Rifkin DB: Latent TGF-β-binding
proteins. Matrix Biol. 47:44–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ehrlich M, Horbelt D, Marom B, Knaus P and
Henis YI: Homomeric and heteromeric complexes among TGF-beta and
BMP receptors and their roles in signaling. Cell Signal.
23:1424–1432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
ten Dijke P, Miyazono K and Heldin CH:
Signaling via hetero-oligomeric complexes of type I and type II
serine/threonine kinase receptors. Curr Opin Cell Biol. 8:139–145.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun D, Han S, Liu C, Zhou R, Sun W, Zhang
Z and Qu J: Microrna-199a-5p functions as a tumor suppressor via
suppressing connective tissue growth factor (CTGF) in follicular
thyroid carcinoma. Med Sci Monit. 22:1210–1217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Das R, Xu S, Nguyen TT, Quan X, Choi SK,
Kim SJ, Lee EY, Cha SK and Park KS: Transforming growth factor
β1-induced apoptosis in podocytes via the extracellular
signal-regulated kinase-mammalian target of rapamycin complex
1-NADPH Oxidase 4 axis. J Biol Chem. 290:30830–30842. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mihaly SR, Ninomiya-Tsuji J and Morioka S:
TAK1 control of cell death. Cell Death Differ. 21:1667–1676. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tvrdík D, Dundr P, Povýsil C, Pytlík R and
Planková M: Up-regulation of p21WAF1 expression is mediated by
Sp1/Sp3 transcription factors in TGFbeta1-arrested malignant B
cells. Med Sci Monit. 12:BR227–BR234. 2006.PubMed/NCBI
|
|
45
|
Stanilova S, Stanilov N, Julianov A,
Manolova I and Miteva L: Transforming growth factor-β1 gene
promoter -509C/T polymorphism in association with expression
affects colorectal cancer development and depends on gender. PLoS
One. 13:e02017752018. View Article : Google Scholar
|
|
46
|
Al Shareef Z, Kardooni H, Murillo-Garzó V,
Domenici G, Stylianakis E, Steel JH, Rabano M, Gorroño-Etxebarria
I, Zabalza I, Vivanco MD, et al: Protective effect of stromal
Dickkopf-3 in prostate cancer: Opposing roles for TGFBI and ECM-1.
Oncogene. 37:5305–5324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang ST, Liu JJ, Wang CZ, Lin B, Hao YY,
Wang YF, Gao S, Qi Y, Zhang SL and Iwamori M: Expression and
correlation of Lewis y antigen and TGF-beta1 in ovarian epithelial
carcinoma. Oncol Rep. 27:1065–1071. 2012. View Article : Google Scholar
|
|
48
|
Zhang N, Bi X, Zeng Y, Zhu Y, Zhang Z, Liu
Y, Wang J, Li X, Bi J and Kong C: TGF-β1 promotes the migration and
invasion of bladder carcinoma cells by increasing fascin1
expression. Oncol Rep. 36:977–983. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wakefield LM, Letterio JJ, Chen T,
Danielpour D, Allison RS, Pai LH, Denicoff AM, Noone MH, Cowan KH,
O'Shaughnessy JA, et al: Transforming growth factor-beta1
circulates in normal human plasma and is unchanged in advanced
metastatic breast cancer. Clin Cancer Res. 1:129–136.
1995.PubMed/NCBI
|
|
50
|
Shuang ZY, Wu WC, Xu J, Lin G, Liu YC, Lao
XM, Zheng L and Li S: Transforming growth factor-β1-induced
epithelial-mesenchymal transition generates ALDH-positive cells
with stem cell properties in cholangiocarcinoma. Cancer Lett.
354:320–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Safina A, Vandette E and Bakin AV: ALK5
promotes tumor angiogenesis by upregulating matrix
metalloproteinase-9 in tumor cells. Oncogene. 26:2407–2422. 2007.
View Article : Google Scholar
|
|
52
|
Moore-Smith L and Pasche B: TGFBR1
signaling and breast cancer. J Mammary Gland Biol Neoplasia.
16:89–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rosman DS, Phukan S, Huang CC and Pasche
B: TGFBR1*6A enhances the migration and invasion of MCF-7 breast
cancer cells through RhoA activation. Cancer Res. 68:1319–1328.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Slattery ML, Lundgreen A, Herrick JS,
Wolff RK and Caan BJ: Genetic variation in the transforming growth
factor-β signaling pathway and survival after diagnosis with colon
and rectal cancer. Cancer. 117:4175–4183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Javle M, Li Y, Tan D, Dong X, Chang P, Kar
S and Li D: Biomarkers of TGF-β signaling pathway and prognosis of
pancreatic cancer. PLoS One. 9:e859422014. View Article : Google Scholar
|
|
56
|
Bian Y, Knobloch TJ, Sadim M, Kaklamani V,
Raji A, Yang GY, Weghorst CM and Pasche B: Somatic acquisition of
TGFBR1*6A by epithelial and stromal cells during head and neck and
colon cancer development. Hum Mol Genet. 16:3128–3135. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pasche B, Pennison MJ, Jimenez H and Wang
M: TGFBR1 and cancer susceptibility. Trans Am Clin Climatol Assoc.
125:300–312. 2014.PubMed/NCBI
|
|
58
|
Myers ER, Moorman P, Gierisch JM,
Havrilesky LJ, Grimm LJ, Ghate S, Davidson B, Mongtomery RC,
Crowley MJ, McCrory DC, et al: Benefits and harms of breast cancer
screening: A systematic review. JAMA. 314:1615–1634. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Oeffinger KC, Fontham ET, Etzioni R,
Herzig A, Michaelson JS, Shih YC, Walter LC, Church TR, Flowers CR,
LaMonte SJ, et al: Breast cancer screening for women at average
risk: 2015 guide-line update from the American cancer society.
JAMA. 314:1599–1614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017 racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Park SJ, Kim JG, Kim ND, Yang K, Shim JW
and Heo K: Estradiol, TGF-β1 and hypoxia promote breast cancer
stemness and EMT-mediated breast cancer migration. Oncol Lett.
11:1895–1902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Menezes ME, Shen XN, Das SK, Emdad L,
Sarkar D and Fisher PB: MDA-9/Syntenin (SDCBP) modulates small
GTPases RhoA and Cdc42 via transforming growth factor β1 to enhance
epithelial-mesenchymal transition in breast cancer. Oncotarget.
7:80175–80189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang S, Huang M, Wang Z, Wang W, Zhang Z,
Qu S and Liu C: MicroRNA-133b targets TGFβ receptor I to inhibit
TGF-β-induced epithelial-to-mesenchymal transition and metastasis
by suppressing the TGF-β/SMAD pathway in breast cancer. Int J
Oncol. 55:1097–1109. 2019.PubMed/NCBI
|
|
64
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar :
|
|
65
|
Ye Z, Zhao L, Li J, Chen W and Li X:
MiR-30d blocked transforming growth Factor beta1-induced
epithelial-mesenchymal transition by targeting snail in ovarian
cancer cells. Int J Gynecol Cancer. 25:1574–1581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dai X, Fang M, Li S, Yan Y, Zhong Y and Du
B: MiR-21 is involved in transforming growth factor β1-induced
chemoresistance and invasion by targeting PTEN in breast cancer.
Oncol Lett. 14:6929–6936. 2017.PubMed/NCBI
|
|
67
|
Li C, Zhou D, Hong H, Yang S, Zhang L, Li
S, Hu P, Ren H, Mei Z and Tang H: TGFβ1-miR-140-5p axis mediated
up-regulation of Flap Endonuclease 1 promotes
epithelial-mesenchymal transition in hepatocellular carcinoma.
Aging (Albany NY). 11:5593–5612. 2019. View Article : Google Scholar
|
|
68
|
Chen Y, Huang S, Wu B, Fang J, Zhu M, Sun
L, Zhang L, Zhang Y, Sun M, Guo L and Wang S: Transforming growth
factor-β1 promotes breast cancer metastasis by downregulating
miR-196a-3p expression. Oncotarget. 8:49110–49122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao XP, Huang YY, Huang Y, Lei P, Peng
JL, Wu S, Wang M, Li WH, Zhu HF and Shen GX: Transforming growth
factor-beta1 upregulates the expression of CXC chemokine receptor 4
(CXCR4) in human breast cancer MCF-7 cells. Acta Pharmacol Sin.
31:347–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chen HS, Bai MH, Zhang T, Li GD and Liu M:
Ellagic acid induces cell cycle arrest and apoptosis through
TGF-β/Smad3 signaling pathway in human breast cancer MCF-7 cells.
Int J Oncol. 46:1730–1738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mishra AK, Parish CR, Wong ML, Licinio J
and Blackburn AC: Leptin signals via TGFB1 to promote metastatic
potential and stemness in breast cancer. PLoS One. 12:e01784542017.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fallone F, Deudon R, Muller C and Vaysse
C: Breast cancer, obesity and adipose tissue: A high-risk
combination. Med Sci (Paris). 34:1079–1086. 2018.In French.
View Article : Google Scholar
|
|
73
|
Lee K, Kruper L, Dieli-Conwright CM and
Mortimer JE: The impact of obesity on breast cancer diagnosis and
treatment. Curr Oncol Rep. 21:412019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Catteau X, Simon P and Noël JC:
Myofibroblastic stromal reaction and lymph node status in invasive
breast carcinoma: Possible role of the TGF-β1/TGF-βR1 pathway. BMC
Cancer. 14:4992014. View Article : Google Scholar
|
|
75
|
Cox DG, Penney K, Guo Q, Hankinson SE and
Hunter DJ: TGFB1 and TGFBR1 polymorphisms and breast cancer risk in
the Nurses' Health Study. BMC Cancer. 7:1752007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Benson AB, Venook AP, Al-Hawary MM,
Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: NCCN guidelines insights: Colon cancer, version
2. 2018.J Natl Compr Canc Netw. 16:359–369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xu Y and Pasche B: TGF-beta signaling
alterations and susceptibility to colorectal cancer. Hum Mol Genet.
16(Spec 1 SPEC): R14–R20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kong J, Du J, Wang Y, Yang M, Gao J, Wei
X, Fang W, Zhan J and Zhang H: Focal adhesion molecule Kindlin-1
mediates activation of TGF-β signaling by interacting with TGF-βRI,
SARA and Smad3 in colorectal cancer cells. Oncotarget.
7:76224–76237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen K, Wei H, Ling S and Yi C: Expression
and significance of transforming growth factor-beta1 in epithelial
ovarian cancer and its extracellular matrix. Oncol Lett.
8:2171–2174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Engle SJ, Hoying JB, Boivin GP, Ormsby I,
Gartside PS and Doetschman T: Transforming growth factor beta1
suppresses nonmetastatic colon cancer at an early stage of
tumorigenesis. Cancer Res. 59:3379–3386. 1999.PubMed/NCBI
|
|
82
|
Schmidt-Weber CB and Blaser K: Regulation
and role of transforming growth factor-beta in immune tolerance
induction and inflammation. Curr Opin Immunol. 16:709–716. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bierie B and Moses HL: Transforming growth
factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth
Factor Rev. 21:49–59. 2010. View Article : Google Scholar
|
|
84
|
Vrba L and Futscher BW: Epigenetic
silencing of lncRNA MORT in 16 TCGA cancer types. F1000Res.
7:2112018. View Article : Google Scholar :
|
|
85
|
Zhou T, Wu L, Zong Z, Ma N, Li Y, Jiang Z,
Wang Q and Chen S: Long non-coding RNA mortal obligate RNA
transcript inhibits the migration and invasion of colon cancer
cells by inactivating transforming growth factor β1. Oncol Lett.
19:1131–1136. 2020.PubMed/NCBI
|
|
86
|
Townsend PA, Cutress RI, Sharp A, Brimmell
M and Packham G: BAG-1: A multifunctional regulator of cell growth
and survival. Biochim Biophys Acta. 1603:83–98. 2003.PubMed/NCBI
|
|
87
|
Skeen VR, Collard TJ, Southern SL,
Greenhough A, Hague A, Townsend PA, Paraskeva C and Williams AC:
BAG-1 suppresses expression of the key regulatory cytokine
transforming growth factor β (TGF-β1) in colorectal tumour cells.
Oncogene. 32:4490–4499. 2013. View Article : Google Scholar
|
|
88
|
Dumond A, Demange L and Pagès G:
Neuropilins: Relevant therapeutic targets to improve the treatment
of cancers. Med Sci (Paris). 36:487–496. 2020.In French. View Article : Google Scholar
|
|
89
|
Huang Y, Fang W, Wang Y, Yang W and Xiong
B: Transforming growth factor-β1 induces glutathione peroxidase-1
and protects from H2O2-induced cell death in colon cancer cells via
the Smad2/ERK1/2/HIF-1α pathway. Int J Mol Med. 29:906–912.
2012.PubMed/NCBI
|
|
90
|
Lei XG, Cheng WH and McClung JP: Metabolic
regulation and function of glutathione peroxidase-1. Annu Rev Nutr.
27:41–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li Y, Zhu G, Zhai H, Jia J, Yang W, Li X
and Liu L: Simultaneous stimulation with tumor necrosis factor-α
and transforming growth factor-β1 induces epithelial-mesenchymal
transition in colon cancer cells via the NF-κB pathway. Oncol Lett.
15:6873–6880. 2018.PubMed/NCBI
|
|
92
|
Tomsic J, Guda K, Liyanarachchi S, Hampel
H, Natale L, Markowitz SD, Tanner SM and de la Chapelle A:
Allele-specific expression of TGFBR1 in colon cancer patients.
Carcinogenesis. 31:1800–1804. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhou R, Huang Y, Cheng B, Wang Y and Xiong
B: TGFBR1*6A is a potential modifier of migration and invasion in
colorectal cancer cells. Oncol Lett. 15:3971–3976. 2018.PubMed/NCBI
|
|
94
|
Luyimbazi D, Nelson RA, Choi AH, Li L,
Chao J, Sun V, Hamner JB and Kim J: Estimates of conditional
survival in gastric cancer reveal a reduction of racial disparities
with long-term follow-up. J Gastrointest Surg. 19:251–257. 2015.
View Article : Google Scholar
|
|
95
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Pennison M and Pasche B: Targeting
transforming growth factor-beta signaling. Curr Opin Oncol.
19:579–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ijichi H, Ikenoue T, Kato N, Mitsuno Y,
Togo G, Kato J, Kanai F, Shiratori Y and Omata M: Systematic
analysis of the TGF-beta-Smad signaling pathway in gastrointestinal
cancer cells. Biochem Biophys Res Commun. 289:350–357. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ma GF, Miao Q, Zeng XQ, Luo TC, Ma LL, Liu
YM, Lian JJ, Gao H and Chen SY: Transforming growth factor-β1 and
-β2 in gastric precancer and cancer and roles in tumor-cell
interactions with peripheral blood mononuclear cells in vitro. PLoS
One. 8:e542492013. View Article : Google Scholar
|
|
100
|
Zhou Y, Jin GF, Jiang GJ, Wang HM, Tan YF,
Ding WL, Wang LN, Chen WS, Ke Q, Shen J, et al: Correlations of
polymorphisms of TGFB1 and TGFBR2 genes to genetic susceptibility
to gastric cancer. Ai Zheng. 26:581–585. 2007.In Chinese.
PubMed/NCBI
|
|
101
|
Yanagihara K and Tsumuraya M: Transforming
growth factor beta 1 induces apoptotic cell death in cultured human
gastric carcinoma cells. Cancer Res. 52:4042–4045. 1992.PubMed/NCBI
|
|
102
|
Wang KS, Hu ZL, Li JH, Xiao DS and Wen JF:
Enhancement of metastatic and invasive capacity of gastric cancer
cells by transforming growth factor-beta1. Acta Biochim Biophys Sin
(Shanghai). 38:179–186. 2006. View Article : Google Scholar
|
|
103
|
Takeuchi Y and Nishikawa H: Roles of
regulatory T cells in cancer immunity. Int Immunol. 28:401–409.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Deng B, Zhu JM, Wang Y, Liu TT, Ding YB,
Xiao WM, Lu GT, Bo P and Shen XZ: Intratumor hypoxia promotes
immune tolerance by inducing regulatory T cells via TGF-β1 in
gastric cancer. PLoS One. 8:e637772013. View Article : Google Scholar
|
|
105
|
Lee MS, Kim TY, Kim YB, Lee SY, Ko SG,
Jong HS, Kim TY, Bang YJ and Lee JW: The signaling network of
transforming growth factor beta1, protein kinase Cdelta, and
integrin underlies the spreading and invasiveness of gastric
carcinoma cells. Mol Cell Biol. 25:6921–6936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhou H, Wang K, Hu Z and Wen J: TGF-β1
alters microRNA profile in human gastric cancer cells. Chin J
Cancer Res. 25:102–111. 2013.PubMed/NCBI
|
|
107
|
Zhu Y, Kong F, Zhang C, Ma C, Xia H, Quan
B and Cui H: CD133 mediates the TGF-β1-induced activation of the
PI3K/ERK/P70S6K signaling pathway in gastric cancer cells. Oncol
Lett. 14:7211–7216. 2017.
|
|
108
|
Zhao Y, Xia S, Cao C and Du X: Effect of
TGF-β1 on apoptosis of colon cancer cells via the ERK signaling
pathway. J BUON. 24:449–455. 2019.PubMed/NCBI
|
|
109
|
Jin S, Gao J, Qi Y, Hao Y, Li X, Liu Q,
Liu J, Liu D, Zhu L and Lin B: TGF-β1 fucosylation enhances the
autophagy and mitophagy via PI3K/Akt and Ras-Raf-MEK-ERK in ovarian
carcinoma. Biochem Biophys Res Commun. 524:970–976. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Cascione M, Leporatti S, Dituri F and
Giannelli G: Transforming growth factor-β promotes morphomechanical
effects involved in epithelial to mesenchymal transition in living
hepatocellular carcinoma. Int J Mol Sci. 20:1082018. View Article : Google Scholar
|
|
111
|
Sun SL and Wang XY: TGF-β1 promotes
proliferation and invasion of hepatocellular carcinoma cell line
HepG2 by activating GLI-1 signaling. Eur Rev Med Pharmacol Sci.
22:7688–7695. 2018.PubMed/NCBI
|
|
112
|
Qu Z, Feng J, Pan H, Jiang Y, Duan Y and
Fa Z: Exosomes derived from HCC cells with different invasion
characteristics mediated EMT through TGF-β/Smad signaling pathway.
Onco Targets Ther. 12:6897–6905. 2019. View Article : Google Scholar :
|
|
113
|
Zhang C, Chen B, Jiao A, Li F, Sun N,
Zhang G and Zhang J: MiR-663a inhibits tumor growth and invasion by
regulating TGF-β1 in hepatocellular carcinoma. BMC Cancer.
18:11792018. View Article : Google Scholar
|
|
114
|
Tang YH, He GL, Huang SZ, Zhong KB, Liao
H, Cai L, Gao Y, Peng ZW and Fu SJ: The long noncoding RNA AK002107
negatively modulates miR-140-5p and targets TGFBR1 to induce
epithelial-mesenchymal transition in hepatocellular carcinoma. Mol
Oncol. 13:1296–1310. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang Y, Li B, Li X, Tan H, Cheng D and
Shi H: An imaging target TGF-β1 for hepatocellular carcinoma in
mice. Hell J Nucl Med. 20:76–78. 2017.PubMed/NCBI
|
|
116
|
Balzarini P, Benetti A, Invernici G,
Cristini S, Zicari S, Caruso A, Gatta LB, Berenzi A, Imberti L,
Zanotti C, et al: Transforming growth factor-beta1 induces
microvascular abnormalities through a down-modulation of neural
cell adhesion molecule in human hepatocellular carcinoma. Lab
Invest. 92:1297–1309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yao S, Tian C, Ding Y, Ye Q, Gao Y, Yang N
and Li Q: Down-regulation of Krüppel-like factor-4 by
microRNA-135a-5p promotes proliferation and metastasis in
hepatocellular carcinoma by transforming growth factor-β1.
Oncotarget. 7:42566–42578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li W, Liu M, Su Y, Zhou X, Liu Y and Zhang
X: The Janus-faced roles of Krüppel-like factor 4 in oral squamous
cell carcinoma cells. Oncotarget. 6:44480–44494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Tian C, Yao S, Liu L, Ding Y, Ye Q, Dong
X, Gao Y, Yang N and Li Q: Klf4 inhibits tumor growth and
metastasis by targeting microRNA-31 in human hepatocellular
carcinoma. Int J Mol Med. 39:47–56. 2017. View Article : Google Scholar
|
|
120
|
Zhang X, Fan Q, Li Y, Yang Z, Yang L, Zong
Z, Wang B, Meng X, Li Q, Liu J and Li H: Transforming growth
factor-beta1 suppresses hepatocellular carcinoma proliferation via
activation of Hippo signaling. Oncotarget. 8:29785–29794. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhang Y, Shi K, Liu H, Chen W, Luo Y, Wei
X and Wu Z: MiR-4458 inhibits the epithelial-mesenchymal transition
of hepatocellular carcinoma cells by suppressing the TGF-β
signaling pathway via targeting TGFBR1. Acta Biochim Biophys Sin
(Shanghai). 52:554–562. 2020. View Article : Google Scholar
|
|
122
|
Perrier ND, Brierley JD and Tuttle RM:
Differentiated and anaplastic thyroid carcinoma: Major changes in
the American Joint Committee on Cancer eighth edition cancer
staging manual. CA Cancer J Clin. 68:55–63. 2018. View Article : Google Scholar
|
|
123
|
Saini S, Tulla K, Maker AV, Burman KD and
Prabhakar BS: Therapeutic advances in anaplastic thyroid cancer: A
current perspective. Mol Cancer. 17:1542018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kebebew E: Anaplastic thyroid cancer:
Rare, fatal, and neglected. Surgery. 152:1088–1089. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Li Y, Chen D, Hao FY and Zhang KJ:
Targeting TGF-β1 and AKT signal on growth and metastasis of
anaplastic thyroid cancer cell in vivo. Eur Rev Med Pharmacol Sci.
20:2581–2587. 2016.PubMed/NCBI
|
|
126
|
Zhang X, Liu L, Deng X, Li D, Cai H, Ma Y,
Jia C, Wu B, Fan Y and Lv Z: MicroRNA 483-3p targets Pard3 to
potentiate TGF-β1-induced cell migration, invasion, and
epithelial-mesenchymal transition in anaplastic thyroid cancer
cells. Oncogene. 38:699–715. 2019. View Article : Google Scholar
|
|
127
|
Yin Q, Liu S, Dong A, Mi X, Hao F and
Zhang K: Targeting transforming growth factor-Beta1 (TGF-β1)
inhibits tumorigenesis of anaplastic thyroid carcinoma cells
through ERK1/2-NFκB-PUMA signaling. Med Sci Monit. 22:2267–2277.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhong J, Liu C, Zhang QH, Chen L, Shen YY,
Chen YJ, Zeng X, Zu XY and Cao RX: TGF-β1 induces HMGA1 expression:
The role of HMGA1 in thyroid cancer proliferation and invasion. Int
J Oncol. 50:1567–1578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Cui M, Chang Y, Du W, Liu S, Qi J, Luo R
and Luo S: Upregulation of lncRNA-ATB by transforming growth
factor-β1 (TGF-β1) promotes migration and invasion of papillary
thyroid carcinoma cells. Med Sci Monit. 24:5152–5158. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li T, Zhao N, Lu J, Zhu Q, Liu X, Hao F
and Jiao X: Epigallocatechin gallate (EGCG) suppresses
epithelial-mesenchymal transition (EMT) and invasion in anaplastic
thyroid carcinoma cells through blocking of TGF-β1/Smad signaling
pathways. Bioengineered. 10:282–291. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
He J, Jin Y, Zhou M, Li X, Chen W, Wang Y,
Gu S, Cao Y, Chu C, Liu X and Zou Q: Solute carrier family 35
member F2 is indispensable for papillary thyroid carcinoma
progression through activation of transforming growth factor-β type
I receptor/apoptosis signal-regulating kinase 1/mitogen-activated
protein kinase signaling axis. Cancer Sci. 109:642–655. 2018.
View Article : Google Scholar :
|
|
132
|
Bonnet D: Cancer stem cells: Lessons from
leukaemia. Cell Prolif. 38:357–361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Xie W, Wang X, Du W, Liu W, Qin X and
Huang S: Detection of molecular targets on the surface of
CD34+CD38-bone marrow cells in myelodysplastic syndromes. Cytometry
A. 77:840–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lyengar V and Shimanovsky A: Leukemia.
StatPearls Publishing, StatPearls Publishing LLC; Treasure Island,
FL: 2020
|
|
135
|
Hunger SP, Lu X, Devidas M, Camitta BM,
Gaynon PS, Winick NJ, Reaman GH and Carroll WL: Improved survival
for children and adolescents with acute lymphoblastic leukemia
between 1990 and 2005: A report from the children's oncology group.
J Clin Oncol. 30:1663–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Huang F, Wan J, Hu W and Hao S:
Enhancement of anti-leukemia immunity by leukemia-derived exosomes
via downregulation of TGF-β1 expression. Cell Physiol Biochem.
44:240–254. 2017. View Article : Google Scholar
|
|
137
|
Geyh S, Rodríguez-Paredes M, Jäger P, Koch
A, Bormann F, Gutekunst J, Zilkens C, Germing U, Kobbe G, Lyko F,
et al: Transforming growth factor β1-mediated functional inhibition
of mesenchymal stromal cells in myelodysplastic syndromes and acute
myeloid leukemia. Haematologica. 103:1462–1471. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Taetle R, Payne C, Dos Santos B, Russell M
and Segarini P: Effects of transforming growth factor beta 1 on
growth and apoptosis of human acute myelogenous leukemia cells.
Cancer Res. 53:3386–3393. 1993.PubMed/NCBI
|
|
139
|
Verheyden S and Demanet C: NK cell
receptors and their ligands in leukemia. Leukemia. 22:249–257.
2008. View Article : Google Scholar
|
|
140
|
Nursal AF, Pehlivan M, Sahin HH and
Pehlivan S: The Associations of IL-6, IFN-γ, TNF-α, IL-10, and
TGF-β1 functional variants with acute myeloid leukemia in turkish
patients. Genet Test Mol Biomarkers. 20:544–551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Rouce RH, Shaim H, Sekine T, Weber G,
Ballard B, Ku S, Barese C, Murali V, Wu MF, Liu H, et al: The
TGF-β/SMAD pathway is an important mechanism for NK cell immune
evasion in childhood B-acute lymphoblastic leukemia. Leukemia.
30:800–811. 2016. View Article : Google Scholar
|
|
142
|
Gong Y, Zhao M, Yang W, Gao A, Yin X, Hu
L, Wang X, Xu J, Hao S, Cheng T and Cheng H: Megakaryocyte-derived
excessive transforming growth factor β1 inhibits proliferation of
normal hematopoietic stem cells in acute myeloid leukemia. Exp
Hematol. 60:40–46.e2. 2018. View Article : Google Scholar
|
|
143
|
Wang H, Wu Q, Zhang Y, Zhang HN, Wang YB
and Wang W: TGF-β1-induced epithelial-mesenchymal transition in
lung cancer cells involves upregulation of miR-9 and downregulation
of its target, E-cadherin. Cell Mol Biol Lett. 22:222017.
View Article : Google Scholar
|
|
144
|
Xue C, Hu Z, Jiang W, Zhao Y, Xu F, Huang
Y, Zhao H, Wu J, Zhang Y, Zhao L, et al: National survey of the
medical treatment status for non-small cell lung cancer (NSCLC) in
China. Lung Cancer. 77:371–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yano T, Okamoto T, Fukuyama S and Maehara
Y: Therapeutic strategy for postoperative recurrence in patients
with non-small cell lung cancer. World J Clin Oncol. 5:1048–1054.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Li L, Yan S, Zhang H, Zhang M, Huang G and
Chen M: Interaction of hnRNP K with MAP 1B-LC1 promotes
TGF-β1-mediated epithelial to mesenchymal transition in lung cancer
cells. BMC Cancer. 19:8942019. View Article : Google Scholar
|
|
147
|
Shi S, Zhao J, Wang J, Mi D and Ma Z: HPIP
silencing inhibits TGF-β1-induced EMT in lung cancer cells. Int J
Mol Med. 39:479–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Zhang HW, Wang EW, Li LX, Yi SH, Li LC, Xu
FL, Wang DL, Wu YZ and Nian WQ: A regulatory loop involving miR-29c
and Sp1 elevates the TGF-β1 mediated epithelial-to-mesenchymal
transition in lung cancer. Oncotarget. 7:85905–85916. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Jiang W, Xu Z, Yu L, Che J, Zhang J and
Yang J: MicroRNA-144-3p suppressed TGF-β1-induced lung cancer cell
invasion and adhesion by regulating the Src-Akt-Erk pathway. Cell
Biol Int. 2019.Epub ahead of print.
|
|
150
|
Zhao X, Liu Y and Yu S: Long noncoding RNA
AWPPH promotes hepatocellular carcinoma progression through YBX1
and serves as a prognostic biomarker. Biochim Biophys Acta Mol
Basis Dis. 1863:1805–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhu F, Zhang X, Yu Q, Han G, Diao F, Wu C
and Zhang Y: LncRNA AWPPH inhibits SMAD4 via EZH2 to regulate
bladder cancer progression. J Cell Biochem. 119:4496–4505. 2018.
View Article : Google Scholar
|
|
152
|
Tang L, Wang T, Zhang Y, Zhang J, Zhao H,
Wang H, Wu Y and Liu K: Long non-coding RNA AWPPH promotes
postoperative distant recurrence in resected non-small cell lung
cancer by upregulating transforming growth factor beta 1 (TGF-β1).
Med Sci Monit. 25:2535–2541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Chae DK, Park J, Cho M, Ban E, Jang M, Yoo
YS, Kim EE, Baik JH and Song EJ: MiR-195 and miR-497 suppress
tumorigenesis in lung cancer by inhibiting SMURF2-induced TGF-β
receptor I ubiquitination. Mol Oncol. 13:2663–2678. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hypes MK, Pirisi L and Creek KE:
Mechanisms of decreased expression of transforming growth
factor-beta receptor type I at late stages of HPV16-mediated
transformation. Cancer Lett. 282:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Xu F, Zhang J, Hu G, Liu L and Liang W:
Hypoxia and TGF-β1 induced PLOD2 expression improve the migration
and invasion of cervical cancer cells by promoting
epithelial-to-mesenchymal transition (EMT) and focal adhesion
formation. Cancer Cell Int. 17:542017. View Article : Google Scholar
|
|
156
|
Li MY, Liu JQ, Chen DP, Li ZY, Qi B, Yin
WJ and He L: p68 prompts the epithelial-mesenchymal transition in
cervical cancer cells by transcriptionally activating the TGF-β1
signaling pathway. Oncol Lett. 15:2111–2116. 2018.PubMed/NCBI
|
|
157
|
Yang L, Yu Y, Xiong Z, Chen H, Tan B and
Hu H: Downregulation of SEMA4C inhibit epithelial-mesenchymal
transition (EMT) and the invasion and metastasis of cervical cancer
cells via inhibiting transforming growth factor-beta 1
(TGF-β1)-induced Hela cells p38 mitogen-activated protein kinase
(MAPK) activation. Med Sci Monit. 26:e9181232020. View Article : Google Scholar
|
|
158
|
Cheng Y, Guo Y, Zhang Y, You K, Li Z and
Geng L: MicroRNA-106b is involved in transforming growth factor
β1-induced cell migration by targeting disabled homolog 2 in
cervical carcinoma. J Exp Clin Cancer Res. 35:112016. View Article : Google Scholar
|
|
159
|
Finkielstein CV and Capelluto DG:
Disabled-2: A modular scaffold protein with multifaceted functions
in signaling. Bioessays. 38(Suppl 1): S45–S55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Tao MZ, Gao X, Zhou TJ, Guo QX, Zhang Q
and Yang CW: Effects of TGF-beta1 on the proliferation and
apoptosis of human cervical cancer Hela cells in vitro. Cell
Biochem Biophys. 73:737–741. 2015. View Article : Google Scholar
|
|
161
|
Wang H, Wang J, Liu H and Wang X: TGF-β1
activates NOX4/ROS pathway to promote the invasion and migration of
cervical cancer cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
35:121–127. 2019.In Chinese. PubMed/NCBI
|
|
162
|
Deng M, Cai X, Long L, Xie L, Ma H, Zhou
Y, Liu S and Zeng C: CD36 promotes the epithelial-mesenchymal
transition and metastasis in cervical cancer by interacting with
TGF-β. J Transl Med. 17:3522019. View Article : Google Scholar
|
|
163
|
Wongnoppavich A, Dukaew N, Choonate S and
Chairatvit K: Upregulation of maspin expression in human cervical
carcinoma cells by transforming growth factor β1 through the
convergence of Smad and non-Smad signaling pathways. Oncol Lett.
13:3646–3652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Ju W, Luo X and Zhang N: LncRNA NEF
inhibits migration and invasion of HPV-negative cervical squamous
cell carcinoma by inhibiting TGF-β pathway. Biosci Rep. Apr
26–2019.Epub ahead of print. View Article : Google Scholar
|
|
165
|
Levovitz C, Chen D, Ivansson E, Gyllensten
U, Finnigan JP, Alshawish S, Zhang W, Schadt EE, Posner MR, Genden
EM, et al: TGFβ receptor 1: An immune susceptibility gene in
HPV-associated cancer. Cancer Res. 74:6833–6844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhu L, Zhang Q, Li S, Jiang S, Cui J and
Dang G: Interference of the long noncoding RNA CDKN2B-AS1
upregulates miR-181a-5p/TGFβI axis to restrain the metastasis and
promote apoptosis and senescence of cervical cancer cells. Cancer
Med. 8:1721–1730. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wu T, Chen X, Peng R, Liu H, Yin P, Peng
H, Zhou Y, Sun Y, Wen L, Yi H, et al: Let-7a suppresses cell
proliferation via the TGF-β/SMAD signaling pathway in cervical
cancer. Oncol Rep. 36:3275–3282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Fang F, Huang B, Sun S, Xiao M, Guo J, Yi
X, Cai J and Wang Z: MiR-27a inhibits cervical adenocarcinoma
progression by downregulating the TGF-βRI signaling pathway. Cell
Death Dis. 9:3952018. View Article : Google Scholar
|
|
169
|
Wu M, Chen X, Lou J, Zhang S, Zhang X,
Huang L, Sun R, Huang P, Wang F and Pan S: TGF-β1 contributes to
CD8+ Treg induction through p38 MAPK signaling in ovarian cancer
microenvironment. Oncotarget. 7:44534–44544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Wang YQ, Li YM, Li X, Liu T, Liu XK, Zhang
JQ, Guo JW, Guo LY and Qiao L: Hypermethylation of TGF-β1 gene
promoter in gastric cancer. World J Gastroenterol. 19:5557–5564.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Ji M, Shi H, Xie Y, Zhao Z, Li S, Chang C,
Cheng X and Li Y: Ubiquitin specific protease 22 promotes cell
proliferation and tumor growth of epithelial ovarian cancer through
synergy with transforming growth factor β1. Oncol Rep. 33:133–140.
2015. View Article : Google Scholar
|
|
172
|
Teng Y, Zhao L, Zhang Y, Chen W and Li X:
Id-1, a protein repressed by miR-29b, facilitates the TGFβ1-induced
epithelial-mesenchymal transition in human ovarian cancer cells.
Cell Physiol Biochem. 33:717–730. 2014. View Article : Google Scholar
|
|
173
|
Facciabene A, Motz GT and Coukos G:
T-regulatory cells: Key players in tumor immune escape and
angiogenesis. Cancer Res. 72:2162–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zhang J, Liu W, Shen F, Ma X, Liu X, Tian
F, Zeng W, Xi X and Lin Y: The activation of
microRNA-520h-associated TGF-β1/c-Myb/Smad7 axis promotes
epithelial ovarian cancer progression. Cell Death Dis. 9:8842018.
View Article : Google Scholar
|
|
175
|
Wang YQ, Qi XW, Wang F, Jiang J and Guo
QN: Association between TGFBR1 polymorphisms and cancer risk: A
meta-analysis of 35 case-control studies. PLoS One. 7:e428992012.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Eli Lilly: Company: A study in
participants with diabetic kidney disease. ClinicalTrials.gov. 2010, https://clinicaltrials.gov/ct2/show/NCT01113801.
Accessed Sep 17, 2019.
|
|
177
|
Zhang Q, Hou X, Evans BJ, VanBlaricom JL,
Weroha SJ and Cliby WA: LY2157299 monohydrate, a TGF-βR1 inhibitor,
suppresses tumor growth and ascites development in ovarian cancer.
Cancers (Basel). 10. pp. 2602018, View Article : Google Scholar
|
|
178
|
Herbertz S, Sawyer JS, Stauber AJ,
Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba
SC, Benhadji KA, et al: Clinical development of galunisertib
(LY2157299 monohydrate), a small molecule inhibitor of transforming
growth factor-beta signaling pathway. Drug Des Devel Ther.
9:4479–4499. 2015.PubMed/NCBI
|
|
179
|
Fujiwara Y, Nokihara H, Yamada Y, Yamamoto
N, Sunami K, Utsumi H, Asou H, TakahashI O, Ogasawara K,
Gueorguieva I and Tamura T: Phase 1 study of galunisertib, a
TGF-beta receptor I kinase inhibitor, in Japanese patients with
advanced solid tumors. Cancer Chemother Pharmacol. 76:1143–1152.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Brandes AA, Carpentier AF, Kesari S,
Sepulveda-Sanchez JM, Wheeler HR, Chinot O, Cher L, Steinbach JP,
Capper D, Specenier P, et al: A Phase II randomized study of
galunisertib monotherapy or galunisertib plus lomustine compared
with lomustine monotherapy in patients with recurrent glioblastoma.
Neuro Oncol. 18:1146–1156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Ikeda M, Takahashi H, Kondo S, Lahn MMF,
Ogasawara K, Benhadji KA, Fujii H and Ueno H: Phase 1b study of
galunisertib in combination with gemcitabine in Japanese patients
with metastatic or locally advanced pancreatic cancer. Cancer
Chemother Pharmacol. 79:1169–1177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Rodón J, Carducci M, Sepulveda-Sánchez JM,
Azaro A, Calvo E, Seoane J, Braña I, Sicart E, Gueorguieva I,
Cleverly A, et al: Pharmacokinetic, pharmacodynamic and biomarker
evaluation of transforming growth factor-β receptor I kinase
inhibitor, galunisertib, in phase 1 study in patients with advanced
cancer. Invest New Drugs. 33:357–370. 2015. View Article : Google Scholar
|
|
183
|
MedPacto: Dose escalation and
proof-of-concept studies of vactosertib (TEW-7197) monotherapy in
patients with MDS. ClinicalTrials.gov. 2017,
https://clinicaltrials.gov/ct2/show/NCT03074006.
Accessed Mar 24, 2020.
|
|
184
|
MedPacto: First in human dose escalation
study of vactosertib (TEW-7197) in subjects with advanced stage
solid tumors. ClinicalTrials.gov. 2014, https://clinicaltrials.gov/ct2/show/NCT02160106
Accessed Sep 5, 2019.
|
|
185
|
Eli Lilly: Company: A study of LY3200882
in participants with solid tumors. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT02937272.
Accessed Aug 19, 2020.
|
|
186
|
Callahan JF, Burgess JL, Fornwald JA,
Gaster LM, Harling JD, Harrington FP, Heer J, Kwon C, Lehr R,
Mathur A, et al: Identification of novel inhibitors of the
transforming growth factor beta1 (TGF-beta1) type 1 receptor
(ALK5). J Med Chem. 45:999–1001. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Inman GJ, Nicolás FJ, Callahan JF, Harling
JD, Gaster LM, Reith AD, Laping NJ and Hill CS: SB-431542 is a
potent and specific inhibitor of transforming growth factor-beta
super-family type I activin receptor-like kinase (ALK) receptors
ALK4, ALK5, and ALK7. Mol Pharmacol. 62:65–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Tanaka H, Shinto O, Yashiro M, Yamazoe S,
Iwauchi T, Muguruma K, Kubo N, Ohira M and Hirakawa K: Transforming
growth factor β signaling inhibitor, SB-431542, induces maturation
of dendritic cells and enhances anti-tumor activity. Oncol Rep.
24:1637–1643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Melisi D, Ishiyama S, Sclabas GM, Fleming
JB, Xia Q, Tortora G, Abbruzzese JL and Chiao PJ: LY2109761, a
novel transforming growth factor beta receptor type I and type II
dual inhibitor, as a therapeutic approach to suppressing pancreatic
cancer metastasis. Mol Cancer Ther. 7:829–840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zhang ZH, Miao YY, Ke BL, Liu K and Xu X:
LY2109761, transforming growth factor β receptor type I and type II
dual inhibitor, is a novel approach to suppress endothelial
mesenchymal transformation in human corneal endothelial cells. Cell
Physiol Biochem. 50:963–972. 2018. View Article : Google Scholar
|
|
191
|
Tandon M, Salamoun JM, Carder EJ, Farber
E, Xu S, Deng F, Tang H, Wipf P and Wang QJ: SD-208, a novel
protein kinase D inhibitor, blocks prostate cancer cell
proliferation and tumor growth in vivo by inducing G2/M cell cycle
arrest. PLoS One. 10:e01193462015. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Araujo SC, Maltarollo VG, Almeida MO,
Ferreira LL, Andricopulo AD and Honorio KM: Structure-based virtual
screening, molecular dynamics and binding free energy calculations
of Hit candidates as ALK-5 inhibitors. Molecules. 25:2642020.
View Article : Google Scholar :
|
|
193
|
de Gouville AC, Boullay V, Krysa G, Pilot
J, Brusq JM, Loriolle F, Gauthier JM, Papworth SA, Laroze A,
Gellibert F and Huet S: Inhibition of TGF-beta signaling by an ALK5
inhibitor protects rats from dimethylnitrosamine-induced liver
fibrosis. Br J Pharmacol. 145:166–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Park CY, Kim DK and Sheen YY: EW-7203, a
novel small molecule inhibitor of transforming growth factor-β
(TGF-β) type I receptor/activin receptor-like kinase-5, blocks
TGF-β1-mediated epithelial-to-mesenchymal transition in mammary
epithelial cells. Cancer Sci. 102:1889–1896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Ehata S, Hanyu A, Fujime M, Katsuno Y,
Fukunaga E, Goto K, Ishikawa Y, Nomura K, Yokoo H, Shimizu T, et
al: Ki26894, a novel transforming growth factor-beta type I
receptor kinase inhibitor, inhibits in vitro invasion and in vivo
bone metastasis of a human breast cancer cell line. Cancer Sci.
98:127–133. 2007. View Article : Google Scholar
|
|
196
|
Suzuki E, Kim S, Cheung HK, Corbley MJ,
Zhang X, Sun L, Shan F, Singh J, Lee WC, Albelda SM and Ling LE: A
novel small-molecule inhibitor of transforming growth factor beta
type I receptor kinase (SM16) inhibits murine mesothelioma tumor
growth in vivo and prevents tumor recurrence after surgical
resection. Cancer Res. 67:2351–2359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Moore-Smith LD, Isayeva T, Lee JH, Frost A
and Ponnazhagan S: Silencing of TGF-β1 in tumor cells impacts MMP-9
in tumor microenvironment. Sci Rep. 7:86782017. View Article : Google Scholar
|
|
198
|
Li XF, Yan PJ and Shao ZM: Downregulation
of miR-193b contributes to enhance urokinase-type plasminogen
activator (uPA) expression and tumor progression and invasion in
human breast cancer. Oncogene. 28:3937–3948. 2009. View Article : Google Scholar : PubMed/NCBI
|