Introduction
Severe acute respiratory syndrome (SARS)
coronavirus-2 (SARS-CoV-2) is the causative viral agent for the
ongoing COVID-19 pandemic, with >75 million infected individuals
and >2.38 million deaths worldwide, up to the writing of the
present study (February 14, 2021) (1,2).
During the writing of the present manuscript, a glimpse of hope
appeared on the horizon with vaccinations being developed and
administered globally (3-7).
It is now well-established that the entry of
SARS-CoV-2 into cells is facilitated by its spike (S) proteins,
mainly through binding to the angiotensin-converting enzyme 2
(ACE2) (8,9). Moreover, the SARS-CoV-2 S proteins
are primed/activated by the transmembrane protease serine 2
(TMPRSS2), which appears to also play a key role in this viral
infection (8,10,11). As such, there is increasing
interest in identifying additional molecular mediators that may
also facilitate the SARS-CoV-2 infection of host cells and promote
further adverse COVID-19 symptoms. Accordingly, neuropilin-1 (NRP1)
has been identified as a novel cellular mediator implicated in the
SARS-CoV-2 infection (12-14), whilst similar evidence has also
more recently emerged for TMPRSS4, another transmembrane protease.
Indeed, TMPRSS4 - along with TMPRSS2 - appear to activate the
SARS-CoV-2 S proteins, and enhance subsequent viral infection of
human small intestinal enterocytes (15). These data from Zang et al,
provide evidence that the intestine is an additional target organ
for SARS-CoV-2 (15). Moreover,
a recent study demonstrated that both ACE2 and TMPRSS2 genes were
abundantly expressed in enterocytes of the lower gastrointestinal
(GI) tract, whilst also exhibiting co-expression with TMPRSS4,
particularly in the small intestine (16). Of note, whereas COVID-19
presentation usually includes fever, cough, pulmonary/respiratory
symptoms, and loss of taste and smell (17,18), an increasing number of studies
has also reported GI-related symptoms (e.g., diarrhoea, nausea and
vomiting) in patients with COVID-19 (19-21). Indeed, a proportion of these
patients with extra-pulmonary symptoms do not exhibit other
respiratory symptoms (22).
Apart from respiratory and GI-related symptoms, it
is now evident that patients with COVID-19 may experience a wide
repertoire of neurological symptoms and complications (23-28), suggesting that SARS-CoV-2 can
also attack the central nervous system (CNS), potentially via
additional cell entry mediators, including NRP1 (14,29). Indeed, in silico analysis
indicated that certain key mediators which facilitate the entry of
SARS-CoV-2 into host cells, including ACE2, Cathepsin L (CTSL),
TMPRSS2 and TMPRSS4, are expressed in the CNS, with the two
transmembrane proteases being highly expressed in neurons (30).
Finally, increasing evidence suggests that cancer
constitutes an additional risk factor for severe COVID-19
infection, with hospitalised patients with COVID-19 (particularly
males and those receiving treatment with chemotherapy) exhibiting a
poor prognosis and being associated with a high case-fatality rate
(31). The authors of the
present study, as well as other researchers have documented the
differential expression of SARS-CoV-2 infection mediators in
malignant states (11,32). TMPRSS2 in particular, was
previously found to be significantly upregulated in rectum
adenocarcinoma, as well as in prostate, breast and lung cancer
(11). Notably, compared to
never-smokers, a similar ACE2 and TMPRSS2 expression, but a higher
TMPRSS4 expression, has been found in the human bronchial
epithelial cells of current smokers when compared to never-smokers
(33).
To date, there is a paucity of published data about
the expression profile of TMPRSS4 in cancer. Therefore, present
study curated pan-cancer data in terms of TMPRSS4 gene expression
using in silico approaches. The TMPRSS4 expression profile
is also further expanded by providing more evidence regarding the
presence of this transmembrane protease in the CNS and GI
track.
Data and methods
Bioinformatics analysis
The expression analysis of TMPRSS4 was validated
through the Genotype-Tissue Expression (GTEx, www.gtexportal.org) and GEPIA (gepia.cancer-pku.cn).
Information regarding the TCGA cohort pan-cancer data was acquired
through cBioPortal (www.cbioportal.org/) and single cell analysis was done
by using the Single Cell Portal (singlecell.broadinstitute.org/single_cell).
Datasets accessed for pan-cancer analysis were the following: ACC,
adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA,
breast invasive carcinoma; CESC, cervical squamous cell carcinoma
and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD,
colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B cell
lymphoma; ESCA, oesophageal carcinoma; GBM, glioblastoma
multiforme; HNSC, head and neck squamous cell carcinoma; KICH,
kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP,
kidney renal papillary cell carcinoma; LAML, acute myeloid
leukaemia; LGG, brain lower grade glioma; LIHC, liver
hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung
squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous
cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG,
pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma;
READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous
melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell
tumours; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine
corpus endometrial carcinoma; UCS, uterine carcinosarcoma; and UVM,
uveal melanoma. The visualisation of TMPRSS4 expression in the
human brain was performed using the Allen brain atlas with 6 human
donors, as assessed by microarray and presented as a heatmap
(34). These data were acquired
from a publicly available source which underwent all appropriate
approvals by the Human Investigation Committees and Institutional
Ethics Committees of each institute from which samples were
obtained.
Statistical analysis
The method used for differential analysis in the
present study was one-way ANOVA, using disease state (Tumour or
Normal) as variables for calculating differential expression: Gene
expression ~disease state. The expression data are first
log2(TPM+1) transformed for differential analysis and the log2FC is
defined as median (Tumor) – median (Normal). Genes with higher
|log2FC| values and lower q values than pre-set thresholds are
considered differentially expressed genes. For more information
please visit: http://gepia.cancer-pku.cn/help.html.
Results
Using the TCGA and GTEX datasets, it was
demonstrated that, compared to the normal control, TMPRSS4 was
upregulated in 11 cancer datasets (i.e., CESC, COAD, LUAD, LUSC,
OV, PAAD, READ, STAD, THCA, UCEC and UCS) (Fig. 1), while it was significantly
downregulated in 6 datasets (i.e., KICH, KIRC, KIRP, LAML, SKCM and
TGCT), with three of the latter consisting kidney tumours (i.e.,
KICK, KIRC and KIRP) (Fig.
2).
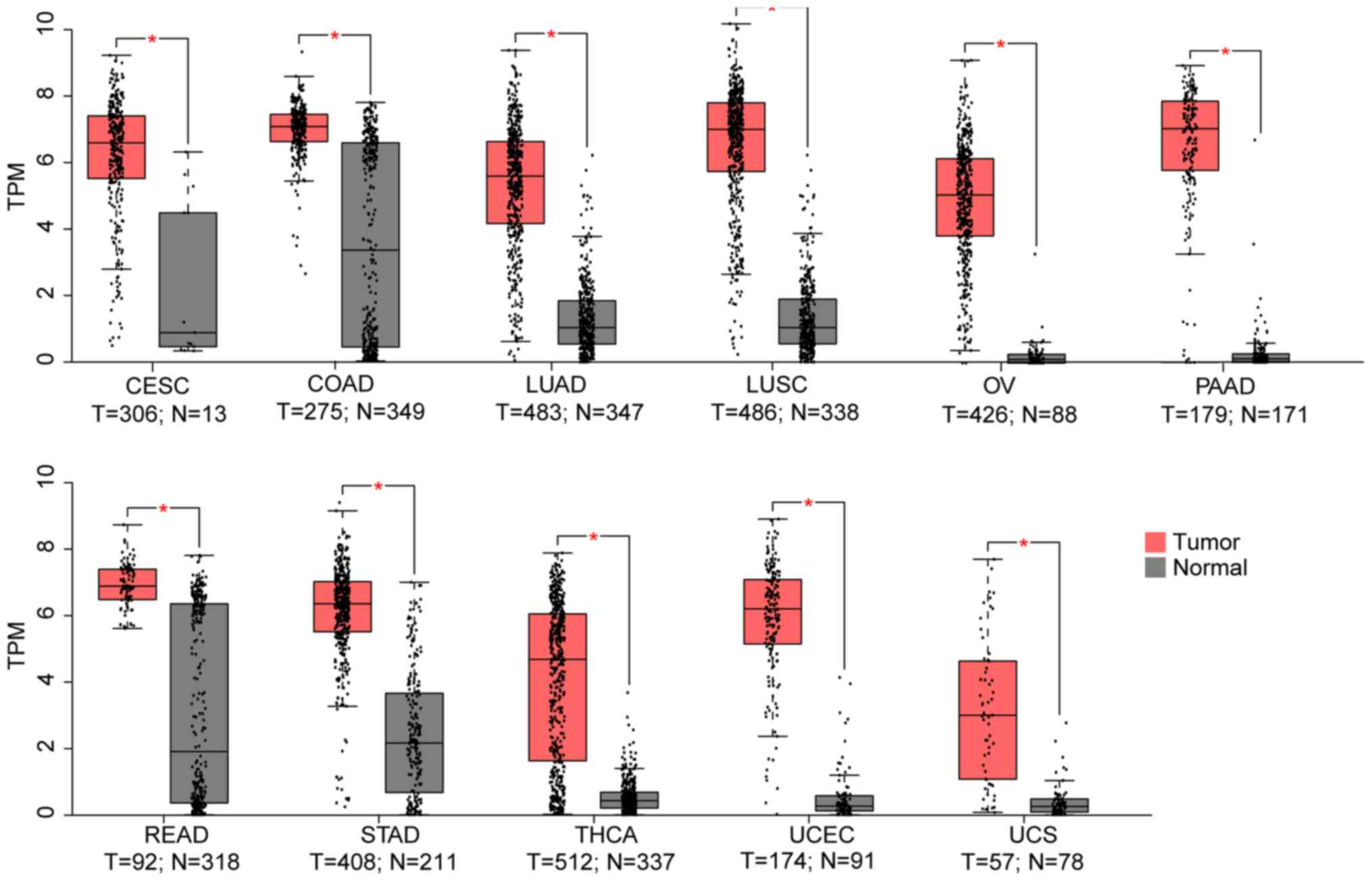 | Figure 1From the 33 TCGA cancer datasets, 11
exhibited a higher TMPRSS4 expression in tumour samples. TMPRSS4
was highly expressed in lung cancers (LUAD and LUSC), gynecological
cancers (CESC, OV, UCEC and UCS), pancreatic carcinoma (PAAD),
colon adenocarcinoma (COAD), rectum carcinoma (READ), stomach
adenocarcinoma (STAD) and thyroid cancer (THCA). T, tumour; N,
normal; TPM, transcripts per million; LUAD, lung adenocarcinoma;
LUSC, lung squamous cell carcinoma; CESC, cervical squamous cell
carcinoma and endocervical adenocarcinoma; OV, ovarian serous
cystadenocarcinoma; UCEC, uterine corpus endometrial carcinoma;
UCS, uterine carcinosarcoma. |
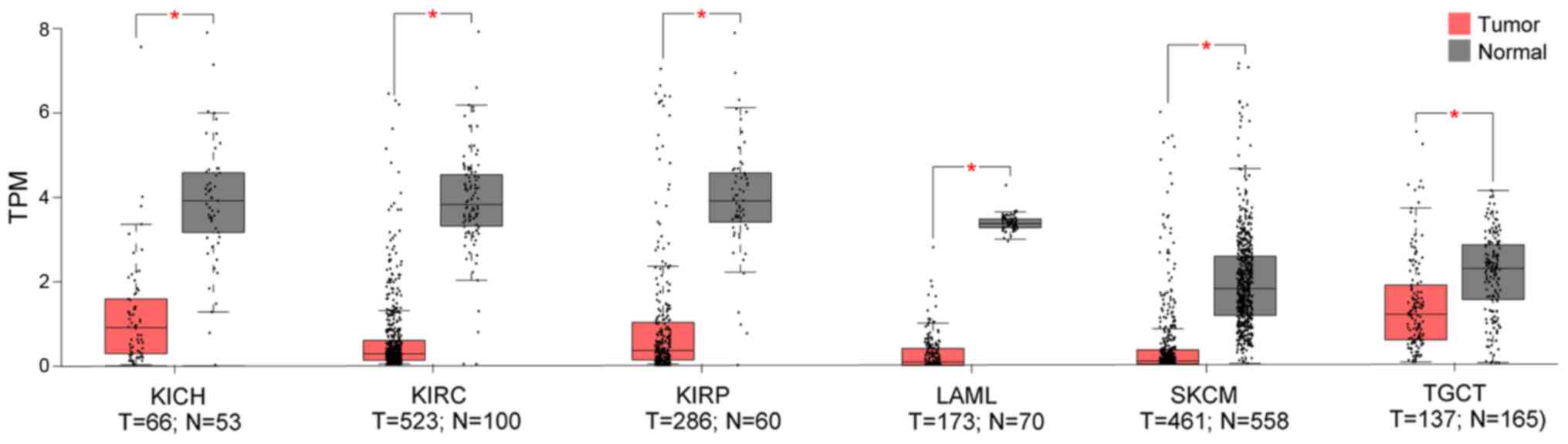 | Figure 2Out of the 33 TCGA cancer datasets,
TMPRSS4 was significantly downregulated in 6 datasets (i.e., KICH,
KIRC, KIRP, LAML, SKCM and TGCT), with three of these consisting
kidney tumours (i.e., KICK, KIRC and KIRP) compared to normal
tissues. KICH, kidney chromophobe; KIRC, kidney renal clear cell
carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute
myeloid leukaemia; SKCM, skin cutaneous melanoma; TGCT, testicular
germ cell tumours; T, tumour; N, normal. |
Furthermore, using the cBioportal pan-cancer panel,
the present study identified specific cancer types with a number of
TMPRSS4 amplifications in LGG, LUAD, STAD, deep deletions in BRCA,
HNSC, and shallow deletions in BLCA, BRCA, CESC, ESCA, LUAD, LUSC,
OV and TGCT. Furthermore, there were two datasets for thyroid
cancers with a number of patients presenting diploid and not
mutated versions of the gene, namely for THYM and THCA. Of note, in
the majority of the studied cancers, the majority of the patients
had deletions and partly some gains and amplifications (Fig. 3).
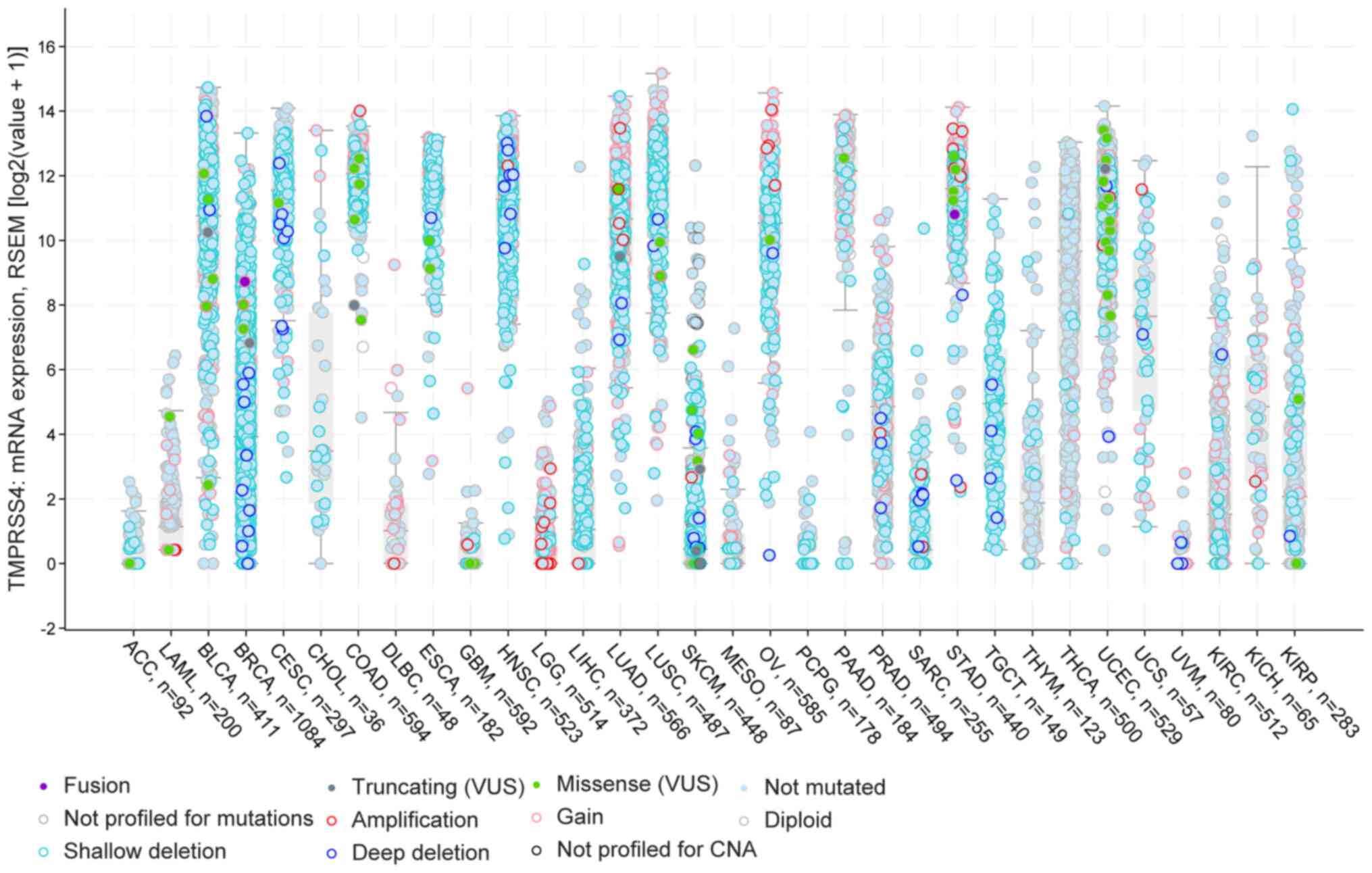 | Figure 3cBioportal analysis of the 32 TCGA
datasets with information about the gene dysrégulation. In BLCA,
LUAD and LUSC there are a number of shallow deletions, while in
BRCA and HNSC, high expression and more deep deletions are
observed. In gynaecological cancers (CESC, OV, UCEC and UCS), there
is high expression of the TMPRSS4 and a few patients present Gains
and Missense 'variants of uncertain significant' (VUS). BLCA,
bladder urothelial carcinoma; BRCA, breast invasive carcinoma;
CESC, cervical squamous cell carcinoma and endocervical
adenocarcinoma; HNSC, head and neck squamous cell carcinoma; LUAD,
lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV,
ovarian serous cystadenocarcinoma; UCEC, uterine corpus endometrial
carcinoma; UCS, uterine carcinosarcoma. |
As aforementioned, there is emerging evidence
suggesting abundant SARS-CoV-2 infection of the GI tract in severe
COVID-19 cases (35). Herein,
these observations were expanded upon using single cell analysis
from 51 cell subsets (366,650 cells in total) in the colon mucosa
of 18 ulcerative colitis and 12 healthy individuals using the
Single Cell Portal (36). Using
T-distributed Stochastic Neighbour Embedding (tSNE) - a machine
learning algorithm for visualization - distinct cell
sub-populations express TMPRSS4 (Fig. 4). Notably, a high TMPRSS4
expression was noted in immature enterocytes, BEST4-expressing
enterocytes and enterocytes, as presented in Fig. 4C.
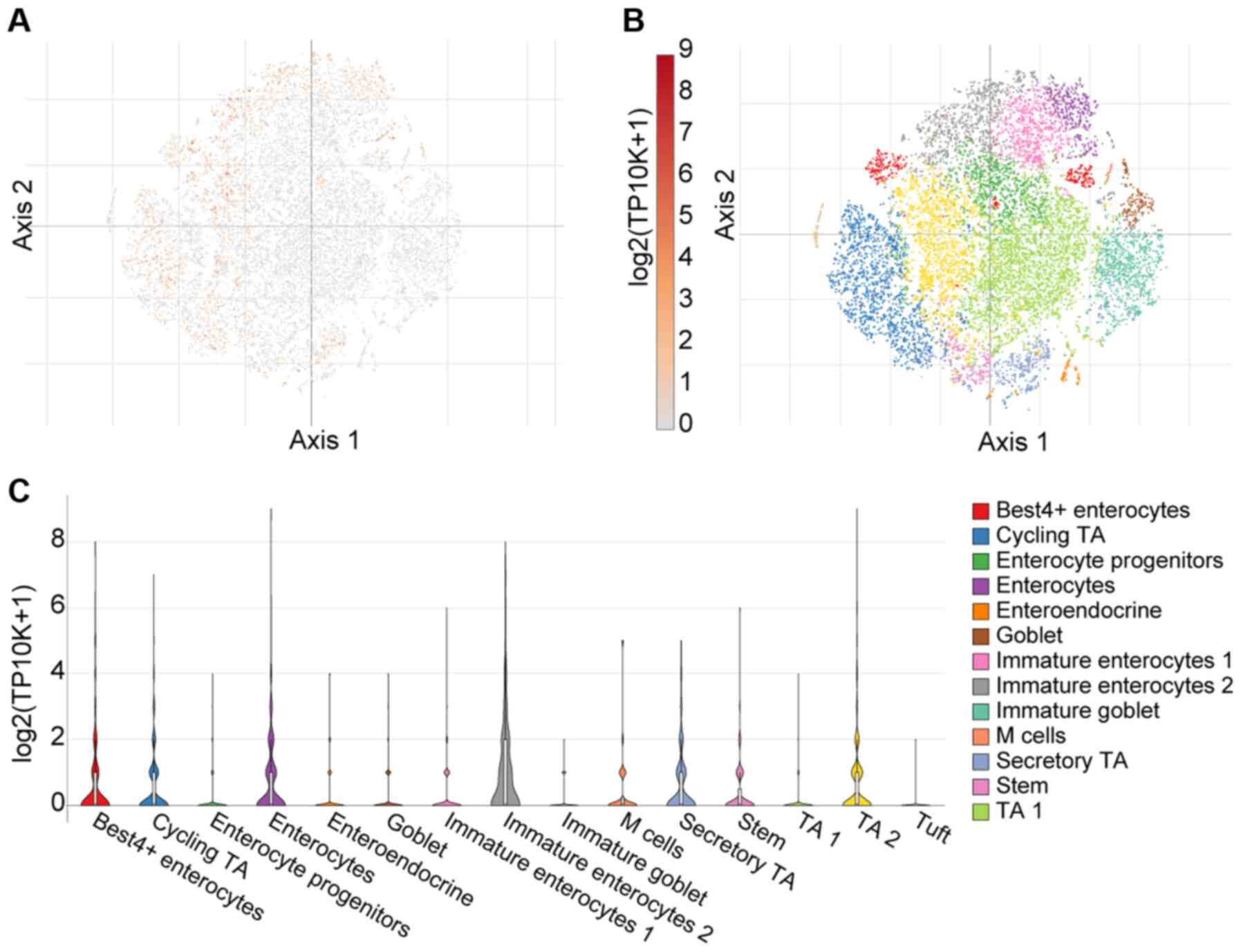 | Figure 4Single cell analysis of colon mucosa,
using the Single Cell Portal, revealed widespread expression of
TMPRSS4. (A) Single-cell transcriptomics data are displayed as a
spectral tSNE (T-distributed Stochastic Neighbour Embedding) plot
of 366,650 cells, annotated according to known cell types; (B)
sub-populations of cell types which are enriched for TMPRSS4
expression, with expression intensity demonstrated by a heat map;
(C) single cell analysis using the Single Cell Portal, enriched for
population subtypes, represented as violin plots. Notably, high
TMPRSS4 expression was noted in immature enterocytes,
BEST4-expressing enterocytes and enterocytes. TA1, TA2;
transit-amplifying (TA) cells. |
Finally, TMPRSS4 expression was also observed in
various brain regions, based on a human microarray data set of 6
human brains (Fig. 5). These
data were acquired from a publicly available source which underwent
all appropriate approvals by the Human Investigation Committees and
Institutional Ethics Committees of each institute from which
samples were obtained.
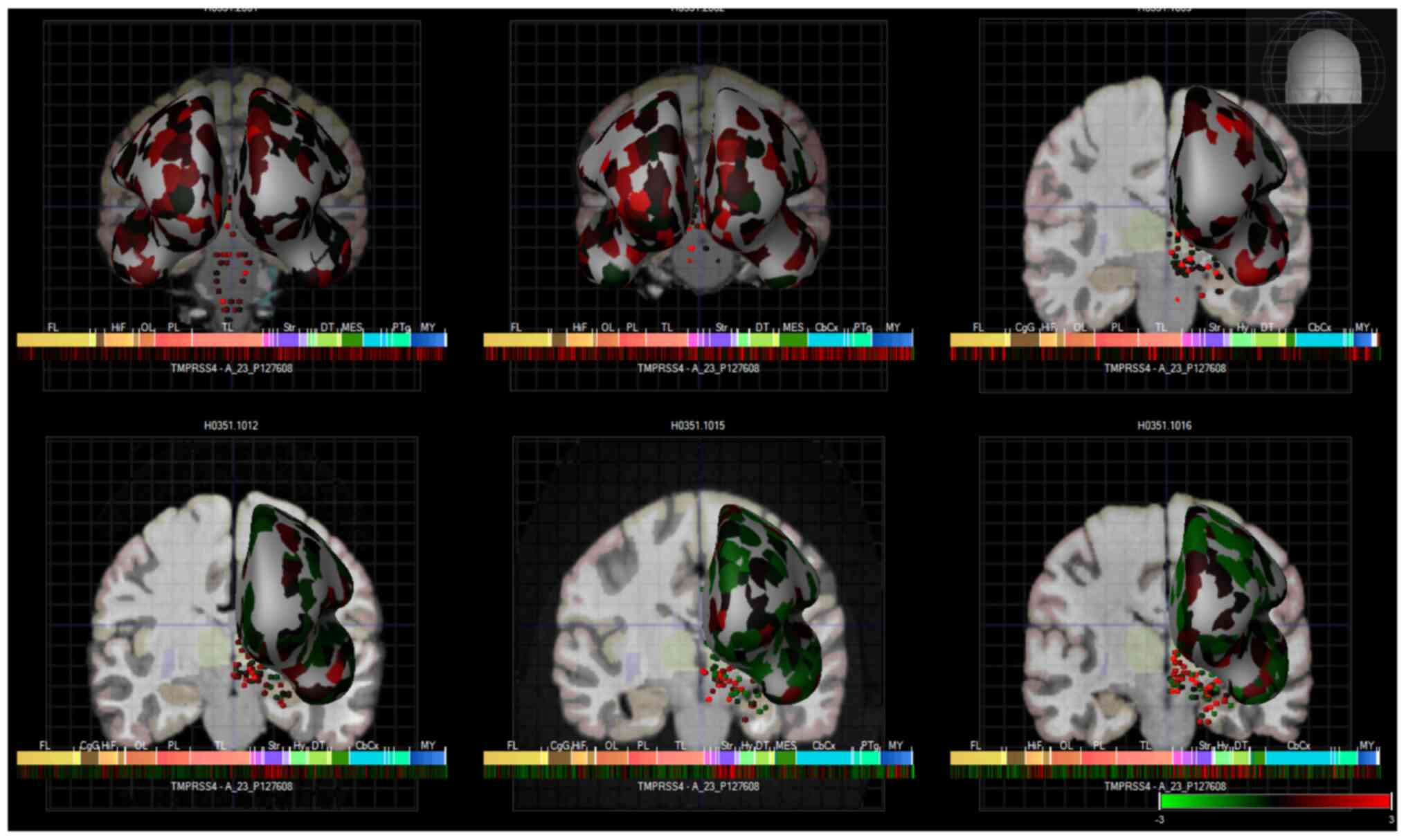 | Figure 5Heatmap expression of TMPRSS4 in the
brains of 6 human donors, as assessed by microarray (Allen brain
atlas). Global brain expression of TMPRSS4, as detected by
microarray probes, with expression of cerebral areas indicated on
the hemispheres and expression in brain nuclei indicated as dots.
Heatmaps of expression are displayed below individual brains and
organised by anterior to posterior regions with the frontal lobe
(Fl), hippocampal formation (HiF), occipital lobe (Ol), parietal
lobe (Pl), temporal lobe (Tl), striatum (Str), dorsal thalamus
(dT), cerebral cortex (cbcx) and myelencephalon (MY) marked on the
heatmap. |
Fig. 5 presents
these data as heatmaps of log2 expression values, showing that
TMPRSS4 is expressed throughout the human brain. Of note, anosmia
represents one of the common first symptoms of SARS-CoV-19
infection (37,38), and although there is global brain
expression of TMPRSS4, a high TMPRSS4 expression is noted in brain
regions important for the sense of smell and taste, namely in the
olfactory tubercle (Log2 expression, 2.1±1), paraolfactory gyrus
(Log2 expression, 1.3±0.3) and frontal operculum (Log2 expression,
1.5±0.5). These Log2 expression values are provided by the Allen
Brain Atlas. Previous research has also demonstrated that TMPRSS4
exhibited the highest expression in neurons, with the neuronal rich
cerebral cortex exhibiting a high TMPRSS4 expression (39). TMPRSS4 protein expression is 1.5
protein-transcripts per million (pTPM) in this region with the
highest expression localised to the neuronal cells
(ProteinAtlas_ENSG00000137648).
Discussion
In the present study, comprehensive evidence is
presented regarding the peripheral tissue and CNS distribution of a
new potential mediator of SARS-CoV-2 infection, namely TMPRSS4. Of
note, it was demonstrated that TMPRSS4 is overexpressed in lung
cancer; a condition that predisposes to severe COVID-19 (33,40,41). The current findings are in
agreement with those of a previous study demonstrating the
co-expression of TMPRSS4 with other key SARS-CoV-2 cell entry
mediators (i.e., ACE2, ADAM17 and TMPRSS2) in bronchial epithelial
cells from never smokers and current smokers (33). Notably, that study also
demonstrated that the TMPRSS4 levels were elevated in smokers,
suggesting that this may be an additional risk factor for
SARS-CoV-2 infection (33). In
the lung cancer cohorts investigated in the present study, a
marginal difference was note in LUAD with a marked downregulation
of TMPRSS4 in current smokers, whilst in patients with LUSC an
increase in TMPRSS4 was noted in smokers compared to non-smokers
(Fig. S1A and B). This is
suggestive of potential tissue-specific effects, given that
patients with LUAD have poorer prognosis than those with LUSC
(42).
Another recent study also demonstrated a high
expression of TMPRSS4 in the human endometrium, which increased
with age, particularly in the early phases of the cycle (43). This is of increasing importance
given that SARS-CoV-2 infectivity increases with age (44). The present study expanded on
these observations and provided evidence of TMPRSS4 expression in
other gynaecological tissues, demonstrating that TMPRSS4 is
expressed in the cervix, vagina, fallopian tubes, ovaries, breast
and uterus, with the first two being the primary organs in terms of
a high expression (Fig. S1D).
Moreover, it was demonstrated that TMPRSS4 was significantly
upregulated in gynaecological malignancies, namely CESC, OV, UCEC
and UCS. As age appears to play a role in the risk of COVID-19, the
present study further investigated the expression of TMPRSS4 in the
above-mentioned malignancies with age. In this respect, TMPRSS4
expression increased with age only in the case of UCEC (Fig. S1C). One of the major limitations
of the present study was that in silico data were accessed,
that did not allow us to perform statistical analysis with a post
hoc test. Another limitation is the absence of survival analysis
data. Future studies are required to determine whether TMPRSS4 can
be of prognostic value in terms of overall- and/or progression free
survival.
Recently, two different groups have described the
involvement of TMPRSS4 as a SARS-CoV-2 entry mediator in the GI
(15,16). In particular, ACE2, TMPRSS2 and
TMPRSS4 have been shown to be co-expressed primarily in the small
intestine (16). In another
study, through a series of elegant experiments, it was shown that,
apart from TMPRSS2, TMPRSS4 also enhanced SARS-CoV-2 infectivity in
gut epithelial cells (15),
suggesting that a leaky gut may allow SARS-CoV-2 to spread to other
organs, including the liver. Future studies are required to focus
on the role of these key cell entry mediators in GI tract organs,
particularly in relation to extra-pulmonary manifestations of
severe COVID-19.
Finally, it was demonstrated that TMPRSS4 is present
in a number of anterior and posterior regions of the brain. This
corroborates previous finding indicating expression in the cerebral
cortex, hippocampus and caudate (45). Of note, other SARS-CoV-2 cell
entry mediators (e.g., ACE2, TMPRSS2, NRP1 and CTSL) are also
expressed in the CNS, raising the possibility of a synergy between
multiple such proteins which may further drive the SARS-CoV-2
neurotropism (29,30,46).
In conclusion, the present study documents
widespread TMPRSS4 protein expression in the CNS and GI tract, and
provides further evidence supporting the potential involvement of
TMPRSS4 in facilitating adverse COVID-19 outcomes in patients with
certain cancers. Collectively, these data suggest that TMPRSS4 may
be implicated in the symptomatology/complications of COVID-19,
acting as another SARS-CoV-2 cell entry mediator responsible for
the tropism of this novel coronavirus in the periphery and the
CNS.
Supplementary Data
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
PK, RK, JD, EK, JD, JLR, IK consulted the
literature, and produced the figures and manuscript. MH, HSR, KC,
JD, VA, DAS, JLR and AP contributed to the literature search,
interpretation of the data and the critical revision of the
manuscript. PK, JD, HSR, DAS, IK and EK contributed to the writing
of the manuscript and final edits. IK and EK contributed equally to
the conception of the study, data and literature analysis, as well
as interpretation. All authors read and approved the final
manuscript. JD is an alumnus of Brunel University London. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
World Health Organization (WHO): Weekly
Epidemiological Update on COVID-19. WHO; Geneva: 2021
|
|
2
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re-emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI
|
|
3
|
Calina D, Hartung T, Docea AO, Spandidos
DA, Egorov AM, Shtilman MI, Carvalho F and Tsatsakis A: COVID-19
vaccines: Ethical framework concerning human challenge studies.
Daru. 28:807–812. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kostoff RN, Kanduc D, Porter AL, Shoenfeld
Y, Calina D, Briggs MB, Spandidos DA and Tsatsakis A: Vaccine- and
natural infection-induced mechanisms that could modulate vaccine
safety. Toxicol Rep. 7:1448–1458. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F,
Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calina D, Sarkar C, Arsene AL, Salehi B,
Docea AO, Mondal M, Islam MT, Zali A and Sharifi-Rad J: Recent
advances, approaches and challenges in targeting pathways for
potential COVID-19 vaccines development. Immunol Res. 68:315–324.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voysey M, Clemens SAC, Madhi SA, Weckx LY,
Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE,
et al Oxford COVID Vaccine Trial Group: Safety and efficacy of the
ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim
analysis of four randomised controlled trials in Brazil, South
Africa, and the UK. Lancet. 397:99–111. 2021. View Article : Google Scholar
|
|
8
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara
H, Geng Q, Auerbach A and Li F: Structural basis of receptor
recognition by SARS-CoV-2. Nature. 581:221–224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwata-Yoshikawa N, Okamura T, Shimizu Y,
Hasegawa H, Takeda M and Nagata N: TMPRSS2 contributes to virus
spread and immunopathology in the airways of murine models after
coronavirus infection. J Virol. 93:e01815–e01818. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katopodis P, Anikin V, Randeva HS,
Spandidos DA, Chatha K, Kyrou I and Karteris E: Pan-cancer analysis
of transmembrane protease serine 2 and cathepsin L that mediate
cellular SARS-CoV-2 infection leading to COVID-19. Int J Oncol.
57:533–539. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bittmann S, Weissenstein A, Villalon G,
Moschuring-Alieva E and Luchter E: Simultaneous treatment of
COVID-19 with serine protease inhibitor camostat and/or cathepsin L
inhibitor? J Clin Med Res. 12:320–322. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cantuti-Castelvetri L, Ojha R, Pedro LD,
Djannatian M, Franz J, Kuivanen S, Kallio K, Kaya T, Anastasina M,
Smura T, et al: Neuropilin-1 facilitates SARS-CoV-2 cell entry and
provides a possible pathway into the central nervous system.
bioRxiv. doi: https://doi.org/10.1101/2020.06.07.137802.
|
|
14
|
Daly JL, Simonetti B, Klein K, Chen KE,
Williamson MK, Anton-Plagaro C, Shoemark DK, Simon-Gracia L, Bauer
M, Hollandi R, et al: Neuropilin-1 is a host factor for SARS-CoV-2
infection. Science. 370:861–865. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zang R, Gomez Castro MF, McCune BT, Zeng
Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB,
et al: TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human
small intestinal enterocytes. Sci Immunol. 5:1–15. 2020. View Article : Google Scholar
|
|
16
|
Lee JJ, Kopetz S, Vilar E, Shen JP, Chen K
and Maitra A: Relative abundance of SARS-CoV-2 entry genes in the
enterocytes of the lower gastrointestinal tract. Genes (Basel).
11:6452020. View Article : Google Scholar :
|
|
17
|
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He
JX, Liu L, Shan H, Lei CL, Hui DSC, et al: China Medical Treatment
Expert Group for Covid-19: Clinical characteristics of coronavirus
disease 2019 in China. N Engl J Med. 382:1708–1720. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kerslake R, Hall M, Randeva HS, Spandidos
DA, Chatha K, Kyrou I and Karteris E: Co-expression of peripheral
olfactory receptors with SARS-CoV-2 infection mediators: Potential
implications beyond loss of smell as a COVID-19 symptom. Int J Mol
Med. 46:949–956. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ng SC and Tilg H: COVID-19 and the
gastrointestinal tract: More than meets the eye. Gut. 69:973–974.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Almeida JFM and Chehter EZ: COVID-19 and
the gastrointestinal tract: What do we already know? Einstein (Sao
Paulo). 18:eRW59092020. View Article : Google Scholar
|
|
21
|
Syed A, Khan A, Gosai F, Asif A and
Dhillon S: Gastrointestinal pathophysiology of SARS-CoV2 - a
literature review. J Community Hosp Intern Med Perspect.
10:523–528. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin X, Lian JS, Hu JH, Gao J, Zheng L,
Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, et al: Epidemiological,
clinical and virological characteristics of 74 cases of
coronavirus-infected disease 2019 (COVID-19) with gastrointestinal
symptoms. Gut. 69:1002–1009. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Felice FG, Tovar-Moll F, Moll J, Munoz
DP and Ferreira ST: Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) and the central nervous system. Trends Neurosci.
43:355–357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q,
Chang J, Hong C, Zhou Y, Wang D, et al: Neurologic manifestations
of hospitalized patients with coronavirus disease 2019 in Wuhan,
China. JAMA Neurol. 77:683–690. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Needham EJ, Chou SH-Y, Coles AJ and Menon
DK: Neurological implications of COVID-19 infections. Neurocrit
Care. 32:667–671. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao XY and Jin WL: The COVID-19 pandemic:
consideration for brain infection. Neuroscience. 437:130–131. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Q, Fan X, Hong H, Gu Y, Liu Z, Fang S,
Wang Q, Cai C and Fang J: Comprehensive assessment of side effects
in COVID-19 drug pipeline from a network perspective. Food Chem
Toxicol. 145:1117672020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sidiropoulou P, Docea AO, Nikolaou V,
Katsarou MS, Spandidos DA, Tsatsakis A, Calina D and Drakoulis N:
Unraveling the roles of vitamin D status and melanin during
Covid-19 (Review). Int J Mol Med. 47:92–100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davies J, Randeva HS, Chatha K, Hall M,
Spandidos DA, Karteris E and Kyrou I: Neuropilin-1 as a new
potential SARS-CoV-2 infection mediator implicated in the
neurologic features and central nervous system involvement of
COVID-19. Mol Med Rep. 22:4221–4226. 2020.PubMed/NCBI
|
|
30
|
Matschke J, Lütgehetmann M, Hagel C,
Sperhake JP, Schröder AS, Edler C, Mushumba H, Fitzek A, Allweiss
L, Dandri M, et al: Neuropathology of patients with COVID-19 in
Germany: A post-mortem case series. Lancet Neurol. 19:919–929.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang K, Sheng Y, Huang C, Jin Y, Xiong N,
Jiang K, Lu H, Liu J, Yang J, Dong Y, et al: Clinical
characteristics, outcomes, and risk factors for mortality in
patients with cancer and COVID-19 in Hubei, China: A multicentre,
retrospective, cohort study. Lancet Oncol. 21:904–913. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chai P, Yu J, Ge S, Jia R and Fan X:
Genetic alteration, RNA expression, and DNA methylation profiling
of coronavirus disease 2019 (COVID-19) receptor ACE2 in
malignancies: A pan-cancer analysis. J Hematol Oncol. 13:432020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Voinsky I and Gurwitz D: Smoking and
COVID-19: Similar bronchial ACE2 and TMPRSS2 expression and higher
TMPRSS4 expression in current versus never smokers. Drug Dev Res.
81:1073–1080. 2020. View Article : Google Scholar
|
|
34
|
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts
AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert
A, Riley ZL, et al: An anatomically comprehensive atlas of the
adult human brain transcriptome. Nature. 489:391–399. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma C, Cong Y and Zhang H: COVID-19 and the
digestive system. Am J Gastroenterol. 115:1003–1006. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smillie CS, Biton M, Ordovas-Montanes J,
Sullivan KM, Burgin G, Graham DB, Herbst RH, Rogel N, Slyper M,
Waldman J, et al: Intra- and inter-cellular rewiring of the human
colon during ulcerative colitis. Cell. 178:714–730.e22. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walker A, Pottinger G, Scott A and Hopkins
C: Anosmia and loss of smell in the era of covid-19. BMJ.
370:m28082020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Veldhuizen MG and Small DM:
Modality-specific neural effects of selective attention to taste
and odor. Chem Senses. 36:747–760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Darmanis S, Sloan SA, Zhang Y, Enge M,
Caneda C, Shuer LM, Hayden Gephart MG, Barres BA and Quake SR: A
survey of human brain transcriptome diversity at the single cell
level. Proc Natl Acad Sci USA. 112:7285–7290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Aberasturi AL, Redrado M, Villalba M,
Larzabal L, Pajares MJ, Garcia J, Evans SR, Garcia-Ros D, Bodegas
ME, Lopez L, et al: TMPRSS4 induces cancer stem cell-like
properties in lung cancer cells and correlates with ALDH expression
in NSCLC patients. Cancer Lett. 370:165–176. 2016. View Article : Google Scholar
|
|
41
|
Larzabal L, Nguewa PA, Pio R, Blanco D,
Sanchez B, Rodríguez MJ, Pajares MJ, Catena R, Montuenga LM and
Calvo A: Overexpression of TMPRSS4 in non-small cell lung cancer is
associated with poor prognosis in patients with squamous histology.
Br J Cancer. 105:1608–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suzuki K, Nagai K, Yoshida J, Nishimura M,
Takahashi K, Yokose T and Nishiwaki Y: Conventional
clinicopathologic prognostic factors in surgically resected
nonsmall cell lung carcinoma. A comparison of prognostic factors
for each pathologic TNM stage based on multivariate analyses.
Cancer. 86:1976–1984. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Henarejos-Castillo I, Sebastian-Leon P,
Devesa-Peiro A, Pellicer A and Diaz-Gimeno P: SARS-CoV-2 infection
risk assessment in the endometrium: Viral infection-related gene
expression across the menstrual cycle. Fertil Steril. 114:223–232.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nikolich-Zugich J, Knox KS, Rios CT, Natt
B, Bhattacharya D and Fain MJ: SARS-CoV-2 and COVID-19 in older
adults: What we may expect regarding pathogenesis, immune
responses, and outcomes. Geroscience. 42:505–514. 2020. View Article : Google Scholar : PubMed/NCBI
Erratum. Geroscience. 42:10132020.
|
|
45
|
Guadarrama-Ortiz P, Choreño-Parra JA,
Sánchez-Martínez CM, Pacheco-Sánchez FJ, Rodríguez-Nava AI and
García-Quintero G: Neurological aspects of SARS-CoV-2 infection:
mechanisms and manifestations. Front Neurol. 11:10392020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Frank S: Catch me if you can: SARS-CoV-2
detection in brains of deceased patients with COVID-19. Lancet
Neurol. 19:883–884. 2020. View Article : Google Scholar : PubMed/NCBI
|



















