Introduction
Radiotherapy, which has been used to treat malignant
tumors for over a century, is still the most commonly used
technique clinically for the management of cancer (1). However, the efficacy of
radiotherapy comes at the expense of damage to adjacent normal
tissues, including tissues of the skeletal system (2-4).
Irradiation initiates bone loss accompanied by bone fractures or
even increases the risk of bone metastasis due to bone matrix
degradation, which seriously impairs a patient's quality of life
and is a major dose-limiting factor in the use of radiotherapy
(5-7). Thus, further investigations to
elucidate the mechanisms responsible for radiation-induced
collateral damage of the skeleton should be performed. Osteocytes
are the most abundant type of cells in the skeleton, are embedded
within the mineralized bone matrix, and have long been deemed to be
passive, metabolically inactive cells (8). However, studies have shown that
osteocytes are multifunctional cells that both compose bone and
respond to mechanical loads for weight support, and also exhibit
key roles in bone remodeling and mineral homeostasis through
secretory regulation of both osteoclasts and osteoblasts (9-12). Targeting osteocytes and
osteocyte-mediated pathways may hold promise in treating
irradiation-related bone damage, therefore it is essential to
investigate the effects of irradiation on osteocytes, and the
underlying mechanisms.
The characteristic dendrite-like morphology of
osteocytes is determined by the expression of genes associated with
dendritic formation and branching, such as E11/gp38, and dendrites
are involved in the ability of osteocytes to communicate with other
cells in the surrounding environment (13,14). Existing research has shown E11 is
involved in early osteocytogenesis and the formation of osteocyte
dendrites (15). Sclerostin
(SOST), a marker of mature osteocytes and a negative modulator of
bone formation, is specifically secreted by osteocytes (16). Alternatively, osteocytes may
generate a variety of functional pro-osteoclastogenic molecules,
such as receptor activator of nuclear factor-κB ligand (RANKL) or
inhibitors, such as osteoprotegerin (OPG), to regulate bone
resorption. As a major source of RANKL, osteocyte was also reported
to release RANKL to the cell surface for stimulation with RANKL
(17).
Radiation may result in notable DNA damage and place
various stresses on cells, resulting in the initiation of typical
cellular senescence, in which cells remain metabolically active,
yet undergo irreversible cell cycle arrest and develop distinct
phenotypic alterations, including chromatin organization,
upregulation of p16, p21 and acquisition of a secretory phenotype
(5,18,19). Despite their maintained metabolic
activity and resistance to apoptosis, which serve roles in
preserving cellular integrity and function, dysfunctional intra-
and extra-cellular effects in cellular senescence cannot be
ignored. The dysfunctional secretion of pro-inflammatory cytokines,
chemokines and matrix metalloproteinases, termed
senescence-associated secretory phenotype (SASP), is a key feature
of cellular senescence, and is considered a modulatory mechanism
for surrounding cells (20).
Previous research showed that radiation could generate senescent
cells as well as the SASP in a chronic manner similar to aging,
which influences fracture healing after radiation treatment. In
addition, several factors derived from the accumulation of
senescent cells are implicated as causal in age-related
osteoporosis and imbalance of bone metabolism (21). Findings of previous studies of
cellular senescence have primarily focused on stem cells (22,23). By contrast, there are a limited
number of studies on post-mitotic cells, such as the terminally
differentiated osteocytes (24-26). Therefore, the biological
functions and cellular senescence phenotypes regarding this type of
bone cell should be explored to determine its relevance. Osteocytes
can survive for decades within the bone matrix, making them one of
the longest living cells in the body (9). However, the consequence of
osteocyte senescence and its role in the pathophysiology of
irradiation-related bone loss is poorly understood. Despite the
existence of several cell lines that have been used to study
osteocytes in vitro, the differences between primary
osteocytes and the immortalized cell lines have not been studied,
to the best of our knowledge. However, in vitro experiments,
albeit important in the preliminary stages, cannot substitute for
primary cells or in vivo models.
Taken together, osteocytes act as important
orchestrators of bone remodeling, and may provide novel targets and
strategies for intervention and treatment of related bone diseases,
including cancer radiotherapy-induced bone loss. In the present
study, the effect of γ-rays on the biological function of primary
osteocytes was assessed. Subsequently, irradiation-induced
senescence and its secretory phenotype were further explored,
particularly regarding regulation of osteoclastogenesis through the
corresponding SASP paracrine pathway. The results of the present
study may provide a novel insight into the underlying mechanism,
and highlight the clinical potential to manage cancer
treatment-induced bone loss.
Materials and methods
Isolation of primary osteocytes
Primary osteocytes were isolated from 7-week-old
male BALB/c mice (weight, ~25 g) purchased from the Department of
Experimental Animals at Fudan University (Shanghai). All
experimental procedures were approved by the Committee for Ethical
Use of Experimental Animals at Fudan University (approval no.
2017-03-FYS-ZGY-01). A modified protocol of sequential
collagenase/pancreatin digestion was utilized to isolate primary
osteocytes (27-29). The femur and tibia of BALB/c male
mice were aseptically dissected after sacrificing mice by cervical
dislocation. The bones were placed in sterile PBS supplemented with
10% penicillin and streptomycin, followed by a series of digestion
processes, as shown in Table I.
Collagenase solution was prepared as 0.001 g/ml collagenase type-I
(Sigma-Aldrich; Merck KGaA) and 0.001 g/ml collagenase type-II
(Sigma-Aldrich; Merck KGaA) dissolved in PBS. EDTA solution (5 mM;
pH, 7.4) was prepared in magnesium and calcium-free PBS with 1%
calf serum (Gibco; Thermo Fisher Scientific, Inc.). Trypsin (Gibco;
Thermo Fisher Scientific, Inc.) was used directly, and all
instruments were autoclaved in advance. Cell suspensions as well as
the bone pieces were directly plated and cultured in α-minimum
essential medium (α-MEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 5% FBS (Gibco; Thermo Fisher Scientific, Inc.),
5% calf serum and 1% penicillin/streptomycin (Sigma-Aldrich; Merck
KGaA). Cells were cultured at 37°C with 5% CO2 in a
humidified incubator for 7 days until cell confluency reached
>90%.
 | Table IStepwise procedure for isolating
osteocytes from mouse long bones. |
Table I
Stepwise procedure for isolating
osteocytes from mouse long bones.
| Step number | Step
description |
|---|
| 1 | Soft tissue
(adherent muscle and connective tissue) were cut off and the
periosteum was scraped off using a surgical blade. |
| 2 | Epiphyses were cut
off and the marrow was rinsed with PBS using a syringe. |
| 3 | After extensive
washing, bones were cut into 1-2 mm fragments in PBS. |
| 4 | The remaining PBS
was removed, and bone pieces were digested using trypsin solution
for 20 min in an incubator. |
| 5 | Bone pieces were
digested with collagenase type-II solution at 37°C for 1 h in an
incubator. |
| 6 | Bone pieces were
washed twice with PBS. |
| 7 | Bone pieces were
incubated with EDTA solution at 37°C for 30 min in an incubator,
and the EDTA solution was collected. The bone pieces were once
again rinsed in PBS, and the PBS was subsequently added to the EDTA
solution. |
| 8 | The mixed EDTA
solution obtained in the step 7 was centrifuged at room temperature
at 200 × g for 10 min and the sedimented cells were resuspended in
2 ml culture medium (α Minimum Essential Medium supplemented with
10% FBS, calf serum and Pen/Strep). |
| 9 | Bone pieces were
incubated with collagenase type-I solution at 37°C for 30 min in an
incubator, and the collagenase type-I solution was collected. The
bone pieces were once again rinsed in PBS, and the PBS was
subsequently added to the collagenase solution. |
| 10 | The mixed
collagenase solution obtained in step 9 was centrifuged at room
temperature at 200 × g for 10 min and the sediment cells were
resuspended in 2 ml culture medium. |
| 11 | Step 7 was
repeated. |
| 12 | The mixed solution
obtained in step 11 was centrifuged at room temperature at 200 × g
for 10 min and the sedimented cells were resuspended in 2 ml
culture medium. |
| 13 | Step 9 was
repeated. |
| 14 | The mixed solution
obtained in step 13 was centrifuged at 200 × g for 10 min at room
temperature and the sedimented cells were resuspended in 2 ml
culture medium. |
| 15 | Sedimented cells in
steps 8, 10, 12 and 14 were mixed and cultured together with
digested bone pieces at 37°C with 5% CO2 in a humidified
incubator for 7 days until cell confluency reached >90%. |
Isolation of osteoblasts
Osteoblasts were isolated mechanically from calvaria
of newborn BALB/c mice, purchased from the Department of
Experimental Animals at Fudan University (Shanghai). All
experimental procedures in mice were approved by the Committee for
Ethical Use of Experimental Animals at Fudan University (approval
no. 2017-03-FYS-ZGY-01). After carefully stripping off the
periosteal layers and soft tissue on bone surface in PBS, the
calvaria were then cut into 1-2 mm fragments. Subsequently, these
bone pieces were digested with Trypsin solution for 20 min in an
incubator, then pre-digested bone pieces were digested twice in
collagenase type-II solution for 1 h. Finally, the collagenase
digestion was collected and centrifuged at 200 × g for 10 min at
room temperature and the sediment osteoblasts were resuspended in 4
ml DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin
for further culture.
Identification of primary osteocytes
Alkaline phosphatase (ALP) staining was used to
identify primary osteocytes. ALP staining is weak in osteocytes
obtained from long bones (28,30). After removing the media, the
cells were washed using PBS and then incubated with ALP staining
solution for 2 h at room temperature, which was provided in a
staining kit (Beyotime Biotechnology). Images of random fields of
view were taken at ×100 magnification using a light microscope
(Leica Microsystems).
Expression levels of markers associated with
osteocytes were also analyzed. Primary osteocyte lysates were
obtained using RIPA lysis buffer (Beyotime Institute of
Biotechnology), loaded on a 10% SDS-gel, resolved using SDS-PAGE
and transferred to a PVDF membrane (EMD Millipore). After blocking
with 5% skimmed milk in TBS supplemented with 0.1% Tween 20 (TBST)
for 1 h, the membrane was incubated with primary antibodies
specific to E11 (cat. no. ab11936, Abcam; 1:1,000), P16 (cat. no.
ab51243, Abcam; 1:1,000), P21 (cat. no. ab188224, Abcam; 1:1,000),
RANKL (cat. no. ab45039, Abcam; 1:1,000), OPG (cat. no. ab183910,
Abcam; 1:1,000), SOST (cat. no. ab63097, Abcam; 1:1,000) or β-actin
(cat. no. 4970S, Cell Signaling Technology, Inc.; 1:10,000 at 4°C
overnight. Subsequently, the membrane was washed three times with
TBST, each for 10 min, followed by incubation with an
anti-mouse-IgG-HRP-conjugated or anti-rabbit-IgG-HRP-conjugated
secondary antibody (cat. no. SA00001-2, Proteintech; 1:1,000) at
room temperature for 1 h. Signals were visualized using an ECL kit
(Beyotime Institute of Biotechnology) and an Omega Lum™ C Imaging
System (Gel Company). Densitometry analysis was performed using
Image J Software (NIH).
Cell culture and irradiation
treatment
Primary osteocytes were cultured as described above.
For ionizing radiation, 1 day after cell plating, osteocytes were
subjected to 2, 4, 6 or 8 Gy irradiation with 137Cs
γ-rays (Nordion). The dose rate at the centre of the chamber was
66.7 cGy/min, which was annually calibrated by the Shanghai
Institute of Measurement and Testing Technology. LiF (Mg, Cu, P)
thermoluminescent dosimeters were used to measure the actual
radiation dose at different distances from the chamber centre
(31,32). Untreated cells (0 Gy) were used
as the control. Subsequent analysis was performed 3 days after
irradiation.
Irradiation-induced morphological and
functional changes in osteocytes
The cell viability of irradiated osteocytes was
detected using a Cell Counting Kit-8 assay (CCK8; Dojindo Molecular
Technologies, Inc.). Briefly, osteocytes seeded in 96-well plates
(3×103 cells/well) were subsequently treated with
irradiation (0, 2, 4 or 8 Gy), followed by incubation for 1, 3 or 5
days. CCK8 reagent was added at 10%, and incubated for 2 h at 37°C.
The absorbance at 450 nm was examined using a microplate reader
(Bioteck), and was considered to indicate cell viability.
The morphological changes including dendrite-like
synapse and cytoskeleton in the irradiated osteocytes were examined
by staining with tetramethyl rhodamine-phalloidin (Beijing Solarbio
Science & Technology Co., Ltd.) for F-actin and with DAPI
(Dojindo Molecular Technologies, Inc.) for nuclei. Each staining
step was processed at room temperature and incubated for 2 h in the
dark. The number and dendritic length of osteocytes were
quantitatively measured using SimplePCI software (HCImage,
SimplePCI 6.6). Six random fields were chosen in three biological
replicates, and representative images were captured using a Leica
fluorescent microscope (Leica Microsystems, GmbH) with a
magnification of ×100. The length of the dendrites was calculated
by total length of dendrites/number of dendrites per cell.
Irradiation-induced osteocyte DNA
damage
A total of 7 h after radiation treatment, osteocytes
were fixed using 4% formaldehyde at room temperature for 20 min.
After washing with PBS, cells were permeabilized and blocked using
1% Triton X-100, 1% BSA and 10% FBS in PBS buffer for 1 h at room
temperature, and then incubated with primary antibodies against
γ-H2AX (cat. no. ab81299, Abcam; 1:250) overnight at 4°C. Cells
were washed with PBS twice and then incubated in the dark with
anti-mouse-IgG-HRP-conjugated or anti-rabbit-IgG-HRP-conjugated
secondary antibodies (cat. no. SA00001-2, Proteintech; 1:1,000) and
DAPI (1:500; Beyotime Institute of Biotechnology) for 60 min. The
number of γ-H2AX foci was quantified using a laser confocal
microscope (Zeiss LSM 880; Carl Zeiss AG) with a magnification of
×60.
Irradiation-induced osteocyte senescence
and its secretory phenotype
Senescence-associated β-galactosidase (SA-β-gal)
expression was visualized using an SA-β-gal staining kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. Cells were washed with PBS twice and fixed using a
fixative solution at room temperature for 15 min, and then stained
with X-gal solution for 24 h at 37°C (without CO2).
Cells were observed using a light microscope (Leica Microsystems,
GmbH) with a magnification of ×100, and the percentage of
SA-β-gal-positive cells in 10 random fields was calculated.
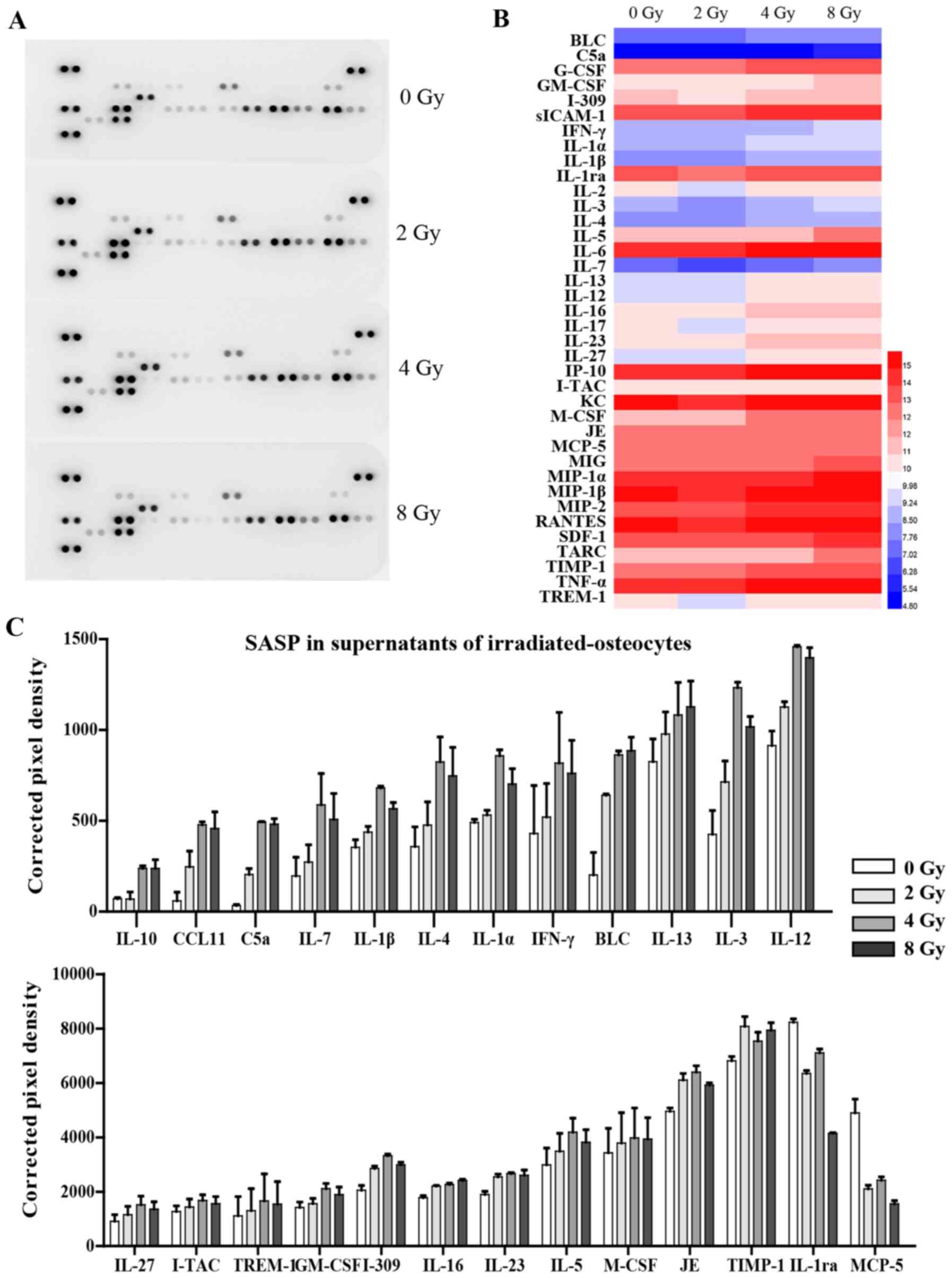
The levels of 40 different cytokines and chemokines
in 700 µl supernatant of cultured osteocytes 3 days after
irradiation were simultaneously detected using R&D Systems
Mouse Cytokine Array, Panel A (cat. no. ARY006; R&D Systems,
Inc.), according to the manufacturer's protocol. Signals were
detected using chemiluminescence (Chemi Scope 6300) and
subsequently quantitated with HLImage++ computer vision systems
(1997, Western Vision Software).
Irradiation-induced senescent
osteocyte-mediated osteoclastogenesis
Primary osteocytes were inoculated in a 24-well
plate (4×103 cells/well), cultured for 24 h and
subsequently irradiated (0, 2, 4, and 8 Gy). RAW264.7 cells
(2×103 cells/well) were plated on top of the irradiated
osteocytes and co-cultured in α-MEM supplemented with 10% FBS and
25 ng/ml RANKL (PeproTech, Inc.). The induction medium was changed
every 2 days. After 7 days, cells were fixed in 2.5% glutaraldehyde
for 10 min at room temperature and stained for tartrate-resistant
acid phosphatase (TRAP) activity using a TRAP staining kit
according to the manufacturer's protocol (Sigma-Aldrich, Merck
KGaA). TRAP-positive multinucleated cells with >3 nuclei were
counted as osteoclasts and the area was analyzed using ImageJ.
Reverse transcription-quantitative PCR
(RT-qPCR)
Gene expression analysis was performed using
RT-qPCR. Three days after irradiation, cells were washed with cold
PBS three times and lysed using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) to obtain total RNA.
Reverse transcription was performed using a Quantscript RT kit
(Tiangen Biotech) at 45°C for 15 min and 95°C for 3 min, and PCR
was performed in an ABI QuantStudio 3 (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using PowerUp SYBR-Green MasterMix
(Invitrogen; Thermo Fisher Scientific, Inc.) to a final volume of
10 µl. The thermocycling conditions used were: 40 cycles of
95°C for 15 sec followed by 55°C for 15 sec and 72°C for 1 min. The
primer sequences are listed in Table II. The relative mRNA expression
levels of the indicated genes were quantified using the
2-ΔΔCq method (33).
GAPDH was used as the loading control.
 | Table IIPrimer sequences for reverse
transcription-quantitative PCR. |
Table II
Primer sequences for reverse
transcription-quantitative PCR.
| Genes | Sequence
(5'-3') |
|---|
| E11 | F:
ACCGTGCCAGTGTTGTTCTG |
| R:
AGCACCTGTGGTTGTTATTTTGT |
| P16 | F:
CGCAGGTTCTTGGTCACTGT |
| R:
TGTTCACGAAAGCCAGAGCG |
| P21 | F:
CCTGGTGATGTCCGACCTG |
| R:
CCATGAGCGCATCGCAATC |
| NF-κB | F:
TGCGATTCCGCTATAAATGCG |
| R:
ACAAGTTCATGTGGATGAGGC |
| TNF-α | F:
TCAGAATGAGGCTGGATAAG |
| R:
GGAGGCAACAAGGTAGAG |
| MMP13 | F:
CCTTGATGCCATTACCAGTCTC |
| R:
TCCACATGGTTGGGAAGTTCT |
| IL-1α | F:
CTGAAGAAGAGACGGCTGAGT |
| R:
CTGGTAGGTGTAAGGTGCTGAT |
| IL-6 | F:
ATGAACAACGATGATGCACTTG- |
| R:
GGTACTCCAGAAGACCAGAGG |
| GAPDH | F:
GGAGTCTACTGGTGTCTTC |
| R:
TCATCATACTTGGCAGGTT |
Statistical analysis
Data were analyzed by one-way ANOVA using SPSS 16.0
(SPSS, Inc.) and GraphPad Prism 5.0 (GraphPad Software, Inc.).
Following each one-way ANOVA, Tukey's post hoc test was performed
to compare all treatments against the control. Data are presented
as the mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of primary osteocytes
Osteocytes, traditionally viewed as a descendant of
osteoblasts, exhibit distinct characteristics from osteoblasts
(30,34). In order to determine whether the
isolated cells could reflect osteocyte metabolism traits in
vitro, cellular dendrite-like synapse morphology, expression of
ALP and expression levels of marker proteins were observed. Three
days after isolation, digested primary osteocytes were present and
were suspended in the medium, whereas on the 5th day, primary
osteocytes emerged from the bone pieces, and cell populations
obtained by direct digestion were observed. In the following
culture process, it was noted that primary osteocytes proliferated
continuously and presented a characteristic stellate shape with the
formation of specific dendritic processes, characteristic to
osteocytes, which are essential for osteocyte function (Fig. 1A).
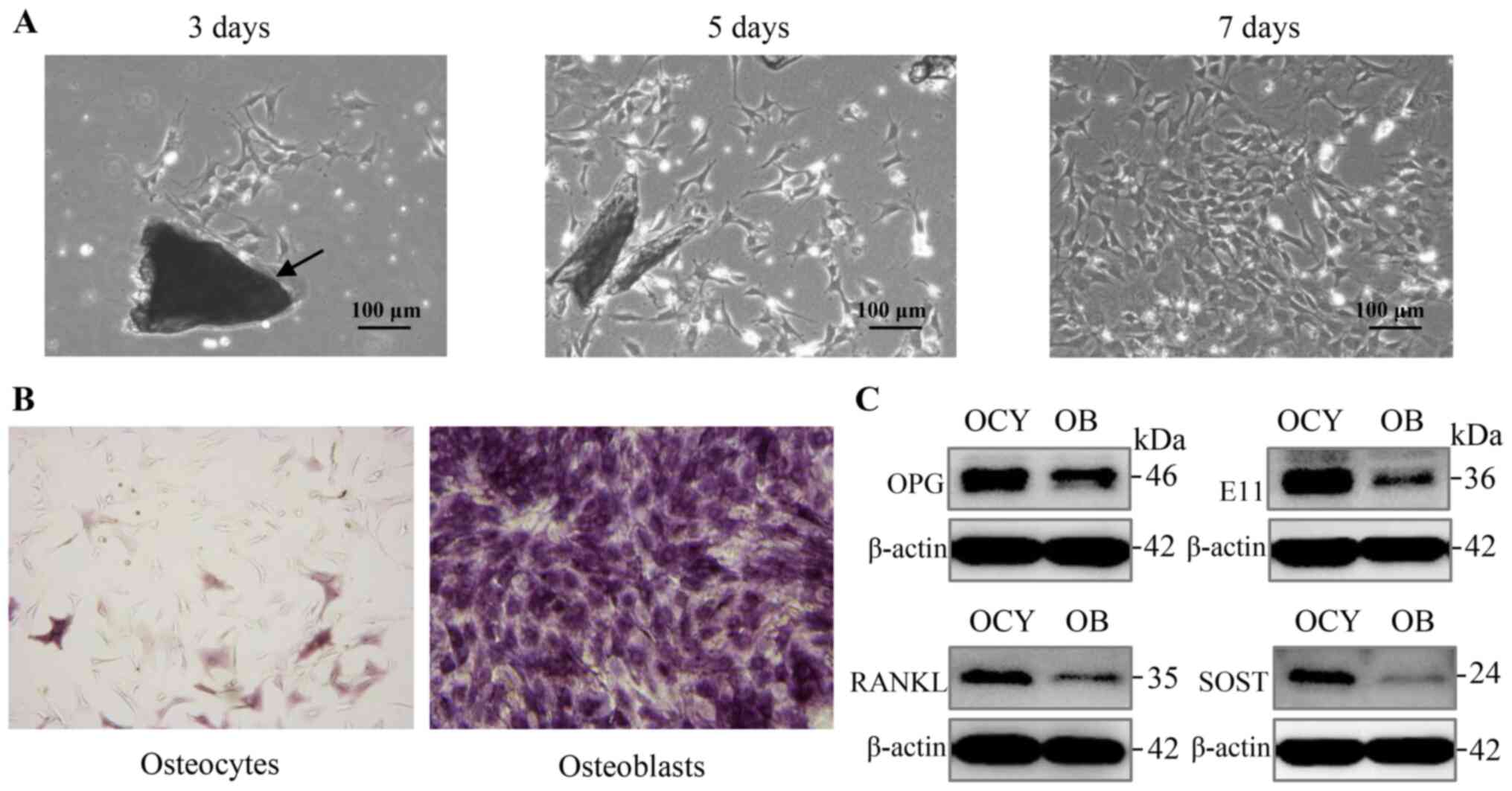 | Figure 1Proliferative primary osteocytes
exhibit a typical morphology and characteristic marker protein
expression. (A) Proliferation and characteristic morphology of
primary osteocytes was observed using bright-field microscopy, 3, 5
and 7 days after isolation. Bone pieces are indicated by the
arrows. Scale bar, 100 µm. Magnification, ×100. (B) ALP
staining of primary osteocytes and osteoblasts, respectively. Scale
bar, 100 µm. Magnification, ×100. (C) Analysis of expression
of the osteocyte markers, E11, SOST, RANKL and OPG in primary
osteocytes using western blot analysis. ALP, alkaline phosphatase;
SOST, sclerostin; RANKL, receptor activator of nuclear factor-κB
ligand; OPG, osteoprotegerin. |
In addition to the above morphological
characteristics, osteocytes showed negative or only weak positive
staining for ALP expression, whereas osteoblasts exhibited strong
staining (Fig. 1B). Furthermore,
primary osteocytes were confirmed by the expression of several
marker proteins, including OPG, E11, RANKL, and SOST, which were
produced almost exclusively in osteocytes (Fig. 1C). Overall, the process of
isolation detailed in Table I
was effectively used to obtain and identify primary osteocytes
isolated from femur and tibia.
Irradiation reduces cell viability and
directly disrupts fundamental biological functions
Taking into consideration the essential role of
osteocytes in bone homeostasis, the fact that they account for
>90% of the composition of all bone cells and function in
mechanical induction, a decreased number of osteocytes would result
in bone loss (16). Viability
and survival are therefore extremely important to ensure optimal
function of the osteocyte network. Irradiation significantly
affected the viability of primary osteocytes, which eventually
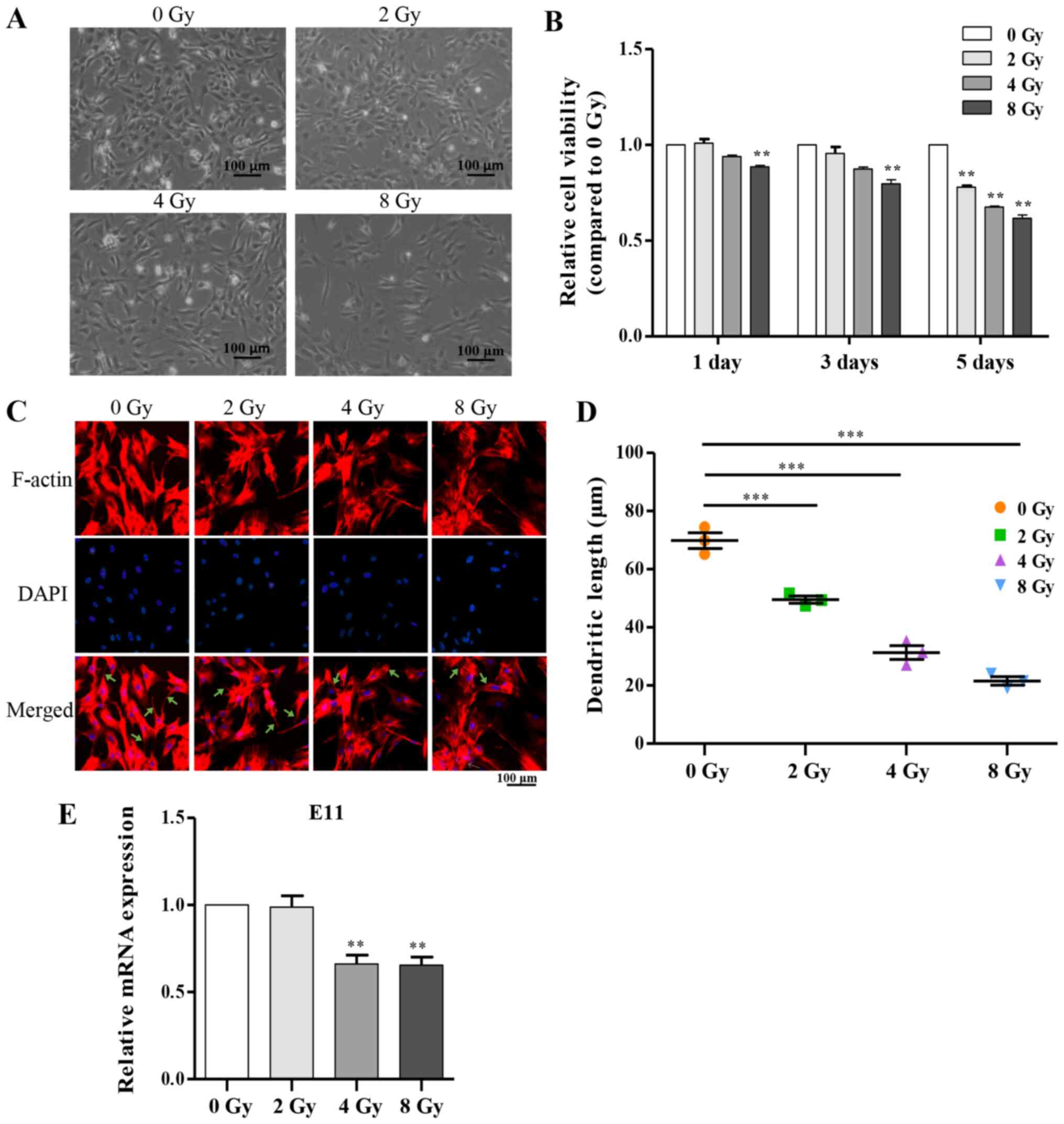
resulted in a decreased number of osteocytes (Fig. 2A and B). Additionally, the
multi-dendritic structure of osteocytes is a key feature closely
associated with physiological function, including communication
with other cells on the bone surface, and itself (15,35). To determine whether irradiation
influenced the dendritic morphology of primary osteocytes, cells
were stained after irradiation for F-actin, and notable
dose-dependent morphological alterations were observed including
sparsely distributed cells, shortened or disappeared dendritic
branches and disordered cytoskeleton compared with the control
group (Fig. 2C and D).
Accordingly, serving as an important marker in dendrite expansion,
E11 mRNA expression was attenuated as the dose of irradiation was
increased (Fig. 2E).
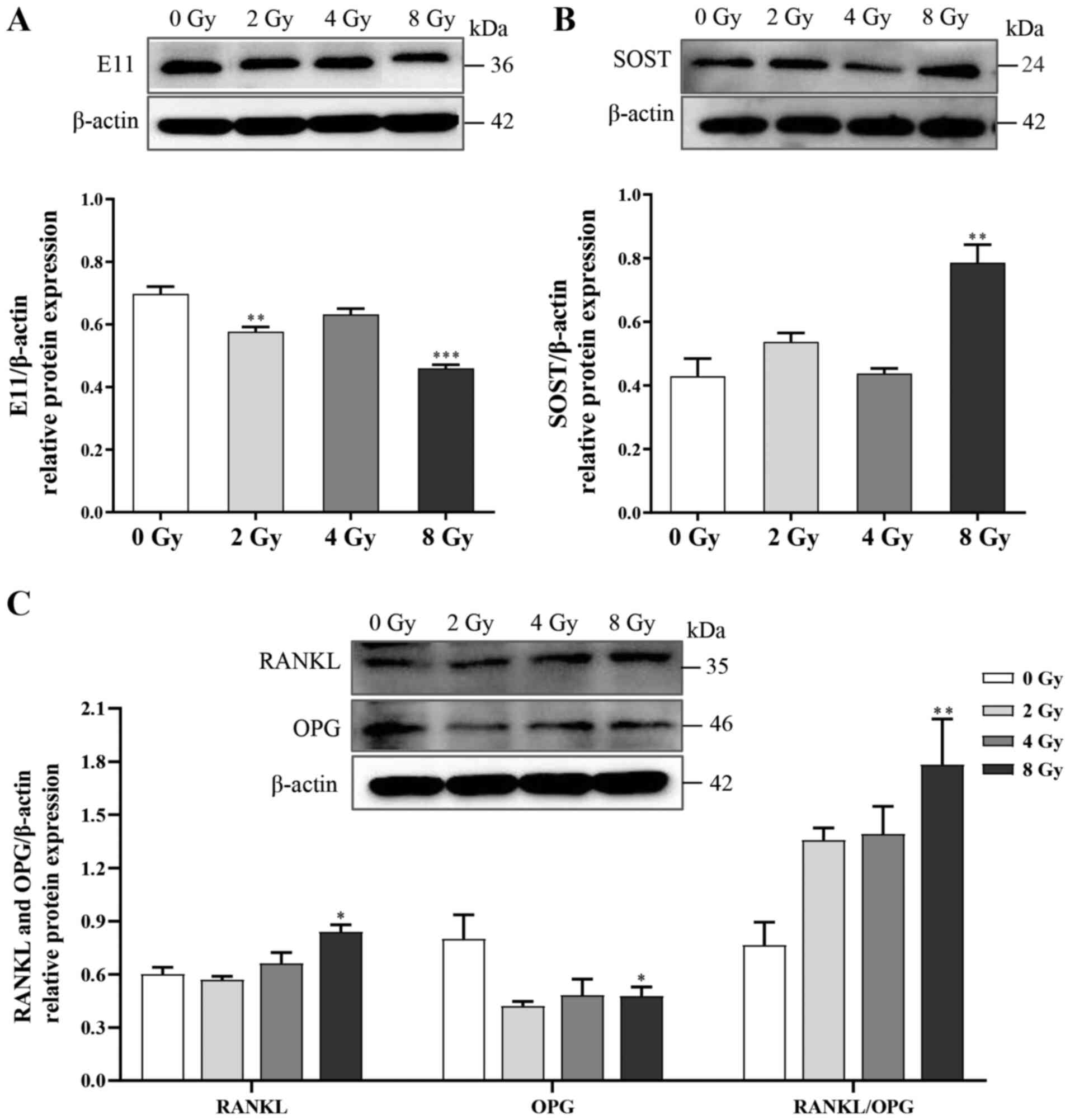
Furthermore, to investigate the effects of irradiation on
biological functions in osteocytes, changes in the expression of
several regulatory factors, including E11, RANKL, OPG and SOST were
examined. Following irradiation, the expression levels of E11 and
OPG were attenuated, whereas the expression levels of both RANKL
and SOST, which respectively activate bone resorption and inhibit
bone formation, were significantly increased (Fig. 3). Thus, the unbalanced function
of osteocytes could be the cellular basis for disturbances in bone
remodeling following irradiation.
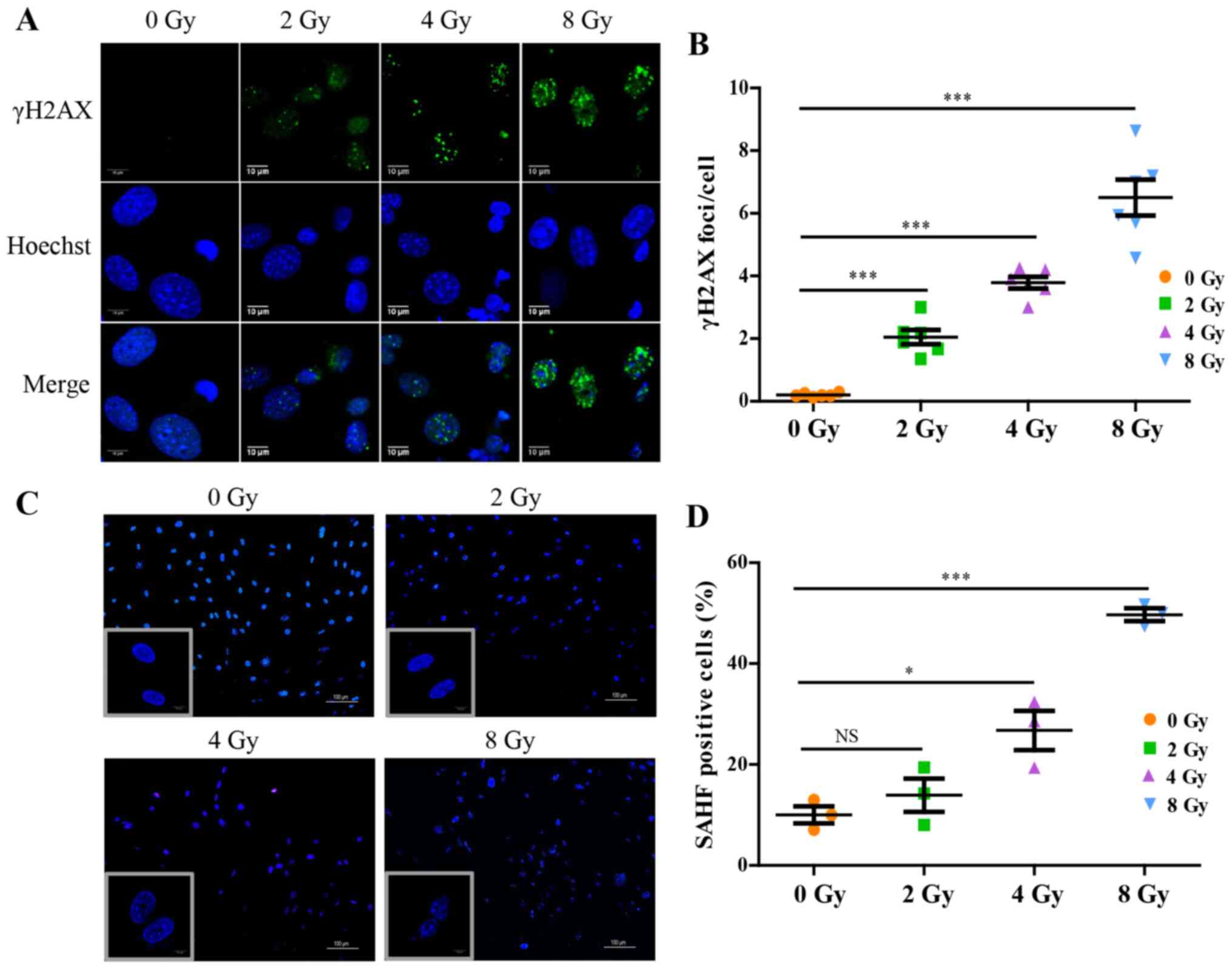
Irradiation induces DNA damage and
cellular senescence
At the primary cell level, irradiation disrupted
osteocyte viability and the expression of proteins associated with
their biological functions. Focusing on the dysregulated
intracellular molecular biology caused by irradiation and the
underlying mechanism, it was shown that irradiation significantly
promoted accumulation of γH2AX in osteocytes, a hallmark of DNA
double-stranded breaks (Fig. 4A and
B). To further confirm the changes in chromatin structure in
irradiation-induced senescent cells, senescence-associated
heterochromatic foci (SAHF) formation in nuclei was assessed and
visualized using DAPI staining (Fig.
4C and D), which exhibited nucleolus enlargement, and
significant loss and compromised nuclear integrity indicative of
nuclear chromatin structure injury, and the effects were
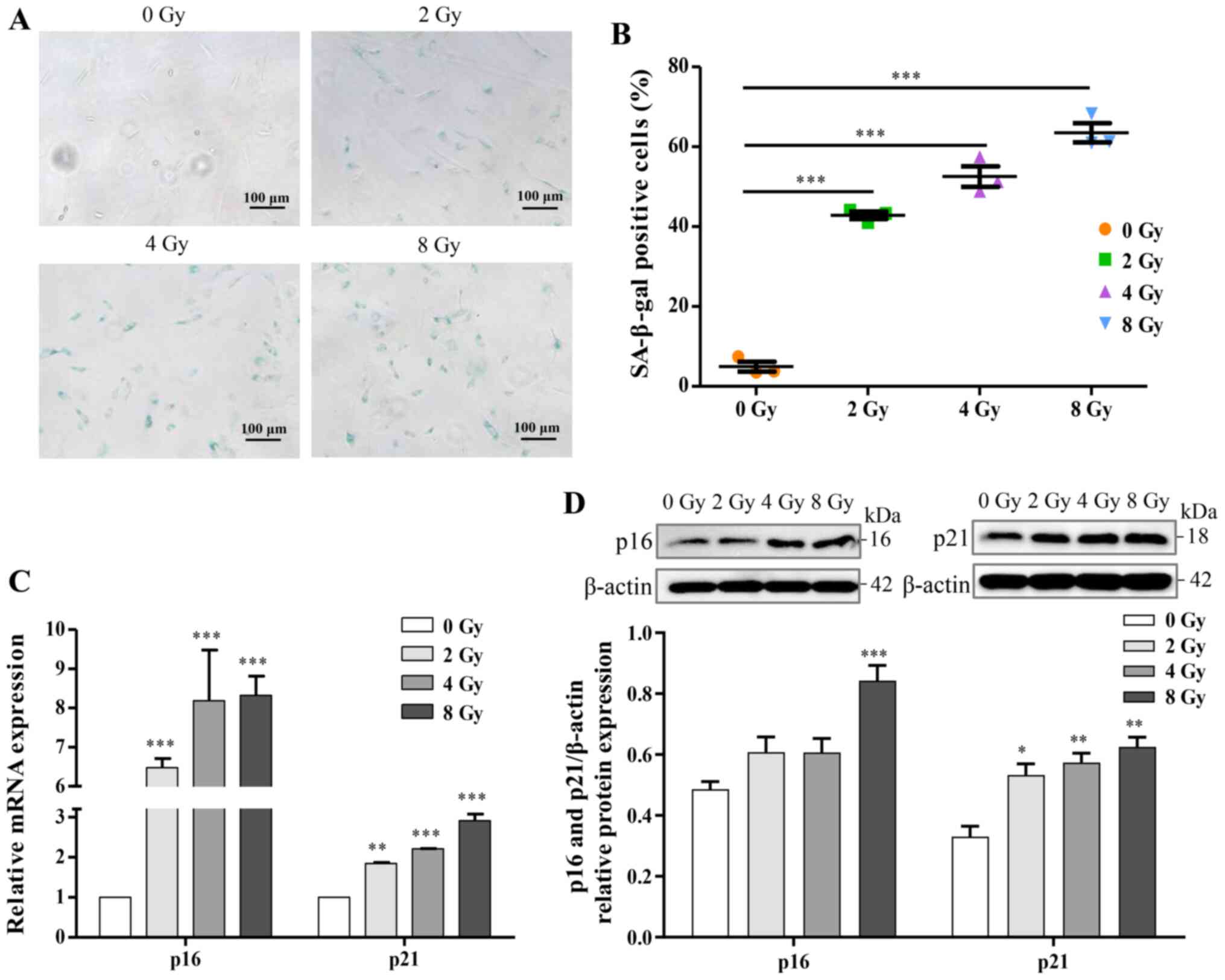
dose-dependent. The expression of SA-β-gal, a marker product of
senescent cells, was increased in a dose-dependent manner (Fig. 5A and B). Additionally, for
further identification of osteocyte senescence induced by
irradiation, upregulation of aging-pathway proteins, p16 and p21,
were assayed by western blotting and RT-qPCR analysis, and the
results were consistent with cellular senescence (Fig. 5C and D). Taken together, the
results suggested that irradiation induced DNA damage and may thus
stimulate cellular senescence in primary osteocytes.
Irradiation induces prematurely senescent
osteocytes and regulates osteoclastogenesis via a paracrine
pathway
Accumulating evidence has suggested that SASP
production is typically a consequence of activated downstream
pathways of senescence, and it serves a pivotal role in specific
pathologies of aging diseases (36,37). However, the underlying mechanism
of the senescent phenotype in the premature aging of
irradiation-induced cells in the bone microenvironment,
particularly for osteocytes, is still incompletely understood.
Thus, in the present study the SASP factors from osteocyte culture
supernatants were investigated using a Mouse Cytokine Array panel
(Fig. 6A). The analysis showed
that the release of 23 cytokines associated with SASP were
significantly increased in supernatants from radiation-induced
senescent osteocytes, including pro-inflammatory interleukin family
members (IL-4, IL-7 and IL-3), chemokines (CCL11 and I-309),
interferon-γ, and various other cytokines. Interestingly, certain
cytokines, such as IL-1ra and MCP-5, were downregulated following
irradiation (Fig. 6B and C).
Expression of several chemokines were not significantly altered and
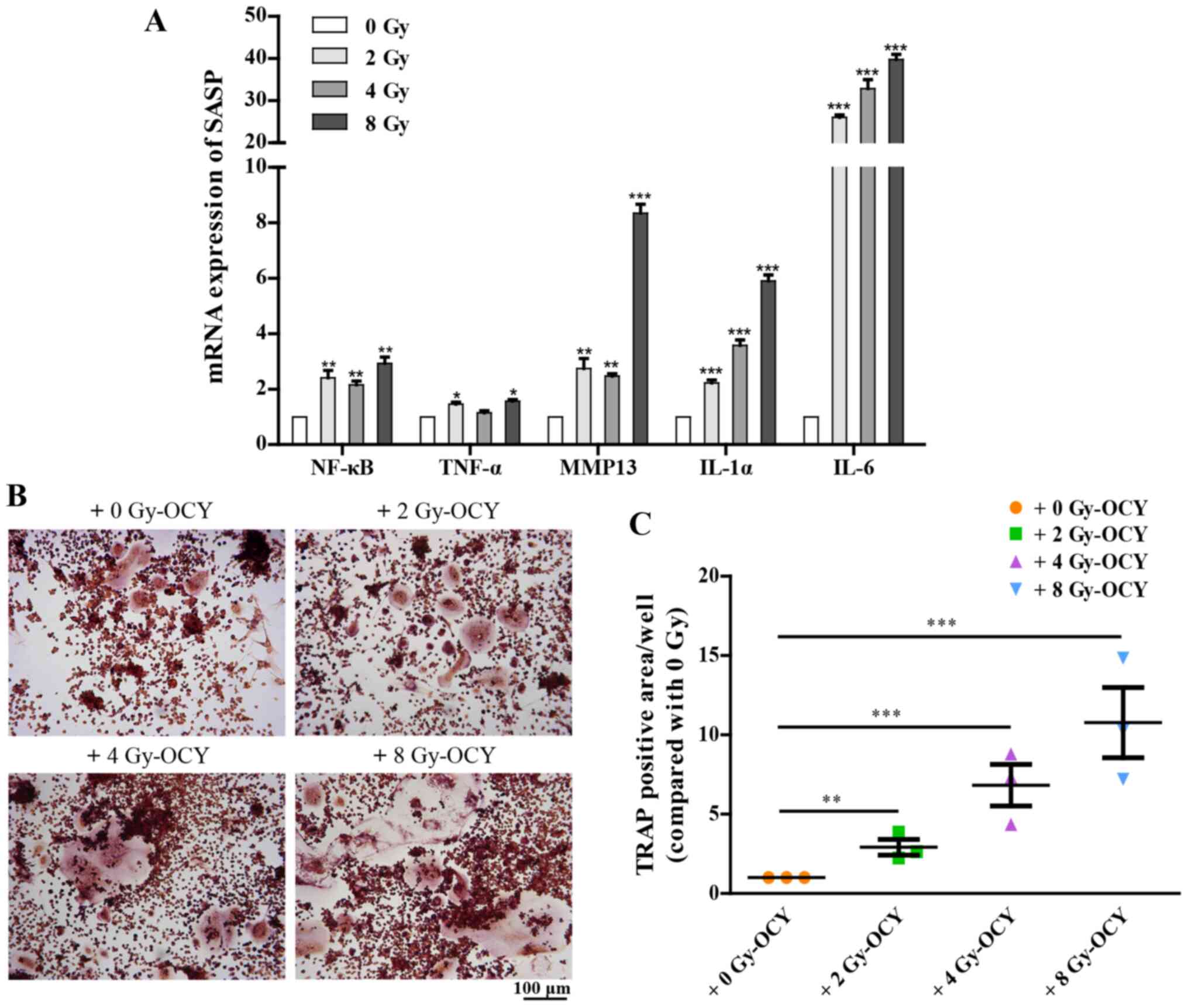
are thus not listed. Additionally, RT-qPCR analysis further
confirmed that irradiation-induced prematurely senescent osteocytes
could activate multiple SASP components, including NF-κB, TNF-α,
MMP13, IL-1α and IL-6 (Fig. 7A),
all of which are associated with bone metabolism (38-41). These results suggest that
radiation-induced prematurely senescent osteocytes may produce and
secrete SASP components, which in turn may regulate other cells in
the surrounding medium, and may also activate macrophages.
Osteocytes, embedded within the bone matrix, are
considered a type of endocrine cell which function to orchestrate
the bone microenvironment via secretion of functional proteins
targeting osteoclasts and osteoblasts (12,17,42). Significant induction of SASP
components in irradiated osteocytes emphasizes their potential role
in radiation-induced bone imbalances in remodeling. Subsequently,
the effects of SASP-mediated osteoclastogenesis, which depends on
activation of bone marrow macrophages, were characterized. As an
osteoclast precursor cell line, RAW264.7 cells, a commonly used
bone marrow macrophage cell line, were co-cultured with osteocytes
previously irradiated with 2, 4 or 8 Gy γ-rays, and treated with 25
ng/ml RANKL in order to observe their osteoclastogenesis potential.
The results showed that compared with the non-irradiated osteocyte
co-culture, irradiated osteocytes significantly promoted the
differentiation of osteoclast precursors as evidenced by TRAP
staining (Fig. 7B and C), in a
dose-dependent manner. Together, these results suggest that the
secretion of SASP components is influenced by irradiation, and
thus, at least in part, mediate osteoclastogenesis imbalances in
the bone microenvironment.
Discussion
Previous studies investigating radiation-induced
bone loss have primarily focused on either the activation of
osteoblasts or the inhibition of osteoclasts (7,43). However, changes in osteocyte
function and molecular communication in response to irradiation
have not been assessed, to the best of our knowledge. Osteocytes
are abundantly present within the bone matrix and orchestrate both
osteoblasts and osteoclasts; osteocytes were previously obtained
from the long bones through a process of collagenase digestions
combined with EDTA-based multiple decalcifications (28,29,44). The above sequential digestion
method was unsuitable for cellular research due to the
time-consuming nature, therefore murine osteocyte cell lines such
as MLO-Y4 have been almost exclusively used in in vitro
studies of osteocytes (45,46). In the present study, an optimized
method based on these protocols was used to obtain primary
osteocytes from the long bone tissue, and then subcultured.
Subsequently, the identity of the obtained cells was further
confirmed based on a lack of ALP staining and the analysis of
protein marker expression. Osteocyte was traditionally viewed as a
descendant of osteoblast (34).
Previous research has indicated that with the transition process
from osteoblasts to osteocytes, alkaline phosphatase is remarkably
reduced, so that osteocytes showed negative or only weakly positive
for ALP expression which was different from osteoblasts (30). In addition, during the isolation
of osteocytes, a small number of osteoblasts are still inevitably
mixed into the osteocytes population. Considering these factual
evidences, the presence of ALP in Fig. 1B is caused by either
contaminating osteoblasts or weakly-expressed osteocytes. However,
compared with the strong expression of osteoblasts in Fig. 1B, identification of osteocytes
and effectiveness for isolation remain to be elucidated. Overall, a
detailed methodology was adapted to enable the isolation and
identification of primary osteocytes from mice skeletal tissue.
This novel primary osteocyte model possesses several advantages
over cell lines and may assist in improving our understanding of
the roles of mature osteocytes in skeletal homeostasis and
remodeling.
Concerning the direct damage of irradiation to
biological function in osteocytes, irradiation can markedly inhibit
the viability and proliferation of osteocytes, which ultimately
leads to a decreased number of osteocytes in a dose-dependent
manner. Although a high dose of radiation tends to induce more
apoptosis and necrosis, previous research had demonstrated that the
initial apoptotic cells tend to die rapidly, and the majority of
surviving cells maintain senescence (46-48). Given these evidences, at 5 days
after radiation a small number of apoptosis or necrosis osteocytes
were inevitably mixed into exiting populations, while the majority
of surviving cells maintain senescence. Additionally, it was shown
that irradiated primary osteocytes presented obvious morphological
changes, including sparsely distributed cells, shortened or
disappeared dendritic branches and disordered cytoskeleton, which
were consistent with reduced mRNA and protein expression levels of
E11, leading to the dysfunction of direct communication between
embedded osteocytes and the surrounding cells. Accordingly, this
study also demonstrated that irradiation upregulated the expression
of SOST and RANKL, whereas OPG expression was decreased. In
conclusion, the above results reflect the functional damage of
osteocytes by irradiation in the modulatory process of bone
homeostasis.
Furthermore, increasing in vitro and in
vivo evidence has shown that bone loss is a normal consequence
associated with aging (49,50). More recently, several molecular
mechanisms, including oxidative stress and DNA damage have been
observed to be increased in senescence and irradiation (21). Interestingly, the present study
demonstrated that irradiated osteocytes showed several markers
associated with DNA damage and cellular senescence, including the
elevated accumulation of γH2AX, positive SA-β-gal staining, and
upregulated expression of p16 and p21 at both the mRNA and protein
level, as well as a novel SAHF indicator. These results suggested
that irradiation, as an alternative promoter of oxidative stress
and DNA damage, may also initiate cellular senescence of osteocytes
in vitro. Moreover, the methods used in the present study
may improve the identification of senescence, and allow for easier
differentiation between stimulator-induced premature senescence
compared with age-related natural aging.
It was previously reported that SASP triggers
senescence of the surrounding normal cells (51,52). Many studies have focused on these
secreted factors and their proposed mechanism in bone metabolism
(12,53). IL-17A was found to mediate the
promotion of osteoclastogenesis by osteocyte paracrine pathways
(54), and MCP-1 has been
indicated to play an important role in bone remodelling and
bone-related cancers (55).
Additionally, drug intervention targeting the SASP profile may be a
hopeful approach that can potentially improve bone integrity and
function (19,50,56). For example, it was confirmed that
inhibition of C5a/C5aR axis could alleviate osteoclast degradation
(57). In the current study, we
indicated that besides the direct dysfunctional damage, osteocytes
undergoing irradiation-induced senescence may significantly
increase the expression and secretion of SASP in the surrounding
environment. Following co-culture, irradiated osteocytes promoted
the differentiation of nearby RAW264.7 cells, highlighting the
paracrine effects of senescent osteocytes in co-ordinating other
cell activities in the bone microenvironment. These results suggest
that senescent osteocytes modulate bone remodeling via a paracrine
pathway targeting the associated bone-metabolizing cells. A recent
study demonstrated that targeted reduction of senescent cells could
alleviate focal radiotherapy-related bone loss (27), consistent with the results of the
present study, and this may partially be explained by the possible
cellular senescence mechanism. Taken together, these results
suggest that the accumulation of senescent osteocytes and increased
SASP secretion induced by radiation are likely one of the primary
initiators of bone damage, or even increased risk of bone
metastasis in post-radiotherapy cancer patients.
Collectively, these results suggested that
irradiation decreased cell viability and disrupted fundamental
biological functions of osteocytes, as well as altered the crucial
dendritic morphology and the regulatory function via changes in the
expression levels of E11, RANKL, OPG and SOST. Additionally, it was
shown that irradiation resulted in the accumulation of DNA damage
and thereby initiated senescence in osteocytes. Furthermore,
irradiation induced prematurely senescent osteocytes to obtain a
SASP, which in turn, indirectly participated in osteoclastogenesis
imbalance. The paracrine modulatory process of osteocytes was also
involved in a series of molecular mechanisms. However, it remains
to be determined which specific upstream and downstream responses
SASP and senescent osteocytes modulate to exhibit their effects in
radiation-induced bone loss.
In conclusion, the present study provides insights
into the mechanisms underlying irradiation-induced bone loss by
showing that irradiated osteocytes prematurely senescent damage and
SASP secretion serve as crucial regulators of bone metabolism.
Availability of data and materials
The datasets generated and/or analyzed in the
present study are all included in this published article.
Authors' contributions
GZ designed the study and revised the article. YW
and JW performed the experiments. JB, LX and JZ analyzed the data.
YW wrote the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
All the animal experimental procedures were approved
by the Committee for Ethical Use of Experimental Animal at Fudan
University (approval no. 201703FYSZGY01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This study was funded by Shanghai Municipal Health Commission
under contract number GWV-10.1-XK10.
References
|
1
|
Allen C, Her S and Jaffray DA:
Radiotherapy for cancer: Present and future. Adv Drug Deliv Rev.
109:1–2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mendes EM, Irie MS, Rabelo GD, Borges JS,
Dechichi P, Diniz RS and Soares PBF: Effects of ionizing radiation
on woven bone: Influence on the osteocyte lacunar network, collagen
maturation, and microarchitecture. Clin Oral Investig.
24:2763–2771. 2020. View Article : Google Scholar
|
|
3
|
Schmeler KM, Jhingran A, Iyer RB, Sun CC,
Eifel PJ, Soliman PT, Ramirez PT, Frumovitz M, Bodurka DC and Sood
AK: Pelvic fractures after radiotherapy for cervical cancer:
Implications for survivors. Cancer. 116:625–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Qiu X, Xi K, Hu W, Pei H, Nie J,
Wang Z, Ding J, Shang P, Li B and Zhou G: Therapeutic ionizing
radiation induced bone loss: A review of in vivo and in vitro
findings. Connect Tissue Res. 59:509–522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saintigny Y, Cruet-Hennequart S, Hamdi DH,
Chevalier F and Lefaix JL: Impact of therapeutic irradiation on
healthy articular cartilage. Radiat Res. 183:135–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alwood JS, Shahnazari M, Chicana B,
Schreurs AS, Kumar A, Bartolini A, Shirazi-Fard Y and Globus RK:
Ionizing radiation stimulates expression of pro-osteoclastogenic
genes in marrow and skeletal tissue. J Interferon Cytokine Res.
35:480–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia D, Gaddy D, Suva LJ and Corry PM:
Rapid loss of bone mass and strength in mice after abdominal
irradiation. Radiat Res. 176:624–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bonewald LF: The role of the osteocyte in
bone and nonbone disease. Endocrinol Metab Clin North Am. 46:1–18.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dallas SL, Prideaux M and Bonewald LF: The
osteocyte: An endocrine cell … and more. Endocr Rev. 34:658–690.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klein-Nulend J, Bakker AD, Bacabac RG,
Vatsa A and Weinbaum S: Mechanosensation and transduction in
osteocytes. Bone. 54:182–190. 2013. View Article : Google Scholar
|
|
11
|
Robling AG and Bonewald LF: The osteocyte:
New insights. Annu Rev Physiol. 82:485–506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitaura H, Marahleh A, Ohori F, Noguchi T,
Shen WR, Qi J, Nara Y, Pramusita A, Kinjo R and Mizoguchi I:
Osteocyte-related cytokines regulate osteoclast formation and bone
resorption. Int J Mol Sci. 21:51692020. View Article : Google Scholar :
|
|
13
|
Staines KA, Javaheri B, Hohenstein P,
Fleming R, Ikpegbu E, Unger E, Hopkinson M, Buttle DJ, Pitsillides
AA and Farquharson C: Hypomorphic conditional deletion of
E11/Podoplanin reveals a role in osteocyte dendrite elongation. J
Cell Physiol. 232:3006–3019. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bellido T: Osteocyte-driven bone
remodeling. Calcif Tissue Int. 94:25–34. 2014. View Article : Google Scholar :
|
|
15
|
Staines KA, Hopkinson M, Dillon S, Stephen
LA, Fleming R, Sophocleous A, Buttle DJ, Pitsillides AA and
Farquharson C: Conditional deletion of E11/Podoplanin in bone
protects against ovariectomy-induced increases in osteoclast
formation and activity. Biosci Rep. 40:BSR201903292020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bonewald LF: The amazing osteocyte. J Bone
Miner Res. 26:229–238. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Honma M, Ikebuchi Y, Kariya Y, Hayashi M,
Hayashi N, Aoki S and Suzuki H: RANKL subcellular trafficking and
regulatory mechanisms in osteocytes. J Bone Miner Res.
28:1936–1949. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mas-Bargues C, Viña-Almunia J, Inglés M,
Sanz-Ros J, Gambini J, Ibáñez-Cabellos JS, García-Giménez JL, Viña
J and Borrás C: Role of p16INK4a and BMI-1 in oxidative
stress-induced premature senescence in human dental pulp stem
cells. Redox Biol. 12:690–698. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pignolo RJ, Samsonraj RM, Law SF, Wang H
and Chandra A: Targeting cell senescence for the treatment of
age-related bone loss. Curr Osteoporos Rep. 17:70–85. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P,
Wangensteen KJ, Simithy J, Lan Y, Lin Y, Zhou Z, et al: Cytoplasmic
chromatin triggers inflammation in senescence and cancer. Nature.
550:402–406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chandra A, Park SS and Pignolo RJ:
Potential role of senescence in radiation-induced damage of the
aged skeleton. Bone. 120:423–431. 2019. View Article : Google Scholar
|
|
22
|
Cmielova J, Havelek R, Soukup T, Jiroutová
A, Visek B, Suchánek J, Vavrova J, Mokry J, Muthna D, Bruckova L,
et al: Gamma radiation induces senescence in human adult
mesenchymal stem cells from bone marrow and periodontal ligaments.
Int J Radiat Biol. 88:393–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bai J, Wang Y, Wang J, Zhai J, He F and
Zhu G: Irradiation-induced senescence of bone marrow mesenchymal
stem cells aggravates osteogenic differentiation dysfunction via
paracrine signaling. Am J Physiol Cell Physiol. 318:C1005–C1017.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sapieha P and Mallette FA: Cellular
senescence in postmitotic cells: Beyond growth arrest. Trends Cell
Biol. 28:595–607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anderson R, Lagnado A, Maggiorani D,
Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik
M, Jurk D, et al: Length-independent telomere damage drives
post-mitotic cardiomyocyte senescence. EMBO J. 38:e1004922019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riessland M, Kolisnyk B, Kim TW, Cheng J,
Ni J, Pearson JA, Park EJ, Dam K, Acehan D, Ramos-Espiritu LS, et
al: Loss of SATB1 induces p21-dependent cellular senescence in
post-mitotic dopaminergic neurons. Cell Stem Cell. 25:514–530.e8.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu G, Nars M, Hentunen TA, Metsikkö K and
Väänänen HK: Isolated primary osteocytes express functional gap
junctions in vitro. Cell Tissue Res. 323:263–271. 2006. View Article : Google Scholar
|
|
28
|
Stern AR, Stern MM, Van Dyke ME, Jähn K,
Prideaux M and Bonewald LF: Isolation and culture of primary
osteocytes from the long bones of skeletally mature and aged mice.
Biotechniques. 52:361–373. 2012.PubMed/NCBI
|
|
29
|
Stern AR and Bonewald LF: Isolation of
osteocytes from mature and aged murine bone. Methods Mol Biol.
1226:3–10. 2015. View Article : Google Scholar
|
|
30
|
Franz-Odendaal TA, Hall BK and Witten PE:
Buried alive: How osteoblasts become osteocytes. Dev Dyn.
235:176–190. 2006. View Article : Google Scholar
|
|
31
|
Fartaria MJ, Reis C, Pereira J, Pereira
MF, Cardoso JV, Santos LM, Oliveira C, Holovey V, Pascoal A and
Alves JG: Assessment of the mean glandular dose using LiF:Mg, Ti,
LiF:Mg, Cu, P, Li2B4O7:Mn and Li2B4O7:Cu TL detectors in
mammography radiation fields. Phys Med Biol. 61:6384–6399. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lucas PA, Aubineau-Lanièce I, Lourenço V,
Vermesse D and Cutarella D: Using LiF:Mg, Cu, P TLDs to estimate
the absorbed dose to water in liquid water around an 192Ir
brachytherapy source. Med Phys. 41:0117112014. View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
Manolagas SC: Birth and death of bone
cells: Basic regulatory mechanisms and implications for the
pathogenesis and treatment of osteoporosis. Endocr Rev. 21:115–137.
2000.PubMed/NCBI
|
|
35
|
Ikpegbu E, Basta L, Clements DN, Fleming
R, Vincent TL, Buttle DJ, Pitsillides AA, Staines KA and
Farquharson C: FGF-2 promotes osteocyte differentiation through
increased E11/podoplanin expression. J Cell Physiol. 233:5334–5347.
2018. View Article : Google Scholar :
|
|
36
|
Childs BG, Durik M, Baker DJ and van
Deursen JM: Cellular senescence in aging and age-related disease:
From mechanisms to therapy. Nat Med. 21:1424–1435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Childs BG, Gluscevic M, Baker DJ, Laberge
RM, Marquess D, Dananberg J and van Deursen JM: Senescent cells: An
emerging target for diseases of ageing. Nat Rev Drug Discov.
16:718–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Huang H, Zhao G, Yokoyama T, Vega
H, Huang Y, Sood R, Bishop K, Maduro V, Accardi J, et al: ATP6V1H
deficiency impairs bone development through activation of MMP9 and
MMP13. PLoS Genet. 13:e10064812017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hameister R, Lohmann CH, Dheen ST, Singh G
and Kaur C: The effect of TNF-α on osteoblasts in metal
wear-induced periprosthetic bone loss. Bone Joint Res. 9:827–839.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dinarello CA: The IL-1 family of cytokines
and receptors in rheumatic diseases. Nat Rev Rheumatol. 15:612–632.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim MH, Lee H, Ha IJ and Yang WM:
Zanthoxylum piperitum alleviates the bone loss in osteoporosis via
inhibition of RANKL-induced c-fos/NFATc1/NF-κB pathway.
Phytomedicine. 80:1533972021. View Article : Google Scholar
|
|
42
|
Maré A, D'Haese PC and Verhulst A: The
role of sclerostin in bone and ectopic calcification. Int J Mol
Sci. 21:31992020. View Article : Google Scholar :
|
|
43
|
Zhang J, Wang Z, Wu A, Nie J, Pei H, Hu W,
Wang B, Shang P, Li B and Zhou G: Differences in responses to X-ray
exposure between osteoclast and osteoblast cells. J Radiat Res.
58:791–802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Metzger CE and Narayanan SA: The role of
osteocytes in inflammatory bone loss. Endocrinol (Lausanne).
10:2852019. View Article : Google Scholar
|
|
45
|
Bonewald LF: Establishment and
characterization of an osteocyte-like cell line, MLO-Y4. J Bone
Miner Metab. 17:61–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He F, Bai J, Wang J, Zhai J, Tong L and
Zhu G: Irradiation-induced osteocyte damage promotes HMGB1-mediated
osteoclastogenesis in vitro. J Cell Physiol. 234:17314–17325. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Alessio N, Esposito G, Galano G, De Rosa
R, Anello P, Peluso G, Tabocchini MA and Galderisi U: Irradiation
of mesenchymal stromal cells with low and high doses of alpha
particles induces senescence and/or apoptosis. J Cell Biochem.
118:2993–3002. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Alessio N, Capasso S, Di Bernardo G,
Cappabianca S, Casale F, Calarco A, Cipollaro M, Peluso G and
Galderisi U: Mesenchymal stromal cells having inactivated RB1
survive following low irradiation and accumulate damaged DNA: Hints
for side effects following radiotherapy. Cell Cycle. 16:251–258.
2017. View Article : Google Scholar :
|
|
49
|
Tiede-Lewis LM, Xie Y, Hulbert MA, Campos
R, Dallas MR, Dusevich V, Bonewald LF and Dallas SL: Degeneration
of the osteocyte network in the C57BL/6 mouse model of aging. Aging
(Albany NY). 9:2190–2208. 2017. View Article : Google Scholar
|
|
50
|
Farr JN, Xu M, Weivoda MM, Monroe DG,
Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM,
et al: Targeting cellular senescence prevents age-related bone loss
in mice. Nat Med. 23:1072–1079. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hitomi K, Okada R, Loo TM, Miyata K,
Nakamura AJ and Takahashi A: DNA damage regulates
senescence-associated extracellular vesicle release via the
ceramide pathway to prevent excessive inflammatory responses. Int J
Mol Sci. 21:37202020. View Article : Google Scholar :
|
|
52
|
Faget DV, Ren Q and Stewart SA: Unmasking
senescence: Context-dependent effects of SASP in cancer. Nat Rev
Cancer. 19:439–453. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Farr JN, Fraser DG, Wang H, Jaehn K,
Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK,
Kirkland JL, et al: Identification of senescent cells in the bone
microenvironment. J Bone Miner Res. 31:1920–1929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liao C, Cheng T, Wang S, Zhang C, Jin L
and Yang Y: Shear stress inhibits IL-17A-mediated induction of
osteoclastogenesis via osteocyte pathways. Bone. 101:10–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mulholland BS, Forwood MR and Morrison NA:
Monocyte chemoattractant protein-1 (MCP-1/CCL2) drives activation
of bone remodelling and skeletal metastasis. Curr Osteoporos Rep.
17:538–547. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chandra A, Lagnado AB, Farr JN, Monroe DG,
Park S, Hachfeld C, Tchkonia T, Kirkland JL, Khosla S, Passos JF
and Pignolo RJ: Targeted reduction of senescent cell burden
alleviates focal radiotherapy-related bone loss. J Bone Miner Res.
35:1119–1131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
D'Angelo R, Mangini M, Fonderico J, Fulle
S, Mayo E, Aramini A and Mariggiò S: Inhibition of osteoclast
activity by complement regulation with DF3016A, a novel
small-molecular-weight C5aR inhibitor. Biomed Pharmacother.
123:1097642020. View Article : Google Scholar : PubMed/NCBI
|