Introduction
Mucin (MUC)5AC is a major component of gel-forming
mucus in human airways, which is understood to contribute to
various functions, ranging from lubrication to hydration. Mucins,
including MUC5AC, are secreted in the airways both at a low
baseline rate and at a high, stimulated rate. Under healthy
conditions, the constant baseline level of mucin secretion supports
the steady-state mucociliary clearance of inhaled particles and
pathogens that enter the airways during breathing (1). During the progression of chronic
airway diseases, particularly during acute exacerbations, massive
levels of acidic viscous mucin have been shown to be produced in a
rapid burst phase (2,3). Once stimulated with mucus-promoting
factors, a significant and rapid increase in both MUC5AC mRNA and
protein expression in goblet cells results in an excessive
accumulation of unfolded MUC5AC protein in the endoplasmic
reticulum (ER) lumen. Previous studies have focused predominantly
on the molecular mechanisms underlying MUC5AC gene transcription
and protein synthesis (4,5).
However, the mechanisms through which the protein load capacity of
the ER may be improved, and those through which protein folding and
the handling efficiency of the ER may be increased, remain unclear;
thus, further experiments are required to elucidate the underlying
mechanisms.
An excessive level of mucin synthesis results in an
accumulation of misfolded proteins in the ER, a condition termed as
'ER stress', which triggers a compensatory cellular response termed
as the 'unfolded protein response' (UPR) (6). The aim of the UPR is to reverse ER
stress by enhancing the protein secretion ability of the ER, as
well as increasing the capacity of the ER for protein folding and
processing. However, whether factors promoting MUC5AC production
are able to induce ER stress, and the effect of ER stress-signaling
pathways on the regulation of the production of airway MUC5AC, have
yet to be elucidated. It has been reported that the overproduction
of interleukin (IL)-13 and allergen-induced MUC5AC is partly
dependent on ER stress (7);
however, the underlying molecular signaling mechanisms have not yet
been fully elucidated. Previous studies have demonstrated that the
ER-stress transducer, inositol-requiring kinase 1α (IRE1α), is
required for normal secretory protein metabolism and cytokine
production (8,9).
In the present study, neutrophil elastase (NE) was
selected to induce MUC5AC mRNA and protein expression. The aim of
the present study was to investigate the role of ER stress in
NE-regulated MUC5AC expression, ultimately to better understand the
mechanisms of airway mucus secretion. The results obtained may aid
in the identification of therapeutic strategies with which to
alleviate airway mucus hypersecretion.
Materials and methods
Cells and reagents
The immortalized human bronchial epithelial cell
line, 16HBE14o-, was provided by Procell Life Science &
Technology. Small interfering RNA (siRNA) for IRE1α, X-box-binding
protein 1 (XBP1) and control siRNA were obtained from Santa Cruz
Biotechnology, Inc. The IRE1α siRNA sequence was: GGA CGU GAG CGA
CAG AAU Adtdt and the XBP1 siRNA sequence was: GCC UGU CUG UAC UUC
AUU Ctt. Mouse anti-human β-actin monoclonal antibody (cat. no.
sc-517582), as well as goat anti-rabbit IgG-HRP (cat. no. sc-2004)
and goat anti-mouse IgG-HRP (cat. no. sc-2005) antibodies were
purchased from Santa Cruz Biotechnology, Inc. Rabbit anti-human
phosphorylated (p-) protein kinase R-like endoplasmic reticulum
kinase (PERK) monoclonal antibody (Thr-980) (no. AP328) was
obtained from the Beyotime Institute of Biotechnology. Rabbit
anti-human polyclonal antibodies against glucose-regulated protein
78 (GRP78) (cat. no. ab230508), PERK (cat. no. ab77654), activating
transcription factor 6 (ATF6; cat. no. ab37149), IRE1α (cat. no.
ab37073), p-IRE1α (Ser724; cat. no. ab48187) and XBP1 {cat. no.
ab37152; note that this antibody recognizes both active spliced
(XBP1s) and inactive unspliced XBP1 [XBP1(u) were purchased from
Abcam. The reactive oxygen species (ROS) assay and MTT cell
viability kits were obtained from the Beyotime Institute of
Biotechnology. The human MUC5AC ELISA kit was purchased from
R&D Systems, Inc. Mouse anti-human MUC5AC monoclonal antibody
was from Neomarkers, Inc. 4-Phenylbutyric acid (4-PBA), an
ER-stress inhibitor, and N-acetylcysteine (NAC), an
inhibitor of ROS, were obtained from Merck KGaA. XtremeGENE siRNA
transfection reagent was obtained from Roche Diagnostics.
Microplate readers were from Tecan Group, Ltd. The confocal laser
scanning microscopy was purchased from Leica Microsystems GmbH,
whereas the fluorometer was from Molecular Devices, LLC.
Cell viability assay
The appropriate concentration of NE was determined
using an MTT assay, as previously described (10). Briefly, a total of
1×104/ml cells in 200 µl cell suspension was
seeded into each well of a 96-well plate. The cells were
subsequently treated with 25, 50, 100 or 200 ng/ml NE, and
incubated at 37°C in an atmosphere of 5% CO2. Cell
viability was determined using an MTT assay, according to the
manufacturer's instructions. At 8, 12, 24, 36 and 48 h following
treatment, 20 µl 5 mg/ml MTT were added to each well, and
the cells were incubated at 37°C for a further 4 h. The
supernatants were subsequently discarded, and dimethyl sulfoxide
was added (150 µl/well). The plate was then placed on an
orbital shaker at room temperature for 15 min. The absorbance was
read at 570 nm using a microplate reader (sunrise F039300; Tecan
Group, Ltd.).
Cell transfection
The immortalized human bronchial epithelial cell
line 16HBE14o-was used in these experiments. The cells were
incubated at a density of 1.5×105/ml in 24-well plates,
and cultured with 0.45 ml serum-free RPMI-1640 in each well. siRNA
transfection reagent (5 µl) was diluted with 45 µl
serum-free medium to reach a final volume of 50 µl. Aliquots
(1 µg) of IRE1α siRNA, XBP1 siRNA or control siRNA were
diluted with 50 µl serum-free medium. Subsequently, the
diluted siRNA and transfection reagent were mixed and incubated for
a further 20 min at room temperature. The transfection mixture was
added in a drop-wise manner to each well, and vortex-mixed gently
for 10 sec, followed by an incubation at room temperature for 16 h.
After carefully removing the supernatant, the cells were washed 3
times with PBS and subsequently incubated with fresh RPMI-1640
medium containing 10% fetal bovine serum, prior to further
treatment with 100 ng/ml NE for a further 24 h.
Cell culture and grouping
The 16HBE14o-cells were grown in RPMI-1640 medium
and incubated at 37°C in an atmosphere of 5% CO2 in a
humidified incubator. The cells were grouped as follows: i) The
control group, where cells were cultured under normal conditions
without any additional interventions; ii) the NE group, where cells
were treated with 100 ng/ml NE; iii) the NE and 4-PBA group, where
cells were pre-treated with 10 mmol/l 4-PBA, as previously
described (11), for 30 min
prior to NE exposure; iv) the NE and NAC group, where cells were
pre-treated with 3 mmol/l NAC, as previously described (12), for 30 min prior to NE exposure;
v) the NE and control siRNA group, where cells were treated with NE
following transfection with control siRNA; vi) the NE and IRE1α
siRNA group, where cells were treated with NE following
transfection with IRE1α siRNA; and vii) the NE and XBP1 siRNA
group, where cells were treated with NE following transfection with
XBP1 siRNA. After grouping, all the cells were incubated at 37°C
for a further 24 h subsequent to further experiments being
performed.
Measurement of ROS production
The 16HBE14o-cells were treated with various
concentrations of NE (25, 50 and 100 ng/ml) for 24 h, and the ROS
detection assay kit was then used to measure intracellular
oxidative stress, according to the manufacturer's instructions. The
collected cells were incubated with serum-free medium-diluted
DCFH-DA (1:1,000; final concentration, 10 µmol/l) at a
density of 5×106/ml for 20 min in the dark at 37°C.
After washing with serum-free medium, the ROS fluorescence
intensity was observed using a fluorometer (Flexstation 3;
Molecular Devices, LLC).
Quantification of MUC5AC protein
expression by ELISA
The cell culture supernatants and 16HBE14o-cells
were collected separately. Cell lysates at multiple dilutions (1:0,
1:1, 1:2 and 1:5) were prepared with phosphate-buffered saline
(PBS). MUC5AC protein expression in the culture supernatants and
cytoplasm were measured following the instructions provided with
the ELISA kit. Optical densities were measured at 450 nm
(Flexstation 3; Molecular Devices, LLC), and the results are
expressed as percentages of the baseline controls.
Quantification of protein expression by
western blot analysis
Total protein was isolated from the cells using RIPA
buffer reagent containing PMSF. Total protein was determined by BCA
assay. The total protein of each sample was 40 µg. Protein
samples were separated on a 5-10% SDS-PAGE gel, and the separated
proteins were then transferred onto a polyvinylidene difluoride
(PVDF) membrane. Subsequent to being blocked with PBS containing
0.05% Tween-20 and 5% skimmed milk for 1 h, the membranes were
incubated for 2 h at room temperature with antibodies against
GRP78, p-PERK, PERK, ATF6, p-IRE1α, IRE1α, XBP1 (all dilution,
1:500) and β-actin (dilution, 1:500). After washing, the membrane
was incubated with an HRP-conjugated secondary antibody (dilution,
1:1,000) for 2 h at room temperature. Peroxidase activity was
detected using an enhanced chemiluminescence (ECL) reagent
(Applygen Technologies Inc.). The relative band density of the
target protein compared with β-actin was quantified using Quantity
One Analysis software (Bio-Rad Laboratories, Inc.).
Quantification of XBP1 mRNA expression by
reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the cultured
16HBE14o-cells in each group using TRIzol® reagent. The
RNA samples were stored at -20°C following initial quantification.
RT-qPCR analysis of XBP1s mRNA levels was performed using a
two-step RT-PCR kit according to the manufacturer's instructions.
The specific primers for PCR were as follows: XBP1s forward,
5′-GGAGTTAAGACAGCGCTTGG-3′ and reverse, 5′-GTC AAT ACC GCC AGA ATC
C-3′; and GAPDH forward, 5′-GGG AAG GTG AAG GTG GGA GTG-3′ and
reverse, 5′-AGC AGA GGG GGC AGA GAT GAT-3′. The amplification
process was performed for 40 cycles under the following conditions:
Pre-denaturation at 95°C for 10 min; denaturation at 95°C for 35
sec; annealing at 56°C for 55 sec, and extension at 72°C for 3 min.
The relative mRNA levels of XBP1s were analyzed using the
2−ΔΔCq method (13).
Quantification of MUC5AC expression by
immunofluorescence assay
The 16HBE14o-cells were seeded on glass coverslips
in 24-well plates at a density of 1×105/ml, and fixed
with 4% paraformaldehyde at room temperature for 30 min.
Subsequently, the cells were permeabilized with 0.1% Triton X-100
for 15 min and were blocked with goat serum for 45 min. The cells
were then incubated with 150 µl primary monoclonal antibody
against MUC5AC (mouse anti-human; dilution, 1:200) overnight at
4°C, followed by incubation in fluorescein
isothiocyanate-conjugated secondary antibody for 1 h. Cell nuclei
were stained with propidium iodide (PI) for 5 min, and images were
captured using a fluorescence confocal microscope (TCS-SP2; Leica
Microsystems GmbH). Finally, the relative fluorescence intensity of
the cells was analyzed using Image Pro Plus software 6.0.
Statistical analysis
All data are presented as the means ± SD.
Statistical analyses were performed with SPSS 17.0 software (SPSS,
Inc.). All experiments were performed with at least 6 cell
cultures, in duplicate or triplicate. One-way analysis of variance
(ANOVA) was used followed by Bonferroni analysis. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
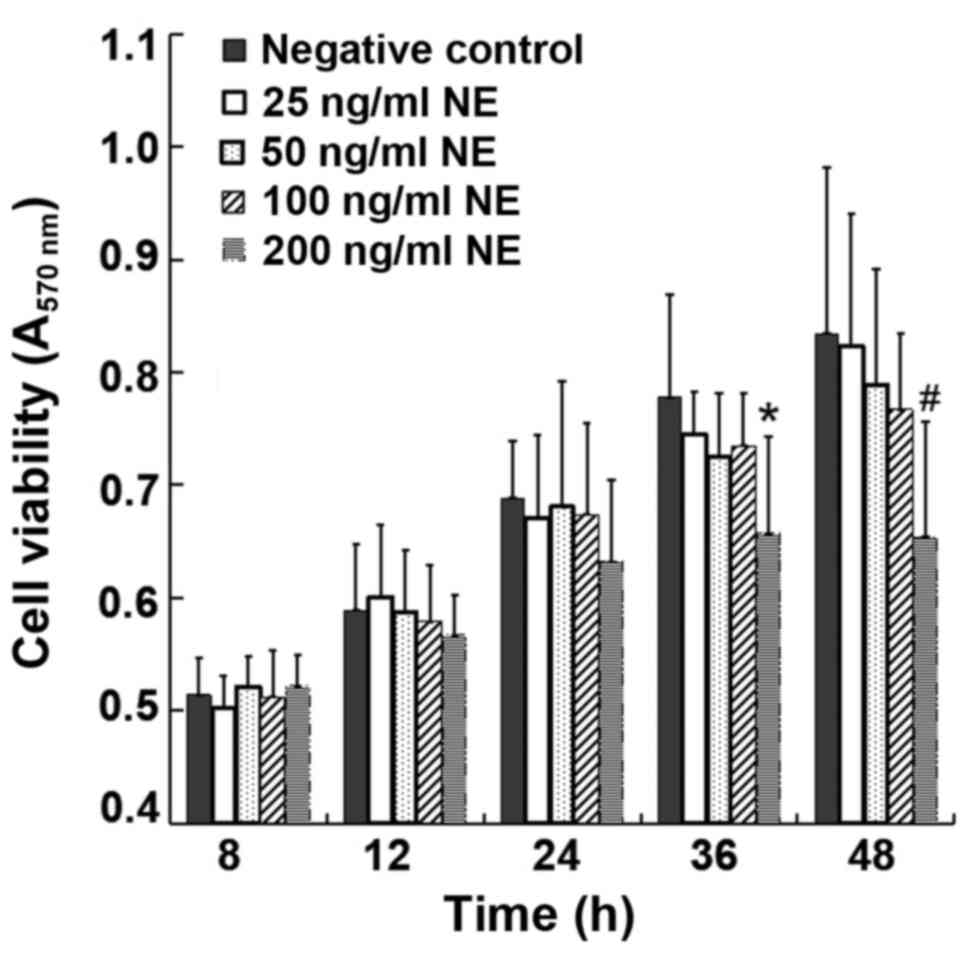
Cell viability upon incubation with
NE
To select the proper concentration and treatment
duration for NE incubation, various concentrations of NE (25, 50,
100 and 200 ng/ml) were used for the treatment of the cells. MTT
assay was used to detect the absorbance from 8 to 36 h in each
group. MTT assay revealed similar levels of cell viability from 8
to 36 h between the control group and the NE-treated groups
following treatment with 25-100 ng/ml NE, indicating that exposure
to NE within this concentration range up to 36 h was essentially
non-toxic to the 16HBE14o-cells. However, treatment with 200 ng/ml
NE for 36-48 h evidently reduced cell viability (P<0.05)
(Fig. 1). Therefore, 100 ng/ml
NE was selected as the intervention condition.
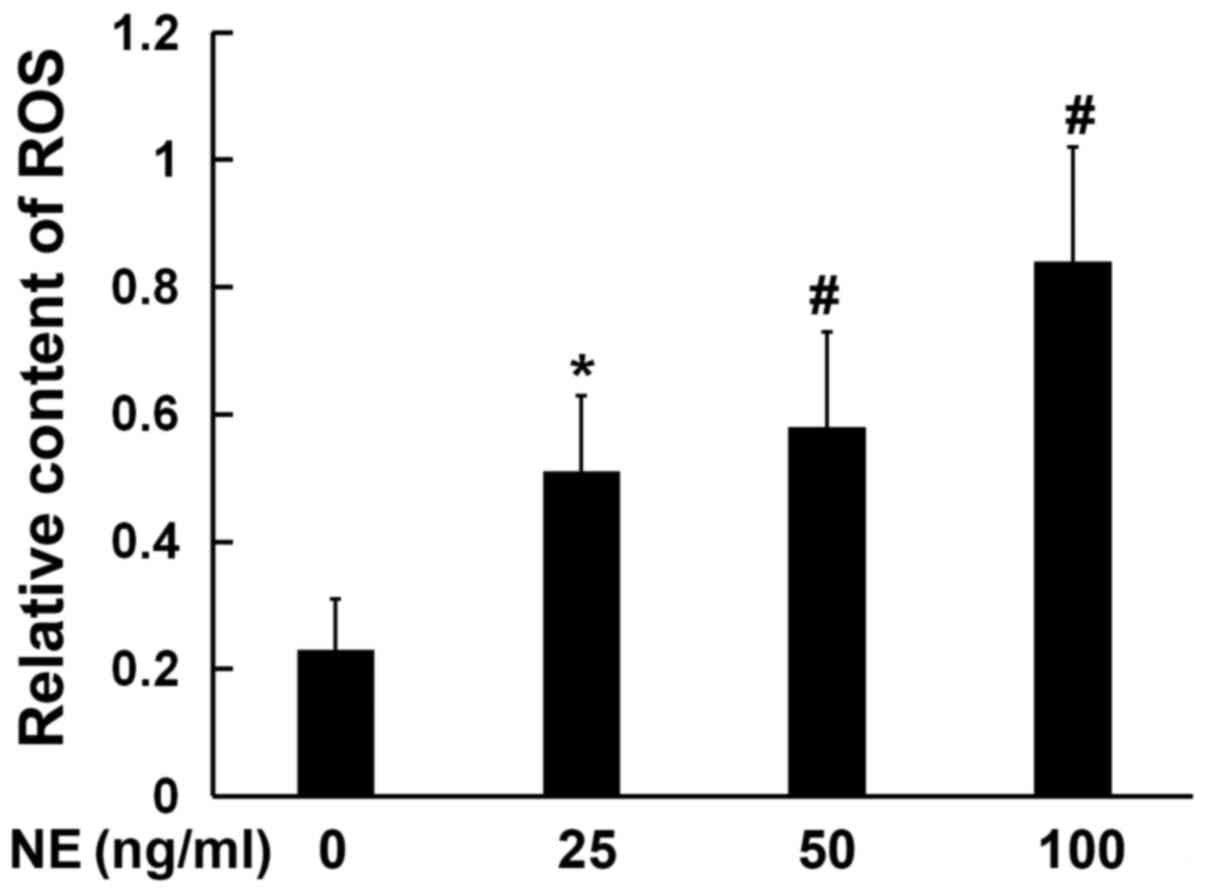
NE promotes ROS production
ER stress may be activated by ROS; therefore, the
levels of ROS production in cells were detected following
incubation with NE. The production of ROS in the cultured
16HBE14o-cells was detected using DCFH-DA. The results revealed a
significant increase in ROS production in the NE treatment group
compared with the control group. Furthermore, the production of ROS
was found to increase with the increasing NE concentration; ROS
production in the 100 ng/ml NE treatment group was found to be
markedly higher, as compared with the negative control group
(Fig. 2).
ROS activates the UPR and increases the
expression of ER stress-related proteins
To investigate whether NE activates the ER stress
pathway, the levels of ER stress-related proteins were assayed
following NE stimulation. Western blot analysis was performed to
examine the p-PERK, p-IRE1α, ATF6 and GRP78 protein expression
levels in the 16HBE14o-cells. As shown in Fig. 3, the protein expression levels of
p-PERK, p-IRE1α, ATF6 and GRP78 were significantly increased
following treatment of the cells with 100 ng/ml NE (all P<0.01).
To further explore the involvement of ROS in the NE-induced
increase in the expression of ER-related proteins, the
16HBE14o-cells were pre-incubated with NAC, an inhibitor of ROS. It
was found that NAC markedly attenuated the NE-induced upregulation
in the protein expression of p-PERK, p-IRE1α, ATF6 and GRP78 (all
P<0.01). These data demonstrated that the generation of ROS was
primarily involved in NE-induced ER stress-associated protein
production. When the cells were pre-treated with the ER stress
inhibitor, 4-PBA, the protein expression levels of p-PERK, p-IRE1α,
ATF6 and GRP78 were also markedly decreased, compared with the
group treated with NE only (all P<0.01).
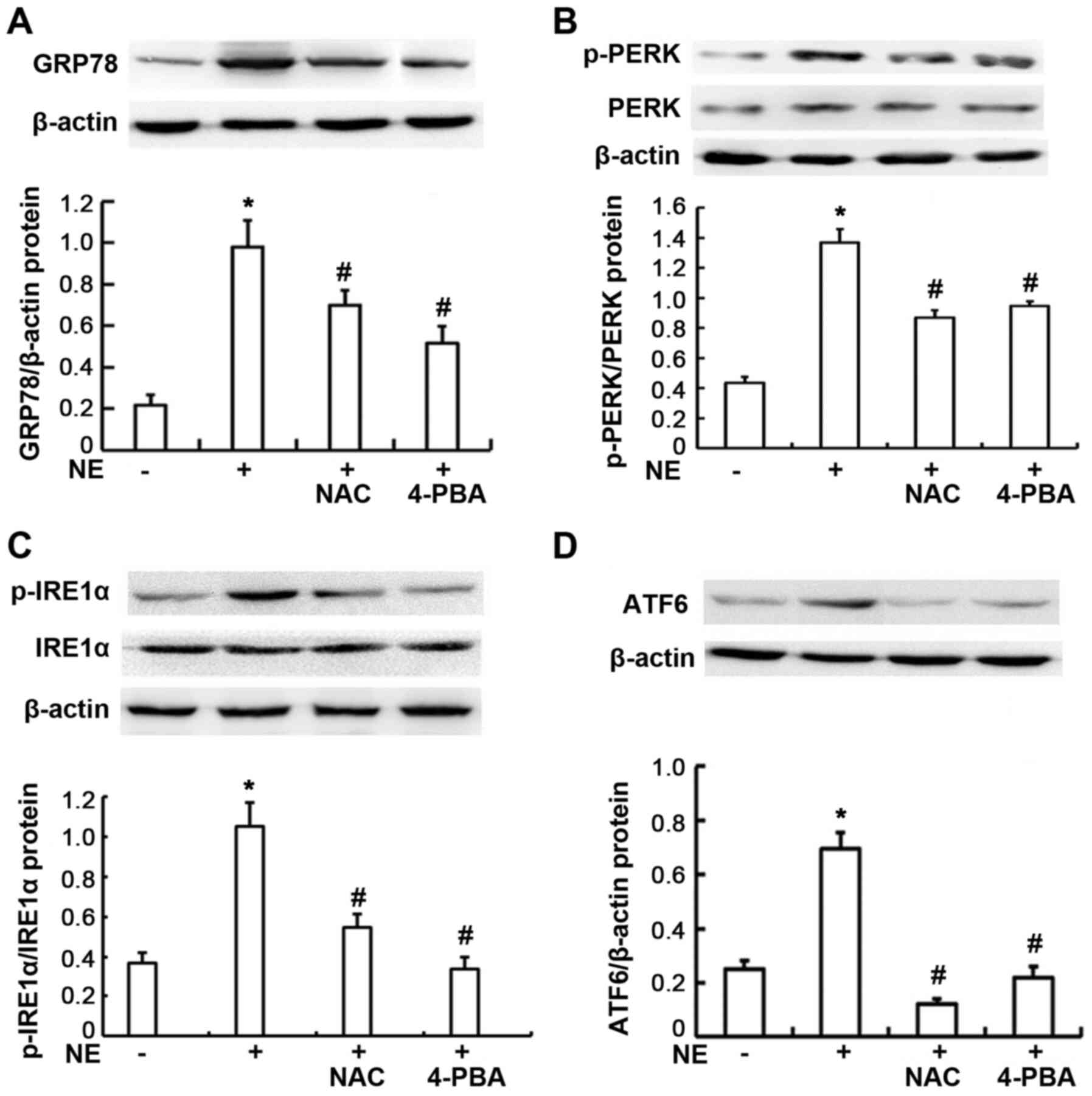 | Figure 3Expression of endoplasmic reticulum
stress-related proteins in 16HBE14o-cells. Cells were exposed to
100 ng/ml NE or pre-treated with NAC and 4-PBA prior to NE
exposure. GRP78, PERK, p-PERK, IRE1α, p-IRE1α, and ATF6 proteins
were assayed by western blot analysis. (A) Relative GRP78 protein
expression levels are presented as the ratio of GRP78 to β-actin.
(B) Relative p-PERK protein expression levels are presented as the
ratio of p-PERK to PERK, β-actin blots are for the loading control.
(C) Relative p-IRE1α protein expression levels are presented as the
ratio of pIRE1α to IRE1α, β-actin blots are for the loading
control. (D) Relative ATF6 protein expression levels are presented
as the ratio of ATF6 to β-actin. Data are presented as the means ±
SD (n=4 samples per group). *P<0.01 vs. negative
control; #P<0.01 vs. the NE group. NE, neutrophil
elastase; NAC, N-acetylcysteine; ATF6, activating transcription
factor 6; 4-PBA, 4-phenylbutyric acid; GRP78, glucose-regulated
protein 78; IRE1α, inositol-requiring kinase 1α; p, phosphorylated;
PERK, protein kinase R-like endoplasmic reticulum kinase. |
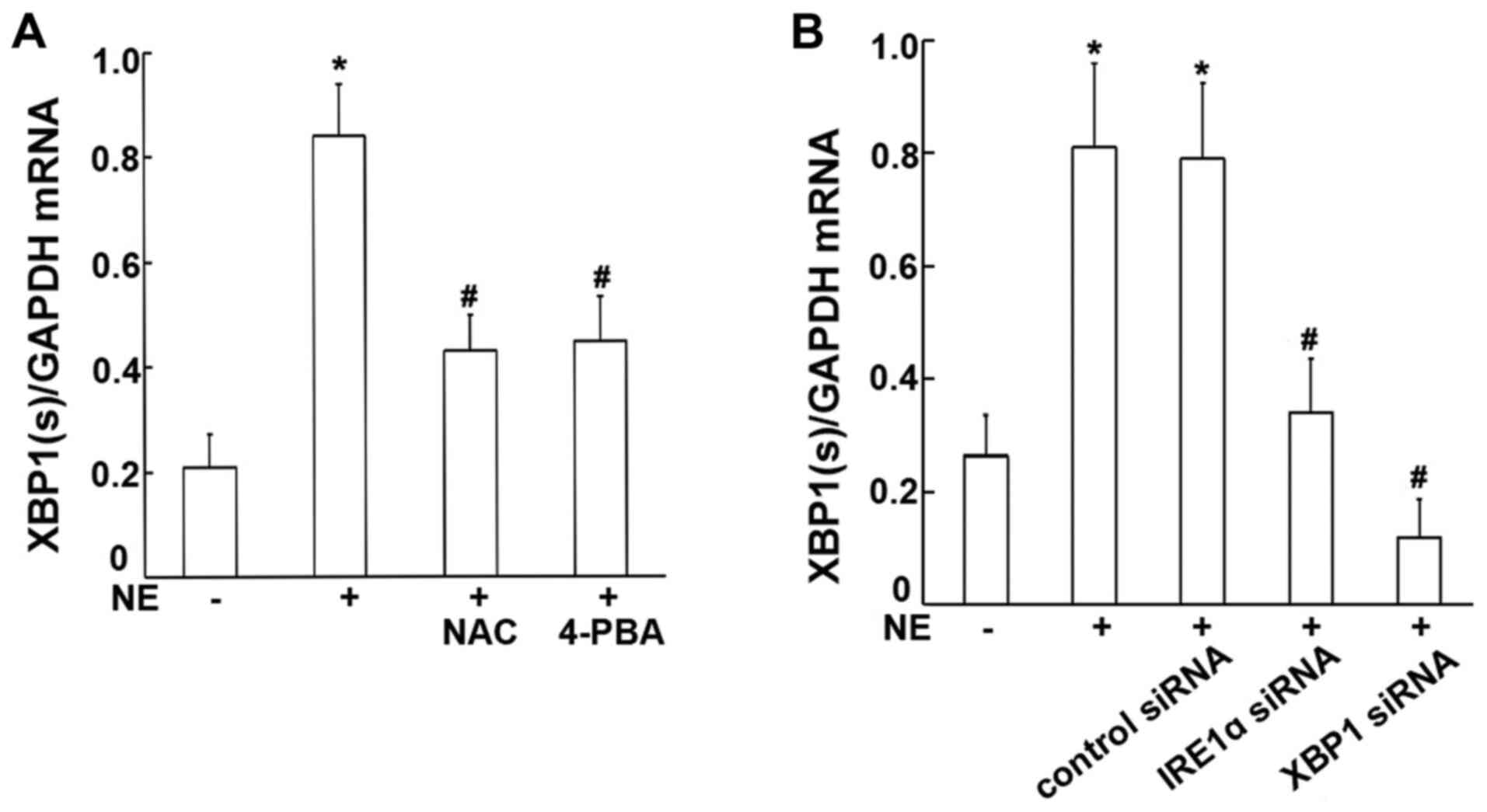
XBP1s expression is increased by NE
stimulation, and may be suppressed by treatment with NAC and
4-PBA
To assess whether XBP1 is involved in the NE-induced
process, RT-qPCR and western blot analysis were performed to
examine the mRNA and protein expression levels of XBP1s in the
16HBE14o-cells. The ROS scavenger, NAC, and the ER stress
inhibitor, 4-PBA, were used to verify the probable signaling
factors. The results obtained revealed significant increases in the
mRNA and protein levels of XBP1s in the 16HBE14o-cells incubated
with 100 ng/ml NE (Figs. 4C and
5A). Moreover, such increases
induced by NE were markedly attenuated by pre-treatment with either
the ROS scavenger, NAC, or the ER stress inhibitor, 4-PBA (all
P<0.01; Figs. 4C and 5A), suggesting that NE may increase
XBP1 expression, and that this may be mediated via ROS generation
and ER stress activation.
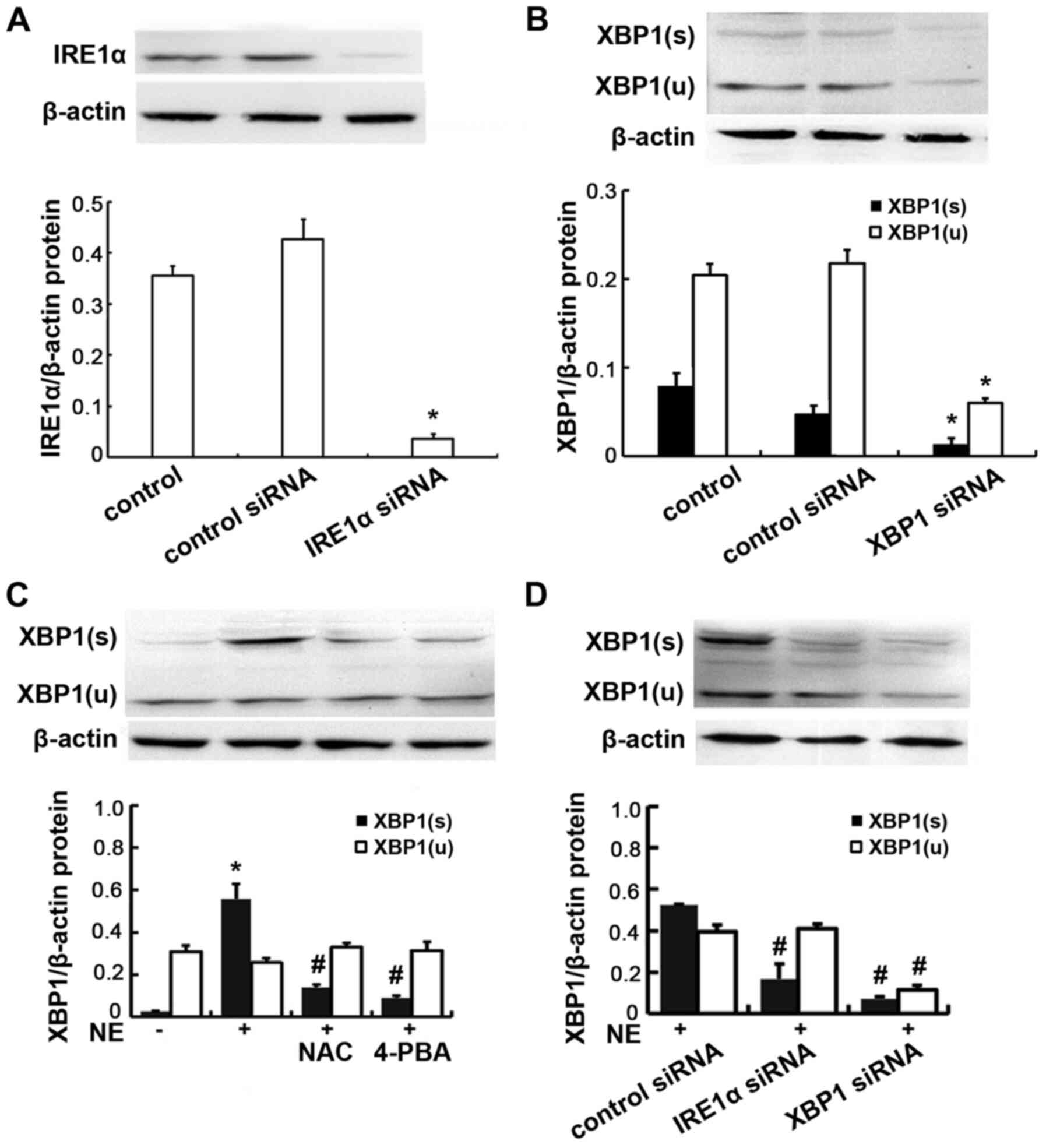 | Figure 4Protein expression of IRE1α and XBP1
in 16HBE14o-cells. (A) Cells were transfected with IRE1α siRNA or
negative control siRNA, IRE1α protein was assayed by western blot
analysis. Relative IRE1α protein expression levels are presented as
the ratio of IRE1α to β-actin. Data are presented as the means ± SD
(n=4 samples per group). *P<0.01 vs. negative control
and control siRNA (B) Cells were transfected with XBP1 siRNA or
negative control siRNA, XBP1 protein was assayed by western blot
analysis. Relative XBP1(s) protein expression levels are presented
as the ratio of XBP1(s) to β-actin. Relative XBP1(u) protein
expression levels are presented as the ratio of XBP1(u) to β-actin.
Data are presented as the means ± SD (n=4 samples per group).
*P<0.01 vs. negative control and control siRNA. (C)
Cells were exposed to 100 ng/ml NE or pre-treated with NAC and
4-PBA prior to NE exposure. XBP1 protein was assayed by western
blot analysis. Data are presented as the means ± SD (n=4 samples
per group). *P<0.01 vs. negative control;
#P<0.01 vs. the NE group. (D) Cells were transfected
with IRE1α siRNA or XBP1 siRNA, and then incubated with 100 ng/ml
NE. Relative XBP1(s) protein expression levels are presented as the
ratio of XBP1(s) to β-actin. Relative XBP1(u) protein expression
levels are presented as the ratio of XBP1(u) to β-actin. Data are
presented as the means ± SD (n=4 samples per group).
#P<0.01 vs. the NE + control siRNA group. IRE1α,
inositol-requiring kinase 1α; NE, neutrophil elastase; NAC,
N-acetylcysteine; 4-PBA, 4-phenylbutyric acid; XBP1, X-box-binding
protein 1; XBP1(s), spliced XBP1; XBP1(u), unspliced XBP1. |
XBP1 expression levels are reduced
following transfection with IRE1α siRNA, XBP1 siRNA
To further evaluate the role of XBP1 and whether
IRE1α is responsible for XBP1 activation, the cells were further
transfected with IRE1α siRNA, XBP1 siRNA or negative control siRNA,
and subsequently stimulated with NE. The transfection efficiency
was verified by detecting the protein expression levels of IRE1α
and XBP1 by western blot analysis following transfection. The
antibody to XBP1 used in the experiment is able to recognize total
XBP1 protein including both isoforms (inactive non-spliced and
active spliced) of XBP1. The results obtained revealed that minimal
IRE1α protein was detected in the IRE1α siRNA-transfected cells. In
addition minimal XBP1 protein (both unspliced and spliced form) was
detected in the XBP1 siRNA-transfected cells, confirming the
successful transfection (Fig. 4A and
B). Following NE stimulation, the XBP1s mRNA and protein
expression levels were significantly decreased in the cells
transfected with IRE1α and XBP1 siRNA, compared with the normal
control siRNA-transfected cells (all P<0.01; Figs. 4D and 5B). These results demonstrate that
IRE1α may be responsible for XBP1 activation.
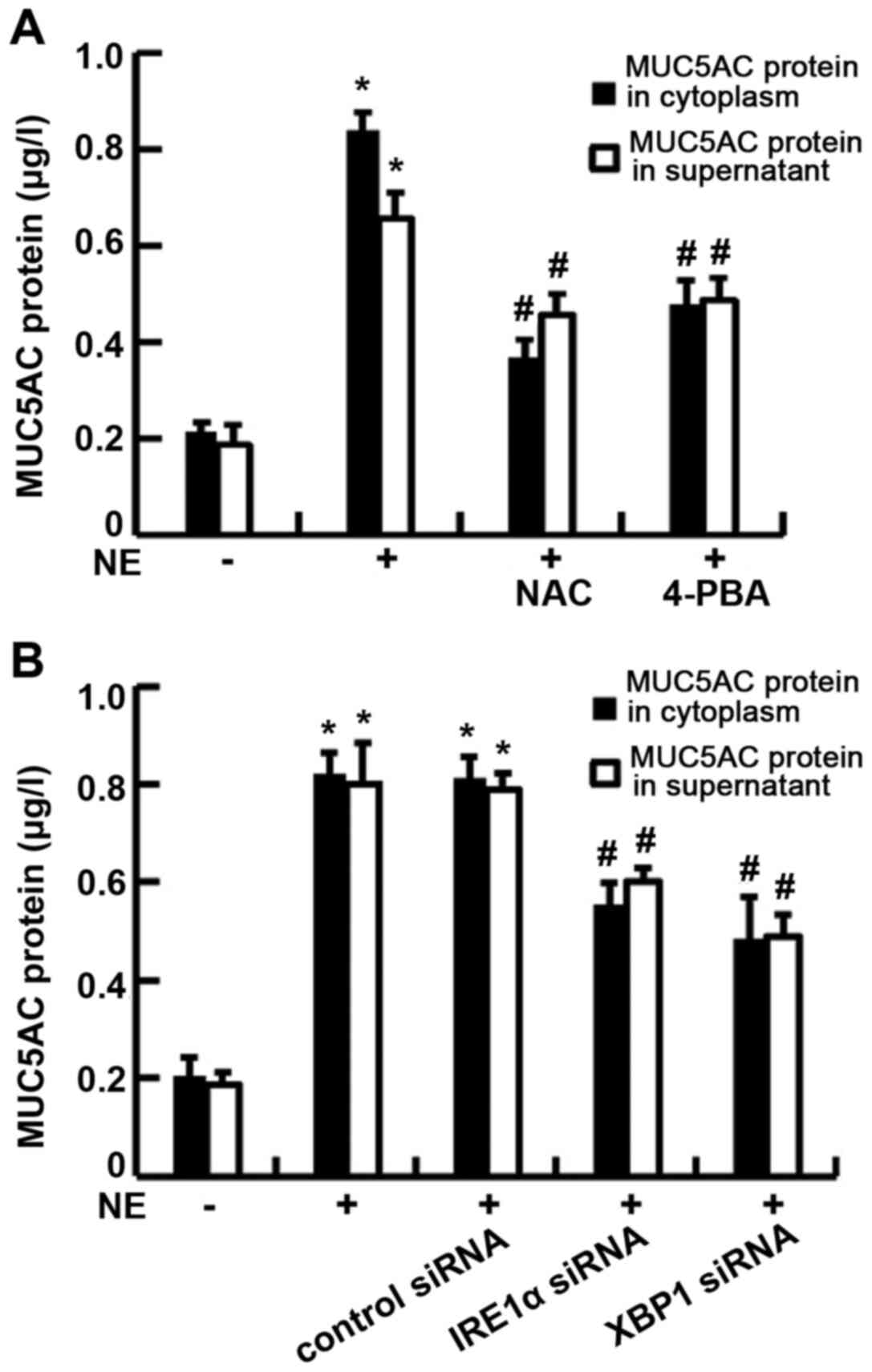
The IRE1α-XBP1 pathway is involved in
NE-induced MUC5AC expression
In order to measure the mucin content during
pathological conditions, the major airway mucin MUC5AC protein and
mRNA expression were assayed following NE stimulation. The roles of
the ROS and ER stress pathways in the process of MUC5AC production
were also assessed by pre-treating the cells with NAC and 4-PBA.
The results revealed that the exposure of 16HBE14o-cells to 100
ng/ml NE led to an increase in MUC5AC protein production in both
the supernatant and cytoplasm, although these increases in the
level of MUC5AC protein were clearly attenuated by pre-treatment
with NAC or 4-PBA (P<0.01) (Fig.
6A), indicating that oxidative stress and ER stress may be
involved in the MUC5AC production process. Moreover, to investigate
whether IRE1α and XBP1 participate in the MUC5AC production, the
cells were transfected with IRE1α siRNA or XBP1 siRNA prior to NE
stimulation. The data indicated that NE-induced MUC5AC mRNA
expression and protein production were significantly decreased in
both the supernatant and cytoplasm (P<0.01; Fig. 6B). The results of
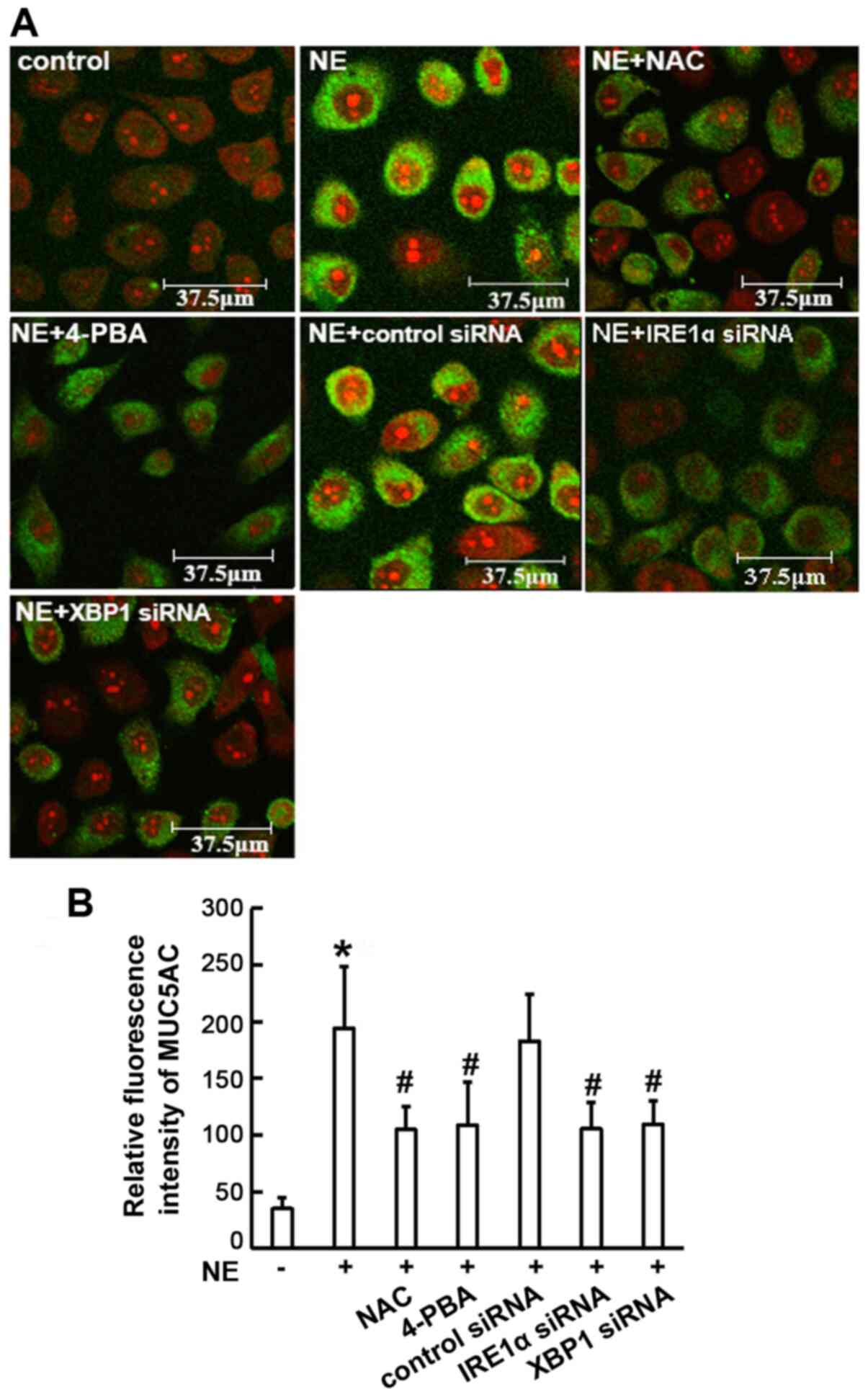
immunofluorescence assay revealed that MUC5AC staining was
diminished in all the experimental groups that were pre-treated
with NAC or 4-PBA, or transfected with IRE1α or XBP1 siRNA, when
compared with the NE-treated group (P<0.01; Fig. 7). Taken together, these results
suggest that the IRE1α/XBP1 pathway may be involved in NE-induced
MUC5AC expression.
Discussion
In the present study, it was demonstrated that NE
stimulation resulted in the activation of the ER stress-associated
proteins, PERK, IRE1α and ATF6, and MUC5AC mucin secretion.
Treatment with the ROS scavenger, NAC, and the ER stress inhibitor,
4-PBA, led to a decrease in ER stress-associated protein expression
and MUC5AC production. In addition, it was observed that MUC5AC
secretion was notably reduced by the knockdown of XBP1 expression
through IRE1α siRNA and XBP1 siRNA transfection, suggesting that ER
stress signaling, particularly that involved with the IRE1α-XBP1
pathway, may play an important role in MUC5AC production induced by
NE.
Airway mucus hypersecretion is a major
pathophysiological feature in numerous patients with chronic
inflammatory airway diseases. NE is a serine protease secreted by
neutrophils that is highly expressed in the airway secretions of
these patients (14). It is
recognized as a potent and significant agonist that gives rise to
MUC5AC overexpression and mucin hypersecretion (15,16). A previous study demonstrated that
NE-induced MUC5AC mRNA and protein expression was associated with
various signaling molecular and pathways that involve the
activation of protein kinase C (PKC), epidermal growth factor
receptor (EGFR) and mitogen-activated protein kinase (MAPK)
(17). In the present study, the
potential role of ER stress induced by NE in the secretion of
airway mucin was investigated, and the results obtained may provide
novel insight into the mechanisms and regulation of airway mucin
secretion; these findings may also lead to the identification of
additional therapeutic strategies to alleviate airway mucus
hypersecretion.
ER stress is an important reaction of airway
epithelia in response to noxious stimuli. Various stimuli involving
hypoxemia (18), oxidative
stress (19), infection
(20-22), cigarette smoke (23,24) and hyperglycemia (25) can disrupt ER function, resulting
in the accumulation of unfolded and misfolded proteins in the ER
lumen, a condition known as ER stress (26). The ER is mainly responsible for
synthesizing, folding and processing secretory and transmembrane
proteins correctly. Previous studies have confirmed that ER stress
is involved in the occurrence and progression of airway
inflammation and various pulmonary diseases, including chronic
obstructive pulmonary disease (COPD), asthma and cystic fibrosis
(27,28).
ROS are one of the major factors causing ER stress
(29,30). Moreover, it is an important
inducing factor in airway inflammation and mucin secretion
(31). In the present study,
intracellular ROS levels, as well as the protein levels of the ER
chaperone, GRP78, p-PERK, p-IRE1α, ATF6 and XBP1s, were
significantly increased by NE exposure, suggesting that NE can
trigger both oxidative and ER stress. Subsequently, co-treatment
with NAC, a ROS scavenger, significantly attenuated the production
and activation of the above-mentioned ER stress-associated
proteins. Taken together, all these results indicate that
NE-induced ER stress is, at least partly, ROS-dependent.
ER stress activates a complex signaling network
referred as the UPR to reduce ER stress and restore homeostasis
(32). Generally, the UPR is
initiated by three ER stress transducers: PERK, IRE1 and ATF6. In
unstressed cells, IRE1, PERK and ATF6 remain in an inactive state
through association with GRP78 (33). Three pathways, namely the
IRE1/XBP1 pathway, the PERK/eIF2α pathway and the ATF6/CHOP
pathway, collectively constitute the ER-specific UPR (34). Upon the ER receiving a stress
stimulus, these UPR transducers are dissociated from GRP78, and
subsequently become activated. PERK is activated by the
autophosphorylation of its kinase domain, which leads to the
subsequent phosphorylation of eukaryotic initiation factor 2
substrate α (eIF2α), specifically at Ser51. IRE1α dimerization and
autophosphorylation activates its RNase activity to splice XBP1
mRNA, thereby generating the mature spliced form of XBP1 known as
XBP1s (35). It has been
reported that XBP1s is a key initiator of ER stress-induced protein
expression (36-38). IRE-XBP1 signaling is one of the
most important pathways in ER stress, which is involved in numerous
pathological processes, including tumor growth, metastasis and
inflammation (35,39).
IRE1 has two isoforms, IRE1α and IRE1β. IRE1α is
ubiquitously expressed, whereas IRE1β is limited to the epithelial
cells, mainly at the gastrointestinal tract (40). Previous studies have demonstrated
that the IRE1α pathway is required for normal secretory protein
metabolism (8). Park et
al (41) have reported that
kaempferol alleviates ovalbumin-induced airway mucus hypersecretion
by inhibiting the activation of IRE1α and c-Jun N-terminal kinase
in human bronchial airway epithelial BEAS-2B cells. It is therefore
possible to propose that an IRE1α-XBP1 dependent signaling cascade
is involved in the synthesis and secretion of MUC5CA. To examine
this hypothesis, siRNA was used to silence the IRE1α and XBP1
genes. The results obtained demonstrated that both siRNAs caused a
significant decrease in the mRNA and protein level of XBP1s, which
was accompanied by decreases in MUC5AC synthesis and secretion. It
has been reported that IRE1β, the other specific isoform of IRE1,
was able to directly induce mucin production in Calu-3 cells by
promoting XBP1 mRNA splicing-dependent transcription of AGR2, which
was not mediated by an ER-stress response (6). Furthermore, IRE1α-dependent XBP1
mRNA splicing was found to be involved in the secretion of airway
MUC5AC in the present set of experiments, suggesting a vital role
of XBP1 in the IRE1 pathway.
XBP1 is a basic leucine zipper constitutional
protein which belongs to the cAMP response element binding protein
(CREB)/activating transcription factors (ATF) family (42). It is involved in several normal
physiological processes, and is an important component of the UPR.
In the UPR after ER stress, XBP1s functions as a transcription
factor to initiate the expression of many target genes that
regulate the unfolding of proteins (43). In a previous study, XBP1 was
reported as one of the MUC5AC-associated core genes in healthy
nonsmokers with high MUC5AC expression (44). In the present study, the human
bronchial epithelial 16HBE14o-cell line was used, and cells that
were cultured in vitro were subsequently exposed to NE. The
results revealed that MUC5AC secretion was reduced following
transfection with XBP1 siRNA, indicating that XBP1 may be
indispensable for MUC5AC production under NE-induced ER stress. It
will be interesting to examine the potential transcriptional
effects of XBP1 on mucin secretion in human airways featuring other
stimuli, such as smoke or fine particles.
Moreover, 4-PBA, an inhibitor of ER stress, was
found to significantly decrease the expression of MUC5AC, and IRE1α
siRNA also markedly reduced (but did not completely inhibit) the
expression of MUC5AC, indicating that other ER stress-associated
proteins may also be involved in airway mucus secretion through
XBP1. Indeed, all the three pathways that participate in ER stress
have been shown to interact with each other. For example, XBP1 mRNA
can be induced by ATF6, and ATF6 expression can be enhanced by PERK
(45). A recent study reported
that ATF6 and XBP1 may reduce colorectal cancer cell proliferation
and stemness by activating PERK-eIF2α signaling (46). The limitation of the present
study is that animal experiments in vivo were not performed
for verification; only the ER stress inhibitor, 4-PBA, was used
while no specific inhibitors of PERK, ATF6 or IRE1α were used.
Among the three branches of the UPR, PERK is a most widely studied
pathway with numerous functions (47). It seems difficult to act as a
relatively specific blocking target. The expression of ATF6 was
relatively weak in the present study, suggesting that the role of
ATF6 may be less important. IRE1α-dependent XBP1 splicing will lead
to production of the XBP1 transcription factor, this is also an
important ER stress signaling pathway. MUC5AC expression in the
human small airway epithelium was linked to several mucus
production/secretion-related transcription factors including XBP1.
Therefore, the present study mainly focused on the IRE1α/XBP1
pathway. However, further studies still need to be performed. The
authors aim to establish animal models and select specific
inhibitors or RNA interference for PERK and ATF6 in the future, in
order to fully elucidate the underlying mechanism(s).
In conclusion, the present study demonstrates that
ER stress is involved in NE-induced MUC5AC secretion in human
airway epithelial cells, and that this is achieved mainly through
the activation of the IRE1α and XBP1 signaling pathways.
Availability of data and materials
All data generated or analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
XZ and ZC designed the study. XX, QL, LL and MZ
performed the experiments. All authors contributed to the critical
data analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Hainan Provincial Natural
Science Fundation of China (grant nos. 820CXTD448 and ZDYF2020223)
and the National Natural Science Foundation of China (grant nos.
81860001, 31660329 and 82011530049).
Abbreviations:
|
ATF6
|
activating transcription factor 6
|
|
4-PBA
|
4-phenylbutyric acid
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
glucose-regulated protein 78
|
|
IRE1α
|
inositol-requiring kinase 1α
|
|
MUC
|
mucin
|
|
NE
|
neutrophil elastase
|
|
NAC
|
N-acetylcysteine
|
|
PERK
|
protein kinase R-like endoplasmic
reticulum kinase
|
|
UPR
|
unfolded protein response
|
|
XBP1
|
X-box-binding protein 1
|
References
|
1
|
Ma J, Rubin BK and Voynow JA: Mucins,
mucus, and goblet cells. Chest. 154:169–176. 2018. View Article : Google Scholar
|
|
2
|
Bonser LR and Erle DJ: Airway mucus and
asthma: The role of MUC5AC and MUC5B. J Clin Med. 6:1122017.
View Article : Google Scholar :
|
|
3
|
Thornton DJ, Rousseau K and McGuckin MA:
Structure and function of the polymeric mucins in airways mucus.
Annu Rev Physiol. 70:459–486. 2008. View Article : Google Scholar
|
|
4
|
Bae CH, Na HG, Choi YS, Song SY and Kim
YD: Clusterin induces MUC5AC expression via activation of NF-κB in
human airway epithelial cells. Clin Exp Otorhinolaryngol.
11:124–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song KS, Yoon JH, Kim KS and Ahn DW:
c-Ets1 inhibits the interaction of NF-κB and CREB, and
downregulates IL-1β-induced MUC5A coverproduction during airway
inflammation. Mucosal Immunol. 5:207–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martino MB, Jones L, Brighton B, Ehre C,
Abdulah L, Davis CW, Ron D, O'Neal WK and Ribeiro CM: The ER stress
transducer IRE1β is required for airway epithelial mucin
production. Mucosal Immunol. 6:639–654. 2013. View Article : Google Scholar
|
|
7
|
Wang X, Yang X, Li Y, Wang X, Zhang Y, Dai
X, Niu B, Wu J, Yuan X, Xiong A, et al: Lyn kinase represses mucus
hypersecretion by regulating IL-13-induced endoplasmic reticulum
stress in asthma. EBioMedicine. 15:137–149. 2017. View Article : Google Scholar :
|
|
8
|
Safra M, Ben-Hamo S, Kenyon C and
Henis-Korenblit S: The IRE-1 ER stress-response pathway is required
for normal secretory-protein metabolism in C. elegans J Cell Sci.
126:4136–4146. 2013. View Article : Google Scholar
|
|
9
|
Kim S, Joe Y, Kim HJ, Kim YS, Jeong SO,
Pae HO, Ryter SW, Surh YJ and Chung HT: Endoplasmic reticulum
stress-induced IRE1α activation mediates cross-talk of GSK-3β and
XBP1 to regulate inflammatory cytokine production. J Immunol.
194:4498–4506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deo SH, Jenkins NT, Padilla J, Parrish AR
and Fadel PJ: Norepinephrine increases NADPH oxidase-derived
superoxide in human peripheral bloodmonuclear cells via
α-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol.
305:R1124–R1132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Basseri S, Lhoták S, Sharma AM and Austin
RC: The chemical chaperone 4-phenylbutyrate inhibits adipogenesis
by modulating the unfolded protein response. J Lipid Res.
50:2486–2501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Halasi M, Wang M, Chavan TS, Gaponenko V,
Hay N and Gartel AL: ROS inhibitor N-acetyl-L-cysteine antagonizes
the activity of proteasome inhibitors. Biochem J. 454:201–208.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Kohri K, Ueki IF and Nadel JA: Neutrophil
elastase induces mucin production by ligand-dependent epidermal
growth factor receptor activation. Am J Physiol Lung Cell Mol
Physiol. 283:L531–L540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shao MX and Nadel JA: Neutrophil elastase
induces MUC5AC mucin production in human airway epithelial cells
via a cascade involving protein kinase C, reactive oxygen species,
and TNF-alpha-converting enzyme. J Immunol. 175:4009–4016. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Voynow JA, Fischer BM, Malarkey DE, Burch
LH, Wong T, Longphre M, Ho SB and Foster WM: Neutrophil elastase
induces mucus cell metaplasia in mouse lung. Am J Physiol Lung Cell
Mol Physiol. 287:L1293–L1302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JA, Sharif AS, Shiomi T, Kobzik L,
Kasahara DI, Tschumperlin DJ, Voynow J and Drazen JM: Human
neutrophil elastase-mediated goblet cell metaplasia is attenuated
in TACE-deficient mice. Am J Physiol Lung Cell Mol Physiol.
304:L701–L707. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mennerich D, Kellokumpu S and Kietzmann T:
Hypoxia and reactive oxygen species as modulators of endoplasmic
reticulum and golgi homeostasis. Antioxid Redox Signal. 30:113–137.
2019. View Article : Google Scholar
|
|
19
|
Zeeshan HM, Lee GH, Kim HR and Chae HJ:
Endoplasmic reticulum stress and associated ROS. Int J Mol Sci.
17:3272016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng M, Sang W, Chen S, Chen R, Zhang H,
Xue F, Li Z, Liu Y, Gong Y, Zhang H and Kong X: 4-PBA inhibits
LPS-induced inflammation through regulating ER stress and autophagy
in acute lung injury models. Toxicol Lett. 5(271): 26–37. 2017.
View Article : Google Scholar
|
|
21
|
Martinon F, Chen X, Lee AH and Glimcher
LH: Toll-like receptor activation of XBP1 regulates innate immune
responses in macrophages. Nat Immunol. 11:411–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keestra-Gounder AM, Byndloss MX, Seyffert
N, Young BM, Chávez-Arroyo A, Tsai AY, Cevallos SA, Winter MG, Pham
OH, Tiffany CR, et al: NOD1 and NOD2 signalling links ER stress
with inflammation. Nature. 532:394–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Wu ZZ and Wang W: Inhibition of
endoplasmic reticulum stress alleviates cigarette smoke-induced
airway inflammation and emphysema. Oncotarget. 8:77685–77695. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kunchithapautham K, Atkinson C and Rohrer
B: Smoke exposure causes endoplasmic reticulum stress and lipid
accumulation in retinal pigment epithelium through oxidative stress
and complement activation. J Biol Chem. 289:14534–14546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li XZ, Xu C and Yang PX: c-Jun
NH2-terminal kinase 1/2 and endoplasmic reticulum stress as
interdependent and reciprocal causation in diabetic embryopathy.
Diabetes. 62:599–608. 2013. View Article : Google Scholar :
|
|
26
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015. View Article : Google Scholar
|
|
27
|
Cao SS, Luo KL and Shi L: Endoplasmic
reticulum stress interacts with inflammation in human diseases. J
Cell Physiol. 231:288–294. 2016. View Article : Google Scholar
|
|
28
|
Mijošek V, Lasitschka F, Warth A, Zabeck
H, Dalpke AH and Weitnauer M: Endoplasmic reticulum stress is a
danger signal promoting innate inflammatory responses in bronchial
epithelial cells. J Innate Immun. 8:464–478. 2016. View Article : Google Scholar
|
|
29
|
Liu ZW, Zhu HT, Chen KL, Dong X, Wei J,
Qiu C and Xue JH: Protein kinase RNA-like endoplasmic reticulum
kinase (PERK) signaling pathway plays a major role in reactive
oxygen species (ROS)-mediated endoplasmic reticulum stress-induced
apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol.
12:1582013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ochoa CD, Wu RF and Terada LS: ROS
signaling and ER stress in cardiovascular disease. Mol Aspects Med.
63:18–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saco TV, Breitzig MT, Lockey RF and
Kolliputi N: Epigenetics of mucus hypersecretion in chronic
respiratory diseases. Am J Respir Cell Mol Biol. 58:299–309. 2018.
View Article : Google Scholar :
|
|
32
|
Peñaranda Fajardo NM, Meijer C and Kruyt
FA: The endoplasmic reticulum stress/unfolded protein response in
gliomagenesis, tumor progression and as a therapeutic target in
glioblastoma. Biochem Pharmacol. 118:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bright MD, Itzhak DN, Wardell CP, Morgan
GJ and Davies FE: Cleavage of BLOC1S1 mRNA by IRE1 is sequence
specific, temporally separate from XBP1 splicing, and dispensable
for cell viability under acute endoplasmic reticulum stress. Mol
Cell Biol. 35:2186–2202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang D, Niwa M and Koong AC: Targeting
the IRE1α-XBP1 branch of the unfolded protein response in human
diseases. Semin Cancer Biol. 33:48–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao SS and Kaufman RJ: Unfolded protein
response. Curr Biol. 22:R622–R626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu R, Zhang QH, Lu YJ, Ren K and Yi GH:
Involvement of the IRE1α-XBP1 pathway and XBP1s-dependent
transcriptional reprogramming in metabolic diseases. DNA Cell Biol.
34:6–18. 2015. View Article : Google Scholar :
|
|
39
|
Chen C and Zhang X: IRE1α-XBP1 pathway
promotes melanoma progression by regulating IL-6/STAT3 signaling. J
Transl Med. 15:422017. View Article : Google Scholar
|
|
40
|
Urano F, Bertolotti A and Ron D: IRE1 and
efferent signaling from the endoplasmic reticulum. J Cell Sci.
21:3697–3702. 2000.
|
|
41
|
Park SH, Gong JH, Choi YJ, Kang MK, Kim YH
and Kang YH: Kaempferol inhibits endoplasmic reticulum
stress-associated mucus hypersecretion in airway epithelial cells
and ovalbumin-sensitized mice. PLoS One. 10:e01435262015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iwakoshi NN, Lee AH, Vallabhajosyula P,
Otipoby KL, Rajewsky K and Glimcher LH: Plasma cell differentiation
and the unfolded protein response intersect at the transcription
factor XBP1. Nat Immunol. 4:321–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Glimcher LH: XBP1: The last two decades.
Ann Rheum Dis. 69(Suppl 1): S67–S71. 2010. View Article : Google Scholar
|
|
44
|
Wang G, Xu Z, Wang R, Al-Hijji M, Salit J,
Strulovici-Barel Y, Tilley AE, Mezey JG and Crystal RG: Genes
associated with MUC5AC expression in small airway epithelium of
human smokers and non-smokers. BMC Med Genomics. 5:212012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoshida H, Okada T, Haze K, Yanagi H, Yura
T, Negishi M and Mori K: ATF6 activated by proteolysis binds in the
presence of NF-Y (CBF) directly to the cis-acting element
responsible for the mammalian unfolded protein response. Mol Cell
Biol. 20:6755–6767. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spaan CN, Smit WL, van Lidth de Jeude JF,
Meijer BJ, Muncan V, van den Brink GR and Heijmans J: Expression of
UPR effector proteins ATF6 and XBP1 reduce colorectal cancer cell
proliferation and stemness by activating PERK signaling. Cell Death
Dis. 10:4902019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gonen N, Sabath N, Burge CB and Shalgi R:
Widespread PERK-dependent repression of ER targets in response to
ER stress. Sci Rep. 9:43302019. View Article : Google Scholar : PubMed/NCBI
|