Introduction
Bone marrow (BM)-derived mesenchymal stem cells
(MSCs) are multipotent cells that have self-renewal capabilities
and multilineage differentiation potential including bone, fat,
cartilage and muscle (1).
Understanding the process and molecular mechanisms of MSC
differentiation to bone holds significant promise to provide new
therapeutic strategy for bone-related diseases (2-5).
To date, a number of clinical trials using MSCs for the treatment
of bone-related diseases have been performed (6-9).
A number of studies have focused on intrinsic transcription factors
that regulate the differentiation of MSCs into osteocytes (10-12). However, their therapeutic utility
still requires a more in-depth understanding of the molecular
mechanisms that regulate MSC differentiation into osteoblasts.
There is evidence to suggest that Wnt signaling
regulates osteoblast differentiation. The secreted glycoproteins,
Wnts, and their receptors include at least 19 Wnts, 10 Fzd
receptors, the two co-receptors, low-density lipoprotein
receptor-related protein (LRP)5/LRP6 and several inhibitors, such
as Dickkopf (Dkk)s, Frizzled (Fz)-related proteins (Frps) and Wif
(13,14). The canonical Wnt signaling is
where Wnt binds to frizzled receptors and LRP-5 and/or LRP-6
co-receptors, promotes disheveled activation, and then blocks the
function of glycogen synthase kinase (GSK)-3β (15). The inactivation of GSK-3β induces
the cytoplasmic accumulation of β-catenin, which translocates to
the nucleus and activates T-cell factor/lymphoid enhancer factor
(TCF/LEF) family, leading to transcriptional activation of target
genes (16). Previous research
has indicated that the homozygous Wnt1 mutation gives rise to
severe bone fragility and that the heterozygous Wnt1 mutation in
family members tends to cause dominant early-onset osteoporosis;
four children with homozygous or heterozygous Wnt1 mutations
treated with bisphosphonate exhibited increased bone mineral
density (17-20). In the study by Keller et
al (21), the expression of
Wnt5a significantly increased at day 7 and the expression of Wnt3a
was observed at a later stage than that of Wnt5a during osteogenic
induction. During mouse embryonic stem cell osteogenesis in
vitro, the supplementation of Wnt5a from 5 to 7 days
significantly enhanced osteogenic yield, although treatment with
Wnt5a for the duration of the osteogenic induction period inhibited
osteogenesis. Treatment with Wnt3a inhibited osteogenic
differentiation from 5 to 7 days, but enhanced osteogenesis from 7
to 9 days. These intriguing results confirmed that Wnt5a and Wnt3a
act sequentially in the osteogenic differentiation of mouse
embryonic stem cell (21). A
previous study using an engineered mouse model revealed that
activation of Wnt7b dramatically enhanced bone mass (22). These results suggested the role
of individual Wnts in osteogenic differentiation and bone
formation; this warrants further investigations.
Of note, various individual Wnts have displayed
distinct expression patterns in different bones. Wnt10b is
expressed in all bones, whereas Wnt4 expression is higher in the
trabecular endosteum, and Wnt7b is highly expressed in the
perichondrium, indicating that there are various individual Wnt
functions in different bones (23). In ST2 cells or 3T3-L1 cells, the
overexpression of Wnt10a and Wnt10b has been shown to inhibit
adipogenesis and promote osteogenesis; however, the depletion of
Wnt6 increased adipocyte differentiation and reduced osteogenesis
compared with the knockdown of Wnt10a or Wnt10b (24). These previous findings
demonstrate that further research is required to focus on
clarifying the roles of individual Wnts in osteogenic
differentiation and bone formation.
Previously, msh homeobox 2 (Msx2) was found to be a
regulator of osteogenic differentiation, and to upregulate Wnt7a
expression; the knockdown of Wnt7a significantly reduced
Msx2-induced alkaline phosphatase (25). To further explore the role of
Wnt7a in the differentiation of MSCs into osteocytes, the present
study examined the expression of Wnt7a in MSCs subjected to
osteogenic- and adipogenic-induced differentiation. It was found
that osteogenic induction medium increased Wnt7a expression,
whereas adipogenic medium downregulated Wnt7a expression. The
knockdown of Wnt7a in MSCs impaired osteogenic commitment in
vitro and the enforced expression of Wnt7a in MSCs enhanced
osteocyte formation in vitro. Mechanistically, it was found
that Wnt7a significantly upregulated the expression of the
osteogenic regulator, Runt-related transcription factor 2
(RUNX2).
Materials and methods
Ethics statement
Human bone tissues were obtained from following the
orthopedic surgery of five 20- to 30-year-old male patients in 2016
at the Department of Orthopedics of the Affiliated Hospital of
Guiyang Medical University. All experiments and protocols were
approved by the Ethics Committee of Guizhou Medical University.
Each patient provided written informed consent prior to the
preparation of the bone tissues.
Cells and cell culture
Aseptic bone samples were minced with blades and
then transferred to a digestion medium containing 200 U/ml
collagenase I (Sigma-Aldrich; Merck KGaA) for 2 h at 37°C with
intermittent agitation. The cell suspension was filtered through a
40-μm strainer (BD Biosciences) and rinsed with D-Hanks
solution twice. The cells were cultured in DMEM containing 10%
fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml
streptomycin. MSCs were used in the 6th passage. MSCs were treated
with 150 ng/ml bone morphogenetic protein (BMP)4/7 (R&D
Systems) for 24 h and mRNA levels were determined by reverse
transcription-quantitative PCR (RT-qPCR) at 24 h and protein levels
by western blot analysis at 48 h as previously described (26).
Vector construction and viral
infection
shRNA was purchased from Sigma-Aldrich; Merck KGaA
and the target sequence for shWnt7a was as follows:
5′-CGTGCTCAAGGACAAGTACAA-3′. Viral synthesis was performed
according to manufacturer's instructions. Briefly, 2 μg the
shWnt7a or control vector were co-transfected with the packaging
vectors, 2 μg psPAX2 and 1 μg pMD2G, into 293T cells
(ATCC) in each well of a 6-well plate. For viral infection, cells
were plated overnight and the MSCs were then infected in the
presence of 8 μg/ml polybrene for 6 h. The cells were
selected with 2 μg/ml puromycin for 2 days. The sequence
encoding human Wnt7a was amplified by RT-qPCR (primers:
5′-TTGGCGCGCCGCCACCATGAACCGGAAAGCGCGGCG-3′ and
5′-CCTTAATTAATCACTTGCACGTGTACATCT-3′) from pcDNA-Wnt7A-V5 (Addgene)
(27) and inserted into the
AscI/PacI sites of pCDF1-MCS2-EF1-copGFP (SBI).
pCDF1-Wnt7a or pCDF1 were co-transfected with packaging vector
pFIV34N and pVSV-G overnight. At 48 h following transfection, the
virus was collected and used to infect the MSCs in the presence of
8 μg/ml polybrene for 6 h. The Wnt7a-positive cells were
isolated by FACS analysis using GFP. Briefly, 1×106 pC
DF1-Wnt7a or pC DF1 infected MSCs were digested by 0.05%
trypsin/0.02%.
EDTA and filtered with a 40 μm strainer. The
cells were rinsed 3 times by D-Hanks and sorted using a BD FACSAria
III cell sorter. The sorted cells were cultured in a 6-cm culture
dish (Corning, Inc.).
Immunofluorescence staining
MSCs were fixed in 4% PFA at room temperature for 15
min and permeabilized with 0.5% Triton X-100 for 5 min. The cells
were blocked with 2.5% goat serum for 1 h and then stained with
1:200 anti-Ki-67 mouse monoclonal antibody (M7240; Dako; Agilent
Technologies, Inc.) overnight at 4°C. Antibody binding was
visualized by incubation with a FITC-conjugated goat anti-mouse
secondary antibody (1:200, sc-2010; Santa Cruz Biotechnology, Inc.)
at room temperature for 1 h. Mouse IgG2a (1:200, sc-2856; Santa
Cruz Biotechnology, Inc.) was used as the isotype control for Ki-67
antibody and FITC-conjugated goat anti-mouse antibody (1:200,
sc-2010; Santa Cruz Biotechnology, Inc.) as a negative control to
assess non-specific binding of the secondary antibody. MSCs were
then stained with DAPI at room temperature for 3 min and imaged
using an Olympus BX51 fluorescence microscope.
Flow cytometry
MSCs were digested with 0.05% trypsin/0.02% EDTA and
filtered with a 70-μm strainer. The cells were blocked with
2.5% goat serum for 30 min and stained with anti-CD44 (555478),
-CD90 (561969), -CD105 (561443), -CD34 (555821) and -CD45 (555482)
antibodies at a dilution of 1:200 for 45 min. Rat IgG2a was used as
an isotype control. All antibodies were directly conjugated to FITC
and obtained from BD Biosciences. MSCs were rinsed 5 times and
analyzed on an Epics XL flow cytometer (Beckman Coulter, Inc.).
Alizarin Red S staining
To induce osteogenic differentiation, MSCs were
grown in DMEM supplemented with 10% FCS, 10 mM β-glycerophosphate,
10−7 M dexamethasone and 0.2 mM ascorbic acid (all from
Sigma-Aldrich; Merck KGaA). The medium was changed every three
days. Following two weeks of culture in vitro, the cells
were fixed in 10% formalin for 15 min at room temperature and
stained by Alizarin Red S stain solution for 30 min at room
temperature using the Osteogenesis Assay kit according to the
manufacturer's instructions (EMD Millipore).
Oil Red O staining
To induce adipogenic differentiation, MSCs were
grown in DMEM containing 0.5 μM hydrocortisone, 0.5 mM
isobutylmethylxanthine and 50 μg/ml indomethacin (all from
Sigma-Aldrich; Merck KGaA) (28). The medium was changed every three
days. Following two weeks of culture, the cells were fixed with 10%
formalin and stained with fresh Oil Red O solution for 10 min at
room temperature (Sigma-Aldrich; Merck KGaA) to detect lipid
droplets.
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate total RNA from the
MSCs. cDNA was synthesized using the PrimeScript™ RT Reagent kit
(Takara Bio, Inc.) according to the manufacturer's protocol. qPCR
was performed using SYBR Premix Ex Taq II (Takara Bio, Inc.) and
the CFX96 Real-time PCR Detection system (Bio-Rad Laboratories,
Inc.). Each sample is done in triplicate. The primers used were as
follows: β-actin forward, 5′-gtaccactggcatcgtgatggact-3′ and
reverse, 5′-ccgctcattgc- caatggtgat-3′; osteocalcin (OCN) forward,
5′-AGCAAAGGTGCAGCCTTTGT-3′ and reverse, 5′-GCGCCTGGGTCTCTTCACT-3′;
osteopontin (OPN) forward, 5′-ATGATGGCCGAGGTGATAGT-3′ and reverse,
5′-ACCATTCAACTCCTCGCTTT-3′; peroxisome proliferator-activated
receptor G2 (PPARG2) forward, 5′-TCCATGCTGTTATGGGTGAA-3′ and
reverse, 5′-TCAAAGGAGTGGGAGTGGTC-3′; lipoprotein lipase (Lpl)
forward, 5′-AGTGGCCAAATAGCACATCC-3′ and reverse,
5′-CCGAAAGATCCAGAATTCCA-3′; Wnt7a forward,
5′-CTGTGGCTGCGACAAAGAGAA-3′ and reverse, 5′-GCCGTGGCACTTACATTCC-3′;
RUNX2 forward, 5′-CAGTAGATGGACCTCGGGAA-3′ and reverse,
5′-CCTAAATCACTGAGGCGGTC-3′; and osterix (OSX) forward,
5′-AGCCTCAGGATGGCGTC-3′ and reverse, 5′-AGAGTTGTTGAGTCCCGCAG-3′.
The results were analyzed using the 2−ΔΔCq method
(29).
Western blot analysis
To obtain protein extracts, MSCs were washed with
chilled PBS and lysed with RIPA buffer containing a protease
inhibitor cocktail (Roche Diagnostics). The protein concentration
was measured using BCA methods according to the manufacturer's
instruction (P0012S, Beyotime) and 30 μg were separated by
10% SDS-PAGE gel and transferred to PVDF membranes. The protein was
blocked by 5% milk for 1 h at room temperature. The membranes were
incubated with specific primary antibodies against β-actin
(sc-47778, Santa Cruz Biotechnology, Inc.), Wnt7a (ab100792;
Abcam), OCN (ab13418; Abcam), OPN (ab214050; Abcam), RUNX2 (12556,
Cell Signaling Technology, Inc.) and OSX (ab94744, Abcam) at
1:1,000 overnight at 4°C. The membranes were rinsed three times by
TBST and incubated with 1:1,000 goat against rabbit (sc-2357; Santa
Cruz Biotechnology, Inc.) or 1:1,000 m-IgGκBP-HRP (sc-516102; Santa
Cruz Biotechnology, Inc.) horseradish peroxidase-conjugated anti-
bodies for 1 h at room temperature. The bands were detected by
Immobilon Western Chemiluminescent HRP Substrate (ECL, WBKLS0100;
Thermo Fisher Scientific, Inc.).
Luciferase assays
The TCF1-binding region (-900 to -1,200 bp upstream
of RUNX2 exon 1) was cloned by PCR amplification from 293T genomic
DNA and inserted into the SacI/XhoI sites of the
pGL3-control vector (Promega Corporation). The primers for cloning
the TCF1-binding region in RUNX2 promoter were as follows: Forward,
5′-GCGCGAGCTCAGGGGCAAAAAAGGAGATAGTT-3′; and reverse,
5′-GCGCCTCGAGGAGTTTCTGATAGCAGATCTTCTAT-3′. pRL-SV40 (Promega
Corporation) was co-transfected as an internal control to assess
the transfection efficiency. Wnt7a silencing/control or Wnt7a
overexpressing/control MSCs were grown in osteogenic-inducing
medium for 7 days. A total of 100 μg the reporter constructs
were co-transfected with 10 ng pRL-SV40 into MSCs in each well of
96-well plate in which Wnt7a was silenced or overexpressed with
internal control plasmids. Cells were collected following 24 h of
transfection, and luciferase activities were detected using the
Dual-Luciferase Reporter assay (Promega Corporation) according to
the manufacturer's protocol. The relative promoter activities were
expressed as the fold change in Firefly luciferase activity
following normalization to Renilla luciferase activity. A
total of three independent experiments were performed for each
sample.
ChIP assays
The assays were performed using a ChIP assay kit
according to the manufacturer's protocol (P2078; Beyotime Institute
of Biotechnology). The antibodies used for CHIP assays were
purchased from the following companies: TCF1 (2203, Cell Signaling
Technology, Inc.) and IgG (sc-2003; Santa Cruz Biotechnology, Inc.
as an IP control). A total of 2 μg TCF1 or IgG were
incubated with the cell lysates overnight at 4°C as previously
reported (30). As previously
reported, TCF1 recognizes and binds to the consensus motif TTCAAAG
(31,32). PCR generated a product from the
RUNX2 promoter containing a TCF1-binding site (-1,056 to -1,062 bp
upstream of exon 1) or a product from the distal region 8 kb
downstream for RUNX2 without the TCF1-binding site. Each experiment
was performed in three samples. The primers used for RT-qPCR for
the TCF1-binding element in the RUNX2 promoter were as follows:
Forward, 5′-CTGAATCAGAATTAGCAAATCG-3′ and reverse,
5′-GTTTCTAATGGAAGCTTTGA-3′; and 8 kb downstream for RUNX2 forward,
5′-GATTGCAAAAAACAGATATTAA-3′ and reverse,
5′-GAAAGTCTTGTGTGACTAAATA-3′.
Statistical analysis
All experiments were performed in triplicate. The
variables were compared between groups by a paired samples t-test.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was carried out using SSPS 13.0
software.
Results
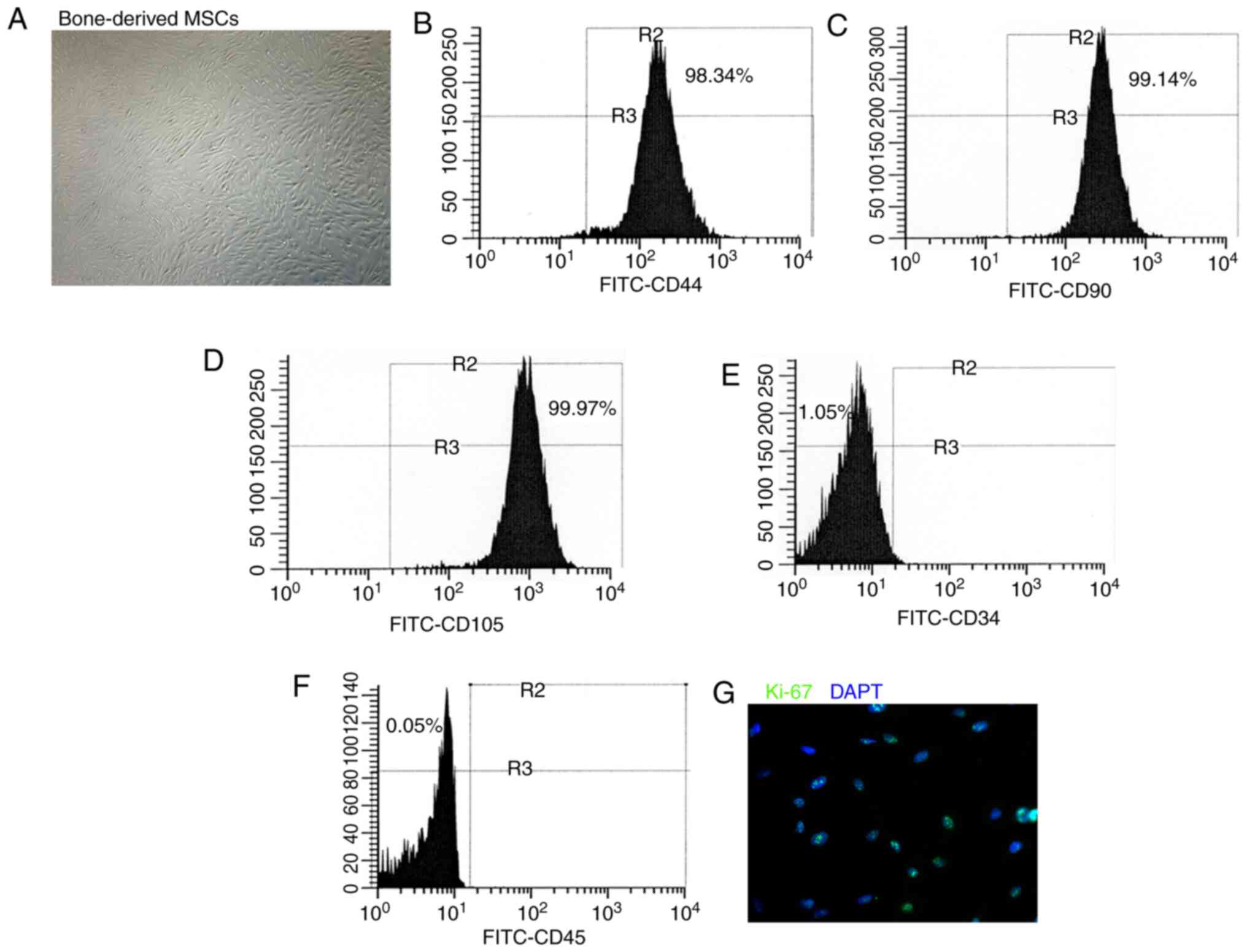
Phenotype of cultured BM-derived
MSCs
The BM-derived cells were cultured in DMEM and the
phenotype was detected in the sixth passage. The adherent
BM-derived cells exhibited a fibroblast-like morphology (Fig. 1A). Flow cytometry was performed
to characterize the phenotype of the BM-derived cells used in the
present study. These BM-derived cells expressed the MSC markers,
CD44, CD90 and CD105 (Fig.
1B-D), whereas they did not express the hematopoietic lineage
markers, CD34 and CD45 (Fig. 1E and
F). Immunofluorescence staining revealed that the BM-derived
cells comprised more Ki-67-labeled proliferative cells (Fig. 1G). These findings suggested that
the BM-derived cells used in the present study acquired the
phenotype and characteristics of MSCs.
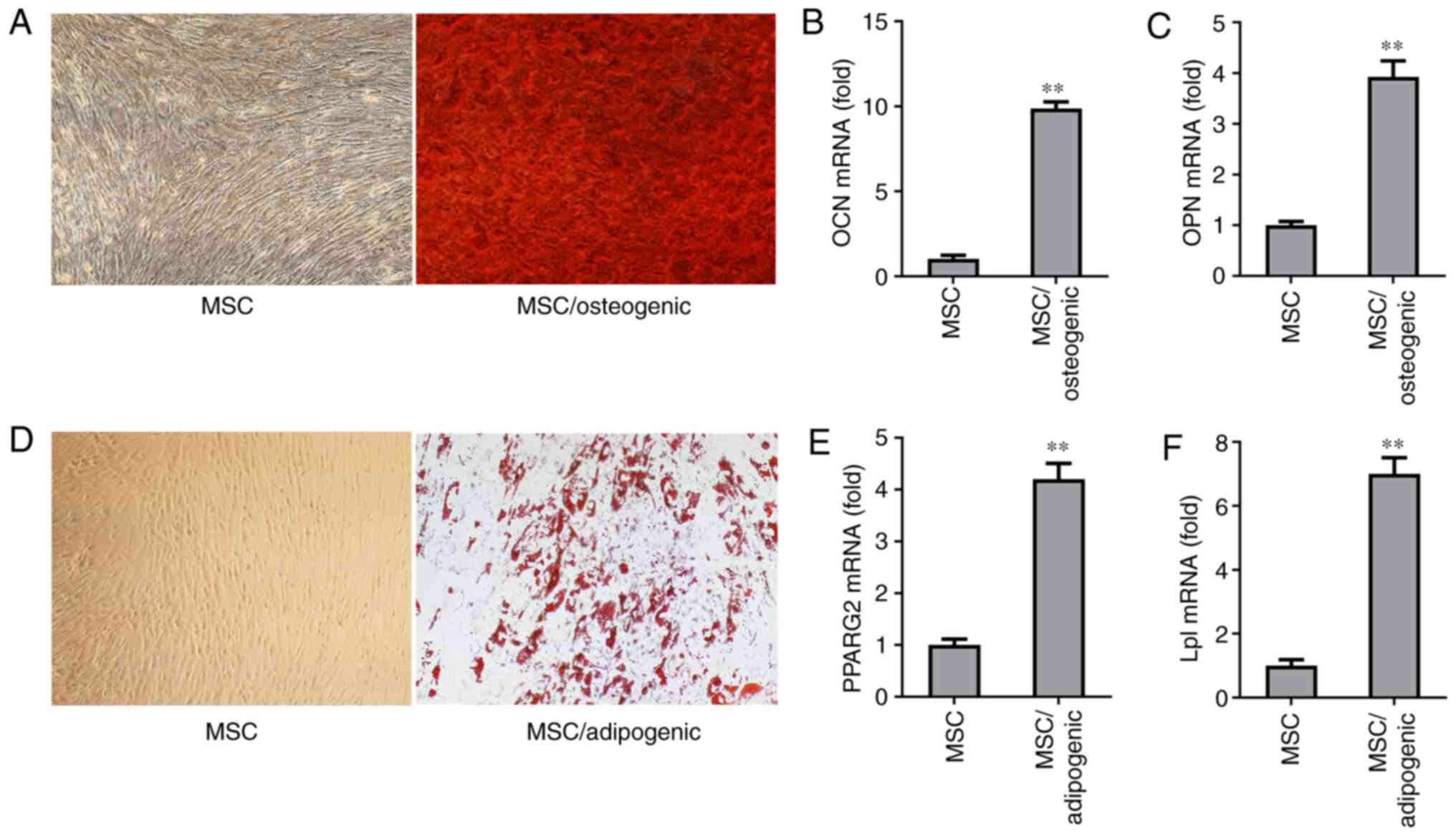
In vitro differentiation potential of
MSCs
To evaluate the differentiation potential of the
MSCs used in the present study, osteogenic medium and
adipocyte-specific induction medium were used to culture the MSCs.
Following two weeks of induction with osteogenic medium, it was
demonstrated that the MSCs cultured with osteogenic medium
exhibited osteogenic differentiation capacity compared with the
MSCs without osteogenic induction, as evidenced by mineralized
nodule formation assessed by Alizarin Red S staining (Fig. 2A). The results of RT-qPCR
confirmed that the MSCs cultured with osteogenic medium for 6 days
expressed higher levels of the osteogenic markers, OCN and OPN
(Fig. 2B and C). The MSCs also
exhibited adipogenic differentiation capacity, as confirmed by Oil
Red O staining of adipocytes induced by growing MSCs in
adipocyte-specific induction medium for two weeks (Fig. 2D). The results of RT-qPCR
revealed that the expression levels of the adipogenic markers,
PPARG2 and Lpl were higher after the MSCs were grown in
adipocyte-specific induction medium for six days (Fig. 2E and F). These findings indicated
that the MSCs used in the present study acquired multilineage
differentiation potential.
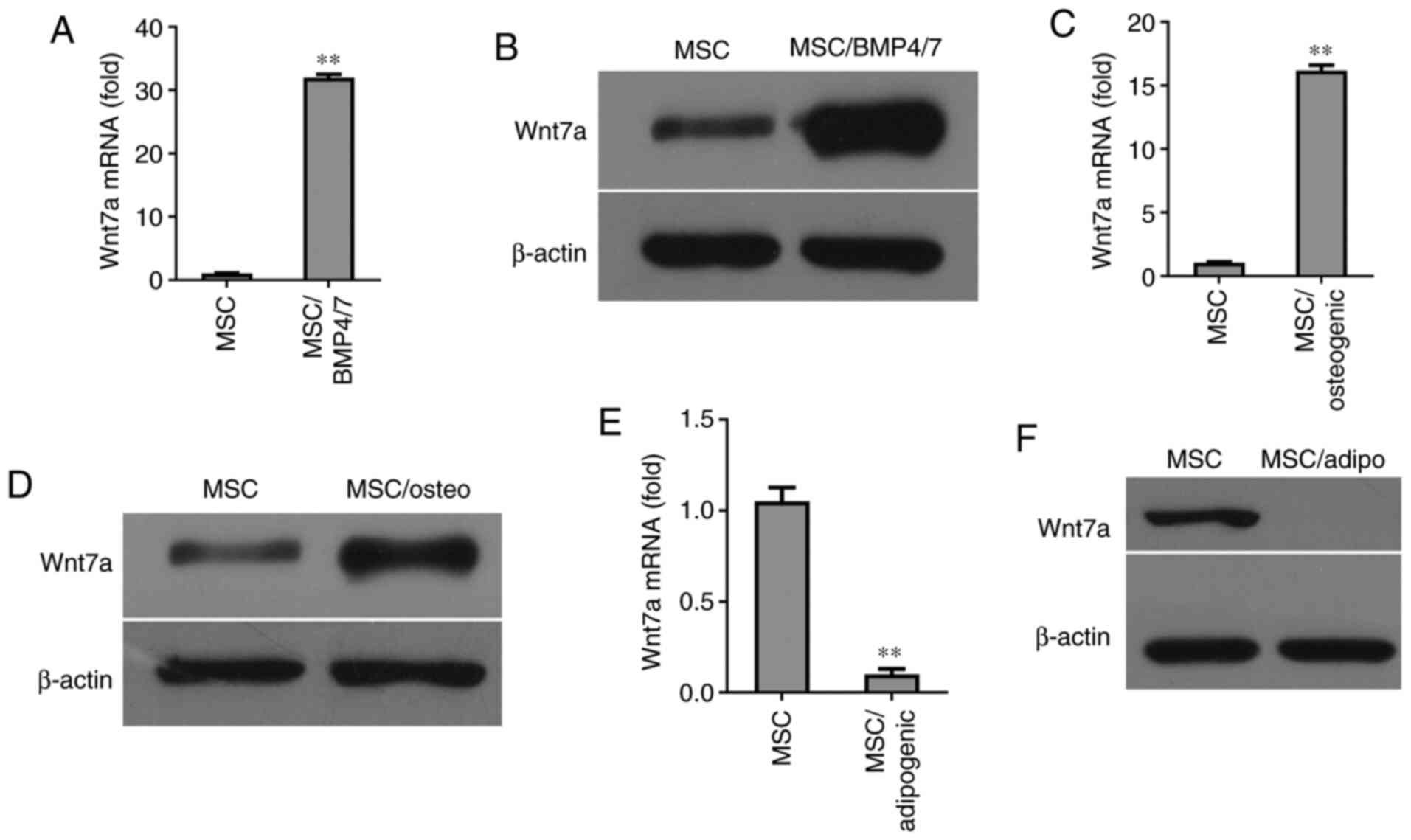
Induction of Wnt7a expression by the
osteogenic differentiation of MSCs
Since BMP-4/7 are capable of inducing MSC
differentiation into osteocytes, the present investigated whether
the expression of Wnt7a was induced by BMP-4/7 in MSCs. The results
of RT-qPCR revealed that Wnt7a expression was strongly induced by
BMP4/7 (Fig. 3A). Western blot
analysis revealed that the expression of Wnt7a was elevated at the
protein level in BMP4/7-treated MSCs (Fig. 3B). The expression of Wnt7a was
also examined in MSCs cultured in osteogenic-inducing medium.
Notably, after six days of culture, the expression of Wnt7a was
found to be elevated in MSCs cultured in osteogenic-inducing medium
compared with the MSCs without osteogenic-inducing medium, as shown
by RT-qPCR and western blot analysis (Fig. 3C and D). Since the dynamic
balance between osteogenesis and adipogenesis exists in MSC
differentiation, the regulators that promote an osteogenic lineage
commitment could be inhibited under the condition of adipogenic
differentiation. To explore whether the expression of Wnt7a was
regulated during the adipogenic differentiation of MSCs, Wnt7a
expression was determined at the mRNA and protein level in the MSCs
grown in adipogenic-inducing medium for six days. The results of
RT-qPCR revealed that Wnt7a expression was significantly inhibited
in MSCs grown in adipogenic-inducing medium (Fig. 3E). Consistently, western blot
analysis revealed that the expression of Wnt7a was inhibited in
MSCs grown in adipogenic-inducing medium (Fig. 3F). Since the Wnt signal pathway
plays a central role in the osteogenic differentiation of MSCs,
these findings suggested that Wnt7a plays a critical role in MSC
differentiation into osteocytes.
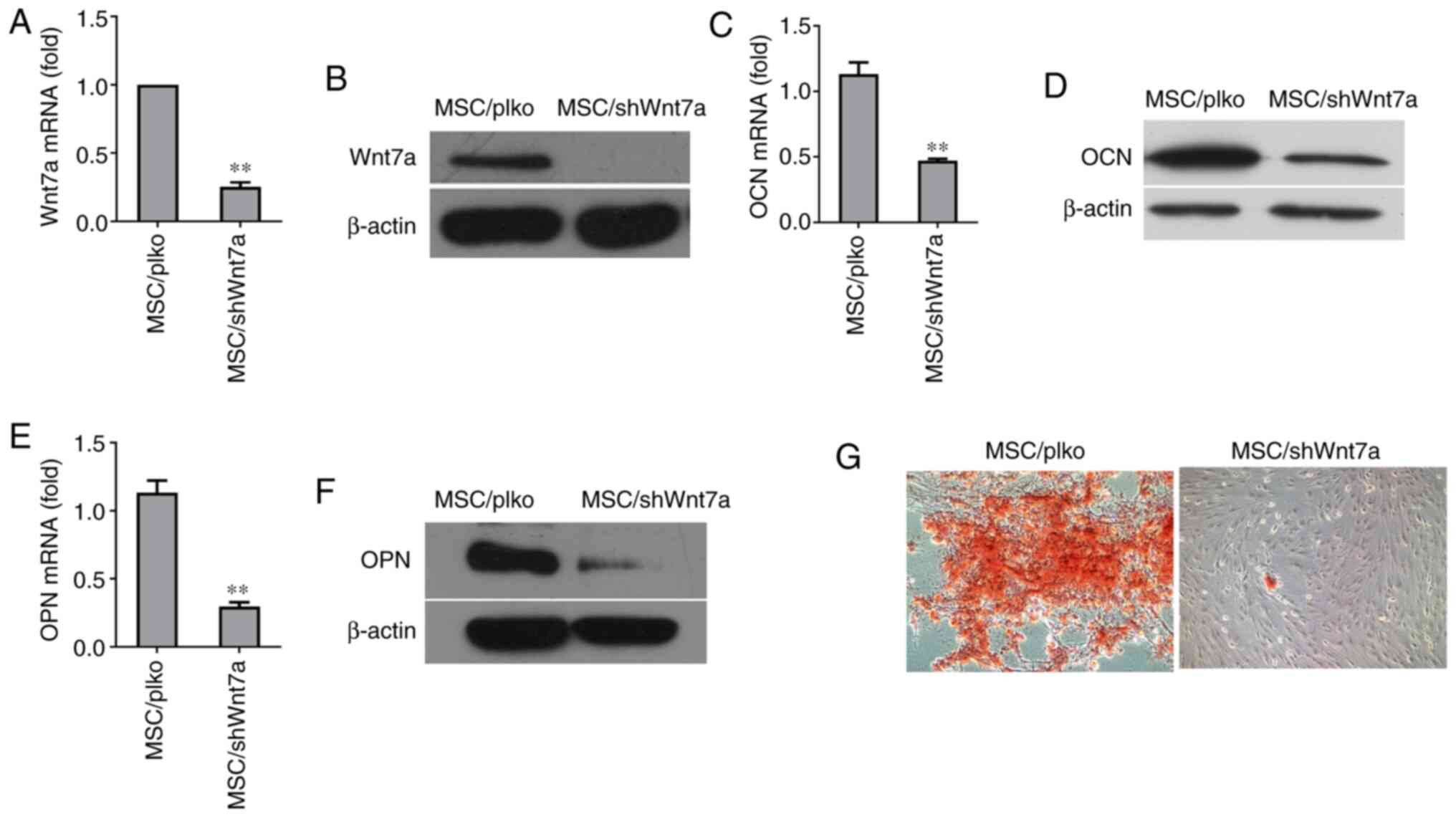
Knockdown of Wnt7a reduces the osteogenic
differentiation of MSCs
To directly determine whether Wnt7a plays a critical
role in the osteogenic differentiation of MSCs, lentivirus was used
to stably silence its expression in MSCs. The knockdown of Wnt7a in
MSCs was confirmed by RT-qPCR (Fig.
4A). Consistently, western blot analysis revealed that
lentivirus expressing Wnt7a shRNA reduced Wnt7a expression at the
protein level (Fig. 4B).
Following osteogenic induction for 6 days, RT-qPCR revealed that
the knockdown of Wnt7a significantly reduced the expression of OCN,
an early marker of osteogenic differentiation (Fig. 4C). Consistently, western blot
analysis revealed that knockdown of Wnt7a reduced the expression of
OCN at the protein level (Fig.
4D). Similarly, the knockdown of Wnt7a reduced the mRNA and
protein expression of OPN, another early marker of osteogenic
differentiation (Fig. 4E and F).
Furthermore, the results revealed that the knockdown of Wnt7a in
MSCs reduced the formation of mineralized nodules after two weeks
of culture in osteogenic-inducing medium (Fig. 4G). These findings implied that
the suppression of Wnt7a expression inhibited the osteogenic
differentiation of MSCs in vitro.
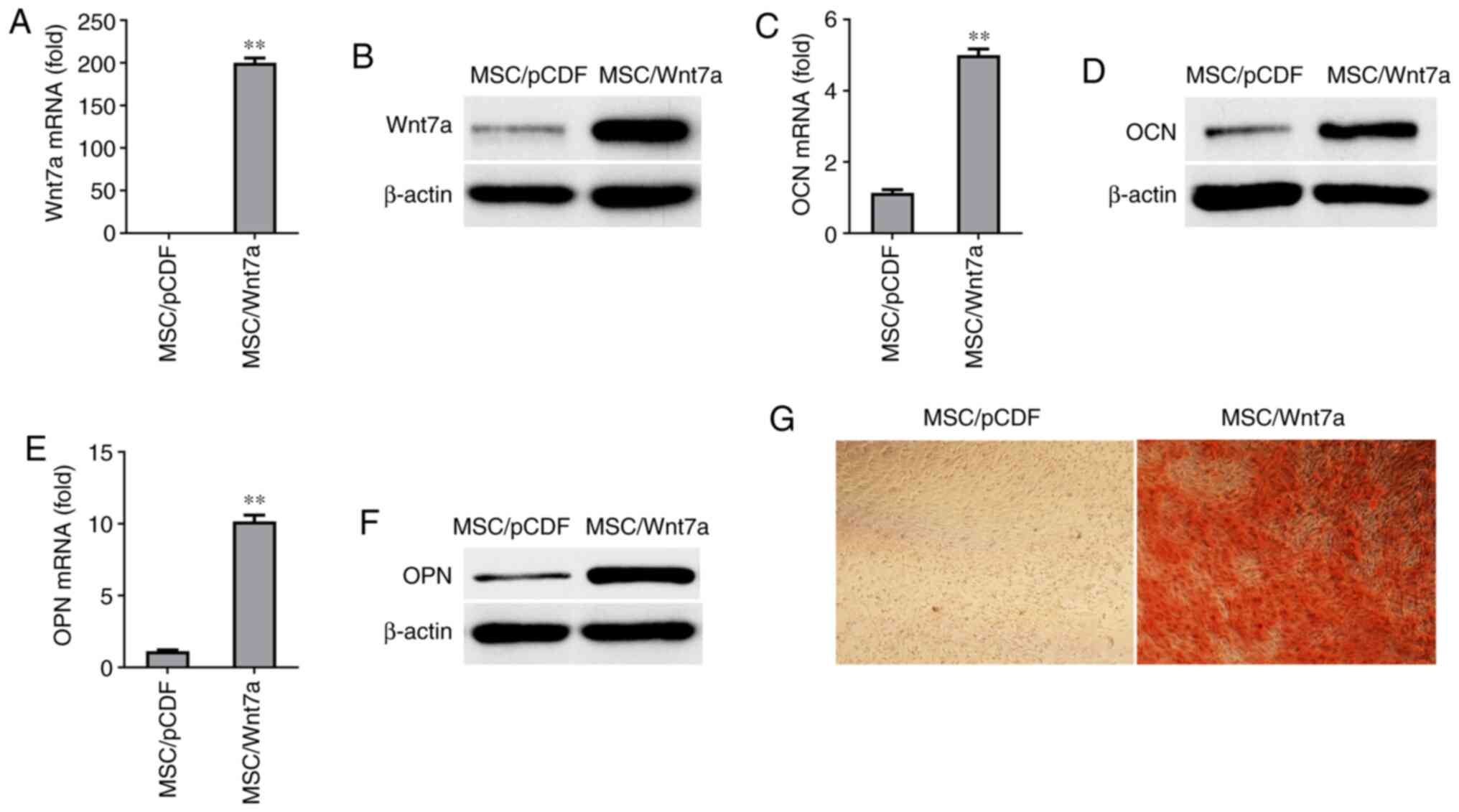
Overexpression of Wnt7a promotes
osteogenic differentiation
To further examine whether Wnt7a plays a critical
role in the osteogenic differentiation of MSCs, Wnt7a was
overexpressed in MSCs using lentivirus. The overexpression of Wnt7a
in MSCs was confirmed at the mRNA level and protein level by
RT-qPCR and western blot analysis, respectively (Fig. 5A and B). Following growth in
osteogenic-inducing medium for six days, the Wnt7a-overexpressing
MSCs exhibited a enhanced expression of OCN and ONN at the mRNA
level and protein level (Fig.
5C-F). Moreover, it was found that the overexpression of Wnt7a
in MSCs increased mineralization in vitro for 9 days, based
on Alizarin Red S staining (Fig.
5G). These findings suggested that overexpression of Wnt7a
enhanced the osteogenic differentiation of MSCs in
vitro.
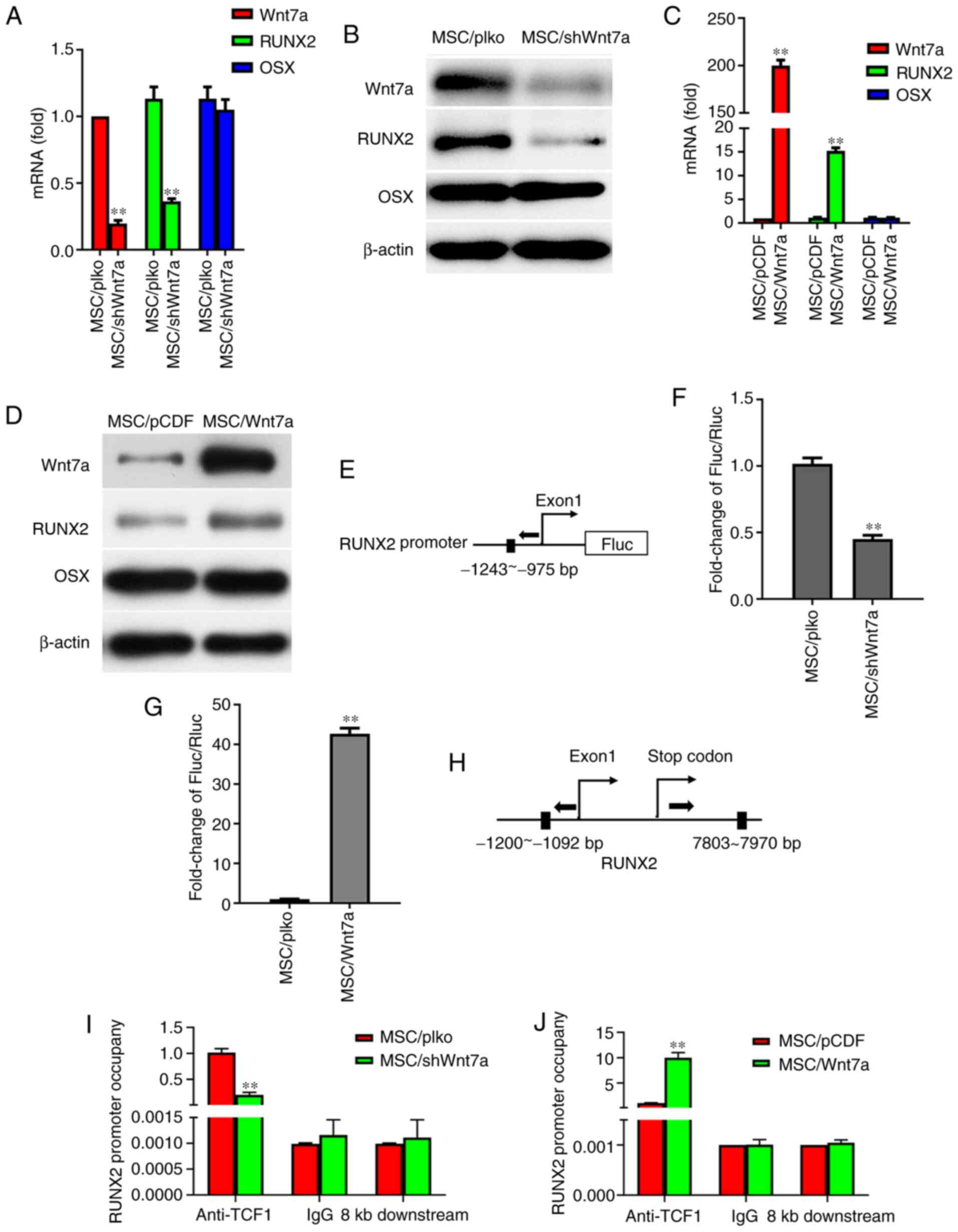
Wnt7a regulates osteogenic
differentiation through RUNX2
To further explore the mechanisms underlying the
regulation of osteogenic differentiation by Wnt7a, the present
study examined the effects of Wnt7a on the expression of RUNX2 and
OSX, which were previously confirmed to be the key regulators of
osteogenic differentiation (33-36). Notably, following the growth of
MSCs in osteogenic-inducing medium for six days, it was found that
the knockdown of Wnt7a decreased the expression of RUNX2 at the
mRNA level and protein level (Fig.
6A and B). However, RT-qPCR and western blot analysis revealed
that the knockdown of Wnt7a in MSCs did not affect OSX expression
at the mRNA level and protein level (Fig. 6A and B). The effects of Wnt7a
overexpression on the expression of RUNX2 and OSX in MSCs were
furthermore determined. Following the growth of MSCs in
osteogenic-inducing medium for six days, RT-qPCR and western blot
analysis revealed that the overexpression of Wnt7a in MSCs
increased RUNX2 expression at the mRNA level and protein level, but
did not affect OSX expression at the mRNA level and protein level
(Fig. 6C and D). To determine
whether Wnt7a regulates MSC differentiation through RUNX2, the
putative TCF1-binding site in the promoter of the RUNX2 gene was
identified and a reporter construct was generated. Transient
transfection assays demonstrated that the knockdown of Wnt7a in
MSCs decreased RUNX2 promoter activity and that Wnt7a
overexpression increased RUNX2 promoter activity (Fig. 6E-G). CHIP assays were further
performed to assess the binding of TCF1-binding site to the
promoter of RUNX2. Indeed, RT-qPCR revealed that the knockdown of
Wnt7a in MSCs reduced TCF-1 binding to the RUNX2 regulatory region
and that the overexpression of Wnt7a increased TCF1 binding to the
RUNX2 regulatory region, indicating that Wnt7a regulates RUNX2
expression by TCF1 binding to the RUNX2 promoter (Fig. 6H-J). These findings suggest that
Wnt7a regulates osteogenic differentiation through the key
osteogenic regulator RUNX2.
Discussion
MSCs acquire multiple differentiation potential,
including the capacity to differentiate into osteocytes or
adipocytes (1,37-39). The associated regulators include
the Wnt/β-catenin, transforming growth factor (TGF)β/BMP,
fibroblast growth factor (FGF), Notch and Hedgehog signaling
pathways, and RUNX2, OSX, activating transcription factor 4 (ATF4),
Tafazzin (TAZ) and nuclear factor of activated T-cells (NFATc)1
transcriptional factors have been identified as being involved in
MSC differentiation into osteocytes (40-43). Although there has been
significant progress made in understanding the molecular framework
in which MSCs differentiate into osteocytes, the underlying
mechanisms controlling this process remain unclear. In the present
study, it was demonstrated that Wnt7a played a critical role in MSC
commitment to bone formation. Wnt7a was found to be upregulated
during the osteogenic differentiation of MSCs but downregulated
following MSC adipogenic differentiation. It was further confirmed
that the knockdown of Wnt7a in MSCs suppressed the expression of
osteocyte molecular markers and inhibited osteogenesis in
vitro. Conversely, the overexpression of Wnt7a in MSCs promoted
osteocytes markers expression and osteogenesis in vitro. The
finding that Wnt7a regulates bone formation provides the
opportunity to understand the pathogenic causes of bone-related
diseases and to develop target therapies for these diseases.
Increasing evidence has demonstrated that Wnt
signaling plays an important role in regulating MSC osteogenic
differentiation. This concept is supported by the fact that Wnt1,
Wnt3a and Wnt10b stimulate bone formation by activating β-catenin,
while Dkk1, which suppresses the Wnt pathway, reduces osteocyte
differentiation. The demonstration that Msx2 increased osteogenic
differentiation and that the depletion of Msx2 reduced Wnt7a mRNA
levels implied that Wnt7a may be implicated in the regulation of
osteogenic differentiation (25). The results confirmed that the
downregulation of Wnt7a expression decreased bone formation in
vitro and that the enforced expression of Wnt7a promoted
osteogenesis in vitro, suggesting that Wnt7a plays a key
regulator role in osteocyte development. RUNX2 and OSX are
essential regulators of osteogenic differentiation. Nakashima et
al (44) demonstrated that
OSX acted genetically downstream of RUNX2 in mesenchymal
differentiation into osteocytes. Artigas et al (45) found that p53 inhibited OSX
expression in osteoblast differentiation, but did not affect RUNX2
expression. The present study demonstrated Wnt7a regulated RUNX2
expression but did not affect the OSX level following osteogenic
differentiation. Upon Wnt interaction with the receptors, FZD and
LRP5/6, β-catenin accumulates in the nucleus, which releases
histone deacetylases (HDACs) from the TCF and recruits histone
acetylase CBP/p300 to activate downstream gene expression (46,47). Previous research has indicated
that Wnt signaling promotes osteogenic differentiation and
bone-related gene expression through TCF binding to the consensus
motif A/TA/TCAAAG of the promoters of these genes (32,48). The present study also found that
Wnt7a enhanced TCF-1 binding to the consensus motif TTCAAAG of the
RUNX2 promoter. These results support the findings that Wnt7a
promotes MSC differentiation into osteocytes possibly by regulating
RUNX2 expression.
In conclusion, the findings of the present study
demonstrated the role of Wnt7a in the differentiation of MSCs into
osteocytes in vitro. Since Wnt7a is secreted, Wnt7a-specific
inhibitors can terminate osteogenic differentiation. Further
investigations are required to explore Wnt7a inhibitors in
vivo and in in vitro. The data presented herein provide
an opportunity to explore the role of Wnt7a in pathogenic
mechanisms of bone-related cancer and therapeutic intervention for
bone damage repair.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, PH and XD conceived and designed the
experiments. LY, QL, JZ, PL, CW, PA, CW and XZ performed the
experiments. XD analyzed the data. LZ, PH and XD wrote the
manuscript. LY, XD and LZ confirm the authenticity of all the raw
data. All authors reviewed the paper and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments and protocols were approved by the
Ethics Committee of Guizhou Medical University. Each patient
provided written informed consent prior to the preparation of the
bone tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by funding from the National
Natural Science Foundation of China (no. 81860516), the start-up
grant from the Affiliated Hospital of Guizhou Medical University
(no. I201802), Guizhou science and Technology Department (no.
2016J7234), Guizhou science and Technology Department (no.
2014LH7149), the National Natural Science Foundation of China (no.
81460448), the R&D infrastructure and facility Development and
Program of Guizhou (no. 20154005).
References
|
1
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin RZ, Moreno-Luna R, Li D, Jaminet SC,
Greene AK and Melero-Martin JM: Human endothelial colony-forming
cells serve as trophic mediators for mesenchymal stem cell
engraftment via paracrine signaling. Proc Natl Acad Sci USA.
111:10137–10142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caplan AI and Bruder SP: Mesenchymal stem
cells: Building blocks for molecular medicine in the 21st century.
Trends Mol Med. 7:259–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang N, Wang G, Hu C, Shi Y, Liao L, Shi
S, Cai Y, Cheng S, Wang X, Liu Y, et al: Tumor necrosis factor α
suppresses the mesenchymal stem cell osteogenesis promoter miR-21
in estrogen deficiency–induced osteoporosis. J Bone Miner Res.
28:559–573. 2013. View Article : Google Scholar
|
|
6
|
Alford AI, Kozloff KM and Hankenson KD:
Extracellular matrix networks in bone remodeling. Int J Biochem
Cell Biol. 65:20–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beck GR Jr, Khazai NB, Bouloux GF,
Camalier CE, Lin Y, Garneys LM, Siqueira J, Peng L, Pasquel F,
Umpierrez D, et al: The effects of thiazolidinediones on human bone
marrow stromal cell differentiation in vitro and in
thiazolidinedione-treated patients with type 2 diabetes. Transl
Res. 161:145–155. 2013. View Article : Google Scholar
|
|
8
|
Tang Y, Xie H, Chen J, Geng L, Chen H, Li
X, Hou Y, Lu L, Shi S, Zeng X and Sun L: Activated NF-κB in bone
marrow mesenchymal stem cells from systemic lupus erythematosus
patients inhibits osteogenic differentiation through downregulating
Smad signaling. Stem Cells Dev. 22:668–678. 2012. View Article : Google Scholar
|
|
9
|
D'Amelio P, Tamone C, Sassi F, D'Amico L,
Roato I, Patanè S, Ravazzoli M, Veneziano L, Ferracini R,
Pescarmona GP and Isaia GC: Teriparatide increases the maturation
of circulating osteoblast precursors. Osteoporos Int. 23:1245–1253.
2012. View Article : Google Scholar
|
|
10
|
Zhang S, Chen X, Hu Y, Wu J, Cao Q, Chen S
and Gao Y: All-trans retinoic acid modulates Wnt3A-induced
osteogenic differentiation of mesenchymal stem cells via activating
the PI3K/AKT/GSK3β signalling pathway. Mol Cell Endocrinol.
422:243–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noronha-Matos JB and Correia-de-Sá P:
Mesenchymal stem cells ageing: Targeting the 'Purinome' to promote
osteogenic differentiation and bone repair. J Cell Physiol.
231:1852–1861. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Xia X and Li B: Mesenchymal stem
cell aging: Mechanisms and influences on skeletal and non-skeletal
tissues. Exp Biol Med (Maywood). 240:1099–1106. 2015. View Article : Google Scholar
|
|
13
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar
|
|
14
|
Zhou L and Liu Y: Wnt/beta-catenin
signalling and podocyte dysfunction in proteinuric kidney disease.
Nat Rev Nephrol. 11:535–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hynes NE, Ingham PW, Lim WA, Marshall CJ,
Massagué J and Pawson T: Signalling change: Signal transduction
through the decades. Nat Rev Mol Cell Biol. 14:393–398. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Palomo T, Al-Jallad H, Moffatt P, Glorieux
FH, Lentle B, Roschger P, Klaushofer K and Rauch F: Skeletal
characteristics associated with homozygous and heterozygous WNT1
mutations. Bone. 67:63–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laine CM, Joeng KS, Campeau PM, Kiviranta
R, Tarkkonen K, Grover M, Lu JT, Pekkinen M, Wessman M, Heino TJ,
et al: WNT1 mutations in early-onset osteoporosis and osteogenesis
imperfecta. N Engl J Med. 368:1809–1816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rauch F and Glorieux FH: Osteogenesis
imperfecta. Lancet. 363:1377–1385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keupp K, Beleggia F, Kayserili H, Barnes
AM, Steiner M, Semler O, Fischer B, Yigit G, Janda CY, Becker J, et
al: Mutations in WNT1 cause different forms of bone fragility. Am J
Hum Genet. 92:565–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Keller KC, Ding H, Tieu R, Sparks NR,
Ehnes DD and Zur Nieden NI: Wnt5a supports osteogenic lineage
decisions in embryonic stem cells. Stem Cells Dev. 25:1020–1032.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Tu X, Esen E, Joeng KS, Lin C,
Arbeit JM, Rüegg MA, Hall MN, Ma L and Long F: WNT7B promotes bone
formation in part through mTORC1. PLoS Genet. 10:e10041452014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan SH, Senarath-Yapa K, Chung MT,
Longaker MT, Wu JY and Nusse R: Wnts produced by Osterix-expressing
osteolineage cells regulate their proliferation and
differentiation. Proc Natl Acad Sci USA. 111:E5262–E5271. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati
N, Martinez-Santibañez G and MacDougald OA: Wnt6, Wnt10a and Wnt10b
inhibit adipogenesis and stimulate osteoblastogenesis through a
β-catenin-dependent mechanism. Bone. 50:477–489. 2012. View Article : Google Scholar
|
|
25
|
Cheng SL, Shao JS, Cai J, Sierra OL and
Towler DA: Msx2 exerts bone anabolism via canonical Wnt signaling.
J Biol Chem. 283:20505–20522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K,
Zhou X, Park NH and Wang CY: Histone demethylases KDM4B and KDM6B
promotes osteogenic differentiation of human MSCs. Cell Stem Cell.
11:50–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Najdi R, Proffitt K, Sprowl S, Kaur S, Yu
J, Covey TM, Virshup DM and Waterman ML: A uniform human Wnt
expression library reveals a shared secretory pathway and unique
signaling activities. Differentiation. 84:203–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao Y, Sun Z, Liao L, Meng Y, Han Q and
Zhao RC: Human adipose tissue-derived stem cells differentiate into
endothelial cells in vitro and improve postnatal neovascularization
in vivo. Biochem Biophys Res Commun. 332:370–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Dou XW, Liang YK, Lin HY, Wei XL, Zhang
YQ, Bai JW, Chen CF, Chen M, Du CW, Li YC, et al: Notch3 maintains
luminal phenotype and suppresses tumorigenesis and metastasis of
breast cancer via trans-activating estrogen receptor-α.
Theranostics. 7:4041–4056. 2017. View Article : Google Scholar :
|
|
31
|
Oosterwegel MA, van de Wetering ML,
Holstege FC, Prosser HM, Owen MJ and Clevers HC: TCF-1, a T
cell-specific transcription factor of the HMG box family, interacts
with sequence motifs in the TCRβ and TCRδ enhancers. Int Immunol.
3:1189–1192. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Beest M, Dooijes D, van De Wetering M,
Kjaerulff S, Bonvin A, Nielsen O and Clevers H: Sequence-specific
high mobility group box factors recognize 10-12-base pair minor
groove motifs. J Biol Chem. 275:27266–27273. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaur T, Lengner CJ, Hovhannisyan H, Bhat
RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS
and Lian JB: Canonical WNT signaling promotes osteogenesis by
directly stimulating Runx2 gene expression. J Biol Chem.
280:33132–33140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh
CF, Kuo ML and Yen ML: Resveratrol promotes osteogenesis of human
mesenchymal stem cells by upregulating RUNX2 gene expression via
the SIRT1/FOXO3A axis. J Bone Miner Res. 26:2552–2563. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baglìo SR, Devescovi V, Granchi D and
Baldini N: MicroRNA expression profiling of human bone marrow
mesenchymal stem cells during osteogenic differentiation reveals
Osterix regulation by miR-31. Gene. 527:321–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li E, Zhang J, Yuan T and Ma B: MiR-143
suppresses osteogenic differentiation by targeting Osterix. Mol
Cell Biochem. 390:69–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
AlAmer N, Bondalapati A, Garcia-Godoy F
and Kandalam U: Osteogenic differentiation of orofacial
tissue-derived mesenchymal stem cells-A review. Curr Tissue Eng.
5:11–20. 2016. View Article : Google Scholar
|
|
38
|
Atashi F, Modarressi A and Pepper MS: The
role of reactive oxygen species in mesenchymal stem cell adipogenic
and osteogenic differentiation: A review. Stem Cells Dev.
24:1150–1163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fakhry M, Hamade E, Badran B, Buchet R and
Magne D: Molecular mechanisms of mesenchymal stem cell
differentiation towards osteoblasts. World J Stem Cells. 5:136–148.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deng ZL, Sharff KA, Tang N, Song WX, Luo
J, Luo X, Chen J, Bennett E, Reid R, Manning D, et al: Regulation
of osteogenic differentiation during skeletal development. Front
Biosci. 13:2001–2021. 2008. View
Article : Google Scholar
|
|
41
|
Harada SI and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Komori T: Regulation of bone development
and maintenance by Runx2. Front Biosci. 13:898–903. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
James AW: Review of signaling pathways
governing MSC osteogenic and adipogenic differentiation.
Scientifica (Cairo). 2013:6847362013.
|
|
44
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Artigas N, Gámez B, Cubillos-Rojas M,
Sánchez-de Diego C, Valer JA, Pons G, Rosa JL and Ventura F: p53
inhibits SP7/Osterix activity in the transcriptional program of
osteoblast differentiation. Cell Death Differ. 24:2022–2031. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leucht P and Helms JA: Wnt signaling: An
emerging target for bone regeneration. J Am Acad Orthop Surg.
23:67–68. 2015. View Article : Google Scholar
|
|
47
|
Yin X, Li J, Salmon B, Huang L, Lim WH,
Liu B, Hunter DJ, Ransom RC, Singh G, Gillette M, et al: Wnt
signaling and its contribution to craniofacial tissue homeostasis.
J Dental Res. 94:1487–1494. 2015. View Article : Google Scholar
|
|
48
|
van de Wetering M, Oosterwegel M, Dooijes
D and Clevers H: Identification and cloning of TCF-1, a T
lymphocyte-specific transcription factor containing a
sequence-specific HMG box. EMBO J. 10:123–132. 1991. View Article : Google Scholar : PubMed/NCBI
|