The incidence of oesophageal cancer has rapidly
increased over the past years and it is currently the fifth most
common type of cancer worldwide with a very high mortality rate
(1,2). Oesophageal cancer is subdivided
into two groups according to its histological appearance:
Oesophageal squamous cell carcinoma (predominant in western
countries) and oesophageal adenocarcinoma (most common form in
Asia) (3,4). Thus far, no molecular markers for
prognosis or treatment efficacy have been discovered for squamous
cell carcinoma. For oesophageal adenocarcinoma, the human epidermal
growth factor receptor-2 (HER-2) status has been proven to be an
efficient biomarker. HER-2 is scored by immunohistochemistry for
protein expression or fluorescent in situ hybridization for
HER2 gene amplification (5). If
positive, a targeted therapy option with trastuzumab for HER-2 is
the treatment of choice (6,7).
Nevertheless, oesophageal cancer is mostly treated by radiation in
combination with chemotherapy or surgery. However, the 5-year
overall survival rate remains very poor and is only at 5-10%
(8,9). Nonetheless, surgery is not
applicable in approximately half of patients as distant metastases
are already present at the time of diagnosis (10). The most commonly used
chemotherapeutic agents for oesophageal cancer treatment are
5′-fluorouracil and platinum agents in combination with
radiotherapy (11,12).
The major risk factors for oesophageal cancer are
represented by smoking, the consumption of hot tea, red meat
consumption, poor oral health, low intake of fresh fruits and
vegetables, alcohol abuse, obesity, nass use (a chewing tobacco
product), opium consumption and low socioeconomic status (13-24). The majority of these risk factors
induce gene mutations which can be recognized by the immune system
(25). In addition, a minority
of oesophageal cancers belong to the spectrum of Lynch
syndrome-associated cancers and are characterized by microsatellite
instability (MSI) (26).
Therefore, the use of immunotherapy approaches in oesophageal
cancer appears to be an attractive novel therapeutic strategy.
The identified main reasons for the high mortality
rate of patients with oesophageal cancer are mainly the late stage
of diagnosis (13) and the key
role of tumour microenvironment in this type of cancer (27), where the surrounding stromal
cells seem to exert an important influence in supporting tumour
cell survival (27). Apart from
cancer-associated fibroblasts, that are able to support tumour
growth and metastasis by altering the extracellular matrix by
secreting growth factors and cytokines, several immune cells [e.g.,
myeloid-derived suppressor cells, tumour-associated macrophages and
regulatory T-cells (TREGS)] are involved in support the development
of oesophageal cancer (27).
Therapies targeting the tumour microenvironment and/or the immune
system may thus be able to increase the survival of patients with
oesophageal cancer. Over the past years, immunotherapy in
particular has revolutionized the management and outcome of several
types of cancer, such as melanoma, lung, gastric and kidney cancer
(28). Therefore, it may be
advantageous to explore the benefits from immunotherapy for
oesophageal cancer. The identification and selection of robust
biomarkers predicting clinical benefit are also mandatory before
commencing immunotherapy treatment, as even though generally
well-tolerated compared to standard therapies, immunotherapy is
associated occasionally with severe toxic side-effects, such as
cutaneous, gastrointestinal, endocrine and hepatic toxicity. Thus,
only patients with oesophageal cancer who have the highest
likelihood of benefit from immunotherapy should be offered this
therapeutic regimen. For example, it is well-established that
immune checkpoint inhibitors are particularly effective against
mismatch repair-deficient tumours (29). In general, tumours with MSI have
a higher mutation rate, which increases the probability for the
immune system to recognize tumour cells (29-31). Recently, several reviews have
summarized the current knowledge on immunotherapy and cancer
(32-34). The present review focuses on the
current state of the use of immunotherapy in oesophageal
cancer.
The immune system is a highly complex and
specialised biological network including specific cells, protein
and organs and is usually composed of two types: Adaptive
(specific) and innate (non-specific) (35). In recognising and preventing the
spread of cancer cells, the innate immunity components, such as
natural killer (NK) cells, dendritic cells and macrophages are of
pivotal importance; nevertheless, T-cells from the adaptive immune
are recruited in order to track and kill tumour cells (35,36). Recently, a new model that
provides a mechanistic explanation of this interaction termed
'cancer-immunity cycle' has been suggested (35). According to this model, dead
cancer cells release antigens that in turn are recognised by
antigen-presenting cells (particularly by dendritic cells). This
results in the priming and activation of dendritic cells and
T-cells in lymph nodes, followed by the recruitment of helper
T-cells [cluster of differentiation (CD)4+-T-cells] and
cytotoxic T-lymphocytes (CD8+-T-cells) at the tumour
site. Following the infiltration of the tumour microenvironment,
immune cells recognize and attack tumour cells that results in the
release of further tumour antigens. The whole cancer-immunity cycle
is fine-tuned by different stimulating and inhibitory factors, such
as chemokines, cytokines, metabolic compounds, surface proteins and
immune checkpoint receptors to prevent autoimmunity (37).
Cancer cells use different strategies to escape the
immune system, and to capture and reprogram immune cells, leading
to immune evasion. Among these strategies is the mechanisms of
shedding of MHC class I chain-related protein A and B (MICA and
MICB) from tumour cells into the tumour microenvironment as
protection against NK cell-mediated killing (38-40). In addition, tumour cells express
immune checkpoint proteins, such as programmed cell death 1 ligand
1 (PD-L1) and receptors, such as cytotoxic T lymphocyte-associated
antigen-4 (CTLA-4) on the surface, but also secrete exosomes which
contains these immune checkpoint regulators. After binding to
proteins expressed on the immune cells (T-cells, B-cells and
myeloid cells) the checkpoint regulators exert an inhibitory signal
and lead to the suppression of the immune response (41-43).
Furthermore, cancer cells, as well as
tumour-associated macrophages are able to secrete chemokines, such
as chemokine (C-C motif) ligand (CCL)-17 and CCl-22 which attract a
subpopulation of T-cells, the so-called TREGs. TREGs are known to
regulate and suppress the activity of other immune cells and to
help preventing autoimmune reactions under healthy conditions
(44,45). In tumour tissue, TREGs protect
cancer cells and foster tumour growth (46,47). Moreover, CD8+-T-cells
are inhibited directly by myeloid-derived suppressor cells (MDSCs)
which are stimulated by tumour-derived growth factors (48,49). In addition, stromal cells in the
tumour microenvironment inhibit the function of the immune system
further supporting tumour progression and metastasis (50).
The immune system is a complex network of
interacting cells and biochemical signals that orchestrate the
recognition and attack of external antigens, whilst preventing
autoimmune reactions. Under physiological conditions, this is
guaranteed by a fine-tuned interplay between immune cells and a
balance between stimulatory and inhibitory signals (51,52). Cancer cells often find a way to
de-regulate the balanced immune system by manipulating signalling
pathways to evade from immune surveillance. To overcome the
mechanisms of tumour immune evasion and use the immune system as
weapon against cancer, either agonists of stimulatory receptors or
antagonists of inhibitory signals can be used (41). Nevertheless, according to
currently available study results, only a subset of oesophageal
cancer patients may benefit from immunotherapy (53). Therefore, there is an urgent need
to identify biomarkers for the prediction of the benefit from
immunotherapy, so that patients can be selected for treatment and
those who have no benefit from immunotherapy are spared from
side-effects (e.g., cutaneous, gastrointestinal, endocrine and
hepatic toxicity) and therapy failure. In light of this scenario,
currently, several clinical trials are underway to evaluate the
efficacy of different immunotherapies combined with other treatment
options in oesophageal cancer patients (Table I) with the aim to increase the
therapeutic option for oesophageal cancer patients. The majority of
these studies are ongoing Phase 2 studies and the results have not
been published yet.
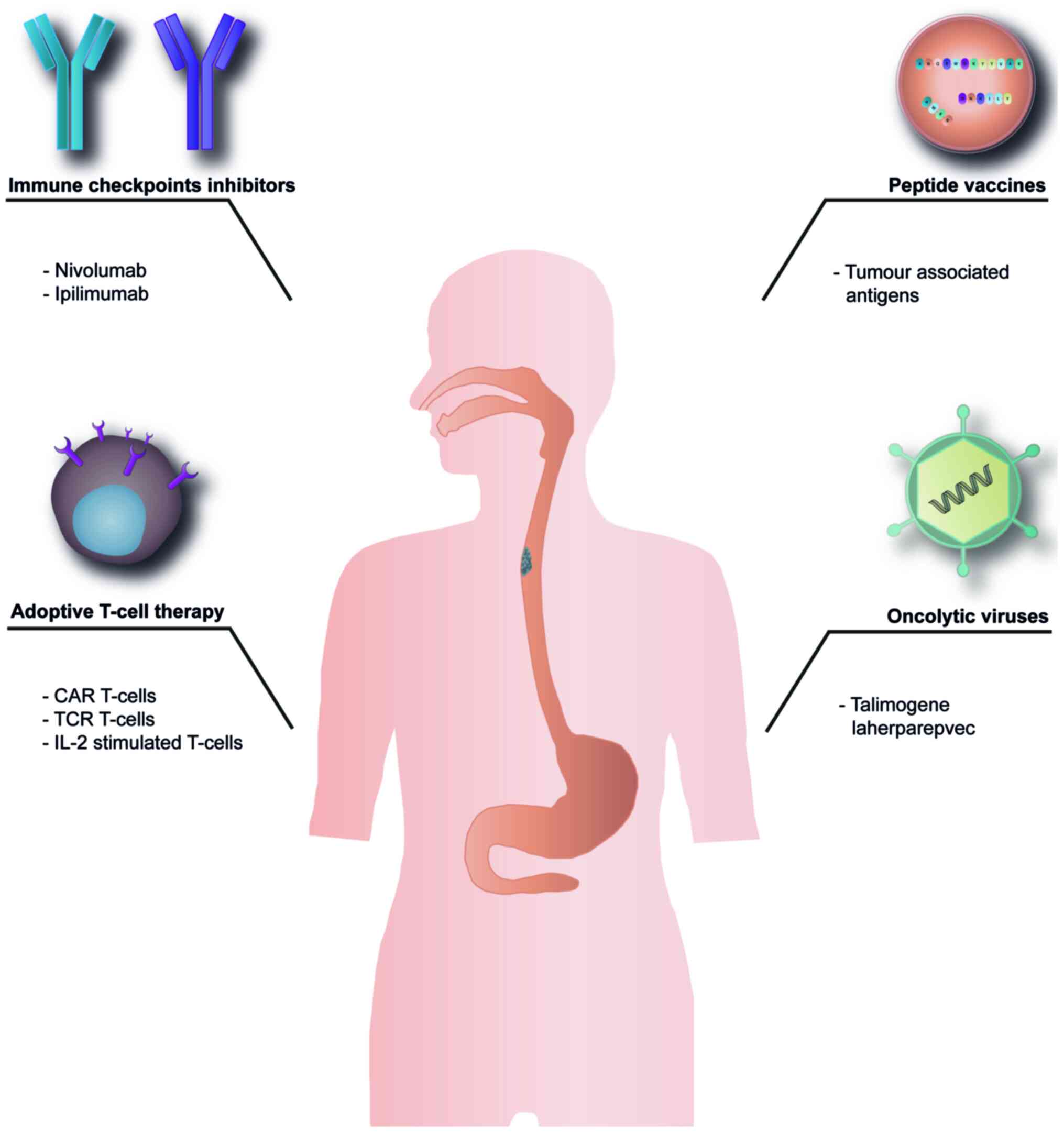
In the following section, the main immunotherapy
approaches that have been studied thus far will be discussed
(Fig. 1).
Immune checkpoints are of pivotal importance to
prevent autoimmunity reactions by the inhibition of antigen
recognition via T-cell receptors (TCRs) (41,54,55). Cancer cells use immune checkpoint
proteins to inactivate the adaptive immune system by blocking
tumour specific T-cells and escape from immune surveillance. Thus
far, the immune checkpoint receptors programmed cell death protein
1 (PD-1; also known as CD279) and CTLA-4 (also known as CD152) have
been found to be associated with the inhibition and downregulation
of T-cell activity (41,54,55).
PD-1 receptor is highly expressed on T-cells,
B-cells and NK cells. The ligand for PD-1 receptor is PD-L1 often
also termed B7-homolog 1 (B7-H1) or CD274. This molecule is
expressed in peripheral tissues following exposure to inflammatory
cytokines and limits T-cell activity (56). Furthermore, interleukin (IL)-18,
an inflammatory cytokine that accumulates in the tumour
microenvironment, results in the upregulation of PD-L1 in activated
mature NK cells and triggers immunosuppression (57). In melanoma, lung, breast,
pancreatic, gastric, colon, ovarian and oesophageal cancers, PD-L1
is often found overexpressed on cancer cells (58). This enables tumour cells to
interact with PD-1 receptors on T-cells and this interaction
prevents T-cell activation, proliferation and ultimately leading to
T-cell apoptosis (41).
The expression of CTLA-4 receptor is restricted to
activated T-cells (e.g., TREGs), whereas the homolog CD28 is also
expressed on non-activated T-cells. Ligands for both receptors are
the immunoglobulin proteins B7-1 (CD80) and B7-2 (CD86), which are
expressed early during the immune response on antigen-presenting
cells, such as macrophages and dendritic cells or on B-cells and
monocytes, respectively. CTLA-4 receptor has a higher affinity for
ligands and competing with CD28 on ligand binding; the interaction
between B7-1 or B7-2 with CD28 results in T-cell activation,
whereas the interaction with CTLA-4 inhibits T-cell activation at
an early stage (59,60).
It has been widely proven that PD-L1 expression is
one of the key mechanisms through which several cancers evade the
immune response; thus, it is not surprising that inhibitors of
PD-L1 and PD-1 have been identified thus far as one of most
efficient and broadly used immunotherapies for cancer (61-71). Recently, a monoclonal antibody
targeting PD-1, pembrolizumab, has been approved for the treatment
of oesophageal and oesophagogastric junction adenocarcinoma by the
US Food and Drug Administration (FDA) (8). The prerequisite for the treatment
of oesophageal cancer with pembrolizumab is either a proven PD-L1
expression on the cancer cells and a high MSI, or a proven
defective mismatch repair system. Therefore, most probably, the
subgroup of Lynch syndrome-associated oesophageal cancers patients
may benefit from this new treatment option. According to a previous
study, it is possible to predict the efficacy of pembrolizumab in
patients with oesophageal cancer by using a six-gene interferon-γ
gene expression signature (72).
This offers the possibility to stratify oesophageal cancer patients
and limit the targeted treatment to the group that will most
probably benefit from the anti-PD-L1 therapy.
Earlier in 2020, the FDA approved nivolumab, a fully
human monoclonal antibody against PD-1 (73) for patients with unresectable
advanced, recurrent or metastatic oesophageal squamous cell
carcinoma as a second line following 5′-fluorouracil- and
platinum-based chemotherapy. The overall survival benefit is 2.5
months according to a phase 3 clinical study (74).
Currently, combination therapies with anti-PD1 and
anti-CTLA-4 antibodies are forthcoming (75). According to the first preliminary
results from clinical studies (NCT02743494, CheckMate 648 and
CheckMate 649) the combination of nivolumab with the anti CDLA-4
antibody, ipilimumab, led to an improved clinical response in
oesophageal cancer compared to treatment with nivolumab alone
(76,77). The combination of nivolumab and
ipilimumab appears to be safe; nevertheless, it must be considered
that CTLA-4 blockade results in more severe and more common
side-effects than it is the case for targeting PD-1/PD-L1 alone.
Therefore, the development of novel strategies for reducing serious
adverse side-effects is an urgent need and the first steps need to
be carefully controlled (78).
As a potential biomarker for prediction of the
response to immune checkpoint inhibitor therapy, the total amount
of PD-1+ CD4+ T-cells in the tumour
microenvironment is discussed. According to the presence or absence
of CD4+ T-cells and PD-1 expression in the tumour
microenvironment, a stratification of patients is possible. The
absence of CD4+ T-cells and PD-1 expression results in
immunological ignorance; in a situation where only one component
(either CD4+ T-cells or PD-1) is expressed,
immunological tolerance exists and only in the case of a
PD-1+ tumour microenvironment containing CD4+
T-cells an adoptive immune resistance is present that is most
likely to respond to immune checkpoint inhibitor therapy (79).
Adoptive T-cell therapy is a personalized approach
of immunotherapy. T-cells are collected from the tumour or
peripheral blood of a patient and the isolated T-cells are
stimulated in vitro with IL-2. After this ex-vivo
expansion, the cancer patient receives his own autologous immune
cells as an infusion (80). In
addition, T-cells can be also genetically modified after collection
from the patient either by introducing chimeric antigen receptor
(CAR T-cells) or transducing antigen-specific TCR cells (TCR
T-cells). In all cases, the expanded or modified T-cells exert an
improved tumour-specific immunity (81-83). In several trials, a regression of
tumours has been demonstrated following persistent adoptive T-cell
therapy (84,85). In a first clinical trial based on
adoptive T-cell therapy for patients with recurrent or advanced
oesophageal cancer, the patients received (on a fortnight basis)
activated T-cells administered into primary tumours or metastatic
lymph nodes; this therapy was found to be safe and in one third of
the patients, a significant tumour regression was observed
(86). In another study, based
on TCR T-cells, oesophageal cancer patients with minimal tumours
survived >27 months; nevertheless, after 2 months of treatment,
several patients exhibited tumour progression even if the
autologous T-cells persist for a long period of time; therefore,
TCR T-cell therapy appears to have a benefit only for oesophageal
cancer patients with minimal lesions (87).
Peptide vaccines are therapeutic cancer vaccines
which aim to increase immunogenic cancer-specific antigens, leading
to the activation of cancer antigen-specific T-cells in vivo
(59,76,88). For the successful use of peptide
vaccines, the characterization of tumour-specific T-cells and the
use of immunogenic tumour-associated antigens are a prerequisite
(89). As tumour-associated
antigens, either recombinant short peptides, whole-cell tumour
lysates or full-length proteins can be used (90,91). The length of the used peptide has
at least in part an influence on the efficiency of the immune
response (92). It has been
well-established that short peptides composed of 8-11 amino acids
induce major histocompatibility complex (MHC) class-I-restricted
antigen-specific CD8+ T-cell reaction via direct binding
to human leukocyte antigen (HLA)-I molecules (93). By contrast, longer peptides
(25-50 amino acids) are usually presented by MHC class-I and
class-II molecules on antigen-presenting cells to CD8+
or CD4+ T-cell, respectively (94). This results in a broader and
longer lasting immune response by generating cytotoxic
T-lymphocytes as well as long-living memory CD8+ T-cells
(95).
In a modified approach, dendritic cells isolated
from the peripheral blood of a cancer patient are presented to
tumour-associated antigens ex vivo and after loading with
the antigens the dendritic cells, are re-injected into patients
(91,96). This strategy was evaluated in a
pre-clinical study as possible novel treatment option for
oesophageal tumours (97).
Dendritic cells from oesophageal cancer patients have been pulsed
with Wilms' tumour 1 peptide ex vivo and used as a vaccine.
The patients were treated in parallel with the chemotherapeutic
agent, picibanil. In this exploratory study, 15 patients were
included; the median progression-free survival and overall survival
were 4.1 and 7.0 months, respectively. This treatment was
well-tolerated and no severe adverse events related to the
vaccinations were observed (97). Based on this promising result, a
phase II clinical trial is in preparation.
Even with the first-generation of peptide vaccines
which have been based on highly expressed non-mutant
tumour-associated antigens of tumour cells [such as melanoma
antigen gene (MAGE) and New York oesophageal squamous cell
carcinoma-1 (NY-ESO-1) proteins] an immune response was induced and
led to clinical positive effect (98-100). The advantage of these peptides
is that they are only expressed in male germ-line cells and
placenta under physiological conditions; however, a number of
tumours, among these oesophageal cancer, express these proteins as
well. Therefore, they represent very promising targets for cancer
immunotherapy (101-103).
The second-generation of peptide vaccines is an
effort for a more personalized medicine with the aim of targeting
mutated antigens that are patient-specific. In this approach,
mutations which have been accumulated during tumour development are
the basis for the vaccine generation (104). In the context of oesophageal
cancer, a large number of genetic mutations are present which
result in specific neo-antigens (105). The main challenge is to
identify mutated epitopes derived from tumour neo-antigens for
developing a patient-specific vaccine (106,107). The vaccine peptides are
patient-specific and they differ completely among patients.
Therefore, batch production will not be possible and it will never
become a conventional drug (104). The advantage is that
neo-antigen vaccines result in a potent T-cell response and induce
a new population of specific T-cells in cancer patients that are
able to kill cancer cells without damaging healthy tissues
(104,108,109). Furthermore, pre-clinical trials
are forthcoming with an aim to induce the T-cell response by
ribonucleic acid (RNA)-based vaccine coding for multiple
neo-epitopes (110). Another
novel strategy combines the use of long-peptide vaccines with
checkpoint inhibitor administration (111). The aim in both cases, is to
increase the repertoire of CD8+ and CD4+
T-cell directed against the tumour. These personalized approaches
have the potential to offer novel therapeutic options with high
specificity and low toxicity for cancer patients who are resistant
to current therapies.
Peptide vaccines have been used in several clinical
trials in patients with oesophageal squamous carcinoma. Different
peptides have been administered simultaneously to patients, which
resulted in a significant induced CD8+ T-cell response.
Clinical benefit, as well as an increased overall survival was
observed in the majority of patients (112,113). Peptide vaccinations can be
combined with other therapeutic options in patients with
oesophageal tumours. One example is the use of a peptide vaccine to
suppress the recurrence of oesophageal cancer following curative
resection. In a previous study, the 5-year relapse-free survival of
oesophageal cancer patients was 44.6% in patients that received the
vaccination compared to the ones that did not receive the
vaccination (31.6% relapse-free survival) (114). Of special interest is the
peptide vaccine, S-588410, which is composed of 5
HLA-A*2402-restricted epitope peptides derived from the
onco-antigens, DEPDC1, MPHOSPH1, URLC10, CDCA1 and KOC1. All these
antigens are up-regulated in the context of oesophageal cancer
(115,116). In previous studies, it was
proven that each of these 5 peptides has the capacity to induce a
peptide-specific activation of CD8+ T-cells in different
tumours, among these oesophageal cancer (112,113,117,118). In an exploratory study based on
15 patients with oesophageal tumours, an increased immune response
in tumour tissue was observed following vaccination with S-588410.
Following a median of 5 injections of S-588410, peptide-specific
CD8+ T-cells for all peptides included in this
vaccination were induced in all patients. The number of functional
T-lymphocytes (CD8+ and CD4+ T-cells) was
found to be increased in blood, as well as in tumour biopsies. In
parallel, a higher PD-L1 expression in the tumour microenvironment
was observed (115). Most
probably, the increased PD-L1 expression was related to interferon
(IFN)-γ produced by infiltrated CD8+ T-cells into the
tumour area. The accumulation of effective T-cells and IFN-γ
production in the tumour microenvironment most probably favour the
change from an immune 'desert' into an immune-inflamed tumour
microenvironment (93). It is
tempting to speculate about the therapeutic potential of combining
peptide vaccines, such as S-588410 with immune-checkpoint
inhibitors in patients with oesophageal cancer (54,79).
Oncolytic virus therapy is still in its infancy, but
it has already proven its potential. In general, oncolytic viruses
infect and replicate selectively in tumour cells and induce tumour
cell lysis (119,120). Talimogene laherparepvec is the
first FDA-approved oncolytic viral therapy for the treatment of
patients with advanced melanoma (121). Recently, the efficacy of a
telomerase-specific oncolytic virus (telomelysin OBP-301) in
combination with radiotherapy was investigated in a Phase I/II
study for the treatment of elderly patients with oesophageal
squamous cell carcinoma. According to the first results, this viral
therapy was well-tolerated and demonstrated efficient tumour
regression (122,123). Based on this success, several
other clinical trials with various oncolytic viruses for the
treatment of patients with oesophageal cancer are ongoing (Table I).
In oesophageal cancer, as in most other tumour
diseases, the therapeutic options are limited and therapeutic
success is only achieved for a short period of time before
resistance appears. Therefore, novel therapeutic options, such as
the addition of immunotherapy to the treatment of tumours are an
urgent need. Albeit some success of immunotherapy in oesophageal
cancer treatment and the approval of pembrolizumab and nivolumab by
the FDA, it is noteworthy to mention that immunotherapy is often
associated with severe toxic side-effects; the most frequent ones
are cutaneous, gastrointestinal, endocrine and hepatic toxicity.
Therefore, a careful monitoring and follow-up of patients under
immunotherapy is required and if necessary, the patient must
receive effective measures to manage the side-effects. An advantage
for patients with oesophageal cancer could be a combination of
immunotherapy with surgery, chemotherapy and radiotherapy.
Recently, the advantage from radiotherapy in parallel with immune
checkpoint inhibitor treatment was already demonstrated (124).
A prerequisite for improving the success and
efficiency of immunotherapy is the knowledge about robust
biomarkers predicting clinical benefit before treatment and
enabling stratification of oesophageal cancer patients in such a
manner that the best possible immunotherapy can be applied to each
patient. One possibility could be the multiplexed
immunohistochemical staining of adaptive immune (CD3, CD4, CD8 and
CD45RO) and immune checkpoint biomarkers [inducible T-cell
costimulatory molecule (ICOS), indoleamine-2,3-di- oxygenase-1
(IDO-1), PD-L1 and PD-1] in combination with digital pathology
quantitation (125).
Furthermore, it is well-established that immunotherapies are
resulting in an increased tumour burden and/or emergence of new
tumour lesions in the short-term. Therefore, the currently used
evaluation system for therapeutically success is most probably not
applicable for immunotherapies; thus, it may be prudent to consider
a different system for this novel type of therapy.
Not applicable.
JCH, AL and NV were involved in the
conceptualization of the study. JCH, MG, MR and AL were involved in
the writing and preparation of the original draft. JCH, NV, MBM and
AFO were involved in the writing, reviewing and editing of the
manuscript. All authors have read and agreed to the published
version of the manuscript.
Not applicable.
Not applicable.
NV received speaker honorarium from the companies,
Bayer, Eli-Lilly, Pfizer and Merck. The funders had no role in the
design of the study; in the collection, analyses, or interpretation
of data; in the writing of the manuscript, or in the decision to
publish the results. All other authors (AL, MR, MG, MBM, AFO and
JCH) declare that they have no competing interests.
Not applicable.
No funding was received.
|
1
|
de Vos-Geelen J, Hoebers FJP, Geurts SME,
Hoeben A, de Greef BTA, Voncken FEM, Bogers JHA, Braam PM, Muijs
CKT, de Jong MA, et al: A national study to assess outcomes of
definitive chemoradiation regimens in proximal esophageal cancer.
Acta Oncol. 59:895–903. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. Feb 4–2021.Epub ahead
of print. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A Review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartley AN, Washington MK, Ventura CB,
Ismaila N, Colasacco C, Benson AB III, Carrato A, Gulley ML, Jain
D, Kakar S, et al: HER2 testing and clinical decision making in
gastroesophageal adenocarcinoma: Guideline from the college of
American pathologists American society for clinical pathology, and
American society of clinical oncology. Arch Pathol Lab Med.
140:1345–1363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lordick F, Mariette C, Haustermans K,
Obermannova R and Arnold D; ESMO Guidelines Committee: Oesophageal
cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27(Suppl 5): v50–v57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brar G and Shah MA: The role of
pembrolizumab in the treatment of PD-L1 expressing gastric and
gastroesophageal junction adenocarcinoma. Therap Adv Gastroenterol.
12:17562848198697672019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Le Bras GF, Farooq MH, Falk GW and Andl
CD: Esophageal cancer: The latest on chemoprevention and state of
the art therapies. Pharmacol Res. 113(Pt A): 236–244. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Short MW, Burgers KG and Fry VT:
Esophageal cancer. Am Fam Physician. 95:22–28. 2017.PubMed/NCBI
|
|
11
|
Kitagawa Y, Uno T, Oyama T, Kato K, Kato
H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, et al:
Esophageal cancer practice guidelines 2017 edited by the Japan
esophageal society: Part 2. Esophagus. 16:25–43. 2019. View Article : Google Scholar :
|
|
12
|
Wang T, Yu J, Liu M, Chen Y, Zhu C, Lu L,
Wang M, Min L, Liu X, Zhang X, et al: The benefit of taxane-based
therapies over fluoropyrimidine plus platinum (FP) in the treatment
of esophageal cancer: A meta-analysis of clinical studies. Drug Des
Devel Ther. 13:539–553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blot WJ: Invited commentary: More evidence
of increased risks of cancer among alcohol drinkers. Am J
Epidemiol. 150:1138–1140; discussion 1141. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guanrei Y and Songliang Q: Endoscopic
surveys in high-risk and low-risk populations for esophageal cancer
in China with special reference to precursors of esophageal cancer.
Endoscopy. 19:91–95. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lagergren J and Lagergren P: Recent
developments in esophageal adenocarcinoma. CA Cancer J Clin.
63:232–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duggan C, Onstad L, Hardikar S, Blount PL,
Reid BJ and Vaughan TL: Association between markers of obesity and
progression from Barrett's esophagus to esophageal adenocarcinoma.
Clin Gastroenterol Hepatol. 11:934–943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carr JS, Zafar SF, Saba N, Khuri FR and
El-Rayes BF: Risk factors for rising incidence of esophageal and
gastric cardia adenocarcinoma. J Gastrointest Cancer. 44:143–151.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lofdahl HE, Lu Y, Lagergren P and
Lagergren J: Risk factors for esophageal adenocarcinoma after
antireflux surgery. Ann Surg. 257:579–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mao WM, Zheng WH and Ling ZQ:
Epidemiologic risk factors for esophageal cancer development. Asian
Pac J Cancer Prev. 12:2461–2466. 2011.
|
|
21
|
D'Onofrio V, Bovero E and Iaquinto G:
Characterization of acid and alkaline reflux in patients with
Barrett's esophagus. G.O.S.P.E. Operative Group for the study of
Esophageal Precancer. Dis Esophagus. 10:16–22; discussion 22-3.
1997.PubMed/NCBI
|
|
22
|
Fassan M, Realdon S, Cascione L, Hahne JC,
Munari G, Guzzardo V, Arcidiacono D, Lampis A, Brignola S, Dal
Santo L, et al: Circulating microRNA expression profiling revealed
miR-92a-3p as a novel biomarker of Barrett's carcinogenesis. Pathol
Res Pract. 216:1529072020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin J, Zeng R, Cao W, Luo R, Chen J and
Lin Y: Hot beverage and food intake and esophageal cancer in
southern China. Asian Pac J Cancer Prev. 12:2189–2192. 2011.
|
|
24
|
Nieman KM, Romero IL, Van Houten B and
Lengyel E: Adipose tissue and adipocytes support tumorigenesis and
metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lawrence MS, Stojanov P, Polak P, Kryukov
GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH,
Roberts SA, et al: Mutational heterogeneity in cancer and the
search for new cancer-associated genes. Nature. 499:214–218. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ratti M, Lampis A, Hahne JC, Passalacqua R
and Valeri N: Microsatellite instability in gastric cancer:
Molecular bases, clinical perspectives, and new treatment
approaches. Cell Mol Life Sci. 75:4151–4162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin EW, Karakasheva TA, Hicks PD, Bass AJ
and Rustgi AK: The tumor microenvironment in esophageal cancer.
Oncogene. 35:5337–5349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ammannagari N and Atasoy A: Current status
of immunotherapy and immune biomarkers in gastro-esophageal
cancers. J Gastrointest Oncol. 9:196–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 Blockade in tumors with Mismatch-Repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim WK, Park M, Park M, Kim YJ, Shin N,
Kim HK, You KT and Kim H: Identification and selective degradation
of neopeptide-containing truncated mutant proteins in the tumors
with high microsatellite instability. Clin Cancer Res.
19:3369–3382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Puzzoni M, Silvestris N, Leone F,
Giampieri R, Faloppi L, Demurtas L, Dell'Aquila E, Marino D,
Brunetti O, Garattini SK, et al: The immune revolution in
gastrointestinal tumours: Leading the way or just following? Target
Oncol. 11:593–603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li P, Xu W, Liu F, Zhu H, Zhang L, Ding Z,
Liang H and Song J: The emerging roles of IDO2 in cancer and its
potential as a therapeutic target. Biomed Pharmacother.
137:1112952021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou H, Jiang M, Yuan H, Ni W and Tai G:
Dual roles of myeloid-derived suppressor cells induced by Toll-like
receptor signaling in cancer. Oncol Lett. 21:1492021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abou Khouzam R, Brodaczewska K, Filipiak
A, Zeinelabdin NA, Buart S, Szczylik C, Kieda C and Chouaib S:
Tumor hypoxia regulates immune Escape/Invasion: Influence on
angiogenesis and potential impact of hypoxic biomarkers on cancer
therapies. Front Immunol. 11:6131142021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gajewski TF, Schreiber H and Fu YX: Innate
and adaptive immune cells in the tumor microenvironment. Nat
Immunol. 14:1014–1022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ando M, Ito M, Srirat T, Kondo T and
Yoshimura A: Memory T cell, exhaustion, and tumor immunity. Immunol
Med. 43:1–9. 2020. View Article : Google Scholar
|
|
37
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hahne JC, Meyer SR, Gambaryan S, Walter U,
Dietl J, Engel JB and Honig A: Immune escape of AKT overexpressing
ovarian cancer cells. Int J Oncol. 42:1630–1635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boutet P, Aguera-Gonzalez S, Atkinson S,
Pennington CJ, Edwards DR, Murphy G, Reyburn HT and Valés-Gómez M:
Cutting edge: The metalloproteinase ADAM17/TNF-alpha-converting
enzyme regulates proteolytic shedding of the MHC class I-related
chain B protein. J Immunol. 182:49–53. 2009. View Article : Google Scholar
|
|
40
|
Waldhauer I, Goehlsdorf D, Gieseke F,
Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG
and Steinle A: Tumor-associated MICA is shed by ADAM proteases.
Cancer Res. 68:6368–6376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud
AB, Speranza MC, Nakashima H, Hayes JL, Lee K, Balaj L, Passaro C,
et al: Immune evasion mediated by PD-L1 on glioblastoma-derived
extracellular vesicles. Sci Adv. 4:eaar27662018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Theodoraki MN, Yerneni SS, Hoffmann TK,
Gooding WE and Whiteside TL: Clinical significance of PD-L1(+)
exosomes in plasma of head and neck cancer patients. Clin Cancer
Res. 24:896–905. 2018. View Article : Google Scholar
|
|
44
|
Corthay A: How do regulatory T cells work?
Scand J Immunol. 70:326–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Romano M, Fanelli G, Albany CJ, Giganti G
and Lombardi G: Past, present, and future of regulatory T Cell
therapy in transplantation and autoimmunity. Front Immunol.
10:432019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Togashi Y, Shitara K and Nishikawa H:
Regulatory T cells in cancer immunosuppression-implications for
anticancer therapy. Nat Rev Clin Oncol. 16:356–371. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Engel JB, Honig A, Kapp M, Hahne JC, Meyer
SR, Dietl J and Segerer SE: Mechanisms of tumor immune escape in
triple-negative breast cancers (TNBC) with and without mutated BRCA
1. Arch Gynecol Obstet. 289:141–147. 2014. View Article : Google Scholar
|
|
48
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gabrilovich DI: Myeloid-Derived suppressor
cells. Cancer Immunol Res. 5:3–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Karakasheva TA, Dominguez GA, Hashimoto A,
Lin EW, Chiu C, Sasser K, Lee JW, Beatty GL, Gabrilovich DI and
Rustgi AK: CD38+ M-MDSC expansion characterizes a subset of
advanced colorectal cancer patients. JCI Insight. 3:e970222018.
View Article : Google Scholar :
|
|
51
|
Shi T, Ma Y, Yu L, Jiang J, Shen S, Hou Y
and Wang T: Cancer immunotherapy: A focus on the regulation of
immune check- points. Int J Mol Sci. 19:13892018. View Article : Google Scholar
|
|
52
|
Kim ES, Kim JE, Patel MA, Mangraviti A,
Ruzevick J and Lim M: Immune checkpoint modulators: An emerging
anti-glioma armamentarium. J Immunol Res. 2016:46836072016.
View Article : Google Scholar
|
|
53
|
Vivaldi C, Catanese S, Massa V, Pecora I,
Salani F, Santi S, Lencioni M, Vasile E, Falcone A and Fornaro L:
Immune check-point inhibitors in esophageal cancers: Are we finally
finding the right path in the mist? Int J Mol Sci. 21:16582020.
View Article : Google Scholar
|
|
54
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Disis ML: Mechanism of action of
immunotherapy. Semin Oncol. 41(Suppl 5): S3–S13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Senju H, Kumagai A, Nakamura Y, Yamaguchi
H, Nakatomi K, Fukami S, Shiraishi K, Harada Y, Nakamura M, Okamura
H, et al: Effect of IL-18 on the expansion and phenotype of human
natural killer cells: Application to cancer immunotherapy. Int J
Biol Sci. 14:331–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mimura K, Yamada L, Ujiie D, Hayase S,
Tada T, Hanayama H, Thar Min AK, Shibata M, Momma T, Saze Z, et al:
Immunotherapy for esophageal squamous cell carcinoma: A review.
Fukushima J Med Sci. 64:46–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Redman JM, Gibney GT and Atkins MB:
Advances in immunotherapy for melanoma. BMC Med. 14:202016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Figueroa-Protti L, Soto-Molinari R,
Calderon-Osorno M, Mora J and Alpizar-Alpizar W: Gastric cancer in
the Era of immune checkpoint blockade. J Oncol. 2019:10797102019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Marchetti A, Di Lorito A and Buttitta F:
Why anti-PD1/PDL1 therapy is so effective? Another piece in the
puzzle. J Thorac Dis. 9:4863–4866. 2017. View Article : Google Scholar
|
|
64
|
Akinleye A and Rasool Z: Immune checkpoint
inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol.
12:922019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Robert C, Ribas A, Wolchok JD, Hodi FS,
Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et
al: Anti-progra mmed-death-receptor-1 treatment with pembrolizumab
in ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Maleki Vareki S, Garrigos C and Duran I:
Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol
Hematol. 116:116–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kato R, Yamasaki M, Urakawa S, Nishida K,
Makino T, Morimoto-Okazawa A, Kawashima A, Iwahori K, Suzuki S,
Ueda R, et al: Increased Tim-3(+) T cells in PBMCs during nivolumab
therapy correlate with responses and prognosis of advanced
esophageal squamous cell carcinoma patients. Cancer Immunol
Immunother. 5467:1673–1683. 2018. View Article : Google Scholar
|
|
69
|
Brahmer JR, Rodriguez-Abreu D, Robinson
AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A,
Cuffe S, et al: Health-related quality-of-life results for
pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC
(KEYNOTE-024): A multicentre, international, randomised, open-label
phase 3 trial. Lancet Oncol. 18:1600–1609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Seiwert TY, Burtness B, Mehra R, Weiss J,
Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et
al: Safety and clinical activity of pembrolizumab for treatment of
recurrent or metastatic squamous cell carcinoma of the head and
neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial.
Lancet Oncol. 17:956–965. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 4:e1800132018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Doi T, Piha-Paul SA, Jalal SI, Saraf S,
Lunceford J, Koshiji M and Bennouna J: Safety and antitumor
activity of the Anti-Programmed Death-1 antibody pembrolizumab in
patients with advanced esophageal carcinoma. J Clin Oncol.
36:61–67. 2018. View Article : Google Scholar
|
|
73
|
Hamanishi J, Mandai M, Ikeda T, Minami M,
Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S,
et al: Safety and antitumor activity of Anti-PD-1 Antibody,
Nivolumab, in patients with platinum-resistant ovarian cancer. J
Clin Oncol. 33:4015–4022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kato K, Cho BC, Takahashi M, Okada M, Lin
CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, et al:
Nivolumab versus chemotherapy in patients with advanced oesophageal
squamous cell carcinoma refractory or intolerant to previous
chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:1506–1517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Das R, Verma R, Sznol M, Boddupalli CS,
Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R,
Dhodapkar MV and Dhodapkar KM: Combination therapy with anti-CTLA-4
and anti-PD-1 leads to distinct immunologic changes in vivo. J
Immunol. 194:950–959. 2015. View Article : Google Scholar
|
|
76
|
Tanaka T, Nakamura J and Noshiro H:
Promising immunotherapies for esophageal cancer. Expert Opin Biol
Ther. 17:723–733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Janjigian YY, Bendell J, Calvo E, Kim JW,
Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, et al:
CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab
plus ipilimumab in patients with metastatic esophagogastric cancer.
J Clin Oncol. 36:2836–2844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhao Y, Yang W, Huang Y, Cui R, Li X and
Li B: Evolving roles for Targeting CTLA-4 in cancer immunotherapy.
Cell Physiol Biochem. 47:721–734. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Darvin P, Toor SM, Sasidharan Nair V and
Elkord E: Immune checkpoint inhibitors: Recent progress and
potential biomarkers. Exp Mol Med. 50:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Alsina M, Moehler M and Lorenzen S:
Immunotherapy of esophageal cancer: Current status, many trials and
innovative strategies. Oncol Res Treat. 41:266–271. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Miliotou AN and Papadopoulou LC: CAR
T-cell Therapy: A New Era in cancer immunotherapy. Curr Pharm
Biotechnol. 19:5–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Walseng E, Koksal H, Sektioglu IM, Fåne A,
Skorstad G, Kvalheim G, Gaudernack G, Inderberg EM and Wälchli S: A
TCR-based Chimeric antigen receptor. Sci Rep. 7:107132017.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Robbins PF, Dudley ME, Wunderlich J,
El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ Jr and Rosenberg SA:
Cutting edge: Persistence of transferred lymphocyte clonotypes
correlates with cancer regression in patients receiving cell
transfer therapy. J Immunol. 173:7125–7130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dudley ME, Yang JC, Sherry R, Hughes MS,
Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et
al: Adoptive cell therapy for patients with metastatic melanoma:
Evaluation of intensive myeloablative chemoradiation preparative
regimens. J Clin Oncol. 26:5233–5239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Toh U, Yamana H, Sueyoshi S, Tanaka T,
Niiya F, Katagiri K, Fujita H, Shirozou K and Itoh K: Locoregional
cellular immunotherapy for patients with advanced esophageal
cancer. Clin Cancer Res. 6:4663–4673. 2000.
|
|
87
|
Kageyama S, Ikeda H, Miyahara Y, Imai N,
Ishihara M, Saito K, Sugino S, Ueda S, Ishikawa T, Kokura S, et al:
Adoptive Transfer of MAGE-A4 T-cell Receptor Gene-Transduced
lymphocytes in patients with recurrent esophageal cancer. Clin
Cancer Res. 21:2268–2277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li W, Joshi MD, Singhania S, Ramsey KH and
Murthy AK: Peptide vaccine: Progress and challenges. Vaccines
(Basel). 2:515–536. 2014. View Article : Google Scholar
|
|
89
|
Masopust D and Schenkel JM: The
integration of T cell migration, differentiation and function. Nat
Rev Immunol. 13:309–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Aranda F, Vacchelli E, Eggermont A, Galon
J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G and Galluzzi
L: Trial Watch: Peptide vaccines in cancer therapy. Oncoimmunology.
2:e266212013. View Article : Google Scholar
|
|
91
|
Tacken PJ, de Vries IJ, Torensma R and
Figdor CG: Dendritic-cell immunotherapy: From ex vivo loading to in
vivo targeting. Nat Rev Immunol. 7:790–802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hos BJ, Tondini E, van Kasteren SI and
Ossendorp F: Approaches to improve chemically defined synthetic
peptide vaccines. Front Immunol. 9:8842018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Blander JM: Regulation of the cell biology
of antigen cross-presentation. Annu Rev Immunol. 36:717–753. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Quakkelaar ED and Melief CJ: Experience
with synthetic vaccines for cancer and persistent virus infections
in nonhuman primates and patients. Adv Immunol. 114:77–106. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kono K: Current status of cancer
immunotherapy. J Stem Cells Regen Med. 10:8–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fujiwara S, Wada H, Miyata H, Kawada J,
Kawabata R, Nishikawa H, Gnjatic S, Sedrak C, Sato E, Nakamura Y,
et al: Clinical trial of the intratumoral administration of labeled
DC combined with systemic chemotherapy for esophageal cancer. J
Immunother. 35:513–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Robbins PF, Kassim SH, Tran TL, Crystal
JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR,
Sherry RM, et al: A pilot trial using lymphocytes genetically
engineered with an NY-ESO-1-reactive T-cell receptor: Long-term
follow-up and correlates with response. Clin Cancer Res.
21:1019–1027. 2015. View Article : Google Scholar
|
|
99
|
Daudi S, Eng KH, Mhawech-Fauceglia P,
Morrison C, Miliotto A, Beck A, Matsuzaki J, Tsuji T, Groman A,
Gnjatic S, et al: Expression and immune responses to MAGE antigens
predict survival in epithelial ovarian cancer. PLoS One.
9:e1040992014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kawabata R, Wada H, Isobe M, Saika T, Sato
S, Uenaka A, Miyata H, Yasuda T, Doki Y, Noguchi Y, et al: Antibody
response against NY-ESO-1 in CHP-NY-ESO-1 vaccinated patients. Int
J Cancer. 120:2178–2184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Veit JA, Heine D, Thierauf J, Lennerz J,
Shetty S, Schuler PJ, Whiteside T, Beutner D, Meyer M, Grünewald I,
et al: Expression and clinical significance of MAGE and NY-ESO-1
cancer-testis antigens in adenoid cystic carcinoma of the head and
neck. Head Neck. 38:1008–1016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Thomas R, Al-Khadairi G, Roelands J,
Hendrickx W, Dermime S, Bedognetti D and Decock J: NY-ESO-1 based
immunotherapy of cancer: Current perspectives. Front Immunol.
9:9472018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bujas T, Marusic Z, Peric Balja M, Mijic
A, Kruslin B and Tomas D: MAGE-A3/4 and NY-ESO-1 antigens
expression in metastatic esophageal squamous cell carcinoma. Eur J
Histochem. 55:e72011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang H, Zhou X, Liu D, Zhu Y, Ma Q and
Zhang Y: Progress and challenges of poersonalized neoantigens in
the clinical treatment of tumors. Med Drug Disc. 6:1000302020.
View Article : Google Scholar
|
|
105
|
Huang TX and Fu L: The immune landscape of
esophageal cancer. Cancer Commun (Lond). 39:792019. View Article : Google Scholar
|
|
106
|
Tran E, Robbins PF and Rosenberg SA:
'Final common pathway' of human cancer immunotherapy: Targeting
random somatic mutations. Nat Immunol. 18:255–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Butterfield LH: Cancer vaccines. BMJ.
350:h9882015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Han XJ, Ma XL, Yang L, Wei YQ, Peng Y and
Wei XW: Progress in neoantigen targeted cancer immunotherapies.
Front Cell Dev Biol. 8:7282020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei
J, Ren S and Zhou C: Tumor neoantigens: From basic research to
clinical applications. J Hematol Oncol. 12:932019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sahin U, Derhovanessian E, Miller M, Kloke
BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B,
et al: Personalized RNA mutanome vaccines mobilize poly-specific
therapeutic immunity against cancer. Nature. 547:222–226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J,
Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al: An
immunogenic personal neoantigen vaccine for patients with melanoma.
Nature. 547:217–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Iinuma H, Fukushima R, Inaba T, Tamura J,
Inoue T, Ogawa E, Horikawa M, Ikeda Y, Matsutani N, Takeda K, et
al: Phase I clinical study of multiple epitope peptide vaccine
combined with chemoradiation therapy in esophageal cancer patients.
J Transl Med. 12:842014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kono K, Iinuma H, Akutsu Y, Tanaka H,
Hayashi N, Uchikado Y, Noguchi T, Fujii H, Okinaka K, Fukushima R,
et al: Multicenter, phase II clinical trial of cancer vaccination
for advanced esophageal cancer with three peptides derived from
novel cancer-testis antigens. J Transl Med. 10:1412012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yasuda T, Nishiki K, Yoshida K, Shiraishi
O, Iwama M, Kato H, Yasuda A, Shinkai M, Chiba Y, Okuno K and
Nakamura Y: Cancer peptide vaccine to suppress postoperative
recurrence in esophageal SCC patients with induction of
antigen-specific CD8+T cell. J Clin Oncol. 35(Suppl 15):
e146352017. View Article : Google Scholar
|
|
115
|
Murahashi M, Hijikata Y, Yamada K, Tanaka
Y, Kishimoto J, Inoue H, Marumoto T, Takahashi A, Okazaki T, Takeda
K, et al: Phase I clinical trial of a five-peptide cancer vaccine
combined with cyclophosphamide in advanced solid tumors. Clin
Immunol. 166-167:48–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Harao M, Hirata S, Irie A, Senju S,
Nakatsura T, Komori H, Ikuta Y, Yokomine K, Imai K, Inoue M, et al:
HLA-A2-restricted CTL epitopes of a novel lung cancer-associated
cancer testis antigen, cell division cycle associated 1, can induce
tumor-reactive CTL. Int J Cancer. 123:2616–2625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Obara W, Eto M, Mimata H, Kohri K,
Mitsuhata N, Miura I, Shuin T, Miki T, Koie T, Fujimoto H, et al: A
phase I/II study of cancer peptide vaccine S-288310 in patients
with advanced urothelial carcinoma of the bladder. Ann Oncol.
28:798–803. 2017. View Article : Google Scholar
|
|
118
|
Yoshitake Y, Fukuma D, Yuno A, Hirayama M,
Nakayama H, Tanaka T, Nagata M, Takamune Y, Kawahara K, Nakagawa Y,
et al: Phase II clinical trial of multiple peptide vaccination for
advanced head and neck cancer patients revealed induction of immune
responses and improved OS. Clin Cancer Res. 21:312–321. 2015.
View Article : Google Scholar
|
|
119
|
Ungerechts G, Engeland CE, Buchholz CJ,
Eberle J, Fechner H, Geletneky K, Holm PS, Kreppel F, Kühnel F,
Lang KS, et al: Virotherapy research in Germany: From engineering
to translation. Hum Gene Ther. 28:800–819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Moehler M, Goepfert K, Heinrich B,
Breitbach CJ, Delic M, Galle PR and Rommelaere J: Oncolytic
virotherapy as emerging immunotherapeutic modality: Potential of
parvovirus h-1. Front Oncol. 4:922014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Conry RM, Westbrook B, McKee S and Norwood
TG: Talimogene laherparepvec: First in class oncolytic virotherapy.
Hum Vaccin Immunother. 14:839–846. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tanabe S, Tazawa H, Kagawa S, Noma K,
Takehara K, Koujima T, Kashima H, Kato T, Kuroda S, Kikuchi S, et
al: Phase I/II trial of endoscopic intratumoral administration of
OBP-301, a novel telomerase-specific oncolytic virus, with
radiation in elderly esophageal cancer patients. Cancer Res.
75:Abstract CT1232015.
|
|
123
|
Nemunaitis J, Tong AW, Nemunaitis M,
Senzer N, Phadke AP, Bedell C, Adams N, Zhang YA, Maples PB, Chen
S, et al: A phase I study of telomerase-specific replication
competent oncolytic adenovirus (telomelysin) for various solid
tumors. Mol Ther. 18:429–434. 2010. View Article : Google Scholar :
|
|
124
|
Sharabi AB, Lim M, DeWeese TL and Drake
CG: Radiation and checkpoint blockade immunotherapy:
Radiosensitisation and potential mechanisms of synergy. Lancet
Oncol. 16:e498–e509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Humphries MP, Craig SG, Kacprzyk R, Fisher
NC, Bingham V, McQuaid S, Murray GI, McManus D, Turkington RC,
James J and Salto-Tellez M: The adaptive immune and immune
checkpoint landscape of neoadjuvant treated esophageal
adenocarcinoma using digital pathology quantitation. BMC Cancer.
20:5002020. View Article : Google Scholar : PubMed/NCBI
|