Introduction
Proliferative vitreoretinopathy (PVR) is a disease
that develops as a complication following retinal detachment
surgery (1). It is characterized
by the presence of epiretinal membranes (ERM) that exert traction
by re-detachment of the retina (2). The epithelial-mesenchymal
transition (EMT) of retinal pigment epithelial (RPE) cells has been
recognized as an important mechanism that contributes to ERM
formation (3,4). EMT can be triggered by a number of
molecules, including fibroblast growth factor (5) and epidermal growth factor (6). However, transforming growth
factor-β1 (TGF-β1) is still considered to be the primary trigger of
EMT (7-9). Our previous study also provided
evidence that TGF-β1 plays a vital role in the EMT of the human RPE
cell line, ARPE-19 (10,11).
Peroxisome proliferator-activated receptor γ (PPARγ)
is a member of the peroxisome proliferator-activated receptor
(PPAR) family. Previous studies have demonstrated that PPARs play
important roles in the regulation of proinflammatory cytokine
expression (12) and tissue
fibrosis (13,14). Furthermore, PPARγ inhibits the
induction of EMT via TGF-β1 in alveolar epithelial cells in humans
(15). In RPE cells, PPARγ
regulates inflammation through monocyte chemoattractant protein-1
(16) and major
histocompatibility complex class II molecule expression (17). Nevertheless, to the best of our
knowledge, there has been no investigation of the role of PPARγ in
the EMT of RPE cells.
L-carnitine
(β-hydroxy-γ-N-trimethylammoniumbutyrate, LC) is essential for
lipid energy metabolism via β-oxidation of long-chain fatty acids
(18). Using metabolomics
approaches, we previously found that LC was significantly reduced
in the vitreous of patients with PVR (19). Baci et al (20) reported that LC had
anti-angiogenic and anti-inflammatory effects via nuclear factor-κB
(NF-κB), and inhibition of vascular endothelial growth factor
(VEGF) and VEGF receptor 2. Of note, LC has also been illustrated
to attenuate kidney fibrosis in hypertensive rats by upregulating
PPARγ (21). However, whether LC
has an effect on the EMT of RPE cells has not yet been elucidated,
and if there is an effect the underlying mechanism is not known. In
the present study, it was found that LC attenuated EMT induced by
TGF-β1 via inhibition of the Erk1/2 and JNK pathways and
upregulation of PPAR-γ expression.
Materials and methods
Cell culture
Human RPE cells were obtained from the healthy eyes
of donors according to a previously published report (22). Primary RPE cells were cultured
and 2-5 generation cells were used in this study. ARPE-19 cells
were purchased from NewGainBio. Primary RPE cells and ARPE-19 cells
were routinely cultured in DMEM/F12 (Gibco; Thermo Fisher
Scientific, Inc.) with 100 U/ml penicillin and 100 µg/ml
streptomycin (Beijing Solarbio Science & Technology Co., Ltd.)
at 37°C in a cell incubator containing 5% CO2. The
medium was changed every 2 days. ARPE-19 cells and human RPE cells
were starved in serum-free medium for 16 h. Cells were then
incubated with 10 ng/ml TGF-β1 with or without LC (Sigma-Aldrich;
Merck KGaA) at the indicated concentrations (0.1, 1 and 10
µM) at 37°C for 24 or 48 h. This study was approved by the
Ethics Committee of Shanghai Tenth People's Hospital (approval no.
SHSY-IEC-KY-4.0/17-79/01; Shanghai, China) and was in compliance
with the Declaration of Helsinki. Donors' eyes were obtained from
the Eye Bank of Shanghai Tenth People's Hospital.
Transwell migration assay
A total of 1×106 RPE cells were plated into the
upper chambers of Transwell plates (8-mm pore size; Costar, Inc.)
in 100 ml DMEM with 0.5% FBS (Invitrogen; Thermo Fisher Scientific,
Inc.). Medium with 10% FBS were added into the lower chambers.
After 24 or 48 h, the upper chambers of the Transwell plates were
fixed with 4% paraformaldehyde for 30 min at room temperature and
stained with 0.1% crystal violet for 20 min at room temperature.
Five fields of migrated cell numbers were counted in each chamber
with a fluorescence microscope (Olympus Corporation).
Immunofluorescence analysis
After TGF-β1 and LC treatment, RPE cells were fixed
with 4% paraformaldehyde for 10 min at room temperature and blocked
with 10% bovine serum albumin (MP Biomedicals, LLC) for 1 h at room
temperature. Then, RPE cells were stained with the primary
antibodies at 4°C overnight, followed by incubation with the
FITC-conjugated secondary antibody (1:500; cat. no. ab8503; Abcam)
at room temperature for 1 h. The nuclei were stained with DAPI for
5 min at room temperature, and subsequently cell images were
captured using a fluorescence microscope at ×400 magnification
(Olympus Corporation). The following primary antibodies were used:
Rabbit anti-cellular retinaldehyde-binding protein (CRALBP; 1:500;
cat. no. ab243664; Abcam), anti-retinoid isomerohydrolase (RPE-65;
1:250; cat. no. ab231782; Abcam), mouse anti-α-smooth muscle actin
(α-SMA; 1:50; cat. no. ab7817; Abcam), anti-zonula occludens-1
(ZO-1; 1:1,000; cat. no. ab276131; Abcam) and anti-E-Cadherin
(1:500; cat. no. AF748; R&D Systems, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was
synthesized using a reverse transcription kit (Takara Bio, Inc.),
according to the manufacturer's protocols. RT-qPCR was performed
using a 7500 Fast Real-time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with SYBR Green (Beijing Solarbio Science
& Technology Co., Ltd.) Specific primers were purchased from
Gentec (Shanghai) Corporation, with GAPDH used as an internal
control. The thermocycling conditions were as follows: 94°C for 30
sec, 40 cycles of 94°C for 5 sec and 60°C for 30 sec. The
2−ΔΔCq method (23)
was used to measure the relative expression of each gene. All
reactions were repeated three times. The primers are presented in
Table I.
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Genes | Primer sequences
(5′→3′) |
|---|
| ZO-1 | F:
TGAGGCAGCTCACATAATGC |
| R:
GGTCTCTGCTGGCTTGTTTC |
| E-cadherin | F:
TGCCCAGAAAATGAAAAAGG |
| R:
GTGTATGTGGCAATGCGTTC |
| α-SMA | F:
AGCAGGCCAAGGGGCTATATAA |
| R:
CGTAGCTGTCTTTTTGTCCCATT |
| N-cadherin | F:
GACAATGCCCCTCAAGTGTT |
| R:
CCATTAAGCCGAGTGATGGT |
| Vimentin | F:
GAGAACTTTGCCGTTGAAGC |
| R:
TCCAGCAGCTTCCTGTAGGT |
| FN | F:
ACCAACCTACGGATGACTCG |
| R:
GCTCATCATCTGGCCATTTT |
| Snail | F:
ACCCCACATCCTTCTCACTG |
| R:
TACAAAAACCCACGCAGACA |
| GAPDH | F:
AGAAGGCTGGGGCTCATTTG |
| R:
AGGGGCCATCCACAGTCTTC |
Western blotting
RPE cells were lysed with RIPA buffer (Beyotime
Institute of Biotechnology) on ice for 30 min and then centrifuged
at 10,000 × g for 10 min at 4°C. The total protein of the
supernatant was quantified using a bicinchoninic acid protein assay
(Pierce; Thermo Fisher Scientific, Inc.). Then, 350 µg
protein/lane was separated via SDS-PAGE on 6-12% gels, and
subsequently separated proteins were transferred to PVDF membranes
(EMD Millipore) and blocked in 5% non-fat milk for 1 h at room
temperature. Membranes were then incubated overnight at 4°C with
the following primary antibodies: Anti-α-SMA (1:200; cat. no.
ab7817; Abcam), anti-ZO-1 (1:1,000; cat. no. ab276131; Abcam),
anti-E-Cadherin (1:500; cat. no. AF748; R&D Systems, Inc.),
anti-fibronectin (FN; 1:500; cat. no. ab2413; Abcam), anti-JNK
(1:1,000; cat. no. ab179461; Abcam), anti-phosphorylated (p)-JNK
(1:1,000; cat. no. ab124956; Abcam), anti-Erk1/2 (1:10,000; cat.
no. ab184699; Abcam), anti-p-Erk1/2 (1:400; cat. no. ab218017;
Abcam), anti-p38 (1:1,000; cat. no. ab170099; Abcam), anti-p-p38
(1:1,000; cat. no. A1984; BioVision, Inc.), p-p105 (1:1,000; cat.
no. bs-0465R; BIOSS), anti-p-p65 (1:1,000; cat. no. AF5881;
Beyotime Institute of Biotechnology), anti-p-IκBα (1:1,000; cat.
no. 4814; Cell Signaling Technology, Inc.), anti-PPARγ (1:10,000;
cat. no. ab178860; Abcam), anti-N-cadherin (1:1,000; cat. no.
13116; Cell Signaling Technology, Inc.), anti-vimentin (1:1,000;
cat. no. 5741; Cell Signaling Technology, Inc), anti-Snail
(1:1,000; cat. no. 3879; Cell Signaling Technology, Inc.) and
anti-GAPDH (1:1,000; cat. no. 5174; Cell Signaling Technology,
Inc). The membrane was then incubated with the secondary antibodies
(1:10,000; cat. nos. w4011 and S3721; Promega Corporation) at room
temperature for 1 h. SB203580 (25 mM), SP600125 (100 mM),
BAY11-7082 (100 mM) and GW9662 (50 mM) were purchased from Abcam.
U0126 (100 mM) was purchased from Beyotime Institute of
Biotechnology. The blots were scanned using Image Quant LAS 4000
(Cytiva) and analyzed with ImageJ version 2 software (National
Institutes of Health).
Statistics
At least three independent experiments were
performed. Data are presented as the mean ± standard deviation, and
were analyzed using SPSS 20.0 software (IBM Corp.). An unpaired
Student's t-test was used for comparisons between two groups.
One-way ANOVA followed by Tukey's post hoc test were used to
compare differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Concentration-dependent effects of LC on
the migratory activity of ARPE-19 cells
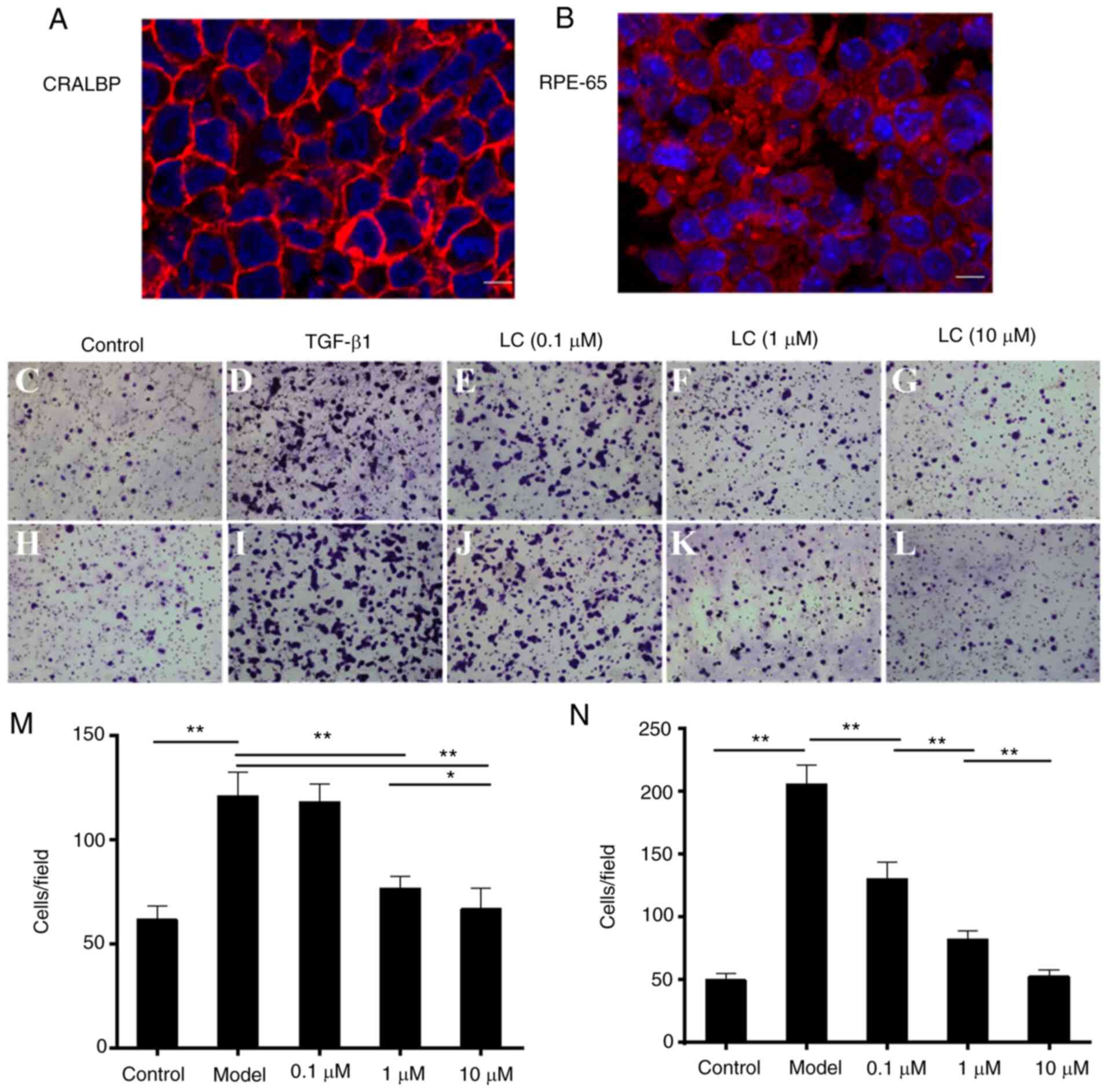
ARPE-19 cells used in this study showed expression
of CRALBP (Fig. 1A) and RPE-65
(Fig. 1B). The number of
migrated cells was significantly lower with increasing
concentrations of LC (Fig.
1C-N). After 24 h of treatment, the number of migrated cells
was lowered by co-culture with the LC concentrations of 1 µM
(Fig. 1F and M) and 10 µM
(Fig. 1G and M). Furthermore,
the number of migrated cells was reduced significantly at an LC
concentration of 0.1 µM (Fig.
1J and N) after 48 h of treatment.
LC prevents TGF-β1-induced EMT in RPE
cells
To investigate whether LC prevented TGF-β1-induced
EMT in RPE cells, the expression levels of epithelial markers
(E-cadherin and ZO-1) and mesenchymal markers (α-SMA, vimentin, FN
and N-cadherin) were determined. As shown in Fig. 2A and B, 10 ng/ml TGF-β1 led to an
increase in the expression levels of α-SMA and FN, and decreased
the expression levels of E-cadherin and ZO-1. These effects were
reversed by treatment with 10 µM LC in ARPE-19 cells. There
were no significant differences between 1 and 10 µM LC on
E-cadherin, ZO-1 and FN expression. However, the expression of
α-SMA and Snail in the 10 µM LC group was significantly
lower than the 1 µM LC group. Furthermore, following
treatment with increasing concentrations of LC, ARPE-19 cells
displayed higher expression of E-cadherin and ZO-1 than in cells of
the control group. Expression of α-SMA, FN, vimentin, N-cadherin
and transcription factor Snail were notably lower at the mRNA
(Fig. 2C) and protein (Fig. 2D) levels. The human primary RPE
cells are presented in Fig. 2E.
The same trend was observed in human RPE cells, as shown in
Fig. 2F and G, TGF-β1
significantly promoted the expression of α-SMA and reduced the
expression of ZO-1, and this process could be reversed by LC
(Fig. 2F and G).
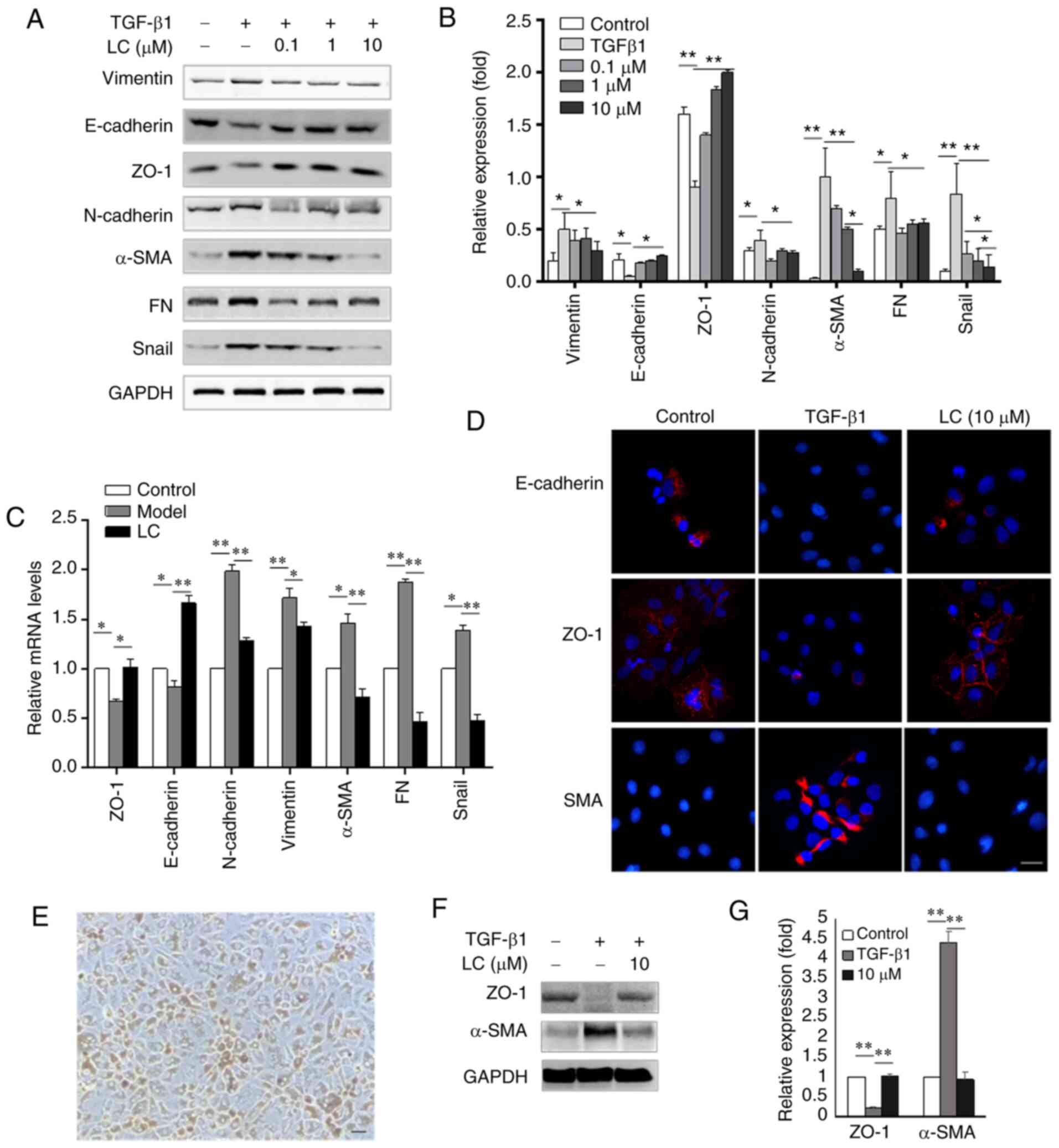 | Figure 2LC attenuates TGF-β1-induced
epithelial-mesenchymal transition in ARPE-19 cells and human
primary RPE cells. ARPE-19 cells were treated with 10 ng/ml TGF-β1
with or without LC (0.1, 1 and 10 µM) for 48 h. Human RPE
cells were treated with 10 ng/ml TGF-β1 with or without 10
µM LC for 48 h. (A) Protein levels of vimentin, E-cadherin,
ZO-1, N-cadherin, α-SMA, FN and Snail were detected using the
corresponding antibodies in ARPE-19 cells. (B) Semi-quantification
of protein levels from three independent experiments. (C) The mRNA
expression levels of ZO-1, E-cadherin, N-cadherin, vimentin, α-SMA,
FN and Snail were evaluated via reverse transcription-quantitative
PCR. (D) The slides were observed by phase contrast microscopy.
Magnification, ×400. Nuclei were stained with DAPI. (E) The
cultured human primary RPE cells. Magnification, ×400. (F) Protein
levels of ZO-1 and α-SMA were detected using the corresponding
antibodies in human primary RPE cells. (G) Semi-quantification of
protein levels from three independent experiments.
*P<0.05, **P<0.01. LC, L-carnitine;
ZO-1, zonula occludens-1; α-SMA, α-smooth muscle actin; FN,
fibronectin; RPE, retinal pigment epithelial. |
LC inhibits TGF-β1-induced EMT by
suppressing MAPK pathways
The effects of LC on TGF-β1 signaling pathways in
ARPE-19 cells (Fig. 3A-C) and
human primary RPE cells (Fig.
3D-F) were investigated. LC had no effect on the canonical
Smad2/3 signaling pathway. p-Erk1/2 and p-JNK expression levels
were significantly lower in 10 µM LC-treated cells than in
the TGF-β1 group, whereas the total Erk1/2, JNK, p38 and p-p38
expression were not affected.
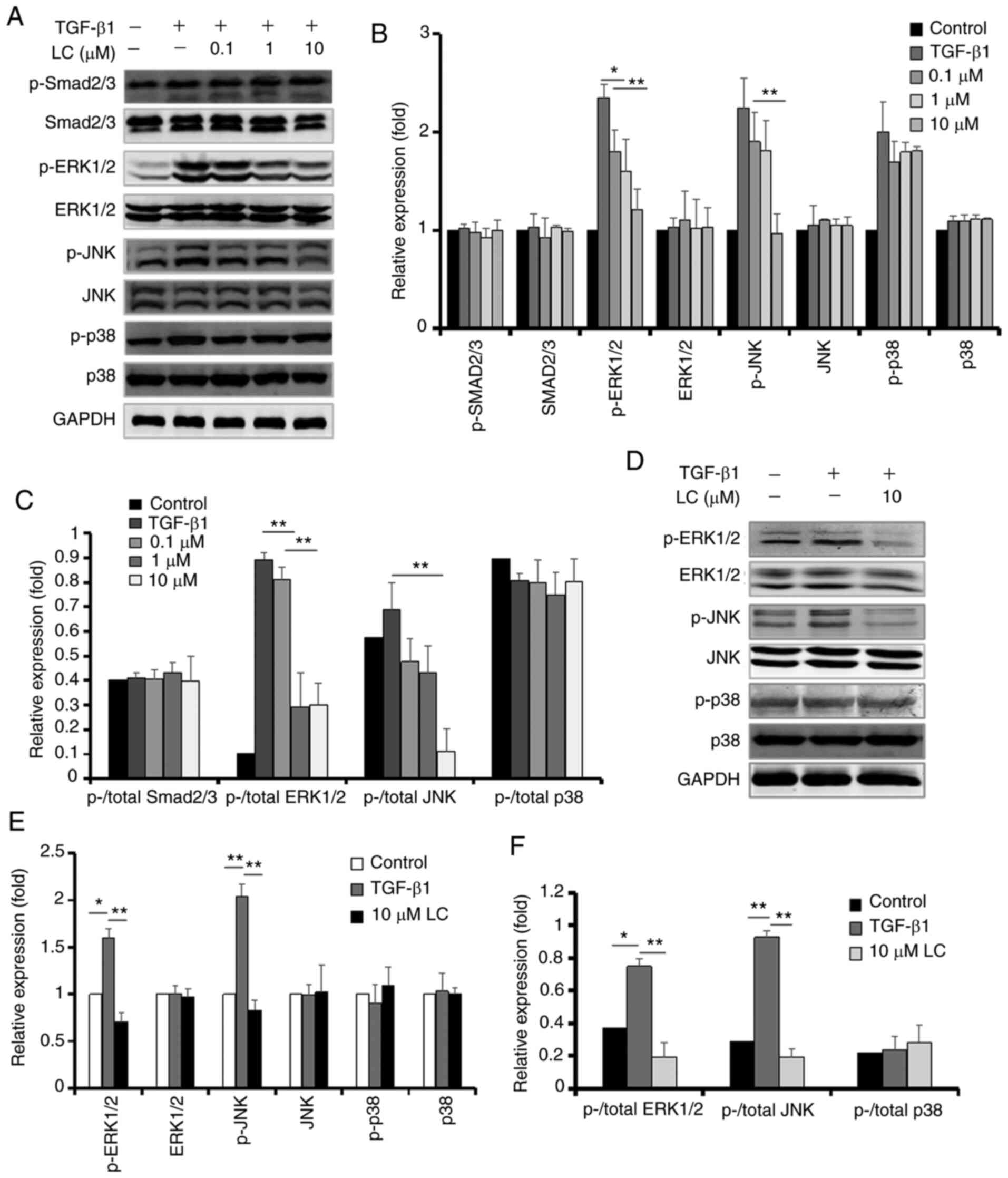 | Figure 3Protein levels of p-Smad2/3 and MAPK
pathway-related markers in ARPE-19 cells and human primary RPE
cells. (A) After ARPE-19 cells were treated with 10 ng/ml TGF-β1
with or without LC (0.1, 1 and 10 µM) for 48 h, p-Smad2/3,
Smad2/3, ERK1/2, p-ERK1/2, JNK, p-JNK, p38 and p-p38 were detected
using the corresponding antibody. (B) Semi-quantification of
protein levels from three independent experiments. (C) The ratio of
p-/total Smad2/3 protein, p-/total ERK1/2 protein, p-/total JNK
protein and p-/total p38 protein. (D) After human RPE cells were
treated with 10 ng/ml TGF-β1 with or without 10 µM LC for 48
h, p-ERK1/2, ERK1/2, p-JNK, JNK, p-p38 and p38 were detected. (E)
Semi-quantification of protein levels from three independent
experiments. (F) The ratio of p-/total ERK1/2 protein, p-/total JNK
protein and p-/total p38 protein in the graph.
*P<0.05, **P<0.01. LC, L-carnitine; p-,
phosphorylated; RPE, retinal pigment epithelial. |
PPARγ and NF-κB are involved in the
inhibitory effect of LC on the EMT of RPE cells
To the best of our knowledge, LC inhibits the EMT of
renal tubular epithelial cells via a PPARγ-dependent mechanism
(13). NF-κB also plays a vital
role in the EMT of RPE cells (24). Therefore, the present study next
investigated the role of PPARγ and NF-κB in the inhibitory effect
of LC on the EMT of RPE cells. As presented in Fig. 4A-C, treatment of ARPE-19 cells
with TGF-β1 resulted in a marked increase in the expression of
p-p105, p-p65 and p-IκB. These effects, with the exception of
p-p105, were suppressed by LC. PPARγ expression was lower in
ARPE-19 cells after treatment with TGF-β1, which was reversed by
LC. In human primary RPE cells, TGF-β1 upregulated the expression
of p-p65 and downregulated the expression of PPARγ, and LC also
inhibited these changes (Fig.
4D-F).
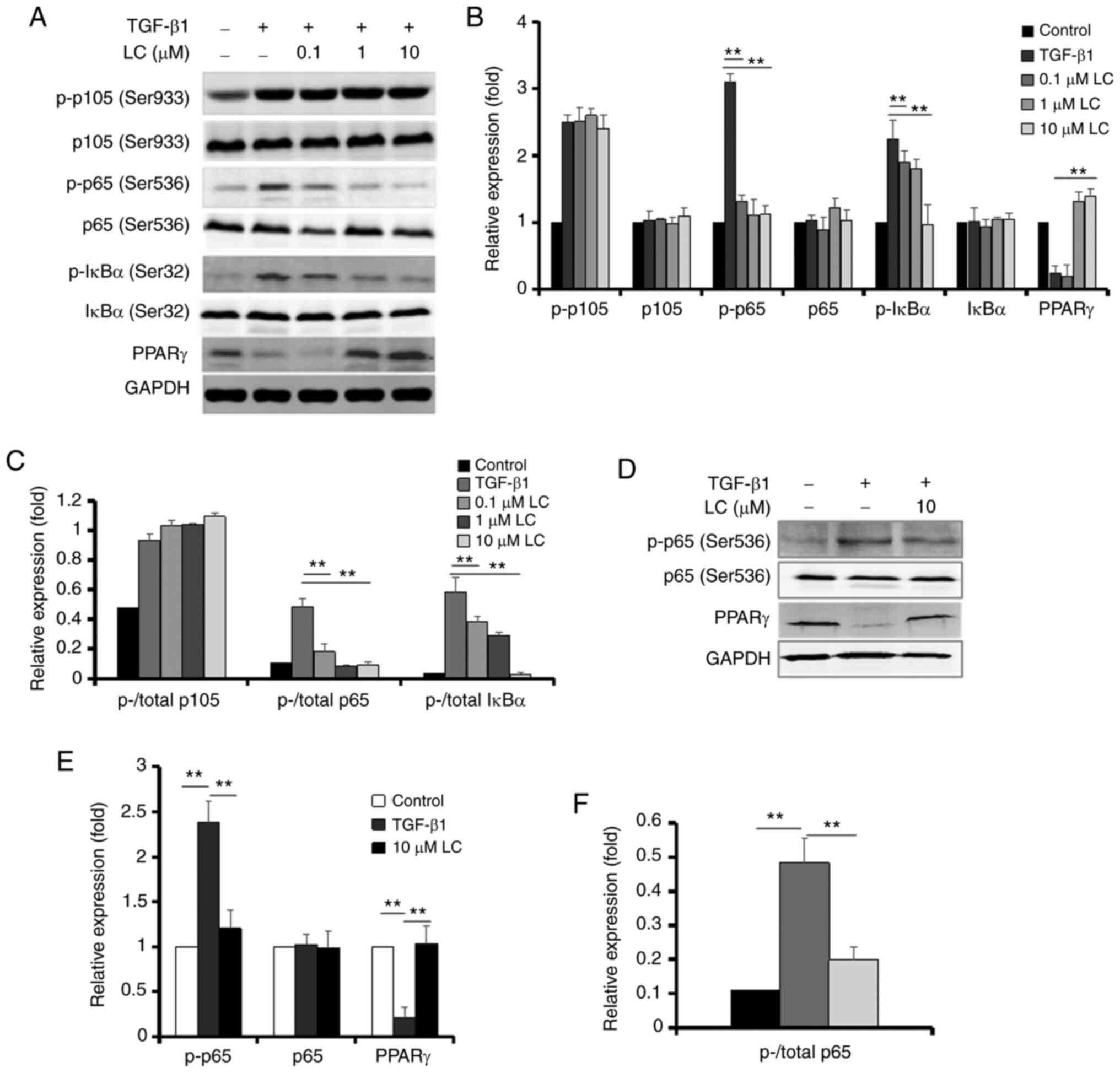 | Figure 4Protein levels of NF-κB and PPARγ in
ARPE-19 cells and human primary RPE cells treated with TGF-β1 and
LC. (A) After ARPE-19 cells were treated with 10 ng/ml TGF-β1 with
or without LC (0.1, 1 and 10 µM) for 48 h, p-p105, p105,
p-p65, p65, p-IκBα, IκBα and PPARγ were detected using the
corresponding antibody. (B) Semi-quantification of protein levels
from three independent experiments. (C) The ratio of p-/total p105
protein, p-/total p65 protein and p-/total IκB protein in the
graph. (D) After human RPE cells were treated with 10 ng/ml TGF-β1
with or without 10 µM LC for 48 h, p-p65, p65 and PPARγ were
detected. (E) Semi-quantification of protein levels from three
independent experiments. (F) The ratio of p-/total p65 protein in
the graph. **P<0.01. NF-κB, nuclear factor-κB; PPARγ,
peroxisome proliferator-activated receptor γ; LC, L-carnitine; p-,
phosphorylated; RPE, retinal pigment epithelial. |
Inhibitory effect of LC on EMT is blocked
by a PPAR-γ inhibitor
To further explore whether PPAR-γ was the critical
factor of LC on EMT of RPE cells, a PPAR-γ inhibitor (GW9662) was
used. ARPE-19 cells showed the typical mesenchymal markers after
treatment with TGF-β1 (Fig. 2A and
D), as shown by the upregulation of Vimentin, N-Cadherin, α-SMA
and FN expression. LC attenuated the EMT effect of TGF-β1 on RPE
cells. Inhibition of LC on EMT was blocked by GW9662 (Fig. 5A and B). A similar trend was
detected in human primary RPE cells (Fig. 5C and D).
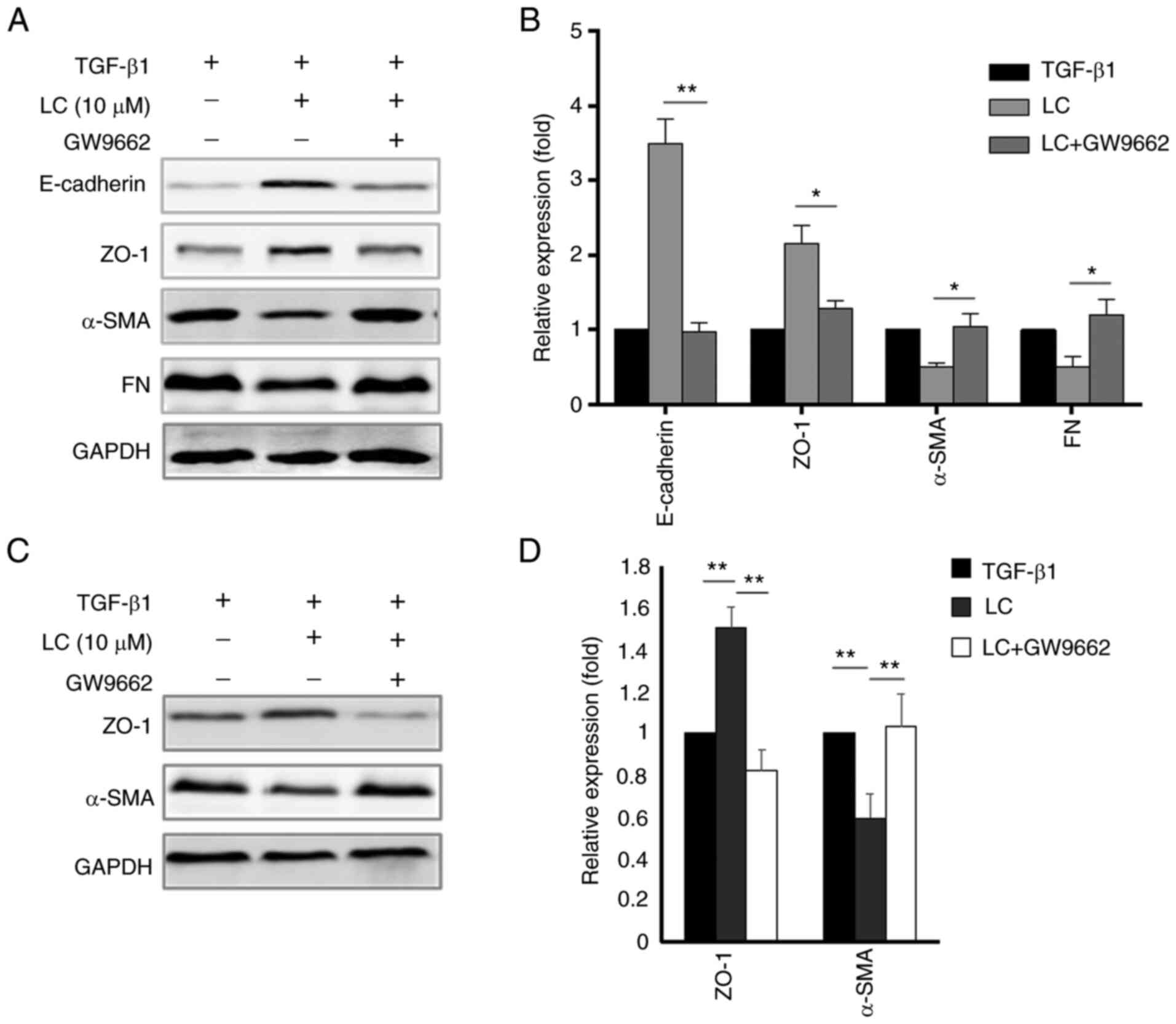 | Figure 5Effect of GW9662 on the
epithelial-mesenchymal transition of RPE cells induced by TGF-β1.
(A) After ARPE-19 cells were treated with 10 ng/ml TGF-β1 with LC
(10 µM) and GW9662 for 48 h, E-cadherin, ZO-1, α-SMA and FN
were detected using the corresponding antibody. (B)
Semi-quantification of protein levels from three independent
experiments. (C) After human primary RPE cells were treated with 10
ng/ml TGF-β1 with LC (10 µM) and GW9662 for 48 h, ZO-1 and
α-SMA were detected. (D) Semi-quantification of protein levels from
three independent experiments. *P<0.05,
**P<0.01. LC, L-carnitine; ZO-1, zonula occludens-1;
α-SMA, α-smooth muscle actin; FN, fibronectin; RPE, retinal pigment
epithelial. |
Relationships among the JNK signaling
pathway, ERK signaling pathway, PPARγ and NF-κB on EMT of RPE
cells
To investigate the underlying signaling pathways,
inhibitors of JNK, ERK and PPARγ were used to explore their
associations with each other. As shown in Fig. 6A-C, p-JNK and p-ERK protein
expression were significantly lower after treatment with JNK and
ERK inhibitors (SP600125 and U0126, respectively), leading to the
upregulation of PPAR-γ and downregulation of p-p65. Furthermore,
GW9662 did not affect upregulation of p-JNK and p-ERK induced by
TGF-β1, but it downregulated the expression of p-p65.
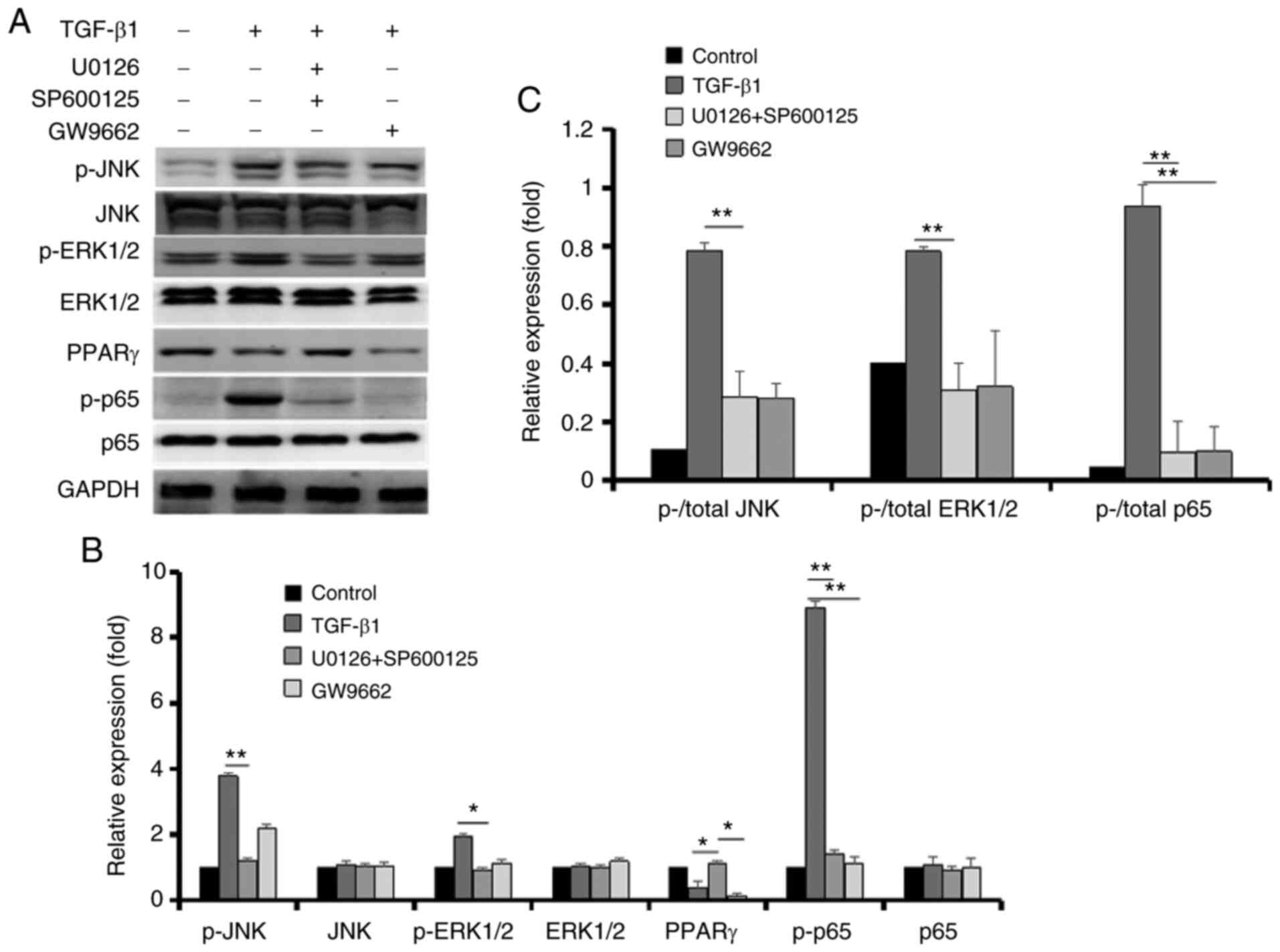 | Figure 6Effects of U0126, SP600125 and GW9662
on the expression of p-JNK, JNK, p-ERK1/2, ERK1/2, PPARγ, p-p65 and
p65 in retinal pigment epithelial cells. (A) After ARPE-19 cells
were treated with 10 ng/ml TGF-β1 with or without U0126, SP600125
and GW9662 for 48 h, p-JNK, JNK, p-ERK1/2, ERK1/2, PPARγ, p-p65 and
p65 were detected using the corresponding antibody. (B)
Semi-quantification of protein levels from three independent
experiments. (C) The ratio of p-/total JNK protein, p-/total ERK1/2
protein and p-/total p65 protein in the graph.
*P<0.05, **P<0.01. LC, L-carnitine; p-,
phosphorylated; PPARγ, peroxisome proliferator-activated receptor
γ. |
Discussion
EMT in RPE cells is proposed to be a vital trigger
in PVR (3,4,10,11). A number of studies have
demonstrated this effect in response to various cytokines,
especially TGF-β1, in the process of PVR formation (3,4,10,11). In our previous study, LC reduced
EMT in RPE cells. LC, a quaternary ammonium compound, is
synthesized from methionine and lysine. It transports fatty acids
from the cytosol to the mitochondria for processing in lipid
catabolism (25). Recently, LC
was found to play vital roles in oxidative (26) and fibrotic diseases (21). To the best of our knowledge, the
present study was the first to show that LC inhibited
TGF-β1-induced EMT in RPE cells. Moreover, it was also found that
LC prevented TGF-β1-induced EMT via controlling the JNK and ERK1/2
pathways, not the classical Smad signaling pathway. Finally, it was
demonstrated that PPARγ was a critical factor in the underlying
mechanism of LC on EMT in RPE cells.
Recent reports have shown that LC is associated with
various pathological conditions. Chou et al (13) found that L-carnitine reversed the
EMT induction caused by perfluorooctanesulfonate in renal tubular
epithelial cells and alleviated cell migration by activating PPARγ.
LC was also reported to attenuate cardiac fibrosis caused by
sunitinib in hypertensive rats, which was dependent on NF-κB
(27). In another previous
report, LC attenuated liver fibrosis via upregulation of the
mitochondrial pathway (25).
Nevertheless, to our knowledge, there has been no report concerning
the role of LC in the EMT of RPE cells. To test the hypothesis that
LC abrogates TGF-β1-induced EMT in RPE cells, ARPE-19 cells and
human primary RPE cells were cultured with TGF-β1 and LC in the
present study. It was found that the expression levels of
epithelial markers were significantly increased, whereas
mesenchymal markers and mobility of RPE cells were significantly
decreased with increasing concentrations of LC. Taken together, the
data suggested that 10 µM LC could be useful for abrogating
EMT in RPE cells.
The downstream pathways of TGF-β1 include not only
the canonical Smad2/3 signaling pathway, but also the non-canonical
p38/MAPK, JNK and ERK1/2 pathways (28,29). Although the Smad2/3 signaling
pathway plays an important role in TGF-β1-induced RPE cell EMT,
several lines of evidence have shown that MAPK pathways integrate
with the Smad2/3 signaling pathway to mediate EMT (28,30). In RPE cells, the present study
also demonstrated that the non-canonical JNK and ERK1/2 pathways
were activated by TGF-β1, as well as the canonical Smad2/3
signaling pathway. Finally, LC was found to attenuate
TGF-β1-induced phosphorylation of JNK and ERK1/2, but not Smad2/3
directly.
Several studies have reported that LC alleviates EMT
and tissue fibrosis via a PPARγ-dependent mechanism in the kidney
and heart (13,21,27). Of note, another previous study
demonstrated that GW9662 was a poor inhibitor of
fibroblast-to-myofibroblast differentiation (31). Therefore, in order to explore
whether this inhibition of TGF-β1-induced EMT on RPE cells was
PPARγ-dependent, the PPARγ antagonist GW9662 was used in
combination with LC. It was found that, without PPARγ, LC did not
inhibit RPE cell EMT. That is to say, the inhibitory effect of LC
on RPE cells was PPARγ-dependent, similar to results from Zambrano
et al (21) and Blanca
et al (27).
NF-κB-Snail signaling is hypothesized to play a
vital role in EMT, tumor cell invasion and metastasis. It regulates
the EMT process via decreasing expression of various epithelial
markers (32) and by increasing
expression of mesenchymal markers (33). In fact, we previously showed that
Snail activation was important in the EMT of RPE cells induced by
TGF-β1 (34,35). In the present study, it was
demonstrated that LC significantly decreased NF-κB and Snail
expression at the protein level. These results may partly explain
the underlying mechanism of the inhibitory effect of LC on the EMT
process.
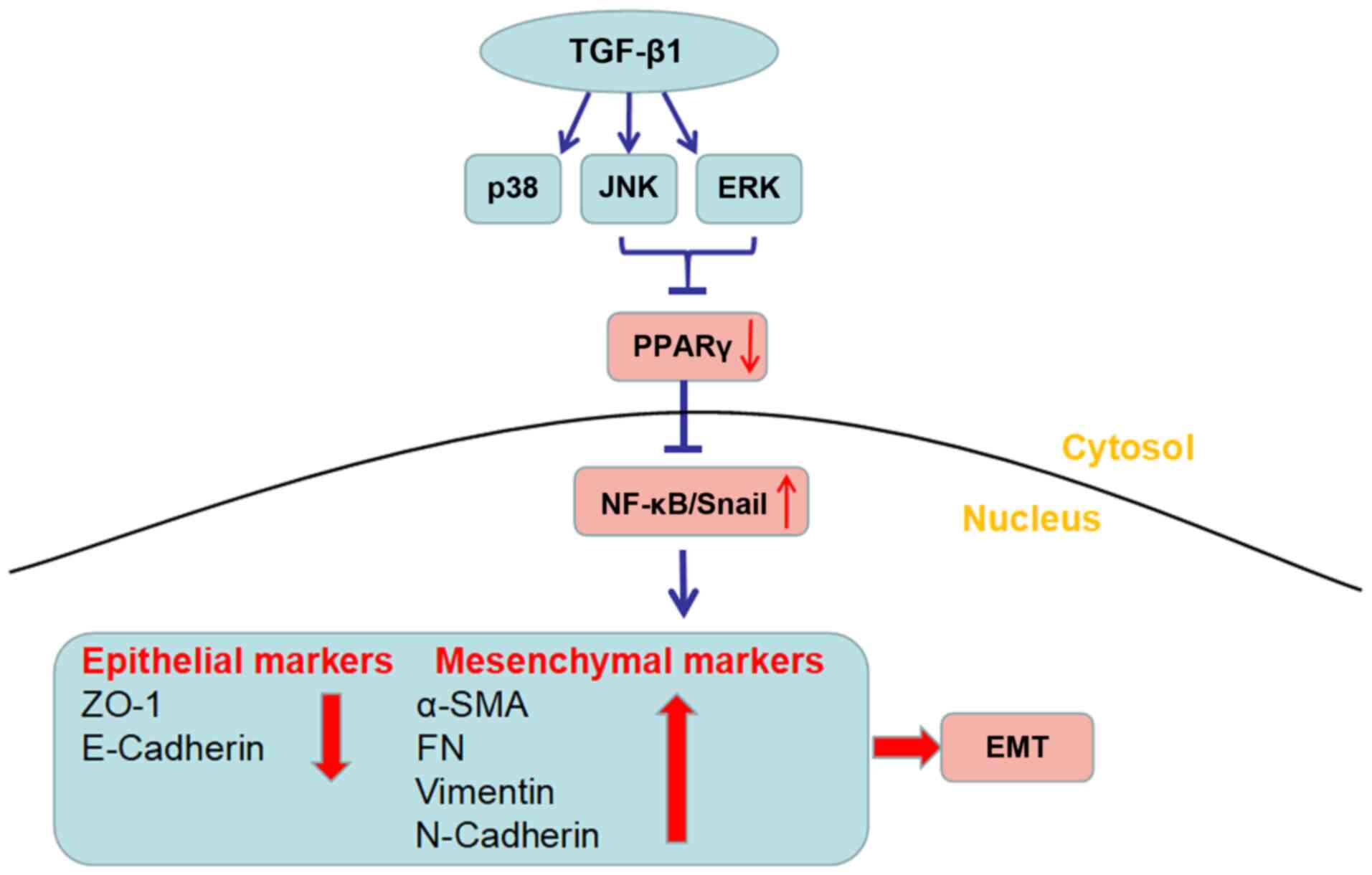
In summary, the current study provided evidence for
the first time that LC inhibited TGF-β1-induced EMT in RPE cells.
The mechanism underlying this process was found to be inactivation
of non-Smad pathways, including ERK1/2 and JNK pathways. Moreover,
the inhibitory effect of LC on RPE cells was revealed to be
PPARγ-dependent. Fig. 7 presents
an outline of the proposed underlying mechanism. The present study
supports the notion that LC has inhibitory effects on EMT in RPE
cells. However, there are limitations of the present study as most
assays in this study were performed using ARPE-19 cells due to the
limited number of primary human RPE cells. Also, no control cell
line was used in this study. Based on these results, we are also
exploring further effects on the primary human RPE cells and the
effects of LC injected into PVR model rat eyes. The in vivo
data will be presented in another future article.
To conclude, this study suggested that LC attenuated
EMT via inhibition of the Erk1/2 and JNK pathways, which was
dependent on PPAR-γ expression. LC may have potential value in the
prevention and treatment of PVR.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and FW designed this study. ML, HL and SY
performed the experiments. XL and CZ analyzed the data. ML and HL
drafted this article. CZ and FW revised the article. ML, HL and SY
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Shanghai Tenth People's Hospital (Shanghai, China) and was in
compliance with the Declaration of Helsinki. Donors' eyes were
obtained from the Eye Bank of Shanghai Tenth People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This study was funded by a grant from the National Natural
Science Foundation of China (grant no. 81500727) and the
Fundamental Research Funds for the Central Universities (grant no.
22120180053).
References
|
1
|
Gartry DS, Chignell AH, Franks WA and Wong
D: Pars plana vitrectomy for the treatment of rhegmatogenous
retinal detachment uncomplicated by advanced proliferative
vitreoretinopathy. Br J Ophthalmol. 77:199–203. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leiderman YI and Miller JW: Proliferative
vitreoretinopathy: Pathobiology and therapeutic targets. Semin
Ophthalmol. 24:62–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pastor JC, de la Rúa ER and Martín F:
Proliferative vitreoretinopathy: Risk factors and pathobiology.
Prog Retin Eye Res. 21:127–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu J, Liu F, Cui SJ, Liu Y, Song ZY, Cao
H, Chen FE, Wang WJ, Sun T and Wang F: Vitreous proteomic analysis
of proliferative vitreoretinopathy. Proteomics. 8:3667–3678. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kurimoto R, Iwasawa S, Ebata T, Ishiwata
T, Sekine I, Tada Y, Tatsumi K, Koide S, Iwama A and Takiguchi Y:
Drug resistance originating from a TGF-β/FGF-2-driven
epithelial-to-mesenchymal transition and its reversion in human
lung adenocarcinoma cell lines harboring an EGFR mutation. Int J
Oncol. 48:1825–1836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim J, Kong J, Chang H, Kim H and Kim A:
EGF induces epithelial-mesenchymal transition through
phospho-Smad2/3-Snail signaling pathway in breast cancer cells.
Oncotarget. 7:85021–85032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garweg JG, Tappeiner C and Halberstadt M:
Pathophysiology of proliferative vitreoretinopathy in retinal
detachment. Surv Ophthalmol. 58:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamouille S and Derynck R: Cell size and
invasion in TGF-beta-induced epithelial to mesenchymal transition
is regulated by activation of the mTOR pathway. J Cell Biol.
178:437–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Charteris DG: Proliferative
vitreoretinopathy: Pathobiology, surgical management, and
adjunctive treatment. Br J Ophthalmol. 79:953–960. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li M, Li H, Liu X, Xu D and Wang F:
MicroRNA-29b regulates TGF-β1-mediated epithelial-mesenchymal
transition of retinal pigment epithelial cells by targeting AKT2.
Exp Cell Res. 345:115–124. 2016. View Article : Google Scholar
|
|
11
|
Yao H, Li H, Yang S, Li M, Zhao C, Zhang
J, Xu G and Wang F: Inhibitory effect of bone morphogenetic protein
4 in retinal pigment epithelial-mesenchymal transition. Sci Rep.
2:321822016. View Article : Google Scholar
|
|
12
|
Martin H: Role of PPAR-gamma in
inflammation. Prospects for therapeutic intervention by food
components. Mutat Res. 690:57–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chou HC, Wen LL, Chang CC, Lin CY, Jin L
and Juan SH: From the Cover: l-Carnitine via PPARγ- and
Sirt1-dependent mechanisms attenuates epithelial-mesenchymal
transition and renal fibrosis caused by perfluorooctanesulfonate.
Toxicol Sci. 160:217–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferguson HE, Kulkarni A, Lehmann GM,
Garcia-Bates TM, Thatcher TH, Huxlin KR, Phipps RP and Sime PJ:
Electrophilic peroxisome proliferator-activated receptor-gamma
ligands have potent antifibrotic effects in human lung fibroblasts.
Am J Respir Cell Mol Biol. 41:722–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kao HF, Chang-Chien PW, Chang WT, Yeh TM
and Wang JY: Propolis inhibits TGF-β1-induced
epithelial-mesenchymal transition in human alveolar epithelial
cells via PPARγ activation. Int Immunopharmacol. 15:565–574. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang IM, Yang CH and Yang CM:
Docosahexaenoic acid reduces linoleic acid induced monocyte
chemoattractant protein-1 expression via PPARγ and nuclear
factor-κB pathway in retinal pigment epithelial cells. Mol Nutr
Food Res. 58:2053–2065. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willermain F, Dulku S, Gonzalez NS, Blero
D, Driessens G, De Graef C, Caspers L and Bruyns C:
15-Deoxy-12,14-prostaglandin J2 inhibits interferon gamma induced
MHC class II but not class I expression on ARPE cells through a
PPAR gamma independent mechanism. Prostaglandins Other Lipid
Mediat. 80:136–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rolim LC, da Silva EM, Flumignan RL, Abreu
MM and Dib SA: Acetyl-L-carnitine for the treatment of diabetic
peripheral neuropathy. Cochrane Database Syst Rev.
6:CD0112652019.PubMed/NCBI
|
|
19
|
Li M, Li H, Jiang P, Liu X, Xu D and Wang
F: Investigating the pathological processes of rhegmatogenous
retinal detachment and proliferative vitreoretinopathy with
metabolomics analysis. Mol Biosyst. 10:1055–1062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baci D, Bruno A, Bassani B, Tramacere M,
Mortara L, Albini A and Noonan DM: Acetyl-L-carnitine is an
anti-angiogenic agent targeting the VEGFR2 and CXCR4 pathways.
Cancer Lett. 429:100–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zambrano S, Blanca AJ, Ruiz-Armenta MV,
Miguel-Carrasco JL, Arévalo M, Mate A and Vázquez CM: L-carnitine
attenuates the development of kidney fibrosis in hypertensive rats
by upregulating PPAR-γ. Am J Hypertens. 27:460–470. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parapuram SK, Ganti R, Hunt RC and Hunt
DM: Vitreous induces components of the prostaglandin E2
pathwayaglandin E2 pathway in human retinal pigment epithelial
cells. Invest Ophthalmol Vis Sci. 44:1767–1774. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Chen Z, Mei Y, Lei H, Tian R, Ni N, Han F,
Gan S and Sun S: LYTAK1, a TAK1 inhibitor, suppresses proliferation
and epithelial-mesenchymal transition in retinal pigment epithelium
cells. Mol Med Rep. 14:145–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishikawa H, Takaki A, Tsuzaki R, Yasunaka
T, Koike K, Shimomura Y, Seki H, Matsushita H, Miyake Y, Ikeda F,
et al: L-carnitine prevents progression of non-alcoholic
steatohepatitis in a mouse model with upregulation of mitochondrial
pathway. PLoS One. 9:e1006272014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferreira GC and McKenna MC: L-Carnitine
and Acetyl-L-carnitine roles and neuroprotection in developing
brain. Neurochem Res. 42:1661–1675. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blanca AJ, Ruiz-Armenta MV, Zambrano S,
Miguel-Carrasco JL, Arias JL, Arévalo M, Mate A, Aramburu O and
Vázquez CM: Inflammatory and fibrotic processes are involved in the
cardiotoxic effect of sunitinib: Protective role of L-carnitine.
Toxicol Lett. 241:9–18. 2016. View Article : Google Scholar
|
|
28
|
Ling G, Ji Q, Ye W, Ma D and Wang Y:
Epithelial-mesenchymal transition regulated by p38/MAPK signaling
pathways participates in vasculogenic mimicry formation in SHG44
cells transfected with TGF-β cDNA loaded lentivirus in vitro and in
vivo. Int J Oncol. 49:2387–2398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park JH, Yoon J, Lee KY and Park B:
Effects of geniposide on hepatocytes undergoing
epithelial-mesenchymal transition in hepatic fibrosis by targeting
TGFβ/Smad and ERK-MAPK signaling pathways. Biochimie. 113:26–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou X, Kuang X, Long C, Liu W, Tang Y,
Liu L, Liu H, He J, Huang Z, Fan Y, et al: Curcumin inhibits
proliferation and epithelial-mesenchymal transition of retinal
pigment epithelial cells via multiple pathways. Curr Mol Med.
17:312–319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou BY, Buckley ST, Patel V, Liu YX, Luo
J, Krishnaveni MS, Ivan M, DeMaio L, Kim KJ, Ehrhardt C, et al:
Troglitazone attenuates TGF-β1-induced EMT in alveolar epithelial
cells via a PPARγ-independent mechanism. PLoS One. 7:e388272012.
View Article : Google Scholar
|
|
32
|
Han D, Wu G, Chang C, Zhu F, Xiao Y, Li Q,
Zhang T and Zhang L: Disulfiram inhibits TGF-β-induced
epithelial-mesenchymal transition and stem-like features in breast
cancer via ERK/NF-κB/Snail pathway. Oncotarget. 6:40907–40919.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho IH, Jang EH, Hong D, Jung B, Park MJ
and Kim JH: Suppression of LPS-induced epithelial-mesenchymal
transition by aqueous extracts of Prunella vulgaris through
inhibition of the NF-κB/Snail signaling pathway and regulation of
EMT-related protein expression. Oncol Rep. 34:2445–2450. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li H, Li M, Xu D, Zhao C, Liu G and Wang
F: Overexpression of Snail in retinal pigment epithelial triggered
epithelial-mesenchymal transition. Biochem Biophys Res Commun.
446:347–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H, Chang HM, Shi Z and Leung PCK: SNAIL
mediates TGF-β1-induced downregulation of pentraxin 3 expression in
human granulosa cells. Endocrinology. 159:1644–1657. 2018.
View Article : Google Scholar : PubMed/NCBI
|