Introduction
Preeclampsia is a systemic disease during pregnancy,
with a global incidence of ~8% in 2019 (1-3).
Severe complications of preeclampsia, such as eclampsia and HELLP
syndrome, are the most common causes of maternal and perinatal
infant death (4). However, to
date, the etiology and underlying pathogenic mechanism of
preeclampsia remain elusive. Termination of pregnancy currently the
only effective treatment measure, especially for severe early-onset
preeclampsia, which may cause maternal complications and iatrogenic
preterm birth (5,6). Early diagnosis and specific
treatment of preeclampsia are severely restricted owning to its
unclear pathogenic mechanism (7).
Circular (circ)RNAs participate in the occurrence
and development of a variety of diseases, such as Alzheimer's
disease, hypertrophic osteoarthropathy, and atherosclerosis
(8). Previous studies have
demonstrated that abnormal expression of circRNAs is associated
with tumor occurrence, invasion, proliferation and metastasis
(9-12). For instance, hsa_circ_100395
regulates the proliferation, migration and invasion of lung cancer
cells through the microRNA (miRNA/miR)-1228/transcription factor 21
pathway (13). In addition,
circRNA La-ribonucleoprotein 4 is considered to act as a miR-424
sponge to inhibit the biological behavior of gastric cancer
(14). Other biological
functions of circRNAs include chemotherapy resistance and
radiosensitivity (15).
Abnormally expressed circRNAs have been reported to exhibit high
sensitivity and specificity as diagnostic markers (16-18). For instance, hsa_circRNA_103809
and hsa_circRNA_104700 may be involved in the occurrence of
colorectal cancer and can be used as effective biomarkers to
diagnose colorectal cancer (19). In addition, circRNAs affect
cisplatin resistance in non-small cell lung cancer (20).
Mounting evidence has revealed that abnormal
expression of circRNAs serves pivotal roles in preeclampsia. For
instance, hsa_circ_0036877 may be used as a potential biomarker for
early diagnosis of preeclampsia (21). In addition, hsa_circ_0001438,
hsa_circ_0001326 and hsa_circ_32340 serve crucial roles in the
progression of preeclampsia (22). Compared with normal pregnancy,
the levels of circRNA-0004904 are increased in the plasma of
patients with preeclampsia and may be a potential biomarker for
diagnosing preeclampsia (23).
However, the role of circRNA-0004904 in preeclampsia has not been
fully elucidated. Therefore, the present study aimed to determine
the role of circRNA-0004904 in preeclampsia and to clarify its
underlying pathogenic mechanism of action.
Materials and methods
Study subjects and placental tissue
collection
The present study was approved by the Ethics
Committee of the West China Hospital of Sichuan University
(Chengdu, China), and all patients signed written informed consent
forms prior to the commencement of the study. The placenta tissues
were obtained from pregnant women who were admitted to the West
China Hospital of Sichuan University between January 2018 and
January 2019. The diagnosis of patients with preeclampsia complied
with The American College of Obstetricians and Gynecologists
Practice Bulletin 2019 (3). The
control group comprised women with normal pregnancy, and those with
pregnancy diseases were excluded. The ages of pregnant women in the
two groups ranged between 24-38 years, with a mean age of 28 years.
There was no statistical difference in the age of pregnant women
between the two groups. Placental tissues (1 cm3) and
serum samples were collected from 30 pregnant women with
preeclampsia and 30 normal parturients with the same gestational
age (control group).
Cell culture, transfection and
reagents
HTR-8 (cat. no. CRL-3271) and JEG-3 (cat. no.
HTB-36) cells were obtained from the American Type Culture
Collection and cultured in Dulbecco's modified Eagle's medium (cat.
no. D5796; MilliporeSigma), containing 10% fetal bovine serum (cat.
no. 16000-044; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (cat. no. C0222; Beyotime Institute of
Biotechnology). The cells were incubated at 37°C in presence of 5%
CO2. The circRNA-0004904-targeting small interfering
(si)RNA (siRNA-001 and siRNA-002), negative control siRNA (siNC),
and lentivirus-mediated circRNA-0004904 overexpression (OE) vector
with its corresponding negative control (OE-NC) were supplied by
Shanghai GenePharma Co., Ltd. The sequences of the siRNAs were as
follows: siRNA-001, 5′-GCA AAT TCC AGA GAG CAC GTT-3′; siRNA-002,
5′-AAA TTC CAG AGA GCA CGT TTT-3′; and siNC, 5′-UUC UUC GAA GGU GUC
ACG UTT-3′. Plasmid expression vectors for the overexpression of
fused in sarcoma (FUS) and autophagy-related 12 (ATG12) (accession
nos. NM_004960 and NM_004707, respectively) were constructed by
GeneCopoeia, Inc. In addition, siATG7 (5′-GGU CAA AGG ACG AAG AUA
ACA-3′), siFUS-1 (5′-GGC UAU GGA ACU CAG UCA ACU-3′), siFUS-2
(5′-GGA CAG CAG CAA AGC UAU AAU-3′), hsa-miR-570-3p mimics (cat.
no. HmiR-SN0669), mimics control (cat. no. CmiR-SN0001-SN),
hsa-miR-570-3p inhibitor (cat. no. HmiR-AN0669-SN-10) and inhibitor
control (cat. no. CmiR-AN0001-SN) were obtained from GeneCopoeia,
Inc. For transfections, cells were seeded in a 6-well plate
(1×105 cells/well). When the cells were ~80% confluent,
2 µg plasmids were transfected using the EndoFectin™ MAX
transfection reagent (cat. no. EFM1004-01; GeneCopoeia, Inc.) for
24 h at 37°C. The mRNA or protein levels were detected at 48 h
post-transfection.
RNA pull-down assay
A circRNA-0004904 probe was used for RNA pull-down
assay (cat. no. A04001; GenePharma Co., Ltd.) as previously
described (24). Cells were
lysed using RIPA Lysis Buffer (cat. no. P0013D, Beyotime
Biotechnology). RNA pulldown assay was performed using Pierce™
Magnetic RNA-Protein Pull-Down Kit (cat. no. 20164; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions with
20 µl lysate per reaction. The miRNA level was detected by
reverse transcription-quantitative (RT-q)PCR.
RT-qPCR
RT-qPCR was performed as previously described
(24). Total RNA was extracted
from cells and tissues using TRIzol® reagent (Takara
Biotechnology Co., Ltd.) according to the manufacturer's
instructions. For miRNA analysis, cDNA was obtained using the
All-in-One™ miRNA First-Strand cDNA Synthesis kit (cat. no. QP013;
GeneCopoeia, Inc.), and RT-qPCR was performed using the All-in-One™
miRNA qRT-PCR Detection kit (cat. no. QP015; GeneCopoeia, Inc.). U6
small nuclear RNA was used as an endogenous control for
normalization. For circRNA analysis, cDNA was synthesized from 1
µg total RNA using the All-in-One First Strand Synthesis kit
(cat. no. AORT-0020; GeneCopoeia, Inc.). The real-time PCR kit was
purchased from GeneCopoeia, Inc. qPCR was performed under the
following conditions: Initial denaturation at 95°C for 10 min,
followed by 35 cycles of 95°C for 10 sec and 60°C for 20 sec, and a
final extension at 72°C for 10 sec. qPCR was conducted using the
PCR System (Bio-Rad Laboratories, Inc.) according to the
manufacturer's instructions. Each experiment was performed in
triplicate. Gene expression analysis was performed using the
2−ΔΔCq method (25).
The circ-0004904 primers (5′-CCT CCG GTG ATA GGT TCT CA-3′ and
3′-TAA ATT TGG TGC AGG GTG GT-5′) were synthesized by Shanghai
GenePharma Co., Ltd. Primers for ATG12 (cat. no. HQP055328), GAPDH
(cat. no. HQP064347), miR-570 (cat. no. HmiRQP0669) and U6 (cat.
no. HmiRQP9001) were purchased from GeneCopoeia, Inc.
Western blot assay
Protein expression levels of ATG12, GAPDH, LC3-II,
P62, FUS and vascular endothelial growth factor (VEGF) were
detected by western blotting. The transfected HTR-8 and JEG-3 cells
were cultured in 6-well plates and lysed at 48 h post-transfection
with radio-immunoprecipitation assay lysis buffer (MilliporeSigma)
containing phosphatase and protease inhibitors. Protein
quantification was performed using BCA assay. Proteins (20
µg/lane) were separated by 10% SDS-PAGE and transferred to
PVDF membranes. The membranes were blocked with 5% BSA for 1 h at
room temperature and incubated overnight at 4°C with the following
primary antibodies (dilution, 1:1,000): Rabbit monoclonal
anti-ATG12 (cat. no. ab109491; Abcam), rabbit recombinant
monoclonal GAPDH (cat. no. ab181602; Abcam), rabbit recombinant
monoclonal LC3-II (cat. no. ab192890; Abcam), rabbit recombinant
monoclonal anti-P62 (cat. no. ab109012; Abcam), rabbit recombinant
monoclonal FUS (cat. no. ab124923; Abcam) and rabbit recombinant
monoclonal VEGF (cat. no. AB1876-I; Sigma-Aldrich; Merck KGaA).
Following washing for 3×10 min using the Western Wash Buffer (cat.
no. P0023C3; Beyotime Institute of Biotechnology) at room
temperature, HRP-conjugated secondary antibodies (1:2,000; cat. no.
7074; Cell Signaling Technology, Inc.) were applied to the
membranes for 1 h at room temperature, and chemiluminescent signals
of membranes were measured by using an ImageQuant LAS system (GE
Healthcare). Densitometric analysis was performed using Image Lab
(version 3.0, Bio-Rad Laboratories). All experiments were performed
in triplicate and repeated three times.
Matrigel invasion assay
Matrigel invasion assay was performed to assess the
invasive ability of HTR8 or JEG3 cells as previously described
(26,27). The upper chamber of the 24-well
Transwell insert was pre-coated with 100 µl diluted Matrigel
at 37°C for 5 h. A total of 1×105 HTR8 or JEG3 cells
resuspended in serum-free medium were seeded into the upper
chamber. The lower chambers contained medium with 10% fetal bovine
serum. The cells were incubated at 37°C for 24 h. Subsequently,
cotton swabs were used to remove the non-invasive cells. The
migrated cells attached to the lower surface of the membrane were
fixed with 4% paraformaldehyde and stained with 0.5% crystal violet
for 15 min at room temperature. The cells that migrated to the
lower chamber were observed under an optical microscope (×20
magnification). Finally, the crystal violet on the membrane was
solubilized with 500 µl 33% acetic acid solution. The
optical density of the plates was measured at 595 nm wavelength
using a microplate reader.
Fluorescence in situ hybridization (FISH)
assay
The source of template was total RNA of
circRNA0004904 (sequence, 5′-AGA GCA CGT TTT CAA TAT CAT AGG AGC
ATT TGA TAT TCC ACG CTT TGT GTA CAA TTC AGA AAG AAA AAA ATT TCTT CC
TCTGTTAATGAC CAA CCA CCC TGC ACC AAATTTATT TGG AAC ACC AAG AGA TAA
AGC AGA GAT GTT TCG TGA GCG ATA TAC CAT TTT GCA CCA GAG GAC CCA CAG
GCA TGA ATT ATT TAC TCC TCC GGT GAT AGG TTC TCA CCC TGA TGA AAG CGG
AAG CAA ATT CCA G-3′). A Cy3-labeled probe against circRNA0004904
(sequence, 5′-CUC CUA UGA UAU UGA AAA CGU GCU CUC UGG AAU UUG CUUC
CGC UUU CAU C-3′) and a FITC-labeled probe against miR-570
(sequence, 5′-CAA AGG UAA UUG CUG UUU UCG-3′) were synthesized by
Shanghai GeneChem Co., Ltd. The FISH assay was conducted according
to the manufacturer's instructions provided with a FISH kit (cat.
no. F32201; Shanghai GeneChem Co., Ltd.) (28,29). HTR8 cells (2×105) were
fixed with absolute ethanol to in a 48-well plate at room
temperature for 15 min, followed by treatment with 100 µl
0.1% Triton x-100 at room temperature for 15 min. Gradient ethanol
dehydration (70, 85 and 100%). Subsequently, denaturation was
performed at 73°C for 5 min. A total of 100 µl probe mixture
was added and incubated overnight at 37°C. The next day, the probe
mixture was removed, 100 µl 0.4X saline sodium citrate/0.3%
Tween-20 was added, and the cells were washed twice at room
temperature for 5 min. Finally, 100 µl Hoechst stain was
added to each well for 15 min at room temperature. Confocal images
were acquired using a laser-scanning confocal microscope (×400
magnification). A total of five fields were imaged per sample. All
experiments were performed in triplicate and repeated three
times.
Dual-luciferase reporter assay
Dual-luciferase receptor assay was performed using
the Dual-Luciferase Reporter Assay system (Promega Corporation). In
brief, 2.5×105 HTR8 cells were harvested in a 24-well
plate. A total of 2 µg wild-type or mutant circ-000490
reporter was constructed by Shanghai GeneChem Co., Ltd. and
co-transfected with 100 nM miR-570 or miR-NC and the Renilla
plasmid using EndoFectin™ MAX transfection reagent (cat. no.
EFM1004-01; GeneCopoeia, Inc.) for 24 h at 37°C. Luciferase
activity was detected at 48 h post-transfection. Firefly luciferase
activity was normalized to that of Renilla luciferase. All
experiments were performed in triplicate and repeated three
times.
Tandem fluorescent-tagged LC3
(mRFP-EGFP-LC3)
A total of 1×105 JEG3 cells were
co-transfected with mRFP-GFP-LC3 and siNC, siRNA-001 or siRNA-002
using the EndoFectin™ MAX transfection reagent for 24 h at 37°C and
cultured for 22 h. Subsequently, the cells were treated with
Earle's balanced salts solution for 2 h at 37°C. HTR8 cells were
co-transfected with mRFP-GFP-LC3 and OE or OE-NC using the
EndoFectin™ MAX transfection reagent for 24 h at 37°C and analyzed
by confocal microscopy after 24 h. LC3 dots were quantified using
the image pro-plus 6.0 software. All experiments were performed in
triplicate and repeated three times. To evaluate the autophagic
flux, 3-MA and chloroquine (CQ) were used. At 48 h
post-transfection, 5 mM 3-MA or 50 µM CQ were added to the
medium for 12 h at 37°C, and LC3 protein expression levels were
detected by western blotting.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
A total of 1×105 HTR-8 cells were seeded
into a 24-well plate and treated with 200 µl EdU (dilution,
1:1,000) in the dark for 1 h at 37°C using the BeyoClick™ EdU Cell
Proliferation kit (Beyotime Institute of Biotechnology) according
to the manufacturer's instructions. The cells were washed with PBS
(5 min three times). Hoechst 33342 (Beyotime Institute of
Biotechnology) was used for nuclear staining (30). Subsequently, the cells were
visualized under a fluorescence microscope (×200 magnification;
Olympus Corporation). A total of five fields were analyzed per
sample.
Bioinformatics
The interaction between circ-0004904 and miRNAs was
predicted using the CircInteractome database (https://circinteractome.nia.nih.gov/)
(31). The binding site between
ATG12 and miR-570 was predicted by TargetScan (32,33).
Statistical analysis
All statistical analyses were performed using SPSS
software version 17.0 (SPSS, Inc.). Data are presented as the mean
± SD or SEM. Statistical analysis was performed by the unpaired
Student's t-test or one-way ANOVA with Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
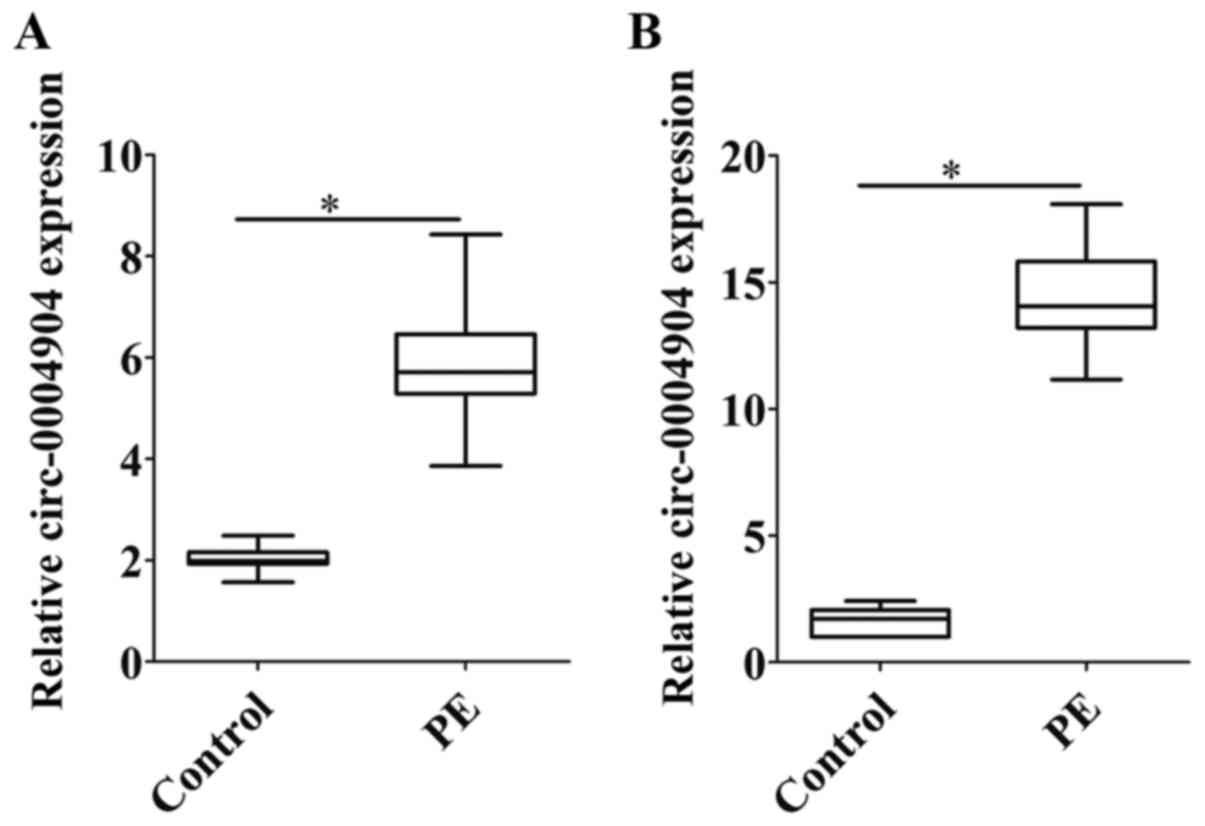
Expression levels of circ-0004904 are
increased in placental tissues and plasma samples of patients with
preeclampsia
The expression levels of circ-0004904 were detected
in the placental tissues and plasma samples of 30 patients with
preeclampsia and 30 normal parturients with the same gestational
age. The results demonstrated that the expression levels of
circ-0004904 were upregulated in the placental tissues and plasma
samples of patients with preeclampsia compared those in the control
group (Fig. 1A and B).
circ-0004904 regulates autophagy,
proliferation and migration of HTR8 and JEG3 cells
The roles of circ-0004904 were investigated in HTR8
and JEG3 cells. First, the expression levels of circ-0004904 were
detected in HTR8 and JEG3 cells. The results revealed that the
expression levels of circ-0004904 in JEG3 cells were higher
compared with those in HTR8 cells. Silencing or ectopic expression
of circ-0004904 significantly inhibited or elevated the expression
levels of circ-0004904, respectively (Fig. S1A and B). By contrast, silencing
or ectopic expression of circ-0004904 did not affect apoptosis
(data not shown). The results also demonstrated that silencing of
circ-0004904 repressed LC3-II expression, and promoted the
proliferation and migration of JEG3 cells (Figs. 2A, C and S2). By contrast, ectopic expression of
circ-0004904 upregulated the levels of LC3-II expression, and
inhibited the proliferation and migration of HTR8 cells (Figs. 2B, D and S2). In order to evaluate the effects
of circ-0004904 on autophagic flux, 3-MA and CQ were used; the
results demonstrated that autophagy mediated by circ-0004904 was
inhibited by 3-MA, but not CQ in HTR8 cells (Fig. 2E and F), which suggested that
circ-0004904 affected autophagosomes rather than
autophagolysosomes. In addition, mRFP-GFP-LC3 was used to detect
autophagic flux, and the results revealed that silencing of
circ-0004904 inhibited autophagic flux in JEG3 cells (Fig. 2G), whereas overexpression of
circ-0004904 promoted autophagic flux in HTR8 cells (Fig. 2H).
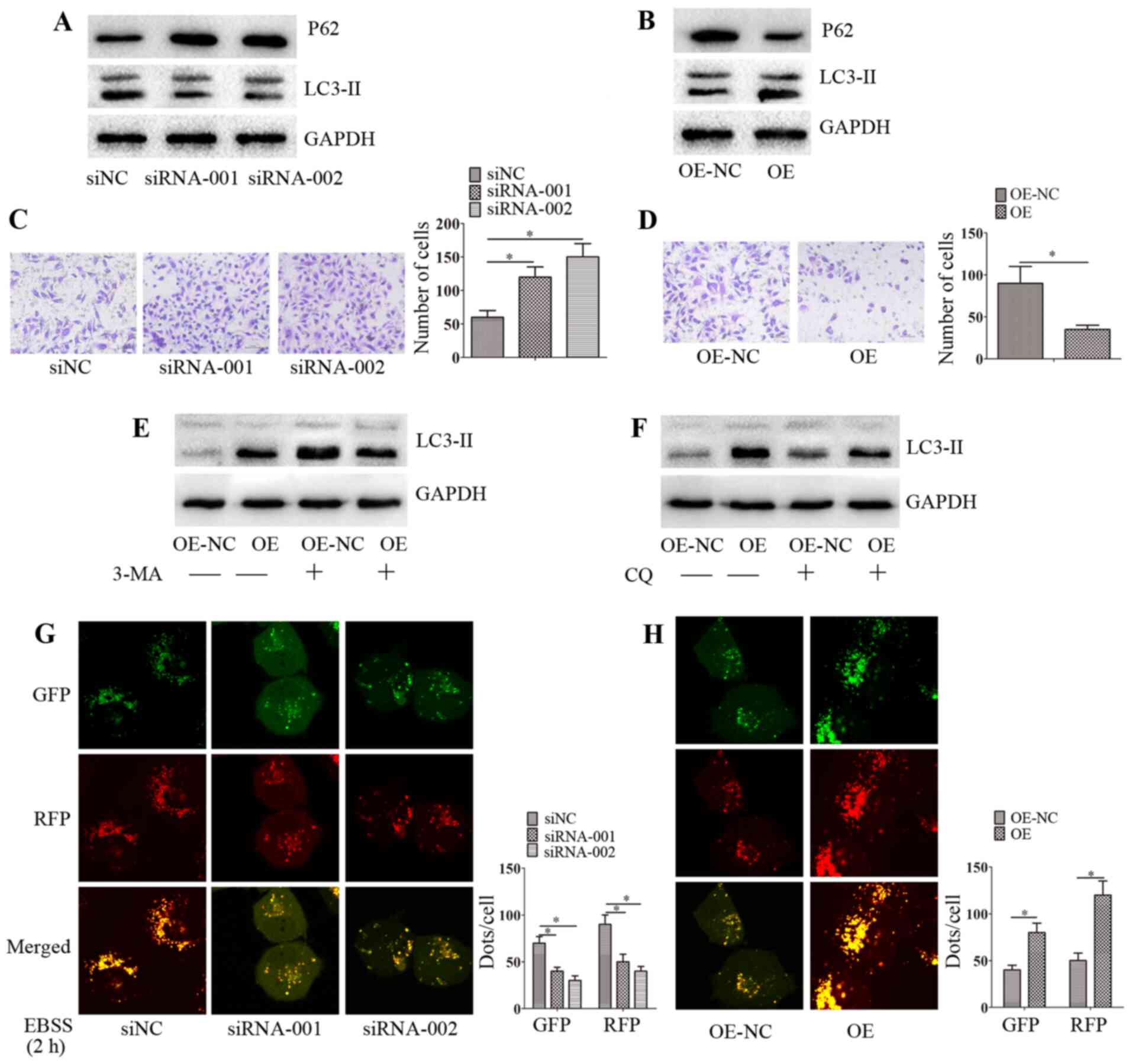 | Figure 2circ-0004904 promotes autophagy and
inhibits migration in HTR8 cells. (A and B) The expression levels
of LC3-II and P62 were determined by western blotting in (A) JEG3
cells transfected with siNC, siRNA-001 or siRNA-002 and (B) HTR8
cells transfected with OE-NC or OE. (C and D) The invasive ability
was assessed by Matrigel invasion assay in (C) JEG3 cells
transfected with siNC, siRNA-001 or siRNA-002 and (D) HTR8 cells
transfected with OE-NC or OE. (E and F) HTR8 cells were transfected
with OE-NC or OE were treated with (E) 3-MA or (F) CQ for 24 h, and
the expression levels of LC3-II were detected by western blotting.
(G and H) The distribution of mRFP-GFP-LC3 in (G) JEG3 cells
transfected with siNC, siRNA-001 or siRNA-002 and (H) HTR8 cells
transfected with OE-NC or OE was analyzed by confocal microscopy.
Data are presented as the mean ± SEM. *P<0.05. circ,
circular RNA; siRNA, small interfering RNA; siNC, negative control
siRNA; OE, overexpression vector; OE-NC, negative control vector;
GFP, green fluorescent protein; RFP, red fluorescent protein; EBSS,
Earle's balanced salt solution. |
circ-0004904-mediated autophagy inhibits
the proliferation and invasion of HTR8 cells
Since circ-0004904 promoted autophagic flux in HTR8
cells, the present study further assessed whether
circ-0004904-mediated autophagy may regulate cell proliferation and
invasion. The results demonstrated that ectopic expression of
circ-0004904 inhibited the proliferation and invasion of HTR8
cells. However, following the silencing of ATG7 or the addition of
3-MA, cell proliferation and invasion was partially promoted
(Fig. 3A-D). These results
suggested that circ-0004904-mediated autophagy inhibited cell
proliferation and invasion.
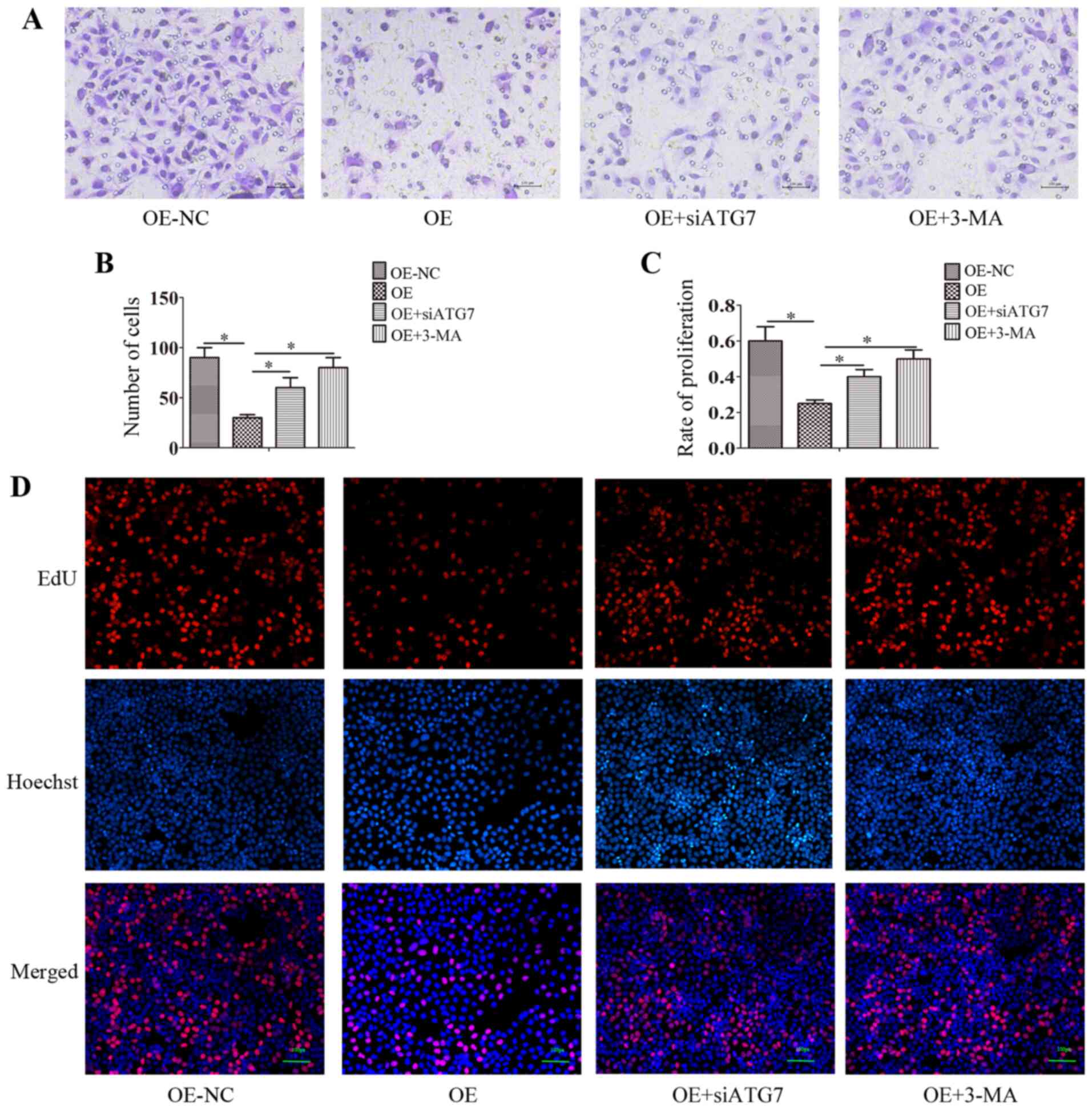 | Figure 3circ-0004904-mediated autophagy
inhibits the proliferation and invasion of HTR8 cells. (A and B)
HTR8 cells were transfected with OE or OE-NC plasmids,
co-transfected with OE + siATG7 or transfected with the OE plasmid
and treated with 3-MA, and the cell invasive ability was assessed
by Matrigel invasion assay. (A) representative images; (B) mean
number of invasive cells. (C and D) HTR8 cells were transfected
with OE, OE-NC or OE + siATG7, or transfected with OE and treated
with 3-MA, and the cell proliferative ability was determined by EdU
assay. (C) Mean number of proliferation cells; (D) representative
images. Data are presented as the mean ± SEM.
*P<0.05. circ, circular RNA; si, small interfering
RNA; OE, overexpression vector; OE-NC, negative control vector;
ATG7, autophagy-related 7. |
circ-0004904 interacts with miR-570
Bioinformatics analysis predicted that circ-0004904
interacted with a number of miRNAs, including miR-494, miR-513a-3p,
miR-570, miR-572, miR-589 and miR-876-3p. The results of the RNA
pull-down assays confirmed that circ-0004904 directly bound miR-570
(Fig. 4A). In addition,
circ-0004904 was mainly located in the cytoplasm, and circ-0004904
and miR-570 were co-localized in the cytoplasm in HTR8 cells
(Fig. 4B). Following silencing
of circ-0004904, the expression levels of miR-570 were increased in
JEG3 cells compared with those in the cells transfected with siNC
(Fig. 4C). By contrast, ectopic
expression of circ-0004904 inhibited the expression levels of
miR-570 in HTR8 cells compared with those in cells transfected with
the OE-NC vector (Fig. 4D).
Co-transfection with miR-570 mimics and a wild-type circ-0004904
vector inhibited luciferase activity compared with that observed
following transfection with the NC mimics in HTR8 cells (Fig. 4E).
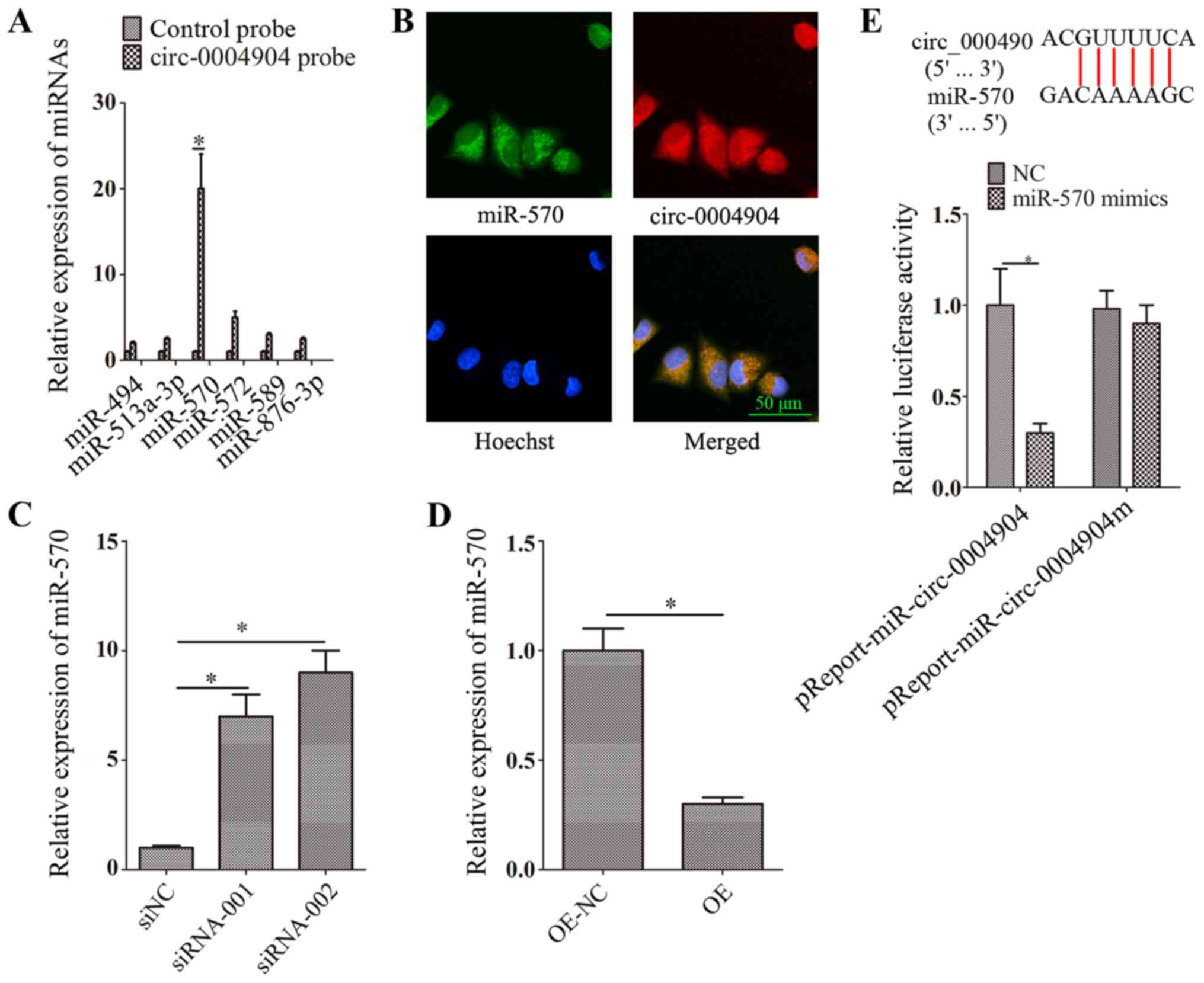 | Figure 4circ-0004904 interacts with miR-570.
(A) Bioinformatics analysis predicted that circ-0004904 interacted
with miR-494, miR-513a-3p, miR-570, miR-572, miR-589 and
miR-876-3p. The interaction between circ-0004904 and miR-570 was
assessed using RNA pull-down assay. Expression levels of miRNAs
were detected by RT-qPCR. (B) Fluorescence in situ
hybridization assay was used to identify the colocalization of
circ-0004904 and miR-570. Green, FITC-labeled miR-570 probe; red,
Cy3-labeled circ-0004904 probe. (C and D) The expression levels of
miR-570 were determined by RT-qPCR in (D) JEG3 cells transfected
with siNC, siRNA-001 or siRNA-002 and (D) HTR8 cells transfected
with OE-NC or OE. (E) Dual-luciferase reporter assay was used to
confirm the interaction between circ-0004904 and miR-570. Data are
presented as the mean ± SEM. *P<0.05. circ, circular
RNA; miR, microRNA; siRNA, small interfering RNA; siNC, negative
control siRNA; OE, overexpression vector; NC, negative control;
RT-qPCR, reverse transcription-quantitative PCR. |
circ-0004904 regulates ATG12 via
miR-570
Based on the results of the bioinformatics analysis,
ATG12 was selected as a potential target of miR-570. Transfection
with miR-570 inhibitors or mimics significantly inhibited or
elevated the levels of miR-570 compared with those in the
corresponding NC groups (Fig. S1C
and D). The results also revealed that transfection with the
miR-570 mimics downregulated the mRNA and protein expression levels
of ATG12 in HTR8 cells compared with those in the NC group
(Fig. 5A), whereas transfection
with the miR-570 inhibitors upregulated the expression levels of
ATG12 (Fig. 5B). The
dual-luciferase reporter assay confirmed that ATG12 was the target
of miR-570 in HTR8 cells (Fig.
5C). In addition, circ-0004904 positively regulated the
expression levels of ATG12 in HTR8 cells (Fig. 5D and E). However, following
transfection with the miR-570 inhibitors, this regulation was
partly blocked in HTR8 cells (Fig.
5F and G).
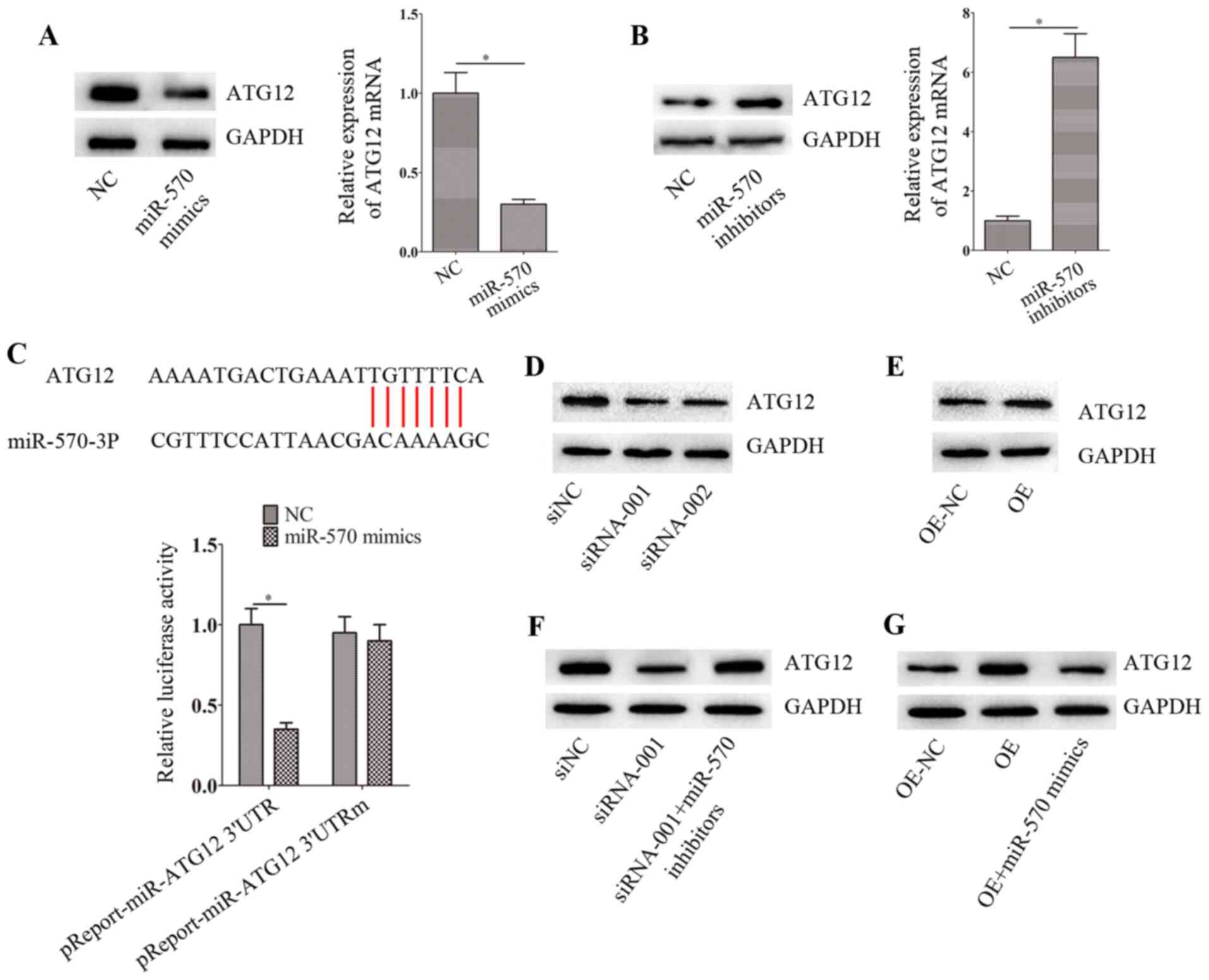 | Figure 5circ-0004904 regulates ATG12 via
miR-570. (A and B) The mRNA and protein levels of ATG12 were
determined by reverse transcription-quantitative PCR and western
blotting, respectively, in HTR8 cells transfected with (A) NC or
miR-570 mimics and (B) NC or miR-570 inhibitors. (C)
Dual-luciferase reporter assay was used to confirm the interaction
between the ATG12 3′-untranslated region and miR-570. (D and E) The
expression levels of ATG12 were determined by western blotting in
(D) JEG3 cells transfected with siNC, siRNA-001 or siRNA-002 and
(E) HTR8 cells transfected with OE-NC or OE. (F and G) The
expression levels of ATG12 were determined by western blotting in
(F) JEG3 cells transfected with siNC, siRNA-001 or siRNA-001 +
miR-570 inhibitors and (G) HTR8 cells transfected with OE-NC, OE or
OE + miR-570 mimics. Data are presented as the mean ± SEM.
*P<0.05. circ, circular RNA; miR, microRNA; siRNA,
small interfering RNA; siNC, negative control siRNA; OE,
overexpression vector; NC, negative control; RT-qPCR, reverse
transcription-quantitative PCR. |
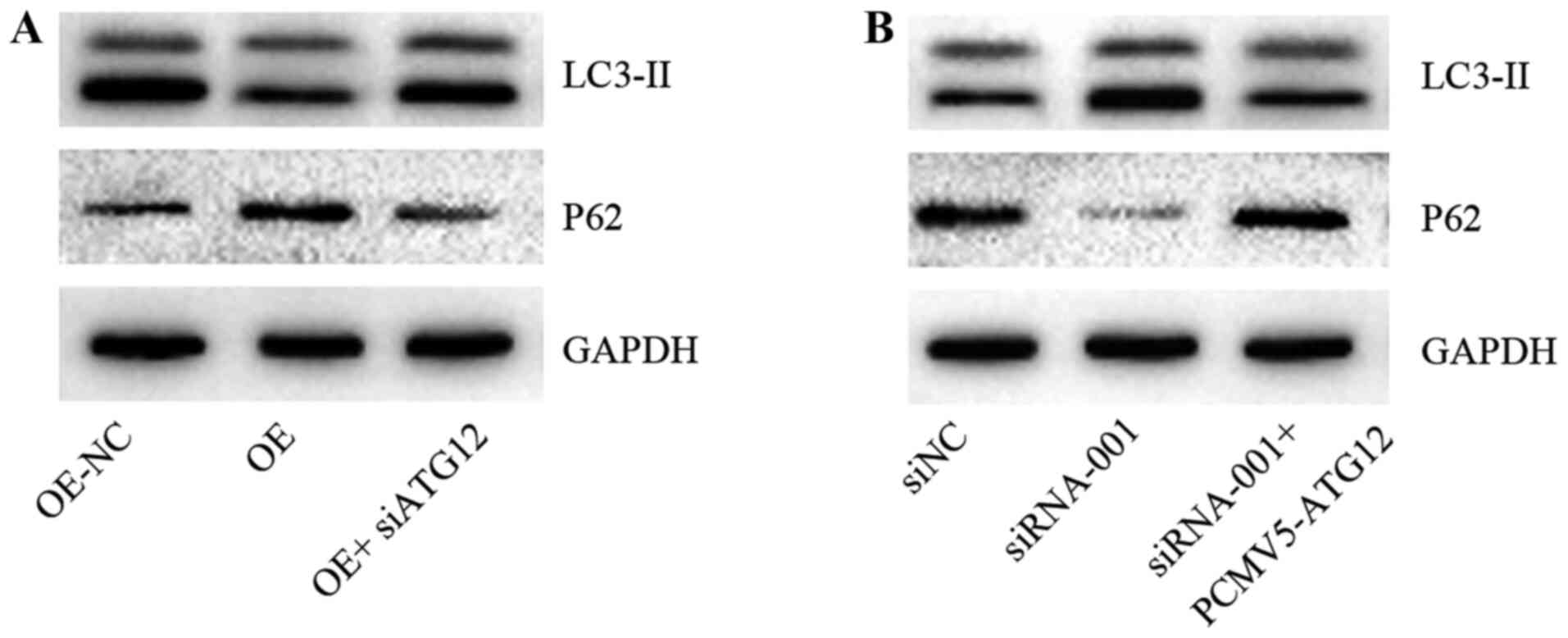
circ-0004904 regulates autophagy via
ATG12
The present study further attempted to determine
whether circ-0004904 regulated autophagy via ATG12. Silencing or
ectopic expression of ATG12 significantly inhibited or elevated,
respectively, the mRNA and protein levels of ATG12 compared with
those in the corresponding NC groups (Fig. S1E and F). Further results
demonstrated that overexpression of circ-0004904 promoted the
expression of LC3-II in HTR8 cells compared with that in the NC
group, whereas following silencing of ATG12, this regulation was
partly suppressed (Fig. 6A). In
addition, silencing of circ-0004904 inhibited the expression of
LC3-II in JEG3 cells compared with that in the cells transfected
with siNC; however, following overexpression of ATG12, the
downregulation was reversed (Fig.
6B).
circ-0004904 regulates the FUS/VEGF
axis
The present study identified that circ-0004904
directly bound to the FUS protein by bioinformatics analysis. A
previous study has reported that FUS inhibits VEGF expression
(34); thus, we hypothesized
that circ-0004904 may regulate the FUS/VEGF axis. RNA pull-down
assay confirmed that circ-0004904 directly bound to the FUS protein
(Fig. 7A). Additionally,
following silencing of circ-0004904, the expression levels of FUS
decreased, whereas the expression levels of VEGF increased in JEG3
cells compared with those in cells transfected with siNC (Fig. 7B). Following ectopic expression
of circ-0004904 in HTR8 cells, the expression levels of FUS
increased, whereas the levels of VEGF reduced compared with those
in the NC group (Fig. 7C). The
results also demonstrated that silencing or ectopic expression of
FUS significantly inhibited or elevated, respectively, the mRNA and
protein levels of FUS compared with those in the corresponding NC
groups (Fig. S1G and H). In
addition, silencing of FUS increased the expression levels of VEGF
compared with those in the siNC group, whereas overexpression of
FUS exerted an opposite effect (Fig.
7D and E). When FUS was silenced or overexpressed, this
regulation was partially reversed, suggesting that the regulatory
effect of circ-0004904 on VEGF protein was dependent on FUS
(Fig. 7F and G).
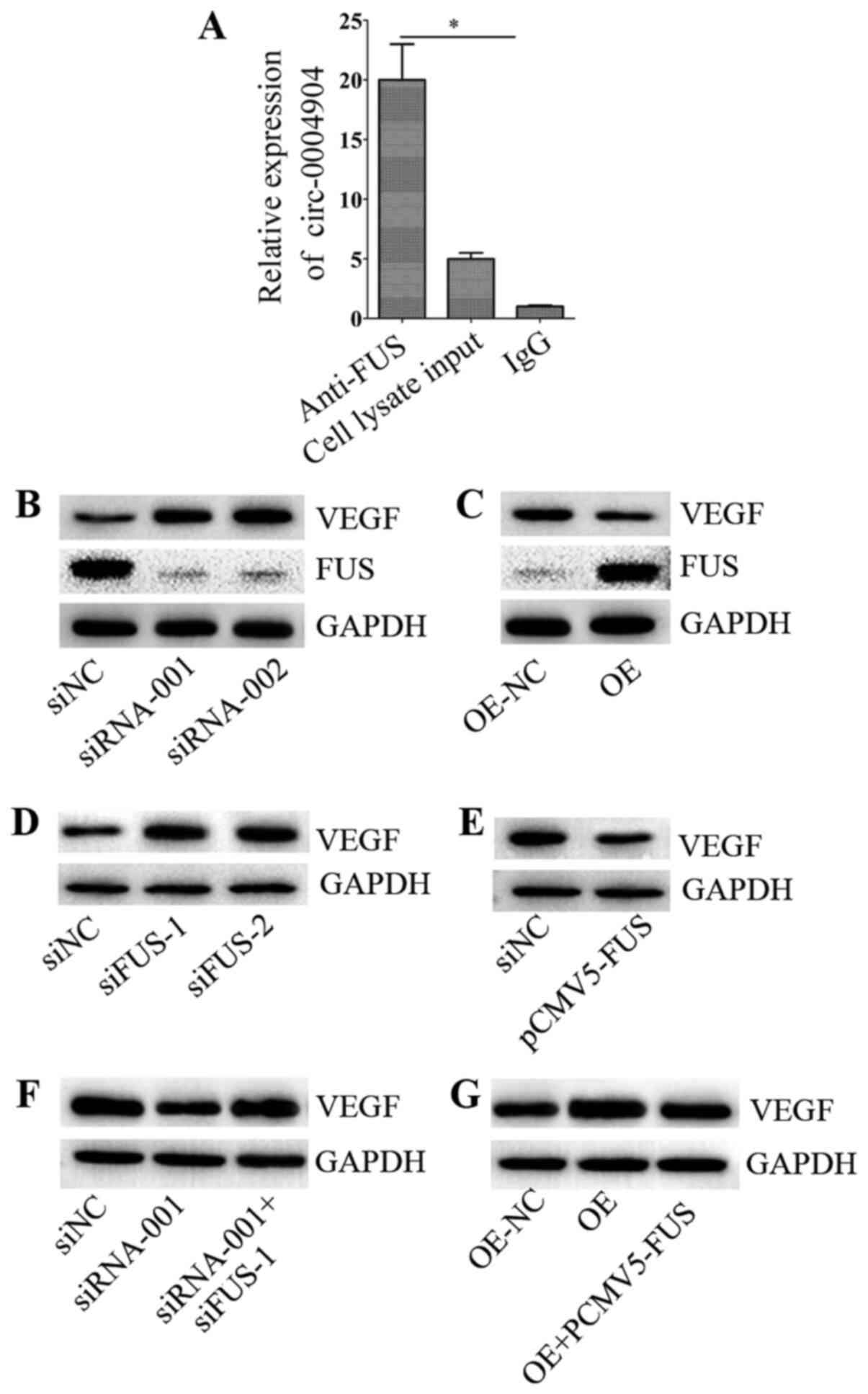 | Figure 7circ-0004904 regulates the FUS/VEGF
axis. (A) RNA pull-down assay was used to assess the interaction
between circ-0004904 and FUS protein. The expression levels of
circ-0004904 were determined by reverse transcription-quantitative
PCR. Data are presented as the mean ± SD. (B and C) The expression
levels of VEGF and FUS were detected by western blotting in (B)
JEG3 cells transfected with siNC, siRNA-001 or siRNA-002 and (C)
HTR8 cells transfected with OE-NC or OE. (D and E) The expression
levels of VEGF were detected by western blotting in HTR8 cells
transfected with (D) NC, siFUS-1 or siFUS-2 and (E) NC or
pCMV5-FUS. (F and G) The expression levels of VEGF were detected by
western blotting in (F) JEG3 cells transfected with siRNA-001, siNC
or siRNA-001 + siFUS-1 and (G) HTR8 cells transfected with OE-NC,
OE or OE + pCMV5-FUS. Data are presented as the mean ± SEM.
*P<0.05. circ, circular RNA; miR, microRNA; siRNA/si,
small interfering RNA; siNC, negative control siRNA; OE,
overexpression vector; NC, negative control; FUS, fused in
sarcoma. |
Discussion
Although there are currently reports that circRNA
serves a role in the pathogenesis of preeclampsia, the function of
circRNA in the occurrence and development of preeclampsia still
requires in-depth studies. The results of the present study
demonstrated increased expression levels of circ-0004904 in the
placental tissues and plasma samples of patients with preeclampsia
compared with those in samples from the control subjects. In
addition, circ-0004904 activated autophagy and promoted cellular
invasion in the HTR8 and JEG-3 cell lines. It also was revealed
that circ-0004904 positively regulated the expression of ATG12 via
miR-570. Additionally, circ-0004904 regulated the FUS/VEGF axis
(Fig. S3). These results
suggested that circ-0004904 may be used as a candidate diagnostic
biomarker for preeclampsia and a potential therapeutic target.
Previous studies have demonstrated that autophagy is
involved in the occurrence and development of preeclampsia
(35-37). The expression levels of LC3,
Beclin-1 and total numbers of autophagosomes are upregulated in the
placental tissues of patients with preeclampsia compared with those
in healthy control subjects, as well as in HTR8 cells and human
umbilical vein endothelial cells treated with glucose oxidase
compared with those in untreated cells (38,39), suggesting a certain association
between autophagy activation and the occurrence of preeclampsia. In
the current study, high expression levels of circ-0004904 promoted
autophagy, which suggested that highly expressed circ-0004904 may
promote the occurrence and progression of preeclampsia. A previous
study has confirmed that excessive autophagy inhibits trophoblast
invasion and vasculature, thereby causing the onset of preeclampsia
(40). In the current study,
ectopic expression of circ-0004904 inhibited the invasion of HTR8
cells compared with that in the NC group. Therefore, we
hypothesized that the abnormally expressed circ-0004904-mediated
autophagy may inhibit the invasion of trophoblasts, thereby
participating in the occurrence and development of
preeclampsia.
ATG12 is crucial for autophagosome formation, basal
autophagy, late endosome-to-lysosome trafficking and exosome
release (41,42). In the current study, circ-0004904
promoted autophagic flux, and this regulation was dependent on
ATG12. A previous study has reported that miR-570-3p regulates the
metastatic effects of metformin on human osteosarcoma by directly
targeting ATG12 (43). In the
present study, miR-570-3p was demonstrated to mediate the
expression of ATG12 by directly targeting ATG12 in HTR8 cells,
which was consistent with the findings of a previous study
(43). In addition, the results
of the present study demonstrated that circ-0004904 regulated ATG12
by sponging miR-570-3p. These results further validated the
potential roles of circ-0004904 in promoting autophagy.
A recent study has reported that circRNA fibronectin
type III domain-containing 3B directly interacts with FUS and
regulates its expression levels (34). In the current study, circ-0004904
directly bound to FUS protein and promoted its expression. Another
study has demonstrated that FUS significantly represses tumor
growth via inhibiting angiogenesis by reducing the expression
levels of VEGF (44). The
present results demonstrated that circ-0004904 suppressed the
expression level of VEGF, which was dependent on FUS. VEGF is a
potential therapeutic target for angiogenesis (45). In placental tissues of patients
with preeclampsia, the expression levels of VEGF are downregulated
compared with those in healthy subjects (46). Therefore, we hypothesized that
high expression levels of circ-0004904 may promote the occurrence
and development of preeclampsia.
In conclusion, the results of the present study
demonstrated that circ-0004904 was upregulated in the serum samples
and placental tissues of patients with preeclampsia compared with
those in samples from healthy subjects. High expression levels of
circ-0004904 promoted autophagic flux and inhibited cellular
invasion in vitro. In addition, circ-0004904 regulated the
expression levels of ATG12 and VEGF. Therefore, circ-0004904 may be
used as a molecular marker for the diagnosis of preeclampsia and a
potential therapeutic target. In our future studies, animal models
of preeclampsia we will be established to assess the roles and
mechanisms of circ-0004904 in vivo.
Supplementary Data
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
WD designed the study, acquired, analyzed and
interpreted the data, drafted and revised the manuscript. XL
designed the study, acquired, analyzed and interpreted the data. WD
and XL confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the West China Hospital of Sichuan University
(Chengdu, China), and all patients signed written informed consent
forms prior to the commencement of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Chilumula K, Saha PK, Muthyala T, Saha SC,
Sundaram V and Suri V: Prognostic role of uterine artery Doppler in
early- and late-onset preeclampsia with severe features. J
Ultrasound. Aug 24–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dyess NF and Kinsella JP: Cardiovascular
implications for offspring born to mothers with preeclampsia. J
Pediatr. 228:11–12. 2021. View Article : Google Scholar
|
|
3
|
ACOG Practice Bulletin No. 202:
Gestational hypertension and preeclampsia. Obstet Gynecol.
133:12019.
|
|
4
|
Bakrania BA, George EM and Granger JP:
Animal models of preeclampsia: Investigating pathophysiology and
therapeutic targets. Am J Obstet Gynecol. Nov 23–2020.Epub ahead of
print. View Article : Google Scholar
|
|
5
|
Jia Y, Xie H, Zhang J and Ying H:
Induction of TGF-β receptor I expression in a DNA
methylation-independent manner mediated by DNMT3A downregulation is
involved in early-onset severe preeclampsia. FASEB J.
34:13224–13238. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reddy M, Fenn S, Rolnik DL, Mol BW, da
Silva Costa F, Wallace EM and Palmer KR: The impact of the
definition of preeclampsia on disease diagnosis and outcomes: A
retrospective cohort study. Am J Obstet Gynecol.
224:217.e1–217.e11. 2021. View Article : Google Scholar
|
|
7
|
Saghafi N, Pourali L, Ghavami Ghanbarabadi
V, Mirzamarjani F and Mirteimouri M: Serum heat shock protein 70 in
preeclampsia and normal pregnancy: A systematic review and
meta-analysis. Int J Reprod Biomed. 16:1–8. 2018.PubMed/NCBI
|
|
8
|
D'Ambra E, Capauto D and Morlando M:
Exploring the regulatory role of circular RNAs in neurodegenerative
disorders. Int J Mol Sci. 20:54772019. View Article : Google Scholar :
|
|
9
|
Bai S, Wu Y, Yan Y, Shao S, Zhang J, Liu
J, Hui B, Liu R, Ma H, Zhang X and Ren J: Construct a
circRNA/miRNA/mRNA regulatory network to explore potential
pathogenesis and therapy options of clear cell renal cell
carcinoma. Sci Rep. 10:136592020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai J, Zhuang Y, Tang M, Qian Q and Chen
JP: CircRNA UBAP2 facilitates the progression of colorectal cancer
by regulating miR-199a/VEGFA pathway. Eur Rev Med Pharmacol Sci.
24:7963–7971. 2020.PubMed/NCBI
|
|
11
|
Deepthi K and Jereesh AS: An ensemble
approach for CircRNA-Disease association prediction based on
Autoencoder and deep neural network. Gene. 762:1450402020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong G, Han Z, Wang W, Xu Q and Zhang J:
Silencing hsa_circRNA_0008035 exerted repressive function on
osteosarcoma cell growth and migration by upregulating
microRNA-375. Cell Cycle. 19:2139–2147. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen D, Ma W, Ke Z and Xie F: CircRNA
hsa_circ_100395 regulates miR-1228/TCF21 pathway to inhibit lung
cancer progression. Cell Cycle. 17:2080–2090. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Liu H, Hou L, Wang G, Zhang R,
Huang Y, Chen X and Zhu J: Circular RNA_LARP4 inhibits cell
proliferation and invasion of gastric cancer by sponging miR-424-5p
and regulating LATS1 expression. Mol Cancer. 16:1512017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Zhang J, Li J, Gui R, Nie X and
Huang R: CircRNA_014511 affects the radiosensitivity of bone marrow
mesenchymal stem cells by binding to miR-29b-2-5p. Bosn J Basic Med
Sci. 19:155–163. 2019.PubMed/NCBI
|
|
16
|
Ding C, Yi X, Wu X, Bu X, Wang D, Wu Z,
Zhang G, Gu J and Kang D: Exosome-mediated transfer of circRNA
CircNFIX enhances temozolomide resistance in glioma. Cancer Lett.
479:1–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Zhang H, Yang H, Bai M, Ning T,
Deng T, Liu R, Fan Q, Zhu K, Li J, et al: Exosome-delivered circRNA
promotes glycolysis to induce chemoresistance through the
miR-122-PKM2 axis in colorectal cancer. Mol Oncol. 14:539–555.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao G, Huang W, Zhan Y, Li J and Tong W:
CircRNA_103762 promotes multidrug resistance in NSCLC by targeting
DNA damage inducible transcript 3 (CHOP). J Clin Lab Anal.
34:e232522020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bian L, Zhi X, Ma L, Zhang J, Chen P, Sun
S, Li J, Sun Y and Qin J: Hsa_circRNA_103809 regulated the cell
proliferation and migration in colorectal cancer via
miR-532-3p/FOXO4 axis. Biochem Biophys Res Commun. 505:346–352.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Y, Zheng R, Chen J and Ning D:
CircRNA CDR1as/miR-641/HOXA9 pathway regulated stemness contributes
to cisplatin resistance in non-small cell lung cancer (NSCLC).
Cancer Cell Int. 20:2892020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu X, Ao J, Li X, Zhang H, Wu J and Cheng
W: Competing endogenous RNA expression profiling in pre-eclampsia
identifies hsa_circ_0036877 as a potential novel blood biomarker
for early pre-eclampsia. Clin Epigenetics. 10:482018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ou Y, Liu M, Zhu L, Deng K, Chen M, Chen H
and Zhang J: The expression profile of circRNA and its potential
regulatory targets in the placentas of severe pre-eclampsia. Taiwan
J Obstet Gynecol. 58:769–777. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang M, Lash GE, Zhao X, Long Y, Guo C
and Yang H: CircRNA-0004904, CircRNA-0001855, and PAPP-A: Potential
novel biomarkers for the prediction of preeclampsia. Cell Physiol
Biochem. 46:2576–2586. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han D, Li J, Wang H, Su X, Hou J, Gu Y,
Qian C, Lin Y, Liu X, Huang M, et al: Circular RNA circMTO1 acts as
the sponge of microRNA-9 to suppress hepatocellular carcinoma
progression. Hepatology. 66:1151–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Wang Z, Yu Z, Wang GH, Zhou YM, Deng JP,
Feng Y, Chen JQ and Tian L: AURKB promotes the metastasis of
gastric cancer, possibly by inducing EMT. Cancer Manag Res.
12:6947–6958. 2020. View Article : Google Scholar :
|
|
27
|
Xu Y, Ye S, Zhang N, Zheng S, Liu H, Zhou
K, Wang L, Cao Y, Sun P and Wang T: The FTO/miR-181b-3p/ARL5B
signaling pathway regulates cell migration and invasion in breast
cancer. Cancer Commun (Lond). 40:484–500. 2020. View Article : Google Scholar
|
|
28
|
Shang A, Gu C, Wang W, Wang X, Sun J, Zeng
B, Chen C, Chang W, Ping Y, Ji P, et al: Exosomal circPACRGL
promotes progression of colorectal cancer via the
miR-142-3p/miR-506-3p-TGF-β1 axis. Mol Cancer. 19:1172020.
View Article : Google Scholar
|
|
29
|
Zhang HD, Jiang LH, Hou JC, Zhong SL, Zhou
SY, Zhu LP, Li J, Wang DD, Sun DW, Ji ZL and Tang JH: Circular RNA
hsa_circ_0052112 promotes cell migration and invasion by acting as
sponge for miR-125a-5p in breast cancer. Biomed Pharmacother.
107:1342–1353. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xin Y, Min P, Xu H, Zhang Z and Zhang Y
and Zhang Y: CD26 upregulates proliferation and invasion in keloid
fibroblasts through an IGF-1-induced PI3K/AKT/mTOR pathway. Burns
Trauma. 8:tkaa0252020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Panda AC, Dudekula DB, Abdelmohsen K and
Gorospe M: Analysis of circular RNAs using the web tool
circinteractome. Methods Mol Biol. 1724:43–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia L, Li F, Qiu J, Feng Z, Xu Z, Chen Z
and Sun J: Oncogenic miR-20b-5p contributes to malignant behaviors
of breast cancer stem cells by bidirectionally regulating CCND1 and
E2F1. BMC Cancer. 20:9492020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu YP, Lin XD, Chen SH, Ke ZB, Lin F, Chen
DN, Xue XY, Wei Y, Zheng QS, Wen YA and Xu N: Identification of
prostate cancer-related circular RNA through bioinformatics
analysis. Front Genet. 11:8922020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garikipati VNS, Verma SK, Cheng Z, Liang
D, Truongcao MM, Cimini M, Yue Y, Huang G, Wang C, Benedict C, et
al: Circular RNA CircFndc3b modulates cardiac repair after
myocardial infarction via FUS/VEGF-A axis. Nat Commun. 10:43172019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cornelius DC and Wallace K: Autophagy in
preeclampsia: A new target? EBioMedicine. 57:1028642020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakashima A, Cheng SB, Ikawa M, Yoshimori
T, Huber WJ, Menon R, Huang Z, Fierce J, Padbury JF, Sadovsky Y, et
al: Evidence for lysosomal biogenesis proteome defect and impaired
autophagy in preeclampsia. Autophagy. 16:1771–1785. 2020.
View Article : Google Scholar
|
|
37
|
Zhao H, Gong L, Wu S, Jing T, Xiao X, Cui
Y, Xu H, Lu H, Tang Y, Zhang J, et al: The inhibition of protein
kinase C β contributes to the pathogenesis of preeclampsia by
activating autophagy. EBioMedicine. 56:1028132020. View Article : Google Scholar
|
|
38
|
Akcora Yildiz D, Irtegun Kandemir S,
Agacayak E and Deveci E: Evaluation of protein levels of autophagy
markers (Beclin 1 and SQSTM1/p62) and phosphorylation of cyclin E
in the placenta of women with preeclampsia. Cell Mol Biol
(Noisy-le-grand). 63:51–55. 2017. View Article : Google Scholar
|
|
39
|
Oh SY, Choi SJ, Kim KH, Cho EY, Kim JH and
Roh CR: Autophagy-related proteins, LC3 and Beclin-1, in placentas
from pregnancies complicated by preeclampsia. Reprod Sci.
15:912–920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao L, Qi HB, Kamana KC, Zhang XM, Zhang H
and Baker PN: Excessive autophagy induces the failure of
trophoblast invasion and vasculature: Possible relevance to the
pathogenesis of preeclampsia. J Hypertens. 33:106–117. 2015.
View Article : Google Scholar
|
|
41
|
Chen ZH, Cao JF, Zhou JS, Liu H, Che LQ,
Mizumura K, Li W, Choi AM and Shen HH: Interaction of caveolin-1
with ATG12-ATG5 system suppresses autophagy in lung epithelial
cells. Am J Physiol Lung Cell Mol Physiol. 306:L1016–L1025. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Murrow L and Debnath J: Atg12-Atg3
coordinates basal autophagy, endolysosomal trafficking, and exosome
release. Mol Cell Oncol. 5:e10391912018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bao X, Zhao L, Guan H and Li F: Inhibition
of LCMR1 and ATG12 by demethylation-activated miR-570-3p is
involved in the anti-metastasis effects of metformin on human
osteosarcoma. Cell Death Dis. 9:6112018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Deng WG, Kawashima H, Wu G, Jayachandran
G, Xu K, Minna JD, Roth JA and Ji L: Synergistic tumor suppression
by coexpression of FUS1 and p53 is associated with down-regulation
of murine double minute-2 and activation of the apoptotic
protease-activating factor 1-dependent apoptotic pathway in human
non-small cell lung cancer cells. Cancer Res. 67:709–717. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin L, Wang Q, Xu F, Luo X, Xu J, Yan L,
Li Q and Hao H: BML-111, the lipoxin A4 agonist,
modulates VEGF or CoCl2-induced migration, angiogenesis
and permeability in tumor-derived endothelial cells. Immunol Lett.
230:27–35. 2021. View Article : Google Scholar
|
|
46
|
Sahay AS, Jadhav AT, Sundrani DP, Wagh GN,
Mehendale SS, Chavan-Gautam P and Joshi SR: VEGF and VEGFR1 levels
in different regions of the normal and preeclampsia placentae. Mol
Cell Biochem. 438:141–152. 2018. View Article : Google Scholar
|