Introduction
Intervertebral disc (IVD) degeneration (IVDD) has
long been considered to contribute to lower back pain, which has a
prevalence ranging between 1.4 and 20.0% (1) and has been one of the three leading
causes of non-fatal health loss for nearly three decades (2), leading to a considerable economic
burden on health care systems (3). Therefore, novel treatments that
effectively prevent and treat IVDD are urgently needed.
Understanding the mechanism underlying the pathogenesis of IVDD is
of great significance for developing effective therapeutics to
treat IVDD.
Epidemiological studies have reported that excessive
mechanical stress is one of the major factors that induce IVDD,
that the IVD is always affected by stress even in a resting state,
and that overloaded compressive force accelerates the initiation
and progression of IVDD (4,5).
The IVD contains a nucleus pulposus (NP) core,
surrounded by an outer ring of the annulus fibrosus (AF) between
two cartilaginous endplates. A previous study has demonstrated that
NP fibrosis triggers and serves a prominent role in the progression
of IVDD (6). In addition to the
degradation and gradual loss of aggrecan and type II collagen, and
the enhanced production of fibrotic proteins, the progression of
tissue fibrosis is characterized by the excessive remodeling and
abnormal deposition of extracellular matrix (ECM) components and
the subsequent accentuated distortion of the architecture, which
has been observed in the NP of degenerative discs (7,8).
Previous studies have demonstrated that the Rho
GTPase family and its downstream signaling pathways are involved in
the basic cellular regulatory mechanisms associated with biological
activities and diseases, including proliferation, differentiation,
migration and gene expression (9,10). The role of the mechanical
stress/Ras homolog family member A (RhoA)/myocardin-related
transcription factor A (MRTF-A)-mediated pathway in cellular
mechanical stress responses and the fibrotic process of a number of
organ systems, including the heart, lung, eye and kidney, has been
previously established (11-15). Mechanical stimulus-initiated
signals derived from the ECM are detected by cognate transmembrane
receptor proteins termed integrins, which are the main
structure-organizing components of focal adhesions; subsequently,
RhoA signaling is activated through selective Rho guanine
nucleotide exchange factors (9).
Consequently, monomeric globular actin (G-actin) subunits in the
cytoplasm assemble into long filamentous F-actin polymers.
Following RhoA-induced actin polymerization, MRTF-A is released
from G-actin and translocated to the nucleus, where it acts as a
transcriptional cofactor for serum response factor (SRF) by binding
to the CArG regulatory sequence [CC(A/T)6GG] in the promoter
regions of its target genes, resulting in the increased expression
levels of type I collagen, connective tissue growth factor (CTGF),
smooth muscle cell actin (SMA) and other fibrosis-related factors
(9,16). To the best of our knowledge, no
relevant studies have been conducted to determine whether this
molecular mechanism serves a dominant role in the process of the
fibrotic changes observed in NP cell types to date.
A previous study has demonstrated that CCG-1423 is a
small molecule inhibitor that specifically binds to the N-terminal
basic domain (which acts as a functional nuclear localization
signal) of MRTF-A and inhibits the nuclear import of MRTF-A
(17). Therefore, the present
study aimed to determine the role of the RhoA/MRTF-A signaling
pathway in mechanically induced NP fibrosis in rats in vivo
and in vitro, and to investigated whether pharmacological
inhibition by the MRTF-A chemical inhibitor CCG-1423 may alleviate
NP fibrogenesis.
Materials and methods
Materials
The primary antibodies against RhoA (cat. no.
A15641), MRTF-A (cat. no. A8504), α-SMA (cat. no. A7248), CTGF
(cat. no. A11067), collagen type I (cat. no. A1352) and II (cat.
no. A1560) were purchased from ABclonal Biotech Co., Ltd. The
anti-F-actin antibody (cat. no. ab205) was purchased from Abcam,
the anti-β-actin antibody (cat. no. AF5003) was purchased from
Beyotime Institute of Biotechnology, and CCG-1423 was purchased
from Cayman Chemical Company.
Ex vivo rat NP explant preparation and
treatment
The present study was approved by the Laboratory
Animal Care and Use Committee of the Affiliated Hospital of Qingdao
University (Qing'dao, China; approval no. QYFYWZLL 26037).
Cell culture
NP explants were obtained from one female
Sprague-Dawley rat (age, 1 month) as previously described (18). In brief, the rat was euthanized
by intraperitoneal injection of sodium pentobarbital (100 mg/kg);
the spinal column was removed under aseptic conditions, and the
gel-like NP was separated from the AF. The NP tissues were digested
with 0.01% trypsin (HyClone; Cytiva) for ~30 min at 37°C, followed
by treatment with 0.125% collagenase II (MilliporeSigma) for ~4 h.
The digested tissue was filtered using a 100-µm cell
strainer and washed twice with phosphate-buffered saline (PBS;
Gibco; Thermo Fisher Scientific, Inc.). The isolated NPCs were
maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin under hypoxic conditions with 5%
O2 and 5% CO2 at 37°C, and the culture media
were exchanged every 3 days. All experiments were completed with
low-passage (<3) cells cultured in monolayers.
Polyacrylamide (PA) substrate
preparation
PA hydrogels are a well-established matrix substrate
system used for matrix stiffness assays (11). PA gels (PAGs) with defined
mechanical stiffness levels were prepared by mixing polymerizing
acrylamide (8% final concentration, prepared from a 40% stock
solution; Bio-Rad Laboratories, Inc.) with two concentrations of
bis-acrylamide (0.048 and 0.48%, prepared from 2% stock solution;
Bio-Rad Laboratories, Inc.) to create substrates of two different
stiffnesses (19). The
procedures for preparing the substrates and determining the modulus
values were adopted from previous studies (20,21). Briefly, the gels were prepared
between two glass coverslips respectively coated with 3-aminopropyl
trimethoxy silane and octadecyl-trichlorosilane. The thickness of
the gel was controlled by adjusting the volume of the solution
placed between the coverslips. Following gelation, the nonadherent
plate was removed. Fibronectin (10 µg/ml; MilliporeSigma),
which is an abundant ECM ligand in the NP to which NPCs adhere
(20), was added dropwise to the
surface of the treated gel using sulfo-SANPAH-based conjugation.
Before seeding the cells, the polyacrylamide gels were washed twice
with precooled sterile HEPES (pH 8.5) and equilibrated with media
for 1 h at room temperature. To prevent the stimulation of SRF
signaling by serum and the potential paracrine signaling induced by
cell adherence to the surrounding plastic coverslip, prior to
transferring the coverslip and hydrogel to a new 6-well plate
containing minimal FBS (0.5%), the NPCs were allowed to attach to
the prepared matrix for 2 h.
For the inhibitor experiments, CCG-1423 was diluted
in vehicle (0.1% DMSO) to obtain a final concentration of 10
µM according to a previous study (22). NP cells were seeded on either
stiff or soft substrate in reduced-serum (0.5% FBS) DMEM to reduce
the serum-induced activation of gene expression (23). According to the manufacturer's
instructions, 10 µM CCG-1423 and an equivalent volume of
0.1% DMSO were added to the cells and cultured for 72 h at 37°C. A
total of four experimental groups were established in the
subsequent experiments: i) Soft, NPCs cultured on a soft substrate
without intervention; ii) stiff, NPCs cultured on a stiff substrate
without intervention; iii) soft + CCG-1423, NPCs cultured on a soft
substrate with CCG-1423; and iv) stiff + CCG-1423, NPCs cultured on
a stiff substrate with CCG-1423. Subsequently, the cytotoxicity of
the inhibitor and solvent was assessed using the Cell Counting
Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology) by adding
10% CCK-8 solution to the cells for 2 h at 37°C, and the viability
of the cells in the four groups was determined by measuring the
optical density value at 450 nm. Images of the cells were captured
under an inverted Olympus BX51 light microscope (magnification,
×20; Olympus Corporation) for identification, and the cells
harvested for protein detection or fixed for immunofluorescence
staining and confocal microscopy.
Immunofluorescence imaging
To visualize MRTF-A, following NPC incubation and
treatment, the gels were washed with PBS, and the NPCs were fixed
with 4% paraformaldehyde at room temperature for 10 min, followed
by permeabilization with 0.2% Triton X-100 in PBS for 15 min at
room temperature. Subsequently, the gels were washed 3 times with
precooled PBS and blocked with PBS containing 1% BSA (Beyotime
Institute of Biotechnology). The gels were incubated with rabbit
anti-MRTF-A (1:200) and anti-F-actin (1:200) primary antibodies at
room temperature for 2 h. Following washing three times with PBS,
the NPCs were incubated with FITC-conjugated anti-rabbit IgG (cat.
no. F9887, MilliporeSigma) and TRITC-conjugated anti-mouse IgG
secondary antibodies (cat. no. T5393, MilliporeSigma) diluted
1:1,000 in PBS/0.1% Triton for 30 min at room temperature. After
three times of washes with PBS, the cell nuclei were counterstained
with 4′,6-diamidino-2-phenylindole (DAPI; MilliporeSigma). The
nuclear localization of MRTF-A and F-actin was analyzed by a Nikon
A1 confocal microscope (magnification, ×20; Nikon Corporation) and
quantified by ImageJ software (version 1.52v; National Institutes
of Health) to determine the integral optical density. The analyses
were performed in a blinded manner.
Western blotting
The protein concentrations of the cell lysates
obtained using RIPA lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.) were detected with the BCA protein assay kit
(Beijing Solarbio Science & Technology Co., Ltd). Then, samples
with equal amounts of proteins (30 µg/lane) were resolved by
10% SDS-polyacrylamide gel electrophoresis, electrotransferred onto
nitrocellulose membranes (MilliporeSigma), blocked with 5% BSA at
room temperature for 1 h and incubated with primary antibodies
against RhoA (1:1,000), F-actin (1:1,000), type I collagen
(1:5,000), type II collagen (1:5,000), β-actin (1:1,000), α-SMA
(1:1,000) and CTGF (1:1,000) overnight at 4°C. Subsequently, the
blots were washed with Tris-buffered saline with 1% Tween-20 and
incubated with appropriate anti-rabbit (cat. no. ZB-2301) or mouse
(cat. no. ZB-2305) IgG HRP-conjugated antibodies (both 1;3,000;
OriGene Technologies, Inc.) at 37°C for 2 h. The membranes were
developed with a chemiluminescence reagent (Thermo Fisher
Scientific, Inc.) using the ChemiDoc™ MP Imaging System (Bio-Rad
Laboratories, Inc.), and densitometry was performed using ImageJ
software.
IVDD animal model and surgical
techniques
A total of 60 healthy female Sprague-Dawley rats
(Charles River Laboratories, Inc.; specific pathogen-free; initial
weight, 80-100 g) aged one month were provided by the Experimental
Animal Center of The School of Medicine at Qingdao University. The
animals were housed in an environmentally controlled room with a
12-h light cycle. Initially, two experimental groups were
established: The intact control group (n=15) and the IVDD model
group (n=45). Establishment of the animal model was conducted as
previously described (24,25). Briefly, the animals were
anesthetized with sodium pentobarbital (40 mg/kg,
intraperitoneally) and aseptically treated during the experiments.
Transverse circular incisions were made over the axilla of the
bilateral forelimbs of each rat. Both brachial plexus rhizotomy as
well as distal skeleton and musculature amputation procedures were
performed. The height of the food and water was gradually increased
from 15 (standard height) to 30 cm (maximum height) as the length
and weight of the rats increased to induce bipedalism. Within 6
months, this procedure led to the development of IVDD. Age-matched
untreated rats served as the intact controls.
CCG-1423 treatment
To assess the therapeutic effects of CCG-1423 in
vivo, at 3 months post-surgery, the animals in the IVDD group
were randomly divided into three subgroups: i) IVDD model, bipedal
rats without treatment; ii) CCG-1423 treatment, bipedal rats
injected with CCG-1423 dissolved in DMSO; and iii) vehicle (DMSO),
bipedal rats injected with DMSO alone. Through a retroperitoneal
approach and with the exposure of the lumbar disc, 2 µl
CCG-1423 (effective dose of 5 µg dissolved in DMSO) or the
same volume of DMSO was injected into the NP of L4/5 IVD using a
microliter syringe (5 µl; Shanghai Gaoge Industrial and
Trading CO., Ltd.) attached to a 31-G needle.
MRI and data processing
At 3 months post-injection, the rats were sedated
with 10% chloral hydrate (300 mg/kg, intraperitoneally) for the MRI
scans. The lumbar spines of the rats were assessed in a 3.0 T
Varian MR scanner (MAGNETOM Skyra; Siemens AG) to obtain
T2-weighted images (Water: SAG IDEAL fat-suppression sequence;
repletion time, 3,000 ms; echo time, 80 ms; field of view, 150×150
mm; slice thickness, 1.2 mm) in the midsagittal plane. None of the
animals exhibited signs of peritonitis, pain or discomfort.
According to the modified Thompson classification (26,27), the discs in the T2-weighted
images were scored from grade I to IV.
All the assessments and quantitative data collection
were performed by three independent, blinded observers and
calculated as the mean of the three evaluations.
Histological analyses
Following the MRI scans, the rats were euthanized
using the aforementioned method, and histological examinations were
performed on samples from the four groups. The L4/5 IVD along with
the adjacent vertebrae dissected from the four groups (n≥5 per
group) were fixed in 10% buffered formalin (pH 7.4) for 24 h,
decalcified in a 10% EDTA solution for 3 weeks, embedded in
paraffin and sectioned transversely at 3-µm thickness with a
microtome. The sections were stained with hematoxylin and eosin
(H&E). All procedures were conducted at room temperature. The
cellularity and morphology of the NPs were independently examined
by two experienced histology researchers in a blinded manner using
a panoramic scanning microscope (magnification, ×20; Pannoramic
DESK, P-MIDI, P250, P1000; 3DHISTECH, Ltd.) with the accompanying
CaseViewer software (version 2.3; 3DHISTECH, Ltd.). The disc
degeneration scores in all groups were assessed and calculated
according to a modified grading system as previously described
(28,29).
Immunohistochemical staining
Immunohistochemical staining was performed to
examine the expression of MRTF-A (1:200), CTGF (1:100), α-SMA
(1:100) and type I collagen (1:200) in the tissues. Following
deparaffinization, each specimen was incubated for 5 min at room
temperature with 3% hydrogen peroxide to eliminate the endogenous
peroxidase activity. Subsequently, 20% goat serum (OriGene
Technologies, Inc.) was used to block the nonspecific protein
binding sites. This step was followed by incubation with the
corresponding antibodies at 4°C overnight. The following day, the
tissue sections were incubated for 30 min with horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibodies
(1:200; cat. no. ZB-2301; OriGene Technologies, Inc.) at 37°C. The
color was developed by incubation with chromogen
3,3′-diaminobenzidine (DAB) tetrahydrochloride, followed by
counterstaining with hematoxylin for 3 min at room temperature. An
Olympus IX71 light microscope was used to capture the images
(magnification, ×60) in a total of five randomly selected regions
in each immunohistochemical section, and the positive cells were
counted with ImageJ software. NPCs in which the nucleus was stained
and the cytoplasm exhibited brown staining were considered to be
positive for the target proteins.
Tissue immunofluorescence
The whole L4/5 disc along with the adjacent
vertebrae of the remaining rats (n≥5 per group) were frozen and
coronally sectioned into 5-µm sections using a Leica CM1950
cryostat (Leica Microsystems GmbH); subsequently, the sections were
stained as aforementioned.
Statistical analysis
All experiments were replicated three times.
Quantitative data are presented as the mean ± standard error.
Statistical analysis was performed using GraphPad Prism 7 software
(GraphPad Software, Inc.). Data were analyzed using one-way ANOVA
with Tukey's multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Stiffness activates RhoA in nucleus
pulposus cells
Soft and stiff PAGs that mimic the physical
properties of normal and overloaded microenvironments,
respectively, were established for the in vitro experiments
in the present study. Dynamic fatigue testing machine measurements
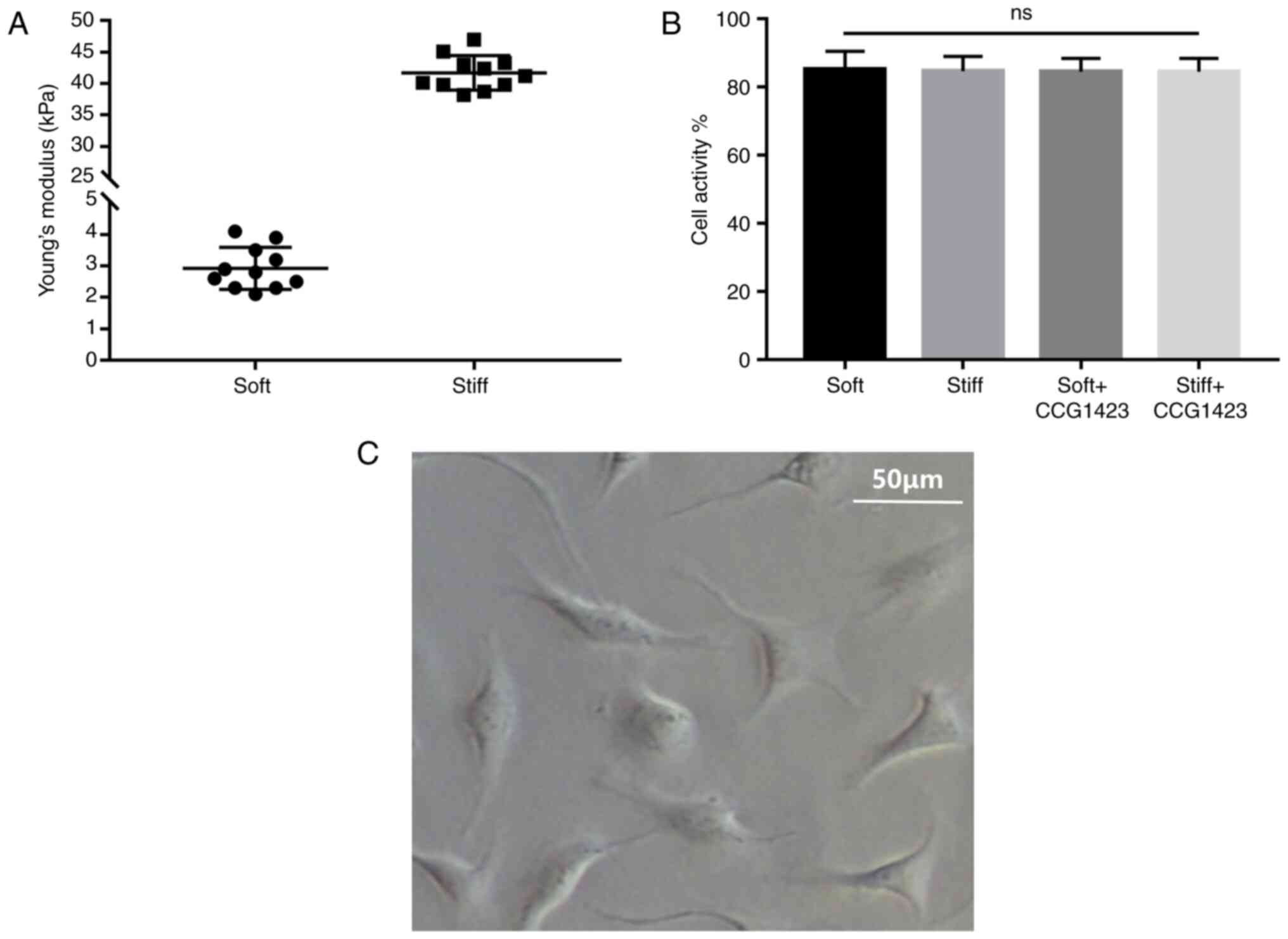
demonstrated that the soft PAGs had a mean stiffness value (Young's
modulus) of 2.9±0.2 kPa, whereas the stiff PAGs had a mean value of
41.7±0.8 kPa (Fig. 1A).
Following seeding, within 72 h, the NPCs gradually
adhered to the bottom of the culture dish (Fig. 1C). The results of the CCK-8 assay
revealed that the cell viability was comparable in the four groups,
suggesting that neither stiff nor soft substrates exerted
significant effects on the proliferation of NPCs in 72 h (Fig. 1B).
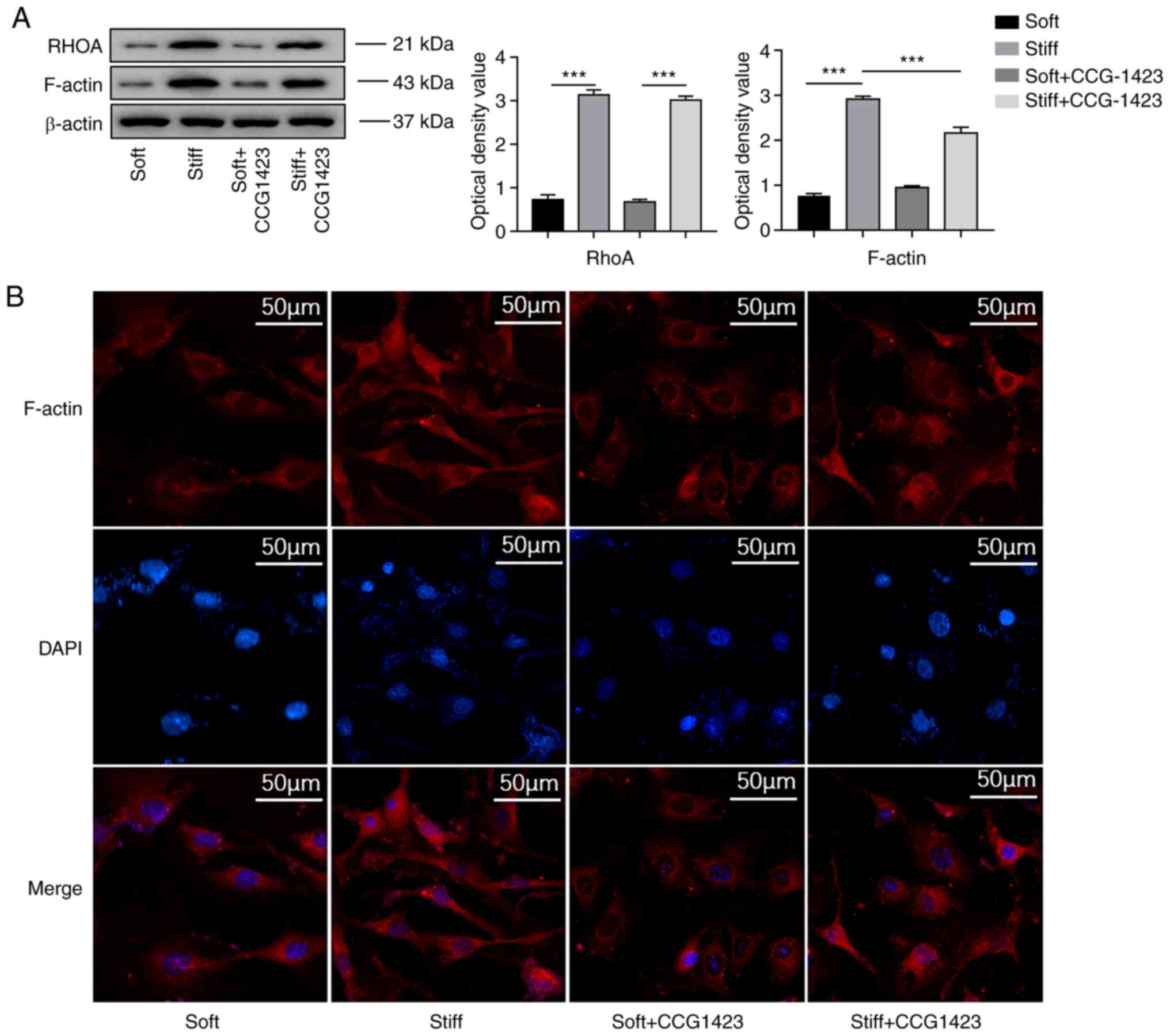
Under serum-free conditions, the western blotting
results demonstrated that the protein levels of RhoA and F-actin
were upregulated in the NPCs in the stiff group compared with those
in the NPCs in the soft group (Fig.
2A). The results of the immunofluorescence analysis also
confirmed that a stiff matrix induced changes in actin dynamics, as
more intense F-actin staining was observed in the cytoplasm of rat
NPCs cultured on a stiff matrix compared with that in the cytoplasm
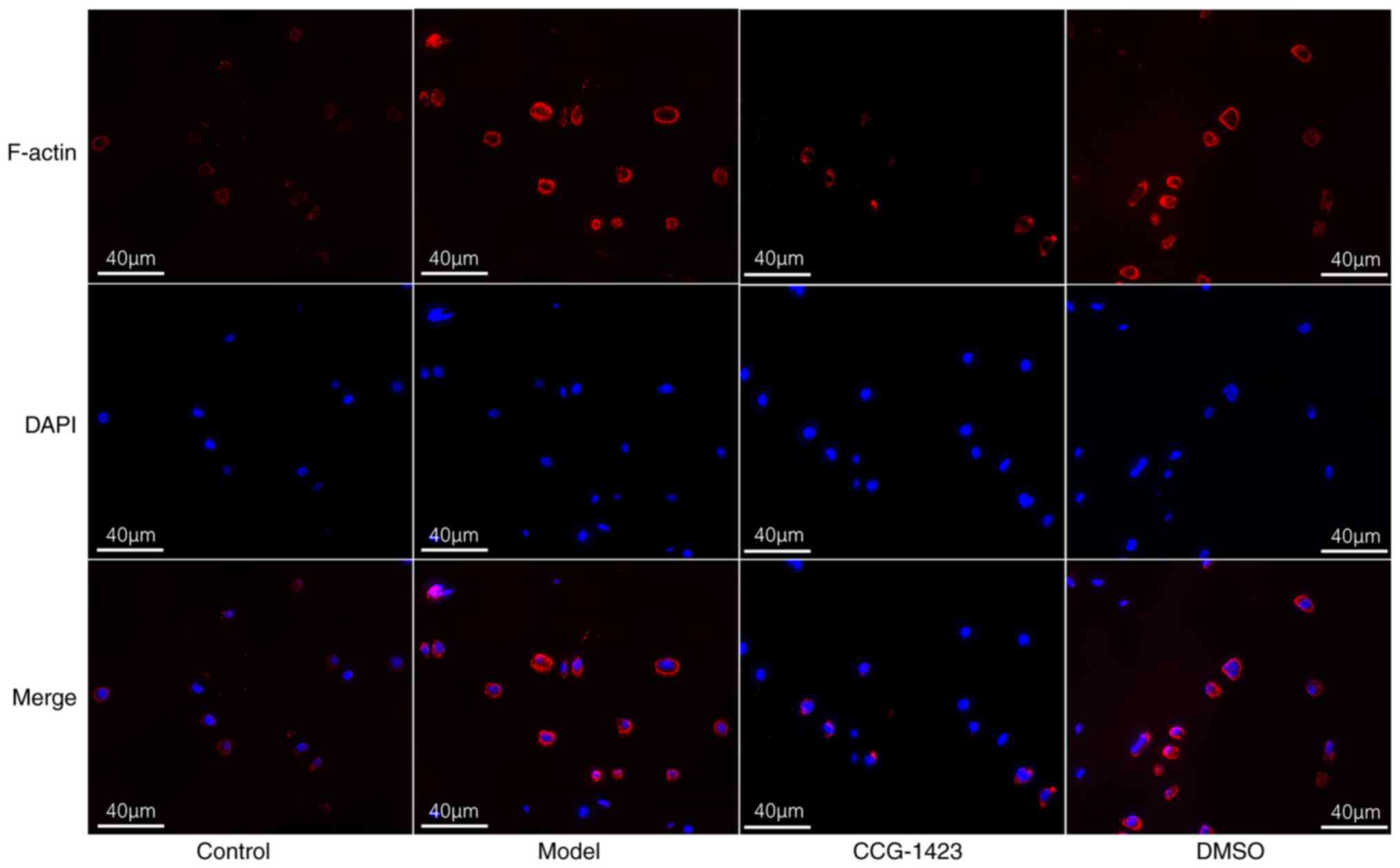
of rat NPCs cultured on a soft matrix (Fig. 2B).
Stiff matrix induces nuclear-cytoplasmic
trafficking of MRTF-A and expression of associated factors
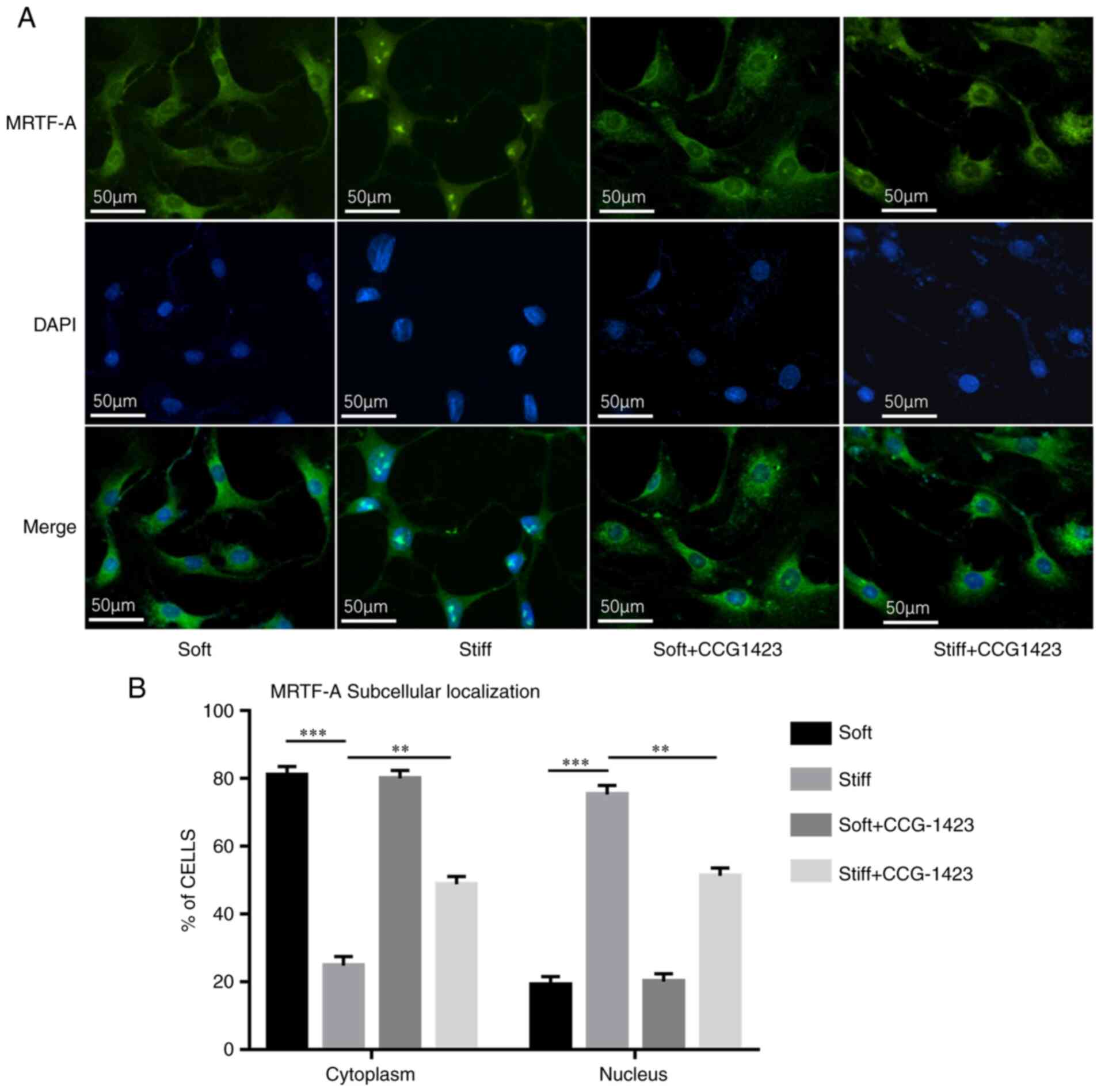
The impact of mechanical cues on the subcellular
localization of MRTF-A was further determined. Based on the
localization of MRTF-A-GFP in fluorescence microscopy images, the
NP cells were classified into two categories: Mainly nuclear or
mainly cytoplasmic. MRTF-A was mainly located in the cytoplasm in
81% of the cells in the soft group, with a mean nuclear state of
13-25%. As demonstrated by immunofluorescence, culture on a stiff
matrix resulted in notable nuclear staining of MRTF-A, and the
proportion of MRTF-A in the nuclei of the NPCs was significantly
increased compared with that in the soft group (75% nuclear and 25%
cytoplasmic; Fig. 3).
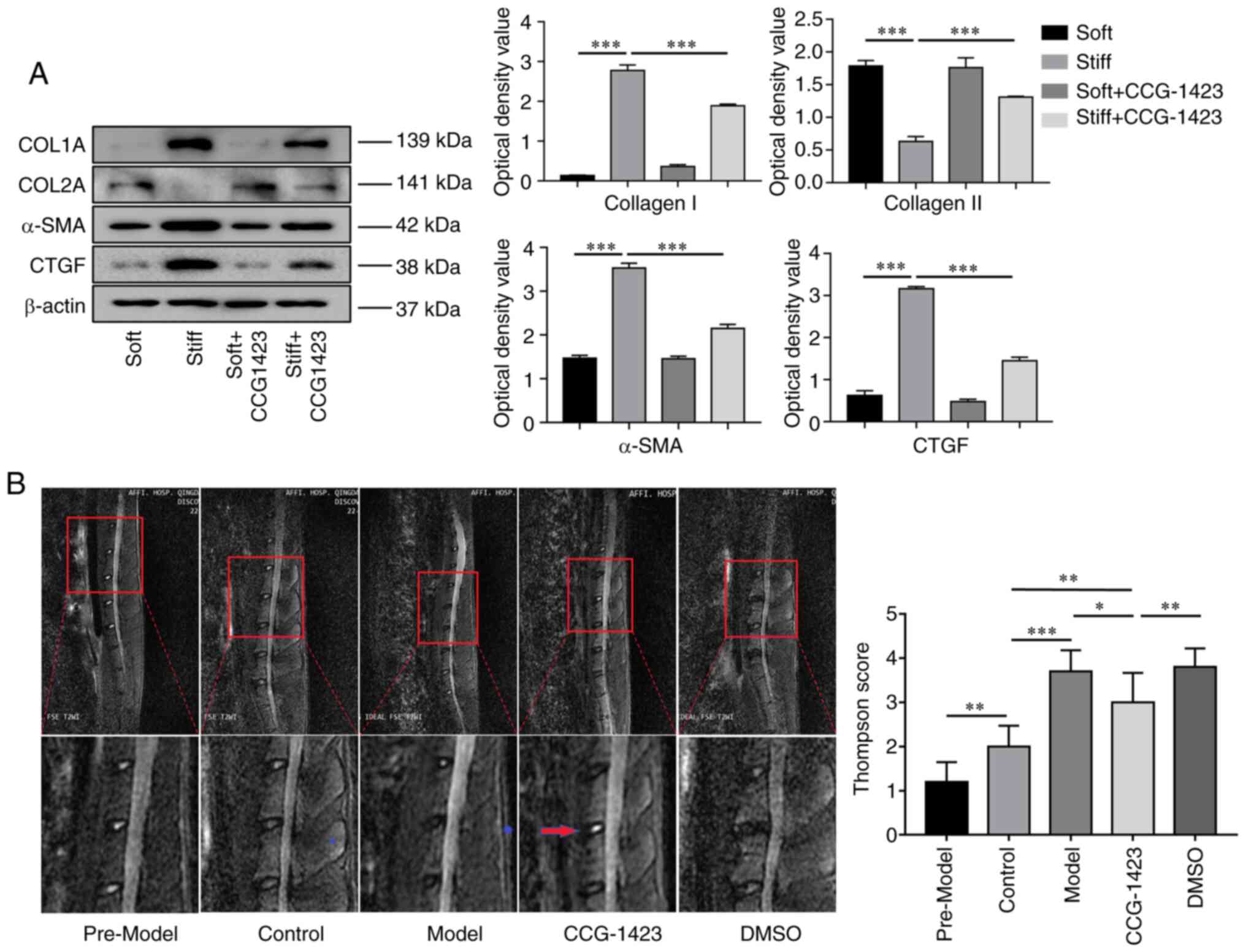
Since high levels of α-SMA and type I collagen are
major characteristics of fibrogenic activation (30), the present study examined the
discrepancy in their expression in NPCs cultured on soft and stiff
matrices. As demonstrated by western blotting, the protein levels
of targeted fibrogenic cytokines, such as type I collagen and CTGF,
were nearly undetectable in the NPCs cultured on the soft
substrate, whereas increasing matrix stiffness caused a significant
increase in the levels of the two proteins. In addition, elevated
α-SMA and downregulated collagen II expression levels were also
observed in the stiff matrix-cultured cells compared with those in
the soft group (Fig. 4A).
Inhibition of MRTF-A alleviates the
levels of fibrogenic factor expression in NP cells exposed to
mechanical stress
Treatment with 10 µM CCG-1423 for 72 h
partially blocked the stiff matrix-induced nuclear accumulation of
MRTF-A in the NP cells (Fig. 3).
Consistently, the protein levels of F-actin were reduced in the
stiff + CCG-1423 compared with those in the stiff group (Fig. 2). However, no significant
differences were observed in the levels of RhoA with or without
CCG-1423 treatment in NPCs (Fig.
2A). Treatment with CCG-1423 significantly abrogated matrix
stiffness-induced α-SMA and type I collagen expression, as well as
CTGF expression to levels below those observed in cells that
received no treatment. These results suggested that the nuclear
translocation of MRTF-A induced by a stiff matrix stimulated the
expression of the SRF-induced proteins α-SMA, type I collagen and
CTGF. Another protein, type II collagen, exhibited an opposite
trend compared with that of the three aforementioned markers
(Fig. 4A).
Changes in the histopathology of
intervertebral discs with increasing modeling time and CCG-1423
treatment
In the in vivo experiments, the present study
first aimed to assess the progression of IVDD. Fig. 4B demonstrates representative
T2-weighted midsagittal images of the approached rat lumbar disc
acquired by MRI. At 6 months after the initial modeling surgery,
all bipedal rats exhibited weaker signal densities in the L4/5
discs compared with those in the intact controls, and the MRI
images of the NP in the CCG-1423-treated group revealed stronger
signal intensities compared with those in the IVDD model and DMSO
groups. The perturbed disc degeneration grades were determined by
the modified Thompson grading evaluation revealed that the rats in
the three IVDD groups exhibited higher grades compared with those
in the control group. The CCG-1423-treated group exhibited lower
grades compared with those in the IVDD or DMSO groups, suggesting
that CCG-1423 treatment inhibited the reduction in the MRI signal
intensity in the IVDD model discs.
The fibrotic changes in the NP were subsequently
verified, and the results demonstrated that long-term (6 months)
upright posture resulted in notable NP fibrosis. As demonstrated by
the H&E staining (Fig. 5A),
in the control group, the NP remained relatively normal
cellularity, and large numbers of vacuolated notochordal cells and
round chondrocyte-like cells were distributed between the cartilage
end plate and AF, suggesting low exposure to mechanical stress.
Degeneration of the IVDs in the IVDD model and DMSO groups was more
severe compared with that in the control group. The qualitative
signs were as follows: The volume of gelatinous NP was reduced;
clefts were observed; the primary NP was replaced with disorganized
collagen fibers; the density and volume of the NPCs low; and
spindle-shaped fibroblast-like cells appeared. The indistinct
boundary between the AF and NP indicated the formation of NP
fibrosis (Fig. 5A). However,
compared with IVDD model and DMSO group, fibrosis was relieved, and
more even and regular cell distribution, relatively reduced
disorganization of collagen fibers, and intact extracellular matrix
alignment full of proteoglycan and water were identified in
CCG-1423-treated IVDD group. The histological scores based on the
H&E staining results demonstrated that the model group
exhibited higher degeneration scores compared with those in the
control group, and the structure of the degenerative IVDD was
improved by CCG-1423, but not by DMSO alone (Fig. 5C).
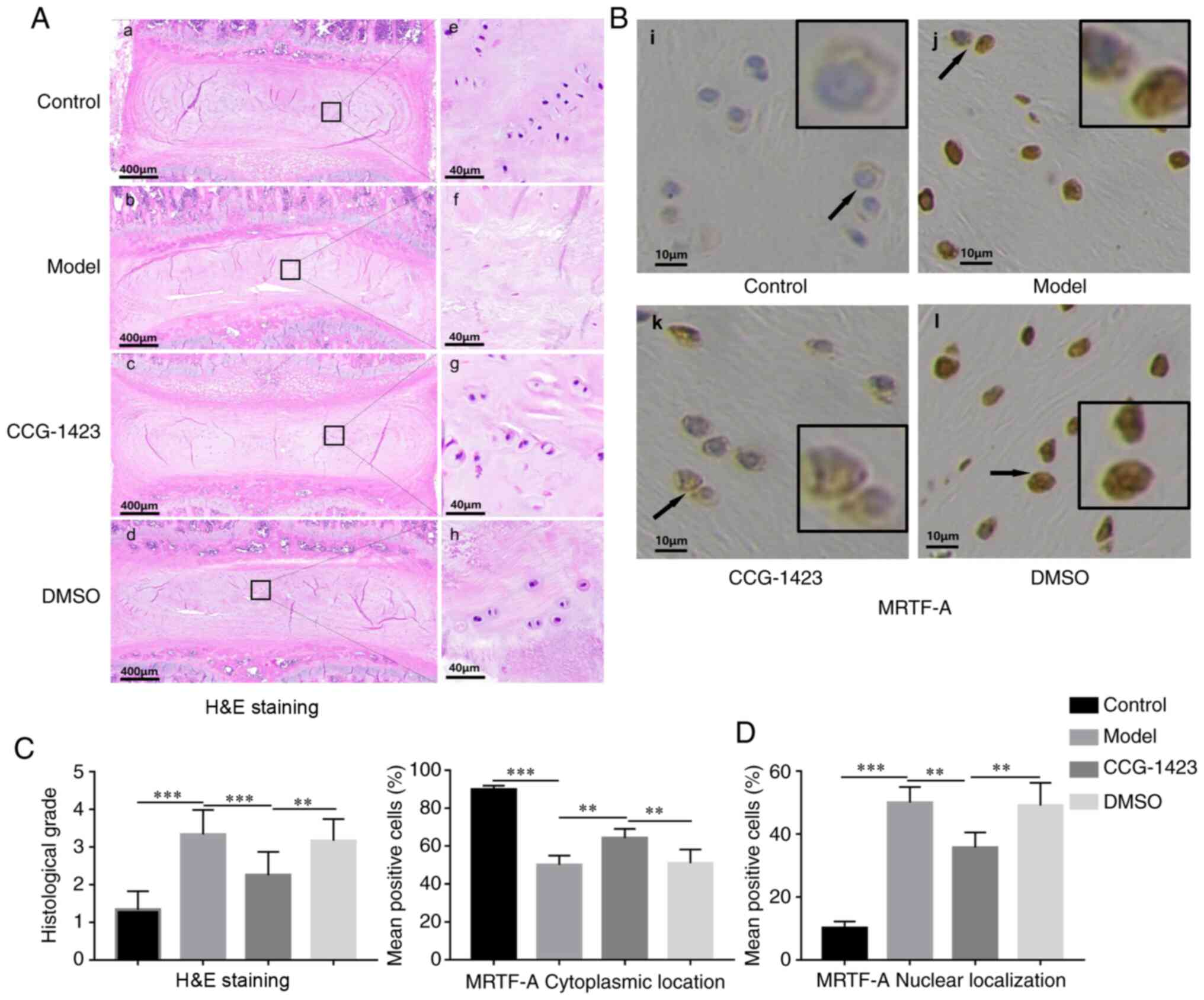 | Figure 5H&E and immunohistochemical
staining of intervertebral disc in the four groups of rats. (A-a-d)
Overview of morphological changes in the discs of the four groups.
(A-a) In the control group, the intervertebral altitude was
reserved; the NP was highly hydrated; the boundary between the AF
and the NP was distinct. (A-b and d) In the (A-b) model and (A-d)
DMSO groups, the intervertebral space was narrow, and the boundary
between the AF and the NP became indistinct. (A-c) In the
CCG-1423-treated group, a reserved boundary was observed between
the AF and the atrophic NP. (A-e-h) Magnified images of selected
regions of the NP. (A-e) In the control group, the NP was highly
cellular with notochordal and chondrocyte-like cells. (A-f and h)
In the (A-f) model and (A-e) DMSO groups, the cell density was low;
spindle-shaped fibroblast-like cells and filamentous collagen
fibers with clefts were observed. (A-g) Low levels of hydration and
cluster-distributed cells were observed in the CCG-1423-treated
group, and clefts were rare. (B) Subcellular expression of MRTF-A
in various disc tissues was detected by the immunohistochemical
staining. Regions indicated by black arrows are magnified in
black-bordered images. (C) The degree of IVDD in the model and DMSO
groups was increased compared with that in the control group. Rats
in the CCG-1423 group exhibited relatively a lower grade of IVDD
compared with the model and DMSO groups. (D) Quantitative analysis
of the subcellular localization of MRTF-A in the four groups based
on the results of immunohistochemical staining. n≥3.
**P<0.01 and ***P<0.001. IVDD,
intervertebral disc degeneration; AF, annulus fibrosus; NP, nucleus
pulposus; MRTF-A, myocardin-related transcription factor A. |
Long-term upright posture induces MRTF-A
nuclear translocation, changes in F-actin expression and fibrosis
biomarker expression, which is attenuated by CCG-1423 in the rat
IVD
The present study further analyzed whether long-term
mechanical stress altered MRTF-A distribution in rat NP samples.
Immunohistochemical staining (Fig.
5B and C) revealed that sustained mechanical load provoked the
nuclear accumulation of MRTF-A labeling in more NPCs compared with
that in the normal control rats, which was consistent with the
results demonstrated in vitro. In the CCG-1423 group, the
number of NPCs exhibiting MRTF-A nuclear localization decreased
compared with that in the model group, suggesting that orthotopic
injection of CCG-1423 mitigated the mechanical load-induced MRTF-A
nuclear translocation in initiated fibrotic NPs. These results
suggested that MRTF-A nuclear-cytoplasmic shuttling may participate
in NP fibrosis.
To determine whether the MRTF-A that accumulated in
the nucleus was transferred from the cytoplasm, the expression of
F-actin, a product of MRTF-A nuclear shuttling, was determined in
the cytoplasm by immunofluorescence, and increased cytoplasmic
staining intensity of F-actin was observed in the NP tissues of
IVDD model rats compared with that in the control animals. In NP
tissues treated with CCG-1423, cytoplasmic F-actin levels were
reduced (Fig. 6). These results
further confirmed the association between mechanical load stress
and actin/MRTF-A signaling in the NP of rats.
The statistical analysis of the immunohistochemical
staining results (Fig. 7)
revealed that prolonged upright positioning led to heterogeneous
trends of upregulated levels of type I collagen, CTGF and α-SMA
expression in the model group compared with those in the control
group. The modelling-enhanced immunoreactivity was downregulated by
CCG-1423 injection to varying degrees. These results suggested that
type I collagen, CTGF and α-SMA may serve important roles in the
process of NP fibrosis in IVDD and validated the positive effects
of CCG-1423 on fibrosis in a rat model of IVDD.
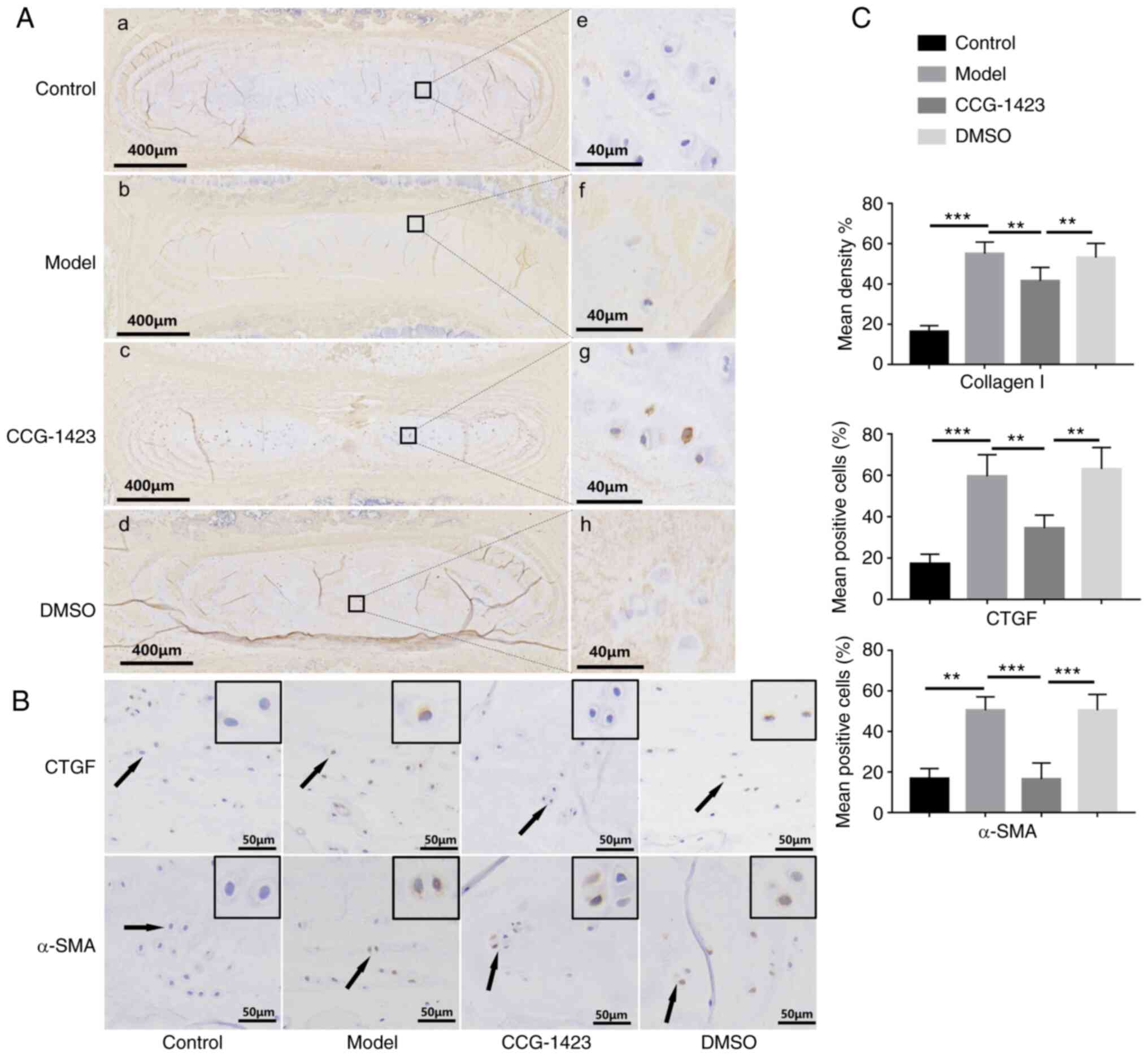 | Figure 7Inhibition of myocardin-related
transcription factor A translocation alleviates the NP fibrosis
phenotype in vivo. (A) Overview and magnified images of
immunohistochemical staining for type I collagen in the
intravertebral discs of rats in the four groups. (A-a and e) In the
control group, type I collagen (brown) was mainly present around
the NP, and most normal NP (blue) was retained. (A-b, f, d and h)
In the model and DMSO groups, the expression of type I collagen
covered a large area of NP, and positive staining was detected in
the intercellular substance of NP cells. (A-c and g) The area of NP
with positive type I collagen staining was smaller following
treatment with CCG-1423 compared with that in the model and DMSO
groups, and the boundary between the AF and the NP remained
distinct. (B) Following 6 months of upright posture, upregulated
expression levels of CTGF and α-SMA were observed in the NP of
bipedal rats compared with those in the control group, which were
reduced by CCG-1423 treatment. (C) Quantitative analysis of the
positive area of type I collagen, CTGF and α-SMA in the four groups
based on the immunohistochemical staining. **P<0.01
and ***P<0.001. NP, nucleus pulposus; AF, annulus
fibrosus; SMA, α-smooth muscle actin; CTGF, connective tissue
growth factor. |
Discussion
NP fibrosis is a key complication of IVDD for which
there is currently no available treatment (31,32). Numerous in vivo studies in
other organ systems have demonstrated the role of mechanoregulation
in fibrosis initiation, ECM remodeling and subsequent tissue
stiffening (33,34).
It has been established in vitro that the Rho
signaling pathway serves multiple functions ranging from cellular
mechanosensing and motility to signal transduction and
transcriptional regulation (35), and the Rho signaling pathway is a
key pathway in the fibrosis of numerous types of solid organs at
the organism level (15,36). In light of the essential roles of
Rho GTPases in cellular activity, the present study focused on a
classic signaling pathway, namely, the mechanical force-stimulated
Rho GTPase cascade, followed by actin filament recombination and
pivotal MRTF-A nuclear translocation, which contributes to fibrotic
gene expression; thus, this pathway links external stress to
MRTF-A-mediated transcriptional activation and subsequent α-SMA,
type I collagen and CTGF expression, providing evidence for a
mechanical force-induced transcriptional response that may
contribute to the development of NP fibrosis.
Comprehensive research findings have indicated that
multiple regimens of mechanosensing may interconnect to form a
synergistic mechanosensing system upstream of MRTF-A; these studies
have demonstrated that Rho signaling includes a variety of
intracellular molecules, such as Rho family small G protein
subfamilies RhoA, Rac1, RhoC and Ras, and their downstream target
Rho kinase, which are important regulators of the response to
mechanical stress (37-39). For example, CTGF is a hallmark of
fibrotic diseases, is upregulated in numerous pathological
processes involving mechanically challenged organs, and promotes
ECM accumulation and, notably, macromolecule synthesis (i.e., type
I collagen) (40). Blomme et
al (41) silenced RhoA, RhoC
and Rac1 expression using small interfering RNAs in valvular
interstitial cells and reported that Rac1 and RhoA were involved in
regulating the basal level of CTGF, whereas RhoC potentially
participated in the mechanical signaling-induced upregulation of
CTGF gene expression. Therefore, the identification of the
systematic mechanism in NP tissue is complicated. However, it has
been demonstrated that the type I collagen, α-SMA and CTGF gene
transcription levels are directly mediated by cooperation between
SRF and the strain-associated nuclear translocation of MRTF-A
(42-44). Thus, the actin
treadmill-dependent nuclear accumulation of MRTF-A appears to be a
key mediator of the upstream signals in the progression of
fibrogenesis.
Since the Rho signaling pathway is involved in
various pathological processes in addition to fibrosis, such as the
cell cycle, morphology and motility along with transcription
regulation and reactive oxygen species production (45,46), the multiple effects of the Rho
signaling pathway have limited selective drug discovery efforts.
RhoA inhibitors, which alter cytoskeletal dynamics, such as
Y-27632, have limited clinical utility due to their potential side
effects and weak efficacy (47).
MRTF-A is sequestered in the cytoplasm by preferentially forming a
complex with G-actin, which significantly attenuates the
interaction between MRTF-A and importin α/β1, a regulator of the
nuclear import of MRTF family members (48). In response to mechanical force,
the activated Rho signaling pathway induces rapid depletion of the
G-actin pool, thus increasing the binding of G-actin-free MRTF-A to
importin α/β1 and its nuclear localization (49). External force-reduced G-actin
monomers in the cytoplasm assembly promote the accumulation of
F-actin subjacent to the plasma membrane (50). Currently, pharmacological
development has shifted from focusing on the upstream locus
inhibition to targeting fibrotic transcription activation
(MRTF-A/SRF) (51). Studies have
identified that CCG-1423 acts as an inhibitor of the binding
between MRTF-A and importin α/β1 protein (49,52) due to the N-terminal basic domain
of G-actin-free MRTF-A, which functions as a nuclear localization
signal (17). Therefore, the
present study investigated MRTF-A nuclear-cytoplasmic trafficking
downstream of the Rho signaling pathway and demonstrated it to be a
potential clinical target for the treatment of mechanical
stimulus-induced NP fibrogenesis. In addition, the beneficial
antifibrotic efficacy of CCG-1423 was assessed both in vitro
and in vivo by the functional inhibition of MRTF-A nuclear
localization.
The present study used two approaches to test the
role of stress-induced MRTF-A translocation and NP fibrosis in the
process of IVDD. First, since PAG substrates with different
stiffness (2.9 and 41.7 kPa in the present study) effectively mimic
various magnitudes of static tension sustained by NP cells from the
mechanical environment (53,54), the existence of this pathway in
NPC was assessed in vitro, and the present study further
examined whether NPCs cultured on soft and stiff matrix substrates
expressed different fibrogenic proteins, and whether interference
with MRTF-A signaling may alter the production of mechanically
induced cytokines. Furthermore, using a brachial plexus rhizotomy
approach, a bipedal rat model was established to imitate the
upright posture of humans and exacerbate the transmitted force
input to the IVD. Th present study tested whether long-term upright
posture induced rat lumbar disc NP fibrogenesis associated with the
upregulation of CTGF, α-SMA and type I collagen; the results
demonstrated that the disruption of MRTF-A postponed the fibrogenic
process in the NP.
The NP tissue is the inner gel-like portion of the
IVD that it is composed primarily of small cartilage-like NPCs
stemming from notochord cells and other ECM components, and large
aggregating proteoglycans loosely held together by a network of
type II collagen and elastin fibers (55). The influx of water molecules to
the NP is created by osmotic pressure, which is maintained by
sulfated glycosaminoglycans in the NP system and provides the
elasticity required for the NP to keep the body stable against
compressive loads (56).
Previous studies have reported that type II collagen, the levels of
which gradually decrease from the inner NP to the outer AP, is
replaced by high expression levels of type I collagen in
degenerative discs (57,58). Consistently, type II collagen
protein expression levels were decreased in NPCs cultured on a
stiff substrate in the present study, which was opposite to the
changes observed in type I collagen expression. These pathological
alterations may be responsible for the NP degeneration and
dehydration that occurred in the bipedal rat model, as observed by
T2-weighted MR imaging. These results suggested that the IVDD
models were successfully established, which laid the foundation for
subsequent experiments.
The nuclear translocation of MRTF-A in response to
high mechanical force has been identified in primary studies with
various human cell types, such as human colonic fibroblasts and
intestinal cells (59-61). Consistent with previous data in
the fibrosis of other organs, the present study first demonstrated
that mechanical stiffness of the culture substrate affected the
nucleocytoplasmic shuttling of MRTF-A in rat NPCs in vitro.
Consistent with a previous study (62), the results of the present study
demonstrated upregulated expression levels of fibrotic markers of
the ECM, such as α-SMA, type I collagen and CTGF, in the NP samples
of a bipedal rat IVDD model, and the levels of these markers were
significantly attenuated in the CCG-1423-treated animals. These
results suggested the involvement of MRTF-A nuclear-cytoplasmic
trafficking in the regulation of the expression of a series of
fibrosis-associated proteins induced by excessive external
stress.
In the present study, CCG-1423 treatment partly
abolished the matrix stiffness-dependent upregulation of fibrotic
protein expression levels in vitro. Notably, no further
suppression of fibrotic protein expression levels was observed in
NPCs co-treated with physiological stiffness and inhibitors in the
stiff + CCG-1423 group, and these levels may represent the basal
level. These results were consistent with a previous study, which
demonstrated the basal expression of CTGF observed in a static
condition (41). In addition,
CCG-1423 treatment had no effect on cell viability, indicating that
the downregulation of fibrotic protein expression in NPCs was not
associated with the cytotoxicity of CCG-1423. Finally, F-actin was
studied to determine the role of MRTF-A in regulating the
differentiation fate of NPCs by mediating the actin dynamics. The
results demonstrated that MRTF-A inhibition significantly decreased
the intensity of F-actin staining; a similar inhibitory effect on
F-actin has been recently demonstrated in human adipose stem cells
treated with CCG-1423 (63).
These results further suggested that MRTF-A together with fibrotic
proteins participate in the fibrogenesis initiated by mechanical
overload by coupling actin. In conclusion, the results of the
present study suggested that strain-derived MRTF-A
translocation-regulated fibrotic protein expression may be a key
genetic switch in the development of NP fibrosis, and provide a
theoretical basis for targeting this signaling pathway as an
effective therapeutic approach. In a model of bipedal rats, the
therapeutic administration of CCG-1423 significantly limited the
extent of NP fibrosis. Thus, these findings may aid in the
formulation of mechanotransduction-based therapies or the
identification of drug targets for treating IVDD at the molecular
level to prevent or reverse the deleterious effects of mechanical
overload.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MK and YZ performed the experiments, analyzed data
and drafted the manuscript. MS performed some of the experiments.
MK, YZ and MS confirm the authenticity of all the raw data. WC and
CG contributed to the data analysis and interpretation. JZ, SH and
QT performed the in vivo experiments, data collection and
analysis. XM designed the study, supervised data analysis and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Laboratory Animal Care
and Use Committee of the Affiliated Hospital of Qingdao University
(Qing'dao, China; approval no. QYFYWZLL 26037).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant nos. 81672200 and 81871804) and the
National Key Research and Development Project of China (grant no.
2019YFC0121400).
References
|
1
|
Fatoye F, Gebrye T and Odeyemi I:
Real-world incidence and prevalence of low back pain using
routinely collected data. Rheumatol Int. 39:619–626. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2017 Disease and Injury Incidence and
Prevalence Collaborators: Global, regional, and national incidence,
prevalence, and years lived with disability for 354 diseases and
injuries for 195 countries and territories, 1990-2017: A systematic
analysis for the Global Burden of Disease Study 2017. Lancet.
392:1789–1858. 2018. View Article : Google Scholar
|
|
3
|
Kleinman N, Patel AA, Benson C, Macario A,
Kim M and Biondi DM: Economic burden of back and neck pain: Effect
of a neuropathic component. Popul Health Manag. 17:224–232. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neidlinger-Wilke C, Galbusera F, Pratsinis
H, Mavrogonatou E, Mietsch A, Kletsas D and Wilke HJ: Mechanical
loading of the intervertebral disc: From the macroscopic to the
cellular level. Eur Spine J. 23(Suppl 3): S333–S343. 2014.
View Article : Google Scholar
|
|
5
|
Setton LA and Chen J: Mechanobiology of
the intervertebral disc and relevance to disc degeneration. J Bone
Joint Surg Am. 88(Suppl 2): S52–S57. 2006.
|
|
6
|
Pengb Y and Lv FJ: Fibrosis in
intervertebral disc degeneration: Knowledge and gaps. Austin J
Orthopade Rheumatol. 1:32014.
|
|
7
|
Yee A, Lam MP, Tam V, Chan WC, Chu IK,
Cheah KS, Cheung KM and Chan D: Fibrotic-like changes in degenerate
human intervertebral discs revealed by quantitative proteomic
analysis. Osteoarthritis Cartilage. 24:503–513. 2016. View Article : Google Scholar
|
|
8
|
Nanthakumar CB, Hatley RJ, Lemma S,
Gauldie J, Marshall RP and Macdonald SJ: Dissecting fibrosis:
Therapeutic insights from the small-molecule toolbox. Nat Rev Drug
Discov. 14:693–720. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olson EN and Nordheim A: Linking actin
dynamics and gene transcription to drive cellular motile functions.
Nat Rev Mol Cell Biol. 11:353–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marinissen MJ, Chiariello M, Tanos T,
Bernard O, Narumiya S and Gutkind JS: The small GTP-binding protein
RhoA regulates c-jun by a ROCK-JNK signaling axis. Mol Cell.
14:29–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu F, Mih JD, Shea BS, Kho AT, Sharif AS,
Tager AM and Tschumperlin DJ: Feedback amplification of fibrosis
through matrix stiffening and COX-2 suppression. J Cell Biol.
190:693–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimizu Y, Dobashi K, Iizuka K, Horie T,
Suzuki K, Tukagoshi H, Nakazawa T, Nakazato Y and Mori M:
Contribution of small GTPase Rho and its target protein rock in a
murine model of lung fibrosis. Am J Respir Crit Care Med.
163:210–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Satoh S, Ueda Y, Koyanagi M, Kadokami T,
Sugano M, Yoshikawa Y and Makino N: Chronic inhibition of Rho
kinase blunts the process of left ventricular hypertrophy leading
to cardiac contractile dysfunction in hypertension-induced heart
failure. J Mol Cell Cardiol. 35:59–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bei Y, Hua-Huy T, Nicco C, Duong-Quy S,
Le-Dong NN, Tiev KP, Chéreau C, Batteux F and Dinh-Xuan AT:
RhoA/Rho-kinase activation promotes lung fibrosis in an animal
model of systemic sclerosis. Exp Lung Res. 42:44–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okumura N, Koizumi N, Ueno M, Sakamoto Y,
Takahashi H, Hirata K, Torii R, Hamuro J and Kinoshita S:
Enhancement of corneal endothelium wound healing by Rho-associated
kinase (ROCK) inhibitor eye drops. Br J Ophthalmol. 95:1006–1009.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miralles F, Posern G, Zaromytidou AI and
Treisman R: Actin dynamics control SRF activity by regulation of
its coactivator MAL. Cell. 113:329–342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayashi K, Watanabe B, Nakagawa Y, Minami
S and Morita T: RPEL proteins are the molecular targets for
CCG-1423, an inhibitor of Rho signaling. PLoS One. 9:e890162014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiyama A, Arai F, Sakai D, Yokoyama K and
Mochida J: The effects of oxygen tension and antiaging factor
Klotho on Wnt signaling in nucleus pulposus cells. Arthritis Res
Ther. 14:R1052012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pelham RJ Jr and Wang Yl: Cell locomotion
and focal adhesions are regulated by substrate flexibility. Proc
Natl Acad Sci USA. 94:13661–13665. 1997. View Article : Google Scholar
|
|
20
|
Tse JR and Engler AJ: Preparation of
hydrogel substrates with tunable mechanical properties. Curr Protoc
Cell Biol. 10:Unit 10.16. 2010.PubMed/NCBI
|
|
21
|
Gilchrist CL, Darling EM, Chen J and
Setton LA: Extracellular matrix ligand and stiffness modulate
immature nucleus pulposus cell-cell interactions. PLoS One.
6:e271702011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wallace MA, Della Gatta PA, Ahmad Mir B,
Kowalski GM, Kloehn J, McConville MJ, Russell AP and Lamon S:
Overexpression of striated muscle activator of Rho signaling
(STARS) increases C2C12 skeletal muscle cell differentiation. Front
Physiol. 7:72016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Esnault C, Stewart A, Gualdrini F, East P,
Horswell S, Matthews N and Treisman R: Rho-actin signaling to the
MRTF coactivators dominates the immediate transcriptional response
to serum in fibroblasts. Genes Dev. 28:943–958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao J, Qiu GX, Wu ZH, Xu J, Wang T, Wang
YP and Weng XS: An improved operation approach for bipedal rat
model construction. Zhonghua Yi Xue Za Zhi. 86:2781–2785. 2006.In
Chinese.
|
|
25
|
Liang X, Shen H, Shi WD, Ren S, Jiang W,
Liu H, Yang P, Sun ZY, Lin J and Yang HL: Effect of axial vertical
vibration on degeneration of lumbar intervertebral discs in
modified bipedal rats: An in-vivo study. Asian Pac J Trop Med.
10:714–717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thompson JP, Pearce RH, Schechter MT,
Adams ME, Tsang IK and Bishop PB: Preliminary evaluation of a
scheme for grading the gross morphology of the human intervertebral
disc. Spine (Phila Pa 1976). 15:411–415. 1990. View Article : Google Scholar
|
|
27
|
Ge J, Cheng X, Yuan C, Qian J, Wu C, Cao
C, Yang H, Zhou F and Zou J: Syndecan-4 is a novel therapeutic
target for intervertebral disc degeneration via suppressing JNK/p53
pathway. Int J Biol Sci. 16:766–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang F, Leung VY, Luk KD, Chan D and
Cheung KM: Injury-induced sequential transformation of notochordal
nucleus pulposus to chondrogenic and fibrocartilaginous phenotype
in the mouse. J Pathol. 218:113–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tam V, Chan WCW, Leung VYL, Cheah KSE,
Cheung KMC, Sakai D, McCann MR, Bedore J, Séguin CA and Chan D:
Histological and reference system for the analysis of mouse
intervertebral disc. J Orthop Res. 36:233–243. 2018.
|
|
30
|
Manohar M, Kandikattu HK, Verma AK and
Mishra A: IL-15 regulates fibrosis and inflammation in a mouse
model of chronic pancreatitis. Am J Physiol Gastrointest Liver
Physiol. 315:G954–G965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui L, Wei H, Li ZM, Dong XB and Wang PY:
TGF-β1 aggravates degenerative nucleus pulposus cells inflammation
and fibrosis through the upregulation of angiopoietin-like protein
2 expression. Eur Rev Med Pharmacol Sci. 24:12025–12033.
2020.PubMed/NCBI
|
|
32
|
Meng X, Zhuang L, Wang J, Liu Z, Wang Y,
Xiao D and Zhang X: Hypoxia-inducible factor (HIF)-1alpha knockout
accelerates intervertebral disc degeneration in mice. Int J Clin
Exp Pathol. 11:548–557. 2018.PubMed/NCBI
|
|
33
|
Eckes B, Nischt R and Krieg T: Cell-matrix
interactions in dermal repair and scarring. Fibrogenesis Tissue
Repair. 3:42010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hinz B: The myofibroblast: Paradigm for a
mechanically active cell. J Biomech. 43:146–155. 2010. View Article : Google Scholar
|
|
35
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken). 67:545–554. 2010. View Article : Google Scholar
|
|
36
|
Bond JE, Kokosis G, Ren L, Selim MA,
Bergeron A and Levinson H: Wound contraction is attenuated by
fasudil inhibition of Rho-associated kinase. Plast Reconstr Surg.
128:438e–450e. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brown JH, Del Re DP and Sussman MA: The
Rac and Rho hall of fame: A decade of hypertrophic signaling hits.
Circ Res. 98:730–742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Holle AW and Engler AJ: More than a
feeling: Discovering, understanding, and influencing mechanosensing
pathways. Curr Opin Biotechnol. 22:648–654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Geneste O, Copeland JW and Treisman R: LIM
kinase and Diaphanous cooperate to regulate serum response factor
and actin dynamics. J Cell Biol. 157:831–838. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leask A, Parapuram SK, Shi-Wen X and
Abraham DJ: Connective tissue growth factor (CTGF, CCN2) gene
regulation: A potent clinical bio-marker of fibroproliferative
disease? J Cell Commun Signal. 3:89–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Blomme B, Deroanne C, Hulin A, Lambert C,
Defraigne JO, Nusgens B, Radermecker M and Colige A: Mechanical
strain induces a pro-fibrotic phenotype in human mitral valvular
interstitial cells through RhoC/ROCK/MRTF-A and Erk1/2 signaling
pathways. J Mol Cell Cardiol. 135:149–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Johnson LA, Rodansky ES, Haak AJ, Larsen
SD, Neubig RR and Higgins PD: Novel Rho/MRTF/SRF inhibitors block
matrix-stiffness and TGF-β-induced fibrogenesis in human colonic
myofibroblasts. Inflamm Bowel Dis. 20:154–165. 2014. View Article : Google Scholar
|
|
43
|
Shiwen X, Stratton R, Nikitorowicz-Buniak
J, Ahmed-Abdi B, Ponticos M, Denton C, Abraham D, Takahashi A, Suki
B, Layne MD, et al: A role of myocardin related transcription
factor-A (MRTF-A) in scleroderma related fibrosis. PLoS One.
10:e01260152015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shao J, Xu H, Wu X and Xu Y: Epigenetic
activation of CTGF transcription by high glucose in renal tubular
epithelial cells is mediated by myocardin-related transcription
factor A. Cell Tissue Res. 379:549–559. 2020. View Article : Google Scholar
|
|
45
|
Arthur WT, Noren NK and Burridge K:
Regulation of Rho family GTPases by cell-cell and cell-matrix
adhesion. Biol Res. 35:239–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim JG, Islam R, Cho JY, Jeong H, Cap KC,
Park Y, Hossain AJ and Park JB: Regulation of RhoA GTPase and
various transcription factors in the RhoA pathway. J Cell Physiol.
233:6381–6392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takahara A, Sugiyama A, Satoh Y, Yoneyama
M and Hashimoto K: Cardiovascular effects of Y-27632, a selective
Rho-associated kinase inhibitor, assessed in the
halothane-anesthetized canine model. Eur J Pharmacol. 460:51–57.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hinson JS, Medlin MD, Lockman K, Taylor JM
and Mack CP: Smooth muscle cell-specific transcription is regulated
by nuclear localization of the myocardin-related transcription
factors. Am J Physiol Heart Circ Physiol. 292:H1170–1180. 2007.
View Article : Google Scholar
|
|
49
|
Pawłowski R, Rajakylä EK, Vartiainen MK
and Treisman R: An actin-regulated importin α/β-dependent extended
bipartite NLS directs nuclear import of MRTF-A. EMBO J.
29:3448–3458. 2010. View Article : Google Scholar
|
|
50
|
Dai J, Qin L, Chen Y, Wang H, Lin G, Li X,
Liao H and Fang H: Matrix stiffness regulates
epithelial-mesenchymal transition via cytoskeletal remodeling and
MRTF-A translocation in osteosarcoma cells. J Mech Behav Biomed
Mater. 90:226–238. 2019. View Article : Google Scholar
|
|
51
|
Hahmann C and Schroeter T: Rho-kinase
inhibitors as therapeutics: From pan inhibition to isoform
selectivity. Cell Mol Life Sci. 67:171–177. 2010. View Article : Google Scholar
|
|
52
|
Nakamura S, Hayashi K, Iwasaki K, Fujioka
T, Egusa H, Yatani H and Sobue K: Nuclear import mechanism for
myocardin family members and their correlation with vascular smooth
muscle cell phenotype. J Biol Chem. 285:37314–37323. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang YH, Zhao CQ, Jiang LS and Dai LY:
Substrate stiffness regulates apoptosis and the mRNA expression of
extracellular matrix regulatory genes in the rat annular cells.
Matrix Biol. 30:135–144. 2011. View Article : Google Scholar
|
|
54
|
Hoffman BD, Grashoff C and Schwartz MA:
Dynamic molecular processes mediate cellular mechanotransduction.
Nature. 475:316–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Waxenbaum JA, Reddy V and Futterman B:
Anatomy, Back, Intervertebral Discs. StatPearls. StatPearls
Publishing. Copyright© 2020, StatPearls Publishing LLC; Treasure
Island, FL: 2020
|
|
56
|
Sivan SS, Roberts S, Urban JP, Menage J,
Bramhill J, Campbell D, Franklin VJ, Lydon F, Merkher Y, Maroudas A
and Tighe BJ: Injectable hydrogels with high fixed charge density
and swelling pressure for nucleus pulposus repair: Biomimetic
glycosaminoglycan analogues. Acta Biomater. 10:1124–1133. 2014.
View Article : Google Scholar
|
|
57
|
Chelberg MK, Banks GM, Geiger DF and
Oegema TR Jr: Identification of heterogeneous cell populations in
normal human intervertebral disc. J Anat. 186:43–53.
1995.PubMed/NCBI
|
|
58
|
Zhang YG, Sun ZM, Liu JT, Wang SJ, Ren FL
and Guo X: Features of intervertebral disc degeneration in rat's
aging process. J Zhejiang Univ Sci B. 10:522–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang X, Yang N, Fiore VF, Barker TH, Sun
Y, Morris SW, Ding Q, Thannickal VJ and Zhou Y: Matrix
stiffness-induced myofibroblast differentiation is mediated by
intrinsic mechanotransduction. Am J Respir Cell Mol Biol.
47:340–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao XH, Laschinger C, Arora P, Szászi K,
Kapus A and McCulloch CA: Force activates smooth muscle alpha-actin
promoter activity through the Rho signaling pathway. Cell Sci.
120:1801–1809. 2007. View Article : Google Scholar
|
|
61
|
Johnson LA, Rodansky ES, Sauder KL,
Horowitz JC, Mih JD, Tschumperlin DJ and Higgins PD: Matrix
stiffness corresponding to strictured bowel induces a fibrogenic
response in human colonic fibroblasts. Inflamm Bowel Dis.
19:891–903. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zheng L, Qin J, Sun L, Gui L, Zhang C,
Huang Y, Deng W, Huang A, Sun D and Luo M: Intrahepatic
upregulation of MRTF-A signaling contributes to increased hepatic
vascular resistance in cirrhotic rats with portal hypertension.
Clin Res Hepatol Gastroenterol. 41:303–310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hyväri L, Vanhatupa S, Halonen HT,
Kääriäinen M and Miettinen S: Myocardin-related transcription
factor A (MRTF-A) regulates the balance between adipogenesis and
osteogenesis of human adipose stem cells. Stem Cells Int.
2020:88535412020. View Article : Google Scholar : PubMed/NCBI
|