Polychlorinated biphenyls (PCBs) are a class of
synthetic organic compounds, which contain 209 congeners (1). Based on their three-dimensional
structure, PCBs can be divided into two main categories:
Dioxin-like PCBs (DL-PCBs) and non-dioxin-like PCBs (NDL-PCBs).
Owing to their chemical and thermal stability, PCBs were used
widely in various industrial and commercial applications, including
lubricating oils, plasticizers, hydraulic fluids, paint and ink
(2-4). Commercial production of PCBs began
in 1929 in the United States and were sold worldwide as commercial
mixtures, such as Aroclor®, Clophen® and
Phenclor® in the 20th century before stopping in the
late 1970s (1,5,6).
PCBs are often used in long-life products, with some reaching
>30 years, such as capacitors and sealants (7). For the PCBs used in closed
electrical systems, large release of chemicals do not occur as long
as the electrical equipment remains intact during use or storage;
however, significant release may occur if these systems are not
properly managed during the waste and recovery phases (8). Persistent PCB emitters are likely
to be released continuously and/or intermittently over the next few
decades (9). The non-degradable
property of PCBs makes them persistent organic pollutants, which
exist in food chains, water, soil and even in air circulation
(2,10).
Maternal PCB accumulation can be transferred to the
offspring via the placenta and breast milk (33,34). For the mother, breastfeeding is
the main method of excreting PCBs, while for the offspring, it is
the main source of PCB accumulation. Takagi et al (33) used (14C)PCBs to
investigate the association between maternal and progeny PCBs via
intragastric feeding in a rat model. In the fetus, the highest PCB
concentration was in the fetal placenta, followed by the liver,
heart, skin, muscles, blood, lungs and the brain. In suckling
offspring, the highest concentration was found in the adipose
tissue, while intermediate concentrations existed in the skin,
adrenal gland and the liver. The concentration of PCBs in the fetal
blood [0.24 parts-per million (ppm)] was similar to that in the
maternal blood (0.26 ppm) and was much lower compared with that in
the milk (1.84 ppm). Furthermore, the PCB content in nursing rats
was significantly lower compared with that in pregnant and virgin
rats. However, exposure to PCBs in multiple pregnancies was not
lower compared with that in the first pregnancy. The levels of PCBs
in the maternal body and breast milk was associated with age, diet,
parity, self-nutrition during pregnancy and smoking habits
(35,36). The concentration of PCBs was
reduced by previous lactation; however, older parturients could
accumulate PCBs for longer periods of time. In contrast, younger
mothers exhibited a shorter lifetime exposure to environmental
pollutants (37).
Language is considered as an indicator of a child's
cognitive development and language retardation may be the earliest
sign of one or more neurodevelopmental disorders (38,39). Furthermore, in a cohort study,
with a large group of mother-child pairs, high exposure to DL-PCBs
during pregnancy increased the risk of language delay at age 3
years according to the parental report and Ages and Stages
Questionnaire (40). However,
due to the neurotoxicity of methylmercury, the neurotoxic effects
of PCB cannot be assessed when individuals are exposed to both
methylmercury and PCB (41).
Intrauterine PCB exposure could have a long-term
impact on intellectual function. The effects of PCBs on
intelligence seem to vary with age. Negative effects could develop
or progress over time. A study by Berghuis et al (42) analyzed the association between
the blood concentration of PCBs in pregnant women in the second
and/or third trimester and intelligence using Touwen examination.
They found that higher gestational exposure to several PCBs was
positively associated with neurological functioning in 3-month-old
babies. In addition, an early study revealed no statistically
significant association between perinatal exposure to PCBs and the
abilities of the children at 3-5 years, which were examined using
the McCarthy Scales (43).
However, as children become older, the negative effects of PCB on
intelligence are becoming more notable (21,44,45). Lower levels of PCBs might be
associated with higher intelligence in infants by stimulating the
neuronal and/or hormonal processes, which leads to positive
effects, while higher exposure levels might exert negative effects
(42), suggesting the effects
were dose-dependent. This is consistent with the way PCBs are
transferred from the mother to the offspring. Since breastfeeding
is the primary source of PCB exposure for newborns, from their
mothers, it is possible that breastfeeding children have higher PCB
accumulation (33).
It remains controversial whether cochlear function
is immature in the first few months of human life or whether
perinatal PCB exposure affects the auditory function in children. A
collaborative perinatal project in the United States (22) suggested no association between
PCB levels in serum from pregnant women and sensorineural hearing
loss (based on hearing threshold) in 8-year-old children.
Conversely, in fish-eating populations of the Faroe Islands, higher
PCB content in the cord tissue was associated with increased
hearing thresholds in infants (46). Jusko et al (47) found that PCB-153 concentrations
in the maternal and cord serum were not associated with distortion
product otoacoustic emissions (DPOAEs) in 45-month-old children,
while high levels of PCB-153 in the serum from children at 6, 16
and 45 months were associated with poor DPOAE amplitudes,
suggesting that continued PCB exposure was more harmful to auditory
function compared with that for a specific period of exposure.
Behavioral problems are also symptoms or signs of
neurodevelopmental abnormalities, including externalizing and
internalizing behavior problems (48). Internal behavior problems,
defined as a lack of control of emotions, seem to be more easily
affected by prenatal PCB exposure. Conversely, parental
child-rearing attitudes around the birth order may play a more
important role in child behavior compared with that in prenatal PCB
exposure itself (49).
Meanwhile, epidemiological investigations have not revealed a
potential association between PCBs and externalizing behavior
problems, which include oppositional, hyperactivity and aggressive
behaviors according to Behavioral Assessment System for Children-2
at age 8 years (20). Several
studies using zebrafish, an ideal model for toxicological research,
have confirmed that embryonic exposure to PCBs was associated with
anxious behavior and altered reactions to visual threats (50-52).
Autism, also known as autism spectrum disorder
(ASD), is a type of neurodevelopmental condition characterized by
different degrees of impaired social interaction and communication,
repetitive or stereotypic behaviors, narrow interests, and abnormal
perceptions (53). The etiology
of ASD has not been fully elucidated; however, a previous study has
shown that PCB exposure alters the endogenous axis and
hormone-dependent neurodevelopment, thereby increasing the risk of
ASD (53). However, such
associations have not been unanimously supported in all
literatures. Granillo et al (23) enrolled high-risk cohort families,
with at least one child with ASD and planned to have another baby.
They found that there was no significant association between total
PCBs and ASD. Furthermore, DL-PCBs decreased the risk of ASD with
borderline significance, whereas NDL-PCBs significantly elevated
the risk of ASD. In another study, which included 546 mother-infant
pairs, in a pregnancy and birth cohort, there was no association
between 6 PCB congeners (PCB118, PCB138, PCB158, PCB170, PCB180 and
PCB187) in the maternal serum in the first trimester of pregnancy
and ASD in their children at 3-4 years of age (54).
The effects of prenatal exposure to PCBs on
offspring shows large interindividual variability. This
inconsistency in epidemiological investigations may be attributable
to a number of reasons, described below.
Genetic polymorphism refers to the presence of two
or more alleles, at a particular locus. Depending on the allele and
the gene, these polymorphisms may either protect the individual
from pesticides-induced oxidative damage, or conversely, makes its
more vulnerable (55,56). For example, two important
polymorphisms (Q192R and L55M) in the human paraoxonase 1 (PON1)
gene, a hydrolytic enzyme, which protects the toxicity of
organophosphates insecticides, have opposing roles. The PON1 Q192R
polymorphism enhanced the role of PON1, while PON1 L55M was
hypothesized to have the opposite effect (57). Cytochrome P450s (CYPs) plays a
key role in detoxification or activation of numerous xenobiotics
(55). DL-PCBs bind and activate
the aryl hydrocarbon receptor (AhR) to regulate three members of
the CYP family: CYP1A1, CYP1A2 and CYP1B1 (58), which play an important role in
the detoxification of PCB (59).
Poor-affinity AhRs and high protein levels of CYP1A2
in maternal liver cells provided important protection to the
offspring against the sensitivity to gestational PCBs exposure
(60-62). Conversely, high-affinity AhRs
were found to respond to low levels of DL-PCBs, while the
CYP1A2-mediated detoxification pathway could sequester DL-PCBs to
prevent transfer to the offspring (60). The affinity of AhR and the
expression of CYP1A2 in the liver varies in the population, which
indicates that there are large individual differences in the
susceptibility to PCBs (63).
The toxicological effect of NDL-PCBs has been
associated with the ryanodine receptor (RyR). Compared with that in
wild-type mice, double mutant (functional mutation in the RyR1 and
a human CGG repeat expansion in the fragile X mental retardation
gene 1) mice were more susceptible to PCBs. Perinatal exposure to
PCBs in the maternal diet caused dysbiosis of the gut microbiota,
resulting in behavioral deficits in the double mutant mice
(64), which represents a
potential role for protein digestion and microbial putrefaction in
the gut-brain axis in patients with ASD (65).
Prenatal PCB exposure is usually detected in the
maternal and umbilical cord blood, which may not be reflected in
fetal suffering. In practice, cord blood is normally collected at
birth, which does not include the sensitive window in early
pregnancy associated with progeny health. Amniotic fluid provides
another possible fetal environment, which can be analyzed, and
amniocentesis is usually performed in the second trimester, during
the prenatal diagnosis of advanced chromosomal abnormalities or
fetal deformities (66,67). However, amniocentesis is an
invasive procedure and is not used for routine prenatal
examinations. Therefore, it is not realistic to use it for
prospective studies.
Different evaluation endpoints are another reason
for the uncertainty with respect to the different results of
epidemiological studies, even within the same cohort. As
aforementioned, the negative effects of PCBs exposure during
pregnancy on the intelligence of the child became more pronounced
with age (21,42,68). Furthermore, the age of the
mother, education, race, preconception body mass index, fat mass,
birth order, birth weight, number of pregnancies, duration of
breastfeeding and diet, confound the effects of PCBs on the health
of the offspring (69,70).
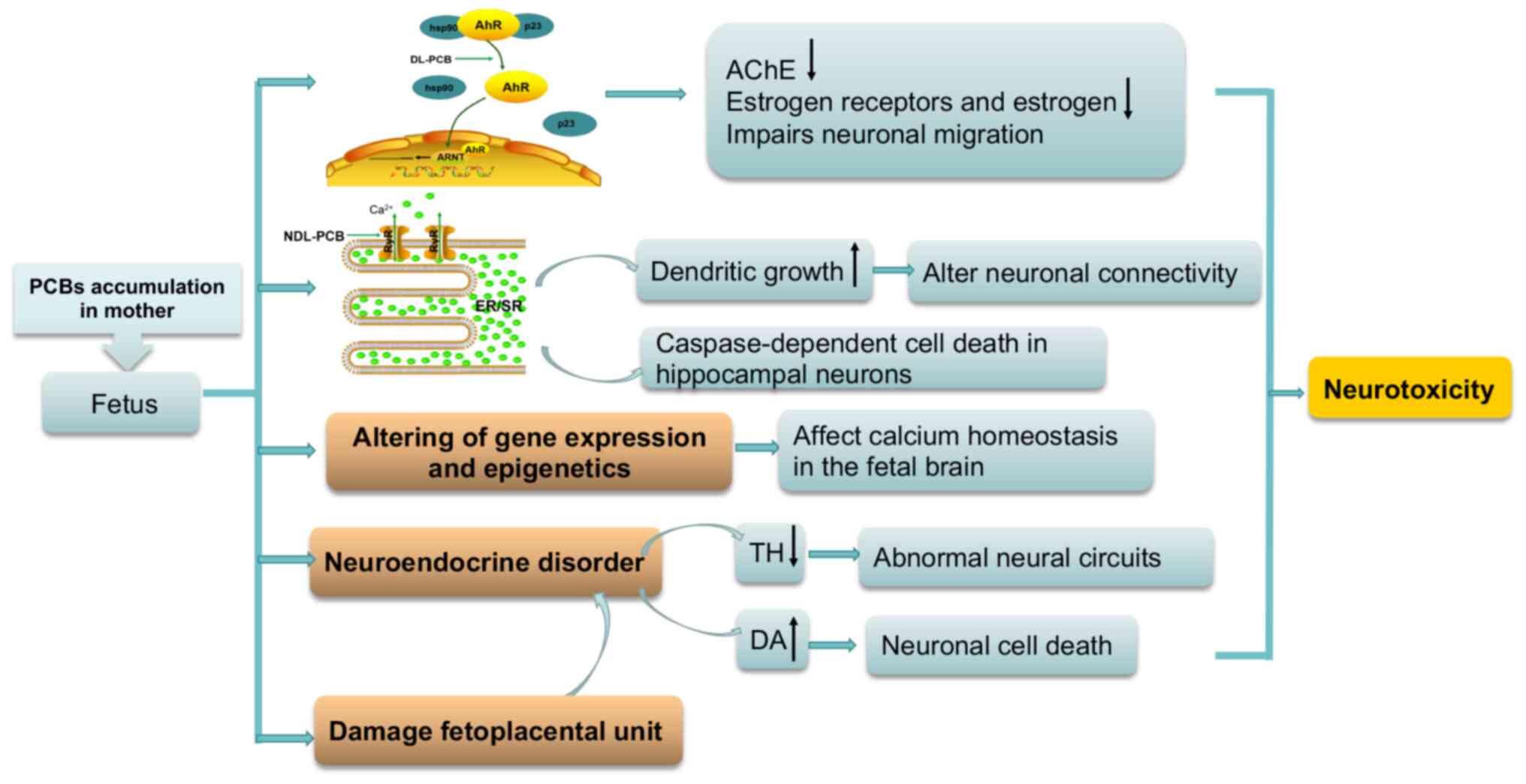
The possible mechanisms of PCB on the development of
the nervous system are described in Fig. 1. The toxicological mechanism of
DL-PCBs has been associated with the activation of AhR (60,71), while NDL-PCBs have been
associated with RyR-mediated calcium (Ca2+) ion channels
(72,73). Both DL- and NDL-PCBs may damage
the fetoplacental unit (74,75), alter gene expression (76) and epigenetics (77), and affect neuroendocrine function
(78).
AhR is a member of the eukaryotic Per-Ah receptor
nuclear translocator (ARNT)-Sim domain protein family, which is
located in the cytoplasm and binds to chaperone proteins hsp90, and
co-chaperone protein p23 (79).
Following ligand activation by DL-PCBs, the AhR separates and is
translocated into the nucleus, where it binds to DNA response
elements with an ARNT and begins transcription (71). In several animal models of
development, AhR has been demonstrated to be widely distributed in
the cerebral cortex, cerebellum, hippocampus and olfactory bulb
neurons (80,81). Increasing evidence has also
indicated that the neurotoxicity of prenatal exposure to DL-PCBs
was associated with AhR activation in a dose-dependent manner
(58,62,82), suggesting it plays an important
role in neural development.
The following mechanisms may be involved in the
effect of the DL-PCBs on the development of the nervous system via
AhR: i) AhR might act as a common upstream regulatory molecule,
that inhibits acetylcholinesterase (AChE) activity via
transcriptional regulation of dioxin-response element sites in
human AChE promoters and post-translational regulation of
AChE-targeting microRNAs (83).
AChE is an enzyme with high catalytic activity in the hydrolysis of
acetylcholine, an important neurotransmitter. It not only plays a
key role in regulating cholinergic neurotransmission, but is also
an important enzyme in neurodevelopment (84); ii) DL-PCBs affect estrogen
signaling via AhR, thereby reducing estrogen receptors and estrogen
levels to disrupt the neuroprotective effect of estrogen in the
cerebral cortex (83,85) and iii) over-activated AhR
activity impairs neuronal migration during hippocampal development
(86).
Epigenetic markers are dynamic and can be affected
by the environment, particularly during critical periods of
embryonic development and early life (97). Several studies have highlighted
the importance of parental exposure to PCBs, which could affect the
epigenome of the offspring and the susceptibility of the offspring
to disease (97-99). DNA methylation refers to the
addition of a methyl group to DNA using the enzyme, DNA
methyltransferase, which can regulate genetic expression without
changing the DNA sequence (17).
In early life, DNA methylation can be affected by environmental
factors and can persistent even after removal of these factors
(99,100). In primordial germ cells, DNA
methylation decreased from 92% in post implantation embryos to 6.0%
at week 10 in females and 7.8% at week 11 in males. DNA methylation
levels gradually increased after 19 weeks until they reached the
level of mature germ cells after birth (101). The placenta provides nutrients
and oxygen to the fetus. It also contains an epigenome, which
enables heritable and sustained changes to gene expression levels
without altering the DNA sequence. The placenta remains
hypomethylated at the genome level; however, site-specific
epigenetic patterns are preserved (77). Imprinted genes are a subset of
genes which undergo epigenetic programming during early development
and also undergo remodeling during pregnancy, highlighting their
potential sensitivity to environmental factors (102). Several studies have
demonstrated that changes in placental DNA methylation were
associated with environmental exposure (77,102-104). In a previous study, errors in
maintaining epigenetic markers affected DNA methylation and was
associated with an increased risk of developing ASD (105).
In zebrafish, developmental exposure to low levels
of AhR agonist, PCB-126 upregulated genes associated with
Ca2+ channels and downregulated genes associated with
oxidative phosphorylation, suggesting that DL-PCBs could affect
Ca2+ homeostasis in the brain in vivo, and one of
the pathways directly affected by altered Ca2+ signaling
was the MAPK signaling pathway (76). Both Ca2+ and MAPK
signaling play important roles in neurodevelopment and cognitive
functions, such as learning and memory (76,106).
PCBs easily penetrate the placental and blood-brain
barriers, resulting in PCB accumulation in the offspring's brain
(107). Early exposure to
dioxins and PCBs could alter basic cellular signaling processes and
endocrine functions, thereby affecting the synthesis and activity
of important neurotransmitters in the central nervous system, as
well as the development of brain tissue (108,109).
Thyroid hormones, which regulate the migration and
maturation of γ-aminobutyric acidergic interneurons, are crucial
during fetal development, particularly in the nervous system
(110,111). The disruption of this process
causes the formation of abnormal neural circuits, which has been
hypothesized to underlie some neurodevelopmental disorders in
humans, such as ASD (112). In
early pregnancy, the fetal thyroid hormone is completely dependent
on transport from the mother prior to fetal self-synthesis
(113). An increasing number of
epidemiological studies have confirmed that gestational PCB
exposure was associated with disturbances in the thyroid function
of neonates (114-116). This destruction has
long-lasting effects in the offspring, potentially lasting until
the child is 8 years old (116). The effects of PCBs and OH-PCBs
on thyroid function may involve the following mechanisms: i) PCBs
may competitively bind to transthyretin, particularly OH-PCBs,
which have stronger binding affinity compared with that in their
parent compounds; ii) PCBs may interact with thyroxine receptors or
suppress DNA transcription and iii) OH-PCBs inhibit thyroid hormone
sulfation, affecting the peripheral metabolism of thyroid hormones
(114,117).
Dopaminergic systems are another potential target of
PCB exposure during critical periods of neuronal development. For
example, DL-PCBs may elevate dopamine (DA) concentrations in the
prefrontal cortex via an estrogenic effect and alter behavior
(78). A coculture model of
developing rat striatum and ventral mesencephalon (VM) revealed
that the neural toxicity of PCBs increased neuronal cell death and
reduced the number of DA neurons in the VM (118). PCBs disturb DA transport into
vesicles in the presynaptic terminal by inhibiting the activity of
the DA transporter and vesicular monoamine transporter 2, leading
to an accumulation of unsequestered DA, and increased production of
the DA metabolites, which results in free-radical formation and
caspase-mediated neuronal cell death (118,119).
The fetal placental unit connects maternal and fetal
circulation and plays an important role in nutrient metabolism and
endocrine systems (120).
Lipophilic EDCs can accumulate in the placenta and can damage the
fetoplacental unit and affect placental endocrine function
(121,122). Angiogenesis, in the
fetoplacental unit, is the result of cross-communication between
different cells, such as invading trophoblasts, endothelial cells
and specialized natural killer cells (119). The binding of δ-like (Dll)-4 to
Notch receptors induces the proteolytic release of the Notch
intracellular domain and regulates VEGF expression, forming a
primary vascular network and secondary angiogenesis at the
maternal-fetal interface (123,124). The Dll4-Notch4-VEGFR2 signaling
axis is a potential target for PCBs, particularly when the IL-10
gene is knocked out, which leads to poor spiral artery remodeling
and reduced angiogenesis in the placenta (74). Animal studies have shown that
gestational PCB126 exposure leads to some histopathological changes
in the placental tissue, which manifests as hyperemia, hemorrhage,
degeneration, apoptosis in the labyrinth layer and spiral arteries
of the placenta, resulting in fetal hypothyroidism and endocrine
disruption. The presence of hypothyroidism negatively affected the
fetal pituitary thyroid axis, the growth hormone/insulin-like
growth factor-I axis and cytokine levels, such as leptin, IL-1β,
TGF-β and tumor necrosis factor (TNF)-α (75). This fetoplacental unit
disruption, caused by maternal PCB exposure, might reduce normal
biological function and the general health of the offspring.
PCBs are persistent environmental EDCs, and have
environmental impacts, even though they have been banned for
decades. There are still limitations with respect to understanding
of PCB neurotoxicity. The novelty of the present review firstly
systematically analyzed prenatal PCB exposure, particularly that
gestational exposure affected the development of the nervous system
in the offspring and even had long-term effects on the brain. Due
to multiple contradictory factors, such as different types of PCB
exposure, different exposure doses, different follow-up ages, and
individual genetic susceptibility, there is not a consistent
conclusion from epidemiology research. The relevant reasons of
epidemiological investigation were analyzed, providing areas of
future epidemiological investigations on intrauterine PCB exposure.
The underlying mechanism of different PCBs congeners, including the
activation of AhR, via RyR-mediated Ca2+ ion channels,
and the epigenetic changes that can occur have been discussed;
however, further investigation is required to fully understand the
mechanisms involved. Furthermore, there is still no effective
method to intervene or block the neurotoxicity of PCBs; therefore,
the establishment of an ideal animal model is important. Despite
these limitations and challenges, increasing attention should be
made to PCB environmental pollution to avoid the potential adverse
effects in the offspring.
Not applicable.
YFW wrote the manuscript. CCH investigated the
association between gestational PCBs exposure and progeny nervous
system development. TF contributed to the mechanisms of PCBs. YJ
contributed to analysis of epidemiological differences. RJW
supervised and revised the manuscript. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Klocke C, Sethi S and Lein PJ: The
developmental neurotoxicity of legacy vs. contemporary
polychlorinated biphenyls (PCBs): Similarities and differences.
Environ Sci Pollut Res Int. 27:8885–8896. 2020. View Article : Google Scholar :
|
|
2
|
Garmash O, Hermanson MH, Isaksson E,
Schwikowski M, Divine D, Teixeira C and Muir DC: Deposition history
of polychlorinated biphenyls to the Lomonosovfonna Glacier,
Svalbard: A 209 congener analysis. Environ Sci Technol.
47:12064–12072. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinberg RM, Walker DM, Juenger TE,
Woller MJ and Gore AC: Effects of perinatal polychlorinated
biphenyls on adult female rat reproduction: Development,
reproductive physiology, and second generational effects. Biol
Reprod. 78:1091–1101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malisch R and Kotz A: Dioxins and PCBs in
feed and food-review from European perspective. Sci Total Environ.
491-492:2–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adams EM, von Hippel FA, Hungate BA and
Buck CL: Polychlorinated biphenyl (PCB) contamination of
subsistence species on Unalaska Island in the Aleutian Archipelago.
Heliyon. 5:e029892019. View Article : Google Scholar :
|
|
6
|
Sharma JK, Gautam RK, Misra RR, Kashyap
SM, Singh SK and Juwarkar AA: Degradation of Di-Through
Hepta-Chlorobiphenyls in clophen oil using microorganisms isolated
from long term PCBs contaminated soil. Indian J Microbiol.
54:337–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kohler M, Tremp J, Zennegg M, Seiler C,
Minder-Kohler S, Beck M, Lienemann P, Wegmann L and Schmid P: Joint
sealants: An overlooked diffuse source of polychlorinated biphenyls
in buildings. Environ Sci Technol. 39:1967–1973. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han W, Feng J, Gu Z, Wu M, Sheng G and Fu
J: Polychlorinated biphenyls in the atmosphere of Taizhou, a major
e-waste dismantling area in China. J Environ Sci (China).
22:589–597. 2010. View Article : Google Scholar
|
|
9
|
Arp HPH, Morin NAO, Andersson PL, Hale SE,
Wania F, Breivik K and Breedveld GD: The presence, emission and
partitioning behavior of polychlorinated biphenyls in waste,
leachate and aerosols from Norwegian waste-handling facilities. Sci
Total Environ. 715:1368242020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang CJ, Terrell ML, Marcus M, Marder ME,
Panuwet P, Ryan PB, Pearson M, Barton H and Barr DB: Serum
concentrations of polybrominated biphenyls (PBBs), polychlorinated
biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in the
Michigan PBB Registry 40 years after the PBB contamination
incident. Environ Int. 137:1055262020. View Article : Google Scholar :
|
|
11
|
Hales CN and Barker DJ: Type 2
(non-insulin-dependent) diabetes mellitus: The thrifty phenotype
hypothesis. Diabetologia. 35:595–601. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Magalhães ESDS, Méio MDBB and Moreira MEL:
Hormonal biomarkers for evaluating the impact of fetal growth
restriction on the development of chronic adult disease. Rev Bras
Ginecol Obstet. 41:256–263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Preston JD, Reynolds LJ and Pearson KJ:
Developmental origins of health span and life span: A mini-review.
Gerontology. 64:237–245. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rice D and Barone S Jr: Critical periods
of vulnerability for the developing nervous system: Evidence from
humans and animal models. Environ Health Perspect. 108(Suppl 3):
S511–S533. 2000.
|
|
15
|
Chu CP, Wu SW, Huang YJ, Chiang MC, Hsieh
ST and Guo YL: Neuroimaging signatures of brain plasticity in
adults with prenatal exposure to polychlorinated biphenyls: Altered
functional connectivity on functional MRI. Environ Pollut.
250:960–968. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perera F and Herbstman J: Prenatal
environmental exposures, epigenetics, and disease. Reprod Toxicol.
31:363–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Z, Cao F and Li X: Epigenetic
programming and fetal metabolic programming. Front Endocrinol
(Lausanne). 10:7642019. View Article : Google Scholar
|
|
18
|
Casati L, Sendra R, Colciago A, Negri-Cesi
P, Berdasco M, Esteller M and Celotti F: Polychlorinated biphenyls
affect histone modification pattern in early development of rats: A
role for androgen receptor-dependent modulation? Epigenomics.
4:101–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vermeir G, Covaci A, Van Larebeke N,
Schoeters G, Nelen V, Koppen G and Viaene M: Neurobehavioural and
cognitive effects of prenatal exposure to organochlorine compounds
in three year old children. BMC Pediatr. 21:992021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Yolton K, Webster GM, Sjödin A,
Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP and
Chen A: Prenatal PBDE and PCB exposures and reading, cognition, and
externalizing behavior in children. Environ Health Perspect.
125:746–752. 2017. View
Article : Google Scholar :
|
|
21
|
Berghuis SA, Van Braeckel KNJA, Sauer PJJ
and Bos AF: Prenatal exposure to persistent organic pollutants and
cognition and motor performance in adolescence. Environ Int. 121(Pt
1): 13–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Longnecker MP, Hoffman HJ, Klebanoff MA,
Brock JW, Zhou H, Needham L, Adera T, Guo X and Gray KA: In utero
exposure to polychlorinated biphenyls and sensorineural hearing
loss in 8-year-old children. Neurotoxicol Teratol. 26:629–637.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Granillo L, Sethi S, Keil KP, Lin Y,
Ozonoff S, Iosif AM, Puschner B and Schmidt RJ: Polychlorinated
biphenyls influence on autism spectrum disorder risk in the MARBLES
cohort. Environ Res. 171:177–184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Macdonal RW, Barrie LA, Bidleman TF,
Diamond ML, Gregor DJ, Semkin RG, Strachan WM, Li YF, Wania F,
Alaee M, et al: Contaminants in the Canadian arctic: 5 years of
progress in understanding sources, occurrence and pathways. Sci
Total Environ. 254:93–234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Desforges JP, Hall A, McConnell B,
Rosing-Asvid A, Barber JL, Brownlow A, De Guise S, Eulaers I,
Jepson PD, Letcher RJ, et al: Predicting global killer whale
population collapse from PCB pollution. Science. 361:1373–1376.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lohmann R, Gioia R, Jones KC, Nizzetto L,
Temme C, Xie Z, Schulz-Bull D, Hand I, Morgan E and Jantunen L:
Organochlorine pesticides and PAHs in the surface water and
atmosphere of the North Atlantic and Arctic Ocean. Environ Sci
Technol. 43:5633–5639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Te B, Yiming L, Tianwei L, Huiting W,
Pengyuan Z, Wenming C and Jun J: Polychlorinated biphenyls in a
grassland food network: Concentrations, biomagnification, and
transmission of toxicity. Sci Total Environ. 709:1357812020.
View Article : Google Scholar
|
|
28
|
Pajewska-Szmyt M, Sinkiewicz-Darol E and
Gadzala-Kopciuch R: The impact of environmental pollution on the
quality of mother's milk. Environ Sci Pollut Res Int. 26:7405–7427.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Portoles T, Sales C, Abalos M, Saulo J and
Abad E: Evaluation of the capabilities of atmospheric pressure
chemical ionization source coupled to tandem mass spectrometry for
the determination of dioxin-like polychlorobiphenyls in
complex-matrix food samples. Anal Chim Acta. 937:96–105. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Son MH, Kim JT, Park H, Kim M, Paek OJ and
Chang YS: Assessment of the daily intake of 62 polychlorinated
biphenyls from dietary exposure in South Korea. Chemosphere.
89:957–963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ebadi Fathabad A, Tajik H, Jafari K,
Hoseinzadeh E, Mirahmadi SS, Conti GO and Miri M: Evaluation of
dioxin-like polychlorinated biphenyls in fish of the Caspian Sea.
MethodsX. 7:1008032020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borrell LN, Factor-Litvak P, Wolff MS,
Susser E and Matte TD: Effect of socioeconomic status on exposures
to polychlorinated biphenyls (PCBs) and
dichlorodiphenyldichloroethylene (DDE) among pregnant
African-American women. Arch Environ Health. 59:250–255. 2004.
View Article : Google Scholar
|
|
33
|
Takagi Y, Aburada S, Hashimoto K and
Kitaura T: Transfer and distribution of accumulated
(14C)polychlorinated biphenyls from maternal to fetal and suckling
rats. Arch Environ Contam Toxicol. 15:709–715. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Uemura H, Arisawa K, Hiyoshi M, Satoh H,
Sumiyoshi Y, Morinaga K, Kodama K and Suzuki T, Nagai M and Suzuki
T: PCDDs/PCDFs and dioxin-like PCBs: Recent body burden levels and
their determinants among general inhabitants in Japan. Chemosphere.
73:30–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ulaszewska MM, Zuccato E and Davoli E:
PCDD/Fs and dioxin-like PCBs in human milk and estimation of
infants' daily intake: A review. Chemosphere. 83:774–782. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Simic I, Jovanovic G, Herceg Romanic S,
Klincic D, Matek Saric M and Popovic A: Optimization of Gas
Chromatography-electron Ionization-tandem mass spectrometry for
determining Toxic Non-ortho polychlorinated biphenyls in breast
milk. Biomed Environ Sci. 33:58–61. 2020.
|
|
37
|
Cok I, Donmez MK, Uner M, Demirkaya E,
Henkelmann B, Shen H, Kotalik J and Schramm KW: Polychlorinated
dibenzo-p-dioxins, dibenzofurans and polychlorinated biphenyls
levels in human breast milk from different regions of Turkey.
Chemosphere. 76:1563–1571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miniscalco C, Nygren G, Hagberg B, Kadesjo
B and Gillberg C: Neuropsychiatric and neurodevelopmental outcome
of children at age 6 and 7 years who screened positive for language
problems at 30 months. Dev Med Child Neurol. 48:361–366. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Clegg J, Hollis C, Mawhood L and Rutter M:
Developmental language disorders-a follow-up in later adult life.
Cognitive, language and psychosocial outcomes. J Child Psychol
Psychiatry. 46:128–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Caspersen IH, Haugen M, Schjølberg S,
Vejrup K, Knutsen HK, Brantsæter AL, Meltzer HM, Alexander J,
Magnus P and Kvalem HE: Maternal dietary exposure to dioxins and
polychlorinated biphenyls (PCBs) is associated with language delay
in 3year old Norwegian children. Environ Int. 91:180–187. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grandjean P, Weihe P, Nielsen F, Heinzow
B, Debes F and Budtz-Jorgensen E: Neurobehavioral deficits at age 7
years associated with prenatal exposure to toxicants from maternal
seafood diet. Neurotoxicol Teratol. 34:466–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Berghuis SA, Soechitram SD, Sauer PJ and
Bos AF: Prenatal exposure to polychlorinated biphenyls and their
hydroxylated metabolites is associated with neurological
functioning in 3-month-old infants. Toxicol Sci. 142:455–462. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gladen BC and Rogan WJ: Effects of
perinatal polychlorinated biphenyls and dichlorodiphenyl
dichloroethene on later development. J Pediatr. 119(1 Pt 1): 58–63.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jacobson JL and Jacobson SW: Intellectual
impairment in children exposed to polychlorinated biphenyls in
utero. N Engl J Med. 335:783–789. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Newman J, Gallo MV, Schell LM, DeCaprio
AP, Denham M and Deane GD; Akwesasne Task Force on Environment:
Analysis of PCB congeners related to cognitive functioning in
adolescents. Neurotoxicology. 30:686–696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Grandjean P, Weihe P, Burse VW, Needham
LL, Storr-Hansen E, Heinzow B, Debes F, Murata K, Simonsen H,
Ellefsen P, et al: Neurobehavioral deficits associated with PCB in
7-year-old children prenatally exposed to seafood neurotoxicants.
Neurotoxicol Teratol. 23:305–317. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jusko TA, Sisto R, Iosif AM, Moleti A,
Wimmerová S, Lancz K, Tihányi J, Sovčiková E, Drobná B, Palkovičová
L, et al: Prenatal and postnatal serum PCB concentrations and
cochlear function in children at 45 months of age. Environ Health
Perspect. 122:1246–1252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Perry KJ and Price JM: Concurrent child
history and contextual predictors of children's internalizing and
externalizing behavior problems in foster care. Child Youth Serv
Rev. 84:125–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tatsuta N, Nakai K, Murata K, Suzuki K,
Iwai-Shimada M, Yaginuma-Sakurai K, Kurokawa N, Nakamura T,
Hosokawa T and Satoh H: Prenatal exposures to environmental
chemicals and birth order as risk factors for child behavior
problems. Environ Res. 114:47–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gonzalez ST, Remick D, Creton R and
Colwill RM: Effects of embryonic exposure to polychlorinated
biphenyls (PCBs) on anxiety-related behaviors in larval zebrafish.
Neurotoxicology. 53:93–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lovato AK, Creton R and Colwill RM:
Effects of embryonic exposure to polychlorinated biphenyls (PCBs)
on larval zebrafish behavior. Neurotoxicol Teratol. 53:1–10. 2016.
View Article : Google Scholar
|
|
52
|
Glazer L, Hahn ME and Aluru N: Delayed
effects of developmental exposure to low levels of the aryl
hydrocarbon receptor agonist 3,3′,4,4′,5-pentachlorobiphenyl
(PCB126) on adult zebrafish behavior. Neurotoxicology. 52:134–143.
2016. View Article : Google Scholar
|
|
53
|
Lyall K, Croen LA, Sjödin A, Yoshida CK,
Zerbo O, Kharrazi M and Windham GC: Polychlorinated biphenyl and
organochlorine pesticide concentrations in maternal mid-pregnancy
serum samples: Association with autism spectrum disorder and
intellectual disability. Environ Health Perspect. 125:474–480.
2017. View
Article : Google Scholar :
|
|
54
|
Bernardo BA, Lanphear BP, Venners SA,
Arbuckle TE, Braun JM, Muckle G, Fraser WD and McCandless LC:
Assessing the relation between plasma PCB concentrations and
elevated autistic behaviours using bayesian predictive odds ratios.
Int J Environ Res Public Health. 16:4572019. View Article : Google Scholar :
|
|
55
|
Teodoro M, Briguglio G, Fenga C and Costa
C: Genetic polymorphisms as determinants of pesticide toxicity:
Recent advances. Toxicol Rep. 6:564–570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Costa C, Briguglio G, Giambò F, Catanoso
R, Teodoro M, Caccamo D and Fenga C: Association between oxidative
stress biomarkers and PON and GST polymorphisms as a predictor for
susceptibility to the effects of pesticides. Int J Mol Med.
45:1951–1959. 2020.PubMed/NCBI
|
|
57
|
Costa C, Gangemi S, Giambo F, Rapisarda V,
Caccamo D and Fenga C: Oxidative stress biomarkers and paraoxonase
1 polymorphism frequency in farmers occupationally exposed to
pesticides. Mol Med Rep. 12:6353–6357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Colter BT, Garber HF, Fleming SM, Fowler
JP, Harding GD, Hooven MK, Howes AA, Infante SK, Lang AL,
MacDougall MC, et al: Ahr and Cyp1a2 genotypes both affect
susceptibility to motor deficits following gestational and
lactational exposure to polychlorinated biphenyls. Neurotoxicology.
65:125–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Docea AO, Vassilopoulou L, Fragou D,
Arsene AL, Fenga C, Kovatsi L, Petrakis D, Rakitskii VN, Nosyrev
AE, Izotov BN, et al: CYP polymorphisms and pathological conditions
related to chronic exposure to organochlorine pesticides. Toxicol
Rep. 4:335–341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Curran CP, Nebert DW, Genter MB, Patel KV,
Schaefer TL, Skelton MR, Williams MT and Vorhees CV: In utero and
lactational exposure to PCBs in mice: Adult offspring show altered
learning and memory depending on Cyp1a2 and Ahr genotypes. Environ
Health Perspect. 119:1286–1293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hufgard JR, Sprowles JLN, Pitzer EM, Koch
SE, Jiang M, Wang Q, Zhang X, Biesiada J, Rubinstein J, Puga A, et
al: Prenatal exposure to PCBs in Cyp1a2 knock-out mice interferes
with F1 fertility, impairs long-term potentiation, reduces acoustic
startle and impairs conditioned freezing contextual memory with
minimal transgenerational effects. J Appl Toxicol. 39:603–621.
2019. View Article : Google Scholar
|
|
62
|
Klinefelter K, Hooven MK, Bates C, Colter
BT, Dailey A, Infante SK, Kania-Korwel I, Lehmler HJ, López-Juárez
A, Ludwig CP and Curran CP: Genetic differences in the aryl
hydrocarbon receptor and CYP1A2 affect sensitivity to developmental
polychlorinated biphenyl exposure in mice: Relevance to studies of
human neurological disorders. Mamm Genome. 29:112–127. 2018.
View Article : Google Scholar
|
|
63
|
Nebert DW and Dalton TP: The role of
cytochrome P450 enzymes in endogenous signalling pathways and
environmental carcinogenesis. Nat Rev Cancer. 6:947–960. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rude KM, Pusceddu MM, Keogh CE, Sladek JA,
Rabasa G, Miller EN, Sethi S, Keil KP, Pessah IN, Lein PJ and
Gareau MG: Developmental exposure to polychlorinated biphenyls
(PCBs) in the maternal diet causes host-microbe defects in weanling
offspring mice. Environ Pollut. 253:708–721. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sanctuary MR, Kain JN, Angkustsiri K and
German JB: Dietary considerations in autism spectrum disorders: The
potential role of protein digestion and microbial putrefaction in
the gut-brain axis. Front Nutr. 5:402018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dai R, Yu Y, Xi Q, Hu X, Zhu H, Liu R and
Wang R: Prenatal diagnosis of 4953 pregnant women with indications
for genetic amniocentesis in Northeast China. Mol Cytogenet.
12:452019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Long M, Ghisari M, Kjeldsen L, Wielsøe M,
Nørgaard-Pedersen B, Mortensen EL, Abdallah MW and
Bonefeld-Jørgensen EC: Autism spectrum disorders, endocrine
disrupting compounds, and heavy metals in amniotic fluid: A
case-control study. Mol Autism. 10:12019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Berghuis SA, Soechitram SD, Hitzert MM,
Sauer PJ and Bos AF: Prenatal exposure to polychlorinated biphenyls
and their hydroxylated metabolites is associated with motor
development of three-month-old infants. Neurotoxicology.
38:124–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tatsuta N, Kurokawa N, Nakai K, Suzuki K,
Iwai-Shimada M, Murata K and Satoh H: Effects of intrauterine
exposures to polychlorinated biphenyls, methylmercury, and lead on
birth weight in Japanese male and female newborns. Environ Health
Prev Med. 22:392017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sonneborn D, Park HY, Petrik J, Kocan A,
Palkovicova L, Trnovec T, Nguyen D and Hertz-Picciotto I: Prenatal
polychlorinated biphenyl exposures in eastern Slovakia modify
effects of social factors on birthweight. Paediatr Perinat
Epidemiol. 22:202–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Calo M, Licata P, Bitto A, Lo Cascio P,
Interdonato M and Altavilla D: Role of AHR, AHRR and ARNT in
response to dioxin-like PCBs in Spaurus aurata. Environ Sci Pollut
Res Int. 21:14226–14231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pessah IN, Cherednichenko G and Lein PJ:
Minding the calcium store: Ryanodine receptor activation as a
convergent mechanism of PCB toxicity. Pharmacol Ther. 125:260–285.
2010. View Article : Google Scholar :
|
|
73
|
Roegge CS, Morris JR, Villareal S, Wang
VC, Powers BE, Klintsova AY, Greenough WT, Pessah IN and Schantz
SL: Purkinje cell and cerebellar effects following developmental
exposure to PCBs and/or MeHg. Neurotoxicol Teratol. 28:74–85. 2006.
View Article : Google Scholar
|
|
74
|
Kalkunte S, Huang Z, Lippe E, Kumar S,
Robertson LW and Sharma S: Polychlorinated biphenyls target
Notch/Dll and VEGF R2 in the mouse placenta and human trophoblast
cell lines for their anti-angiogenic effects. Sci Rep. 7:398852017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ahmed RG, El-Gareib AW and Shaker HM:
Gestational 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) exposure
disrupts fetoplacental unit: Fetal thyroid-cytokines dysfunction.
Life Sci. 192:213–220. 2018. View Article : Google Scholar
|
|
76
|
Aluru N, Karchner SI and Glazer L: Early
life exposure to low levels of AHR Agonist PCB126
(3,3′,4,4′,5-Pentachlorobiphenyl) reprograms gene expression in
adult brain. Toxicol Sci. 160:386–397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kappil MA, Li Q, Li A, Dassanayake PS, Xia
Y, Nanes JA, Landrigan PJ, Stodgell CJ, Aagaard KM, Schadt EE, et
al: In utero exposures to environmental organic pollutants disrupt
epigenetic marks linked to fetoplacental development. Environ
Epigenet. 2:dvv0132016. View Article : Google Scholar :
|
|
78
|
Seegal RF, Brosch KO and Okoniewski RJ:
Coplanar PCB congeners increase uterine weight and frontal cortical
dopamine in the developing rat: Implications for developmental
neurotoxicity. Toxicol Sci. 86:125–131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pappas B, Yang Y, Wang Y, Kim K, Chung HJ,
Cheung M, Ngo K, Shinn A and Chan WK: p23 protects the human aryl
hydrocarbon receptor from degradation via a heat shock protein
90-independent mechanism. Biochem Pharmacol. 152:34–44. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jain S, Maltepe E, Lu MM, Simon C and
Bradfield CA: Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and
Ah receptor mRNAs in the developing mouse. Mech Dev. 73:117–123.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kimura E and Tohyama C: Embryonic and
postnatal expression of aryl hydrocarbon receptor mRNA in mouse
brain. Front Neuroanat. 11:42017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shi H, Hardesty JE, Jin J, Head KZ,
Falkner KC, Cave MC and Prough RA: Concentration dependence of
human and mouse aryl hydrocarbon receptor responsiveness to
polychlorinated biphenyl exposures: Implications for aroclor
mixtures. Xenobiotica. 49:1414–1422. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Juricek L and Coumoul X: The aryl
hydrocarbon receptor and the nervous system. Int J Mol Sci.
19:25042018. View Article : Google Scholar :
|
|
84
|
Chatonnet F, Boudinot E, Chatonnet A,
Taysse L, Daulon S, Champagnat J and Foutz AS: Respiratory survival
mechanisms in acetylcholinesterase knockout mouse. Eur J Neurosci.
18:1419–1427. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Desaulniers D, Xiao GH, Leingartner K, Chu
I, Musicki B and Tsang BK: Comparisons of brain, uterus, and liver
mRNA expression for cytochrome p450s, DNA methyltransferase-1, and
catechol-o-methyltransferase in prepubertal female Sprague-Dawley
rats exposed to a mixture of aryl hydrocarbon receptor agonists.
Toxicol Sci. 86:175–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kimura E, Kubo KI, Endo T, Nakajima K,
Kakeyama M and Tohyama C: Excessive activation of AhR signaling
disrupts neuronal migration in the hippocampal CA1 region in the
developing mouse. J Toxicol Sci. 42:25–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Fritsch EB, Stegeman JJ, Goldstone JV,
Nacci DE, Champlin D, Jayaraman S, Connon RE and Pessah IN:
Expression and function of ryanodine receptor related pathways in
PCB tolerant Atlantic killifish (Fundulus heteroclitus) from New
Bedford Harbor, MA, USA. Aquat Toxicol. 159:156–166. 2015.
View Article : Google Scholar :
|
|
88
|
Sethi S, Morgan RK, Feng W, Lin Y, Li X,
Luna C, Koch M, Bansal R, Duffel MW, Puschner B, et al: Comparative
analyses of the 12 most abundant PCB congeners detected in human
maternal serum for activity at the thyroid hormone receptor and
ryanodine receptor. Environ Sci Technol. 53:3948–3958. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Libersat F and Duch C: Mechanisms of
dendritic maturation. Mol Neurobiol. 29:303–320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pittenger C and Kandel ER: In search of
general mechanisms for long-lasting plasticity: Aplysia and the
hippocampus. Philos Trans R Soc Lond B Biol Sci. 358:757–763. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wayman GA, Yang D, Bose DD, Lesiak A,
Ledoux V, Bruun D, Pessah IN and Lein PJ: PCB-95 promotes dendritic
growth via ryanodine receptor-dependent mechanisms. Environ Health
Perspect. 120:997–1002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yang D, Kania-Korwel I, Ghogha A, Chen H,
Stamou M, Bose DD, Pessah IN, Lehmler HJ and Lein PJ: PCB 136
atropselectively alters morphometric and functional parameters of
neuronal connectivity in cultured rat hippocampal neurons via
ryanodine receptor-dependent mechanisms. Toxicol Sci. 138:379–392.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Feng W, Zheng J, Robin G, Dong Y, Ichikawa
M, Inoue Y, Mori T, Nakano T and Pessah IN: Enantioselectivity of
2,2′,3,5′,6-Pentachlorobiphenyl (PCB 95) atropisomers toward
ryanodine receptors (RyRs) and their influences on hippocampal
neuronal networks. Environ Sci Technol. 51:14406–14416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wayman GA, Bose DD, Yang D, Lesiak A,
Bruun D, Impey S, Ledoux V, Pessah IN and Lein PJ: PCB-95 modulates
the calcium-dependent signaling pathway responsible for
activity-dependent dendritic growth. Environ Health Perspect.
120:1003–1009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Howard AS, Fitzpatrick R, Pessah I,
Kostyniak P and Lein PJ: Polychlorinated biphenyls induce
caspase-dependent cell death in cultured embryonic rat hippocampal
but not cortical neurons via activation of the ryanodine receptor.
Toxicol Appl Pharmacol. 190:72–86. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sethi S, Keil KP, Chen H, Hayakawa K, Li
X, Lin Y, Lehmler HJ, Puschner B and Lein PJ: Detection of
3,3′-Dichlorobiphenyl in human maternal plasma and its effects on
axonal and dendritic growth in primary rat neurons. Toxicol Sci.
158:401–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Casati L, Sendra R, Poletti A, Negri-Cesi
P and Celotti F: Androgen receptor activation by polychlorinated
biphenyls: Epigenetic effects mediated by the histone demethylase
Jarid1b. Epigenetics. 8:1061–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kanherkar RR, Bhatia-Dey N and Csoka AB:
Epigenetics across the human lifespan. Front Cell Dev Biol.
2:492014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Su KY, Li MC, Lee NW, Ho BC, Cheng CL,
Chuang YC, Yu SL and Guo YL: Perinatal polychlorinated biphenyls
and polychlorinated dibenzofurans exposure are associated with DNA
methylation changes lasting to early adulthood: Findings from
Yucheng second generation. Environ Res. 170:481–486. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Leung YK, Ouyang B, Niu L, Xie C, Ying J,
Medvedovic M, Chen A, Weihe P, Valvi D, Grandjean P and Ho SM:
Identification of sex-specific DNA methylation changes driven by
specific chemicals in cord blood in a Faroese birth cohort.
Epigenetics. 13:290–300. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Guo F, Yan L, Guo H, Li L, Hu B, Zhao Y,
Yong J, Hu Y, Wang X, Wei Y, et al: The transcriptome and DNA
methylome landscapes of human primordial germ cells. Cell.
161:1437–1452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Pozharny Y, Lambertini L, Ma Y, Ferrara L,
Litton CG, Diplas A, Jacobs AR, Chen J, Stone JL, Wetmur J and Lee
MJ: Genomic loss of imprinting in first-trimester human placenta.
Am J Obstet Gynecol. 202:391.e1–e8. 2010. View Article : Google Scholar
|
|
103
|
Zhao Y, Song Q, Ge W, Jin Y, Chen S, Zhao
Y, Xiao X and Zhang Y: Associations between in utero exposure to
polybrominated diphenyl ethers, pathophysiological state of fetal
growth and placental DNA methylation changes. Environ Int. 133(Pt
B): 1052552019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Maghbooli Z, Hossein-Nezhad A, Adabi E,
Asadollah-Pour E, Sadeghi M, Mohammad-Nabi S, Zakeri Rad L, Malek
Hosseini AA, Radmehr M, Faghihi F, et al: Air pollution during
pregnancy and placental adaptation in the levels of global DNA
methylation. PLoS One. 13:e01997722018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dunaway KW, Islam MS, Coulson RL, Lopez
SJ, Vogel Ciernia A, Chu RG, Yasui DH, Pessah IN, Lott P, Mordaunt
C, et al: Cumulative impact of polychlorinated biphenyl and large
chromosomal duplications on DNA methylation, chromatin, and
expression of autism candidate genes. Cell Rep. 17:3035–3048. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wefers B, Hitz C, Hölter SM, Trümbach D,
Hansen J, Weber P, Pütz B, Deussing JM, de Angelis MH, Roenneberg
T, et al: MAPK signaling determines anxiety in the juvenile mouse
brain but depression-like behavior in adults. PLoS One.
7:e350352012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Naveau E, Pinson A, Gérard A, Nguyen L,
Charlier C, Thomé JP, Zoeller RT, Bourguignon JP and Parent AS:
Alteration of rat fetal cerebral cortex development after prenatal
exposure to polychlorinated biphenyls. PLoS One. 9:e919032014.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Seegal RF: Epidemiological and laboratory
evidence of PCB-induced neurotoxicity. Crit Rev Toxicol.
26:709–737. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Caspersen IH, Aase H, Biele G, Brantsæter
AL, Haugen M, Kvalem HE, Skogan AH, Zeiner P, Alexander J, Meltzer
HM and Knutsen HK: The influence of maternal dietary exposure to
dioxins and PCBs during pregnancy on ADHD symptoms and cognitive
functions in Norwegian preschool children. Environ Int. 94:649–660.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Morreale de Escobar G, Obregon MJ and
Escobar del Rey F: Role of thyroid hormone during early brain
development. Eur J Endocrinol. 151(Suppl 3): U25–U37. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Gilbert ME, O'Shaughnessy KL and Axelstad
M: Regulation of thyroid-disrupting chemicals to protect the
developing brain. Endocrinology. 161:bqaa1062020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Levitt P, Eagleson KL and Powell EM:
Regulation of neocortical interneuron development and the
implications for neurodevelopmental disorders. Trends Neurosci.
27:400–406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chan SY, Vasilopoulou E and Kilby MD: The
role of the placenta in thyroid hormone delivery to the fetus. Nat
Clin Pract Endocrinol Metab. 5:45–54. 2009. View Article : Google Scholar
|
|
114
|
Li ZM, Hernandez-Moreno D, Main KM,
Skakkebæk NE, Kiviranta H, Toppari J, Feldt-Rasmussen U, Shen H,
Schramm KW and De Angelis M: Association of in utero persistent
organic pollutant exposure with placental thyroid hormones.
Endocrinology. 159:3473–3481. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Itoh S, Baba T, Yuasa M, Miyashita C,
Kobayashi S, Araki A, Sasaki S, Kajiwara J, Hori T, Todaka T, et
al: Association of maternal serum concentration of hydroxylated
polychlorinated biphenyls with maternal and neonatal thyroid
hormones: The Hokkaido birth cohort study. Environ Res.
167:583–590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Su PH, Chen HY, Chen SJ, Chen JY, Liou SH
and Wang SL: Thyroid and growth hormone concentrations in
8-year-old children exposed in utero to dioxins and polychlorinated
biphenyls. J Toxicol Sci. 40:309–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Boas M, Feldt-Rasmussen U and Main KM:
Thyroid effects of endocrine disrupting chemicals. Mol Cell
Endocrinol. 355:240–248. 2012. View Article : Google Scholar
|
|
118
|
Lyng GD, Snyder-Keller A and Seegal RF:
Polychlorinated biphenyl-induced neurotoxicity in organotypic
cocultures of developing rat ventral mesencephalon and striatum.
Toxicol Sci. 97:128–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kalkunte SS, Mselle TF, Norris WE, Wira
CR, Sentman CL and Sharma S: Vascular endothelial growth factor C
facilitates immune tolerance and endovascular activity of human
uterine NK cells at the maternal-fetal interface. J Immunol.
182:4085–4092. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Osol G, Ko NL and Mandala M: Plasticity of
the maternal vasculature during pregnancy. Annu Rev Physiol.
81:89–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chen ZJ, Liu HY, Cheng Z, Man YB, Zhang
KS, Wei W, Du J, Wong MH and Wang HS: Polybrominated diphenyl
ethers (PBDEs) in human samples of mother-newborn pairs in South
China and their placental transfer characteristics. Environ Int.
73:77–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Brucker-Davis F, Ferrari P, Boda-Buccino
M, Wagner-Mahler K, Pacini P, Gal J, Azuar P and Fenichel P: Cord
blood thyroid tests in boys born with and without cryptorchidism:
Correlations with birth parameters and in utero xenobiotics
exposure. Thyroid. 21:1133–1141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hellstrom M, Phng LK and Gerhardt H: VEGF
and Notch signaling: The yin and yang of angiogenic sprouting. Cell
Adh Migr. 1:133–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Hunkapiller NM, Gasperowicz M, Kapidzic M,
Plaks V, Maltepe E, Kitajewski J, Cross JC and Fisher SJ: A role
for Notch signaling in trophoblast endovascular invasion and in the
pathogenesis of pre-eclampsia. Development. 138:2987–2998. 2011.
View Article : Google Scholar : PubMed/NCBI
|