The association between sarcopenia, frailty and
locomotive syndrome is complex; however, sarcopenia is a
muscle-specific concept that is relatively easy to approach in
research. At the organ level, it is known that specific changes in
the muscles of the elderly involve a decrease in fast-twitch muscle
components and the accumulation of fat in muscles, and at the
cellular level, a mitochondrial dysfunction occurs (5-7).
Age-related sarcopenia is termed primary sarcopenia, and
disease-related sarcopenia is termed secondary sarcopenia (8,9).
Sarcopenia can be a primary component of physical frailty.
Sarcopenia is also a main health concern in the era of the COVID-19
pandemic. Sarcopenia can be an adverse predictor in elderly
patients with COVID-19 infection (10,11).
The present review article focuses on age-related
primary sarcopenia and outlines its pathogenesis and
mechanisms.
Multiple factors have been proposed to explain the
pathogenesis of primary sarcopenia. Myofibers are multinucleated
cells formed by the fusion of satellite cells. Skeletal muscle is
an organ that is susceptible to damage from overload and trauma;
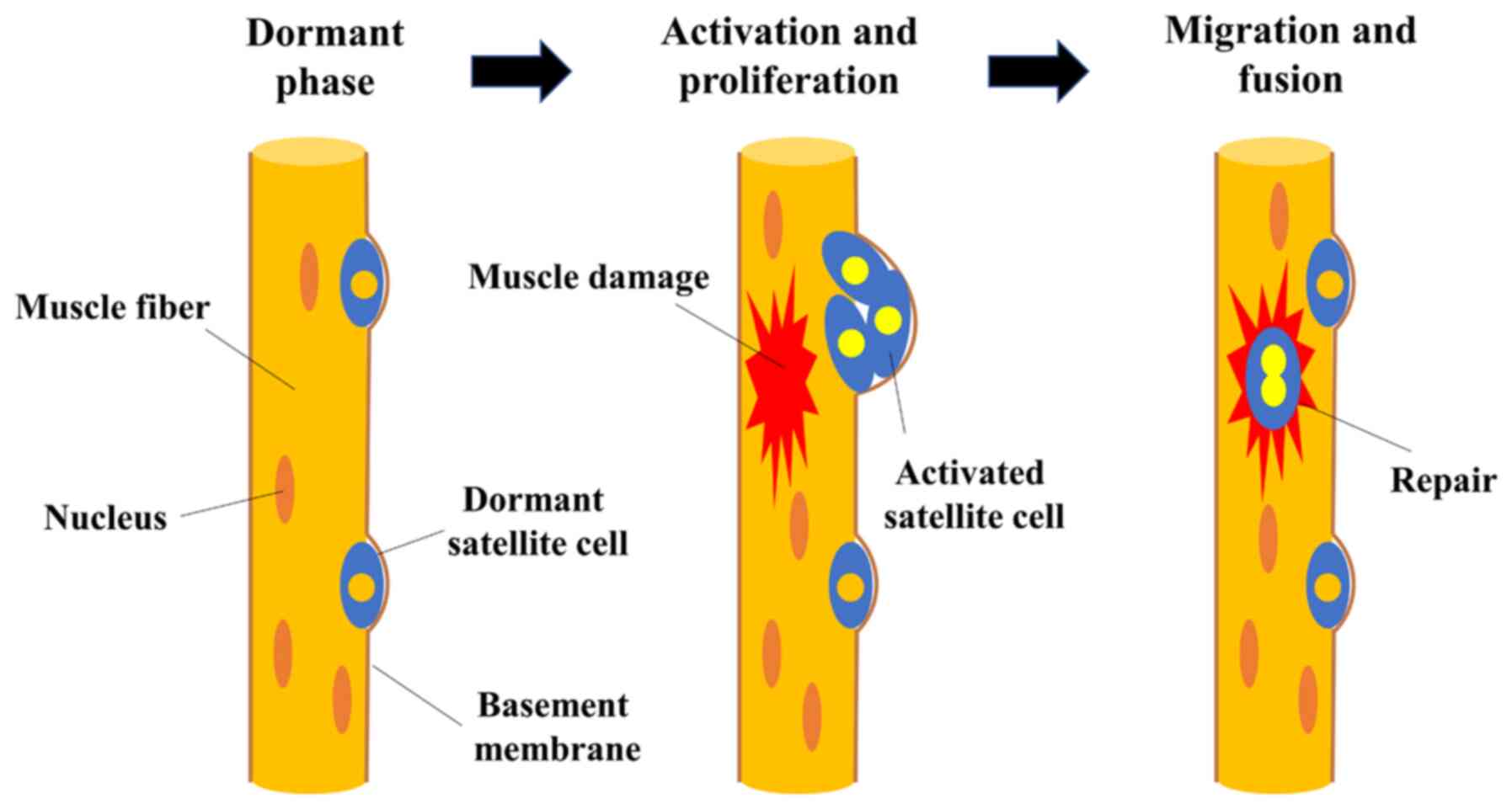
however, it has a notable ability to regenerate. Satellite cells,
known as skeletal muscle-specific somatic stem cells, play a
central role in the process of muscle regeneration (12-15). Satellite cells are normally
dormant; however, when nearby muscle fibers are damaged, they are
stimulated by damaged myofibers to become active and form muscle
progenitor cells. Cells that proliferate by division fuse with each
other or with existing muscle fibers, contributing to the
formation, repair and hypertrophy of new myofibers (12-15). Myofibers are classified into two
major types (four subtypes) according to the isoform of myosin
heavy chain: Type I, IIa, IIx and IIb (16). Myofibers are commanded to
contract and relax by neuromuscular junctions, and receive blood
flow from surrounding capillaries (5-7).
Damaged myofibers are repaired and regenerated by satellite cells,
which are bone marrow stem cells (5-7).
In addition, mitochondria are abundant in the cells and are
involved not only in energy production, mainly through fatty acid
beta-oxidation, but also in metabolic regulation, such as insulin
sensitivity (15).
The age-related loss of skeletal muscle mass is
caused by a decrease in the number of myofibers and the atrophy of
individual myofibers, while disuse muscle atrophy due to a
long-term bed ridden status and related disuse, which is the cause
of secondary sarcopenia, is mainly due to a decrease in the
cross-sectional area of myofibers (Table I) (5,14). In disuse atrophy, the time course
is acute, the degree is severe, the recovery is often reversible,
and the slow-twitch muscles are mainly affected, whereas in primary
sarcopenia, the time course is chronic, the degree is mild, the
recovery is sometimes irreversible, and the fast-twitch muscles are
mainly affected (Table I)
(17). As mentioned above,
skeletal myofibers are classified into two major types: Type I
(slow-twitch fibers) and type II (fast-twitch fibers) fibers, and a
decrease in the number of type II fibers is observed from an early
stage with aging, eventually resulting in a decrease in the number
of both types of myofibers (5,6).
The motor neurons that innervate myofibers are located in the
spinal cord, and the nerve fibers that emerge from these neurons
branch out in multiple directions to reach the muscle fibers
(7). The motor neurons and the
myofibers they innervate are collectively called motor units, and
it is known that these motor units decrease with aging (7). In addition, it has been reported
that aging causes morphological changes in neuromuscular synapses,
resulting in the functional decline of skeletal muscles and muscle
atrophy (18). Muscle satellite
cells exist between the plasma membrane and basement membrane of
muscle fibers and are normally dormant; however, they are activated
by stimulation, proliferate, differentiate and fuse with existing
muscle fibers, playing an important role in muscle regeneration
(5-7). Aging causes a loss of function of
muscle satellite cells, a decrease in the regenerative capacity of
myofibers, and a decrease in the number of myofibers (12,19). Muscle regeneration is maintained
by the infiltration of macrophages and the subsequent activation of
satellite cells (12). The
expression of notch ligand (Delta) is decreased in senescent muscle
satellite cells, which may be involved in the decreased
proliferative potential of satellite cells (20). In addition, it has been reported
that Wnt signaling is also enhanced in senescent satellite cells,
which promotes their differentiation into fibrogenic cells
(21). The repair process of
damaged skeletal muscle from the perspective of muscle satellite
cells is illustrated in Fig.
1.
The atrophy or hypertrophy of myofibers is dependent
on their protein content. Over 80% of the dry weight of muscle is
comprised of protein (22).
Theoretically, muscle hypertrophy occurs when muscle protein
synthesis is increased and degradation is inhibited, while muscle
atrophy occurs when degradation is increased and synthesis is
inhibited. Muscle protein anabolism in muscle cells is known to be
mediated by the following: i) Amino acids (branched chain amino
acids, such as leucine); ii) exercise; iii) insulin and
insulin-like growth factor-1 (IGF-1); and iv) hormones (23-26). All these factors induce the
phosphorylation of mammalian target of rapamycin (mTOR) in myocytes
(27). They also exhibit protein
anabolism through the activation of 70-kDa ribosomal protein S6
kinase (p70S6K) and eukaryotic initiation factor 4E binding
protein-1 (4E-BP1) (27).
The mTOR complex 1 (mTORC1) signaling pathway is a
major regulator of protein metabolism (28). mTORC1 regulates protein synthesis
and degradation by integrating a number of intracellular signals
(28). For example, leucine
intake and exercise activate mTORC1, leading to increased protein
synthesis. On the other hand, during fasting, mTORC1 is inactivated
and protein degradation is enhanced (28). The age-related loss of skeletal
muscle mass is less likely to lead to the diet-induced enhancement
of protein synthesis in the elderly due to the decreased
sensitivity of mTORC1 to leucine (29). It has been shown that leucine is
not only an organelle of muscle proteins, but also acts directly on
muscle cells to induce protein synthesis (13). In addition, IGF-1, a potent
anabolic factor, is regulated by growth hormone (GH) and is
produced mainly in the liver (30). Ghrelin, a GH-promoting peptide,
not only promotes GH secretion, but also has the function of
promoting central or peripheral feeding (31). IGF-1 is involved in a number of
anabolic pathways in skeletal muscle, including cell proliferation,
differentiation and metabolism and muscle regeneration (32,33). As mentioned above, one of the
causes of sarcopenia is decreased muscle synthesis; IGF-I activates
the intracellular signaling pathways of phosphoinositide3-kinase
(PI3K) and Akt, and further activates downstream mTOR, which
enhances protein synthesis (34,35). The IGF-1/PI3K/mTOR system is
important in muscle hypertrophy; however, its activity decreases
with aging (36). The second is
the enhancement of muscle breakdown. Ubiquitin is an approximately
8.5-kDa protein with a high degree of sequence conservation among
different species and exists in a ubiquitinated (ubiquitylation, a
type of protein modification) state (37,38). When a protein is ubiquitinated in
the cell, the proteasome is able to degrade it. In 2001, the
muscle-specific ubiquitin ligase genes, muscle-specific RING finger
protein 1 (MuRF1) and Atrogin-1 (muscle atrophy-related factors),
were identified (37,38). Atrorgin-1 is encoded by the
Fbxo32 gene, which is also referred to as a muscle atrophy-related
factor, and is upregulated in a wide range of pathological
conditions, such as neurectomy and disuse; however, mice in which
Atrorgin-1 is knocked out are less susceptible to
neurectomy-induced muscle atrophy (37).
It has also been reported that muscle
atrophy-related factor is increased in skeletal muscle of elderly
individuals and aging rats (39,40). Protein synthesis in muscle
decreases with aging, and protein anabolism is suppressed in the
muscles of the elderly even when the same amounts of amino acids
are present in the blood (i.e., anabolic resistance) (41). The mTOR activation response to
amino acids, such as leucin that can stimulate mTOR phosphorylation
is reduced in the elderly (41,42).
Elderly individuals are more likely to develop
chronic inflammation, which is a persistent mild inflammation, due
to the decline in immune function caused by aging. The risk of
developing inflammatory diseases, such as infections and collagen
diseases is increased in the elderly with an impaired immune
function (43). These chronic
inflammations are characterized by mildly elevated blood levels of
pro-inflammatory cytokines, such as tumor necrosis factor-α
(TNF-α), interleukin (IL)-1β, IL-6 and IL-18. C-reactive protein
(CRP), an acute-phase protein produced by the liver in response to
IL-6, is also upregulated during chronic inflammation (44). Blood levels of TNF-α, IL-1 β, and
IL-6 have been reported to increase 2 to 4-fold in the elderly
compared with healthy young adults (45). It has been also shown that the
administration of IL-6 and TNF-α to rats causes the degradation of
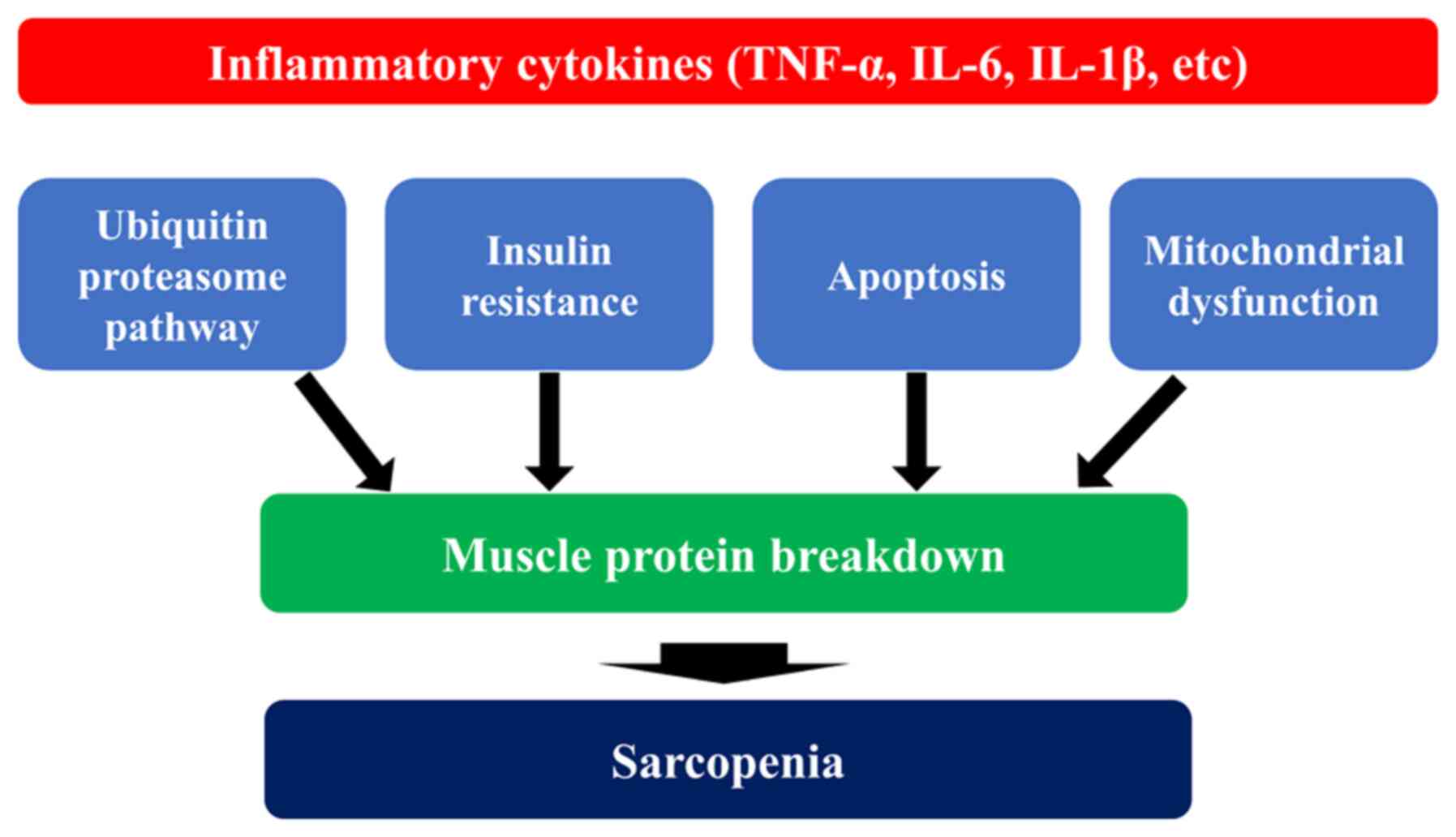
skeletal muscle (46,47). Inflammatory cytokines cause the
dysfunction of mitochondria, which are involved in energy
production, resulting in a decreased ATP production, as well as in
the excessive production of reactive oxygen species (ROS) (48,49). Excessive ROS production further
exacerbates mitochondrial damage and subsequent metabolic
abnormalities, and induces proteolysis by enhancing the
ubiquitin-proteasome system, one of the major pathways for protein
degradation as described above, resulting in skeletal muscle
atrophy (50,51). Proteins labeled with ubiquitin
are degraded by the proteasome, a large enzyme complex (52). Apoptosis, on the other hand, is a
cell death mechanism that removes unnecessary cells. The activation
of caspases, proteolytic enzymes, rapidly degrades intracellular
proteins, which are ultimately phagocytosed by macrophages and
other phagocytic cells (53).
TNF-α is a major regulator of the apoptotic signaling pathway.
TNF-α binds to TNF-α receptors in skeletal muscle and activates
caspases through the Fas-associated death domain (FADD), thereby
inducing apoptosis (54).
Excessive apoptosis in skeletal muscle leads to increased
degradation of muscle proteins, resulting in muscle atrophy
(55).
Obesity is another important factor in the
development of chronic inflammation. In recent years, it has been
shown that adipose tissue interacts with immune cells, such as
macrophages and neutrophils to induce chronic inflammation in obese
individuals (56). TNF-α
secreted by macrophages increases free fatty acids by promoting
lipolysis through the Toll-like receptor 4 (TLR4) signaling pathway
in adipose tissue (56). In
addition, the macrophage response to free fatty acids increases the
secretion of pro-inflammatory cytokines, such as TNF-α, IL-1β and
IL-6, further exacerbating chronic inflammation (57). Inflammation-associated immune
cell infiltration is found not only in adipose tissue, but also in
skeletal muscle; in a study on critically ill hospitalized patients
aged 50-59 years, the increased infiltration of CD68-positive
macrophages into skeletal muscle was observed with atrophy of the
rectus femoris muscle after 7 days of hospitalization (58). Thus, chronic inflammation induced
by various factors in aging is considered to reduce muscle strength
and function by increasing macrophage infiltration into skeletal
muscle, decreasing muscle mass and increasing the accumulation of
ectopic fat (59). Recently,
sarcopenic obesity, a condition that involves both sarcopenia and
obesity, has been attracting attention. Patients with sarcopenic
obesity have a poorer prognosis than those with sarcopenia alone or
obesity alone (60). The
association between inflammatory cytokines and sarcopenia is
illustrated in Fig. 2.
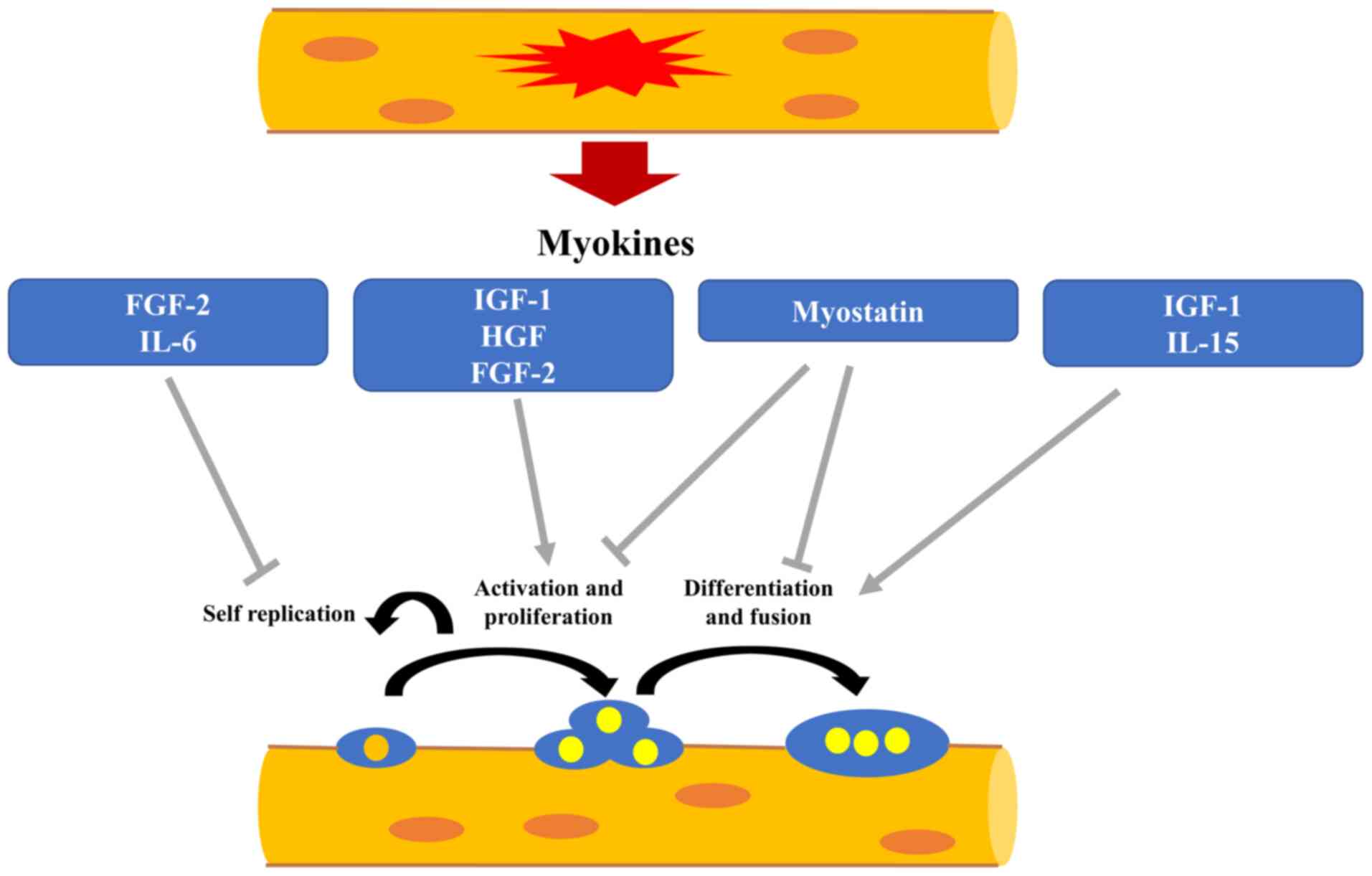
Biologically active substances produced by muscle
cells are termed myokines, and IGF-1, IL-6, fibroblast growth
factor 2 (FGF-2), hepatocyte growth factor (HGF) and IL-15 are
representative myokines (61,62). Some myokines act
endocrinologically on organs throughout the body (e.g., pancreas,
brain, adipose tissue), while others act paracrine or autocrine on
skeletal muscle itself (61,62). Pedersen et al (63) defined myokines as 'cytokines and
peptides expressed in and secreted from skeletal myofibers that act
in a paracrine and endocrine manner'. Myokines released from
damaged myofibers act as messengers in the process of muscle
regeneration by satellite cells upon muscle injury. When myofibers
are damaged, cytokines and chemokines are first secreted by
macrophages that migrate to the damaged area, and growth factors
are also released from the damaged myofibers, which act on
satellite cells to initiate muscle regeneration (64). Growth factors play a role in
regulating the proliferation and differentiation of satellite cells
(64). The expression of IGF-1
has also been found in skeletal muscle, where it is released from
myofibers upon stimuli that damage the cell membrane, such as
muscle overload (65). IL-6 is
the oldest known myokine molecule, and its physiological effects
include systemic metabolic regulation (66). HGF is also released
extracellularly upon muscle injury and activates satellite cells
(67). HGF activates mTOR
signaling (68). FGF-2 is
another growth factor that is secreted upon cell membrane damage
(69,70). FGF-2 plays a role in regulating
cell proliferation and differentiation by activating the
mitogen-activated protein kinase (MAPK) signaling pathway in many
cells (71). In satellite cells,
p38α/βMAPK is activated upon entry from quiescence into the cell
cycle, which is triggered by FGF-2 (62). It has also been shown that the
activation of the Erk1/2 pathway by FGF-2 is essential in
proliferating myocytes between G1 and S phases of the cell cycle
(72). IL-15 is a cytokine that
is abundantly expressed in skeletal muscle and is recognized as a
myokine that acts endocrinologically on adipose tissue and
regulates whole body energy metabolism (73). On the other hand, IL-15 has
anabolic effects and is considered to be involved in skeletal
muscle hypertrophy (74). It has
been shown that the muscle hypertrophic effect of IL-15 occurs in a
pathway independent of IGF-1 (75). When cultured skeletal muscle
cells are treated with IL-15, protein synthesis is increased and
protein degradation is inhibited, resulting in hypertrophy of
muscle fibers (75,76). While, it has been reported that
the number of satellite cells decreases with aging, suggesting a
link to reduced muscle regeneration capacity (77). This is attributed to the reduced
self-replication capacity of satellite cells due to aging and the
inability to secure the number of stem cells. On the other hand,
myokines are also considered to play a part in the mechanism of
inhibiting cancer growth by exercise, and one myokine that has
actually been shown to inhibit cancer growth is secreted protein
acidic and rich in cysteine (SPARC) (78).
Myostatin is a myokine that belongs to the
TGF-family. In 1997, it was reported that skeletal muscle mass
markedly increased in myostatin gene-knockout mice, which attracted
attention as a factor regulating muscle mass (79). Myostatin binds to activin type
IIB receptor and ALK4/ALK5 coreceptor, promotes phosphorylation of
Smad2 and Smad3 proteins, and suppresses the expression of genes
involved in skeletal muscle differentiation (80,81). Myostatin has also been reported
to inhibit the PI3K/Akt signaling pathway (82). It has also been reported that
myostatin secretion from muscle and adipocytes is increased in
patients with severe obesity (83), and that weight loss decreases the
expression of myostatin in muscle (84). Sarcopenic obesity can be
associated with these observations. Follistatin and
follistatin-related genes are known to be molecules that bind to
and inhibit the function of myostatin, and it is expected that
these molecules can be used to increase muscle mass (85). During high-intensity exercise,
myostatin is suppressed and muscle hypertrophy can occur through
activation of the mTOR/IGF-1 system (86). The schematic explanation between
myokines associated with the regulation for the functions of muscle
satellite cells and the repair of damaged myofiber is illustrated
in Fig. 3.
In recent years, it has also become clear that the
myostatin gene is involved in the 'appropriateness for the running
distance' of racing horses (87). There are three genetically
distinct types of myostatin (C/C, C/T and T/T) in Thoroughbreds
(88). It has been found that
the difference of genetic types is associated with muscle mass and
appropriate-ness for the running distance. In the C/C type muscle
mass tends to increase slightly, in the T/T type it tends to
decrease slightly, and in the C/T type it tends to be in the middle
(88). Therefore, racing horses
with the C/C type tends to be suitable for a short distance, while
those with the T/T type tends to be suitable for medium and long
distances. Those with the C/T type tends to be suitable for a
medium distance (88).
The renin-angiotensin system (RAS) is known from a
report published in the Lancet in 2002, which demonstrated that
continuous angiotensin-converting enzyme (ACE) inhibitor treatment
suppressed knee extensor strength decline and walking speed decline
(89). This report attracted
attention to the suppression of the RAS. RAS activation is thought
to cause sarcopenia through the following: i) Indirect effects,
such as angiotensin II-induced decrease in anabolic hormones,
induction of proinflammatory cytokines and increased muscle protein
degradation via increased myostatin; and ii) direct oxidative
stress via angiotensin II type 1 receptors (90,91). RAS suppression may contribute to
the prevention of sarcopenia.
Age-related changes in reproductive endocrine organs
are considered to be one of the most important functional changes
associated with aging. In general, thyroid hormones and
glucocorticoids maintain relatively constant levels in response to
aging, whereas blood levels of sex steroid hormones, such as
testosterone, are known to decrease with age in adults (92,93). The decline in blood testosterone
levels with aging is considered to be associated with geriatric
diseases and functional disabilities. In a cross-sectional study on
men aged 24-90 years, serum testosterone levels were reported to be
positively associated with skeletal muscle mass and muscle strength
(94). In post-menopausal women,
estrogen decline can cause endocrine and metabolic dysfunction,
resulting in a predisposition to osteoporosis, metabolic syndrome
and sarcopenia (95).
Osteosarcopenia, a combined condition of osteoporosis and
sarcopenia, increases the risk of developing frailty (96).
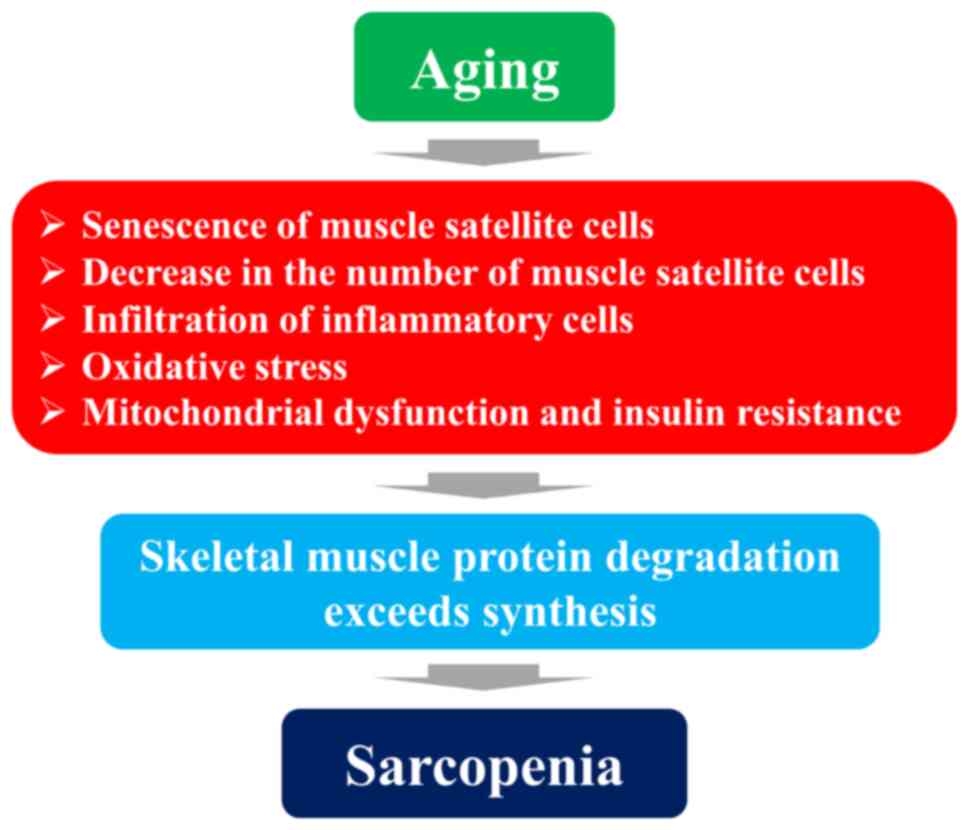
The present review outlined the pathogenesis of
primary sarcopenia from the following viewpoints: i) Myofibers and
muscle satellite cells; ii) protein synthesis and degradation; iii)
immunocompetence and inflammation; iv) myokines; v) RAS; and vi)
sex hormones. In the elderly, a lack of exercise, malnutrition and
hormonal changes lead to neuromuscular junction insufficiency, an
impaired capillary blood flow, a reduced repair and regeneration
capacity due to the senescence of muscle satellite cells, a
decrease in the number of muscle satellite cells, the infiltration
of inflammatory cells and oxidative stress, resulting in muscle
protein degradation exceeding synthesis. In addition, mitochondrial
dysfunction causes metabolic abnormalities, such as insulin
resistance, which may lead to quantitative and qualitative
abnormalities in skeletal muscle, resulting in sarcopenia. A
schematic diagram of the pathogenesis of sarcopenia during aging
process is illustrated in Fig.
4. Skeletal muscle has been the subject of a great amount of
research in recent years, and it is hoped that further drug
discovery for sarcopenia based on pathological conditions will be
developed in the future. The authors consider that the novelty of
the present review article is that it outlines the pathogenesis of
sarcopenia based on the latest evidence, with the aim of assisting
in the development of novel drugs for sarcopenia.
Not applicable.
HN wrote the review article. SF, AA, KY, SN and KH
were involved in the editing and reviewing of the article. HN and
KH confirm the authenticity of all the raw data. All authors have
read and approved the final article.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Rosenberg IH: Summary comments. Am J Clin
Nutr. 50:1231–1233. 1989. View Article : Google Scholar
|
|
2
|
Lexell J, Taylor CC and Sjostrom M: What
is the cause of the ageing atrophy? Total number, size and
proportion of different fiber types studied in whole vastus
lateralis muscle from 15to 83-year-old men. J Neurol Sci.
84:275–294. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuzuya M: Aging-related frailty and
sarcopenia. The concepts and diagnostic criteria of frailty. Clin
Calcium. 28:1171–1176. 2018.In Japanese.
|
|
4
|
Tournadre A, Vial G, Capel F, Soubrier M
and Boirie Y: Sarcopenia. Joint Bone Spine. 86:309–314. 2019.
View Article : Google Scholar
|
|
5
|
Ciciliot S, Rossi AC, Dyar KA, Blaauw B
and Schiaffino S: Muscle type and fiber type specificity in muscle
wasting. Int J Biochem Cell Biol. 45:2191–2199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nilwik R, Snijders T, Leenders M, Groen
BB, van Kranenburg J, Verdijk LB and van Loon LJ: The decline in
skeletal muscle mass with aging is mainly attributed to a reduction
in type II muscle fiber size. Exp Gerontol. 48:492–498. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deschenes MR: Effects of aging on muscle
fibre type and size. Sports Med. 34:809–824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen LK, Woo J, Assantachai P, Auyeung TW,
Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al: Asian
Working group for sarcopenia: 2019 consensus update on sarcopenia
diagnosis and treatment. J Am Med Dir Assoc. 21:300–307.e2. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishikawa H, Shiraki M, Hiramatsu A,
Moriya K, Hino K and Nishiguchi S: Japan Society of Hepatology
guidelines for sarcopenia in liver disease (1st edition):
Recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 46:951–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim WS, Liang CK, Assantachai P, Auyeung
TW, Kang L, Lee WJ, Lim JY, Sugimoto K, Akishita M, Chia SL, et al:
COVID-19 and older people in Asia: Asian Working Group for
Sarcopenia calls to actions. Geriatr Gerontol Int. 20:547–558.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang PY, Li Y and Wang Q: Sarcopenia: An
underlying treatment target during the COVID-19 pandemic.
Nutrition. 84:1111042021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shang M, Cappellesso F, Amorim R, Serneels
J, Virga F, Eelen G, Carobbio S, Rincon MY, Maechler P, De Bock K,
et al: Macrophage-derived glutamine boosts satellite cells and
muscle regeneration. Nature. 587:626–631. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Xiang L, Jia G, Liu G, Zhao H and
Huang Z: Leucine regulates slow-twitch muscle fibers expression and
mitochondrial function by Sirt1/AMPK signaling in porcine skeletal
muscle satellite cells. Anim Sci J. 90:255–263. 2019. View Article : Google Scholar
|
|
14
|
Verdijk LB, Koopman R, Schaart G, Meijer
K, Savelberg HH and van Loon LJ: Satellite cell content is
specifically reduced in type II skeletal muscle fibers in the
elderly. Am J Physiol Endocrinol Metab. 292:E151–E157. 2007.
View Article : Google Scholar
|
|
15
|
Fochi S, Giuriato G, De Simone T,
Gomez-Lira M, Tamburin S, Del Piccolo L, Schena F, Venturelli M and
Romanelli MG: Regulation of microRNAs in satellite cell renewal,
muscle function, sarcopenia and the role of exercise. Int J Mol
Sci. 21:67322020. View Article : Google Scholar :
|
|
16
|
Schiaffin S, Reggiani C and Murgia M:
Fiber type diversity in skeletal muscle explored by mass
spectrometry-based single fiber proteomics. Histol Histopathol.
35:239–246. 2020.
|
|
17
|
Wang Y and Pessin JE: Mechanisms for
fiber-type specificity of skeletal muscle atrophy. Curr Opin Clin
Nutr Metab Care. 16:243–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lepore E, Casola I, Dobrowolny G and
Musarò A: Neuromuscular Junction as an Entity of Nerve-Muscle
Communication. Cells. 8:9062019. View Article : Google Scholar :
|
|
19
|
Yamakawa H, Kusumoto D, Hashimoto H and
Yuasa S: Stem cell aging in skeletal muscle regeneration and
disease. Int J Mol Sci. 21:18302020. View Article : Google Scholar :
|
|
20
|
Liu L, Charville GW, Cheung TH, Yoo B,
Santos PJ, Schroeder M and Rando TA: Impaired notch signaling leads
to a decrease in p53 activity and mitotic catastrophe in aged
muscle stem cells. Cell Stem Cell. 23:544–556.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ,
Keller C and Rando TA: Increased Wnt signaling during aging alters
muscle stem cell fate and increases fibrosis. Science. 317:807–810.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilkinson DJ, Piasecki M and Atherton PJ:
The age-related loss of skeletal muscle mass and function:
Measurement and physiology of muscle fibre atrophy and muscle fibre
loss in humans. Ageing Res Rev. 47:123–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sartori R, Romanello V and Sandri M:
Mechanisms of muscle atrophy and hypertrophy: Implications in
health and disease. Nat Commun. 12:3302021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilkinson DJ, Hossain T, Hill DS, Phillips
BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen
L, Phillips SM, et al: Effects of leucine and its metabolite
β-hydroxy-β-methylbutyrate on human skeletal muscle protein
metabolism. J Physiol. 591:2911–2923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kruse R, Petersson SJ, Christensen LL,
Kristensen JM, Sabaratnam R, Ørtenblad N, Andersen M and Højlund K:
Effect of long-term testosterone therapy on molecular regulators of
skeletal muscle mass and fibre-type distribution in aging men with
subnormal testosterone. Metabolism. 112:1543472020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Alcantara Borba D, da Silva Alves E,
Rosa JPP, Facundo LA, Costa CMA, Silva AC, Narciso FV, Silva A and
de Mello MT: Can IGF-1 serum levels really be changed by acute
physical exercise? A systematic review and meta-analysis. J Phys
Act Health. 17:575–584. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan KT, Ang SJ and Tsai SY: Sarcopenia:
Tilting the balance of protein homeostasis. Proteomics.
20:e18004112020. View Article : Google Scholar
|
|
28
|
Laplante M and Sabatini DM: mTOR signaling
at a glance. J Cell Sci. 122:3589–3594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Antona G and Nisoli E: mTOR signaling as
a target of amino acid treatment of the age-related sarcopenia.
Interdiscip Top Gerontol. 37:115–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giovannini S, Marzetti E, Borst SE and
Leeuwenburgh C: Modulation of GH/IGF-1 axis: Potential strategies
to counteract sarcopenia in older adults. Mech Ageing Dev.
129:593–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akalu Y, Molla MD, Dessie G and Ayelign B:
physiological effect of ghrelin on body systems. Int J Endocrinol.
2020:13851382020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu Y, Bradley JS, McCoski SR, Gonzalez JM,
Ealy AD and Johnson SE: Reduced skeletal muscle fiber size
following caloric restriction is associated with calpainmediated
proteolysis and attenuation of IGF-1 signaling. Am J Physiol Regul
Integr Comp Physiol. 312:R806–R815. 2017. View Article : Google Scholar
|
|
33
|
Matheny RW Jr, Carrigan CT, Abdalla MN,
Geddis AV, Leandry LA, Aguilar CA, Hobbs SS and Urso ML: RNA
transcript expression of IGF-I/PI3K pathway components in
regenerating skeletal muscle is sensitive to initial injury
intensity. Growth Horm IGF Res. 32:14–21. 2017. View Article : Google Scholar
|
|
34
|
Bodine SC, Stitt TN, Gonzalez M, Kline WO,
Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC,
Glass DJ and Yancopoulos GD: Akt/mTOR pathway is a crucial
regulator of skeletal muscle hypertrophy and can prevent muscle
atrophy in vivo. Nat Cell Biol. 3:1014–1019. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rommel C, Bodine SC, Clarke BA, Rossman R,
Nunez L, Stitt TN, Yancopoulos GD and Glass DJ: Mediation of
IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and
PI(3) K/Akt/GSK3 pathways. Nat Cell Biol. 3:1009–1013. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Parkington JD, LeBrasseur NK, Siebert AP
and Fielding RA: Contraction-mediated mTOR, p70S6k, and ERK1/2
phosphorylation in aged skeletal muscle. J Appl Physiol 1985.
97:243–248. 2004.PubMed/NCBI
|
|
37
|
Gomes MD, Lecker SH, Jagoe RT, Navon A and
Goldberg AL: Atrogin-1, a muscle-specific F-box protein highly
expressed during muscle atrophy. Proc Natl Acad Sci USA.
98:14440–14445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bodine SC, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giresi PG, Stevenson EJ, Theilhaber J,
Koncarevic A, Parkington J, Fielding RA and Kandarian SC:
Identification of a molecular signature of sarcopenia. Physiol
Genomics. 21:253–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Clavel S, Coldefy AS, Kurkdjian E, Salles
J, Margaritis I and Derijard B: Atrophy-related ubiquitin ligases,
atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior
muscle. Mech Ageing Dev. 127:794–801. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Burd NA, Gorissen SH and van Loon LJ:
Anabolic resistance of muscle protein synthesis with aging. Exerc
Sport Sci Rev. 41:169–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marshall RN, Smeuninx B, Morgan PT and
Breen L: Nutritional strategies to offset disuse-induced skeletal
muscle atrophy and anabolic resistance in older adults: From
whole-foods to isolated ingredients. Nutrients. 12:15332020.
View Article : Google Scholar :
|
|
43
|
Wilson D, Jackson T, Sapey E and Lord JM:
Frailty and sarcopenia: The potential role of an aged immune
system. Ageing Res Rev. 36:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee JS, Auyeung TW, Kwok T, Lau EM, Leung
PC and Woo J: Associated factors and health impact of sarcopenia in
older Chinese men and women: A cross-sectional study. Gerontology.
53:404–410. 2008. View Article : Google Scholar
|
|
45
|
Baylis D, Bartlett DB, Patel HP and
Roberts HC: Understanding how we age: Insights into inflammaging.
Longev Healthspan. 2:82013. View Article : Google Scholar
|
|
46
|
Goodman MN: Tumor necrosis factor induces
skeletal muscle protein breakdown in rats. Am J Physiol.
260:E727–E730. 1991.PubMed/NCBI
|
|
47
|
Goodman MN: Interleukin-6 induces skeletal
muscle protein breakdown in rats. Proc Soc Exp Biol Med.
205:182–185. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ko F, Abadir P, Marx R, Westbrook R, Cooke
C, Yang H and Walston J: Impaired mitochondrial degradation by
autophagy in the skeletal muscle of the aged female interleukin 10
null mouse. Exp Gerontol. 73:23–27. 2016. View Article : Google Scholar :
|
|
49
|
Correia-Melo C, Marques FD, Anderson R,
Hewitt G, Hewitt R, Col J, Carroll BM, Miwa S, Birch J, Merz A, et
al: Mitochondria are required for pro-ageing features of the
senescent phenotype. EMBO J. 35:724–742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sriram S, Subramanian S, Sathiakumar D,
Venkatesh R, Salerno MS, McFarlane CD, Kambadur R and Sharma M:
Modulation of reactive oxygen species in skeletal muscle by
myostatin is mediated through NF-κB. Aging Cell. 10:931–948. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tang H, Inoki K, Brooks SV, Okazawa H, Lee
M, Wang J, Kim M, Kennedy CL, Macpherson PCD, Ji X, et al: mTORC1
underlies age-related muscle fiber damage and loss by inducing
oxidative stress and catabolism. Aging Cell. 18:e129432019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bodine SC and Baehr LM: Skeletal muscle
atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am
J Physiol Endocrinol Metab. 307:E469–E484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Povea-Cabello S, Oropesa-Ávila M, de la
Cruz-Ojeda P, Villanueva-Paz M, de la Mata M, Suárez-Rivero JM,
Álvarez-Córdoba M, Villalón-García I, Cotán D, Ybot-González P and
Sánchez-Alcázar JA: Dynamic reorganization of the cytoskeleton
during apoptosis: The two coffins hypothesis. Int J Mol Sci.
18:pii: E23932017. View Article : Google Scholar
|
|
54
|
Phillips T and Leeuwenburgh C: Muscle
fiber specific apoptosis and TNF-alpha signaling in sarcopenia are
attenuated by life-long calorie restriction. FASEB J. 19:668–670.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dupont-Versteegden EE: Apoptosis in muscle
atrophy: Relevance to sarcopenia. Exp Gerontol. 40:473–481. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu H and Ballantyne CM: Skeletal muscle
inflammation and insulin resistance in obesity. J Clin Invest.
127:43–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tornatore L, Thotakura AK, Bennett J,
Moretti M and Franzoso G: The nuclear factor kappa B signaling
pathway: Integrating metabolism with inflammation. Trends Cell
Biol. 22:557–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Puthucheary ZA, Rawal J, McPhail M,
Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T,
Sidhu PS, et al: Acute skeletal muscle wasting in critical illness.
JAMA. 310:1591–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Merritt EK, Stec MJ, Thalacker-Mercer A,
Windham ST, Cross JM, Shelley DP, Craig Tuggle S, Kosek DJ, Kim JS
and Bamman MM: Heightened muscle inflammation susceptibility may
impair regenerative capacity in aging humans. J Appl Physiol.
115:937–948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nishikawa H, Enomoto H, Nishiguchi S and
Iijima H: Sarcopenic obesity in liver cirrhosis: Possible mechanism
and clinical impact. Int J Mol Sci. 22:19172021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pedersen BK and Febbraio MA: Muscles,
exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev
Endocrinol. 8:457–465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Giudice J and Taylor JM: Muscle as a
paracrine and endocrine organ. Curr Opin Pharmacol. 34:49–55. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pedersen BK, Akerström TC, Nielsen AR and
Fischer CP: Role of myokines in exercise and metabolism. J Appl
Physiol. 103:1093–1098. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ten Broek RW, Grefte S and Von den Hoff
JW: Regulatory factors and cell populations involved in skeletal
muscle regeneration. J Cell Physiol. 224:7–16. 2010.PubMed/NCBI
|
|
65
|
Adams GR: Invited Review:
Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl
Physiol. 93:1159–1167. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pedersen BK and Febbraio MA: Muscle as an
endocrine organ: Focus on muscle-derived interleukin-6. Physiol
Rev. 88:1379–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tatsumi R, Anderson JE, Nevoret CJ, Halevy
O and Allen RE: HGF/SF is present in normal adult skeletal muscle
and is capable of activating satellite cells. Dev Biol.
194:114–128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rodgers JT, King KY, Brett JO, Cromie MJ,
Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai CR, et
al: mTORC1 controls the adaptive transition of quiescent stem cells
from G0 to G(Alert). Nature. 509:393–396. 2014. View Article : Google Scholar
|
|
69
|
Clarke MS and Feeback DL: Mechanical load
induces sarcoplasmic wounding and FGF release in differentiated
human skeletal muscle cultures. Faseb J. 10:502–509. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yablonka-Reuveni Z, Seger R and Rivera AJ:
Fibroblast growth factor promotes recruitment of skeletal muscle
satellite cells in young and old rats. J Histochem Cytochem.
47:23–42. 1999. View Article : Google Scholar
|
|
71
|
Jones NC, Tyner KJ, Nibarger L, Stanley
HM, Cornelison DD, Fedorov YV and Olwin BB: The p38alpha/beta MAPK
functions as a molecular switch to activate the quiescent satellite
cell. J Cell Biol. 169:105–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jones NC, Fedorov YV, Rosenthal RS and
Olwin BB: ERK1/2 is required for myoblast proliferation but is
dispensable for muscle gene expression and cell fusion. J Cell
Physiol. 186:104–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Quinn LS, Anderson BG, Strait-bodey L,
Stroud AM and Argile M: Oversecretion of interleukin-15 from
skeletal muscle reduces adiposity. Am J Physiol Endocrinal Metab.
296:E191–E202. 2009. View Article : Google Scholar
|
|
74
|
Furmanczyk PS and Quinn LS: Interleukin-15
increases myosin accretion in human skeletal myogenic cultures.
Cell Biol Int. 27:845–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Quinn LS, Anderson BG, Drivdahl RH,
Alvarez B and Argilés JM: Overexpression of interleukin-15 induces
skeletal muscle hypertrophy in vitro: Implications for treatment of
muscle wasting disorders. Exp Cell Res. 280:55–63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Quinn LS, Haugk KL and Damon SE:
Interleukin-15 stimulates C2 skeletal myoblast differentiation.
Biochem Biophys Res Commun. 239:6–10. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shefer G, Rauner G, Yablonka-Reuveni Z and
Benayahu D: Reduced satellite cell numbers and myogenic capacity in
aging can be alleviated by endurance exercise. PLoS One.
5:e133072010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Aoi W, Naito Y, Takagi T, Tanimura Y,
Takanami Y, Kawai Y, Sakuma K, Hang LP, Mizushima K, Hirai Y, et
al: A novel myokine, secreted protein acidic and rich in cysteine
(SPARC), suppresses colon tumorigenesis via regular exercise. Gut.
62:882–889. 2013. View Article : Google Scholar
|
|
79
|
McPherron AC, Lawler AM and Lee SJ:
Regulation of skeletal muscle mass in mice by a new TGF-beta super
family member. Nature. 387:83–90. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Saneyasu T, Honda K and Kamisoyama H:
Myostatin Increases Smad2 phosphorylation and atrogin-1 expression
in chick embryonic myotubes. J Poult Sci. 56:224–230. 2019.
View Article : Google Scholar
|
|
81
|
Nikooie R, Jafari-Sardoie S, Sheibani V
and Nejadvaziri Chatroudi A: Resistance training-induced muscle
hypertrophy is mediated by TGF-β1-Smad signaling pathway in male
Wistar rats. J Cell Physiol. 235:5649–5665. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu L, Hu R, You H, Li J, Liu Y, Li Q, Wu
X, Huang J, Cai X, Wang M and Wei L: Formononetin ameliorates
muscle atrophy by regulating myostatin-mediated PI3K/Akt/FoxO3a
pathway and satellite cell function in chronic kidney disease. J
Cell Mol Med. 25:1493–1506. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hill M, Wernig A and Goldspink G: Muscle
satellite stem cell activation during local tissue injury and
repair. J Anat. 203:89–99. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Milan G, Dalla Nora E, Pilon C, Pagano C,
Granzotto M, Manco M, Mingrone G and Vettor R: Changes in muscle
myostatin expression in obese subjects after weight loss. J Clin
Endocrinol Metab. 89:2724–2727. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Mafi F, Biglari S, Ghardashi Afousi A and
Gaeini AA: Improvement in skeletal muscle strength and plasma
levels of follistatin and myostatin induced by an 8-week resistance
training and epicatechin supplementation in sarcopenic older
adults. J Aging Phys Act. 27:384–391. 2019. View Article : Google Scholar
|
|
86
|
Biglari S, Afousi AG, Mafi F and Shabkhiz
F: High-intensity interval training-induced hypertrophy in
gastrocnemius muscle via improved IGF-I/Akt/FoxO and myostatin/Smad
signaling pathways in rats. Physiol Int. Jul 7–2020.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hill EW, McGivney BA, Rooney MF, Katz LM,
Parnell A and MacHugh DE: The contribution of myostatin (MSTN) and
additional modifying genetic loci to race distance aptitude in
Thoroughbred horses racing in different geographic regions. Equine
Vet J. 51:625–633. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
McGivney BA, Browne JA, Fonseca RG, Katz
LM, Machugh DE, Whiston R and Hill EW: MSTN genotypes in
Thoroughbred horses influence skeletal muscle gene expression and
racetrack performance. Anim Genet. 43:810–812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Onde G, Penninx BW, Balkrishnan R, Fried
LP, Chaves PH, Williamson J, Carter C, Di Bari M, Guralnik JM and
Pahor M: Relation between use of angiotensin-converting enzyme
inhibitors and muscle strength and physical function in older
women: An observational study. Lancet. 359:926–930. 2002.
View Article : Google Scholar
|
|
90
|
Mogi M: Effect of renin-angiotensin system
on senescence. Geriatr Gerontol Int. 20:520–525. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yoshida T, Tabony AM, Galvez S, Mitch WE,
Higashi Y, Sukhanov S and Delafontaine P: Molecular mechanisms and
signaling pathways of angiotensin II-induced muscle wasting:
Potential therapeutic targets for cardiac cachexia. Int J Biochem
Cell Biol. 45:2322–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
van den Beld AW, Kaufman JM, Zillikens MC,
Lamberts SWJ, Egan JM and van der Lely AJ: The physiology of
endocrine systems with ageing. Lancet Diabetes Endocrinol.
6:647–658. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Roy TA, Blackman MR, Harman SM, Tobin JD,
Schrager M and Metter EJ: Interrelationships of serum testosterone
and free testosterone index with FFM and strength in aging men. Am
J Physiol Endocrinol Metab. 283:E284–E294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sipila S, Narici M, Kjaer M, Pollanen E,
Atkinson RA, Hansen M and Kovanen V: Sex hormones and skeletal
muscle weakness. Biogerontology. 14:231–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ikeda K, Horie-Inoue K and Inoue S:
Functions of estrogen and estrogen receptor signaling on skeletal
muscle. J Steroid Biochem Mol Biol. 191:1053752019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yoshimura N, Muraki S, Iidaka T, Oka H,
Horii C, Kawaguchi H, Akune T, Nakamura K and Tanaka S: Prevalence
and co-existence of locomotive syndrome, sarcopenia, and frailty:
The third survey of research on osteoarthritis/osteoporosis against
disability (ROAD) study. J Bone Miner Metab. 37:1058–1066. 2019.
View Article : Google Scholar : PubMed/NCBI
|