Introduction
Endometrial cancer (EC) is considered one of the
most common gynecological malignancies in industrialized countries
(1). Primarily due to relevant
risk factors, including obesity, diabetes and aging, among others,
its incidence is gradually increasing (2,3).
Recurrent or advanced EC responds poorly to treatment. Few
therapeutic options are available for such patients, who therefore
have low survival rates and a poor prognosis (4). The 5-year survival rate for
patients with stage IV disease is limited only at 0-10% (5). Accordingly, EC is increasingly
recognized as a serious health and epidemiological concern. Thus,
novel therapeutic approaches that can effectively reduce morbidity
and mortality in EC are urgently required.
In recent years, natural compounds for cancer
prevention and treatment have gained attention due to their
anticancer activity and safety, and are a rich source of
phytochemicals. They are an integral part of the human diet
(6,7). The use of natural compounds
combined with targeted drugs may provide new perspectives for the
development of anticancer drugs. Chrysin (5,7-dihydroxyflavone;
chemical structure shown in Fig.
1A) is a natural dietary flavonoid, commonly present in various
plant extracts, including honey and propolis. It has a notable
medicinal functions and economic value (8). Additionally, chrysin has diverse
biological properties, specifically including anticancer,
antioxidant, anti-inflammatory, antibacterial, anti-diabetic and
anti-allergenic effects (9,10). Recently, several studies have
reported that chrysin exerts its cancer-suppressive effects on
breast, lung, cervical and bladder cancer cells through regulating
multiple cell signaling pathways selectively (11-15). However, the anti-EC possible
mechanisms of chrysin have not yet been fully elucidated.
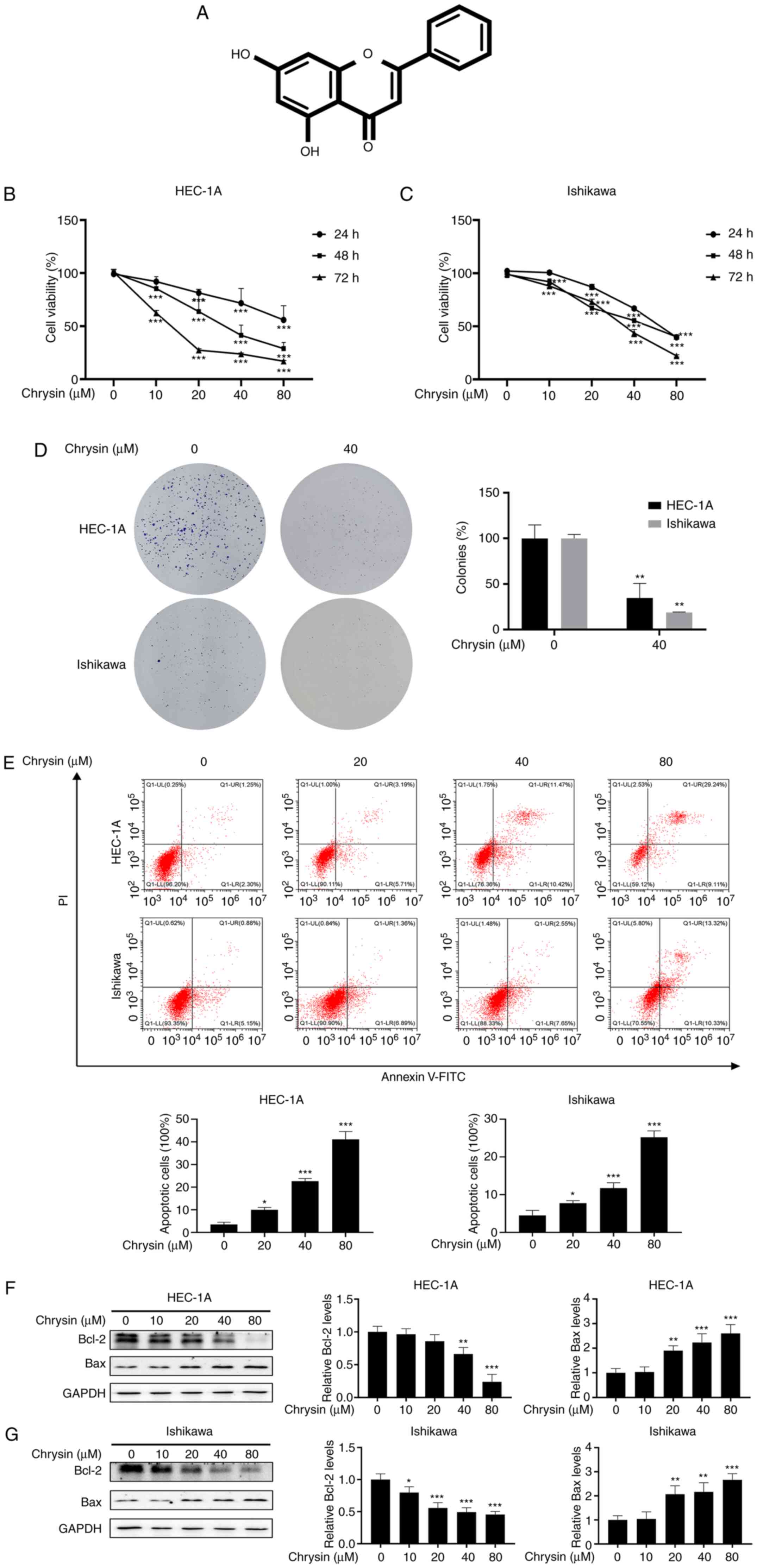 | Figure 1Chrysin inhibits the proliferation
and induces the apoptosis of endometrial cancer cells. (A) Chemical
structure of chrysin. (B and C) HEC-1A and Ishikawa cells were
treated with chrysin (0, 10, 20, 40 and 80 µM), and Cell
Counting Kit-8 solution was added at 24, 48 and 72 h. (D) Following
40 µM chrysin treatment for 48 h in HEC-1A and Ishikawa
cells, colony formation assay was performed. (E) Following
treatment with chrysin (0, 20, 40 and 80 µM) for 48 h, cell
apoptosis was analyzed by flow cytometry using Annexin
V-fluorescein isothiocyanate and 10 µl propidium iodide
staining in HEC-1A and Ishikawa cells. (F and G) HEC-1A and
Ishikawa cells were exposed to chrysin (0, 10, 20, 40 and 80
µM) for 48 h, and the Bcl-2 and Bax levels were confirmed by
western blot analysis. Data are expressed as the mean ± standard
deviation (n=3). *P<0.05, **P<0.01,
***P<0.001 vs. control group. |
Cancer cell death is often caused by apoptosis,
which is considered to be the principal anticancer mechanism
(16). However, it has been
documented that autophagy and apoptosis are intertwined processes
(17). Autophagy is the process
of engulfing and degrading cytosolic proteins and damaged
organelles (18). It has been
reported that the inhibition of autophagy increases the anticancer
efficiency by inducing the apoptotic process (19). In addition, reactive oxygen
species (ROS) have been found to be the associated factors in the
anticancer effects of chrysin-induced autophagy. For example, Lin
et al (20) indicated
that chrysin induced ROS production and then autophagy, resulting
in the attenuation of the viability of human colorectal cancer
cells. This suggests that there may be a link among anticancer
drugs, such as chrysin, ROS levels and autophagy pathways in EC.
However, to date, at least to the best of our knowledge, there are
no reports on the pharmacological mechanisms of chrysin in EC,
particularly concerning its role in the regulation of cell ROS
levels and autophagy pathways, which remains unclear.
The present study thus aimed to investigate the
molecular mechanisms of the anticancer role of chrysin and focused
on how the compound regulates the autophagy pathway in EC
cells.
Materials and methods
Reagents and antibodies
Chrysin and LY294002 were purchased from
MedChemExpress. N-acetylcysteine (NAC) and chloroquine (CQ)
were obtained from Sigma-Aldrich (Sigma-Aldrich; Merck KGaA). The
cells were pretreated with 5 µM CQ, or 10 µM
LY294002, or 10 mM NAC for 1 h and were then treated with chrysin
for additional 24 or 48 h. The antibodies used for western blotting
included monoclonal anti-LC3 (dilution 1:1,000; cat. no. ab192890;
Abcam), monoclonal anti-sequestosome-1/p62 (dilution 1:1,000; cat.
no. ab207305; Abcam), monoclonal anti-autophagy-related gene 5
(ATG5; dilution 1:1,000, cat. no. ab108327; Abcam), polyclonal
anti-Beclin 1 (dilution 1:1,000; cat. no. AF5128; Affinity
Biosciences), polyclonal anti-Bcl-2 (dilution 1:1,000; cat. no.
AF6139; Affinity Biosciences), polyclonal anti-Bax (dilution
1:1,000; cat. no. AF0120; Affinity Biosciences), monoclonal
anti-Akt (dilution 1:1,000; cat. no. 4691; Cell Signaling
Technology, Inc.), monoclonal anti-phosphorylated (p)-Akt (Ser473;
dilution 1:1,000; cat. no. 4060; Cell Signaling Technology, Inc.),
monoclonal anti-mTOR (dilution 1:1,000; cat. no. 2983; Cell
Signaling Technology, Inc.), monoclonal anti-p-Mtor (Ser2448;
dilution 1:1,000; cat. no. 5536; Cell Signaling Technology, Inc.)
and polyclonal anti-GAPDH (dilution 1:10,000; cat. no. AF7021;
Affinity Biosciences). Horseradish peroxidase-conjugated goat
anti-rabbit IgG (dilution 1:10,000; cat. no. ZB-2301) and goat
anti-mouse IgG (dilution 1:10,000; cat. no. ZB-2305) secondary
antibodies were purchased from Beijing Zhongshan Jinqiao
Biotechnology, Co., Ltd.
Cell lines and cell culture
The human endometrioid adenocarcinoma cell line,
HEC-1A (cat. no. HTB-112), was purchased from the American Type
Culture Collection (ATCC) and the endometrioid adenocarcinoma cell
line, Ishikawa (cat. no. 99040201), was purchased from the European
Collection of Authenticated Cell Cultures (ECACC). Cells were
maintained in high-glucose DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% v/v FBS (Gibco; Thermo Fisher
Scientific, Inc.) and penicillin/streptomycin (100 U/ml penicillin
and 100 µg/ml streptomycin; Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in a 95% O2 and 5% CO2
atmosphere in a humidified incubator.
Cell viability assay
The Cell Counting Kit-8 (CCK-8; Biosharp Life
Sciences) was used to analyze the viability of the HEC-1A and
Ishikawa cells following treatment with chrysin. Briefly,
5×103 cells in 100 µl medium were plated into
each well of a 96-well plate until they became adherent. In
experiments evaluating the effect of chrysin alone, cells were
treated with chrysin (0, 10, 20, 40 and 80 µM) for 24, 48 or
72 h. In the experiments that evaluated the combined effect of
chrysin and CQ, cells were pretreated with 5 µM CQ for 1 h
prior to exposure to 40 µM chrysin, and the cells were then
further incubated for 48 h at 37°C. Subsequently, 10% CCK-8
solution (the ratio of volume of medium and CCK-8 was 9:1) was
added to the culture medium. Following further incubation for 1 h
at 37°C in the dark, a Varioskan LUX microplate reader (Thermo
Fisher Scientific, Inc.) was used to measure the absorbance at 450
nm in each well.
Colony formation assay
HEC-1A and Ishikawa cells were inoculated into
6-well plates at a low cell density (1,000 cells per well) and
incubated at 37°C overnight. The cells were the exposed to 40
µM chrysin and cultured for ~1 week. The medium was replaced
every 3 days, in order to maintain stable chrysin concentration
levels. The plates were periodically observed until distinct
colonies were formed and then fixed with 4% paraformaldehyde. After
staining with 0.1% crystal violet solution (Beyotime Institute of
Biotechnology) for 15 min at room temperature, the colonies (>50
cells per colony) were counted with ImageJ software (version 1.38;
National Institutes of Health).
Flow cytometric analysis
Apoptotic cells were detected by flow cytometry and
quantified according to the percentage of apoptotic cells in each
group. Briefly, HEC-1A and Ishikawa cells that had been treated
with the indicated concentrations of chrysin with or without 5
µM CQ were digested with trypsin without EDTA and harvested.
Subsequently, the cells were resuspended in 100 µl binding
buffer, and incubated with 5 µl Annexin V-FITC and 10
µl propidium iodide (PI) for 15 min at room temperature in
the dark. The apoptotic ratio was measured using a Navios flow
cytometer (Beckman Coulter, Inc.). The upper and lower right
quadrants on the dot-plot graphs represented late and early
apoptotic cells, respectively.
Transmission electron microscopy (TEM)
examination
To observe the cell morphological changes of
autophagosomes and autolysosomes, TEM was performed. Briefly,
HEC-1A and Ishikawa cells, following exposure to 40 µM
chrysin for 48 h, were fixed with 2.5% glutaraldehyde at 4°C
overnight, and then post-fixed with 1% osmium tetroxide
(OsO4) for 1 h followed by incubation with 2% uranyl
acetate at room temperature for a further 1 h. After washing with
phosphate-buffered saline (PBS; Gibco; Thermo Fisher Scientific,
Inc.), the cells were dehydrated in an ethanol series, infiltrated
with propylene oxide and finally embedded in epoxy resin. Ultrathin
sections (70 nm) were prepared, stained with uranyl acetate and
lead citrate, and then examined in a JEM-1400 TEM system (JEOL,
Ltd.).
Western blotting
The cells were lysed with RIPA lysis buffer
(Beyotime Institute of Biotechnology), and the supernatant was
collected by centrifugation at 13,000 × g for 30 min at 4°C in
order to extract total cellular protein. After quantifying the
protein concentration in the supernatant using a BCA protein assay
kit (Beyotime Institute of Biotechnology), total protein was mixed
by loading buffer and boiled at 100°C for 5 min. The denatured cell
proteins (10-20 µg) were separated on 6-15% SDS-PAGE
according to the molecular weight of the target proteins, and
subsequently transferred onto polyvinylidene difluoride membranes.
The membranes were then blocked for 2 h at room temperature in 5%
skimmed milk, and then incubated with the aforementioned diluted
primary antibodies at 4°C overnight, following by incubation with
the aforementioned secondary antibodies for 1 h at room
temperature. The protein bands were visualized with an enhanced
chemiluminescent reagent (Thermo Fisher Scientific, Inc.), and
analyzed using ImageJ version 1.38 (National Institutes of
Health).
Immunofluorescence assay
Following treatment with 40 µM chrysin for 48
h, HEC-1A and Ishikawa cells were fixed with 4% paraformaldehyde
for 15 min. Following permeabilization with 0.5% (v/v) Triton X-100
for 15 min at room temperature, the cells were blocked with 5%
bovine serum albumin (Beyotime Institute of Biotechnology) for 30
min and incubated with anti-LC3 antibody (dilution 1:200; cat. no.
ab192890; Abcam) overnight at 4°C. Finally, the cells were
incubated with the FITC-labeled goat anti-rabbit IgG (H+L)
secondary antibody (dilution 1:400; cat. no. A0562; Beyotime
Institute of Biotechnology) for 1 h at room temperature, and the
cell nuclei finally were stained with DAPI for 10 min in the dark.
All samples were imaged using an Axio Scope A1 fluorescence
microscope (Zeiss GmbH).
Intracellular reactive oxygen species
(ROS) analysis
Intracellular ROS levels were determined using a ROS
assay kit (Beyotime Institute of Biotechnology);
2′,7′-dichlorofluorescein diacetate (DCFH-DA), a ROS-sensitive
fluorescent dye, was used as the ROS detection probe. DCFH-DA was
deacetylated to non-fluorescent DCFH, and ROS then oxidized DCFH to
produce fluorescent DCF. The fluorescence intensity was
proportional to the oxidant production levels. HEC-1A and Ishikawa
cells were treated with 5 µM CQ or 10 mM NAC for 1 h prior
to treatment with 40 µM chrysin for 48 h. Processed cells
were incubated with 10 µM DCFH-DA diluted for 30 min at 37°C
in the dark. The cells were then washed three times with serum-free
DMEM to fully remove the probe. The levels of ROS, represented by
the green fluorescence signal, were photographed with the use of a
DP80 fluorescence microscope (Olympus Corporation).
Small interfering RNAs (siRNAs/si) and
transfection
The target siRNA sequence was as follows: siATG5,
5′-GCA ACU CUG GAU GG-3′; and negative control siRNA, 5′-UUC UCC
GAA CGU GUC ACG UTT-3′. All siRNAs were designed and synthesized by
Shanghai GenePharma Co., Ltd. Prior to transfection, HEC-1A and
Ishikawa cells were seeded into 6-well plates and cultured to
60-70% confluence. Cells were transfected with 50 nM siRNA using 4
µl jetPRIME® Transfection Reagent
(Polyplus-transfection® SA) in 200 µl
jetPRIME® buffer. These mixtures were incubated for 15
min at room temperature and added to 2 ml fresh medium per well of
a 6-well plate. The cells were cultured for 24 h, and then treated
with 0-80 µM chrysin for 24 or 48 h.
Statistical analysis
Data are represented as the mean ± standard
deviation. The experiments were performed ≥3 times. One-way ANOVA
was used to compare the experimental groups to the control values,
whereas comparisons between multiple groups were performed using
Tukey's multiple comparison test. Statistical analysis was
performed with GraphPad Prism 8.0 software (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Chrysin inhibits the proliferation and
induces the apoptosis of EC cells
CCK-8 assay revealed that chrysin exerted an
inhibitory effect on the proliferation of HEC-1A and Ishikawa
cells, in a concentration- and time-dependent manner (Fig. 1B and C). In addition, the colony
formation activity of the HEC-1A and Ishikawa cells was
significantly inhibited by chrysin (Fig. 1D). These results confirmed that
chrysin inhibited the proliferation of EC cells.
To investigate whether chrysin induces the apoptosis
of EC cells, cell apoptosis was examined by flow cytometry after
double staining with Annexin V-FITC and PI. As shown in Fig. 1E, chrysin notably increased the
proportion of apoptotic cells. Bax is a pro-apoptotic protein that
leads to cell death, whereas Bcl-2 is an anti-apoptotic protein
that promotes cell survival (21). Western blotting revealed that the
treatment of HEC-1A and Ishikawa cells with chrysin for 48 h
increased the level of Bax and decreased the expression of Bcl-2
(Fig. 1F and G). These results
demonstrated that chrysin induced the apoptosis of EC cells.
Chrysin initiates the autophagy of EC
cells
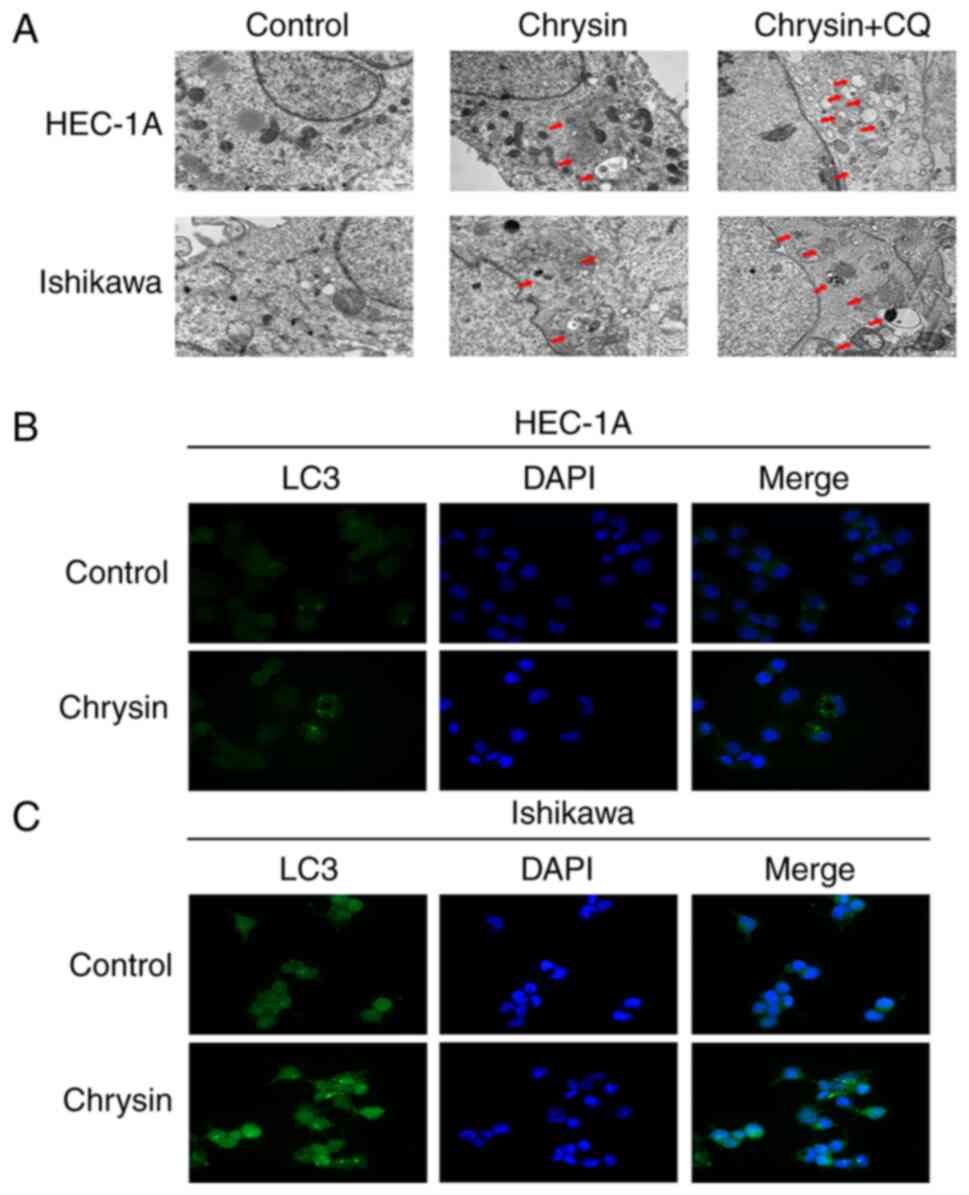
As shown in Fig.
2A, TEM images indicated autophagosomes and autophagolysosomes
in the chrysin group, which are characteristics of autophagic
cells. In cells treated with 5 µM CQ for 1 h, prior to the
application of 40 µM chrysin for 48 h, the aforementioned
effects intensified. Immunofluorescence assay was used to detect
the distribution of endogenous LC3 in HEC-1A and Ishikawa cells. In
chrysin-treated cells, the intracellular localization of LC3 and
abundant LC3 puncta were detected, while the control cells did not
exhibit any noticeable fluorescence intensity (Fig. 2B and C). The results of western
blot analysis revealed that, with an increased concentration of
chrysin, the level of LC3II and Beclin 1 increased, which was
accompanied by a decrease in p62 expression (Fig. 3A and B).
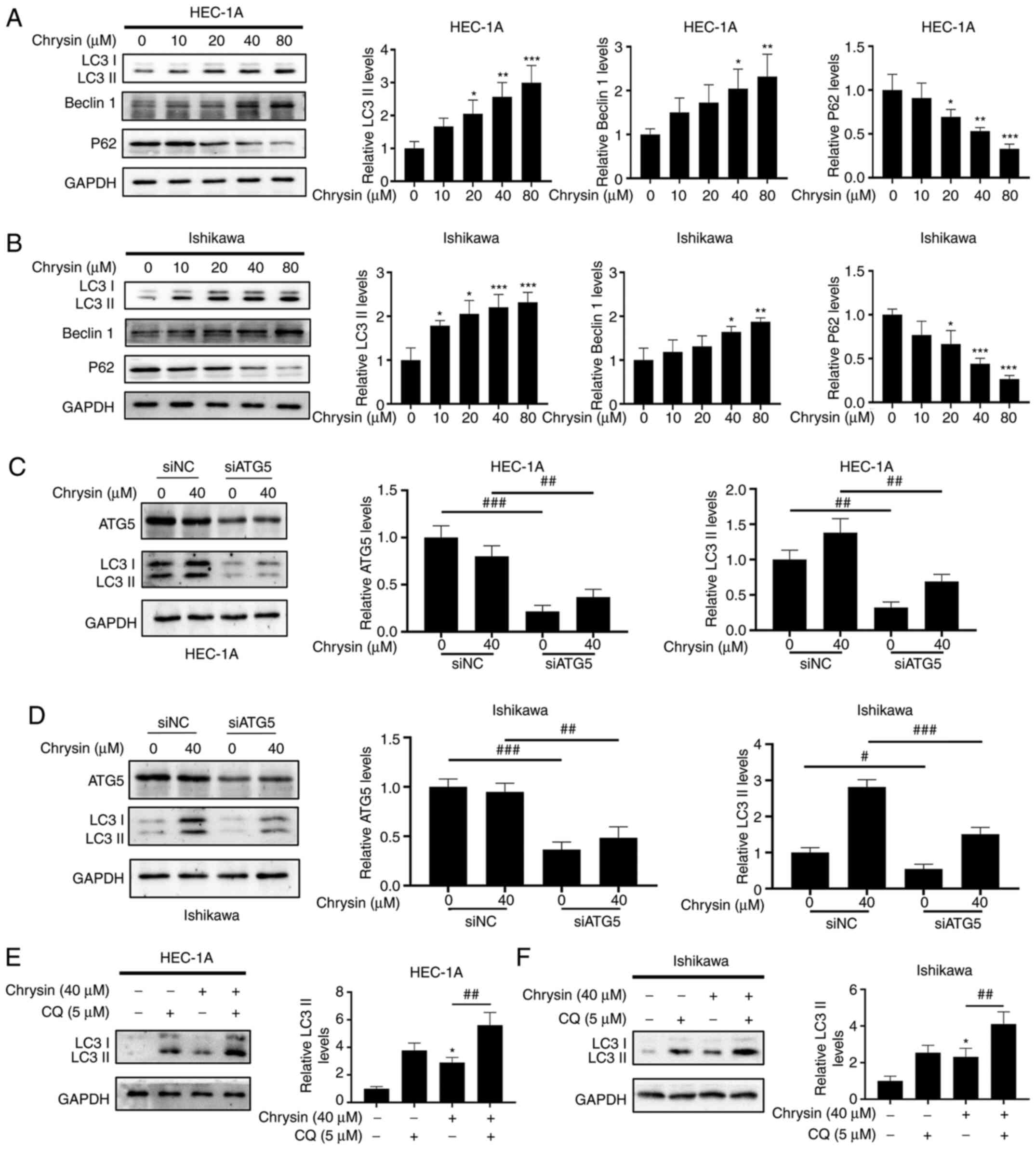 | Figure 3Concentration-dependent effect of
chrysin affects the expression of the autophagy-related protein
LC3II in endometrial cancer cells. (A and B) Following treatment of
HEC-1A and Ishikawa cells with various concentrations of chrysin
(0, 10, 20, 40 and 80 µM) for 48 h, the levels of LC3II,
Beclin 1 and p62 were examined by western blotting. (C and D)
HEC-1A and Ishikawa cells were transfected with si-negative control
or siATG5 for 24 h, and then treated with chrysin at 40 µM
for 24 h. The expression of ATG5 and LC3 was detected by western
blotting. #P<0.05, ##P<0.01,
###P<0.001 vs. siNC. (E and F) HEC-1A and Ishikawa
cells were untreated or treated with 5 µM CQ for 1 h,
followed by chrysin treatment at 40 µM for 24 h. The
expression levels of LC3II were then examined by western blotting.
Values are reported as the mean ± standard deviation (n=3).
*P<0.05, **P<0.01,
***P<0.001 vs. control group; #P<0.05,
##P<0.01, ###P<0.001 vs. chrysin group.
si, small interfering RNA; NC, negative control; ATG5,
autophagy-related gene 5; CQ, chloroquinone; LC3,
Microtubule-associated proteins 1A/1B light chain 3B. |
ATG5 participates in the vesicle elongation step of
autophagy and plays critical roles in the process of autophagy
(22). The present study
demonstrated that the knockdown of ATG5 by siRNA reduced the
chrysin-induced LC3II accumulation in HEC-1A and Ishikawa cells
(Fig. 3C and D). Furthermore,
the LC3II levels were markedly increased in the HEC-1A and Ishikawa
cells pretreated with CQ, in comparison with the group treated with
chrysin alone (Fig. 3E and F),
thus confirming that chrysin promoted the autophagic flux. These
results revealed that chrysin induced autophagy and increased the
autophagic flux in EC cells.
The role of autophagy in chrysin-induced cell
apoptosis was also assessed. The inhibition of autophagy by CQ
significantly enhanced the cytotoxicity of chrysin to EC cells,
which was demonstrated via the inhibition of proliferation
(Fig. 4A-C) and the induction of
apoptosis (Fig. 4D-F). Autophagy
inhibition by CQ potentiated the chrysin-induced inhibition of cell
proliferation and promoted the chrysin-induced apoptosis of EC
cells. Thus, chrysin induced cytoprotective autophagy in EC
cells.
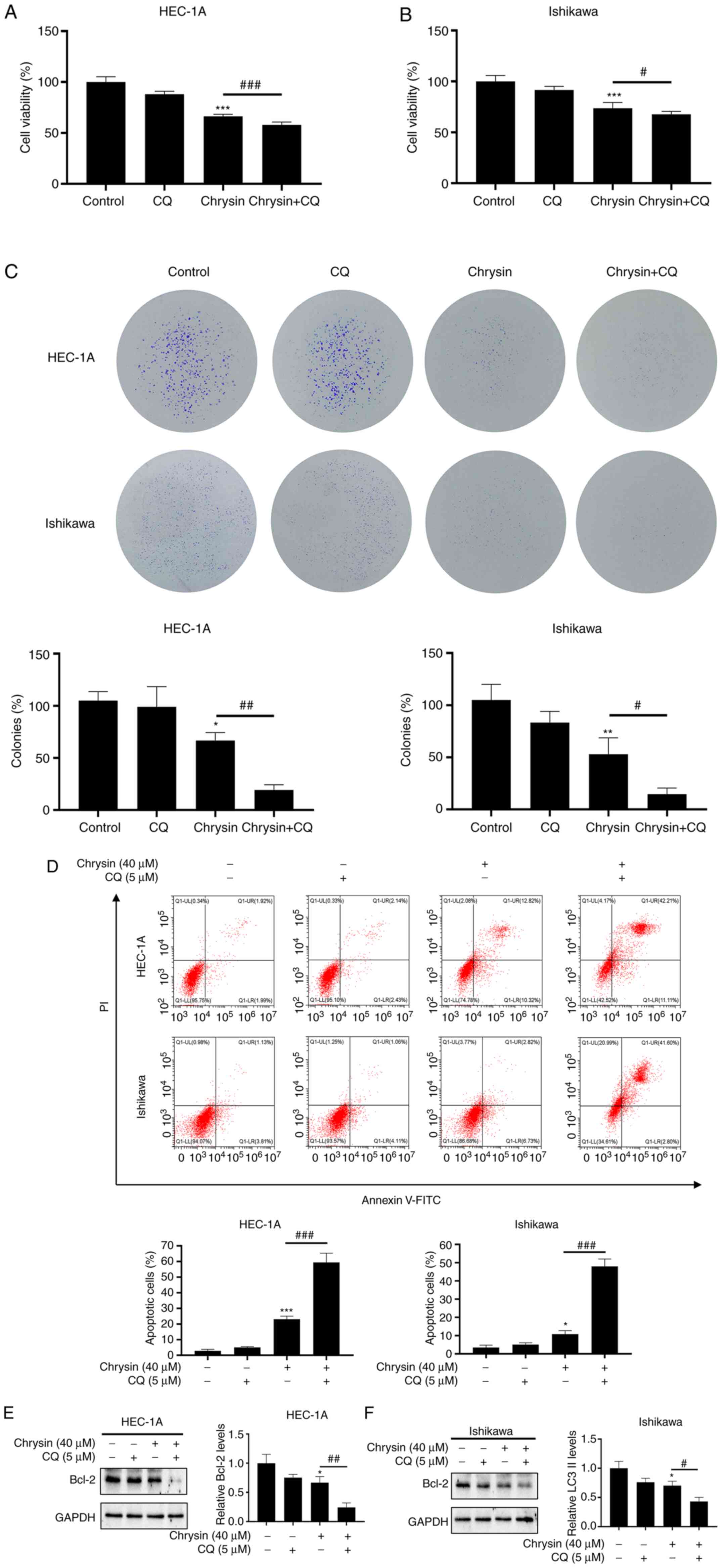 | Figure 4Chrysin induces cytoprotective
autophagy in endometrial cancer cells. (A and B) HEC-1A and
Ishikawa cells were treated with 40 µM chrysin without or
with 5 µM CQ for 48 h, and cell viability was then assessed
using a CCK-8 assay. (C) HEC-1A and Ishikawa cells were pretreated
with CQ (5 µM) for 1 h before being exposed to 40 µM
chrysin for colony formation assay. (D) HEC-1A and Ishikawa cells
were pretreated without or with 5 µM CQ for 1 h, followed by
treatment with chrysin (40 µM) for 48 h. Cell apoptosis was
then analyzed by flow cytometry using Annexin V-FITC/PI staining.
(E and F) HEC-1A and Ishikawa cells were untreated or treated with
5 µM CQ for 1 h, followed by chrysin treatment at 40
µM for 24 h. Subsequently, the expression levels of Bcl-2
were examined by western blotting. Values are presented as the mean
± standard deviation (n=3). *P<0.05,
**P<0.01, ***P<0.001 vs. control group;
#P<0.05, ##P<0.01,
###P<0.001 vs. chrysin group. CQ, chloroquine; CCK-8,
Cell Counting Kit-8; FITC, fluorescein isothiocyanate; PI,
propidium iodide. |
Chrysin induces autophagy via ROS in EC
cells
The association between ROS and autophagy is
complex. To clarify whether autophagy induced by chrysin in EC
cells is regulated by ROS or not, HEC-1A and Ishikawa cells were
treated with chrysin for 48 h, and the level of ROS in cells was
detected by DCF fluorescence intensity. Fluorescence microscopy
demonstrated that chrysin-induced intracellular ROS accumulation
was markedly increased, which was clearly inhibited by the ROS
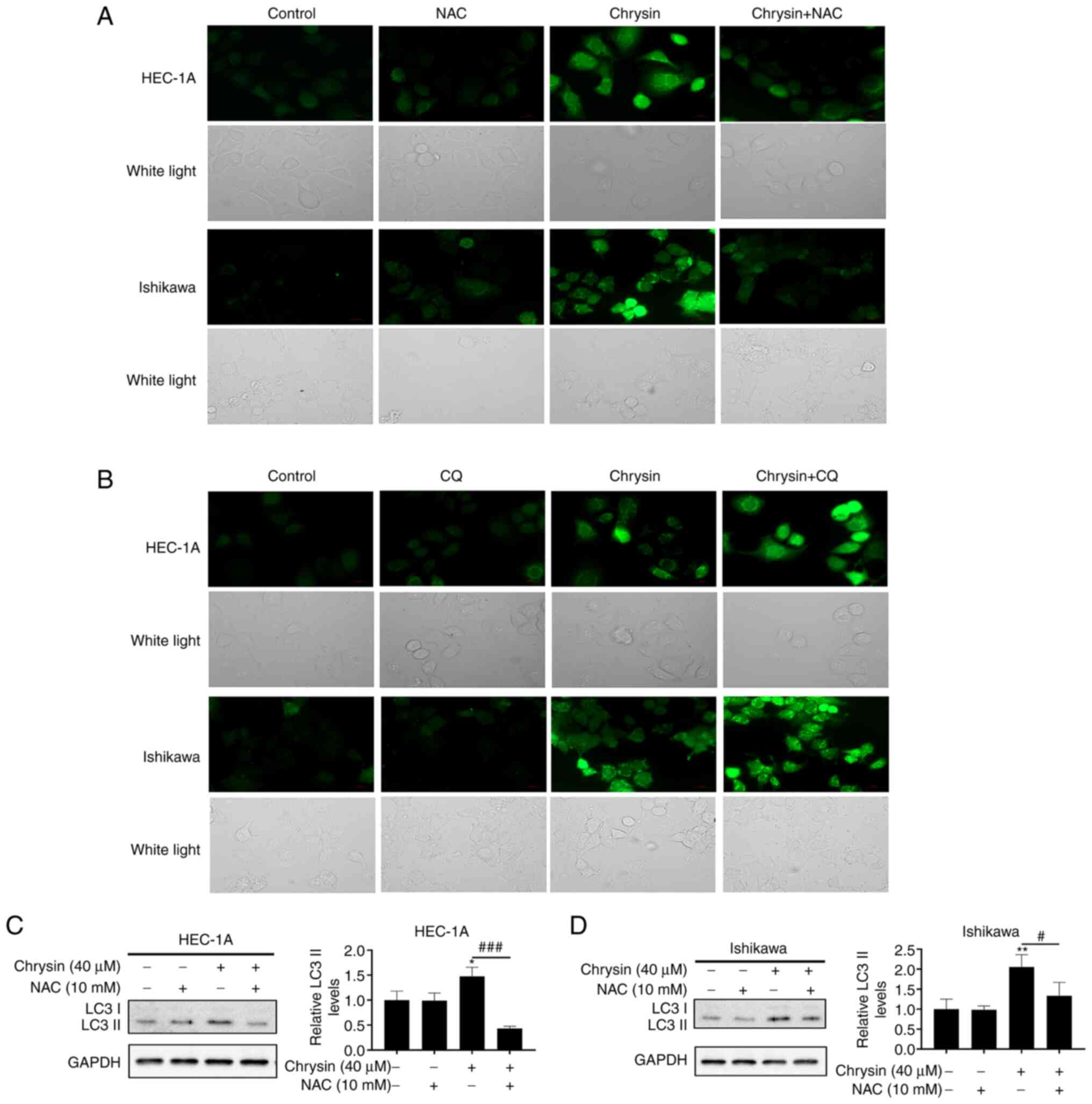
scavenger, NAC (Fig. 5A). ROS
levels were increased to a greater extent in cells treated with a
combination of chrysin and CQ, as compared with that of cells
treated with chrysin alone (Fig.
5B). Notably, NAC pretreatment inhibited the increase in the
LC3II level induced by chrysin (Fig.
5C and D). Collectively, it was revealed that chrysin induces
cellular ROS accumulation, which may be one of the reasons for
autophagy induced by chrysin in EC cells.
ROS-mediated inactivation of the Akt/mTOR
signaling pathway is involved in chrysin-induced autophagy in EC
cells
To further investigate the potential mechanisms of
autophagy induced by chrysin, the effect of chrysin on the
expression of Akt/mTOR in autophagy-related signaling pathways in
EC was analyzed in the present study (23). The increase in chrysin
concentration resulted in the decrease of p-Akt and p-mTOR levels
in a concentration-dependent manner (Fig. 6A and B), suggesting that chrysin
caused the inactivation of the Akt/mTOR signaling pathway.
Pretreatment with LY294002, an inhibitor of PI3K, further decreased
p-Akt and p-mTOR expression levels (Fig. 6C and D). Furthermore, LY294002
markedly increased the expression of LC3II induced by chrysin
(Fig. 6E and F). These results
indicated that the inactivation of the Akt/mTOR signaling pathway
by chrysin contributed to autophagy activation in EC cells.
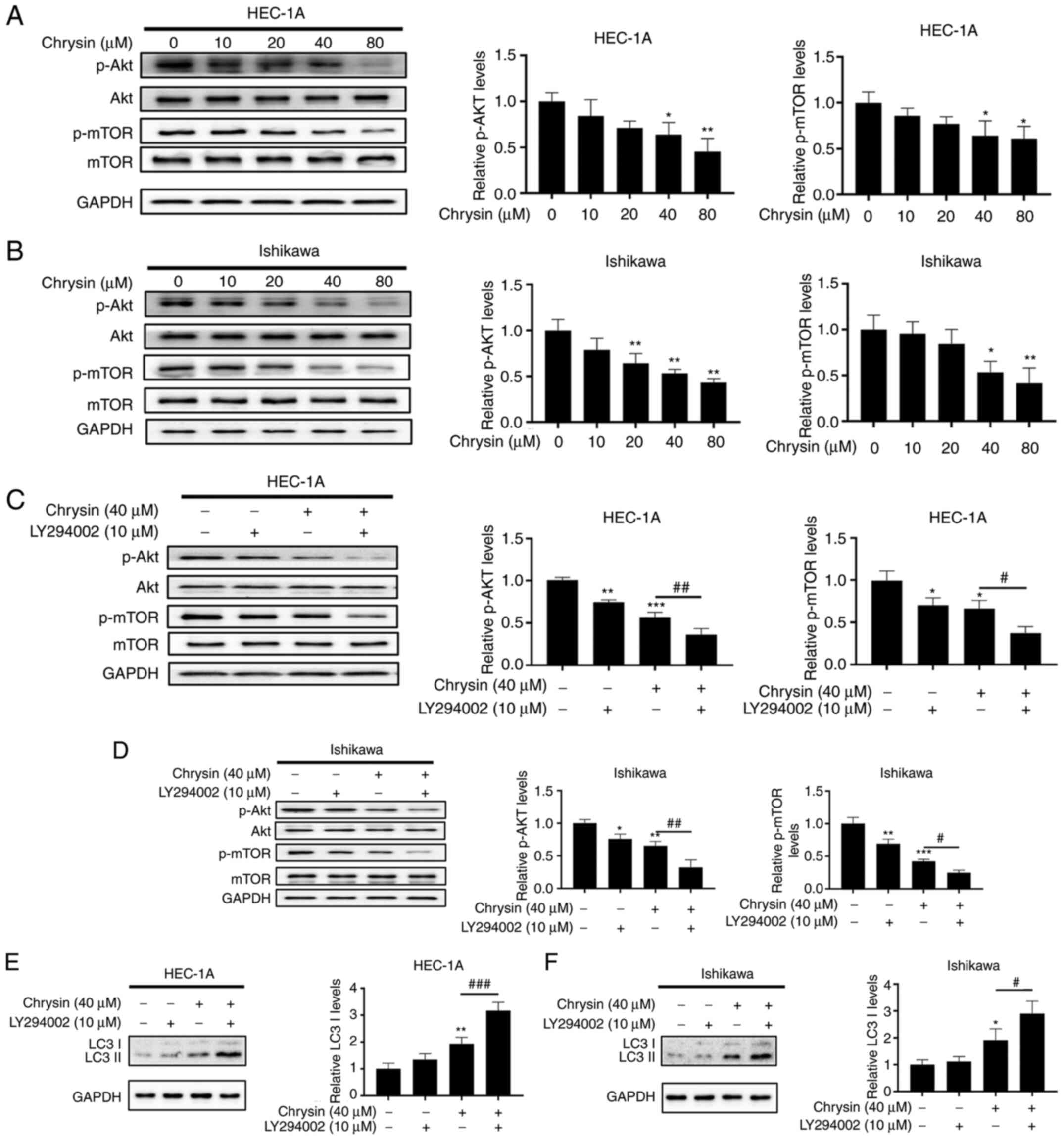 | Figure 6The Akt/mTOR pathway is involved in
chrysin-induced autophagy in endometrial cancer cells. (A and B)
HEC-1A and Ishikawa cells were exposed to chrysin at different
concentrations (0, 10, 20, 40 and 80 µM) for 48 h, and the
effects of chrysin on the levels of Akt, p-Akt, mTOR, p-mTOR were
examined by western blotting. (C-F) HEC-1A and Ishikawa cells were
either not treated, or treated with 10 µM LY294002 for 1 h,
and then incubated with 40 µM chrysin for 48 h, Next, Akt,
p-Akt, mTOR, p-mTOR and LC3II protein expression was analyzed by
western blotting. Values are presented as the mean ± standard
deviation of three independent experiments. *P<0.05,
**P<0.01, ***P<0.001 vs. control group;
#P<0.05, ##P<0.01,
###P<0.001 vs. chrysin group. p-, phosphorylated. |
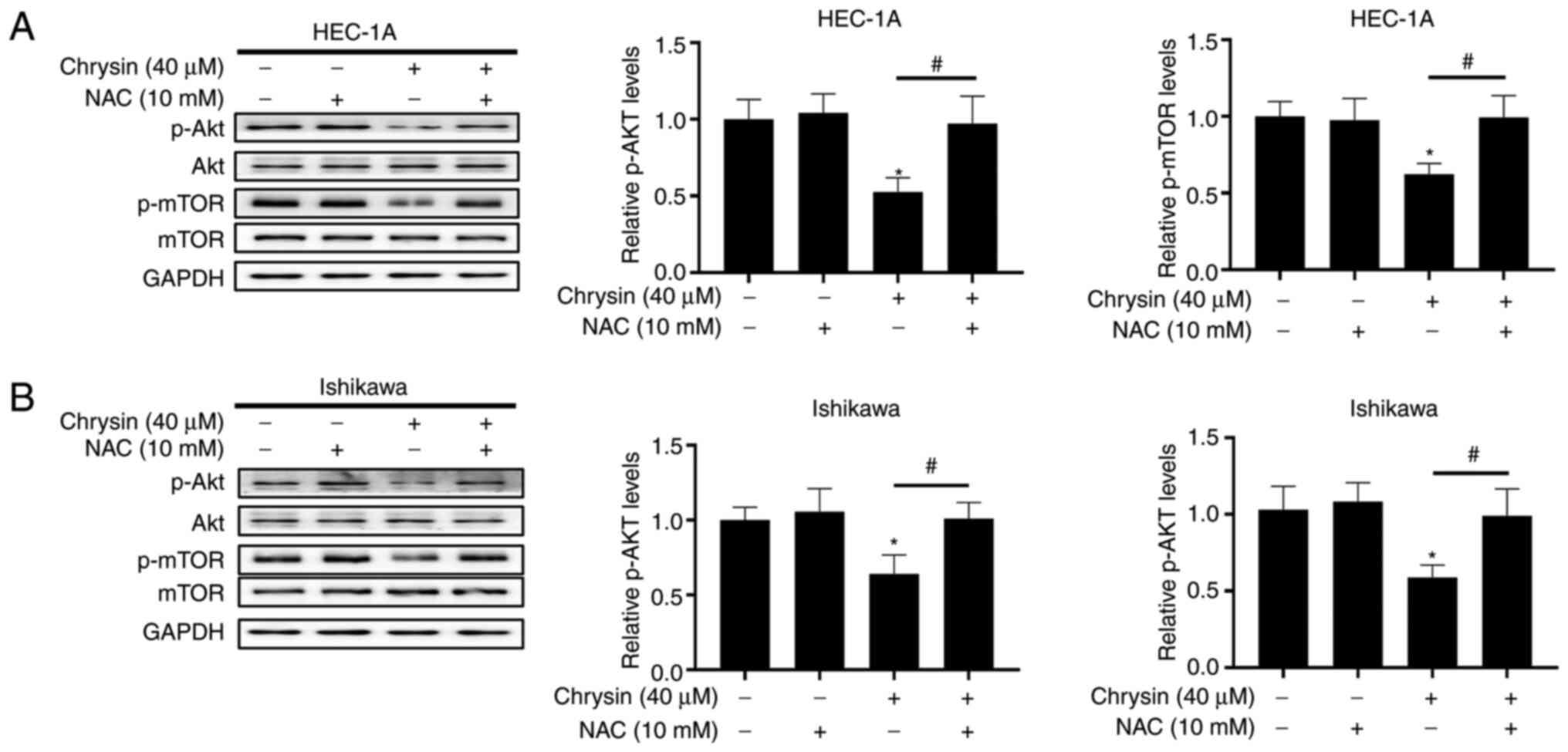
Furthermore, HEC-1A and Ishikawa cells were
pre-treated with NAC and subsequently treated with chrysin, in
order to evaluate the association between Akt/mTOR signal
suppression and ROS accumulation. The results of western blotting
reveaeld that NAC pretreatment upregulated the expression of p-Akt
and p-mTOR in chrysin-treated cells (Fig. 7A and B). Overall, these findings
indicated that chrysin inhibited the activity of the Akt/mTOR
signaling pathway by inducing the accumulation of intracellular ROS
in EC cells.
Discussion
At present, cisplatin and paclitaxel, which are the
first-line chemotherapeutics applied for EC therapy, exert an
inhibitory effect on cancer cell growth. However, chemoresistance
remains a major obstacle in EC therapy (24). Chrysin has been reported as a
potent inhibitor of breast cancer resistance protein, which is one
of the ATP-binding cassette (ABC) transporters, and is commonly
involved in the multidrug resistance of chemotherapy (25). TNF-related apoptosis-inducing
ligand (TRAIL) has been regarded as an anti-cancer agent. However,
certain types of cancer, including gliomas, are resistant to
TRAIL-induced cell death (26).
Chrysin has been shown to overcome TRAIL resistance in breast,
pancreatic, cervical, colon, prostate and bladder cancer, as well
as in hepatoma and melanoma cells (27). Thus, chrysin represents a
potential therapeutic target for the development of clinical
applications.
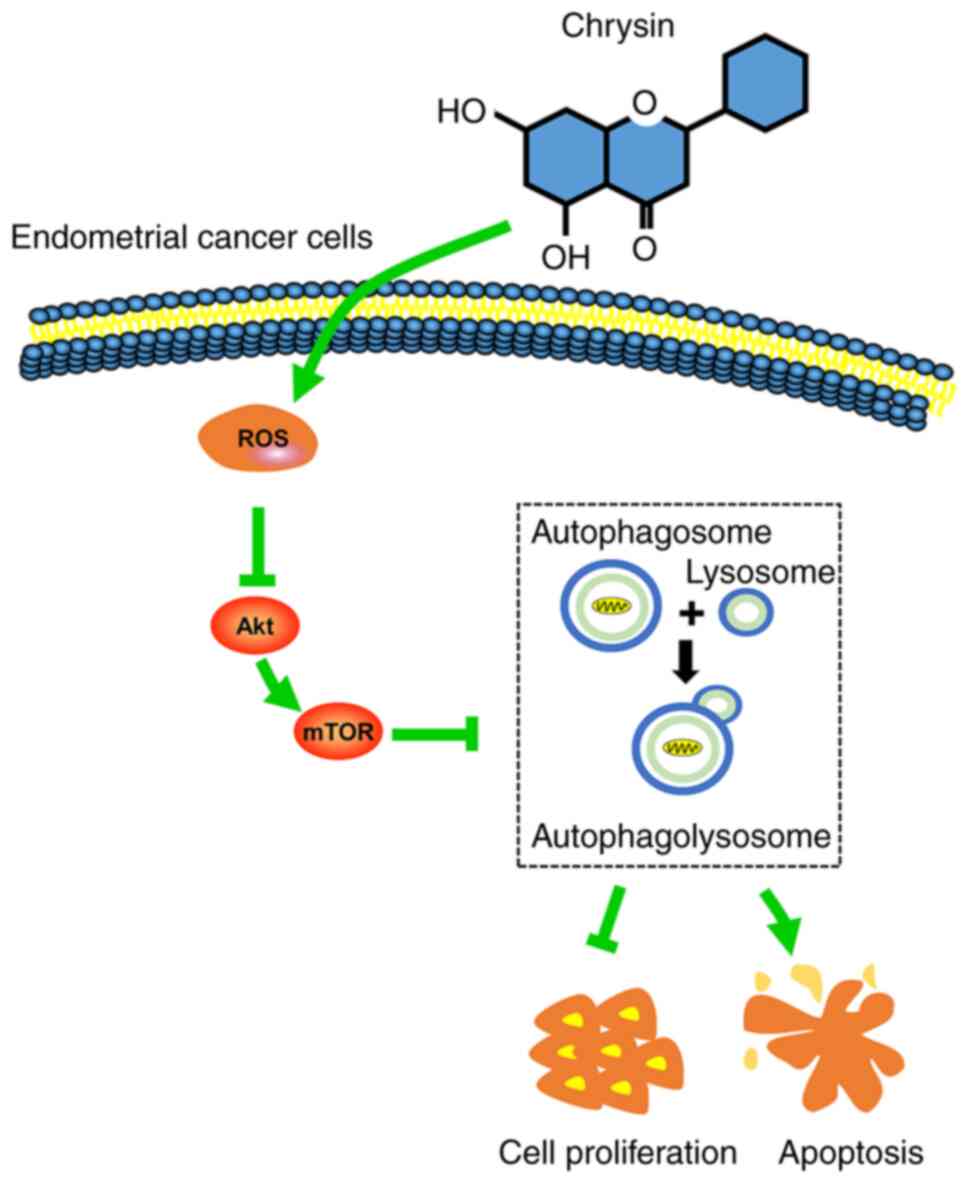
In the present study, it was revealed that chrysin
induced protective autophagy through the ROS/Akt/mTOR signaling
pathway in EC cells. More specifically, chrysin induced autophagy
through the upregulation of the intracellular accumulation of ROS,
and the subsequent downregulation of p-Akt and p-mTOR protein
levels in EC cells. Suppression of autophagy by CQ potentiated
chrysin-induced apoptosis in EC cells.
Targeting autophagy is considered a promising
anti-cancer therapy (28).
According to previous studies, it has been demonstrated that
certain anticancer natural compounds and extracts cab activate
autophagy and exert anticancer effects. Dou et al (29) revealed that ivermectin induced
autophagy in breast cancer cells, while Yao et al (30) demonstrated that crocin inhibited
the growth of hepatocellular carcinoma through activating
autophagy. Current attempts to modulate autophagy in a clinical
setting are focused on using autophagy inhibition combined with
other anti-cancer drugs, which are the focus of >36 clinical
trials (31). In the present
study, it was demonstrated that chrysin induced a protective
autophagy response in EC cells. Additionally, the concurrent
inhibition of autophagy along with chrysin application suppressed
cell proliferation and induced cell apoptosis to a much greater
extent than that observed with chrysin treatment alone. The
knockdown of ATG5 by siRNA reduced the chrysin-induced accumulation
of LC3II in EC cells, demonstrating that chrysin-induced autophagy
was ATG5-dependent. The expression level of LC3II, which is closely
associated with the number of intracellular autophagosomes, is
regarded as an indicator of autophagy (32). CQ, a lysosomal degradation
blocker, can improve the pH-value of lysosomes and then destroy the
function of lysosomes, inhibit fusion of lysosomes and
autophagosomes, thus conversely increasing LC3II expression
(33). Consequently, it was
deduced that the suppression of chrysin-activated autophagy
enhanced the anticancer effect of chrysin in EC cells, and may be a
robust candidate strategy for EC treatment.
The balance between ROS production and ROS
scavenging plays an important in cell homeostasis (34). Notably, ROS may play a dual role
in cancer biology, depending on their concentration. At low
concentrations, ROS act as signaling molecules involved in
promoting cell survival and proliferation, whereas at high
concentrations, they induce programmed cell apoptosis and necrosis
(35,36). Previously published studies noted
that the excessive accumulation of high levels of ROS may trigger
cancer cell apoptosis and death and may inhibit cancer progression
during enhanced oxidative stress injury (37-39). Thus, it was concluded that, in
the process of cancer progression, the analysis of ROS level
fluctuation may be of importance for cancer prevention and
treatment. Furthermore, autophagy is triggered in response to all
types of biological stress, including increased oxidative stress
(40). The fluorescence
microscopy results of the present study revealed that NAC
pretreatment may act as an antioxidant which remarkably inhibits
ROS production and the transition from LC3I to LC3II in treated
cells, thus suggesting that NAC exhibited a considerable blocking
effect on chrysin-induced autophagy. Contrarily, ROS levels were
notably higher when chrysin and CQ were applied simultaneously.
Therefore, it was hypothesized that chrysin-induced autophagy
played a protective role; however, when oxidative stress reached a
degree beyond the control of the protective response of autophagy,
cell death may occur by apoptosis in EC cells. These results were
also consistent with the results of the aforementioned apoptotic
studies. Collectively, these results implied that chrysin-induced
ROS accumulation may act as an autophagy inducer in EC cells
(Fig. 8).
The Akt/mTOR signaling pathway is frequently
activated in human tumors and is one of the main growth regulatory
pathways (41). Akt is a key
upstream regulator of mTOR, and Akt activation may lead to the
phosphorylation of the downstream effector mTOR, thereby negatively
regulating autophagy (42,43). In the present study, chrysin
suppressed Akt/mTOR signaling, indicating that chrysin induced
autophagy by targeting Akt/mTOR signaling. In previously published
studies, it was indicated that cellular oxidative stress is
associated with the Akt/mTOR signaling pathway, and Akt and mTOR
are two kinases regulated by ROS (44,45). The present study revealed that
NAC reversed the decrease of p-Akt and p-mTOR in chrysin-treated
cells, which demonstrated the association between Akt/mTOR signal
suppression and ROS accumulation by chrysin. Chrysin inhibited the
growth of cancer cells by increasing oxidative stress and
subsequently inhibiting the Akt/mTOR signaling pathway. Overall,
the results suggested that chrysin-induced autophagy was triggered
by inactivation of the ROS-mediated Akt/mTOR signaling pathway.
However, there were certain limitations to the
present study. Firstly, only in vitro experiments were
conducted. Therefore, animal experiments should be carried out to
confirm the effectiveness of chrysin in vivo. Furthermore,
our findings were limited to EC and did not verify the effect of
chrysin in tumors other than EC, which should be investigated in
future studies. Subsequently, the present study demonstrated that
chrysin inhibited cell proliferation by CCK-8 and colony formation
assay. It is preferable to detect the expression of cell
cycle-related proteins including cyclins or CDKs in order to
strengthen these conclusions. And finally, it is well known that
ROS are produced from peroxisomes, the endoplasmic reticulum, and
mitochondria, which is their primary source (46). Regrettably, the present study did
not detect the production of ROS in mitochondria.
In conclusion, the present study confirmed for the
first time, at least to the best of our knowledge, that the
ROS-mediated Akt/mTOR signaling pathway played a key role in
chrysin-induced autophagy in EC cells. Chrysin also induced the
apoptosis of EC cells. It was also demonstrated that the inhibition
of autophagy by CQ enhanced the chrysin-induced inhibition of cell
proliferation and promoted chrysin-induced apoptosis, indicating
that chrysin-induced autophagy played a pro-survival role in EC
cells. Thus, combination treatment with an autophagy inhibitor may
be a promising intervention strategy for EC treatment.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The present study was conceived and designed by YH,
YY, LZ and BW. The experiments were conducted by YH, YS, YY, HH, YF
and YW. YH and YS wrote the manuscript with support from BW and YY,
HH, YF, YW LZ and BW critically revised the manuscript for
important intellectual content. All authors agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved. YH and BW confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Urick ME and Bell DW: Clinical
actionability of molecular targets in endometrial cancer. Nat Rev
Cancer. 19:510–521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Troisi J, Raffone A, Travaglino A, Belli
G, Belli C, Anand S, Giugliano L, Cavallo P, Scala G, Symes S, et
al: Development and validation of a serum metabolomic signature for
endometrial cancer screening in postmenopausal women. JAMA Netw
Open. 3:e20183272020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clarke MA, Long BJ, Del Mar Morillo A,
Arbyn M, Bakkum-Gamez JN and Wentzensen N: Association of
endometrial cancer risk with postmenopausal bleeding in women: A
systematic review and meta-analysis. JAMA Intern Med.
178:1210–1222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Korets SB, Czok S, Blank SV, Curtin JP and
Schneider RJ: Targeting the mTOR/4E-BP pathway in endometrial
cancer. Clin Cancer Res. 17:7518–7528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rai R, Essel KG, Benbrook DM, Garland J,
Zhao YD and Chandra V: Preclinical efficacy and involvement of AKT,
mTOR, and ERK kinases in the mechanism of sulforaphane against
endometrial cancer. Cancers (Basel). 12:12732020. View Article : Google Scholar
|
|
6
|
Ma L, Zhang M, Zhao R, Wang D, Ma Y and Li
A: Plant natural products: Promising resources for cancer
chemoprevention. Molecules. 26:9332021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sauter ER: Cancer prevention and treatment
using combination therapy with natural compounds. Expert Rev Clin
Pharmacol. 13:265–285. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song S, Gao K, Niu R, Wang J, Zhang J, Gao
C, Yang B and Liao X: Inclusion complexes between chrysin and
amino-appended β-cyclodextrins (ACDs): Binding behavior, water
solubility, in vitro antioxidant activity and cytotoxicity. Mater
Sci Eng C Mater Biol Appl. 106:1101612020. View Article : Google Scholar
|
|
9
|
Mani R and Natesan V: Chrysin: Sources,
beneficial pharmacological activities, and molecular mechanism of
action. Phytochemistry. 145:187–196. 2018. View Article : Google Scholar
|
|
10
|
Kasala ER, Bodduluru LN, Madana RM, Athira
KV, Gogoi R and Barua CC: Chemopreventive and therapeutic potential
of chrysin in cancer: Mechanistic perspectives. Toxicol Lett.
233:214–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moghadam ER, Ang HL, Asnaf SE, Zabolian A,
Saleki H, Yavari M, Esmaeili H, Zarrabi A, Ashrafizadeh M and Kumar
AP: Broad-spectrum preclinical antitumor activity of chrysin:
Current trends and future perspectives. Biomolecules. 10:13742020.
View Article : Google Scholar :
|
|
12
|
Roy S, Sil A and Chakraborty T:
Potentiating apoptosis and modulation of p53, Bcl2, and Bax by a
novel chrysin ruthenium complex for effective chemotherapeutic
efficacy against breast cancer. J Cell Physiol. 234:4888–4909.
2019. View Article : Google Scholar
|
|
13
|
Brechbuhl HM, Kachadourian R, Min E, Chan
D and Day BJ: Chrysin enhances doxorubicin-induced cytotoxicity in
human lung epithelial cancer cell lines: The role of glutathione.
Toxicol Appl Pharmacol. 258:1–9. 2012. View Article : Google Scholar :
|
|
14
|
Zhang T, Chen X, Qu L, Wu J, Cui R and
Zhao Y: Chrysin and its phosphate ester inhibit cell proliferation
and induce apoptosis in hela cells. Bioorg Med Chem. 12:6097–6105.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lima APB, Almeida TC, Barros TMB, Rocha
LCM, Garcia CCM and da Silva GN: Toxicogenetic and
antiproliferative effects of chrysin in urinary bladder cancer
cells. Mutagenesis. 13:geaa0212020.
|
|
16
|
Koff JL, Ramachandiran S and
Bernal-Mizrachi L: A time to kill: Targeting apoptosis in cancer.
Int J Mol Sci. 16:2942–2955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pang X, Zhang X, Jiang Y, Su Q, Li Q and
Li Z: Autophagy: Mechanisms and therapeutic potential of flavonoids
in cancer. Biomolecules. 11:1352021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crawley O, Opperman KJ, Desbois M, Adrados
I, Borgen MA, Giles AC, Duckett DR and Grill B: Autophagy is
inhibited by ubiquitin ligase activity in the nervous system. Nat
Commun. 10:50172019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ranieri R, Ciaglia E, Amodio G, Picardi P,
Proto MC, Gazzerro P, Laezza C, Remondelli P, Bifulco M and Pisanti
S: N6-isopentenyladenosine dual targeting of AMPK and rab7
prenylation inhibits melanoma growth through the impairment of
autophagic flux. Cell Death Differ. 25:353–367. 2018. View Article : Google Scholar :
|
|
20
|
Lin YM, Chen CI, Hsiang YP, Hsu YC, Cheng
KC, Chien PH, Pan HL, Lu CC and Chen YJ: Chrysin attenuates cell
viability of human colorectal cancer cells through autophagy
induction unlike 5-fluorouracil/oxaliplatin. Int J Mol Sci.
19:17632018. View Article : Google Scholar :
|
|
21
|
Garcia-Martinez T, Vendrell-Flotats M,
Martinez-Rodero I, Ordóñez-León EA, Álvarez-Rodríguez M,
López-Béjar M, Yeste M and Mogas T: Glutathione ethyl ester
protects in vitro-maturing bovine oocytes against oxidative stress
induced by subsequent vitrification/warming. Int J Mol Sci.
21:75472020. View Article : Google Scholar :
|
|
22
|
An Z, Tassa A, Thomas C, Zhong R, Xiao G,
Fotedar R, Tu BP, Klionsky DJ and Levine B: Autophagy is required
for G1/G0 quiescence in response to nitrogen starvation in
Saccharomyces cerevisiae. Autophagy. 10:1702–1711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nunez-Olvera SI, Gallardo-Rincon D,
Puente-Rivera J, Salinas-Vera YM, Marchat LA, Morales-Villegas R
and López-Camarillo C: Autophagy machinery as a promising
therapeutic target in endometrial cancer. Front Oncol. 9:13262019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo F, Zhang H, Jia Z, Cui M and Tian J:
Chemoresistance and targeting of growth factors/cytokines
signalling pathways: Towards the development of effective
therapeutic strategy for endometrial cancer. Am J Cancer Res.
8:1317–1331. 2018.PubMed/NCBI
|
|
25
|
Sharom FJ: ABC multidrug transporters:
Structure, function and role in chemoresistance. Pharmacogenomics.
9:105–127. 2008. View Article : Google Scholar
|
|
26
|
Crommentuijn MH, Maguire CA, Niers JM,
Vandertop WP, Badr CE, Würdinger T and Tannous BA: Intracranial
AAV-sTRAIL combined with lanatoside C prolongs survival in an
orthotopic xenograft mouse model of invasive glioblastoma. Mol
Oncol. 10:625–634. 2016. View Article : Google Scholar
|
|
27
|
Bronikowska J, Szliszka E,
Kostrzewa-Susłow E, Jaworska D, Czuba ZP, Bednarski P and Król W:
Novel structurally related flavones augment cell death induced by
rhsTRAIL. Int J Mol Sci. 18:12112017. View Article : Google Scholar :
|
|
28
|
Rybstein MD, Bravo-San Pedro JM, Kroemer G
and Galluzzi L: The autophagic network and cancer. Nat Cell Biol.
20:243–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dou QH, Chen HN, Wang K, Yuan K, Lei Y, Li
K, Lan J, Chen Y, Huang Z, Xie N, et al: Ivermectin induces
cytostatic autophagy by blocking the PAK1/akt axis in breast
cancer. Cancer Res. 76:4457–4469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao C, Liu BB, Qian XD, Li LQ, Cao HB, Guo
QS and Zhou GF: Crocin induces autophagic apoptosis in
hepatocellular carcinoma by inhibiting Akt/mTOR activity. Onco
Targets Ther. 11:2017–2028. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amaravadi RK, Lippincott-Schwartz J, Yin
XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT and White E:
Principles and current strategies for targeting autophagy for
cancer treatment. Clin Cancer Res. 17:654–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohnishi K, Yano S, Fujimoto M, Sakai M,
Harumoto E, Furuichi A, Masuda M, Ohminami H, Yamanaka-Okumura H,
Hara T and Taketani Y: Identification of dietary phytochemicals
capable of enhancing the autophagy flux in HeLa and Caco-2 human
cell lines. Antioxidants (Basel). 9:11932020. View Article : Google Scholar
|
|
33
|
Lu Y, Xiao L, Liu Y, Wang H, Li H, Zhou Q,
Pan J, Lei B, Huang A and Qi S: MIR517C inhibits autophagy and the
epithelial-to-mesenchymal (-like) transition phenotype in human
glioblastoma through KPNA2-dependent disruption of TP53 nuclear
translocation. Autophagy. 11:2213–2232. 2016. View Article : Google Scholar :
|
|
34
|
Kim K, Dayem AA, Gil M, Yang GM, Lee SB,
Kwon OH, Choi S, Kang GH, Lim KM, Kim D and Cho SG:
3,2′-Dihydroxyflavone improves the proliferation and survival of
human pluripotent stem cells and their differentiation into
hematopoietic progenitor cells. J Clin Med. 9:6692020. View Article : Google Scholar
|
|
35
|
Lewinska A, Adamczyk-Grochala J,
Deregowska A and Wnuk M: Sulforaphane-induced cell cycle arrest and
senescence are accompanied by DNA hypomethylation and changes in
microRNA profile in breast cancer cells. Theranostics. 7:3461–3477.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ye S, Zhao T, Zhang W, Tang Z, Gao C, Ma
Z, Xiong JW, Peng J, Tan WQ and Chen J: p53 isoform Δ113p53
promotes zebrafish heart regeneration by maintaining redox
homeostasis. Cell Death Dis. 11:5682020. View Article : Google Scholar
|
|
37
|
Deng L, Gao X, Liu B, He X, Xu J, Qiang J,
Wu Q and Liu S: NMT1 inhibition modulates breast cancer progression
through stress-triggered JNK pathway. Cell Death Dis. 9:11432018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang CC, Tsai MH, Li KY, Hou CH and Lin
FH: Carbon-doped TiO2 activated by X-ray irradiation for
the generation of reactive oxygen species to enhance photodynamic
therapy in tumor treatment. Int J Mol Sci. 20:20722019. View Article : Google Scholar
|
|
39
|
Lim W, Ryu S, Bazer FW, Kim SM and Song G:
Chrysin attenuates progression of ovarian cancer cells by
regulating signaling cascades and mitochondrial dysfunction. J Cell
Physiol. 233:3129–3140. 2018. View Article : Google Scholar
|
|
40
|
Ru JY and Wang YF: Osteocyte apoptosis:
The roles and key molecular mechanisms in resorption-related bone
diseases. Cell Death Dis. 11:8462020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guglielmelli P, Barosi G, Rambaldi A,
Marchioli R, Masciulli A, Tozzi L, Biamonte F, Bartalucci N,
Gattoni E, Lupo ML, et al: Safety and efficacy of everolimus, a
mTOR inhibitor, as single agent in a phase 1/2 study in patients
with myelofibrosis. Blood. 118:2069–2076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou Y, Chen X, Qu N, Zhang B and Xia C:
Chondroprotection of PPARα activation by WY14643 via autophagy
involving akt and ERK in LPS-treated mouse chondrocytes and
osteoarthritis model. J Cell Mol Med. 23:2782–2793. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang YX, Yang L, Wang HQ, Zhao XQ, Liu T,
Li YH, Zeng QX, Li YH and Song DQ: Synthesis and evolution of
berberine derivatives as a new class of antiviral agents against
enterovirus 71 through the MEK/ERK pathway and autophagy.
Molecules. 23:20842018. View Article : Google Scholar :
|
|
44
|
Ives A, Nomura J, Martinon F, Roger T,
LeRoy D, Miner JN, Simon G, Busso N and So A: Xanthine
oxidoreductase regulates macrophage IL1β secretion upon NLRP3
inflammasome activation. Nat Commun. 6:65552015. View Article : Google Scholar
|
|
45
|
Su Z, Burchfield JG, Yang P, Humphrey SJ,
Yang G, Francis D, Yasmin S, Shin SY, Norris DM, Kearney AL, et al:
Global redox proteome and phosphoproteome analysis reveals redox
switch in akt. Nat Commun. 10:54862019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang CR, Chang TW, Lee CT, Shen CJ, Chang
WC and Chen BK: ARNT deficiency represses pyruvate dehydrogenase
kinase 1 to trigger ROS production and melanoma metastasis.
Oncogenesis. 10:112021. View Article : Google Scholar : PubMed/NCBI
|