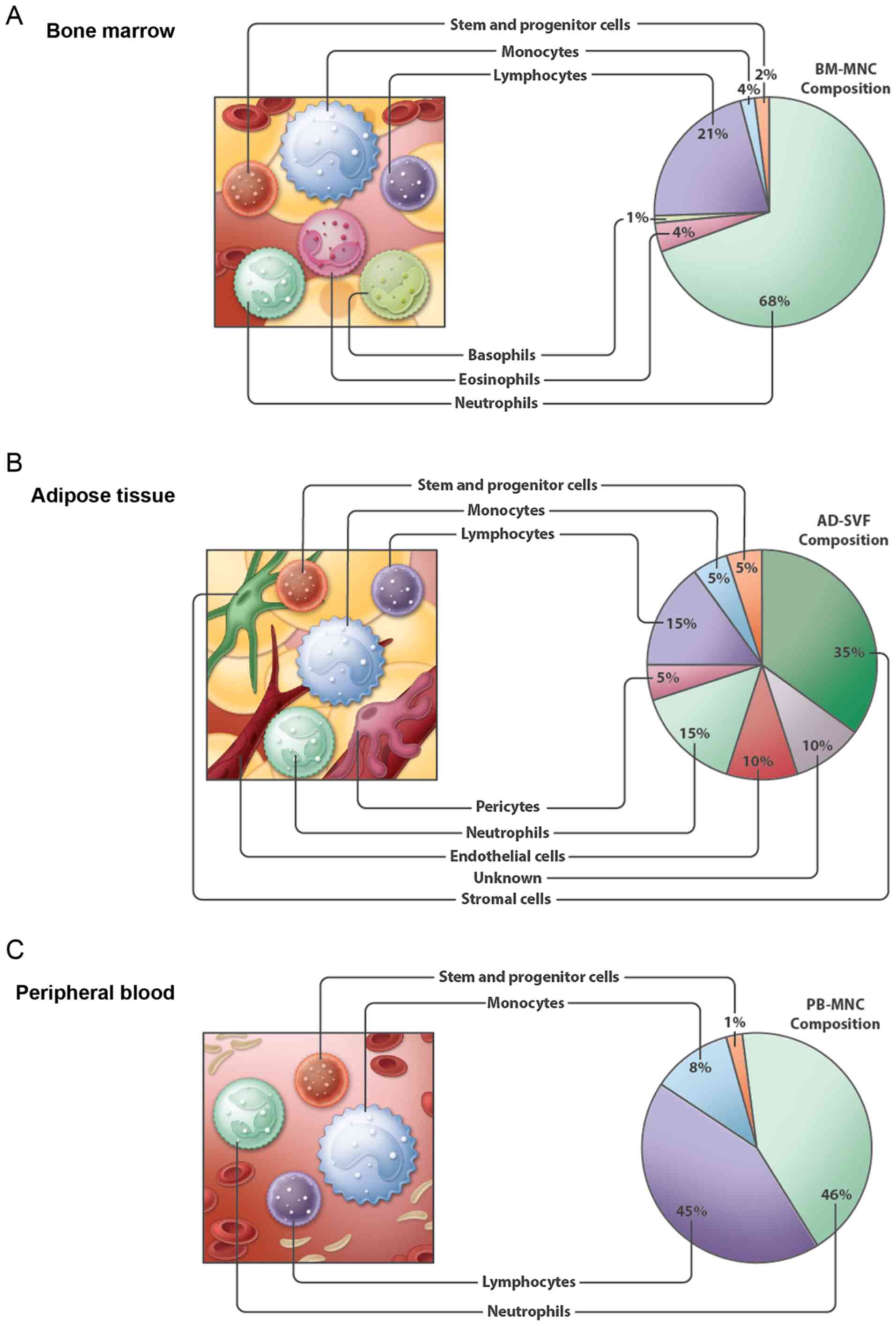
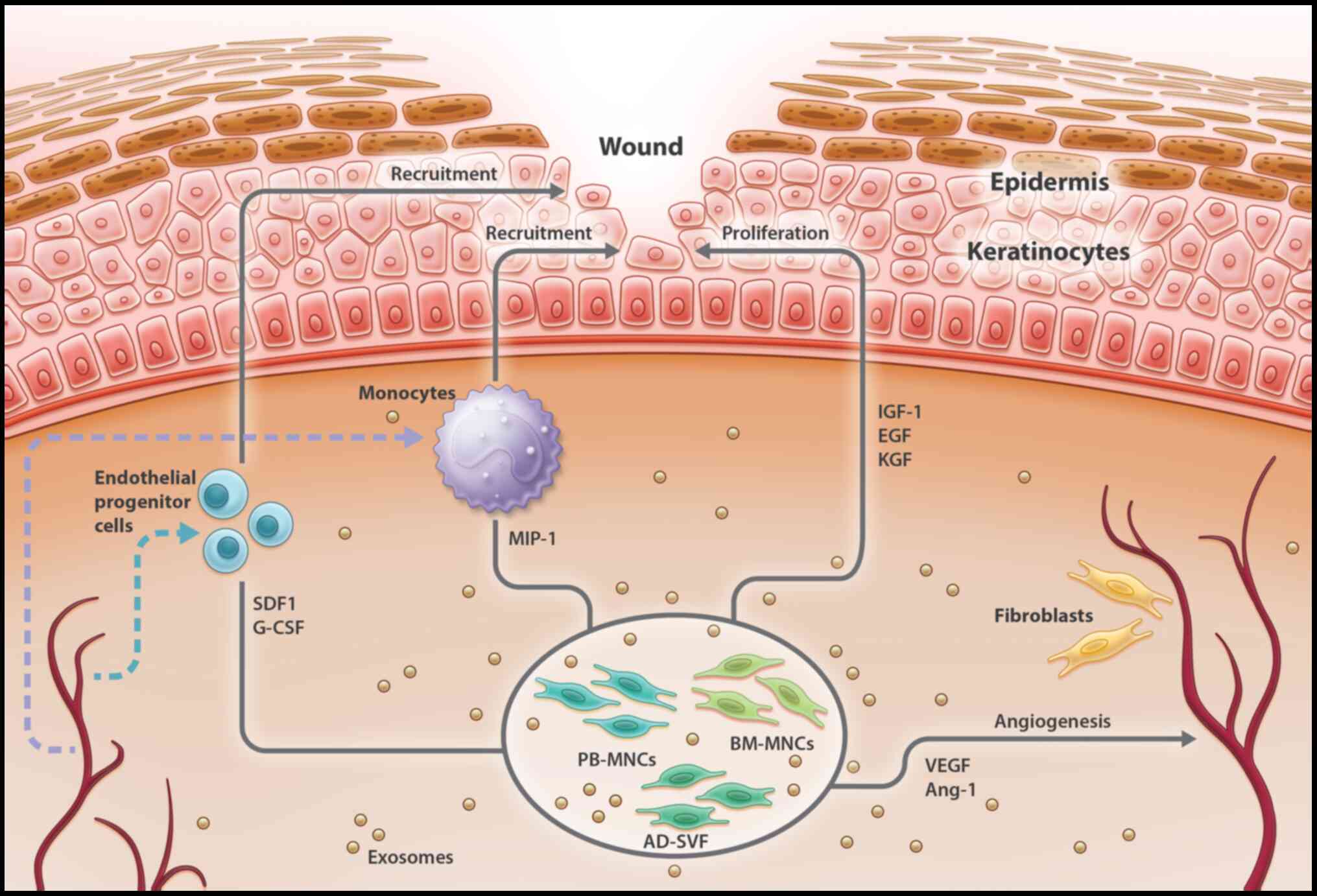
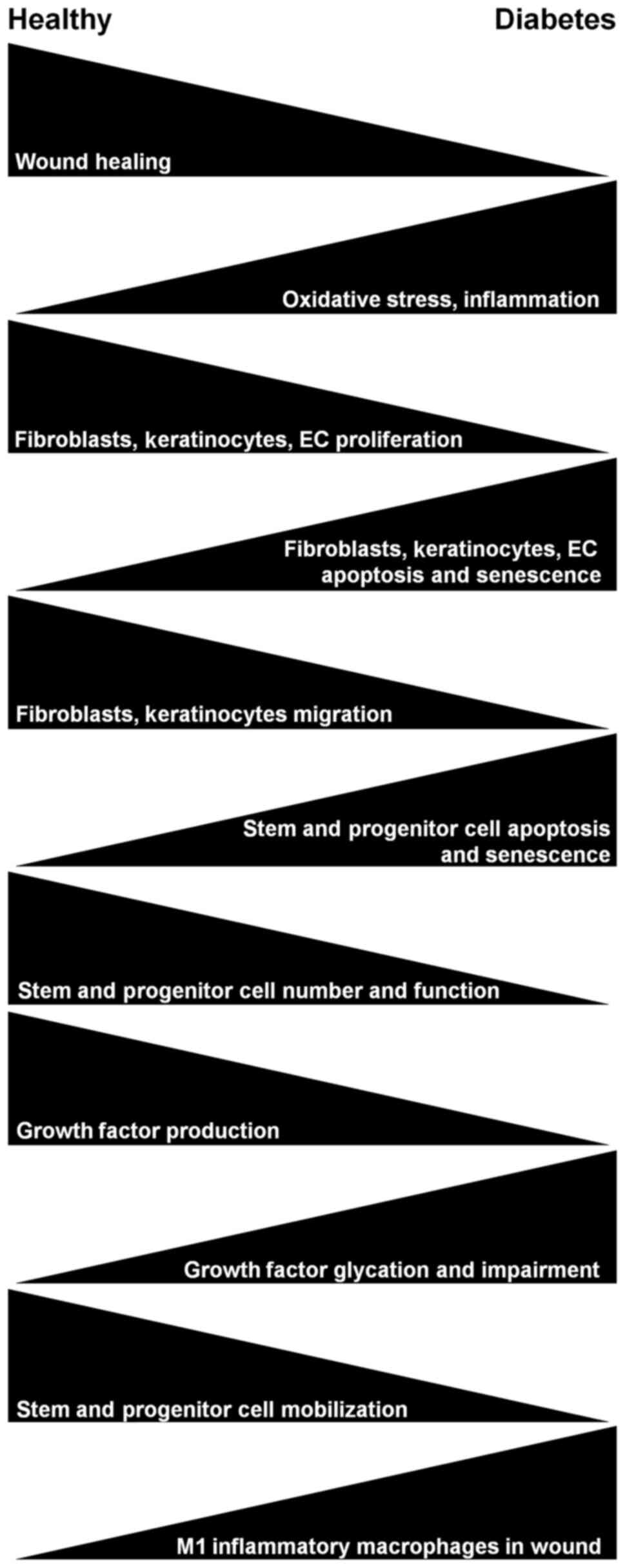
|
1
|
Fowkes FGR, Rudan D, Rudan I, Aboyans V,
Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ,
Mensah GA and Criqui MH: Comparison of global estimates of
prevalence and risk factors for peripheral artery disease in 2000
and 2010: A systematic review and analysis. Lancet. 382:1329–1340.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cooke JP and Losordo DW: Modulating the
vascular response to limb ischemia: Angiogenic and cell therapies.
Circ Res. 116:1561–1578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Criqui MH and Aboyans V: Epidemiology of
peripheral artery disease. Circ Res. 116:1509–1526. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teraa M, Conte MS, Moll FL and Verhaar MC:
Critical limb ischemia: Current trends and future directions. J Am
Heart Assoc. 5:e0029382016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reinecke H, Unrath M, Freisinger E,
Bunzemeier H, Meyborg M, Lüders F, Gebauer K, Roeder N, Berger K
and Malyar NM: Peripheral arterial disease and critical limb
ischaemia: Still poor outcomes and lack of guideline adherence. Eur
Heart J. 36:932–938. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freisinger E, Malyar NM, Reinecke H and
Lawall H: Impact of diabetes on outcome in critical limb ischemia
with tissue loss: A large-scaled routine data analysis. Cardiovasc
Diabetol. 16:412017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan J, Tie G, Wang S, Tutto A, DeMarco N,
Khair L, Fazzio TG and Messina LM: Diabetes impairs wound healing
by Dnmt1-dependent dysregulation of hematopoietic stem cells
differentiation towards macrophages. Nat Commun. 9:332018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rigato M, Monami M and Fadini GP:
Autologous cell therapy for peripheral arterial disease: Systematic
review and meta-analysis of randomized, nonrandomized, and
noncontrolled studies. Circ Res. 120:1326–1340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qadura M, Terenzi DC, Verma S, Al-Omran M
and Hess DA: Concise review: Cell therapy for critical limb
ischemia: An integrated review of preclinical and clinical studies.
Stem Cells. 36:161–171. 2018. View Article : Google Scholar
|
|
10
|
Dubský M, Jirkovská A, Bem R, Nemcová A,
Fejfarová V and Jude EB: Cell therapy of critical limb ischemia in
diabetic patients-State of art. Diabetes Res Clin Pract.
126:263–271. 2017. View Article : Google Scholar
|
|
11
|
Dubský M, Jirkovská A, Bem R, Fejfarova V,
Pagacova L, Sixta B, Varga M, Langkramer S, Sykova E and Jude EB:
Both autologous bone marrow mononuclear cell and peripheral blood
progenitor cell therapies similarly improve ischaemia in patients
with diabetic foot in comparison with control treatment. Diabetes
Res Clin Pract. 29:369–376. 2013.
|
|
12
|
Weck M, Slesaczeck T, Rietzsch H, Münch D,
Nanning T, Paetzold H, Florek HJ, Barthel A, Weiss N and Bornstein
S: Noninvasive management of the diabetic foot with critical limb
ischemia: Current options and future perspectives. Ther Adv
Endocrinol Metab. 2:247–255. 2011. View Article : Google Scholar
|
|
13
|
Parikh PP, Liu ZJ and Velazquez OC: A
molecular and clinical review of stem cell therapy in critical limb
ischemia. Stem Cell Int. 2017:37508292017.
|
|
14
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murray IR, Chahla J, Safran MR, Krych AJ,
Saris DBF, Caplan AI, LaPrade RF, Cell Therapies Communication and
Expert Group: International expert consensus on a cell therapy
communication tool: DOSES. J Bone Joint Surg Am. 101:904–911. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Silvestre JS, Smadja DM and Levy BI:
Postischemic revascularization: From cellular and molecular
mechanisms to clinical applications. Physiol Rev. 93:1743–1802.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verfaillie C, Blakolmer K and McGlave P:
Purified primitive human hematopoietic progenitor cells with
long-term in vitro repopulating capacity adhere selectively to
irradiated bone marrow stroma. J Exp Med. 172:509–602. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Finney MR, Greco NJ, Haynesworth SE,
Martin JM, Hedrick DP, Swan JZ, Winter DG, Kadereit S, Joseph ME,
Fu P, et al: Direct comparison of umbilical cord blood versus bone
marrow-derived endothelial precursor cells in mediating
neovascularization in response to vascular ischemia. Biol Blood
Marrow Transplant. 12:585–593. 2005. View Article : Google Scholar
|
|
20
|
Xiang Y, Zheng Q, Jia BB, Huang GP, Xu YL,
Wang JF and Pan ZJ: Ex vivo expansion and pluripotential
differentiation of cryopreserved human bone marrow mesenchymal stem
cells. J Zhejiang Univ Sci B. 8:136–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veronesi F, Giavaresi G, Tschon M, Borsari
V, Nicoli Aldini N and Fini M: Clinical use of bone marrow, bone
marrow concentrate, and expanded bone marrow mesenchymal stem cells
in cartilage disease. Stem Cells Dev. 22:181–192. 2013. View Article : Google Scholar
|
|
22
|
D'souza N, Rossignoli F, Golinelli G,
Grisendi G, Spano C, Candini O, Osturu S, Catani F, Paolucci P,
Horwitz EM and Dominici M: Mesenchymal stem/stromal cells as a
delivery platform in cell and gene therapies. BMC Med. 13:1862015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang X, Ding Y, Zhang Y, Tse HF and Lian
Q: Paracrine mechanisms of mesenchymal stem cell-based therapy:
Current status and perspectives. Cell Transplant. 23:1045–1059.
2014. View Article : Google Scholar
|
|
24
|
Spees JL, Lee RH and Gregory CA:
Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res
Ther. 7:1252016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liew A and O'Brien T: Therapeutic
potential for mesenchymal stem cell transplantation in critical
limb ischemia. Stem Cell Res Ther. 3:282012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Najar M, Krayem M, Merimi M, Burny A,
Meuleman N, Bron D, Raicevic G and Lagneaux L: Insights into
inflammatory priming of mesenchymal stromal cells: Functional
biological impacts. Inflamm Res. 67:467–477. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naji A, Suganuma N, Espagnolle N, Yagyu
KI, Baba N, Sensebé L and Deschaseaux F: Rationale for determining
the functional potency of mesenchymal stem cells in preventing
regulated cell death for therapeutic use. Stem Cells Transl Med.
6:713–719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma S, Xie N, Li W, Yuan B, Shi Y and Wang
Y: Immunobiology of mesenchymal stem cells. Cell Death Differ.
21:216–225. 2014. View Article : Google Scholar :
|
|
29
|
Kusuma GD, Carthew J, Lim R and Frith JE:
Effect of the micro-environment on mesenchymal stem cell paracrine
signaling: Opportunities to engineer the therapeutic effect. Stem
Cells Dev. 26:617–631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim N and Cho SG: Overcoming
immunoregulatory plasticity of mesenchymal stem cells for
accelerated clinical applications. Int J Hematol. 103:129–137.
2016. View Article : Google Scholar
|
|
31
|
Hoffmann J, Glassford AJ, Doyle TC,
Robbins RC, Schrepfer S and Pelletier MP: Angiogenic effects
despite limited cell survival of bone marrow-derived mesenchymal
stem cells under ischemia. Thorac Cardiovasc Surg. 58:136–142.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kinnaird T, Stabile E, Burnett MS, Shou M,
Lee CW, Barr S, Fuchs S and Epstein SE: Local delivery of
marrow-derived stromal cells augments collateral perfusion through
paracrine mechanisms. Circulation. 109:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ben-Mordechai T, Holbova R, Landa-Rouben
N, Harel-Adar T, Feinberg MS, Abd Elrahman I, Blum G, Epstein FH,
Silman Z, Cohen S and Leor J: Macrophage subpopulations are
essential for infarct repair with and without stem cell therapy. J
Am Coll Cardiol. 62:1890–1901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pinto AR, Godwin JW and Rosenthal NA:
Macrophages in cardiac homeostasis, injury responses and progenitor
cell mobilisation. Stem Cell Res. 13:705–714. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Forbes SJ and Rosenthal N: Preparing the
ground for tissue regeneration: From mechanism to therapy. Nat Med.
20:857–869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Julier Z, Park AJ, Briquez PS and Martino
MM: Promoting tissue regeneration by modulating the immune system.
Acta Biomater. 53:13–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Naik S, Larsen SB, Cowley CJ and Fuchs E:
Two to tango: Dialog between immunity and stem cells in health and
disease. Cell. 175:908–920. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Anton K, Banerjee D and Glod J:
Macrophage-associated mesenchymal stem cells assume an activated,
migratory, pro-inflammatory phenotype with increased IL-6 and
CXCL10 secretion. PLoS One. 7:e350362012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Faulknor RA, Olekson MA, Ekwueme EC,
Krzyszczyk P, Freeman JW and Berthiaume F: Hypoxia impairs
mesenchymal stromal cell-induced macrophage M1 to M2 transition.
Technology (Singap World Sci). 5:81–86. 2017.
|
|
40
|
Kajiguchi M, Kondo T, Izawa H, Kobayashi
M, Yamamoto K, Shintani S, Numaguchi Y, Naoe T, Takamatsu J, Komori
K and Murohara T: Safety and efficacy of autologous progenitor cell
transplantation for therapeutic angiogenesis in patients with
critical limb ischemia. Circ J. 71:196–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matoba S, Tatsumi T, Murohara T, Imaizumi
T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, et
al: Long-term clinical outcome after intramuscular implantation of
bone marrow mononuclear cells [therapeutic angiogenesis by cell
transplantation (TACT) trial] in patients with chronic limb
ischemia. Am Heart J. 156:1010–1018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tateishi-Yuyama E, Matsubara H, Murohara
T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto
K, Akashi H, et al: Therapeutic angiogenesis for patients with limb
ischaemia by autologous transplantation of bone-marrow cells: A
pilot study and a randomised controlled trial. Lancet. 360:427–435.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Di Trapani M, Bassi G, Midolo M, Gatti A,
Kamga PT, Cassaro A, Carusone R, Adamo A and Krampera M:
Differential and transferable modulatory effects of mesenchymal
stromal cell-derived extracellular vesicles on T, B and NK cell
functions. Sci Rep. 6:241202016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kamihata H, Matsubara H, Nishiue T,
Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi
E, et al: Implantation of bone marrow mononuclear cells into
ischemic myocardium enhances collateral perfusion and regional
function via side supply of angioblasts, angiogenic ligands, and
cytokines. Circulation. 104:1046–1052. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qin SL, Li TS, Takahashi M and Hamano K:
In vitro assessment of the effect of interleukin-1beta on
angiogenic potential of bone marrow cells. Circ J. 70:1195–1199.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu Y, Zhao RC and Tredget EE: Concise
review: Bone marrow-derived stem/progenitor cells in cutaneous
repair and regeneration. Stem Cell. 28:905–915. 2010.
|
|
47
|
Okuno Y, Nakamura-Ishizu A, Kishi K, Suda
T and Kubota Y: Bone marrow-derived cells serve as proangiogenic
macrophages but not endothelial cells in wound healing. Blood.
117:5264–5272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang ZX, Li D, Cao JX, Liu YS, Wang M,
Zhang XY, Li JL, Wang HB, Liu JL and Xu BL: Efficacy of autologous
bone marrow mononuclear cell therapy in patients with peripheral
arterial disease. J Atheroscler Thromb. 21:1183–1196. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Powell RJ, Comerota AJ, Berceli SA, Guzman
R, Henry TD, Tzeng E, Velazquez O, Marston WA, Bartel RL, Longcore
A, et al: Interim analysis results from the RESTORE-CLI, a
randomized, double-blind multicenter phase II trial comparing
expanded autologous bone marrow-derived tissue repair cells and
placebo in patients with critical limb ischemia. J Vasc Surg.
54:1032–1041. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bourin P, Bunnell BA, Casteilla L,
Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K and
Gimble JM: Stromal cells from the adipose tissue-derived stromal
vascular fraction and culture expanded adipose tissue-derived
stromal/stem cells: A joint statement of the International
Federation for Adipose Therapeutics and Science (IFATS) and the
international society for cellular therapy (ISCT). Cytotherapy.
15:641–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guillaume-Jugnot P, Daumas A, Magalon J,
Sautereau N, Veran J, Magalon G, Sabatier F and Granel B: State of
the art. Autologous fat graft and adipose tissue-derived stromal
vascular fraction injection for hand therapy in systemic sclerosis
patients. Curr Res Transl Med. 64:35–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ong WK and Sugii S: Adipose-derived stem
cells: Fatty potentials for therapy. Int J Biochem Cell Biol.
45:1083–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guo J, Nguyen A, Banyard DA, Fadavi D,
Toranto JD, Wirth GA, Paydar KZ, Evans GR and Widgerow AD: Stromal
vascular fraction: A regenerative reality? Part 2: Mechanisms of
regenerative action. J Plast Reconstr Aesthetic Surg. 69:180–188.
2016. View Article : Google Scholar
|
|
55
|
Parker AM and Katz AJ: Adipose-derived
stem cells for the regeneration of damaged tissues. Expert Opin
Biol Ther. 6:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Park YB, Ha CW, Rhim JH and Lee HJ: Stem
cell therapy for articular cartilage repair: Review of the entity
of cell populations used and the result of the clinical application
of each entity. Am J Sports Med. 46:2540–2552. 2018. View Article : Google Scholar
|
|
57
|
Strong AL, Cederna PS, Rubin JP, Coleman
SR and Levi B: The current state of fat grafting: A review of
harvesting, processing, and injection techniques. Plast Reconstr
Surg. 136:897–912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bianchi F, Maioli M, Leonardi E, Olivi E,
Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M,
Tremolada C and Ventura C: A new nonenzymatic method and device to
obtain a fat tissue derivative highly enriched in pericyte-like
elements by mild mechanical forces from human lipoaspirates. Cell
Transplant. 22:2063–2077. 2013. View Article : Google Scholar
|
|
59
|
Harada Y, Yamamoto Y, Tsujimoto S,
Matsugami H, Yoshida A and Hisatome I: Transplantation of freshly
isolated adipose tissue-derived regenerative cells enhances
angiogenesis in a murine model of hind limb ischemia. Biomed Res.
34:23–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rehman J, Traktuev D, Li J, Merfeld-Clauss
S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV
and March KL: Secretion of angiogenic and antiapoptotic factors by
human adipose stromal cells. Circulation. 109:1292–1298. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim Y, Kim H, Cho H, Bae Y, Suh K and Jung
J: Direct comparison of human mesenchymal stem cells derived from
adipose tissues and bone marrow in mediating neovascularization in
response to vascular ischemia. Cell Physiol Biochem. 20:867–876.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Navarro A, Marín S, Riol N,
Carbonell-Uberos F and Miñana MD: Human adipose tissue-resident
monocytes exhibit an endothelial-like phenotype and display
angiogenic properties. Stem Cell Res Ther. 5:502014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cai J, Feng J, Liu K, Zhou S and Lu F:
Early macrophage infiltration improves fat graft survival by
inducing angiogenesis and hematopoietic stem cell recruitment.
Plast Reconstr Surg. 141:376–386. 2018. View Article : Google Scholar
|
|
64
|
Dong Z, Peng Z, Chang Q and Lu F: The
survival condition and immunoregulatory function of adipose stromal
vascular fraction (SVF) in the early stage of nonvascularized
adipose transplantation. PLoS One. 8:e803642013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Spaltro G, Straino S, Gambini E, Bassetti
B, Persico L, Zoli S, Zanobini M, Capogrossi MC, Spirito R, Quarti
C and Pompilio G: Characterization of the pall celeris system as a
point-of-care device for therapeutic angiogenesis. Cytotherapy.
17:1302–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rennert RC, Sorkin M, Januszyk M, Duscher
D, Kosaraju R, Chung MT, Lennon J, Radiya-Dixit A, Raghvendra S,
Maan ZN, et al: Diabetes impairs the angiogenic potential of
adipose-derived stem cells by selectively depleting cellular
subpopulations. Stem Cell Res Ther. 5:792014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li J, Tan J, Martino MM and Lui KO:
Regulatory T-cells: Potential regulator of tissue repair and
regeneration. Front Immunol. 9:5852018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Aurora AB and Olson EN: Immune modulation
of stem cells and regeneration. Cell Stem Cell. 15:14–25. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Spiller KL and Koh TJ: Macrophage-based
therapeutic strategies in regenerative medicine. Adv Drug Deliv
Rev. 122:74–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang J, Yang Y, Yang Z, Li T and Chen F:
Snapshot: Targeting macrophages as a candidate for tissue
regeneration. Curr Issues Mol Biol. 29:37–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tauber AI: Metchnikoff and the
phagocytosis theory. Nat Rev Mol Cell Biol. 4:897–901. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Huang PP, Yang XF, Li SZ, Wen JC, Zhang Y
and Han ZC: Randomised comparison of G-CSF-mobilized peripheral
blood mononuclear cells versus bone marrow-mononuclear cells for
the treatment of patients with lower limb arteriosclerosis
obliterans. Thromb Haemost. 98:1335–1342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gurevich DB, Severn CE, Twomey C,
Greenhough A, Cash J, Toye AM, Mellor H and Martin P: Live imaging
of wound angiogenesis reveals macrophage orchestrated vessel
sprouting and regression. EMBO J. 37:e977862018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Moriya J, Minamino T, Tateno K, Shimizu N,
Kuwabara Y, Sato Y, Saito Y and Komuro I: Long-term outcome of
therapeutic neovascularization using peripheral blood mononuclear
cells for limb ischemia. Circ Cardiovasc Interv. 2:245–254. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Krishnasamy K, Limbourg A, Kapanadze T,
Gamrekelashvili J, Beger C, Häger C, Lozanovski VJ, Falk CS, Napp
LC, Bauersachs J, et al: Blood vessel control of macrophage
maturation promotes arteriogenesis in ischemia. Nat Commun.
8:9522017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Beer L, Zimmermann M, Mitterbauer A,
Ellinger A, Gruber F, Narzt MS, Zellner M, Gyöngyösi M, Madlener S,
Simader E, et al: Analysis of the secretome of apoptotic peripheral
blood mononuclear cells: Impact of released proteins and exosomes
for tissue regeneration. Sci Rep. 5:166622015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Blomgran P, Blomgran R, Ernerudh J and
Aspenberg P: A possible link between loading, inflammation and
healing: Immune cell populations during tendon healing in the rat.
Sci Rep. 6:298242016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kirana S, Stratmann B, Prante C, Prohaska
W, Koerperich H, Lammers D, Gastens MH, Quast T, Negrean M, Stirban
OA, et al: Autologous stem cell therapy in the treatment of limb
ischaemia induced chronic tissue ulcers of diabetic foot patients.
Int J Clin Pract. 66:384–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tateno K, Minamino T, Toko H, Akazawa H,
Shimizu N, Takeda S, Kunieda T, Miyauchi H, Oyama T, Matsuura K, et
al: Critical roles of muscle-secreted angiogenic factors in
therapeutic neovascularization. Circ Res. 98:1194–1202. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
De Angelis B, Gentile P, Orlandi F,
Bocchini I, Di Pasquali C, Agovino A, Gizzi C, Patrizi F, Scioli
MG, Orlandi A and Cervelli V: Limb rescue: A new
autologous-peripheral blood mononuclear cells technology in
critical limb ischemia and chronic ulcers. Tissue Eng Part C
Methods. 21:423–435. 2015. View Article : Google Scholar
|
|
82
|
Persiani F, Paolini A, Camilli D,
Mascellari L, Platone A, Magenta A and Furgiuele S: Peripheral
blood mononuclear cells therapy for treatment of lower limb
ischemia in diabetic patients: A single-center experience. Ann Vasc
Surg. 53:190–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mutirangura P, Ruangsetakit C, Wongwanit
C, Chinsakchai K, Porat Y, Belleli A and Czeiger D: Enhancing limb
salvage by non-mobilized peripheral blood angiogenic cell
precursors therapy in patients with critical limb ischemia. J Med
Assoc Thail. 92:320–327. 2009.
|
|
84
|
Liotta F, Annunziato F, Castellani S,
Boddi M, Alterini B, Castellini G, Mazzanti B, Cosmi L, Acquafresca
M, Bartalesi F, et al: Therapeutic efficacy of autologous
non-mobilized enriched circulating endothelial progenitors in
patients with critical limb ischemia-the SCELTA trial. Circ J.
82:1688–1698. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Arras M, Ito WD, Scholz D, Winkler B,
Schaper J and Schaper W: Monocyte activation in angiogenesis and
collateral growth in the rabbit hindlimb. J Clin Invest. 101:40–50.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Anghelina M, Krishnan P, Moldovan L and
Moldovan NI: Monocytes/macrophages cooperate with progenitor cells
during neovascularization and tissue repair: Conversion of cell
columns into fibrovascular bundles. Am J Pathol. 168:529–541. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Capoccia BJ, Gregory AD and Link DC:
Recruitment of the inflammatory subset of monocytes to sites of
ischemia induces angiogenesis in a monocyte chemoattractant
protein-1-dependent fashion. J Leukoc Biol. 84:760–768. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Schaper J, König R, Franz D and Schaper W:
The endothelial surface of growing coronary collateral arteries.
Intimal margination and diapedesis of monocytes. A combined SEM and
TEM study. Virchows Arch A Pathol Anat Histol. 370:193–205. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Scholz D, Ito W, Fleming I, Deindl E,
Sauer A, Wiesnet M, Busse R, Schaper J and Schaper W:
Ultrastructure and molecular histology of rabbit hind-limb
collateral artery growth (arteriogenesis). Virchows Arch.
436:257–270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bergmann CE, Hoefer IE, Meder B, Roth H,
van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S and
Buschmann IR: Arteriogenesis depends on circulating monocytes and
macrophage accumulation and is severely depressed in op/op mice. J
Leukoc Biol. 80:59–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ito WD, Arras M, Winkler B, Scholz D,
Schaper J and Schaper W: Monocyte chemotactic protein-1 increases
collateral and peripheral conductance after femoral artery
occlusion. Circ Res. 80:829–837. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fantin A, Vieira JM, Gestri G, Denti L,
Schwarz Q, Prykhozhij S, Peri F, Wilson SW and Ruhrberg C: Tissue
macrophages act as cellular chaperones for vascular anastomosis
downstream of VEGF-mediated endothelial tip cell induction. Blood.
116:829–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Barnett FH, Rosenfeld M, Wood M, Kiosses
WB, Usui Y, Marchetti V, Aguilar E and Friedlander M: Macrophages
form functional vascular mimicry channels in vivo. Sci Rep.
6:366592016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu C, Wu C, Yang Q, Gao J, Li L, Yang D
and Luo L: Macrophages mediate the repair of brain vascular rupture
through direct physical adhesion and mechanical traction. Immunity.
44:1162–1176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Baer C, Squadrito ML, Iruela-Arispe ML and
De Palma M: Reciprocal interactions between endothelial cells and
macrophages in angiogenic vascular niches. Exp Cell Res.
319:1626–1634. 2013. View Article : Google Scholar
|
|
96
|
Minutti CM, Knipper JA, Allen JE and Zaiss
DMW: Tissue-specific contribution of macrophages to wound healing.
Semin Cell Dev Biol. 61:3–11. 2017. View Article : Google Scholar
|
|
97
|
Ding J, Lei L, Liu S, Zhang Y, Yu Z, Su Y
and Ma X: Macrophages are necessary for skin regeneration during
tissue expansion. J Transl Med. 17:362019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Krzyszczyk P, Schloss R, Palmer A and
Berthiaume F: The role of macrophages in acute and chronic wound
healing and interventions to promote pro-wound healing phenotypes.
Front Physiol. 9:4192018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kimball A, Schaller M, Joshi A, Davis FM,
den Dekker A, Boniakowski A, Bermick J, Obi A, Moore B, Henke PK,
et al: Ly6C Hi blood monocyte/macrophage drive chronic inflammation
and impair wound healing in diabetes mellitus. Arterioscler Thromb
Vasc Biol. 38:1102–1114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Boniakowski AE, Kimball AS, Jacobs BN,
Kunkel SL and Gallagher KA: Macrophage-mediated inflammation in
normal and diabetic wound healing. J Immunol. 199:17–24. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Awad O, Dedkov EI, Jiao C, Bloomer S,
Tomanek RJ and Schatteman GC: Differential healing activities of
CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb
Vasc Biol. 26:758–764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rodero MP, Licata F, Poupel L, Hamon P,
Khosrotehrani K, Combadiere C and Boissonnas A: In vivo imaging
reveals a pioneer wave of monocyte recruitment into mouse skin
wounds. PLoS One. 9:e1082122014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Willenborg S and Eming SA:
Macrophages-sensors and effectors coordinating skin damage and
repair. J Dtsch Dermatol Ges. 12:214–221. 2014.
|
|
105
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Van Weel V, Toes RE, Seghers L, Deckers
MM, de Vries MR, Eilers PH, Sipkens J, Schepers A, Eefting D, van
Hinsbergh VW, et al: Natural killer cells and CD4+ T-cells modulate
collateral artery development. Arterioscler Thromb Vasc Biol.
27:2310–2318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Hellingman AA, Zwaginga JJ, Van Beem RT;
TeRM/Smart Mix Consortium; Hamming JF, Fibbe WE, Quax PH and
Geutskens SB: T-cell-pre-stimulated monocytes promote
neovascularisation in a murine hind limb ischaemia model. Eur J
Vasc Endovasc Surg. 41:418–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Stabile E, Burnett MS, Watkins C, Kinnaird
T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE and Fuchs S:
Impaired arteriogenic response to acute hindlimb ischemia in
CD4-knockout mice. Circulation. 108:205–210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Stabile E, Kinnaird T, La Sala A, Hanson
SK, Watkins C, Campia U, Shou M, Zbinden S, Fuchs S, Kornfeld H, et
al: CD8+ T lymphocytes regulate the arteriogenic response to
ischemia by infiltrating the site of collateral vessel development
and recruiting CD4+ mononuclear cells through the expression of
interleukin-16. Circulation. 113:118–124. 2006. View Article : Google Scholar
|
|
110
|
Zouggari Y, Ait-Oufella H, Waeckel L,
Vilar J, Loinard C, Cochain C, Récalde A, Duriez M, Levy BI,
Lutgens E, et al: Regulatory T cells modulate postischemic
neovascularization. Circulation. 120:1415–1425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lee H, Schlereth SL, Park EY, Emami-Naeini
P, Chauhan SK and Dana R: A novel pro-angiogenic function for
interferon-Y-secreting natural killer cells. Invest Ophthalmol Vis
Sci. 55:2885–2892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Leung OM, Li J, Li X, Chan VW, Yang KY, Ku
M, Ji L, Sun H, Waldmann H, Tian XY, et al: Regulatory T cells
promote apelin-mediated sprouting angiogenesis in type 2 diabetes.
Cell Rep. 24:1610–1626. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Burzyn D, Kuswanto W, Kolodin D, Shadrach
JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C and
Mathis D: A special population of regulatory T cells potentiates
muscle repair. Cell. 155:1282–1295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ingram DA, Caplice NM and Yoder MC:
Unresolved questions, changing definitions, and novel paradigms for
defining endothelial progenitor cells. Blood. 106:1525–1531. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Basile DP and Yoder MC: Circulating and
tissue resident endothelial progenitor cells. J Cell Physiol.
229:10–16. 2014.
|
|
116
|
Rehman J, Li J, Orschell CM and March KL:
Peripheral blood 'endothelial progenitor cells' are derived from
monocyte/macrophages and secrete angiogenic growth factors.
Circulation. 107:1164–1169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Romagnani P, Annunziato F, Liotta F,
Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L,
Scheffold A, et al: CD14+CD34low cells with stem cell phenotypic
and functional features are the major source of circulating
endothelial progenitors. Circ Res. 97:314–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Rookmaaker MB, Verhaar MC, Loomans CJ,
Verloop R, Peters E, Westerweel PE, Murohara T, Staal FJ, van
Zonneveld AJ, Koolwijk P, et al: CD34+cells home, proliferate, and
participate in capillary formation, and in combination with
CD34-cells enhance tube formation in a 3-dimensional matrix.
Arterioscler Thromb Vasc Biol. 25:1843–1850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Dong Z, Pan T, Fang Y, Wei Z, Gu S, Fang
G, Liu Y, Luo Y, Liu H, Zhang T, et al: Purified
CD34+cells versus peripheral blood mononuclear cells in
the treatment of angiitis-induced no-option critical limb
ischaemia: 12-Month results of a prospective randomised
single-blinded non-inferiority trial. EBioMedicine. 35:46–57. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Albiero M, Poncina N, Ciciliot S,
Cappellari R, Menegazzo L, Ferraro F, Bolego C, Cignarella A,
Avogaro A and Fadini GP: Bone marrow macrophages contribute to
diabetic stem cell mobilopathy by producing oncostatin M. Diabetes.
64:2957–2968. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Fadini GP, Albiero M, Vigili de
Kreutzenberg S, Boscaro E, Cappellari R, Marescotti M, Poncina N,
Agostini C and Avogaro A: Diabetes impairs stem cell and
proangiogenic cell mobilization in humans. Diabetes Care.
36:943–949. 2013. View Article : Google Scholar :
|
|
122
|
Fadini GP, Spinetti G, Santopaolo M and
Madeddu P: Impaired regeneration contributes to poor outcomes in
diabetic peripheral artery disease. Arterioscler Thromb Vasc Biol.
40:34–44. 2020. View Article : Google Scholar
|
|
123
|
Loomans CJM, De Koning EJP, Staal FJ,
Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B,
Rabelink TJ and van Zonneveld AJ: Endothelial progenitor cell
dysfunction: A novel concept in the pathogenesis of vascular
complications of type 1 diabetes. Diabetes. 53:195–199. 2004.
View Article : Google Scholar
|
|
124
|
Tepper OM, Galiano RD, Capla JM, Kalka C,
Gagne PJ, Jacobowitz GR, Levine JP and Gurtner GC: Human
endothelial progenitor cells from type II diabetics exhibit
impaired proliferation, adhesion, and incorporation into vascular
structures. Circulation. 106:2781–2786. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Facchiano F, Lentini A, Fogliano V,
Mancarella S, Rossi C, Facchiano A and Capogrossi MC: Sugar-induced
modification of fibroblast growth factor 2 reduces its angiogenic
activity in vivo. Am J Pathol. 161:531–541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Di Stefano V, Cencioni C, Zaccagnini G,
Magenta A, Capogrossi MC and Martelli F: p66ShcA modulates
oxidative stress and survival of endothelial progenitor cells in
response to high glucose. Cardiovasc Res. 82:421–429. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Jarajapu YP, Hazra S, Segal M, Li Calzi S,
Jadhao C, Qian K, Mitter SK, Raizada MK, Boulton ME and Grant MB:
Vasoreparative dysfunction of CD34+ cells in diabetic individuals
involves hypoxic desensitization and impaired autocrine/paracrine
mechanisms. PLoS One. 9:e939652014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Tchaikovski V, Olieslagers S, Böhmer FD
and Waltenberger J: Diabetes mellitus activates signal transduction
pathways resulting in vascular endothelial growth factor resistance
of human monocytes. Circulation. 120:150–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Torres-Castro I, Arroyo-Camarena ÚD,
Martínez-Reyes CP, Gómez-Arauz AY, Dueñas-Andrade Y, Hernández-Ruiz
J, Béjar YL, Zaga-Clavellina V, Morales-Montor J, Terrazas LI, et
al: Human monocytes and macrophages undergo M1-type inflammatory
polarization in response to high levels of glucose. Immunol Lett.
176:81–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Khanna S, Biswas S, Shang Y, Collard E,
Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK and Roy S:
Macrophage dysfunction impairs resolution of inflammation in the
wounds of diabetic mice. PLoS One. 5:e95392010. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Bannon P, Wood S, Restivo T, Campbell L,
Hardman MJ and Mace KA: Diabetes induces stable intrinsic changes
to myeloid cells that contribute to chronic inflammation during
wound healing in mice. Dis Model Mech. 6:1434–1447. 2013.PubMed/NCBI
|
|
132
|
Arnold L, Henry A, Poron F, Baba-Amer Y,
van Rooijen N, Plonquet A, Gherardi RK and Chazaud B: Inflammatory
monocytes recruited after skeletal muscle injury switch into
antiinflammatory macrophages to support myogenesis. J Exp Med.
204:1057–1069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Di Pardo A, Cappello E, Pepe G, Marracino
F, Carrieri V, Maglione V and Pompeo F: P-0717-Infusion of
autologous-peripheral blood mononuclear cells: A new approach for
limb salvage in patients with diabetes. In: Proceedings of the
International Diabetes Federation 2017 Congress; Abu Dhabi.
2017
|
|
134
|
Cianfarani F, Toietta G, Di Rocco G,
Cesareo E, Zambruno G and Odorisio T: Diabetes impairs adipose
tissue-derived stem cell function and efficiency in promoting wound
healing. Wound Repair Regen. 21:545–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kornicka K, Houston J and Marycz K:
Dysfunction of mesenchymal stem cells isolated from metabolic
syndrome and type 2 diabetic patients as result of oxidative stress
and autophagy may limit their potential therapeutic use. Stem Cell
Rev. 14:337–345. 2018. View Article : Google Scholar :
|
|
136
|
Inoue O, Usui S, Takashima SI, Nomura A,
Yamaguchi K, Takeda Y, Goten C, Hamaoka T, Ootsuji H, Murai H, et
al: Diabetes impairs the angiogenic capacity of human
adipose-derived stem cells by reducing the CD271+
subpopulation in adipose tissue. Biochem Biophys Res Commun.
517:369–375. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Benoit E, O'donnell TF and Patel AN:
Safety and efficacy of autologous cell therapy in critical limb
ischemia: A systematic review. Cell Transplant. 22:545–562. 2013.
View Article : Google Scholar
|
|
138
|
Liew A, Bhattacharya V, Shaw J and Stansby
G: Cell therapy for critical limb ischemia: A meta-analysis of
randomized controlled trials. Angiology. 67:444–455. 2016.
View Article : Google Scholar
|
|
139
|
Jiang X, Zhang H and Teng M: Effectiveness
of autologous stem cell therapy for the treatment of lower
extremity ulcers: A systematic review and meta-analysis. Medicine
(Baltimore). 95:e27162016. View Article : Google Scholar
|
|
140
|
Ai M, Yan CF, Xia FC, Zhou SL, He J and Li
CP: Safety and efficacy of cell-based therapy on critical limb
ischemia: A meta-analysis. Cytotherapy. 18:712–724. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Peeters Weem SM, Teraa M, De Borst GJ,
Verhaar MC and Moll FL: Bone marrow derived cell therapy in
critical limb ischemia: A meta-analysis of randomized placebo
controlled trials. Eur J Vasc Endovasc Surg. 50:775–783. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wang SK, Green LA, Motaganahalli RL,
Wilson MG, Fajardo A and Murphy MP: Rationale and design of the
MarrowStim PAD Kit for the treatment of critical limb ischemia in
subjects with severe peripheral arterial disease (MOBILE) trial
investigating autologous bone marrow cell therapy for critical limb
ischemia. J Vasc Surg. 65:1850–1857.e2. 2017. View Article : Google Scholar
|
|
143
|
Lehalle B, Jan P and Stoltz JF: Diabetic
patients on Rutherford's stage 5 is the best indication of stem
cell therapy in peripheral artery disease: A retrospective study on
367 patients. J Cell Immunother. 4:18–21. 2018. View Article : Google Scholar
|
|
144
|
Shu X, Shu S, Tang S, Yang L, Liu D, Li K,
Dong Z, Ma Z, Zhu Z and Din J: Efficiency of stem cell based
therapy in the treatment of diabetic foot ulcer: A meta-analysis.
Endocr J. 65:403–413. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Guo J, Dardik A, Fang K, Huang R and Gu Y:
Meta-analysis on the treatment of diabetic foot ulcers with
autologous stem cells. Stem Cell Res Ther. 8:2282017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Gao W, Chen D, Liu G and Ran X: Autologous
stem cell therapy for peripheral arterial disease: A systematic
review and meta-analysis of randomized controlled trials. Stem Cell
Res Ther. 10:1402019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Hao C, Shintani S, Shimizu Y, Kondo K,
Ishii M, Wu H and Murohara T: Therapeutic angiogenesis by
autologous adipose-derived regenerative cells: Comparison with bone
marrow mononuclear cells. Am J Physiol Heart Circ Physiol.
307:H869–H879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Lee HC, An SG, Lee HW, Park JS, Cha KS,
Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, et al: Safety and effect
of adipose tissue-derived stem cell implantation in patients with
critical limb ischemia: A pilot study. Circ J. 76:1750–1760. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Bura A, Planat-Benard V, Bourin P,
Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA,
Fleury S, Gadelorge M, et al: Phase I trial: The use of autologous
cultured adipose-derived stroma/stem cells to treat patients with
non-revascularizable critical limb ischemia. Cytotherapy.
16:245–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Darinskas A, Paskevicius M, Apanavicius G,
Vilkevicius G, Labanauskas L, Ichim TE and Rimdeika R: Stromal
vascular fraction cells for the treatment of critical limb
ischemia: A pilot study. J Transl Med. 15:1432017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Moon KC, Chung HY, Han SK, Jeong SH and
Dhong ES: Possibility of injecting adipose-derived stromal vascular
fraction cells to accelerate microcirculation in ischemic diabetic
feet: A pilot study. Int J Stem Cells. 12:107–113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Lonardi R, Leone N, Gennai S, Trevisi
Borsari G, Covic T and Silingardi R: Autologous micro-fragmented
adipose tissue for the treatment of diabetic foot minor
amputations: A randomized controlled single-center clinical trial
(MiFrAADiF). Stem Cell Res Ther. 10:2232019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Teraa M, Sprengers RW, Schutgens RE,
Slaper-Cortenbach IC, van der Graaf Y, Algra A, van der Tweel I,
Doevendans PA, Mali WP, Moll FL and Verhaar MC: Effect of
repetitive intra-arterial infusion of bone marrow mononuclear cells
in patients with no-option limb ischemia: The randomized,
double-blind, placebo-controlled rejuvenating endothelial
progenitor cells via transcutaneous intra-arterial supplementation
(JUVENTAS) trial. Circulation. 131:851–860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Walter DH, Krankenberg H, Balzer JO, Kalka
C, Baumgartner I, Schlüter M, Tonn T, Seeger F, Dimmeler S,
Lindhoff-Last E and Zeiher AM; PROVASA Investigators: Intraarterial
administration of bone marrow mononuclear cells in patients with
critical limb ischemia a randomized-start, placebo-controlled pilot
trial (PROVASA). Circ Cardiovasc Interv. 4:26–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Molavi B, Zafarghandi MR, Aminizadeh E,
Hosseini SE, Mirzayi H, Arab L, Baharvand H and Aghdami N: Safety
and efficacy of repeated bone marrow mononuclear cell therapy in
patients with critical limb ischemia in a pilot randomized
controlled trial. Arch Iran Med. 19:388–396. 2016.PubMed/NCBI
|
|
156
|
Kang WC, Oh PC, Lee K, Ahn T and Byun K:
Increasing injection frequency enhances the survival of injected
bone marrow derived mesenchymal stem cells in a critical limb
ischemia animal model. Korean J Physiol Pharmacol. 20:657–667.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Beugels J, De Munter JPJM, Van Der Hulst
R, Kramer BW and Wolters ECH: Efficacy of different doses of human
autologous adult bone marrow stem cell transplantation on
angiogenesis in an immune deficient rat model with hind limb
ischemia. J Stem Cells Res Dev Ther. 5:1–6. 2019. View Article : Google Scholar
|
|
158
|
Kolvenbach R, Kreissig C, Cagiannos C,
Afifi R and Schmaltz E: Intraoperative adjunctive stem cell
treatment in patients with critical limb ischemia using a novel
point-of-care device. Ann Vasc Surg. 24:367–372. 2010. View Article : Google Scholar
|
|
159
|
Furgiuele S: Trattamento combinato della
arteriopatia periferica al IV stadio mediante rivascolarizzazione
endovascolare e terapia cellulare rigenerativa. Ital J Vasc
Endovasc Surg. 23:672016.
|
|
160
|
Shiraki T, Iida O, Takahara M, Soga Y,
Yamauchi Y, Hirano K, Kawasaki D, Fujihara M, Utsunomiya M, Tazaki
J, et al: Predictors of delayed wound healing after endovascular
therapy of isolated infrapopliteal lesions underlying critical limb
ischemia in patients with high prevalence of diabetes mellitus and
hemodialysis. Eur J Vasc Endovasc Surg. 49:565–573. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Okazaki J, Matsuda D, Tanaka K, Ishida M,
Kuma S, Morisaki K, Furuyama T and Maehara Y: Analysis of wound
healing time and wound-free period as outcomes after surgical and
endovascular revascularization for critical lower limb ischemia. J
Vasc Surg. 67:817–825. 2018. View Article : Google Scholar
|
|
162
|
Robinson WP, Loretz L, Hanesian C, Flahive
J, Bostrom J, Lunig N, Schanzer A and Messina L: Society for
vascular surgery wound, ischemia, foot infection (WIfI) score
correlates with the intensity of multimodal limb treatment and
patient-centered outcomes in patients with threatened limbs managed
in a limb preservation center. J Vasc Surg. 66:488–498.e2. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Reed GW, Salehi N, Giglou PR, Kafa R,
Malik U, Maier M and Shishehbor MH: Time to wound healing and major
adverse limb events in patients with critical limb ischemia treated
with endovascular revascularization. Ann Vasc Surg. 36:190–198.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Vagnozzi RJ, Maillet M, Sargent MA, Khalil
H, Johansen AKZ, Schwanekamp JA, York AJ, Huang V, Nahrendorf M,
Sadayappan S and Molkentin JD: An acute immune response underlies
the benefit of cardiac stem cell therapy. Nature. 577:405–409.
2020. View Article : Google Scholar :
|
|
165
|
Dort J, Fabre P, Molina T and Dumont NA:
Macrophages are key regulators of stem cells during skeletal muscle
regeneration and diseases. Stem Cells Int. 2019:47614272019.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Chisari E, Rehak L, Khan WS and Maffulli
N: The role of the immune system in tendon healing: A systematic
review. Br Med Bull. 133:49–64. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Shabbir A, Cox A, Rodriguez-Menocal L,
Salgado M and Van Badiavas E: Mesenchymal stem cell exosomes induce
proliferation and migration of normal and chronic wound
fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev.
24:1635–1647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q,
Xie Z, Zhang C and Wang Y: Exosomes released from human induced
pluripotent stem cells-derived MSCs facilitate cutaneous wound
healing by promoting collagen synthesis and angiogenesis. J Transl
Med. 13:492015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Baldari S, Di Rocco G, Magenta A, Picozza
M and Toietta G: Extracellular vesicles-encapsulated MicroRNA-125b
produced in genetically modified mesenchymal stromal cells inhibits
hepatocellular carcinoma cell proliferation. Cells. 8:15602019.
View Article : Google Scholar
|
|
170
|
Woodell-May JE, Tan ML, King WJ, Swift MJ,
Welch ZR, Murphy MP and McKale JM: Characterization of the cellular
output of a point-of-care device and the implications for
addressing critical limb ischemia. Biores Open Access. 4:417–424.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Sugaya H, Yoshioka T, Kato T, Taniguchi Y,
Kumagai H, Hyodo K, Ohneda O, Yamazaki M and Mishima H: Comparative
analysis of cellular and growth factor composition in bone marrow
aspirate concentrate and platelet-rich plasma. Bone Marrow Res.
2018:15498262018. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
O'Connor SL, Sepulveda CA, Kaur I, Sumari
RD and McMannis JD: Characterization of BioSafe SEPAX manufactured
stem cell intended for cardiac cell therapy. Blood. 110:40642007.
View Article : Google Scholar
|