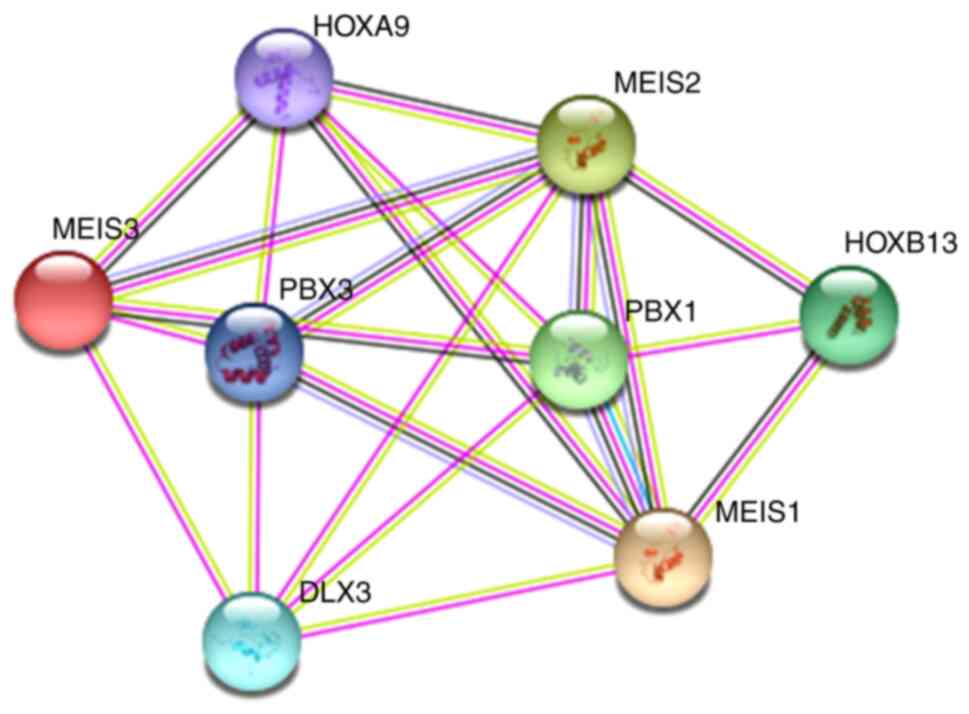
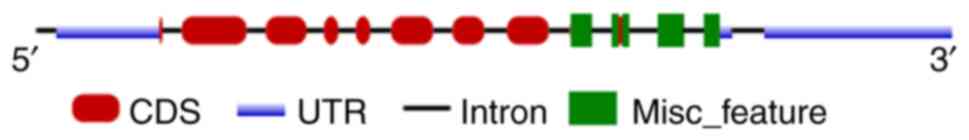
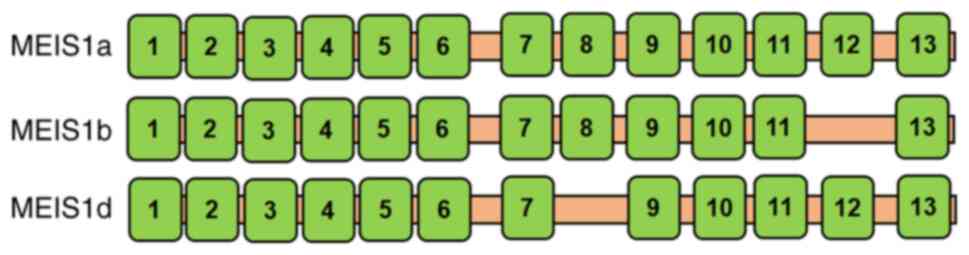
Homeobox protein MEIS1 belongs to the three amino
acid loop extension (TALE) homeodomain transcription factor family,
whose members also include MEIS2 and MEIS3 (5). MEIS2 protein comprises of 477 amino
acids and has a molecular weight of 51,790 Da. This protein is
coded by the MEIS2 gene, that has a length of 212,108 bp.
The MEIS3 protein is coded by the MEIS3 gene with a length
of 19,110 bp and is composed of 375 amino acids. The potential
interplay between the two proteins and MEIS1, is illustrated in
Fig. 1 (modified from www.string-db.org/).
MEIS1 is involved in a number of physiological and
pathophysiological processes; most notably, in cell migration,
apoptosis and metabolism. The present review article summarizes the
results of previous studies that have implicated MEIS1 in cancer
cell proliferation. The potential use of MEIS1 as a novel biomarker
and therapeutic target in cancer was also discussed.
Full-length MEIS1 consists of 390 amino acids and
has a molecular weight of 43,016 Da (6). MEIS1 contains a pre-B-cell leukemia
homeobox (PBX)1 interaction domain, serine/threonine-rich domain,
aspartic acid/glutamic acid-rich domain, poly-aspartic acid domain,
homeodomain and transcriptional activation domain (10). The conserved PBX interaction
motif, homeodomain and C-terminal region are critical for leukemic
transformation (11).
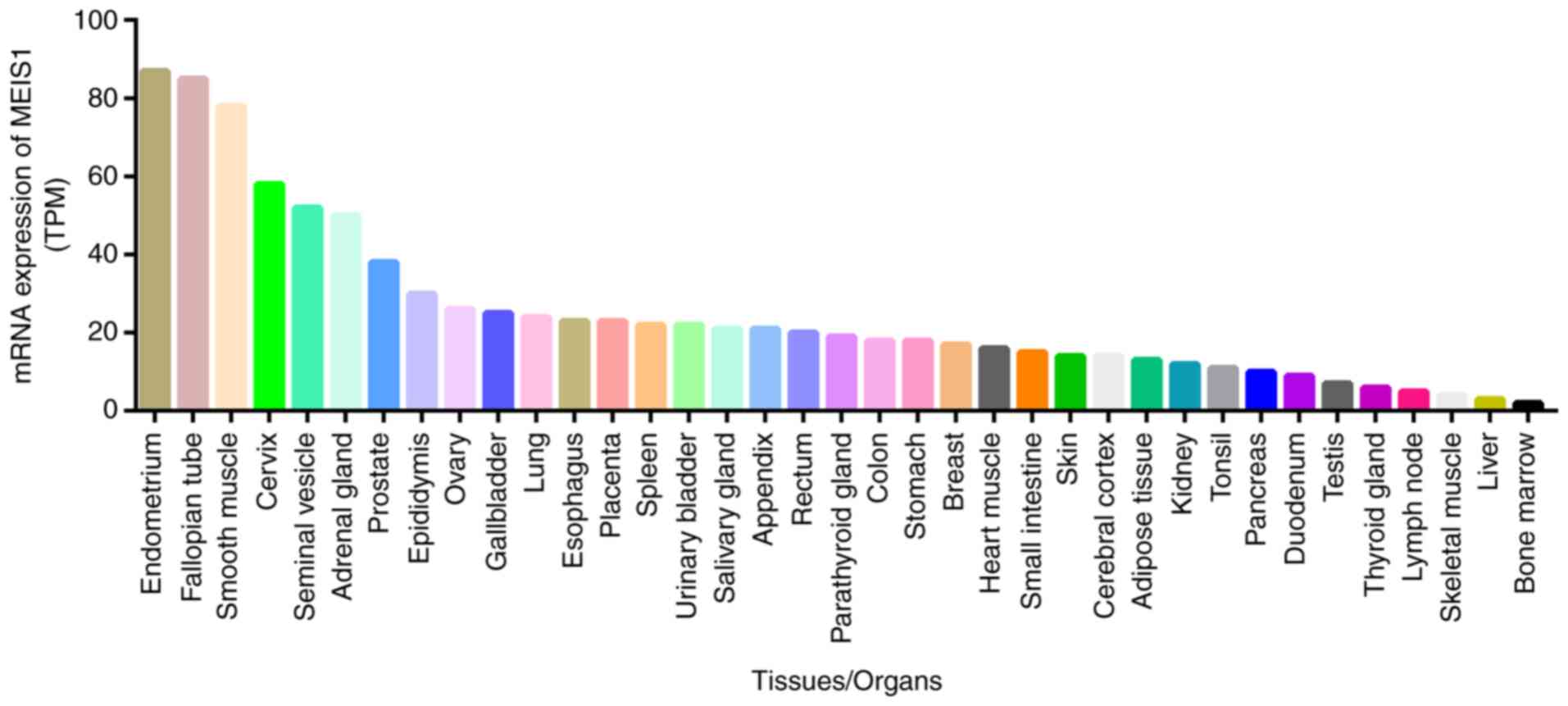
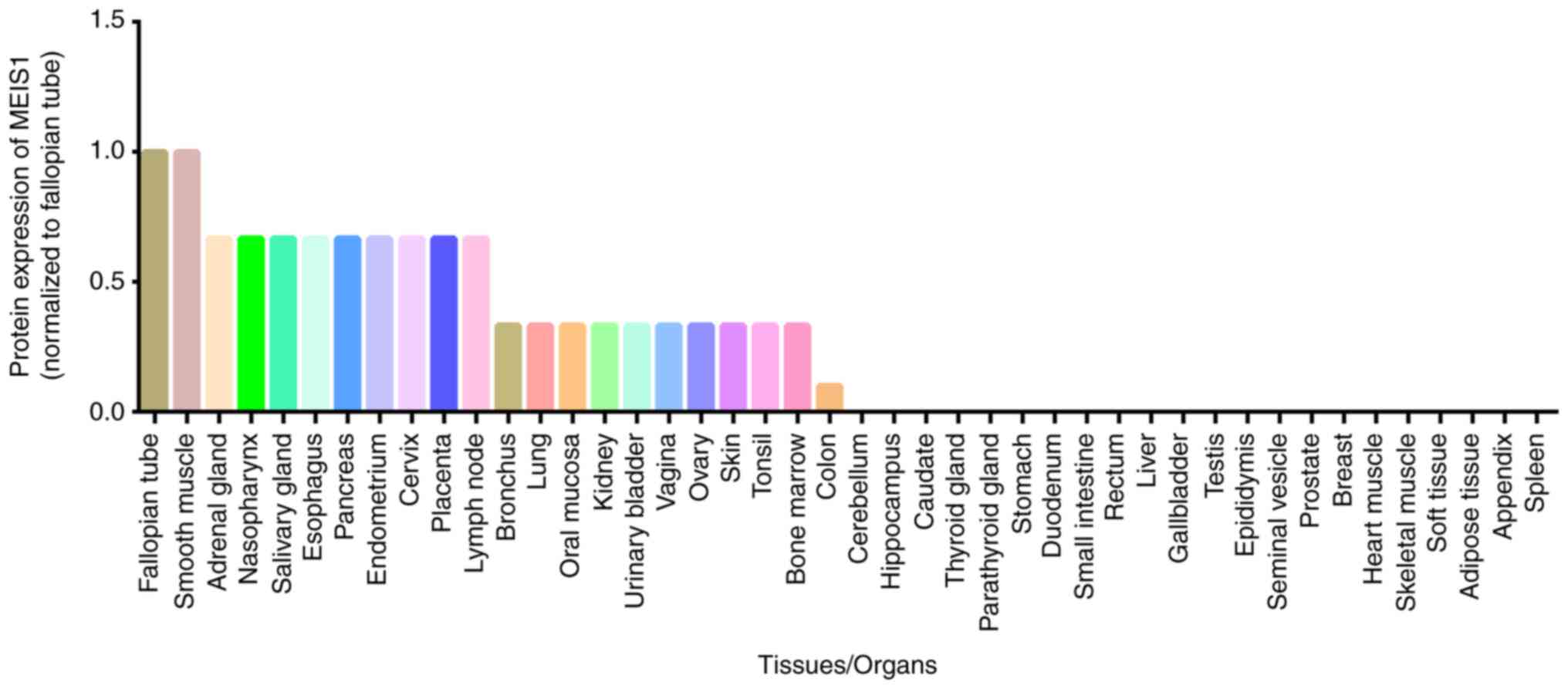
Relative MEIS1 expression at the protein level in
different human tissues, according to the Human Protein Atlas, is
illustrated in Fig. 5. The
highest expression of MEIS1 occurs in the fallopian tubes and
smooth muscle. However, a previous study suggested that MEIS1 was
expressed at highest levels in the endo- and myometrium among 87
types of normal tissues (15).
In addition to the pathological state, other factors
such as cell type, age and environmental conditions also affect
MEIS1 expression. MEIS1 is highly expressed in all cell lines with
a mixed-lineage leukemia (MLL) mutation, but not in wild-type MLL
cell lines (16). The expression
of MEIS1 in solid tumors is somewhat paradoxical, as it is
overexpressed in certain tumor types, whereas it is downregulated
in others (5). The cell types
from the different organ sites under investigation (5) and the types of metabolism (10) the cancer cells require may be
responsible for these contradicting findings. MEIS1 tends to be
expressed at higher levels in fetal hearts than in adult hearts
(17); however, another study
reported a lower MEIS1 expression in postnatal hearts than in adult
hearts (18). Among pediatric
patients with acute lymphoblastic leukemia (ALL), MEIS1 expression
is more frequent in infant ALL than in childhood ALL (19). The discrepancies among these
studies, particularly, the association of MEIS1 with age, requires
further investigation. Hypoxia has been shown to reduce the
expression of MEIS1 in the heart (20), and to downregulate MEIS1
expression in pulmonary artery smooth muscle cells in both primary
culture and animals (21).
MEIS1 related modifications include DNA methylation
and protein ubiquitination. These are discussed below.
Protein-protein interactions play crucial roles in
cellular functions and biological processes. MEIS1 is known to
interact with several partners, including homeobox (HOX) and PBX
proteins.
MEIS1 and HOX can also bind to a third protein to
form trimers. For example, MEIS1 forms a trimer with HOXA9 and PBX2
in myeloid leukemia cells, which then binds to the target DNA
(36). Trimers formed by MEIS1,
HOXA10 and PBX2 mediate the expression of target genes in the human
endometrium (37).
PBX proteins located in the nucleus are
significantly associated with MEIS1 expression (38). PBX1 and MEIS1 form a dynamic
dimer, with each protein binding to its respective target DNA site
(39). A cyclic adenosine
monophosphate (cAMP)-responsive sequence (CRS1) in the bovine
cytochrome P450 family 17 (CYP17) gene is a transcriptional
regulatory element that contains binding sites for PBX and MEIS1.
PBX1 and MEIS1 bind cooperatively to CRS1 to regulate
cAMP-dependent transcription, whereas neither protein can bind this
element on its own (40). The
dimers formed by PBX1 and MEIS1 can also bind to the PBX/MEIS
binding site in the SRY-box transcription factor 3 (SOX3)
promoter, where they regulate SOX3 expression during
development (41).
PBX-regulating protein-1 (PREP1) controls the
expression of MEIS1 through post-transcriptional regulation: PREP1
inhibits the interaction between PBX1 and MEIS1 in mouse embryonic
fibroblasts, destabilizing MEIS1 and inhibiting the interaction
between MEIS1 and DEAD-box helicase 3 x-linked (DDX3x) and DDX5,
and ultimately decreasing MEIS1 tumorigenicity (42). Dimerization with PBX3 stabilizes
MEIS1, allowing it to upregulate target genes, such as FMS related
receptor tyrosine kinase 3 (FLT3) and tribbles pseudokinase
2 (TRIB2), thereby enhancing HOX9-mediated transformation.
MEIS1 protein that does not bind to PBX3 is prone to ubiquitination
and subsequent degradation. Mutations in the PBX binding region in
MEIS1 can also prevent the ubiquitination of MEIS1, as PBX3 and the
responsible E3 ubiquitin ligase share common binding requirements
within MEIS1 (30,43).
MEIS1 can form trimers with PBX protein, as well as
HOX protein. MEIS1 in megakaryocytes binds to PBX1b and PBX2 to
form MEIS1/PBX complexes, which in turn binds to the TGACAG
sequence in tandem repeats of the MEIS1 binding element (TME) of
the platelet factor 4 (PF4) promoter, inducing the
expression of the gene. The simultaneous overexpression of MEIS1
and PBX2 potentiates the activation of the PF4 promoter, and
is abrogated when the MEIS1 binding site on the TME is destroyed
(44).
As a transcription factor, MEIS1 functions as a
positive regulator of the proliferation of leukemia cells (45), as well as in certain solid
tumors, such as esophageal squamous cell carcinoma (46), malignant peripheral nerve sheath
(47) and Ewing sarcoma
(48); however, it has also been
shown to function as a negative regulator of several other solid
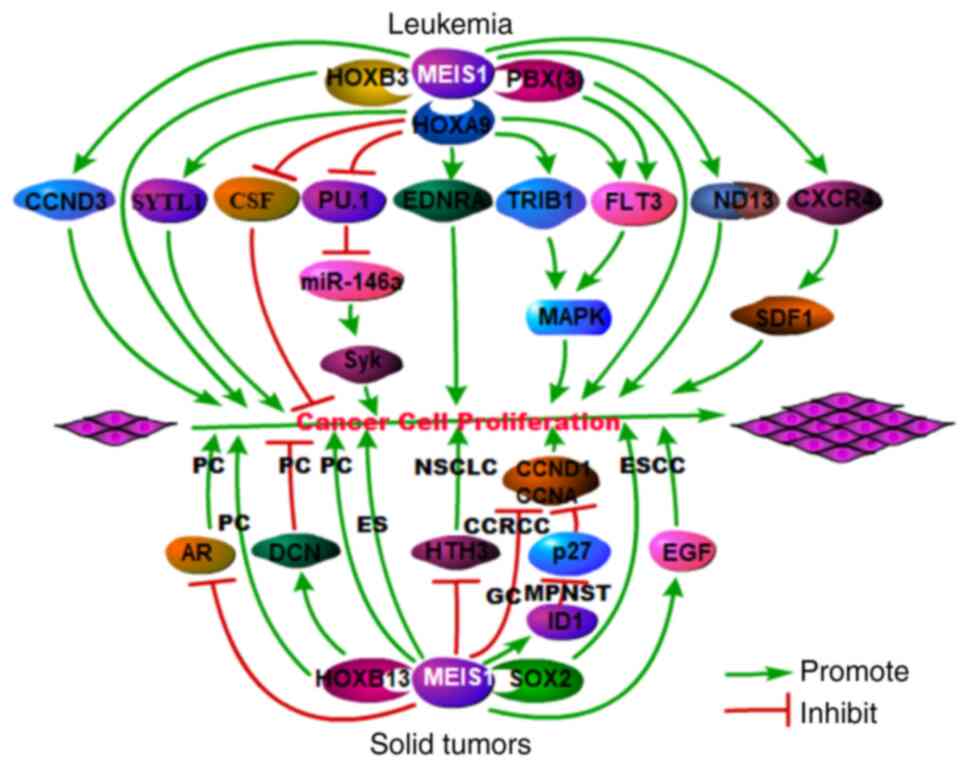
tumors (49) (Fig. 6 and Table I). A schematic diagram of the
promoting and inhibitory roles of MEIS1 in various types of cancer
is presented in Fig. 6.
MEIS1 interacts with a variety of partners to
promote the proliferation of leukemia cells. Recent studies have
demonstrated that MEIS1 interacts with HOXA9 to promote cell
proliferation in leukemia via synaptotagmin-like 1 (SYTL1)
(50), cyclin D3 (CCND3)
(51) or spleen tyrosine kinase
(Syk) (52). In patients with
acute AML, the interaction between MEIS1 and HOXA9 induces the
proliferation of leukemia cells via the simultaneous overexpression
of MEIS1 and HOXA9 (31,52,53), suppresses granulocyte
colony-stimulating factor (G-CSF)-induced granulocytic
differentiation and promotes cell proliferation via stem cell
factor (SCF) (54). In addition,
the complex formed by MEIS1 and HOXA9 is recruited to the
FLT3 promoter to turn on the gene (55), and FLT3 induces mitogen-activated
protein kinase (MAPK) phosphorylation, inhibits apoptosis and
promotes leukemia cell proliferation (56). The MEIS1-HOXA9 complex also
interacts with TRIB1 to induce MAPK phosphorylation, and to
stimulate leukemia cell proliferation (57). A recent study demonstrated that
the MEIS1-HOXA9 complex bound to the endothelin receptor type A
(EDNRA, a tumor promotor) promoter in leukemia cells,
resulting in cell proliferation and in resistance to apoptosis
(58). HOXD13 also helps
regulate the pro-proliferative function of MEIS1 in AML. The human
leukemia-specific fusion gene NUP98-HOXD13 (ND13) has
myeloproliferative activity, although it does not directly induce
AML in mice (59). However, when
MEIS1 expression is upregulated, ND13 can induce cell proliferation
and generate AML in transplanted mice (59). PBX also functions as a cofactor
of MEIS1 to regulate leukemia cell proliferation. Immortalized
progenitor cells induced by HOXA9 typically do not undergo leukemic
transformation; however, the co-expression of MEIS1 and PBX can
induce FLT3 expression, increase MAPK phosphorylation and promote
leukemic transformation (60).
The co-expression of MEIS1 and PBX3 can transform normal
hematopoietic stem/progenitor cells in vitro and induce AML
(leukemia cell proliferation) in mice (61). MEIS1 is also involved in MLL.
MEIS1 regulates the differentiation arrest, cycle activity, in
vivo invasion and self-renewal of MLL cells, and thus
represents a key limiting factor of MLL stem cell potential
(62). The downregulation of
MEIS1 and HOXA alters C-X-C motif chemokine receptor 4
(CXCR4)/stromal cell derived factor 1 (SDF-1) signaling to inhibit
the proliferation of transplanted mutant MLL-rearranged acute
leukemia cells (63), again
supporting a pro-proliferative role of MEIS1 in MLL.
MEIS1 has been associated with a variety of solid
cancers, including prostate, non-small cell lung and gastric
cancer, as well as clear cell renal cell carcinoma, esophageal
squamous cell carcinoma, malignant peripheral nerve sheath tumors
and Ewing sarcoma. The MYC-mediated downregulation of MEIS1
upregulates the androgen receptor (AR) to promote prostate cancer
cell proliferation (64). AR
transcription is inhibited by the overexpression of MEIS1 and is
promoted by MEIS1 knockdown with small interfering RNA (49). A proposed mechanism is that MEIS1
interacts with AR to affect the trafficking of androgen between the
cytoplasm and nucleus. In this manner, MEIS1 may inhibit prostate
cancer cell proliferation by regulating AR, since this receptor
plays a key role in proliferation of human prostate cancer cells.
MEIS1 also participates in prostate cancer through mechanisms
independent of AR. Recent studies have indicated that MEIS1
regulates the proliferation of prostate cancer cells by interacting
with MEIS-interacting domains in HOXB13 (65), which induces decorin (DCN), a
multi-RTK inhibitor (66). MEIS1
knockdown has been shown to significantly increase the
proliferation of non-small cell lung cancer cells through a
mechanism related to DNA synthesis and histone H3 phosphorylation
(67). MEIS1 expression is
reduced in human gastric cancer and clear cell renal cell
carcinoma, and MEIS1 overexpression inhibits cell proliferation by
decreasing CCND1 and CCNA expression (68,69). In contrast to its inhibitory
roles, MEIS1 maintains the stem cell status and cell proliferation
through interaction with SOX2 in esophageal squamous cell carcinoma
cells (46). The downregulation
of MEIS1 upregulates epithelial markers and downregulates epidermal
growth factor (EGF), a marker of cell proliferation (70). In malignant peripheral nerve
sheath tumors, MEIS1 expression is increased and promotes cell
proliferation and maintains cell survival by inhibiting the cell
cycle suppressor, p27, via transcription factor inhibitor of DNA
binding 1 (ID1) (47). In Ewing
sarcoma, MEIS1 collaborates with EWS-FLI1 to stimulate cell
proliferation (48).
Since MEIS1 plays an important pro-proliferative
role in leukemia and certain solid tumors, it has the potential for
use as a therapeutic target. MEIS1 inhibitors under development for
treatment of cancer are reviewed and listed in Table II.
Menin-MLL inhibitors have an anti-proliferative
function via MEIS1 in leukemia cells. MI-2 is a first-generation
small molecule inhibitor of the Menin-MLL interaction, but has poor
pharmacological profiles (71).
The second-generation inhibitor, MI-503, is highly potent and
orally bioavailable, and has been shown to exert profound
anti-proliferative effects in MLL cells (72). The cytotoxic concentration of
these compounds in cells was >2 µM and the relatively
modest effect in vivo suggested limited druggability
(73). The third generation
inhibitor, MI-3454, is well-tolerated and does not impair normal
hematopoiesis in mice; however, it inhibits the proliferation and
induces the differentiation of acute leukemia cells by
downregulating MEIS1 and FLT3 indirectly (74). VTP-50469, another type of highly
selective oral Menin-MLL inhibitor, appears to promote leukemia
cell differentiation and inhibit cell proliferation through the
indirect downregulation of MEIS1 (75-77). All Menin-MLL inhibitors mentioned
above are reversible in nature. To achieve an optimal anti-leukemic
activity, extended drug exposure is required (78). M-525, a highly potent and
irreversible Menin-MLL inhibitor, has been shown to inhibit the
proliferation of and suppress MEIS1 expression in MLL cells at a
sub-nanomolar concentration (78), indicating that the downregulation
of MEIS1 with Menin-MLL inhibitors may represent a promising
therapeutic strategy for MLL. In addition to Menin-MLL inhibitors,
certain other agents that downregulate MEIS1 indirectly can exert
anti-proliferative effects in leukemia cells. For instance, the
proton pump inhibitor, rabeprazole, selectively suppresses the
proliferation and induces the apoptosis of leukemia cells harboring
MLL fusion proteins by downregulating MEIS1 (79). The DOT1-like histone lysine
methyltransferase (DOT1L) inhibitor compound, 9e (80), and the MLL-rearranged leukemia
inhibitor, CCI-007 (81), also
produce similar effects via MEIS1-dependent mechanism.
In a recent study, two small molecule MEIS1
inhibitors (MEISi-1 and MEISi-2) were identified using
high-throughput in silico screening, and induced
hematopoietic stem cell expansion (82).
The present review article summarized the
characteristics of MEIS1, its role in cancer cell proliferation and
the current status on the development of MEIS1 inhibitors as
therapeutic agents. MEIS1 is upregulated and mediates cell
proliferation in leukemia and certain solid tumors (e.g.,
esophageal squamous cell carcinoma and malignant peripheral nerve
sheath tumors), whereas it inhibits cell proliferation in other
solid tumors. Agents that inhibit MEIS1 directly or indirectly are
being developed. MEIS1 is increasingly recognized as a marker of
cancer diagnosis and a therapeutic target.
The paradoxical role of MEIS1 among solid tumors
(stimulatory in some but inhibitory in others) warrants further
investigation. Illustrating the potential association between the
Warburg effect, cancer cell proliferation and MEIS1 may resolve
this contradiction, as the Warburg effect plays an important role
in cell proliferation (83).
Designing MEIS1 inhibitors based on the molecular structure of
MEIS1 may expedite the developmental process of anticancer
agents.
Not applicable.
MY drafted the manuscript. ZC conceived the study
and participated in the manuscript preparation. ZY and KZ assisted
in the literature search and edited the manuscript. YG revised the
manuscript. ZC and MY confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Moskow JJ, Bullrich F, Huebner K, Daar IO
and Buchberg AM: Meis1, a PBX1-related homeobox gene involved in
myeloid leukemia in BXH-2 mice. Mol Cell Biol. 15:5434–5443. 1995.
View Article : Google Scholar
|
|
2
|
Smith JE, Bollekens JA, Inghirami G and
Takeshita K: Cloning and mapping of the MEIS1 gene, the human
homolog of a murine leukemogenic gene. Genomics. 43:99–103. 1997.
View Article : Google Scholar
|
|
3
|
Nakamura T, Largaespada DA, Shaughnessy JD
Jr, Jenkins NA and Copeland NG: Cooperative activation of hoxa and
Pbx1-related genes in murine myeloid leukaemias. Nat Genet.
12:149–153. 1996. View Article : Google Scholar
|
|
4
|
Steelman S, Moskow JJ, Muzynski K, North
C, Druck T, Montgomery JC, Huebner K, Daar IO and Buchberg AM:
Identification of a conserved family of Meis1-related homeobox
genes. Genome Res. 7:142–156. 1997. View Article : Google Scholar
|
|
5
|
Jiang M, Xu S, Bai M and Zhang A: The
emerging role of MEIS1 in cell proliferation and differentiation.
Am J Physiol Cell Physiol. 320:C264–C269. 2021. View Article : Google Scholar
|
|
6
|
Torres-Flores J and Jave-Suárez L: MEIS1
(Meis homeobox 1). Atlas Genet Cytogenet Oncol Haematol:. 424–429.
2013.
|
|
7
|
Su ZH, Si WX, Li L, Zhou BS, Li XC, Xu Y,
Xu CQ, Jia HB and Wang QK: MiR-144 regulates hematopoiesis and
vascular development by targeting meis1 during zebrafish
development. Int J Biochem Cell Biol. 49:53–63. 2014. View Article : Google Scholar
|
|
8
|
Knoepfler PS, Calvo KR, Chen H,
Antonarakis SE and Kamps MP: Meis1 and pKnox1 bind DNA
cooperatively with Pbx1 utilizing an interaction surface disrupted
in oncoprotein E2a-Pbx1. Proc Natl Acad Sci USA. 94:14553–14558.
1997. View Article : Google Scholar
|
|
9
|
Crist RC, Roth JJ, Waldman SA and Buchberg
AM: A conserved tissue-specific homeodomain-less isoform of MEIS1
is downregulated in colorectal cancer. PLoS One. 6:e236652011.
View Article : Google Scholar
|
|
10
|
Aksoz M, Turan RD, Albayrak E and Kocabas
F: Emerging roles of Meis1 in cardiac regeneration, stem cells and
cancer. Curr Drug Targets. 19:181–190. 2018. View Article : Google Scholar
|
|
11
|
Mamo A, Krosl J, Kroon E, Bijl J, Thompson
A, Mayotte N, Girard S, Bisaillon R, Beslu N, Featherstone M and
Sauvageau G: Molecular dissection of Meis1 reveals 2 domains
required for leukemia induction and a key role for hoxa gene
activation. Blood. 108:622–629. 2006. View Article : Google Scholar
|
|
12
|
Dintilhac A, Bihan R, Guerrier D,
Deschamps S, Bougerie H, Watrin T, Bonnec G and Pellerin I: PBX1
intracellular localization is independent of Meis1 in epithelial
cells of the developing female genital tract. Int J Dev Biol.
49:851–858. 2005. View Article : Google Scholar
|
|
13
|
Crijns APG, de Graeff P, Geerts D, Hoora
KAT, Hollemac H, van der Sluis T, Hofstrad RMW, de Bock GH, de Jong
S, van der Zeea AGJ and de Vries EGE: MEIS and PBX homeobox
proteins in ovarian cancer. Eur J Cancer. 43:2495–2505. 2007.
View Article : Google Scholar
|
|
14
|
Li HX, Guo XY, Xie Y, Yuan QL, Ge MX and
Zhang JY: Study of the dynamic expression of Meis1 in mice. Iran J
Reprod Med. 11:139–144. 2013.
|
|
15
|
Xu B, Geerts D, Qian K, Zhang H and Zhu G:
Myeloid ecotropic viral integration site 1 (MEIS) 1 involvement in
embryonic implantation. Hum Reprod. 23:1394–1406. 2008. View Article : Google Scholar
|
|
16
|
Quentmeier H, Dirks WG, Macleod RAF,
Reinhardt J, Zaborski M and Drexler HG: Expression of HOX genes in
acute leukemia cell lines with and without MLL translocations. Leuk
Lymphoma. 45:567–574. 2004. View Article : Google Scholar
|
|
17
|
Locatelli P, Belaich MN, López AE, Olea
FD, Vega MU, Giménez CS, Simonin JA, Bauzá MDR, Castillo MG,
Cuniberti LA, et al: Novel insights into cardiac regeneration based
on differential fetal and adult ovine heart transcriptomic
analysis. Am J Physiol Heart Circ Physiol. 318:H994–H1007. 2020.
View Article : Google Scholar
|
|
18
|
Mahmoud AI, Kocabas F, Muralidhar SA,
Kimura W, Koura AS, Thet S, Porrello ER and Sadek HA: Meis1
regulates postnatal cardiomyocyte cell cycle arrest. Nature.
497:249–253. 2013. View Article : Google Scholar
|
|
19
|
Imamura T, Morimoto A, Takanashi M, Hibi
S, Sugimoto T, Ishii E and Imashuku S: Frequent co-expression of
HoxA9 and Meis1 genes in infant acute lymphoblastic leukaemia with
MLL rearrangement. Br J Haematol. 119:119–121. 2002. View Article : Google Scholar
|
|
20
|
Kimura W, Xiao F, Canseco DC, Muralidhar
S, Thet SW, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM,
et al: Hypoxia fate mapping identifies cycling cardiomyocytes in
the adult heart. Nature. 523:226–230. 2015. View Article : Google Scholar
|
|
21
|
Yao MZ, Ge XY, Liu T, Huang N, Liu H, Chen
Y, Zhang Z and Hu CP: MEIS1 regulated proliferation and migration
of pulmonary artery smooth muscle cells in hypoxia-induced
pulmonary hypertension. Life Sci. 255:1178222020. View Article : Google Scholar
|
|
22
|
Ferreira HJ, Heyn H, Vizoso M, Moutinho C,
Vidal E, Gomez A, Martínez-Cardús A, Simó-Riudalbas L, Moran S,
Jost E and Esteller M: DNMT3A mutations mediate the epigenetic
reactivation of the leukemogenic factor MEIS1 in acute myeloid
leukemia. Oncogene. 35:3079–3082. 2016. View Article : Google Scholar
|
|
23
|
Lasa A, Carnicer MJ, Aventín A, Estivill
C, Brunet S, Sierra J and Nomdedéu JF: MEIS 1 expression is
downregulated through promoter hypermethylation in AML1-ETO acute
myeloid leukemias. Leukemia. 18:1231–1237. 2004. View Article : Google Scholar
|
|
24
|
Musialik E, Bujko M, Kober P, Grygorowicz
MA, Libura M, Przestrzelska M, Juszczynski P, Borg K, Florek I,
Jakóbczyk M and Siedlecki JA: Promoter DNA methylation and
expression levels of HOXA4, HOXA5 and MEIS1 in acute myeloid
leukemia. Mol Med Rep. 11:3948–3954. 2015. View Article : Google Scholar
|
|
25
|
Ropa J, Saha N, Chen Z, Serio J, Chen W,
Mellacheruvu D, Zhao L, Basrur V, Nesvizhskii AI and Muntean AG:
PAF1 complex interactions with SETDB1 mediate promoter H3K9
methylation and transcriptional repression of Hoxa9 and Meis1 in
acute myeloid leukemia. Oncotarget. 9:22123–22136. 2018. View Article : Google Scholar
|
|
26
|
Beukers W, Hercegovac A, Vermeij M,
Kandimalla R, Blok AC, van der Aa MMN, Zwarthoff EC and Zuiverloon
TCM: Hypermethylation of the polycomb group target gene PCDH7 in
bladder tumors from patients of all ages. J Urol. 190:311–316.
2013. View Article : Google Scholar
|
|
27
|
Dihal AA, Boot A, van Roon EH, Schrumpf M,
Fariña-Sarasqueta A, Fiocco M, Zeestraten CM, Peter JK, Kuppen PJK,
Morreau H, et al: The homeobox gene Meis1 is methylated in BRAF
(V600E) mutated colon tumors. PLoS One. 8:e798982013. View Article : Google Scholar
|
|
28
|
Soltani N, Karimiani EG, Farzanehfar M,
Mashkani B, Jafarian A, Ashraf H, Rezyat AA and Soukhtanloo M:
Evaluation of the methylation status of the MEIS1 promoter gene in
colorectal cancer. Middle East J Cancer. 7:203–207. 2016.
|
|
29
|
Popovic D, Vucic D and Dikic I:
Ubiquitination in disease pathogenesis and treatment. Nat Med.
20:1242–1253. 2014. View Article : Google Scholar
|
|
30
|
Liu X, Zhang F, Zhang Y, Li X, Chen C,
Zhou M, Zhuo Yu, Liu Y, Zhao Y, Hao X, et al: PPM1K regulates
hematopoiesis and leukemogenesis through CDC20-mediated
ubiquitination of MEIS1 and p21. Cell Rep. 23:1461–1475. 2018.
View Article : Google Scholar
|
|
31
|
Garcia-Cuellar MP, Steger J, Füller E,
Hetzner K and Slany RK: Pbx3 and Meis1 cooperate through multiple
mechanisms to support hox-induced murine leukemia. Haematologica.
100:905–913. 2015. View Article : Google Scholar
|
|
32
|
Lawrence HJ, Rozenfeld S, Cruz C,
Matsukuma K, Kwong A, Kömüves L, Buchberg AM and Largman C:
Frequent co-expression of the HOXA9 and MEIS1 homeobox genes in
human myeloid leukemias. Leukemia. 13:1993–1999. 1999. View Article : Google Scholar
|
|
33
|
Shen WF, Montgomery JC, Rozenfeld S,
Moskow JJ, Lawrence HJ, Buchberg AM and Largman C: AbdB-like hox
proteins stabilize DNA binding by the Meis1 homeodomain proteins.
Mol Cell Biol. 17:6448–6458. 1997. View Article : Google Scholar
|
|
34
|
Williams TM, Williams ME and Innis JW:
Range of HOX/TALE superclass associations and protein domain
requirements for HOXA13: MEIS interaction. Dev Biol. 277:457–471.
2005. View Article : Google Scholar
|
|
35
|
Wermuth PJ and Buchberg AM: Meis1-mediated
apoptosis is caspase dependent and can be suppressed by
coexpression of HoxA9 in murine and human cell lines. Blood.
105:1222–1230. 2005. View Article : Google Scholar
|
|
36
|
Shen WF, Rozenfeld S, Kwong A, Köm ves LG,
Lawrence HJ and Largman C: HOXA9 forms triple complexes with PBX2
and MEIS1 in myeloid cells. Mol Cell Biol. 19:3051–3061. 1999.
View Article : Google Scholar
|
|
37
|
Sarno JL, Kliman HJ and Taylor HS: HOXA10,
Pbx2, and Meis1 protein expression in the human endometrium:
Formation of multimeric complexes on HOXA10 target genes. J Clin
Endocrinol Metab. 90:522–528. 2005. View Article : Google Scholar
|
|
38
|
Toresson H, Parmar M and Campbell K:
Expression of Meis and Pbx genes and their protein products in the
developing telencephalon: Implications for regional
differentiation. Mech Dev. 94:183–187. 2000. View Article : Google Scholar
|
|
39
|
Chang CP, Jacobs Y, Nakamura T, Jenkins
NA, Copeland NG and Cleary ML: Meis proteins are major in vivo DNA
binding partners for wild-type but not chimeric Pbx proteins. Mol
Cell Biol. 17:5679–5687. 1997. View Article : Google Scholar
|
|
40
|
Bischof LJ, Kagawa N, Moskow JJ, Takahashi
Y, Iwamatsu A, Buchberg AM and Waterman MR: Members of the Meis1
and pbx homeodomain protein families cooperatively bind a
cAMP-responsive sequence (CRS1) from bovine CYP17. J Biol Chem.
273:7941–7948. 1998. View Article : Google Scholar
|
|
41
|
Mojsin M and Stevanovic M: PBX1 and MEIS1
up-regulate SOX3 gene expression by direct interaction with a
consensus binding site within the basal promoter region. Biochem J.
425:107–116. 2009. View Article : Google Scholar
|
|
42
|
Dardaei L, Longobardi E and Blasi F: Prep1
and Meis1 competition for Pbx1 binding regulates protein stability
and tumorigenesis. Proc Natl Acad Sci USA. 111:E896–E905. 2014.
View Article : Google Scholar
|
|
43
|
Thorne RMW and Milne TA: Dangerous
liaisons: Cooperation between Pbx3, Meis1 and Hoxa9 in leukemia.
Haematologica. 100:850–853. 2015. View Article : Google Scholar
|
|
44
|
Okada Y, Nagai R, Sato T, Matsuura E,
Minami T, Morita I and Doi T: Homeodomain proteins MEIS1 and PBXs
regulate the lineage-specific transcription of the platelet factor
4 gene. Blood. 101:4748–4756. 2003. View Article : Google Scholar
|
|
45
|
Rosales-Aviña JA, Torres-Flores J,
Aguilar-Lemarroy A, Gurrola-Díaz C, Hernández-Flores G,
Ortiz-Lazareno PC, Lerma-Díaz JM, de Celis R, González-Ramella Ó,
Barrera-Chaires E, et al: MEIS1, PREP1, and PBX4 are differentially
expressed in acute lymphoblastic leukemia: Association of MEIS1
expression with higher proliferation and chemotherapy resistance. J
Exp Clin Cancer Res. 30:1122011. View Article : Google Scholar
|
|
46
|
Rad A, Farshchian M, Forghanifard MM,
Matin MM, Bahrami AR, Geerts D, A'rabi A, Memar B and Abbaszadegan
MR: Predicting the molecular role of MEIS1 in esophageal squamous
cell carcinoma. Tumour Biol. 37:1715–1725. 2016. View Article : Google Scholar
|
|
47
|
Patel AV, Chaney KE, Choi K, Largaespada
DA, Kumar AR and Ratner N: An shRNA screen identifies MEIS1 as a
driver of malignant peripheral nerve sheath tumors. EBioMedicine.
9:110–119. 2016. View Article : Google Scholar
|
|
48
|
Lin LH, Huang ML, Shi XP, Mayakonda A, Hu
KS, Jiang YY, Guo X, Chen L, Pang B, Doan N, et al:
Super-enhancer-associated MEIS1 promotes transcriptional
dysregulation in ewing sarcoma in co-operation with EWS-FLI1.
Nucleic Acids Res. 47:1255–1267. 2019. View Article : Google Scholar
|
|
49
|
Cui L, Li M, Feng F, Yang Y, Hang X, Cui J
and Gao J: MEIS1 functions as a potential AR negative regulator.
Exp Cell Res. 328:58–68. 2014. View Article : Google Scholar
|
|
50
|
Yokoyama T, Nakatake M, Kuwata T, Couzinet
A, Goitsuka R, Tsutsumi S, Aburatani H, Valk PJM, Delwel R and
Nakamura T: MEIS1-mediated transactivation of synaptotagmin-like 1
promotes CXCL12/CXCR4 signaling and leukemogenesis. J Clin Invest.
126:1664–1678. 2016. View Article : Google Scholar
|
|
51
|
Argiropoulos B, Yung E, Xiang P, Lo C,
Kuchenbauer F, Palmqvist L, Reindl C, Heuser M, Sekulovic S, Rosten
P, et al: Linkage of the potent leukemogenic activity of Meis1 to
cell-cycle entry and transcriptional regulation of cyclin D3.
Blood. 115:4071–4082. 2010. View Article : Google Scholar
|
|
52
|
Mohr S, Doebele C, Comoglio F, Berg T,
Beck J, Bohnenberger H, Alexe G, Corso J, Ströbel P, Wachter A, et
al: Hoxa9 and Meis1 cooperatively induce addiction to syk signaling
by suppressing miR-146a in acute myeloid leukemia. Cancer Cell.
31:549–562. 2017. View Article : Google Scholar
|
|
53
|
Thorsteinsdottir U, Kroon E, Jerome L,
Blasi F and Sauvageau G: Defining roles for HOX and MEIS1 genes in
induction of acute myeloid leukemia. Mol Cell Biol. 21:224–234.
2001. View Article : Google Scholar
|
|
54
|
Calvo KR, Knoepfler PS, Sykes DB, Pasillas
MP and Kamps MP: Meis1a suppresses differentiation by G-CSF and
promotes proliferation by SCF: Potential mechanisms of
cooperativity with Hoxa9 in myeloid leukemia. Proc Natl Acad Sci
USA. 98:13120–13125. 2001. View Article : Google Scholar
|
|
55
|
Wang GG, Pasillas MP and Kamps MP:
Persistent transactivation by Meis1 replaces hox function in
myeloid leukemogenesis models: Evidence for co-occupancy of
Meis1-pbx and hox-pbx complexes on promoters of leukemia-associated
genes. Mol Cell Biol. 26:3902–3916. 2006. View Article : Google Scholar
|
|
56
|
Kiyoi H, Kawashima N and Ishikawa Y: FLT3
mutations in acute myeloid leukemia: Therapeutic paradigm beyond
inhibitor development. Cancer Sci. 111:312–322. 2020. View Article : Google Scholar
|
|
57
|
Jin G, Yamazaki Y, Takuwa M, Takahara T,
Kaneko K, Kuwata T, Miyata S and Nakamura T: Trib1 and evi1
cooperate with Hoxa and Meis1 in myeloid leukemogenesis. Blood.
109:3998–4005. 2007. View Article : Google Scholar
|
|
58
|
Arabanian LS, Johansson P, Staffas A,
Nilsson T, Rouhi A, Fogelstrand L and Palmqvist L: The endothelin
receptor type A is a downstream target of Hoxa9 and Meis1 in acute
myeloid leukemia. Leuk Res. 75:61–68. 2018. View Article : Google Scholar
|
|
59
|
Pineault N, Buske C, Feuring-Buske M,
Abramovich C, Rosten P, Hogge DE, Aplan PD and Humphries RK:
Induction of acute myeloid leukemia in mice by the human
leukemia-specific fusion gene NUP98-HOXD13 in concert with Meis1.
Blood. 101:4529–4538. 2003. View Article : Google Scholar
|
|
60
|
Wang GG, Pasillas MP and Kamps MP: Meis1
programs transcription of FLT3 and cancer stem cell character,
using a mechanism that requires interaction with Pbx and a novel
function of the Meis1 C-terminus. Blood. 106:254–264. 2005.
View Article : Google Scholar
|
|
61
|
Li ZJ, Chen P, Su R, Hu C, Li Y, Elkahloun
AG, Zuo Z, Gurbuxani S, Arnovitz S, Weng H, et al: PBX3 and MEIS1
cooperate in hematopoietic cells to drive acute myeloid leukemias
characterized by a core transcriptome of the MLL-rearranged
disease. Cancer Res. 76:619–629. 2016. View Article : Google Scholar
|
|
62
|
Wong P, Iwasaki M, Somervaille TCP, So
CWE, So CWE and Cleary ML: Meis1 is an essential and rate-limiting
regulator of MLL leukemia stem cell potential. Genes Dev.
21:2762–2774. 2007. View Article : Google Scholar
|
|
63
|
Orlovsky K, Kalinkovich A, Rozovskaia T,
Shezen E, Itkin T, Alder H, Ozer HG, Carramusa L, Avigdor A,
Volinia S, et al: Down-regulation of homeobox genes MEIS1 and HOXA
in MLL-rearranged acute leukemia impairs engraftment and reduces
proliferation. Proc Natl Acad Sci USA. 108:7956–7961. 2011.
View Article : Google Scholar
|
|
64
|
Whitlock NC, Trostel SY, Wilkinson S,
Terrigino NT, Hennigan ST, Lake R, Carrabba NV, Atway R, Walton ED,
Gryder BE, et al: MEIS1 down-regulation by MYC mediates prostate
cancer development through elevated HOXB13 expression and AR
activity. Oncogene. 39:5663–5674. 2020. View Article : Google Scholar
|
|
65
|
Johng D, Torga G, Ewing CM, Jin K, Norris
JD, McDonnell DP and Isaacs WB: HOXB13 interaction with MEIS1
modifies proliferation and gene expression in prostate cancer.
Prostate. 79:414–424. 2019. View Article : Google Scholar
|
|
66
|
VanOpstall C, Perike S, Brechka H, Gillard
M, Lamperis S, Zhu BZ, Brown R, Bhanvadia R and Griend DJ:
MEIS-mediated suppression of human prostate cancer growth and
metastasis through HOXB13-dependent regulation of proteoglycans.
ELife. 9:e536002020. View Article : Google Scholar
|
|
67
|
Li W, Huang K, Guo H and Cui G: Meis1
regulates proliferation of non-small-cell lung cancer cells. J
Thorac Dis. 6:850–855. 2014.
|
|
68
|
Song F, Wang H and Wang Y: Myeloid
ecotropic viral integration site 1 inhibits cell proliferation,
invasion or migration in human gastric cancer. Oncotarget.
8:90050–90060. 2017. View Article : Google Scholar
|
|
69
|
Zhu J, Cui L, Xu A, Yin X, Li F and Gao J:
MEIS1 inhibits clear cell renal cell carcinoma cells proliferation
and in vitro invasion or migration. BMC cancer. 17:1762017.
View Article : Google Scholar
|
|
70
|
Mahmoudian RA, Bahadori B, Rad A,
Abbaszadegan MR and Forghanifard MM: MEIS1 knockdown may promote
differentiation of esophageal squamous carcinoma cell line KYSE-30.
Mol Genet Genomic Med. 7:e007462019. View Article : Google Scholar
|
|
71
|
Grembecka J, He S, Shi A, Purohit T,
Muntean AG, Sorenson RJ, Showalter HD, Murai MJ, Belcher AM,
Hartley T, et al: Menin-MLL inhibitors reverse oncogenic activity
of MLL fusion proteins in leukemia. Nat Chem Biol. 8:277–284. 2012.
View Article : Google Scholar
|
|
72
|
Borkin D, He S, Miao H, Kempinska K,
Pollock J, Chase J, Purohit T, Malik B, Zhao T, Wang J, et al:
Pharmacologic inhibition of the Menin-MLL interaction blocks
progression of MLL leukemia in vivo. Cancer Cell. 27:589–602. 2015.
View Article : Google Scholar
|
|
73
|
Kühn MWM, Song E, Feng Z, Sinha A, Chen
CW, Deshpande AJ, Cusan M, Farnoud N, Mupo A, Grove C, et al:
Targeting chromatin regulators inhibits leukemogenic gene
expression in NPM1 mutant leukemia. Cancer Discov. 6:1166–1181.
2016. View Article : Google Scholar
|
|
74
|
Klossowski S, Miao H, Kempinska K, Wu T,
Purohit T, Kim E, Linhares BM, Chen D, Jih G, Perkey E, et al:
Menin inhibitor MI-3454 induces remission in MLL1-rearranged and
NPM1-mutated models of leukemia. J Clin Invest. 130:981–997. 2020.
View Article : Google Scholar
|
|
75
|
Krivtsov AV, Evans K, Gadrey JY, Eschle
BK, Hatton C, Uckelmann HJ, Ross KN, Perner F, Olsen SN, Pritchard
T, et al: A menin-MLL inhibitor induces specific chromatin changes
and eradicates disease in models of MLL-rearranged leukemia. Cancer
Cell. 36:660–673. 2019. View Article : Google Scholar
|
|
76
|
Gundry MC, Goodell MA and Brunetti L: It's
all about MEis: Menin-MLL inhibition eradicates NPM1-mutated and
MLL-rearranged acute leukemias in mice. Cancer Cell. 37:267–269.
2020. View Article : Google Scholar
|
|
77
|
Uckelmann HJ, Kim SM, Wong EM, Hatton C,
Giovinazzo H, Gadrey JY, Krivtsov AV, Rücker FG, Döhner K, McGeehan
GM, et al: Therapeutic targeting of preleukemia cells in a mouse
model of NPM1 mutant acute myeloid leukemia. Science. 367:586–590.
2020. View Article : Google Scholar
|
|
78
|
Xu S, Aguilar A, Xu T, Zheng K, Huang L,
Stuckey J, Chinnaswamy K, Bernard D, Fernández-Salas E, Liu L, et
al: Design of the first-in-class, highly potent irreversible
inhibitor targeting the Menin-MLL protein-protein interaction.
Angew Chem Int Ed Engl. 57:1601–1605. 2018. View Article : Google Scholar
|
|
79
|
Chen WL, Li DD, Chen X, Wang YZ, Xu JJ,
Jiang ZY, You QD and Guo XK: Proton pump inhibitors selectively
suppress MLL rearranged leukemia cells via disrupting MLL1-WDR5
protein-protein interaction. Eur J Med Chem. 188:1120272020.
View Article : Google Scholar
|
|
80
|
Zhang L, Chen Y, Liu N, Li L, Xiao S, Li
X, Chen K, Luo C, Chen S and Chen H: Design, synthesis and anti
leukemia cells proliferation activities of pyrimidylaminoquinoline
derivatives as DOT1L inhibitors. Bioorg Chem. 80:649–654. 2018.
View Article : Google Scholar
|
|
81
|
Somers K, Chudakova DA, Middlemiss SMC,
Wen VW, Clifton M, Kwek A, Liu B, Mayoh C, Bongers A, Karsa M, et
al: CCI-007, a novel small molecule with cytotoxic activity against
infant leukemia with MLL rearrangements. Oncotarget. 7:46067–46087.
2016. View Article : Google Scholar
|
|
82
|
Turan RD, Albayrak E, Uslu M, Siyah P,
Alyazici LY, Kalkan BM, Aslan GS, Yuce DC, Aksoz M, Tuysuz EC, et
al: Development of small molecule MEIS inhibitors that modulate HSC
activity. Sci Rep. 10:79942020. View Article : Google Scholar
|
|
83
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar
|