Introduction
Prostate cancer (PCa) is the most common type of
cancer among males and the second leading cause of cancer-related
mortality worldwide (1). In
recent decades, the 5-year survival rate for patients with primary
PCa has increased due to the significant progress made in the
development of treatment methods for primary PCa; however, the
5-year survival rate for patients with advanced PCa remains
unsatisfactory, due to the occurrence of distant metastases
(2). Thus, it remains an urgent
priority to determine the underlying mechanisms contributing to the
development and metastasis of PCa.
Long non-coding RNAs (lncRNAs) are non-coding RNAs
of >200 nucleotides in length (3). It has been revealed that lncRNAs
play important roles in the onset, progression and metastasis of
the majority of types of cancer (4). Numerous lncRNAs, including long
intergenic non-protein coding 844 (5), maternally expressed 3 (6), colon cancer associated transcript 1
(7) and zinc finger E-box
binding homeobox 1 antisense RNA 1 (8), have been reported to be involved in
the development and metastasis of PCa. Notably, it was recently
demonstrated that lncRNA small nucleolar RNA host gene 11 (SNHG11)
may be involved in the regulation of the progression of various
tumor types. For example, Liu et al (9) demonstrated that the upregulated
expression of SNHG11 predicted a poor prognosis of patients with
lung cancer and that the overexpression of SNHG11 facilitated the
development of lung cancer. In gastric cancer, SNHG11 has been
reported to promote gastric cancer cell proliferation and
metastasis (10). In addition,
SNHG11 expression has been discovered to be upregulated in
colorectal cancer cells and to be associated with increased levels
of cell proliferation (11).
However, to the best of our knowledge, to date, limited information
is available on the expression and function of SNHG11 in PCa.
In the present study, SNHG11 expression in PCa was
investigated, and the aim of the study was to determine whether
SNHG11 knockdown inhibits the proliferation, migration and invasion
of PCa cells. Furthermore, the present study was investigated
whether the suppressive effects of SNHG11 knockdown on PCa
progression are achieved through the downregulation of insulin-like
growth factor 1 receptor (IGF-1R) expression via sponging microRNA
(miRNA/miR)-184. Taken together, in the present study, it was
revealed that SNHG11 may serve as a promising therapeutic target
for PCa.
Materials and methods
Patient samples
A total of 30 PCa and adjacent normal tissue samples
from patients with PCa were obtained. Written informed consent was
signed by all patients and the study protocol was approved by The
First Affiliated Hospital of University of South China Ethnics
Committee (approval no. LL20201103017).
Cell lines and culture
A normal human prostate epithelial cell line
(RWPE-1, cat. no. SCSP-5025) and human PCa cell lines (LNCaP, cat.
no. TCHu173; C4-2, cat. no. CL-0046; PC3, cat. no. TCHu158; and
DU145, cat. no. TCHu222) were purchased from The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences. RWPE-1 cells
were cultured in 1X Defined Keratinocyte SFM (Gibco; Thermo Fisher
Scientific, Inc.). All PCa cell lines were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and maintained in
5% CO2 at 37°C.
Cell transfection
A lentiviral short hairpin RNA (shRNA/sh) vector
targeting SNHG11 (sh-SNHG11#1, 5′-GGA GTG GTC TTC CCA AGA A-3′; and
sh-SNHG11#2, 5′-CCT CTC ACC CAC TCA ATA A-3′) and sh-negative
control (NC; 5′-UUC UCC GAA CGU GUC ACG UU-3′) were purchased from
Shanghai GenePharma Co., Ltd. miR-184 mimic (5′-GGC AUUCUG UAU ACA
UCG GAG -3′), miR-184 inhibitor (5′-CAG UAC UUU UGU GUA GUA CAA
-3′), mimic-NC (5′-UUC UCC GAA CGU GUC ACG UTT -3′) and
inhibitor-NC (5′-CAG UAC UUU UGU GUA GUA CAA -3′) were synthesized
by Guangzhou RiboBio Co., Ltd. IGF-1R overexpression plasmid
[pcDNA3.1 (+) IGF-1R] and its NC [pcDNA3.1 (+)] were constructed by
Shanghai GenePharma Co., Ltd. RNAs (100 nM) or miR-184 mimics (50
nM) or miR-184 inhibitor (150 nM) or plasmids (1.5 µg per
well) were transfected into the cells. All cell transfections were
performed using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Transfection was performed at room
temperature for 30 min. Following incubation for 48 h at 37°C, the
cells were collected for use in subsequent experiments by
ultracentrifugation (4°C; 1,000 x g; 10 min). The lentivirus was
prepared according to the User Manual of the Lenti-Pac™ HIV
Expression Packaging Kit (GeneCopoeia, Inc.). Viral packaging was
performed in 293T cells (SCSP-502; The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences) following
co-transfection of the Lv3-si-SNHG11#2, or empty lentiviral vector,
and Lenti-Pac™ HIV packaging mix (GeneCopoeia, Inc.) using
EndoFectin™ Lenti transfection reagent (GeneCopoeia, Inc.). The
medium containing the retroviral supernatant was harvested at 48-72
h following transfection. The retroviruses were added into the
cells for infection. The cells were selected by culture in the
presence of puromycin (5 µg/ml; Invitrogen; Thermo Fisher
Scientific, Inc.) for up to 2 weeks. All cell transfections were
performed using Lipofectamine® LTX reagent (Invitrogen;
Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using the phenol-chloroform
method. Total RNA was reverse transcribed into cDNA using a One
Step PrimeScript miRNA cDNA Synthesis kit (Takara Bio, Inc.).
RT-qPCR was subsequently performed using a SYBR-Green PCR kit
(Takara Bio, Inc.). The PCR protocol consisted of cycling at 94°C
for 3 min, followed by 30 cycles of 94°C for 30 sec, 55°C for 30
sec and 72°C for 60 sec and a final extension at 72°C for 5 min.
GAPDH or U6 served as the internal reference controls. The primer
sequences (Shanghai GenePharma Co. Ltd.) used for RT-qPCR are
listed in Table I. The ΔCt data
and ΔΔCt data were calculated using the following formula: ΔCt=Ct
of target gene-Ct of reference gene. ΔΔCt=ΔCt of the experimental
group-ΔCt of the control group. Target gene relative expression
levels were calculated using the 2−ΔΔCq method (12).
 | Table IPrimers used for RT-qPCR. |
Table I
Primers used for RT-qPCR.
| Gene | Primer sequences
(5′-3′) |
|---|
| SNHG11 | F:
5′-TGGGAGTTGTCATGTTGGGA-3′ |
| R:
5′-ACTCGTCACTCTTGGTCTGT-3′ |
| miR-184 | F:
5′-GCATGCCTAAATGTTGACAGCC-3′ |
| R:
5′-GTGCAGGGTCCGAGGT-3′ |
| U6 | F:
5′-CTCGCTTCGGCAGCACATATACT-3′ |
| R:
5′-ACGCTTCACGAATTTGCGTGTC-3′ |
| IGF-1R | F:
5′-GGAGGCTGAATACCGCAAAGTC-3′ |
| R:
5′-AAAGACGAAGTTGGAGGCGCT-3′ |
| GAPDH | F:
5′-ATGTTCGTCATGGGTGTGAA-3′ |
| R:
5′-CAGTGATGGCATGGACTGT-3′ |
Colony formation assay
A total of 800 cells per well were plated in 6-well
plates and cultured in growth medium (RPMI-1640 medium supplemented
with 10% FBS) for 2 weeks. Following incubation for 2 weeks at
37°C, the colonies were fixed using paraformaldehyde and stained
using 0.1% crystal violet solution (Sigma-Aldrich Merck KGaA).
Images of the 6-well plates were obtained and colonies containing
>50 cells were counted using ImageJ software V1.8.0 (National
Institutes of Health).
Transwell assays
Migration and invasion assays were conducted using
Transwell chambers (8-µm pore size; BD Biosciences) with
(invasion assay) or without (migration assay) Matrigel precoating.
Briefly, the upper chamber was filled with 200 µl serum-free
RPMI-1640 medium containing 6×104 cells, while the lower
chamber was filled with 750 µl RPMI-1640 10%
serum-containing medium. Following 48 h of incubation at 37°C, the
cells remaining on the upper surface of the membrane were removed,
while the cells on the lower surface were fixed with ethanol at
room temperature for 10 min and stained with 0.1% crystal violet at
room temperature for 10 min. The migratory or invasive cells were
visualized using a microscope (magnification, ×200; CKX41, Olympus
Corporation).
Dual luciferase reporter assay
StarBase software V2.0 (http://starbase.sysu.edu.cn) was used to predict
potential target miRNAs of SNHG11. Subsequently, the target gene of
miR-184 was predicted using the bioinformatics tools, ComiRNet
(http://comirnet.di.uniba.it:8080/).
The wild-type (WT) or mutant (MUT) 3′-untranslated region of SNHG11
was cloned into the pmirGLO Dual Luciferase vector (Promega
Corporation). pmirGLO-SNHG11-WT/MUT (1.0 µg per well)
vectors were co-transfected into PC3 and DU145 cells alongside the
mimic-NC (40 nM) or miR-184 mimic (40 nM). Similarly,
pmirGLO-IGF-1R-WT or pmirGLO-IGF-1R-MUT vectors (1.0 µg per
well) were established and co-transfected with miR-184 mimic (40
nM) or NC-mimic (40 nM) into PC3 and DU145 cells using
Lipofectamine® LTX (Invitrogen; Thermo Fisher
Scientific, Inc.). Following 48 h of transfection, relative
luciferase activity was measured using a Dual Luciferase Reporter
assay system (Promega Corporation).
RNA immunoprecipitation (RIP) assay
RIP assay was performed using a Magna RIP kit (cat.
no. 17-700, MilliporeSigma). Briefly, cells were lysed in RIPA
lysis buffer containing magnetic beads conjugated with human
anti-argonaute (Ago2) antibody (cat. no. ab186733, 1:300; Abcam) or
anti-IgG antibody (cat. no. MA5-27548, 1:200; MilliporeSigma).
SNHG11 and miR-184 expression levels were determined using
RT-qPCR.
Western blotting
Western blotting was performed as previously
described (13). Briefly, total
protein was extracted from the cells using RIPA lysis buffer. Total
protein was quantified using the BCA protein assay kit (cat. no.
23225, Pierce; Thermo Fisher Scientific, Inc.) and 40 µg
protein/lane was separated via 10% SDS-PAGE. The proteins were
subsequently transferred onto PVDF membranes and blocked with 5%
skimmed milk for 2 h at room temperature. The membranes were then
incubated with the following primary antibodies at 4°C overnight:
Anti-IGF-1R (1:1,000, cat. no. ab182408; Abcam) and anti-GAPDH
(1:4,000; cat. no. ab181602, Abcam). Following incubation with the
primary antibodies, the membranes were incubated with an
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:2,000;
cat. no. ab6728, Abcam) for 1 h at room temperature. Protein bands
were visualized using the LI-COR Odyssey® CLX Two-colour
infrared laser imaging system (LI-COR Biosciences). And
densitometric analysis was performed using ImageJ V1.8.0 software
(National Institutes of Health).
Hem a tox ylin a n d eosin (H& E)
staining a n d immunohistochemistry (IHC)
Tissue slices were deparaffinized in xylene and
hydrated in decreasing concentrations of alcohol prior to
hematoxylin and eosin (H&E) staining. IHC was performed
according to a standard IHC protocol. Briefly, the sections were
deparaffinized, rehydrated and endogenous peroxidase activity was
blocked in 3% fresh H2O2 for 10 min.
Specimens on the slides were microwaved in citrate buffer antigen
retrieval solution (Vector Laboratories, Inc.) twice for 5 min each
and washed with PBS for 5 min. After the slides were blocked with
10% normal goat serum in PBS for 30 min, the sections were
incubated with the primary antibodies, mouse anti-IGF-1R (1:1,000,
cat. no. ab182408; Abcam) or mouse anti-Ki-67 (1:1,000, cat. no.
ab279653; Abcam) overnight at 4°C, and the appropriate
HRP-conjugated secondary antibody (cat. no. BM3894; Wuhan Boster
Biological Technology, Ltd.) was applied at a dilution of 1:100 for
1 h at room temperature. Immunoreactivity was subsequently detected
using DAB (Vector Laboratories, Inc.). The percentage of positive
cells was scored as follows: 0 (0-5%), 1 (6-25%), 2 (26-50%), 3
(51-75%) and 4 (>75%). The staining intensity was scored as
follows: 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). The
final immunoreactivity score was calculated by multiplying the
percentage score with the intensity score.
Establishment of xenograft and lung
metastasis models
A total of 20 male nude mice (age, 5 weeks; weight,
18-20 g) were obtained from the Experimental Animal Center of
Guangdong Province and were housed five per cage in wire-top cages
with sawdust bedding in an isolated, clean, air-conditioned room at
a temperature of 25-26°C and a relative humidity of ~50%, with
light for 12 h/day and with free access to fresh water and a solid
pellet diet. All procedures were performed in strict accordance
with guidelines issued by and following the approval of the Animal
Care Commission of The First Affiliated Hospital of University of
South China (Hengyang, China; approval no. LL20201103017).
For the tumorigenesis assay, 100 µl PC-3
cells (2×106 cells) infected with sh-SNHG11 or sh-NC
were subcutaneously injected into the right side of the axillary
region of the mice. The mice were randomly divided into 2 groups
(n=5/group). Tumor volume was detected every 4 days based on the
following equation: Volume (mm3)=length x
width2 (mm)/2. Following 4 weeks of tumor growth, the
mice were euthanized by cervical dislocation, under anesthesia with
1% pentobarbital (50 mg/kg; intraperitoneal).
For the metastasis assay, 3×106
sh-SNHG11- or sh-NC-transfected PC-3 cells were transfected with
Firefly luciferase vector and then injected into the tail vein of
mice (n=5/group) in 150 µl PBS. Lung metastases were
monitored by bioluminescent imaging for 8 consecutive weeks. After
8 weeks, tumor cell metastasis was imaged using a Xenogen IVIS
Spectrum Imaging system (PerkinElmer, Inc.). The mice were then
euthanized by cervical dislocation under anesthesia with
intraperitoneal 1% pentobarbital (50 mg/kg) and the lungs were
harvested. The following signs were used to verify the death of the
mice: The eyes had turned pale, no heartbeat, and no response to
external stimuli. Prior to collecting the lungs, the mice were
euthanized by cervical dislocation under anesthesia with 1%
pentobarbital (50 mg/kg) by intraperitoneal injection.
Statistical analysis
Statistical analyses were performed using SPSS 18.0
software (SPSS, Inc.). All experiments were conducted in triplicate
and data are expressed as the mean ± SD. For the comparison of
matched samples (PCa and normal adjacent tissues), a paired
Student's t-test was performed. For the other comparisons between
two groups, an unpaired t-test was used. For the comparison of
multiple groups, one-way ANOVA analysis was performed followed by a
Bonferroni's post hoc test. Pearson's correlation analysis was
applied, to assess the correlation between the expression levels of
different genes. The analyses of ordinal variable were performed
using the Mann-Whitney test (also known as the Wilcoxon rank-sum
test). A Levene test was used to assess the equality of variances.
P<0.05 was considered to indicate a statistically significant
difference.
Results
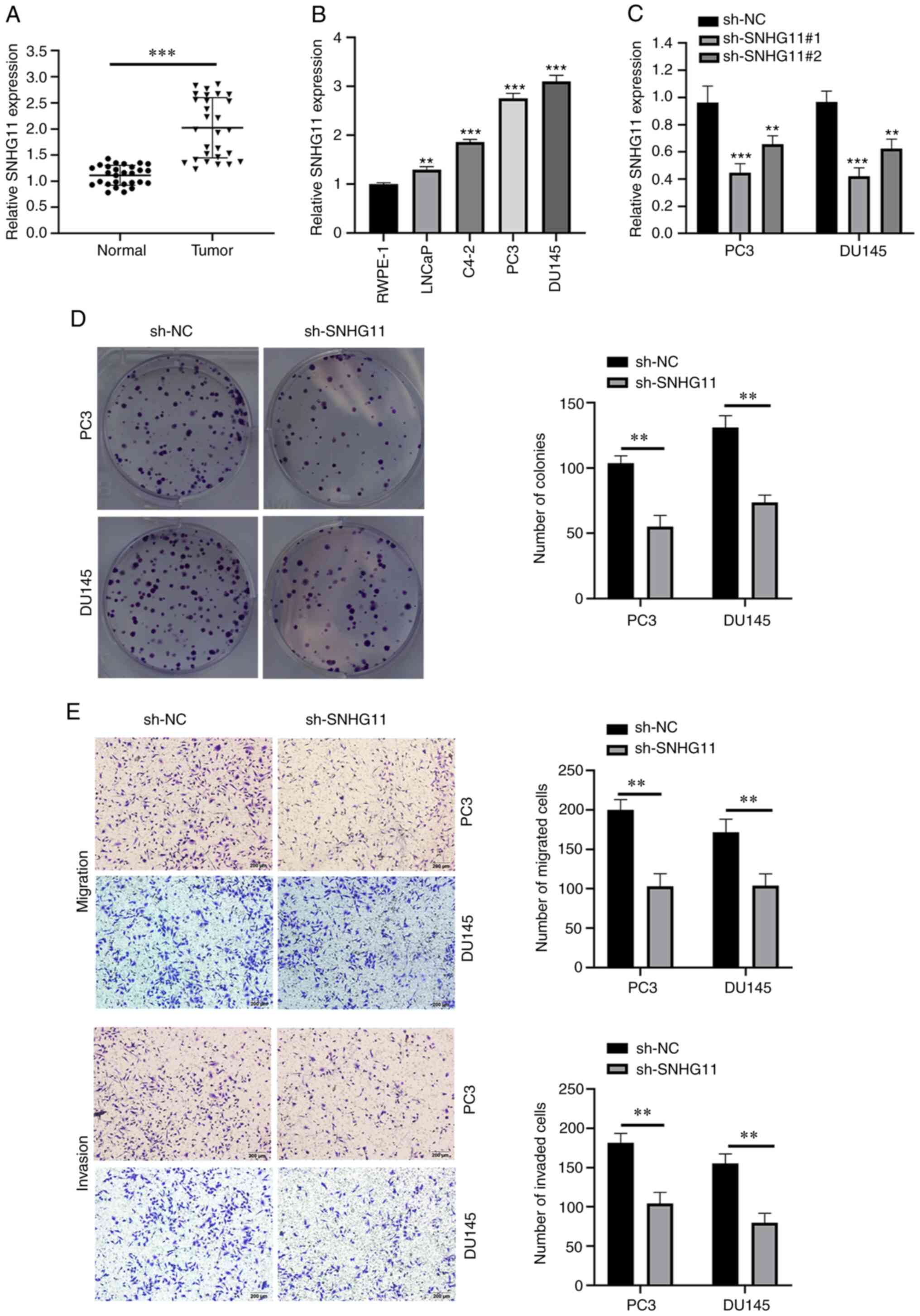
SNHG11 expression levels are upregulated
in PCa tissues and cell lines
As shown in Fig.
1A, the SNHG11 expression levels were markedly upregulated in
PCa tissues compared with those in adjacent normal tissues. In
addition, SNHG11 expression was notably upregulated in the PCa cell
lines, LNCaP, C4-2, PC3 and DU145 compared with the normal cell
line, RWPE-1 (Fig. 1B).
Silencing SNHG11 inhibits the colony
formation, migration and invasion of PCa cells
To determine the roles of SNHG11 in PCa development,
a lentiviral vector silencing SNHG11 expression was constructed
(Fig. 1C). Since sh-SNHG11#1
exhibited the most prominent inhibitory efficiency, sh-SNHG11#1 was
selected for use in subsequent experiments and referred to as
sh-SNHG11 henceforth. Notably, the silencing of SNHG11 expression
in PC3 and DU145 cells significantly suppressed their proliferation
(Fig. 1D). In addition, SNHG11
knockdown significantly weakened the migratory and invasive
abilities of the PC3 and DU145 cells (Fig. 1E).
SNHG11 directly targets the
miR-184/IGF-1R signaling axis
StarBase software V2.0 (http://starbase.sysu.edu.cn) was used to predict
potential target miRNAs of SNHG11, which identified miR-184 as a
candidate target of SNHG11 (Fig.
2A). The expression levels of miR-184 were found to be
downregulated in PCa tissues and cell lines (LNCaP, C4-2, PC3 and
DU145) (Fig. 2B and C). To
determine whether SNHG11 regulates miR-184 expression, miR-184
expression was measured in sh-SNHG11-transfected PC3 and DU145
cells and a negative group. Notably, miR-184 expression was
significantly upregulated following SNHG11 knockdown (Fig. 2D). The results of dual luciferase
activity assay revealed that co-transfection with miR-184 mimic
attenuated the relative luciferase activity of pmirGLO-SNHG11-WT,
but not that of pmirGLO-SNHG11-MUT (Fig. 2E). Furthermore, a negative
correlation was identified between SNHG11 and miR-184 expression in
PCa tissues (Fig. 2F). Moreover,
the results of RIP assay demonstrated that SNHG11 and miR-184 were
more abundant in the anti-Ago2 group (Fig. 2G).
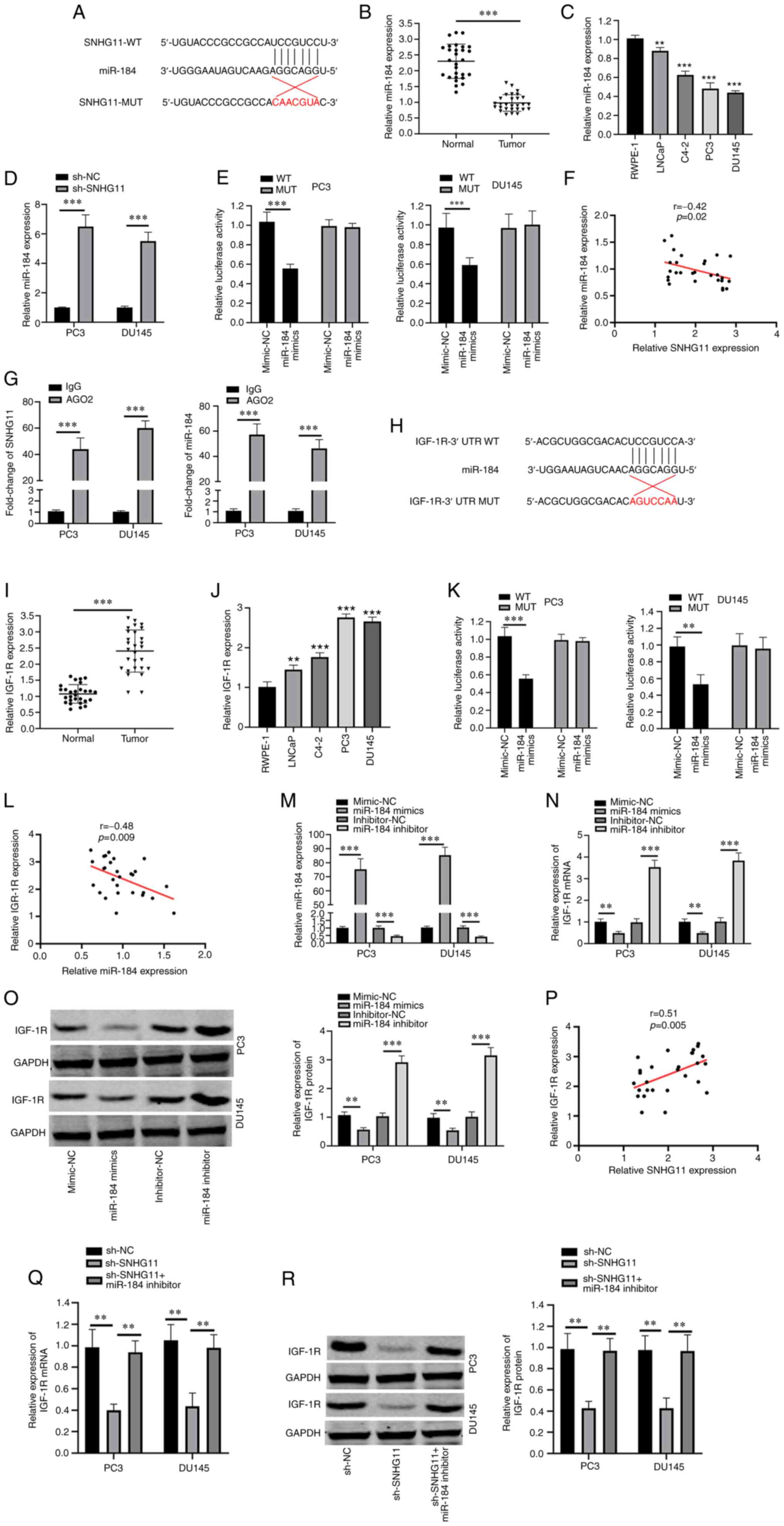 | Figure 2SNHG11 acts as a sponge for miR-495.
(A) Predicted binding site with miR-184 in SNHG11 through
bioinformatics analysis. (B) miR-184 expression levels were
markedly reduced in PCa tissues compared with that in normal
tissues. (C) miR-184 expression levels were markedly decreased in
PCa cell lines (LNCaP, C4-2, PC3, DU145) compared with RWPE-1 cell.
(D) Silencing SNHG11 increased miR-184 relative expression levels
in PC3 and DU145 cells. (E) Luciferase reporter assay revealed that
miR-184 mimics inhibited the SNHG20-WT activity in PC3 and DU145
cells. (F) SNHG11 expression negatively correlated with miR-184
expression in PCa tissues. (G) RIP assay results suggested the
enrichment of SNHG11 and miR-184 in Ago2-containing beads. (H)
Predicted binding sites between miR-184 and IGF-1R through
bioinformatics analysis. (I) IGF-1R levels in PCa tissues were
significantly increased in comparison with normal tissues. (J)
IGF-1R expression was significantly increased in PCa cell lines
(LNCaP, C4-2, PC3, DU145), as compared with RWPE-1 cell. (K)
miR-184 mimics significantly reduced IGF-1R-WT luciferase activity
in PC3 and DU145 cells. (L) IGF-1R expression was negatively
associated with miR-184 expression in PCa tissues. (M) Effects of
miR-184 mimics or miR-184 inhibitor on miR-184 expression in PC3
and DU145 cells as detected using RT-qPCR. (N and O) miR-184
markedly reduced IGF-1R mRNA and protein levels in PC3 and DU145
cells; however, this was reversed with the use of miR-184
inhibitor. (P) SNHG11 expression positively correlated with IGF-1R
expression in PCa tissues. (Q and R) SNHG11 knockdown inhibited the
IGF-1R mRNA and protein expression levels, and these effects were
reversed with the addition of the miR-184 inhibitor in PC3 and
DU145 cells. **P<0.01 and ***P<0.001,
in comparison with the control group. SNHG11, lncRNA small
nucleolar RNA host gene 11; PCa, prostate cancer; IGF-R1,
insulin-like growth factor 1 receptor. |
Subsequently, the target gene of miR-184 was
predicted using the bioinformatics tools, ComiRNet (http://comirnet.di.uniba.it:8080/). IGF-1R has
been reported to be overexpressed in a number of human cancers and
to play a vital role in the cancer progression (14). IGF-1R signaling is important for
prostate cancer progression and development. Therefore, IGF-R1 was
selected as a target candidate. The results demonstrated that
IGF-1R was a candidate target of miR-184 (Fig. 2H). IGF-1R expression levels in
PCa tissues and cell lines (LNCaP, C4-2, PC3 and DU145) were found
to be upregulated, in comparison with those in adjacent normal
tissue and RWPE-1 cells, respectively (Fig. 2I and J). In addition, the
relative luciferase activity of pmirGLO-IGF-1R-WT was suppressed
following co-transfection with miR-184 mimic (Fig. 2K). Furthermore, a negative
correlation was observed between the miR-184 and IGF-1R expression
levels in PCa tissues (Fig. 2L).
To determine whether IGF-1R expression was targeted by miR-184, a
miR-184 mimic and miR-184 inhibitor were transfected into PC3 and
DU145 cells (Fig. 2M).
Transfection with the miR-184 mimic suppressed the IGF-1R mRNA and
protein expression levels, while transfection with the miR-184
inhibitor resulted in an increase in IGF-1R expression levels
(Fig. 2N and O). As was
expected, a positive correlation was identified between SNHG11 and
IGF-1R expression in the PCa tissues (Fig. 2P). Of note, IGF-1R mRNA and
protein expression levels were significantly decreased following
SNHG11 knockdown, while this suppressive effect was reversed by
transfection with miR-184 inhibitor (Fig. 2Q and R). According to the
aforementioned data, it was suggested that SNHG11 may act as a
sponge for miR-184 to upregulate IGF-1R expression.
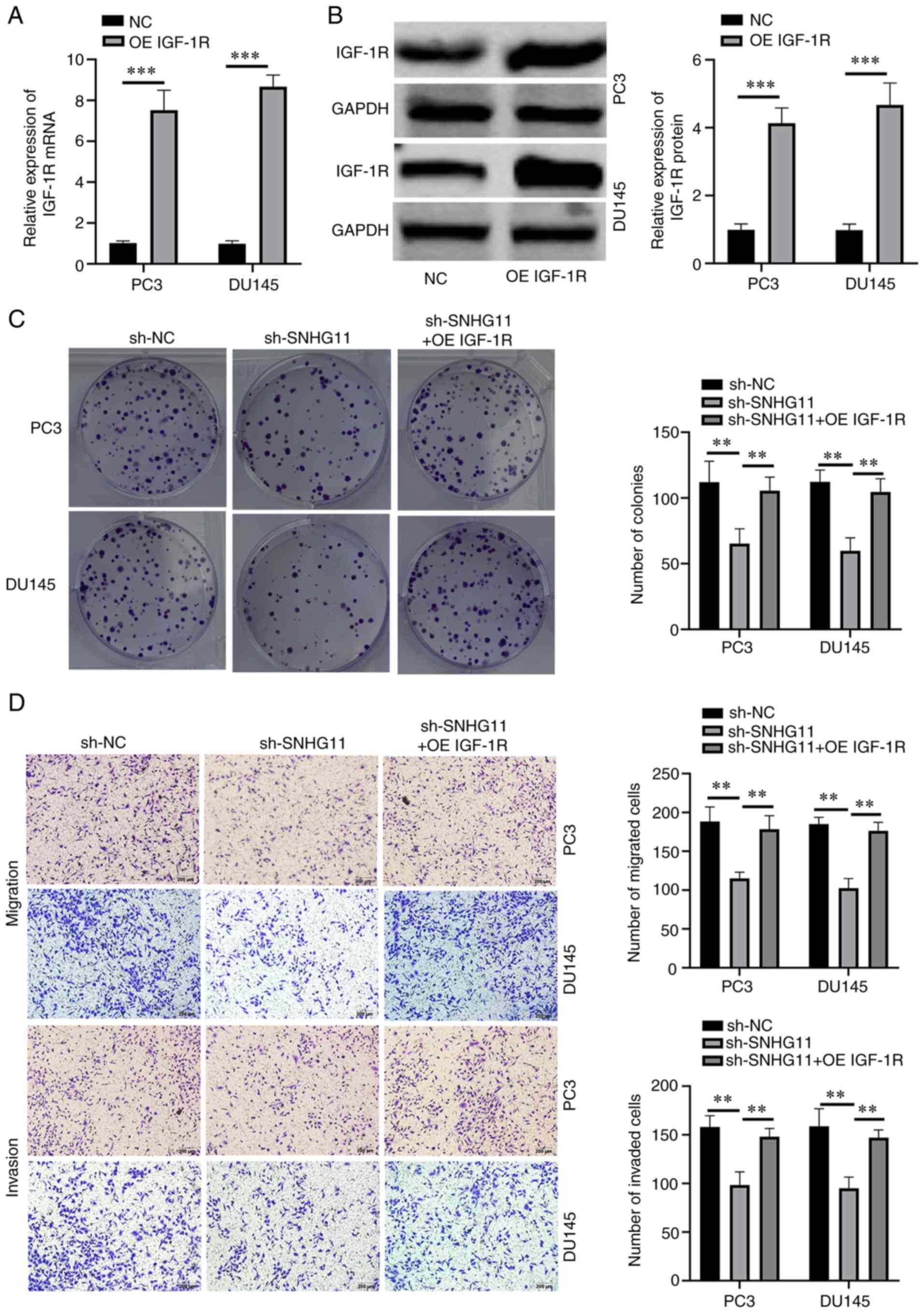
IGF-1R overexpression reverses the
effects of SNHG11 knockdown in PCa cells
To investigate whether SNHG11 exerts its effects via
IGF-1R, functional rescue assays were performed. First, IGF-1R
overexpression was successfully induced in PC3 and DU145 cells
through transfection with a pcDNA3.1 (+) IGF-1R vector (Fig. 3A and B). Functional rescue assays
were then performed using IGF-1R-overexpressing PC3 and DU145 cells
following SNHG11 stable knockdown. Rescue experiments indicated
that the overexpression of IGF-1R reversed the suppressive effects
of SNHG11 knockdown on the proliferative, migratory and invasive
abilities of PCa cells (Fig. 3C and
D). These data thus indicated that SNHG11 may promote PCa
progression by targeting the miR-184/IGF-1R signaling axis.
Silencing SNHG11 inhibits tumor growth
and metastasis in vivo
The effects of SNHG11 on tumor growth in vivo
were further analyzed. It was revealed that tumor volume and weight
were both markedly decreased in the mice injected with
sh-SNHG11-transfected PC3 cells, as compared with those injected
with sh-NC-transfected PC3 cells (Fig. 4A-C). In the sh-NC group, the
maximum tumor volume observed was 826 mm3, while that in
the sh-SNHG11 group was 398 mm3. In addition, IHC
staining revealed more positive staining for Ki-67 protein,
representing cell proliferative ability, in the sh-NC group; in
addition, IGF-1R expression was significantly reduced in the
xenografts of mice injected with sh-SNHG11-transfected PC3 cells
(Fig. 4D). These data suggested
that silencing SNHG11 inhibited PCa growth in vivo.
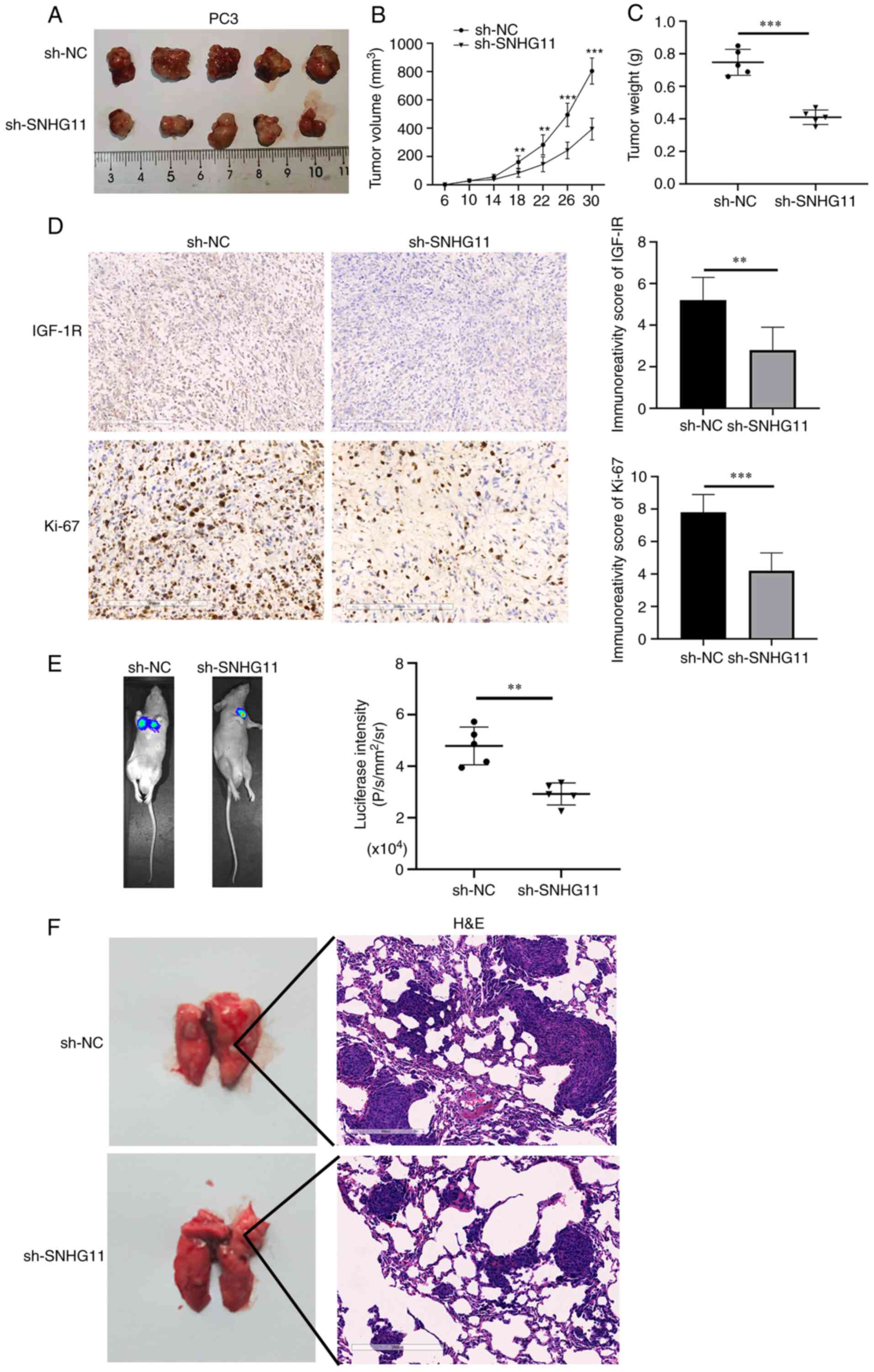 | Figure 4SNHG11-knockdown inhibits PCa growth
and metastasis in vivo. (A and B) The mean tumor volume of
the xenograft tumor in the PC3 SNHG11 knockdown group was
significantly reduced, in comparison with control group tumor
volumes. (C) The average weight of xenograft tumors in the PC3
SNHG11 knockdown group was markedly decreased, in comparison with
the control group weights. (D) IGF-1R and Ki-67 expression were
markedly decreased in tumor samples from the PC3 SNHG11 knockdown
group, in comparison with the control group. (E) Bioluminescence
imaging showing that SNHG11 knockdown markedly inhibited PCa cell
lung metastatic ability, in comparison with that of the control
group. (F) SNHG11-knockdown significantly reduced the tumor burden
in the lungs, in comparison with that of the control group.
**P<0.01, ***P<0.001, in comparison
with the control group. SNHG11, lncRNA small nucleolar RNA host
gene 11; PCa, prostate cancer; IGF-R1, insulin-like growth factor 1
receptor. |
Finally, the roles of SNHG11 in PCa metastasis in
vivo were investigated. As shown in Fig. 4E, the mice injected with
sh-SNHG11-transfected PC3 cells exhibited fewer lung metastases, as
compared with those injected with sh-NC-transfected PC3 cells.
H&E staining also suggested that the silencing of SNHG11
markedly reduced the metastatic burden in the lungs (Fig. 4F). According to these findings,
it was indicated that the silencing of SNHG11 may suppress the
metastasis of PCa in vivo.
Discussion
Numerous studies have reported that lncRNAs are
implicated in the development and metastasis of several types of
cancer. For example, Huang et al (15) found that lncRNA CASC2 expression
was significantly decreased in colorectal cancer (CRC) tissues and
CRC cell lines, and a decreased expression was significantly more
frequent in patients with advanced tumor-node-metastasis stage
disease. Chen et al (16)
observed that the expression of lncRNA CDKN2B antisense RNA 1 was
notably upregulated in metastatic hepatocellular carcinoma (HCC)
tissues, and facilitated disease progression by regulating the
miR-153-5p/Rho GTPase activating protein 18 signaling axis in HCC
cells. Kong et al (17)
reported that the overexpression of lncRNA cell division cycle 6 in
breast cancer cells markedly increased their proliferation and
invasion by regulating miR-215. Furthermore, He et al
(18) revealed that lncRNA
abhydrolase domain containing 11 antisense RNA 1 (ABHD11-AS1)
expression was upregulated in colorectal cancer and that the
overexpression of lncRNA ABHD11-AS1 markedly enhanced cell
proliferation and invasion by modulating the miR-1254/Wnt family
member 11 signaling axis.
SNHG11 (GenBank accession no. NR_003239.1) is a
recently identified lncRNA; however, little is known with regard to
its functional roles in cancer. It has been previously reported
that SNHG11 expression is frequently upregulated in several types
of cancer (9-11). In HCC, SNHG11 overexpression has
been reported to facilitate cell proliferation and migration
(19). However, the roles and
potential underlying molecular mechanisms of SNHG11 regulation in
PCa have not yet been fully elucidated, at least to the best of our
knowledge. Therefore, the present study aimed to investigate the
molecular mechanisms underlying the involvement of SNHG11 in PCa
progression. It was revealed that the SNHG11 expression levels were
significantly upregulated in PCa tissues and cells. Functional
experiments demonstrated that the silencing of SNHG11 expression
inhibited the proliferation, migration and invasion of PCa cells
in vitro. Moreover, SNHG11 silencing culminated in a
reduction in tumor growth and metastasis in vivo. These
results further confirmed the oncogenic role of SNHG11 in PCa.
Accumulating studies have indicated that lncRNAs may
act as sponges to regulate miRNAs. For example, Dong et al
(20) found that the
overexpression of lncRNA NEAT1 in endometrial cancer prooted
aggressive endometrial cancer progression. Zhu et al
(21) found that the
overexpression of lncRNA forkhead box D2 antisense RNA 1 in
colorectal cancer cells promoted cellular proliferation by sponging
miR-185-5p. Xu et al (22) reported that the overexpression of
lncRNA DiGeorge syndrome critical region gene 5 inhibited the
growth and metastasis of gastric cancer via targeting miR-23b. Long
et al (23) indicated
that silencing the lncRNA lysyl oxidase like 1-AS1 suppressed cell
cycle progression in PCa via inhibiting miR-541-3p. In the present
study, it was demonstrated that miR-184 was a downstream target of
SNHG11, as the relative luciferase activity of pmirGLO-SNHG11-WT
was suppressed by co-transfection with the miR-184 mimic.
Additionally, it was observed that the miR-184 expression levels
were significantly upregulated after the silencing of SNHG11.
Additionally, SNHG11 expression in PCa tissues was found to
negatively correlate with miR-184 expression. Collectively, these
findings indicated that SNHG11 may directly interact with miR-184
in PCa. Previous studies have also reported that miR-184 suppresses
the progression of numerous types of cancer, including renal cell
carcinoma (24), glioma
(25) and colorectal cancer
(26). However, miR-184 has also
been identified as an oncogene in HCC (27). In the present study, it was
revealed that miR-184 was sponged by SNHG11 and inhibited PCa
development.
In the present study, the target genes of miR-184
were predicted using the ComiRNet online tool. The relative
luciferase activity of pmirGLO-IGF-1R-WT was inhibited by
co-transfection with miR-184 mimic. Additionally, miR-184
overexpression significantly decreased the IGF-1R expression level
in PCa cells. miR-184 expression in PCa tissues was also observed
to negatively correlate with IGF-1R expression. Notably,
transfection with miR-184 inhibitor abolished the suppressive
effects of SNHG11 knockdown on the expression levels of IGF-1R. A
positive correlation between SNHG11 and IGF-1R expression levels
was also revealed in PCa tissues. IGF-1R overexpression reversed
the suppressive effects induced by SNHG11 knockdown on the
malignant phenotypes of PCa cells. Previous studies have also
demonstrated that IGF-1R plays an oncogenic role in several types
of cancer, including PCa (28),
bladder cancer (29), colorectal
cancer (26) and glioma
(30). Similarly, the data of
the present study also suggested that IGF-1R may act as an oncogene
in PCa.
In conclusion, according to the findings of the
present study, it was suggested that SNHG11 facilitates the
progression and metastasis of PCa by directly binding with miR-184,
in order to upregulate IGF-1R expression. Therefore, the
SNHG11/miR-184/IGF-1R signaling axis was revealed as a novel
molecular mechanism underlying PCa progression, which indicated
that SNHG11 may represent a possible promising therapeutic target
for PCa.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QX and XW were responsible for the conception of the
study and the drafting of the manuscript. SZ and RK assisted in the
design of the study and performed the statistical analysis. QX
revised the manuscript. QX and XW confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
All patients signed an informed consent form prior
to participation and the patient study protocol was approved by The
First Affiliated Hospital of University of South China Ethnics
Committee. The animal experiments in the present study were
performed in compliance with the guidelines and following the
approval of the Institute for Laboratory Animal Research at the
First Affiliated Hospital of University of South China (approval
no. LL20201103017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar
|
|
3
|
Kung JTY, Colognori D and Lee JT: Long
noncoding RNAs: Past, present, and future. Genetics. 193:651–669.
2013. View Article : Google Scholar
|
|
4
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar
|
|
5
|
Lingadahalli S, Jadhao S, Sung YY, Chen M,
Hu L, Chen X and Cheung E: Novel lncRNA LINC00844 regulates
prostate cancer cell migration and invasion through AR signaling.
Mol Cancer Res. 16:1865–1878. 2018. View Article : Google Scholar
|
|
6
|
Wu M, Huang Y, Chen T, Wang W, Yang S, Ye
Z and Xi X: LncRNA MEG3 inhibits the progression of prostate cancer
by modulating miR-9-5p/QKI-5 axis. J Cell Mol Med. 23:29–38. 2019.
View Article : Google Scholar
|
|
7
|
You Z, Liu C, Wang C, Ling Z, Wang Y, Wang
Y, Zhang M, Chen S, Xu B, Guan H and Chen M: LncRNA CCAT1 promotes
prostate cancer cell proliferation by interacting with DDX5 and
MIR-28-5P. Mol Cancer Ther. 18:2469–2479. 2019. View Article : Google Scholar
|
|
8
|
Su W, Xu M, Chen X, Chen N, Gong J, Nie L,
Li L, Li X, Zhang M and Zhou Q: Long noncoding RNA ZEB1-AS1
epigenetically regulates the expressions of ZEB1 and downstream
molecules in prostate cancer. Mol Cancer. 16:1422017. View Article : Google Scholar
|
|
9
|
Liu S, Yang N, Wang L, Wei B, Chen J and
Gao Y: IncRNA SNHG11 promotes lung cancer cell proliferation and
migration via activation of Wnt/β-catenin signaling pathway. J Cell
Physiol. 235:7541–7553. 2020. View Article : Google Scholar
|
|
10
|
Wu Q, Ma J, Wei J, Meng W, Wang Y and Shi
M: LncRNA SNHG11 promotes gastric cancer progression by activating
Wnt/β-catenin pathway and oncogenic autophagy. Mol Ther.
29:1258–1278. 2021. View Article : Google Scholar
|
|
11
|
Huang W, Dong S, Cha Y and Yuan X: SNHG11
promotes cell proliferation in colorectal cancer by forming a
positive regulatory loop with c-Myc. Biochem Biophys Res Commun.
527:985–992. 2020. View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
13
|
Kang R, Zhao S, Liu L, Li F, Li E, Luo L,
Xu L, Wan S and Zhao Z: Knockdown of PSCA induces EMT and decreases
metastatic potentials of the human prostate cancer DU145 cells.
Cancer Cell Int. 16:202016. View Article : Google Scholar
|
|
14
|
Chen HX and Sharon E: IGF-1R as an
anti-cancer target-trials and tribulations. Chin J Cancer.
32:242–252. 2013. View Article : Google Scholar
|
|
15
|
Huang G, Wu X, Li S, Xu X, Zhu H and Chen
X: The long noncoding RNA CASC2 functions as a competing endogenous
RNA by sponging miR-18a in colorectal cancer. Sci Rep. 6:265242016.
View Article : Google Scholar
|
|
16
|
Chen J, Huang X, Wang W, Xie H, Li J, Hu
Z, Zheng Z, Li H and Teng L: LncRNA CDKN2BAS predicts poor
prognosis in patients with hepatocellular carcinoma and promotes
metastasis via the miR-153-5p/ARHGAP18 signaling axis. Aging
(Albany NY). 10:3371–3381. 2018. View Article : Google Scholar
|
|
17
|
Kong X, Duan Y, Sang Y, Li Y, Zhang H,
Liang Y, Liu Y, Zhang N and Yang Q: LncRNA-CDC6 promotes breast
cancer progression and function as ceRNA to target CDC6 by sponging
microRNA-215. J Cell Physiol. 234:9105–9117. 2019. View Article : Google Scholar
|
|
18
|
He D, Yue Z, Liu L, Fang X, Chen L and Han
H: Long noncoding RNA ABHD11-AS1 promote cells proliferation and
invasion of colorectal cancer via regulating the miR-1254-WNT11
pathway. J Cell Physiol. 234:12070–12079. 2019. View Article : Google Scholar
|
|
19
|
Huang W, Huang F, Lei Z and Luo H: LncRNA
SNHG11 promotes proliferation, migration, apoptosis, and autophagy
by regulating hsa-miR-184/AGO2 in HCC. Onco Targets Ther.
13:413–421. 2020. View Article : Google Scholar
|
|
20
|
Dong P, Xiong Y, Yue J, Xu D, Ihira K,
Konno Y, Kobayashi N, Todo Y and Watari H: Long noncoding RNA NEAT1
drives aggressive endometrial cancer progression via
miR-361-regulated networks involving STAT3 and tumor
microenvironment-related genes. J Exp Clin Cancer Res. 38:2952019.
View Article : Google Scholar
|
|
21
|
Zhu Y, Qiao L, Zhou Y, Ma N, Wang C and
Zhou J: Long non-coding RNA FOXD2-AS1 contributes to colorectal
cancer proliferation through its interaction with microRNA-185-5p.
Cancer Sci. 109:2235–2242. 2018. View Article : Google Scholar
|
|
22
|
Xu Y, Zhang G, Zou C, Gong Z, Wang S, Liu
J, Ma G, Liu X, Zhang W and Jiang P: Long noncoding RNA DGCR5
suppresses gastric cancer progression by acting as a competing
endogenous RNA of PTEN and BTG1. J Cell Physiol. 234:11999–12010.
2019. View Article : Google Scholar
|
|
23
|
Long B, Li N, Xu XX, Li XX, Xu XJ, Liu JY
and Wu ZH: Long noncoding RNA LOXL1-AS1 regulates prostate cancer
cell proliferation and cell cycle progression through miR-541-3p
and CCND1. Biochem Biophys Res Commun. 505:561–568. 2018.
View Article : Google Scholar
|
|
24
|
Su Z, Chen D, Li Y, Zhang E, Yu Z, Chen T,
Jiang Z, Ni L, Yang S, Gui Y, et al: MicroRNA-184 functions as
tumor suppressor in renal cell carcinoma. Exp Ther Med. 9:961–966.
2015. View Article : Google Scholar
|
|
25
|
Cheng Z, Wang HZ, Li X, Wu Z, Han Y, Li Y,
Chen G, Xie X, Huang Y, Du Z and Zhou Y: MicroRNA-184 inhibits cell
proliferation and invasion, and specifically targets TNFAIP2 in
glioma. J Exp Clin Cancer Res. 34:272015. View Article : Google Scholar
|
|
26
|
Wu G, Liu J, Wu Z, Wu X and Yao X:
MicroRNA-184 inhibits cell proliferation and metastasis in human
colorectal cancer by directly targeting IGF-1R. Oncol Lett.
14:3215–3222. 2017. View Article : Google Scholar
|
|
27
|
Wu GG, Li WH, He WG, Jiang N, Zhang GX,
Chen W, Yang HF, Liu QL, Huang YN, Zhang L, et al: Mir-184
post-transcriptionally regulates SOX7 expression and promotes cell
proliferation in human hepatocellular carcinoma. PLoS One.
9:e887962014. View Article : Google Scholar
|
|
28
|
Aleksic T, Verrill C, Bryant RJ, Han C,
Worrall AR, Brureau L, Larré S, Higgins GS, Fazal F, Sabbagh A, et
al: IGF-1R associates with adverse outcomes after radical
radiotherapy for prostate cancer. Br J Cancer. 117:1600–1606. 2017.
View Article : Google Scholar
|
|
29
|
Liao G, Chen F, Zhong J and Jiang X:
MicroRNA-539 inhibits the proliferation and invasion of bladder
cancer cells by regulating IGF-1R. Mol Med Rep. 17:4917–4924.
2018.
|
|
30
|
Jiang J, Wang W, Fang D, Jin X, Ding L and
Sun X: MicroRNA-186 targets IGF-1R and exerts tumor-suppressing
functions in glioma. Mol Med Rep. 16:7821–7828. 2017. View Article : Google Scholar
|