Introduction
The occurrence of defects and deformity of bone
tissue affects normal function and patient mobility (1). Severe cases may also result in
psychological problems for patients (1). Multiple treatment options have been
available in the past, including bone transplantation (either
autologous or allogeneic) and the application of bone substitute
materials (2). However, these
methods have disadvantages, including rejection, high surgery
costs, limited availability of bone, surgical trauma and
complicated surgery (3), which
result in limited clinical application. Two fundamental processes,
bone formation and resorption, are involved in bone metabolic
formation, which leads to decreased bone formation and mass; the
application of β-receptor blocker propranolol significantly
increases bone formation and bone mass (4,5).
As the effects of the sympathetic nervous system on
bone remodeling and healing are complex and extensive, the
underlying mechanism has not yet been fully recognized (6). Numerous experiments have
investigated the effect of propranolol, a sympathetic blocker drug,
on bone metabolism (7-9). To the best of our knowledge,
however, few reports have illustrated the effect and mechanism of
propranolol on proliferation of osteoblasts (OBs) and bone
resorption of osteoclasts (OCs). OBs are derived from bone
marrow-derived stem cells (BMSCs); OBs generate bone matrix during
bone formation and release diverse bioactive substances to regulate
and control the function of OCs (10,11). The stimulation of the sympathetic
nervous system increases bone resorption and inhibits bone
formation (12). This mechanism
is associated with the activity of β2-adrenergic receptors (ARs) on
OBs. Takeda et al (13)
confirmed the presence of β2-ARs (and absence of other adrenoceptor
subtypes) on OBs using reverse transcription-quantitative (RT-q)PCR
and northern blotting in OBs. The most frequently administered
drugs for hypertension and cardiovascular disease are β2-AR
blockers. A previous study demonstrated the effect of β2-AR
blockers in skeleton tissue (7).
AR agonists promote bone resorption in mice (7). Systemic application of β-AR
agonists decreases bone formation and mass, while the opposite
result has been obtained using the β-AR blocker propranolol
(14-16). To the best of our knowledge,
however, the effects and regulatory mechanisms of propranolol on
OBs and MSCs have not been fully explored. Therefore, the present
study aimed to investigate whether the β-AR blocker propranolol
promotes osteogenic differentiation of OBs and MSCs and enhances
osseointegration of implants. OBs and MSCs were treated with
propnaolol and the effects on cell proliferation and osteogenic
differentiation and expression levels of osteoblast-associated
proteins were detected. The present study also aimed to serve as a
basis for further investigation of the mechanisms and effects of
propranolol on implant osseointegration.
Materials and methods
Model establishment of implant
osseointegration
The present animal experiment was performed in
strict accordance with the regulations of the Ethics Committee of
The Affiliated Hospital of Qingdao University (approval no.
201905036). A total of 32 healthy male New Zealand white rabbits
(age, 5-6 months; weight ~3.5 kg), purchased from Qingdao Kangda
Biological Technology Co., Ltd., were randomly divided into four
groups (n=8/group): Control and low-(0.1 mg/kg), medium-(1 mg/kg)
and high-dose propranolol (10 mg/kg). Rabbits were housed in
sterile cages at 22°C and relative humidity of 50-60%, with 12 h
light/dark cycle and free access to fodder and sterile water. All
rabbits were acclimatized for 7 days before the operation. The
anesthetic protocol was based on the Guide for Care and Use of
Laboratory Animals (17). For
anesthesia, xylazine hydrochloride (Jilin Province Huamu Animal
Health Products Co., Ltd.) at 5 mg/kg was intramuscularly injected,
followed by slow intravenous injection of 3% pentobarbital sodium
(Sigma-Aldrich; Merck KGaA) at 24 mg/kg. Subcutaneous propoanolol
injections were administered to the propranolol groups close to the
surgical site preoperatively and an equal amount of normal saline
was given to the control group for 28 successive days. Animals from
each group subsequently received conventional surgical cavity
preparation at the metaphysis of the tibia and two pure titanium
implants (diameter, 3.4 mm; length; 8.0 mm) were inserted using an
implant machine, one in each hindleg. On days 1-3 postoperatively,
rabbits in each group were subcutaneously injected with
buprenorphine hydrochloride (0.03 mg/kg) and doxycycline (3.2
mg/kg) and all healed well following surgery. Bone tissue binding
to implants and absorption was observed through X-ray examinations.
Then, rabbits in each group were sacrificed with a lethal dose of
pentobarbital sodium (100 mg/kg) by intravenous injection and the
intact tibia tissue was removed for subsequent experiments.
Extraction of MSCs
Isolation, culture and characterization of MSCs by
cell surface markers detection and multi-lineage differentiation
were performed as described previously by Farahzadi et al
(8,9) and Fathi et al (18,19). Two laboratory rabbits were
sacrificed and placed in 75% alcohol at room temperature for 15
min. Following separation on a clean bench under aseptic
conditions, the tibia and femur of rabbits were placed into a
beaker supplemented with PBS solution. The connective tissue and
periosteum were removed immediately and the medullary cavity was
washed with serum-free Dulbecco's modified eagle medium (DMEM) in a
syringe and transferred into a centrifuge tube containing DMEM with
15% fetal bovine serum (FBS; both HyClone; Cytiva). When the
medullary cavity turned white, ultrasound shaking at room
temperature was performed at 240 × g for 5 min. The supernatant was
discarded and high-glucose DMEM containing 15% FBS was added for
cell suspension preparation. The samples were planted in a culture
flask while being blown evenly. The culture medium was refreshed
after 24 h and replaced every 2-3 days. Experiments were performed
using third passage cells.
OBs isolation and culture
Isolation and culture of OBs were performed as
described by Zhao et al (20). New Zealand rabbits were
anesthetized by intravenous injection of pentobarbital sodium. The
femur was subsequently removed and treated as aforementioned. The
marrow was extracted from cleaned bones using DMEM supplemented
with 15% FBS (HyClone; Cytiva) and incubated at 37°C with the
medium replaced every 3 days. Once the cells were 50% confluent,
the medium was replaced with DMEM containing 10% FBS and 1%
penicillin + streptomycin (all HyClone; Cytiva). The cells were
harvested after reaching 80-90% confluence using 0.25% trypsin.
Experiments were performed using third passage cells.
Alizarin red staining
MSCs or OBs were cultured in DMEM containing 50 nM
isoproterenol to simulate the effects of the sympathetic nerve
(21). Following 24 h culture in
DMEM, MSCs or OBs were cultured in osteogenic differentiation
medium [5 mM β-glycerophosphate, 50 µg/ml ascorbic acid
phosphate and 10 nM dexamethasone (all Sigma-Aldrich; Merck KGaA)
in DMEM supplemented with 15% FBS] containing 50 nM isoproterenol
and propranolol at different concentrations for 7-day induction at
37°C. Mineralized nodule alizarin red staining was subsequently
performed. The original culture medium was discarded and PBS
solution was used for three cycles of washing. Subsequently, 4%
paraformaldehyde was used for fixation at room temperature for 20
min. Finally, the PBS solution was used to rinse cells. Cells were
stained with 0.1% alizarin red dye for 30 min at 37°C, washed with
running water and examined under a light microscope at ×200
magnification.
Alkaline phosphatase (ALP) staining
ALP staining was performed using the Gomori modified
calcium-cobalt method, as previously described Huang et al
(22). Following 3 weeks of
induction, MSCs or OBs were inoculated into a petri dish (density,
2×104). Following removal of the original culture
medium, cells were fixed with 95% ethanol at room temperature for
10 min, and then incubated with incubation buffer (5 3% sodium
β-glycerophosphate, 5 2% sodium barbiturate, 10 distilled water, 10
2% CaCl2 and 1 ml 2% MgSO4) at 37°C for 4 h.
Substrate solution was subsequently added to the petri dish,
covered with hydrophobic membrane, incubated in a 37°C oven in the
dark for 15 min and washed. Finally, dye solution was added for
staining at room temperature for 3 min and samples were washed with
running water and examined under a light microscope at ×200
magnification.
Cell proliferation assessment via EdU
assay
EdU assay was performed as described by Shen et
al (23). Briefly, MSCs or
OBs (1×107) were cultured at 37°C in 6-well plates
overnight. Cells were supplemented with EdU (10 µM) and
incubated at 37°C for 2 h. After DMEM was removed, the cells were
fixed with 1 ml 4% paraformaldehyde for 15 min at room temperature.
After fixing medium was removed, cells were washed 3 times by
adding 1 ml/well washing solution (3-5 min each). The washing
solution was discarded. Then, 1 ml PBS containing 0.3% Triton X-100
was added to each well and incubated at room temperature for 10-15
min. The cells in each well were washed with 1 ml washing solution
1-2 times (3-5 min each). Then, endogenous peroxidase blocking
solution was added for incubation at room temperature for 20 min to
inactivate endogenous peroxidase activity. The cells were then
rinsed with washing solution 3 times (2 min each) and visualized
via a fluorescent microscope (Olympus Corporation) at ×200
magnification.
Cell proliferation assessment via flow
cytometry
To determine cell proliferation, BrdU assay was
performed as described by Heo et al (24). The MSCs or OBs (density,
1.5×105/ml) were inoculated in a 35-mm-diameter culture
dish for 1 day at 37°C and synchronized in DMEM containing 0.4% FBS
for 3 days until most cells entered the G0 phase. Cell
proliferation was measured by a BrdU Staining kit for Flow
Cytometry (cat. no. 8811-6600-42; Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Anti-BrdU FITC was added and incubated at 37°C for 40 min. The
culture medium was discarded. The cells were collected into a flow
tube and centrifugated at room temperature and 350 × g for 5 min.
The supernatant was discarded. Each tube was fixed at room
temperature with 1 ml 4% paraformaldehyde for 15 min and
centrifugated at room temperature and 600 × g for 10 min. The
supernatant was discarded. After washing, 2 glycine and 1 ml 0.5%
triton was added to each tube and incubated at 37°C for 10 min.
Following washing with PBS, the cells were resuspended and detected
by BD LSRFortessa Flow Cytometer (BD Biosciences). Data was
analyzed using CytExpert 2.3 software (Beckman Coulter, Inc.).
Wound healing assay
MSCs or OBs were seeded into 6-well plates at a cell
density of 5×105/ml (500 µl/well) and cultured at
37°C for 24 h in RPMI-1640 complete growth medium (Invitrogen;
Thermo Fisher Scientific, Inc.) to form an 80% confluent monolayer,
which was scratched using the tip of a 10-µl pipetting gun.
Cells were washed three times with PBS and incubated for 48 h
(37°C; 5% CO2) in serum-free DMEM (HyClone; Cytiva). The
cells were observed and photographed at 24 and 48 h under an
inverted fluorescence microscope (Olympus Corporation) at ×200
magnification and quantification of scratch closure was evaluated
using the wound healing measurement tool of ImageJ V1.8.0.112
(National Institutes of Health).
RT-qPCR
Total RNA was isolated from MSCs or OBs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RT-qPCR was performed as described by Yin et al
(25). RT was performed using a
PrimeScript™ RT reagent kit (cat. no. RR047A; Takara Biotechnology
Co., Ltd.) and a reaction system was established containing 2.2
µg RNA, 2.0 µl OligodT, 4.0 µl dNTP, 4.0
µl 5X buffer, 1.0 µl reverse transcriptase, 0.5
µl RNAase inhibitor and ≤20.0 µl RNAase-free
ddH2O. The reaction conditions were as follows: 25°C for
5 min, 50°C for 15 min, 85°C for 5 min and 4°C for 10 min. qPCR was
performed using a 2xT5 SYBR Green Fast qPCR Mix kit (cat. no.
TSE202; TsingKe Biological Technology) according to the
manufacturer's instructions. The reaction system contained 0.4
forward primer, 0.4 reverse primer, 10.0 SYBR Green and 5.2
µl H2O. The thermocycling conditions were as
follows: 50°C for 2 min, 95°C for 10 min, 95°C for 30 sec and 60°C
for 30 sec for 40 cycles. The 2−ΔΔCq method was used to
calculate the relative expression levels (26). The primers were as follows
(5′-3′): BMP2 forward, TGG CCC ATT TAG AGG AGA ACC and reverse, AGG
CAT GAT AGC CCG GAG G; RUNX family transcription factor (RunX)2
forward, GAG ACT ACT GCC GAC CAC and reverse, TAC CTC TCC GAG GGC
TAC C; collagen (COL)-1 forward, AGG GCC AAG ACG AAG ACA TC and
reverse, AGA TCA CGT CAT CGC ACA ACA ; osteocalcin (OCN) forward,
CTC ACA CTC CTC GCC CTA TT and reverse, CGC CTG GGT CTC TTC ACT AC;
β2-AR forward, GGA CAA CCT CAT CCC TAA and reverse, GGA CAA CCT CAT
CCC TAA and GAPDH forward, CAC ATG GCC TCC AAG GAG TAA and reverse,
GTA CAT GAC AAG GTG CGG CTC. GAPDH was used as the control for
normalization.
Protein expression level detection by
western blot analysis
Western blotting was performed as described by Yin
et al (25). Total
protein was extracted from MSCs or OBs and bone tissue using RIPA
reagent (Beyotime Institute of Biotechnology), and the
concentration of extracted proteins was measured by BCA. Proteins
(20 µg/lane) were isolated using SDS-PAGE (12% separating
gel and 5% stacking gel) and transferred to polyvinylidene
difluoride membranes. The membranes were blocked with 5% BSA
(Beyotime Institute of Biotechnology) at 37°C for 1 h. The
membranes were incubated with primary antibodies against BMP2,
RunX2, COL-1, OCN and β2-AR overnight at 4°C, and using
corresponding secondary antibodies incubated for 1.5 h at room
temperature. Protein bands were detected by an enhanced
chemiluminescent kit (Thermo Fisher Scientific, Inc.). The
antibodies (all Chongqing Boai Madison Biotechnology Co., Ltd.)
were as follows: BMP2 (1:500; cat. no. BM19970), RunX2 (1:500; cat.
no. BM16360), COL-1 (1:500; cat. no. BM2319), OCN (1:500; cat. no.
BM18692), β2-AR (1:500; cat. no. BMP0265), β-actin (1:2,000; cat.
no. BMC026) and horseradish peroxidase-conjugated goat anti-rabbit
IgG (1:2,000; cat. no. BMS014). Results were normalized yo β-actin.
Western blots were quantified using Image J V1.8.0.112 (National
Institutes of Health).
Immunofluorescence detection
Immunofluorescence detection was performed as
described by Fathi et al (18). The MSCs or OBs were fixed with 4%
paraformaldehyde at 4°C overnight. Paraffin-embedded sections
(thickness, 4 µm) were baked at 60°C for 2 h, dewaxed and
hydrated with xylene and alcohol. The sections were placed in
citrate buffer solution (pH 6.0) for antigen repair, heated at 98°C
in a microwave oven for 20 min and left to cool to room
temperature. Three cycles of washing were performed with PBS (5 min
each) followed by blocking with 5% BSA for 30 min at room
temperature. The aforementioned primary antibodies (1:100) were
added and incubated overnight at 4°C. Antibodies are described in
western blot. Following reheating at 37°C for 30 min, PBS was used
for washing (3 times; 5 min each). Corresponding secondary
antibodies conjugated to Alexa594 (cat. no. R37117, 1:100; Thermo
Fisher Scientific, Inc.) were added and incubated at room
temperature for 1 h. PBS washing was repeated 3 times (5 min each).
Nuclei were visualized by staining with 0.3 µM DAPI in the
dark for 5-10 min at 37°C and then PBS was used to wash 3 times (1
min each). The sections were observed and photographed under a
fluorescence microscope at ×200 magnification.
Statistical analysis
The experimental data were statistically analyzed
using SPSS 23.0 software (IBM Corp.) and are expressed as the mean
± SD of three independent experiments. A paired t-test was used to
compare groups before and after treatment. One-way ANOVA with
Tukey's post hoc test was used for comparison between multiple
groups. P<0.05 and P<0.01 were considered to indicate a
statistically significant difference.
Results
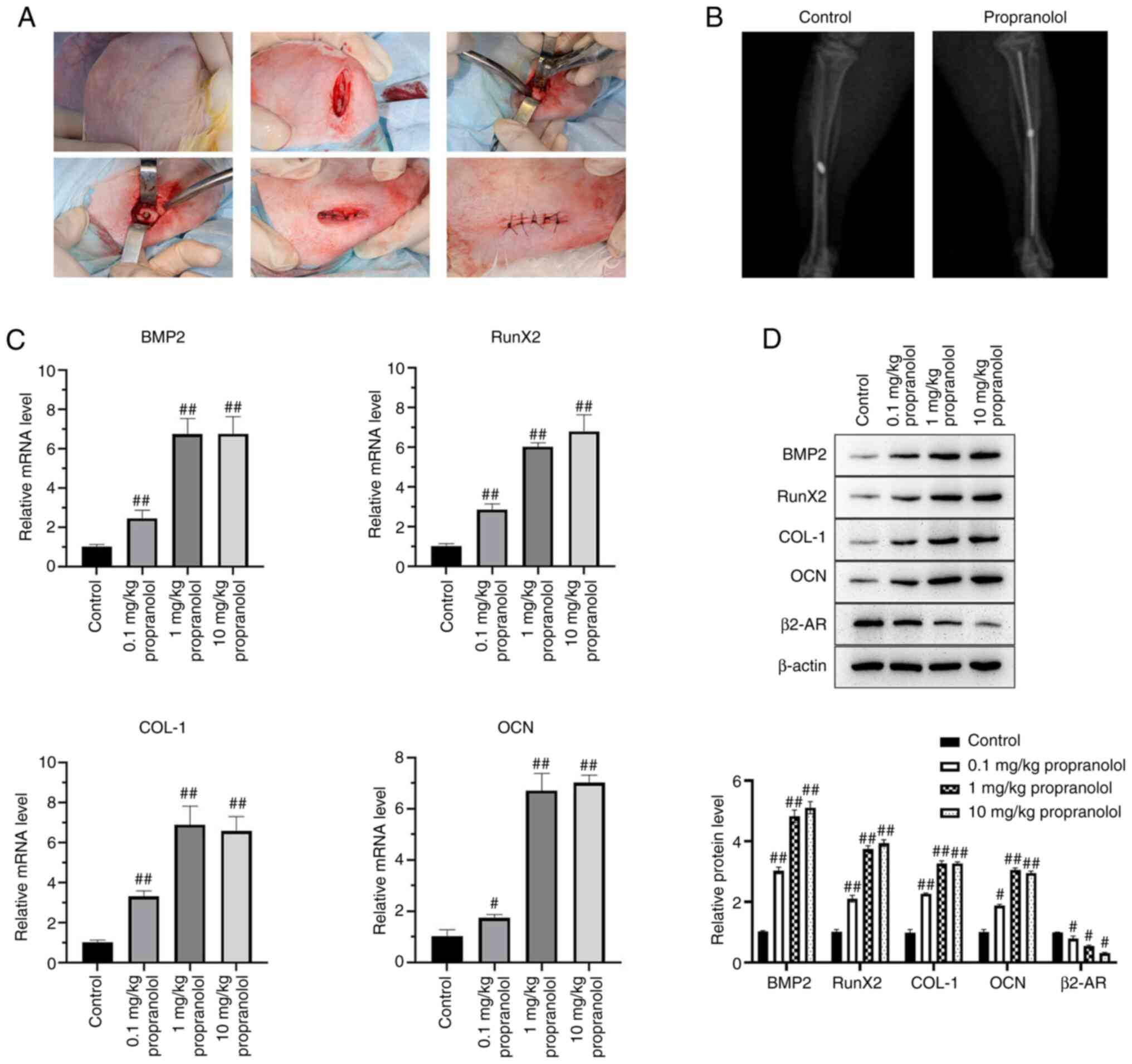
Propranolol promotes osseointegration of
implants
A New Zealand white rabbit model of implant
osseointegration was established and injected with different doses
of propranolol (0.1, 1.0 and 10.0 mg/kg). At 14 days post-surgery,
bone tissue binding to implants and absorption were examined by
X-ray scanning (Fig. 1A and B).
Bone tissue samples of rabbits near the implants were collected and
mRNA expression of BMP2, RunX2, COL-1, OCN, and β2-AR was detected
by qPCR. Propranolol increased mRNA expressions of BMP2, RunX2,
COL-1 and OCN and decreased that of β2-AR. The protein expression
of BMP2, RunX2, COL-1, OCN and β2-AR was detected by western
blotting; propranolol increased protein levels of BMP2, RunX2,
COL-1 and OCN and decreased β2-AR levels. (Fig. 1C and D).
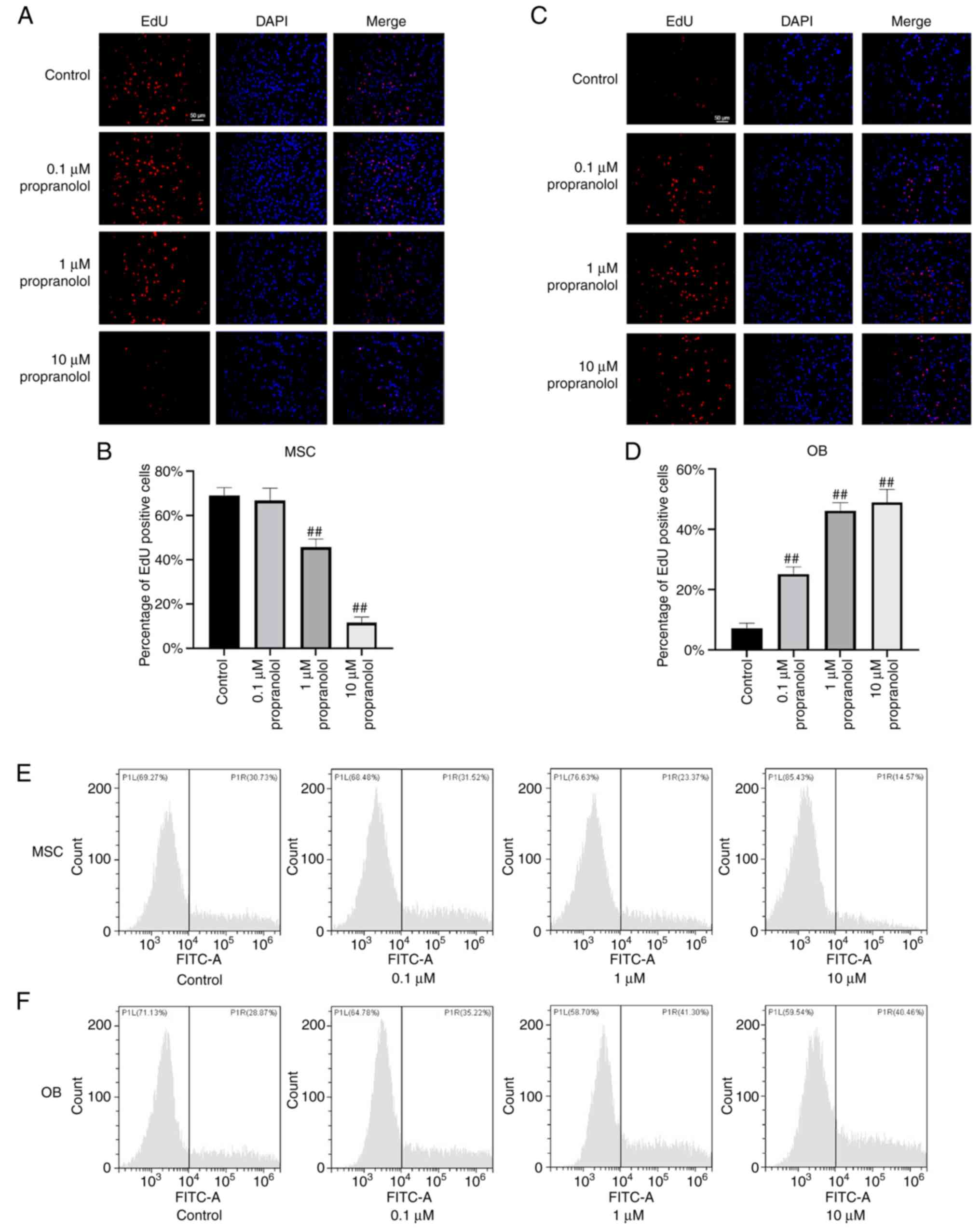
Propranolol inhibits proliferation of
MSCs and promotes proliferation of OBs
To determine the effect of propranolol on
proliferation of MSCs and OBs, MSCs and OBs were cultured in
vitro using complete medium containing isoproterenol and
treated with propranolol for 24 h. Cell proliferation was detected
by EdU staining. The number of EdU-positive MSCs decreased markedly
following the addition of propranolol and was lowest in the 10
µM propranolol group, indicating that propranolol inhibited
proliferation of MSCs (Fig. 2A and
B). In OBs, all concentrations of propranolol significantly
promoted cell proliferation and the number of Edu-positive cells in
the 1 and 10 µM propranolol groups were similar (Fig. 2C and D). These results suggested
that propranolol inhibited proliferation of MSCs and promoted
proliferation of OBs.
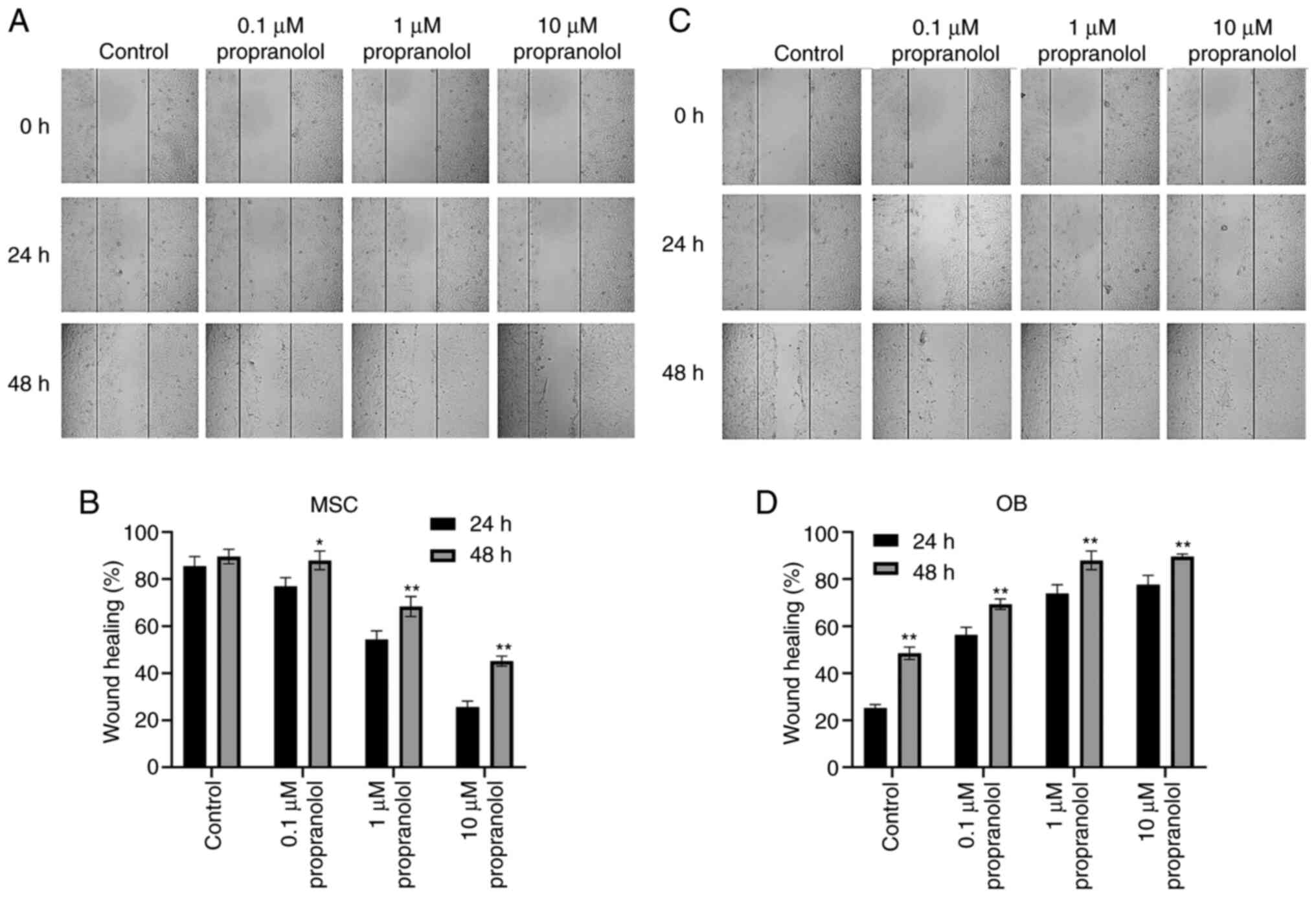
Propranolol inhibits migration of MSCs
and promotes migration of OBs
The effect of propranolol on cell migration was
detected by wound healing at 24 and 48 h. MSC migration was
inhibited following the addition of propranolol to complete medium
containing isoproterenol. Moreover, the healing rate of scratches
was markedly lower in propranolol-treated MSCs compared with the
control group; the higher the concentration, the lower the healing
rate (Fig. 3A and B).
Conversely, propranolol improved the healing rate of OBs and cell
migration was higher than that of the control group (Fig. 3C and D). These results suggested
that propranolol inhibited MSC migration and promoted OB
migration.
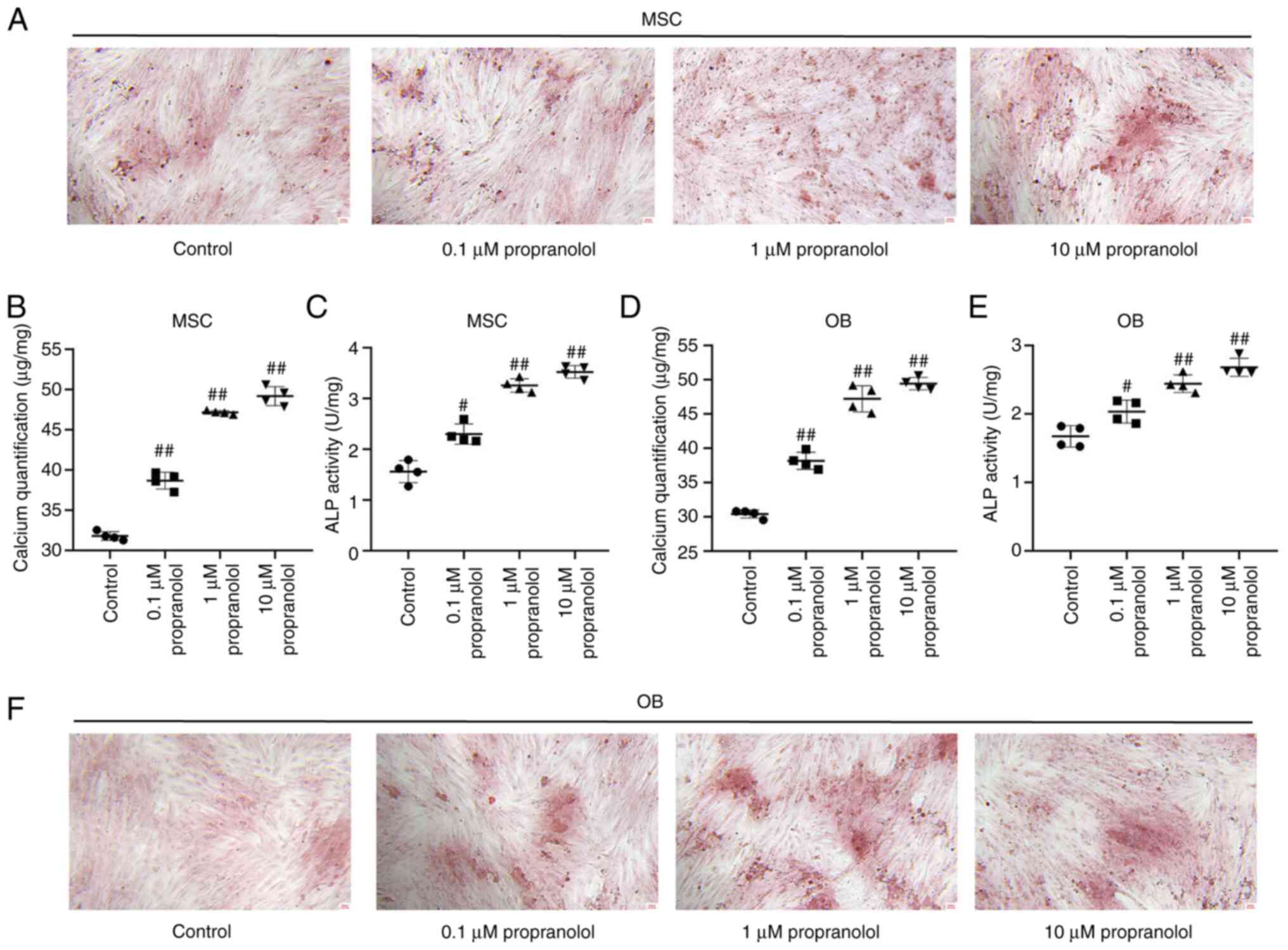
Propranolol promotes osteogenic
differentiation of MSCs and OBs
To determine the effect of propranolol on osteogenic
differentiation of MSCs and OBs, MSCs and OBs cultured in
vitro with osteogenic differentiation medium containing
isoproterenol and propanolol. Following induction for 7 days,
osteogenic differentiation of cells was detected by alizarin red
staining. Propranolol promoted osteogenic differentiation of both
MSCs and OBs and increased the number of intracellular calcium
nodules following induction. The calcium content and ALP activity
of cells treated with propranolol were significantly higher
compared with the control group (Fig. 4A-F).
Regulatory effect of propranolol on
osteogenesis-associated genes
To elucidate the osteogenic mechanism of propranolol
in regulating MSCs (Fig. 5A-H)
and OBs (Fig. 6A-H), expression
levels of osteogenesis-associated genes BMP2, RunX2, COL-1, OCN and
β2-AR were detected by immunofluorescence, RT-qPCR and western
blotting. The findings demonstrated that propranolol significantly
increased protein expression levels of BMP2, RunX2, COL-1 and OCN
and decreased expression of β2-AR in a dose-dependent manner. The
results of RT-qPCR and western blotting were consistent with those
of immunofluorescence analysis, indicating that propranolol
upregulated expression levels of osteogenesis-associated genes
(BMP2, RunX2, COL-1 and OCN), thereby promoting osteogenic
differentiation of MSCs and OBs (Fig. 7A-D).
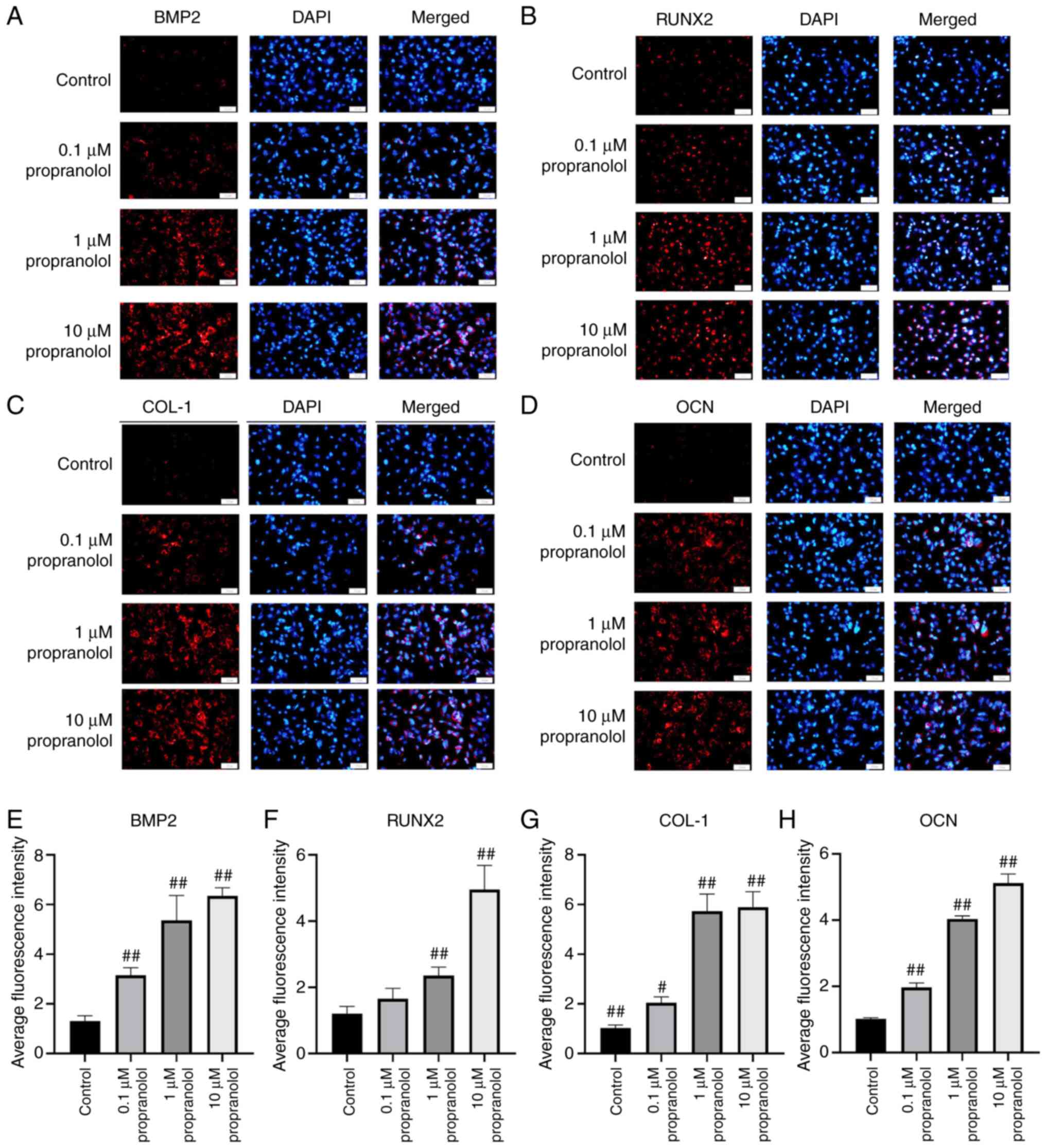 | Figure 5Regulatory effect of propranolol on
osteogenesis-associated genes in MSCs. Immunofluorescence was used
to detect (A) BMP2, (B) RunX2, (C) COL-1 and (D) OCN expression
levels in MSCs. Relative immunofluorescence intensity of (E) BMP2,
(F) RunX2, (G) COL-1 and (H) OCN was measured by Image J.
Magnification, ×200. Scale bar, 50 µm. Statistical
significance was assessed by ANOVA with Tukey's post hoc test.
#P<0.05, ##P<0.01. BMP2, bone
morphogenetic protein 2; RunX2, RUNX family transcription factor 2;
COL-1, collagen 1; OCN, osteocalcin; MSC, mesenchymal stem cell;
OB, osteoblast. |
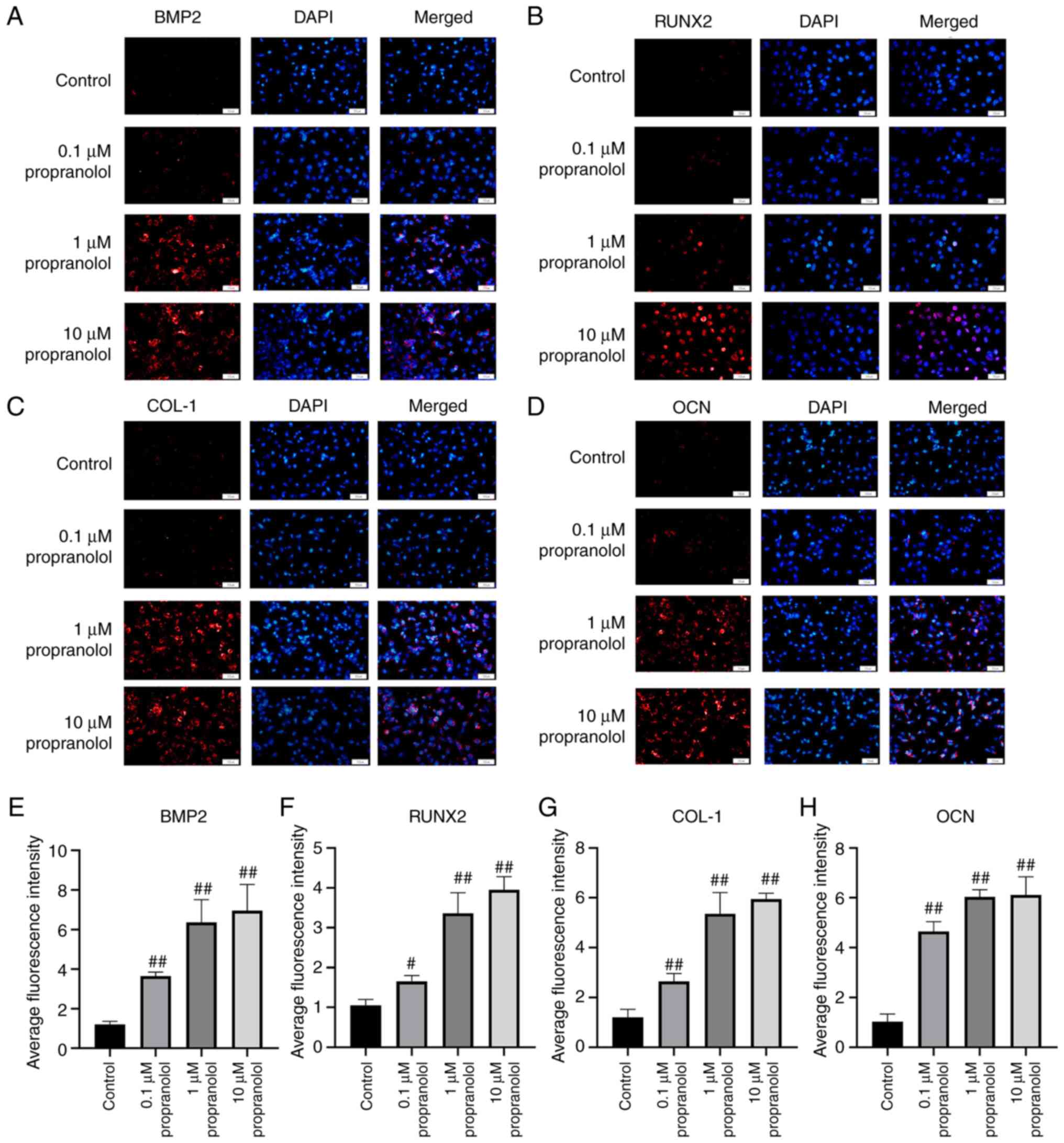 | Figure 6Regulatory effect of propranolol on
osteogenesis-associated genes in OBs. Immunofluorescence was used
to detect (A) BMP2, (B) RunX2, (C) COL-1 and (D) OCN expression in
OBs. Relative immunofluorescence intensity of (E) BMP2, (F) RunX2,
(G) COL-1 and (H) OCN was measured by Image J. Magnification, ×200.
Scale bar, 50 µm. Statistical significance was assessed by
ANOVA with Tukey's post hoc test. #P<0.05,
##P<0.01, vs. control. BMP2, bone morphogenetic
protein 2; RunX2, RUNX family transcription factor 2; COL-1,
collagen 1; OCN, osteocalcin; MSC, mesenchymal stem cell; OB,
osteoblast. |
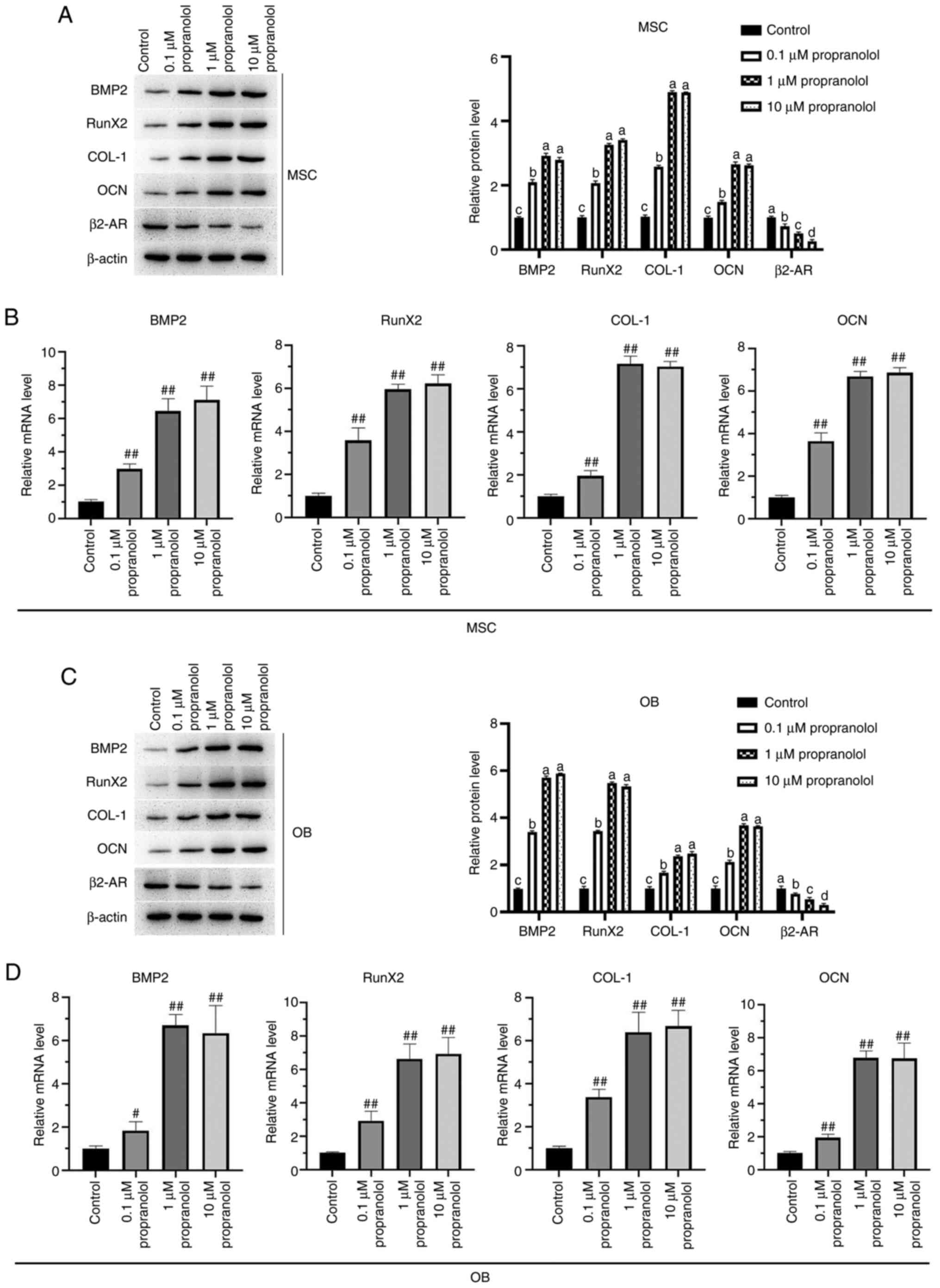 | Figure 7Expression levels of BMP2, RunX2,
COL-1, OCN and β2-AR in MSCs and OBs detected by RT-qPCR and
western blotting. Expression levels of BMP2, RunX2, COL-1, OCN and
β2-AR in MSC were detected by (A) western blotting and (B) RT-qPCR.
Expression levels of BMP2, RunX2, COL-1, OCN and β2-AR in OB were
detected by (C) western blotting and (D) RT-qPCR. Statistical
significance was assessed by ANOVA with Tukey's test.
#P<0.05, ##P<0.01 vs. control. BMP2,
bone morphogenetic protein 2; RunX2, RUNX family transcription
factor 2; COL-1, collagen 1; OCN, osteocalcin; MSC, mesenchymal
stem cell; OB, osteoblast; AR, adrenergic receptor; RT-q, reverse
transcription-quantitative. |
Discussion
Early rapid osseointegration is a key factor for
successful implantation but is limited by biological inertia, high
elastic modulus and limited biological effects of the material
surface (27). An effective
technique to improve the performance of titanium implants and to
promote and accelerate osseointegration has been the focus of
research in recent years (28-30). Despite titanium and titanium
alloy implants are widely used in the fields of dentistry and oral
and maxillofacial surgery, postoperative infection and poor
osseointegration remain obstacles to implant surgery (31). Drugs, such as zoledronic acid,
osteoprotegerin, and kaempferol, also promote early
osseointegration of implants (32-34).
As an integrated part of the autonomic nervous
system of the human body, the sympathetic nervous system serves a
key role in regulating homeostasis. Growing evidence has shown that
the sympathetic nervous system is involved in bone remodeling
(6,35). In 1977, Duncan and Shim (36) demonstrated that the surface of
intraosseous vessels is covered with rich adrenergic nerve fibers
by observing the bone tissue of rabbits using histochemistry and
fluorescence electron microscopy. Mach et al (37) demonstrated that sympathetic nerve
fibers are primarily located in the bone marrow cavity, favors
areas of abundant blood flow and are rare in the periosteum. When
the sympathetic nerve is excited, bone resorption is promoted and
bone formation is decreased via β2-ARs on the surface of OBs
(38). β2-AR has been visualized
on the surface of human OBs by immunofluorescence and studies using
β2-AR agonists indicated that they serve a role in inhibiting
proliferation of OBs (38,39). Although regulation of sympathetic
nerve on bone turnover via β2-ARs expressed on OBs has been
demonstrated, little is known about the effect of β-AR blocker
propranolol on osteogenic differentiation of OBs. In the study,
propranolol promoted the proliferation and differentiation of OBs,
which is consistent with previous studies (40,41).
Propranolol is a recognized drug for treatment of
hypertension and cardiovascular disease (42-44). Propranolol is a non-selective β1
and β2-AR blocker that has been used since 1964 to treat coronary
artery insufficiency (45).
Propranolol competitively inhibits the action of epinephrine and
norepinephrine on β1- and β2-ARs (46). Multiple studies have shown that
propranolol inhibits expression of β2-AR (47,48). Previously, the effect of
propranolol on bone metabolism has received attention (41,49,50). Minkowitz et al (49) reported that mineral deposition
and bone formation increase in a rat model of surgical fracture
treated with propranolol for 9 weeks. Bonnet et al (41) found that low-dose propranolol
improved bone formation and prevented osteoclasts proliferation in
ovariectomized rats. Epidemiological studies have also demonstrated
that β2-AR blockers serve as potential candidate drugs for
treatment of osteoporosis and fractures (51,52). β-blockers enhanced bone healing
and improved bone metabolism (53). The present study investigated the
effects of β-AR blocker propranolol on osteogenesis in an animal
model. The results demonstrated that propranolol promoted
osseointegration of implants in rabbits, which is consistent with
conclusions of previous studies (7,50), suggesting that propranolol
enhances bone regeneration and implant osseointegration.
No consensus on the mechanism of β2-AR in regulating
osteogenesis and osteoclast has been reached. By investigating the
osteogenic mechanism of MSCs, certain scholars have reported that
β-AR activators inhibit osteogenesis of MSCs, while blockers
promote osteogenesis of MSCs (54,55). The β-AR activator also inhibits
the signaling pathway associated with osteogenesis by regulating
expression of MEK and ERK1/2 phosphorylation, thereby inhibiting
differentiation of BMSCs into osteoblast-like cells in vitro
(56,57). The osteogenic capacity of MSCs
has been established (58). MSCs
serve as a source of osteochondral progenitors that invade bone
sites, proliferate and differentiate into cartilage and bones
(59). β-AR antagonists promote
MSC osteogenesis (50,55). In the present study, propranolol
promoted osteogenic differentiation of MSCs while inhibiting their
proliferation. This may be due to inhibition of MSC proliferation
during differentiation; this has been reported in previous studies,
which illustrated that stem cell differentiation is inhibited but
proliferation is promoted (60,61). Osteoblastic differentiation of
cells is accompanied by upregulation of osteoblast marker genes,
including BMP2, RunX2, COL-1 and OCN (62,63). The present study demonstrated
that propranolol increased the mRNA and protein expression levels
of BMP2, RunX2, COL-1 and OCN in tissue and cells and decreased
expression of β2-AR in OBs and MSCs. The increased calcium content
and ALP activity in propranolol-treated OBs and MSCs also indicated
osteoblastic differentiation of cells. The findings provide a basis
for further studies on the mechanisms underlying the regulatory
effects of propranolol.
There effects and mechanisms of propranolol on
osteogenesis at different concentrations are disputed (64). Smitham et al (2014)
(65) demonstrated that low-dose
propranolol (0.1 mg/kg) has little effect on bone defect healing,
while Bonnet et al (2008) (41) suggested that high-dose
propranolol (10-100 mg/kg) produces no additional positive effect
on osteogenesis compared with low-dose propranolol. The present
study demonstrated that the osteogenic effect of propranolol at
medium (1 mg/kg) and high (10 mg/kg) doses was markedly enhanced
compared with the low dose (0.1 mg/kg), whereas no superior effect
was revealed in the high-compared with the medium-dose group.
However, further investigation of the clinical use of propanolol in
this context is required.
The present study demonstrated that the β-AR blocker
propranolol promoted osteogenic differentiation of OBs and MSCs and
enhanced osseointegration of implants by regulating expression
levels of osteogenic-associated proteins, including BMP2, RunX2,
COL-1, OCN and β2-AR. The present study therefore provided a novel
insight into the application and regulatory mechanisms of
propranolol.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
All authors contributed to study conception and
design. YW wrote the manuscript. YW, QZ, BZ and XW performed
experiments and collected and analyzed data. YW and XW reviewed and
edited the manuscript. YW and XW confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
ethical review committee of the Ethics Committee of The Affiliated
Hospital of Qingdao University (approval no. 201905036).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by Open Project (grant no.
2017KB03) from State Key Laboratory of Military Stomatology, the
Project QDFY + X2021002 and Project QDFY + X2021004 from the
Affiliated Hospital of Qingdao University.
References
|
1
|
Yamakawa D, Kawase-Koga Y, Fujii Y, Kanno
Y, Sato M, Ohba S, Kitaura Y, Kashiwagi M and Chikazu D: Effects of
helioxanthin derivative-treated human dental pulp stem cells on
fracture healing. Int J Mol Sci. 21:91582020. View Article : Google Scholar :
|
|
2
|
Warzecha J, Seebach C, Flinspach A, Wenger
F, Henrich D and Marzi I: Effect of sonic hedgehog/β-TCP composites
on bone healing within the critical-sized rat femoral defect. Exp
Ther Med. 5:1035–1039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu J, Hu X, Jiang S, Wang Y, Parungao R,
Zheng S, Nie Y, Liu T and Song K: The application of multi-walled
carbon nanotubes in bone tissue repair hybrid scaffolds and the
effect on cell growth in vitro. Polymers (Basel). 11:2302019.
View Article : Google Scholar
|
|
4
|
Banfi G, Lombardi G, Colombini A and Lippi
G: Bone metabolism markers in sports medicine. Sports Med.
40:697–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leffers D and Collins L: An overview of
the use of bone scintigraphy in sports medicine. Sports Med
Arthrosc Rev. 17:21–24. 2009. View Article : Google Scholar
|
|
6
|
Niedermair T, Straub RH, Brochhausen C and
Grässel S: Impact of the sensory and sympathetic nervous system on
fracture healing in ovariectomized mice. Int J Mol Sci. 21:21–24.
2020. View Article : Google Scholar
|
|
7
|
Tomlinson RE, Christiansen BA, Giannone AA
and Genetos DC: The role of nerves in skeletal development,
adaptation, and aging. Front Endocrinol (Lausanne). 11:6462020.
View Article : Google Scholar
|
|
8
|
Farahzadi R, Mesbah-Namin SA, Zarghami N
and Fathi E: L-carnitine effectively Induces hTERT gene expression
of human adipose tissue-derived mesenchymal stem cells obtained
from the aged subjects. Int J Stem Cells. 9:107–114. 2016.
View Article : Google Scholar :
|
|
9
|
Farahzadi R, Fathi E and Vietor I:
Mesenchymal stem cells could be considered as a candidate for
further studies in cell-based therapy of alzheimer's disease via
targeting the signaling pathways. ACS Chem Neurosci. 11:1424–1435.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Zheng Y, Hou L, Zhou Z, Huang Y,
Zhang Y, Jia L and Li W: Exosomes derived from maxillary BMSCs
enhanced the osteogenesis in iliac BMSCs. Oral Dis. 26:131–144.
2020. View Article : Google Scholar
|
|
11
|
Yu L, Wu Y, Liu J, Li B, Ma B, Li Y, Huang
Z, He Y, Wang H, Wu Z and Qiu G: 3D culture of bone marrow-derived
mesenchymal stem cells (BMSCs) could improve bone regeneration in
3D-printed porous Ti6Al4V scaffolds. Stem Cells Int.
2018:20740212018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lombardi G, Ziemann E, Banfi G and
Corbetta S: Physical activity-dependent regulation of parathyroid
hormone and calcium-phosphorous metabolism. Int J Mol Sci.
21:53882020. View Article : Google Scholar :
|
|
13
|
Takeda S, Elefteriou F, Levasseur R, Liu
X, Zhao L, Parker KL, Armstrong D, Ducy P and Karsenty G: Leptin
regulates bone formation via the sympathetic nervous system. Cell.
111:305–317. 2002. View Article : Google Scholar
|
|
14
|
Hamano S, Tomokiyo A, Hasegawa D, Yuda A,
Sugii H, Yoshida S, Mitarai H, Wada N and Maeda H: Functions of
beta2-adrenergic receptor in human periodontal ligament cells. J
Cell Biochem. 2020.Online Ahead of Print. View Article : Google Scholar
|
|
15
|
Nijhuis LE, Olivier BJ, Dhawan S, Hilbers
FW, Boon L, Wolkers MC, Samsom JN and de Jonge WJ: Adrenergic β2
receptor activation stimulates anti-inflammatory properties of
dendritic cells in vitro. PLoS One. 9:e850862014. View Article : Google Scholar
|
|
16
|
Mauro LJ, Wenzel SJ and Sindberg GM:
Regulation of chick bone growth by leptin and catecholamines. Poult
Sci. 89:697–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council Committee for
the Update of the Guide for the Care and use of Laboratory Animals:
Guide for the Care and Use of Laboratory Animals The National
Academies Collection: Reports funded by National Institutes of
Health. National Academies Press; Washington, DC: 2011
|
|
18
|
Fathi E, Valipour B, Sanaat Z, Nozad
Charoudeh H and Farahzadi R: Interleukin-6, -8, and TGF-β secreted
from mesenchymal stem cells show functional role in reduction of
telomerase activity of leukemia cell via Wnt5a/β-catenin and P53
pathways. Adv Pharm Bull. 10:307–314. 2020. View Article : Google Scholar
|
|
19
|
Fathi E, Farahzadi R, Javanmardi S and
Vietor I: L-carnitine extends the telomere length of the cardiac
differentiated CD117+-expressing stem cells. Tissue
Cell. 67:1014292020. View Article : Google Scholar
|
|
20
|
Zhao ZQ, Liu WL, Guo SB, Bai R and Yan JL:
Mechanism of methylprednisolone-induced primary cilia formation
disorder and autophagy in osteoblasts. Orthop Surg. 12:645–652.
2020. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dasu MR, Ramirez SR, La TD, Gorouhi F,
Nguyen C, Lin BR, Mashburn C, Stewart H, Peavy TR, Nolta JA and
Isseroff RR: Crosstalk between adrenergic and toll-like receptors
in human mesenchymal stem cells and keratinocytes: A recipe for
impaired wound healing. Stem Cells Transl Med. 3:745–759. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang X, Zhu B, Wang X, Xiao R and Wang C:
Three-dimensional co-culture of mesenchymal stromal cells and
differentiated osteoblasts on human bio-derived bone scaffolds
supports active multi-lineage hematopoiesis in vitro: Functional
implication of the biomimetic HSC niche. Int J Mol Med.
38:1141–1151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen C, Yang C, Xu S and Zhao H:
Comparison of osteogenic differentiation capacity in mesenchymal
stem cells derived from human amniotic membrane (AM), umbilical
cord (UC), chorionic membrane (CM), and decidua (DC). Cell Biosci.
9:172019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heo SK, Noh EK, Gwon GD, Kim JY, Jo JC,
Choi Y, Koh S, Baek JH, Min YJ and Kim H: LIGHT (TNFSF14) increases
the survival and proliferation of human bone marrow-derived
mesenchymal stem cells. PLoS One. 11:e01665892016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin Y, Chen P, Yu Q, Peng Y, Zhu Z and
Tian J: The effects of a pulsed electromagnetic field on the
proliferation and osteogenic differentiation of human
adipose-derived stem cells. Med Sci Monit. 24:3274–3282. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Chang B, Song W, Han T, Yan J, Li F, Zhao
L, Kou H and Zhang Y: Influence of pore size of porous titanium
fabricated by vacuum diffusion bonding of titanium meshes on cell
penetration and bone ingrowth. Acta Biomater. 33:311–321. 2016.
View Article : Google Scholar
|
|
28
|
Fares C, Hsu SM, Xian M, Xia X, Ren F,
Mecholsky JJ Jr, Gonzaga L and Esquivel-Upshaw J: Demonstration of
a SiC protective coating for titanium implants. Materials (Basel).
13:33212020. View Article : Google Scholar
|
|
29
|
Cardona MJ, Turner C, Ross C, Baird E and
Black RA: An improved process for the fabrication and surface
treatment of custom-made titanium cranioplasty implants informed by
surface analysis. J Biomater Appl. 35:602–614. 2021. View Article : Google Scholar
|
|
30
|
Scarano A, Lorusso F, Orsini T, Morra M,
Iviglia G and Valbonetti L: Biomimetic surfaces coated with
covalently immobilized collagen type I: An X-ray photoelectron
spectroscopy, atomic force microscopy, micro-CT and
histomorphometrical study in rabbits. Int J Mol Sci. 20:7242019.
View Article : Google Scholar
|
|
31
|
Gong T, Xie J, Liao J, Zhang T, Lin S and
Lin Y: Nanomaterials and bone regeneration. Bone Res. 3:150292015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dikicier E, Karaçaylı Ü, Dikicier S and
Günaydın Y: Effect of systemic administered zoledronic acid on
osseointegration of a titanium implant in ovariectomized rats. J
Craniomaxillofac Surg. 42:1106–1111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Hu J, Liu B, Jiang X and Li Y: The
effect of osteoprotegerin on implant osseointegration in
ovariectomized rats. Arch Med Sci. 13:489–495. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsuchiya S, Sugimoto K, Kamio H, Okabe K,
Kuroda K, Okido M and Hibi H: Kaempferol-immobilized titanium
dioxide promotes formation of new bone: Effects of loading methods
on bone marrow stromal cell differentiation in vivo and in vitro.
Int J Nanomedicine. 13:1665–1676. 2018. View Article : Google Scholar
|
|
35
|
Carnagarin R, Matthews V, Zaldivia MTK,
Peter K and Schlaich MP: The bidirectional interaction between the
sympathetic nervous system and immune mechanisms in the
pathogenesis of hypertension. Br J Pharmacol. 176:1839–1852. 2019.
View Article : Google Scholar :
|
|
36
|
Duncan CP and Shim SS: J. Edouard Samson
address: The autonomic nerve supply of bone. An experimental study
of the intraosseous adrenergic nervi vasorum in the rabbit. J Bone
Joint Surg Br. 59:323–330. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mach DB, Rogers SD, Sabino MC, Luger NM,
Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O'Leary P
and Mantyh PW: Origins of skeletal pain: Sensory and sympathetic
innervation of the mouse femur. Neuroscience. 113:155–166. 2002.
View Article : Google Scholar
|
|
38
|
Mediero A, Wilder T, Shah L and Cronstein
BN: Adenosine A2A receptor (A2AR) stimulation modulates
expression of semaphorins 4D and 3A, regulators of bone
homeostasis. Faseb J. 32:3487–3501. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Emet M, Ozcan H, Ozel L, Yayla M, Halici Z
and Hacimuftuoglu A: A review of melatonin, its receptors and
drugs. Eurasian J Med. 48:135–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Teong B, Kuo SM, Chen CH, Chen YK, Cheng
ZJ and Huang HH: Characterization and human osteoblastic
proliferation- and differentiation-stimulatory effects of
phosphatidylcholine liposomes-encapsulated propranolol
hydrochloride. Biomed Mater Eng. 24:1875–1887. 2014.PubMed/NCBI
|
|
41
|
Bonnet N, Benhamou CL, Malaval L,
Goncalves C, Vico L, Eder V, Pichon C and Courteix D: Low dose
beta-blocker prevents ovariectomy-induced bone loss in rats without
affecting heart functions. J Cell Physiol. 217:819–827. 2008.
View Article : Google Scholar
|
|
42
|
Srinivasan AV: Propranolol: A 50-year
historical perspective. Ann Indian Acad Neurol. 22:21–26. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Woroń J, Siwek M and Gorostowicz A:
Adverse effects of interactions between antidepressants and
medications used in treatment of cardiovascular disorders.
Psychiatr Pol. 53:977–995. 2019. View Article : Google Scholar
|
|
44
|
Zhou HM, Zhong ML, Wang RH, Long CL, Zhang
YF, Cui WY and Wang H: Synergisms of cardiovascular effects between
iptakalim and amlodipine, hydrochlorothiazide or propranolol in
anesthetized rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
31:532–540. 2015.
|
|
45
|
Stapleton MP: Sir James Black and
propranolol. The role of the basic sciences in the history of
cardiovascular pharmacology. Tex Heart Inst J. 24:336–342.
1997.
|
|
46
|
Wolter JK, Wolter NE, Blanch A, Partridge
T, Cheng L, Morgenstern DA, Podkowa M, Kaplan DR and Irwin MS:
Anti-tumor activity of the beta-adrenergic receptor antagonist
propranolol in neuroblastoma. Oncotarget. 5:161–172. 2014.
View Article : Google Scholar :
|
|
47
|
Bustamante P, Miyamoto D, Goyeneche A, de
Alba Graue PG, Jin E, Tsering T, Dias AB, Burnier MN and Burnier
JV: Beta-blockers exert potent anti-tumor effects in cutaneous and
uveal melanoma. Cancer Med. 8:7265–7277. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bravo-Calderón DM, Assao A, Garcia NG,
Coutinho-Camillo CM, Roffé M, Germano JN and Oliveira DT: Beta
adrenergic receptor activation inhibits oral cancer migration and
invasiveness. Arch Oral Biol. 118:1048652020. View Article : Google Scholar
|
|
49
|
Minkowitz B, Boskey AL, Lane JM, Pearlman
HS and Vigorita VJ: Effects of propranolol on bone metabolism in
the rat. J Orthop Res. 9:869–975. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu H, Song Y, Li J, Lei X, Zhang S, Gao Y,
Cheng P, Liu B, Miao S, Bi L, et al: Blockade of adrenergic
β-receptor activation through local delivery of propranolol from a
3D collagen/polyvinyl alcohol/hydroxyapatite scaffold promotes bone
repair in vivo. Cell Prolif. 53:e127252020. View Article : Google Scholar
|
|
51
|
Sato T, Arai M, Goto S and Togari A:
Effects of propranolol on bone metabolism in spontaneously
hypertensive rats. J Pharmacol Exp Ther. 334:99–105. 2010.
View Article : Google Scholar
|
|
52
|
Sato T, Miyazawa K, Suzuki Y, Mizutani Y,
Uchibori S, Asaoka R, Arai M, Togari A and Goto S: Selective
β2-adrenergic antagonist butoxamine reduces orthodontic tooth
movement. J Dent Res. 93:807–812. 2014. View Article : Google Scholar
|
|
53
|
Actis L, Gaviria L, Guda T and Ong JL:
Antimicrobial surfaces for craniofacial implants: State of the art.
J Korean Assoc Oral Maxillofac Surg. 39:43–54. 2013. View Article : Google Scholar
|
|
54
|
Jenei-Lanzl Z, Grässel S, Pongratz G, Kees
F, Miosge N, Angele P and Straub RH: Norepinephrine inhibition of
mesenchymal stem cell and chondrogenic progenitor cell
chondrogenesis and acceleration of chondrogenic hypertrophy.
Arthritis Rheumatol. 66:2472–2481. 2014. View Article : Google Scholar
|
|
55
|
Li H, Fong C, Chen Y, Cai G and Yang M:
beta2- and beta3-, but not beta1-adrenergic receptors are involved
in osteogenesis of mouse mesenchymal stem cells via cAMP/PKA
signaling. Arch Biochem Biophys. 496:77–83. 2010. View Article : Google Scholar
|
|
56
|
Li XL, Zeng D, Chen Y, Ding L, Li WJ, Wei
T, Ou DB, Yan S, Wang B and Zheng QS: Role of alpha- and
beta-adrenergic receptors in cardiomyocyte differentiation from
murine-induced pluripotent stem cells. Cell Prolif. 50:e123102017.
View Article : Google Scholar
|
|
57
|
Xiao L, Pimental DR, Amin JK, Singh K,
Sawyer DB and Colucci WS: MEK1/2ERK1/2 mediates alpha1-adrenergic
receptor-stimulated hypertrophy in adult rat ventricular myocytes.
J Mol Cell Cardiol. 33:779–787. 2001. View Article : Google Scholar
|
|
58
|
Marolt Presen D, Traweger A, Gimona M and
Redl H: Mesenchymal stromal cell-based bone regeneration therapies:
From cell transplantation and tissue engineering to therapeutic
secretomes and extracellular vesicles. Front Bioeng Biotechnol.
7:3522019. View Article : Google Scholar :
|
|
59
|
Zhang W, Zhou L, Dang J, Zhang X, Wang J,
Chen Y, Liang J, Li D, Ma J, Yuan J, et al: Human gingiva-derived
mesenchymal stem cells Ameliorate Streptozoticin-induced T1DM in
mice via suppression of T effector cells and Up-regulating Treg
subsets. Sci Rep. 7:152492017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Reumann MK, Linnemann C, Aspera-Werz RH,
Arnold S, Held M, Seeliger C, Nussler AK and Ehnert S: Donor site
location is critical for proliferation, stem cell capacity, and
osteogenic differentiation of adipose mesenchymal stem/stromal
cells: Implications for bone tissue engineering. Int J Mol Sci.
19:18682018. View Article : Google Scholar
|
|
61
|
Givogri MI, de Planell M, Galbiati F,
Superchi D, Gritti A, Vescovi A, de Vellis J and Bongarzone ER:
Notch signaling in astrocytes and neuroblasts of the adult
subventricular zone in health and after cortical injury. Dev
Neurosci. 28:81–91. 2006. View Article : Google Scholar
|
|
62
|
Masaoutis C and Theocharis S: The role of
exosomes in bone remodeling: Implications for bone physiology and
disease. Dis Markers. 2019:94179142019. View Article : Google Scholar
|
|
63
|
Wong SK, Chin KY and Ima-Nirwana S: The
osteoprotective effects of kaempferol: The evidence from in vivo
and in vitro studies. Drug Des Devel Ther. 13:3497–3514. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rodrigues WF, Madeira MF, da Silva TA,
Clemente-Napimoga JT, Miguel CB, Dias-da-Silva VJ, Barbosa-Neto O,
Lopes AH and Napimoga MH: Low dose of propranolol down-modulates
bone resorption by inhibiting inflammation and osteoclast
differentiation. Br J Pharmacol. 165:2140–2151. 2012. View Article : Google Scholar
|
|
65
|
Smitham P, Crossfield L, Hughes G,
Goodship A, Blunn G and Chenu C: Low dose of propranolol does not
affect rat osteotomy healing and callus strength. J Orthop Res.
32:887–893. 2014. View Article : Google Scholar : PubMed/NCBI
|