Introduction
Osteoarthritis (OA) is a degenerative joint disorder
causing disability in the elderly population worldwide (1), and is estimated to potentially
affect ~400 million individuals in China by 2030 (2). OA has caused a heavy socioeconomic
burden, which costs almost 2.5% of the gross domestic product of
developed countries (3).
Generally speaking, joints (especially the knee joint) are
flexible, act as a functional motif to withstand compressive
forces, and allow for a multi-directional range of motion for
movement. Joints are composed of articular cartilage, subchondral
bone and synovium, which are all severely compromised by OA, but
especially the cartilage. However, the etiology of OA is
complicated, particularly that which is associated with anatomic
hip dysplasia or joint morphology (4), or that caused by immune factors,
including rheumatoid arthritis (5). Thus, the management of OA is
currently focused on pain relief and functional reconstruction.
These conventional strategies include oral non-steroidal
anti-inflammatory drugs (6),
intra-articular injection of glucocorticoids (2) or hyaluronic acid (7), and surgical methods such as
arthroscopic management and total arthroplasty. Stem cell injection
therapy has gained significant attention in basal research and
clinical trials (8); however,
based on challenges such as cell leakage, osteogenic transformation
of mesenchymal stem cells and other safety concerns, long-term
complications and the cost-effectiveness of the procedure should be
addressed (9,10). Therefore, from the prospective of
the underlying molecular mechanism of OA progression, inhibiting
inflammatory pathways, such as the NF-κB or MAPK cascades, may help
to alleviate the progression of joint degeneration.
Curcumenol is a bioactive ingredient isolated from
edible rhizome of Curcuma zedoaria (zedoary, Zingiberaceae),
which is an important constituent of Chinese Traditional Medicine
(11,12). Subsequently, curcumenol was found
to be one of the primary constituents in numerous other plants,
such as various Piper species, Torilis japonica and
Neolitsea pallens (13,14). Such plants exhibit various
functions, including anti-inflammatory, hepatoprotective,
neuroprotective and antioxidant activities (15,16). Thus, curcumenol may be considered
a potential option for treating inflammation.
The aim of the present study was to investigate the
potential use of curcumenol to treat OA in vitro and in
vivo. First, the inhibitory effect of curcumenol on the NF-κB
and MAPK pathways was determined in ATDC5 chondrocytes and primary
chondrocytes in vitro, after which the rescue function of
curcumenol in destabilization of medial meniscus (DMM)-induced knee
joint OA in mice was evaluated in vivo.
Materials and methods
Reagents
Curcumenol was purchased from Selleck Chemicals, and
according to the manufacturer's protocol, was isolated from
Curcuma zedoary, with the following characteristics: High
performance liquid chromatography, purity=99.89%; nuclear magnetic
resonance, consistent structure. Then, 10 mg curcumenol was
dissolved in 0.4268 ml DMSO (Sigma-Aldrich; Merck KGaA) to a
concentration of 100 mM, and stored at -20°C. Recombinant TNF-α
(PeproTech China) and IL-1β (R&D Systems, Inc.) were dissolved
in sterile PBS containing 0.1% BSA (Beyotime Institute of
Biotechnology) to a concentration of 10 µg/ml.
Primary antibodies against IKKα (cat. no. D3W6N;
rabbit monoclonal), phosphorylated (p)-IKKα/β (Ser176/180; cat. no.
16A6; rabbit monoclonal), P65 (cat. no. D14E12; rabbit monoclonal),
p-P65 (Ser536; cat. no. 93H1; rabbit monoclonal), IκBα (cat. no.
L35A5; mouse monoclonal), p-IκBα (Ser32; cat. no. 14D4; rabbit
monoclonal), Akt (cat. no. 11E7; rabbit monoclonal), p-Akt (Ser473;
cat. no. D9E; rabbit monoclonal), SAPK/JNK (cat. no. 9252; rabbit
monoclonal), p-SAPK/JNK (Thr183/Tyr185, G9; cat. no. 81E11; rabbit
monoclonal), P38 (cat. no. D13E1; rabbit monoclonal), p-P38
(Thr180/Tyr182; cat. no. D13.14.4E; rabbit monoclonal), p44/42
(cat. no. 137F5; rabbit monoclonal), p-p44/42 (Thr202/Tyr204; cat.
no. D3F9; rabbit monoclonal) and β-actin (cat. no. D6A8; rabbit
monoclonal) were purchased from Cell Signaling Technology, Inc.
Primary antibodies against collagen type II α 1 chain (Col2a1; cat.
no. ab188570; rabbit monoclonal), MMP3 (cat. no. ab52915; rabbit
monoclonal), MMP7 (cat. no. ab5706; rabbit monoclonal) and MMP13
(cat. no. ab51072; rabbit monoclonal) were obtained from Abcam.
Isolation and culture of primary mouse
chondrocytes
For each isolation process, three 5-day-old mice
(weight, 2-4 g; Shanghai Lab, Animal Research Center Co., Ltd.;
housed under pathogen-free conditions at 26-28°C and 50-65%
humidity with a 12-h day/night cycle.) were sacrificed via
decapitation and immersed in 75% ethanol for 10 min. Both of the
lower limbs were dissected, and the skin removed, and the whole
knee joint was extracted with the synovial and muscle tissue
stripped. These six cartilage samples were cut into pieces (0.5-1
mm) and then soaked in 1% collagenase II solution for 2 h at 37°C,
followed by centrifugation (in 300 × g, 37°C for 5 min) and
resuspension in complete medium (DMEM/F12 with 5% FBS, 1%
penicillin-streptomycin). The primary chondrocytes were cultured in
DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
5% FBS, 1% penicillin-streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) and 1% insulin-transferrin-selenium (ITS)
solution at 37°C with 5% CO2.
ATDC5 cell culture
Mouse ATDC5 immortalized chondrocytes (17) were purchased from Shaanxi Fuheng
(FH) Biotechnology Co., Ltd., and were maintained in DMEM/F12
supplemented with 5% FBS and 1% penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2.
RNA extraction and reverse
transcription-quantitative PCR (qPCR)
ATDC5 and primary chondrocytes were stimulated with
TNF-α and IL-1β (both 10 ng/ml) with or without curcumenol (50
µM), and the control group was cultured in medium with
1:2,000 DMSO. After 24 h at 37°C, total RNA was isolated from cells
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Extracted RNA was reverse
transcribed to first strand cDNA using the cDNA Synthesis kit
(Takara Bio, Inc.). qPCR was conducted using the TB Green Premix Ex
Taq kit (Takara Bio, Inc.) on an Applied Biosystems QuantStudio 6
Flex Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) per the following conditions: Denaturation at
95°C for 30 sec; 40 cycles of 95°C for 3 sec and 60°C for 34 sec;
and then 95°C for 15 sec, 60°C for 60 sec and finally, 95°C for 15
sec. Primers were designed using NCBI BLAST (18), the sequences of which are
provided in Table I. Target gene
expression levels were determined using the 2−ΔΔCq
method (19), with GAPDH as the
internal reference control.
 | Table IPCR primer information. |
Table I
PCR primer information.
| Gene | Accession
number | Description | Sequence
(5′-3′) |
|---|
| MMP3 | NM_010809.2 | Forward |
CCCTGCAACCGTGAAGAAGA |
| Reverse |
GACAGCATCCACCCTTGAGT |
| MMP7 | NM_010810.5 | Forward |
CCCTGTTCTGCTTTGTGTGTC |
| Reverse |
AGGGGGAGAGTTTTCCAGTCA |
| MMP13 | NM_008607.2 | Forward |
AGAAGTGTGACCCAGCCCTA |
| Reverse |
GGTCACGGGATGGATGTTCA |
| ADAMTS4 | NM_172845.3 | Forward |
GAGTCCCATTTCCCGCAGA |
| Reverse |
GCAGGTAGCGCTTTAACCCT |
| ADAMTS5 | NM_011782.2 | Forward |
GAGAACCCTGCAAAACAGCC |
| Reverse |
AACCATACAAGTGCCTTTTCTCT |
| Col2a1 | NM_053593.2 | Forward |
GTGTGACACTGGGAATGTCCTCT |
| Reverse |
TGGCCCTAATTTTCCACTGGC |
| GAPDH | NM_008084.3 | Forward |
CGACTTCAACAGCAACTCCCACTCTTCC |
| Reverse |
TGGGTGGTCCAGGGTTTCTTACTCCTT |
Cell viability analysis
Cell viability was evaluated using the Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Laboratories, Inc.). ATDC5
chondrocytes were seeded into a 96-well plate at a density of
3×103 cells/well. The next day, the cells were treated
with increasing concentrations of curcumenol (12.5, 25, 50 and 100
µM, dissolved in DMSO) for 24, 48 and 72 h at 37°C; the
control group was cultured in medium containing 1:1,000 DMSO. Media
were refreshed every 2 days. Subsequently, the cells were incubated
with fresh complete media containing 10 µl CCK-8 reagent,
for 2 h at 37°C. Complete medium containing CCK-8 reagent, with no
cells or untreated cells, were used as the blank and mock controls,
respectively. Absorbance at 450 nm (mean optical density; OD) was
measured using an Infinite M200 Pro multimode microplate reader
(Tecan Group, Ltd.).
High-density culture and pellet
culture
To assess chondrogenic differentiation,
1.5×105 ATDC5 or primary chondrocytes were resuspended
in 10 µl incomplete MEM/F12 (Gibco; Thermo Fisher
Scientific, Inc.) and seeded as micromasses in the bottom of a
24-well plate. The cells were allowed to adhere for 1 h at 37°C,
after which 0.5 ml MEM/F12 containing 10 ng/ml ITS and 2% FBS were
added. After 24 h at 37°C, the cells were stimulated with TNF-α and
IL-1β (both 10 ng/ml) with or without curcumenol (50 µM),
and the control groups were cultured in a medium with DMSO only
(1:2,000) for 9 days at 37°C. All media were refreshed every other
day, and after 9 days the micromasses were stained with alcian blue
for 24 h at room temperature (RT).
For pellet culture, 1.5×107 ATDC5 were
pelleted in 15-ml centrifuge tubes (200 × g, 37°C for 5 min)
supplemented with mesenchymal stem cell chondrogenic
differentiation medium (Cyagen Biosciences, Inc.). After 48 h at
37°C, the ATDC5 and primary chondrocyte pellets were stimulated
with TNF-α and IL-1β (both 10 ng/ml) with or without curcumenol (50
µM), and the control group was cultured in medium containing
DMSO only (1:2,000) for 21 days at 37°C. The media were refreshed
every 3 days. After 21 days of culture, the pellets were collected
and fixed at RT in 4% paraformaldehyde (PFA) for 5 h, and then
embedded in optimal cutting temperature compound (Sakura Finetek
USA, Inc.). The samples were then stored at -80°C overnight and cut
to a 20-µm thickness using a freezing microtome (Leica
Microsystems GmbH).
Digital images were captured under a light
microscope at a ×7.8 magnification (Leica DM4000 B; Leica
Microsystems GmbH). Alcian blue staining intensity was analyzed
using Image Pro Plus 6.0 software (20) to evaluate the ratio of integrated
(I)OD (expressed as the IOD/area for each sample).
Senescence assays
The senescence of primary chondrocytes was analyzed
using the Senescence β-Galactosidase Staining kit (Beyotime
Institute of Biotechnology). Primary chondrocytes were seeded into
a 12-well plate at a density of 4×105 cells/well,
following stimulation with TNF-α and IL-1β (10 ng/ml each) with or
without curcumenol (50 µM). After 24 h at 37°C, the cells
were fixed with Beyotime Fixative Solution for 15 min at room
temperature, and then incubated with Beyotime β-Galactosidase
Staining buffer at 37°C overnight. Digital images were captured
under a light microscope at ×10× and ×20 magnification (Leica
DM4000 B; Leica Microsystems GmbH) and the percentage of positive
cells was calculated.
Western blot analysis
ATDC5 and primary chondrocytes were stimulated with
TNF-α and IL-1β (10 ng/ml each) with or without curcumenol (50
µM). After 24 h at 37°C, total cellular proteins were
extracted for detection of MMP family and Col2a1 protein
expression. For preventive analysis of the NF-κB and MAPK pathways,
ATDC5 and primary chondrocytes were pretreated with increasing
concentrations of curcumenol (6.25, 12.5, 25 and 50 µM,
dissolved in DMSO) for 2 h at 37°C, and then stimulated with TNF-α
and IL-1β for 10 min at 37°C, then total cellular proteins were
extracted. For reactive analysis of the NF-κB and MAPK pathways,
ATDC5 chondrocytes were pretreated with serum-free medium for 2 h
at 37°C and then stimulated with TNF-α with or without curcumenol
for 10 min at 37°C; total cellular proteins were then
extracted.
Cultured cells were lysed using RIPA lysis buffer
supplemented with phosphatase and protease inhibitors (Roche
Diagnostics). The protein was quantified by BCA assay (Thermo
Fisher Scientific, Inc.) and then equal quantities of extracted
protein (20-30 µg) were separated via 10 or 12.5% SDS-PAGE
and electroblotted onto 0.22-µm PVDF membranes
(MilliporeSigma). The membranes were blocked with 5% BSA-PBS
(Beyotime Institute of Biotechnology) at room temperature for 1 h,
and then incubated with primary antibodies against IKKα,
phosphorylated (p)-IKKα/β, P65, p-P65, IκBα, p-IκBα, Akt, p-Akt,
SAPK/JNK, p-SAPK/JNK, P38, p-P38, p44/42, p-p44/42 and β-actin
overnight (≥16 h) at 4°C. The membranes were washed with TBS-0.1%
Tween20 (TBST) and subsequently incubated with anti-rabbit IgG
(H+L) secondary antibody (cat. no. 5151; DyLight™ 800 4X PEG
Conjugate; Cell Signaling Technology, Inc.; 1:5,000) for 1 h at
room temperature in the dark. After washing in TBST, protein
immunoreactivity was detected using the Odyssey Fluorescence
Imaging system (LI-COR Biosciences). Semi-quantitative analysis of
protein band intensity was conducted using ImageJ V1.8.0 software
(National Institutes of Health) and normalized to the internal
loading control, β-actin.
Animals and surgical procedures
All animal experiments were approved by the
Institutional Animal Care and Ethics Committee of Ninth People's
Hospital, Shanghai Jiaotong University School of Medicine
(Shanghai, China), and performed in accordance with the principles
and procedures of the National Institutes of Health Guide for the
Care and Use of Laboratory Animals, and the Guidelines for Animal
Treatment of Shanghai Jiaotong University. A total of 18 8-week-old
male C57/BL mice (weight, 18-22 g; Shanghai Lab, Animal Research
Center Co., Ltd.) were housed under pathogen-free conditions at
26-28°C and 50-65% humidity with a 12-h day/night cycle. Animals
were fed standard rodent chow and had access to fresh water ad
libitum. Before the surgical procedures, mice were anesthetized
by intraperitoneal injection of pentobarbital sodium (50 mg/kg of
body weight). In the control group (n=6; underwent sham surgery and
were treated with corn oil; however, during research, one mouse was
lost from the control group), the fur on the skin was shaved, a
0.5-cm incision was made near the right knee joint, and the
ligamentum patellae was exposed and stretched. The remaining 12
mice were assigned to the DMM group (n=6; underwent DMM surgery and
were treated with corn oil; 1 mouse was removed from the DMM group
to equalize the numbers) and the curcumenol group (n=6; underwent
DMM surgery and were treated with curcumenol; one was removed from
curcumenol group to equalize the numbers). After exposure, the
medial collateral ligaments were transected, and the medial
meniscus of the tibia was partially removed using a 5-mm blade
micro-surgical knife (Beyotime Institute of Biotechnology)
(21). After the operation, the
incisions were sutured and the mice were initially treated two days
after surgery, and then for another 2 months with intraperitoneal
injections of curcumenol (50 mg curcumenol pre-dissolved in 1 ml
DMSO and then diluted in 100 ml corn oil) for the curcumenol group,
and corn oil (cat. no. C8267; Sigma-Aldrich; Merck KGaA; 1 ml DMSO
diluted in 100 ml corn oil) for the control and DMM groups, at 4
mg/kg/time twice a week. At the end of the experimental period, all
mice were sacrificed by cervical dislocation and the right lower
limbs were extracted, cleaned of soft tissues, stretched and fixed
in 4% PFA at RT for 48 h.
Histology and immunofluorescence
staining
Fixed lower limb samples were embedded in paraffin
and subjected to histological sectioning (5-µm thickness).
For histological assessment, paraffin-embedded tissue sections were
processed for Safranin O-Fast Green and hematoxylin and eosin
(H&E) staining (Servicebio) at RT for 2-5 min, in accordance
with the manufacturer's instructions. Sections were examined for
tissue thickness, which was quantified by measuring the Safranin
O-positive thickness in the center of the medial tibial plateau
(22,23), and the OA Research Society
Internationall histological (OARSI) score system: 0, normal; 0.5,
loss of Safranin-O without structural changes; 1, small
fibrillations without loss of cartilage; 2, vertical clefts down to
the layer immediately below the superficial layer and some loss of
surface lamina; 3, vertical clefts/erosion to the calcified
cartilage extending to <25% of the articular surface; 4,
vertical clefts/erosion to the calcified cartilage extending to
25-50% of the articular surface; 5, vertical clefts/erosion to the
calcified cartilage extending to 50-75% of the articular surface;
and 6, vertical clefts/erosion to the calcified cartilage extending
>75% of the articular surface.
For immunofluorescence assessment, ATDC5 cells were
cultured on slides added to a 6-well plate. At 10% confluence, the
cells were stimulated with TNF-α and IL-1β for 20 min at 37°C, with
or without curcumenol pretreatment for 2 h at 37°C. Then the slides
were fixed with 4% PFA at RT for 48 h, and then immersed in PBS (pH
7.4) and washed three times for 5 min each. Auto-fluorescence
quencher was added to the sections for 5 min, which were then
blocked with blocking buffer (Cell Signaling Technology, Inc.) for
30 min at RT. The slides were subsequently incubated with primary
antibodies in a wet box at 4°C overnight. Anti-pp65 primary
antibody was used at a 1:100 dilution. The following day, the
slides were washed with PBS and incubated with N Alexa Fluor
594-conjugated secondary antibody (cat. no. 8889; anti-rabbit;
1:500; Cell Signaling Technology, Inc.) for 50 min at RT in the
dark. Subsequently, the slides were washed with PBS and then
incubated with DAPI solution (Sigma-Aldrich; Merck KGaA) for 10 min
at RT in the dark to stain cell nuclei. After a final wash with
PBS, the samples were air-dried and sealed with anti-fluorescence
quenching tablets. Digital fluorescence images were captured under
a Leica DM4000 B epifluorescence microscope (Leica Microsystems
GmbH) at a ×10 and ×20 magnification, and IOD measurements were
obtained using Image Pro Plus 6.0 software (Media Cybernetics,
Inc.).
For tissue staining, the sections were
de-paraffinized in graded xylene, rehydrated in graded alcohol
solutions, and then incubated in antigen retrieval buffer (Roche
Diagnostics) at 37°C for 30 min. After cooling to RT, the sections
were immersed in PBS (pH 7.4) and washed three times for 5 min
each, and then processed as slides as aforementioned. Anti-TNF-α
(cat. no. ab183218; Abcam), anti-IL-1β (cat. no. ab234437; Abcam)
and anti-Col2a1 (cat. no. AF0135; Affinity) primary antibodies were
used at a 1:100 dilution.
Immunohistochemistry
Fixed lower limb samples were embedded in paraffin
as aforementioned, and cut into slices (8 µm), then
subjected to immunohistochemistry using a kit (cat. no. G1215-200T;
Wuhan Servicebio Technology Co., Ltd.) according to the
manufacturer's instructions. Briefly, tissue sections were
incubated with rabbit anti-TNF-α (cat. no. ab9579; Abcam),
anti-IL-1β (cat. no. ab283818; Abcam) and anti-Col2a1 (cat. no.
ab34712; Abcam) overnight at 4°C (1:100 dilution). The following
day, the slides were washed with PBS and incubated with goat
anti-mouse/rabbit IgG HRP-polymer (cat. no. 91196; anti-rabbit;
1:500; Cell Signaling Technology, Inc.) for 30 min at RT using
3,3′-diaminobenzidin as the chromogen. Digital images were captured
under a Leica DM4000 B microscope at ×10 and ×20 magnification, and
positively-stained cell measurements were obtained using Image Pro
Plus 6.0 software.
Radiographic analysis
Digital X-ray imaging of the right lower limbs was
conducted per the manufacturer's instructions (24) in the anteroposterior axis with a
21 lp/mm detector that provides up to ×5 geometric magnification
(Faxitron VersaVision; Faxitron Bioptics LLC).
Statistical analysis
A total of three independent experiments or repeated
measurements were conducted for all data. Data are presented as the
mean ± SD. Differences between study groups were analyzed by
one-way ANOVA with Tukey's post hoc test. Significant differences
in ordinal data between study groups were assessed by
Kruskal-Wallis test with a Dunn's post hoc test. Analyses were
conducted using SPSS 19.0 software (IBM Corp.), and P<0.05 was
considered to indicate a statistically significant difference.
Results
Curcumenol inhibits MMP family
upregulation induced by TNF-α and IL-1β in ATDC5 chondrocytes
Curcumenol is a bioactive ingredient isolated from
Curcuma zedoaria, and its chemical structure is shown in
Fig. 1A. Considering its safety
for the treatment of OA, CCK-8 assays were conducted to evaluate
curcumenol cytotoxicity in ATDC5 chondrocytes. Concentrations of
12.5, 25, 50 and 100 µM curcumenol were not cytotoxic
towards ATDC5 chondrocytes, and did not affect the proliferation
rate of these cells between 24 and 72 h (Fig. 1B); 50 µM was selected for
further experimentation, as in our preliminary studies, RT-qPCR and
western blotting revealed that the effects of 100 µM
curcumenol were not considerably different to those after 50
µM treatment (data not shown). To further investigate the
anti-inflammatory effects of curcumenol on ATDC5 chondrocytes,
TNF-α and IL-1β (10 ng/ml, 24 h) were used to activate the
inflammatory response. The expression of the MMP3, MMP13, ADAM
metallopeptidase with thrombospondin type 1 motif 4 (ADAMTS4) and
ADAMTS5 genes was increased following TNF-α (Fig. 1C and E) and IL-1β (Fig. 1D and E) stimulation. However,
following treatment with 50 µM curcumenol, the expression
levels of these genes were significantly decreased; ADAMTS gene
expression was also downregulated, but not to a significant degree
(Fig. 1C-E). Western blotting
was conducted to determine the effects of curcumenol on protein
expression levels in ATDC5 chondrocytes. After stimulation with
TNF-α and IL-1β, MMP3 expression was increased, and 50 µM
curcumenol effectively mitigated the upregulation of MMP3 protein
(Figs. S1E-H). Moreover, Col2a1
expression decreased following inflammation induction, and was
subsequently increased by curcumenol treatment, though not
significantly so. In conclusion, curcumenol safely and effectively
inhibited the TNF-α- and IL-1β-induced upregulation of MMP family
proteins in ATDC5 chondrocytes.
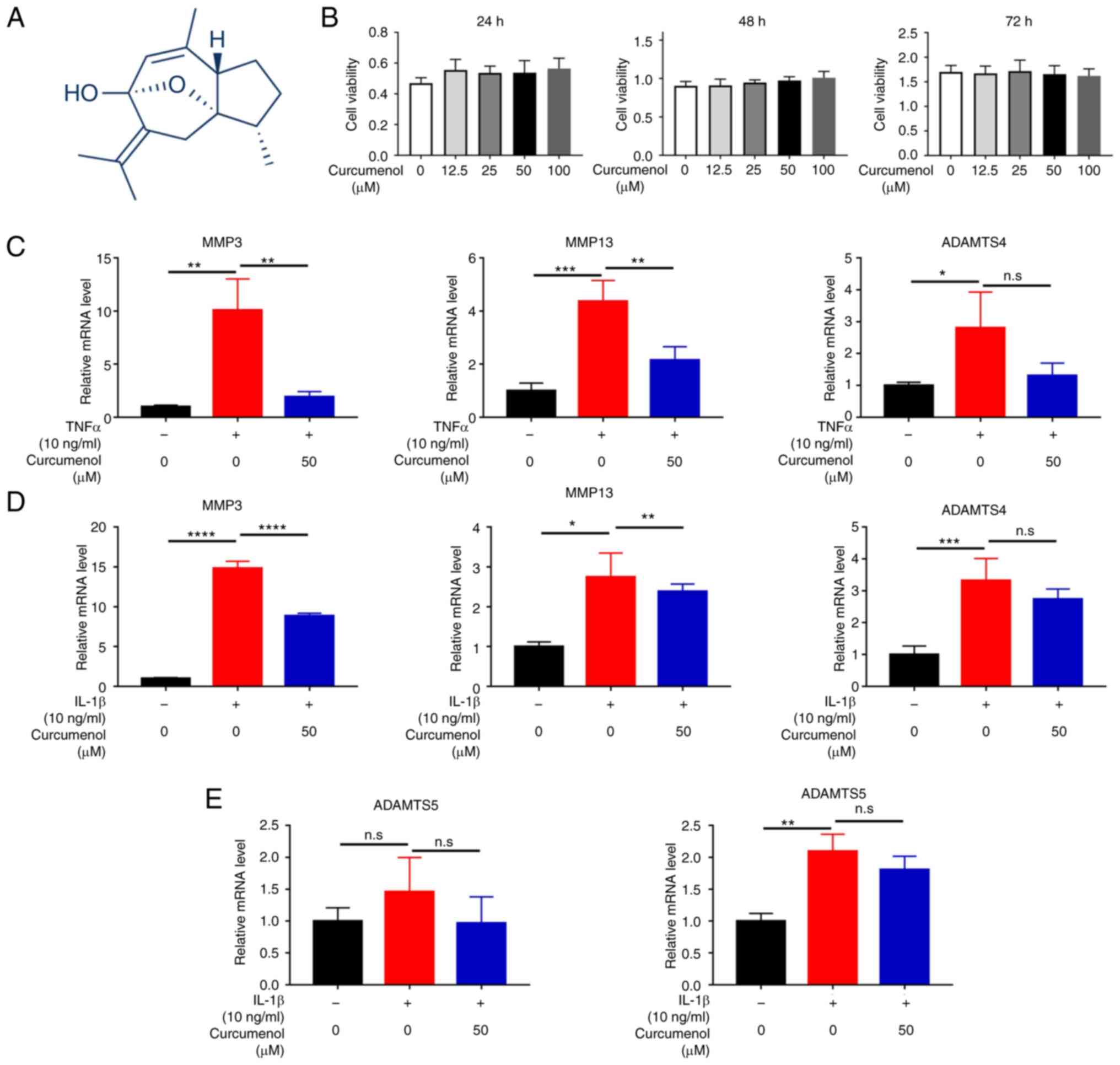 | Figure 1Curcumenol is not cytotoxic to ATDC5
chondrocytes, and inhibits TNF-α and IL-1β-induced MMP family
upregulation in vitro. (A) Chemical structure of curcumenol.
(B) Cell Counting Kit-8 assay results of ATDC5 chondrocytes
stimulated with curcumenol at different concentrations (0, 12.5,
25, 50 and 100 µM) and over different time periods (24-72
h). (C) RT-qPCR analysis of relative mRNA expression levels of
MMP3, MMP13 and ADAMTS4 in ATDC5 chondrocytes with TNF-α (10 ng/ml)
or/and curcumenol (50 µM) administration. (D) RT-qPCR
analysis of relative mRNA expression levels of MMP3, MMP13 and
ADAMTS4 in ATDC5 chondrocytes with IL-1β (10 ng/ml) or/and
curcumenol (50 µM) administration for 24 h. (E) RT-qPCR
analysis of relative mRNA expression levels of ADAMTS5 in ATDC5
chondrocytes with TNF-α (10 ng/ml) and IL-1β (10 ng/ml) with or
without curcumenol (50 µM) administration. All data are
presented as the mean ± SD from three experiments.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. RT-qPCR,
reverse transcription-quantitative PCR; ADAMTS4, ADAM
metallopeptidase with thrombospondin type 1 motif 4; ADAMTS5, ADAM
metallopeptidase with thrombospondin type 1 motif 5. |
Curcumenol mitigates TNF-α and IL-1β
induced inflammation in ATDC5 cells by inhibiting the
phosphorylation of NF-κB and MAPK pathway components
To further investigate the underlying mechanisms by
which curcumenol inhibits inflammation, ATDC5 cells were treated
with various curcumenol concentrations following stimulation with
TNF-α and IL-1β. For NF-κB pathway analysis, the levels of p-IKKα,
p-P65 and p-IκBα were significantly increased 10 min after TNF-α
and IL-1β stimulation (Fig. 2A and
E). Although curcumenol had little effect on p-IKKα, it
effectively decreased the upregulation of p-P65 and p-IκBα.
Moreover, curcumenol increased the total inflammation-induced
protein expression level of IκBα (Fig. 2A and E). The quantification
analysis revealed a significant rescue effect of curcumenol on
p-P65 and p-IκBα, following both TNF-α (Fig. 2B-D) and IL-1β (Fig. 2F-H) stimulation.
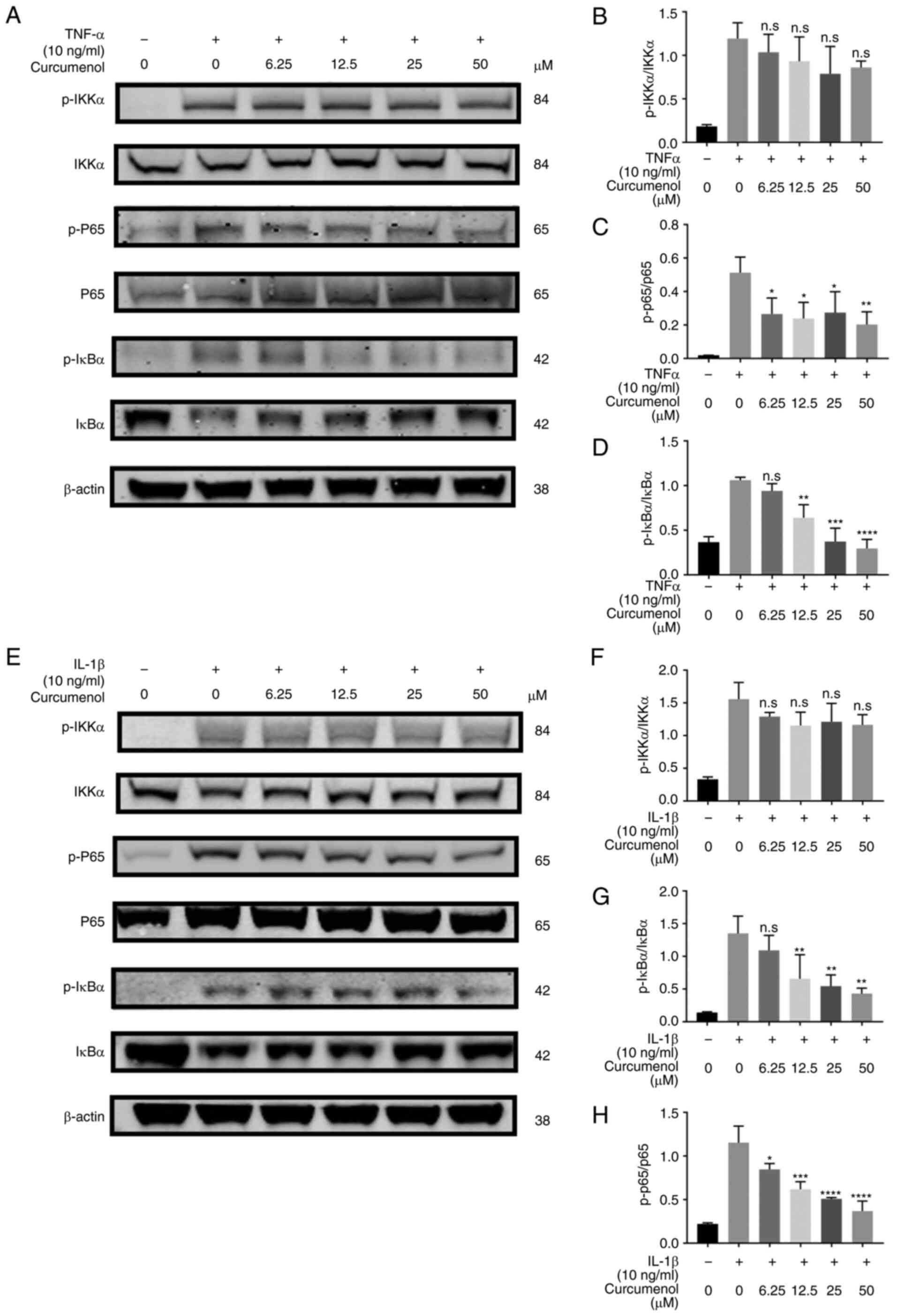 | Figure 2Curcumenol inhibits TNF-α and
IL-1β-induced phosphorylation of NF-κB pathway in ATDC5 cells.
(A-D) Western blot analysis of p-IKKα, IKKα, p-P65, P65, p-IκBα and
IκBα expression in ATDC5 chondrocytes stimulated with TNF-α (10
ng/ml) for 10 min (E-H) Western blot analysis of p-IKKα, IKKα,
p-P65, P65, p-IκBα and IκBα expression in ATDC5 chondrocytes
stimulated with IL-1β (10 ng/ml) for 10 min. Cells were pretreated
with 0, 6.25, 12.5, 25 and 50 µM curcumenol. Grey scale
values were generated using β-actin as the internal reference. All
data are presented as mean ± SD from three experiments.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. p-,
phosphorylated. |
For MAPK pathway analysis (10 min is the only
timepoint used in this study), curcumenol effectively inhibited the
phosphorylation of SAPK/JNK, but showed a minimal inhibitory effect
on ERK and P38 phosphorylation in a short time period (Figs. S1A and S1C). Quantification also
revealed a significant rescue effect of curcumenol on p-SAPK/JNK,
but not on p-ERK/ERK and p-p38/p38 (Fig. S1B and S1D). Based on
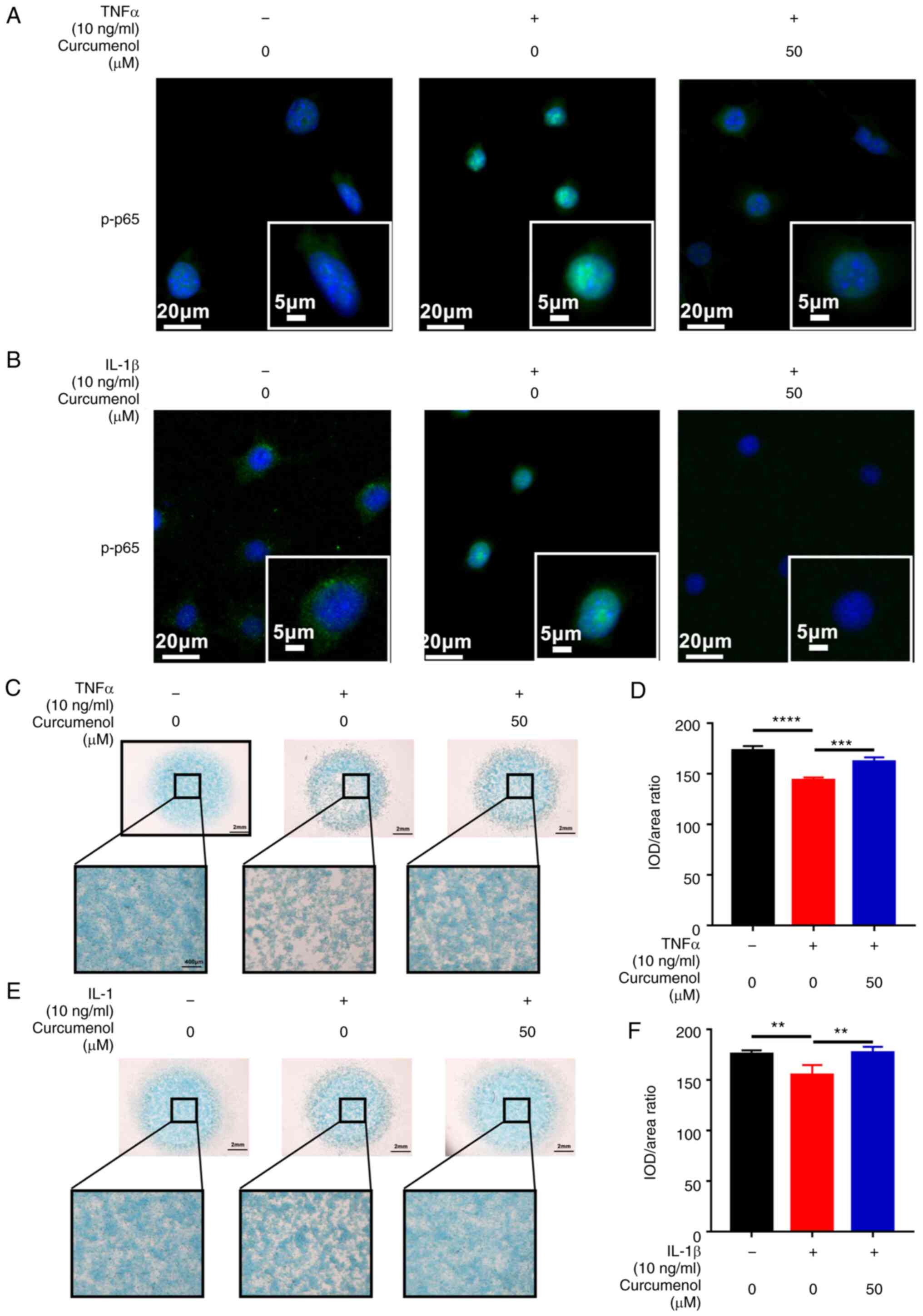
immunofluorescence analysis, after stimulation with TNF-α and
IL-1β, P65 was phosphorylated and translocated into the cell
nucleus within 20 min, but curcumenol treatment was able to
effectively block the phosphorylation and translocation of P65
(Fig. 3A and B). The results
indicated that in ATDC5 chondrocytes, curcumenol exerted an
inhibitory effect on NF-κB and MAPK pathway activation induced by
TNF-α and IL-1β.
Curcumenol modifies TNF-α and
IL-1β-induced catabolic status in high-density culture and pellet
culture
The dynamic status between catabolism and metabolism
was analyzed using alcian blue staining of high-density and pellet
cultures. ATDC5 chondrocytes were cultured at high-density culture,
and TNF-α and IL-1β stimulation was found to disrupt the
extracellular matrix (ECM) of the micromass (Fig. 3C and E). However, the damage to
the ECM was significantly reversed by curcumenol treatment
(Fig. 3D and F).
In pellet culture, the ATDC5 pellets of the control
group showed abundant ECM, while in the TNF-α and IL-1β groups, the
pellets were shrunken and the ECM was degraded, with decreased
Safranine O staining. Moreover, curcumenol was able to rescue this
disruption, recovering the ECM to near normal status (Fig. S2A and C), with the ratio of
Safranin O-Fast Green decreased in the TNF-α and IL-1β group, and
increased in the curcumenol group (Fig. S2B and D). Therefore, curcumenol
effectively altered catabolism status following deterioration by
inflammatory cytokines, and partially rescued micromass and pellet
damage.
Curcumenol exerts an anti-inflammatory
effect on primary chondrocytes by inhibiting the NF-κB and MAPK
pathway in vitro
Considering the prospect of using curcumenol in
clinical practice, the current study aimed to isolate mouse primary
chondrocytes to further confirm the anti-inflammatory function of
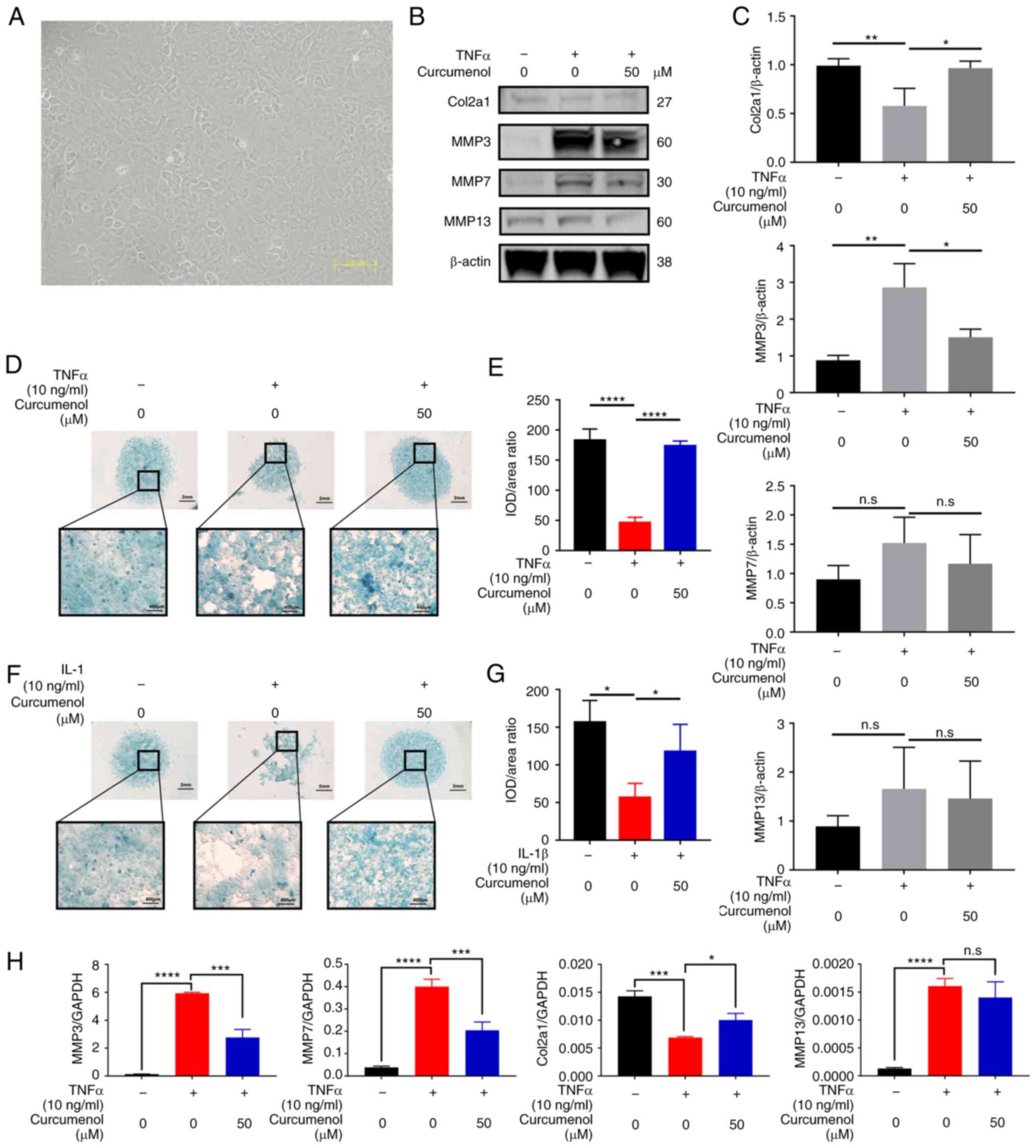
curcumenol (Fig. 4A). Curcumenol
effectively mitigated the ECM degradation induced by TNF-α and
IL-1β (Fig. 4D-G). Moreover, the
TNF-α- and IL-1β-induced senescence of primary chondrocytes was
rescued by curcumenol treatment (Fig. S3A-D). Following stimulation with
inflammatory cytokines, the number of cells stained with β-gal
(blue stain) increased, suggesting that these cells aged under
stress; however, with curcumenol treatment number of aging cells
decreased compared with the TNF-α or IL-1β group. The results
demonstrated that TNF-α stimulation activated the classical
inflammation (NF-κB and MAPK) pathways, and immediately increased
the phosphorylation of IKKα, P65, IκBα, Akt, SAPK/JNK, P44/P42 and
P38. However, after pre-treatment with curcumenol, the
phosphorylation of P65, IκBα and SAPK/JNK was significantly
inhibited (Fig. S3E and F).
Furthermore, the degradation of IκBα was rescued by curcumenol
treatment (Fig. S3E). After
these pathways were inhibited with curcumenol, the catabolic MMP
family genes were similarly downregulated compared with the
inflammation-induced cells alone (Fig. 4H), as shown by the reduced
expression of MMP3, MMP7 and MMP13 (Fig. 4B and C), which further confirmed
the current hypothesis. With regards to the chondrogenic marker
Col2a1, curcumenol significantly rescued its downregulation
following inflammatory stimulation (Fig. 4B, C and H). Based on the
aforementioned results, curcumenol was not only functional in ATDC5
cells, but also exerted anti-inflammatory effects in primary
chondrocytes.
Curcumenol alleviates DMM-induced OA in
mice by inhibiting TNF-α expression
In addition to its preventative effects in
vitro, curcumenol also significantly inhibited the
phosphorylation of P65, IκBα and SAPK/JNK (Fig. S4A and B). Thus, to further
investigate the possibility of clinical curcumenol use, a
DMM-induced OA model was established in mice, and
intraperitoneally-injected curcumenol was used to mitigate this
degeneration (the exact number of mice in every group was 6;
however, during the research, 1 mouse was lost from the control
group, and 1 was removed from each of the other two groups to
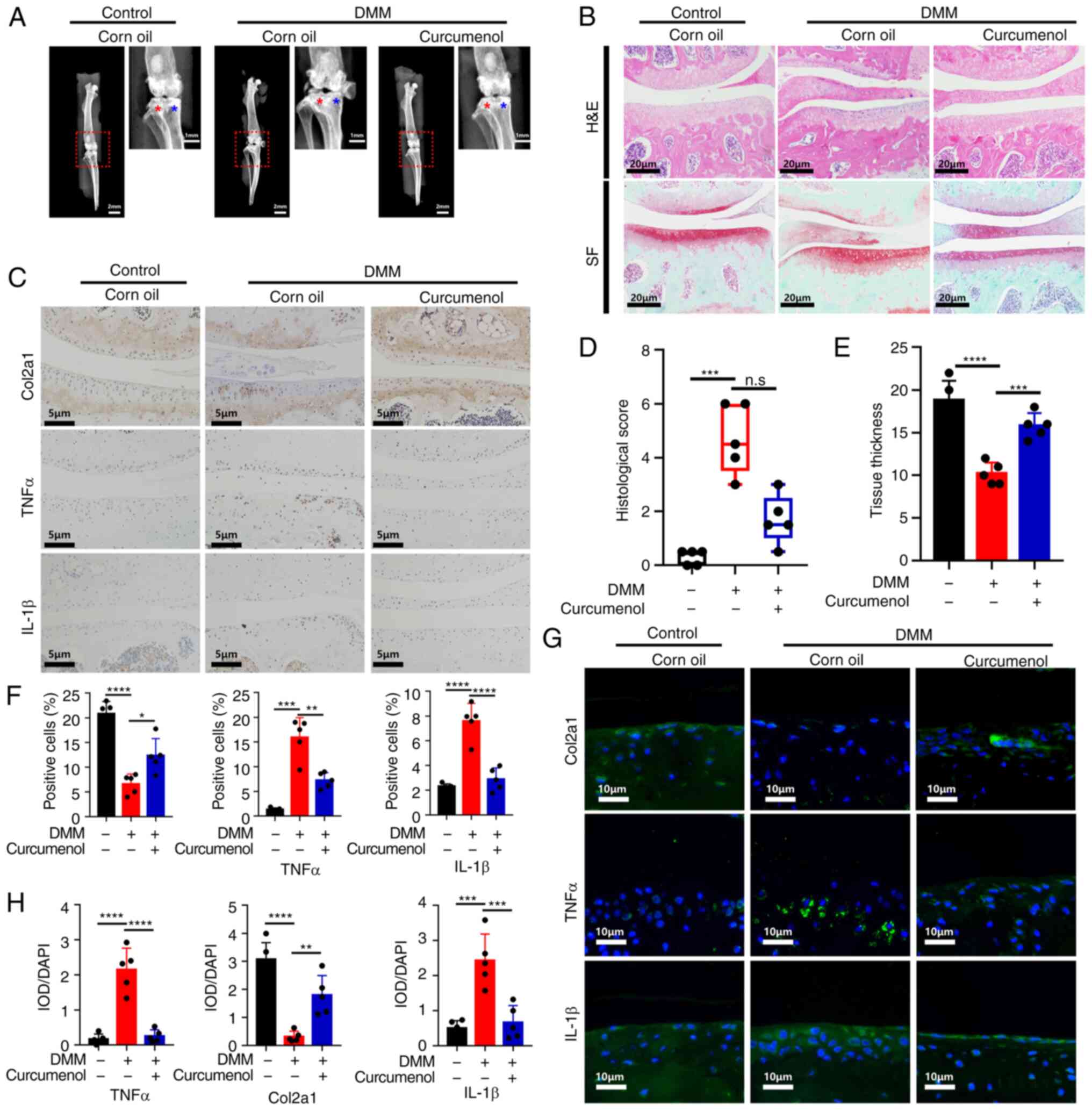
equalize the numbers). As presented in Fig. 5A, DMM-induced OA was severe, with
additional osteophyte formation and the collapse of the joint
space. As shown by the H&E and Safranine O-Fast green staining,
curcumenol prevented further degeneration of the cartilage
surrounding the tibia and femur (Fig. 5B). Cartilage tissue thickness was
also significantly restored (Fig.
5E), and the OARIS histological score was decreased by
curcumenol compared with the DMM group (Fig. 5D). The immunohistochemistry
results demonstrated that TNF-α and IL-1β expression was activated
in the DMM group, but that the levels of these inflammatory
cytokines were decreased by curcumenol treatment. By contrast,
Col2a1 was decreased in the DMM group, but was recovered by
curcumenol treatment (Fig. 5C and
G). In line with the immunohistochemistry results,
immunofluorescence revealed an increase in TNFα and IL-1β, and a
decrease in Col2a1 in the DMM group, which were decreased by
curcumenol treatment (Fig. 5F and
H). Collectively, the results indicated that curcumenol exerted
an inhibitory effect on inflammatory cascades such as the NF-κB and
MAPK pathways in vitro, and was involved in the rescue of
DMM-induced osteoarthritis in vivo.
Discussion
OA, associated with age-related degeneration, immune
reactivity and trauma, affects an increasing number of individuals
worldwide, and leads to suboptimal health status and disability
(25). At present, the primary
treatment for OA is surgical intervention, which aims to achieve
symptomatic relief, and while this treatment is effective as an
end-stage choice, it can also be traumatic and with numerous
side-effects (26). Thus, OA
lacks early and mid-term treatment options to ameliorate and cure
this chronic disease.
Non-steroidal anti-inflammatory drugs, and some
analgesics, are the conventional medicines used to manage OA
(27,28), but the side-effects of these
drugs, such as hepatic damage and gastrointestinal injury, are
concerns for their long-term usage (29). Due their reduced side-effects,
abundant production capacities and anti-inflammatory functions,
plant-derived traditional medicines have gained increasing
attention in previous years (30-32). Therefore, the present study
investigated whether traditional medicines could be used to
ameliorate the symptoms and slow the progression of OA.
Curcumenol is a bioactive compound isolated from
edible rhizome of Curcuma zedoaria, with potential
anti-inflammatory effects (33).
Curcumenol belongs to the Curcuma genus, and one of the most
well-known members is Curcuma longa, of which the bioactive
extraction curcumin is used to treat synovitis experienced by
patients with knee OA (34).
Considering the broad application range of the Curcuma
genus, and the role of proinflammatory cytokines such as TNF-α and
IL-1β in the pathophysiology of OA (35), the current study aimed to
investigate the anti-inflammatory effects of curcumenol to treat
TNF-α- and IL-1β-induced inflammation in ATDC5 and primary
chondrocytes. The present results also demonstrated that curcumenol
ameliorated the effects of OA in the knee joints of DMM-model mice
in vivo.
DMM surgery was used to generate a mechanical OA
model. During joint degeneration, especially that of the cartilage,
the levels of some inflammatory cytokines, (including TNF-α and
IL-1β) are increased, and subsequently cause a progressive,
cell-mediated cascade of molecular and structural deterioration
(36,37). TNF-α and IL-1β exert their
detrimental functions via multiple important intracellular
cascades, such as the NF-κB and MAPK pathways (38,39), In general, TNF-α and IL-1β
transmit inflammatory signals via their receptors and activate the
phosphorylation of the IKK complex (40), which then phosphorylates IκBα
(41). Subsequently, IκBα is
unbound and phosphorylates NF-κB, which translocates into the
nucleus to initiate the transcription of inflammatory products,
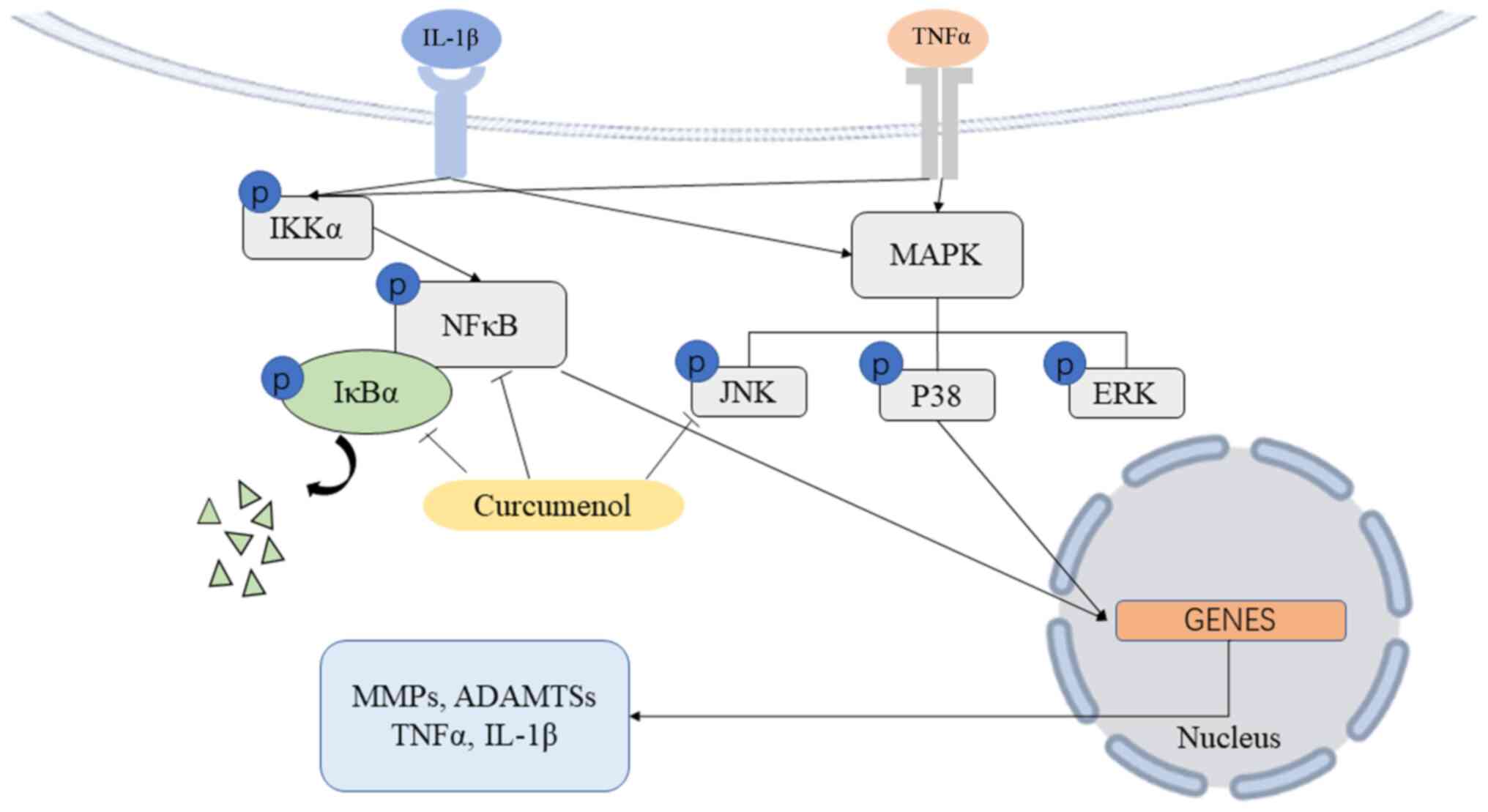
catabolic enzymes and apoptotic mediators (42-45). Curcumenol blocks the
phosphorylation and translocation of NF-κB, and inhibits the
phosphorylation of IκBα, to block the anti-inflammatory function.
With regards to the MAPK pathway, curcumenol prevents the
phosphorylation of JNK, which belongs to a large family of
serine/threonine kinases and crosslinks with numerous developmental
pathways, such as Hippo signaling (46), promoting proteoglycan metabolism
and inhibiting the production of catabolic enzymes and inflammatory
mediators in chondrocytes (47)
and the nucleus pulposus (48).
After TNF-α stimulation, the expression levels of MMPs are
increased and the production of ECM is inhibited, which promotes
the ECM to change to a catabolic and degradable status (49,50). This imbalance causes the
senescence of primary chondrocytes, followed by instability and
cartilage loss in joints (51).
In the present study, curcumenol was used to successfully inhibit
the phosphorylation of IκBα, NF-κB P65 subunit and SAPK/JNK
(Fig. 6). At the same time, the
nuclear translocation of p-P65 was blocked, which lead to a
subsequent decrease in MMP expression and the rescue of type 2
collagen in ATDC5 and primary chondrocytes. Thus, it was suggested
that inhibiting the function of these cytokines may be beneficial
in the DMM-induced OA model.
The DMM-induced OA model in mice is an effective
animal model first established in 2007 (21), whereby removing the medial
meniscus can pathologically disrupt the stability status of normal
knee joints, which can restrict the range of motion (52). Using the widely recognized
DMM-induced model, the present study demonstrated the rescue effect
of curcumenol in OA of the knee joint, as it effectively mitigated
inflammation in the cartilage of the tibia and femur, as well as
preventing joint space collapse and osteophyte formation. The
mechanism underlying this damage was consistent with the in
vitro data. The immunohistochemistry results revealed an
increase in TNF-α and IL-1β expression in the DMM group, which
triggered the subsequent detrimental molecular cascade and
degeneration. In the curcumenol-treated group, TNF-α and IL-1β
expression was downregulated and Col2a1 expression was restored,
demonstrating the curative effect of curcumenol in DMM-induced OA
in vivo. Another important consideration is how to
reconstitute and administer curcumenol, as it is insoluble in
water. Therefore, DMSO was used in the present study to
pre-dissolve curcumenol, which was then diluted in
non-pharmaceutical grade corn oil, which showed little adverse
effect during intraperitoneal injection in mice (53,54). With regard to the administration
method, intraperitoneal injection was selected rather than
intraarticular administration, based on the following: i)
Intraperitoneal injection of corn oil is widely used in mouse
models and the only administration method of curcumenol in mice
appears to be intraperitoneal injection (55); and ii) there is a possibility of
cartilage injury (56,57) and difficulties associated with
injecting corn oil via multiple micro-injections into the knee
joint.
The current study confirmed the efficiency and
safety of curcumenol, but there are still some concerns and
limitations to our studies. For efficiency, the systemic
distribution of curcumenol after intraperitoneal injection was not
assessed, thus there was a lack of direct evidence that the optimal
concentration of curcumenol reached the joint. In subsequent
studies, the concentration of curcumenol in in the blood will first
be assessed, and then preliminary investigations of curcumenol
distribution in the knee joint tissues will be conducted. For
safety, although the intraperitoneal injection of curcumenol is
relatively safe with corn oil (53), treatment-induced injury was not
evaluated. In subsequent studies, relevant experiments will be
conducted, such as the evaluation of paraffin sections of lung,
liver, heart, kidney and spleen, to further confirm the safety of
curcumenol in vivo. Furthermore, the administration method
was via intraperitoneal injection, which in clinical use, may be
more difficult when treating patients. Therefore, the efficiency
and safety of oral administration in DMM-induced osteoarthritis or
type II collagen (UC-II) diminished deterioration of articular
cartilage will be investigated in mice (58,59).
In conclusion, the current study presented a novel
plant-derived bioactive medicine, curcumenol, which was
demonstrated to serve as a potential anti-inflammatory agent for
the management of OA. The low cytotoxicity, reduced side-effects
and high production capacity are also considerable advantages of
future curcumenol use in the clinic.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and TZ guided the study, making substantial
contributions to conception and design. XY and YZ performed the
experiments. XY, YZ, CH and XL interpreted the data and drafted the
manuscript. KZ, ZC and CC performed the statistical analysis and
reviewed the manuscript critically for important intellectual
content. HT and XC confirmed the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and informed consent
Animal Ethics approval was received from the
Institutional Animal Ethics Review Board of Shanghai Ninth People's
Hospital, Shanghai Jiao Tong University School of Medicine
(approval no. SH9H-2020-A559-1). Written informed consent was
obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81871790, 81572768
and 81972136) and the fundamental research program funding of Ninth
People's Hospital Affiliated to Shanghai Jiao Tong university
School of Medicine (grant no. JYZZ003G).
Abbreviations:
|
OA
|
osteoarthritis
|
|
DMM
|
destabilization of medial meniscus
|
|
ECM
|
extracellular matrix
|
References
|
1
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z, Huang C, Jiang Q, Zheng Y, Liu Y,
Liu S, Chen Y, Mei Y, Ding C, Chen M, et al: Guidelines for the
diagnosis and treatment of osteoarthritis in China (2019 edition).
Ann Transl Med. 8:12132020. View Article : Google Scholar
|
|
3
|
Hiligsmann M, Cooper C, Arden N, Boers M,
Branco JC, Luisa Brandi M, Bruyère O, Guillemin F, Hochberg MC,
Hunter DJ, et al: Health economics in the field of osteoarthritis:
An expert's consensus paper from the European society for clinical
and economic aspects of osteoporosis and osteoarthritis (ESCEO).
Semin Arthritis Rheum. 43:303–313. 2013. View Article : Google Scholar
|
|
4
|
Agricola R, Heijboer MP, Roze RH, Reijman
M, Bierma-Zeinstra SMA, Verhaar JAN, Weinans H and Waarsing JH:
Pincer deformity does not lead to osteoarthritis of the hip whereas
acetabular dysplasia does: Acetabular coverage and development of
osteoarthritis in a nationwide prospective cohort study (CHECK).
Osteoarthritis Cartilage. 21:1514–1521. 2013. View Article : Google Scholar
|
|
5
|
Tsuchiya H, Ota M, Sumitomo S, Ishigaki K,
Suzuki A, Sakata T, Tsuchida Y, Inui H, Hirose J, Kochi Y, et al:
Parsing multiomics landscape of activated synovial fibroblasts
highlights drug targets linked to genetic risk of rheumatoid
arthritis. Ann Rheum Dis. Nov 2–2020.Epub ahead of print.
|
|
6
|
Myers J, Wielage RC, Han B, Price K, Gahn
J, Paget MA and Happich M: The efficacy of duloxetine,
non-steroidal anti-inflammatory drugs, and opioids in
osteoarthritis: A systematic literature review and meta-analysis.
BMC Musculoskelet Disord. 15:762014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McAlindon TE, Bannuru RR, Sullivan MC,
Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y,
Hunter DJ, Kawaguchi H, et al: OARSI guidelines for the
non-surgical management of knee osteoarthritis. Osteoarthritis
Cartilage. 22:363–388. 2014. View Article : Google Scholar
|
|
8
|
Pas HI, Winters M, Haisma HJ, Koenis MJ,
Tol JL and Moen MH: Stem cell injections in knee osteoarthritis: A
systematic review of the literature. Br J Sports Med. 51:1125–1133.
2017. View Article : Google Scholar
|
|
9
|
Hached F, Vinatier C, Le Visage C, Gondé
H, Guicheux J, Grimandi G and Billon-Chabaud A:
Biomaterial-assisted cell therapy in osteoarthritis: From
mesenchymal stem cells to cell encapsulation. Best Pract Res Clin
Rheumatol. 31:730–745. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Liao T, Wan C, Yang X, Zhao J, Fu
R, Yao Z, Huang Y, Shi Y, Chang G, et al: Efficient generation of
human primordial germ cell-like cells from pluripotent stem cells
in a methylcellulose-based 3D system at large scale. PeerJ.
6:e61432019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hikino H, Sakurai Y, Numabe S and Takemoto
T: Structure of curcumenol. Chem Pharm Bull (Tokyo). 16:39–42.
1968. View Article : Google Scholar
|
|
12
|
Xu J, Ji F, Kang J, Wang H, Li S, Jin DQ,
Zhang Q, Sun H and Guo Y: Absolute configurations and NO inhibitory
activities of terpenoids from curcuma longa. J Agric Food Chem.
63:5805–5812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Assis A, Brito V, Bittencourt M, Silva L,
Oliveira F and Oliveira R: Essential oils composition of four Piper
species from Brazil. J Essential Oil Res. 25:203–209. 2013.
View Article : Google Scholar
|
|
14
|
Saikia AK, Sarma SK, Strano T and Ruberto
G: Essential oil from piper pedicellatum C. DC. Collected in
North-East India. J Essential Oil Bearing Plants. 18:314–319. 2015.
View Article : Google Scholar
|
|
15
|
Sun DX, Fang ZZ, Zhang YY, Cao YF, Yang L
and Yin J: Inhibitory effects of curcumenol on human liver
cytochrome P450 enzymes. Phytother Res. 24:1213–1216. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pintatum A, Maneerat W, Logie E, Tuenter
E, Sakavitsi ME, Pieters L, Berghe WV, Sripisut T, Deachathai S and
Laphookhieo S: In Vitro anti-inflammatory, anti-oxidant, and
cytotoxic activities of four species and the isolation of compounds
from rhizome. Biomolecules. 10:7992020. View Article : Google Scholar
|
|
17
|
Oh CD, Im HJ, Suh J, Chee A, An H and Chen
D: Rho-associated kinase inhibitor immortalizes rat nucleus
pulposus and annulus fibrosus cells: Establishment of
intervertebral disc cell lines with novel approaches. Spine (Plila
Pa 1976). 41:E255–E261. 2016. View Article : Google Scholar
|
|
18
|
Johnson M, Zaretskaya I, Raytselis Y,
Merezhuk Y, McGinnis S and Madden TL: NCBI BLAST: A better web
interface. Nucleic Acids Res. 36(Web Server issue): W5–W9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Shi JW, Zhang TT, Liu W, Yang J, Lin XL,
Jia JS, Shen HF, Wang SC, Li J, Zhao WT, et al: Direct conversion
of pig fibroblasts to chondrocyte-like cells by c-Myc. Cell Death
Discov. 5:552019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Glasson SS, Blanchet TJ and Morris EA: The
surgical destabilization of the medial meniscus (DMM) model of
osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage.
15:1061–1069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang M, Sampson ER, Jin H, Li J, Ke QH, Im
HJ and Chen D: MMP13 is a critical target gene during the
progression of osteoarthritis. Arthritis Res Ther. 15:R52013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loeser RF, Kelley KL, Armstrong A, Collins
JA, Diekman BO and Carlson CS: Deletion of JNK enhances senescence
in joint tissues and increases the severity of age-related
osteoarthritis in mice. Arthritis Rheumatol. 72:1679–1688. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sohara Y, Shimada H, Scadeng M, Pollack H,
Yamada S, Ye W, Reynolds CP and DeClerck YA: Lytic bone lesions in
human neuroblastoma xenograft involve osteoclast recruitment and
are inhibited by bisphosphonate. Cancer Res. 63:3026–3031.
2003.PubMed/NCBI
|
|
25
|
Sharma L: Osteoarthritis of the knee. N
Engl J Med. 384:51–59. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thorlund JB, Juhl CB, Roos EM and
Lohmander LS: Arthroscopic surgery for degenerative knee:
Systematic review and meta-analysis of benefits and harms. BMJ.
350:h27472015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rasmussen-Barr E, Held U, Grooten WJA,
Roelofs PDDM, Koes BW, van Tulder MW and Wertli MM: Nonsteroidal
anti-inflammatory drugs for sciatica: An updated cochrane review.
Spine (Phila Pa 1976). 42:586–594. 2017. View Article : Google Scholar
|
|
28
|
Derry S, Wiffen PJ, Kalso EA, Bell RF,
Aldington D, Phillips T, Gaskell H and Moore RA: Topical analgesics
for acute and chronic pain in adults-an overview of cochrane
reviews. Cochrane Database Syst Rev. 5:CD0086092017.
|
|
29
|
Marmon P, Owen SF and Margiotta-Casaluci
L: Pharmacology-informed prediction of the risk posed to fish by
mixtures of non-steroidal anti-inflammatory drugs (NSAIDs) in the
environment. Environ Int. 146:1062222021. View Article : Google Scholar :
|
|
30
|
Chen J, Xuan J, Gu YT, Shi KS, Xie JJ,
Chen JX, Zheng ZM, Chen Y, Chen XB, Wu YS, et al: Celastrol reduces
IL-1β induced matrix catabolism, oxidative stress and inflammation
in human nucleus pulposus cells and attenuates rat intervertebral
disc degeneration in vivo. Biomed Pharmacother. 91:208–219. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Deng SJ, Zhang Z, Gu Y, Xia SN, Bao
XY, Cao X and Xu Y: 6-Gingerol attenuates microglia-mediated
neuroinflammation and ischemic brain injuries through
Akt-mTOR-STAT3 signaling pathway. Eur J Pharmacol. 883:1732942020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Lin S, Liu P, Huang J, Qiu J, Wen Z,
Yuan J, Qiu H, Liu Y, Liu Q, et al: Carnosol suppresses
RANKL-induced osteoclastogenesis and attenuates titanium
particles-induced osteolysis. J Cell Physiol. 236:1950–1966. 2021.
View Article : Google Scholar
|
|
33
|
Lo JY, Kamarudin MNA, Hamdi OAA, Awang K
and Kadir HA: Curcumenol isolated from curcuma zedoaria suppresses
Akt-mediated NF-κB activation and p38 MAPK signaling pathway in
LPS-stimulated BV-2 microglial cells. Food Funct. 6:3550–3559.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Jones G, Winzenberg T, Cai G,
Laslett LL, Aitken D, Hopper I, Singh A, Jones R, Fripp J, et al:
Effectiveness of extract for the treatment of symptoms and
effusion-synovitis of knee osteoarthritis : A randomized trial. Ann
Intern Med. 173:861–869. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar
|
|
36
|
Rodriguez-Trillo A, Mosquera N, Pena C,
Rivas-Tobío F, Mera-Varela A, Gonzalez A and Conde C: Non-Canonical
WNT5A signaling through RYK contributes to aggressive phenotype of
the rheumatoid fibroblast-like synoviocytes. Front Immunol.
11:5552452020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao X, Meng F, Hu S, Yang Z, Huang H,
Pang R, Wen X, Kang Y and Zhang Z: The synovium attenuates
cartilage degeneration in KOA through activation of the
Smad2/3-Runx1 cascade and chondrogenesis-related miRNAs. Mol Ther
Nucleic Acids. 22:832–845. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moqbel SAA, Xu K, Chen Z, Xu L, He Y, Wu
Z, Ma C, Ran J, Wu L and Xiong Y: Tectorigenin alleviates
inflammation, apoptosis, and ossification in rat tendon-derived
stem cells modulating NF-Kappa B and MAPK pathways. Front Cell Dev
Biol. 8:5688942020. View Article : Google Scholar
|
|
40
|
Zhang Q, Lenardo MJ and Baltimore D: 30
Years of NF-κB: A blossoming of relevance to human pathobiology.
Cell. 168:37–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhongyi S, Sai Z, Chao L and Jiwei T:
Effects of nuclear factor kappa B signaling pathway in human
intervertebral disc degeneration. Spine (Phila Pa 1976).
40:224–232. 2015. View Article : Google Scholar
|
|
42
|
Baldwin AS: The NF-kappa B and I kappa B
proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun Z, Yin Z, Liu C, Liang H, Jiang M and
Tian J: IL-1β promotes ADAMTS enzyme-mediated aggrecan degradation
through NF-κB in human intervertebral disc. J Orthop Surg Res.
10:1592015. View Article : Google Scholar
|
|
44
|
Tu J, Li W, Zhang Y, Wu X, Song Y, Kang L,
Liu W, Wang K, Li S, Hua W and Yang C: Simvastatin inhibits
IL-1β-induced apoptosis and extracellular matrix degradation by
suppressing the NF-kB and MAPK pathways in nucleus pulposus cells.
Inflammation. 40:725–734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen J, Garssen J and Redegeld F: The
efficacy of bortezomib in human multiple myeloma cells is enhanced
by combination with omega-3 fatty acids DHA and EPA: Timing is
essential. Clin Nutr. 40:1942–1953. 2021. View Article : Google Scholar
|
|
46
|
Pham TH, Hagenbeek TJ, Lee HJ, Li J, Rose
CM, Lin E, Yu M, Martin SE, Piskol R, Lacap JA, et al: Machine
learning and chemico-genomics approach defines and predicts
cross-talk of Hippo and MAPK pathways. Cancer Discov. 11:778–793.
2021. View Article : Google Scholar
|
|
47
|
Cao C, Wu F, Niu X, Hu X, Cheng J, Zhang
Y, Li C, Duan X, Fu X, Zhang J, et al: Cadherin-11 cooperates with
inflammatory factors to promote the migration and invasion of
fibroblast-like synoviocytes in pigmented villonodular synovitis.
Theranostics. 10:10573–10588. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Séguin CA, Bojarski M, Pilliar RM,
Roughley PJ and Kandel RA: Differential regulation of matrix
degrading enzymes in a TNFalpha-induced model of nucleus pulposus
tissue degeneration. Matrix Biol. 25:409–418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang G, Chen S, Xie Z, Shen S, Xu W, Chen
W, Li X, Wu Y, Li L, Liu B, et al: TGFβ attenuates cartilage
extracellular matrix degradation via enhancing FBXO6-mediated MMP14
ubiquitination. Ann Rheum Dis. 79:1111–1120. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He L, He T, Xing J, Zhou Q, Fan L, Liu C,
Chen Y, Wu D, Tian Z, Liu B and Rong L: Bone marrow mesenchymal
stem cell-derived exosomes protect cartilage damage and relieve
knee osteoarthritis pain in a rat model of osteoarthritis. Stem
Cell Res Ther. 11:2762020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Inoue R, Ishibashi Y, Tsuda E, Yamamoto Y,
Matsuzaka M, Takahashi I, Danjo K, Umeda T, Nakaji S and Toh S:
Knee osteoarthritis, knee joint pain and aging in relation to
increasing serum hyaluronan level in the Japanese population.
Osteoarthritis Cartilage. 19:51–57. 2011. View Article : Google Scholar
|
|
52
|
Lamo-Espinosa JM, Blanco JF, Sánchez M,
Moreno V, Granero-Moltó F, Sánchez-Guijo F, Crespo-Cullel I, Mora
G, Vicente DDS, Pompei-Fernández O, et al: Phase II multicenter
randomized controlled clinical trial on the efficacy of
intra-articular injection of autologous bone marrow mesenchymal
stem cells with platelet rich plasma for the treatment of knee
osteoarthritis. J Transl Med. 18:3562020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hubbard JS, Chen PH and Boyd KL: Effects
of repeated intraperitoneal injection of pharmaceutical-grade and
nonpharmaceutical-grade corn oil in female C57BL/6J mice. J Am
Assoc Lab Anim Sci. 56:779–785. 2017.PubMed/NCBI
|
|
54
|
Alsina-Sanchis E, Mülfarth R, Moll I,
Mogler C, Rodriguez-Vita J and Fischer A: Intraperitoneal oil
application causes local inflammation with depletion of resident
peritoneal macrophages. Mol Cancer Res. 19:288–300. 2021.
View Article : Google Scholar
|
|
55
|
Wang S, Ma Q, Xie Z, Shen Y, Zheng B,
Jiang C, Yuan P, An Q, Fan S and Jie Z: An antioxidant
sesquiterpene inhibits osteoclastogenesis via blocking IPMK/TRAF6
and counteracts OVX-induced osteoporosis in mice. J Bone Miner Res.
May 6–2021.Epub ahead of print. View Article : Google Scholar
|
|
56
|
Kompel AJ, Roemer FW, Murakami AM, Diaz
LE, Crema MD and Guermazi A: Intra-articular corticosteroid
injections in the hip and knee: Perhaps not as safe as we thought?
Radiology. 293:656–663. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mehta PN and Ghadially FN: Articular
cartilage in corn oil-induced lipoarthrosis. Ann Rheum Dis.
32:75–82. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bagi CM, Berryman ER, Teo S and Lane NE:
Oral administration of undenatured native chicken type II collagen
(UC-II) diminished deterioration of articular cartilage in a rat
model of osteoarthritis (OA). Osteoarthritis Cartilage.
25:2080–2090. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Runhaar J, Rozendaal RM, van Middelkoop M,
Bijlsma HJW, Doherty M, Dziedzic KS, Lohmander LS, McAlindon T,
Zhang W and Zeinstra SB: Subgroup analyses of the effectiveness of
oral glucosamine for knee and hip osteoarthritis: A systematic
review and individual patient data meta-analysis from the OA trial
bank. Ann Rheum Dis. 76:1862–1869. 2017. View Article : Google Scholar : PubMed/NCBI
|